Abstract
Extrinsic factors can adversely affect immune responses, producing states of secondary immunodeficiency and consequent increased risk of infections. These immunodeficiencies, which can be encountered in routine clinical practice, arise from a number of conditions, such as treatment with glucocorticoids and immunomodulatory drugs, surgery and trauma, extreme environmental conditions, and chronic infections, such as those caused by HIV. The most common cause of immunodeficiency is malnutrition, affecting many communities around the world with restricted access to food resources. Protein-calorie deficiency and micronutrient deficiencies have been shown to alter immune responses; of note, recent progress has been made in the influence of vitamin D deficiency in causing failure of immune activation. Other categories of disease that might present with secondary immunodeficiency include metabolic diseases and genetic multisystemic syndromes. The immune defects observed in secondary immunodeficiency are usually heterogeneous in their clinical presentation, and their prognosis depends on the severity of the immune defect. Management of the primary condition often results in improvement of the immunodeficiency; however, this is sometimes not possible, and the risk of infections can be reduced with prompt antimicrobial treatment and prophylaxis.
Keywords: Secondary immunodeficiency, immunosuppression, lymphopenia, AIDS
Secondary immunodeficiencies are far more common than primary immunodeficiencies, which are, by definition, caused by genetic defects affecting cells of the immune system.1 Secondary immunodeficiencies result from a variety of factors that can affect a host with an intrinsically normal immune system, including infectious agents, drugs, metabolic diseases, and environmental conditions. These deficiencies of immunity are clinically manifested by an increased frequency or unusual complications of common infections and occasionally by the occurrence of opportunistic infections (Fig 1). The secondary immunodeficiencies have a wide spectrum of presentation, depending on the magnitude of the offending external condition and on the host susceptibility. For example, the immunodeficiency induced by the use of corticosteroids and other immunosuppressive drugs depends on the dose used2,3 and, to a lesser degree, on concomitant disease processes of the host, such as the presence of sepsis. AIDS, resulting from infection by HIV, is the best known secondary immunodeficiency largely because of its prevalence and its high mortality rate if not treated. However, the most common immunodeficiency worldwide results from severe malnutrition, affecting both innate and adaptive immunity.4 The restoration of immunity in secondary immunodeficiencies is generally achieved with the management of the primary condition or the removal of the offending agent. We summarize reports of immune defects occurring in a variety of clinical scenarios (Table I), with special emphasis on HIV infection. We selected diseases and conditions based on their frequent presentation in general medical practice and their relevance for allergists and immunologists. We do not discuss immunomodulating mAbs and fusion proteins, which are covered in Chapter 28 of this primer.5
FIG 1.
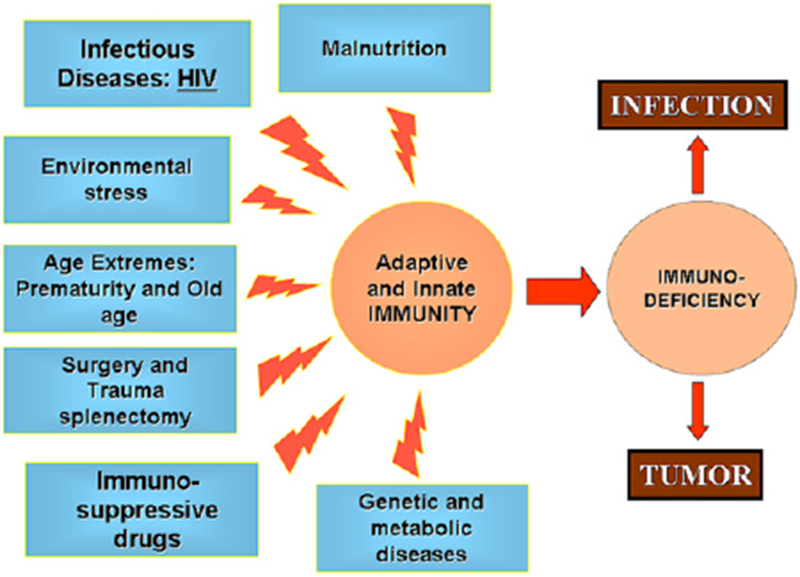
Extrinsic factors leading to defects of immune function.
TABLE I.
Selected causes of secondary immunodeficiencies
| Condition | Effect on immune function |
|---|---|
| Extremes of age | |
| Newborn period | Immature lymphoid organs Absent memory immunity Low maternal IgG levels in premature infants Decreased neutrophil storage pool Decreased neutrophil function Decreased natural killer activity |
| Advanced age | Decreased antigen-specific cellular immunity T-cell oligoclonality Restricted B-cell repertoire |
| Malnutrition | Decreased cellular immune response Weakened mucosal barriers |
| Metabolic diseases | |
| Diabetes mellitus | Decreased mitogen-induced lymphoproliferation Defective phagocytosis Decreased chemotaxis |
| Chronic uremia | Decreased cellular immune response Decreased generation of memory antibody responses Decreased chemotaxis |
| Genetic syndromes: trisomy 21 | Defective phagocytosis Defective chemotaxis Variable defects of antigen-specific immune responses |
| Anti-inflammatory, immunomodulatory, and immunosuppressive drug therapy: corticosteroids, calcineurin inhibitors, cytotoxic agents | Lymphopenia Decreased cellular immune response and anergy Decreased proinflammatory cytokines Decreased phagocytosis Decreased chemotaxis Neutropenia (cytotoxic agents) Weakened mucosal barriers (cytotoxic agents) |
| Surgery and trauma | Disruption of epithelial and mucosal barriers T-cell anergy caused by nonspecific immune activation |
| Environmental conditions UV light, radiation, hypoxia, space Flight | Increased lymphocyte apoptosis Increased secretion of tolerogenic cytokines Cytopenias Decreased cellular immunity and anergy Stress-induced nonspecific immune activation |
| Infectious diseases: HIV infection | T-cell lymphopenia Decreased cellular immune response and anergy Defective antigen-specific antibody responses |
EXTREMES OF AGE: NEWBORN PERIOD AND ADVANCED AGE
Newborn period
Neonates have an increased susceptibility to common and opportunistic infections and sepsis compared with older children.6 There is an inverse association of infection susceptibility and the age of prematurity. In early life there are fewer marginal-zone B cells in lymphoid tissue and a decreased expression of CD21 on B cells, thus limiting the ability of B cells to develop specific responses.7 Although they can develop humoral responses to some antigens after exposure in utero, impaired immunity in newborns can be attributed to the relative lack of maturity of secondary lymphoid organs, including the lymphoid tissue associated to mucosa in the gastrointestinal and respiratory tracts. This immaturity is related to the absence of memory cell development because of the relative isolation provided by the maternal environment. In addition, premature infants are more vulnerable to infections because of the absence of maternal IgG transfer before 32 weeks of gestational age. Other significant recent observations described at this early age are related to innate immunity mechanisms, such as a decreased neutrophil storage pool, as defined by the ability of neutrophilia to develop in response to an infection; decreased in vitro neutrophil functions (ie, phagocytosis, oxidative burst, chemotaxis, and adhesion); capacity to develop a neutrophil extracellular trap8; decreased natural killer cell activity; decreased Toll-like receptor signaling; decreased production of cytokines; and reduced complement components.
Advanced age
Among the elderly, some subjects experience malignancies and an excessive number of infections caused by viruses and bacteria, reflecting a decrease in the immune defenses, particularly in the cellular compartment. Decreased delayed-type hypersensitivity skin reactions and decreased lymphocyte proliferative responses to mitogens can be demonstrated in this patient population. This relative impairment of the immune response has been linked to the development of T-cell oligoclonality together with a limited capacity of the thymus to generate naive T cells and therefore reduced responses to new antigens. Oligoclonal expansion of CD8+ T cells begins in the seventh decade of life, which results in the skewing of the T-cell repertoire and an increased number of differentiated memory CD8+ T cells.9 Advanced age is similarly associated with a restricted B-cell diversity repertoire and a limited response to vaccines; however, there is also an increased number of total memory B cells and increased total IgG levels. The innate immunity might be compromised in the elderly, with increased breakdown of skin and mucosal barriers and slow healing processes caused by metabolic and endocrinologic changes associated with aging. A diminished production of hematopoietic growth factors has been postulated to occur in the elderly, resulting in decreased ability to upregulate the production and function of macrophages and neutrophils.10 Some subjects are at higher risk of infections when these immunologic defects are combined with other environmental factors, such as malnutrition or the concomitant presence of chronic inflammation caused by autoimmunity or persistent infections.11 Progress in understanding the aging-associated immune defect is of importance to optimize protective immunity against preventable infectious diseases.12
MALNUTRITION
Worldwide, protein-calorie malnutrition is the most common cause of immunodeficiency.13 Malnutrition can result from limited access to food sources and chronic diseases that induce cachexia, such as neoplastic diseases. Diarrhea caused by infections and respiratory tract infections are common. T-cell production and function decrease in proportion to the severity of hypoproteinemia; however, specific antibody titers and immune responses to vaccines can be detected in a malnourished subject for a relatively prolonged period. Eventually, these immune responses decrease if malnutrition persists. The deficiency of micronutrients (eg, zinc and ascorbic acid) contributes to increased susceptibility to infections through the weakening of barrier mucosa, therefore facilitating a pathogen’s invasiveness.14,15 Other essential molecules have been shown to have specific roles in the immune system; for example, vitamin D appears to be necessary in the macrophage activity against intracellular pathogens, remarkably Mycobacterium tuberculosis (Fig 2).16 Correction of the nutritional deficiencies often results in the resolution of these immunologic defects.
FIG 2.
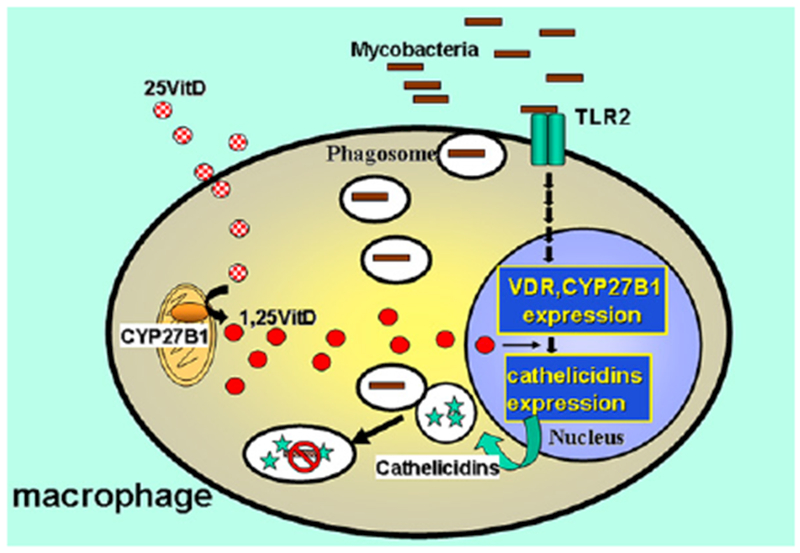
Role of vitamin D (VitD) in macrophage activation. Toll-like receptor 2 (TLR2) activation increases expression of CYP21B1, a mitochondrial enzyme that converts vitamin D into its active form, 1,25OH vitamin D, and vitamin D nuclear receptor (VDR) expression, which when bound to 1,25OH vitamin D promotes cathelicidin synthesis. Cathelicidins are intracellular bactericidal proteins.
METABOLIC DISEASES: DIABETES MELLITUS AND UREMIA
Many disease processes originating from dysfunctional metabolic pathways significantly affect the cells involved in the immune response. Diabetes mellitus and uremia resulting from kidney or liver disease are 2 common metabolic disorders with known deleterious effects on immunity. Optimal control of the metabolic abnormality usually leads to improved immune function. The defective immune functions reported in patients with diabetes mellitus include defective phagocytosis and macrophage chemotaxis in vitro, T-cell anergy demonstrated by delayed hypersensitivity skin tests, and poor lymphoproliferative response to mitogens caused by chronic exposure to hyperglycemia.17 Impaired glucose metabolism, insufficient blood supply, and denervation are other factors that contribute to the increased susceptibility to infection in patients with diabetes, who present most commonly with skin sores, bacterial and fungal respiratory tract infections, and systemic viral diseases.
Uremic patients experience increased incidence and severity of infections compared with the general population. Even when disparities in age, sex, race, and diabetes mellitus were taken into account, mortality rates in patients undergoing dialysis attributed to sepsis were higher by a factor of 100 to 300.18 The need for dialysis procedures and use of vascular devices are independent risk factors for invasive infections. Multiple defects of the innate and adaptive immunity have been described to have a role in the increased frequency of infections, summarized as immune hyporesponsiveness and a state of chronic activation. The diminished capacity to generate memory antibody responses, regardless of repeated vaccination, and defective phagocyte chemotaxis and microbicidal activity in vitro are examples of the immune defects present in uremic patients.19,20
INHERITED DEFECTS OTHER THAN PRIMARY IMMUNODEFICIENCIES
Diseases caused by genetic defects might not primarily affect the immune system, but they can present with impaired immunity to infections resulting from metabolic and cellular dysfunction, such as poor expression of adhesion molecules or defects in the DNA repair machinery. The molecular mechanisms leading to immunologic defects remain not well defined. Genetic syndromes are relatively rare, and usually only a subset of patients present with an immune defect of clinical severity that increases their risk of infections or malignancies. The disease processes caused by chromosomal number abnormalities are the most common within the genetic disorders. As an example, patients with Down syndrome or trisomy of chromosome 21 present with increased incidence of infections, although they are usually not severe, including skin abscesses, periodontitis, and upper respiratory tract infections. T- and B-cell number and function are variably affected.21 Neutrophils isolated from patients with Down syndrome have shown defects in chemotaxis and phagocytosis in vitro. Most recent studies have focused on the overexpression of the gene Down syndrome critical region 1 (DSCR1) and its role in contributing to phagocyte dysfunction.22 Patients with Turner syndrome (complete or partial absence of the second X chromosome) also have an increased number of respiratory tract infections, and hypogammaglobulinemia can be identified, although this immune defect is not consistently demonstrated in these patients. The gene defects involved in the decrease of immunoglobulin production are not known.
In other genetic diseases, such as cystic fibrosis, caused by deleterious mutations in the cystic fibrosis transmembrane conductance regulator, the increased susceptibility to sinusitis and pneumonia is explained by defective mechanisms of innate immunity.23 Patients with cystic fibrosis have an impaired airway mucous clearance caused by the thickness of the mucous secretions, which favors the development of respiratory infections caused by Pseudomonas species. It is recommended that patients receive prompt antibiotic therapy when infection is suspected, and antibiotic prophylaxis should be prescribed to those patients with recurrent infections to reduce the number of infectious episodes.
ANTI-INFLAMMATORY, IMMUNOMODULATORY, AND IMMUNOSUPPRESSIVE DRUG THERAPY
The use of drugs to ameliorate undesirable immune responses is common in clinical practice as a consequence of the increasing prevalence of inflammatory conditions. These diseases include the categories of autoimmune disorders, allergic disorders, transplant rejection, and graft-versus-host disease (GvHD). Broadly, we can study these drugs by dividing them into biologic, physical, and chemical categories. The chemical agents are the most available clinically and have in common their ability to inhibit lymphocyte proliferation and their lack of specificity for the immune response causing the particular illness of interest. Biologic immunosuppressive drugs have been developed to increase the immune specificity by targeting specific components of the immune response, such as cytokines or a particular lymphocyte subset. Physical agents (ie, UV light and ionizing radiation) can also be used to ablate immune responses.
In addition, there are drugs that might have an immunosuppressive effect that is not clearly related to the pharmacologic activity of the molecule. Its occurrence is not predictable and varies within different patient populations. Well-known examples of this drug mechanism are the development of hypogammaglobulinemia in patients receiving antiepileptic drugs and the leukopenia seen in patients taking trimethoprim-sulfamethoxazole.
Based on their structure and mechanism of action, most molecules with immunosuppressive activity can be grouped into corticosteroids, calcineurin inhibitors, and cytotoxic drugs. The adverse side effect of these drugs is that they tend to weaken the cellular immune response, rendering patients more susceptible to fungal and viral infections (acute, chronic, and reactivated).
Corticosteroids
The corticosteroids include both glucocorticoid and mineral-ocorticoid molecules. Only the glucocorticoids have significant anti-inflammatory activity. Glucocorticoids are well known for their variety of applications in both general and subspecialty medicine to reduce tissue damage caused by an excessive inflammatory response. The range of potency of the different molecules of this group and their routes of administration is diverse, each designed to different applications. For example, betamethasone is 25 times more potent than cortisol and can be used in topical, oral, and injectable preparations. Glucocorticoids bind a cytosolic receptor, which then translocates to the nucleus to act as a transcription factor affecting the expression of a number of genes, resulting in an anti-inflammatory effect (Fig 3).24 The bound complex-glucocorticoid receptor modulates signal transduction pathways, resulting in the activation of the transcription factors nuclear factor κB, nuclear factor of activated T cells, and activator protein 1. It has been suggested that glucocorticoids might also cause an effect on cell function by interacting with the cell membranes, which could explain observed clinical benefits when used as “pulse therapy,” with doses higher than required for receptor saturation. The overall results are decreased cytokine production (IL-1, IL-6, and TNF-α) and impaired leukocyte chemotaxis, cell adhesion, phagocytosis, and lymphocyte anergy. Lymphopenia occurs as a result of the proapoptotic activity and inhibition of IL-2–mediated proliferative responses. When used at large doses, antibody responses and delayed-type hypersensitivity responses are reversibly suppressed. This wide range of immune defects renders the patient susceptible to viral, bacterial, and fungal infections, according to the degree of immunosuppression and the administration route. Examples of these are oral candidiasis, a frequent complication of the use of inhaled steroids, and herpes zoster disease, which often presents with chronic use of systemic corticosteroids.
FIG 3.
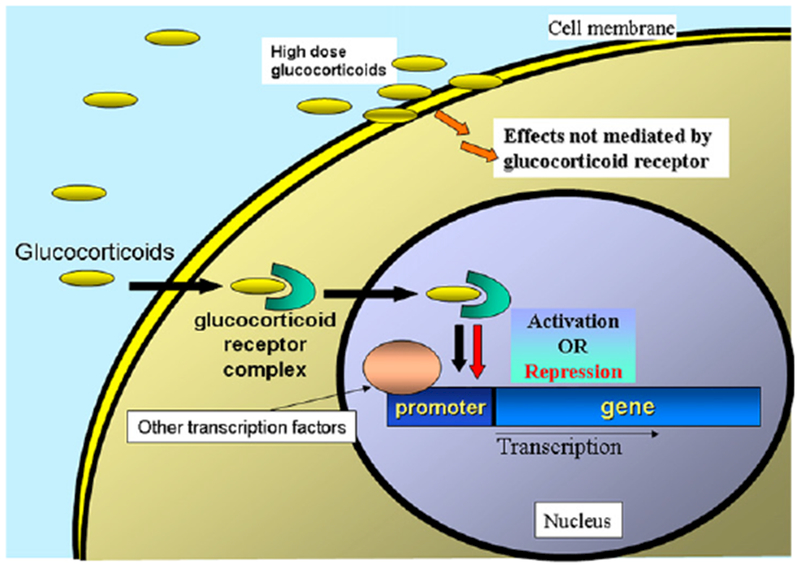
Molecular mechanism of action of glucocorticoids. A cytosolic receptor binds glucocorticoids and translocates them to the nucleus, where they either activate anti-inflammatory genes or inhibit proinflammatory genes. At high doses, corticosteroids can also affect cell function by non–receptor-dependent mechanisms.
Calcineurin inhibitors
Calcineurin inhibitors bind cytoplasmic proteins from the immunophilin family and inhibit their interaction with calcineurin, which is essential for the activation of IL-2 transcription and T-cell function (Fig 4). The advantage of these drugs over corticosteroids and cytotoxic drugs is to spare macrophage and neutrophil functions, reducing the spectrum of susceptibilities to infections. However, these drugs cause respiratory tract and skin infections, usually of viral cause, to occur with increased frequency. The most common adverse effects of calcineurin inhibitors are hypertension and renal dysfunction; less common but more serious is the increased frequency of lymphoproliferative disorders and skin neoplasias. The first drug in this category was cyclosporine, which has been extensively used to prevent organ transplant rejection,25 GvHD, and corticosteroid-resistant autoimmune disorders. Other agents with a similar mechanism of action and immune selectivity are tacrolimus and pimecrolimus. The latter is the most recent member of this group, and it was developed for topical use in the treatment of severe atopic dermatitis. An agent with a similar name, sirolimus or rapamycin, also binds an immunophilin but does not inhibit calcineurin. Instead, sirolimus inhibits the IL-2–induced response by inhibiting the mammalian target of rapamycin, a protein essential for cell activation and proliferation.
FIG 4.
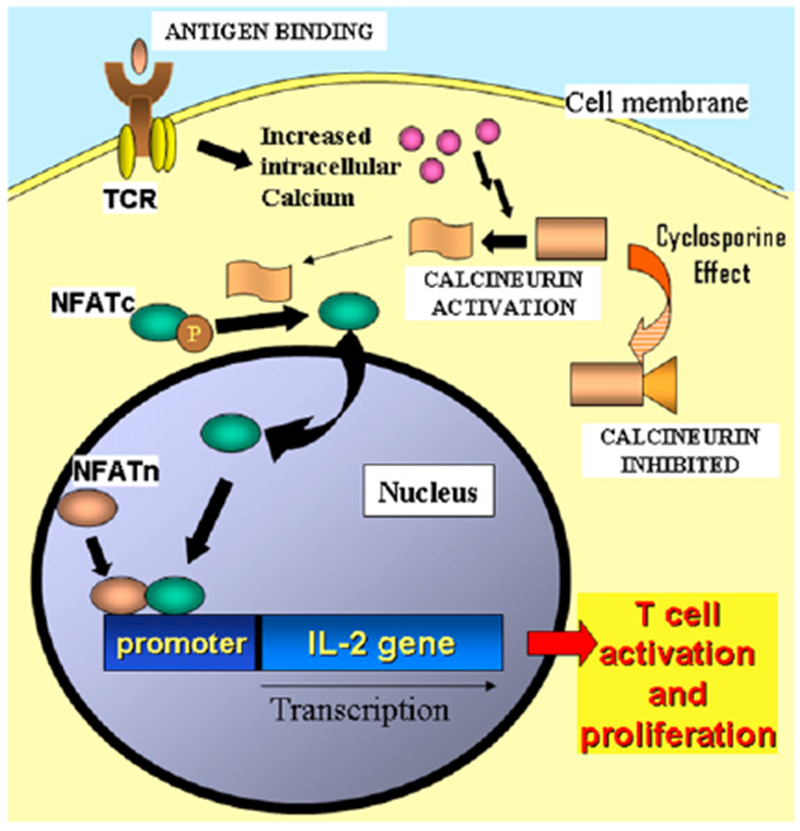
Effect of cyclosporine on T cells. Inhibition of calcineurin activity by cyclosporine results in decreased activation of IL-2 transcription. TCR, T-cell receptor; NFAT, nuclear factor of activated T cells; NFATc, cytoplasmic monomer; NFATn, nuclear monomer.
Cytotoxic agents
Cytotoxic agents were conceived to control neoplastic cell growth and ablate the bone marrow for transplantation. They have progressively found their niche in the immunosuppressive drugs category because of the selectivity conferred by the proliferative nature of the immune response, and their application has extended to autoimmune and inflammatory disorders, including GvHD and the prevention of graft rejection.26 The most common drugs used for these applications are the alkylating agent cyclophosphamide and the antimetabolites methotrexate, mycophenolate, azathioprine, and 6-mercaptopurine. Other drugs with predominant use in autoimmune disorders are sulfasalazine, hydroxychloroquine, and leflunomide.26 These compounds interfere with the synthesis of DNA, arresting the cell cycle and inducing apoptosis. Generally, they inhibit both T- and B-cell proliferation and therefore any new immune responses. In addition, depending on the dose used, they inhibit cellular and antibody responses resulting from previous sensitizations. The major limitation of the use of these agents is their toxicity to other hematopoietic and nonhematopoietic cells, with development of cytopenias, gastrointestinal mucosa, and skin deterioration. These cytopenias contribute to the state of secondary immunodeficiency and susceptibility to infections.
SURGERY AND TRAUMA
Surgery and trauma cause disruption of epithelial barriers and cell destruction that triggers an inflammatory response to promote healing and local microbicidal activity.27,28 Microorganisms contain surface pathogen–derived molecules that activate pattern-recognition receptors expressed on antigen-presenting cells and other immune cells to induce cytokine and chemokine release and recruitment of the adaptive immune system.29 Massive tissue injury further increases activation of proinflammatory mechanisms in response to the presence of toxic byproducts of cell death.30 In this inflammatory response Toll-like receptors play a central role in activating immune cells, resulting in the release of inflammatory cytokines, such as IL-6 and TNF-α. If this response is severe, trauma patients might experience the adult inflammatory respiratory syndrome in the lung or the systemic inflammatory response syndrome when there is multiorgan failure. The inflammatory response observed in patients with severe trauma develops gradually: loss of epithelial barriers, vasodilatation and increased vascular permeability, cellular activation and increased adhesion to endothelia, and a neuroendocrine stress response. At the same time, injured patients are relatively immunosuppressed because of nonspecific cell activation leading to an anergic immune state and because of increased levels of cortisol induced by stress in addition to the loss of containment provided by epithelial barriers. This process occurs within the context of a delicate balance of inflammatory and counterinflammatory mechanisms.31
Patients who have undergone splenectomy deserve special consideration because they are particularly susceptible to infections by encapsulated bacteria, such as Streptococcus pneumoniae. The mortality for sepsis in splenectomized patients is between 50% and 70%, emphasizing the need to avoid splenectomy when possible. Patients who are scheduled for elective splenectomy should receive antipneumococcal, anti–Haemophilus influenzae, and antimeningococcal immunizations at least 2 weeks before surgical intervention.32
ENVIRONMENTAL CONDITIONS: UV LIGHT, IONIZING RADIATION, HIGH ALTITUDE, CHRONIC HYPOXIA, AND SPACE FLIGHTS
There is increased awareness of potential adverse effects caused by chronic exposure to inhospitable environmental conditions, such as extreme cold or high altitude. It has been recommended to avoid exposure to sunlight because of increased risk of malignancies; however, beneficial effects of sunlight have also been observed, particularly in patients with skin inflammatory conditions, such as psoriasis.33 The biologic effect of sunlight in inflammation is mediated by UV light, which induces T-cell apoptosis, nonspecific release of tolerogenic cytokines from antigen-presenting cells in the epidermis, and differentiation of regulatory T cells; hence UV light is used in the treatment of eczema and the skin manifestations of autoimmune disorders.
The immunosuppressive effect of ionizing radiation affects all blood cell lineages by depleting the bone marrow and inducing cytopenias, whereas the humoral response and phagocytosis are considered radioresistant.34 However, continuous exposure to radiation eventually weakens all immune functions. Animal experiments of space radiation similar to that human subjects would experience during long-duration space flights have demonstrated a weakness of T cell–mediated immunity and reactivation of latent viral infections.35 Other adverse conditions, such as chronic hypoxia at high-altitude locations and long-duration space flights, might affect immunity by causing physical and mental stress. Confinement, isolation, and sleep-cycle alterations induce chronic stress, which disturbs the corticoadrenal regulation and increases cortisol levels. In human subjects space flight–equivalent models, including acute sleep deprivation, have been shown to increase blood levels of inflammatory cytokines and suppression of IL-10 secretion.36 Prolonged bedrest (ie, 60 days) with head-down tilt, a model of microgravity in space, has produced a significant increase of serum TNF-α soluble receptor levels in female volunteers (Fig 5).37 Interestingly, vigorous exercise served as an effective countermeasure in negating this effect.
FIG 5.
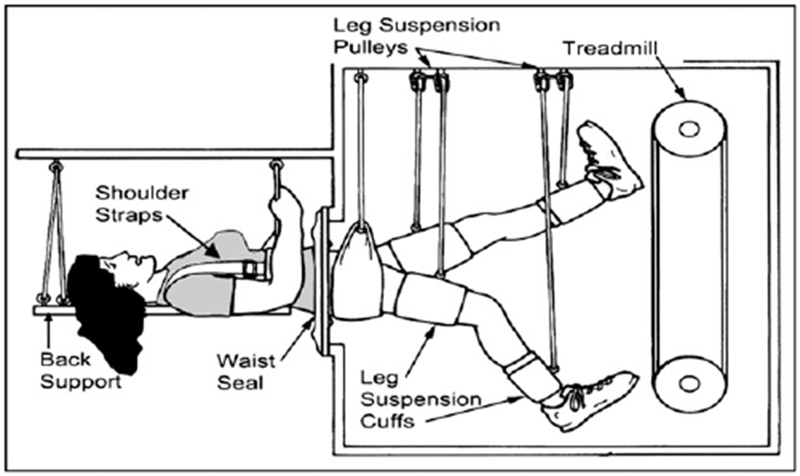
Human model to test the effects of microgravity. Volunteers are maintained in bedrest position for 60 days to mimic the affects of microgravity in space. Exercise is used as a countermeasure.
INFECTIOUS DISEASES
Transient periods of immunosuppression have been associated with viral infections since the 1900s, when it was observed that tuberculin skin test results became negative in patients with measles during the acute phase of the infection. Some infectious agents or their toxins and metabolites might be present in excess amounts to activate the immune system, leading to a nonresponsive state, such as the T-cell anergy observed after toxic shock syndrome induced by staphylococcal superantigen. Tissue destruction caused by microbial-induced damage or inflammatory reaction to a particular infection facilitates access for other microbes to develop secondary infections. Infections with measles virus, CMV, and influenza virus can induce lymphopenia and also T-cell anergy; however, these are transient and usually less severe than the immunodeficiency seen in AIDS. One additional mechanism of immune compromise is infection of the bone marrow by viral and bacterial organisms producing neutropenia or pancytopenia, particularly in immunocompromised hosts.38
HIV INFECTION: AIDS
Background
Without antiretroviral drug treatment, HIV infection almost always progresses to the advanced stage of the disease called AIDS that is characterized by profound lymphopenia and susceptibility to infections with opportunistic pathogens. HIV is transmitted sexually, for the most part, but it is also transmitted parenterally among intravenous drug users and vertically from mothers to their infants.39 Initially recognized during the early 1980s in a handful of cases, it is currently estimated that more than 30 million persons are infected with HIV worldwide. Two thirds of these subjects are living in the sub-Saharan region of Africa, and approximately half of them are women and children (Fig 6).40 The HIV epidemics in North America and Europe have shown decreasing trends in the last decade, thanks to massive education campaigns and the use of potent anti-HIV drugs. However, more than 56,000 new cases of HIV infection were reported in the United States in the last HIV infection survey by the Centers for Disease Control and Prevention, and approximately half of these were in subjects younger than 25 years.41 There is an increasing number of reports of viral multidrug resistance and clinical complications caused by the chronic use of antiretroviral drugs.42
FIG 6.
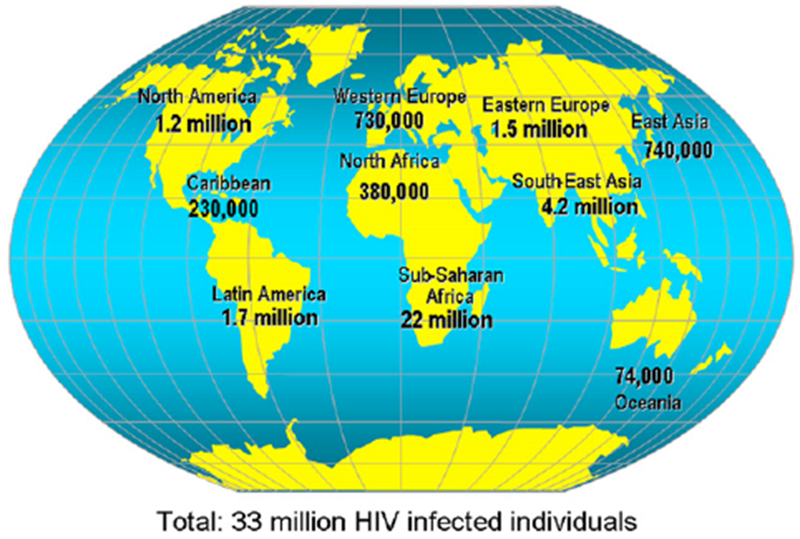
Worldwide prevalence of HIV infection. Adapted from the United Nations Programme on HIV/AIDS.40
Virology
HIV is a double-stranded, enveloped RNA retrovirus from the group lentiviruses, with a tropism for human CD4+ expressing cells, including T cells and macrophages.41 Two HIV types have been identified, HIV-1 and HIV-2, and both cause human disease. HIV-2 is more prevalent in West Africa and might take more time from infection to the development of immunodeficiency than HIV-1. The HIV genome contains 3 structural genes (gag, pol, and env) and 6 regulatory genes (tat, rev, nef, vif, vpr, and vpu). Gag protein is split by the HIV protease into the proteins named capsid (p24), matrix, nucleocapsid, p6, and p2, all of which form the viral particle and stabilize the viral genome. Pol protein is also split to produce 3 enzymes: integrase, reverse transcriptase, and the protease that cleaves the viral proteins. After the viral genomic RNA is converted into DNA by the reverse transcriptase, the integrase facilitates the incorporation of the viral DNA into the host genome and uses the host cell’s replication mechanisms to produce more virions. The Env protein is also cleaved to produce 2 envelope proteins named gp120 and gp41, which are involved in the binding to CD4 and the chemokine receptors CXCR4 and CCR5 on the cell surface. Tat protein increases the transcription of HIV genes by 100-fold, whereas Rev protein allows the expression of the different HIV genes by regulating mRNA splicing.
The roles of the other regulatory genes have only been clarified in the last few years. Nef protein downregulates CD4 and MHC class I surface expression on the membranes of infected cells, probably facilitating escape from immune surveillance. Vif is a protein that induces the degradation of APOBEC3 G, a cytosine deaminase that causes mutations during viral transcription. Vpr and Vpu proteins seem to facilitate the intracellular transport of viral proteins for viral particle formation.
Immunopathogenesis
HIV infection begins with the binding of the HIV gp120 protein to the CD4 molecule and the chemokine receptor CCR5 on target cells. Infected cells migrate to the lymph nodes, where initial replication and infection of nearby CD4+ T cells occur.43 During acute HIV infection, the gut-associated lymphoid tissue is severely depleted, with predominant loss of memory CD4+ T cells and with high viremia and immune activation.44,45 HIV induces T-cell lymphopenia through several mechanisms: HIV-induced apoptosis, viral cytopathic effect, apoptosis caused by nonspecific immune activation, and cytotoxicity to HIV-infected cells. An additional form of cell death named autophagy, in which organelles are sequestered and directed toward lysosomal pathways, has been shown to be induced by HIV Env protein in uninfected T cells.46 The acute phase of HIV infection occurs 1 to 6 weeks after infection, with nonspecific symptoms, such as fever, fatigue, myalgia, and headaches. The period of clinical latency that follows is characterized by a virtual absence of signs or symptoms until symptomatic disease occurs and can last as long as 10 years. Levels of several cytokines are increased and contribute to determine the degree of control of HIV viremia. Higher viral loads at the initial stage predict shorter clinical latency. Without anti-HIV drug treatment, CD4+ T-cell counts progressively decrease, and the host usually succumbs to infections with opportunistic organisms that take place because of the immune deficiency. Investigators have been able to demonstrate the production of specific anti-HIV CD4+ T cells and CD8+ T cells, as well as neutralizing anti-HIV antibodies; however, these immune responses are eventually overcome by viral escape strategies. At this stage, patients present with fever, weight loss, diarrhea, lymphadenopathy, and fungal and viral skin infections, indicating compromise of the immune system. When the peripheral CD4+ T-cell count is less than 200 cells/mL, the patient can present with any of a number of infections that define AIDS, such as Pseudomonas jiroveci–induced pneumonia, histoplasmosis, toxoplasmosis, and coccidioidomycosis.47 If the patient does not receive antiretroviral treatment, repeated infections that are difficult to manage lead to the patient’s death. A small proportion of HIV-infected patients remain asymptomatic and do not have AIDS. These patients are called long-term nonprogressors and have been the focus of multiple studies to understand the basis of their protection. Those who maintain low levels of HIV (ie, <50 RNA copies/mL) without treatment are called elite controllers.48 This immunity appears to be explained by different viral and host factors. The best known of these factors is the inherited defect in the gene encoding the CCR5 receptor, a T-cell surface molecule that is necessary for HIV cell entry. CCR5 gene mutations have been found with significant prevalence in persons of Northern European ancestry. Other factors identified in long-term nonprogressors include a low number of activated CD8+ T cells,49 the presence of particular HLA haplotypes, and viral mutations that result in low virulence. The diagnosis of HIV infection is made by using a sensitive ELISA to detect antibodies against the HIV protein p24. A positive HIV ELISA result is confirmed by using the more specific Western blot, which detects antibodies to several HIV proteins, or the detection of HIV DNA sequences by PCR. Rapid diagnostic tests to rule out HIV infection use serum, saliva, or urine with similar sensitivity and specificity to the ELISA and can be performed in the office or at home. Infants and children up to 18 months of age born to HIV-infected mothers should be evaluated with an HIV DNA PCR test because the presence of passively acquired maternal antibodies in the serum of the child can result in a positive HIV ELISA test result, even if the child is not infected with HIV. Other useful laboratory tests are genotyping and phenotyping assays. Genotyping identifies HIV mutations that confer viral resistance to antiretroviral drugs. Phenotyping measures the inhibitory action of anti-HIV drugs on the isolated HIV strain, which is similar to a bacterial susceptibility assay. These assays define anti-HIV drug susceptibility profiles of viral strains isolated from infected patients and help in the design of the combination of drugs with the most probability to have a therapeutic effect in a particular patient.
Treatment
In adults specific anti-HIV therapy is recommended when the patient has an AIDS-defining illness, the CD4+ T-cell count is less than 350 cells/mm3, or the HIV viral load is greater than 100,000 copies/mL. Caution should be exercised in other clinical situations because of the development of viral resistance to the antiretroviral agents and significant drug-induced adverse effects, including allergic and metabolic syndromes.50,51 In children treatment is considered for any HIV-infected infant because disease progresses faster than in older children. For children older than 12 months, the criteria are similar to those in adults: presence of an AIDS-defining illness, CD4+ T-cell count of less than 15% of PBMCs, or viral load greater than 100,000 copies/mL.52 Anti-HIV drug classes are defined according to their mechanism of action: nucleoside reverse transcriptase inhibitor, nonnucleoside reverse transcriptase inhibitor, protease inhibitor, and cell fusion inhibitor. In the last 2 years, CCR5 inhibitors and integrase inhibitors have been added to the arsenal of anti-HIV medications.53,54 Combinations of 3 synergistic anti-HIV drugs from 2 different classes are known as highly active antiretroviral therapy (HAART). HAART protocols have been effective in reducing viremia and restoring normal T-cell counts, with drastic reduction of mortality and number of infections; however, they do not eradicate HIV and need to be administered continuously for life. As an adjuvant treatment to improve baseline immunity, the administrations of IL-7 and IL-2 have been independently tested to increase CD4+ T-cell counts, with promising results.55,56
Immunologic reactions associated with anti-HIV treatment
The immune reconstitution inflammatory syndrome (IRIS) is a severe inflammatory response to existing opportunistic infections that can be observed in 15% to 25% of patients with AIDS 2 to 3 weeks after starting HAART treatment.57 The management of IRIS consists of corticosteroid therapy and simultaneous treatment of the opportunistic infections; however, IRIS might not occur if these infections are recognized and treated before starting the HAART therapy. A similar clinical observation is the increased incidence of asthma in HIV-infected patients receiving HAART, up to 3 times the rate of HIV-negative control subjects.58
Drug-allergic reactions have an increased prevalence in this patient population. Urticarial or maculopapular rashes, which occasionally present as the Steven-Johnson syndrome, occur in as many as 60% of patients with HIV receiving trimethoprim-sulfamethoxazole and in 17% of those receiving the antiretroviral nevirapine.59 Abacavir is a nucleoside reverse transcriptase inhibitor that causes a multiorgan hypersensitivity syndrome characterized by fever, rash, diarrhea, myalgia, and arthralgia in as many as 14% of patients who take this drug. This has a strong association with the presence of HLA B5701. This syndrome presents within the first weeks of treatment and can be fatal; however, it usually resolves after 72 hours of discontinuing the drug.
HIV vaccine
The failure of current antiretroviral therapy to eliminate the HIV virus emphasizes the need of preventive measures to control the HIV pandemic. Research for an effective anti-HIV vaccine has yielded several lessons; perhaps the most important is the need to demonstrate the development of specific cellular immunity and humoral responses and include mucosal protective immunity.60 The first vaccine candidates were based on strategies that had worked for other infectious diseases, such as inactivated virus and HIV proteins conjugated to adjuvants. These were able to induce only weak neutralizing antibody activity and did not provide significant protection against HIV infection in clinical trials. Live attenuated simian immunodeficiency virus strains have been demonstrated to protect macaques from simian immunodeficiency virus challenge; however, there are safety concerns related to the extraordinary capacity of HIV for recombination, which might lead to wild-type revertant strains. A novel approach using an adenovirus-based vaccine expressing HIV proteins elicited strong anti-HIV immunity; however, it was unable to demonstrate a protective effect over placebo in a phase I/II clinical trial with more than 3,000 subjects.61
Prevention measures
Considerable resources have been placed on educational campaigns to control the HIV epidemics. Preventive interventions that have been useful are using condoms, providing intravenous drug users with free sterile needles, screening blood products, and administering antiretroviral agents to HIV-infected pregnant women and their infants. Avoidance of breast-feeding has been recommended on the basis of the increased risk of transmitting the virus through breast milk; however, this might be revised in communities with poor resources, where it has been demonstrated that breast-feeding up to 1 month in combination with antiretroviral therapy does not increase early transmission and provides immune and nutritional support to the newborn.62 Other preventative interventions are male circumcision, with a reduction of the risk of HIV infection in heterosexual males by 50% to 60%,63 and topical anti-HIV microbicidals as an alternative to the use of condoms.64 The control of this deadly disease will only result from a combined effort of researchers and physicians developing and using anti-HIV drugs effectively and educators working in the promotion of safe behavioral practices in communities at risk.
CONCLUSION
There is an increased awareness of the variety of factors that can affect the immune response. When evaluating a patient with increased frequency or severity of infections suggesting immunodeficiency, physicians should consider that secondary immunodeficiencies are far more common than primary immune defects of genetic cause. A detailed clinical history might uncover the condition affecting the immune system, such as infection, malnutrition, age extremes, concomitant metabolic or neoplastic diseases, use of immunosuppressive drugs, surgery and trauma, and exposure to harsh environmental conditions. Because of its prevalence and clinical progression, HIV infection should be considered and ruled out. The specific immune defects and clinical presentation in other secondary immunodeficiencies are usually heterogeneous, affecting both the innate and the adaptive immunity. The immune impairment improves with the resolution of the primary condition.
Acknowledgments
Supported by National Institutes of Health grants AI27551, AI36211, AI6944I, HD41983, RR0188, HD79533, HL72705, and HD78522 and the David Fund, the Pediatrics AIDS Fund, and the Immunology Research Fund, Texas Children’s Hospital.
Abbreviations used
- GvHD:
Graft-versus-host disease
- HAART:
Highly active antiretroviral therapy
- IRIS:
Immune reconstitution inflammatory syndrome
Footnotes
Disclosure of potential conflict of interest: J. Chinen and W. T. Shearer have declared that they have no conflict of interest.
REFERENCES
- 1.Notarangelo LD. Primary immunodeficiencies. J Allergy Clin Immunol 2010;125: S182–94. [DOI] [PubMed] [Google Scholar]
- 2.Annane D, Bellissant E, Bollaert PE, Briegel J, Confalonieri M, De Gaudio R, et al. Corticosteroids in the treatment of severe sepsis and septic shock in adults: a systematic review. JAMA 2009;301:2362–75. [DOI] [PubMed] [Google Scholar]
- 3.Tan HP, Smaldone MC, Shapiro R. Immunosuppressive preconditioning or induction regimens, evidence to date. Drugs 2006;66:1535–45. [DOI] [PubMed] [Google Scholar]
- 4.Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, et al. Maternal and Child Undernutrition Study Group. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 2008;371:243–60. [DOI] [PubMed] [Google Scholar]
- 5.Lee SJ, Chinen J, Kavanaugh A. Immunomodulator therapy: Monoclonal antibodies, fusion proteins, cytokines, and immunoglobulins. J Allergy Clin Immunol 2010;125:S314–23. [DOI] [PubMed] [Google Scholar]
- 6.Lewis DB. Development of the fetal and neonatal immune system In: Rich RR, editor. Clinical immunology. Principles and practice. 3th ed. Philadelphia: Elsevier Saunders; 2008. p. 493–502. [Google Scholar]
- 7.Siegrist CA, Aspinalli R. B cell responses to vaccination at the extremes of age. Nat Rev Immunol 2009;9:185–94. [DOI] [PubMed] [Google Scholar]
- 8.Yost CC, Cody MJ, Harris ES, Thornton NL, McInturff AM, Martinez ML, et al. Impaired neutrophil extracellular trap (NET) formation: a novel innate immune deficiency of human neonates. Blood 2009;113:6419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dorshkind K, Montecino-Rodriguez E, Signer RA. The ageing immune system: is it ever too old to become young again? Nat Rev Immunol 2009;9:57–62. [DOI] [PubMed] [Google Scholar]
- 10.Gomez CR, Nomellini V, Faunce DE, Kovacs EJ. Innate immunity and aging. Exp Gerontol 2008;43:718–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castle SC, Uyemura K, Fulop T, Makinodan T. Host resistance and immune responses in advanced age. Clin Geriatr Med 2007;23:463–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinberger B, Herndler-Brandstetter D, Schwanninger A, Weiskopf D, Grubeck-Loebenstein B. Biology of immune responses to vaccines in elderly persons. Clin Infect Dis 2008;46:1078–84. [DOI] [PubMed] [Google Scholar]
- 13.Roth DE, Caulfield LE, Ezzati M, Black RE. Acute lower respiratory infections in childhood: opportunities for reducing the global burden through nutritional interventions. Bull World Health Organ 2008;86:356–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamer DH, Sempértegui F, Estrella B, Tucker KL, Rodríguez A, Egas J, et al. Micronutrient deficiencies are associated with impaired immune response and higher burden of respiratory infections in elderly Ecuadorians. J Nutr 2009;139:113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunningham-Rundles S, McNeeley DF, Moon A. Mechanisms of nutrient modulation of the immune response. J Allergy Clin Immunol 2005;115:1119–28. [DOI] [PubMed] [Google Scholar]
- 16.Adams JS, Liu PT, Chun R, Modlin RL, Hewison M. Vitamin D in defense of the human immune response. Ann N Y Acad Sci 2007;1117:94–105. [DOI] [PubMed] [Google Scholar]
- 17.Daoud AK, Tayyar MA, Fouda IM, Harfeil NA. Effects of diabetes mellitus vs. in vitro hyperglycemia on select immune cell functions. J Immunotoxicol 2009;6:36–41. [DOI] [PubMed] [Google Scholar]
- 18.Foley RN. Infectious complications in chronic dialysis patients. Perit Dial Int 2008; 28(suppl 3):S167–71. [PubMed] [Google Scholar]
- 19.Lim WH, Kireta S, Leedham E, Russ GR, Coates PT. Uremia impairs monocyte and monocyte-derived dendritic cell function in hemodialysis patients. Kidney Int 2007;72:1138–48. [DOI] [PubMed] [Google Scholar]
- 20.Raff AC, Meyer TW, Hostetter TH. New insights into uremic toxicity. Curr Opin Nephrol Hypertens 2008;17:560–5. [DOI] [PubMed] [Google Scholar]
- 21.De Hingh YCM, van der Vossen VW, Gemen EFB, Mulder AB, Hop WCJ, Brus F, et al. Intrinsic abnormalities of lymphocyte counts in children with Down syndrome. J Pediatr 2005;147:744–7. [DOI] [PubMed] [Google Scholar]
- 22.Douglas SD. Down syndrome: immunologic and epidemiologic association enigmas remain. J Pediatr 2005;147:723–5. [DOI] [PubMed] [Google Scholar]
- 23.Brennan S Innate immune activation and cystic fibrosis. Paediatr Respir Rev 2008; 9:271–9. [DOI] [PubMed] [Google Scholar]
- 24.Stahn C, Buttgereit F. Genomic and nongenomic effects of glucocorticoids. Nat Clin Pract Rheumatol 2008;4:525–33. [DOI] [PubMed] [Google Scholar]
- 25.Opelz G, Döhler B. Collaborative Transplant Study. Influence of immunosuppressive regimens on graft survival and secondary outcomes after kidney transplantation. Transplantation 2009;87:795–802. [DOI] [PubMed] [Google Scholar]
- 26.Sagg KG, Teng GG, Patkar NM, Anuntiyo J, Finney C, Curtis JR, et al. American College of Rheumatology. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum 2008;59:762–4. [DOI] [PubMed] [Google Scholar]
- 27.Flohé SB, Flohé S, Schade FU. Deterioration of the immune system after trauma: signals and cellular mechanisms. Innate Immun 2008;14:333–44. [DOI] [PubMed] [Google Scholar]
- 28.Heizmann O, Koeller M, Muhr G, Oertli D, Schinkel C. Th1- and Th2-type cytokines in plasma after major trauma. J Trauma 2008;65:1374–8. [DOI] [PubMed] [Google Scholar]
- 29.Joffre O, Nolte MA, Sporri R, Reis e Sousa C. Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol Rev 2009;227:234–47. [DOI] [PubMed] [Google Scholar]
- 30.Lenz A, Franklin GA, Cheadle WG. Systemic inflammation after trauma. Injury 2007;38:1336–45. [DOI] [PubMed] [Google Scholar]
- 31.Tschoeke SK, Ertel W. Immunoparalysis after multiple trauma. Injury 2007;38: 1346–57. [DOI] [PubMed] [Google Scholar]
- 32.American Academy of Pediatrics. Pneumococcal infections In: Pickering LK, editor. RedBook. 2009 Report of the Committee of Infections Diseases. 28th ed. Elk Grove Village (IL): American Academy of Pediatrics; 2009. p. 534–5. [Google Scholar]
- 33.Patel RV, Clark LN, Lebwohl M, Weinberg JM. Treatments for psoriasis and the risk of malignancy. J Am Acad Dermatol 2009;60:1001–17. [DOI] [PubMed] [Google Scholar]
- 34.Kusunoki Y, Hayashi T. Long-lasting alterations of the immune system by ionizing radiation exposure: implications for disease development among atomic bomb survivors. Int J Radiat Biol 2008;84:1–14. [DOI] [PubMed] [Google Scholar]
- 35.Shearer WT, Zhang S, Reuben JM, Lee BM, Butel JS. Effects of radiation and latent virus on immune responses in a space flight model. J Allergy Clin Immunol 2005;115:1297–303. [DOI] [PubMed] [Google Scholar]
- 36.Shearer WT, Lee BN, Cron SG, Rosenblatt HM, Smith EO, Lugg DJ, et al. Suppression of human anti-inflammatory plasma cytokines IL-10 and IL-1RA with elevation of proinflammatory cytokine IFN-gamma during the isolation of the Antarctic winter. J Allergy Clin Immunol 2002;109:854–7. [DOI] [PubMed] [Google Scholar]
- 37.Shearer WT, Ochs HD, Lee BN, Cohen EN, Reuben JM, Cheng I, et al. Immune responses in adult female volunteers during the bed-rest model of spaceflight: antibodies and cytokines. J Allergy Clin Immunol 2009;123:900–5. [DOI] [PubMed] [Google Scholar]
- 38.Lindblom A, Heyman M, Gustafsson I, Norbeck O, Kaldensjö T, Vernby A, et al. Parvovirus B19 infection in children with acute lymphoblastic leukemia is associated with cytopenia resulting in prolonged interruptions of chemotherapy. Clin Infect Dis 2008;46:528–36. [DOI] [PubMed] [Google Scholar]
- 39.Baliga CS, Paul ME, Chine J, Shearer WT. HIV infection and the acquired immunodeficiency syndrome In: Rich RR, editor. Clinical immunology. Principles and practice. 3th ed. Philadelphia: Elsevier Saunders; 2008. p. 553–60. [Google Scholar]
- 40.UNAIDS. 2008 Report on the global AIDS epidemic. Geneva, Switzerland: United Nations Programme on HIV/AIDS (UNAIDS) and World Health Organization (WHO); 2008. [Google Scholar]
- 41.Centers for Disease Control and Prevention. HIV/AIDS surveillance report. Vol 19 Department of Health and Human Services; 2007. [Google Scholar]
- 42.Hammer SM, Eron JJ Jr, Reiss P, Schooley RT, Thompson MA, Walmsley S, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA 2008;300:555–70. [DOI] [PubMed] [Google Scholar]
- 43.Shen L, Siliciano RF. Viral reservoirs, residual viremia, and the potential of highly active antiretroviral therapy to eradicate HIV infection. J Allergy Clin Immunol 2008;122:22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, et al. CD4 T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med 2004;200:749–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Norris PJ, Pappalardo BL, Custer B, Spotts G, Hecht FM, Busch MP, et al. Elevations in IL-10, TNF-alpha, and IFN-gamma from the earliest point of HIV Type 1 infection. AIDS Res Hum Retroviruses 2006;22:757–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Espert L, Denizot M, Grimaldi M, Robert-Hebmann V, Gay B, Varbanov M, et al. Autophagy is involved in T cell death after binding of HIV-1 envelope proteins to CXCR4. J Clin Invest 2006;116:2161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodriguez B, Sethi AK, Cheruvu VK, Mackay W, Bosch RJ, Kitahata M, et al. Predictive value of plasma HIV RNA level on rate of CD4 T-cell decline in untreated HIV infection. JAMA 2006;296:1498–506. [DOI] [PubMed] [Google Scholar]
- 48.Baker BM, Block BL, Rothchild AC, Walker BD. Elite control of HIV infection: implications for vaccine design. Expert Opin Biol Ther 2009;9:55–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paul ME, Mao C, Charurat M, Serchuck L, Foca M, Hayani K, et al. Predictors of immunologic long-term non progression in HIV-infected children: implications for initiating therapy. J Allergy Clin Immunol 2005;115:848–55. [DOI] [PubMed] [Google Scholar]
- 50.Kitahata MM, Gange SJ, Abraham AG, Merriman B, Saag MS, Justice AC, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med 2009;360:1815–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Panel on Antiretroviral Guidelines for Adult and Adolescents. Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents. Department of Health and Human Services; November 3, 2008. Available at: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentsGL.pdf Accessed May 30, 2009. [Google Scholar]
- 52.Working Group on Antiretroviral Therapy and Medical Management of HIV Infected Children. Guidelines for the use of antiretroviral agents in pediatric HIV infection. February 23, 2009. Available at: http://aidsinfo.nih.gov/ContentFiles/PediatricGuidelines.pdf Accessed May 30, 2009.
- 53.MacArthur RD, Novak RM. Reviews of anti-infective agents: maraviroc: the first of a new class of antiretroviral agents. Clin Infect Dis 2008;47:236–41. [DOI] [PubMed] [Google Scholar]
- 54.Hicks C, Gulick RM. Raltegravir: the first HIV type 1 integrase inhibitor. Clin Infect Dis 2009;48:931–9. [DOI] [PubMed] [Google Scholar]
- 55.Pahwa S, Muresan P, Sleasman J, Fenton T, Moye J, Deveikis A, et al. Phase I/II trial of intermittent subcutaneous IL-2 administration in pediatric patients with moderate immune suppression: results of Pediatric AIDS Clinical Trials Study 402. J Allergy Clin Immunol 2007;119:1538–41. [DOI] [PubMed] [Google Scholar]
- 56.Camargo JF, Kulkarni H, Agan BK, Gaitan AA, Beachy LA, Srinivas S, et al. Responsiveness of T cells to interleukin-7 is associated with higher CD4 + T cell counts in HIV-1-positive individuals with highly active antiretroviral therapy-induced viral load suppression. J Infect Dis 2009;199:1872–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.French MA. HIV/AIDS: immune reconstitution inflammatory syndrome: a reappraisal. Clin Infect Dis 2009;48:101–7. [DOI] [PubMed] [Google Scholar]
- 58.Foster SB, McIntosh K, Thompson B, Lu M, Yin W, Rich KC, et al. Increased incidence of asthma in HIV-infected children treated with highly active antiretroviral therapy in the National Institutes of Health Women and Infants Transmission Study. J Allergy Clin Immunol 2008;122:159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davis CM, Shearer WT. Diagnosis and management of HIV drug hypersensitivity. J Allergy Clin Immunol 2008;121:826–32. [DOI] [PubMed] [Google Scholar]
- 60.Haynes BF, Shattock RJ. Critical issues in mucosal immunity for HIV-1 vaccine development. J Allergy Clin Immunol 2008;122:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McElrath MJ, De Rosa SC, Moodie Z, Dubey S, Kierstead L, Janes H, et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet 2008;372:1894–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shapiro RL, Smeaton L, Lockman S, Thior I, Rossenkhan R, Wester C, et al. Risk factors for early and late transmission of HIV via breast-feeding among infants born to HIV-infected women in a randomized clinical trial in Botswana. J Infect Dis 2009;199:414–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Newell ML, Barninghausen T. Male circumcision to cut HIV risk in the general population. Lancet 2007;369:617–8. [DOI] [PubMed] [Google Scholar]
- 64.Cutler B, Justman J. Vaginal microbicides and the prevention of HIV transmission. Lancet Infect Dis 2008;8:685–97. [DOI] [PMC free article] [PubMed] [Google Scholar]


