Abstract
Prostate cancer is a substantial public health burden and a leading cause of cancer—related morbidity and mortality in the United States despite the observation that annual prostate cancer—specific mortality rates have been declining during the previous decade. Although the reasons for this positive development are unclear, a combination of factors may have contributed. This update will review ongoing developments and summarize therapeutic advances in prostate cancer treatment on the basis of the current understanding of prostate cancer biology. Literature for this review was selected in 2009 by searching PubMed for the following keywords: prostatic neoplasms, castration, androgen receptor, hormonal, and chemotherapy. Emphasis is placed on published clinical studies in advanced prostate cancer therapeutics in the past 5 to 10 years. Also included in the review are novel hormonal agents targeting the androgen receptor currently in development for the treatment of advanced prostate cancer.
5αR = 5α-reductase; ADT = androgen deprivation therapy; AR = androgen receptor; DHT = dihydrotestosterone; ET = endothelin; OR = odds ratio; PSA = prostate-specific antigen; SNP = single-nucleotide polymorphism
Prostate cancer substantially contributes to cancer-related morbidity and mortality in the industrialized world. In 2008, it ranked as the most common malignancy in men in the United States and resulted in 28,660 deaths,1 making it the second most common cause of cancer-related mortality in US men. The annual prostate cancer—specific mortality rate has been declining during the previous decade. Although the reason for this positive development is unclear, a combination of factors may have contributed. Temporally associated with this decline has been a greater use of novel therapeutic strategies that target biological pathways associated with prostate cancer initiation and progression. This time period is also coincident with extensive use of prostate-specific antigen (PSA) for detection, diagnosis, management, and monitoring of prostate cancer. The use of PSA for screening has increased the chance that potentially curative treatments will be offered earlier for localized cancer stages.2,3 Moreover, evidence shows that radical prostatectomy reduces prostate cancer mortality and the risk of developing subsequent metastases when compared with watchful waiting, at least in patients in whom prostate cancer is diagnosed without PSA screening.4 The confluence of these developments does not provide direct evidence that PSA screening has affected annual mortality rates in the United States. Cumulatively, however, these factors suggest that the use of the PSA test and early treatments for localized disease may have contributed to the observed decline in annual mortality rates in the United States. This update will focus on reviewing ongoing developments and therapeutic advances in prostate cancer treatment based on the current understanding of prostate cancer biology.
PROSTATE-SPECIFIC ANTIGEN SCREENING
Prostate-specific antigen is a serine protease that has been used extensively in clinical practice since 1988. It is the best-known member of the kallikrein family, which is the largest contiguous group of proteases in the human genome that are clustered in a 300-kilobase region on chromosome 19q13.4. In practice, PSA has been used extensively for diagnosis of prostate cancer. Levels of PSA are also used for monitoring disease recurrence after initial treatments and for evaluating response to cancer treatments. Despite its use for cancer diagnosis since 1988, the value of PSA as a screening test remains mired in controversy, as no study has shown that PSA screening has decreased prostate cancer—specific mortality. This has led to conflicting recommendations regarding the use of PSA as a screening test.
Recently, revealing results were reported from 2 large, randomized clinical trials that evaluated PSA as a screening test. The PLCO (Prostate, Lung, Cervical, Ovarian screening) trial5 randomized 76,693 US men (50-74 years) to either annual screening using PSA and digital rectal examination or usual care, with a primary end point of cause-specific mortality during 7 years of follow-up. The study found that a greater number of prostate cancers were diagnosed in patients undergoing regular screening with PSA. However, PSA screening was not found to significantly reduce death from prostate cancer. Of note, nearly 40% of the participants in the control arm undergoing usual care received a routine PSA test. The second trial, ERSPC (European Randomized Study for reducing Prostate Cancer),6 randomized 162,387 European men (55-69 years) into either a screening group or a control group and used an end point of reducing mortality due to prostate cancer by 25% in PSA-screened patients. The follow-up period was 8.8 years in the screening group and 9.0 years in the control group. The ERSPC investigators concluded that PSA screening reduces prostate cancer—specific mortality, but at a significant cost of overdiagnosis and overtreatment. The absolute difference between screening and control groups was 0.71 prostate cancer deaths per 1000 men. Thus, to prevent 1 prostate cancer death, 1410 men would need to be screened during a 9-year period. The ERSPC investigators also noted that 48 patients with prostate cancer would need to be treated to prevent 1 death from prostate cancer.
These trial results have again highlighted a controversial area with wide public health ramifications, one that is inevitable during the initial formulation of a cancer-screening strategy. In the initial days of screening for breast cancer, the use of film mammography for breast cancer screening (in average-risk women) led to an absolute risk reduction of 0.05% from cancer-related death. Screening with film mammography at that time was similarly found to lead to overdiagnosis and overtreatment, with an estimated increase of 30% (absolute risk increase, 0.5%). This meant that for every 2000 women screened during the course of 10 years, only 1 woman would have had her life prolonged. In addition, 10 healthy women would have been diagnosed as having breast cancer with film mammography and would have been treated unnecessarily.7 These statistics improved with the advent of digital mammography. Thus, the value of PSA screening may improve with the advent of additional biomarkers. Apart from PSA-based screening programs that continue to evolve, considerable progress has been made in our understanding of tumor biology during the past decade, with powerful implications for clinical use in prostate cancer detection and prognosis.
MOLECULAR PATHOGENESIS
Although no single molecular event has yet been identified for prostate cancer initiation, fusion chromosomal rearrangements with oncogenic transcription factor genes specific for prostate cancer appear to be implicated in the development of invasive prostate tumors.8 Recurrent gene fusions involving 2 members of the oncogenic ETS (see glossary at end of article for expansion of all gene symbols) family of transcription factors, ERG and ETV1, and the 5′ untranslated region of TMPRSS2 on chromosome 21 have been identified in 50% to 70% of malignant cells of localized tumors. Fusion of TMPRSS2 with ERG results in androgen regulation of ERG and is detected in essentially all the malignant cells within a focus of tumor, suggesting a role in cancer initiation. However, such fusion rearrangements are not universally detected in preneoplastic prostate intraepithelial neoplasia, the morphological noninvasive precursor lesion for prostate cancer. It also remains unclear whether cooperation exists between fusion gene rearrangements and multiple molecular pathways, such as PTEN loss, PI3 kinase pathway activation, and MYC amplification, which are relatively common abnormalities in prostate cancer leading to prostate tumorigenesis.9
The potential role that aberrant gene fusions may play in prostate cancer development is independent of other well-known risk factors, such as family history. In a metaanalysis, the impact of family history showed a pooled odds ratio (OR) of 2.5 for developing prostate cancer in men with a first-degree relative with the disease10,11; these findings led to the evaluation of variation in the human genome as a predisposition for developing prostate cancer. Large genome-wide association studies conducted in different geographic populations have identified the presence of several common risk alleles in germline DNA, including single-nucleotide polymorphisms (SNPs) in the 8q24 region,12,13 as well as other groups of SNPs. The presence of these risk alleles is associated with a higher OR for prostate cancer development.14 Furthermore, it has recently been calculated that the mere presence of a single SNP carries only a marginal and insignificant increased risk attribute (estimated OR, 1.64; 95% confidence interval, 1.34-2.00), which increases in the presence of multiple disease risk alleles, resulting in a cumulative OR for cancer development of 11.26 (95% confidence interval, 4.74-24.75) when more than 5 risk SNPs are present.14,15
Risk assessment for prostate cancer has also resulted in devising prevention strategies in “at-risk” populations; thus far, however, ethnicity, family history, and the presence of morphology-based precancerous lesions, along with PSA measurements and variations in the human genome or presence of gene fusions, have formed the basis of targeted chemopreventive interventions. Most of these chemopreventive strategies have targeted the testosterone production axis as a greater understanding of the active role of transcription factors in prostate tumor biology, and specifically the hormonally activated AR, have evolved. A broad overview of the importance of the testosterone axis and AR follows.
ANDROGEN RECEPTOR BIOLOGY
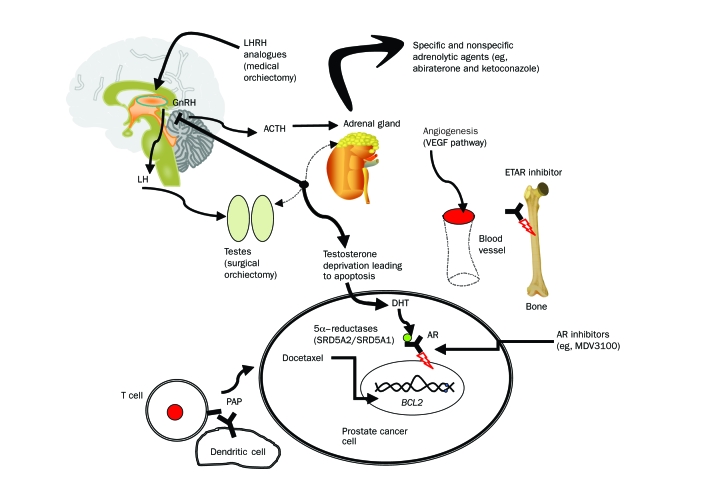
Treatment of prostate cancer with androgen deprivation therapy (ADT) remains a cornerstone intervention for advanced-stage disease management. The Figure summarizes key pathophysiological pathways and therapeutic targets (both those that are already established and those that are currently in development) in the management of advanced prostate cancer. An initial insight into the management of prostate cancer emerged from the seminal observations of Huggins and Hodges,16 who showed that orchiectomy slowed tumor growth. Their finding led to the realization that prostate cancer growth is dependent on the predominant male hormone testosterone. Since these early observations, the central role of the androgen axis and the AR in normal prostate development has been recognized, as has the necessary role of stromal-epithelial interactions in androgen-dependent growth stimulus of epithelial differentiation17 and the organizational failure of the epithelial-stromal signals during prostate tumorigenesis and progression.
FIGURE.
Pathophysiological pathways and therapeutic targets in prostate cancer. ACTH = adrenocorticotropic hormone; AR = androgen receptor; DHT = dihydrotestosterone; GnRH = gonadotropin-releasing hormone; LH = luteinizing hormone; LHRH = luteinizing hormone—releasing hormone; PAP = prostate acid phosphatase. See glossary at end of article for expansion of all gene symbols.
Testosterone is produced by the testicular Leydig cells under the influence of pulsatile secretion of luteinizing hormone—releasing hormone from the anterior hypothalamus, which in turn is regulated by negative feedback inhibition of the pituitary-gonadal axis by circulating testosterone. Most of the testosterone in circulation is bound to sex hormone—binding globulin and albumin. Only 1% to 2% of testosterone exists in the free, unbound form that diffuses into target cells of the prostate, testis, adrenal gland, skin, muscle, bone, and adipose tissue, where it is irreversibly converted into a more potent biologically active metabolite, dihydrotestosterone (DHT), by action of 5α-reductase (5αR) in some, but not all, tissue types (depending on the presence of type 1 or 2 isoenzyme).18 Both DHT and testosterone exert their biological activities by binding to AR, a 110-kDa member of the nuclear receptor superfamily of ligand-activated transcription factors, which in its unliganded state resides primarily in the cytoplasmic compartment of prostate epithelial and stromal cells. Dihydrotestosterone binds to the AR with higher affinity, has greater biological activity, and dissociates more slowly than does testosterone. Before ligand activation, cytoplasmic AR exists as a stable complex bound to heat shock proteins in an inactivated form. Ligand activation triggers dissociation of the AR—heat shock protein complex, receptor dimerization, and translocation of AR to the nucleus.19 In the nucleus, the AR binds to androgen response elements on promoter and enhancer regions of target genes, such as PSA, leading to transcription of messenger RNAs. Elucidating this structural and functional interaction of AR in prostate biology has permitted the development of preventive and therapeutic strategies that target the androgen axis during different stages of tumor progression. Novel AR inhibitors (eg, MDV3100,20 a second-generation antiandrogen for treating advanced prostate cancer) are now in phase 3 clinical trials. These new diarylthiohydantoin compounds target AR by binding overexpressed AR in advanced-stage disease with an affinity that is several-fold greater than that previously obtained with antiandrogens (bicalutamide and flutamide). These drugs also disrupt the nuclear translocation of AR and impair DNA binding to androgen response elements and recruitment of coactivators20 and thus have multifunctional antitumor capabilities.
TARGETING THE TESTOSTERONE-AR AXIS
The physiologic basis for hormonal action in prostate cancer is to deprive cancer cells of androgens. Apoptotic regression of an androgen-dependent tumor is induced either chemically with luteinizing hormone—releasing hormone analogues or surgically by orchiectomy, which initially reduces the intracellular concentration of DHT,21 resulting in the death (by apoptosis) of androgen-sensitive cancer cells. Although noncurative, androgen deprivation slows disease progression, enhances quality of life, modestly increases survival,22 and is associated with a biochemical response of decreased PSA levels. After a biochemical response, serial increases in PSA levels during ongoing androgen deprivation are generally accepted as a sensitive measure of disease progression.23 The median time to progression to a “castration-recurrent” stage for patients who initially exhibit a biochemical response to androgen deprivation is 18 to 30 months.24-26 However, although pretreatment serum PSA levels correlate roughly with tumor burden, PSA levels have no readily defined prognostic or predictive value,23 likely because the biological heterogeneity of prostate cancer results in varying degrees of PSA production. As a consequence, the absolute level of pretreatment PSA has yet to be confirmed to correlate with long-term response to treatment or survival. Nevertheless, some evidence shows that PSA's ability to predict an early response after initiation of androgen deprivation is clinically useful.26 Overall, in metastatic-stage disease, 76% of patients treated with ADT are likely to have an early decrease in PSA levels to less than 4.0 ng/mL (to convert to μg/L, multiply by 1.0). A higher number of patients with minimal metastatic disease experience this early response compared with those with clinically detected metastatic disease (92% vs 70%). In general, a PSA nadir level is reached at 2.5 to 4.0 months after starting androgen deprivation.26,27 Other PSA-based variables, such as time to normalization of an increasing PSA level and PSA kinetics (PSA doubling time, PSA velocity), have also been evaluated in clinical studies and appear to have some prognostic relevance, but consensus does not exist whether the predictive value for ADT is best demonstrated by absolute PSA measurements or by PSA kinetics.28 The lack of prognostic value using PSA for predicting long-term outcome has led to scientific interest in the development and application of additional tumor biomarkers in prostate cancer. The development of promising genomic- and proteomic-based biomarkers for this purpose will help better identify groups of patients who will benefit from hormonal treatments,29,30 an important advantage given that ADT is not without adverse effects. Androgen deprivation induces an andropausal state, leading to long-term adverse effects, including osteoporosis, loss of libido, increased rates of major depression, increased risk of diabetes and cardiovascular disease,31,32 andropausal symptoms, and fatigue33; the duration of symptoms varies, depending on how long treatment is maintained.
Although testosterone depletion remains an unchallenged standard for advanced-stage hormone-sensitive disease, evidence has emerged that “castration-recurrent” prostate cancer remains androgen receptor (AR)—dependent and is neither hormone refractory nor androgen-independent, terms once commonly used to define progression of advanced-stage disease after ADT. That ARs function despite the paucity of circulating androgens is evidenced by the elevation of AR messenger RNA in castration-recurrent tumor tissue relative to androgen-dependent tumors and re-expression of some androgen-regulated genes during clinical castration resistance,34,35 indicative of a persistently activated AR cell—signaling pathway. Furthermore, although testosterone and DHT are depleted in serum after ADT, substantial levels of androgens have been measured in locally recurrent prostate cancer tissue,36 as well as in intratumoral metastatic castration-recurrent prostate cancer tissue.37 These androgens continue to regulate AR transcriptional activity and stimulate expression of androgen-regulated genes such as PSA, which commonly precedes clinical failure.36,37 Indeed, a number of molecular and cellular changes have been shown to facilitate activation of the AR under these conditions: amplification of the AR gene allows the receptor to respond to lower levels of androgens; increased expression of several coactivator proteins enhances activation of the AR; other signaling pathways, including the AKT1-PI3 kinase and MAPK pathways, activate the AR; and gain-of-function sequence variations in the AR allow the receptor to become “promiscuous” and bind to ligands other than DHT, including antiandrogens38 and weaker sex steroids such as androstenedione. Thus, although the source of the tissue androgens may be either adrenal or intratumoral, targeting AR signaling in castration-recurrent prostate cancer after ADT by further blocking adrenal enzymes engaged in the biosynthesis of testosterone and DHT is receiving renewed interest. In the recent past, one such second-line hormonal maneuver, which used a combination of nonspecific adrenolytic agents including ketoconazole and hydrocortisone,39 resulted in a greater than 50% reduction in PSA levels in 27% of patients but was associated with adverse effects, including lethargy, rash, hypothyroidism, myelotoxicity, nausea, and vomiting.39-41 More recently, abiraterone, a selective, steroidal irreversible inhibitor of CYP17 (17 hydroxylase/C17,20-lyase) that blocks 2 important enzymatic activities in the synthesis of testosterone,42 has been evaluated in patients with castration-recurrent prostate cancer. Early results with abiraterone, a prodrug, demonstrate greater than 50% declines in PSA level in 67% of patients with advanced, castration-recurrent prostate cancer and objective radiologic responses in patients deriving no benefit from standard androgen ablation; adverse effects, which were reasonably well tolerated, included hypertension, hypokalemia, and fluid retention.43,44 These observations appear promising compared with historical attempts to target adrenal testosterone production in castration-recurrent tumors using such adrenolytic drugs as aminoglutethimide and ketoconazole, which are nonselective weak inhibitors of CYP17 adrenal enzymes involved in adrenal androgen biosynthesis.41
Another means of decreasing active androgen levels in target tissues is provided by the overexpression by prostate cells of types 1 and 2 5αR isoenzymes, which are critical for the conversion of testosterone to DHT. Cytoplasmic DHT allows nuclear translocation of the activated AR, resulting in transcriptional activation of androgen-regulated target genes, including PSA, kallikrein 2, and genes involved in cell cycle regulation and cell survival.38 At the molecular level, inhibition leads to down-regulation of cellular pathways involved in metabolism, catalytic activity, cell growth, protein metabolism, and signal transduction, as well as expression of proapoptotic proteins, such as caspase 7 and 8.45 At the tissue morphological level, 5αR inhibitors lead to decreased microvessel density,46 increased percentages of atrophic epithelium, and increased stroma-to-gland ratio.47 Two medications that have been successfully used to inhibit 5αR in prostatic tissue are finasteride, which primarily targets type 2 5αR, and dutasteride, which inhibits both isoenzymes. These medications have been used more to prevent than to treat prostate cancer. The clinical benefits of these medications became apparent with the results from the PCPT (Prostate Cancer Prevention Trial),48 a 7-year study involving 18,882 men, showing that finasteride significantly decreased (by 24.8%) the risk of prostate cancer vs placebo. More recently, the REDUCE (Reduction by Dutasteride of Prostate Cancer Events) trial, a 4-year randomized placebo-controlled trial evaluating the reduction by dutasteride of prostate cancer events in approximately 8000 men at increased risk of prostate cancer,49 showed promising results that the inhibition of both types 1 and 2 5αR inhibitors could help prevent prostate cancer.
The AR signaling pathway is also affected by other nuclear receptor ligands, such as vitamin D, which is known to promote growth inhibitory effects in prostate cancer.50 Vitamin D is a steroid hormone that modulates calcium homeostasis and is involved primarily in bone and mineral metabolism through the vitamin D receptor, a nuclear receptor expressed in the intestinal tract, kidney, and bone. Vitamin D is synthesized in the skin from 7-dehydrocholesterol in response to sunlight and hydroxylated in the liver to 25-hydroxycholecalciferol, which is further hydroxylated to 1, 25-dihydroxycholecalciferol or calcitriol in the kidneys. Epidemiological evidence suggesting that low vitamin D levels play a role in prostate cancer tumorigenesis and progression51 led to investigations for prevention and treatment of prostate cancer with vitamin D. At a preclinical level, calcitriol was found to exert many antitumor activities in several tumor types, including prostate cancer,52 by binding to the vitamin D receptor expressed in the malignant prostate epithelium.53 This treatment resulted in inhibition of proliferation, stimulation of apoptosis, and promotion of cell cycle arrest in malignant tissue. In vitro treatment of prostate cancer cells with calcitriol also resulted in upregulation of AR messenger RNA and protein, increased AR nuclear localization, and increased ligand binding,54,55 although it is less clear that the AR is a direct target of vitamin D. Thus, the exact molecular mechanism of calcitriol's antitumor activities remains undefined. Clinical treatment of patients with recurrent prostate cancer with calcitriol resulted in the lowering of PSA levels but was also associated with serious adverse effects of hypercalcemia.56 Subsequent clinical trials with calcitriol and its analogues (eg, DN-101, a high-dose oral formulation of calcitriol designed for cancer therapy) have demonstrated the safety and efficacy of these compounds in treating castration-recurrent prostate cancer.57 Initial results from these early trials led to a larger randomized phase 2 clinical trial of weekly docetaxel and prednisone, with or without DN-101, in castration-recurrent prostate cancer, which showed an improved hazard ratio for death in patients who received chemotherapy with DN-101.57 The phase 3 clinical trial of the same combination was recently stopped before study completion out of concern for excess deaths observed in the DN-101 combination treatment arm. Thus, further development of this agent in castration-recurrent prostate cancer may be in jeopardy; however, clinical trials are ongoing to determine the therapeutic benefit and toxicity from other vitamin D analogue combinations in the prevention and treatment of prostate cancer at various stages.
CHEMOTHERAPY
Apart from nuclear receptors and coregulatory proteins associated with prostate cancer biology, survival pathways for prostate cancer cell growth include deregulated expression and/or sequence variations of the PTEN gene that occur with high frequency in advanced prostate cancer, leading to aberrant activation of AKT kinase activity and of its down-stream targets MTOR58-60 and FOX, which in turn promote tumor growth.61 Loss of PTEN also permits activated AKT to phosphorylate the intracellular protein BAD, resulting in the release of the antiapoptotic protein BCL2, which then leads to cancer cell survival61 and significantly contributes to cancer progression at this stage of the disease. This axis has been the focus of therapeutic targeting using several strategies. For example, MTOR inhibitors (eg, RAD-001 or everolimus), used either as single agents or in combination,62 are currently being explored in the laboratory for efficacy in advanced castration-recurrent prostate cancer, a stage associated with an activated EPHB2 (formerly known as ERK)-MAPK pathway63 and aberrant PTEN sequence variations with deregulation of the PTEN-AKT-MTOR axis; however, results from human trials are not yet mature.
The most successful targeting of the BCL2 axis in prostate cancer, however, has been achieved using chemotherapy. Success of docetaxel chemotherapy in prolonging survival of patients with castration-recurrent prostate cancer was first demonstrated in 2004. Docetaxel is a taxoid that inhibits the de polymerization of microtubules, leading to disruption of the mitotic process and cell cycle arrest at the G2M phase and apoptosis. Two large randomized clinical trials64,65 found an increased median survival with docetaxel-based combinations in patients with castration-recurrent prostate cancer, compared with therapy with mitoxantrone and prednisone, the previous standard of treatment for castration-recurrent prostate cancer and a regimen that has been used primarily for palliation of symptoms during advanced-stage disease.66,67 In addition to its apoptotic effect via microtubule stabilization, docetaxel also induces apoptosis by inhibiting BCL2, a key mechanism for cancer cell survival achieved by the effect of PTEN sequence variations on AKT signaling. Androgen-independent cells may overexpress BCL2 independent of the PTEN-AKT signaling effects.61 Docetaxel phosphorylates the serine residues of BCL2, resulting in its inactivation and consequent activation of the caspase cascade and apoptosis. Although 2 different docetaxel-based regimens both increased median survival vs therapy with mitoxantrone and prednisone, their toxicity profiles differed. Combining docetaxel with estramustine, an oral conjugate of an alkylating mustard of estradiol, led to a 20% incidence of nausea and a 15% incidence of cardiovascular or clotting adverse effects64 not observed with docetaxel combined with prednisone alone,65 suggesting a greater therapeutic acceptability for using docetaxel and prednisone in castration-recurrent prostate cancer. For patients whose disease progresses during first-line chemotherapy, satraplatin, an oral platinum analogue, was evaluated as a second-line therapeutic agent on the basis of promising results of preclinical68 antitumor activity in human prostate cancer models and from efficacy in early clinical trials.69 For the primary end point of median progression-free survival, therapy with satraplatin and prednisone for 11 weeks was only marginally more effective than prednisone alone for 9.7 weeks. Currently, approval for use of satraplatin as a second-line chemotherapeutic agent is pending while further results of survival analyses are awaited.
After decades without substantial progress in treating advanced prostate cancer, the encouraging results with cytotoxic chemotherapy have led to the testing of combinations of docetaxel and prednisone with targeted therapy in castration-recurrent prostate cancer. Angiogenesis has emerged as a viable target, with the rationale that several angiogenic proteins, including those in the VEGF family70 and endothelin (ET)-A,71 are overexpressed in prostate cancer tissues and are associated with adverse outcomes in metastatic prostate cancer.72 Preliminary results using a combination of bevacizumab, a humanized monoclonal antibody against VEGFA, and docetaxel, were encouraging in patients experiencing docetaxel failure.73 Currently, a large phase 3 clinical trial is under way, led by the Cancer and Leukemia Group B (CALGB) in the United States (CALGB 90401; clinical trials identifier: NCT00110214), which will test if addition of bevacizumab to docetaxel and prednisone improves overall survival. Beyond targeting the VEGF pathway, other promising novel angiogenic targets include the endothelin axis. Endothelin, a potent vasoconstrictor protein produced by the vascular endothelium, plays an important role in vascular homeostasis and mediation of osteoblast growth and function.74 In the normal prostate gland, ET-1 is produced by the epithelial cells, and its clearance is regulated by binding to the ET-B receptor (ETBR) and neural endopeptidase, which is a metalloendo-peptidase responsible for the metabolism of several bioactive peptides, including ETs. In prostate cancer, dysregulation of key ET-1 clearance components, including reduced ETBR binding and neural endopeptidase activity,75 results in overexpression of ET-1. Also, increased ET-1/ET-A receptor (ET-1/ETAR) expression is observed during advancing tumor stages of primary and metastatic prostate cancer. This dysregulated pathway has an important and relevant role in progression of prostate cancer to bone metastases,76,77 because osteoblasts express ETAR in high density, and tumor-derived ET-1 promotes osteoblast proliferation and new bone formation through this receptor. Osteoblast proliferation generates other growth factors that appear to promote local metastatic bone formation.
Atrasentan blocks the ET-1/ETAR pathway and may therefore block the role of osteoblasts in promoting bone metastases in prostate cancer. A randomized phase 3 clinical trial comparing daily oral atrasentan with placebo in patients with castration-recurrent prostate cancer with increasing PSA levels or PSA greater than 20 ng/mL in chemotherapy-naive patients was unable to demonstrate a significant difference in the primary end point of time to disease progression.78 Despite these results, patients receiving atrasentan showed a lesser increase in levels of bone alkaline phosphatase, a marker for bone formation. Currently, atrasentan with or without docetaxel is being evaluated in a randomized study led by the Southwest Oncology Group in men with castration-recurrent prostate cancer, with the end point of prolonging progression-free survival in this population. Docetaxel combinations with other antiangiogenic agent combinations in castration-recurrent disease include early efficacy trials with thalidomide79 and exploratory trials with sorafenib, a potent multityrosine kinase inhibitor that blocks BRAF, RAF1 (formerly c-Raf), KIT (formerly c-KIT), KDR (formerly VEGFR2), and PDGFRB.
IMMUNOTHERAPY
Independent of cytotoxic approaches, prostate cancer has been considered as an ideal candidate for immunotherapy development because its natural history allows sufficient time for the generation of potentially effective antitumor immune responses. Thus, attempts to treat prostate cancer by immunotherapy have begun to yield encouraging results.80,81 Treatment of advanced, metastatic disease (in both chemotherapy-naive and chemotherapy-treated patients) with dendritic cells expressing human prostatic acid phosphatase fused to GM-CSF (sipuleucel-T; Dendreon, Seattle, WA) resulted in a short survival benefit, indicating the safety and efficacy of the approach in advanced-stage prostate cancer.80 The updated effects of sipuleucel-T on metastatic castration-recurrent prostate cancer were presented at the 2009 annual conference of the American Urology Association, showing a 4-month survival benefit in this population of patients who had received the vaccine.82 This is the only phase 3 cancer vaccine trial that has shown a survival advantage. Single antigen—targeting of prostate cancer is attractive because prostate cancer cells, even with relapse after hormonal treatment, usually continue to express the characteristic levels of PSA, prostatic acid phosphatase, and prostate-specific membrane antigen. However, targeting single vs multiple antigens carries a higher risk that the tumor will escape immune recognition, leading to investigations of the targeting of a broader spectrum of tumor-associated antigens. The efficacy and safety of GM-CSF gene—transduced, irradiated prostate cancer cellular vaccine (GVAX; Cell Genesys, San Francisco, CA) that uses exogenous tumor cells programmed to secrete GM-CSF for enhancing dendritic cell presentation of antigens to the immune system had initially produced promising results, but these results have yet to be confirmed in comparative phase 3 clinical trials with chemotherapy.
Another immune modulation approach explored in clinical trials has been to block CTLA4 using an anti-CTLA4 antibody. A costimulatory molecule on the surface of T cells, CTLA4 (together with CD28) binds to the same ligands present on antigen-presenting cells. Unlike CD28 binding, which leads to T-cell stimulation, CTLA4 binding leads to inhibition of T-cell stimulation. Blockade of CTLA4 has been shown to potentiate T-cell responses, representing a potential antitumoral immunotherapy strategy, and is being tested in multiple malignancies, including castration-recurrent prostate cancer, with promising results.82
Despite the initial success of immunotherapy, many men still develop progressive disease, and cancer vaccine trial results need to be validated before the widespread use of cancer vaccines to treat prostate cancer is instituted. Immunotherapy remains an attractive option in view of its documented antitumor activity, low toxicity, and the potential to alter the natural history of the disease.
SUMMARY
Understanding prostate cancer biology has yielded meaningful results, some of which have translated into a clinical benefit in the medical management of prostate cancer. Current therapeutic strategies based on novel chemotherapeutic agents, immunotherapy, biologic agents, or a combination of these at different stages of cancer progression focus on enhancing quality of life and prolonging longevity as well as on developing prognostic and predictive biomarkers. Key current and previous therapeutic interventions are summarized in the Table. Results from these trials are likely to set the stage for individualized prostate cancer treatments in the future decade.
TABLE.
Summary of Therapeutic Agents Targeting Prostate Cancer Pathways
Supplementary Material
Glossary of Genetics Terminology
- AKT1
akt murine thymoma viral oncogene homolog 1
- AR
androgen receptor
- BAD
BCL2-associated agonist of cell death
- BCL2
B-cell CLL/lymphoma 2
- BRAF
v-raf murine sarcoma viral oncogene homolog B1
- CTLA4
cytotoxic T-lymphocyte-associated protein 4
- EPHB2 (formerly wERK)
EPH receptor B2
- ERG
ETS-related gene
- ETS
rythroblastosis virus E26 transforming sequence
- ETV1
ETS variant 1
- FOX
forkhead box
- GM-CSF
granulocyte-macrophage colony—stimulating factor
- KIT (formerly c-Kit)
v-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog
- KDR (formerly VEGFR)
kinase insert domain receptor (a type III receptor tyrosine kinase)
- MAPK
mitogen-activated protein kinase
- MTOR
mammalian target of rapamycin
- MYC
v-myc myelocytomatosis viral oncogene homolog (avian)
- PDGFRB
platelet-derived growth factor receptor, β polypeptide
- PI3
peptidase inhibitor 3, skin-derived
- PTEN
phosphatase and tensin homolog
- RAF1 (formerly c-Raf)
v-raf-1 murine leukemia viral oncogene homolog 1
- TMPRSS2
transmembrane protease, serine 2
- VEGF
vascular endothelial growth factor
Footnotes
A glossary of genetics terminology appears at the end of this article.
This work was funded, in part, by National Cancer Institute Grant No. CA091956.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin 2008Mar–Apr;58(2):71-96 Epub 2008 Feb 20 [DOI] [PubMed] [Google Scholar]
- 2.Concato J, Wells CK, Horwitz RI, et al. The effectiveness of screening for prostate cancer: a nested case-control study. Arch Intern Med 2006;166(1):38-43 [DOI] [PubMed] [Google Scholar]
- 3.Oberaigner W, Horninger W, Klocker H, Schönitzer D, Stühlinger W, Bartsch G. Reduction of prostate cancer mortality in Tyrol, Austria, after introduction of prostate-specific antigen testing. Am J Epidemiol 2006August15;164(4):376-384 Epub 2006 Jul 7 [DOI] [PubMed] [Google Scholar]
- 4.Bill-Axelson A, Holmberg L, Filén F, et al. Scandinavian Prostate Cancer Group Study Number 4 Radical prostatectomy versus watchful waiting in localized prostate cancer: the Scandinavian prostate cancer group-4 randomized trial. J Natl Cancer Inst 2008August20;100(16):1144-1154 Epub 2008 Aug 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andriole GL, Crawford ED, Grubb RL, III, et al. PLCO Project Team Mortality results from a randomized prostate-cancer screening trial [published correction appears in N Engl J Med. 2009;360(17):1797] N Engl J Med 2009March26;360(13):1310-1319 Epub 2009 Mar 18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schröder FH, Hugosson J, Roobol MJ, et al. ERSPC Investigators Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009March26;360(13):1320-1328 Epub 2009 Mar 18 [DOI] [PubMed] [Google Scholar]
- 7.Gøtzsche PC, Nielsen M. Screening for breast cancer with mammography. Cochrane Database Syst Rev. 2006;(4):CD001877 [DOI] [PubMed] [Google Scholar]
- 8.Tomlins SA, Rhodes DR, Perner S, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 2005;310(5748):644-648 [DOI] [PubMed] [Google Scholar]
- 9.King JC, Xu J, Wongvipat J, et al. Cooperativity of TMPRSS2-ERG with PI3-kinase pathway activation in prostate oncogenesis. Nat Genet 2009May;41(5):524-526 Epub 2009 Apr 29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grönberg H. Prostate cancer epidemiology. Lancet 2003;361(9360):859-864 [DOI] [PubMed] [Google Scholar]
- 11.Johns LE, Houlston RS. A systematic review and meta-analysis of familial prostate cancer risk. BJU Int 2003;91(9):789-794 [DOI] [PubMed] [Google Scholar]
- 12.Amundadottir LT, Sulem P, Gudmundsson J, et al. A common variant associated with prostate cancer in European and African populations. Nat Genet 2006June;38(6):652-658 Epub 2006 May 7 [DOI] [PubMed] [Google Scholar]
- 13.Gudmundsson J, Sulem P, Manolescu A, et al. Genome-wide association study identifies a second prostate cancer susceptibility variant at 8q24. Nat Genet 2007May;39(5):631-637 Epub 2007 Apr 1 [DOI] [PubMed] [Google Scholar]
- 14.Zheng SL, Sun J, Wiklund F, et al. Cumulative association of five genetic variants with prostate cancer. N Engl J Med 2008February28;358(9):910-919 Epub 2008 Jan 16 [DOI] [PubMed] [Google Scholar]
- 15.Sun J, Chang BL, Isaacs SD, et al. Cumulative effect of five genetic variants on prostate cancer risk in multiple study populations. Prostate 2008;68(12):1257-1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huggins C, Hodges CV. Studies on prostatic cancer, I: the effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate: 1941. J Urol 2002;168:9-12 [DOI] [PubMed] [Google Scholar]
- 17.Arnold JT, Isaacs JT. Mechanisms involved in the progression of androgen-independent prostate cancers: it is not only the cancer cell's fault. Endocr Relat Cancer 2002;9(1):61-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russell DW, Wilson JD. Steroid 5 α-reductase: two genes/two enzymes. Annu Rev Biochem 1994;63:25-61 [DOI] [PubMed] [Google Scholar]
- 19.Edwards J, Bartlett JM. The androgen receptor and signal-transduction pathways in hormone-refractory prostate cancer, Part 1: modifications to the androgen receptor. BJU Int 2005;95(9):1320-1326 [DOI] [PubMed] [Google Scholar]
- 20.Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 2009May8;324(5928):787-790 Epub 2009 Apr 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Isaacs JT, Furuya Y, Berges R. The role of androgen in the regulation of programmed cell death/apoptosis in normal and malignant prostatic tissue. Semin Cancer Biol 1994;5(5):391-400 [PubMed] [Google Scholar]
- 22.The Medical Research Council Prostate Cancer Working Party Investigators Group Immediate versus deferred treatment for advanced prostatic cancer: initial results of the Medical Research Council Trial. Br J Urol 1997;79(2):235-246 [DOI] [PubMed] [Google Scholar]
- 23.Vicini FA, Vargas C, Abner A, Kestin L, Horwitz E, Martinez A. Limitations in the use of serum prostate specific antigen levels to monitor patients after treatment for prostate cancer. J Urol 2005;173(5):1456-1462 [DOI] [PubMed] [Google Scholar]
- 24.Crawford ED, Eisenberger MA, McLeod DG, et al. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med 1989;321(7):419-424 [DOI] [PubMed] [Google Scholar]
- 25.Denis LJ, Keuppens F, Smith PH, et al. EORTC Genito-Urinary Tract Cancer Cooperative Group and the EORTC Data Center Maximal androgen blockade: final analysis of EORTC phase III trial 30853. Eur Urol 1998;33(2):144-151 [DOI] [PubMed] [Google Scholar]
- 26.Eisenberger MA, Blumenstein BA, Crawford ED, et al. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med 1998;339(15):1036-1042 [DOI] [PubMed] [Google Scholar]
- 27.Smith JA, Jr, Lange PH, Janknegt RA, Abbou CC, deGery A. Serum markers as a predictor of response duration and patient survival after hormonal therapy for metastatic carcinoma of the prostate. J Urol 1997;157(4):1329-1334 [PubMed] [Google Scholar]
- 28.Small EJ, Roach M., III Prostate-specific antigen in prostate cancer: a case study in the development of a tumor marker to monitor recurrence and assess response. Semin Oncol 2002;29(3):264-273 [DOI] [PubMed] [Google Scholar]
- 29.Quinn DI, Henshall SM, Sutherland RL. Molecular markers of prostate cancer outcome. Eur J Cancer 2005;41(6):858-887 [DOI] [PubMed] [Google Scholar]
- 30.van Gils MPMQ, Stenman UH, Schalken JA, et al. Innovations in serum and urine markers in prostate cancer: current European research in the P-Mark project. Eur Urol 2005December;48(6):1031-1041 Epub 2005 Jul 1 [DOI] [PubMed] [Google Scholar]
- 31.Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol 2006;24(27):4448-4456 [DOI] [PubMed] [Google Scholar]
- 32.Saigal CS, Gore JL, Krupski TL, Hanley J, Schonlau M, Litwin MS, Urologic Diseases in America Project Androgen deprivation therapy increases cardiovascular morbidity in men with prostate cancer. Cancer 2007;110(7):1493-1500 [DOI] [PubMed] [Google Scholar]
- 33.Klotz L. Hormone therapy for patients with prostate carcinoma. Cancer 2000;88(12 suppl):3009-3014 [DOI] [PubMed] [Google Scholar]
- 34.Balk SP. Androgen receptor as a target in androgen-independent prostate cancer. Urology 2002;60(3, suppl 1):132-138 [DOI] [PubMed] [Google Scholar]
- 35.Holzbeierlein J, Lal P, LaTulippe E, et al. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am J Pathol 2004;164(1):217-227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mohler JL. Castration-recurrent prostate cancer is not androgen-independent. Adv Exp Med Biol 2008;617:223-234 [DOI] [PubMed] [Google Scholar]
- 37.Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res 2008;68(11):4447-4454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dehm SM, Tindall DJ. Molecular regulation of androgen action in prostate cancer. J Cell Biochem 2006;99(2):333-344 [DOI] [PubMed] [Google Scholar]
- 39.Small EJ, Halabi S, Dawson NA, et al. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583). J Clin Oncol 2004;22:1025-1033 [DOI] [PubMed] [Google Scholar]
- 40.Kruit WH, Stoter G, Klijn JG. Effect of combination therapy with aminoglutethimide and hydrocortisone on prostate-specific antigen response in metastatic prostate cancer refractory to standard endocrine therapy. Anticancer Drugs 2004;15(9):843-847 [DOI] [PubMed] [Google Scholar]
- 41.Yap TA, Carden CP, Attard G, de Bono JS. Targeting CYP17: established and novel approaches in prostate cancer. Curr Opin Pharmacol 2008August;8(4):449-457 Epub 2008 Jul 28 [DOI] [PubMed] [Google Scholar]
- 42.Reid AH, Attard G, Barrie E, de Bono JS. CYP17 inhibition as a hormonal strategy for prostate cancer. Nat Clin Pract Urol 2008;5(11):610-620 [DOI] [PubMed] [Google Scholar]
- 43.Attard G, Reid AH, Yap TA, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol 2008October1;26(28):4563-4571 Epub 2008 Jul 21 [DOI] [PubMed] [Google Scholar]
- 44.Attard G, Reid AH, A'Hern R, et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2009August10;27(23):3742-3748 Epub 2009 May 26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmidt LJ, Murillo H, Tindall DJ. Gene expression in prostate cancer cells treated with the dual 5 alpha-reductase inhibitor dutasteride. J Androl 2004;25(6):944-953 [DOI] [PubMed] [Google Scholar]
- 46.Andriole GL, Humphrey P, Ray P, et al. Effect of the dual 5α-reductase inhibitor dutasteride on markers of tumor regression in prostate cancer. J Urol 2004;172(3):915-919 [DOI] [PubMed] [Google Scholar]
- 47.Iczkowski KA, Qiu J, Qian J, et al. The dual 5-alpha-reductase inhibitor dutasteride induces atrophic changes and decreases relative cancer volume in human prostate. Urology 2005;65(1):76-82 [DOI] [PubMed] [Google Scholar]
- 48.Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med 2003July17;349(3):215-224 Epub 2003 Jun 24 [DOI] [PubMed] [Google Scholar]
- 49.Andriole G, Bostwick D, Brawley O, et al. REDUCE Study Group Chemoprevention of prostate cancer in men at high risk: rationale and design of the Reduction by Dutasteride of Prostate Cancer Events (REDUCE) trial. J Urol 2004;172(4, pt 1):1314-1317 [DOI] [PubMed] [Google Scholar]
- 50.Krishnan AV, Peehl DM, Feldman D. Inhibition of prostate cancer growth by vitamin D: regulation of target gene expression. J Cell Biochem 2003;88(2):363-371 [DOI] [PubMed] [Google Scholar]
- 51.Ahonen MH, Tenkanen L, Teppo L, Hakama M, Tuohimaa P. Prostate cancer risk and prediagnostic serum 25-hydroxyvitamin D levels (Finland). Cancer Causes Control 2000;11(9):847-852 [DOI] [PubMed] [Google Scholar]
- 52.Hershberger PA, Yu WD, Modzelewski RA, Rueger RM, Johnson CS, Trump DL. Calcitriol (1,25-dihydroxycholecalciferol) enhances paclitaxel antitumor activity in vitro and in vivo and accelerates paclitaxel-induced apoptosis. Clin Cancer Res 2001;7(4):1043-1051 [PubMed] [Google Scholar]
- 53.Skowronski RJ, Peehl DM, Feldman D. Vitamin D and prostate cancer: 1,25 dihydroxyvitamin D3 receptors and actions in human prostate cancer cell lines. Endocrinology 1993;132(5):1952-1960 [DOI] [PubMed] [Google Scholar]
- 54.Zhao XY, Ly LH, Peehl DM, Feldman D. 1α,25-dihydroxyvitamin D3 actions in LNCaP human prostate cancer cells are androgen-dependent. Endocrinology 1997;138(8):3290-3298 [DOI] [PubMed] [Google Scholar]
- 55.Hsieh T, Wu JM. Induction of apoptosis and altered nuclear/cytoplasmic distribution of the androgen receptor and prostate-specific antigen by 1α,25-dihydroxyvitamin D3 in androgen-responsive LNCaP cells. Biochem Biophys Res Commun 1997;235(3):539-544 [DOI] [PubMed] [Google Scholar]
- 56.Gross C, Stamey T, Hancock S, Feldman D. Treatment of early recurrent prostate cancer with 1,25-dihydroxyvitamin D3 (calcitriol) [published correction appears in J Urol. 1998;160(3, pt 1):840] J Urol 1998;159(6):2035-2039 [DOI] [PubMed] [Google Scholar]
- 57.Beer TM, Ryan CW, Venner PM, et al. Double-blinded randomized study of high-dose calcitriol plus docetaxel compared with placebo plus docetaxel in androgen-independent prostate cancer: a report from the ASCENT Investigators. J Clin Oncol 2007;25(6):669-674 [DOI] [PubMed] [Google Scholar]
- 58.Malik SN, Brattain M, Ghosh PM, et al. Immunohistochemical demonstration of phospho-Akt in high Gleason grade prostate cancer. Clin Cancer Res 2002;8(4):1168-1171 [PubMed] [Google Scholar]
- 59.McMenamin ME, Soung P, Perera S, Kaplan I, Loda M, Sellers WR. Loss of PTEN expression in paraffin-embedded primary prostate cancer correlates with high Gleason score and advanced stage. Cancer Res 1999;59(17):4291-4296 [PubMed] [Google Scholar]
- 60.Kremer CL, Klein RR, Mendelson J, et al. Expression of mTOR signaling pathway markers in prostate cancer progression. Prostate 2006;66(11):1203-1212 [DOI] [PubMed] [Google Scholar]
- 61.Huang H, Cheville JC, Pan Y, Roche PC, Schmidt LJ, Tindall DJ. PTEN induces chemosensitivity in PTEN-mutated prostate cancer cells by suppression of Bcl-2 expression. J Biol Chem 2001October19;276(46):38830-38836 Epub 2001 Aug 8 [DOI] [PubMed] [Google Scholar]
- 62.Morgan TM, Pitts TE, Gross TS, Poliachik SL, Vessella RL, Corey E. RAD001 (Everolimus) inhibits growth of prostate cancer in the bone and the inhibitory effects are increased by combination with docetaxel and zoledronic acid. Prostate 2008;68(8):861-871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gioeli D. Signal transduction in prostate cancer progression. Clin Sci (Lond) 2005;108(4):293-308 [DOI] [PubMed] [Google Scholar]
- 64.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 2004;351(15):1513-1520 [DOI] [PubMed] [Google Scholar]
- 65.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004;351(15):1502-1512 [DOI] [PubMed] [Google Scholar]
- 66.Tannock IF, Osoba D, Stockler MR, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol 1996;14(6):1756-1764 [DOI] [PubMed] [Google Scholar]
- 67.Kantoff PW, Halabi S, Conaway M, et al. Hydrocortisone with or without mitoxantrone in men with hormone-refractory prostate cancer: results of the cancer and leukemia group B 9182 study. J Clin Oncol 1999;17(8):2506-2513 [DOI] [PubMed] [Google Scholar]
- 68.Wosikowski K, Lamphere L, Unteregger G, et al. Preclinical antitumor activity of the oral platinum analog satraplatin. Cancer Chemother Pharmacol 2007September;60(4):589-600 Epub 2007 May 31 [DOI] [PubMed] [Google Scholar]
- 69.Sternberg CN, Whelan P, Hetherington J, et al. Phase III trial of satraplatin, an oral platinum plus prednisone vs. prednisone alone in patients with hormone-refractory prostate cancer. Oncology 2005;68(1):2-9 Epub 2005 Feb 28 [DOI] [PubMed] [Google Scholar]
- 70.Kaushal V, Mukunyadzi P, Dennis RA, Siegel ER, Johnson DE, Kohli M. Stage-specific characterization of the vascular endothelial growth factor axis in prostate cancer: expression of lymphangiogenic markers is associated with advanced-stage disease. Clin Cancer Res 2005;11(2, pt 1):584-593 [PubMed] [Google Scholar]
- 71.Guise TA, Mohammad KS. Endothelins in bone cancer metastases. Cancer Treat Res 2004;118:197-212 [DOI] [PubMed] [Google Scholar]
- 72.George DJ, Halabi S, Shepard TF, et al. Prognostic significance of plasma vascular endothelial growth factor levels in patients with hormone-refractory prostate cancer treated on Cancer and Leukemia Group B 9480. Clin Cancer Res 2001;7(7):1932-1936 [PubMed] [Google Scholar]
- 73.Di Lorenzo G, Figg WD, Fossa SD, et al. Combination of bevacizumab and docetaxel in docetaxel-pretreated hormone-refractory prostate cancer: a phase 2 study. Eur Urol 2008November;54(5):1089-1094 Epub 2003 Feb 5 [DOI] [PubMed] [Google Scholar]
- 74.Nelson J, Bagnato A, Battistini B, Nisen P. The endothelin axis: emerging role in cancer. Nat Rev Cancer 2003;3(2):110-116 [DOI] [PubMed] [Google Scholar]
- 75.Papandreou CN, Usmani B, Geng Y, et al. Neutral endopeptidase 24.11 loss in metastatic human prostate cancer contributes to androgen-independent progression. Nat Med 1998;4(1):50-57 [DOI] [PubMed] [Google Scholar]
- 76.Mundy GR. Endothelin-1 and osteoblastic metastasis. Proc Natl Acad Sci U S A 2003September16;100(19):10588-10589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yin JJ, Mohammad KS, Käkönen SM, et al. A causal role for endothelin-1 in the pathogenesis of osteoblastic bone metastases. Proc Natl Acad Sci U S A 200September16;100(19):10954-10959 Epub 2003 Aug 26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Carducci MA, Saad F, Abrahamsson PA, et al. Atrasentan Phase III Study Group Institutions A phase 3 randomized controlled trial of the efficacy and safety of atrasentan in men with metastatic hormone-refractory prostate cancer. Cancer 2007;110(9):1959-1966 [DOI] [PubMed] [Google Scholar]
- 79.Dahut WL, Gulley JL, Arlen PM, et al. Randomized phase II trial of docetaxel plus thalidomide in androgen-independent prostate cancer. J Clin Oncol 2004;22(13):2532-2539 [DOI] [PubMed] [Google Scholar]
- 80.Small EJ, Schellhammer PF, Higano CS, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol 2006;24(19):3089-3094 [DOI] [PubMed] [Google Scholar]
- 81.Simons JW, Sacks N. Granulocyte-macrophage colony-stimulating factor-transduced allogeneic cancer cellular immunotherapy: the GVAX vaccine for prostate cancer. Urol Oncol 2006;24(5):419-424 [DOI] [PubMed] [Google Scholar]
- 82.Schellhammer PF, Higano C, Berger ER, et al. IMPACT Study Investigators Arandomized, double-blind, placebo-controlled, multi-center, phase III trial of sipuleucel-T in men with metastatic, androgen independent prostatic adenocarcinoma (AIPC) Abstract presented at: Late-Breaking Science Forum; American Urological Association 104th Annual Scientific Meeting; April28, 2009; Chicago, IL Abstract 9 [Google Scholar]
- 83.Small EJ, Tchekmedyian NS, Rini BI, Fong L, Lowy I, Allison JP. A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clin Cancer Res 2007;13(6):1810-1815 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.