Conspectus
Recent work at the interface of stem biology and biomaterials science has demonstrated that while our ability to mimic the natural stem cell microenvironment is rather limited, our control over stem cell behavior with artificial microenvironments is quite advanced. Embryonic and adult stem cells are potentially useful platforms for tissue regeneration, cell based therapeutics and disease-in-a-dish models for drug screening. The major challenge in this field has been to reliably control their behavior outside the body. Common biological control schemes often ignore physicochemical parameters, such as substrate topography and mechanical properties, rheological properties and others that materials scientists and engineers commonly control. With appropriate attention to these parameters novel synthetic microenvironments have been designed to control stem cell behavior in rather unnatural ways.
In this Account we review synthetic microenvironments that try to overcome the limitations of natural niches rather than attempt to mimic them. The major limitation of a biomimetic stem cell control strategy is an incomplete understanding of the complex signaling pathways that drive stem cell behavior from early embryogenesis to late adulthood. The stem cell extracellular environment presents a miscellany of competing biological signals that keep the cell in a state of unstable equilibrium. Synthetic polymers have been used to design synthetic microenvironments with an uncluttered array of cell signals, both specific and non-specific, that are motivated rather modeled after biology. These have proven useful in maintaining cell potency, studying asymmetric cell division, and controlling cellular differentiation.
We discuss recent works that highlight important biomaterials properties for controlling stem cell behavior as well as advanced selection processes, such as combinatorial and high throughput screening. Much of this work utilized micro- and nanoscale fabrication tools for controlling material properties and generating diversity in both 2D and 3D. Hydrogels have become a biomaterial of choice for generating 3D microenvironments due to their ease of synthesis and similarity to biological soft matter. These are presented in the framework of synthetic biology with the goal the future researchers may exploit synthetic polymers in creating microenvironments that control stem cell behavior in ways that are clinically relevant.
1. Introduction
Embryonic stem cells (ESCs) can differentiate into any adult cell type1, while adult stem cells (ASCs) are restricted to certain lineages2. Both offer powerful new tools for regenerating lost tissue as well as advancing our understanding of early human development, pathophysiology and epigenetics. Our ability to exploit the power of stem cells has been limited by poor control over the complex signaling events that influence their differentiation. Recently there has been great progress in engineering polymeric biomaterials that control stem cell fate3. The insight they provide on stem cell behavior in vivo depends on how closely they mimic naturally occurring stem cell microenvironment, or `niche'. But it may not be true that only by reconstituting elements of the natural niche can we truly derive therapeutic benefits from stem cells. A biomimetica approach to creating synthetic microenvironments comes with formidable challenges because there is much we don't yet know about the natural stem cell microenvironment. Therapeutically, it may be more useful to take a bio-inspiredb approach to design, where the synthetic niche acts on the stem cells in an unnatural way to achieve a therapeutic goal.
The stem cell niche is a dynamic ensemble of physicochemical and biological cues that provide the cell with vital decision making information. It can be broken down into three major components: cell-cell contacts, cell-extracellular matrix (ECM) and cell-soluble factor interactions4. These components can be broken down further. For example, the ECM provides topographical, mechanical and biochemical input to stem cell. Cell-cell communication can be heterologous (e.g., neural input), and homologous, such as with daughter cells. Likewise, soluble factors can be endocrine or paracrine in origin (Fig. 1). Modeling these components ex vivo can be a difficult feat while integrating them in a controlled way has proven to be exceedingly complex. While all of these components may control in vivo stem cell differentiation, they all may not be necessary in vitro. In this review we demonstrate that we can compensate for a lack of natural materials in our design of synthetic microenvironments by exerting greater control over the natural materials present or by engineering synthetic materials with enhanced signaling properties. In this way we can create synthetic stem cell niches that are more bio-inspired than biomimetic5 and potentially more efficient than those observed in nature.
Figure 1.

Control Elements of the stem cell niche. The adult stem cell (ASC) can receive both long and short range paracrine and endocrine signals, neural input, and architectural, mechanical and trophic cues from the ECM. Cell-cell communication can occur between the ASC and heterologous niche cells (HNC) or daughter cells (DC). Reprinted by permission from Nature Publishing Group: ref. 4 Copyright 2006.
2. Engineering the Stem Cell Niche: Mimicking What We Know
In order to appropriately model the in vivo stem cell niche we must first know all necessary niche components along with individual and synergistic effects on cell behavior. Due to a lack of this knowledge, many of the in vitro methods currently used to control stem cell behavior are poor biomimics. A large amount of the work up until now has been based on single stem cell types exposed to a range of experimental conditions, without regard for cell source or interspecies variations. ESCs potentially provide a more uniform platform to study cell-material interactions. But there have been documented differences between ESC lines in the same species6. ASCs may be more attractive to use therapeutically, for both ethical and immunological reasons, but they also present great challenges. For example, ASCs can undergo lineage transitions we are only beginning to understand, such as transdifferentiation7 and malignant transformation8. The fact that naturally occurring ASC niches are as varied as the stem cells they nurture adds an additional layer of complexity. Furthermore, synthetic materials with similar properties may have dissimilar effects on stem cells of different species or with different degrees of potency9. Thus, before any feasible model is developed for the rational design of biomimetic stem cell niches, further attention to cell source variation will be needed.
Stem cells may be crucial in regenerating lost or damaged tissue beyond the human body's natural ability to heal itself. But there is an inherent causality dilemma in trying to design biomimetic niches to control stem cell behavior. The natural niche is developmentally derived from stem cell activity, whereas adult stem cell activity is directed by the niche. It is unclear exactly when during development ASC niches are established and by which cell types. But the microenvironments where these cells reside have certain similarities, such as their close proximity to basement membranes and vasculature, and relative protection from damage10. Their numbers, along with their resident cells, also decline with age10. This process is accelerated by damage and disease, further limiting the body's natural regenerative capacity. Biomaterials could potentially alleviate this decline through the targeted delivery of bioactive compounds and cells or by providing endogenous stem cells with additional niches (Fig. 2).
Figure 2.
Biomaterials can be used to modulate the natural stem cell microenvironment. (a) Local delivery of bioactive niche components or inhibitory/stimulatory molecules from a solid (injectable) biomaterial scaffold. (b) Targeting the niche via micro- or nanoparticles that carry and delivery bioactive molecules to manipulate the niche. (c) Local delivery of support cells to augment or manipulate stem cell fates in vivo. Cell delivery could be facilitated using (injectable) biomaterials carriers that likely improve the survival and engraftment of the transplanted cells. (d) Implanted, multicomponent artificial niche that could possibly attract stem cells to populate it (d). Reprinted with permission from Ref. 3 Copyright 2009 Wiley-VCH.
The pharmaceutical field began with a focus on understanding the activity of naturally occurring therapeutic compounds and eventually moved towards studying unnatural drugs with greater therapeutic potential. The same shift is occurring in the stem cell community. Good evidence of this is the recent identification of (−)-indolactam, a small molecule that directs ESCs towards a pancreatic lineage11. Biomaterials have continued this shift by giving the stem cell researcher new ways to control the major components of the stem cell milieu.
3. Synthetic Polymer Microenvironments: Design Considerations
Many of the new biomaterials used to develop synthetic stem cell microenvironments are based on synthetic polymers. These can be procured from commercial sources or freshly synthesized. They come with ample control over size and shape, and possess tailorable material properties. Synthetic polymer production can also be more easily scaled up than bioderived materials. A wide variety of synthetic polymers have been evaluated as cell substrates and scaffolds for tissue regeneration, with varying degrees of success12. Before these materials can be effectively used in artificial microenvironments an integrated expertise in both stem cell biology and polymer science is needed. This includes an understanding of the mutual interplay between cells and materials and how they may change each other's properties. Many of the works we have reviewed here show that material properties can be considered biological signals. Therefore the limited range of properties inherent in many biomaterials needs to be considered before they are used.
3.1 Modeling the Extracellular Matrix
The most basic function of synthetic microenvironments is to act as a physical substrate for stem cell attachment and migration, similar to the natural ECM. The natural ECM is a heterogeneous, self-assembled network of biological macromolecules with structural hierarchy. The molecular constituents of the ECM bind specifically to cells, other matrix molecules and soluble factors, with spatiotemporal control. Stem cells can bind to different components of the ECM either directly or via intermediate factors, with varying adhesion affinity. The ECM can influence stem cell behavior via specific interactions with cells, such as with certain adhesive ligands or nonspecifically through its physicochemical properties.
The diverse biological functions of the ECM are still being uncovered. But lack of knowledge has not limited the success of synthetic ECM analogues. Gerecht and coworkers13 used one constituent of the ECM present during the early stages of embryogenesis, hyaluronic acid (HA), to develop microenvironments that inhibited human ESC differentiation. This was achieved by encapsulating the ESCs in a photopolymerized HA hydrogel, a configuration with homogeneity and structural coherence otherwise not encountered during embryogenesis. Other groups have also controlled stem cell behavior by reconfiguring single elements of the natural ECM, such as collagen14, fibronectin, and laminin15 orby utilizing cooperative effects of multiple ECM molecules. For example, Bhatia and co-workers16 analyzed combinatorial mixtures of ECM molecules for cooperative control over murine ESC differentiation, and rapidly identified key mixtures with synergistic properties. Nakajima et al.17 screened combinatorial mixtures of ECM proteins combined with immobilized growth factors for controlling neural stem cell differentiation, uncovering synergistic effects between certain matrix components and soluble factors. This work demonstrates the rigorous and potentially protracted empirical work needed to find the right niche for the right stem cell, particularly for the stem cell researcher without access to microfabrication technology. One way around this is to use bioderived ECM as starting materials, such as small intestine submucosa18, or Matrigel™ 19, which have both proven superior to synthetic substrates in certain applications. However, these materials are generally xenogenic and prone to batch-to-batch inconsistency. Also, as poorly understood materials they provide little mechanistic information for controlling stem cells.
3.1.1 Synthetic Extracellular Matrices
What is fascinating about many of the synthetic biomaterials that have been developed to control stem fate is both how much and how little they mimic the natural ECM. For example, poly(ethylene glycol) (PEG) hydrogels are a popular biomaterial for encapsulating stem cells. They mimic the natural ECM in their ability to imbibe large amounts of water and can be tailored to possess mechanical properties similar to various natural tissue types. But these nonionic and covalently crosslinked networks are very different from the self-assembled polyelectrolytes that comprise the bulk of natural ECMs. Likewise, synthetically degradable biomaterials often have non-biologically controlled degradation rates, whereas the turnover of the natural ECM is largely dictated by needs of the resident cell types. Similarly, inorganic surfaces and other highly crystalline materials are substrates that stem cells generally do not contact in vivo.
There are those biomaterials that control stem cell behavior primarily because of their biomimicry. For example, Engler et al.20 showed that MSCs differentiate into tissues that most closely match the mechanical properties of the polyacrylamide substrate upon which they were cultured (Fig. 3). Thus, MSCs that were cultured on stiff (bone-like) gels differentiated into osteoblasts, those that were cultured on medium stiffness (muscle-like) gels differentiated into muscle cells and those that were cultured on compliant gels (neural-like) differentiated into neural cells. Due to the heterogeneity of MSCs, it is unclear if the same cell type responded differently to substrate stiffness or stiffness resulted in the differential growth of pre-committed progenitor cells. Benoit et al.21 modeled the unique chemical environments present in various tissue types and the potential cues these provide to resident stem cells. In their work PEG hydrogels were doped with small amounts of pendant carboxyl groups to mimic glycosaminoglycans in cartilage, phosphate groups for their role in bone mineralization, or tert-butyl groups to mimic the lipid rich environment in adipose tissue. True to their model, these gels were successfully able to direct the differentiation of human MSCs down chondrogenic, osteogenic or adipogenic pathways, respectively.
Figure 3.
Substrate elasticity directs the differentiation of MSCs. (A) The range of elastic modulus, E for select tissues. (B) MSCs placed on substrates with varied stiffness are initially small and round but overtime change morphology according to substrate elasticity. (i) cell branching per length of primary mouse neurons, MSCs, and blebbistatin-treated MSCs and (ii) spindle morphology of MSCs, blebbistatin-treated MSCs, and mitomycin-C treated MSCs (open squares) compared to C2C12 myoblasts (dashed line). Reprinted with permission from ref. 20 Copyright 2006 Elsevier.
While isolated ECM proteins and mixtures thereof can provide a more controlled ECM mimic, a more reductionist approach to controlling stem cell fate is to use individual peptide sequences and epitopes derived from ECM molecules or by modeling the basic physicochemical properties of the ECM itself. In this way, ECM signaling cues can be utilized without the redundancy and complexity of the natural material. Hybrid microenvironments that consist of both natural and synthetic components can allow certain biological cues to be used in otherwise non-natural ways. A good example of this is the use of substrates with covalently attached peptide sequences such as matrix metalloproteinase sensitive peptide sequences22 within stem cell microenvironments. This allows for `cell demanded' matrix degradation without the additional signaling cues present in many of the natural proteins from which these peptides are derived. Recently Martino et al.23 were able to isolate peptide fragments from fibronectin that were able to enhance the osteogenic differentiation of human MSCs compared to the full length protein. The peptide showed increased specificity for integrin α5β1 in contrast to the relatively promiscuous integrin binding of fibronectin, which provides both pro- and anti-differentiative cues in the natural MSC niche. Certain peptides can be designed to self-assemble in situ to form synthetic microenvironments. They can be designed to assemble into 3D ECM-like supramolecular structures but with greater control over both architecture and cell signaling. They are an attractive alternative to poorly understood substrates such as Matrigel™. In certain cases, they have been shown to perform as well or better. Gelain et al.24 designed self-assembling peptides scaffolds with pre-programmed cell adhesion, differentiation and bone marrow homing motifs for controlling NSC differentiation compared with Matrigel™. The peptide scaffold was similar to Matrigel™ in its ability to induce neuronal differentiation but caused a different and more uniform gene expression. Silva et al.25 have also engineered amphiphilic, self-assembling peptides of particle interest in stem cell differentiation. These are comprised of a peptide component and a lipophilic hydrocarbon tail. In physiological conditions these molecules undergo an ordered arrangement of entangled nanofibers, similar to the nanofibrous architecture of the native ECM. These peptides can be tailor made with biological specificity. When these were designed to display the laminin-derived IKVAV peptide sequence they proved effective at directing mNSCs towards a neuronal fate.
3.2 Combinatorial and High Throughput Screening
Combinatorial and high throughput screening (CHTS) can be extremely useful for selecting polymers for biomedical applications, particularly where there is a lack of mechanistic information. Combined with micro- and nanofabrication, CTHS can be performed using small amounts of sample and at the cellular size scale. For example, Langer and coworkers26,27 used CTHS to identify polymers that supported the attachment and proliferation of mesenchymal stem cells (MSCs) and ESCs (Fig. 4). Others have similarly used CTHS to analyze stem cells exposed to libraries of synthetic polymers, mixtures of ECM proteins, and soluble factors28–30. To date, most of the work done investigating stem cell-biomaterial interactions using CTHS has been conducted using 2D surfaces. This limits the amount of mechanistic information that be can be derived from the results these studies and therefore makes it difficult to determine how closely they model natural stem cell niche.
Figure 4.
Biomaterial array designed for high throughput analysis of hESC-substrate interactions. (a) monomers used for array synthesis, (b) combinations for the major monomer 1 with monomers A–F, (c) a polymer microarray in triplicate with a close-up of 8 spots, (d) merged fluorescence and laser scattering images showing three representative cell attachments (high, intermediate, low) on the polymer spots. (scale bar = 100mm). (e) Reproduced large-scale stem cell adhesion on polymer film (scale bar = 200mm) Reprinted with permission from ref. 27 Copyright 2009 Wiley-VCH.
3.3 Hydrogel Microenvironments
Hydrogels have become a biomaterial of choice for 3D stem cell work. These materials are used as substrates, scaffolds and encapsulants for stem cells due to their tissue-like tunable material properties. These networks can be held together via physical or chemical crosslinks, can be made biodegradable, and can be engineered to controllably deliver soluble factors31. While hydrogel crosslinking is controllable, it may not be straightforward to modify other gel properties independently. The swelling ratio, mesh size and mechanical properties of hydrogels all can vary with crosslinking and changes in these properties have been correlated with changes in stem cell behavior20,32.
Covalently crosslinked hydrogels offer greater structural integrity and can be easily synthesized using methods such as photopolymerization33, thermally initiated polymerization32,34, and Michael addition23,35. Hydrogels niches can also be self-assembled (i.e., physically crosslinked) from polymers such as polysaccharides and custom-made peptides31. Care should be taken in the fabrication of hydrogels niches formed for the purpose of stem cell encapsulation or in situ polymerization. In case of free radical polymerization, the chemical environment around the cell changes drastically throughout the course of the reaction. This process has been shown to negatively affect the viability, proliferation and differentiation of ASCs during encapsulation36. In many cases it is unclear how close the reaction comes to completion or how many residual active groups are leftover. This deserves further attention in light of recent work which showed that low concentrations of small functional groups can affect the lineage commitment of encapsulated MSCs 21. An alternative approach is to preform porous hydrogels capable of stem cell infiltration. Recently such a method was developed using gaseous CO2 as a porogen37. These PEG scaffolds possessed interconnected pores ranging in sizes from 100 to 600 μm.
4. Geometric Control of Stem Cell fate
A spatial relationship is known to exist between stem cells and their niche. The microarchitecture of the in vivo stem cell niche can maintain concentration gradients of growth factors, modulate cell-cell adhesion and determine proximity to the vasculature or basement membrane. For example, MSCs are known to show a gradient pattern of differentiation depending on their distance from the endosteal surface38. Similarly, intestinal epithelial cells display a crypt-to-villus pattern of differentiation39, and rodent incisor stem cells show a apical to incisal pattern40. Recently, micro- and nanofabrication schemes have allowed for the design of artificial microenvironments that may reductively shed light on these mechanisms and offer novel new ways to control stem cell fate41,42. Some of the first studies in controlling behavior of individual cells on surfaces were performed by immobilizing cells on micropatterned substrates that were coated with regions of adhesive and non-adhesive molecules. By using this technology it was determined that primary cells on smaller surfaces tended to undergo apoptosis while those that were patterned on larger substrates tended to proliferate43. Similar studies on ASCs demonstrated that changes in cell substrate geometry caused changes in cytoskeletal tension which influenced their differentiation20,44. Recently micropatterned microenvironments have been used to control the differentiation of stem cells exposed to a mixture of pro-differentiative signals. Ruiz et al.44 cultured MSC sheets on micropatterns of controlled shape and exposed them to a mixture of pro-osteogenic and pro-adipogenic morphogens. The cells showed a pattern of differentiation dictated by their spatial arrangement and corresponding cytoskeletal stress. The authors used this mechanism to created multicellular stem cell constructs which mimicked the architecture of normal; a bony tube filled with a fatty core (Fig. 5).
Figure 5.
Spatially controlled differentiation of mesenchymal stem cells into bony (blue) and fatty tissue (red). Planar cell adhesive micropatterns, such as a square (top left) or an offset annulus (top right) provide controlled regions of high and low cytoskeletal stress, thereby influencing differentiation. Scale bar = 250 μm. (bottom right, left) Multicellular 3D constructs differentiate into a fatty core surrounded by bony tissue, similar to natural long bones. Reprinted with permission from reference 44 Copyright 2008 Wiley-VCH.
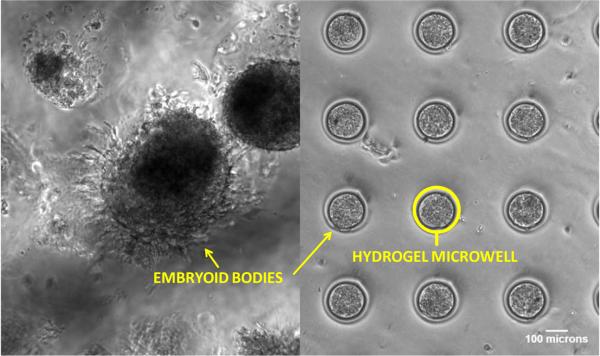
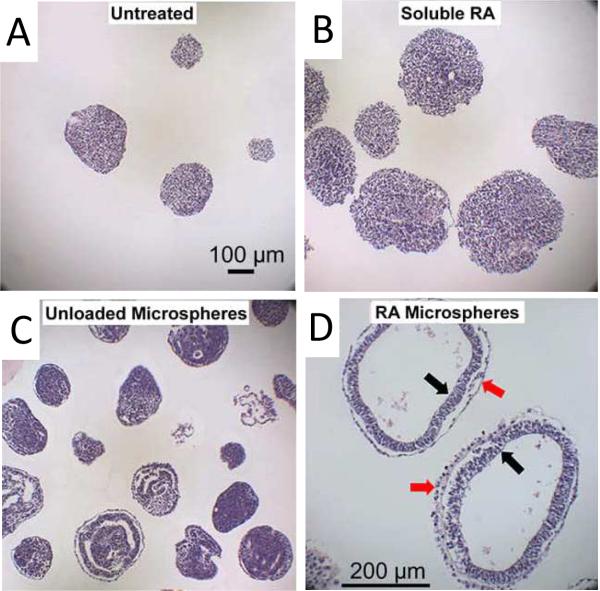
Geometric control is also useful for controlling cell-cell interactions. For example, the aggregation of ESCs in vitro into embryoid bodies (EB) is a preliminary step towards differentiation. One issue with culturing embryoid bodies using conventional methods is poor control over EB formation and a resulting broad range of sizes. Spatial control over the formation of EBs can lead to a more homogeneous, and thereby more efficiently controlled differentiation. Karp et al.45 developed microfabricated hydrogel microwells as a way to control the shape of EBs. Their approach resulted in synthetic microenvironments that enhanced the differentiation of ESCs and significantly reduced variability in the expression of differentiation markers. They were also able to pattern EBs into shapes that do not naturally occur, such a triangles and curves (Fig. 6). The biological implications of this have yet to become clear. In another approach, Carpenedo et al.46 designed morphogen releasing spherical templates to both control the shape and direct the differentiation of EBs. In their work embryonic stem cells were cultured on biodegradable microspheres loaded with retinoic acid. This resulted in the homogenous differentiation of cystic spheroids with a bi-epithelial morphology (Fig. 7). This approach could be useful for applications such as regenerating intestinal tissue for the treatment of short bowel syndrome, where a hollow epithelial construct is required prior to implantation.
Figure 6.
Microfabricated culture wells designed to control the shape of embryoid bodies derived from murine ESCs. (A) Confocal microscopy images of fluorescent labeled EBs within microwells 40–150 in diameter. The technique was also used to create EBs with synthetic shapes, such as triangles (B), and curves (C). Reprinted with permission from ref. 45 Copyright 2007 The Royal Society of Chemistry.
Figure 7.
Controlled delivery of retinoic acid (RA) to embryoid bodies. Untreated EBs (A) as well as EBs treated with soluble RA (B), unloaded microspheres (C), or RA-loaded microspheres (D) were formalin-fixed, sectioned and stained with hematoxylin and eosin after 10 days of differentiation. EBs cultured of morphogen loaded microspheres contained a bi-epithelial morphology, with a columnar, pseudo-stratified inner cell layer (black arrows) and an adjacent, flattened outermost cell layer (red arrows). Reprinted with permission from ref. 46 Copyright 2009 Elsevier Inc.
5. The Right Timing
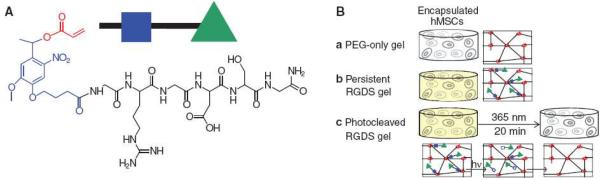
It is well understood that time varying cues are important components of the stem cell niche, particularly in the inherently transient process of embryogenesis. But the bulk of bioengineered microenvironments have relied on sustained signaling schemes for stem cell control. One reason for this is poor knowledge of how signaling events vary in time in the natural niche. Another is that the design of synthetic microenvironments with time varying properties can be quite difficult. An exception is the delivery of soluble factors. For example, the transient delivery of transforming growth factor beta-3 enhanced the mechanical properties of synthetic cartilage derived from MSCs.47 Yet these were still mechanically inferior to constructs derived from chondrocytes. The need for time varying substrate properties is supported by evidence that the effective use of cell adhesion ligands depends on the stage of stem cell commitment.48 More recent work has shown that transient substrate properties can be engineered very effectively into synthetic niches with the right amount of innovation. Anseth and coworkers49 designed hydrogel microenvironments for encapsulating MSCs that contained the pendant photocleavable peptide sequence, Arg-Gly-Asp-Ser (RGDS) (Fig. 8). Taking a cue from nature, these pendant groups were cleaved after ten days of cell culture. This resulted in significantly enhanced chondrogenic differentiation as indicated by a dramatic increase in the production of glycosaminoglycan and type II collagen. This use of light to initiate changes in the biochemical composition of a synthetic microenvironment demonstrates a type of control scheme that is highly effective yet does not naturally occur.
Figure 8.
Hydrogel microenvironments with phototunable properties for stem cell encapsulation. The RGDS peptide sequence was coupled to a photodegradable nitrobenzyl ether acrylate to create a photocleavable monomer (A). The monomer was polymerized into a nondegradable gel which upon light exposure releases the tethered peptide (B). Human MSCs were encapsulated in nondegradable PEG gels (b) with or (a) without photoreleasable RGDS. The presentation of RGDS was temporally altered by (c) photocleavage of RGDS from the gel on day 10 in culture. Reprinted with permission from ref. 49 Copyright © 2009 by the American Association for the Advancement of Science.
6. Conclusions
In this Account we discuss how biomaterials with their controllable physical, chemical and biological properties may be a potentially enabling tool in directing stem cells towards various lineages for a variety of therapeutic applications. Clearly, this field is at its earliest stages, but as has been demonstrated by a number of seminal studies, there is a huge potential in using materials for stem cell biology applications. In particular with advances in the biological understanding of the stem cell niche, it may be possible to merge biomimicry and bioinspiration to form advanced biomaterials that direct stem cell fate in novel ways.
Acknowledgments
This work is supported in part by the National Institutes of Health (Grants EB000246, DE016516, HL60435, HL092836, DE019024), and the Pratt Foundation (Grant 09-398).
Biographies
Omar Fisher is currently a Ford Foundation Postdoctoral Fellow at the David H. Koch Institute for Integrative Cancer Research at MIT. He obtained a B.S. in Biomedical Engineering from Drexel University in 2003, then a Ph.D. in Biomedical Engineering from the University of Texas at Austin in 2008. His current research is focused on developing synthetic macromolecular assemblies to control and understand cell behavior.
Ali Khademhosseini is an Assistant Professor of Medicine and Health Sciences and Technology at Harvard-MIT's Division of Health Sciences and Technology and Harvard Medical School. His research is focused on developing micro- and nanoscale technologies to control cellular behavior with particular emphasis on developing microscale biomaterials for tissue engineering. He has published 1 edited book, 80 peer reviewed papers, 25 book chapters, 100 abstracts, and 17 patent applications. Select awards include Technology Review Magazine's “Top Young Innovator” (TR35) award (2007), the BMW Scientific Award (2007), the ACS Victor K. LaMer Award (2008) and the IEEE-EMBS Early Career Award (2008).
Robert Langer is the David H. Koch Institute Professor at MIT. He has authored over 1,050 articles, has over 750 issued or pending patents, and holds 11 honorary doctorates. He has received over 150 awards including the Charles Stark Draper Prize, considered the engineering equivalent of the Nobel Prize; the Albany Medical Prize, the nation's largest medical prize; the National Medal of Science; and the American Chemical Society's (ACS) Polymer Chemistry, Applied Polymer Science, and Chemistry of Materials Awards. He has been elected to the National Academy of Science, the Institute of Medicine of the National Academy of Science, and the National Academy of Engineering.
Nicholas A. Peppas is the Fletcher S. Pratt Chair in Chemical Engineering, Biomedical Engineering, and Pharmacy, and is the director of the Center for Biomaterials, Drug Delivery and Bionanotechnology at the University of Texas at Austin. He is a member of the National Academy of Engineering, the Institute of Medicine of the National Academies, and the National Academy of Pharmacy of France. He received his Diploma in Engineering (D. Eng.) from the National Technical University of Athens, Greece in 1971 and his Sc.D. from MIT in 1973, both in chemical engineering.
Footnotes
Biomimicry relies on first learning the mechanistic principle used by a living system to achieve a particular function, then attempting to adapt that principle to achieve similar function in a synthetic material (see reference 5).
Bioinspiration relies on merely knowing that a task can be achieved by a living system and developing synthetic systems to achieve the same function, even if this requires a different scheme from that of the biological system (see reference 5).
References
- (1).Odorico JS, Kaufman DS, Thomson JA. Multilineage differentiation from human embryonic stem cell lines. Stem Cells. 2001;19:193–204. doi: 10.1634/stemcells.19-3-193. [DOI] [PubMed] [Google Scholar]
- (2).Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- (3).Lutolf MP, Blau HM. Artificial Stem Cell Niches. Advanced Materials. 2009;9999:NA. doi: 10.1002/adma.200802582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- (5).National Research Council (U.S.) Inspired by biology : from molecules to materials to machines. National Academies Press; Washington, D.C.: 2008. Committee on Biomolecular Materials and Processes. [PubMed] [Google Scholar]
- (6).Osafune K, Caron L, Borowiak M, Martinez RJ, Fitz-Gerald CS, Sato Y, Cowan CA, Chien KR, Melton DA. Marked differences in differentiation propensity among human embryonic stem cell lines. Nature Biotechnology. 2008;26:313–315. doi: 10.1038/nbt1383. [DOI] [PubMed] [Google Scholar]
- (7).Song L, Tuan RS. Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. Faseb J. 2004;18:980–2. doi: 10.1096/fj.03-1100fje. [DOI] [PubMed] [Google Scholar]
- (8).Miura M, Miura Y, Padilla-Nash HM, Molinolo AA, Fu B, Patel V, Seo BM, Sonoyama W, Zheng JJ, Baker CC, Chen W, Ried T, Shi S. Accumulated chromosomal instability in murine bone marrow mesenchymal stem cells leads to malignant transformation. Stem Cells. 2006;24:1095–103. doi: 10.1634/stemcells.2005-0403. [DOI] [PubMed] [Google Scholar]
- (9).Neuss S, Apel C, Buttler P, Denecke B, Dhanasingh A, Ding XL, Grafahrend D, Groger A, Hemmrich K, Herr A, Jahnen-Dechent W, Mastitskaya S, Perez-Bouza A, Rosewick S, Salber J, Woltje M, Zenke M. Assessment of stem cell/biomaterial combinations for stem cell-based tissue engineering. Biomaterials. 2008;29:302–313. doi: 10.1016/j.biomaterials.2007.09.022. [DOI] [PubMed] [Google Scholar]
- (10).Jones DL, Wagers AJ. No place like home: anatomy and function of the stem cell niche. Nature Reviews Molecular Cell Biology. 2008;9:11–21. doi: 10.1038/nrm2319. [DOI] [PubMed] [Google Scholar]
- (11).Chen S, Borowiak M, Fox JL, Maehr R, Osafune K, Davidow L, Lam K, Peng LF, Schreiber SL, Rubin LL, Melton D. A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nature Chemical Biology. 2009;5:258–265. doi: 10.1038/nchembio.154. [DOI] [PubMed] [Google Scholar]
- (12).Langer R, Peppas NA. Advances in biomaterials, drug delivery, and bionanotechnology. Aiche Journal. 2003;49:2990–3006. [Google Scholar]
- (13).Gerecht S, Burdick JA, Ferreira LS, Townsend SA, Langer R, Vunjak-Novakovic G. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:11298–303. doi: 10.1073/pnas.0703723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Hosseinkhani H, Hosseinkhani M, Gabrielson NP, Pack DW, Khademhosseini A, Kobayashi H. DNA nanoparticles encapsulated in 3D tissue-engineered scaffolds enhance osteogenic differentiation of mesenchymal stem cells. J Biomed Mater Res A. 2008;85:47–60. doi: 10.1002/jbm.a.31327. [DOI] [PubMed] [Google Scholar]
- (15).Goetz AK, Scheffler B, Chen HX, Wang S, Suslov O, Xiang H, Brustle O, Roper SN, Steindler DA. Temporally restricted substrate interactions direct fate and specification of neural precursors derived from embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103:11063–8. doi: 10.1073/pnas.0510926103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Flaim CJ, Teng D, Chien S, Bhatia SN. Combinatorial signaling microenvironments for studying stem cell fate. Stem Cells Dev. 2008;17:29–39. doi: 10.1089/scd.2007.0085. [DOI] [PubMed] [Google Scholar]
- (17).Nakajima M, Ishimuro T, Kato K, Ko IK, Hirata I, Arima Y, Iwata H. Combinatorial protein display for the cell-based screening of biomaterials that direct neural stem cell differentiation. Biomaterials. 2007;28:1048–60. doi: 10.1016/j.biomaterials.2006.10.004. [DOI] [PubMed] [Google Scholar]
- (18).Tan MY, Zhi W, Wei RQ, Huang YC, Zhou KP, Tan B, Deng L, Luo JC, Li XQ, Xie HQ, Yang ZM. Repair of infarcted myocardium using mesenchymal stem cell seeded small intestinal submucosa in rabbits. Biomaterials. 2009;30:3234–3240. doi: 10.1016/j.biomaterials.2009.02.013. [DOI] [PubMed] [Google Scholar]
- (19).Hakala H, Rajala K, Ojala M, Panula S, Areva S, Kellomaki M, Suuronen R, Skottman H. Comparison of Biomaterials and Extracellular Matrices as a Culture Platform for Multiple, Independently Derived Human Embryonic Stem Cell Lines. Tissue Eng Part A. 2009 doi: 10.1089/ten.tea.2008.0316. [DOI] [PubMed] [Google Scholar]
- (20).Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- (21).Benoit DSW, Schwartz MP, Durney AR, Anseth KS. Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells. Nature Materials. 2008;7:816–823. doi: 10.1038/nmat2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Kraehenbuehl TP, Zammaretti P, Van der Vlies AJ, Schoenmakers RG, Lutolf MP, Jaconi ME, Hubbell JA. Three-dimensional extracellular matrix-directed cardioprogenitor differentiation: Systematic modulation of a synthetic cell-responsive PEG-hydrogel. Biomaterials. 2008;29:2757–66. doi: 10.1016/j.biomaterials.2008.03.016. [DOI] [PubMed] [Google Scholar]
- (23).Martino MM, Mochizuki M, Rothenfluh DA, Rempel SA, Hubbell JA, Barker TH. Controlling integrin specificity and stem cell differentiation in 2D and 3D environments through regulation of fibronectin domain stability. Biomaterials. 2009;30:1089–97. doi: 10.1016/j.biomaterials.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Gelain F, Bottai D, Vescovi A, Zhang S. Designer self-assembling Peptide nanofiber scaffolds for adult mouse neural stem cell 3-dimensional cultures. PLoS ONE. 2006;1:e119. doi: 10.1371/journal.pone.0000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA, Stupp SI. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 2004;303:1352–5. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- (26).Anderson DG, Levenberg S, Langer R. Nanoliter-scale synthesis of arrayed biomaterials and application to human embryonic stem cells. Nature Biotechnology. 2004;22:863–866. doi: 10.1038/nbt981. [DOI] [PubMed] [Google Scholar]
- (27).Mei Y, Gerecht S, Taylor M, Urquhart AJ, Bogatyrev SR, Cho S, Davies MC, Alexander MR, Langer RS, Anderson DG. Mapping the Interactions among Biomaterials, Adsorbed Proteins, and Human Embryonic Stem Cells. Advanced Materials. 2009;21:2781–2786. doi: 10.1002/adma.200803184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Meredith JC. Advances in combinatorial and high-throughput screening of biofunctional polymers for gene delivery, tissue engineering and anti-fouling coatings. Journal of Materials Chemistry. 2009;19:34–45. [Google Scholar]
- (29).Peters A, Brey DM, Burdick JA. High-throughput and Combinatorial Technologies for Tissue Engineering Applications. Tissue Eng Part B Rev. 2009 doi: 10.1089/ten.TEB.2009.0049. [DOI] [PubMed] [Google Scholar]
- (30).Goldberg M, Mahon K, Anderson D. Combinatorial and rational approaches to polymer synthesis for medicine. Adv Drug Deliv Rev. 2008;60:971–8. doi: 10.1016/j.addr.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA. Hydrogels in Regenerative Medicine. Advanced Materials. 2009 doi: 10.1002/adma.200802106. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Park H, Guo X, Temenoff JS, Tabata Y, Caplan AI, Kasper FK, Mikos AG. Effect of Swelling Ratio of Injectable Hydrogel Composites on Chondrogenic Differentiation of Encapsulated Rabbit Marrow Mesenchymal Stem Cells In Vitro. Biomacromolecules. 2009 doi: 10.1021/bm801197m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Nuttelman CR, Rice MA, Rydholm AE, Salinas CN, Shah DN, Anseth KS. Macromolecular monomers for the synthesis of hydrogel niches and their application in cell encapsulation and tissue engineering. Progress in Polymer Science. 2008;33:167–179. doi: 10.1016/j.progpolymsci.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Park H, Temenoff JS, Tabata Y, Caplan AI, Raphael RM, Jansen JA, Mikos AG. Effect of dual growth factor delivery on chondrogenic differentiation of rabbit marrow mesenchymal stem cells encapsulated in injectable hydrogel composites. Journal of Biomedical Materials Research Part A. 2009;88A:889–897. doi: 10.1002/jbm.a.31948. [DOI] [PubMed] [Google Scholar]
- (35).Lutolf MP, Doyonnas R, Havenstrite K, Koleckar K, Blau HM. Perturbation of single hematopoietic stem cell fates in artificial niches. Integrative Biology. 2009;1:59–69. doi: 10.1039/b815718a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Fedorovich NE, Oudshoorn MH, van Geemen D, Hennink WE, Alblas J, Dhert WJ. The effect of photopolymerization on stem cells embedded in hydrogels. Biomaterials. 2009;30:344–53. doi: 10.1016/j.biomaterials.2008.09.037. [DOI] [PubMed] [Google Scholar]
- (37).Keskar V, Marion NW, Mao JJ, Gemeinhart RA. In Vitro Evaluation of Macroporous Hydrogels to Facilitate Stem Cell Infiltration, Growth, and Mineralization. Tissue Eng Part A. 2009 doi: 10.1089/ten.tea.2008.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Ashton BA, Eaglesom CC, Bab I, Owen ME. Distribution of Fibroblastic Colony-Forming Cells in Rabbit Bone-Marrow and Assay of Their Osteogenic Potential by an Invivo Diffusion Chamber Method. Calcified Tissue International. 1984;36:83–86. doi: 10.1007/BF02405298. [DOI] [PubMed] [Google Scholar]
- (39).van der Flier LG, Clevers H. Stem Cells, Self-Renewal, and Differentiation in the Intestinal Epithelium. Annu Rev Physiol. 2008 doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- (40).Mitsiadis TA, Omella B, Rochat A, Barrandon Y, Bari C. Stem cell niches in mammals. Experimental Cell Research. 2007;313:3377–3385. doi: 10.1016/j.yexcr.2007.07.027. [DOI] [PubMed] [Google Scholar]
- (41).Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Microscale technologies for tissue engineering and biology. Proc Natl Acad Sci U S A. 2006;103:2480–7. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Ferreira L, Karp JM, Nobre L, Langer R. New opportunities: The use of Nanotechnologies to manipulate and track stem cells. Cell Stem Cell. 2008;3:136–146. doi: 10.1016/j.stem.2008.07.020. [DOI] [PubMed] [Google Scholar]
- (43).Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–8. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- (44).Ruiz SA, Chen CS. Emergence of patterned stem cell differentiation within multicellular structures. Stem Cells. 2008;26:2921–7. doi: 10.1634/stemcells.2008-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Karp JM, Yeh J, Eng G, Fukuda J, Blumling J, Suh KY, Cheng J, Mahdavi A, Borenstein J, Langer R, Khademhosseini A. Controlling size, shape and homogeneity of embryoid bodies using poly(ethylene glycol) microwells. Lab Chip. 2007;7:786–94. doi: 10.1039/b705085m. [DOI] [PubMed] [Google Scholar]
- (46).Carpenedo RL, Bratt-Leal AM, Marklein RA, Seaman SA, Bowen NJ, McDonald JF, McDevitt TC. Homogeneous and organized differentiation within embryoid bodies induced by microsphere-mediated delivery of small molecules. Biomaterials. 2009;30:2507–15. doi: 10.1016/j.biomaterials.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Huang AH, Stein A, Tuan RS, Mauck RL. TRANSIENT EXPOSURE TO TGF-beta3 IMPROVES THE MECHANICAL PROPERTIES OF MSC-LADEN CARTILAGE CONSTRUCTS IN A DENSITY DEPENDENT MANNER. Tissue Eng Part A. 2009 doi: 10.1089/ten.tea.2009.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Hsiong SX, Carampin P, Kong HJ, Lee KY, Mooney DJ. Differentiation stage alters matrix control of stem cells. J Biomed Mater Res A. 2008;85:145–56. doi: 10.1002/jbm.a.31521. [DOI] [PubMed] [Google Scholar]
- (49).Kloxin AM, Kasko AM, Salinas CN, Anseth KS. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science. 2009;324:59–63. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]