Abstract
A variety of iodo-substituted isochromenes, dihydroisobenzofurans, and pyranopyridines are readily prepared in good to excellent yields under mild conditions by the iodocyclization of readily available 2-(1-alkynyl)benzylic alcohols or 2-(1-alkynyl)-3-(hydroxymethyl)pyridines. Reactions are carried out in MeCN at 15 °C using 3 equiv of I2 as the iodine source and NaHCO3 (3 equiv) as the base. The regiochemical outcome of the reaction strongly depends on the substitution pattern of the starting material. In particular, the 5-exo-dig cyclization mode, leading to dihydroisobenzofurans, is observed in the case of substrates bearing a tertiary alcoholic group, owing to the gem-dialkyl effect, while the 6-endo-dig cyclization mode, leading to isochromene or pyranopyridines, is the usually preferred pathway in the case of substrates bearing a primary or secondary alcoholic group
Introduction
The iodocyclization of alkynes has emerged as a powerful tool in organic synthesis.1 Recently, we and others have utilized this methodology to accomplish efficient syntheses of a wide variety of interesting carbocyclic and heterocyclic compounds.2–26 In general, these electrophilic cyclization reactions are very clean and efficient, and tolerate a wide variety of functional groups. Furthermore, the iodine-containing products can be further diversified using a number of subsequent palladium-catalyzed processes.
Herein we report a simple and efficient method for the synthesis of 1H-isochromene and/or (Z)-1-(1-iodoalkylidene)-1,3-dihydroisobenzofuran derivatives, based on the iodocyclization of readily available 2-(1-alkynyl)benzylic alcohols (Eq 1). Isochromenes and 1,3-dihydroisobenzofurans are important heterocyclic compounds, and there are several examples of naturally occurring and biologically active compounds containing these ring systems.27, 28
 |
(1) |
Over the years, several groups have reported a variety of synthetic approaches to isochromene and dihydroisobenzofuran derivatives by the heterocyclization of acyclic precursors.29 In particular, we30 and the Barluenga group31 have independently reported the formation of 4-iodo-1H-isochromenes by the iodocyclization of 2-(1-alkynyl)benzaldehydes in the presence of suitable nucleophiles (Eq 2).
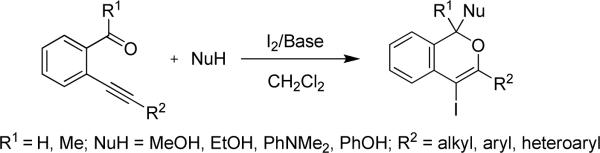 |
(2) |
We have also previously disclosed the Pd(II)-catalyzed cycloisomerization of 2-(1-alkynyl)benzylic alcohols to 1H-isochromenes and/or (Z)-1-alkylidene-1,3-dihydroisobenzofurans, depending on the nature of the substrate and the reaction conditions (Eq 3).29k
 |
(3) |
The new methodology reported in this work is complementary to those previously reported procedures, and allows for the direct synthesis of 3-iodo-1H-isochromenes, (Z)-1-(1-iodoalkylidene)-1,3-dihydroisobenzofurans, and iodopyranopyridines by the iodocyclization of variously substituted 2-(1-alkynyl)benzylic alcohols or 2-(1-alkynyl)-3-(hydroxymethyl)pyridines under mild conditions (see Eq 1).
Results and Discussion
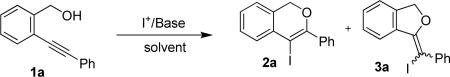
2-(2-Phenylethynyl)benzyl alcohol (1a, Y = CH, R1 = R2 = R3 = H, R4 = Ph) was chosen as a model substrate for determining the optimum conditions for the iodocyclization reaction, using I2 or ICl as the iodine source, MeCN, CH2Cl2 or EtOH as the solvent, in the presence of an inorganic (NaHCO3, K2CO3, KHCO3, NaH) or organic (morpholine) base. The results obtained are shown in Table 1.32
Table 1.
Iodocyclization of 2-(2-Phenylethynyl)benzyl Alcohol: Optimization Studiesa
| entry | I+ | base | time (h) | solvent | total yield (2a + 3a)b (%) | 2a/3a ratioc |
|---|---|---|---|---|---|---|
| 1 | 2.0 I2 | 2.0 NaHCO3 | 72 | CH3CN | 56 | 75/25 |
| 2 | 2.0 I2 | 3.0 NaHCO3 | 72 | CH3CN | 56 | 71/29 |
| 3 | 3.0 I2 | 3.0 NaHCO3 | 15 | CH3CN | 71 | 91/9 |
| 4 | 3.0 I2 | 3.0 NaHCO3 | 15 | CH2Cl2 | 45 | 100/0 |
| 5 | 3.0 I2 | 3.0 NaHCO3 | 15 | EtOH | 45 | 100/0 |
| 6d | 3.0 I2 | 3.0 NaHCO3 | 15 | CH3CN | 33 | 64/36 |
| 7 | 3.0 I2 | 3.0 K2CO3 | 24 | CH3CN | 56 | 48/52 |
| 8 | 3.0 I2 | 3.0 KHCO3 | 24 | CH3CN | 46 | 48/52 |
| 9 | 3.0 I2 | 3.0 NaH | 24 | CH3CN | 10 | 100/0 |
| 10 | 3.0 I2 | 3.0 Morpholine | 24 | CH3CN | - | - |
| 11 | 3.0 ICl | 3 NaHCO3 | 1.5 | CH3CN | 38 | 100/0 |
| 12 | 3.0 ICl | 3 NaHCO3 | 1.5 | CH2Cl2 | 25 | 100/0 |
Unless otherwise noted, all reactions were carried out on a 0.3 mmol scale in 6 mL of acetonitrile at 25 °C.
Isolated yield of 2a + 3a, based on starting 1a. In most cases, an inseparable mixture of the two regioisomers was obtained from column chromatography.
The 2:3 ratio is based on 1H NMR spectroscopic data.
The reaction was carried out in 2.5 mL of MeCN.
As can be seen from Table 1, the isochromene derivative 2a derived from a 6-endo-dig cyclization was consistently obtained in higher yield than the dihydroisobenzofuran derivative 3a derived from a 5-exo-dig cyclization. The optimal reaction conditions in terms of total yield for the iodocyclization of 1a are those reported in entry 3 (Substrate: I2: NaHCO3 molar ratio = 1:3:3, T = 25 °C, substrate concentration = 0.05 M in CH3CN, time = 15 h).33 Under these conditions, 1a was converted into approximately an 11:1 mixture of 2a and 3a in a total yield of 71%. On the other hand, no formation of 3a was observed using I2 as the iodine source in CH2Cl2 or EtOH as the solvent (entries 4 and 5) or using ICl as the iodine source either in MeCN or in CH2Cl2 (entries 11 and 12, respectively). However, the yields of 2a obtained under these conditions ranged from only 25 to 45%.
A variety of 2-(1-alkynyl)benzylic alcohols 1b–t were then subjected to iodocyclization under the conditions of Table 1, entry 3. The results obtained are summarized in Table 2.
Table 2.
Synthesis of 3-Iodo-1H-isochromenes and 1-(1-Iodoalkylidene)-1,3-dihydroisobenzofurans by the Iodocyclization of 2-(1-Alkynyl)benzylic alcoholsa

| isolated yield (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| entry | 1 | Y | R1 | R2 | R3 | R4 | 2 | 3 |
| 1 | 1a | CH | H | H | H |  |
71b (11:1)c | |
| 2 | 1b | CH | H | H | H |  |
92 | - |
| 3 | 1c | CH | H | H | H |  |
85 | - |
| 4 | 1d | CH | H | H | H |  |
41 | 35 |
| 5 | 1e | CH | H | H | H |  |
85b (3:1)c | |
| 6 | 1f | CH | H | H | H |  |
88b (1:3)c | |
| 7 | 1g | CH | H | H | H |  |
74b (1:4)c | |
| 8 | 1h | CH | H | H | H |  |
82 | - |
| 9 | 1i | CH | H | H | H |  |
80 | - |
| 10 | 1j | CH | H | H | H | CH3CH2CH2CH2- | 57 | - |
| 11 | 1n | CH | OMe | H | H |  |
51 | - |
| 12 | 1o | CH | OMe | H | H |  |
61 | - |
| 13 | 1p | CH | NO2 | H | H |  |
92 | - |
| 14 | 1q | N | H | H | H |  |
98 | - |
| 15 | 1r | N | H | H | H |  |
92 | - |
| 16 | 1s | CH | H | Bu | H |  |
76b (3:1)c | |
| 17 | 1t | CH | H | Bu | H |  |
72 | |
| 18 | 1u | CH | H | Et | Et |  |
- | 70 |
| 19 | 1v | CH | H | Et | Et | 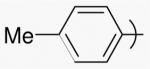 |
- | 70 |
| 20 | 1w | CH | H | Bu | Et |  |
- | 82 |
Unless otherwise noted, all reactions were carried out on a 0.3 mmol scale in 6 mL of acetonitrile. The reactions were allowed to stir at room temperature for 15 h. All yields are isolated yields after column chromatography.
An inseparable mixture of the two regioisomers was obtained from column chromatography.
The 2:3 ratio is based on 1H NMR spectroscopic data.
As can be seen from the results reported in Table 2, the regiochemistry of the process strongly depends on the substitution pattern of the substrate. Interestingly, the presence of a electron-donating group in the para position of a phenyl ring conjugated with the triple bond (R4 = p-MeC6H4 or p-MeOC6H4, substrates 1b and 1c, respectively) led to preferential formation of the isochromene derivative (compare entries 2 and 3 with entry 1). In fact, the electron-donating effect of the para substituent should increase the electron density on C-1 of the arylethynyl group, thus favoring intramolecular nucleophilic attack of the hydroxyl group on C-2. As expected, this effect was not observed when the substituents are in the meta positions, as shown by the results obtained in the case of substrate 1d (R4 = 3,5-dimethoxyphenyl). In fact, iodocyclization of the latter led to a mixure of the isochromene and isobenzofuran derivatives (entry 4). A mixture of 2e and 3e was also obtained in the case of a p-chloro substituent (R4 = p-ClC6H4, substrate 1e, entry 5). On the other hand, the presence of a nitro group in the para position (R4 = p-O2NC6H4, substrate 1f, entry 6) significantly augments the electrophilicity of C-1 of the arylethynyl group, thus reversing the selectivity of the reaction in favor of the 5-membered ring product 3f. A similar effect is observed when R4 is a 3,5-bis(trifluoromethyl)phenyl substituent (substrate 1g, entry 7). When the triple bond is substituted with a 3-thienyl, 1-cyclohexenyl, or butyl group, the reaction consistently follows a 6-endo-dig pathway, with selective formation of the corresponding isochromene derivatives 2h–2j (entries 8–10). Substrates bearing a sterically demanding substituent [R4 = t-butyl (1k) or TMS (1l)] led to complex reaction mixtures. Also, the reaction did not proceed well with a terminal triple bond (R4 = H, 1m), and partial decomposition of the substrate occurred. Interestingly, the presence of either an electron-donating or an electron-withdrawing group meta with respect to the hydroxymethyl group (R1 = OMe or NO2, substrates 1n–1p) also tended to favor selective formation of the 6-membered ring products 2n–2p (entries 11–13). Excellent yields of pyranopyridine derivatives 2q and 2r were obtained by 6-endo-dig iodocyclization of the corresponding 2-(1-alkynyl)-3-(hydroxymethyl)pyridines 1q and 1r (entries 14 and 15).
Substrates bearing a secondary alcoholic group (1s and 1t) behaved similarly to substrates with a primary alcoholic group, as can be seen by comparing entries 16 and 17 (Table 2) with entries 1 and 2 (Table 2). On the other hand, substrates bearing a tertiary alcohol group (such as 1u–1w) selectively proceed by a 5-exo-dig cyclization, with formation of the corresponding dihydroisobenzofurans 3u–3w in good yields (70–82%, entries 18–20). This result is in agreement with what has been previously observed in the Pd(II)-catalyzed cycloisomerization of 2-(1-alkynyl)benzylic alcohols.29k The gem-dialkyl effect34 may be responsible for the observed regioselectivity. In fact, in the presence of α,α-dialkyl substitution, the hydroxyl group is forced closer to the triple bond, thus favoring the 5-exo-dig pathway with respect to the 6-endo-dig pathway.
X-ray crystallographic experiments were performed in order to confirm the regiochemistry of the cyclized products.35 Interestingly, the 1-alkylidene-1,3-dihydroisobenzofuran product 3u was found to be the Z-isomer, instead of the E-isomer that would be expected from an anti-5-exo-dig cyclization (Figure 1). This Z-stereochemistry was also found in other 5-membered ring products (by 1H NMR spectral data correlation with 3u).
Figure 1.
X-ray Evidence for the Structural Assignment of 1-Alkylidene-1,3-dihydroisobenzofurans
Given the unexpected stereochemistry, an immediately reasonable hypothesis was that the predicted stereoisomer is originally formed, and then equilibrates to the observed isomer. This would imply that the observed Z-isomer would need to be more stable than the expected E-isomer. In order to substantiate this idea, computations were carried out at MP2 and B3LYP levels of theory using a 6–31G(d) basis set on carbon and hydrogen atoms, and an approximately equivalent electron core potential and valence basis set on I. (Details are given in the Supporting Information.) At both levels of theory, the Z-isomer was favored, by 3.6 and 3.9 kcal/mol using B3LYP and MP2, respectively.
On the basis of these observations, the following reaction mechanism can be proposed for the iodocyclization of 1 (Scheme 1).36 Coordination of an I+ equivalent to the alkyne leads to electrophilic activation of the alkyne carbon-carbon triple bond1 generating iodonium intermediate A. Nucleophilic attack by the hydroxyl group may then take place by either of two intramolecular cyclization modes (anti-6-endo-dig or anti-5-exo-dig, paths a and b, respectively) to give intermediates B or B' respectively. Deprotonation of intermediate B leads to the isochromene product 2 that is isolated as the major product in most cases. However, dihydroisobenzofuran derivative 3' with E stereochemistry derived from intermediate B' was not isolated. Presumably, isomerization of the initially formed E-isobenzofuran (3') leads to the more stable Z-isomer (3). A few examples of the isomerization of substituted cis-alkenes to the more stable corresponding trans-isomers in the presence of iodine are known.37 In order to demonstrate the feasibility of the isomerization, cis-stilbene was subjected to our reaction conditions and partial isomerization was observed. The ratio of cis:trans stilbene was found to be 1:0.6 at 10 h and 1:0.9 at 19 h by 1H NMR spectral data. The possibility that E → Z isomerization occurs through from the formation of intermediate B" (deriving from B' via proton shift) followed by stereospecific deprotonation cannot be ruled out, however.
Scheme 1.
Proposed Mechanistic Pathways for the Iodocyclization of 2-(1-Alkynyl)benzylic alcohols (1)
Conclusions
A simple and mild synthesis of 6- and 5-membered iodoheterocyclic ether derivatives (2 and 3, respectively) via iodocyclization of readily available 2-(1-alkynyl)benzylic alcohols 1 is reported. The nature of the substituents in the starting material governs the regiochemistry of the reaction products. The 5-membered ring products obtained (1-alkylidene-1,3-dihydroisobenzofurans 3) exhibit unexpected Z-stereochemistry, and are presumably derived from the initially formed less stable E-isomers through iodine-mediated isomerization.
Experimental Section
Substrates 1a–y were prepared by Sonogashira coupling between the appropriate o-halobenzylic alcohol or hydroxymethylpyridine and a terminal alkyne, as described in the Supporting Information. All products have been fully characterized by spectroscopic techniques and, in the case of 4-iodo-3-(4-methoxyphenyl)-1H-isochromene (2c) and 1,1-diethyl-3-(iodophenylmethylene)-1,3-dihydro-isobenzofuran (3u), by X-ray diffraction analysis also, as detailed in the Supporting Information. A typical procedure for iodocyclization is given below.
Typical Procedure for Iodocyclization
We report here a typical procedure for the preparation of 4-iodo-3-p-tolyl-1H-isochromene (Table 2, entry 2). Details for the preparation of all other products can be found in the Supporting Information. To a solution of 2-(p-tolylethynyl)benzyl alcohol (1b, 67 mg, 0.30 mmol) in CH3CN (6.0 mL) was added NaHCO3 (76 mg, 0.90 mmol), followed by I2 (228 mg, 0.90 mmol), at room temperature with stirring. The resulting mixture was allowed to stir at room temperature for 15 h. The excess I2 was removed by adding a satd aq solution of Na2S2O3, followed by stirring for 5–10 min. The phases were separated, and the aqueous phase was extracted with Et2O (2 × 10 mL). The collected organic phases were dried (Na2SO4) and concentrated under vacuum. The crude product was purified by column chromatography on silica gel (hexane-EtOAc from 95:5 to 9:1) to give 4-iodo-3-p-tolyl-1H-isochromene (2b) as a yellow solid (96.0 mg, 92%): mp 43–44 °C; IR (KBr) 1590 (m), 1509 (m), 1477 (m), 1452 (m), 1265 (s), 1183 (w), 1084 (s), 919 (m), 819 (m), 737 (s) cm−1; 1H NMR (400 MHz, CDCl3) δ7.55 (d, J = 8.0 Hz, 2H), 7.45 (d, J = 7.6 Hz, 1H), 7.33 (t, J = 7.7 Hz, 1H), 7.21 (d, J = 7.6 Hz, 3H), 6.97 (d, J = 6.2, 1H), 5.20 (s, 2H), 2.39 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 156.4, 140.0, 133.7, 133.5, 130.3, 128.6, 128.5, 128.4, 127.4, 123.3, 121.2, 72.8, 69.5, 21.5; GC-MS, m/z 348 (M+, 100), 347 (7), 222 (4), 221 (4), 220 (3), 194 (5), 193 (8), 192 (6). HRMS calcd for C16H13IO 348.00111. Found 348. 00173.
Supplementary Material
Acknowledgments
We gratefully acknowledge the National Institute of General Medical Sciences (GM070620 and GM079593) for support of this research. We also thank Dr. Arkady Ellern and the Molecular Structure Laboratory of Iowa State University, and Dr. Armentano Donatella (X-Ray Diffractometry Laboratory of the University of Calabria) for providing X-ray crystallographic data for products 2c and 3u, respectively. Thanks are also extended to Johnson Matthey, Inc. and Kawaken Fine Chemicals Co. for donating the palladium catalysts.
Footnotes
Supporting Information Available: General experimental methods, preparation of substrates, general procedures for the iodocyclization reactions, characterization data, copies of 1H and 13C NMR spectra for all previously unreported compounds, and X-ray crystallographic data for compounds 2c and 3u. This material is available free of charge via the Internet at http://pubs.acs.org.
References and Notes
- (1).(a) Mehta S, Waldo JP, Larock RC. J. Org. Chem. 2009;74:1141. doi: 10.1021/jo802196r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Larock RC. Chapter 2. In: Diederich F, Stang PJ, Tykwinski RR, editors. Acetylene Chemistry. Chemistry, Biology, and Material Science. Wiley-VCH; New York: 2005. pp. 51–99. [Google Scholar]
- (2).(a) Arcadi A, Cacchi S, Fabrizi G, Marinelli F, Moro L. Synlett. 1999:1432. [Google Scholar]; (b) Yue D, Yao T, Larock RC. J. Org. Chem. 2005;70:10292. doi: 10.1021/jo051299c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Yue D, Yao T, Larock RC. J. Comb. Chem. 2005;7:809. doi: 10.1021/cc050062r. [DOI] [PubMed] [Google Scholar]; (d) Manarin F, Roehrs JA, Gay RM, Brandão R, Menezes PH, Nogueira CW, Zeni G. J. Org. Chem. 2009;74:2153. doi: 10.1021/jo802736e. [DOI] [PubMed] [Google Scholar]
- (3).(a) Sniady A, Wheeler KA, Dembinski R. Org. Lett. 2005;7:1769. doi: 10.1021/ol050372i. [DOI] [PubMed] [Google Scholar]; (b) Yao T, Zhang X, Larock RC. J. Org. Chem. 2005;70:7679. doi: 10.1021/jo0510585. [DOI] [PubMed] [Google Scholar]; (c) Liu Y, Zhou S. Org. Lett. 2005;7:4609. doi: 10.1021/ol051659i. [DOI] [PubMed] [Google Scholar]; (d) Yao T, Zhang X, Larock RC. J. Am. Chem. Soc. 2004;126:11164. doi: 10.1021/ja0466964. [DOI] [PubMed] [Google Scholar]; (e) Bew SP, El-Taeb GMM, Jones S, Knight DW, Tan W. Eur. J. Org. Chem. 2007:5759. [Google Scholar]; (f) Arimitsu S, Jacobsen JM, Hammond GB. J. Org. Chem. 2008;73:2886. doi: 10.1021/jo800088y. [DOI] [PubMed] [Google Scholar]; (g) Huang X, Fu W, Miao M. Tetrahedron Lett. 2008;49:2359. [Google Scholar]
- (4).(a) Larock RC, Yue D. Tetrahedron Lett. 2001;42:6011. [Google Scholar]; (b) Yue D, Larock RC. J. Org. Chem. 2002;67:1905. doi: 10.1021/jo011016q. [DOI] [PubMed] [Google Scholar]; (c) Hessian KO, Flynn BL. Org. Lett. 2003;5:4377. doi: 10.1021/ol035663a. [DOI] [PubMed] [Google Scholar]
- (5).Flynn BL, Flynn GP, Hamel E, Jung MK. Bioorg. Med. Chem. Lett. 2001;11:2341. doi: 10.1016/s0960-894x(01)00436-x. [DOI] [PubMed] [Google Scholar]
- (6).Worlikar SA, Kesharwani T, Yao T, Larock RC. J. Org. Chem. 2007;72:1347. doi: 10.1021/jo062234s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).(a) Kesharwani T, Worlikar SA, Larock RC. J. Org. Chem. 2006;71:2307. doi: 10.1021/jo0524268. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Bui CT, Flynn BL. J. Comb. Chem. 2006;8:163. doi: 10.1021/cc050066w. [DOI] [PubMed] [Google Scholar]
- (8).Alves D, Luchese C, Nogueira CW, Zeni G. J. Org. Chem. 2007;72:6726. doi: 10.1021/jo070835t. [DOI] [PubMed] [Google Scholar]
- (9).Zhang X, Sarkar S, Larock RC. J. Org. Chem. 2006;71:236. doi: 10.1021/jo051948k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).(a) Yue D, Larock RC. Org. Lett. 2004;6:1037. doi: 10.1021/ol0498996. [DOI] [PubMed] [Google Scholar]; (b) Amjad M, Knight DW. Tetrahedron Lett. 2004;45:539. [Google Scholar]; (c) Barluenga J, Trincado M, Rubio E, González JM. Angew. Chem., Int. Ed. 2003;42:2406. doi: 10.1002/anie.200351303. [DOI] [PubMed] [Google Scholar]; (d) Yue D, Yao T, Larock RC. J. Org. Chem. 2006;71:62. doi: 10.1021/jo051549p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Zhang X, Campo MA, Yao T, Larock RC. Org. Lett. 2005;7:763. doi: 10.1021/ol0476218. [DOI] [PubMed] [Google Scholar]
- (12).(a) Huang Q, Hunter JA, Larock RC. J. Org. Chem. 2002;67:3437. doi: 10.1021/jo020020e. [DOI] [PubMed] [Google Scholar]; (b) Fischer D, Tomeba H, Pahadi NK, Patil NT, Yamamoto Y. Angew. Chem., Int. Ed. 2007;46:4764. doi: 10.1002/anie.200701392. [DOI] [PubMed] [Google Scholar]
- (13).Yao T, Larock RC. J. Org. Chem. 2003;68:5936. doi: 10.1021/jo034308v. [DOI] [PubMed] [Google Scholar]
- (14).Barluenga J, Vázquez-Villa H, Ballesteros A, González JM. Org. Lett. 2003;5:4121. doi: 10.1021/ol035691t. [DOI] [PubMed] [Google Scholar]
- (15).(a) Yao T, Campo MA, Larock RC. Org. Lett. 2004;6:2677. doi: 10.1021/ol049161o. [DOI] [PubMed] [Google Scholar]; (b) Yao T, Campo MA, Larock RC. J. Org. Chem. 2005;70:3511. doi: 10.1021/jo050104y. [DOI] [PubMed] [Google Scholar]
- (16).(a) Waldo JP, Larock RC. Org. Lett. 2005;7:5203. doi: 10.1021/ol052027z. [DOI] [PubMed] [Google Scholar]; (b) Waldo JP, Larock RC. J. Org. Chem. 2007;72:9643. doi: 10.1021/jo701942e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).(a) Zhou C, Dubrovskiy AV, Larock RC. J. Org. Chem. 2006;71:1626. doi: 10.1021/jo0523722. [DOI] [PubMed] [Google Scholar]; (b) Likhar PR, Subhas MS, Roy M, Roy S, Kantam ML. Helv. Chim. Acta. 2008;91:259. [Google Scholar]
- (18).Ren X-F, Konaklieva MI, Shi H, Dickey S, Lim DV, Gonzalez J, Turos E. J. Org. Chem. 1998;63:8898. [Google Scholar]
- (19).Marshall JA, Yanik MM. J. Org. Chem. 1999;64:3798. [Google Scholar]
- (20).Knight DW, Redfern AL, Gilmore J. J. Chem. Soc., Chem. Commun. 1998:2207. [Google Scholar]
- (21).Arcadi A, Cacchi S, Di Giuseppe S, Fabrizi G, Marinelli F. Org Lett. 2002;4:2409. doi: 10.1021/ol0261581. [DOI] [PubMed] [Google Scholar]
- (22).(a) Zhang X, Larock RC. J. Am. Chem. Soc. 2005;127:12230. doi: 10.1021/ja053079m. [DOI] [PubMed] [Google Scholar]; (b) Tang B-X, Tang D-J, Yu Q-F, Zhang Y-H, Liang Y, Zhong P, Li J-H. Org. Lett. 2008;10:1063. doi: 10.1021/ol703050z. [DOI] [PubMed] [Google Scholar]
- (23).Yao T, Yue D, Larock RC. J. Org. Chem. 2005;70:9985. doi: 10.1021/jo0517038. [DOI] [PubMed] [Google Scholar]
- (24).(a) Crone B, Kirsch SF. J. Org. Chem. 2007;72:5435. doi: 10.1021/jo070695n. [DOI] [PubMed] [Google Scholar]; (b) Just ZW, Larock RC. J. Org. Chem. 2008;73:2662. doi: 10.1021/jo702666j. [DOI] [PubMed] [Google Scholar]
- (25).Barange DK, Batchu VR, Gorja D, Pattabiraman VR, Tatini LK, Babu JM, Pal M. Tetrahedron. 2007;63:1775. [Google Scholar]
- (26).For miscellaneous other examples, see: Hessian KO, Flynn BL. Org. Lett. 2006;8:243. doi: 10.1021/ol052518j.. Bi H-P, Guo L-N, Duan X-H, Gou F-R, Huang S-H, Liu X-Y, Liang Y-M. Org. Lett. 2007;9:397. doi: 10.1021/ol062683e.. Barluenga J, Palomas D, Rubio E, González JM. Org. Lett. 2007;9:2823. doi: 10.1021/ol0710459.. Tellitu I, Serna S, Herrero T, Moreno I, Domínguez E, SanMartin R. J. Org. Chem. 2007;72:1526. doi: 10.1021/jo062320s.. Appel TR, Yehia NAM, Baumeister U, Hartung H, Kluge R, Ströhl D, Fanghänel E. Eur. J. Org. Chem. 2003:47..
- (27).For isochromenes, see: Kang H-S, Jun E-M, Park S-H, Heo S-J, Lee T-S, Yoo I-D, Kim J-P. J. Nat. Prod. 2007;70:1043. doi: 10.1021/np060637h.. Kanokmedhakul S, Kanokmedhakul K, Nasomjai P, Louangsysouphanh S, Soytong K, Isobe M, Kongsaeree P, Prabpai S, Suksamram A. J. Nat. Prod. 2006;69:891. doi: 10.1021/np060051v.. Lin Y-L, Shen C-C, Huang Y-J, Chang Y-Y. J. Nat. Prod. 2005;68:381. doi: 10.1021/np0401819.. Brimble MA, Nairn MR, Prabaharan H. Tetrahedron. 2000;56:1937.. Majumder PL, Guha S, Sen S. Phytochemistry. 1999;52:1365.. Wang W, Li T, Milburn R, Yates J, Hinnant E, Luzzio MJ, Noble SA, Attardo G. Bioorg. Med. Chem. Lett. 1998;8:1579. doi: 10.1016/s0960-894x(98)00274-1.. Biber B, Muske J, Ritzan M, Graft U.J. Antibiot 1998513819589078. Wang W, Breining T, Li T, Milbum R, Attardo G. Tetrahedron Lett. 1998;39:2459.. Thines E, Anke H, Sterner O. J. Nat. Prod. 1998;61:306. doi: 10.1021/np970469g.. Kim J-P, Kim W-G, Koshino H, Jung J, Yoo I-D. Phytochemistry. 1996;43:425. doi: 10.1016/0031-9422(96)00279-8.. Solis P, Lang'at C, Gupta MP, Kirby G, Warhurst D, Phillipson J. Planta Med. 1995;61:62. doi: 10.1055/s-2006-958001.. Ali A, Read RW, Sotheeswaran S. Phytochemistry. 1994;35:1029.. Hari L, De Buyck LF, De Pootert HL. Phytochemistry. 1991;30:1726.. Poch GK, Gloer JB. Tetrahedron Lett. 1989;30:3483.. Hayashi T, Smith FT, Lee K-H. J. Med. Chem. 1987;30:2005. doi: 10.1021/jm00394a013.. Harborne JB, Girija AR, Devi HM, Lakshmi NKM. Phytochemistry. 1983;22:2741.. Papadakis DP, Salemik CA, Alikaridis FJ, Kephalas TA. Tetrahedron. 1983;39:2223.; Marini Bettolo GB, Casinovi CG, Galeffi C. Tetrahedron Lett. 1965;6:4857..
- (28).For isobenzofurans, see: Pahari P, Senapati B, Mal D. Tetrahedron Lett. 2004;45:5109.. Harper JK, Arif AM, Ford E, Strobel G, Porco JA, Jr., Tomer DP, Oneill KL, Heider EM, Grant DM. Tetrahedron. 2003;59:2471.. Strobel G, Ford E, Worapong J, Harper JK, Arif AM, Grant DM, Fung PCW, Chau RMW. Phytochemistry. 2002;60:179. doi: 10.1016/s0031-9422(02)00062-6..
- (29).(a) Butin AV, Abaev VT, Mel'chin VV, Dmitriev AS, Pilipenko AS, Shashkov AS. Synthesis. 2008:1798. [Google Scholar]; (b) Kobayashi K, Nagaoka T, Fukamachi S, Shirai Y, Morikawa O, Konishi H. Synthesis. 2007:3032. [Google Scholar]; (c) Villeneuve K, Tam W. Organometallics. 2007;26:6082. [Google Scholar]; (d) Obika S, Kono H, Yasui Y, Yanada R, Takemoto Y. J. Org. Chem. 2007;72:4462. doi: 10.1021/jo070615f. [DOI] [PubMed] [Google Scholar]; (e) Yao X, Li C-J. Org. Lett. 2006;8:1953. doi: 10.1021/ol060645p. [DOI] [PubMed] [Google Scholar]; (f) Kusama H, Sawada T, Okita A, Shiozawa F, Iwasawa N. Org. Lett. 2006;8:1077. doi: 10.1021/ol052951t. [DOI] [PubMed] [Google Scholar]; (g) Villeneuve K, Tam W. Eur.J. Org. Chem. 2006:5449. doi: 10.1021/jo060864o. [DOI] [PubMed] [Google Scholar]; (h) Butin AV, Abaev VT, Mel'chin VV, Dmitriev AS. Tetrahedron Lett. 2005;46:8439. [Google Scholar]; (i) Patil NT, Yamamoto Y. J. Org. Chem. 2004;69:5139. doi: 10.1021/jo049416b. [DOI] [PubMed] [Google Scholar]; (j) Mondal S, Nogami T, Asao N, Yamamoto Y. J. Org. Chem. 2003;68:9496. doi: 10.1021/jo035016x. [DOI] [PubMed] [Google Scholar]; (k) Gabriele B, Salerno G, Fazio A, Pittelli R. Tetrahedron. 2003;59:6251. [Google Scholar]; (l) Asao N, Nogami T, Takahashi K, Yamamoto Y. J. Am. Chem. Soc. 2002;124:764. doi: 10.1021/ja017366b. [DOI] [PubMed] [Google Scholar]; (m) Mutter R, Campbell IB, Martin de la Nava EM, Merritt AT, Wills M. J. Org. Chem. 2001;66:3284. doi: 10.1021/jo0011991. [DOI] [PubMed] [Google Scholar]; (n) Van TN, Kesteleyn B, De Kimpe N. Tetrahedron. 2001;57:4213. [Google Scholar]; (o) Mutter R, Martin de la Neva EM, Wills M. Chem. Commun. 2000:1675. [Google Scholar]; (p) Giles RGF, Green IR, Taylor CP. Tetrahedron Lett. 1999;40:4871. [Google Scholar]
- (30).(a) Yue D, Della Cá N, Larock RC. Org. Lett. 2004;6:1581. doi: 10.1021/ol049690s. [DOI] [PubMed] [Google Scholar]; (b) Yue D, Della Cá N, Larock RC. J. Org. Chem. 2006;71:3381. doi: 10.1021/jo0524573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).(a) Barluenga J, Vázquez-Villa H, Ballesteros A, González JM. J. Am. Chem. Soc. 2003;125:9028. doi: 10.1021/ja0355372. [DOI] [PubMed] [Google Scholar]; (b) Barluenga J, Vázquez-Villa H, Merino I, Ballesteros A, González JM. Chem. Eur. J. 2006;12:5790. doi: 10.1002/chem.200501505. [DOI] [PubMed] [Google Scholar]
- (32).The reaction did not occur in the absence of base, as shown by blank experiments.
- (33).Complex reaction mixtures were obtained when the reaction was carried out at temperatures higher than 25 °C (40–80 °C)
- (34).Sammes PG, Weller DJ. Synthesis. 1995:1205. [Google Scholar]
- (35).The structures of iodocyclization products 2c and 3u were unequivocally confirmed by X-ray diffraction analysis. The structures of all other regioisomeric products 2 and 3 were determined by spectroscopic techniques, and confirmed by comparison with compounds 2c and 3u, respectively. See the Supporting Information for details.
- (36).The possibility of base-promoted cyclization, followed by iodination of the resulting vinylic ethers, was examined. The 2-(1-alkynyl)benzylic alcohol substrates 1a, 1s and 1w were subjected to our usual cyclization conditions omitting iodine. In all of these cases, no cyclization was observed and the starting materials were recovered.
- (37).Muizebelt WJ, Nivard RJF. Chem. Commun. 1965:148. [Google Scholar]; (b) Windmon N, Dragojlovic V. Tetrahedron Lett. 2008;49:6543. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





