Abstract
Drug addiction is a chronically relapsing disorder that is characterized by a compulsion to take drugs and loss of control in limiting intake. Medications that are on the market for the treatment of drug addiction target either the direct reinforcing effects of abuse (eg, naltrexone) or the consequent protracted abstinence syndrome (eg, acamprosate). Both conceptual and neurobiological advances in research have suggested that brain stress systems contribute to the withdrawal/negative affect and preoccupation/anticipation stages of the addiction cycle that promote the compulsivity of drug-taking in addiction. Validated animal models of the stress component of addiction and improved understanding of the neurocircuitry and neuropharmacological mechanisms involved in perturbations of this component suggest that corticotropin-releasing factor systems are a viable target for the development of future medications for drug addiction.
Keywords: Addiction, corticotropin-releasing factor, drug relapse, drug withdrawal, stress
Introduction
Both conceptual and neurobiological advances in research have suggested that brain stress systems can contribute to the compulsivity of drug-taking and therefore participate in the development and persistence of addiction. Corticotropin-releasing factor (CRF) is a 41-amino acid polypeptide that mediates hormonal, autonomic and behavioral responses to stressors. This review outlines significant progress that has been achieved in understanding how perturbations of neuropharmacological mechanisms involving CRF can drive the addiction process. Based on this research, CRF systems are hypothesized to represent a particularly viable target for the development of medications for treating drug addiction in the withdrawal/negative affect and preoccupation/anticipation stages of the addiction cycle.
Mechanisms of addiction
Three neurobiological circuits have been identified to have heuristic value for the study of neurobiological changes associated with the development and persistence of drug dependence (Figure 1): a binge/intoxication-related circuit, a withdrawal/negative affect-related circuit, and a preoccupation/anticipation (ie, craving)-related circuit. Such mechanisms may be relevant to the use of CRF receptor antagonists in the treatment of drug addiction. The acute reinforcing effects of drugs of abuse that comprise the binge/intoxication stage most likely involve actions with an emphasis on the ventral striatum and extended amygdala reward system, as well as dopaminergic and opioid inputs from the ventral tegmental area (VTA) and arcuate nucleus of the hypothalamus, respectively [1,2]. In contrast, the symptoms of acute withdrawal that are important for addiction, such as dysphoria and increased anxiety associated with the withdrawal/negative affect stage, most likely involve decreases in the reward function of the ventral striatum, but also the recruitment of brain stress neurocircuitry, including CRF and norepinephrine in the extended amygdala. The preoccupation/anticipation stage also involves core elements of the brain stress systems in the extended amygdala (for stress-induced reinstatement), as well as key afferent glutamatergic projections to the extended amygdala and nucleus accumbens, specifically from the prefrontal cortex (for drug-induced reinstatement) and the basolateral amygdala (for cue-induced reinstatement) [1,2]. Compulsive drug-seeking behavior is also hypothesized to activate ventral striatal-ventral pallidal-thalamic-cortical loops that may subsequently engage dorsal striatal-pallidal-thalamic-cortical loops [3], with both effects influenced by concomitant decreases in reward function and the activation of brain stress systems in the extended amygdala [4]. The activation of the brain stress system by CRF that drives dependence and compulsivity in addiction is the conceptual framework for this review article.
Figure 1. The three stages of the addiction cycle and the criteria for substance dependence.
There are three stages in the addiction cycle: binge/intoxication, withdrawal/negative affect and preoccupation/anticipation; these stages are defined from a psychiatric perspective, with different criteria for substance dependence incorporated from the American Psychiatric Association's Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) [92].
(Adapted with permission from The American Association for the Advancement of Science and Koob GF, Le Moal M: Drug abuse: Hedonic homeostatic dysregulation. Science (1997) 278(5335):52-58. © 1997 The American Association for the Advancement of Science)
Corticotropin-releasing factor
The identification of CRF was followed by the discovery of genes encoding three paralogs of CRF (urocortin [Ucn]1, Ucn2 and Ucn3), as well as two GPCRs (CRF1 and CRF2) to which the CRF/Ucn peptides bind and activate with varying affinities [5,6]. Pharmacological and transgenic studies have demonstrated that brain and pituitary CRF1 receptors mediate many of the functional stress-like effects of the CRF systems [7]. Consequently, numerous selective CRF1 receptor antagonists have been designed that are capable of penetrating the blood-brain barrier [8]. Previous reviews have surveyed the biology of CRF systems [5,7].
CRF is widely distributed throughout the brain, with particularly high concentrations of cell bodies occurring in the paraventricular nucleus of the hypothalamus, the basal forebrain (notably the extended amygdala) and the brainstem [9]. The central administration of CRF mimics the behavioral response to activation and stress in rodents, and the administration of competitive CRF receptor antagonists generally has anti-stress effects [10–12] (for reviews, see references [13–16]). CRF1 receptor activation is associated with increased stress responsiveness [17], and CRF2 receptor activation is associated with decreases in feeding [18,19]. CRF2 activation also may decrease stress responsiveness although there is some controversy in this area. The activation of CRF2 receptors has also been implicated in increased stress responsiveness or no change in stress-related behavior depending on the neuropharmacological probe, the dose and the brain site used [6,20–22].
The role of CRF in animal models of addiction
The chronic administration of drugs with potential for dependence dysregulates the stress responses mediated by CRF, including the hypothalamic-pituitary-adrenal axis and the brain extrahypothalamic stress system. During acute withdrawal, certain responses are common for all drugs of abuse and alcohol, including an activated hypothalamic-pituitary-adrenal stress response, reflected by elevated ACTH and corticosteroids; and an activated brain stress response, with increased CRF release from the amygdala. However, with repeated cycles of addiction, a blunted hypothalamic-pituitary-adrenal response occurs, but with a sensitized extrahypothalamic CRF stress system response [2,23].
The neuroanatomical entity referred to as the extended amygdala may represent a common anatomical substrate for acute drug reward, as well as a common neuroanatomical substrate for the negative effects on reward function produced by stress that helps to drive compulsive drug administration. The extended amygdala includes not only the central nucleus of the amygdala, but also the bed nucleus of the stria terminalis and a transition zone in the medial subregion of the nucleus accumbens (shell of the nucleus accumbens) [24]; these regions share certain cytoarchitectural and circuitry similarities. A key neurochemical component of this circuitry that delineates the central division of the extended amygdala is a high density of cell bodies and terminals for CRF. The extended amygdala receives numerous afferent neurons from limbic (emotional) structures, such as the basolateral amygdala and hippocampus, and sends efferent neurons to the medial part of the ventral pallidum and a large projection of neurons to the lateral hypothalamus, thus further defining the specific brain areas that interface classic limbic structures with the extrapyramidal motor system and motivational systems [24].
In rats, in vivo microdialysis during acute withdrawal following the chronic administration or self-administration of drugs of abuse increased extracellular CRF in the extended amygdala. During alcohol withdrawal, extrahypothalamic CRF systems have been observed to become hyperactive, with an increase in extracellular CRF levels within the central nucleus of the amygdala and bed nucleus of the stria terminalis in alcohol-dependent rats [25,26]. Extracellular CRF in rats also increased in the central nucleus of the amygdala during precipitated withdrawal from chronic nicotine [27], withdrawal from binge cocaine self-administration [28], and precipitated withdrawal from opioids [29] and cannabinoids [30]. Amygdala CRF tissue content was reduced during acute withdrawal from ethanol exposure [31,32] and from binge cocaine self-administration [31]. CRF mRNA expression in the central nucleus of the amygdala also increased in rats during withdrawal from cannabinoids [33] or ethanol [34].
Another common response to acute withdrawal and protracted abstinence from all major drugs of abuse is the manifestation of a negative emotional state, including anxiety-like responses. Animal models in which the dependent variable is often a passive response to a novel and/or aversive stimulus, such as the open field, elevated plus maze, defensive withdrawal and social interaction tests, or an active response to an aversive stimulus, such as defensive burying of an electrified metal probe, have demonstrated anxiety-like responses to acute withdrawal from all major drugs of abuse [2]. Withdrawal from the repeated administration of cocaine, alcohol, nicotine, cannabinoids and benzodiazepines produces an anxiogenic-like response in the elevated plus maze, defensive withdrawal test and defensive burying test, and such effects are reversed by the administration of CRF antagonists [27,30,35–40]. Moreover, the aversive state of opiate withdrawal and the decreased brain reward function associated with nicotine withdrawal have both been demonstrated to be CRF1 receptor-dependent [41–44].
The motivational effects of CRF in dependence can be observed in animal models of increased alcohol self-administration in alcohol-dependent animals. A competitive CRF peptide antagonist, which had no effect on alcohol self-administration in non-dependent rats, effectively eliminated excessive drinking in dependent or post-dependent rats [45]. When administered directly into the central nucleus of the amygdala, a CRF1/CRF2 receptor antagonist blocked alcohol self-administration in alcohol-dependent rats [32]. The direct administration of a CRF2 agonist revealed an identical pattern of results, suggesting that, in the amygdala, the activation of CRF2 systems may act in opposition to the activation of CRF1 systems [46]. These data suggest an important role for CRF, primarily within the central nucleus of the amygdala, in mediating the increased self-administration that is associated with drug dependence.
Systemic injections of small-molecule CRF1 antagonists also reduced the increased alcohol intake observed in dependent or post-dependent animals [47–53] (Table 1). The ability of CRF antagonists to block the anxiogenic-like and aversive-like motivational effects of drug withdrawal also predicts the motivational effects of such antagonists in animal models of extended access to drugs. Accordingly, CRF1 small-molecule antagonists selectively reduced the increased self-administration of drugs during extended access to intravenous cocaine [54], nicotine [27] and heroin [55].
Table 1.
Comparison of the effects of CRF antagonists with naltrexone and acamprosate in alcohol addiction.
| Naltrexone | Acamprosate | CRF antagonist | |
|---|---|---|---|
| Baseline drinking | ↓ [93] | −[94] | – (antalarmin, MJL-1-109-2 and R-121919 [48]; LWH-63, MTIP and MPZP [47,49–53]) |
| Binge drinking | ↓ [95,96] | ↓ [95] | – (MPZP [96]) |
|
Dependence-induced drinking |
↓ [97] | ↓ [98] | ↓ (antalarmin, MJL-1-109-2 and R-121919 [48]; MTIP and MPZP [47,49–53]) |
| Cue-induced reinstatement | ↓ [99] | ↓ [100] | – (d- Phe CRF12–41 [99]) |
| Stress-induced reinstatement | − [99] | Not determined | ↓ (d- Phe CRF12–41 [99]) |
Naltrexone and acamprosate, medications that are used to treat alcoholism, and CRF (corticotropin-releasing factor) antagonists were evaluated in various animal models, including rats, mice and primates, reflecting different components of the addiction cycle: binge drinking (binge/intoxication), dependence-induced drinking (withdrawal/negative affect), and cue- and stress-induced reinstatement (preoccupation/anticipation). Dashes (–) indicate no effect.
MPZP N,N-bis(2-methoxyethyl)-3-(4-methoxy-2-methylphenyl)-2,5-dimethyl-pyrazolo [1,5a]pyrimidin-7-amine, MTIP 3-(4-chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl-imidazo[1,2-b]pyridazine
(Adapted with permission from George F Koob. © 2010 George F Koob)
Stress and stressors also have an established association with relapse and vulnerability to relapse [23,56]. In human alcoholics, numerous symptoms that can be characterized by negative affect persist after acute withdrawal from alcohol [57]. These symptoms, referred to as protracted abstinence, tend to be affective in nature, may last months to years, and often precede relapse [58,59]. A factor analysis of Marlatt's relapse taxonomy revealed that negative emotion, including elements of anger, frustration, sadness, anxiety and guilt, was a key factor in relapse [60], and was the leading precipitant of relapse in a large-scale replication of the taxonomy [61]. Exposure to negative affect, stress or withdrawal-related distress also increases drug craving [62–64].
The neuropharmacology of the stress-induced reinstatement of drug-seeking follows a pattern of results that is somewhat parallel to the neuropharmacology of the anxiety-like effects of acute withdrawal and dependence-induced increases in drug intake [65,66]. Mixed CRF1/CRF2 antagonists injected intracerebroventricularly and CRF1 small-molecule antagonists administered systemically have been demonstrated to block the stress-induced reinstatement of cocaine-, opiate-, alcohol- and nicotine-seeking behavior [44,53,65–69]. These effects have been replicated with intracerebral injections of a mixed CRF1/CRF2 antagonist or a small-molecule CRF1 antagonist into the bed nucleus of the stria terminalis, median raphe and VTA, but not the amygdala or nucleus accumbens [65,66]. The foot-shock-induced reinstatement of cocaine-seeking was blocked by the administration of a CRF2 receptor antagonist into the VTA [70,71]. These observations suggest that sites such as the bed nucleus of the stria terminalis, median raphe and VTA may be important for stress-induced relapse, in contrast to the role of CRF in dependence-induced increases in drug self-administration that have been localized to the central nucleus of the amygdala [32]. CRF systems have also been identified in the VTA, and foot-shock stress can release CRF into the VTA [72,73]. Further supporting a role for VTA CRF systems in stress-induced relapse was the observation that the intra-VTA infusion of CRF elicited the reinstatement of cocaine-seeking behavior in rats [73]. The mechanism of reinstatement may involve the activation of the mesolimbic dopamine system; the application of CRF to a VTA slice preparation facilitated excitatory glutamatergic responses of dopamine neurons [70], a potentiation that was greater in cocaine-experienced animals compared with drug-naïve animals [74]. VTA CRF-mediated facilitation of dopamine transmission and reinstatement behavior may also involve the CRF binding protein, a protein that binds free CRF with high affinity [75]. CRF6–33, a fragment without known receptor agonist activity that competitively displaces CRF from the CRF binding protein, prevented CRF from stimulating VTA dopamine transmission or from eliciting cocaine-seeking in rats [70,71]. These results complement the role of the CRF1 system in the extended amygdala in stress-induced reinstatement [68].
Stress-induced reinstatement occurs independently from stress-induced activation of the hypothalamic-pituitary-adrenal axis [67,76,77]. Other brain stress systems that are implicated in stress-induced reinstatement and are possibly linked to brain CRF systems include norepinephrine, orexin, vasopressin and nociceptin [65,66] (Figure 2). CRF1 antagonists are ineffective in blocking cue-induced reinstatement, suggesting the selectivity of these actions on the stress components of the addiction cycle [2]. CRF antagonists failed to block yohimbine-induced reinstatement of cocaine seeking [78], but were capable of reversing yohimbine-induced reinstatement of alcohol and food seeking [69,79], suggesting a specific interaction with cocaine (which also blocks the reuptake of norepinephrine) that is not observed with other drugs of abuse. The hypothesis is not that CRF1 antagonists will reverse all craving but, rather, that CRF1 antagonists may be effective in reversing both state- (protracted abstinence) and stressor-induced reinstatement of drug seeking. Substantial evidence supports the hypothesis that some form of a stress-like (negative emotional) state or stressor exposure contributes to the majority of relapses in humans [60,61].
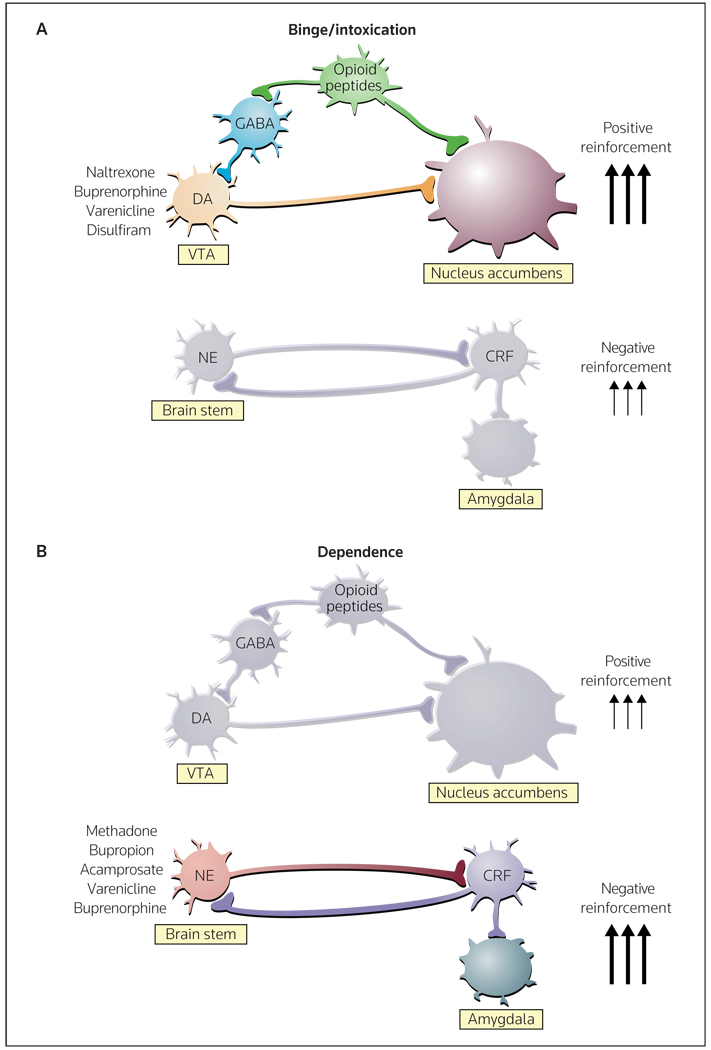
Figure 2. Neurocircuitry associated with the acute positive reinforcing effects of drugs of abuse and the negative reinforcement of dependence, and changes in the transition from non-dependent to dependent drug-taking.
(A) Key elements of the reward circuit include dopamine and opioid peptide neurons that intersect at both the VTA (ventral tegmental area) and the nucleus accumbens that are activated during initial drug use and the early binge/intoxication stage of the addiction cycle. Drugs approved for the treatment of this component of the addiction cycle include naltrexone, buprenorphine, varenicline and disulfiram. (B) Key elements of the stress circuit include CRF (corticotropin-releasing factor) and noradrenergic neurons that converge on GABA interneurons in the central nucleus of the amygdala that are activated during the development of dependence. Drugs approved for the treatment of this component of the addiction cycle include methadone, bupropion, acamprosate, varenicline and buprenorphine.
DA dopamine, NE norepinephrine
(Adapted with permission from Nature Publishing Group and Nestler EJ: Is there a common molecular pathway for addiction? Nat Neurosci (2005) 8(11):1445-1449. © 2005 Nature Publishing Group)
Thus, brain stress systems may have an impact on both the withdrawal/negative affect and preoccupation/anticipation stages of the addiction cycle, albeit by engaging different components of the extended amygdala emotional system (ie, the central nucleus of the amygdala versus the bed nucleus of the stria terminalis). The dysregulations that comprise the negative emotional state of drug dependence persist during protracted abstinence to promote vulnerability to craving by activation of the drug-, cue-and stress-induced reinstatement neurocircuits, driven by a hypofunctioning and possibly reorganized prefrontal system [80].
No laboratory studies or clinical trials have been initiated to explore the effects of CRF antagonists on drug dependence in humans. However, several clinical trials that explored the effects of CRF1 antagonists, including ONO-2333Ms, CP-316311 and R-121919 (NBI-30775), on anxiety and depression measures have been completed. Double-blind, placebo-controlled trials of ONO-2333Ms (ClinicalTrials.gov identifier: NCT00514865) and CP-316311 (NCT00143091) for major depression have yielded negative results [81], in contrast to the results of an earlier open-label trial for R-121919 [82,83]. However, in all trials, the CRF1 antagonists were well tolerated, and no common adverse effects were observed [81,84–86].
Given the negative results that have been observed for CRF1 antagonists in the treatment of depression [84–86], prospects for the efficacy of CRF1 antagonists in addiction remain unknown. However, addiction is not necessarily equated with depression. Antidepressants generally demonstrate poor efficacy in the treatment of addiction. Indeed, the emotional allostatic state that has been argued to occur in addiction appears to have more similarity with the anxiety-dysphoria continuum. In addition, given that CRF has a prominent role in 'fight or flight' responses in animals, and that CRF antagonists have a mixed efficacy in classic animal models of depression, better therapeutic indications for CRF1 antagonists may be stress, anxiety and the stress-like components associated with addiction, rather than depression.
Pharmacogenetic interactions with the CRF1 receptor
Genetic markers may ultimately predict the individuals who will respond to CRF1 antagonists, and association studies have identified a potential genetic interaction. An association has been observed between SNPs of the CRF1 receptor gene and excessive drinking in humans and rats [87–90]. One SNP observed in humans, R1876831, is located in an intron, and may influence the transcription of the CRF1 receptor gene. Adolescents who were homozygous for the C-allele of R1876831 drank more alcohol per occasion, and demonstrated higher lifetime rates of heavy drinking related to negative life events, compared with individuals carrying the T-allele [87].
In preclinical studies, a genetic polymorphism in the corticotropin-releasing hormone receptor 1 (Crhr1) promoter was identified in Marchigian Sardinian alcohol-preferring (msP) rats, which express a phenotype of elevated CRF1 mRNA expression in the extended amygdala, increased anxiety-like behavior, and CRF1 antagonist-reversible elevations in alcohol intake and alcohol-seeking behavior [88]. Expression of the Crhr1 transcript was downregulated following ad libitum access to alcohol in alcohol-preferring msP rats [89]; however, the expression of CRF and the Crhr1 transcript within the amygdala were upregulated in post-dependent animals [52]. Similarly, a Crh haplotype predicted alcohol consumption in rhesus macaques [90]. Altogether, these results suggest that certain SNPs in the human population may predict vulnerability to particular subtypes of excessive-drinking syndromes, and may also predict responsiveness to the use of CRF receptor antagonists in the treatment of alcoholism.
Conclusion
The extrahypothalamic CRF systems appear to be involved in mediating the anxiety-like effects of acute withdrawal from drugs of abuse, the increase in drug-taking associated with dependence, and stress-induced reinstatement for all major drugs of abuse, including psychostimulants, opioids, alcohol, nicotine and (in limited studies) cannabinoids. Many of these effects have been localized to the extended amygdala, and acute withdrawal from major drugs of abuse was demonstrated to increase CRF release in the central nucleus of the amygdala, as measured by in vivo microdialysis.
The majority of studies have implicated CRF and the CRF1 receptor in stress responsivity [84], although CRF2 receptors and Ucn/CRF2 ligands have also been demonstrated to have both stress and anti-stress-like effects [6]. The results suggest a major role for CRF systems in mediating the negative emotional states that have motivational significance in maintaining the drug-dependent state [4,91]. Pharmacogenetic studies suggest that certain CRF system-related SNPs may predict vulnerability to the excessive drinking that is associated with stress, and thus may also predict the efficacy of CRF1 antagonists in addiction. Overall, these results suggest that the CRF system is a significant factor in the withdrawal/negative affect and preoccupation/anticipation stages of the addiction cycle, and pharmacotherapeutic agents directed at the CRF system may be effective in treating some key aspects of addiction.
Acknowledgements
This is publication number 20241 from The Scripps Research Institute. The authors were each supported by the Pearson Center for Alcoholism and Addiction Research, and NIH grants DK26741 from the National Institute of Diabetes and Digestive and Kidney Diseases and AA06420 from the National Institute on Alcohol Abuse and Alcoholism. The authors thank Michael Arends for assistance with the preparation of the manuscript.
References
• • of outstanding interest
• of special interest
- 1.Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- 2.Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59(1):11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vanderschuren LJ, Everitt BJ. Behavioral and neural mechanisms of compulsive drug seeking. Eur J Pharmacol. 2005;526(1–3):77–88. doi: 10.1016/j.ejphar.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 4.Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the 'dark side' of drug addiction. Nat Neurosci. 2005;8(11):1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- 5. Bale TL, Vale WW. CRF and CRF receptors: Role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. •• Excellent review summarizing the role of CRF systems in stress.
- 6.Fekete EM, Zorrilla EP. Physiology, pharmacology, and therapeutic relevance of urocortins in mammals: Ancient CRF paralogs. Front Neuroendocrinol. 2007;28(1):1–27. doi: 10.1016/j.yfrne.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heinrichs SC, Koob GF. Corticotropin-releasing factor in brain: A role in activation, arousal, and affect regulation. J Pharmacol Exp Ther. 2004;311(2):427–440. doi: 10.1124/jpet.103.052092. [DOI] [PubMed] [Google Scholar]
- 8.Zorrilla EP, Zhao Y, Koob GF. Anti-CRF. In: Fink H, editor. Encyclopedia of Stress. Amsterdam, the Netherlands: Elsevier; 2007. pp. 206–214. [Google Scholar]
- 9.Swanson LW, Sawchenko PE, Rivier J, Vale WW. The organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: An immunohistochemical study. Neuroendocrinology. 1983;36(3):165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- 10.Heinrichs SC, Menzaghi F, Merlo Pich E, Baldwin HA, Rassnick S, Britton KT, Koob GF. Anti-stress action of a corticotropin-releasing factor antagonist on behavioral reactivity to stressors of varying type and intensity. Neuropsychopharmacology. 1994;11(3):179–186. doi: 10.1038/sj.npp.1380104. [DOI] [PubMed] [Google Scholar]
- 11.Menzaghi F, Howard RL, Heinrichs SC, Vale W, Rivier J, Koob GF. Characterization of a novel and potent corticotropin-releasing factor antagonist in rats. J Pharmacol Exp Ther. 1994;269(2):564–572. [PubMed] [Google Scholar]
- 12.Spina MG, Basso AM, Zorrilla EP, Heyser CJ, Rivier J, Vale W, Merlo-Pich E, Koob GF. Behavioral effects of central administration of the novel CRF antagonist astressin in rats. Neuropsychopharmacology. 2000;22(3):230–239. doi: 10.1016/S0893-133X(99)00108-6. [DOI] [PubMed] [Google Scholar]
- 13.Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin-releasing factor administration: Is CRF a mediator of anxiety or stress responses? Brain Res Brain Res Rev. 1990;15(2):71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- 14.Koob GF, Heinrichs SC, Menzaghi F, Pich EM, Britton KT. Corticotropin releasing factor, stress and behavior. Semin Neurosci. 1994;6(4):221–229. [Google Scholar]
- 15.Koob GF, Bartfai T, Roberts AJ. The use of molecular genetic approaches in the neuropharmacology of corticotropin-releasing factor. Int J Comp Psychol. 2001;14(3–4):90–110. [Google Scholar]
- 16.Sarnyai Z, Shaham Y, Heinrichs SC. The role of corticotropin-releasing factor in drug addiction. Pharmacol Rev. 2001;53(2):209–243. [PubMed] [Google Scholar]
- 17.Koob GF, Heinrichs SC. A role for corticotropin-releasing factor and urocortin in behavioral responses to stressors. Brain Res. 1999;848(1–2):141–152. doi: 10.1016/s0006-8993(99)01991-5. [DOI] [PubMed] [Google Scholar]
- 18.Spina M, Merlo-Pich E, Chan RKW, Basso AM, Rivier J, Vale W, Koob GF. Appetite-suppressing effects of urocortin, a CRF-related neuropeptide. Science. 1996;273(5281):1561–1564. doi: 10.1126/science.273.5281.1561. [DOI] [PubMed] [Google Scholar]
- 19.Pelleymounter MA, Joppa M, Carmouche M, Cullen MJ, Brown B, Murphy B, Grigoriadis DE, Ling N, Foster AC. Role of corticotropin-releasing factor (CRF) receptors in the anorexic syndrome induced by CRF. J Pharmacol Exp Ther. 2000;293(3):799–806. [PubMed] [Google Scholar]
- 20.Ho SP, Takahashi LK, Livanov V, Spencer K, Lesher T, Maciag C, Smith MA, Rohrbach KW, Hartig PR, Arneric SP. Attenuation of fear conditioning by antisense inhibition of brain corticotropin releasing factor-2 receptor. Brain Res Mol Brain Res. 2001;89(1–2):29–40. doi: 10.1016/s0169-328x(01)00050-x. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi LK, Ho SP, Livanov V, Graciani N, Arneric SP. Antagonism of CRF2 receptors produces anxiolytic behavior in animal models of anxiety. Brain Res. 2001;902(2):135–142. doi: 10.1016/s0006-8993(01)02405-2. [DOI] [PubMed] [Google Scholar]
- 22.Zhao Y, Valdez GR, Fekete EM, Rivier JE, Vale WW, Rice KC, Weiss F, Zorrilla EP. Subtype-selective corticotropin-releasing factor receptor agonists exert contrasting, but not opposite, effects on anxiety-related behavior in rats. J Pharmacol Exp Ther. 2007;323(3):846–854. doi: 10.1124/jpet.107.123208. [DOI] [PubMed] [Google Scholar]
- 23.Koob GF, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164(8):1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heimer L, Alheid G. Piecing together the puzzle of basal forebrain anatomy. In: Napier TC, Kalivas PW, Hanin I, editors. The Basal Forebrain: Anatomy to Function. New York, NY, USA: Plenum Press; 1991. pp. 1–42. [DOI] [PubMed] [Google Scholar]
- 25. Merlo-Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, Weiss F. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995;15(8):5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. • Describes an early study that implicated the activation of the amygdala CRF system in alcohol withdrawal.
- 26.Olive MF, Koenig HN, Nannini MA, Hodge CW. Elevated extracellular CRF levels in the bed nucleus of the stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake. Pharmacol Biochem Behav. 2002;72(1–2):213–220. doi: 10.1016/s0091-3057(01)00748-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. George O, Ghozland S, Azar MR, Cottone P, Zorrilla EP, Parsons LH, O'Dell LE, Richardson HN, Koob GF. CRF-CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proc Natl Acad Sci USA. 2007;104(43):17198–17203. doi: 10.1073/pnas.0707585104. •• Demonstrated the efficacy of a CRF1 antagonist in reducing excessive nicotine self-administration during nicotine withdrawal.
- 28.Richter RM, Weiss F. In vivo CRF release in rat amygdala is increased during cocaine withdrawal in self-administering rats. Synapse. 1999;32(4):254–261. doi: 10.1002/(SICI)1098-2396(19990615)32:4<254::AID-SYN2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 29.Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu X, Zorrilla EP, Valdez GR, Ben-Shahar O, Angeletti S, Richter RR. Compulsive drug-seeking behavior and relapse: Neuroadaptation, stress, and conditioning factors. In: Quinones-Jenab V, editor. The Biological Basis of Cocaine Addiction. New York, NY, USA: New York Academy of Sciences; 2001. pp. 1–26. [DOI] [PubMed] [Google Scholar]
- 30. Rodriguez de Fonseca F, Carrera MR, Navarro M, Koob GF, Weiss F. Activation of corticotropin-releasing factor in the limbic system during cannabinoid withdrawal. Science. 1997;276(5321):2050–2054. doi: 10.1126/science.276.5321.2050. • Describes an early study that implicated the activation of the CRF system in the amygdala in cannabinoid withdrawal.
- 31. Zorrilla EP, Valdez GR, Weiss F. Changes in levels of regional CRF-like-immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacology. 2001;158(4):374–381. doi: 10.1007/s002130100773. • Provided neurobiological evidence that amygdala CRF systems remain functionally active during a protracted abstinence from alcohol dependence.
- 32. Funk CK, O'Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci. 2006;26(44):11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. • Demonstrated a key role for CRF activation in the central nucleus of the amygdala in driving excessive alcohol consumption.
- 33.Caberlotto L, Rimondini R, Hansson A, Eriksson S, Heilig M. Corticotropin-releasing hormone (CRH) mRNA expression in rat central amygdala in cannabinoid tolerance and withdrawal: Evidence for an allostatic shift? Neuropsychopharmacology. 2004;29(1):15–22. doi: 10.1038/sj.npp.1300296. [DOI] [PubMed] [Google Scholar]
- 34.Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, Cottone P, Madamba SM, Souffer DG, Zorrilla EP, Koob GF, et al. CRF-induced amygdala GABA release plays a key role in alcohol dependence. Biol Psychiatry. 2010 doi: 10.1016/j.biopsych.2009.11.007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sarnyai Z, Biro E, Gardi J, Vecsernyes M, Julesz J, Telegdy G. Brain corticotropin-releasing factor mediates 'anxiety-like' behavior induced by cocaine withdrawal in rats. Brain Res. 1995;675(1–2):89–97. doi: 10.1016/0006-8993(95)00043-p. • One of the first studies to demonstrate that CRF mediates the anxiogenic-like effects of psychostimulant withdrawal.
- 36.Basso AM, Spina M, Rivier J, Vale W, Koob GF. Corticotropin-releasing factor antagonist attenuates the 'anxiogeniclike' effect in the defensive burying paradigm but not in the elevated plus-maze following chronic cocaine in rats. Psychopharmacology. 1999;145(1):21–30. doi: 10.1007/s002130051028. [DOI] [PubMed] [Google Scholar]
- 37.Knapp DJ, Overstreet DH, Moy SS, Breese GR. SB242084, flumazenil, and CRA1000 block ethanol withdrawal-induced anxiety in rats. Alcohol. 2004;32(2):101–111. doi: 10.1016/j.alcohol.2003.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Overstreet DH, Knapp DJ, Breese GR. Modulation of multiple ethanol withdrawal-induced anxiety-like behavior by CRF and CRF1 receptors. Pharmacol Biochem Behav. 2004;77(2):405–413. doi: 10.1016/j.pbb.2003.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tucci S, Cheeta S, Seth P, File SE. Corticotropin releasing factor antagonist, α-helical CRF9–41, reverses nicotine-induced conditioned, but not unconditioned, anxiety. Psychopharmacology. 2003;167(3):251–256. doi: 10.1007/s00213-003-1403-4. [DOI] [PubMed] [Google Scholar]
- 40.Skelton KH, Gutman DA, Thrivikraman KV, Nemeroff CB, Owens MJ. The CRF1 receptor antagonist R121919 attenuates the neuroendocrine and behavioral effects of precipitated lorazepam withdrawal. Psychopharmacology. 2007;192(3):385–396. doi: 10.1007/s00213-007-0713-3. [DOI] [PubMed] [Google Scholar]
- 41. Stinus L, Cador M, Zorrilla EP, Koob GF. Buprenorphine and a CRF1 antagonist block the acquisition of opiate withdrawal-induced conditioned place aversion in rats. Neuropsychopharmacology. 2005;30(1):90–98. doi: 10.1038/sj.npp.1300487. • Provided evidence that the CRF system has a key role in the aversive stimulus effects of opioid withdrawal.
- 42.Contarino A, Papaleo F. The corticotropin-releasing factor receptor-1 pathway mediates the negative affective states of opiate withdrawal. Proc Natl Acad Sci USA. 2005;102(51):18649–18654. doi: 10.1073/pnas.0506999102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bruijnzeel AW, Zislis G, Wilson C, Gold MS. Antagonism of CRF receptors prevents the deficit in brain reward function associated with precipitated nicotine withdrawal in rats. Neuropsychopharmacology. 2007;32(4):955–963. doi: 10.1038/sj.npp.1301192. [DOI] [PubMed] [Google Scholar]
- 44.Bruijnzeel AW, Prado M, Isaac S. Corticotropin-releasing factor-1 receptor activation mediates nicotine withdrawal-induced deficit in brain reward function and stress-induced relapse. Biol Psychiatry. 2009;66(2):110–117. doi: 10.1016/j.biopsych.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: Regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26(10):1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. • Provided pharmacological evidence that CRF systems remained functionally active to drive excessive drinking and anxiety during a protracted abstinence from alcohol dependence.
- 46.Valdez GR, Sabino V, Koob GF. Increased anxiety-like behavior and ethanol self-administration in dependent rats: Reversal via corticotropin-releasing factor-2 receptor activation. Alcohol Clin Exp Res. 2004;28(6):865–872. doi: 10.1097/01.alc.0000128222.29875.40. [DOI] [PubMed] [Google Scholar]
- 47.Sabino V, Cottone P, Koob GF, Steardo L, Lee MJ, Rice KC, Zorrilla EP. Dissociation between opioid and CRF1 antagonist sensitive drinking in Sardinian alcohol-preferring rats. Psychopharmacology. 2006;189(2):175–186. doi: 10.1007/s00213-006-0546-5. [DOI] [PubMed] [Google Scholar]
- 48. Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry. 2007;61(1):78–86. doi: 10.1016/j.biopsych.2006.03.063. • Demonstrated efficacy of CRF antagonists in reducing excessive drinking associated with dependence.
- 49.Richardson HN, Zhao Y, Fekete EM, Funk CK, Wirsching P, Janda KD, Zorrilla EP, Koob GF. MPZP: A novel small molecule corticotropin-releasing factor type 1 receptor (CRF1) antagonist. Pharmacol Biochem Behav. 2008;88(4):497–510. doi: 10.1016/j.pbb.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chu K, Koob GF, Cole M, Zorrilla EP, Roberts AJ. Dependence-induced increases in ethanol self-administration in mice are blocked by the CRF1 receptor antagonist antalarmin and by CRF1 receptor knockout. Pharmacol Biochem Behav. 2007;86(4):813–821. doi: 10.1016/j.pbb.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gilpin NW, Richardson HN, Koob GF. Effects of CRF1-receptor and opioid-receptor antagonists on dependence-induced increases in alcohol drinking by alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2008;32(9):1535–1542. doi: 10.1111/j.1530-0277.2008.00745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, Heilig MA. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala Crhr1 expression following a history of dependence. Biol Psychiatry. 2008;63(2):139–145. doi: 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 53. Gehlert DR, Cippitelli A, Thorsell A, Le AD, Hipskind PA, Hamdouchi C, Lu J, Hembre EJ, Cramer JJ, Song M, McKinzie D, et al. 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethylimidazo[1,2-b]pyridazine: A novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. J Neurosci. 2007;27(10):2718–2726. doi: 10.1523/JNEUROSCI.4985-06.2007. • Demonstrated efficacy of a CRF1 antagonist in reducing excessive drinking in different animal models of excessive drinking.
- 54. Specio SE, Wee S, O'Dell LE, Boutrel B, Zorrilla EP, Koob GF. CRF1 receptor antagonists attenuate escalated cocaine self-administration in rats. Psychopharmacology. 2008;196(3):473–482. doi: 10.1007/s00213-007-0983-9. • Demonstrated efficacy of a CRF1 antagonist in reducing excessive cocaine self-administration in animals with cocaine dependence.
- 55. Greenwell TN, Funk CK, Cottone P, Richardson HN, Chen SA, Rice K, Zorrilla EP, Koob GF. Corticotropin-releasing factor-1 receptor antagonists decrease heroin self-administration in long- but not short-access rats. Addict Biol. 2009;14(2):130–143. doi: 10.1111/j.1369-1600.2008.00142.x. • Demonstrated efficacy of a CRF1 antagonist in excessive heroin self-administration in animals with heroin dependence.
- 56.Marlatt GA, Gordon JR. Determinants of relapse: Implications for the maintenance of behavioral change. In: Davidson P, Davidson SH, editors. Behavioral Medicine: Changing Health Lifestyles. New York, NY, USA: Brunner/Mazel; 1980. pp. 410–452. [Google Scholar]
- 57.Alling C, Balldin J, Bokstrom K, Gottfries CG, Karlsson I, Langstrom G. Studies on duration of a late recovery period after chronic abuse of ethanol: A cross-sectional study of biochemical and psychiatric indicators. Acta Psychiatr Scand. 1982;66(5):384–397. doi: 10.1111/j.1600-0447.1982.tb06720.x. [DOI] [PubMed] [Google Scholar]
- 58.Hershon HI. Alcohol withdrawal symptoms and drinking behavior. J Stud Alcohol. 1977;38(5):953–971. doi: 10.15288/jsa.1977.38.953. [DOI] [PubMed] [Google Scholar]
- 59.Annis HM, Sklar SM, Moser AE. Gender in relation to relapse crisis situations, coping, and outcome among treated alcoholics. Addict Behav. 1998;23(1):127–131. doi: 10.1016/s0306-4603(97)00024-5. [DOI] [PubMed] [Google Scholar]
- 60.Zywiak WH, Connors GJ, Maisto SA, Westerberg VS. Relapse research and the Reasons for Drinking Questionnaire: A factor analysis of Marlatt's relapse taxonomy. Addiction. 1996;91 Suppl:S121–S130. [PubMed] [Google Scholar]
- 61.Lowman C, Allen J, Stout RL, The Relapse Research Group Replication and extension of Marlatt' s taxonomy of relapse precipitants: Overview of procedures and results. Addiction. 1996;91 Suppl:S51–S71. [PubMed] [Google Scholar]
- 62.Childress AR, Ehrman R, McLellan AT, MacRae J, Natale M, Natale M, O'Brien CP. Can induced moods trigger drug-related responses in opiate abuse patients? J Subst Abuse Treatment. 1994;11(1):17–23. doi: 10.1016/0740-5472(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 63.Cooney NL, Litt MD, Morse PA, Bauer LO, Gaupp L. Alcohol cue reactivity, negative-mood reactivity, and relapse in treated alcoholic men. J Abnorm Psychol. 1997;106(2):243–250. doi: 10.1037//0021-843x.106.2.243. [DOI] [PubMed] [Google Scholar]
- 64.Sinha R, Fuse T, Aubin LR, O'Malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology. 2000;152(2):140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- 65. Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: History, methodology and major findings. Psychopharmacology. 2003;168(1–2):3–20. doi: 10.1007/s00213-002-1224-x. •• Excellent review summarizing the neurobiology of the reinstatement of drug-seeking behavior in animals.
- 66.Lu L, Shepard JD, Hall FS, Shaham Y. Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: A review. Neurosci Biobehav Rev. 2003;27(5):457–491. doi: 10.1016/s0149-7634(03)00073-3. [DOI] [PubMed] [Google Scholar]
- 67.Le AD, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y. The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology. 2000;150(3):317–324. doi: 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- 68.Shaham Y, Erb S, Leung S, Buczek Y, Stewart J. CP-154,526, a selective, non-peptide antagonist of the corticotropin-releasing factor1 receptor attenuates stress-induced relapse to drug seeking in cocaine- and heroin-trained rats. Psychopharmacology. 1998;137(2):184–190. doi: 10.1007/s002130050608. [DOI] [PubMed] [Google Scholar]
- 69.Marinelli PW, Funk D, Juzytsch W, Harding S, Rice KC, Shaham Y, Lê AD. The CRF1 receptor antagonist antalarmin attenuates yohimbine-induced increases in operant alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology. 2007;195(3):345–355. doi: 10.1007/s00213-007-0905-x. [DOI] [PubMed] [Google Scholar]
- 70.Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39(3):401–407. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- 71.Wang B, You ZB, Rice KC, Wise RA. Stress-induced relapse to cocaine seeking: Roles for the CRF2 receptor and CRF-binding protein in the ventral tegmental area of the rat. Psychopharmacology. 2007;193(2):283–294. doi: 10.1007/s00213-007-0782-3. [DOI] [PubMed] [Google Scholar]
- 72.Tagliaferro P, Morales M. Synapses between corticotropin-releasing factor-containing axon terminals and dopaminergic neurons in the ventral tegmental area are predominantly glutamatergic. J Comp Neurol. 2008;506(4):616–626. doi: 10.1002/cne.21576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: A role in stress-induced relapse to drug seeking. J Neuroscience. 2005;25(22):5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hahn J, Hopf FW, Bonci A. Chronic cocaine enhances corticotropin-releasing factor-dependent potentiation of excitatory transmission in ventral tegmental area dopamine neurons. J Neurosci. 2009;29(20) doi: 10.1523/JNEUROSCI.4773-08.2009. 6535-6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Behan DP, de Souza EB, Lowry PJ, Potter E, Sawchenko P, Vale WW. Corticotropin releasing factor (CRF) binding protein: A novel regulator of CRF and related peptides. Front Neuroendocrinol. 1995;16(4):362–382. doi: 10.1006/frne.1995.1013. [DOI] [PubMed] [Google Scholar]
- 76.Erb S, Shaham Y, Stewart J. The role of corticotropin-releasing factor and corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats. J Neurosci. 1998;18(14):5529–5536. doi: 10.1523/JNEUROSCI.18-14-05529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shaham Y, Funk D, Erb S, Brown TJ, Walker CD, Stewart J. Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. J Neurosci. 1997;17(7):2605–2614. doi: 10.1523/JNEUROSCI.17-07-02605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brown ZJ, Tribe E, D'souza NA, Erb S. Interaction between noradrenaline and corticotrophin-releasing factor in the reinstatement of cocaine seeking in the rat. Psychopharmacology. 2009;203(1):121–130. doi: 10.1007/s00213-008-1376-4. [DOI] [PubMed] [Google Scholar]
- 79.Ghitza UE, Gray SM, Epstein DH, Rice KC, Shaham Y. The anxiogenic drug yohimbine reinstates palatable food seeking in a rat relapse model: A role of CRF1 receptors. Neuropsychopharmacology. 2006;31(10):2188–2196. doi: 10.1038/sj.npp.1300964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: Involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10(3):318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- 81.Binneman B, Feltner D, Kolluri S, Shi Y, Qiu R, Stiger T. A 6-week randomized, placebo-controlled trial of CP-316,311 (a selective CRH1 antagonist) in the treatment of major depression. Am J Psychiatry. 2008;165(5):617–620. doi: 10.1176/appi.ajp.2008.07071199. [DOI] [PubMed] [Google Scholar]
- 82.Zobel AW, Nickel T, Kunzel HE, Ackl N, Sonntag A, Ising M, Holsboer F. Effects of the high-affinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression: The first 20 patients treated. J Psychiatr Res. 2000;34(3):171–181. doi: 10.1016/s0022-3956(00)00016-9. [DOI] [PubMed] [Google Scholar]
- 83.Held K, Künzel H, Ising M, Schmid DA, Zobel A, Murck H, Holsboer F, Steiger A. Treatment with the CRH1-receptor-antagonist R121919 improves sleep-EEG in patients with depression. J Psychiatr Res. 2004;38(2):129–136. doi: 10.1016/s0022-3956(03)00076-1. [DOI] [PubMed] [Google Scholar]
- 84.Zorrilla EP, Koob GF. The therapeutic potential of CRF1 antagonists for anxiety. Expert Opin Invest Drugs. 2004;13(7):799–828. doi: 10.1517/13543784.13.7.799. [DOI] [PubMed] [Google Scholar]
- 85.Steckler T, Dautzenberg FM. Corticotropin-releasing factor receptor antagonists in affective disorders and drug dependence - An update. CNS Neurol Disord Drug Targets. 2006;5(2):47–165. doi: 10.2174/187152706776359619. [DOI] [PubMed] [Google Scholar]
- 86.Holsboer F, Ising M. Central CRH system in depression and anxiety: Evidence from clinical studies with CRH1 receptor antagonists. Eur J Pharmacol. 2008;583(2–3):350–357. doi: 10.1016/j.ejphar.2007.12.032. [DOI] [PubMed] [Google Scholar]
- 87. Blomeyer D, Treutlein J, Esser G, Schmidt MH, Schumann G, Laucht M. Interaction between CRHR1 gene and stressful life events predicts adolescent heavy alcohol use. Biol Psychiatry. 2008;63(2):146–151. doi: 10.1016/j.biopsych.2007.04.026. • Describes results from a study in humans suggesting the involvement of a genetic component within CRF systems in vulnerability to excessive alcohol consumption.
- 88.Hansson AC, Cippitelli A, Sommer WH, Fedeli A, Bjork K, Soverchia L, Terasmaa A, Massi M, Heilig M, Heilig M, Ciccocioppo R. Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proc Natl Acad Sci USA. 2006;103(41):15236–15241. doi: 10.1073/pnas.0604419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hansson AC, Cippitelli A, Sommer WH, Ciccocioppo R, Heilig M. Region-specific down-regulation of Crhr1 gene expression in alcohol-preferring msP rats following ad lib access to alcohol. Addict Biol. 2007;12(1):30–34. doi: 10.1111/j.1369-1600.2007.00050.x. [DOI] [PubMed] [Google Scholar]
- 90.Barr CS, Dvoskin RL, Yuan Q, Lipsky RH, Gupte M, Hu X, Zhou Z, Schwandt ML, Lindell SG, McKee M, Becker ML, et al. CRH haplotype as a factor influencing cerebrospinal fluid levels of corticotropin-releasing hormone, hypothalamic-pituitary-adrenal axis activity, temperament, and alcohol consumption in rhesus macaques. Arch Gen Psychiatry. 2008;65(8):934–944. doi: 10.1001/archpsyc.65.8.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bruijnzeel AW, Gold MS. The role of corticotropin-releasing factor-like peptides in cannabis, nicotine, and alcohol dependence. Brain Res Brain Res Rev. 2005;49(3):505–528. doi: 10.1016/j.brainresrev.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 92.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th edition. Washington DC, USA: American Psychiatric Press; 1994. [Google Scholar]
- 93.Altshuler HL, Phillips PE, Feinhandler DA. Alteration of ethanol self-administration by naltrexone. Life Sci. 1980;26(9):679–688. doi: 10.1016/0024-3205(80)90257-x. [DOI] [PubMed] [Google Scholar]
- 94.Heyser CJ, Moc K, Koob GF. Effects of naltrexone alone and in combination with acamprosate on the alcohol deprivation effect in rats. Neuropsychopharmacology. 2003;28(8):1463–1471. doi: 10.1038/sj.npp.1300175. [DOI] [PubMed] [Google Scholar]
- 95.Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32(10):1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ji D, Gilpin NW, Richardson HN, Rivier CL, Koob GF. Effects of naltrexone, duloxetine, and a corticotropin-releasing factor type 1 receptor antagonist on binge-like alcohol drinking in rats. Behav Pharmacol. 2008;19(1):1–12. doi: 10.1097/FBP.0b013e3282f3cf70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Heilig M, Koob GF, Ekman R, Britton KT. Corticotropin-releasing factor and neuropeptide Y: Role in emotional integration. Trends Neurosci. 1994;17(2):80–85. doi: 10.1016/0166-2236(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 98.Le Magnen J, Tran G, Durlach J, Martin C. Dose-dependent suppression of the high alcohol intake of chronically intoxicated rats by Ca-acetyl homotaurinate. Alcohol. 1987;4(2):97–102. doi: 10.1016/0741-8329(87)90005-x. [DOI] [PubMed] [Google Scholar]
- 99.Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: Exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22(18):7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bachteler D, Economidou D, Danysz W, Ciccocioppo R, Spanagel R. The effects of acamprosate and neramexane on cue-induced reinstatement of ethanol-seeking behavior in rat. Neuropsychopharmacology. 2005;30(6):1104–1110. doi: 10.1038/sj.npp.1300657. [DOI] [PubMed] [Google Scholar]