Abstract
The Gac/Rsm signal transduction pathway positively regulates secondary metabolism, production of extracellular enzymes, and biocontrol properties of Pseudomonas fluorescens CHA0 via the expression of three noncoding small RNAs, termed RsmX, RsmY, and RsmZ. The architecture and function of the rsmY and rsmZ promoters were studied in vivo. A conserved palindromic upstream activating sequence (UAS) was found to be necessary but not sufficient for rsmY and rsmZ expression and for activation by the response regulator GacA. A poorly conserved linker region located between the UAS and the −10 promoter sequence was also essential for GacA-dependent rsmY and rsmZ expression, suggesting a need for auxiliary transcription factors. One such factor involved in the activation of the rsmZ promoter was identified as the PsrA protein, previously recognized as an activator of the rpoS gene and a repressor of fatty acid degradation. Furthermore, the integration host factor (IHF) protein was found to bind with high affinity to the rsmZ promoter region in vitro, suggesting that DNA bending contributes to the regulated expression of rsmZ. In an rsmXYZ triple mutant, the expression of rsmY and rsmZ was elevated above that found in the wild type. This negative feedback loop appears to involve the translational regulators RsmA and RsmE, whose activity is antagonized by RsmXYZ, and several hypothetical DNA-binding proteins. This highly complex network controls the expression of the three small RNAs in response to cell physiology and cell population densities.
Many gammaproteobacteria regulate the expression of extracellular enzymes, secondary metabolites, and some carbon storage compounds via a conserved signal transduction pathway in which the GacS/GacA two-component system activates the transcription of one or several genes for noncoding small RNAs (sRNAs). Repeated, unpaired GGA motifs allow these sRNAs to capture small RNA-binding proteins of the RsmA/CsrA family. Thus, translational repression exerted by RsmA/CsrA proteins can be relieved and target mRNAs can become accessible to ribosomes for translation. High cell population densities appear to favor the expression of these sRNAs (1, 3, 32). Upon interaction with unknown signals, the GacS sensor kinase autophosphorylates and activates the GacA response regulator by phosphorylation (14, 19, 40). Presumably, phosphorylated GacA undergoes dimerization when acting as a transcription factor, by analogy with other response regulators belonging to the same protein family, such as FixJ of Sinorhizobium meliloti and NarL of Escherichia coli (11, 23, 36, 53). Mutations affecting the predicted helix-turn-helix motif of GacA result in reduced GacA activity in vivo, as these mutant proteins are predicted to have a diminished ability to bind to target DNA sequences (7, 53, 58). However, little is known about the mode of DNA recognition by GacA. Although the GacA homologues SirA of Salmonella enterica and LetA of Legionella pneumophila have been reported to bind to the promoters of the sRNA genes csrB and rsmY, respectively, in vitro (44, 54), these studies have not determined the specificity of GacA-DNA interaction experimentally.
The GacS/GacA signal transduction pathway has been studied extensively in Pseudomonas fluorescens CHA0, a root-colonizing soil bacterium that protects plant roots from diseases caused by fungi and nematodes. This protection is afforded by the production and secretion of biocontrol factors, especially a range of antimicrobial compounds and lytic enzymes, and by the induction of systemic resistance in the plant (21, 22, 37, 47, 57). In the GacS/GacA signal transduction pathway of strain CHA0, there are two paralogous RNA-binding proteins, RsmA and RsmE, and three sequestering sRNAs (RsmX, RsmY, and RsmZ), whose transcription strictly depends on GacA (4, 24, 28, 42, 55). A conserved upstream activating sequence (UAS) spanning 18 bp is found in the rsmX, rsmY, and rsmZ promoters (24, 28, 32, 55). The same palindromic UAS generally occurs in promoters of GacA-controlled sRNA genes in a variety of gammaproteobacteria (31, 34), suggesting that the activated GacA response regulator may bind to this UAS. Recent in vivo studies indicated that in Pseudomonas aeruginosa, the GacA protein specifically activates the expression of the rsmY and rsmZ genes, by interacting exclusively with regions located upstream of these genes (6, 29). In the case of the rsmZ promoter of P. fluorescens, a preliminary analysis has shown that a sequence which is located between positions −191 and −160 (relative to the transcription start site) and which includes the UAS is required for rsmZ transcription. Moreover, less-conserved sequences downstream of the UAS also appear to be involved in rsmZ transcription (24).
The rsmX and rsmY genes of P. fluorescens CHA0 are expressed in parallel, showing a gradual increase during growth, whereas rsmZ expression follows a different pattern, characterized by low levels during exponential growth and a sharp increase at the end of the growth phase (28). In the present study, we investigated factors involved in the divergent expression of rsmY and rsmZ. In particular, we studied the role of the UAS in the rsmZ and rsmY promoters of P. fluorescens and have begun to search for accessory elements involved in transcription from these promoters. This has led to the finding that PsrA, a transcriptional activator of the stress and stationary-phase sigma factor RpoS (27, 30), also activates rsmZ transcription in P. fluorescens. Moreover, we have obtained in vitro evidence for specific binding of the integration host factor (IHF) protein to the rsmZ promoter region, which therefore is assumed to undergo bending.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The strains and plasmids used are listed in Table 1. E. coli and P. fluorescens were grown in nutrient yeast broth (NYB) with shaking or on nutrient agar plates (48). When appropriate, antibiotics were added at the following concentrations: ampicillin (Ap), 100 μg ml−1 (only for E. coli); gentamicin (Gm), 10 μg ml−1; kanamycin (Km), 25 μg ml−1; and tetracycline (Tc), 25 μg ml−1 (for E. coli) or 125 μg ml−1 (for P. fluorescens). For pME497-dependent mobilization (57) of suicide plasmids (that is, pME3087 derivatives) from E. coli to P. fluorescens, chloramphenicol (Cm) at 10 μg ml−1 and Tc were used to select for the recipient having integrated the suicide plasmid. Enrichment for Tc-sensitive strains, from which the suicide plasmid had been excised, was performed as previously described (26). Routine incubation temperatures were 30°C for P. fluorescens and 37°C for E. coli. Alternatively, P. fluorescens was grown at 35°C to improve its capacity to accept DNA originating from E. coli. When relevant, 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) was added to plates at a final concentration of 0.02%.
TABLE 1.
Bacterial strains and plasmids used for this study
| Strain or plasmid | Genotype, phenotype, or relevant characteristicsa | Reference or origin |
|---|---|---|
| Bacterial strains | ||
| E. coli | ||
| DH5α, HB101, and JM105 | Laboratory strains | 45 |
| JM105::rsmZ-lacZ | JM105 with λ(Φbla rsmZ-lacZ lacY′); Apr | This study |
| P. fluorescens | ||
| CHA0 | Wild type | 49 |
| CHA89 | gacA::Kmr | 33 |
| CHA1009 | rsmA::Ω-Km rsmE::Ω-Hg Kmr Hgr | 43 |
| CHA1063 | ΔpsrA | This study |
| CHA1144 | ΔrsmX ΔrsmY ΔrsmZ | 28 |
| CHA1306 | Δpfl_5683 | This study |
| Plasmids | ||
| pBluescript KS(−) | E. coli cloning vector; Apr | Stratagene |
| pJL30 | pBR322 derivative carrying bla, ′lacZ, and lacY′; protein fusion vector; Apr | 5 |
| pME497 | Mobilizing plasmid; IncP-1 Tra RepA(Ts) Apr | 57 |
| pME3087 | ColE1-based suicide plasmid; Tcr | 57 |
| pME6016 | pVS1-p15A shuttle vector for transcriptional lacZ fusions; Tcr | 46 |
| pME6088 | pBluescript carrying a 3.0-kb rpoS-rsmZ insert | 25 |
| pME6090 | pUC6S with a transcriptional rsmZ-lacZ fusion; Apr | 23 |
| pME6091 | pME6016 derivative with a transcriptional wild-type rsmZ-lacZ fusion; Tcr | 23 |
| pME6182 | Mini-Tn7 gene delivery vector with HindIII-SmaI-KpnI-NcoI-SphI multiple cloning site, ColE1 replicon; Gmr Apr | 26 |
| pME6894 | pJL30 derivative carrying bla, rsmZ-lacZ, and lacY′; Apr | This study |
| pME6916 | pME6016 derivative with a transcriptional wild-type rsmY-lacZ fusion; Tcr | 55 |
| pME7065 | pME3087 with XbaI-BamHI fragment containing a 648-bp in-frame deletion in the psrA gene | This study |
| pME7317 | pME6016 derivative with a transcriptional rsmX-lacZ fusion; Tcr | 28 |
| pME7654 | pME6916 derivative with a transcriptional rsmY-lacZ fusion containing NcoI and BglII restriction sites in the rsmY promoter; Tcr | This study |
| pME7655 | pME7654 containing a random linker sequence (mut21) in the rsmY promoter; Tcr | This study |
| pME7656 | pME7654 containing a 6-bp deletion (UASΔ6) in the UAS of the rsmY promoter; Tcr | This study |
| pME7659 | pME3087 with 1.8-kb EcoRI-PstI fragment containing a 921-bp deletion in the PFL_5683 gene; Tcr | This study |
| pME7660 | pME6916 containing mutations (mut3) in the −10 box of the rsmY promoter; Tcr | This study |
| pME7662 | pME7654 containing random half-sites (mut12) in the UAS of the rsmY promoter; Tcr | This study |
| pME7667 | pME6091 containing a deletion of the UAS (UASΔ18) in the rsmZ promoter; Tcr | This study |
| pME7677 | pME7654 containing a 6-bp substitution (mut6) in the linker sequence of the rsmY promoter; Tcr | This study |
| pME7678 | pME7654 containing a 3-bp deletion (Δ3) in the linker sequence of the rsmY promoter; Tcr | This study |
| pME7679 | pME6916 containing rsmX linker and −35 sequence (mut44) in the rsmY promoter; Tcr | This study |
| pME7680 | pME7654 containing a 3-bp deletion (Δ3bis) in the linker sequence of the rsmY promoter; Tcr | This study |
| pME7681 | pME6091 containing a 3-bp deletion (ΔTTT) in the rsmZ promoter; Tcr | This study |
| pME7682 | pME6182 with a 3.5-kb fragment containing linker mut6 rsmY-lacZ of pME7677; Gmr Apr | This study |
| pME7683 | pME6182 with a 3.5-kb fragment containing linker Δ3 rsmY-lacZ of pME7678; Gmr Apr | This study |
| pME7684 | pME6182 with a 3.5-kb fragment containing linker mut44 rsmY-lacZ of pME7679; Gmr Apr | This study |
| pME7685 | pME6182 with a 3.5-kb fragment containing linker Δ3bis rsmY-lacZ of pME7680; Gmr Apr | This study |
| pME7687 | pME6091 containing a 3-bp substitution (GGG) in the rsmZ promoter; Tcr | This study |
| pME7689 | pME6182 with a 3.5-kb fragment containing UAS mut12 rsmY-lacZ of pME7662; Gmr Apr | This study |
| pME7694 | pME6182 with a 3.5-kb fragment containing linker mut21 rsmY-lacZ of pME7655; Gmr Apr | This study |
| pME7695 | pME6182 with a 3.5-kb fragment containing UASΔ6 rsmY-lacZ of pME7656; Gmr Apr | This study |
| pME7696 | pME6182 with a 3.5-kb fragment containing −10 box mut3 rsmY-lacZ of pME7660; Gmr Apr | This study |
| pME7699 | pME6182 with a 3.5-kb fragment containing wild-type rsmY-lacZ; Gmr Apr | 26 |
| pUC6S | Cloning vector, ColE1 replicon; Apr | 56 |
| pUK21 | Cloning vector, ColE1 replicon; Kmr | 56 |
| pUK21-65 | pUK21 containing a 4.6-kb insert carrying lexA′, psrA, nagZ, and PFL_1952′; Kmr | This study |
| pUX-BF13 | Helper plasmid containing Tn7 transposition functions, R6K replicon; Apr | 2 |
Apr, ampicillin resistance; Gmr, gentamicin resistance; Kmr, kanamycin resistance; Tcr, tetracycline resistance.
DNA manipulations.
Small- and large-scale plasmid extractions were done with a QIAprep Spin miniprep kit (Qiagen, Basel, Switzerland) and a Jetstar kit (Genomed GmbH, Basel, Switzerland), respectively. Chromosomal DNA was prepared from P. fluorescens as previously described (16). DNA manipulations were carried out by standard techniques (45). DNA fragments were purified from agarose gels with MinElute or QIAquick gel extraction kits (Qiagen, Basel, Switzerland), depending on the fragment size. Electroporation of bacterial cells with plasmid DNA was done as described previously (15). Conditions for amplifying PCR fragments have been detailed elsewhere (26). Primers are listed in Table S1 in the supplemental material. The reaction products were purified on an agarose gel, and the purified fragments were sequenced with an automatic sequencer. Verification of CHA0 nucleotide sequences was facilitated by the fact that strain CHA0 is very similar to the completely sequenced strain Pf-5 of P. fluorescens (39).
Northern hybridization.
RNA preparations used for Northern blots were isolated using a hot acid phenol extraction protocol, based on a method described elsewhere (18). RNAs (5 μg of total RNA per sample) were separated in denaturing urea-polyacrylamide gels and analyzed by Northern blotting as previously described (55). Membranes were hybridized with digoxigenin (DIG)-labeled DNA probes covering the full-length rsmY or rsmZ transcript, as previously described (55). 5S rRNA served as a loading control. For this purpose, a 5S rRNA gene probe was synthesized with primers 5S rRNA-1 and 5S rRNA-2 and then labeled with DIG. DIG labels were detected by an anti-DIG alkaline phosphatase-conjugated antibody and chemiluminescence according to the supplier's instructions (Roche Diagnostics).
Construction of rsmY and rsmZ mutant promoters.
The prototype plasmid pME7654, in which mutagenesis of the rsmY promoter was performed, was the same as pME6916 carrying a transcriptional rsmY-lacZ fusion (55), except for the artificial NcoI and BglII restriction sites (see Fig. S1 in the supplemental material). These were introduced into pME6916 by PCR, using primer PTRRBAM (55), which anneals to the −300 region of the rsmY promoter and introduces a BamHI restriction site, and primer BH5, which anneals to the −75 region of the rsmY promoter and contains a 2-bp change resulting in an NcoI site. The 215-bp BamHI-NcoI fragment obtained was joined to a synthetic 76-mer oligonucleotide, which was bordered by a 5′ NcoI site and a 3′ PstI site and consisted of positions −75 to +1 of the rsmY promoter, except for a 2-bp change creating a BglII site. The resulting BamHI-PstI fragment was inserted into pME6016 cleaved with BamHI and PstI, giving plasmid pME7654, which was checked by sequencing. Several derivatives of pME7654 were constructed by exchanging the 76-mer NcoI-BglII fragment with corresponding fragments carrying different sequence modifications. A 3-bp substitution in plasmid pME7660, which was otherwise identical to pME6916, was generated by amplifying the entire rsmY promoter region with primers PTRRBAM and BH22, which introduced the substitution mutations into the −10 region and inserted a PstI restriction site at the +1 transcription start site. Chromosomal insertion of the mutated rsmY-lacZ fusions was achieved by recruiting them from the relevant plasmids on 3.5-kb EcoRI-XhoI fragments, which were blunted and cloned into the mini-Tn7 vector pME6182 digested with SmaI. The constructs obtained (pME7682 to pME7685, pME7689, pME7694 to pME7697, and pME7699) and pUX-BF13 were introduced into recipient strains by coelectroporation, allowing transposition of the constructs into the chromosomal Tn7 attachment site (26, 59).
pUC6S carrying a transcriptional rsmZ-lacZ fusion (pME6090) was used as a template to perform site-directed mutagenesis. Oligonucleotides containing the designed mutations (primer pairs BH29-BH30, BH31-BH32, and BH33-BH34) were used to generate pME6090 derivatives by inverse PCR (QuikChange site-directed mutagenesis kit; Stratagene). The mutated rsmZ-lacZ fusions were then amplified by PCR, using primers BH27 and BH28, harboring a BamHI site and a PstI site, respectively, restricted, and cloned into pME6016 digested with the same enzymes, to obtain pME7667, pME7681, and pME7687, respectively.
Isolation of the psrA gene as a positive regulator of rsmZ expression.
The rsmZ-lacZ fusion of pME6091 was subcloned into pJL30; in the resulting plasmid, pME6894, the rsmZ-lacZ fusion was flanked by the bla (Apr) and lacY′ genes. E. coli JM105 carrying pME6894 was infected with λRZ5 (20). Homologous recombination between pME6894 and λRZ5 produced a recombinant λ phage carrying bla, rsmZ-lacZ, and lacY′. A lysate containing this phage was prepared and used to lysogenize strain JM105 by standard procedures (20, 38). The resulting recombinant strain, JM105::rsmZ-lacZ, was checked for the Apr and LacZ+ (very light blue colonies on X-Gal plates) phenotypes. Chromosomal DNA of P. fluorescens CHA0 was partially digested with Sau3A, and gel-purified fragments of 2 to 5 kb were ligated into BamHI-cleaved pUK21. The ligation mixture was used to transform JM105::rsmZ-lacZ. Recombinants were selected on nutrient agar plates amended with Km and X-Gal, and dark blue colonies were purified. One candidate (JM105::rsmZ-lacZ/pUK21-65), containing a 4.6-kb insert with a lexA′ psrA nagZ PFL_1952′ fragment in pUK21, was kept for further study.
Construction of psrA and PFL_5683 deletion mutants.
A 1.5-kb fragment carrying lexA′, psrA, and nagZ′ was amplified by PCR, using a standard forward sequencing primer, the PsrA5 primer, and pUK21-65 as the template. This fragment was cloned into pBluescript KS(−). A 648-bp in-frame deletion in psrA was created with NcoI, leaving 19 intact psrA codons at the 3′ end. The ΔpsrA fragment was inserted into the XbaI-BamHI-cleaved suicide vector pME3087, resulting in plasmid pME7065, which served to introduce the ΔpsrA mutation into strain CHA0 via homologous recombination, using E. coli HB101/pME497 as the mobilizing strain in a triparental mating. Clones in which excision of the vector by a second crossover event had occurred were isolated after enrichment for Tc-sensitive cells (26). The ΔpsrA mutation in the resulting strain, CHA1063, was verified by PCR with primers PsrA1 and PsrA5. To generate the PFL_5683 mutant CHA1306, a 921-bp fragment was deleted in frame in the chromosomal PFL_5683 gene. For this purpose, a 1,040-bp EcoRI-XhoI fragment including the downstream region and the 3′-terminal 9 bp of PFL_5683 was amplified by PCR from strain CHA0, using primers BH18 and BH19. An 800-bp XhoI-PstI fragment containing the PFL_5683 upstream region was amplified with primers BH20 and BH21. Both PCR fragments were cloned by triple ligation into the suicide plasmid pME3087 digested with EcoRI and PstI, producing pME7659 (Table 1). This plasmid was integrated into the chromosome of strain CHA0 harboring the wild-type chromosomal rsmY-lacZ fusion as described previously (26). The Δpfl-5683 mutation in the recombinant strain CHA1306 was verified by PCR, using primers BH18 and BH21.
Electrophoretic mobility shift assay.
A 408-bp rsmZ promoter fragment was generated by PCR, using pME6088 as the template and primers rsmZ1 and rsmZ2. The resulting fragment was digested with EcoRI, and the protrusive ends were radioactively labeled by filling in with DNA polymerase (Klenow enzyme) in the presence of [α-33P]ATP as specified by the enzyme supplier (Fermentas). The labeled fragment was separated from nonincorporated nucleotides with a nucleotide removal kit (Qiagen). A gel retardation assay was performed with purified IHF of E. coli (a kind gift from S. Goodman), essentially as described previously (13). IHF at various concentrations was incubated with 20 nM labeled DNA in binding buffer (12 mM HEPES-NaOH, pH 7.9, 4 mM Tris-HCl, pH 7.9, 12% [vol/vol] glycerol, 1 mM EDTA, 60 mM KCl, 1 mM dithiothreitol, 1.0 μg ml−1 bovine serum albumin, 0.2 μg ml−1 sonicated salmon sperm DNA) at 25°C in a final volume of 20 μl for 5 min. The incubation mixture then was applied to a nondenaturing polyacrylamide gel buffered with Tris-borate-EDTA (TBE) buffer (45) and electrophoresed at 160 V for 60 min.
β-Galactosidase assays.
P. fluorescens strains containing lacZ constructs were grown in 20 ml NYB (amended with 0.05% [vol/vol] Triton X-100) in 100-ml Erlenmeyer flasks with shaking. β-Galactosidase activities were quantified by the Miller method (38). All experiments were performed in triplicate.
RESULTS
Quorum sensing regulation of rsmXYZ expression in P. fluorescens CHA0.
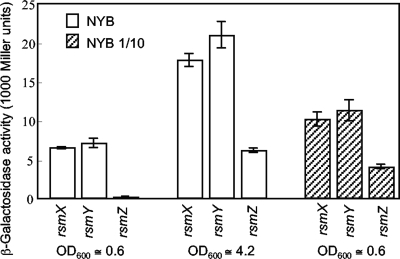
The Gac/Rsm signal transduction pathway is part of the quorum sensing machinery of numerous gammaproteobacteria. In some organisms, such as P. aeruginosa PAO1, Pseudomonas chlororaphis PCL1391, and Pseudomonas syringae pv. tomato DC3000, the Gac/Rsm system regulates the production of N-acyl-homoserine lactone signals (9, 10, 29, 41, 42), whereas in other organisms, such as P. fluorescens CHA0, where N-acyl-homoserine lactones have not been found, the Gac/Rsm cascade itself provides a positively autoregulated quorum sensing system (14, 28). To determine the influence of cell population density on the expression of rsmX, rsmY, and rsmZ, we measured the expression of lacZ fusions to these genes in strain CHA0 grown in rich medium (NYB). All three genes were expressed at basal levels during exponential growth (at on optical density at 600 nm [OD600] of ≈0.6, corresponding to about 6 × 108 cells ml−1) and more strongly at the end of growth (OD600 ≈ 4.2); induction above basal levels was 3- to 4-fold for rsmX and rsmY and about 60-fold for rsmZ (Fig. 1). Thus, the dynamic range of expression was much larger for rsmZ than for rsmX and rsmY. When strain CHA0 was grown in dilute medium (10-fold-diluted NYB), cell populations reached a plateau at an OD600 of ≈0.6, and rsmXYZ expression was significantly lower than that obtained at an OD600 of ≈4.2 in full-strength NYB (Fig. 1), as would be expected of quorum sensing regulation. However, the rsmXYZ expression levels obtained at the end of growth in dilute medium clearly exceeded the basal levels (Fig. 1), indicating that in addition to population density, the nutritional state of cells at the end of growth also plays a role in rsmXYZ expression. Again, the upregulation factor was larger for rsmZ than for rsmX and rsmY.
FIG. 1.
Cell population density-dependent expression of transcriptional lacZ fusions to the rsmX, rsmY, and rsmZ promoters in P. fluorescens CHA0. Cultures of CHA0 carrying either pME7317 (rsmX-lacZ), pME6916 (rsmY-lacZ), or pME6091 (rsmZ-lacZ) were obtained either in full-strength NYB (open bars) or in 10-fold-diluted NYB (hatched bars), and β-galactosidase activities were determined in triplicate (mean ± standard deviation [SD]) at the OD600 values indicated.
Essential role of conserved UAS in rsmZ expression.
The aligned promoter sequences of rsmX, rsmY, and rsmZ of strain CHA0 reveal the conserved palindromic UAS that is typical of GacA-controlled sRNA genes and a conserved −10 hexamer (TAATCT) but otherwise show no evidence of typical motifs (Fig. 2). Whereas the rsmX and rsmY promoter sequences are similar in length and give rise to parallel expression of rsmX and rsmY in cells grown in rich media, the rsmZ promoter sequence is much longer and is characterized by strong activation of rsmZ at the end of growth (Fig. 1 and 2). In the rsmZ-lacZ promoter probe construct pME6091, precise deletion of the UAS resulted in almost complete loss of transcription in strain CHA0 growing in NYB (pME7667) (Table 2), demonstrating an essential role of the UAS in rsmZ expression. Unexpectedly, mutation in or deletion of three nonconserved base pairs located immediately downstream of the UAS also resulted in loss of expression (pME7681 and pME7687) (Table 2). The residual level of expression found with the three mutant constructs was similar to that of the wild-type plasmid pME6091 in a gacA mutant (24). These data indicate that the GacA protein and the UAS are essential but not sufficient for expression and suggest that transcription factors in addition to GacA might be important for the activation of the rsmZ promoter.
FIG. 2.
Alignment of rsmX, rsmY, and rsmZ promoter regions of P. fluorescens CHA0. The consensus UAS (28, 31) and the PsrA box (30) are shown above and below the promoter sequences, respectively. Two sequence elements showing resemblance to the IHF consensus recognition sequence WATCARN4TTR (where W = A or T and R = A or G [51]) or AATCAAN4TTG (35) are shown in bold. Conserved nucleotides are indicated by asterisks. The −10 promoter elements are underlined.
TABLE 2.
Effects of promoter mutations on rsmZ promoter activity
| Construct | Sequencea | lacZ expression level (103 Miller units) (mean ± SD)b |
|---|---|---|
| Wild type (pME6091) | TGTAAGCCAAAGATTACTTTTTCGTACGATTCGGTTCTCGGCTACTTCTCTGCGTTGTCTAGTTTTGCGTAGGCTGTTTTTTATATTATTGATTTATATAATATTTTTTATGAATGGCTATGAGTTTCGACAAAGTTTCACACGAATAGTGCGATTGCTCTTGCCGGGGAACTCTCTTAATATCAGCCCTGT | 6.5 ± 0.9 |
| UAS Δ18 (pME7667) | ------------------TTTTCGTACGATTCGGTTCTCGGCTACTTCTCTGCGTTGTCTAGTTTTGCGTAGGCTGTTTTTTATATTATTGATTTATATAATATTTTTTATGAATGGCTATGAGTTTCGACAAAGTTTCACACGAATAGTGCGATTGCTCTTGCCGGGGAACTCTCTTAATATCAGCCCTGT | 0.5 ± 0.0 |
| PsrA box ΔTTT (pME7681) | TGTAAGCCAAAGATTACT---TCGTACGATTCGGTTCTCGGCTACTTCTCTGCGTTGTCTAGTTTTGCGTAGGCTGTTTTTTATATTATTGATTTATATAATATTTTTTATGAATGGCTATGAGTTTCGACAAAGTTTCACACGAATAGTGCGATTGCTCTTGCCGGGGAACTCTCTTAATATCAGCCCTGT | 0.4 ± 0.0 |
| PsrA box mutTTT (pME7687) | TGTAAGCCAAAGATTACTgggTCGTACGATTCGGTTCTCGGCTACTTCTCTGCGTTGTCTAGTTTTGCGTAGGCTGTTTTTTATATTATTGATTTATATAATATTTTTTATGAATGGCTATGAGTTTCGACAAAGTTTCACACGAATAGTGCGATTGCTCTTGCCGGGGAACTCTCTTAATATCAGCCCTGT | 0.4 ± 0.0 |
The UAS and the −10 hexamer are shown with underlining and in bold, respectively. Lowercase letters indicate mutational changes.
Values were determined for the wild-type strain CHA0 grown in NYB to an OD600 of 4.0 to 4.5.
Identification of PsrA as an activator of the rsmZ promoter.
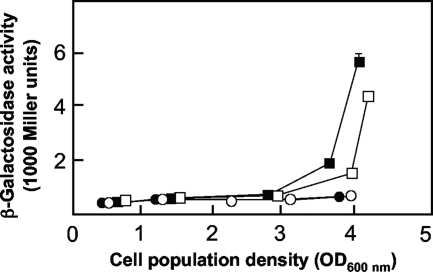
Although E. coli has a functional GacA homologue termed UvrY (40), and although the rsmZ promoter has a −10 hexamer that can be recognized by the housekeeping sigma factor (Fig. 2), a chromosomally inserted rsmZ-lacZ fusion of P. fluorescens gave very little expression (5 ± 2 Miller units; light blue colonies on X-Gal plates) in E. coli strain JM105::rsmZ-lacZ. We searched for P. fluorescens genes allowing enhanced rsmZ expression in this E. coli strain by introducing an expression library of pUK21 derivatives containing P. fluorescens inserts of 2 to 5 kb (see Materials and Methods). One clone (pUK21-65) giving dark blue colonies (corresponding to 110 ± 10 Miller units) was identified; it contained a 4.6-kb insert consisting of a lexA′ psrA nagZ PFL_1952′ fragment. This suggested that the psrA gene, which encodes the Pseudomonas sigma RpoS regulator (27, 30), could be an activator of rsmZ. This expectation was confirmed in that the rsmZ-lacZ fusion (on pME6091) showed reduced and delayed expression in the psrA deletion mutant CHA1063 compared to that in wild-type CHA0 (Fig. 3). This experiment was carried out four times, and a 23% ± 2% reduction of rsmZ expression in the psrA mutant was reproducibly observed. The psrA mutant grew at wild-type rates in rich NYB medium (data not shown). In separate control experiments (data not shown), we verified that PsrA of P. fluorescens acted as a positive regulator of the rpoS gene and as a negative regulator of its own gene, as previously found for PsrA of P. aeruginosa (30), P. chlororaphis (17), and P. syringae (9). We also considered the possibility that the GacS/GacA two-component system might control the expression of psrA, as this appears to occur in P. chlororaphis (10). However, a translational psrA′-′lacZ fusion gave the same levels of expression in wild-type CHA0 and in the gacA mutant CHA89 (data not shown).
FIG. 3.
Expression of rsmZ in the wild-type strain CHA0 and in the psrA mutant CHA1063. Strains CHA0 (filled symbols) and CHA1063 (open symbols), carrying pME6091 (wild-type rsmZ-lacZ; squares) or pME7681 (PsrA box ΔTTT rsmZ-lacZ; circles) were grown in NYB, and β-galactosidase activities were determined in triplicate (mean ± SD). Plasmid pME7687 (PsrA box mutTTT rsmZ-lacZ) gave values that were indistinguishable from those for pME7681.
A potential PsrA recognition sequence was found overlapping with the UAS in the rsmZ promoter region (Fig. 2). When this sequence was mutated either by a 3-bp deletion (PsrA box ΔTTT in pME7681) or by a 3-bp change (PsrA box mutTTT in pME7687), neither of which altered the UAS (Table 2), regulation by PsrA appeared to be lost (Fig. 3). However, the same mutant constructs were poorly expressed, even in the wild type (Table 2; Fig. 3). This strongly decreased expression cannot be due solely to the loss of the relatively moderate activation by PsrA, and it is likely that additional transcription factors help to activate the rsmZ promoter. In conclusion, our results indicate (i) that PsrA acts as an accessory activator of rsmZ, apparently by recognizing a sequence overlapping the UAS, and (ii) that sequences bordering the UAS are crucial for rsmZ transcription.
IHF interacts with the rsmZ linker region.
The fact that the UAS is located about 180 bp upstream of the −10 promoter element in the rsmZ promoter region suggests that DNA bending might be important for regulated expression of rsmZ. We therefore investigated whether the rsmZ linker region contains recognition sequences for IHF, a global regulatory protein known to induce DNA bending (51). We found two potential IHF binding sites, located at about −100 and −40 relative to the rsmZ transcription start site (Fig. 2). The heterodimeric IHF protein is structurally and functionally conserved between enteric bacteria and pseudomonads (8, 12). The fully sequenced strain Pf-5 of P. fluorescens, which is very closely related to strain CHA0, contains homologues of the IHF structural genes himA (ihfA) and himD (ihfB) of E. coli, although the latter gene has not been annotated for strain Pf-5 (39). Thus, although IHF has not been studied in strains Pf-5 and CHA0, we assume that this protein will be very similar to that of E. coli. When we incubated purified E. coli IHF with a 33P-labeled 0.4-kb rsmZ promoter fragment in vitro, the formation of a strong DNA-IHF complex was observed in an electrophoretic mobility shift assay (Fig. 4). The same result was obtained with an rsmZ fragment not containing the UAS (data not shown), suggesting that IHF binds to either or both putative recognition sites present in the linker region. Since the experiment was carried out in the presence of an excess of unlabeled nonspecific competitor DNA (see Materials and Methods) and since the dissociation constant (KD) was estimated to be 2.5 nM, binding of IHF to the rsmZ linker region appears to be highly effective and specific. However, more work will be required to define the IHF recognition sites and the role of IHF in the regulated expression of rsmZ in P. fluorescens.
FIG. 4.
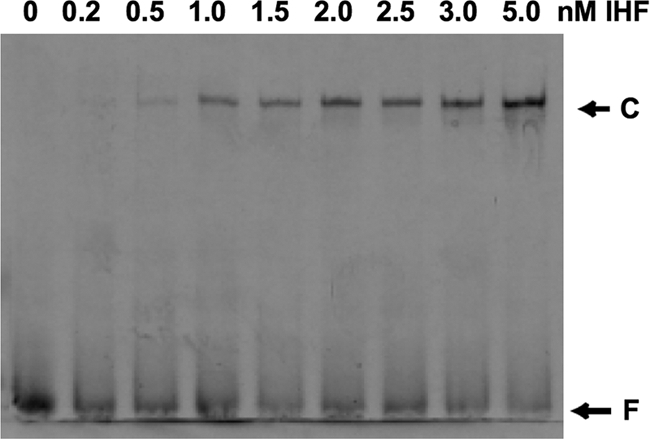
Electrophoretic mobility shift analysis of IHF binding to an rsmZ promoter fragment. Increasing concentrations of purified E. coli IHF protein were incubated with 33P-labeled rsmZ promoter DNA, as specified in Materials and Methods, and separated by gel electrophoresis under nondenaturing conditions. F, free DNA fragment; C, IHF-DNA complex.
Analysis of rsmY promoter elements.
Mutations were introduced into the rsmY promoter region in three steps. First, artificial NcoI and BglII sites were inserted into the rsmY-lacZ construct pME6916, upstream and downstream of the UAS, respectively, resulting in pME7654 (see Fig. S1 in the supplemental material). These restriction sites did not affect rsmY expression in strain CHA0 (data not shown). Second, the 76-bp NcoI-BglII wild-type fragment was replaced by a series of synthetic mutant cassettes; the resulting plasmids are listed in Table 1. Third, the mutated rsmY-lacZ constructs were subcloned into a mini-Tn7 element (Table 3) and inserted into the chromosomal Tn7 attachment site of P. fluorescens strains via site-specific transposition (see Materials and Methods).
TABLE 3.
Effects of promoter mutations on rsmY promoter activity
| Construct | Sequencea |
lacZ expression level (103 Miller units) (mean ± SD)b |
|||
|---|---|---|---|---|---|
| Wild type | gacA mutant | rsmA rsmE mutant | rsmX rsmY rsmZ mutant | ||
| Wild type (pME7699) | TGTAAGTCATCTCTTACATAACGTGCTGCTGATCGTCCATATCGGTAGATCTCGGCTGAAGCTAATCTACTTCACA | 15.5 ± 0.9c | 0.9 ± 0.1 | 2.3 ± 0.1 | 24.0 ± 1.1 |
| UAS Δ6 (pME7695) | ------TCATCTCTTACATAACGTGCTGCTGATCGTCCATATCGGTAGATCTCGGCTGAAGCTAATCTACTTCACA | 0.5 ± 0.0 | 0.5 ± 0.0 | 0.6 ± 0.0 | 0.4 ± 0.0 |
| UAS mut12 (pME7689) | aagtctTCATCTcaatgtTAACGTGCTGCTGATCGTCCATATCGGTAGATCTCGGCTGAAGCTAATCTACTTCACA | 0.6 ± 0.0 | 0.3 ± 0.0 | 0.9 ± 0.0 | 0.4 ± 0.0 |
| −10 box mut3 (pME7696) | TGTAAGTCATCTCTTACATAACGTGCTGCTGATCGTCCATATCGGTAGATCTCGGCTGAAGCTAtaaTACTTCACA | 15.5 ± 1.1 | 1.0 ± 0.0 | 2.8 ± 0.2 | 26.9 ± 1.3 |
| Linker mut6 (pME7682) | TGTAAGTCATCTCTTACATAAtagcCaaCTGATCGTCCATATCGGTAGATCTCGGCTGAAGCTAATCTACTTCACA | 2.0 ± 0.2 | 0.3 ± 0.0 | 0.5 ± 0.0 | 5.0 ± 0.1 |
| Linker mut21 (pME7694) | TGTAAGTCATCTCTTACAgtgatcagacagctggtgaatTATCGGTAGATCTCGGCTGAAGCTAATCTACTTCACA | 0.3 ± 0.0 | 0.2 ± 0.0 | 0.5 ± 0.0 | 0.3 ± 0.0 |
| Linker mut44 (pME7684) | TGTAAGTCATCTCTTACAcgcgctgtaattcaatgcgccttctgcgcaccgggctttgtgttTAATCTACTTCACA | 3.5 ± 0.2 | 0.9 ± 0.1 | 0.9 ± 0.1 | 4.5 ± 0.0 |
| Linker Δ3 (pME7683) | TGTAAGTCATCTCTTACA---CGTGCTGCTGATCGTCCATATCGGTAGATCTCGGCTGAAGCTAATCTACTTCACA | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 |
| Linker Δ3bis (pME7685) | TGTAAGTCATCTCTTACATAACGTGCTGC---TCGTCCATATCGGTAGATCTCGGCTGAAGCTAATCTACTTCACA | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.4 ± 0.0 |
The UAS, artificially introduced BglII sites, and the −10 hexamer are shown in italics, with underlining, and in bold, respectively. Lowercase letters indicate mutational changes.
Values were determined at an OD600 of 2.0 to 2.5 for strains CHA0 (wild type), CHA89 (gacA mutant), CHA1009 (rsmA rsmE mutant), and CHA1144 (rsmX rsmY rsmZ mutant) grown in NYB.
The same value was found with pME6916 (rsmY-lacZ), which does not contain the artificial BglII site.
When either a 6-bp half-site of the UAS was deleted (UAS Δ6) or both half-sites of the UAS were replaced by random nucleotides (UAS mut12), rsmY-lacZ expression was very low and resembled that found in a gacA mutant (Table 3), confirming the essential role of the UAS. Since our analysis of the rsmZ promoter had revealed a crucial role of sequences lying downstream of the UAS (Table 2), we also introduced various mutations into the linker region located between the UAS and the −10 promoter element of rsmY and into the −10 hexamer itself. The consensus TATAAT box, when exchanged for the wild-type TAATCT box, had no significant effect on rsmY-lacZ expression (Table 3, −10 box mut3 construct). In contrast, substitution mutations in a sequence (CGTGNTG) that is weakly conserved within the rsmY linkers of a dozen Pseudomonas spp. (see Fig. S2 in the supplemental material) resulted in a partial loss of expression but remained positively controlled by GacA (Table 3, linker mut6 construct). This result suggests that the linker region interacts with one or several transcriptional activators other than GacA. Random mutations replacing 21 bp of the linker led to a complete loss of rsmY-lacZ expression (Table 3, linker mut21 construct). These data suggest that transcriptional activation of the rsmY promoter results from an intimate interplay between GacA and some additional transcription factor(s). When the linker of rsmY was replaced by that of rsmX, regulation by GacA was restored, although the level of lacZ expression was suboptimal (Table 3, linker mut44 construct). Expression of the rsmY(mut44)-lacZ construct was also about 4 times lower than that of an rsmX-lacZ fusion (28). The reasons for this expression deficit are not clear at this stage. Finally, two different 3-bp deletions in the linker abolished rsmY-lacZ expression (Table 3, linker Δ3 and linker Δ3bis constructs). These strong effects may reflect a loss of promoter geometry and/or a loss of function of the postulated additional activator(s).
As candidates for rsmY activation, we considered PsrA and the putative LysR-type transcriptional activator PFL_5683, which is encoded by a gene located downstream of rsmY (see Fig. S1 in the supplemental material). However, rsmY-lacZ expression was similar in the ΔpsrA mutant CHA1063, in the Δpfl_5683 mutant CHA1306, and in the wild-type strain CHA0 (data not shown), suggesting that neither PsrA nor the PFL_5683 gene product have a role in rsmY expression under the conditions tested in full-strength NYB.
Regulation of rsmY expression by RsmAE and RsmXYZ.
We previously noted that the RNA-binding proteins RsmA and RsmE have positive, probably indirect effects on the rsmX, rsmY, and rsmZ promoters (28, 42). RsmA and RsmE showed the same positive effects on the expression of the series of rsmY-lacZ constructs used here, and in general, these effects were less strong than those due to GacA (Table 3). We verified in a Northern blot experiment that GacA was essential for production of the RsmY and RsmZ sRNAs and that the combined action of RsmA and RsmE was beneficial to the production of both sRNAs (Fig. 5). In an rsmX rsmY rsmZ triple mutant (CHA1144), the expression of the wild-type rsmY-lacZ fusion was significantly higher than that in the wild type, and this overexpression effect was also seen in the experiments using various mutant forms of the rsmY promoter (Table 3). These data are in agreement with the general model that pictures the sRNAs RsmX, RsmY, and RsmZ as antagonists of RsmA and RsmE (28, 32). As a net result, it appears that the three sRNAs strongly and negatively control rsmY expression, and it is likely that similar feedback loops operate in rsmX and rsmZ expression as well.
FIG. 5.
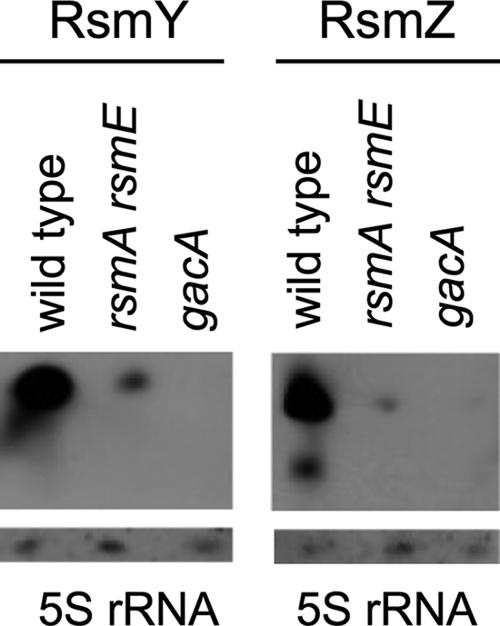
Northern blot revealing RsmY and RsmZ sRNAs. Total RNA was extracted from cells grown at 30°C in NYB to an OD600 of ∼4.2. Experimental conditions are described in Materials and Methods. As a loading control, 5S rRNA was revealed in all samples by use of a specific probe.
DISCUSSION
Our in vivo analysis of the rsmY and rsmZ promoters of P. fluorescens CHA0 led to the following major conclusions. (i) The conserved palindromic UAS, a hallmark of GacA-controlled sRNA genes in gammaproteobacteria (6, 19, 28, 31, 34, 55), is essential for the expression of the rsmY and rsmZ genes and their positive regulation by GacA. (ii) A linker region, which is located between the UAS and the −10 promoter element and which appears to be poorly conserved between the rsmX, rsmY, and rsmZ promoters (Fig. 2), contains information that is strictly required for the expression of rsmY and rsmZ (Tables 2 and 3) as well as for control by GacA, suggesting that various transcriptional activators may bind to these linker regions and thereby facilitate the interactions between GacA protein (presumably in its phosphorylated state) and RNA polymerase. (iii) One transcriptional activator of the rsmZ promoter but not of the rsmY promoter has been identified as PsrA (Fig. 3), previously known as a transcriptional activator of the rpoS gene and as a repressor of a fatty acid degradation operon (27, 30). The deduced PsrA recognition sequence overlaps with the UAS in the rsmZ promoter region (Fig. 2). (iv) Negative feedback control of rsmY transcription by the three sRNAs RsmX, RsmY, and RsmZ (Table 3) is reminiscent of similar feedback mechanisms that have been reported for rsmY and rsmZ expression in P. aeruginosa (29) and for csrB expression in E. coli (50). This feedback control needs RsmA and RsmE in P. fluorescens and is influenced by the sequence of the linker region, as evidenced in particular by the linker mut6 construct (Table 3).
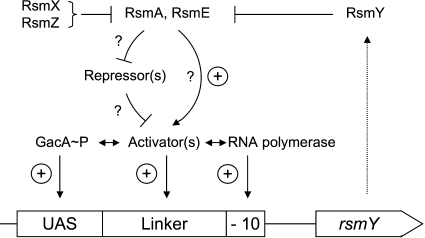
The above findings can be used to assemble a tentative rsmY promoter model (Fig. 6). The most parsimonious assumption is that activated GacA interacts with the UAS. In P. aeruginosa, this UAS is present only in the rsmY and rsmZ promoters, and a recent publication has shown by chromatin immunoprecipitation that these promoters are the only sites to which GacA binds in vivo (6). In the P. fluorescens CHA0 background, an rsmXYZ triple mutant has a pleiotropic phenotype that is very similar to that of a gacA mutant (28), suggesting that GacA recognizes the rsmXYZ promoters specifically, and probably exclusively. A fourth GacA-controlled sRNA gene, rgsA, does not have the UAS, and its expression is indirectly regulated by GacA (18). Our attempts to demonstrate specific binding of purified, phosphorylated GacA protein to the rsmY and rsmZ promoters of P. fluorescens have failed so far (our unpublished observations). Conceivably, the GacA protein might need a scaffold of auxiliary proteins interacting with the linker region in order to give productive DNA binding. These additional transcription factors are probably responsible for the characteristic induction patterns that each of the three sRNA genes exhibits as a function of cell population density and physiological state (Fig. 1). How the RsmA and RsmE proteins achieve activation of the rsmY promoter remains a mystery at present. In principle, these RNA-binding proteins might activate translation of the proposed transcriptional activators. Such translational activation by RsmA and RsmE has not yet been observed in Pseudomonas spp., but there is a precedent in E. coli, where the RsmA homologue CsrA stabilizes flhDC mRNA and thereby enhances translation of this mRNA (1). Alternatively, RsmA and RsmE might act indirectly, by translationally repressing some transcriptional repressors, which in turn would regulate the expression of the auxiliary activators (Fig. 6). However, not all transcriptional activators interacting with the linker region need to be under Gac/Rsm control, as evidenced by PsrA, which is not regulated by GacA.
FIG. 6.
Hypothetical model describing transcriptional regulation of the rsmY promoter. For explanation, see text in the Discussion. ⊥, negative control; arrow with plus sign, positive control; GacA∼P, phosphorylated GacA protein.
PsrA activates rpoS expression primarily at the level of transcription (30). By moderately activating rsmZ expression at the end of the growth phase (Fig. 3), PsrA may also contribute to posttranscriptional activation of rpoS expression via activation of the Gac/Rsm pathway, which acts positively on rpoS translation (25). PsrA might not be an rsmZ activator in all Pseudomonas species. A sequence alignment of rsmZ promoter regions reveals the deduced PsrA binding site only in the two closely related strains CHA0 and Pf-5, not in other Pseudomonas spp. (see Fig. S3 in the supplemental material).
Recently, Brencic et al. (6) provided evidence for repression of rsmZ expression by the DNA-binding protein MvaT in P. aeruginosa, and they speculated that this interaction might occur in an AT-rich part of the rsmZ linker region. However, the resolution of the immunoprecipitation experiment used in that study does not exclude the possibility that MvaT might make further DNA contacts upstream of the UAS and/or within the rsmZ structural gene. In the present work, we hypothesized that the AT-rich part of the rsmZ linker region of P. fluorescens could contain two IHF recognition sites, and we obtained preliminary evidence for high-affinity binding of IHF to this region (Fig. 4). Furthermore, and in contrast to our expectation, a graft of the entire rsmX linker into the rsmY-lacZ construct pME7684 (linker mut44) failed to give wild-type rsmY-lacZ activity (Table 3), suggesting that an rsmX linker combined with an rsmY sequence far upstream does not allow maximal expression, perhaps because accessory activators and repressors of rsmX and rsmY cannot be exchanged ad libitum.
In conclusion, the architecture of the rsmY and rsmZ promoters seems to be more complex than that of most ordinary bacterial promoters. Several transcriptional regulators—mostly activators but, evidently, also repressors—appear to interact with GacA, and these regulators are not the same for all three sRNA genes in P. fluorescens. This complex, multi-input system determines the production of the RsmXYZ sRNAs. It reflects cell physiology as well as cell population density and involves at least three sensors (GacS, RetS, and LadS). In recent work (52), we have found that Krebs cycle intermediates have a key influence on the expression of rsmXYZ. Further complexity of this regulatory system is provided by two additional important parameters not investigated here: the stability of the sRNAs and the cellular concentrations of the antagonistic RNA-binding proteins (RsmA and RsmE). Together, all of these parameters determine the output of the Gac/Rsm pathway at the level of translation. As a result, the Gac/Rsm pathway integrates much greater complexity in regulatory signals than any simple transcriptional control system could do.
Supplementary Material
Acknowledgments
This work was supported by the Swiss National Foundation (projects 3100A0-100180 and 3100A0-120365) and the European Union (QLK3-CT-2002-0286).
We thank S. Goodman for supplying IHF protein and Karine Lapouge for critically reading the manuscript.
Footnotes
Published ahead of print on 4 January 2010.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Babitzke, P., and T. Romeo. 2007. CsrB sRNA family: sequestration of RNA-binding regulatory proteins. Curr. Opin. Microbiol. 10:156-163. [DOI] [PubMed] [Google Scholar]
- 2.Bao, Y., D. P. Lies, H. Fu, and G. P. Roberts. 1991. An improved Tn7-based system for the single-copy insertion of cloned genes into chromosomes of gram-negative bacteria. Gene 109:167-168. [DOI] [PubMed] [Google Scholar]
- 3.Bejerano-Sagie, M., and K. B. Xavier. 2007. The role of small RNAs in quorum sensing. Curr. Opin. Microbiol. 10:189-198. [DOI] [PubMed] [Google Scholar]
- 4.Blumer, C., S. Heeb, G. Pessi, and D. Haas. 1999. Global GacA-steered control of cyanide and exoprotease production in Pseudomonas fluorescens involves specific ribosome binding sites. Proc. Natl. Acad. Sci. U. S. A. 96:14073-14078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blumer, C., A. Kleefeld, D. Lehnen, M. Heintz, U. Dobrindt, G. Nagy, K. Michaelis, L. Emödy, T. Polen, R. Rachel, V. F. Wendisch, and G. Unden. 2005. Regulation of type 1 fimbriae synthesis and biofilm formation by the transcriptional regulator LrhA of Escherichia coli. Microbiology 151:3287-3298. [DOI] [PubMed] [Google Scholar]
- 6.Brencic, A., K. A. McFarland, H. R. McManus, S. Castang, I. Mogno, S. L. Dove, and S. Lory. 2009. The GacS/GacA signal transduction system of Pseudomonas aeruginosa acts exclusively through its control over the transcription of the RsmY and RsmZ regulatory small RNAs. Mol. Microbiol. 73:434-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bull, C. T., B. Duffy, C. Voisard, G. Défago, C. Keel, and D. Haas. 2001. Characterization of spontaneous gacS and gacA regulatory mutants of Pseudomonas fluorescens biocontrol strain CHA0. Antonie Van Leeuwenhoek 79:327-336. [DOI] [PubMed] [Google Scholar]
- 8.Calb, R., A. Davidovitch, S. Koby, H. Giladi, D. Goldenberg, H. Margalit, A. Holtel, K. Timmis, J. M. Sanchez-Romero, V. de Lorenzo, and A. B. Oppenheim. 1996. Structure and function of the Pseudomonas putida integration host factor. J. Bacteriol. 178:6319-6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatterjee, A., Y. Cui, H. Hasegawa, and A. K. Chatterjee. 2007. PsrA, the Pseudomonas sigma regulator, controls regulators of epiphytic fitness, quorum-sensing signals, and plant interactions in Pseudomonas syringae pv. tomato strain DC3000. Appl. Environ. Microbiol. 73:3684-3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chin-A-Woeng, T. F. C., D. van den Broek, B. J. J. Lugtenberg, and G. V. Bloemberg. 2005. The Pseudomonas chlororaphis PCL1391 sigma regulator psrA represses the production of the antifungal metabolite phenazine-1-carboxamide. Mol. Plant Microbe Interact. 18:244-253. [DOI] [PubMed] [Google Scholar]
- 11.Da Re, S., J. Schumacher, P. Rousseau, J. Fourment, C. Ebel, and D. Kahn. 1999. Phosphorylation-induced dimerization of the FixJ receiver domain. Mol. Microbiol. 34:504-511. [DOI] [PubMed] [Google Scholar]
- 12.Delic-Attree, I., B. Toussaint, A. Froger, J. C. Willison, and P. M. Vignais. 1996. Isolation of an IHF-deficient mutant of a Pseudomonas aeruginosa mucoid isolate and evaluation of the role of IHF in algD gene expression. Microbiology 142:2785-2793. [DOI] [PubMed] [Google Scholar]
- 13.Drapal, N., and G. Sawers. 1995. Purification of ArcA and analysis of its specific interaction with the pfl promoter-regulatory region. Mol. Microbiol. 16:597-607. [DOI] [PubMed] [Google Scholar]
- 14.Dubuis, C., C. Keel, and D. Haas. 2007. Dialogues of root-colonizing biocontrol pseudomonads. Eur. J. Plant Pathol. 119:311-328. [Google Scholar]
- 15.Farinha, M. A., and A. M. Kropinski. 1990. High efficiency electroporation of Pseudomonas aeruginosa using frozen cell suspensions. FEMS Microbiol. Lett. 58:221-225. [DOI] [PubMed] [Google Scholar]
- 16.Gamper, M., B. Ganter, M. R. Polito, and D. Haas. 1992. RNA processing modulates the expression of the arcDABC operon in Pseudomonas aeruginosa. J. Mol. Biol. 226:943-957. [DOI] [PubMed] [Google Scholar]
- 17.Girard, G., S. Barends, S. Rigali, E. T. van Rij, B. J. J. Lugtenberg, and G. V. Bloemberg. 2006. Pip, a novel activator of phenazine biosynthesis in Pseudomonas chlororaphis PCL1391. J. Bacteriol. 188:8283-8293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.González, N., S. Heeb, C. Valverde, E. Kay, C. Reimmann, T. Junier, and D. Haas. 2008. Genome-wide search reveals a novel GacA-regulated small RNA in Pseudomonas species. BMC Genomics 9:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodman, A. L., M. Merighi, M. Hyodo, I. Ventre, A. Filloux, and S. Lory. 2009. Direct interaction between sensor kinase proteins mediates acute and chronic disease phenotypes in a bacterial pathogen. Genes Dev. 23:249-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grove, C. L., and R. P. Gunsalus. 1987. Regulation of the aroH operon of Escherichia coli by the tryptophan repressor. J. Bacteriol. 169:2158-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haas, D., and G. Défago. 2005. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat. Rev. Microbiol. 3:307-319. [DOI] [PubMed] [Google Scholar]
- 22.Haas, D., and C. Keel. 2003. Regulation of antibiotic production in root-colonizing Pseudomonas spp. and relevance for biological control of plant disease. Annu. Rev. Phytopathol. 41:117-153. [DOI] [PubMed] [Google Scholar]
- 23.Heeb, S., and D. Haas. 2001. Regulatory roles of the GacS/GacA two-component system in plant-associated and other gram-negative bacteria. Mol. Plant Microbe Interact. 14:1351-1363. [DOI] [PubMed] [Google Scholar]
- 24.Heeb, S., C. Blumer, and D. Haas. 2002. Regulatory RNA as mediator in GacA/RsmA-dependent global control of exoproduct formation in Pseudomonas fluorescens CHA0. J. Bacteriol. 184:1046-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heeb, S., C. Valverde, C. Gigot-Bonnefoy, and D. Haas. 2005. Role of the stress sigma factor RpoS in GacA/RsmA-controlled secondary metabolism and resistance to oxidative stress in Pseudomonas fluorescens CHA0. FEMS Microbiol. Lett. 243:251-258. [DOI] [PubMed] [Google Scholar]
- 26.Humair, B., N. González, D. Mossialos, C. Reimmann, and D. Haas. 2009. Temperature-responsive sensing regulates biocontrol factor expression in Pseudomonas fluorescens CHA0. ISME J. 3:955-965. [DOI] [PubMed] [Google Scholar]
- 27.Kang, Y., V. V. Lunin, T. Skarina, A. Savchenko, M. J. Schurr, and T. T. Hoang. 2009. The long-chain fatty acid sensor, PsrA, modulates the expression of rpoS and the type III secretion exsCEBA operon in Pseudomonas aeruginosa. Mol. Microbiol. 73:120-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kay, E., C. Dubuis, and D. Haas. 2005. Three small RNAs jointly ensure secondary metabolism and biocontrol in Pseudomonas fluorescens CHA0. Proc. Natl. Acad. Sci. U. S. A. 102:17136-17141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kay, E., B. Humair, V. Dénervaud, K. Riedel, S. Spahr, L. Eberl, C. Valverde, and D. Haas. 2006. Two GacA-dependent small RNAs modulate the quorum sensing response in Pseudomonas aeruginosa. J. Bacteriol. 188:6026-6033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kojic, M., B. Jovcic, A. Vindigni, F. Odreman, and V. Venturi. 2005. Novel target genes of PsrA transcriptional regulator of Pseudomonas aeruginosa. FEMS Microbiol. Lett. 246:175-181. [DOI] [PubMed] [Google Scholar]
- 31.Kulkarni, P. R., X. Cui, J. W. Williams, A. M. Stevens, and R. V. Kulkarni. 2006. Prediction of CsrA-regulating small RNAs in bacteria and their experimental verification in Vibrio fischeri. Nucleic Acids Res. 34:3361-3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lapouge, K., M. Schubert, F. H. Allain, and D. Haas. 2008. Gac/Rsm signal transduction pathway of γ-proteobacteria: from RNA recognition to regulation of social behaviour. Mol. Microbiol. 67:241-253. [DOI] [PubMed] [Google Scholar]
- 33.Laville, J., C. Voisard, C. Keel, M. Maurhofer, G. Défago, and D. Haas. 1992. Global control in Pseudomonas fluorescens mediating antibiotic synthesis and suppression of black root rot of tobacco. Proc. Natl. Acad. Sci. U. S. A. 89:1562-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lenz, D. H., M. B. Miller, J. Zhu, R. V. Kulkarni, and B. L. Bassler. 2005. CsrA and three redundant small RNAs regulate quorum sensing in Vibrio cholerae. Mol. Microbiol. 58:1186-1202. [DOI] [PubMed] [Google Scholar]
- 35.Mangan, M. W., S. Lucchini, V. Danino, T. Ó. Cróinín, J. C. D. Hinton, and C. J. Dorman. 2006. The integration host factor (IHF) integrates stationary-phase and virulence gene expression in Salmonella enterica serovar Typhimurium. Mol. Microbiol. 59:1831-1847. [DOI] [PubMed] [Google Scholar]
- 36.Maris, A. E., M. R. Sawaya, M. Kaczor-Grzeskowiak, M. R. Jarvis, S. M. D. Bearson, M. L. Kopka, I. Schröder, R. P. Gunsalus, and R. E. Dickerson. 2002. Dimerization allows DNA target site recognition by the NarL response regulator. Nat. Struct. Biol. 9:771-778. [DOI] [PubMed] [Google Scholar]
- 37.Maurhofer, M., C. Hase, P. Meuwley, J.-P. Métraux, and G. Défago. 1994. Induction of systemic resistance of tobacco to tobacco necrosis virus by the root-colonizing Pseudomonas fluorescens strain CHA0: influence of the gacA gene and of pyoverdine production. Phytopathology 84:139-146. [Google Scholar]
- 38.Miller, J. H. 1992. A short course in bacterial genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 39.Paulsen, I. T., C. M. Press, J. Ravel, D. Y. Kobayashi, G. S. Myers, D. V. Mavrodi, R. T. DeBoy, R. Seshadri, Q. Ren, R. Madupu, R. J. Dodson, A. S. Durkin, L. M. Brinkac, S. C. Daugherty, S. A. Sullivan, M. J. Rosovitz, M. L. Gwinn, L. Zhou, D. J. Schneider, S. W. Cartinhour, W. C. Nelson, J. Weidman, K. Watkins, K. Tran, H. Khouri, E. A. Pierson, L. S. Pierson III, L. S. Thomashow, and J. E. Loper. 2005. Complete genome sequence of the plant commensal Pseudomonas fluorescens Pf-5. Nat. Biotechnol. 23:873-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pernestig, A. K., O. Melefors, and D. Georgellis. 2001. Identification of UvrY as the cognate response regulator for the BarA sensor kinase in Escherichia coli. J. Biol. Chem. 276:225-231. [DOI] [PubMed] [Google Scholar]
- 41.Pessi, G., and D. Haas. 2001. Dual control of hydrogen cyanide biosynthesis by the global activator GacA in Pseudomonas aeruginosa PAO1. FEMS Microbiol. Lett. 200:73-78. [DOI] [PubMed] [Google Scholar]
- 42.Reimmann, C., M. Beyeler, A. Latifi, H. Winteler, M. Foglino, A. Lazdunski, and D. Haas. 1997. The global activator GacA of Pseudomonas aeruginosa PAO1 positively controls the production of the autoinducer N-butyryl-homoserine lactone and the formation of the virulence factors pyocyanin, cyanide, and lipase. Mol. Microbiol. 24:309-319. [DOI] [PubMed] [Google Scholar]
- 43.Reimmann, C., C. Valverde, E. Kay, and D. Haas. 2005. Posttranscriptional repression of GacS/GacA-controlled genes by the RNA-binding protein RsmE acting together with RsmA in the biocontrol strain Pseudomonas fluorescens CHA0. J. Bacteriol. 187:276-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sahr, T., H. Brüggemann, M. Jules, M. Lomma, C. Albert-Weissenberger, C. Cazalet, and C. Buchrieser. 2009. Two small ncRNAs govern virulence and transmission in Legionella pneumophila. Mol. Microbiol. 72:741-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 46.Schnider-Keel, U., A. Seematter, M. Maurhofer, C. Blumer, B. Duffy, C. Gigot-Bonnefoy, C. Reimmann, R. Notz, G. Défago, D. Haas, and C. Keel. 2000. Autoinduction of 2,4-diacetylphloroglucinol biosynthesis in the biocontrol agent Pseudomonas fluorescens CHA0 and repression by the bacterial metabolites salicylate and pyoluteorin. J. Bacteriol. 182:1215-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siddiqui, I. A., D. Haas, and S. Heeb. 2005. Extracellular protease of Pseudomonas fluorescens CHA0, a biocontrol factor with activity against the root-knot nematode Meloidogyne incognita. Appl. Environ. Microbiol. 71:5646-5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stanisich, V. A., and B. W. Holloway. 1972. A mutant sex factor of Pseudomonas aeruginosa. Genet. Res. 19:91-108. [DOI] [PubMed] [Google Scholar]
- 49.Stutz, E. W., G. Défago, and H. Kern. 1986. Naturally occurring fluorescent pseudomonads involved in suppression of black root rot of tobacco. Phytopathology 76:181-185. [Google Scholar]
- 50.Suzuki, K., X. Wang, T. Weilbacher, A.-K. Pernestig, Ö. Melefors, D. Georgellis, P. Babitzke, and T. Romeo. 2002. Regulatory circuits of the CsrA/CsrB and BarA/UvrY systems of Escherichia coli. J. Bacteriol. 184:5130-5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swinger, K. K., and P. A. Rice. 2004. IHF and HU: flexible architects of bent DNA. Curr. Opin. Struct. Biol. 14:28-35. [DOI] [PubMed] [Google Scholar]
- 52.Takeuchi, K., P. Kiefer, C. Reimmann, C. Keel, C. Dubuis, J. Rolli, J. A. Vorholt, and D. Haas. 2009. Small RNA-dependent expression of secondary metabolism is controlled by Krebs cycle function in Pseudomonas fluorescens. J. Biol. Chem. 284:34976-34985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Teplitski, M., and B. M. Ahmer. 2005. The control of secondary metabolism, motility, and virulence by the two-component regulatory system BarA/SirA of Salmonella and other γ-proteobacteria, p. 107-132. In B. M. Prüss (ed.), Global regulatory networks in enteric bacteria. Research Signpost, Trivandrum, India.
- 54.Teplitski, M., R. I. Goodier, and B. M. Ahmer. 2003. Pathways leading from BarA/SirA to motility and virulence gene expression in Salmonella. J. Bacteriol. 185:7257-7265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Valverde, C., S. Heeb, C. Keel, and D. Haas. 2003. RsmY, a small regulatory RNA, is required in concert with RsmZ for GacA-dependent expression of biocontrol traits in Pseudomonas fluorescens CHA0. Mol. Microbiol. 50:1361-1379. [DOI] [PubMed] [Google Scholar]
- 56.Vieira, J., and J. Messing. 1991. New pUC-derived cloning vectors with different selectable markers and DNA replication origins. Gene 100:189-194. [DOI] [PubMed] [Google Scholar]
- 57.Voisard, C., C. T. Bull, C. Keel, J. Laville, M. Maurhofer, M. Schnider, G. Défago, and D. Haas. 1994. Biocontrol of root diseases by Pseudomonas fluorescens CHA0: current concepts and experimental approaches, p. 67-89. In F. O'Gara, D. N. Dowling, and B. Boesten (ed.), Molecular ecology of rhizosphere microorganisms. VCH Publishers, Weinheim, Germany.
- 58.Whistler, C. A., N. A. Corbell, A. Sarniguet, W. Ream, and J. E. Loper. 1998. The two-component regulators GacS and GacA influence accumulation of the stationary-phase sigma factor sigma S and the stress response in Pseudomonas fluorescens Pf-5. J. Bacteriol. 180:6635-6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zuber, S., F. Carruthers, C. Keel, A. Mattart, C. Blumer, G. Pessi, C. Gigot-Bonnefoy, U. Schnider-Keel, S. Heeb, C. Reimmann, and D. Haas. 2003. GacS sensor domains pertinent to the regulation of exoproduct formation and to the biocontrol potential of Pseudomonas fluorescens CHA0. Mol. Plant Microbe Interact. 16:634-644. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.