Abstract
Background and purpose:
Stimulation of muscarinic receptors in intestinal smooth muscle cells results in suppression of voltage-gated Ca2+ channel currents (ICa). However, little is known about which receptor subtype(s) mediate this effect.
Experimental approach:
The effect of carbachol on ICa was studied in single intestinal myocytes from M2 or M3 muscarinic receptor knockout (KO) and wild-type (WT) mice.
Key results:
In M2KO cells, carbachol (100 µM) induced a sustained ICa suppression as seen in WT cells. However, this suppression was significantly smaller than that seen in WT cells. Carbachol also suppressed ICa in M3KO cells, but with a phasic time course. In M2/M3-double KO cells, carbachol had no effect on ICa. The extent of the suppression in WT cells was greater than the sum of the ICa suppressions in M2KO and M3KO cells, indicating that it is not a simple mixture of M2 and M3 receptor responses. The Gi/o inhibitor, Pertussis toxin, abolished the ICa suppression in M3KO cells, but not in M2KO cells. In contrast, the Gq/11 inhibitor YM-254890 strongly inhibited only the ICa suppression in M2KO cells. Suppression of ICa in WT cells was markedly reduced by either Pertussis toxin or YM-254890.
Conclusion and implications:
In intestinal myocytes, M2 receptors mediate a phasic ICa suppression via Gi/o proteins, while M3 receptors mediate a sustained ICa suppression via Gq/11 proteins. In addition, another pathway that requires both M2/Gi/o and M3/Gq/11 systems may be operative in inducing a sustained ICa suppression.
Keywords: M2 receptors, M3 receptors, knockout mice, intestinal smooth muscle, calcium channel, Pertussis toxin, YM-254890
Introduction
In gastrointestinal smooth muscle, stimulation of muscarinic receptors by the parasympathetic neurotransmitter, acetylcholine (ACh) or other muscarinic agonists produces a rise in the intracellular concentration of Ca2+ ([Ca2+]i), followed by a contractile response (Unno et al., 2003). The muscarinic mobilization of cytosolic Ca2+ is generally believed to arise mainly via intracellular Ca2+ released by the second messenger, inositol-1,4,5-trisphosphate (IP3). Additionally, Ca2+ entry into the cell through the L-type voltage-gated Ca2+ channels (Cav1.2; nomenclature follows Alexander et al., 2008) is achieved by depolarization due to activation of the muscarinic cationic channels (Inoue and Isenberg, 1990; Komori et al., 1992; Yan et al., 2003). Previous work suggests that muscarinic receptor stimulation also produces inhibition of currents through L-type Ca2+ channels (ICa) (Unno et al., 1995). This inhibitory effect is supposed to represent a negative feedback mechanism to prevent cytosolic Ca2+ overload induced by the muscarinic Ca2+ mobilization.
In guinea pig ileal smooth muscle cells, carbachol (CCh) suppressed ICa in a biphasic manner where an initial rapid suppression lasting 10 s was followed by a more sustained suppression lasting until CCh removal (Unno et al., 1995). The initial transient ICa suppression is known to involve IP3-induced Ca2+ release that occurs upon muscarinic stimulation (Unno et al., 1995). Evidence indicates that Ca2+ release directly inhibits the activity of L-type Ca2+ channels through a Ca2+-induced Ca2+ channel inactivation mechanism, which is dependent upon the binding of Ca2+ to the inactivation site of the Ca2+ channels (Ganitkevich et al., 1987). However, the mechanisms underlying the later sustained suppression remain to be elucidated. Unno et al. (1995), studying CCh-induced ICa suppression in guinea pig ileal myocytes, reported that both the initial transient and the subsequent sustained ICa suppression were insensitive to Pertussis toxin (PTX), an agent known to inactivate Gi/o G proteins. Conversely, Pucovsky et al. (1998) (who investigated the effect of PTX under conditions involving constant [Ca2+]i levels in the same type of smooth muscle cells) concluded that the CCh-induced sustained suppression of ICa involves Gi/o proteins. These contradictory results may have arisen from the different experimental conditions under which the CCh-induced ICa suppression was observed.
In the present study, we used M2 and M3 muscarinic receptor single knockout (KO) and M2/M3 receptor double KO mice as novel experimental tools. Specifically, we investigated the effect of CCh on ICa in longitudinal smooth muscle cells isolated from the small intestine. We also used YM-254890, a selective inhibitor of Gq/11 G proteins, as well as the Gi/o inhibitor PTX. Our results suggest the existence of three distinct pathways linked to muscarinic receptor-mediated ICa suppression in these cells that are in addition to the M3-mediated transient ICa suppression involving the Ca2+-induced Ca2+ channel inactivation mechanisms. We propose that an M2/Gi/o pathway mediates phasic ICa suppression, that an M3/Gq/11 pathway leads to sustained suppression and that a third pathway mediates sustained suppression requiring both M2/Gi/o and M3/Gq/11 systems for its operation.
Methods
Experimental animals
All animal care and experimental procedures described below complied with the guidelines approved by the local animal ethics committee of the Faculty of Applied Biological Science, Gifu University.
The generation of the M2 and M3 muscarinic receptor single KO and M2/M3-double KO mice has been described previously (Gomeza et al., 1999; Yamada et al., 2001; Struckmann et al., 2003). The genomic background of the mice used in the present study was 129J1 (50%) × CF1 (50%) for the M2KO and their corresponding wild-type (WT) mice, 129SvEv (50%) × CF1 (50%) for the M3KO and their corresponding WT mice, and 129J1 (25%) × 129SvEv (25%) × CF1 (50%) for the M2/M3-double KO mice and their corresponding WT mice. Animals were housed in polycarbonate ventilated cages. The animal room was maintained at 22–25°C with a relative humidity of 40–60% and a daily light/dark cycle (07:00–19:00). Food (MF or CMF; Oriental Yeast Co., Itabashi Tokyo, Japan) and distilled water were supplied ad libitum. Cells obtained from the three WT strains showed similar functional properties. Data obtained with these cells were therefore pooled (Sakamoto et al., 2007).
Cell preparation
Mice of either sex, aged more than 2 months and weighing 20–50 g were killed by cervical dislocation. A gut segment of 15 cm in length was then removed from a region over the jejunum and ileum (except for the terminal 2.0 cm portion) and placed in physiological salt solution (PSS), consisting of 126 mM NaCl, 6 mM KCl, 2 mM CaCl2, 1.2 mM MgCl2, 14 mM glucose and 10.5 mM HEPES (pH was adjusted to 7.2 with NaOH). The gut segment was cut into 1.0–1.5 cm pieces, from each of which the longitudinal muscle layer was carefully peeled off from the underlying tissue. Single intestinal smooth muscle cells were isolated enzymatically from the longitudinal muscle layers, as described previously (Sakamoto et al., 2006). The cells were suspended in PSS containing 0.5 mM CaCl2, placed on the cover-glass as a small aliquot and stored at 4°C for 2–8 h until use on the same day.
Whole-cell current recording
Cells on a cover-glass were attached to a 1.4 mL chamber on the stage of an inverted microscope (IMT-2, Olympus, Tokyo, Japan). Whole-cell membrane current recordings were made at room temperature (21–25°C) using standard patch-clamp techniques (Hamill et al., 1981) with patch pipettes of 2–7 MΩ. Current signals were amplified by a current amplifier (EPC8, HEKA, Lambrecht, Germany), filtered at 1 kHz and captured at a sampling rate of 4 kHz using an analogue-digital converter (DIGIDATA 1320A, Axon Instruments, Inc., Union city, CA, USA) interfaced to a computer (IMC PC-433, Inter medical Co., Nagoya, Japan) running with the pCLAMP program (version 9, Axon Instruments). The signals were also stored on a digital tape using a PCM data recorder (RD-130T, TEAC, Tokyo, Japan) for later analysis and illustration.
ICa recordings were made from cells bathed in PSS and intracellularly dialysed with a Cs+-rich pipette solution composed of 134 mM CsCl, 1.0 mM Na2GTP, 1.2 mM MgCl2, 1.0 mM MgATP, 14 mM glucose, 0.05 mM EGTA and 10.5 mM HEPES (pH adjusted to 7.2 with CsOH). After being held under whole-cell voltage clamp mode at −60 mV, cells were continuously perfused with PSS at a rate of 5 mL·min−1 using a gravity perfusing device, unless otherwise stated, and 30 ms step pulses to 0 mV generated by the pCLAMP program were applied to the cell repeatedly every 10 s to elicit ICa. If necessary, 20 mM EGTA was included in the pipette solution (in this case CsCl was reduced to 80 mM). When guanosine 5′-(γ-thio) triphosphate tetralithium salt (GTP-γ-S) was added in the pipette solution, Na2GTP was omitted from it.
When currents through Ca2+-activated BK channels (IBK) were recorded, a K+-rich pipette solution of the following composition was used (mM): KCl, 134; Na2GTP, 1.0; MgCl2, 1.2; MgATP, 1.0; glucose, 14; EGTA, 0.05; HEPES, 10.5 (pH adjusted to 7.2). Cells were bathed in PSS and held at a holding potential of 0 mV.
PTX treatment
PTX was injected into mice at a dose of 100 µg·kg−1 body weight (i.p.) and, 70–74 h later the cells were prepared as described above. This protocol has been used previously for effective blockade of M2 muscarinic receptor-mediated contraction of mouse gut smooth muscle (Unno et al., 2005; 2006;).
Data analysis
The amplitude of ICa was measured as the difference between the peak current level and the current level obtained by the step pulse to 0 mV after ICa blockade by nicardipine (1 µM). Current density of ICa was calculated using the measured membrane capacitance obtained by applying hyperpolarizing pulses (10 mV). The extent of ICa inhibition by CCh or other agents was expressed as percentage reduction compared with the control ICa immediately before drug application. If the ICa rundown observed for a period of 1 min before CCh application was more than 10%, the experimental data were not used for evaluation of CCh effects. The amplitude of cationic current and IBK evoked by CCh was measured as the difference from the holding current before application of CCh.
The values in the text are given as means ± SEM. Statistical significance between two groups was tested using a Student's unpaired or paired t-test. When more than three groups were compared, one-way analysis of variance (anova) followed by a post hoc Bonferroni-type multiple comparison test was used. If the data of the multiple group had not equal variance and normality, Kruskal–Wallis (non-parametric anova) followed by a post hoc Steel–Dwass-type multiple comparison test was used. Differences were considered significant when P < 0.05.
Materials
Drugs used were carbamylcholine chloride (CCh), GTP-γ-S, PTX, nicardipine hydrochloride (all from Sigma, St. Louis, MO, USA), atropine sulphate and caffeine (Wako, Osaka, Japan), and YM-254890 (kindly given from Astellas Pharm. Inc., Tokyo, Japan). YM-254890 was dissolved in dimethyl sulphoxide as a stock solution, stored at −20°C and diluted with the pipette solution to the desired final concentrations. Drug concentrations in the text and figures are expressed as final concentrations applied to the cells.
Drugs were applied extracellularly by perfusion of an external solution with drug-containing PSS. Calculations made from a perfusion rate of 5 mL·min−1 and a bath volume of 1.4 mL, gave a time constant of 16.8 s for solution exchange. Thus, about 1 min after the start of perfusion, the entire external solution was replaced with drug-containing PSS. For the recording of IBK, cells were not perfused with PSS, but drugs were rapidly applied by replacing of the external solution with drug-containing PSS within 10 s using a pair of syringes connected to the chamber, one for injection and the other one for suction. EGTA, GTP-γ-S or YM-254890 was applied intracellularly via diffusion from the patch pipette into the cell, as previously described (Komori et al., 1992).
Results
Effects of CCh on ICa in WT mice
Mouse intestinal smooth muscle cells prepared from WT mice were held under voltage clamp at −60 mV using patch pipettes filled with a Cs+-based solution, followed by their continuous perfusion with PSS. Under these conditions, a 30 ms step pulse to 0 mV was repeatedly applied every 10 s in order to elicit ICa. The amplitude of ICa usually reached a steady level 3–4 min after the beginning of cell perfusion (see Methods).
In WT cells, application of CCh (100 µM) produced an initial transient inward current followed by a smaller sustained current due to activation of cationic channels ICCh (Sakamoto et al., 2006). As shown in Figure 1B, ICa was reduced in amplitude after CCh (100 µM) application. The ICa suppression developed progressively with time and reached a plateau about 2–3 min later. The mean suppression of ICa at the end of a 3 min application of CCh (3 min point) was about 60% (Figure 3A and B). Removal of CCh from the perfusate allowed a partial recovery of ICa with a slow time course. Such sustained suppression of ICa was occasionally preceded by a transient suppression (n= 5; data not shown). This effect has previously been observed in guinea pig ileal smooth muscle cells (Unno et al., 1995). The initial transient suppression (47.5 ± 8.5%, n= 5) was seen when the step pulse to 0 mV happened to be applied on the point of the initial transient ICCh reaching a peak.
Figure 1.
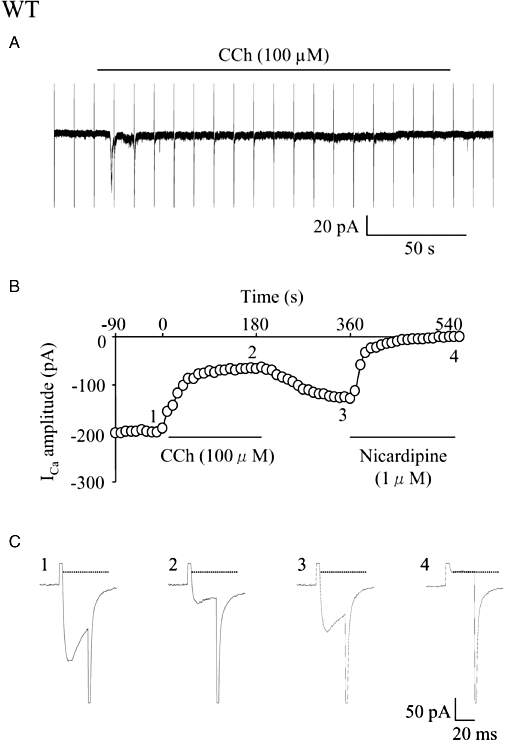
Effects of carbachol (CCh) on the holding current and the voltage-gated Ca2+ channel currents (ICa) in a single longitudinal smooth muscle cell isolated from the small intestine of a wild-type (WT) mouse. ICa was elicited by a 30 ms step pulse to 0 mV from the holding potential of −60 mV at a frequency of 0.1 Hz. CCh (100 µM) was applied extracellularly during application of the depolarizing pulses. (A) CCh induced a cationic inward current (ICCh) consisting of an initial transient component followed by a sustained component. Every sharp current deflection shows overlapped ICa and the capacitive current. (B) Time course of the change in ICa amplitude (○) in the cell plotted against time (the beginning of CCh application was taken as zero). Points 1–4 in the graph correspond to actual ICa records (1–4) in (C). The amplitude of ICa was measured as the difference from the current level (interrupted line) obtained by the depolarizing pulse in the presence of nicardipine (1 µM). In the following figures, measurements and plots of ICa amplitude were made in the same way.
Figure 3.
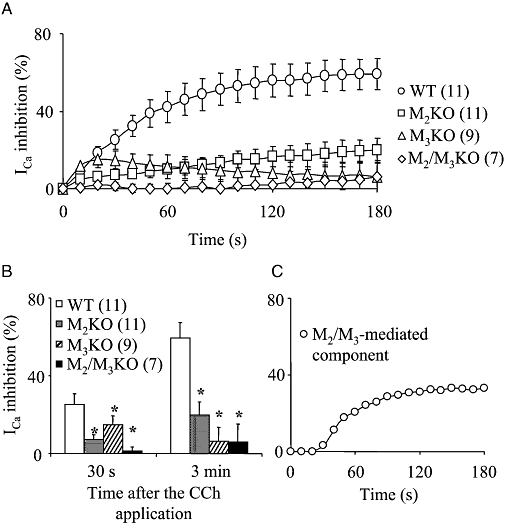
Comparison of the inhibitory effect of carbachol (CCh) on the voltage-gated Ca2+ channel currents (ICa) in cells from wild-type (WT) and muscarinic receptor knockout (KO) mice. (A) Time courses of the change in the mean percentage suppression of ICa[the beginning of CCh (100 µM) application was taken as zero]. Each point indicates mean ± SEM. (B) The mean percentage suppression of ICa measured 30 s (four left-hand columns) and 3 min (four right-hand columns) after CCh application. Each column shows the mean with one SEM. In (A and B), the numbers of cells used are presented in parentheses. Asterisks in (B) represent statistically significant differences from the corresponding value in WT cells (P < 0.05). (C) An assumed sustained component of ICa suppression mediated by M2/M3 pathway. Each point was estimated by subtracting the average percentage suppression curve of ICa in both M2KO and M3KO cells from that in WT cells shown in (A).
The effects of CCh on ICa were concentration-dependent. When 1 or 10 µM CCh was applied, the mean percentage suppression of ICa at the 3 min point was 18.3 ± 6.2% (n= 3) or 25.2 ± 6.6% (n= 4) respectively, which was significantly smaller than the inhibition observed after application of 100 µM CCh (59.2 ± 8.0%, n= 11). Because of the robust response observed with 100 µM CCh, this CCh concentration was used for subsequent experiments.
The ICa suppression and ICCh generation observed after CCh application were abolished by 1 µM atropine (n= 4, data not shown), indicating that both responses were exclusively mediated via muscarinic receptors.
Muscarinic receptor subtypes involved in the ICa suppression
In order to identify the muscarinic receptor subtypes involved in the CCh-induced ICa suppression, we used cells isolated from M2KO or M3KO mice and M2/M3-double KO mice and compared data from these mutant mice with data from WT mice. The mean current densities of ICa measured just before CCh application were not significantly different among WT and the three mutant strains or any pair of them (WT: 4.3 ± 0.6 pA/pF, n= 16; M2KO: 3.6 ± 0.7 pA/pF, n= 15; M3KO: 4.3 ± 0.5 pA/pF, n= 15; M2/M3KO: 3.9 ± 1.8 pA/pF, n= 12).
In cells from M2KO mice, CCh (100 µM) induced a suppression of ICa that persisted for the 3 min CCh application period, as seen in WT cells (Figure 2B). However, the mean ICa suppression at the 3 min point was significantly smaller than the corresponding value in WT cells (Figure 3B). In four out of 11 cells tested, the sustained ICa suppression was preceded by an initial, rapid suppression, as seen occasionally in WT cells. However, irrespective of the occurrence of the initial transient suppression, the percentage suppression of ICa in the sustained phase was again smaller than that in WT cells.
Figure 2.
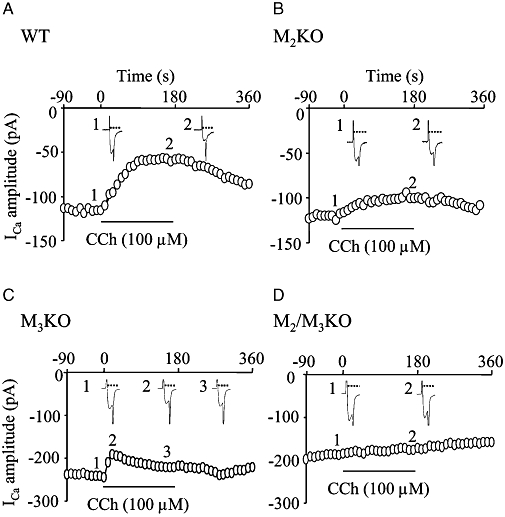
Effects of carbachol (CCh) on the voltage-gated Ca2+ channel currents (ICa) in cells isolated from muscarinic receptor knockout (KO) mice. (A–D) Time courses of the change in ICa amplitude in cells from wild-type (WT) (A), M2KO (B), M3KO (C) and M2/M3-double KO mice (D). Points 1–3 in each graph correspond to actual ICa records in insets 1–3.
In M3KO cells, CCh (100 µM) caused suppression of ICa, but the ICa suppression was observed only during the initial period of the 3 min application of CCh, as illustrated in Figure 2C. As can be seen from an average curve for percentage suppression of ICa (Figure 3A), the peak suppression of ICa was achieved 30 s after the beginning of CCh application (30 s point) and then the ICa amplitude gradually increased to recover from its suppression despite the continued presence of CCh. The mean values of ICa suppression at the 30 s and 3 min points are shown in Figure 3B. These two values were significantly different (P < 0.05, paired t-test) and the latter value was similar to the time-dependent rundown. Therefore, the CCh-induced ICa suppression in M3KO cells was considered to be phasic, but not sustained in nature.
In cells from M2/M3-double KO mice, CCh (100 µM) had no effect on ICa (Figure 2D; mean values in Figure 3A and B). Again, the ICa reduction was within the time-dependent rundown. These data indicated that no other muscarinic receptor subtypes, beside M2 and M3, participated in the CCh-induced ICa suppression.
Figure 3A and B shows summarized results of the CCh-induced ICa suppression in the individual mouse strains. The sum of the mean suppression at the 30 s point in M2KO (7%) and M3KO cells (15%) was 22%, a value comparable with the WT value at this time (25%). Therefore, the CCh-induced ICa suppression in WT cells at the 30 s point can be explained by a combined effect elicited by M2 and M3 receptors. Conversely, the sum of the mean suppression (26%) observed with M2KO (20%) and M3KO cells (6%) at the 3 min point was significantly smaller than the corresponding WT value (Figure 3B). If both the M2- and M3-mediated ICa suppression were subtracted from the WT curve (see Figure 3A), a large component of sustained ICa suppression still existed, as shown in Figure 3C. This component appeared about 30 s after CCh application, gradually developed, and reached a plateau level of about 33% inhibition within 2–3 min. Thus, the sustained ICa suppression evoked by CCh in WT cells is not a simple mixture of M2 and M3 receptor responses.
GTP-γ-S, a direct activator of GTP-binding proteins, has been shown to suppress ICa in guinea pig ileal myocytes (Beech, 1993; Unno et al., 1995). We therefore also investigated the effects of GTP-γ-S on ICa in mouse intestinal smooth muscle cells. After intracellular application of GTP-γ-S (100 µM), the ICa amplitude progressively decreased with a much faster time course than that seen in untreated cells and reached a plateau about 4–10 min after breaking the patch membrane in cells from all mouse strains. As shown in Figure 4, the mean percentage suppression of ICa measured 10 min after breaking the patch membrane was not significantly different among WT and all other mutant strains or any pair of them. Therefore, all cells used showed preserved coupling between G proteins and ICa suppression.
Figure 4.
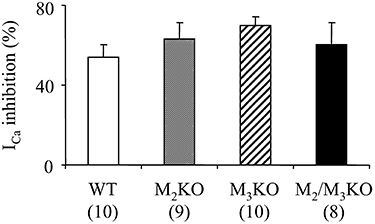
Effect of guanosine 5′-(γ-thio) triphosphate tetralithium salt (GTP-γ-S) on the voltage-gated Ca2+ channel currents (ICa) in cells from wild-type (WT) and muscarinic receptor knockout (KO) mice. GTP-γ-S (200 µM) was applied intracellularly via patch pipettes. The mean percentage suppression of ICa measured 10 min after the establishment of whole-cell clamp configuration are shown. Each column represents the mean with one SEM. The numbers of cells used are shown in parentheses.
Type of G proteins involved in the muscarinic pathways mediating ICa suppression
In order to identify the type of G proteins involved in the muscarinic ICa suppression, we used PTX (which is known to ADP-ribosylate the α-subunit of Gi/o) (Katada and Ui, 1982) and YM-254890 (which has been recently developed as a specific inhibitor of Gq/11) (Takasaki et al., 2004).
Treatment of mice with PTX has been shown to prevent CCh from producing M2-mediated contraction or cation channel currents in gut smooth muscle preparations (Unno et al., 2005; 2006; Sakamoto et al., 2006). So far, the ability of YM-254890 to inhibit M3/Gq/11-mediated responses has not been tested in mouse intestinal smooth muscle cells. Therefore, we first investigated the effect of YM-254890 on the M3/Gq/11-mediated Ca2+ release event, which could be detected as IBK responses (Sakamoto et al., 2007). Cells from M2KO mice were dialysed with a K+-rich pipette solution including dimethyl sulphoxide (0.3%) as control, or YM-254890 (1–10 µM) for 10 min after the establishment of whole-cell configuration at the holding potential of 0 mV (see Material and Methods). As shown in Figure 5A, CCh (100 µM) consistently evoked a brief IBK in control cells (mean values in Figure 5C). Subsequent application of caffeine (10 mM), a potent releaser of Ca2+ from internal stores, had no effect or induced a small IBK (mean values in Figure 5C), indicating the previous depletion of Ca2+ stores by CCh. In M2KO cells treated with YM-254890 (1 µM), CCh induced no noticeable IBK in one cell, but did induce a sizable IBK of 2.1 ± 0.5 nA in three other cells. The overall size of IBK induced by CCh (Figure 5C) was not significantly different from the control value. At higher concentrations of YM-254890 (3 or 10 µM), however, CCh evoked little or no IBK, but subsequent application of 10 mM caffeine induced a sizable IBK, as shown in Figure 5B. The average sizes of IBK induced by CCh and caffeine in cells treated with 3 or 10 µM YM-2548790 are shown in Figure 5C and these values were significantly different from the corresponding control values (Figure 5C). Thus, YM-254890 at a concentration of 3 µM or higher was highly efficient in inhibiting the activity of Gq/11 proteins in mouse intestinal smooth muscle cells.
Figure 5.
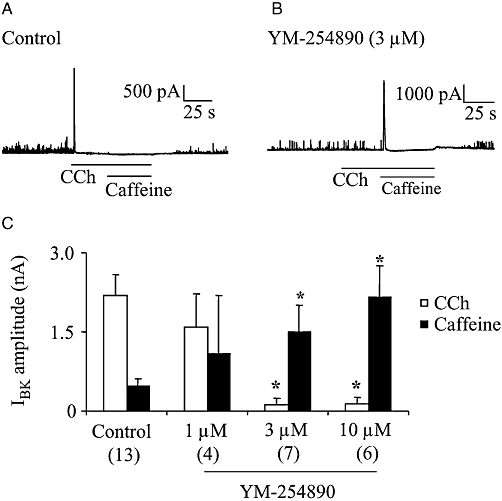
Effect of YM-254890 on BK current (IBK) in cells isolated from M2KO mice. CCh (100 µM)- or caffeine (10 mM)-induced Ca2+ release events were detected as IBK at the holding potential of 0 mV in M2KO cells. Cells were dialysed with a K+-rich pipette solution containing 0.3% DMSO (control) or YM-254890 (1–10 µM). (A and B) Typical current traces from a control cell (A) and YM-254890 (3 µM)-dialysed cell (B). (C) Summary of CCh- and caffeine- induced IBK in the presence or absence of YM-254890. The individual columns indicate the mean with one SEM. The numbers of cells used are shown in parentheses. Asterisks represent significant differences from the corresponding control values (P < 0.05). CCh, carbachol; DMSO, dimethyl sulphoxide; KO, knockout.
Treatment with PTX (100 µg·kg−1) or YM-254890 (3 µM) produced no noticeable effect on ICa in cells from WT, M2KO and M3KO mice. For example, PTX-treated and -untreated WT cells had similar current densities of ICa of 4.7 ± 1.0 pA/pF (n= 12) and 4.3 ± 0.6 pA/pF (n= 16) respectively. Similarly, YM-254890-treated and -untreated WT cells had mean values of 3.4 ± 0.7 (n= 20) and 3.5 ± 0.9 pA/pF (n= 12) respectively.
As shown in Figure 6A, PTX-treated M2KO cells responded to 100 µM CCh with a sustained ICa suppression similar in the percent inhibition and time course to that seen in control cells. The mean suppression at the 3 min point was not different from the control values (Figure 6A and B). Conversely, YM-254890 (3 µM) treatment strongly attenuated the CCh-induced sustained suppression of ICa, as shown in Figure 6C (mean values in Figure 6D). These results suggested the involvement of Gq/11 proteins in the M3-mediated sustained ICa suppression.
Figure 6.
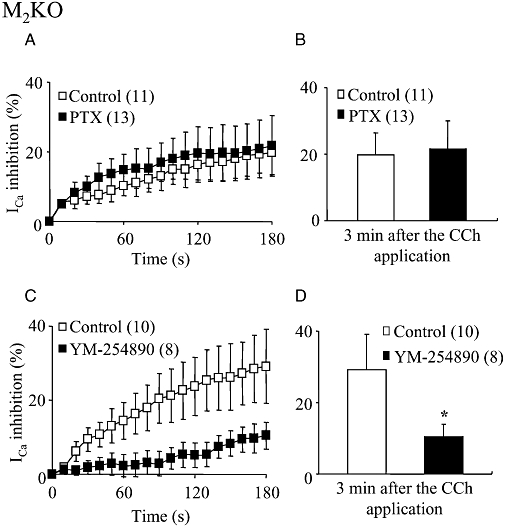
Effects of Pertussis toxin (PTX) and YM-254890 on the CCh-induced sustained ICa suppression in M2KO cells. (A) Time courses of the change in the mean percentage suppressions of ICa mediated by CCh (100 µM) in PTX-untreated (control) and -treated cells (the beginning of CCh application was taken as zero). (C) Time courses of the change in the mean percentage suppressions of ICa in 0.3% DMSO-dialysed (control) and YM-254890 (3 µM)-dialysed cells. Each point indicates mean ± SEM. The numbers of used cells are shown in parentheses. (B and D) Summary of the mean percentage suppression of ICa measured 3 min after the CCh application in control, PTX-treated (B) or YM-254890-dialysed cells (D). Each column shows the mean with one SEM. The asterisk in (D) represents a significant difference from the corresponding control value (P < 0.05). PTX had no effect on the sustained ICa suppression, but YM-254890 markedly diminished it. CCh, carbachol; DMSO, dimethyl sulphoxide; ICa, voltage-gated Ca2+ channel currents; KO, knockout.
In contrast, PTX-treated M3KO cells showed no suppression of ICa in response to CCh (Figure 7A; mean values in Figure 7B), but YM-254890 (3 µM) treatment did not affect the CCh-induced ICa suppression (Figure 7C and D). These results suggest the involvement of Gi/o proteins in the M2-mediated phasic ICa suppression.
Figure 7.
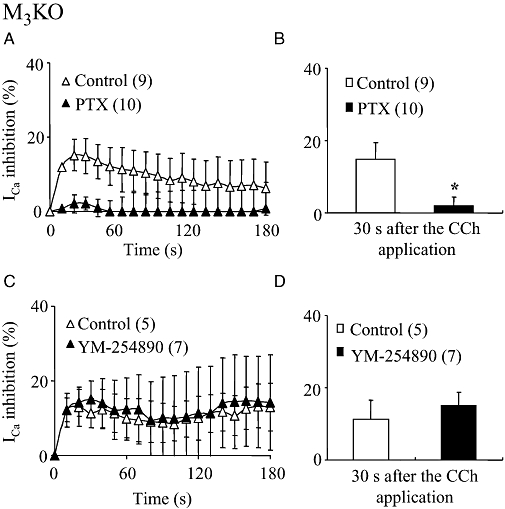
Effects of PTX and YM-254890 on the CCh-induced phasic ICa suppression in M3KO cells. (A and C) Time courses of the change in the mean percentage suppression of ICa induced by CCh (100 µM) in PTX-treated (A) and YM-254890 (3 µM)-dialysed (C) M3KO cells plotted in the same way as in Figure 6. (B and D) Summary of the mean percentage suppression of ICa measured 30 s after CCh application in PTX-treated (B) and YM-254890-dialysed (D) cells. Asterisk in (B) represents significant difference from the corresponding control value (P < 0.05). PTX abolished the phasic ICa suppression in M3KO cells, but YM-254890 had no effect on this response. CCh, carbachol; ICa, voltage-gated Ca2+ channel currents; KO, knockout; PTX, Pertussis toxin.
In WT cells, PTX treatment strongly reduced the sustained ICa suppression (Figure 8A; mean values in Figure 8B). As shown in Figure 8A, the average ICa suppression curve against time after CCh application in PTX-treated WT cells closely resembled that in PTX-untreated M2KO cells.
Figure 8.
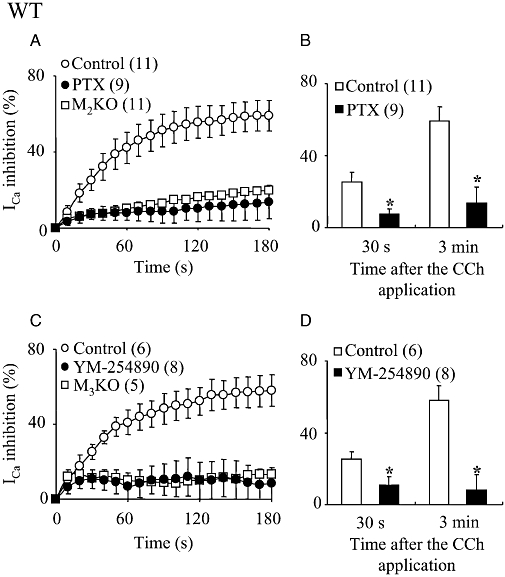
Effects of PTX and YM-254890 on the CCh-induced ICa suppression in WT cells. (A and C) Time courses of the change in the mean percentage suppression of ICa induced by CCh (100 µM) in PTX-treated (A) and YM-254890 (3 µM)-dialysed (C) WT cells. For comparison, the averaged ICa suppression curves against time after CCh application in PTX-untreated M2KO cells (Figure 6) and YM-254890-untreated M3KO cells (Figure 7) were superimposed in (A and C) respectively. (B and D) Summary of the mean percentage suppression of ICa measured 30 s (left-hand columns) and 3 min (right-hand columns) after CCh application in PTX-treated (B) and YM-254890-dialysed (D) WT cells. Asterisks represent significant differences from the corresponding control value (P < 0.05). CCh, carbachol; ICa, voltage-gated Ca2+ channel currents; KO, knockout; PTX, Pertussis toxin; WT, wild-type.
In WT cells treated with YM-254890 (3 µM), CCh induced a suppression that reached a peak inhibition at about 30 s (Figure 8C). The mean suppression values at the 30 s and 3 min points were significantly smaller than the corresponding control values (Figure 8D) but were the same as those in M3KO cells (Figure 8C). The average ICa suppression curve in WT cells treated with YM-254890 (3 µM) overlapped that in M3KO cells (see Figure 8C).
Ca2+ dependency of the muscarinic ICa suppression
In guinea pig ileal smooth muscle cells, a certain level of intracellular Ca2+ is required for the CCh-induced sustained suppression of ICa (Unno et al., 1995). In order to test the Ca2+ dependency of M2- or M3-mediated ICa suppression, cells were dialysed with a pipette solution containing EGTA (20 mM). Intracellular application of EGTA itself caused ICa to increase by approximately two to fourfold in the different mouse strains used. For example, the ICa current density in WT cells was increased from 4.3 ± 1.1 pA/pF (n= 16) to 11.4 ± 2.7 pA/pF (n= 20) by EGTA treatment.
The intracellular application of EGTA had little or no effect on the average ICa suppression curve in M2KO cells (Figure 9A and B) or in M3KO cells (Figure 9C and D). In contrast, EGTA treatment of WT cells caused a marked reduction of the CCh-induced suppression of ICa. The ICa suppression in EGTA treated WT cells reached a peak within 1 min after the beginning of CCh application and then gradually recovered to some extent or maintained its decreased level during the presence of the agonist (Figure 9E). Thus the mean suppression at 30 s was not affected by EGTA but, at 3 min, EGTA significantly reduced the suppression of ICa. Interestingly, the averaged ICa suppression curve obtained in the EGTA-treated WT cells almost overlapped with a combined curve from the M2-mediated suppression in M3KO cells and the M3-mediated suppression in M2KO cells (see Figure 9E).
Figure 9.
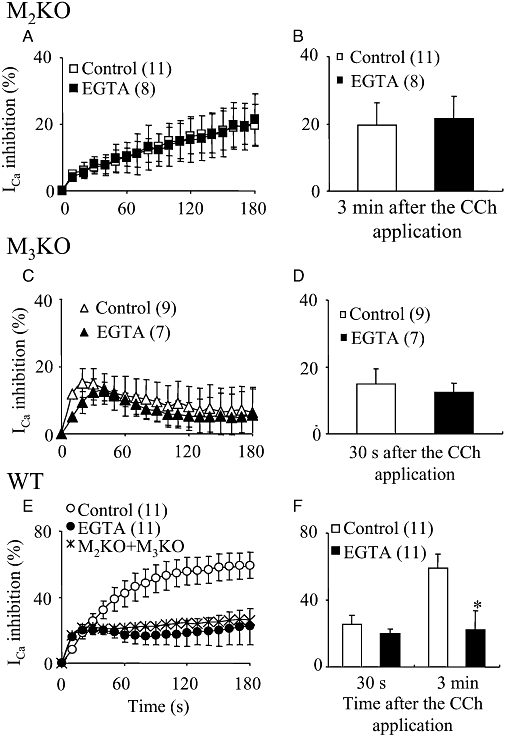
Effects of EGTA on the CCh-induced ICa suppressions in M2KO, M3KO and WT cells. (A, C and E) Time courses of the change in the mean percentage suppression of ICa induced by CCh (100 µM) in 0.05 mM EGTA (control)- and 20 mM EGTA-dialysed cells from M2KO (A), M3KO (C) and WT (E) mice. In (E), the sum of the average ICa suppression curve in M2KO (A) and M3KO (C) control cells is also shown. (B, D and F) Summary of the mean percentage suppression of ICa measured 30 s or 3 min after CCh application in M2KO (B), M3KO (D) and WT (F) cells. The asterisk in (F) represents a significant difference (P < 0.05) from the corresponding control value. CCh, carbachol; ICa, voltage-gated Ca2+ channel currents; KO, knockout; WT, wild-type.
Discussion
The L-type voltage-gated Ca2+ channel represents one of the major pathways for Ca2+ influx into the cytosol and thereby plays an essential role in the regulation of [Ca2+]i in smooth muscle cells. The channel activity is regulated by various neurotransmitters and hormones (see Imaizumi et al., 1991; Pacaud and Bolton, 1991; Beech, 1993; Han et al., 1995; Unno et al., 1995; Wade et al., 1996; Pucovsky et al., 1998), although the precise signal transduction mechanisms involved remains unclear. In the present study, the inhibitory effects of the muscarinic agonist CCh on ICa were investigated in order to elucidate the muscarinic receptor subtypes and possible signal transduction mechanisms involved in intestinal smooth muscle cells from WT mice and mutant mice lacking M2 or M3, or both subtypes of muscarinic receptors.
In WT cells, CCh (100 µM) induced either a sustained suppression of ICa or a biphasic suppression consisting of an early transient component followed by a slowly developing sustained component, as previously described in guinea pig ileal smooth muscle cells (Unno et al., 1995). No appreciable ICa suppression was observed upon CCh application in M2/M3-double KO cells, suggesting that only M2 and M3 receptors participate in the ICa suppression in WT cells. The early transient ICa suppression was detected during the initial transient component of cationic inward current, which reflects release of Ca2+ from intracellular stores (Komori et al., 1993; Unno et al., 1995). Such release of stored Ca2+ by CCh reflects M3 subtype stimulation (Sakamoto et al., 2007), and the transient ICa suppression is likely to be mediated via the M3/Gq/11/PLCβ/IP3/Ca2+ release pathway and Ca2+-induced Ca2+ channel inactivation mechanisms (Figure 10).
Figure 10.
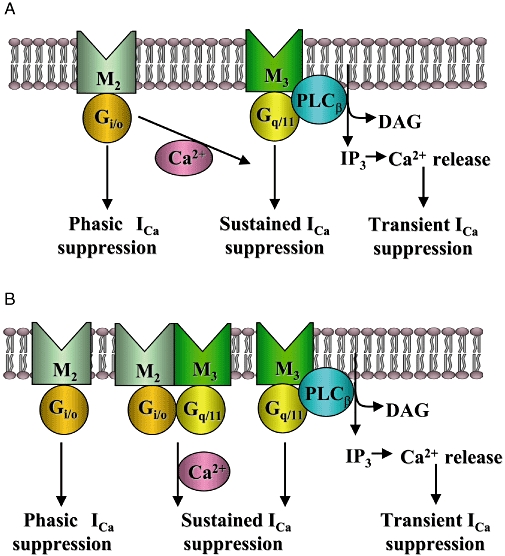
Assignment of muscarinic receptor subtypes and G proteins mediating the suppressions of L-type voltage-gated Ca2+ channels in intestinal smooth muscle cells. M2 muscarinic receptors, via Gi/o proteins, induce a phasic ICa suppression, while M3 receptors, via Gq/11 proteins, induced a transient ICa suppression due to Ca2+ release from intracellular stores and a sustained ICa suppression. In addition, M2 and M3 receptors can cooperatively suppress ICa. The M2/M3-mediated pathway involves both Gi/o and Gq/11 proteins and a Ca2+-dependent process to induce a sustained ICa suppression. The present results are consistent with two possible models for the M2/M3-mediated pathway; one is a M2/M3-synergistic model in which the M3/Gq/11-mediated sustained suppression is potentiated by the M2/Gi/o-mediated, Ca2+-dependent signal (A), and the other one is a M2/M3-complex model in which both the M2 and M3 receptor subtypes and the respective G proteins form a complex to operate a Ca2+-dependent signal and induce a sustained ICa suppression (B), as suggested for the activation mechanisms of muscarinic cationic channels (Sakamoto et al., 2007). ICa, voltage-gated Ca2+ channel currents.
In M2KO cells, CCh elicited a sustained, yet smaller, ICa suppression than that seen in WT cells. In M3KO cells, CCh induced a phasic ICa suppression, the time course of which was clearly different from that of the sustained suppression observed in WT cells. The ICa suppression in M2KO and M3KO cells are likely to be mediated through M3 and M2 receptors. The M3-mediated sustained ICa suppression was inhibited by the Gq/11 inhibitor YM-254890, but was unaffected by the Gi/o inhibitor PTX. In contrast, the M2-mediated phasic ICa suppression was abolished by PTX, but remained unaffected by YM-254890. Also, intracellular application of EGTA (20 mM) had no effect on either M2- or M3-mediated suppression. These results suggest that stimulation of both M2 and M3 receptors induce the phasic and sustained ICa suppression via Gi/o and Gq/11 type G proteins and that intracellular Ca2+ is unlikely to be involved (Figure 10).
In rat sympathetic neurons, substance P (SP) and somatostatin inhibited whole-cell N-type Ca2+ channel currents via PTX-insensitive and -sensitive G proteins (Shapiro and Hille, 1993). The SP-induced suppression developed slowly and then plateaued, while the somatostatin-induced suppression developed more rapidly, peaked and then gradually recovered even in the presence of the agonist. Furthermore, N-type Ca2+ channel suppression was resistant to BAPTA (20 mM). Thus, the characteristics of the SP- and somatostatin-induced suppression are similar to those of the M3-mediated sustained and M2-mediated phasic suppression. In these studies Shapiro and Hille (1993) suggested that both the SP- and somatostatin-induced suppression is not mediated by a diffusible cytosolic messenger, but by a membrane-delimited signal transduction pathway (Shapiro and Hille, 1993), possibly involving phosphatidylinositol 4,5-bisphosphate (PIP2) (Michailidis et al., 2007).
In rabbit colonic smooth muscle cells, Jin et al. (2002) demonstrated that M2 receptor stimulation induced an enhancement of ICa, the effect was Ca2+-independent and required Gi-dependent activation of the c-src kinase pathway. Moreover, in order to demonstrate M2-mediated enhancement of ICa, Jin et al. (2002) had to block M3-mediated signalling pathways by either treatment with 4-DAMP or by intracellular dialysis of an anti-Gαq antibody. Hence, we would have expected a similar result using M3KO cells. However, we failed to detect any enhancement of ICa by muscarinic receptor stimulation, but detected a phasic suppression in M3KO intestinal smooth muscle cells. This finding might be explained by the M2-mediated phasic ICa suppression representing the sum of both an inhibitory and a facilitatory pathway operating in parallel.
In WT cells, some ICa suppression still occurred independent of the combined effect of M2 and M3 receptor responses (as demonstrated by data from M2KO and M3KO cells Figure 3C). While EGTA had no significant effect on the ICa suppression in M2KO or M3KO cells, the Ca2+ chelator strongly inhibited the residual ICa suppression in WT cells (Figure 9E). These results suggest that the component of ICa suppression inhibited by EGTA in WT cells corresponds to a component that requires the simultaneous stimulation of both M2 and M3 receptors and that this M2/M3-mediated pathway may contribute to ICa suppression in WT cells.
The ICa suppression in WT cells was inhibited by either PTX or YM-254890. The CCh-induced ICa suppression curve in PTX-treated WT cells resembled that of M3 receptor stimulation (Figure 8A). Similarly, the average ICa suppression curve in YM-254890-treated WT cells was similar to that of M2 receptor stimulation (Figure 8C). These results suggest that PTX and YM-254890 not only inhibit the M2-mediated and M3-mediated suppression, but also the M2/M3-mediated sustained suppression. Taken together, these observations suggest that the M2/M3-mediated sustained suppression involves both Gi/o and Gq/11 type G proteins and a Ca2+-dependent process.
In our previous work using guinea pig ileal myocytes, we did not detect a PTX-sensitive component of the CCh-induced ICa suppression (Unno et al., 1995). We reported that ICa suppression is Ca2+-dependent and potentiated by an influx of Ca2+ through L-type Ca2+ channels (Unno et al., 1995). However, in this previous study, we used Ba2+ as a charge carrier for the current recording, so that [Ca2+]i was kept at a low level, which probably resulted in a reduced activity of the M2/M3-mediated pathway. As a result, only the PTX-insensitive, M3-mediated sustained component of ICa suppression was detected. However, Pucovsky et al. (1998) did detect a PTX-sensitive component of CCh-induced ICa suppression in guinea pig ileal myocytes, and they used a pipette solution in which the Ca2+ concentration was kept constant at 100 nM. This concentration of Ca2+ may be sufficient to allow the operation of the M2/M3-mediated pathway.
Wade et al. (1996) reported that ACh decreased the open probability of L-type Ca2+ channels in guinea pig gastric myocytes under the cell-attached patch clamp mode. They postulated the involvement of a diffusible cytosolic messenger in the ACh-induced ICa suppression. A similar result was obtained on muscarinic suppression of N- and L-type Ca2+ channels in rat sympathetic neurons (Mathie et al., 1992). This report also demonstrated that oxotremorine-M induced a sustained ICa suppression that was dependent upon intracellular Ca2+. Hence, our findings suggest that the M2/M3-mediated ICa suppression involves a diffusible cytosolic messenger in the signal transduction pathway. Unno et al. (1999) suggested the involvement of polymerization of tubulin, a constituent of the microtubule cytoskeleton, in the sustained muscarinic ICa suppression in guinea pig ileal smooth muscle cells. As polymerization of tubulin is dynamically regulated by cytosolic Ca2+ (Dustin, 1984), this signalling molecule could also regulate the M2/M3-mediated ICa suppression.
Figure 10 depicts possible models for the assignment of muscarinic receptor subtypes and G proteins mediating the ICa suppression. M2 receptors, via Gi/o proteins, induce a phasic ICa suppression, while M3 receptors, via Gq/11 proteins, induce a transient and a sustained ICa suppression. The third pathway (M2/M3) induces a sustained suppression when both M2 and M3 receptors are stimulated.
The M3-mediated transient suppression is due to a Ca2+-induced inactivation mechanism brought about by IP3-induced Ca2+ release from intracellular stores. On the other hand, the M2-mediated phasic and M3-mediated sustained suppression probably occur through Ca2+-independent mechanisms. For the M2/M3-mediated pathway, one possible model is depicted in Figure 10A. In this model, M3 receptors mediate the sustained suppression through a Ca2+-independent process, and this pathway is potentiated by an M2/Gi/o-mediated, Ca2+-dependent signal. Alternatively, the M2/M3-mediated pathway may arise from a receptor complex consisting of both M2 and M3 receptors possibly via the formation of M2/M3 heterodimers (Maggio et al., 1999). Therefore, the M2 and M3 receptor subtypes may form a complex to activate two types of G proteins, Gi/o and Gq/11, and the downstream signals may act to operate the sustained suppression through a Ca2+ dependent process, as shown in Figure 10B. This M2/M3 complex model is identical to that postulated for the activation mechanisms of muscarinic cationic channels in M2 or M3 KO mice (Sakamoto et al., 2007).
Muscarinic receptor stimulation activates cation channels to produce membrane depolarization and thereby increases discharge rate of action potentials, which are accompanied by Ca2+ entry (Inoue and Isenberg, 1990; Komori et al., 1992; Unno et al., 2003). As action potential discharge is accelerated, operation of a process to suppress ICa is accelerated by the increased Ca2+ influx. Since the M2/M3-mediated pathway depends on cytosolic Ca2, this pathway may detect the accumulation of [Ca2+]i and then function to suppress the activity of L-type Ca2+ channels as a negative feedback system. The M2/M3-mediated pathway may involve a diffusible messenger and is sustained, allowing for prolonged inhibitory control of L-type Ca2+ channel activity. Using ileal smooth muscles isolated from muscarinic receptor KO mice, Griffin et al. (2004) reported that the heterologous desensitization induced by ACh is contingent upon activation of both M2 and M3 receptors and that activation of either receptor is insufficient to cause desensitization. Therefore, it is likely that the M2/M3-mediated suppression of L-type Ca2+ channel activity is responsible, at least partly, for the heterologous desensitization caused by muscarinic receptor stimulation.
Acknowledgments
This work was supported by the JSPS Research Fellows for Young Scientists (No. 203080) and a Grant-in-Aid for scientific research (No. 20580322) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Glossary
Abbreviations:
- ICa
voltage-gated Ca2+ channel currents
- KO mice
knockout mice
- PTX
Pertussis toxin
- WT mice
wild-type mice
Conflicts of interest
The authors state no conflict of interest.
References
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edn. Br J Pharmacol. 2008;153(Suppl 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech DJ. Inhibitory effects of histamine and bradykinin on calcium current in smooth muscle cells isolated from guinea-pig ileum. J Physiol. 1993;463:565–583. doi: 10.1113/jphysiol.1993.sp019611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin P. General physiology of tubulins and microtubules. In: Dustin P, editor. Microtubules. New York: Springer-Verlag; 1984. pp. 94–126. [Google Scholar]
- Ganitkevich V, Shuba MF, Smirnov SV. Calcium-dependent inactivation of potential-dependent calcium inward current in an isolated guinea-pig smooth muscle cell. J Physiol. 1987;392:431–449. doi: 10.1113/jphysiol.1987.sp016789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomeza J, Shannon H, Kostenis E, Felder C, Zhang L, Brodkin J, et al. Pronounced pharmacologic deficits in M2 muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:1692–1697. doi: 10.1073/pnas.96.4.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin MT, Matsui M, Shehnaz D, Ansari KZ, Taketo MM, Manabe T, et al. Muscarinic agonist-mediated heterologous desensitization in isolated ileum requires activation of both muscarinic M2 and M3 receptors. J Pharmacol Exp Ther. 2004;308:339–349. doi: 10.1124/jpet.103.055327. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Han X, Shimoni Y, Giles WR. A cellular mechanism for nitric oxide-mediated cholinergic control of mammalian heart rate. J Gen Physiol. 1995;106:45–65. doi: 10.1085/jgp.106.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi Y, Takeda M, Muraki K, Watanabe M. Mechanisms of NE-induced reduction of Ca current in single smooth muscle cells from guinea pig vas deferens. Am J Physiol. 1991;260:C17–C25. doi: 10.1152/ajpcell.1991.260.1.C17. [DOI] [PubMed] [Google Scholar]
- Inoue R, Isenberg G. Effect of membrane potential on acetylcholine-induced inward current in guinea-pig ileum. J Physiol. 1990;424:57–71. doi: 10.1113/jphysiol.1990.sp018055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Morsy N, Shoeb F, Zavzavadjian J, Akbarali HI. Coupling of M2 muscarinic receptor to L-type Ca channel via c-src kinase in rabbit colonic circular smooth muscle. Gastroenterology. 2002;123:827–834. doi: 10.1053/gast.2002.35388. [DOI] [PubMed] [Google Scholar]
- Katada T, Ui M. ADP ribosylation of the specific membrane protein of C6 cells by islet-activating protein associated with modification of adenylate cyclase activity. J Biol Chem. 1982;257:7210–7216. [PubMed] [Google Scholar]
- Komori S, Kawai M, Takewaki T, Ohashi H. GTP-binding protein involvement in membrane currents evoked by carbachol and histamine in guinea-pig ileal muscle. J Physiol. 1992;450:105–126. doi: 10.1113/jphysiol.1992.sp019118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori S, Kawai M, Pacaud P, Ohashi H, Bolton TB. Oscillations of receptor-operated cationic current and internal calcium in single guinea-pig ileal smooth muscle cells. Pflügers Arch. 1993;424:431–438. doi: 10.1007/BF00374905. [DOI] [PubMed] [Google Scholar]
- Maggio R, Barbier P, Colelli A, Salvadori F, Demontis G, Corsini GU. G protein-linked receptors: pharmacological evidence for the formation of heterodimers. J Pharmacol Exp Ther. 1999;291:251–257. [PubMed] [Google Scholar]
- Mathie A, Bernheim L, Hille B. Inhibition of N- and L-type calcium channels by muscarinic receptor activation in rat sympathetic neurons. Neuron. 1992;8:907–914. doi: 10.1016/0896-6273(92)90205-r. [DOI] [PubMed] [Google Scholar]
- Michailidis IE, Zhang Y, Yang J. The lipid connection-regulation of voltage-gated Ca2+ channels by phosphoinositides. Pflügers Arch. 2007;455:147–155. doi: 10.1007/s00424-007-0272-9. [DOI] [PubMed] [Google Scholar]
- Pacaud P, Bolton TB. Relation between muscarinic receptor cationic current and internal calcium in guinea-pig jejunal smooth muscle cells. J Physiol. 1991;441:477–499. doi: 10.1113/jphysiol.1991.sp018763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucovsky V, Zholos AV, Bolton TB. Muscarinic cation current and suppression of Ca2+ current in guinea pig ileal smooth muscle cells. Eur J Pharmacol. 1998;346:323–330. doi: 10.1016/s0014-2999(98)00059-4. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Unno T, Matsuyama H, Uchiyama M, Hattori M, Nishimura M, et al. Characterization of muscarinic receptor-mediated cationic currents in longitudinal smooth muscle cells of mouse small intestine. J Pharmacol Sci. 2006;100:215–226. doi: 10.1254/jphs.fp0050973. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Unno T, Kitazawa T, Taneike T, Yamada M, Wess J, et al. Three distinct muscarinic signalling pathways for cationic channel activation in mouse gut smooth muscle cells. J Physiol. 2007;582:41–61. doi: 10.1113/jphysiol.2007.133165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro MS, Hille B. Substance P and somatostatin inhibit calcium channels in rat sympathetic neurons via different G protein pathways. Neuron. 1993;10:11–20. doi: 10.1016/0896-6273(93)90237-l. [DOI] [PubMed] [Google Scholar]
- Struckmann N, Schwering S, Wiegand S, Gschnell A, Yamada M, Kummer W, et al. Role of muscarinic receptor subtypes in the constriction of peripheral airways: studies on receptor-deficient mice. Mol Pharmacol. 2003;64:1444–1451. doi: 10.1124/mol.64.6.1444. [DOI] [PubMed] [Google Scholar]
- Takasaki J, Saito T, Taniguchi M, Kawasaki T, Moritani Y, Hayashi K, et al. A novel Gαq/11-selective inhibitor. J Biol Chem. 2004;279:47438–47445. doi: 10.1074/jbc.M408846200. [DOI] [PubMed] [Google Scholar]
- Unno T, Komori S, Ohashi H. Inhibitory effect of muscarinic receptor activation on Ca2+ channel current in smooth muscle cells of guinea-pig ileum. J Physiol. 1995;484:567–581. doi: 10.1113/jphysiol.1995.sp020687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unno T, Komori S, Ohashi H. Microtubule cytoskeleton involvement in muscarinic suppression of voltage-gated calcium channel current in guinea-pig ileal smooth muscle. Br J Pharmacol. 1999;127:1703–1711. doi: 10.1038/sj.bjp.0702711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unno T, Kwon SC, Okamoto H, Irie Y, Kato Y, Matsuyama H, et al. Receptor signaling mechanisms underlying muscarinic agonist-evoked contraction in guinea-pig ileal longitudinal smooth muscle. Br J Pharmacol. 2003;139:337–350. doi: 10.1038/sj.bjp.0705267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unno T, Matsuyama H, Sakamoto T, Uchiyama M, Izumi Y, Okamoto H, et al. M2 and M3 muscarinic receptor-mediated contractions in longitudinal smooth muscle of the ileum studied with receptor knockout mice. Br J Pharmacol. 2005;146:98–108. doi: 10.1038/sj.bjp.0706300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unno T, Matsuyama H, Izumi Y, Yamada M, Wess J, Komori S. Roles of M2 and M3 muscarinic receptors in cholinergic nerve-induced contractions in mouse ileum studied with receptor knockout mice. Br J Pharmacol. 2006;149:1022–1030. doi: 10.1038/sj.bjp.0706955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade GR, Barbera J, Sims SM. Cholinergic inhibition of Ca2+ current in guinea-pig gastric and tracheal smooth muscle cells. J Physiol. 1996;491:307–319. doi: 10.1113/jphysiol.1996.sp021217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Miyakawa T, Duttaroy A, Yamanaka A, Moriguchi T, Makita R, et al. Mice lacking the M3 muscarinic acetylcholine receptor are hypophagic and lean. Nature. 2001;410:207–212. doi: 10.1038/35065604. [DOI] [PubMed] [Google Scholar]
- Yan HD, Okamoto H, Unno T, Tsytsyura YD, Prestwich SA, Komori S, et al. Effects of G-protein-specific antibodies and Gβγ subunits on the muscarinic receptor-operated cation current in guinea-pig ileal smooth muscle cells. Br J Pharmacol. 2003;139:605–615. doi: 10.1038/sj.bjp.0705289. [DOI] [PMC free article] [PubMed] [Google Scholar]


