Abstract
Objectives
We describe rates of success for two-stage revision of prosthetic joint infection (PJI), including data on reimplantation microbiology.
Methods
We retrospectively collected data from all the cases of PJI that were managed with two-stage revision over a 4 year period. Patients were managed with an antibiotic-free period before reimplantation, in order to confirm, clinically and microbiologically, that infection was successfully treated.
Results
One hundred and fifty-two cases were identified. The overall success rate (i.e. retention of the prosthesis over 5.75 years of follow-up) was 83%, but was 89% for first revisions and 73% for re-revisions [hazard ratio = 2.9, 95% confidence interval (CI) 1.2–7.4, P = 0.023]. Reimplantation microbiology was frequently positive (14%), but did not predict outcome (hazard ratio = 1.3, 95% CI 0.4–3.7, P = 0.6). Furthermore, most unplanned debridements following the first stage were carried out before antibiotics were stopped (25 versus 2 debridements).
Conclusions
We did not identify evidence supporting the use of an antibiotic-free period before reimplantation and routine reimplantation microbiology. Re-revision was associated with a significantly worse outcome.
Keywords: infected arthroplasty, antibiotic, complications, re-revision
Introduction
Arthroplasty improves the patient's quality of life and is highly cost effective.1–3 Prosthetic joint infection (PJI) complicates up to 2.5%4 of the estimated 90 000 primary arthroplasties performed annually in England and Wales,5 and presents a major challenge to patients, physicians and funding agencies. PJI does not generally respond to antibiotics alone, but can be managed by ‘DAIR’ (debridement, antibiotics and implant retention), or by one- or two-stage exchange revisions.6 Exchange revision is the intervention of choice if the implant has become loose.7
A recent meta-analysis including 926 two-stage knee joint revisions concluded there was an 82%–100% success rate, but the reasons for variable success rates were not clear.8 Success rates described for two-stage exchanges of hip prostheses ranged from 75% among 169 cases9 to 90% among 99 cases,10 29 cases11 and 41 cases.12
However, there are few descriptions of the clinical and microbiological parameters that predict treatment failure, and the role of reimplantation microbiology remains unclear. We present 155 cases of PJI treated by two-stage revision, managed with standardized surgical and microbiological protocols13 and detailed clinical information. Our routine practice during this period was to undertake reimplantation at least 2 weeks after stopping antibiotics. This was done, firstly, so that clinical failures after stopping antibiotics would become evident before reimplantation had taken place and, secondly, so that samples for microbial culture taken at reimplantation would not be falsely negative because of recent antibiotics.
Methods
This was a retrospective series of all cases of PJI managed by two-stage revision surgery in the Bone Infection Unit of the Nuffield Orthopaedic Centre, Oxford, UK between 1 January 1999 and 30 April 2003. Cases were managed by infectious disease physicians and orthopaedic surgeons in a multidisciplinary team. We established a registry and included all cases of PJI using multiple data sources: histopathology and outpatient parenteral antimicrobial therapy (OPAT) databases; hospital activity coding databases; systematically hand-searched diagnostic details listed in outpatient clinic letters; and prospective capture of patients attending follow-up outpatient appointments or readmitted to our unit.
Following a review of the case notes, we excluded patients whose primary management of infection was excision arthroplasty without an intention to reimplant, single-stage revision surgery, DAIR or suppressive antibiotic therapy without debridement.
Two researchers (N. G. and H. P.) extracted data from case notes of included patients for entry into a Microsoft® Access database. Data were cleaned by a senior investigator (P. B.), who examined extreme outlier observations and obvious inconsistencies in the entered data, and then subsequently selected a random set of these case notes (totalling 10% of the case notes), independently extracted data and reconciled the two extractions. Reconciling the differences among randomly selected data confirmed the accuracy of the primary extraction. The hip and knee scores using the 0–48 Oxford scoring system14 were measured at the end of follow-up, a mean of 5.75 years after the original surgery.
Case definition
Infection was defined as those having a clinical syndrome of arthroplasty infection (any of persistent inflammation in the tissues around the implant, wound discharge or implant loosening) with one or more of the following: bacterial growth of an indistinguishable organism from two or more deep periprosthetic tissue samples; histology of periprosthetic tissues indicative of infection; or a persistent sinus tract. Patients with no clinical or radiographic evidence of joint loosening were managed with DAIR.15 Multiple samples were taken for microbiological culture, as previously described.13 The histological diagnosis was made on the basis of the degree of infiltration by neutrophil polymorphonuclear leucocytes, as outlined in previous studies.16–18
Definition of treatment failure
We defined treatment failure as: (i) sinus drainage recurring after reimplantation; (ii) a requirement for further revision surgery (irrespective of the indication); or (iii) amputation of the affected limb. The requirement for ongoing antibiotics or further debridement was not considered treatment failure per se.
Data collection
We collected data from the patient notes on patient demographics, co-morbidities (diabetes, renal failure, immunosuppression, rheumatoid arthritis, malignancy and smoking), the date of primary surgery, symptom onset, clinical features at presentation and surgical findings, microbiology, and antibiotic choice and duration. There was no research-related contact with patients and informed consent was not required (as advised by our institutional review board). All activity was conducted in accordance with the Declaration of Helsinki, and national and institutional standards.
Antibiotic management
After intraoperative sampling at excision of the infected implant, patients received empirical intravenous (iv) vancomycin (1 g; continued as 1 g initially 12 hourly, adjusted according to levels) plus 500 mg of meropenem 8 hourly (increased to 1 g 8 hourly for pseudomonads). Therapy was rationalized once definitive culture results were obtained from the laboratory. Meropenem was discontinued at 48 h if no aerobic Gram-negative pathogens requiring treatment with a carbapenem had been cultured.15,19
Intravenous antibiotic therapy was continued for 6 weeks with a β-lactam or glycopeptide, according to the susceptibility profiles of cultured organisms. It was not our practice to prolong antibiotic treatment based on measurement of biomarkers, unless further debridements were required, in which case iv antibiotic therapy was restarted after the debridements. If patients were suitable for OPAT, the preferred treatment was with ceftriaxone (1 g daily) for most susceptible pathogens or with teicoplanin for coagulase-negative staphylococci and methicillin-resistant Gram-positive organisms. Patients with negative cultures were treated with glycopeptides.20 We used a carbapenem to treat Gram-negative organisms that were resistant to cephalosporins or that were associated with inducible resistance to cephalosporins. Oral antibiotics were not used routinely, but patients experiencing side effects that precluded further iv therapy, or experiencing difficulties in maintaining iv access, were switched to oral therapy to complete 6 weeks. Antibiotics were stopped at 6 weeks, followed by a minimum 2 week antibiotic-free period before reimplantation.
Surgical management
At first-stage surgery, sinuses were excised and the superficial contaminated layers discarded. Intraoperative samples for culture and histology were taken of joint fluid, joint capsule (hip), synovium (knee), infected collections and membrane from each interface as prosthetic components were removed. Each sample was obtained with separate instruments and placed into separate containers for processing, as previously described.13
The bone ends, medullary cavities and joint cavity were debrided of all infected, necrotic and foreign material, following which the operative field was lavaged with several litres of warm saline. Spacers of bone cement containing gentamicin were used routinely in the knee but rarely in the hip. Where plastic surgery was used to achieve soft tissue cover, this was done using medial gastrocnemius flaps with skin grafting over the knee.
At second-stage surgery, a further set of specimens for culture and histology was taken in the same manner prior to the implantation of a new prosthesis. Antibiotic prophylaxis was not given until after the specimens were taken. Gentamicin-impregnated cement was used for cemented implants and allograft bone was used if required. According to our protocol, no pre-operative sampling was undertaken.
Analysis
We used STATA version 10 (Stata Corp., College Station, TX, USA) to run Cox survival analyses. Data were censored from follow-up after recurrence of infection or when lost to follow-up. The endpoint for treatment failure was as defined above. Univariate analysis was conducted on factors relevant to presentation or initial treatment. Subsequently, multivariate analysis was conducted for factors significant or of borderline significance (P < 0.1) on univariate analysis.
Results
Characteristics of the cohort
One hundred and fifty-five cases of PJI treated by two-stage revision were identified. In three instances the case definition for infection was not met and these were excluded from further analysis. In the 152 that remained, the diagnosis was supported by histological criteria in the vast majority (95%). Both microbiology and histology were positive in 56%. The diagnosis was based on microbiological criteria alone in only 3% of cases. One percent was included on the basis of a sinus tract alone, despite negative histology and microbiology results (Table 1).
Table 1.
Patient demographics, operative and antibiotic management
| Factor | n | % |
|---|---|---|
| Case definition | ||
| microbiological criteria | 90 | 59 |
| histological criteria | 145 | 95 |
| microbiology only | 5 | 3 |
| sinus without microbiology/histology | 2 | 1 |
| Age group (years) | ||
| 20–50 | 8 | 5 |
| ≥50–<60 | 23 | 15 |
| ≥60–<70 | 46 | 30 |
| ≥70–<80 | 63 | 42 |
| ≥80 | 12 | 8 |
| Gender | ||
| male | 93 | 61 |
| female | 59 | 39 |
| Joint | ||
| hip | 71 | 46 |
| knee | 77 | 51 |
| elbow | 4 | 3 |
| Co-morbidities | ||
| none | 53 | 35 |
| 1 | 55 | 36 |
| >1 | 44 | 29 |
| Time since first joint replacement | ||
| <90 days | 4 | 3 |
| ≥90 days–<1 year | 21 | 15 |
| ≥1–<2 years | 31 | 22 |
| ≥2–<10 years | 29 | 20 |
| ≥10 years | 58 | 41 |
| missing data | 9 | – |
| Length of symptoms | ||
| <3 months | 37 | 32 |
| ≥3–<12 months | 29 | 25 |
| ≥1 year | 51 | 44 |
| missing data | 35 | – |
| Previous revisions | ||
| 0 | 62 | 45 |
| 1 | 46 | 34 |
| >1 | 29 | 21 |
| missing data | 42 | – |
| Referral route | ||
| tertiary referral | 119 | 78 |
| Oxfordshire patient | 33 | 22 |
| Microbiology | ||
| Staphylococcus aureus | 27 | 18 |
| Gram-negative rods | 11 | 7 |
| coagulase-negative staphylococci | 45 | 30 |
| streptococci | 11 | 7 |
| culture negative | 62 | 41 |
| Debridements between stages | ||
| no second stage | 6 | 4 |
| 0 | 124 | 82 |
| 1 | 18 | 12 |
| 2 | 3 | 2 |
| 3 | 1 | 1 |
| Muscle flaps | ||
| none | 136 | 87 |
| at first stage | 9 | 6 |
| after first stage | 7 | 5 |
| Periprosthetic fracture | ||
| absent | 140 | 95 |
| present | 12 | 5 |
| Gap between stages | ||
| <56 days | 8 | 4 |
| ≥56–<120 days | 70 | 46 |
| ≥120 days–<1 year | 54 | 36 |
| ≥1–<2 years | 10 | 7 |
| ≥2 years | 1 | 1 |
| no second stage | 6 | 4 |
| Outcomes (median follow-up = 5.75 years)a | ||
| not reimplanted | 6 | 4 |
| early excision | 3 | 1 |
| retained | 126 | 83 |
| late excision | 15 | 10 |
| late amputation | 2 | 1 |
| Antibiotics between stages | ||
| <4 weeks iv | 48 | 32 |
| ≥4 weeks iv | 103 | 68 |
| missing | 1 | – |
| Antibiotics after reimplantation | ||
| none | 88 | 58 |
| <1 week | 9 | 6 |
| ≥1 week–<6 weeks | 13 | 15 |
| ≥6 weeks–<6 months | 8 | 5 |
| ≥6 months–<1 year | 6 | 4 |
| ≥1 year | 28 | 18 |
aLate excision includes any operation, after 6 months, that excises the implant, including excision arthroplasty and one- or two-stage re-revisions.
Half of the patients were >70 years old and 65% had one or more co-morbidities. Most of the implants were >2 years old (62%) and most patients had had symptoms for ≥3 months (69%). Previous revisions were common (55%) and were over-represented among tertiary referrals from other centres. Of the patients with complete data for numbers of re-revisions and referral source, 62% of 89 tertiary referrals were re-revisions, compared with 29% of the 28 patients from the Oxford area, P = 0.002. Of 20 patients with more than one previous revision, all were tertiary referrals.
Infecting organisms
The most common infecting organisms were coagulase-negative staphylococci and culture-negative infection was surprisingly frequent (41%), despite 93% of cases having at least a 2 week antibiotic-free gap before primary sampling (Table 1). Among the 11 patients who did not have a 2 week antibiotic-free gap, 8 were culture positive.
Culture-negative infection was not associated: (i) with autoimmune disease (38% of 26 patients, compared with 41% of 126 patients without, P = 0.4); (ii) with age of implant (36% of 56 patients with implants <2 years old, compared with 40% of 87 with implants ≥2 years old, P = 0.6); (iii) with tertiary referral (37% of 119 patients, compared with 52% of local referrals, P = 0.16); or (iv) with previous revisions (48% of 62 primary implants versus 33% of 75 previously revised implants, P = 0.1). Culture-negative infection was, however, more common in knees than hips (28% of 71 hip joints versus 51% of 77 knee joints, P = 0.008).
Operative management
The operative course was complicated for some patients: 11% required a muscle flap; 15% required a further debridement between the first and second stages; and a periprosthetic fracture occurred in 5% (Table 1). The second stage (i.e. reimplantation) was more likely to be delayed past 120 days if these complications occurred (38% were delayed without complications, 70% with, χ2 = 12, P < 0.0005).
Outcomes
Of those who began on the two-stage treatment pathway, 96% eventually progressed to reimplantation. There were no perioperative deaths. One patient was discharged to long-term institutional care, five patients (3%) required transfer to rehabilitation facilities after reimplantation and the rest (96%) were discharged home.
Although 96% proceeded to reimplantation, loss of the prosthesis or of the limb was subsequently recorded in a further 12% over the mean follow-up time of 5.75 years, suggesting an overall success rate of 83%. In two instances, amputation was undertaken. In two instances, debridements with subsequent long-term retention of the prosthesis were performed; this was not considered treatment failure.
Oxford hip and knee scores were available on 76 (50%) of the patients, measured at a median of 5.75 years after surgery. The median score was 27, with an interquartile range of 21–38.
Factors associated with implant survival
On univariate Cox regression, only three factors were significantly associated with an increased rate of implant failure: younger age of the patient; more time elapsed since the original surgery; and a greater number of previous revisions (Table 2). On multivariate analysis, the effect of time since first joint replacement was not significant and the effect of time elapsed appeared to be accounted for by the number of previous revisions (Table 3).
Table 2.
Factors influencing outcomes, univariate Cox regression
| Factor | HR | 95% CI | P value |
|---|---|---|---|
| Age of patient (per 10 years) | 0.58 | 0.4–0.9 | 0.008 |
| Age of implant (per 5 years) | 1.36 | 1.1–1.8 | 0.019 |
| Length of symptoms ≥90 days | 0.61 | 0.2–2.3 | 0.46 |
| Length of symptoms ≥1 year | 0.94 | 0.4–2.7 | 0.9 |
| Gender | 1.6 | 0.7–3.5 | 0.3 |
| Co-morbidity | 0.9 | 0.6–1.4 | 0.6 |
| Knee (versus hip) | 1.4 | 0.6–3.1 | 0.4 |
| Tertiary referral | 1.1 | 0.4–2.8 | 0.8 |
| Previous revision | 2.9 | 1.2–7.4 | 0.023 |
| Muscle flap required | 0.97 | 0.3–3.3 | 0.97 |
| Fracture occurred | 2.2 | 0.8–6.5 | 0.14 |
| Gram-negative bacilli | 0.6 | 0.1–4.7 | 0.7 |
| Streptococci | 0.4 | 0.1–3.1 | 0.4 |
| Staphylococcus aureus | 0.35 | 0.1–1.5 | 0.2 |
| Coagulase-negative staphylococci | 1.3 | 0.6–3.0 | 0.5 |
| Culture negative | 1.7 | 0.8–3.6 | 0.2 |
| Reimplantation microbiology | 1.3 | 0.4–3.7 | 0.6 |
| ≥4 weeks of iv antibiotics between stages | 0.78 | 0.4–1.7 | 0.5 |
| ≥1 week of antibiotics after second stage | 0.73 | 0.3–1.6 | 0.4 |
HR, hazard ratio; CI, confidence interval.
Table 3.
Factors influencing outcomes, multivariate Cox regression
| Factor | HR | 95% CI | P value |
|---|---|---|---|
| Age of patient (per 10 years) | 0.6 | 0.4–0.9 | 0.02 |
| Time since first implant (per 5 years) | 1.0 | 0.8–1.4 | 0.5 |
| Previous revision | 2.9 | 1.1–7.7 | 0.032 |
HR, hazard ratio; CI, confidence interval.
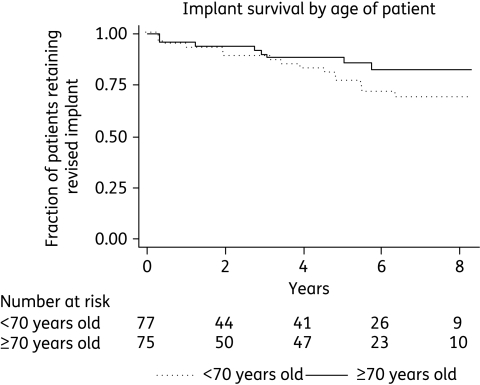
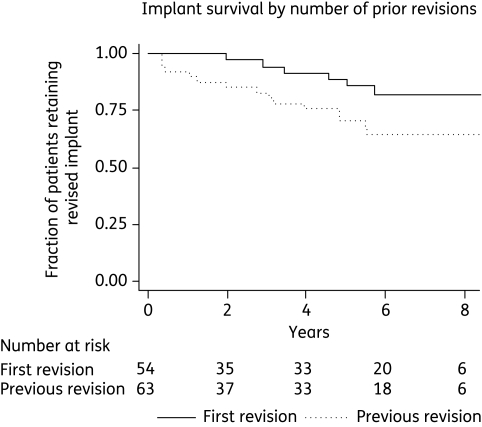
The survival plots of the two factors that remained significant on multivariate analysis show that patient age may have a constant effect over time (Figure 1), but that the effect of previous revisions on implant survival is mostly mediated in the first 6 months (Figure 2).
Figure 1.
Kaplan–Meier plot showing survival of implants by older (≥70 years) versus younger (<70 years) patient age.
Figure 2.
Kaplan–Meier plot showing survival of implants by primary implant versus previously revised implants.
Re-revisions
The overall success rate was 83%, but was 89% for first revisions and 73% for re-revisions. In addition to being associated with greater failure rates, re-revisions were also associated with increased use of muscle flaps (16% versus 4%, P = 0.03), and tendencies towards an increased rate of periprosthetic fracture (11% versus 6%, P = 0.4) and an increased rate of unplanned debridements between stages (20% versus 11%, P = 0.18). The composite complication rates for any of these events were 41% versus 20% (P = 0.015). However, none of these complications was independently associated with a worse outcome and so did not explain the worse outcome of re-revision (Table 2). Re-revision was also associated with more long-term oral antibiotic use after reimplantation (40% versus 19%, P = 0.02).
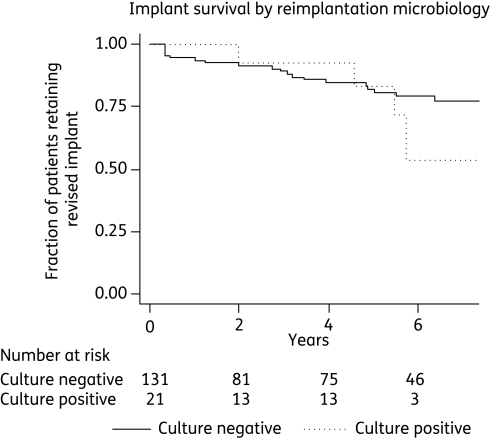
Reimplantation microbiology
Routine cultures sent at reimplantation were positive in 14% of patients. Coagulase-negative staphylococci were most commonly isolated (11%). Staphylococcus aureus (3%) and coliforms (2%) were less common. The same organism, determined by comparing species and antibiotic susceptibility patterns, was isolated at both excision and reimplantation in four cases (3%). In 10 cases (6%) a different organism was isolated and in 7 cases (5%) reimplantation cultures were positive following negative cultures at the first stage.
There was no evidence that positive reimplantation cultures were associated with worse outcome (Table 2 and Figure 3), but more antibiotics were given to these patients. Ninety-one percent of patients with positive reimplantation cultures were treated with antibiotics, compared with 18% of those with negative cultures. Very prolonged antibiotics (i.e. >1 year) were given to 57% of those with positive reimplantation cultures, compared with 12% of those with negative cultures.
Figure 3.
Kaplan–Meier plot showing survival of implants by reimplantation culture results.
Reimplantation was preceded by a 2 week antibiotic-free period in 88% of cases. Microbiology was positive in 3 of 18 patients (16%) without a 2 week antibiotic-free period, compared with 18 out of 134 (13%) with a 2 week antibiotic-free period. Reimplantation cultures were more often positive in knees than in hips (21% versus 6%, P = 0.01).
Unplanned debridements were rarely required during the antibiotic-free period (two instances only). In contrast, 25 unplanned debridements were carried out before the antibiotic-free period.
Discussion
We report 152 cases of PJI managed by two-stage revision, with an overall success rate of 83% over a median follow-up of 5.75 years. This is within the range of reported success rates,8,10,12 but is confounded by the very high rate of tertiary referrals requiring re-revisions in this cohort. Re-revision was associated with three times the risk of implant failure in time-to-event Cox regression analysis, with success rates of 89% for first revisions and 73% for re-revisions.
Re-revision was also associated with a more complicated surgical course, with a greater requirement for muscle flaps to manage soft tissue problems, more frequent unplanned debridement before reimplantation, more frequent periprosthetic fractures and more often required prolonged antibiotics after reimplantation. However, on univariate analysis, none of these factors was associated with increased failure rates except time since original surgery. On multivariate analysis, re-revision was the stronger factor. This suggests that poor outcome was associated with re-revision per se and was not confounded by these other measured factors.
Re-revision for infection was associated with poorer outcomes in previous studies. In a study of 64 two-stage revisions for patients with infected knee replacements, the success rate was 92% for first revision and 41% if previous surgery had been conducted (including previous revision).21 In a study of 24 re-revised knee replacements, only one prosthesis remained uninfected at 2 years,22 and in a study of 11 re-revised hip replacements, only 3 remained infection free.23
Our data demonstrate that re-revision surgery is more complex and less often successful than first revisions for infection. It is therefore particularly important that infection is considered and adequately managed at the first revision. Nevertheless, in our dataset we found a relatively high success rate for re-revisions (73%), using a multidisciplinary team approach, with joint revision surgeons, plastic surgeons and infectious diseases physicians using rigorous diagnostic and treatment protocols.
Younger age was also independently associated with worse outcome. This may be because symptoms of implant failure are more likely to be reported or acted on in younger patients, leading to a surveillance bias, or that the implants are exposed to more mechanical stress because of the younger patients' life styles.
Culture-negative infection has been identified as a particular challenge in managing PJI24 and it is therefore encouraging that, despite being a common problem in our cohort, it was not associated with a poorer outcome. This may be because non-cultured organisms are low-grade or present in low numbers, or were susceptible to our empirical antibiotic therapy (glycopeptides in our cohort). Previous reports of culture-negative PJI found a lower prevalence (11%),24 but this study included PJI managed by a variety of protocols. In our centre, only 3% of the more acute infections managed by DAIR were culture negative,15,19 compared with 41% in this cohort. It may be that the chronic infections referred for two-stage revisions are more likely to be culture negative. Most patients (95%) had a histological confirmation of the diagnosis of infection. Some of the organisms responsible might have been cultured had we used longer incubation periods or techniques designed to disrupt biofilms more effectively than the culture methods that we used.25,26
The Oxford hip and knee scores have not, to our knowledge, been used previously to assess outcome of revision surgery. Nevertheless, these scores are well-validated patient-reported outcome measures14 and have been adopted by the NHS in England for universal outcome assessment. Categories for the Oxford hip score have been developed based on the Harris hip score,27 suggesting >41 excellent, 34–41 good, 27–33 fair and <27 poor. However, these scores relate to outcome following primary hip replacement. Hence, a median score of 27 following a two-stage revision for infection might be considered an acceptable outcome, although we do not have baseline hip and knee scores prior to revision.
Reimplantation cultures were positive surprisingly often (14%), but the organism cultured was frequently unrelated to the organisms cultured at excision, as in previous, smaller studies.28–30 At reimplantation, knee joints were more often culture positive than hip joints, despite being less frequently culture positive at the first-stage excision. Spacers were used in all of the knee joint revisions, but rarely in hips (13%), and may contribute to the higher positive culture rate. Reimplantation microbiology did not correlate with first-stage microbiology, further suggesting that the organisms were acquired at excision. However, there seemed to be few clinical consequences of positive cultures, albeit in the context of broad-spectrum iv antibiotics prior to reimplantation, a low incidence of uncontrolled infection and antibiotic treatment of most positive cultures at reimplantation. Nevertheless, taking these findings together with our previous observations in DAIR that prolonged antibiotics beyond 6 months were of little benefit,15 it seems difficult to justify similarly prolonged courses of antibiotics for microbiological results of uncertain significance. Furthermore, our evidence does not support the use of an antibiotic-free period as a test of clinical cure, since the risk of requiring unplanned debridement was highest during the period of antibiotic use, rather than during the antibiotic-free period before reimplantation.
In conclusion, we report a success rate of 83% over a median follow-up of 5.75 years, comprising success rates of 89% for first revisions and 73% for re-revision. Culture-negative infection was common among these chronic infections treated by two-stage revision, but was not associated with a worse outcome. Cultures taken at reimplantation following an antibiotic-free period did not predict outcome and clinical failure of treatment was more often identified during antibiotic treatment than after antibiotics were stopped. Reimplantation may be considered without an antibiotic-free period, with additional antibiotic prophylaxis before reimplantation.31 Where multiple reimplantation cultures are positive in the absence of clinical indicators of ongoing infection, a limited course of oral antibiotics may be appropriate.
Funding
Specific funding was not used for this study. P. B. is funded by the Biomedical Research Centre, Oxford Radcliffe Hospitals Trust.
Transparency declarations
I. B. has received honoraria for serving on advisory boards (Pfizer) and lecture fees (Pfizer and Nordic Pharma). A. B. has received honoraria for serving on advisory boards (Pfizer and Macrochem), for speakers bureaux (Merck) and for producing sponsored non-promotional educational materials (Merck). P. M.-S. is advisor to Wright Medical Technologies and receives royalties from the Corin Group. P. B., B. L. A., N. G., H. P., S. M. and R. G. have no conflicts of interest.
References
- 1.Lavernia CJ, Guzman JF, Gachupin-Garcia A. Cost effectiveness and quality of life in knee arthroplasty. Clin Orthop Relat Res. 1997;345:134–9. [PubMed] [Google Scholar]
- 2.Chang RW, Pellisier JM, Hazen GB. A cost-effectiveness analysis of total hip arthroplasty for osteoarthritis of the hip. JAMA. 1996;275:858–65. [PubMed] [Google Scholar]
- 3.Ethgen O, Bruyere O, Richy F, et al. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86-A:963–74. doi: 10.2106/00004623-200405000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Phillips CB, Barrett JA, Losina E, et al. Incidence rates of dislocation, pulmonary embolism, and deep infection during the first six months after elective total hip replacement. J Bone Joint Surg Am. 2003;85-A:20–6. doi: 10.2106/00004623-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Sibanda N, Copley LP, Lewsey JD, et al. Revision rates after primary hip and knee replacement in England between 2003 and 2006. PLoS Med. 2008;5:e179. doi: 10.1371/journal.pmed.0050179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silva M, Tharani R, Schmalzried TP. Results of direct exchange or debridement of the infected total knee arthroplasty. Clin Orthop Relat Res. 2002;404:125–31. doi: 10.1097/00003086-200211000-00022. [DOI] [PubMed] [Google Scholar]
- 7.Zimmerli W, Ochsner PE. Management of infection associated with prosthetic joints. Infection. 2003;31:99–108. doi: 10.1007/s15010-002-3079-9. [DOI] [PubMed] [Google Scholar]
- 8.Jamsen E, Stogiannidis I, Malmivaara A, et al. Outcome of prosthesis exchange for infected knee arthroplasty: the effect of treatment approach. Acta Orthop. 2009;80:67–77. doi: 10.1080/17453670902805064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanchez-Sotelo J, Berry DJ, Hanssen AD, et al. Midterm to long-term followup of staged reimplantation for infected hip arthroplasty. Clin Orthop Relat Res. 2009;467:219–24. doi: 10.1007/s11999-008-0480-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsieh PH, Huang KC, Lee PC, et al. Two-stage revision of infected hip arthroplasty using an antibiotic-loaded spacer: retrospective comparison between short-term and prolonged antibiotic therapy. J Antimicrob Chemother. 2009;64:392–7. doi: 10.1093/jac/dkp177. [DOI] [PubMed] [Google Scholar]
- 11.Masri BA, Panagiotopoulos KP, Greidanus NV, et al. Cementless two-stage exchange arthroplasty for infection after total hip arthroplasty. J Arthroplasty. 2007;22:72–8. doi: 10.1016/j.arth.2006.02.156. [DOI] [PubMed] [Google Scholar]
- 12.Whittaker JP, Warren RE, Jones RS, et al. Is prolonged systemic antibiotic treatment essential in two-stage revision hip replacement for chronic Gram-positive infection? J Bone Joint Surg Br. 2009;91:44–51. doi: 10.1302/0301-620X.91B1.20930. [DOI] [PubMed] [Google Scholar]
- 13.Atkins BL, Athanasou N, Deeks JJ, et al. Prospective evaluation of criteria for microbiological diagnosis of prosthetic-joint infection at revision arthroplasty. The OSIRIS Collaborative Study Group. J Clin Microbiol. 1998;36:2932–9. doi: 10.1128/jcm.36.10.2932-2939.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray DW, Fitzpatrick R, Rogers K, et al. The use of the Oxford hip and knee scores. J Bone Joint Surg Br. 2007;89:1010–4. doi: 10.1302/0301-620X.89B8.19424. [DOI] [PubMed] [Google Scholar]
- 15.Byren I, Bejon P, Atkins BL, et al. One hundred and twelve infected arthroplasties treated with ‘DAIR’ (debridement, antibiotics and implant retention): antibiotic duration and outcome. J Antimicrob Chemother. 2009;63:1264–71. doi: 10.1093/jac/dkp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mirra JM, Amstutz HC, Matos M, et al. The pathology of the joint tissues and its clinical relevance in prosthesis failure. Clin Orthop Relat Res. 1976;117:221–40. [PubMed] [Google Scholar]
- 17.Feldman DS, Lonner JH, Desai P, et al. The role of intraoperative frozen sections in revision total joint arthroplasty. J Bone Joint Surg Am. 1995;77:1807–13. doi: 10.2106/00004623-199512000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Spangehl MJ, Masri BA, O'Connell JX, et al. Prospective analysis of preoperative and intraoperative investigations for the diagnosis of infection at the sites of two hundred and two revision total hip arthroplasties. J Bone Joint Surg Am. 1999;81:672–83. doi: 10.2106/00004623-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Moran E, Masters S, Berendt AR, et al. Guiding empirical antibiotic therapy in orthopaedics: the microbiology of prosthetic joint infection managed by debridement, irrigation and prosthesis retention. J Infect. 2007;55:1–7. doi: 10.1016/j.jinf.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Matthews PC, Conlon CP, Berendt AR, et al. Outpatient parenteral antimicrobial therapy (OPAT): is it safe for selected patients to self-administer at home? A retrospective analysis of a large cohort over 13 years. J Antimicrob Chemother. 2007;60:356–62. doi: 10.1093/jac/dkm210. [DOI] [PubMed] [Google Scholar]
- 21.Hirakawa K, Stulberg BN, Wilde AH, et al. Results of 2-stage reimplantation for infected total knee arthroplasty. J Arthroplasty. 1998;13:22–8. doi: 10.1016/s0883-5403(98)90071-7. [DOI] [PubMed] [Google Scholar]
- 22.Hanssen AD, Trousdale RT, Osmon DR. Patient outcome with reinfection following reimplantation for the infected total knee arthroplasty. Clin Orthop Relat Res. 1995;321:55–67. [PubMed] [Google Scholar]
- 23.Pagnano MW, Trousdale RT, Hanssen AD. Outcome after reinfection following reimplantation hip arthroplasty. Clin Orthop Relat Res. 1997;338:192–204. doi: 10.1097/00003086-199705000-00026. [DOI] [PubMed] [Google Scholar]
- 24.Berbari EF, Marculescu C, Sia I, et al. Culture-negative prosthetic joint infection. Clin Infect Dis. 2007;45:1113–9. doi: 10.1086/522184. [DOI] [PubMed] [Google Scholar]
- 25.Schafer P, Fink B, Sandow D, et al. Prolonged bacterial culture to identify late periprosthetic joint infection: a promising strategy. Clin Infect Dis. 2008;47:1403–9. doi: 10.1086/592973. [DOI] [PubMed] [Google Scholar]
- 26.Trampuz A, Piper KE, Hanssen AD, et al. Sonication of explanted prosthetic components in bags for diagnosis of prosthetic joint infection is associated with risk of contamination. J Clin Microbiol. 2006;44:628–31. doi: 10.1128/JCM.44.2.628-631.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalairajah Y, Azurza K, Hulme C, et al. Health outcome measures in the evaluation of total hip arthroplasties—a comparison between the Harris hip score and the Oxford hip score. J Arthroplasty. 2005;20:1037–41. doi: 10.1016/j.arth.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 28.Mont MA, Waldman BJ, Hungerford DS. Evaluation of preoperative cultures before second-stage reimplantation of a total knee prosthesis complicated by infection. A comparison-group study. J Bone Joint Surg Am. 2000;82-A:1552–7. doi: 10.2106/00004623-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Mittal Y, Fehring TK, Hanssen A, et al. Two-stage reimplantation for periprosthetic knee infection involving resistant organisms. J Bone Joint Surg Am. 2007;89:1227–31. doi: 10.2106/JBJS.E.01192. [DOI] [PubMed] [Google Scholar]
- 30.Murillo O, Euba G, Calatayud L, et al. The role of intraoperative cultures at the time of reimplantation in the management of infected total joint arthroplasty. Eur J Clin Microbiol Infect Dis. 2008;27:805–11. doi: 10.1007/s10096-008-0509-3. [DOI] [PubMed] [Google Scholar]
- 31.Classen DC, Evans RS, Pestotnik SL, et al. The timing of prophylactic administration of antibiotics and the risk of surgical-wound infection. N Engl J Med. 1992;326:281–6. doi: 10.1056/NEJM199201303260501. [DOI] [PubMed] [Google Scholar]