Abstract
We describe for the first time fluorescent virus-like particles decorated with biologically active mono- and multisubunit immune receptors of choice and the basic application of such fluorosomes (FSs) to visualize and target immune receptor-ligand interactions. For that purpose, human embryonic kidney (HEK)-293 cells were stably transfected with Moloney murine leukemia virus (MoMLV) matrix protein (MA) GFP fusion constructs. To produce FSs, interleukins (ILs), IL-receptors (IL-Rs), and costimulatory molecules were fused to the glycosyl phosphatidyl inositol anchor acceptor sequence of CD16b and coexpressed along with MoMLV group-specific antigen-polymerase (gag-pol) in MA::GFP+ HEK-293 cells. We show that IL-2 decorated but not control-decorated FSs specifically identify normal and malignant IL-2 receptor-positive (IL-2R+) lymphocytes by flow cytometry. In addition to cytokines and costimulatory molecules, FSs were also successfully decorated with the heterotrimeric IL-2Rs, allowing identification of IL-2+ target cells. Specificity of binding was proven by complete inhibition with nonlabeled, soluble ligands. Moreover, IL-2R FSs efficiently neutralized soluble IL-2 and thus induced unresponsiveness of T cells receiving full activation stimuli via T-cell antigen receptor and CD28. FSs are technically simple, multivalent tools for assessing and blocking mono- and multisubunit immune receptor-ligand interactions with natural constituents in a plasma membrane context.—Kueng, H. J., Manta, C., Haiderer, D., Leb, V. M., Schmetterer, K. G., Neunkirchner, A., Byrne, R. A., Scheinecker, C., Steinberger, P., Seed, B., Pickl, W. F. Fluorosomes: a convenient new reagent to detect and block multivalent and complex receptor-ligand interactions.
Keywords: lipid rafts, viral pseudotyping, glycosyl phosphatidyl inositol anchored proteins, flow cytometry, fluorescent proteins
Virus-like particles (VLPs) are noninfectious enveloped particles that can be induced in mammalian cells by the expression of viral structural proteins in the absence of viral nucleic acid. They have proven to be powerful tools for the display and transport of diverse biologically active immunomodulatory molecules (1,2,3). We have shown previously that VLPs can be engineered to modulate immune responses by judicious incorporation of additional membrane-associated proteins that sustain stimulatory or inhibitory immune responses. We have named the resulting engineered microvesicles immunosomes and anergosomes, respectively (1,2,3). Because direct interaction between leukocyte receptors and VLP-resident ligands was found to be a prerequisite for effective receptor triggering, in this study we have explored whether it would be possible to visualize the interaction between VLPs decorated with specific ligands and cells expressing the cognate receptors. We hypothesized that sufficient quantities of fluorescently labeled VLPs would be detectable once bound to cells, and that such particles might represent both a versatile staining tool and a traceable agent for the efficient modulation of immune responses.
In this study we have focused on VLPs labeled in vivo by recombinant fluorescent proteins of cnidarian origin (4,5,6) and have demonstrated their utility for the visualization of specific immune receptor-ligand interactions. Translational fusions of viral proteins with GFP have been broadly used to elucidate infectious pathways of viruses (7,8,9,10). Because lipid rafts are the meeting points for glycosyl phosphatidyl inositol (GPI)-anchored surface molecules and viral core proteins (11), we hypothesized that fluorescent proteins linked to raft-targeted viral core proteins might accumulate in sufficiently high abundance to generate fluorescently labeled VLPs that could be used to track the interactions of VLPs in different settings. For efficient decoration of VLPs, immune receptors or ligands of choice were fused at their C termini to the GPI-anchor attachment sequence of CD16b, a well-defined GPI-anchored molecule of human granulocytes (12). Previous reports have shown that GPI-anchored molecules are efficiently targeted to the lipid raft regions of producer cells and consequently to VLPs (11).
To illustrate the potential of this approach, we have chosen cytokine/cytokine-receptor interactions as a model system. Cytokines are small- to medium-sized proteins or glycoproteins that mediate potent biological programs on binding to their specific receptors (13). Cytokine receptors are differentially expressed on various cell types and can be visualized and their density determined by receptor specific mAbs. Flow cytometric detection of cytokine receptors on normal and malignant cells using mAbs has been widely applied in the past (14,15,16). However, many growth factor receptors consist of multisubunit receptors, and frequently similar receptor chains (subunits) are used by different receptors, e.g., the common γ-chain (CD132) (17), which is part of the interleukin (IL)-2, IL-4, IL-7, IL-9, IL-13, IL-15, IL-21 receptors, or the IL-2 receptor (IL-2R) β-chain, which is part of the IL-2R as well as the IL-15R (13). Thus, the identification of single receptor components does not always allow determination of overall receptor composition.
Consequently, precise enumeration and visualization of growth factor receptors has required radioligand-, affinity-, or fluorochrome-based (18, 19) binding assays with in vitro modified cytokines in the past. Modification of cytokines requires, for the most part, that the molecule of interest be available in sufficient quantity and purity. The most widely used labeling techniques rely on the existence on the target molecule of a sufficient number of reactive residues susceptible to chemical modification. Alterations to protein sidechains, which in most cases is a random process, can cause changes of the physiological or biophysical properties of the natural protein.
In addition to assessing their staining potential, we were interested in determining whether VLPs could also be decorated with more complex structures, e.g., multichain cytokine receptors, and whether such particles would bind and neutralize the respective cytokines. The potential utility of this approach has been suggested by recent reports that cytokine deprivation may be one of the mechanisms by which CD4+CD25+Foxp3+ regulatory T (Treg) cells (20) and myeloid dendritic cells (21, 22) exert their regulatory function on effector T cells.
MATERIALS AND METHODS
Cell lines and primary cells
The human embryonic kidney (HEK) epithelial cell line 293 and the murine HT-2 cell line (ATCC CRL-1841; American Type Culture Collection, Manassas, VA, USA) were maintained in IMDM (Sigma Chemicals, St. Louis, MO, USA) plus 10% FCS (Invitrogen, Carlsbad, CA, USA) supplemented with 2 mM l-glutamine, 50 μM 2-mercaptoethanol, and 15 μg/ml gentamicin sulfate. HT-2 cells were replated every 48 h at a cell density of 1 × 105 cells/ml in the presence of 100 U/ml human IL-2 (Peprotech, London, UK). BW5147 (23) and BW5147 CTLA-4 cells were described previously (24, 25) and were cultured in RPMI 1640 medium plus 10% FCS (Invitrogen), 2 mM l-glutamine, 50 μM 2-mercaptoethanol, and 15 μg/ml gentamicin sulfate. Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood of healthy adult donors on informed consent by standard density gradient centrifugation with Lymphoprep (Technoclone, Vienna, Austria). Whole-blood samples of patients with chronic lymphocytic leukemia (CLL) obtained after informed consent were collected with EDTA anticoagulant and analyzed in standard whole-blood stainings using ADG-Lyse (An der Grub, Kaumberg, Austria).
Expression constructs
The modified cytokine expression constructs have been described previously (2). Briefly, the specific cDNAs for human IL-2 and IL-7 were amplified with primers as specified in Supplemental Table 1 from clone pTCGF-11 (ATCC 39673) and pHumLP-1 (ATCC 67546), respectively. At the 5′ ends, optimized translational initiation sites preceded by a HindIII restriction site were inserted. The 3′ stop codons were replaced with a polyglycine linker sequence followed by a NheI restriction site for direct fusion to the minimal CD16b GPI-anchor acceptor sequence (IL-2R constructs) or alternatively to the CD16b GPI anchor preceded by 2 Ig-like domains of CD16 (membrane-bound cytokines; unpublished results). The DNA fragments were ligated into the pEAK12-CD80::CD16b plasmid from which the CD80 ectodomain had been released (1). Original Moloney murine leukemia virus (MoMLV) group-specific antigen-polymerase (gag-pol) (OGP) was expressed from pMD.gagpol (26) kindly provided by Dr. R. Mulligan (Childrens’ Hospital, Boston, MA, USA). IL-2R subunits were amplified by RT-PCR using mRNA from PHA plus IL-2 activated (24 h) PB T cells as template and were modified in a similar fashion (primers in Supplemental Table 1). Full-length human CD16b served as a control (12). The matrix protein (MA)::GFP fusion construct was generated by PCR using pMD.gagpol and pEAK12.GFP as a template (27). HindIII and NheI, or NheI and NotI, restriction sites incorporated in the PCR primers allowed the resulting fragments to be inserted into the pEAK12 expression plasmid at the location of the cognate sites in the vector.
Stable HEK-293 transfectants
HEK-293 cells stably expressing MA::GFP, GFP, or IL-2::GPI (single cell clones) were generated by transfection with plasmids linearized by digestion with SfiI (New England Biolabs, Ipswich, MA, USA) (28), selection in puromycin (1 μg/ml; Sigma Chemicals) and analysis by flow cytometry (FACSCalibur; Becton Dickinson, Palo Alto, CA, USA) using CellQuest software (Becton Dickinson).
Generation of GFP-labeled VLPs decorated with cytokines or costimulatory molecules [fluorosomes (FSs)]
For the generation of FSs, HEK-293 cells stably expressing MA::GFP or GFP were transiently transfected using the calcium-phosphate coprecipitation method (28). Briefly, 1 d prior to transfection, HEK-293 cells were seeded onto 100-mm cell culture dishes (3×106 cells/plate) and were transfected the following day with 2 ml transfection mix. For generation of IL-, IL-R-, or CD80-decorated FSs, HEK-293 cells were transfected with a total DNA amount of 30 μg/dish, including 7.5 μg of the MoMLV OGP encoding plasmid pMD.gagpol and 22.5 μg of the indicated IL::GPI or CD80::GPI pEAK12 expression plasmid. For generation of IL-R-decorated FSs, HEK-293 cells were transfected with 30 μg DNA/dish, including 7.5 μg pMD.gagpol and 7.5 μg of each IL-2R subunit expression plasmid. If necessary, DNA amounts were adjusted with control vector (empty pEAK12 vector or, where indicated, full-length CD16b in pEAK12). Control particles were generated by transfection of HEK-293 cells with 7.5 μg pMD.gagpol and 22.5 μg of control vector. The medium was changed 18–24 h after transfection, and transfectants were cultured for another 48 h before FS-containing supernatants were harvested.
Flow cytometric analysis of HEK-293 producer cells
For membrane staining, 5 × 105 HEK-293 cells in 50 μl were incubated at 4°C with fluorochrome-conjugated mAbs (20 μg/ml; Supplemental Table 2) for 30 min. After 2 washes with staining buffer (PBS, 1% BSA, 0.05% NaN3) membrane fluorescence was analyzed by flow cytometry. Treatment of HEK-293 cells with phosphatidyl inositol-specific phospholipase C (PI-PLC) from Bacillus thuringiensis (American Radiolabeled Chemicals, St. Louis, MO, USA) was performed according to the manufacturer’s recommendations. Briefly, HEK-293 cells were washed in PBS without Ca2+ and Mg2+ and resuspended at a concentration of 1 × 107 cells/ml in PBS. Next, 100 mU of PI-PLC was added to 1 × 107 cells, and the cells were incubated at 37°C for 2 h. Subsequently, cells were washed in PBS/1% BSA and subjected to membrane staining.
Isopycnic separation of producer cell lysates
Preparation of lipid raft fractions was performed as described previously (29). Aliquots of 20 μl of individual fractions collected from top to bottom of 5–40% sucrose gradients were analyzed by SDS-PAGE on 4–20% gradient gels. Proteins were transferred to PVDF membranes (Millipore, Billerica, MA, USA) and subjected to Western blotting using mAbs specific for IL-2 (Supplemental Table 2), p30gag, GFP, CD59, and CD147. HRP-conjugated secondary reagents (Supplemental Table 2) were used at a dilution of 1:104. Blots were developed with a luminol-based indicator system (Western Lightning; Perkin Elmer, Boston, MA, USA) and exposed to X-ray films (Eastman Kodak, Rochester, NY, USA).
Purification of VLPs/FSs
Supernatants (10 ml/culture plate) were harvested 72 h after transfection and cleared of cellular debris by filtration through 0.45-μm syringe filters (Millipore), concentrated by ultracentrifugation in a Beckman-Optima LE-80K centrifuge (Beckman Instruments, Palo Alto, CA, USA), using a SW41 Ti rotor, at 100,000 g for 1 h and washed twice in a large volume of PBS. Amounts of VLPs/FSs were determined by standard protein assay (Micro BCA-Pierce, Thermo Scientific, Rockford, IL, USA) and adjusted to protein concentrations as described in individual experiments. For functional tests, VLP/FS preparations were sterile filtered (0.22-μm syringe filters, Millipore) before use.
Confocal microscopy
HEK-293 cells stably transfected with MA::GFP or GFP were seeded at a density of 4 × 104 cells/cm2 in a PeCon POC-R cell cultivation chamber, grown overnight, and analyzed on an Axiovert 100 M confocal microscope using an ×63/1.40 oil-immersion objective (Carl Zeiss, Göttingen, Germany).
FS-based staining assays
HT-2 cells (1×105cells) were resuspended in staining buffer (PBS, 0.5% BSA, and 0.05% NaN3) and were incubated at room temperature with titrated amounts of either cytokine-decorated or nondecorated FSs (starting with 400 μg) in a total volume of 100 μl for >1 h until equilibrium binding was reached. Control stainings were performed with IL-2R α-chain-specific (CD25; Supplemental Table 2) or nonbinding control mAbs, all used at 1 μg in a total volume of 100 μl for 30 min. The ED50 value was calculated from mean fluorescence intensity values using nonlinear regression analysis fitted to classical Michaelis-Menten kinetics (Prism 5.0b; GraphPad Software, San Diego, CA, USA). Specificity of binding reactions was determined by preincubation of HT-2 cells with soluble IL-2 (5×103 U in 100 μl). Off-rates were determined by first labeling HT-2 cells (1×105) with 400 μg IL-2 decorated MA::GFP+VLPs at 4°C for >1 h and by washing them extensively. Following initial binding, HT-2 cells were chased in an excess of staining buffer (4 ml) at 4°C, room temperature, 37°C, and 37°C in the presence of 4 × 104 U soluble IL-2 (Peprotech) for 0, 15, 30, 60, and 120 min and analyzed by flow cytometry. Staining of HEK-293 IL-2R transfectants with VLPs (20 μg/1×105 cells) was performed in a similar fashion, except that control stainings were performed with mAbs against human CD25, CD122, and CD132. BW5147 CTLA-4 transfectants were control stained with a CTLA-4 mAb or negative control mAbs. Subsequently, cells were washed 2 times with 4 ml staining buffer (4°C) by centrifugation (300 g for 5 min), resuspended in 150 μl staining buffer and 20 μl propidium iodide (0.5 μg/ml), and analyzed immediately on a FACSCalibur flow cytometer supported by CellQuest software (Becton Dickinson). Activated T cells were generated by incubation of 1 × 106 PBMCs/ml with anti-mouse IgG magnetic beads (Dynabeads; Invitrogen) coated at 150 fg/bead with CD3 and CD28 mAb at a bead-to-cell ratio of 1:1. After 72 h of stimulation, cells were compared to resting cells for their binding of FSs (20 μg/1×105 cells). Activation status of T cells was determined by double staining for CD3 and CD25 or CD3 plus CD69. Peripheral blood of patients with CLL (n=10) was analyzed by the whole blood method using ADG-Lyse (An der Grub). CD19+ B lymphocytes from patient samples were immunophenotyped with a panel of diagnostic mAbs (e.g., CD5, CD20, CD23, CD25, CD43, surface Ig, surface Ig-κ, surface Ig-λ) and were reacted with IL-2-decorated GFP+ FSs or control FSs (see above). After one wash in PBS, cells were resuspended in 200 μl sheath fluid and immediately analyzed (15×103 cells) by flow cytometry (FACSCalibur, CellQuest software). Lymphocytes were identified by typical forward and right-angle scatter characteristics.
IL-2 sequestration experiments
PBMCs (105) of healthy volunteers were cocultured with αCD3/αCD28 mAb microbeads at a 1:1 ratio and with titrated amounts of IL-2R αβγ::GPI, IL-2R α::GPI, or CD16::GPI (control) expressing FSs (starting with 2 μg/well) or medium alone. Cell proliferation was determined after 4 d by [3H]-thymidine uptake. For antigen-specific experiments Art v 1-specific T cell antigen receptor (TCR) tg T cells were generated from nonallergic HLA-DR1-positive individuals as described previously (30). TCR tg T cells (1×106 cells/well) were cultured in 24-well plates with VLPs (2 μg/ml) decorated with HLA-DRA*0101, HLA-DRB1*0101, Ii::Art v 125–34, CD80::GPI, CD86::GPI, CD58::GPI, CD54::GPI, cathepsin S, or control plasmid combined as indicated or medium alone for 10 d. Proliferation of primary antigen-specific cultures was determined in replica plates containing 1 × 105 T cells and corresponding stimuli. After 10 d, cells from 24-well plates were harvested and washed, and 1 × 105 cells/well were restimulated in 96-well flat-bottom plates with αCD3/CD28 microbeads (2.5×104) alone or in the presence of IL-2 (500 U/ml) for 4 d, and proliferation was determined. Cell proliferation was determined after 4 d by [3H]-thymidine uptake.
The binding capacity of IL-2R αβγ:GPI FSs was determined with the help of radioactively labeled 125I IL-2 (Perkin Elmer). For that purpose, constant amounts of 125I IL-2 corresponding to 25 U IL-2 (i.e., 2,5 ng) were incubated at room temperature with titrated amounts of IL-2R αβγ:GPI FSs, starting from 30 μg for 1 h. FS-bound 125I IL-2 was determined (Packard Cobra II γ counter; Packard, Meriden, CT, USA) from the retentate on size-exclusion filtration using Amicon Microcon YM-100 filter units (Millipore) according to the manufacturer’s recommendations.
Alternatively, 5 ng nonlabeled IL-2 (Peprotech) was used to stimulate proliferation of HT-2 cells (5×103/well) in the presence of titrated amounts of IL-2R αβγ:GPI FSs. Inhibition of HT-2 cell proliferation was taken as a measure to determine the IL-2 binding capacity of IL-2R αβγ:GPI FSs compared to control FSs. Proliferation is expressed as [3H]-thymidine uptake after 64 h of cocultivation.
RESULTS
Generation of fluorescent VLPs
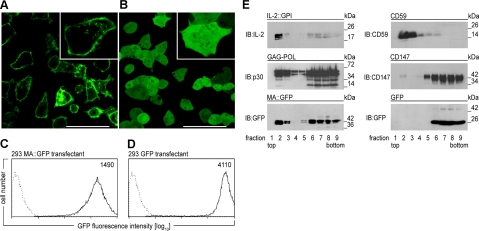
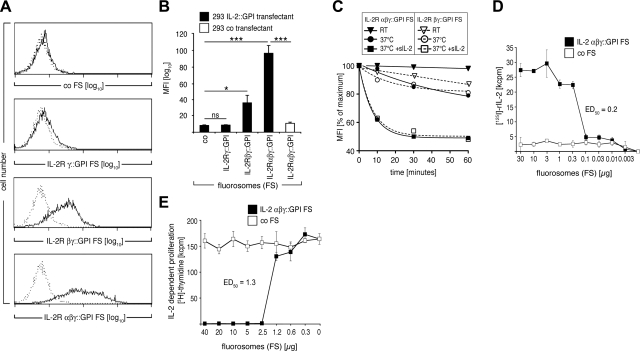
To generate VLPs with high membrane fluorescence, we amplified and fused the coding sequence of MoMLV MA and GFP (Fig. 1A). Expression of MA::GFP in HEK-293 cells resulted in pronounced membrane-associated fluorescence (Fig. 2A), whereas expression of untagged GFP led to evenly distributed cytoplasmic fluorescence (Fig. 2B). MA::GFP expression in HEK-293 cells was ∼3-fold lower than expression of soluble GFP (Fig. 2C, D). As a model ligand for testing specific interactions, we coexpressed human IL-2 fused by a polyglycine spacer to the GPI-anchor acceptor sequence of human CD16b (2) preceded by 2 CD16b Ig-like domains (Fig. 1A). Membrane fractionation experiments with HEK-293 transfectants showed that IL-2::GPI, MA::GFP, the MoMLV capsid proteins, and the GPI-anchored, constitutively expressed CD59 molecule were all targeted to the lipid raft fractions of HEK-293 cell lysates (Fig. 2E), while soluble GFP and the nonlipid raft marker molecule CD147 (31) remained excluded.
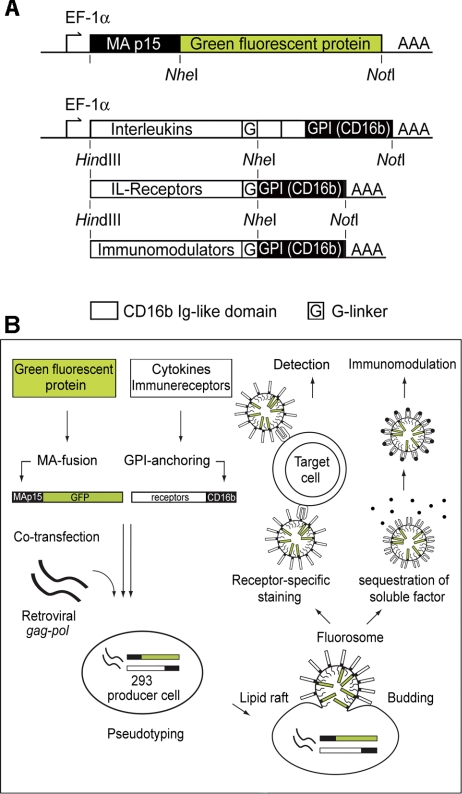
Figure 1.
A) Diagram of expression constructs. DNA sequences encoding MoMLV MA (p15) were PCR amplified and endowed with flanking HindIII and NheI restriction sites. For optimal expression levels, the consensus transcriptional initiation site GCCACC was introduced upstream of the ATG codon. The MA coding region was fused to GFP sequences previously inserted in the pEAK12 expression vector. Cytokine coding sequences were similarly PCR amplified and prepared for vector insertion. At the 3′ ends of the cytokine cDNAs, the intrinsic stop codons were replaced by a polyglycine linker sequence allowing fusion to a CD16b GPI-anchor acceptor sequence (in the case of interleukins preceded by 2 CD16b Ig-like domains) previously inserted in the pEAK12 vector. B) Production scheme for FSs. FS production is initiated by transfection of MoMLV gag-pol. FS bud from the lipid raft regions of HEK-293 producer cell membranes. MA::GFP is targeted to the lipid rafts and consequently to nascent FSs by virtue of its fusion to MA. Similarly, the cytokines, cytokine receptors, and costimulatory molecules fused to the GPI-anchor acceptor sequence of CD16b become enriched in lipid rafts and FSs. After collection and purification from HEK-293 producer cell supernatants, FSs can be used to visualize receptor-ligand interactions by standard flow cytometry and microscopy, or, alternatively, for cytokine sequestration.
Figure 2.
A, B) Analysis of HEK-293 producer cells. HEK-293 cells stably transfected with MA::GFP (A) or GFP (B) were seeded in a cell cultivation chamber, grown overnight, and analyzed by confocal microscopy (×63/1.40 oil immersion). Images show characteristic morphology and fluorescence pattern. Inset: high magnification. Scale bar = 50 μm. C, D) Flow cytometric determination of GFP expression levels in HEK-293 producer cells. Overlay histograms show green fluorescence intensity of HEK-293 cells stably expressing MA::GFP (C) or GFP (D) (solid lines) compared to native HEK-293 cells (dotted lines) as determined by flow cytometry. Numbers indicate geometric MFI. E) GPI anchor attachment or fusion to MA targets modified molecules to lipid rafts. HEK-293 cells transfected with IL-2::GPI, MoMLV gag/pol, MA::GFP, or GFP, respectively, were lysed at 4°C in Triton X-100 and fractionated on 5 to 40% sucrose gradients into 9 fractions (top to bottom). Equal amounts of each fraction were resolved by SDS-PAGE, blotted, and probed by immunoblotting (IB) with mAbs specific for IL-2 (all antibodies used are listed in Supplemental Table 2), p30Gag, GFP, CD59, or CD147, respectively. Binding of antibodies was visualized by HRP-conjugated secondary reagents followed by a luminol-based detection reaction. One of several representative experiments is shown.
Binding of IL-2-decorated VLPs to IL-2R+ target cells
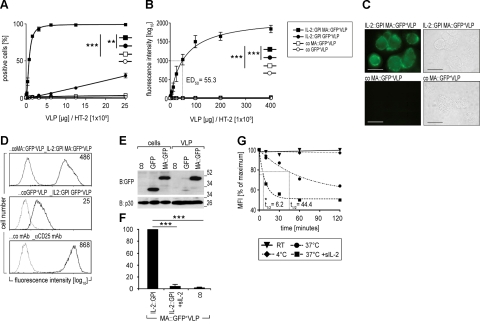
In the next set of experiments, we induced secretion of IL-2-decorated fluorescent VLPs and asked whether such particles would bind to and stain IL-2R+ cells. For that purpose, we induced particle formation by transfection (28) of either HEK-293-MA::GFP or HEK-293-GFP stable transfectants with pEAK12.IL-2::GPI and pMD.gagpol(MoMLV) (production scheme according to Fig. 1B). Subsequently, the IL-2R+ murine T-cell line HT-2 (32) was incubated with varying amounts of IL-2::GPI-decorated VLPs derived from either of the two producer cell lines (1). Binding was analyzed by flow cytometry. Background binding was assessed by comparison to fluorescence resulting from CD16b-decorated VLPs produced by either of the 2 cell lines. Binding to >95% of HT-2 cells was achieved with 4 μg (by protein mass) of MA::GFP+VLPs decorated with IL-2::GPI per 1 × 105 HT-2 cells (Fig. 3A, D), with 50% positive cells obtained with 1 μg VLPs. No binding was observed with control MA::GFP+VLPs [P=0.001; Mann Whitney (MW) U test, 2-tailed], although similar amounts of VLPs were applied (not shown). In marked contrast, IL-2::GPI-decorated VLPs from HEK-293 cells expressing soluble GFP (GFP+VLPs) produced significantly weaker signals, with only 30% positive cells at a concentration of 25 μg (P=0.002; MW U test, 2-tailed). Saturating binding with a geometric mean fluorescence intensity (geo MFI) of 1.842 was reached with 400 μg of IL-2-decorated MA::GFP+VLPs, compared to an MFI of 38 for IL-2-decorated GFP+VLPs (P<0.001; MW U test, 2-tailed; Fig. 3B), and a MFI 35 for VLPs lacking IL-2 (P<0.001; MW U test, 2-tailed). Very similar results showing clear-cut membrane pronounced fluorescence of HT-2 cells preincubated with IL-2::GPI MA::GFP+VLPs but not control MA::GFP+VLPs were obtained by fluorescence microscopy (Fig. 3C). The reduced fluorescence intensity of VLPs bearing soluble GFP compared to MA::GFP was confirmed by GFP-specific immunoblotting (Fig. 3E). Specificity of binding reactions was confirmed by preincubation of HT-2 cells with 5000 U soluble IL-2, which completely inhibited subsequent binding of IL-2 decorated MA::GFP+VLPs (P<0.001; Student’s t test, paired, 2-tailed; Fig. 3F). The interaction of IL-2-decorated MA::GFP+VLPs with HT-2 cells was remarkably stable at 4°C and room temperature (Fig. 3G). At 37°C, clear-cut dissociation rates became apparent, with a dissociation half-life t1/2 = 44.4 min in the absence and t1/2 = 6.2 min in the presence of excess amounts of of soluble IL-2 (4×104 U) used as competitor (Fig. 3G).
Figure 3.
Binding of IL-2-decorated fluorescent VLPs to IL-2R+ HT-2 cells. A, B) Dose-dependent binding. Saturation binding isotherms were generated by reacting 2-fold dilutions of the indicated amounts of purified IL-2::GPI MA::GFP+VLPs (solid squares), control MA::GFP+VLPs (open squares), IL-2::GPI GFP+VLPs (solid circles), and control GFP+VLPs (open circles) with HT-2 cells (starting with 400 μg VLPs/1×105 HT-2 cells) followed by flow cytometric analyses. Percentage positive cells (A) and geometric mean fluorescence intensity (geo MFI) (B) obtained are plotted against the VLP concentrations applied. Native HT-2 cells exhibited a geo MFI of 5 (<2% positive cells). Dotted line indicates ED50. **P < 0.01, ***P < 0.001; 2-tailed Mann Whitney U test. C) VLP-based detection of IL-2R on HT-2 cells by standard microscopy. HT-2 cells were incubated and processed with IL-2::GPI MA::GFP+VLPs or control MA::GFP+VLPs as described above. Cell morphology was determined by light microscopy (right), membrane fluorescence by fluorescence microscopy (left). Scale bar = 10 μm. D) Overlay histograms of HT-2 cells (1×105) incubated with 30 μg IL-2::GPI-MA::GFP+VLPs or control MA::GFP+VLPs (top panel), IL-2::GPI-GFP+VLPs or control GFP+VLPs (middle panel), and αCD25 mAbs or control mAbs (bottom panel). Specific fluorescence (solid lines) is compared to the respective control (dotted lines). Numbers indicate geo MFI values of specific reagents. E) Differential targeting of MA::GFP and GFP to VLPs. HEK-293 cells were transfected with the indicated expression plasmids or control plasmid and cell lysates (cells) and corresponding VLP preparations were analyzed by SDS-PAGE under reducing conditions followed by immunoblotting (IB) with a GFP-specific goat antiserum or the p30 Gag-specific mAb R187, respectively. Bound antibodies were detected by HRP-conjugated secondary reagents and a luminol-based detection system. ***P < 0.001; paired, 2-tailed Student’s t test. F) Exogenous IL-2 inhibits binding of IL-2::GPI MA::GFP+VLPs. Native HT-2 cells (1×105) or HT-2 cells preincubated with recombinant soluble human IL-2 (5×103 U) were incubated with IL-2::GPI-decorated MA::GFP+VLPs and analyzed by flow cytometry. Results show binding of IL-2::GPI-decorated MA::GFP+VLPs to IL-2 preincubated relative to native HT-2 cells (means+sd). G) Temperature-dependent dissociation of IL-2::GPI MA::GFP+VLPs from HT-2 cells. Dissociation of IL-2::GPI MA::GFP+VLPs from HT-2 cells was monitored after removing excess VLPs and chasing at 4°C, room temperature (RT), 37°C, and 37°C in the presence of 4 × 104 U soluble IL-2. MFI was determined and plotted as percentage of maximum, corresponding to the values obtained at start of chase. t1/2, dissociation half-life.
For convenience in subsequent exposition, we will identify the MA::GFP-expressing VLPs as FSs. Notably, IL-2::GPI FSs displayed a high degree of physical stability. Their initial staining activity was reduced by only 8.8 ± 3.0 and 4.0 ± 14.4% (geo MFI) on freezing and thawing or storage at 4°C for 3 wk, respectively (not shown). A rapid decline in staining intensity was evident only on storage at room temperature.
IL-2::GPI FSs discriminate between cells expressing different forms of the IL-2R
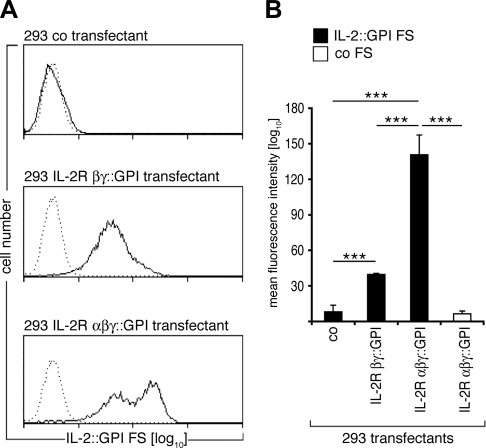
To determine whether IL-2::GPI FSs are also able to discriminate between different IL-2 receptor subunit combinations (16), we evaluated their binding to respective HEK-293 cell transfectants. Figure 4A, B shows that similar amounts of IL-2::GPI FSs derived from the same production batches bound significantly more strongly to IL-2R αβγ-transfectants than to IL-2R βγ-transfectants, and not to control transfected HEK-293 cells (P<0.001; t test), which mirrors the affinities of IL-2 for the different receptor types (16, 33). In contrast, no binding of control FSs to either of the transfectants was evident (P<0.001; t test).
Figure 4.
IL-2::GPI FSs discriminate between target cells expressing low- or high-affinity IL-2Rs. A) Overlay histograms of HEK-293 cells transfected with control plasmid (top panel), IL-2R βγ-chain (middle panel), or IL-2R αβγ-chain (bottom panel) stained with identical batches of either IL-2::GPI (solid line) or control (dotted line) FSs. B) Geometric MFI + sd values of indicated HEK-293 cell transfectants stained with IL-2::GPI (black bars) or control (white bar) FSs from 3 independently performed experiments. ***P < 0.001; paired, 2-tailed Student’s t test.
IL-2::GPI FSs identify activated IL-2R+ T lymphocytes and B non-Hodgkin lymphoma (B-NHL) CLL cells
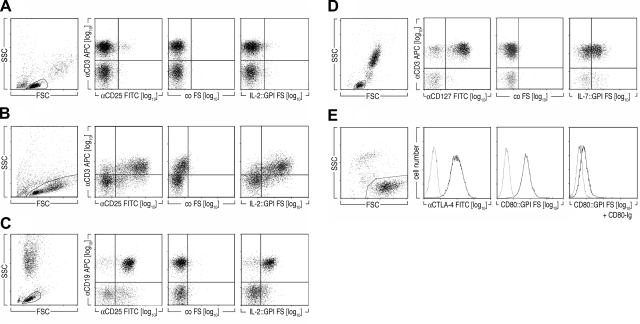
The results above suggest that IL-2::GPI FS could be used to identify distinct leukocyte subpopulations of healthy or diseased individuals. Figure 5A demonstrates that IL-2::GPI FS indeed bound to a small subset of CD3+ T lymphocytes in freshly isolated PBMCs of healthy individuals, likely representing the CD4+CD25+ Treg fraction (34). On polyclonal activation with CD3 plus CD28 coated microbeads for 48 h, binding of IL-2::GPI FSs and CD25 mAbs, but not of control FSs, to the majority of CD3+ T cells was observed (Fig. 5B).
Figure 5.
A, B) IL-2::GPI FSs identify activated T lymphocytes and CLL B cells. Two-parameter dot-plot analyses of freshly isolated (A) or activated (αCD3/αCD28 coated beads, 48 h) PBMCs (B) on incubation with IL-2::GPI FSs, control FSs, or CD25 FITC-conjugated mAbs and counterstained with a CD3 APC-conjugated mAbs. Gating for lymphocytes was performed according to typical FSC/SSC characteristics. Markers were set according to nonbinding control mAbs or control FSs. Data are representative of 3 experiments. C) Whole blood of a CLL patient was stained with IL-2::GPI FSs, control FSs, or CD25 FITC-conjugated mAbs and counterstained with a CD19 APC-conjugated mAb. Lymphocytes were gated according to typical FSC/SSC characteristics. Markers were set according to staining of nonbinding control mAbs or control FSs. Data are representative of multiple individuals (n=11). D) IL-7::GPI FSs or CD80::GPI FSs specifically bind to target cells expressing their respective receptors. Two-parameter dot-plot analyses of whole blood cells gated on lymphocytes of a representative healthy donor incubated with IL-7::GPI FSs, control FSs, or CD127 PE-conjugated mAbs and counterstained with CD3 APC-conjugated mAbs. Lymphocytes were gated according to typical FSC/SSC characteristics. Data are representative of 4 experiments. E) Overlay histograms of CTLA-4 transfected BW cells incubated with CD80::GPI FSs, control FSs, or CTLA-4 mAbs plus anti-mouse-Ig conjugated to OG are shown (bold lines). Dotted lines indicate fluorescence obtained with native BW cells. Preincubation of CTLA-4 transfected BW cells with soluble CD80-Ig fusion protein (2 μg) was used as specificity control. Data are representative of 4 experiments.
In addition to its action on T cells, IL-2 is also an important stimulatory cytokine for B lymphocytes (35, 36). Expression of IL-2R by CLL B cells is one of the distinguishing characteristics of the disease (37,38,39). IL-2::GPI FSs combined with a CD19 APC mAb could clearly identify the IL-2R+ B-cell populations in peripheral blood (Fig. 5C) and bone marrow (not shown) of CLL patients (11 of 11 patients positive). Compared to the standard staining procedure with CD25 mAbs, somewhat lower overall numbers of positive cells were identified with IL-2::GPI FSs (69.4±22.6 vs. 50.3±16.4%). However, in some patients the staining intensity obtained with IL-2::GPI FSs was identical or even exceeded that obtained with CD25 mAb. It will be interesting to explore whether the observed differential binding of IL-2::GPI FSs to CLL B cells correlates with disease progression and/or severity (40) and possibly reflects differences in receptor composition.
Generality of the FS approach shown by specific binding of IL-7::GPI FSs and CD80::GPI FSs to respective target cells
To determine whether the approach can be generalized, we decorated FSs with ligands other than IL-2 and investigated their binding characteristics to respective targets. For this we constructed IL-7::GPI (Fig. 1) and took advantage of a CD80::GPI construct described previously (1). Figure 5D shows that the IL-7::GPI FSs, unlike control FSs, specifically bound to resting CD3+ T cells (P<0.001; t test), and binding could be specifically inhibited by addition of exogenous soluble IL-7 (not shown). The IL-7R is expressed as a low (Ka∼1×10−6 M) and a high (Ka∼5×10−9 M) affinity receptor on lymphocytes (13). The clearly weaker signal obtained with IL-7::GPI FSs as compared to parallel stainings performed with IL-7R α-chain-specific mAbs might be a reflection of the differential binding of IL-7 to these receptors. Moreover, CD80::GPI FSs bound to CTLA-4 transfected (as described in ref. 25) but not to control transfected BW cells (P=0.01; t test, Fig. 5E). Addition of 2 μg soluble CD80-Ig fusion protein or blocking CD80 mAb 7–480 (not shown) inhibited the binding of CD80::GPI FSs to CTLA-4 expressing BW cells to background levels (Fig. 5E).
FS probes comprising functional multisubunit receptors
Conventional labeling approaches typically use monomeric or homomultimeric reagents to identify single epitopes on analyzed cell populations. To establish whether FS probes could be constituted from heteromultimeric reagents, we produced FSs decorated with various combinations of IL-2R chains (modified by GPI-anchor acceptor sequences according to Fig. 1) and analyzed whether they would specifically bind to target cells expressing membrane-anchored IL-2. Figure 6A, B shows that this is the case. FSs expressing the high-affinity IL-2R (IL-2R αβγ-chain) showed strong and highly significant binding to the IL-2+ target cells when compared to control FSs (P<0.001; t test). FSs decorated with the low-affinity IL-2R (IL-2R βγ-chain) revealed intermediate but still significant binding (P=0.03; t test), whereas IL-2R γ-chain decorated FSs similar to the control FSs displayed no significant binding (P=0.2; t test). In contrast to the staining intensities, no significant differences in dissociation rates between IL-2R αβγ::GPI FSs and IL-2R βγ::GPI FSs in the presence or absence of excess amounts of soluble IL-2 (4×104 U) used as competitor were detectable (Fig. 6C). To obtain a more accurate estimate also of the valency of FSs we have resorted to binding studies using IL-2R FSs and 125I-labeled IL-2 as the ligand. In parallel experiments the IL-2 binding capacity of IL-2R αβγ::GPI FSs was tested in functional assays using HT-2 cell proliferation as the indicator system. The experiments with 125I-labeled IL-2 revealed that 1 μg of IL-2R αβγ::GPI FSs bind 6.25 ng IL-2 (Fig. 6D). Assuming a mean mass for C-type retrovirus particles of 6.5 × 107 Da (41), this would indicate that one IL-2R αβγ::GPI FS has ∼26 binding sites for 125I-IL-2. The functional experiments revealed a 3.25-fold lower IL-2 binding capacity, i.e., ∼8 sites per FS (Fig. 6E). However, these experiments had a much longer duration than the binding assays (64 vs. 1 h) and had to be carried out at 37°C (as opposed to room temperature for binding assays), presumably causing considerably higher off-rates (Fig. 3G) making more IL-2 bioavailable. In fact, the obtained data are well in line with our previous findings, in which we detected up to 15 IL-2 molecules on one VLP as determined by immuno-gold staining followed by electron microscopy (2).
Figure 6.
FSs decorated with complex combinations of the IL-2R bind to target cells expressing membrane-bound IL-2 and dose-dependently bind soluble IL-2. A) Overlay histograms of HEK-293 cells expressing membrane-bound IL-2::GPI (single cell clone), which were incubated with control FSs (top panel), IL-2R γ-chain::GPI FSs (second panel), IL-2R βγ-chain::GPI FSs (third panel), or IL-2R αβγ-chain::GPI FSs (bottom panel) (bold lines). Dotted lines represent fluorescence of control transfected HEK-293 cells incubated with the respective FSs. B) Geometric mean + sd fluorescence values of IL-2::GPI (black bars) or control (white bar) HEK-293 transfectants stained with the indicated FSs from 3 independently performed experiments. *P < 0.05, ***P < 0.001; paired, 2-tailed Student’s t test. ns, P > 0.05. C) Dissociation kinetics of IL-R FSs from IL-2+ target cells. Dissociation of IL-2R αβγ::GPI FSs and IL-2R βγ::GPI from IL-2+ HEK-293 cells was monitored after removing excess FSs and chasing at RT, 37°C, and 37°C in the presence of 4 × 104 U soluble IL-2. MFI was determined and plotted as percentage of maximum, corresponding to the values obtained at start of chase. D) Binding capacity of IL-2R αβγ::GPI FSs. D) Constant amounts of 125I IL-2 (2.5 ng) were incubated with titrated amounts of IL-2R αβγ::GPI FSs or control FSs for 1 h at RT. Subsequently, FSs were collected by size-exclusion filtration and FS-bound 125I IL-2 determined by γ-counting. E) Constant amounts of soluble IL-2 (5 ng) were incubated with titrated amounts of IL-2R αβγ::GPI FSs or control FSs and added to 5 × 103 HT-2 cells cultured in 96-well plates. After 48 h, [3H]- thymidine was added; cells were harvested 16 h later, and cell proliferation was determined. Data are mean ± sd values of triplicate cultures (n=2). ED50, effective dose 50%.
IL-2 αβγ-chain::GPI FSs neutralize soluble IL-2 and thereby induce novel functional T-cell programs
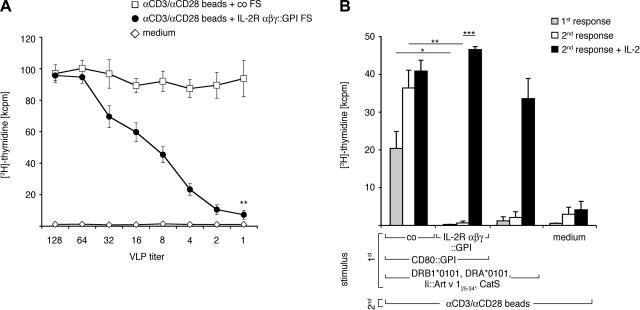
We also examined whether IL-2R-decorated FSs are capable of neutralizing soluble IL-2. Recently IL-2 neutralization/depletion has been shown to be one of the effector mechanisms of Treg cells (20). To determine whether FSs decorated with IL-2R components are able to interfere with T cell activation programs, we stimulated PBMCs of healthy individuals with microbeads coated with CD3 and CD28 mAbs in the presence of IL-2R αβγ-chain::GPI+FSs or control FSs. In marked contrast to control FSs, high concentrations of IL-2R αβγ-chain::GPI+FSs almost completely abrogated T-cell proliferation. (Fig. 7A). Next we asked whether a similar inhibition would take place after antigen-specific T-cell stimulation (3) and whether IL-2 deprivation from T cells receiving a full activation stimulus would render T cells unresponsive during secondary stimulation provided by αCD3 plus αCD28-coated microbeads. Figure 7B shows that allergen-specific T cells react with VLPs expressing Art v 1 in the context of HLA-DR1 and CD80 in both primary and secondary T-cell stimulation assays. In contrast, the presence of IL-2R αβγ-chain::GPI FSs during primary stimulation not only significantly inhibited the primary, antigen-specific but also completely abrogated secondary polyclonal T-cell responses (Fig. 7B, P<0.05 and P<0.01, respectively, t test). T cells did, however, survive the FS-mediated IL-2 deprivation, because they proliferated well on addition of exogenous IL-2 (500 U/ml, P<0.001, t test). Similar results were obtained with antigen-specific T cells receiving only antigen stimulation during the primary stimulation, which is in line with our previous results (3), and served as a control. Thus, immune-receptor-decorated FSs bind and sequester their respective ligands, shaping the evolution of responses in T cells exposed to these agents.
Figure 7.
A) IL-2R αβγ::GPI+FSs neutralize proliferation of polyclonally activated PB T cells. PBMCs (105) were cocultured with αCD3/αCD28 mAb-substituted microbeads and varying amounts of IL-2R αβγ::GPI or CD16::GPI (control) expressing FSs (starting with 2 μg/well) or medium alone. Cell proliferation was determined after 4 d by [3H]-thymidine uptake. Data are mean ± sd values of triplicate cultures (n=3). **P < 0.01 vs. control; paired, 2-tailed Student’s t test. B) Induction of antigen-specific unresponsiveness by FSs. Art v 1 allergen-specific Vα17/Vβ18 TCR tg PB T cells (105) were cultured with the indicated primary stimuli, and proliferation was measured by [3H]-thymidine incorporation for the last 12 h of a 72-h incubation (gray bars). Proliferation of T cells obtained on culture with indicated stimuli for 10 d, washed, and recultured with αCD3/αCD28 mAb-substituted microbeads in the absence (white bars) or presence (black bars) of exogenous IL-2 (500 U/ml). Data show one representative experiment (n=3). *P < 0.05, **P < 0.01, ***P < 0.001; paired, 2-tailed Student’s t test. kcpm, kilocounts per minute.
DISCUSSION
In this work, we introduce a technology for the facile preparation of analytical reagents that have high ligand affinity and functional activity. In addition to their specific binding to cell surface receptors, FSs are also able to sequestrate soluble ligands, such as IL-2, with high efficiency and thereby induce altered cellular programs in T cells that have been activated via their TCR and CD28 molecules.
Production of FSs relies on the preferential incorporation of the MoMLV MA::GFP fusion protein as fluorochrome into the lipid rafts of HEK-293 producer cells. Coexpressed immune receptors/ligands of choice can be targeted to FSs by attachment of a GPI anchor. FSs can be produced quickly (within 4 d) and in large amounts (9.8±4.5 μg/ml producer cell supernatant; not shown). The production procedure relies on transfection of the easy-to-handle HEK-293 epithelial cell line (42) with an optimized Ca2PO4-precipitation method (28). The strong fluorochrome accumulation in VLPs allows direct visualization of fluorescence without the need for secondary reagents, which makes the FS staining procedure quick, typically completed within 1 h. In this study, normal and malignant lymphocyte subsets could be identified by IL-2+FSs. FSs containing membrane-targeted GFP were significantly more efficient than FSs containing soluble GFP. The generality of the approach was demonstrated by successful application of ligands other than IL-2, i.e., IL-7 and the type I, or single-pass, transmembrane T-cell surface expressed costimulatory molecule CD80.
In contrast to labeled antibodies, usually identifying single receptor subunits, FSs directly measure receptor-ligand interactions between natural interaction partners and thereby offer the possibility of a more accurate assessment of activity of the targeted receptor. Interleukin receptor components such as the IL-2R α-chain (CD25) and IL-7R α-chain (CD127) are differentially represented among human T-cell subsets (43), and it has been reported that the CD4+CD25hiCD127lo (IL-2RαhiIL-7Rαlo) phenotype identifies natural Treg cells (43). Determination of IL-2R composition in Treg cells based on both IL-2+FSs and CD25 monoclonal antibodies might lead to new insights into the biology of Treg function, a possibility suggested by a recent report that cell types expressing partial IL-2R with regulatory function can be identified (22). It should also be interesting to explore the possibility of establishing multicolor FSs for double or triple staining purposes in the future. The recently described “fruit” colors developed by Tsien and coworkers (44) and relying on Discosoma sp. appear to be interesting candidates in this respect.
Another potential application of FSs is the characterization of so far unknown receptor-ligand interactions. As shown here, the VLP platform can accommodate multisubunit ligands or receptors, which are otherwise not easy to express in the appropriate configuration. The membrane environment of VLPs should also permit the convenient expression of type III integral membrane proteins, which pass the plasma membrane 2 to 7 times (45) and are associated with lipid rafts (46, 47), for staining purposes and ligand screens. The multivalent nature of FSs as such might facilitate the detection of low-affinity interactions between monomeric receptor-ligand pairs.
Reagents that can neutralize biological activity are frequently useful, and antibodies can often be found with this capacity, although antibodies rarely achieve the extremely high binding affinity exhibited by natural receptors (48). These studies have shown that IL-2R αβγ-chain::GPI+FSs very effectively sequester IL-2 from T-cell cultures on polyclonal and antigen-specific activation. This culminates in a nearly complete inhibition of T-cell proliferation. Similar results have been obtained with nonfluorescent IL-2R αβγ-chain+VLPs (not shown). T cells exposed to these FSs were unresponsive when restimulated in secondary cultures. The state of unresponsiveness could, however, be overcome by the addition of exogenous IL-2. This seems to be of special interest, because Treg cells have been shown to exert their regulatory function on activated effector CD4+ T cells (among other mechanisms) by binding and neutralizing of IL-2 and other common γ-chain (γc, CD132)-associated cytokines (20). Our results indicate that 1) IL-2R αβγ-chain::GPI FSs not only bind but also functionally neutralize soluble IL-2; 2) IL-2R αβγ-chain::GPI FSs have functional capabilities usually associated with regulatory cells; and 3) the regulatory function of FSs is independent of classical transmembrane signaling events, because the intracellular and transmembrane regions of the individual IL-2R chains have been exchanged by GPI-anchor acceptor sequences. An advantage of the VLP platform over other forms of binding reagents is their ability to accommodate several different functionally active molecules at a time, which potentially allows complex regulatory instructions to be imparted to a given cell by a single type of particle. Because FSs are functionally inert otherwise (compared to intact cells), the effects of their administration to target populations are more easily interpreted.
We here describe for the first time fluorescent VLPs decorated with biologically active mono- and multisubunit immune receptors of choice and the basic application of such FSs to visualize and functionally block immune receptor-ligand interactions. Because of their simple generation and broad applicability, FSs might open new avenues in various fields of basic immunological research as well as for diagnostic purposes.
Supplementary Material
Acknowledgments
The authors thank Dr. Otto Majdic (Institute of Immunology, Medical University of Vienna, Vienna, Austria) for providing mAbs. This work was supported by the Austrian Science Foundation (grant SFB F1816-B13); the Österreichische Forschungsförderungsgesellschaft (grant 812079); Biomay (Vienna, Austria); and the Christian Doppler Society. B.S. was supported by grants from the U.S. National Institutes of Health.
References
- Derdak S V, Kueng H J, Leb V M, Neunkirchner A, Schmetterer K G, Bielek E, Majdic O, Knapp W, Seed B, Pickl W F. Direct stimulation of T lymphocytes by immunosomes: virus-like particles decorated with T cell receptor/CD3 ligands plus costimulatory molecules. Proc Natl Acad Sci U S A. 2006;103:13144–13149. doi: 10.1073/pnas.0602283103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kueng H J, Leb V M, Haiderer D, Raposo G, Thery C, Derdak S V, Schmetterer K G, Neunkirchner A, Sillaber C, Seed B, Pickl W F. General strategy for decoration of enveloped viruses with functionally active lipid-modified cytokines. J Virol. 2007;81:8666–8676. doi: 10.1128/JVI.00682-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leb V M, Jahn-Schmid B, Kueng H J, Schmetterer K G, Haiderer D, Neunkirchner A, Fischer G F, Hartl A, Thalhamer J, Steinberger P, Bohle B, Seed B, Pickl W F. Modulation of allergen-specific T-lymphocyte function by virus-like particles decorated with HLA class II molecules. J Allergy Clin Immunol. 2009;124:121–128. doi: 10.1016/j.jaci.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Shimomura O, Johnson F H, Saiga Y. Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J Cell Comp Physiol. 1962;59:223–239. doi: 10.1002/jcp.1030590302. [DOI] [PubMed] [Google Scholar]
- Haas J, Park E C, Seed B. Codon usage limitation in the expression of HIV-1 envelope glycoprotein. Curr Biol. 1996;6:315–324. doi: 10.1016/s0960-9822(02)00482-7. [DOI] [PubMed] [Google Scholar]
- Heim R, Tsien R Y. Engineering green fluorescent protein for improved brightness, longer wavelengths and fluorescence resonance energy transfer. Curr Biol. 1996;6:178–182. doi: 10.1016/s0960-9822(02)00450-5. [DOI] [PubMed] [Google Scholar]
- Larson D R, Johnson M C, Webb W W, Vogt V M. Visualization of retrovirus budding with correlated light and electron microscopy. Proc Natl Acad Sci U S A. 2005;102:15453–15458. doi: 10.1073/pnas.0504812102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingen Y, Conzelmann K K, Finke S. Double-labeled rabies virus: live tracking of enveloped virus transport. J Virol. 2008;82:237–245. doi: 10.1128/JVI.01342-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauber R H, Rulong S, Palm G, Tarasova N I. Direct visualization of HIV-1 entry: mechanisms and role of cell surface receptors. Biochem Biophys Res Commun. 1999;258:695–702. doi: 10.1006/bbrc.1999.0511. [DOI] [PubMed] [Google Scholar]
- Warrington K H, Jr, Gorbatyuk O S, Harrison J K, Opie S R, Zolotukhin S, Muzyczka N. Adeno-associated virus type 2 VP2 capsid protein is nonessential and can tolerate large peptide insertions at its N terminus. J Virol. 2004;78:6595–6609. doi: 10.1128/JVI.78.12.6595-6609.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickl W F, Pimentel-Muinos F X, Seed B. Lipid rafts and pseudotyping. J Virol. 2001;75:7175–7183. doi: 10.1128/JVI.75.15.7175-7183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D, Seed B. The Fc gamma receptor of natural killer cells is a phospholipid-linked membrane protein. Nature. 1988;333:568–570. doi: 10.1038/333568a0. [DOI] [PubMed] [Google Scholar]
- Mire-Sluis A R, Thorpe R. San Diego, CA, USA: Academic Press; Cytokines. 1998 [Google Scholar]
- Hoshino S, Oshimi K, Tsudo M, Miyasaka M, Teramura M, Masuda M, Motoji T, Mizoguchi H. Flow cytometric analysis of expression of interleukin-2 receptor beta chain (p70–75) on various leukemic cells. Blood. 1990;76:767–774. [PubMed] [Google Scholar]
- Lanza F, Castagnari B, Rigolin G, Moretti S, Latorraca A, Ferrari L, Bardi A, Castoldi G. Flow cytometry measurement of GM-CSF receptors in acute leukemic blasts, and normal hemopoietic cells. Leukemia. 1997;11:1700–1710. doi: 10.1038/sj.leu.2400794. [DOI] [PubMed] [Google Scholar]
- Waldmann T A. The structure, function, and expression of interleukin-2 receptors on normal and malignant lymphocytes. Science. 1986;232:727–732. doi: 10.1126/science.3008337. [DOI] [PubMed] [Google Scholar]
- Takeshita T, Asao H, Ohtani K, Ishii N, Kumaki S, Tanaka N, Munakata H, Nakamura M, Sugamura K. Cloning of the gamma chain of the human IL-2 receptor. Science. 1992;257:379–382. doi: 10.1126/science.1631559. [DOI] [PubMed] [Google Scholar]
- Harel-Bellan A, Mishal Z, Willette-Brown J, Farrar W L. Detection of low and high affinity binding sites with fluoresceinated human recombinant interleukin-2. J Immunol Methods. 1989;119:127–133. doi: 10.1016/0022-1759(89)90389-x. [DOI] [PubMed] [Google Scholar]
- Jadus M R, Horansky E, Wepsic H T. Detection of interleukin 2 receptors on murine lymphocytes using fluorescent interleukin 2. Immunol Lett. 1992;34:127–133. doi: 10.1016/0165-2478(92)90238-j. [DOI] [PubMed] [Google Scholar]
- Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo M J. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8:1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- Driesen J, Popov A, Schultze J L. CD25 as an immune regulatory molecule expressed on myeloid dendritic cells. Immunobiology. 2008;213:849–858. doi: 10.1016/j.imbio.2008.07.026. [DOI] [PubMed] [Google Scholar]
- Von Bergwelt-Baildon M S, Popov A, Saric T, Chemnitz J, Classen S, Stoffel M S, Fiore F, Roth U, Beyer M, Debey S, Wickenhauser C, Hanisch F G, Schultze J L. CD25 and indoleamine 2,3-dioxygenase are up-regulated by prostaglandin E2 and expressed by tumor-associated dendritic cells in vivo: additional mechanisms of T-cell inhibition. Blood. 2006;108:228–237. doi: 10.1182/blood-2005-08-3507. [DOI] [PubMed] [Google Scholar]
- Kappler J W, Skidmore B, White J, Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas: lack of independent antigen and H-2 recognition. J Exp Med. 1981;153:1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita S, Su L, Amano M, Timmerman L A, Kaneshima H, Nolan G P. The T cell activation factor NF-ATc positively regulates HIV-1 replication and gene expression in T cells. Immunity. 1997;6:235–244. doi: 10.1016/s1074-7613(00)80326-x. [DOI] [PubMed] [Google Scholar]
- Pfistershammer K, Klauser C, Pickl W F, Stockl J, Leitner J, Zlabinger G, Majdic O, Steinberger P. No evidence for dualism in function and receptors: PD-L2/B7-DC is an inhibitory regulator of human T cell activation. Eur J Immunol. 2006;36:1104–1113. doi: 10.1002/eji.200535344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ory D S, Neugeboren B A, Mulligan R C. A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc Natl Acad Sci U S A. 1996;93:11400–11406. doi: 10.1073/pnas.93.21.11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin J M, Hughes S H, Varmus H E. Cold Spring Harbor, NY, USA: Cold Spring Harbor Laboratory Press; Retroviruses. 1997 [PubMed] [Google Scholar]
- Jordan M, Schallhorn A, Wurm F M. Transfecting mammalian cells: optimization of critical parameters affecting calcium-phosphate precipitate formation. Nucleic Acids Res. 1996;24:596–601. doi: 10.1093/nar/24.4.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier R, Brennan T, Li Q, McCormack C, Seed B. Membrane compartmentation is required for efficient T cell activation. Immunity. 1998;8:723–732. doi: 10.1016/s1074-7613(00)80577-4. [DOI] [PubMed] [Google Scholar]
- Leb V M, Jahn-Schmid B, Schmetterer K G, Kueng H J, Haiderer D, Neunkirchner A, Fischer G F, Nissler K, Hartl A, Thalhamer J, Bohle B, Seed B, Pickl W F. Molecular and functional analysis of the antigen receptor of Art v 1-specific helper T lymphocytes. J Allergy Clin Immunol. 2008;121:64–71. doi: 10.1016/j.jaci.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Noisakran S, Dechtawewat T, Avirutnan P, Kinoshita T, Siripanyaphinyo U, Puttikhunt C, Kasinrerk W, Malasit P, Sittisombut N. Association of dengue virus NS1 protein with lipid rafts. J Gen Virol. 2008;89:2492–2500. doi: 10.1099/vir.0.83620-0. [DOI] [PubMed] [Google Scholar]
- Watson J. Continuous proliferation of murine antigen-specific helper T lymphocytes in culture. J Exp Med. 1979;150:1510–1519. doi: 10.1084/jem.150.6.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb R J, Greene W C, Rusk C M. Low and high affinity cellular receptors for interleukin 2. Implications for the level of Tac antigen. J Exp Med. 1984;160:1126–1146. doi: 10.1084/jem.160.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Pure E, Isakson P C, Takatsu K, Hamaoka T, Swain S L, Dutton R W, Dennert G, Uhr J W, Vitetta E S. Induction of B cell differentiation by T cell factors. I. Stimulation of IgM secretion by products of a T cell hybridoma and a T cell line. J Immunol. 1981;127:1953–1958. [PubMed] [Google Scholar]
- Swain S L, Wetzel G D, Soubiran P, Dutton R W. T cell replacing factors in the B cell response to antigen. Immunol Rev. 1982;63:111–128. doi: 10.1111/j.1600-065x.1982.tb00413.x. [DOI] [PubMed] [Google Scholar]
- Armitage R J, Cawley J C. Normal and certain leukaemic B cells express IL-2 receptors without in vitro activation. Clin Exp Immunol. 1986;63:298–302. [PMC free article] [PubMed] [Google Scholar]
- Jaffe E S, Harris N L, Stein H, Vardiman J W. Lyon, France: IARC Press; World Health Organization Classification of TumoursPathology and Genetics of Tumours of Heamatopoietic and Lymphoid Tissues. 2001 [Google Scholar]
- Hallek M, Cheson B D, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, Hillmen P, Keating M J, Montserrat E, Rai K R, Kipps T J. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montillo M, Hamblin T, Hallek M, Montserrat E, Morra E. Chronic lymphocytic leukemia: novel prognostic factors and their relevance for risk-adapted therapeutic strategies. Haematologica. 2005;90:391–399. [PubMed] [Google Scholar]
- Yu F, Joshi S M, Ma Y M, Kingston R L, Simon M N, Vogt V M. Characterization of Rous sarcoma virus Gag particles assembled in vitro. J Virol. 2001;75:2753–2764. doi: 10.1128/JVI.75.6.2753-2764.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F L, Smiley J, Russell W C, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander S I, Nanan R, Kelleher A, Fazekas de St Groth B. Expression of interleukin (IL) -2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaner N C, Campbell R E, Steinbach P A, Giepmans B N, Palmer A E, Tsien R Y. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol. 2004;22:1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- Zola H, Swart B, Nicholson I, Voss E. Hoboken, NJ, USA: John Wiley & Sons; Leucocyte and Stromal Cell MoleculesThe CD Markers. 2007 [Google Scholar]
- Claas C, Stipp C S, Hemler M E. Evaluation of prototype transmembrane 4 superfamily protein complexes and their relation to lipid rafts. J Biol Chem. 2001;276:7974–7984. doi: 10.1074/jbc.M008650200. [DOI] [PubMed] [Google Scholar]
- Yashiro-Ohtani Y, Zhou X Y, Toyo-Oka K, Tai X G, Park C S, Hamaoka T, Abe R, Miyake K, Fujiwara H. Non-CD28 costimulatory molecules present in T cell rafts induce T cell costimulation by enhancing the association of TCR with rafts. J Immunol. 2000;164:1251–1259. doi: 10.4049/jimmunol.164.3.1251. [DOI] [PubMed] [Google Scholar]
- Mosmann T R, Bond M W, Coffman R L, Ohara J, Paul W E. T-cell and mast cell lines respond to B-cell stimulatory factor 1. Proc Natl Acad Sci U S A. 1986;83:5654–5658. doi: 10.1073/pnas.83.15.5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.