Abstract
Sickle cell trait (HbAS) associates with impaired urinary concentration, hematuria, and renal papillary necrosis, but its prevalence among African Americans with ESRD is unknown. We performed a cross-sectional study reviewing available hemoglobin phenotypes for 188 of 206 adult African-American patients receiving renal replacement therapy in four dialysis units. Results from the state newborn screening program in corresponding counties provided the local population prevalence of sickle trait among African Americans. Compared with the general African-American population, HbAS was twice as common among African Americans with ESRD (15% versus 7%, P < 0.001). Prevalence of hemoglobin C trait (HbAC) was similarly more common (5% versus 2%, P < 0.01). The higher prevalence of HbAS and HbAC in the ESRD population raises the possibility that these hemoglobinopathies contribute to a decline in kidney function, either alone or in conjunction with other known risk factors for renal disease. The potential effect of HbAS on the development and progression of CKD and its effect on the course and management of patients with ESRD deserve further study.
Sickle cell trait (HbAS) is present in 7 to 9% of African Americans1,2 and has typically been described as a benign carrier state with little effect on the health of affected individuals. Although uncommon, several adverse effects of HbAS have been reported in settings of low oxygen tension or high oxygen demand, including splenic infarction at high altitude, sudden death with extreme physical exertion, venous thromboembolism, and glaucoma from anterior chamber hemorrhage.1,3–7 The low oxygen content of the renal medulla provides a propitious setting for intravascular sickling. HbAS has been associated with fewer and disrupted vessels of the vasa recta,8,9 which likely translates clinically to the highly prevalent impaired urinary concentration.3,6,7,10,11 Renal microvascular obstruction also occurs with HbAS, presenting most frequently as asymptomatic hematuria and most dramatically as renal papillary necrosis.3,6,7,10,11The rare renal medullary carcinoma is seen almost exclusively among HbAS patients.6,11,12
In epidemiologic studies, the presence of HbAS has been associated with microalbuminuria and proteinuria, particularly among diabetic men,13,14 and African Americans with autosomal dominant polycystic kidney disease (ADPKD) and HbAS have been shown to progress to ESRD more rapidly than those without the trait.15 With its associated structural and physiologic changes, HbAS could adversely affect renal function, especially in the setting of comorbid disease, and may represent a potential risk factor for kidney disease.16,17 We postulated that HbAS may be more common among African Americans with ESRD and examined the prevalence of HbAS in a group of African Americans receiving renal replacement therapy.
Hemoglobin phenotyping was performed on 188 of the 206 African-American patients receiving treatment through four of our affiliated dialysis centers, 172 of whom were receiving hemodialysis and 21 receiving peritoneal dialysis. We obtained newborn hemoglobinopathy screening results in the corresponding three North Carolina counties from the inception of the newborn screening program to the planned date of the study. This included 6729 African-American individuals born between January 1, 1994 and November 30, 2008 who were used to determine the local population prevalence.
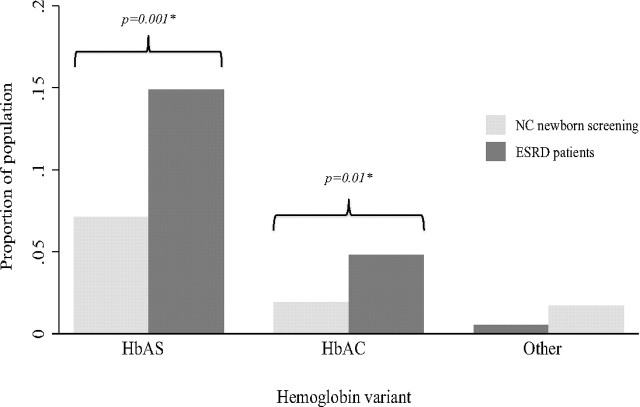
Among the tested African-American ESRD patients, 28 (14.9%) patients had HbAS, 9 (4.8%) were heterozygous for hemoglobin C [hemoglobin C trait (HbAC)], and 1 (0.5%) was heterozygous for β-thalassemia (β-thalassemia minor). In comparison, the local population prevalence among screened newborns was 7.1% (P < 0.001) for HbAS and 1.9% for HbAC (P < 0.01) (Figure 1).
Figure 1.
Prevalence of hemoglobin variants from North Carolina newborn screening data and among ESRD patients. North Carolina screening data from newborn screening of African-American live births (n = 6729) from January 1, 1994 to November 30, 2008 were obtained for the three counties in which African-American ESRD patients (n = 188) from four dialysis clinics were based. *Fisher's exact test with Bonferroni correction for repeat testing. HbAS, sickle cell trait; HbAC, hemoglobin C trait.
We also sought to determine if there were any major differences among the ESRD patients when separated by hemoglobin phenotype (Table 1). Mean age for the entire group was 58.5 (SD 14.6) years, with similar values obtained among all groups. Gender distribution and age of ESRD onset did not differ among the variants of hemoglobin. Median dialysis vintage was greater in both groups with variant hemoglobin by approximately 2.5 years (P = 0.05). Most patients were using in-center hemodialysis as their chosen modality for renal replacement therapy.
Table 1.
Characteristics of patients receiving renal replacement therapy by hemoglobin phenotypea
| Characteristic | HbAAb (%) (n = 150) | HbAS (%) (n = 28) | HbAC (%) (n = 9) | P |
|---|---|---|---|---|
| Age, years (SD) | 58.4 (14.8) | 59.8 (12.5) | 59.7 (16.8) | 0.9 |
| Gender, n (SD) | 0.9 | |||
| female | 71 (47.3) | 13 (46.4) | 5 (55.6) | |
| male | 79 (52.7) | 15 (53.6) | 4 (44.4) | |
| Age of ESRD onset, years (SD) | 54.1 (15.5) | 54.0 (13.8) | 54.5 (18.6) | 1.0 |
| Dialysis vintage, median years (interquartile range) | 3.0 (1.2 to 5.8) | 5.5 (2.2 to 8.5) | 5.5 (1.8 to 6.5) | 0.05 |
| Modality | 0.8 | |||
| in-center hemodialysis | 137 (91.3) | 25 (89.3) | 9 (100) | |
| peritoneal dialysis | 13 (8.7) | 3 (10.7) | 0 | |
| Cause of ESRDc | 0.1 | |||
| diabetes mellitus | 59 (39.3) | 10 (35.7) | 6 (66.7) | |
| hypertension | 52 (34.7) | 15 (53.6) | 1 (11.1) | |
| GN | 24 (16.0) | 1 (3.6) | 0 | |
| cystic disease | 3 (2.0) | 0 | 0 | |
| other | 12 (8.0) | 2 (7.1) | 2 (22.2) |
aOne patient heterozygous for β-thalassemia (β-thalassemia minor, β-thalassemia trait) is not included here. This patient was a 32 year-old female on hemodialysis with hypertension as cause of ESRD and onset of ESRD at age 22.
bHbAA, normal adult hemoglobin phenotype.
cAs reported in Medicare CMS-2728 form.
Differences were noted in ascribed cause of ESRD, although these did not reach statistical significance (P = 0.1). Diabetes mellitus was the cause of ESRD in over one third of patients with normal adult hemoglobin phenotype and HbAS and two thirds of patients with HbAC. More patients with HbAS had hypertension as their cause of ESRD. Only one (4%) patient with HbAS had GN, compared with 24 (16%) from the portion of patients with normal adult hemoglobin phenotype. Only three patients in the entire cohort were identified as having cystic kidney disease, all of whom had normal hemoglobin phenotypes. The one 32 year-old female patient with β-thalassemia minor and hypertensive ESRD at age 22 was not included in the above analyses.
In this study of 188 African-American patients receiving dialysis, we found that the prevalence of HbAS was more than twice that of the general population, present in one in seven ESRD patients. We also found that HbAC was more common in the dialysis patients.
To our knowledge, this study is the first to evaluate the prevalence of these hemoglobinopathies among African-American ESRD patients relative to an appropriate reference population. Our analysis, which was based on an a priori hypothesis, is exploratory in nature. HbAS, as examined in a group of prevalent ESRD patients, could be a risk factor or risk indicator for ESRD or merely associated at a statistical level. Acknowledging that we cannot exclude residual confounding, we propose potential mechanisms for our observation.
First, HbAS may directly lead to loss of renal function. Episodic sickling could lead to chronic parenchymal ischemia and eventually fibrotic changes as reported in autopsy and biopsy studies.18 Although not proven to be causative, the presence of HbAS has even been reported in the setting of GN.19,20 Second, HbAS could accelerate the effects of another process. Medullary structural and physiologic abnormalities provide a background pathology in which a primary disease would have an accelerated effect. Although HbAS may not be sufficient to cause ESRD by itself, it could contribute to progression of renal insufficiency to ESRD in the presence of additional factors, such as diabetes or hypertension. As noted previously, microalbuminuria and proteinuria have been reported in higher frequency among diabetic men with HbAS,14 further suggesting this possibility.
If HbAS is a cofactor for renal disease, identifying HbAS in patients otherwise at risk for chronic kidney disease could be important for preventive measures. Overt sickle cell disease (HbSS) is associated with lesions of glomerular hypertension, and angiotensin converting enzyme inhibitors improve proteinuria in these patients.21 Similar pathology may occur in the HbAS state and identifying these patients would allow aggressive monitoring for albuminuria and early intervention with antiproteinuric agents.
The presence of abnormal hemoglobin, particularly HbAS, may also affect the course and care of ESRD patients. HbAS may be an independent risk factor among African Americans for venous thromboembolism1,5 and could add to the already high risk for pulmonary embolism among ESRD patients.22 Additionally, this predisposition for thrombosis could affect arteriovenous fistula failure and access loss, already known to occur at higher rates among African Americans.23 African Americans with ESRD also appear to require larger doses of erythropoiesis stimulating agents (ESAs) to achieve their hemoglobin targets.24 Results of the Correction of Hemoglobin and Outcomes in Renal Insufficiency study suggested higher target hemoglobin may be associated with more cardiovascular outcomes,25 and adverse outcomes were seen primarily in those who failed to reach their target hemoglobin.26 The presence of HbAS and other hemoglobinopathies may play a role in this relative resistance, placing these patients at higher risk with increasing ESA exposure.
Heterozygosity for HbAC was also twice as common in this cohort compared with the geographically matched African-American population and to the reported national prevalence.2 Hemoglobin C is more likely to precipitate, and when present with hemoglobin S it leads to a syndrome similar to but less severe than HbSS disease. Heterozygosity is thought to be clinically silent.27 The elevated prevalence of HbAC in our cohort is of unclear significance but may have similar consequences as those of HbAS.
Of note, mean age of onset of ESRD in our study population was similar among all groups, whereas dialysis vintage was higher in the groups with either hemoglobinopathy by a median of 2.5 years. The small sample size and borderline statistical significance limit our ability to conjecture a potential explanation or draw meaningful conclusions from this difference. However, the greater length of vintage, in part, could influence our assessment of prevalence. The extended presence of those with hemoglobinopathies in this ESRD population could increase the prevalence of HbAS when surveyed in a cross-sectional manner.
The findings of this study must be interpreted in the context of several limitations. Primarily, the assessment of population prevalence of hemoglobinopathies via newborn screening results may not be accurately reflective of the population from which our ESRD cohort is derived. North Carolina newborn screening results include births occurring after 1994, and our cohort is of a different era. Any large migration into or out of these regions would lead to prevalence discrepancies between the time of our cohort and the advent of newborn screening. However, because our measured prevalence is similar to that found in the general African-American population nationally,1,2 we do feel confident in our assessment of the baseline population prevalence.
Our findings may not translate to all African-American ESRD populations. The aforementioned study of African-American ADPKD patients15 did include an assessment of HbAS in ESRD patients without ADPKD, finding it in only 6 of their 80 patients (7.5%). Although the local prevalence of HbAS was not evaluated, this discrepancy from our study does implicate that our findings should be confirmed in another cohort, perhaps larger and more geographically diverse.
Lastly, the cross-sectional design prevents the determination of the exact nature of the observed associations. Initial cross-sectional findings are primarily useful for informing subsequent prospective studies. The size of the cohort is also relatively small and geographically compact, such that any familial clustering might provide an exaggeration of the prevalence.
The prevalence of abnormal hemoglobin, HbAS and HbAC in this African-American ESRD cohort, was found to be over twice that of the baseline African-American population in the same geographic region. The high prevalence of these hemoglobinopathies suggests that they may contribute to progression to ESRD by providing a background of renal injury. Our findings also raise questions as to how the presence of HbAS or HbAC may affect management of ESRD patients. Longevity of hemodialysis access and response to ESAs may be altered. Although these results require confirmation, knowledge of a patient's hemoglobin status may be important to the management of ESRD patients and before ESRD may even identify individuals who would benefit from aggressive interventions to attenuate progression of renal disease.
Concise Methods
Study Design
We used a cross-sectional design to determine the prevalence of HbAS and other hemoglobin variants among African-American patients with ESRD. Hemoglobin status was determined in June 2008 for the patients attending four University of North Carolina-affiliated dialysis centers as part of their clinical anemia evaluation. All patients receiving hemodialysis in three of the units and all patients receiving hemodialysis or peritoneal dialysis at the fourth unit were included in this evaluation. Blood samples were obtained with the routine monthly laboratory studies. We determined patient-specific characteristics including race, age, gender, modality of dialysis, and date of initial dialysis from review of administrative data from each dialysis center. All African-American patients with available hemoglobin phenotype results were included in our study of prevalence. Age of onset of ESRD was calculated by subtracting the patient's date of birth from the date of first-ever dialysis. Vintage was calculated from the time of first-ever dialysis to July 1, 2008, the first day of the month after hemoglobin electrophoreses were collected. Cause of ESRD was obtained in a similar review and reflected the etiology provided in the Medicare CMS-2728 form. We categorized these causes into a schema similar to that used in the U.S. Renal Data System reporting.28 The University of North Carolina Institutional Review Board approved the extraction of all administrative data and laboratory study results.
The population prevalence of HbAS was determined from hemoglobinopathy screening results of the newborn screening program conducted by the North Carolina State Laboratory of Public Health. Screening for hemoglobinopathies was extended to all newborns in North Carolina in 1994. Specimens are typically collected via heel stick to filter paper and are performed within 2 to 3 days of birth. Data for the three counties in which the four dialysis units are based were used to estimate the population prevalence. All available data for live African-American births from the initiation of the screening program to the time of this study were included (January 1, 1994 to November 30, 2008).
Laboratory Studies
All studies for the ESRD patients were performed at the same laboratory site (Laboratory Corporation of America [LabCorp], Raritan, NJ). Hemoglobin variants were identified using HPLC. The North Carolina State Laboratory of Public Health ascertained hemoglobin status for newborn screening in its own laboratory first by isoelectric focusing; all abnormal variants were then confirmed by HPLC.
Statistical Analysis
In evaluating the prevalence of hemoglobin variants, proportions of variants in the ESRD cohort were compared with those proportions from the newborn screening population data using Fisher's exact test. We evaluated characteristics of ESRD patients using ANOVA for continuous measures and Fisher's exact test for categorical measures. Kruskal–Wallis testing was used for nonparametric variables. A sole patient with β-thalassemia minor in the ESRD group was not included in these comparisons because a single observation would be inappropriate to include in these analyses. Two-sided hypothesis testing with α = 0.05 was used for these statistical inferences. For those categorical variables with multiple groups that were found to have statistically significant differences, Fisher's exact test was repeated to compare specific group pairs. We adjusted the level of significance for these repeated measures using Bonferroni correction (<0.025 for a second level of testing). All statistical analyses were performed using Stata 10.1 (StataCorp, College Station, TX).
Disclosures
None.
Acknowledgments
The authors thank Stephanie McCollum, RD, LDN; Treva Williams, RN; Zoe Davison of Carolina Dialysis and Renal Research Institute; and Patricia Atwood and Patrick Fleming of the North Carolina State Laboratory of Public Health for their assistance in compiling our data. The authors also thank the members of the University of North Carolina K30 Clinical Scholars Program for their initial review of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Time to Recognize an Overlooked Trait,” on pages 385–386.
References
- 1. Heller P, Best WR, Nelson RB, Becktel J: Clinical implications of sickle-cell trait and glucose-6-phosphate dehydrogenase deficiency in hospitalized black male patients. N Engl J Med 300: 1001–1005, 1979 [DOI] [PubMed] [Google Scholar]
- 2. Schneider RG, Hightower B, Hosty TS, Ryder H, Tomlin G, Atkins R, Brimhall B, Jones RT: Abnormal hemoglobins in a quarter million people. Blood 48: 629–637, 1976 [PubMed] [Google Scholar]
- 3. Sears DA: Sickle cell trait. In: Sickle Cell Disease: Basic Principles and Clinical Practice, edited by Embury SH, Hebbel RP, Mohandas N, Steinberg MH. New York, Raven Press, 1994: 381–394 [Google Scholar]
- 4. Kark JA, Posey DM, Schumacher HR, Ruehle CJ: Sickle-cell trait as a risk factor for sudden death in physical training. N Engl J Med 317: 781–787, 1987 [DOI] [PubMed] [Google Scholar]
- 5. Austin H, Key NS, Benson JM, Lally C, Dowling NF, Whitsett C, Hooper WC: Sickle cell trait and the risk of venous thromboembolism among blacks. Blood 110: 908–912, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Tsaras G, Owusu-Ansah A, Boateng FO, Amoateng-Adjepong Y: Complications associated with sickle cell trait: A brief narrative review. Am J Med 122: 507–512, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Mitchell BL: Sickle cell trait and sudden death—Bringing it home. J Natl Med Assoc 99: 300–305, 2007 [PMC free article] [PubMed] [Google Scholar]
- 8. Statius van Eps LW, Pinedo-Veels C, de Vries GH, de Koning J: Nature of concentrating defect in sickle-cell nephropathy: Microradioangiographic studies. Lancet 1: 450–452, 1970 [DOI] [PubMed] [Google Scholar]
- 9. Statius van Eps LW, Earley LE: The kidney in sickle cell disease. In: Strauss and Welt's Diseases of the Kidney, 3rd Ed., edited by Earley LE, Gottschalk CW. Philadelphia, W.B. Saunders, 1979: 1229–1240 [Google Scholar]
- 10. Ataga KI, Orringer EP: Renal abnormalities in sickle cell disease. Am J Hematol 63: 205–211, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Scheinman JI: Sickle cell disease and the kidney. Nat Clin Pract Nephrol 5: 78–88, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Davis CJ, Jr, Mostofi FK, Sesterhenn IA: Renal medullary carcinoma. The seventh sickle cell nephropathy. Am J Surg Pathol 19: 1–11, 1995 [DOI] [PubMed] [Google Scholar]
- 13. Sesso R, Almeida MA, Figueiredo MS, Bordin JO: Renal dysfunction in patients with sickle cell anemia or sickle cell trait. Braz J Med Biol Res 31: 1257–1262, 1998 [DOI] [PubMed] [Google Scholar]
- 14. Ajayi AA, Kolawole BA: Sickle cell trait and gender influence type 2 diabetic complications in African patients. Eur J Intern Med 15: 312–315, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Yium J, Gabow P, Johnson A, Kimberling W, Martinez-Maldonado M: Autosomal dominant polycystic kidney disease in blacks: Clinical course and effects of sickle-cell hemoglobin. J Am Soc Nephrol 4: 1670–1674, 1994 [DOI] [PubMed] [Google Scholar]
- 16. Norris K, Nissenson AR: Race, gender, and socioeconomic disparities in CKD in the United States. J Am Soc Nephrol 19: 1261–1270, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Norris K, Mehrotra R, Nissenson AR: Racial differences in mortality and ESRD. Am J Kidney Dis, 52: 205–208, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wolfe HL, Rios MA: Hypertension, proteinuria, and segmental renal parenchymal fibrosis with sickle cell trait. Tex Med 75: 60–62, 1979 [PubMed] [Google Scholar]
- 19. Nicholson GD, Amin UF, Brooks SE, Alleyne GA: End-stage renal failure in sickle cell trait. West Indian Med J 28: 235–239, 1979 [PubMed] [Google Scholar]
- 20. Ozawa T, Mass MF, Guggenheim S, Strauss J, McIntosh RM: Autologous immune complex nephritis associated with sickle cell trait: Diagnosis of the haemoglobinopathy after renal structural and immunological studies. BMJ 1: 369–371, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Falk RJ, Scheinman J, Phillips G, Orringer E, Johnson A, Jennette JC: Prevalence and pathologic features of sickle cell nephropathy and response to inhibition of angiotensin-converting enzyme. N Engl J Med 326: 910–915, 1992 [DOI] [PubMed] [Google Scholar]
- 22. Casserly LF, Dember LM: Thrombosis in end-stage renal disease. Sem Dial 16: 245–256, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Gibson KD, Gillen DL, Caps MT, Kohler TR, Sherrard DJ, Stehman-Breen CO: Vascular access survival and incidence of revisions: A comparison of prosthetic grafts, simple autogenous fistulas, and venous transposition fistulas from the United States Renal Data System Dialysis Morbidity and Mortality Study. J Vasc Surg 34: 694–700, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Lacson E, Rogus J, Teng M, Lazarus JM, Hakim RM: The association of race with erythropoietin dose in patients on long-term hemodialysis. Am J Kid Dis 52: 1104–1114, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D: Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 355: 2085–2098, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Szczech LA, Barnhart HX, Inrig JK, Reddan DN, Sapp S, Califf RM, Patel UD, Singh AK: Secondary analysis of the CHOIR trial epoetin-alpha dose and achieved hemoglobin outcomes. Kidney Int 74: 791–798, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wintrobe MM, Greer JP: Wintrobe's Clinical Hematology, Philadelphia, Lippincott Williams & Wilkins, 2004 [Google Scholar]
- 28. U.S. Renal Data System: USRDS 2008 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2008 [Google Scholar]