Abstract
Alternative macrophage activation is associated with exacerbated disease in murine models of pulmonary cryptococcosis. The present study evaluated the efficacy of interferon-γ transgene expression by Cryptococcus neoformans strain H99γ in abrogating alternative macrophage activation in infected mice. Macrophage recruitment into the lungs of mice after infection with C. neoformans strain H99γ was comparable with that observed in mice challenged with wild-type C. neoformans. However, pulmonary infection in mice with C. neoformans strain H99γ was associated with reduced pulmonary fungal burden, increased pulmonary Th1-type and interleukin-17 cytokine production, and classical macrophage activation as evidenced by increased inducible nitric oxide synthase expression, histological evidence of enhanced macrophage fungicidal activity, and resolution of inflammation. In contrast, progressive pulmonary infection, enhanced Th2-type cytokine production, and the induction of alternatively activated macrophages expressing arginase-1, found in inflammatory zone 1, Ym1, and macrophage mannose receptor were observed in the lungs of mice infected with wild-type C. neoformans. These alternatively activated macrophages were also shown to harbor highly encapsulated, replicating cryptococci. Our results demonstrate that pulmonary infection with C. neoformans strain H99γ results in the induction of classically activated macrophages and promotes fungal clearance. These studies indicate that phenotype, as opposed to quantity, of infiltrating macrophages correlates with protection against pulmonary C. neoformans infection.
Cryptococcus neoformans is an opportunistic fungal pathogen and frequent cause of life-threatening infection in individuals with suppressed cell-mediated immunity.1 C. neoformans is the most common mycological agent of morbidity and mortality in patients with AIDS with acute mortality rates ranging between 10 and 25% in developed countries worldwide.2 Infection is initiated after the inhalation of desiccated basidiospores or yeast into lung alveoli, resulting in asymptomatic disease or mild bronchopneumonia in immunocompetent individuals.1 However, bronchial infection is severe in immunocompromised patients and often leads to dissemination, resulting in severe meningoencephalitis. As inhalation is the principal route of entry for C. neoformans, clearance from the lungs is largely dependent on the ability of resident alveolar macrophages to degrade the yeast cells, thereby preventing dissemination.
Experimental murine models of pulmonary C. neoformans infection suggest that resolution of infection is associated with the induction of Th1-type cytokine responses characterized by the production of interleukin (IL)-2, IL-12, tumor necrosis factor-α, and interferon (IFN)-γ.3,4,5,6,7,8,9,10,11 These cytokines, in turn, induce lymphocyte and phagocyte recruitment and activation of anticryptococcal delayed-type hypersensitivity responses. In contrast, uncontrolled fungal growth and exacerbation of pulmonary cryptococcosis is associated with Th2-type cytokine responses and the generation of alternatively activated macrophages (aaMac).4,11,12 Specifically, aaMac are induced in high IL-4/IL-13 environments and are thought to contribute to pulmonary pathology by a variety of means.12,13,14,15 First, aaMac up-regulate genes that increase cryptococcal persistence within macrophages, including arginase-1 (Arg1) and the macrophage mannose receptor (CD206). Up-regulation of Arg1 decreases synthesis of fungicidal nitric oxide by competing with inducible nitric oxide synthase (iNOS) for the substrate l-arginine.16 Increased surface expression of CD206 results in increased phagocytosis but is accompanied by decreased intracellular killing and TNF-α production.17,18,19,20 Second, aaMac up-regulate proteins implicated in pulmonary pathology, such as chitinase family proteins Ym1, Ym2, and AMCase as well as found in inflammatory zone 1 (FIZZ1) protein.4,12,13 Pulmonary C. neoformans infection in C57BL/6 mice is characterized by enhanced alternative macrophage activation and disease progression.4,13 Interestingly, C57BL/6 mice deficient in IFN-γ develop augmented Th2-type cytokine production and the induction of aaMac during pulmonary C. neoformans infection.4 Moreover, IL-13 promotes aaMac differentiation, Th2-type cytokine responses, and allergic inflammation during experimental pulmonary cryptococcosis in mice.11 Thus, alternative macrophage activation has a clear role in promoting progressive cryptococcal disease.4,15,21
Experimental pulmonary infection with the wild-type C. neoformans strain H99 results in fatal outcomes associated with overexuberant Th2-type cytokine responses in a variety of mouse models.12,22 In contrast, experimental pulmonary infection of BALB/c mice with a C. neoformans strain H99 engineered to produce murine IFN-γ (designated H99γ) results in the induction of Th1-type cytokines as well as a significant influx of T cells, granulocytes, and antigen-presenting cells into the lungs.23 However, the effect of IFN-γ transgene expression by C. neoformans strain H99γ on the macrophage activation profile in infected lungs remains unknown. The objective of these studies was to determine the activation phenotype of macrophages elicited in response to pulmonary infection with C. neoformans strain H99γ compared with that observed in mice infected with the wild-type C. neoformans.
Materials and Methods
Mice
Female BALB/c (H-2d) mice, 4 to 6 weeks of age (National Cancer Institute/Charles River Laboratories, Wilmington, MA), were used in these studies. Mice were housed at The University of Texas at San Antonio Small Animal Laboratory vivarium and handled according to guidelines approved by the institutional animal care and use committee.
Strains and Media
C. neoformans strains H99 (serotype A, Mat α) and H99γ (an interferon-γ producing C. neoformans strain derived from H9923) were recovered from 15% glycerol stocks stored at −80°C before use in the experiments described herein. The strains were maintained on yeast extract-peptone-dextrose (YPD) medium (BD Diagnostic Systems, Sparks, MD). Yeast cells were grown for 14 to 16 hours at 30°C with shaking in YPD broth (BD Diagnostic Systems), collected by centrifugation, and washed three times with sterile PBS, and viable yeast was quantified using trypan blue dye exclusion in a hemacytometer.
Murine Model
Pulmonary C. neoformans infections were initiated by nasal inhalation as described previously.24,25 In brief, BALB/c mice were anesthetized with 2% isoflurane using a rodent anesthesia device (Eagle Eye Anesthesia, Jacksonville, FL) and then given a yeast inoculum of 1 × 104 colony forming units (CFU) of C. neoformans strains H99 or H99γ in 50 μl of sterile PBS pipetted directly into the nares. The inocula used for nasal inhalation were verified by quantitative culture on YPD agar. The mice were fed ad libitum and monitored by inspection twice daily. Mice were euthanized on predetermined days after inoculation, and lung tissues were excised using aseptic technique, homogenized in 1 ml of sterile PBS, and cultured by 1:10 dilutions on YPD agar supplemented with chloramphenicol (Mediatech, Inc., Herndon, VA). CFU were enumerated after incubation at 30°C for 48 hours.
Pulmonary Leukocyte Isolation
Lungs were excised on days 3, 7, and 14 postinoculation and digested enzymatically at 37°C for 30 minutes in 10 ml of digestion buffer (RPMI 1640 and 1 mg/ml of collagenase type IV [Sigma Chemical Co., St. Louis, MO]) with intermittent (every 10 minutes) stomacher homogenizations. The enzymatically digested tissues were then successively filtered through sterile nylon filters of various pore sizes (70 and 40 μm) (BD Biosciences, San Jose, CA) and washed with sterile Hanks’ balanced salt solution to enrich for leukocytes. Erythrocytes were lysed by incubation in NH4Cl buffer (0.859% NH4Cl, 0.1% KHCO3, and 0.0372% Na2EDTA [pH 7.4], Sigma Chemical Co.) for 3 minutes on ice followed by a 10-fold excess of PBS. The resulting leukocyte population was then collected by centrifugation (800 × g for 5 minutes, washed twice with sterile PBS, resuspended in sterile PBS containing 2% heat-inactivated fetal bovine serum (fluorescence-activated cell sorting [FACS] buffer) and enumerated in a hemacytometer using trypan blue dye exclusion. For gene expression analysis, the leukocyte population was enriched for macrophages by positive selection using magnetic beads labeled with CD11b antibody according to the manufacturer’s recommendations (Miltenyi Biotec, Auburn, CA).
Antibodies
For flow cytometry experiments, rat anti-mouse CD16/CD32 (Fc Block) (BD Pharmingen, San Diego, CA), rat anti-mouse CD45 (BD Pharmingen) conjugated to phycoerythrin (PE), rat anti-mouse F4/80 conjugated to allophycocyanin (Caltag Laboratories, Burlingame, CA) or to phycoerythrin (eBioscience, San Diego, CA), rat anti-mouse IL-4 conjugated to PE-Cy7 (eBioscience), and IL-13 conjugated to Alexa Fluor 647 (eBioscience) were used. For immunohistochemistry experiments, rabbit anti-mouse Arg1 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), rat anti-mouse CD206 (macrophage mannose receptor) (AbD Serotec, Raleigh, NC), rat anti-mouse Ym1 (R&D Systems, Minneapolis, MN), rat anti-mouse F4/80 (AbD Serotec), and rabbit anti-mouse iNOS (Axxora, LLC, San Diego, CA) were used. Primary antibodies were detected using appropriate fluorescence isothiocyanate-conjugated goat anti-rat IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) or goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories, Inc.) secondary antibodies.
Flow Cytometry
Standard methodology was used for the direct and indirect immunofluorescence of pulmonary leukocytes. In brief, in 96-well U-bottom plates 1 × 106 leukocyte-enriched lung cells were incubated with Fc Block in 50 μl of PBS for 5 minutes to block nonspecific binding of antibodies to cellular Fc receptors. Subsequently, optimal concentrations of CD45 and F4/80 antibodies were added to allow for dual staining in 50 μl of FACS buffer. After 30 minutes of incubation on ice, the cells were washed three times with FACS buffer and then were fixed in 200 μl of 2% ultrapure formaldehyde (Polysciences, Inc., Warrington, PA). Cells were incubated with either FACS buffer alone or single fluorochrome-conjugated antibodies to determine positive staining and spillover/compensation calculations, and background fluorescence was determined by the flow cytometer.
Leukocytes were also stained for intracellular cytokine expression. Cells were initially labeled with anti-F4/80 antibodies as outlined above and fixed with 100 μl of 2% ultrapure formaldehyde (Polysciences, Inc.) at room temperature for 10 minutes. The cells were then permeabilized using 0.1% saponin (Sigma Chemical Co.) for 10 minutes at room temperature, and optimal concentrations of anti-IL-4 and IL-13 antibodies were added. Cells were incubated with intracellular antibodies at 4°C for 30 minutes. After staining, the cells were washed three times with 0.1% saponin and then fixed in 200 μl of 2% ultrapure formaldehyde. Samples were analyzed using the software provided with a BD FACSArray flow cytometer (BD Pharmingen). Dead cells were excluded on the basis of forward angle and 90° light scatter. For data analyses, 30,000 events (cells) were evaluated, and the absolute number of CD45+/F4/80+ or the percentage of F4/80+/IL-4, and F4/80+/IL-13 cells was calculated.
Real-Time PCR
Total RNA was isolated from purified CD11b+ cells using TRIzol reagent (Invitrogen, Carlsbad, CA) and then DNase (Invitrogen)-treated to remove possible traces of contaminating DNA according to the manufacturer’s instructions. First-strand cDNA was synthesized from 1 μg of total RNA using the oligo(dT) primer, and reagents supplied in the SuperScript III RT Kit (Invitrogen) according to the manufacturer’s instructions. The cDNA was used as a template for analysis by real-time PCR using the TaqMan Gene Expression Assay (Applied Biosystems, Foster City, CA) according to manufacturer’s instructions. All real-time PCR reactions were performed using the 7300 Real-Time PCR System (Applied Biosystems). For each real-time PCR reaction, a master mix was prepared on ice with TaqMan Gene Expression Assays specific for iNOS, IFN-γ, Ym1, FIZZ1, Arg1, IL-4, IL-13, IL-17, and CD206 (Applied Biosystems). TaqMan rodent glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Applied Biosystems) was used as an internal control. The thermal cycling parameters contained an initial denaturing cycle of 95°C for 10 minutes followed by 40 cycles of 95°C for 15 seconds and 60°C for 60 seconds. Results of the real-time PCR data were derived using the comparative Ct method as described previously26,27,28 to detect relative gene expression. The parameter Ct is defined as the cycle number at which the amplification plot passes a fixed threshold above baseline. Each reaction was run in triplicate in separate wells and normalized to a control endogenous gene, GAPDH. The following formula was used to quantify the fold differential expression of a specific gene in a macrophage-enriched population after pulmonary inoculation with C. neoformans strain H99γ compared with wild-type infected mice: 2−ΔΔCt, where ΔΔCt = [Ct H99γ sample − Ct GAPDH of H99γ sample] − [Ct H99 sample − Ct GAPDH of H99 sample]. Ct represents the mean Ct value of each sample in triplicate. Therefore, the result represents the fold increase or decrease in the expression of the gene in question after infection with C. neoformans strain H99γ compared with wild-type C. neoformans.
Immunohistochemistry and Histology
Mice were continuously sedated at predetermined time points using 2% isoflurane as stated previously. Lungs were perfused with sterile PBS by transcardial perfusion through the right ventricle. The pericardium and trachea were exposed by dissection and an incision was made in the trachea for the insertion of a sterile flexible cannula attached to a 3-ml syringe. The lungs were then slowly inflated with 0.5 to 0.7 ml of a Tissue-Tek optimal cutting temperature (OCT) compound (Sakura Finetek, Torrance, CA)/2 mol/L sucrose (1:1, v/v) solution. The lungs were then excised and immediately preserved in cryomolds containing OCT medium on dry ice and stored at −80°C until use.
Serial frozen tissue sections were cut at a thickness of 10 μm and fixed at −20°C in acetone for 10 minutes. Tissue sections were then stained using H&E (The University of Texas Health Sciences Center at San Antonio Histology and Immunohistochemistry Laboratory) or further processed for immunohistochemical analysis to visualize the leukocyte infiltration. Tissue sections were placed in cold (−20°C) 70% ethanol for 5 minutes and washed in PBS for 3 minutes. Nonspecific binding was inhibited by blocking for 30 minutes at room temperature with serum from the same species from which the fluorochrome-conjugated antibodies were derived. Tissue sections were incubated overnight at 4°C with primary antibodies diluted in species-specific serum (3% in PBS) at preoptimized concentrations. Subsequently, the sections were washed seven times in Tris-NaCl Tween 20 (TNT) buffer solution for 3 minutes each time. Sections were then incubated with secondary antibodies for 30 minutes at room temperature. Slides were then washed seven times in TNT buffer for 3 minutes each time, one time in PBS containing 1% Triton X to minimize background fluorescence (3 minutes) and a final wash in TNT buffer (3 minutes). Sections were then mounted with FluorSave reagent (Calbiochem, La Jolla, CA) containing 0.3 μmol/L 4′,6′-diamidino-2-phenylindole dilactate (DAPI) (Molecular Probes, Eugene, OR). Fluorescence was visualized with a Leica DMR epifluorescence microscope (Leica Microsystems, Wetzlar, Germany). Images were acquired using a cooled SPOT RT charge-coupled device camera (Diagnostic Instruments Inc., Sterling Heights, MI).
Cytokine Analysis
Cytokine production in lung tissues was analyzed using the Bio-Plex Protein Array System (Luminex-based technology) (Bio-Rad Laboratories, Hercules, CA). In brief, lung tissue was excised and homogenized in ice-cold sterile PBS (1 ml). An aliquot (50 μl) was taken to quantify the pulmonary fungal burden, and an anti-protease buffer solution (1 ml) containing PBS, protease inhibitors (inhibiting cysteine, serine, and other metalloproteinases) and 0.05% Triton X-100 was added to the homogenate that was then clarified by centrifugation (800 × g) for 5 minutes. Pulmonary homogenates were assayed undiluted for cytokine production using the Bio-Plex Protein Array System.
Statistical Analysis
An unpaired Student’s t-test (two-tailed) using GraphPad Prism (version 5.00 for Windows, GraphPad Software, Inc., San Diego, CA) was used to detect statistically significant differences. Statistically significant differences were defined as P < 0.05.
Results
Effect of IFN-γ Transgene Expression by C. neoformans Strain H99γ on Pulmonary Fungal Burden and Macrophage Recruitment
Previous results in our laboratory have demonstrated that BALB/c mice given an experimental pulmonary infection with C. neoformans strain H99γ, a transgenic strain expressing murine IFN-γ, were able to resolve the acute infection and generate protective immunity against a second infection with wild-type C. neoformans. 23,29 To verify a reduction in fungal load and to assess macrophage recruitment during C. neoformans strain H99γ infection, BALB/c mice were given an intranasal inoculation with 1 × 104 CFU of either C. neoformans strain H99γ or wild-type C. neoformans strain H99, and the pulmonary fungal burden was quantified on days 3, 7, and 14 postinoculation. Figure 1A demonstrates that pulmonary fungal burden was significantly decreased on day 14 postinoculation in mice given an experimental pulmonary infection with C. neoformans strain H99γ compared with wild-type infected mice (P < 0.01). These results corroborate previous studies demonstrating that BALB/c mice given an experimental pulmonary infection with C. neoformans strain H99γ are able to completely resolve the fungal infection.23,29,30
Figure 1.
Resolution of pulmonary infection with C. neoformans strain H99γ is not associated with increased macrophage recruitment. BALB/c mice received an intranasal inoculum of 1 × 104 CFU of C. neoformans strains H99 or H99γ in 50 μl of sterile PBS. The lungs from each group of mice were excised at days 3, 7, and 14 after secondary inoculation, and the fungal burden was quantified (A). Alternatively, pulmonary leukocytes were enzymatically dispersed and the absolute number of F4/80+/CD45+ macrophages was quantified by flow cytometry (B). Pulmonary fungal burden data are cumulative of three experiments using five mice per time point. Results are expressed as mean log10 CFU per milliliter of lung homogenate ± SEM. Flow cytometry data are cumulative results of five independent experiments using pooled leukocytes from five mice per experiment. Results are expressed as the absolute number of F4/80+/CD45+ dual positive cells. Significant decreases were observed in C. neoformans strain H99γ-infected compared with wild-type C. neoformans H99-infected mice. (**P < 0.01).
Pulmonary leukocytes were isolated from the lungs of infected BALB/c mice on days 3, 7, and 14 postinoculation with C. neoformans strains H99 or H99γ and analyzed by flow cytometry for macrophage infiltration. We observed no significant difference in the absolute number of F4/80+ cells (macrophages) during infection of mice with C. neoformans strain H99γ compared with wild-type infected mice (Figure 1B), consistent with previous studies using 10-fold higher inocula of C. neoformans strains H99 or H99γ.23 Thus, pulmonary infection with C. neoformans strain H99γ results in improved control of pulmonary C. neoformans growth that occurs without an increase in macrophage accumulation in infected lungs.
Outcome of IFN-γ Transgene Expression by C. neoformans Strain H99γ on Macrophage Activation in Mice During Pulmonary C. neoformans Infection
Mice given an experimental pulmonary infection with C. neoformans strain H99γ were able to control the microbe without an increase in macrophage recruitment (Figure 1). We then investigated whether potential differences in macrophage activation phenotype account for the differential response. Lungs were excised from mice given an intranasal inoculation with C. neoformans strains H99 or H99γ on days 3, 7, and 14 postinoculation and evaluated for hallmarks of aaMac and classically activated macrophage (caMac) by immunohistological examination. Tissue sections were immunofluorescently labeled for F4/80+ cells (macrophages) or markers of classical (iNOS) and alternative (Arg1, CD206, and Ym1) macrophage activation. No difference in F4/80 staining was observed in the lungs of mice infected with wild-type H99 cryptococci or C. neoformans strain H99γ at any time point evaluated (Figure 2), a finding that corroborates previous results using flow cytometry (Figure 1B). Staining for iNOS, a marker of caMac, appeared strongest on day 7 postinoculation in mice infected with C. neoformans strain H99γ (Figure 3), consistent with the beginning of clearance of fungi that we observed between days 7 and 14 (Figure 1A). A decline in iNOS staining (Figure 3) appeared consistent with the decline in fungal burden observed in C. neoformans strain H99γ-infected animals on day 14 postinoculation (Figure 1A). In contrast, iNOS protein expression was not detected at any time point evaluated during infection with wild-type C. neoformans (Figure 3), despite progressive yeast growth. The aaMac hallmark proteins, Arg1, CD206, and Ym1, were expressed at low levels on days 3 and 7 postinoculation in tissues derived from mice infected with either C. neoformans strain H99γ or wild-type yeast (Figures 4, 5, and 6, for Arg1, CD206, and Ym1, respectively). However, their expression increased markedly in wild-type C. neoformans strain H99-infected lungs on day 14 postinoculation (Figures 4, 5, and 6, for Arg-1, CD206, and Ym1, respectively). In contrast, lungs from C. neoformans strain H99γ-infected mice showed only very modest aaMac fluorescence on day 14 postinoculation. Thus, pulmonary infection with wild-type C. neoformans resulted in the progressive induction of aaMac proteins that was not observed in mice challenged with C. neoformans strain H99γ. Furthermore, experimental pulmonary infection with C. neoformans strain H99γ resulted in the promotion of iNOS protein expression, a caMac hallmark.
Figure 2.
Mice given an experimental pulmonary infection with C. neoformans strain H99γ or wild-type cryptococci have comparable macrophage recruitment into the lungs during infection. BALB/c mice were given an intranasal inoculum of 1 × 104 CFU of C. neoformans strains H99 or H99γ in 50 μl of sterile PBS. Lungs were excised on days 3, 7, and 14 postinoculation and immediately frozen in OCT medium. Lungs were cryosectioned, and macrophage infiltration was evaluated using immunofluorescence staining with anti-F4/80 antibodies. Nuclei were counterstained with DAPI. Data shown are representative lung sections from three independent experiments (three mice per group and per experiment). Digital photographs show representative areas of lungs: ×20 objective.
Figure 3.
Pulmonary infection with C. neoformans strain H99γ, but not wild-type C. neoformans, results in increased iNOS expression by pulmonary macrophages. BALB/c mice were given an intranasal inoculum of 1 × 104 CFU of C. neoformans strains H99 or H99γ in 50 μl of sterile PBS. Lungs were excised on days 3, 7, and 14 postinoculation and immediately frozen in OCT medium. Lungs were cryosectioned, and iNOS was evaluated using immunofluorescence staining with anti-iNOS antibodies. Nuclei were counterstained with DAPI. Data shown are representative lung sections from three independent experiments (three mice per group and per experiment). Digital photographs show representative areas of lungs: ×20 objective.
Figure 4.
Mice given an experimental pulmonary infection with C. neoformans show increased expression of Arg1 on lung macrophages that is not observed in C. neoformans strain H99γ-infected mice. BALB/c mice were given an intranasal inoculum of 1 × 104 CFU of C. neoformans strains H99 or H99γ in 50 μl of sterile PBS. Lungs were excised on days 3, 7, and 14 postinoculation and immediately frozen in OCT medium. Lungs were subsequently cryosectioned, and Arg1 expression was evaluated using immunofluorescence staining with anti-Arg1 antibodies. Nuclei were counterstained with DAPI. Data shown are representative lung sections from three independent experiments (three mice per group and per experiment). Digital photographs show representative areas of lungs: ×20 objective.
Figure 5.
Pulmonary infection of mice with wild-type C. neoformans strain H99, but not C. neoformans strain H99γ, results in increased CD206 expression on lung macrophages. BALB/c mice were given an intranasal inoculum of 1 × 104 CFU of C. neoformans strains H99 or H99γ in 50 μl of sterile PBS. The lungs were excised on days 3, 7, and 14 postinoculation and immediately frozen in OCT medium. Lungs were subsequently cryosectioned, and CD206 expression was evaluated using immunofluorescence staining with anti-CD206 antibodies. Nuclei were counterstained with DAPI. Data shown are representative lung sections from three independent experiments (three mice per group and experiment). Digital photographs show representative areas of lungs: ×20 objective.
Figure 6.
Pulmonary Ym1 expression is increased in mice after intranasal infection with wild-type C. neoformans but is abrogated in C. neoformans strain H99γ-infected mice. BALB/c mice were given an intranasal inoculum of 1 × 104 CFU of C. neoformans strains H99 or H99γ in 50 μl of sterile PBS. Lungs were excised on days 3, 7, and 14 postinoculation and immediately frozen in OCT medium. Lungs were subsequently cryosectioned, and the level of Ym1 expression was evaluated using immunofluorescence staining with anti-Ym1 antibodies. Nuclei were counterstained with DAPI. Data shown are representative lung sections from three independent experiments (three mice per group and per experiment). Digital photographs show representative areas of lungs: ×20 objective.
Result of IFN-γ Transgene Expression by C. neoformans Strain H99γ on Macrophage Activation and Cytokine Gene Expression in Pulmonary Macrophage-Enriched Cells
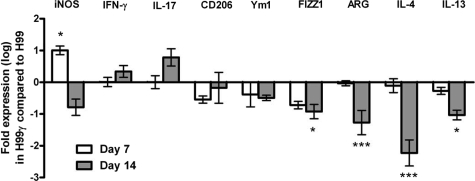
To directly examine macrophage activation phenotype, we quantified relative mRNA expression levels for aaMac and caMac genes in pulmonary macrophage-enriched cell populations obtained from C. neoformans strain H99- or H99γ-infected lungs. Pulmonary leukocytes were isolated from enzymatically dispersed lungs at days 7 and 14 after inoculation with either wild-type cryptococci or C. neoformans strain H99γ. We subsequently extracted total RNA from CD11b+ cell populations and evaluated macrophage activation using real-time PCR (Figure 7). We observed significantly increased transcript levels of FIZZ1, Arg1, IL-4, and IL-13 (aaMac markers) on day 14 after inoculation with C. neoformans strain H99 compared with those in C. neoformans strain H99γ-infected mice (P < 0.05, 0.001, 0.001, and 0.05 for FIZZ1, Arg1, IL-4, and IL-13, respectively). In contrast, iNOS transcript expression (a marker for caMac) was significantly higher in C. neoformans strain H99γ-infected mice on day 7 postinoculation (P < 0.05) compared with that in wild-type infected mice. We observed increased expression of IL-17 transcripts in mice infected with C neoformans strain H99γ on day 14 postinoculation compared with that in wild-type infected mice; however, the increase was not statistically significant (P < 0.058). Thus, the macrophage gene expression profile indicates that C. neoformans strain H99 infection resulted in the differentiation of aaMac and that transgenic IFN-γ expression during infection with C. neoformans strain H99γ opposed this process, promoting caMac polarization.
Figure 7.
Real-time PCR analysis of macrophage-enriched populations show enhanced expression of aaMac and caMac transcripts in C. neoformans strains H99- and H99γ-infected mice, respectively. BALB/c mice were given an intranasal inoculation with 1 × 104 CFU of C. neoformans strain H99 or H99γ in 50 μl of sterile PBS. Pulmonary leukocytes were isolated by enzymatic digestion on days 7 and 14 postinoculation, and macrophages were enriched for by positive selection of CD11b+ cells. Real-time PCR analyses of total mRNA from macrophage populations were conducted for iNOS, IFN-γ, CD206, Ym1, FIZZ1, ARG, IL-4, IL-13, IL-17, and GAPDH. Bars represent the log10 fold change in gene expression during infection with C. neoformans strain H99γ compared with infection with wild-type C. neoformans strain H99. Data shown are cumulative from three independent experiments using pooled leukocytes of five mice per group per experiment. Significant differences were observed at *P < 0.05; ***P < 0.001.
Our real-time PCR studies indicate that macrophages in the lungs of C. neoformans-infected mice express the Th2-type cytokines IL-4 and IL-13. Recent studies have demonstrated that macrophages can serve as a source of IL-4 and IL-13 production,31,32,33,34,35,36,37 suggesting that our findings are not unusual. Nonetheless, we sought to confirm that macrophages isolated from the lungs of mice given an experimental pulmonary C. neoformans infection express IL-4 and IL-13. Flow cytometry analysis demonstrated that F4/80+ cells in the lungs of mice infected with C. neoformans strain H99γ or wild-type cryptococci were positive for IL-4 and IL-13 on days 7 and 14 postinoculation (Figure 8). Results from three independent experiments showed no significant differences between groups (data not shown).
Figure 8.
Lung macrophages are positive for IL-4 and IL-13 protein expression during pulmonary infection with C. neoformans. BALB/c mice received an intranasal inoculum of 1 × 104 CFU of C. neoformans strains H99 or H99γ in 50 μl of sterile PBS. The lungs from each group of mice were excised at days 7 and 14 after secondary inoculation, and a single cell suspension was generated using enzymatic digestion. The leukocytes were stained with anti-mouse F4/80+ antibodies, fixed, permeabilized, and incubated with anti-mouse antibodies specific for IL-4 and IL-13 and quantified by flow cytometry. Flow cytometry data are representative results of three independent experiments using pooled leukocytes from five mice per experiment. Results shown are the percentage of leukocytes expressing the indicated surface markers.
Effect of IFN-γ Transgene Expression by C. neoformans Strain H99γ on Pulmonary Pathology
Our data demonstrate a significant effect of IFN-γ expression by C. neoformans strain H99γ on microbial clearance and the expression pattern of macrophage activation markers. Therefore, we sought to determine whether mice challenged with C. neoformans strain H99γ experienced the severe lung pathology typically associated with wild-type C. neoformans strain H99 infection.12 Uninfected and infected lungs were collected on day 21 after infection with wild-type C. neoformans strain H99 or transgenic C. neoformans strain H99γ and processed for histological analysis. Sections were stained with H&E and analyzed by light microscopy (Figure 9, A–F). Our data show that BALB/c mice infected with wild-type cryptococci developed the severe lung pathology associated with a nonprotective Th2-type cytokine response (Figure 9, B–D). C. neoformans growth was observed to be widespread, replacing most of the pulmonary airspaces (compare Figure 9A with Figure 9, B and C). Many C. neoformans organisms were also found to be unaccompanied by an inflammatory response. However, cryptococcal growth was also observed within infected macrophages (Figure 9C) consistent with the aaMac phenotype demonstrated by immunohistochemical analysis during C. neoformans strain H99 infection. Macrophages harbored multiple C. neoformans cells with some showing evidence of proliferation. We detected Ym1 crystals in some of the wild-type-infected macrophages along with eosinophil infiltrates (Figure 9C), suggesting a prolonged presence of Th2-type responses and aaMac in the lungs. In contrast with the development of severe pulmonary allergic mycosis in wild-type C. neoformans-infected mice, we observed a significant resolution of infection in the lungs of mice challenged with C. neoformans strain H99γ (Figure 9, E–F). Sections from day 21 of C. neoformans strain H99γ infection showed that a majority of the lung was clear of inflammation with few remaining mononuclear infiltrates (Figure 9E). These infiltrates present as bronchovascular neo-lymphoid tissue clusters accompanied by groups of small macrophages, as described previously in Th1-type cytokine responses,13 and occasional giant cells. Extracellular cryptococci were absent and only a few intracellular yeast cells were found. Furthermore, the macrophages contained small inclusion bodies weakly stained with mucicarmine, suggesting that they were the remains of ingested or destroyed cryptococci (Figure 9F). Thus, infection with C. neoformans H99γ is characterized not only by rapid clearance of the microbe but also by rapid resolution of inflammation, in contrast to the pathology that develops in the lungs of mice challenged with wild-type C. neoformans strain H99.
Figure 9.
Pulmonary infection with C. neoformans H99γ results in rapid clearance of the cryptococci and rapid resolution of inflammation, whereas the lungs of mice challenged with wild-type C. neoformans strain H99 show exacerbated disease and inflammation. Lungs were collected on day 21 postinoculation from uninfected mice and mice inoculated with C. neoformans strains H99 or H99γ, frozen in OCT medium, and processed for histological analysis. Tissue sections were stained with H&E and mucicarmine and examined under a light microscope. Digital photographs show representative areas of lungs from uninfected mice (×10 objective, A), C. neoformans strain H99-infected mice (×10, ×40, and ×100 objectives, B–D), and C. neoformans H99γ-infected mice (×10 and ×40 objectives, E and F). Note widespread intra- and extracellular cryptococci (orange arrowheads, B–D), extended macrophages with proliferating cryptococci and Ym1/Ym2 crystal formation (green arrow [Ym], C), and eosinophilic infiltrate (black arrows [Eo], D) in C. neoformans strain H99-infected lungs. Note limited inflammatory response (E), few internalized yeasts (orange arrowheads, F), and small inclusion bodies (purple arrows, F) consistent with elements of destroyed fungi within the macrophages in C. neoformans strain H99γ-infected lungs.
Impact of IFN-γ Transgene Expression by C. neoformans Strain H99γ on Pulmonary Cytokine Production
The local cytokine milieu appears to be critical in determining macrophage activation.19 Our final goal was to determine whether changes in the pulmonary cytokine milieu resulting from infection with C. neoformans strain H99γ could be accountable for changes in macrophage phenotype. We therefore evaluated Th2-type (IL-4, IL-5, and IL-13), Th1-type (IL-2, IL-12, and IFN-γ), and IL-17 cytokine production in total lung homogenates derived from mice infected with wild-type C. neoformans strain H99 or transgenic C. neoformans strain H99γ on days 7 and 14 postinoculation. We observed a significant elevation of the Th2-type cytokines IL-4 and IL-5 (Figure 10, A and B) on day 14 postinoculation in lungs infected with C. neoformans strain H99 (P < 0.001 for both), a timing that is consistent with the development of a Th2-type cytokine response12 and with the strong aaMac polarization shown above (Figures 456–7). In contrast, IL-4 and IL-5 production was abrogated in lungs infected with C. neoformans strain H99γ (Figure 10, A and B). Next, we observed a significant increase in the Th1-type cytokines IL-2 and IL-12 (Figure 10, D and E) and the Th17-type cytokine IL-17 (Figure 10F) on day 7 postinoculation in lungs from C. neoformans strain H99γ-infected mice compared with those obtained from mice infected with wild-type yeast (P < 0.05, P < 0.01, and P < 0.001 for IL-2, IL-12, and IL-17, respectively). The timing of IL-2, IL-12, and IL-17 responses is consistent with the peak of caMac-associated iNOS expression detected in C. neoformans strain H99γ-infected lungs as described above (Figures 3 and 7). Interestingly, we did not observe any significant differences in the presence of the Th2-type cytokine IL-13 (Figure 10C) or the Th1-type cytokine IFN-γ (Figure 10G) in lung homogenates derived from wild-type C. neoformans strain H99-infected compared with H99γ-infected mice, suggesting that these cytokines were not affected in the total environmental milieu. Altogether, these observations indicate that experimental pulmonary infection with C. neoformans strain H99γ resulted in the generation of a predominant Th1-type cytokine environment that was refractory to the development of Th2-type cytokine responses and aaMac polarization.
Figure 10.
Pulmonary infection with C. neoformans strain H99γ results in the generation of a predominantly Th1-type and IL-17 cytokine environment in the lungs. BALB/c mice were given an intranasal inoculation with 1 × 104 CFU of C. neoformans strain H99 or H99γ in 50 μl of sterile PBS. Lung homogenates were prepared from lungs excised on days 7 and 14 postinoculation and assayed for IL-4 (A), IL-5 (B), IL-13 (C), IL-2 (D), IL-12 (E), IL-17 (F), and IFN-γ (G) cytokine production. Data are cumulative of two experiments using five mice each. Significant differences were observed at *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
Previous work in our laboratory has demonstrated that mice given an experimental pulmonary infection with an IFN-γ-producing C. neoformans strain, H99γ, have levels of pulmonary macrophage infiltrates comparable to those of mice infected with wild-type yeast, yet proceed to resolve the infection.23 In the present studies we characterized the activation phenotype of macrophages elicited during pulmonary infection with C. neoformans strain H99γ to elucidate a potential mechanism for the protection developed in these mice. We found that infection with C. neoformans strain H99γ was associated with increased expression of the caMac-associated gene iNOS, augmented pulmonary Th1-type cytokine production, rapid resolution of the inflammatory response, and protection from the severe lung pathology that develops in the lungs of mice infected with wild-type C. neoformans. Furthermore, these studies indicate that the phenotype of infiltrating macrophages is a more important determinant of protection against C. neoformans infection than macrophage quantity.
Indeed, Arora et al4 demonstrated that experimental pulmonary C. neoformans infection of IFN-γ knockout mice resulted in significantly greater macrophage recruitment compared with that in wild-type mice. However, the infected IFN-γ knockout mice displayed Th2-type polarized responses, aaMac differentiation, and reduced fungistasis, leading to progressive cryptococcal infection. Thus, it seems that augmented macrophage recruitment during experimental pulmonary C. neoformans infection is not sufficient to induce protective anti-cryptococcal immune responses. Given that pulmonary challenge with C. neoformans strain H99γ in mice results in the development of a protective Th1-type cytokine response that is conducive to caMac activation,19,23 we hypothesized that caMac are induced during pulmonary challenge with C. neoformans strain H99γ.
In the present study, mice infected with C. neoformans strain H99γ or wild-type yeast developed disparate macrophage activation phenotypes. A robust, progressive increase in hallmark markers of aaMac differentiation (namely Arg1, CD206, FIZZ1, and Ym1) was observed during infection with the wild-type strain of C. neoformans, but these markers were repressed during infection with the transgenic C. neoformans strain H99γ. FIZZ1 expression is enhanced in alveolar epithelial and type II cells during allergic pulmonary inflammation and Ym1 crystallizes in alveolar spaces and in hyperactive lung macrophages and giant cells.17,38,39,40,41 Arg1 competes with iNOS for its substrate, arginine, thus depleting the cell of nitric oxide, an important intracellular fungicide. Up-regulated CD206 expression increases phagocytosis but is accompanied by decreased intracellular microbicidal activity.18,19,20,42 Consistent with these observations, we noted Ym1 crystal formation and C. neoformans yeasts displaying evidence of proliferation within macrophages of wild-type C. neoformans strain H99-infected lungs. Conversely, although we observed abrogated aaMac development during infection with C. neoformans strain H99γ, we also found increased iNOS expression, an important marker of caMac. An increase in iNOS expression by caMac results in improved fungicidal activity due to the generation of reactive oxygen and nitrogen species.16,43,44,45 Furthermore, pulmonary macrophages from mice infected with C. neoformans strain H99γ harbored few intracellular cryptococci and contained vacuoles stained with mucicarmine, indicating cryptococci in latter stages of dissolution.
Polarized cytokine environments are perhaps the most influential determinants of macrophage activation phenotype.15 Mice challenged with wild-type C. neoformans developed a predominantly pulmonary Th2-type cytokine environment consisting of IL-4 and IL-5; conversely, pulmonary infection with transgenic C. neoformans strain H99γ resulted in a predominantly Th1-type and IL-17 cytokine environment consisting of IL-2, IL-12, and IL-17. Thus, the overall cytokine milieu generated in the lungs of mice infected with wild-type C. neoformans promotes aaMac differentiation, whereas the pulmonary cytokine environment in mice challenged with C. neoformans strain H99γ favors caMac development. Interestingly, our studies showed the presence of IL-4 and IL-13 protein expression within lung macrophages of mice after pulmonary C. neoformans infection. These results corroborate several other recent studies, indicating that macrophages can serve as a source of IL-4 and IL-13.31,32,33,34,35,36,37 Also intriguing, IFN-γ protein levels were not significantly different in pulmonary homogenates of mice challenged with C. neoformans strain H99γ, despite observation of significant increases in other Th1-type cytokines compared with those in wild-type-infected mice. Previous studies in mice administered a 10-fold higher inoculum of C. neoformans strain H99γ than that used in the current study showed significantly increased pulmonary expression of IFN-γ and IL-17 compared with that of wild-type-infected mice.23 Recent studies have shown that the proliferation of C. neoformans within macrophages and cryptococcal expulsion rates are significantly lower after treatment with Th1-type cytokines and IL-17.46 Alternatively, IL-4 and IL-13 treatment significantly increased intracellular yeast proliferation. Thus, it appears that the inoculum used in this study was able to induce a Th1-type and IL-17 polarized environment that was not predominated by IFN-γ. The presence of a significant biological effect indicates that production of IFN-γ by the microbe is sufficient to evoke protection without altering the overall IFN-γ production by leukocytes. It is likely that the close interaction between C. neoformans strain H99γ and macrophages during phagocytosis is sufficient to induce the essential effects of IFN-γ production, such as major histocompatibility class II expression,10,11,47 enhanced macrophage respiratory burst, and the release of proinflammatory cytokines by macrophages.18,19,42 These responses are expected to augment macrophage activity toward the resolution of cryptococcal infection. In addition, the transgenic strain is likely to have induced other cellular responses that may influence caMac differentiation.
The question remains whether IFN-γ alone is sufficient to induce caMac differentiation in mice challenged with C. neoformans strain H99γ. High IL-4/13 environments induce aaMac marker expression but are antagonistic to the development of caMac.15 Specifically, IL-4 and IL-13, acting through a common receptor chain (IL-4Rα), enhance the expression of CD206, FIZZ1, Ym1, and Arg1 while suppressing macrophage IFN-γ production and subsequent iNOS-mediated nitric oxide production.15,39,41,48 In contrast, IFN-γ down-regulates CD206 expression and enhances the expression of iNOS that, in turn, enhances nitric oxide synthesis.16,18,19,42,49 Indeed, C. neoformans-infected IFN-γ knockout mice generate aaMac and experience progressive infection.4 In contrast, previous reports using IL-13-deficient mice clearly showed an abrogation of aaMac induction that correlated with increased resistance to pulmonary C. neoformans infection.11 Likewise, the results herein demonstrate a preclusion of aaMac induction in mice after infection with C. neoformans strain H99γ. However, the present study is the first report to show the generation of caMac and sterilizing immunity, in vivo, during experimental pulmonary C. neoformans infection. We were able to demonstrate that pulmonary infection with C. neoformans strain H99γ was resolved in mice and resulted in the induction of a predominant Th1-type and IL-17 cytokine environment in the lungs and caMac differentiation, coupled with the abrogation of Th2-type cytokine production and aaMac. Therefore, it seems that not only is the prevention of aaMac differentiation essential in the protective response to C. neoformans but also that caMac are induced.
Altogether, the results show that pulmonary infection with C. neoformans strain H99γ in mice results in the induction of Th1-type and IL-17 cytokine responses, classical macrophage activation, and the resolution of infection. Pulmonary infection of mice with wild-type cryptococci induced Th2-type polarized responses, alternative macrophage activation, pulmonary eosinophilia, and progressive disease associated with disease in accordance with previous independent studies.4,11 Our studies suggest that therapies targeting the induction of classical macrophage activation have the potential to generate protection against pulmonary cryptococcosis in human patients.
Acknowledgments
We thank Jose Lopez-Ribot, Pharm.D., Ph.D., Sarah Bubeck, Ph.D., and Judy Teale, Ph.D., for critical reading of the manuscript.
Footnotes
Address reprint requests to Floyd L. Wormley Jr, Ph.D., Department of Biology, The University of Texas at San Antonio, One UTSA Circle, San Antonio, TX 78249-0062. E-mail: floyd.wormley@utsa.edu.
Supported by research grants R01-AI071752-03 from the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) (F.L.W.) and a Merit Review grant from the Department of Veterans Affairs (M.A.O.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID, NIH.
References
- Mitchell TG, Perfect JR. Cryptococcosis in the era of AIDS—100 years after the discovery of Cryptococcus neoformans. Clin Microbiol Rev. 1995;8:515–548. doi: 10.1128/cmr.8.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powderly WG. Cryptococcal meningitis and AIDS. Clin Infect Dis. 1993;17:837–842. doi: 10.1093/clinids/17.5.837. [DOI] [PubMed] [Google Scholar]
- Aguirre K, Havell EA, Gibson GW, Johnson LL. Role of tumor necrosis factor and γ interferon in acquired resistance to Cryptococcus neoformans in the central nervous system of mice. Infect Immun. 1995;63:1725–1731. doi: 10.1128/iai.63.5.1725-1731.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora S, Hernandez Y, Erb-Downward JR, McDonald RA, Toews GB, Huffnagle GB. Role of IFN-γ in regulating T2 immunity and the development of alternatively activated macrophages during allergic bronchopulmonary mycosis. J Immunol. 2005;174:6346–6356. doi: 10.4049/jimmunol.174.10.6346. [DOI] [PubMed] [Google Scholar]
- Collins HL, Bancroft GJ. Cytokine enhancement of complement-dependent phagocytosis by macrophages: synergy of tumor necrosis factor-α and granulocyte-macrophage colony-stimulating factor for phagocytosis of Cryptococcus neoformans. Eur J Immunol. 1992;22:1447–1454. doi: 10.1002/eji.1830220617. [DOI] [PubMed] [Google Scholar]
- Flesch IE, Schwamberger G, Kaufmann SH. Fungicidal activity of IFN-γ-activated macrophages. Extracellular killing of Cryptococcus neoformans. J Immunol. 1989;142:3219–3224. [PubMed] [Google Scholar]
- Huffnagle GB, Lipscomb MF. Cells and cytokines in pulmonary cryptococcosis. Res Immunol. 1998;149:387–396. doi: 10.1016/s0923-2494(98)80762-1. discussion 512–514. [DOI] [PubMed] [Google Scholar]
- Levitz SM, DiBenedetto DJ. Differential stimulation of murine resident peritoneal cells by selectively opsonized encapsulated and acapsular Cryptococcus neoformans. Infect Immun. 1988;56:2544–2551. doi: 10.1128/iai.56.10.2544-2551.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loke P, Nair MG, Parkinson J, Guiliano D, Blaxter M, Allen JE. IL-4 dependent alternatively-activated macrophages have a distinctive in vivo gene expression phenotype. BMC Immunol. 2002;3:7. doi: 10.1186/1471-2172-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody CH, Tyler CL, Sitrin RG, Jackson C, Toews GB. Interferon-γ activates rat alveolar macrophages for anticryptococcal activity. Am J Respir Cell Mol Biol. 1991;5:19–26. doi: 10.1165/ajrcmb/5.1.19. [DOI] [PubMed] [Google Scholar]
- Müller U, Stenzel W, Kohler G, Werner C, Polte T, Hansen G, Schutze N, Straubinger RK, Blessing M, McKenzie AN, Brombacher F, Alber G. IL-13 induces disease-promoting type 2 cytokines, alternatively activated macrophages and allergic inflammation during pulmonary infection of mice with Cryptococcus neoformans. J Immunol. 2007;179:5367–5377. doi: 10.4049/jimmunol.179.8.5367. [DOI] [PubMed] [Google Scholar]
- Osterholzer JJ, Surana R, Milam JE, Montano GT, Chen GH, Sonstein J, Curtis JL, Huffnagle GB, Toews GB, Olszewski MA. Cryptococcal urease promotes the accumulation of immature dendritic cells and a non-protective T2 immune response within the lung. Am J Pathol. 2009;174:932–943. doi: 10.2353/ajpath.2009.080673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GH, Olszewski MA, McDonald RA, Wells JC, Paine R, 3rd, Huffnagle GB, Toews GB. Role of granulocyte macrophage colony-stimulating factor in host defense against pulmonary Cryptococcus neoformans infection during murine allergic bronchopulmonary mycosis. Am J Pathol. 2007;170:1028–1040. doi: 10.2353/ajpath.2007.060595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Schaller M, Hogaboam CM, Standiford TJ, Chensue SW, Kunkel SL. TLR9 activation is a key event for the maintenance of a mycobacterial antigen-elicited pulmonary granulomatous response. Eur J Immunol. 2007;37:2847–2855. doi: 10.1002/eji.200737603. [DOI] [PubMed] [Google Scholar]
- Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- Hesse M, Modolell M, La Flamme AC, Schito M, Fuentes JM, Cheever AW, Pearce EJ, Wynn TA. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of l-arginine metabolism. J Immunol. 2001;167:6533–6544. doi: 10.4049/jimmunol.167.11.6533. [DOI] [PubMed] [Google Scholar]
- Chang NC, Hung SI, Hwa KY, Kato I, Chen JE, Liu CH, Chang AC. A macrophage protein. Ym1, transiently expressed during inflammation is a novel mammalian lectin. J Biol Chem. 2001;276:17497–17506. doi: 10.1074/jbc.M010417200. [DOI] [PubMed] [Google Scholar]
- Goerdt S, Politz O, Schledzewski K, Birk R, Gratchev A, Guillot P, Hakiy N, Klemke CD, Dippel E, Kodelja V, Orfanos CE. Alternative versus classical activation of macrophages. Pathobiology. 1999;67:222–226. doi: 10.1159/000028096. [DOI] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176:287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groot PW, Ram AF, Klis FM. Features and functions of covalently linked proteins in fungal cell walls. Fungal Genet Biol. 2005;42:657–675. doi: 10.1016/j.fgb.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Noverr MC, Cox GM, Perfect JR, Huffnagle GB. Role of PLB1 in pulmonary inflammation and cryptococcal eicosanoid production. Infect Immun. 2003;71:1538–1547. doi: 10.1128/IAI.71.3.1538-1547.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormley FL, Jr, Perfect JR, Steele C, Cox GM. Protection against cryptococcosis by using a murine γ interferon-producing Cryptococcus neoformans strain. Infect Immun. 2007;75:1453–1462. doi: 10.1128/IAI.00274-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox GM, McDade HC, Chen SC, Tucker SC, Gottfredsson M, Wright LC, Sorrell TC, Leidich SD, Casadevall A, Ghannoum MA, Perfect JR. Extracellular phospholipase activity is a virulence factor for Cryptococcus neoformans. Mol Microbiol. 2001;39:166–175. doi: 10.1046/j.1365-2958.2001.02236.x. [DOI] [PubMed] [Google Scholar]
- Cox GM, Mukherjee J, Cole GT, Casadevall A, Perfect JR. Urease as a virulence factor in experimental cryptococcosis. Infect Immun. 2000;68:443–448. doi: 10.1128/iai.68.2.443-448.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarskog NK, Vedeler CA. Real-time quantitative polymerase chain reaction. A new method that detects both the peripheral myelin protein 22 duplication in Charcot-Marie-Tooth type 1A disease and the peripheral myelin protein 22 deletion in hereditary neuropathy with liability to pressure palsies. Hum Genet. 2000;107:494–498. doi: 10.1007/s004390000399. [DOI] [PubMed] [Google Scholar]
- Chang JT, Chen IH, Liao CT, Wang HM, Hsu YM, Hung KF, Lin CJ, Hsieh LL, Cheng AJ. A reverse transcription comparative real-time PCR method for quantitative detection of angiogenic growth factors in head and neck cancer patients. Clin Biochem. 2002;35:591–596. doi: 10.1016/s0009-9120(02)00403-4. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young M, Macias S, Thomas D, Wormley FL., Jr A proteomic-based approach for the identification of immunodominant Cryptococcus neoformans proteins. Proteomics. 2009;9:2578–2588. doi: 10.1002/pmic.200800713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak KL, Ravi S, Macias S, Young ML, Olszewski MA, Steele C, Wormley FL. Insights into the mechanisms of protective immunity against Cryptococcus neoformans infection using a mouse model of pulmonary cryptococcosis. PLoS One. 2009;4:e6854. doi: 10.1371/journal.pone.0006854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büttner C, Skupin A, Reimann T, Rieber EP, Unteregger G, Geyer P, Frank KH. Local production of interleukin-4 during radiation-induced pneumonitis and pulmonary fibrosis in rats: macrophages as a prominent source of interleukin-4. Am J Respir Cell Mol Biol. 1997;17:315–325. doi: 10.1165/ajrcmb.17.3.2279. [DOI] [PubMed] [Google Scholar]
- Hancock A, Armstrong L, Gama R, Millar A. Production of interleukin 13 by alveolar macrophages from normal and fibrotic lung. Am J Respir Cell Mol Biol. 1998;18:60–65. doi: 10.1165/ajrcmb.18.1.2627. [DOI] [PubMed] [Google Scholar]
- Hauber HP, Gholami D, Meyer A, Pforte A. Increased interleukin-13 expression in patients with sarcoidosis. Thorax. 2003;58:519–524. doi: 10.1136/thorax.58.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouliot P, Turmel V, Gelinas E, Laviolette M, Bissonnette EY. Interleukin-4 production by human alveolar macrophages. Clin Exp Allergy. 2005;35:804–810. doi: 10.1111/j.1365-2222.2005.02246.x. [DOI] [PubMed] [Google Scholar]
- Prieto J, Lensmar C, Roquet A, van der Ploeg I, Gigliotti D, Eklund A, Grunewald J. Increased interleukin-13 mRNA expression in bronchoalveolar lavage cells of atopic patients with mild asthma after repeated low-dose allergen provocations. Respir Med. 2000;94:806–814. doi: 10.1053/rmed.2000.0826. [DOI] [PubMed] [Google Scholar]
- Shirey KA, Cole LE, Keegan AD, Vogel SN. Francisella tularensis live vaccine strain induces macrophage alternative activation as a survival mechanism. J Immunol. 2008;181:4159–4167. doi: 10.4049/jimmunol.181.6.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymczak WA, Deepe GS., Jr The CCL7-CCL2-CCR2 axis regulates IL-4 production in lungs and fungal immunity. J Immunol. 2009;183:1964–1974. doi: 10.4049/jimmunol.0901316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Johnson RS, Schuh JC. Biochemical characterization of endogenously formed eosinophilic crystals in the lungs of mice. J Biol Chem. 2000;275:8032–8037. doi: 10.1074/jbc.275.11.8032. [DOI] [PubMed] [Google Scholar]
- Loke P, MacDonald AS, Robb A, Maizels RM, Allen JE. Alternatively activated macrophages induced by nematode infection inhibit proliferation via cell-to-cell contact. Eur J Immunol. 2000;30:2669–2678. doi: 10.1002/1521-4141(200009)30:9<2669::AID-IMMU2669>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Raes G, De Baetselier P, Noel W, Beschin A, Brombacher F, Hassanzadeh Gh G. Differential expression of FIZZ1 and Ym1 in alternatively versus classically activated macrophages. J Leukoc Biol. 2002;71:597–602. [PubMed] [Google Scholar]
- Welch JS, Escoubet-Lozach L, Sykes DB, Liddiard K, Greaves DR, Glass CK. TH2 cytokines and allergic challenge induce Ym1 expression in macrophages by a STAT6-dependent mechanism. J Biol Chem. 2002;277:42821–42829. doi: 10.1074/jbc.M205873200. [DOI] [PubMed] [Google Scholar]
- Allen JE, Loke P. Divergent roles for macrophages in lymphatic filariasis. Parasite Immunol. 2001;23:345–352. doi: 10.1046/j.1365-3024.2001.00394.x. [DOI] [PubMed] [Google Scholar]
- Forman HJ, Torres M. Reactive oxygen species and cell signaling: respiratory burst in macrophage signaling. Am J Respir Crit Care Med. 2002;166:S4–S8. doi: 10.1164/rccm.2206007. [DOI] [PubMed] [Google Scholar]
- Hickman-Davis JM, Fang FC, Nathan C, Shepherd VL, Voelker DR, Wright JR. Lung surfactant and reactive oxygen-nitrogen species: antimicrobial activity and host-pathogen interactions. Am J Physiol Lung Cell Mol Physiol. 2001;281:L517–L523. doi: 10.1152/ajplung.2001.281.3.L517. [DOI] [PubMed] [Google Scholar]
- Hickman-Davis JM, O'Reilly P, Davis IC, Peti-Peterdi J, Davis G, Young KR, Devlin RB, Matalon S. Killing of Klebsiella pneumoniae by human alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 2002;282:L944–L956. doi: 10.1152/ajplung.00216.2001. [DOI] [PubMed] [Google Scholar]
- Voelz K, Lammas DA, May RC. Cytokine signaling regulates the outcome of intracellular macrophage parasitism by Cryptococcus neoformans. Infect Immun. 2009;77:3450–3457. doi: 10.1128/IAI.00297-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noël W, Raes G, Hassanzadeh Ghassabeh G, De Baetselier P, Beschin A. Alternatively activated macrophages during parasite infections. Trends Parasitol. 2004;20:126–133. doi: 10.1016/j.pt.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Schindler H, Lutz MB, Rollinghoff M, Bogdan C. The production of IFN-γ by IL-12/IL-18-activated macrophages requires STAT4 signaling and is inhibited by IL-4. J Immunol. 2001;166:3075–3082. doi: 10.4049/jimmunol.166.5.3075. [DOI] [PubMed] [Google Scholar]
- Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]