Lon is an oligomeric ATP-dependent protease that degrades defective or denatured proteins as well as some folded proteins for the control of cellular protein quality and metabolism. Lon from T. onnurineus NA1 has been purified and crystallized at 295 K.
Keywords: Lon, ATP-dependent proteases, Thermococcus onnurineus NA1
Abstract
Lon is an oligomeric ATP-dependent protease that degrades defective or denatured proteins as well as some folded proteins for the control of cellular protein quality and metabolism. Lon from Thermococcus onnurineus NA1 was purified and crystallized at 295 K. A 2.0 Å resolution data set was collected using synchrotron radiation. The crystals belonged to space group P63, with unit-cell parameters a = 121.45, b = 121.45, c = 195.24 Å. Assuming the presence of two monomers in the asymmetric unit, the solvent content was estimated to be about 60.7%.
1. Introduction
In cells, ATP-dependent proteases play key roles in protein quality control and metabolic regulation by eliminating abnormal/damaged proteins and short-lived regulatory proteins (Baker & Sauer, 2006 ▶; Goldberg, 1992 ▶; Gottesman et al., 1997 ▶; Wickner et al., 1999 ▶). At least five bacterial ATP-dependent proteases (Lon, ClpAP, ClpXP, HslUV and FtsH) have been subjected to extensive investigations in order to understand the working mechanism of this type of cellular machine. ATP-dependent proteases are composed of an ATPase component and a protease component and the two components are assembled into a barrel-shaped complex with an internal channel that harbours the proteolytic sites. The ATPase components are responsible for the binding, unfolding and translocation of substrates, while the protease components degrade the ATPase domain-guided substrates.
Lon was the first ATP-dependent protease with a Ser-Lys catalytic dyad to be identified (Botos, Melnikov, Cherry, Tropea et al., 2004 ▶; Goldberg et al., 1994 ▶; Starkova et al., 1998 ▶; Swamy & Goldberg, 1981 ▶) and its orthologues are distributed in all kingdoms of life (Rotanova, 1999 ▶; Wang et al., 1993 ▶, 1994 ▶). Unlike ClpAP, ClpXP and HslUV, which are assembled from independently expressed ATPase and protease domains, the ATPase and protease domains of Lon and FtsH exist in a single polypeptide. Lon plays a major role in the degradation of a wide spectrum of damaged proteins in cells (Tsilibaris et al., 2006 ▶). For example, Lon is responsible for ∼50% of the turnover of proteins resulting from premature translational termination (Kowit & Goldberg, 1977 ▶). Emerging evidence indicates that Lons can be divided into two subgroups: LonA and LonB (Rotanova et al., 2006 ▶). LonA proteases, as represented by Escherichia coli Lon (EcLon), and LonB proteases, which are found only in archaea, differ in domain composition. LonA proteases are composed of an N-terminal domain, an ATPase domain and a protease domain, whereas LonB proteases consist of an ATPase domain and a protease domain. In addition, LonB proteases contain a membrane-anchoring region in their ATPase domains that contributes to their anchoring to the cytoplasmic face of the membrane (Rotanova et al., 2006 ▶).
Detailed structural information provides an important framework for physical and functional interpretation of a protein. However, in the case of Lon only the protease domain and small proteolytic fragments of other domains have been structurally studied to date (Botos, Melnikov, Cherry, Khalatova et al., 2004 ▶; Botos et al., 2005 ▶; Botos, Melnikov, Cherry, Tropea et al., 2004 ▶; Im et al., 2004 ▶; Li et al., 2005 ▶). With only fragmentary structural information, it is impossible to delineate how the ATPase domain and the protease domain in Lon are organized and communicate with each other in order to coordinate ATP hydrolysis with proteolysis. In the full genome sequence of Thermococcus onnurineus NA1 (Lee et al., 2008 ▶), we found a gene (TON_0529) encoding a 635-residue protein that exhibits 90% sequence identity to the LonB protease (TkLon) from Thermococcus kodakaraensis KOD1 (Fukui et al., 2002 ▶). In particular, the TON_0529 gene product has the same membrane-anchoring region as TkLon (Fukui et al., 2002 ▶). We generated a mutant protein with a deletion of the membrane-anchoring region for structural study. Furthermore, we introduced two point mutations (Ser523Ala and Lys566Ala) to remove the catalytic dyad. Hereafter, the mutant protein with both the deletion and the two point mutations will be referred to as TonLon. As a first step towards structure determination, we report the overexpression, crystallization and preliminary X-ray crystallographic analysis of TonLon.
2. Materials and methods
2.1. Cloning, expression and purification
The TON_0529 gene (GenBank accession No. ACJ16016) from T. onnurineus NA1 was amplified by polymerase chain reaction, digested with NdeI and SalI and inserted downstream of the T7 promoter of pET-24a (Novagen, Wisconsin, Wisconsin, USA). The resulting construct contains residues 1–635 with an additional 15 residues including six histidines at its C-terminus (VDKLAAALEHHHHHH). The expression plasmid for a mutant with a deletion of the putative membrane-anchoring region (residues 134–170) was generated in a similar manner with an additional mutagenic primer pair: 5′-CAGTACTCTTAAGGCGCATATTGGAGGATTTTACGCTCTCCTGGCTCTT-3′ and 5′-CAAGAGCCAGGAGAGCGTAAAATCCTCCAATTAGCGCCTTAAGAGTACT-3′. In addition, we substituted alanines for the serine and lysine of the Ser-Lys catalytic dyad at positions 523 and 566 using the following mutagenic primer pairs: forward primer (S523A), 5′-TTGAGGGCGACGCAGCCAGCATAAGC-3′; reverse primer (S523A), 5′-GCTTATGCTGGCTGCGTCGCCCTCAAC-3′; forward primer (K566A), 5′-GGTGCAACACCAGCGATAGAGGCCGCA-3′; reverse primer (K566A), 5′-TGCGGCCTCTATCGCTGGTGTTGCACC-3′. After verifying the DNA sequence, plasmid DNA was transformed into E. coli strain Rosetta (DE3) pLysS (Stratagene, La Jolla, California, USA). The transformed cells were grown in Luria–Bertani medium (Merck) containing 50 µg ml−1 kanamycin to an OD600 of 0.5 at 310 K and expression of TonLon was induced with 1 mM isopropyl β-d-1-thiogalactopyranoside (Duchefa). After 5 h induction at 303 K, the cells were harvested and resuspended in 50 mM Tris–HCl pH 8.0 and 500 mM KCl. The cells were disrupted by sonication and the crude lysate was centrifuged at 20 000g for 60 min at 277 K. The resulting supernatant was loaded onto a nickel–nitrilotriacetic acid column (Qiagen). The column was washed with a wash buffer containing 50 mM Tris–HCl pH 8.0, 500 mM KCl and 10 mM imidazole. TonLon was eluted with the same buffer containing 300 mM imidazole. The eluted faction containing TonLon was concentrated and subsequently loaded onto a Superdex 200 HR 16/60 column (Amersham Biosciences) pre-equilibrated with a buffer containing 50 mM Tris–HCl pH 8.0 and 15 mM MgCl2. The TonLon protein eluted at ∼39.78 min with a flow rate of 1.5 ml min−1. The purified TonLon was dialysed against a buffer containing 50 mM Tris–HCl pH 8.0, 2.5 mM ATP and 0.4 mM MgCl2 and then concentrated to ∼24 mg ml−1 for crystallization.
2.2. Crystallization and X-ray data collection
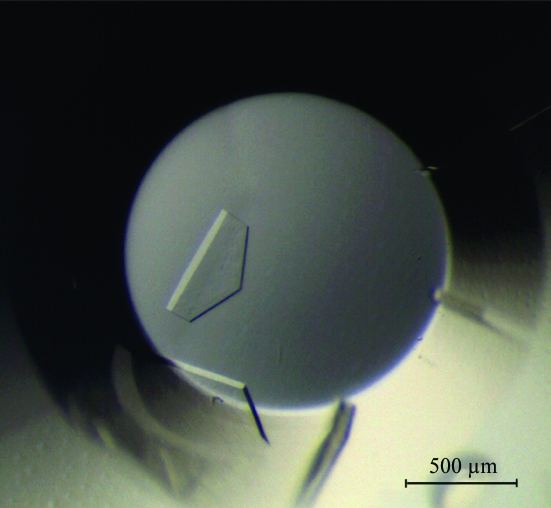
The batch crystallization method was used to screen for crystallization conditions and for optimization at 295 K. Small drops composed of 1 µl protein solution and an equal volume of crystallization reagent were pipetted under a layer of a 1:1 mixture of silicon oil and paraffin oil in 72-well HLA plates (Nunc). Screening for crystallization conditions was performed with all available screening kits from Hampton Research, Axygen Biosciences and Emerald BioSystems. Initial crystals (Fig. 1 ▶) were grown in a precipitant containing 1.2 M ammonium sulfate, 0.1 M sodium succinate pH 7.5 and 0.96% lauryldimethylamine oxide (condition No. A11 of CP-CUSTOM-I from Axygen Biosciences). It took over six months to obtain crystals after crystallization setup and therefore the initial crystals were used for data collection. Crystals were frozen at 100 K using a Cryostream cooler (Oxford Cryosystems) after brief immersion in a cryoprotectant solution containing 15% glucose and 2.5 mM ATP in the same precipitant solution. A 2.0 Å resolution data set was collected using an ADSC Quantum 315 CCD on beamline 4A of Pohang Light Source, Republic of Korea (Table 1 ▶). Diffraction data were processed using DENZO and scaled using SCALEPACK from the HKL-2000 program suite (Otwinowski & Minor, 1997 ▶).
Figure 1.
Crystals of TonLon.
Table 1. Crystal information and data-collection statistics.
Values in parentheses are for the outer shell.
| Radiation source | Beamline 4A, Pohang Light Source |
| Oscillation angle (°) | 1 |
| No. of frames | 240 |
| Crystal-to-detector distance (mm) | 250 |
| Space group | P63 |
| Unit-cell parameters (Å) | a = 121.45, b = 121.45, c = 195.24 |
| Wavelength (Å) | 1.00000 |
| Resolution (Å) | 50–2.0 (2.03–2.0) |
| Completeness (>0σ) (%) | 99.9 (99.9) |
| Rmerge (%)† | 8.8 (41.0) |
| Average I/σ(I) | 24.3 (3.2) |
| Unique reflections | 109495 |
| Average redundancy | 10.7 (7.7) |
R
merge = 
 .
.
3. Results and discussion
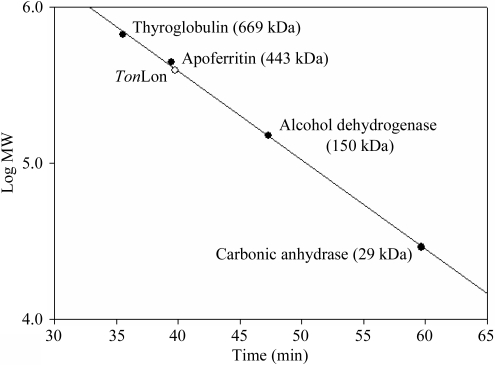
Lon proteases are highly likely to form hexamers in solution (Lee & Suzuki, 2008 ▶). Analytical ultracentrifugation analysis showed that Mycobacterium smegmatis Lon was hexameric (Rudyak et al., 2001 ▶) and electron microscopy of EcLon revealed a hexameric structure (Park et al., 2006 ▶). In addition, the crystal structure of the protease domain of EcLon (Botos, Melnikov, Cherry, Tropea et al., 2004 ▶) showed that it forms a robust hexamer. TonLon eluted at ∼39.78 min in gel-filtration experiments. Calculation of the molecular mass based on the elution time revealed the molecular mass of TonLon to be 403 511 Da (Fig. 2 ▶). The molecular mass of the TonLon monomer (65 856 Da) indicated that it forms a hexamer in solution. Consistent with this, the crystals of TonLon belonged to the hexagonal space group P63, with unit-cell parameters a = 121.45, b = 121.45, c = 195.24 Å. The calculated crystal volume per unit of molecular mass (V M) was 3.2 Å3 Da−1, with a solvent content of 60.7% by volume (Matthews, 1968 ▶), when the asymmetric unit was assumed to contain two TonLon monomers. The data-collection statistics are summarized in Table 1 ▶.
Figure 2.
Plot of the elution time versus the logarithm of the molecular weight of the size markers.
Acknowledgments
This study was supported by the Marine and Extreme Genome Research Center Program from the Ministry of Land, Transport and Maritime Affairs and the KORDI in-house program (PE98402).
References
- Baker, T. A. & Sauer, R. T. (2006). Trends Biochem. Sci.31, 647–653. [DOI] [PMC free article] [PubMed]
- Botos, I., Melnikov, E. E., Cherry, S., Khalatova, A. G., Rasulova, F. S., Tropea, J. E., Maurizi, M. R., Rotanova, T. V., Gustchina, A. & Wlodawer, A. (2004). J. Struct. Biol.146, 113–122. [DOI] [PubMed]
- Botos, I., Melnikov, E. E., Cherry, S., Kozlov, S., Makhovskaya, O. V., Tropea, J. E., Gustchina, A., Rotanova, T. V. & Wlodawer, A. (2005). J. Mol. Biol.351, 144–157. [DOI] [PubMed]
- Botos, I., Melnikov, E. E., Cherry, S., Tropea, J. E., Khalatova, A. G., Rasulova, F., Dauter, Z., Maurizi, M. R., Rotanova, T. V., Wlodawer, A. & Gustchina, A. (2004). J. Biol. Chem.279, 8140–8148. [DOI] [PubMed]
- Fukui, T., Eguchi, T., Atomi, H. & Imanaka, T. (2002). J. Bacteriol.184, 3689–3698. [DOI] [PMC free article] [PubMed]
- Goldberg, A. L. (1992). Eur. J. Biochem.203, 9–23. [DOI] [PubMed]
- Goldberg, A. L., Moerschell, R. P., Chung, C. H. & Maurizi, M. R. (1994). Methods Enzymol.244, 350–375. [DOI] [PubMed]
- Gottesman, S., Wickner, S. & Maurizi, M. R. (1997). Genes Dev.11, 815–823. [DOI] [PubMed]
- Im, Y. J., Na, Y., Kang, G. B., Rho, S. H., Kim, M. K., Lee, J. H., Chung, C. H. & Eom, S. H. (2004). J. Biol. Chem.279, 53451–53457. [DOI] [PubMed]
- Kowit, J. D. & Goldberg, A. L. (1977). J. Biol. Chem.252, 8350–8357. [PubMed]
- Lee, H. S. et al. (2008). J. Bacteriol.190, 7491–7499. [DOI] [PMC free article] [PubMed]
- Lee, I. & Suzuki, C. K. (2008). Biochim. Biophys. Acta, 1784, 727–735. [DOI] [PMC free article] [PubMed]
- Li, M., Rasulova, F., Melnikov, E. E., Rotanova, T. V., Gustchina, A., Maurizi, M. R. & Wlodawer, A. (2005). Protein Sci.14, 2895–2900. [DOI] [PMC free article] [PubMed]
- Matthews, B. W. (1968). J. Mol. Biol.33, 491–497. [DOI] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol.276, 307–326. [DOI] [PubMed]
- Park, S. C., Jia, B., Yang, J. K., Van, D. L., Shao, Y. G., Han, S. W., Jeon, Y. J., Chung, C. H. & Cheong, G. W. (2006). Mol. Cells, 21, 129–134. [PubMed]
- Rotanova, T. V. (1999). Bioorg. Khim.25, 883–891. [PubMed]
- Rotanova, T. V., Botos, I., Melnikov, E. E., Rasulova, F., Gustchina, A., Maurizi, M. R. & Wlodawer, A. (2006). Protein Sci.15, 1815–1828. [DOI] [PMC free article] [PubMed]
- Rudyak, S. G., Brenowitz, M. & Shrader, T. E. (2001). Biochemistry, 40, 9317–9323. [DOI] [PubMed]
- Starkova, N. N., Koroleva, E. P., Rumsh, L. D., Ginodman, L. M. & Rotanova, T. V. (1998). FEBS Lett.422, 218–220. [DOI] [PubMed]
- Swamy, K. H. & Goldberg, A. L. (1981). Nature (London), 292, 652–654. [DOI] [PubMed]
- Tsilibaris, V., Maenhaut-Michel, G. & Van Melderen, L. (2006). Res. Microbiol.157, 701–713. [DOI] [PubMed]
- Wang, N., Gottesman, S., Willingham, M. C., Gottesman, M. M. & Maurizi, M. R. (1993). Proc. Natl Acad. Sci. USA, 90, 11247–11251. [DOI] [PMC free article] [PubMed]
- Wang, N., Maurizi, M. R., Emmert-Buck, L. & Gottesman, M. M. (1994). J. Biol. Chem.269, 29308–29313. [PubMed]
- Wickner, S., Maurizi, M. R. & Gottesman, S. (1999). Science, 286, 1888–1893. [DOI] [PubMed]