A C-terminal truncation construct of human PACSIN 1 (1–344) has been purified and crystallized. Diffraction data were collected to 3.0 Å resolution.
Keywords: PACSIN 1
Abstract
PACSIN 1, which is mainly detected in brain tissue, is one of the PACSIN-family proteins involved in endocytosis and recruitment of synaptic vesicles. It binds to dynamin, synaptojanin 1 and N-WASP, and functions in vesicle formation and transport. However, the mechanisms of action of PACSIN 1 in these processes are largely unknown. Here, full-length and five C-terminal truncation constructs of human PACSIN 1 have been successfully expressed and purified in Escherichia coli. PACSIN 1 (1–344) was crystallized and diffracted to a resolution of 3.0 Å. The crystal belonged to space group C2, with unit-cell parameters a = 158.65, b = 87.38, c = 91.76 Å, α = 90.00, β = 113.61, γ = 90.00°. There were two molecules in the asymmetric unit and the solvent content was estimated to be about 70.47%.
1. Introduction
Intracellular trafficking between different membranous organelles is important and many tubular and vesicular membrane carriers contribute to this process via budding and fusing (Farsad & De Camilli, 2003 ▶). These proteins can either penetrate the outer bilayer leaflet of the membrane or bind the bilayer of the membrane via hydrophilic interfacial interactions or via their property of assembling into curved scaffolds in order to result in curvature of the membrane for related biological functions (Itoh et al., 2005 ▶). However, the precise mechanism of how invagination and fission of the membrane occurs is still elusive.
PACSIN 1, also called syndapin I, is a neurospecific protein which mainly comprises an N-terminal FCH (Fes/CIP4 homology) domain, a coiled-coil (CC) domain and a C-terminal SH3 domain. In synaptic vesicle endocytosis, PACSIN 1 binds to dynamin, synaptojanin 1 and N-WASP via its SH3 domain to participate in this process (Qualmann et al., 1999 ▶). Dynamin I is a guanosine triphosphatase (GTPase) that is enriched in pre-synaptic nerve terminals (Powell & Robinson, 1995 ▶) and is important for vesicle fission in SVE (synaptic vesicle endocytosis; Sweitzer & Hinshaw, 1998 ▶). PACSIN 1 binds the overlapping proline-rich (PRD) regions of dynamin I and functions in SVE (Anggono & Robinson, 2007 ▶); phosphorylation of PACSIN 1 increases this interaction (Hilton et al., 2001 ▶).
By electrical stimulation, PACSIN 1 may stabilize the plasma membrane and/or facilitate bulk endocytosis (Andersson et al., 2008 ▶). The N-terminal F-BAR of PACSIN 1 has 22% sequence identity to the BAR domain of CIP4, which is dimerized and tubulates liposome (Frost et al., 2008 ▶). Therefore, PACSIN 1 may be involved in invagination and fission in endocytosis.
PACSIN 1 is absent from synaptic varicosities in presymptomatic Huntington’s disease brains (Modregger et al., 2002 ▶). However, the precise functions of PACSIN 1 in all these processes remain elusive. In this study, we expressed, purified and crystallized full-length PACSIN 1 and five C-terminal truncation constructs and finally obtained preliminary X-ray crystallographic data for PACSIN 1 (1–344).
2. Methods
2.1. Protein purification
The cDNA of full-length human pacsin1 was cloned into pET21DEST expression vector with the N-terminal tag MASMTGGQQMGSSHHHHHSS using the Gateway cloning system (Invitrogen, USA). After confirmation by DNA sequencing, the expression plasmid was transformed into Escherichia coli strain BL21 (DE3) and plated on a Luria–Bertani broth (LB) agar plate with 100 mg l−1 ampicillin. A single colony was cultured in 200 ml LB medium supplemented with 100 mg l−1 ampicillin overnight at 310 K and then transferred into 6 l LB medium and incubated until the OD600 reached 0.6. The expression of PACSIN 1 was induced with 0.5 mM isopropyl β-d-1-thiogalactopyranoside (IPTG; Sigma) for 6 h at 303 K. Cells were harvested by centrifugation at 5000 rev min−1 for 10 min at 277 K and were suspended in binding buffer containing 50 mM HEPES pH 7.5, 500 mM NaCl and 5 mM imidazole. The cells were then sonicated and the lysate was centrifuged at 18 000 rev min−1 for 30 min at 277 K. The supernatant was collected, filtered through a 0.22 µm filter and applied onto a 5 ml Ni2+-HiTrap affinity column (GE Healthcare, Sweden). The protein peak eluted at 500 mM imidazole. To further purify PACSIN 1, the peak fractions were concentrated to 2 ml and loaded onto a Superdex-75 column (GE Healthcare) which was equilibrated with buffer containing 10 mM HEPES pH 7.5, 500 mM NaCl.
Five truncates of PACSIN 1 (1–333, 1–342, 1–344, 1–346 and 1–384) were also designed based on the degradation of full-length PACSIN 1. The truncation constructs were amplified by PCR, ligated into pET28a vector (Novagen) with N-terminal MGSSHHHHHHSS tags and purified following the same protocol as described above.
2.2. Protein crystallization
The protein solution was centrifuged at 14 000 rev min−1 for 30 min before crystallization setup. Initial and optimized crystallization experiments were performed at 293 K by the hanging-drop vapour-diffusion method.
Initial crystallization conditions were screened using kits from Hampton Research including PEG, Crystal Screen, Crystal Screen 2 and Index, with each drop containing 1 µl protein solution (8 mg ml−1 in 500 mM NaCl, 10 mM HEPES pH 7.5) mixed with 1 µl of reservoir solution and being equilibrated against 200 µl reservoir solution.
For further crystal optimization, five truncation constructs of PACSIN 1, 1–333, 1–342, 1–344, 1–346 and 1–384 (Fig. 1 ▶ b), were designed. Drops were prepared by mixing 2 µl protein solution (8 mg ml−1 in 500 mM NaCl, 10 mM HEPES pH 7.5) with 2 µl reservoir solution and were equilibrated against 500 µl reservoir solution.
Figure 1.
SDS–PAGE analysis of purified PACSIN 1. (a) Lane M, markers (kDa); lane 1, full-length PACSIN 1 with N-terminal tags immediately after purification; lane 2, degraded protein from a microcrystal. (b) Purified truncates of PACSIN 1. Lane M, markers (kDa); lanes 1–5 indicate PACSIN 1 truncation constructs 1–333, 1–342, 1–344, 1–346 and 1–384, respectively.
2.3. Diffraction data collection
For crystal data collection, crystals were transferred into the corresponding reservoir solution containing 20% glycerol as a cryoprotectant. The crystal was mounted in a cryoloop and flash-cooled in a nitrogen stream at 100 K. X-ray diffraction data were collected on a MAR 345 image-plate detector at Beijing Synchrotron Radiation Facility (beamline 3W1A), Institute of High Energy Physics, Chinese Academy of Life Sciences. The crystal-to-detector distance was 200 mm. A total of 180 frames of 1° oscillation were measured with 60 s exposure per frame. Data were processed with MOSFLM (Powell, 1999 ▶) and CCP4 (Collaborative Computational Project, Number 4, 1994 ▶).
3. Results and discussion
Purified full-length PACSIN 1 (or truncated PACSIN 1) protein was expressed in E. coli with a typical measured final yield of 5 mg per litre of culture and a purity of over 95% (Figs. 1 ▶ a and 1 ▶ b).
PACSIN 1 microcrystals were obtained using 200 mM NH4H2PO4 pH 7.9, 20%(w/v) PEG 3350. However, SDS–PAGE analysis showed that these microcrystals were a degraded form of full-length PACSIN 1 with a molecular weight of 38 kDa (Fig. 1 ▶ a, lane 2). N-terminal sequencing analysis showed that the N-terminal amino acids of this degraded band were MSSSY, which corresponded to the first five N-terminal amino acids of PACSIN 1, indicating that the N-terminal tags degraded naturally and the microcrystals mainly degraded from the C-terminus.
The full-length PACSIN microcrystal shown in Fig. 2 ▶(a) diffracted to 4.2 Å resolution. Therefore, in order to improve the resolution, we designed and constructed five truncation constructs of PACSIN 1 and obtained a larger crystal of PACSIN 1 (1–344) (600 × 190 × 200 µm) within two weeks from 200 mM NH4H2PO4 pH 7.5, 16%(w/v) PEG 3350 and 5% glycerol (Fig. 2 ▶ b).
Figure 2.
Crystals of PACSIN 1. (a) Crystal of full-length PACSIN 1. (b) Crystal of truncate PACSIN 1 (1–344).
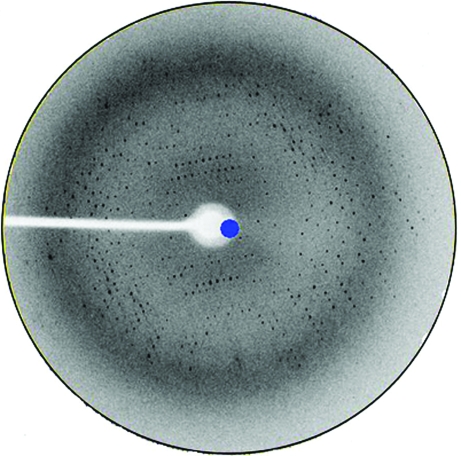
The truncated protein (1–344) diffracted to a higher resolution (3.0 Å; Fig. 3 ▶). The PACSIN 1 (1–344) crystal belonged to space group C2, with unit-cell parameters a = 158.65, b = 87.38, c = 91.76 Å, α = 90.00, β = 113.61, γ = 90.00°. Because it mainly forms dimers in solution and the self-rotation map showed twofold noncrystallographic symmetry (NCS) rotation axes, we presumed there were two molecules in the asymmetric unit and this gave a V M value of 4.16 Å3 Da−1. The crystallographic parameters and data-collection statistics are listed in Table 1 ▶.
Figure 3.
Diffraction pattern of a PACSIN 1 (1–344) crystal.
Table 1. Crystallographic parameters and data-collection statistics.
Values in parentheses are for the last resolution shell.
| Wavelength (Å) | 1.072 |
| Resolution (Å) | 30–3.0 (3.12–3.0) |
| Completeness (%) | 100 (99.7) |
| Rmerge† (%) | 16.3 (51.9) |
| I/σ(I) | 8.6 (2.16) |
| Space group | C2 |
| Unit-cell parameters (Å) | a = 158.65, b = 87.38, c = 91.76 |
| No. of observed reflections | 42008 |
| No. of unique reflections | 22946 |
| Molecules per ASU | 2 |
| VM (Å3 Da−1) | 4.16 |
| Solvent content (%) | 70.47 |
R
merge = 
 .
.
Acknowledgments
We are grateful to Professor Yuhui Dong and Zengqiang Gao at the Institute of High Energy Physics (Chinese Academy of Sciences) for data collection. This work was supported by grants from the National High Technology and Development Program of China (863 Programs, No. 2006AA02A314; 973 Programs, Nos. 2007CB914303 and No. 2010CB911800). Portions of this research involving X-ray diffraction data collection were carried out at the Beijing Synchrotron Radiation Laboratory, a national user facility operated by the Institute of High Energy Physics.
References
- Andersson, F., Jakobsson, J., Low, P., Shupliakov, O. & Brodin, L. (2008). J. Neurosci.28, 3925–3933. [DOI] [PMC free article] [PubMed]
- Anggono, V. & Robinson, P. J. (2007). J. Neurochem.102, 931–943. [DOI] [PubMed]
- Collaborative Computational Project, Number 4 (1994). Acta Cryst. D50, 760–763.
- Farsad, K. & De Camilli, P. (2003). Curr. Opin. Cell Biol.15, 372–381. [DOI] [PubMed]
- Frost, A., Perera, R., Roux, A., Spasov, K., Destaing, O., Egelman, E. H., De Camilli, P. & Unger, V. M. (2008). Cell, 132, 807–817. [DOI] [PMC free article] [PubMed]
- Hilton, J. M., Plomann, M., Ritter, B., Modregger, J., Freeman, H. N., Falck, J. R., Krishna, U. M. & Tobin, A. B. (2001). J. Biol. Chem.276, 16341–16347. [DOI] [PubMed]
- Itoh, T., Erdmann, K. S., Roux, A., Habermann, B., Werner, H. & De Camilli, P. (2005). Dev. Cell, 9, 791–804. [DOI] [PubMed]
- Modregger, J., DiProspero, N. A., Charles, V., Tagle, D. A. & Plomann, M. (2002). Hum. Mol. Genet.11, 2547–2558. [DOI] [PubMed]
- Powell, H. R. (1999). Acta Cryst. D55, 1690–1695. [DOI] [PubMed]
- Powell, K. A. & Robinson, P. J. (1995). Neuroscience, 64, 821–833. [DOI] [PubMed]
- Qualmann, B., Roos, J., DiGregorio, P. J. & Kelly, R. B. (1999). Mol. Biol. Cell, 10, 501–513. [DOI] [PMC free article] [PubMed]
- Sweitzer, S. M. & Hinshaw, J. E. (1998). Cell, 93, 1021–1029. [DOI] [PubMed]