Abstract
Background
Animals carrying genetic mutations have provided powerful insights into the role of interstitial cells of Cajal (ICC) in motility. One classic model is the W/WV mouse which carries loss-of-function mutations in c-kit alleles, but retains minimal function of the tyrosine kinase. Previous studies have documented loss of slow waves and aberrant motility in the small intestine of W/WV mice where myenteric ICC (ICC-MY) are significantly depleted.
Methods
Here we used morphological and electrophysiological techniques to further assess the loss of ICC around the circumference of the small intestine and determine consequences of losing ICC-MY on electrical activity, Ca2+ transients and contractions of the longitudinal muscle (LM).
Key Results
In wild-type mice, there was coherent propagation of Ca2+ transients through the ICC-MY network and spread of this activity to the LM. In short segments of small intestine in vitro and in exteriorized segments, slow waves coordinated smoothly propagating Ca2+ waves and contractions in the LM of wild-type mice. In W/WV mice, Ca2+ waves were initiated at variable sites along and around intestinal segments and propagated without constraint unless they collided with other Ca2+ waves. This activity resulted in abrupt, uncoordinated contractions.
Conclusions & Inferences
These results show how dominance of pacemaking by ICC-MY coordinates propagating contractions and regulates the spontaneous activity of smooth muscle.
Keywords: Ca2+ transients, ICC-MY, longitudinal muscle, slow waves, W/WV
Introduction
Recent evidence suggests that loss of interstitial cells of Cajal (ICC) is associated with numerous gastrointestinal disorders ranging from gastroparesis, pseudo-obstruction and idiopathic constipation 1–5. An animal model of ICC loss, the W/WV mouse, has been used extensively to examine functional changes resulting from lesions in ICC 6–8. A dense network of ICC in the region of the myenteric plexus (ICC-MY) is disrupted in the small intestine of these mice and ICC-MY are largely absent along the anti-mesenteric aspect. However, scattered remnants of ICC-MY networks can be found in the tunica muscularis of the small intestine 7. ICC-IM (which are concentrated in the region of the deep muscular plexus of the mouse and therefore are referred to as ICC-DMP) are preserved in the small bowel of the W/WV mouse 7, making this an ideal model to study the consequences of significant loss of the ICC-MY pacemaker network on motility.
Rhythmic motor activity in the mouse ileum has been shown to be driven by pacemaker activity that is generated and spreads through the ICC-MY network 9–11. Longitudinal muscle (LM) cells are electrically coupled to the ICC-MY network as demonstrated by low resistance electrical pathways between ICC and LM 12,13. In W/WV mice slow waves are not recorded in the CM layer 7, but rhythmic contractile activity with a similar frequency to the frequency of spontaneous contractions in wild-type mice has been observed in strips of muscle from these mice 14, and electrical activity can be recorded in W/Wv mice, consisting of smooth muscle action potentials 15. The prevalence of muscle action potentials appears to be related to the resting membrane potentials of smooth muscle cells, which are usually depolarised by about 10mV in W/WV mice 15. Depolarized membrane potentials moves smooth muscle cells into the window current range for L-type Ca2+ channels and facilitates the development of spontaneous action potentials. Unlike wild-type mice in which rhythmic slow waves organize the activation of L-type Ca2+ channels in smooth muscle cells, spontaneous activation of this conductance would tend to cause more random contractions that may be the cause of the disordered motility and reduced intestinal transit observed by video fluoroscopy in W/WV mice 16. Nevertheless, intestinal transit occurs as a result of the disordered motility in W/WV mice, and there is sufficient digestion and absorption of nutrients, as these mice have normal weights and life expectancies 17. Others have also reported that overall gastrointestinal transit is not significantly reduced in conscious W/WV mice 18, however transit of the small intestine could be only a minor factor in this measurement.
In the present study we have investigated the role of ICC-MY in coordinating rhythmic LM activity and contractile patterns in intact intestinal segments and exteriorized loops of intestine. We used electrophysiological and morphological techniques to verify the pattern of ICC-MY loss around the circumference of the small intestine to determine whether alternative mechanisms can activate the LM in the absence of ICC-MY. Ca2+ imaging was used to investigate ICC-MY and LM activation and video imaging was used to study micro-motions of segments of mouse small intestine and macroscopic movements of exteriorized intestinal loops. Our data document the aberrant motility patterns that result from significant lesions in the ICC-MY network in the small intestine and suggest that these cells exert a powerful organizational drive on the intrinsic excitability of smooth muscle cells.
Materials and Methods
Tissue preparation
Three different strains of mice of either sex (age 30–60 days, Jackson Laboratory, Bar Harbour, MN) were used in this study. BALB/c and WBB6F1/J-Kit+/Kit+ mice were used to assess normal longitudinal muscle (LM) activity and W/Wv mice were used to assess LM behavior when ICC-MY were disrupted. The use and treatment of animals was approved by the Institutional Animal Use and Care Committee at the University of Nevada, in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
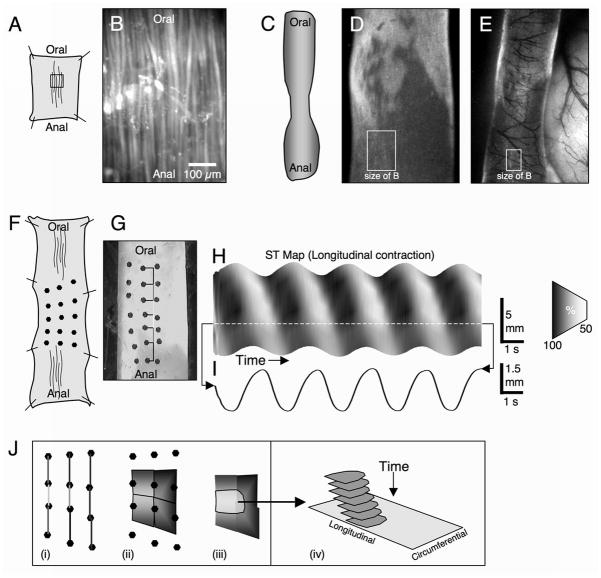
For experiments involving isolated preparations, mice were euthanized by isoflurane inhalation (Baxter, Deerfield, IL) and cervical dislocation. A midline abdominal incision was made and a segment of terminal ileum (3–4 cm in length) was removed, flushed clean with KRB solution (see composition below) and was either opened along the mesenteric border (flat-sheet; Fig 1A, B, F, G) or kept intact (tubular; Fig 1C–E). Flat-sheet preparations were pinned mucosa side down, and tubular segments pinned at either end in a Sylgard lined organ bath continuously perfused with oxygenated KRB solution @ 36.0 ± 0.5 °C.
Figure 1.
Preparations used to study Ca2+ transients in ICC-MY and LM and longitudinal contractions in the mouse small intestine from the cellular to organ level. A & B. show a flat-sheet preparation used to examine the spread of Ca2+ transients at the cellular level where single LM cells and underlying ICC-MY could be resolved. In some experiments the LM was removed to better resolve the activity of ICC-MY. To examine the spread of Ca2+ waves in the longitudinal axis and around the circumference of the small bowel, isolated tubular preparations (D) or exteriorized loops of small intestine (E) were used. LM contractions were monitored using a grid of surface markers positioned on flat-sheet preparations (F&G). The distance between pairs of markers were calculated dynamically, expressed in grayscale (see vertical bars in G), and were used to create spatio-temporal maps (H) showing the change in LM contraction (I) over time. Spatio-temporal cubes were constructed by constructing a grid of quadrangles between surface markers (J). Each corner of each quadrangle was positioned midway between relevant pairs of surface markers (J (i)). The color at each corner corresponded to the longitudinal distance between relevant pairs of surface markers. Individual quadrangles were filled by blending the colors at each corner throughout the quadrangle using linear interpolation (J (ii)). Pixels that were above a certain level of contraction (color) were thresholded (J (iii)) and a marching cubes algorithm was used to construct 3 dimensional spatio-temporal objects (J (iv)) from thresholded areas in frames throughout the movie.
To examine Ca2+ activity and contractions in exteriorized loops of small intestine, mice were anesthetized by a single intraperitoneal dose of Nembutal (Pentobarbitone Sodium; 60mg/mL) for 30–60 minutes prior to surgery. Once the absence of a hind limb pinch withdrawal reflex had been established, a paramedical (left hand side) laparotomy (20mm in length) was performed ~10mm rostral to the pelvic brim. A loop of terminal ileum (40mm in length) was exposed and draped into a neighboring organ bath. The mesenteric attachment of the exposed segment of ileum was gently pinned to the base of a Sylgard-lined organ bath, being sure not to sever any fine mesenteric blood vessels supplying the ileum. The longitudinal muscle and serosal surface of the exteriorized loop of ileum lay on a coverslip that was installed in the base of the organ bath and was fully submerged in oxygenated warm Krebs solution that was constantly perfused at 36°C. To ensure comfort and viability of the animals during any experimentation, animals were placed on a heated moistened blanket to maintain the surface temperatures at 36°C. At the conclusion of the experiment, mice were humanely euthanized by exsanguination under anesthesia, in accordance with approved operating procedures determined by the UNR Institutional Animal Care and Use Committee (IACUC).
Solutions and Drugs
The Krebs solution used in this study contained (in mM): 120.4 NaCl, 5.9 KCl, 15.5 NaHCO3, 11.5 glucose, 1.2 MgCl2, 1.2 NaH2PO4, 2.5 CaCl2. This solution had a pH of 7.3–7.4 at 37°C when bubbled with 97% O2 - 3% CO2. Dimethyl sulfoxide (DMSO), Cremophor EL were obtained from Sigma-Aldrich Co (St. Louis, MO). β-glycyrrhetinic acid (β-GA) nifedipine and nicardipine were purchased from Sigma.
Dye Loading
After an equilibration period (30 minutes), isolated flat-sheet and tubular preparations were loaded with 25μg of fluo-4 (FluoroPure™-AM, Molecular Probes, Eugene, OR) in a solution of 0.02% DMSO and 0.01% non-toxic detergent Cremophor EL for 20 minutes @ 25°C. The dye was preferentially loaded into the outer LM layer, as the mucosa provided a barrier to the solution from diffusing through or underneath these preparations. After incubation, the preparation was perfused with warm KRB solution for 20 minutes to allow for de-esterification of the dye 19, 9. For anesthetized mice, the dye solution was applied directly to the exteriorized loop for 15 minutes at 37°C. Following loading, the exteriorized loop was rinsed with warm KRB solution for 5 minutes, followed by 15 minutes equilibration to allow for de-esterification of the dye.
Kit labeling of ICC-MY
Tissues were fixed in acetone (4°C; 10 min) then were washed overnight in phosphate buffered saline (PBS; 0.01M, pH 7.2) and rewashed with fresh PBS the following day for 4 hours with a change of PBS every hour. Tissues were subsequently incubated in bovine serum albumin (BSA; 1%; 1 hour at room temperature) to reduce non-specific antibody binding. ICC-MY were identified using antibodies raised against hSCF-R (guinea-pig; diluted 1:500 in 0.5% Triton-X 100; R&D Systems Inc, Minneapolis, MN, USA) incubated for 48 hours at 4°C. After washing in PBS, the tissues were then incubated with appropriate Alexa fluor 488 secondary antibodies (Molecular Probes; 1:500 in PBS; 1 hour, room temperature). Control tissues were prepared by omitting either primary or secondary antibodies from the incubation solution.
Imaging Equipment
Ca2+ activity was monitored using either an upright Nikon TS 100 microscope (Nikon Inc., Melville, NY) or water immersion microscopes (Olympus BX50 [Olympus, Center Valley, PA] or Nikon Eclipse E600FN). A number of lenses were used to record the spread of Ca2+ transients and/or contractions at different magnifications (2×, 10×, 20× & 40×). Image sequences were captured using a Cascade 512B camera (Roper Scientific Inc., Trenton, NJ) and MetaMorph 6.26 software (Universal Imaging Corporation™, Molecular Devices Corporation, Downington, PA). Surface marker movements (see below) were viewed using a stereo dissecting microscope (SMZ1000, Nikon) and HD Camera (DMK31AF03, The Imaging Source, Charlotte, NC) and image sequences were recorded using AstroIIDC software (Aupperle Services and Contracting, Calgary, Alberta). The distribution of ICC-MY around the circumference of the small intestine was examined using a Zeiss LSM 510 META confocal microscope (Carl Zeiss Microimaging, Thornwood, NY). All image sequences were visualized and analyzed using custom-written software (Volumetry G7mv, GWH).
Image Analysis
As preparations were not treated with L-type Ca2+ channel inhibitors, there was significant movement of the preparation that needed to be stabilized. Tracking routines were used extract the X, Y coordinates of particles throughout image sequence. Ca2+ transients from individual LM cells were extracted and the frequency, time course, rates-of-rise and amplitude were measured. Ca2+ activity that spread through the LM syncitia (Ca2+ waves) was visualized and measured using spatio-temporal maps (ST Maps; see 19). The velocity (both parallel and perpendicular to the long axis of LM cells), stability, number of initiation sites and size of the wave front during propagation and coherence of propagation of Ca2+ waves were calculated from ST Maps.
Surface Marker Arrays
We used surface marker arrays 20 to determine the coordination of longitudinal and circular contractions in W/WV mice. Long segments (5 cm) of mouse ileum were opened along the mesenteric border and were pinned flat using pins only at each corner, thereby allowing the middle regions to contract in an unimpeded fashion. Small black pieces of glitter (100μm diameter) were placed approximately 3mm apart to form a 2D array (5 × 5) and motor behavior was recorded onto video. The coordinates of each surface marker were tracked using custom built algorithms (see Fig 1G–I), and the distance between pairs of markers was calculated for the duration of the movie. These distances were converted into a grayscale such that whiter shades represent pairs of markers closer together (contraction), and blacker shades represent pairs of markers further apart (elongation/relaxation). If no comparisons were needed between ST maps, the grayscale equates to the % change in separation compared to the maximum separation distance, otherwise the grayscale was converted to Δmm per standardized average separation (1 mm). The calculated distance of separation between 2 markers was placed midway between the markers, and linear interpolation was used to fill in regions between distance of separation points, allowing the construction of spatio-temporal maps of longitudinal contraction (Fig 1I).
Spatio-temporal Cubes
To better visualize the spread of longitudinal contractions both longitudinally and circumferentially, spatio-temporal contraction objects were constructed using the following method. The longitudinal separation between pairs of markers was calculated as described above (Fig 1J (i)). Quadrangles were constructed so that each corner of each quadrangle was positioned midway between pairs of markers (see Fig 1J (ii)) with the color of each corner corresponding to longitudinal distance between the relevant pair of surface markers. The colors at each corner were blended using linear interpolation to fill the quadrangle. This procedure was repeated for all markers in the array to create a continuous grid of colored quadrangles for each frame of the movie. Pixels in the quadrangle grid that had a contraction value (color) above a certain level (0.2 mm.mm−1) were thresholded (see Fig 1J (iii)) and a marching cubes algorithm was used to create an iso-surface between thresholded areas in frames throughout the movie. Spatio-temporal contraction objects were smoothed (Laplacian, 7 iterations) and were rendered in 3D (see Fig 1J (iv) & Fig 5B & E).
Figure 5.
Spread of Ca2+ transients and LM contraction in W/WV small intestine. In flat-sheet preparations (A) the circumferential spread of Ca2+ transients in LM was irregular with dramatic changes in frequency, direction and length of propagation (see asterisks in B & C). In some preparations trains of Ca2+ transients were observed in LM (C). A disordered pattern of Ca2+ transients was also observed in isolated tubular preparations (D & E), and resulted irregular, abrupt movements (E). Ca2+ transients in the LM often propagated for at least several millimeters at high velocity (F–I), however the site of initiation was variable and LM transients were not coordinated around the circumference of the small intestine.
Electrical Recording
Impalements of cells were made with glass microelectrodes having resistances of 80–120 MΩ. Transmembrane potentials were recorded with a standard electrometer (Intra 767; World Precision Instruments, Sarasota, FL, USA) and a PC running AxoScope 8.0 data acquisition software (Axon Instruments). To identify the LM or CM cells from which recordings were made, propridium iodide (0.1% w/v in 3 m KCl) was added to electrode filling solution. After intracellular recordings were performed, tissues were fixed with paraformaldehyde (4% (w/v) in 0.1 m PB) and examined with a confocal microscope with the appropriate excitation for propridium iodide (535 nm). All experiments were performed in the presence of nifedipine (1 μm) to reduce contractions and facilitate impalements of cells for extended periods.
Statistics
Results are expressed as means ± standard error (SE). One-way ANOVA and Neuman-Keuls post-hoc tests were used to compare results from controls and W/WV mutant mice (Prism, GraphPad Software Inc., La Jolla, CA, USA).
Results
Distribution and function of ICC-MY in wild-type and W/WV mice
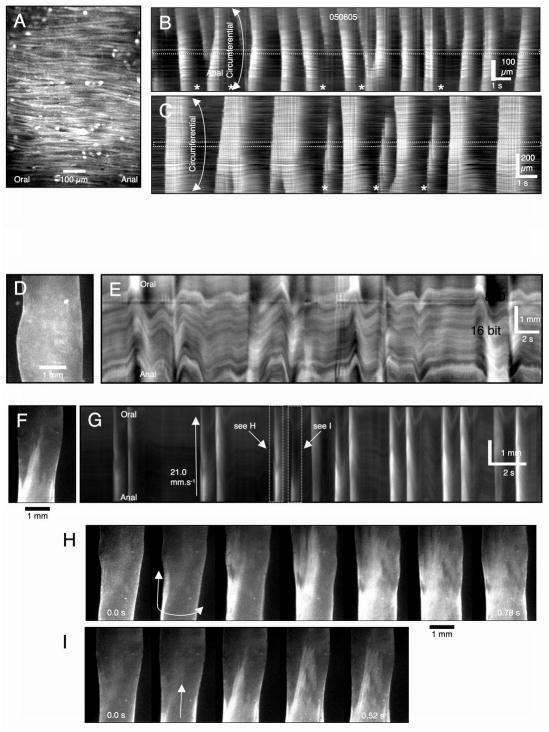
The goal of the present study was to measure the effect of reduced ICC-MY on motor patterns in small intestinal muscles, so we revisited the distribution of ICC-MY in tissues of W/WV mice 7. Previous studies have shown a gradient in the severity of loss of ICC-MY depending upon anatomical location in the stomach 21, but there are no reports about the distribution of ICC-MY in the circumferential axis of the small intestine. In the present study whole mount preparations were made from tissue samples taken from 3 points around the circumference of small bowels of wild-type and W/WV mice, extending from the mesenteric to the anti-mesenteric border. A dense, uniform network of ICC-MY was found in wild-type tissues from each region, demonstrating the lack of a circumferential gradient in ICC-MY (Fig. 2A–C). The ICC-MY network was greatly reduced in all samples of small intestine from W/WV animals (Fig 2D–F), but small clusters of ICC-MY were observed in some samples from the midpoint and in samples from the mesenteric region (Fig. D–E). The clusters of ICC-MY may be poorly connected since there were no visible connections between them, suggesting that while pacemaker cells may still be present in the mesenteric region, there slow wave activity would be poorly coupled. ICC-DMP were present and distributed normally in muscles of W/WV mice (Fig 2D–F).
Figure 2.
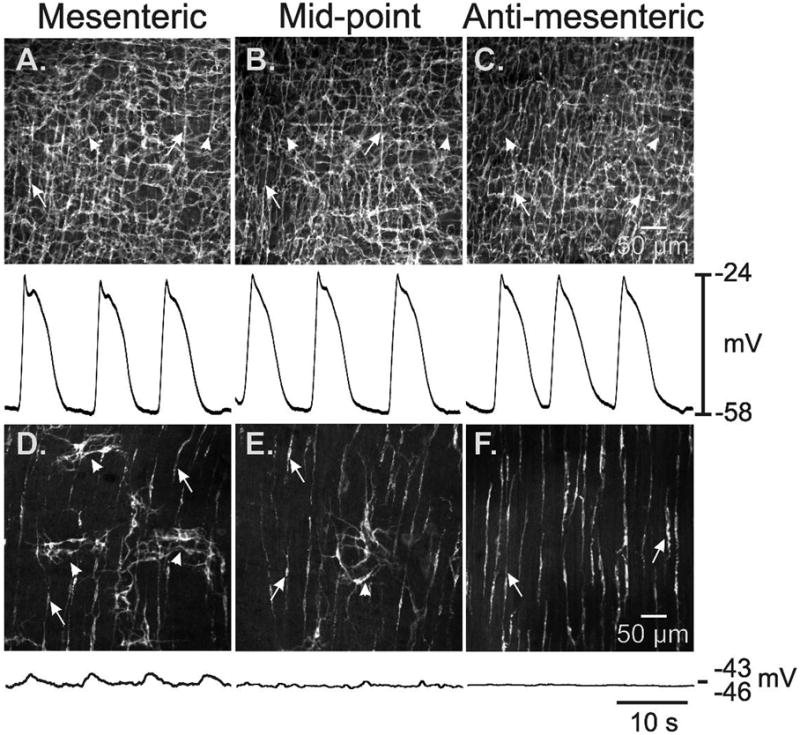
ICC networks and electrical slow waves in the small intestines of W/WV mutants. A dense network of ICC-MY (see arrowheads) and ICC-DMP (see arrows) was observed from the mesenteric to the anti-mesenteric region of wild-type mouse small intestine (A–C). Slow waves with similar characteristics were recorded at each of these positions (below anatomical panels). In W/WV mice isolated patches of ICC-MY (arrowheads) were observed in the mesenteric region (D), and, in some cases, midway between the mesenteric and anti-mesenteric regions (E). ICC-MY were rare in the anti-mesenteric region (F). ICC-DMP (arrows) were unaffected in all three regions, as previously reported 7. In a few impalements in the mesenteric region, very low amplitude slow waves were recorded (D; trace below anatomical panel). Little or no rhythmic slow wave activity was recorded at other positions around the circumference of the small intestine. Scale bars in panels C and F apply to panels A–C and D–F respectively.
We impaled smooth muscle cells to make intracellular electrical recordings in muscles of wild-type and W/WV mice taken from the same regions of the small intestine used for morphological examination. In wild-type tissues, slow waves of similar characteristics were recorded from all regions around the circumference of the bowel (Fig. 2A–C). Recordings from W/WV muscles confirmed that slow waves were absent from most cells impaled. However, small-amplitude slow waves (2.5 ± 0.5 mV amplitude; 33.0 ± 5.1 cpm) were recorded in a few cells in muscles from the myenteric region (Fig 2D–F; 4 of 42 impalements, n=5 muscles). As previously reported 7, no slow waves were observed in impalements of muscles from the anti-mesenteric region (Fig 2F). Thus, this brief morphological and electrophysiological survey suggests that studies to determine the role of ICC-MY on motor activity are best focused on muscles of the anti-mesenteric region where the greatest lesion in ICC-MY and slow wave activity occurs.
There have been reports that a different form of electrical rhythmicity dominates LM, because the pharmacology of phasic contractions differed in muscle strips oriented in the axis of the LM and CM 22. We impaled cells identified by propridium iodide filling in the CM (resting membrane potential −53 ± 2 mV, n = 14 cells from 4 animals) and LM (resting membrane potential −56 ± 2.7 mV, n = 11 cells from 4 animals) layers of W/WV mice to test whether a unique (i.e. non-ICC-MY) pacemaker generates slow waves, activates LM, and remains functional in the absence of ICC-MY. The LM is very active mechanically, so these experiments were conducted in the continuous presence of nifedipine (1μM) to facilitate impalements of sufficient duration to fill cells. These experiments demonstrated that slow waves were present at the same frequency and at the same approximate amplitude in LM and CM of wild-type mice, and slow waves were absent in muscles of W/WV mice (Fig 3). These data suggest that the LM and CM are both paced by ICC-MY in the small intestine.
Figure 3.
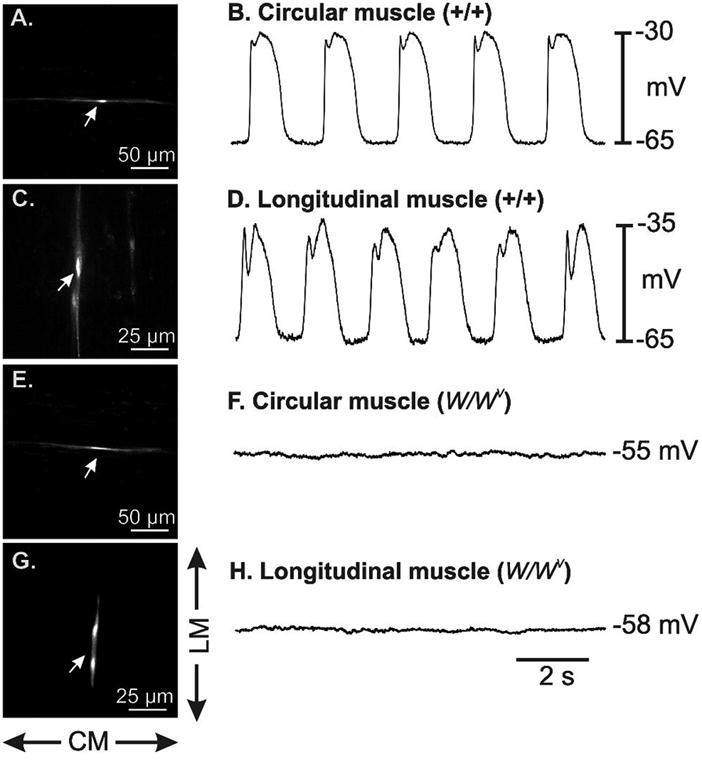
Electrical activity recorded from the circular (CM) and longitudinal muscle (LM) cells of wild-type (+/+) and W/WV mutants. CM and LM displayed similar slow wave activity in wild-type small intestinal muscles (A–D). Panels A and B show a propridium iodide (PI) filled CM cell and slow wave activity recorded from the cell that was filled. Panels C and D show PI filling of a LM cell and slow wave activity recorded from the cell. Slow wave activity was not observed in the CM and LM of W/WV muscles. Panels E and F show PI filled cell from the CM lacking slow wave activity and panels G and H show a PI filled cell from the LM also lacking slow wave activity. Impaled smooth muscles were in the anti-mesenteric region. All recordings were taken in the presence of nifedipine, 1M, to stabilize the muscles during filling. Scale bars are as indicated in each panel. Directions of CM and LM are indicated adjacent to panel G.
LM Ca2+ transients in wild-type and W/Wv mice
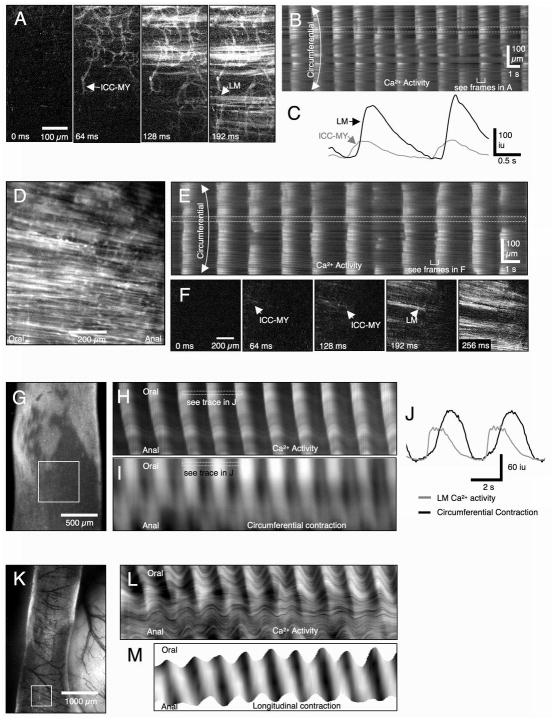
Ca2+ transients in flat-sheets, tubular and exteriorized loops of the small intestine of wild-type mice consisted of rhythmic Ca2+ waves that spread rapidly through the LM layer. In flat-sheet preparations in which the LM was partially dissected away, the ICC-MY network was revealed, and Ca2+ transients in ICC-MY propagated as coherent waves as previously reported 9. The pacemaker role of ICC-MY was clearly demonstrated in these tissues because Ca2+ transients in ICC-MY preceded LM transients (by 66–127 ms) and developed fully before LM transients developed (see Fig 4A–C). In preparations in which the full thickness of the LM was intact, Ca2+-induced fluorescence in ICC-MY networks was not easily resolved but was still visible and preceded LM activation (see Fig 4D–F). In tubular (Fig 4G–J) or excised loop preparations (Fig 4K–M), only LM activity was detectable using low power objectives, but the longitudinal spread of Ca2+ waves in the LM could be measured with this technique (see Supplementary Movies 1 & 2). The circumferential spread of Ca2+ waves was equivalent to activity observed using high power objectives in flat-sheet preparations (see below).
Figure 4.
Spread of Ca2+ transients and LM contractions in wild-type small intestine. In a preparation in which the LM was partially removed, Ca2+transients spread in the ICC-MY network (A). The frequency and direction of propagation of Ca2+ transients in ICC-MY and LM were consistent throughout recording periods (B). Ca2+ increased approximately 0.1s before LM fibers (running horizontally) overlying the ICC-MY network were activated, indicating ICC-MY pace LM (C). Ca2+ waves with consistent frequency and direction were also observed in an preparation with intact LM (D&E), Faint Ca2+ transients were observed in ICC-MY approximately 0.1s before LM was activated (F). In isolated tubular preparations (G), the frequency, velocity and direction of propagating Ca2+ waves was constant during periods of recording (H). Ca2+ transients in the LM preceded circumferential contraction (I) by approximately 0.5–1s (J). In exteriorized loops (K) a similar constant patterns of Ca2+ waves were observed (L) prior to longitudinal contractions (M).
The frequency (~30cpm) and variability in the interval (~7%) of Ca2+ transients in the LM of wild-type mice was similar in flat-sheet, isolated tubular and exteriorized loop preparation of mouse ileum (p > 0.05, Table 1A; see Fig 4B). In 1/5 experiments in flat-sheet preparations, multiple Ca2+ peaks were observed, however the majority of transients consisted of a single peak (average number of peaks = 1.1 ± 0.03, n=4), followed by a slow decay to baseline (Tau = −0.35 ± 0.04 s, n=4). The duration of Ca2+ transients in the LM at 50% amplitude was 0.66 ± 0.04 s (n=4). In isolated tubular or exteriorized loop preparations, Ca2+ transients in the LM propagated along the segment at similar velocities of ~4 mm.s−1 (Table 1A). Ca2+ transients in the LM preceded longitudinal shortening and circular contraction by ~1s (Fig 4C). In exteriorized loop preparations, multiple Ca2+ transients in the LM were observed around the circumference of the preparations but were constrained within a band ~1.5mm long that propagated at ~4 mm.s−1 (see Supplementary Movie 2).
Table 1.
Frequency and velocity of Ca2+ transients in 3 different preparations of wild-type mouse ileum (A). Frequency of contractions in flat-sheet ileum preparations from wild-type and W/Wv mice (B).
| Table 1A: Ca2+ Imaging Data (Wild-type) | |||
|---|---|---|---|
| Parameter | Flat-sheet | Isolated Tube | Exteriorized Loop |
| Number of Animals | 4 | 7 | 6 |
| Frequency (cpm) | 29.8 ± 2.4 | 30.0 ± 5.4 | 30.3 ± 1.5 |
| Interval (s) | 2.01 ± 0.16 | 2.0 ± 0.36 | 1.98 ± 0.1 |
| Δ Interval (s) | 0.15 ± 0.008; 7.5 % | 0.12 ± 0.016; 5.6% | 0.15± 0.03; 7.6% |
| Velocity (mm.s−1) | - | 3.8 ± 0.7 | 4.2 ± 0.5 |
| Δ Velocity (mm.s−1) | - | 0.75; 20% | 0.77; 18% |
| Table 1B: Video Imaging Data (Flat-sheet; Wild-type & W/Wv) | ||
|---|---|---|
| Parameter | Wild-type | W/Wv |
| Number of Animals | 9 | 4 |
| Freq (cpm) | 25.9 ± 0.2 | 35.3 ± 9.3 |
| Interval (s) | 2.31 ± 0.13 | 1.7 ± 0.45 |
| Δ Interval (s) | 0.125 ± 0.04; 5.4 % | 0.62 ± 0.34*; 59.0 % |
Δ Interval is the average change in interval from event to event.
Δ Velocity is the average change in velocity from event to event.
denotes p=0.03
In small intestinal muscles from the anti-mesenteric region of W/Wv mice, the ICC-MY network was not observed under the LM layer. Ca2+ activity in the LM consisted of irregularly occurring Ca2+ waves that propagated at variable velocities and directions and terminated spontaneously or were annihilated when they collided with Ca2+ waves propagating from a different site of origin (see Fig 5A–C: see asterisks). Unlike in wild-type muscles, LM of W/Wv mice also displayed trains of Ca2+ transients that were likely to be due to muscle action potentials as these events were blocked by nicardipine, 2 μM (see Fig 5C). Ca2+ waves occurred irregularly in isolated tubular preparations and were initiated at different sites along and around the preparation (see Supplementary Movie 3). Ca2+ waves did not appear simultaneously around the entire circumference. Provided there were no other Ca2+ waves along the preparation, Ca2+ waves propagated rapidly along the entire segment (Fig 5D–I; see Supplementary Movie 4)
In flat-sheet preparations the frequency of Ca2+ transients in the LM of W/Wv mice was 28.2 ± 1.0 cpm (interval: 2.13 ± 0.8, n=5). The variability in interval was 0.72 ± 0.27 s, which equates to a change in frequency of 34% event-to-event (i.e. ~6 fold greater variability than in wild-type mice). In 3/5 experiments, trains of Ca2+ transients were observed (5.5 ± 1.36 transients, n=3) resulting in an elevated period of Ca2+ fluorescence (average number of peaks = 3.1 ± 1.1, n=5). The duration of single Ca2+ transients at 50% amplitude was 0.47 ± 0.10s (n=5, see Fig 5D). The frequency of Ca2+ transients in the LM of W/Wv mice in isolated tubular preparations was 12.8 ± 3.7 cpm (significantly slower compared to wild-type, p=0.03, n=3). The velocity of propagation of Ca2+ waves in the LM was 22.3 ± 3.3 mm.s−1 (n=3) which is ~6x faster compared to the velocity of Ca2+ waves in the LM of wild-type mice (p<0.001).
Motor patterns in wild-type and W/Wv mice
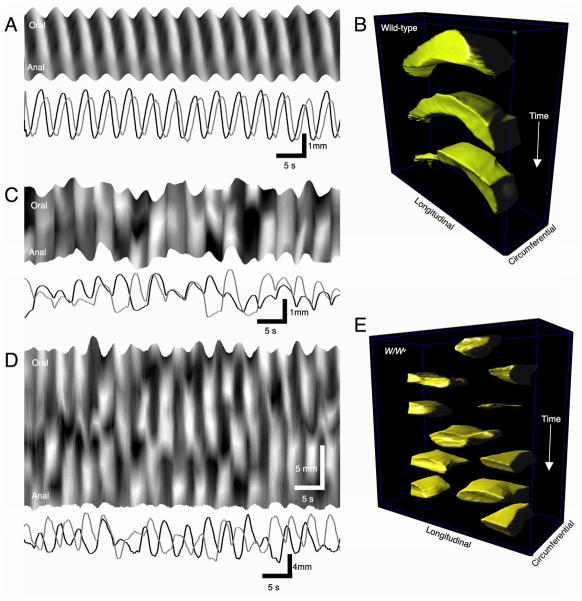
Motility mapping of ileal contractions in wild-type mice using surface marker arrays revealed a stable pattern of rhythmic, propagating contractions. These contractions were most prominent in the longitudinal axis and originated at the oral end of the muscle preparation for the duration of the recordings. The average frequency of ileal contractions was 25.9 ± 0.2 cpm and waves of contraction propagated along the entire length of the ileal segments at an average velocity of 5.61 ± 0.37 mm.s−1 with very little variation in interval or velocity from wave-to-wave (see Table 1B & Fig 6A–B). The direction of propagation was consistent over long periods of time indicating the stability of the dominant pacemaking site (see Supplementary Movie 5).
Figure 6.
Pattern of LM contraction in wild-type and W/WV mice. LM contractions were constant in wild-type mice during recording periods, with contractions originating at the same frequency from the same region of the preparation and propagated in the aboral direction at constant velocities (A). The consistent LM contractions around the circumference of the small bowel are portrayed in the spatio-temporal cubes in B. In W/WV mice, LM contractions were abrupt and originated at variable positions within the preparations (C). Multiple initiation sites were observed in the small intestine of W/WV mice and propagating events collided (D & E).
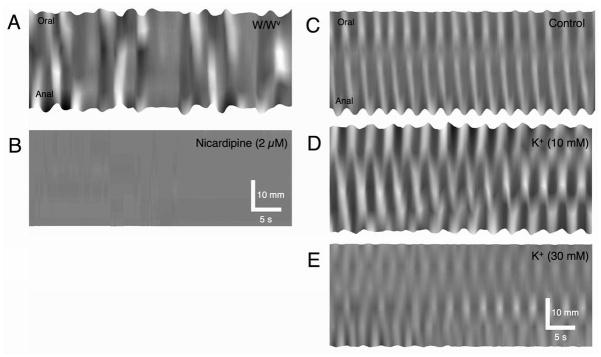
W/WV mice displayed forceful contractions, however this activity was abrupt and uncoordinated in comparison to the activity in wild-type tissues (Fig 6C–E; see Supplementary Movie 6). The loci of initiation of contraction and pattern of propagation were highly unstable, shifting from event-to-event, and like recordings of Ca2+ transients in LM in much smaller regions of muscle (see above), there were many collisions of contractions. The lack of coordination in this activity was reflected by a measurement of stability (see below). Multiple loci of initiation of contractions were observed in segments of the same size that were used to evaluate motility patterns in wild-type tissues (Fig 6D). The overall frequency of 35.3 ± 9.3 cpm was not significantly different from controls (p > 0.05), however the variability in interval was significantly greater (Table 1B). The velocity of propagating contractions in wild-type mice was 5.61 ± 0.37 mm.s−1 (n=9), however it was difficult to delineate the velocity of propagating contractions in W/WV mice due to abruptness of the contractions and short propagation length. To test whether LM muscle movements were reliant on Ca2+ entry through L-type Ca2+ channels, we added nicardipine (1–2 μM). Addition of nicardipine blocked all rhythmic activity (Fig 7A & B).
Figure 7.
Effects of block of L-type Ca2+ channels and depolarization. Contractions in W/WV mice (A) were reliant upon Ca2+ entry through L-Type Ca2+ channels because nicardipine (2μM) blocked all contractions (B). Propagating contractions in wild-type small intestines (C) were disrupted by elevation of external K+ (to 10 mM: D) and the number of pacemaker initiation sites increased. Higher concentrations of external K+ (30 mM: E) compounded this effect, however the amplitude of contractions decreased.
Effect of depolarization
It has been previously reported that loss of ICC-MY from the mouse ileum results in a ~10 mV depolarisation of smooth muscle cells 7. We depolarized wild-type preparations with K+ (10mM & 30mM) to see if a motor pattern similar to that observed in W/Wv mice could be evoked. Longitudinal pendular movements 22 were enhanced by increasing extracellular K+ to 10mM, and the number of sites of initiation increased and appeared less stable. At higher doses (30mM), the amplitude of pendular contractions diminished. At all concentrations of K+, the frequency of pendular movements was similar to controls (Figure 7C–E).
Discussion
This study represents another significant step in the phenotyping of the electrical and mechanical of the W/WV mouse, which has been invaluable in developing an understanding of ICC in GI motor function. Previous studies have shown that tissues lacking ICC-MY lack the slow wave pacemaker mechanism 7,8, and loss of these cells and slow waves results in aberrant motility small intestine in vivo and reduced propagation of spontaneous action potentials in segments in vitro16. In this study we demonstrated in a more rigorous manner that ICC-MY facilitate coordinated, propagated contractions in intestinal muscles. Ca2+ transients, which are coincident with electrical slow wave activity 9, propagate in coherent waves through the network of ICC-MY, and electrical activity spreads to adjacent muscle layers. When ICC-MY are reduced in numbers, smooth muscle cells depolarize and develop intrinsic rhythmicity based on the activation of L-type Ca2+ channels 7,23,15. The electrical activity intrinsic to smooth muscle results in considerably different motility patterns that are the consequence of spontaneous generation of smooth muscle Ca2+ transients and contractions from random sites within the muscle. These changes include i) non-circumferential activation of muscle layers, ii) unimpeded spread of activity, unless collisions with other waves occurs, and iii) lack of ordered directional waves of activity (i.e. failure of pacemaker activity to dominate large areas of muscle). Thus, while W/WV intestinal muscles are still capable of phasic contractile activity, loss of coordination resulting from loss of slow waves has important consequences on patterns and efficiency of intestinal motility 16.
It has been incorrectly assumed by some authors that loss of ICC-MY is absolute in small intestinal muscles of W/WV mice 18,24,25. The first report detailing the status of ICC-MY in the small intestine documented greatly reduced numbers of ICC, but clusters of cells, amounting to about 10% of the ICC-MY population in wild-type animals, were still apparent (see Fig. 1 in 7). As in the stomach 21, we showed in the present study that there are also regional differences in the degree of loss of ICC-MY in the small intestine, with greater reduction in cells in the anti-mesenteric region than in the mesenteric region. There appear to be sufficient ICC-MY in the mesenteric region to generate small amplitude slow waves. Thus, it may be possible to record a variety of electrical patterns from small intestinal tissues of W/WV mice with extracellular or volume electrodes in vivo, however the weak slow wave signals from remaining clusters of ICC-MY are unlikely to entrain smooth muscle activity making it difficult resolve specific ICC/muscle components clearly. In previous electrophysiological studies we confined our recordings to the anti-mesenteric border of the small intestine where the loss of ICC-MY is most extensive to determine the role of these cells in pacemaker activity 7. In the present study we also studied Ca2+ transients and motor activity of the anti-mesenteric region of the bowel, again to understand how substantial loss of ICC-MY affects patterns of activation. We also investigated motility in intact intestinal segments, however, to determine whether the defects we noted in dissected muscles may have been due to tissue manipulation. Our data suggest that the loss of ICC-MY results in the emergence of spontaneous activity from smooth muscle cells in the form of spontaneous action potentials, and this activity can result in phasic muscular contraction. This consequence was manifest in preparations of any size and in intact small intestinal segments. Propagation of action potentials is likely to be the mechanism by which groups of smooth muscle cells can develop phasic contractions in muscles lacking ICC-MY. As shown previously, there is considerable difference in the pattern of propagation of action potentials and slow waves 26,27. Slow waves propagate in an isotropic manner, equally in both the circular and longitudinal axes, while action potentials propagation displays a high degree of anisotropy, with the dominant direction of propagation favoring the long axis of longitudinal muscle fibers. The irregular nature of contractile events we observed in the absence of ICC-MY may be related to the fact that action potentials abruptly die out 26. Thus, tissue wide coordination of activity is poorly accomplished by action potential propagation. In contrast, slow waves propagate continuously over large areas of tissue and are thus more suited to organization of contractile waves in GI muscles.
Electrical slow waves, generated by ICC-MY in the small intestine, naturally organize contractile activity into phasic contractions, and simultaneous recordings of electrical and mechanical activity have demonstrated the relationship between these parameters 9. Slow waves result in membrane depolarization into a potential range that increases the open probability of L-type Ca2+ channels (CaV1.2). Ca2+ action potentials develop in smooth muscle cells, and Ca2+ entry via this mechanism with or without intracellular Ca2+ release initiates excitation-contraction coupling. During, the ‘diastolic’ period between slow waves, membrane potential returns to more negative potentials where the open probability for L-type Ca2+ channel opening decreases. Ca2+ entry is reduced and Ca2+ retrieval/extrusion mechanisms reduce the concentration of cytoplasmic Ca2+, causing relaxation. Thus, the slow wave cycle of ICC-MY exerts a contraction/relaxation cycle that is dictated by the frequency of slow waves. In regions of the small intestine in which ICC-MY are greatly reduced and the slow wave pacemaker apparatus is compromised (as in W/WV mice), membrane potentials of smooth muscle cells depolarize about 10 mV 7,15, bringing these cells into a range of potentials where L-type Ca2+ channels can generate ‘window current’ 28. This is a state determined by the voltage-dependent properties of these channels in which there is a low level of activation and incomplete inactivation. Thus, it is possible for small inward currents via L-type Ca2+ channels to depolarize cells to threshold and generate spontaneous action potentials in smooth muscle cells. Ca2+-dependent outward currents (e.g. large and small-conductance Ca2+-activated K+ channels are expressed in GI muscle cells; 29,30), and activation of these channels, due to Ca2+ entry during action potentials, tends to push threshold toward less negative values. Activation of Ca2+-dependent outward currents might organize smooth muscle activity into rhythmical bursts of action potentials (i.e. phasic activity), as occurs in other excitable cells (see 31). These conditions are not easily duplicated in the continued presence of slow wave activity (i.e. in wild-type muscles), as slow waves are not blocked in ICC-MY by a small increment of depolarization (e.g. 10–15 mV; 32). We have previously shown that depolarization of wild-type muscles by only 10 mV was capable of disrupting normal propagation of slow waves 33, thus the organization of contractions due to propagation of slow waves is affected by depolarization. The effects of depolarization in wild-type tissues, however, did not simulate exactly the patterns of behavior of muscles lacking ICC-MY.
Data from the present study emphasizes the importance of slow waves in providing pacemaker activity that over-rides the natural tendency of smooth muscle cells to generate spontaneous activity. This is analogous to the heart in which cells downstream of the SA node, such as Purkinje cells, are excitable and have the potential for spontaneous rhythmicity, but proper pacemaker frequency and directionality of cardiac action potential propagation are maintained due to higher frequency of SA node pacemakers. While much emphasis has been placed on the activation of muscle layers in an orderly and coherent manner by ICC-MY, this study shows that suppression of spontaneous LM activity by slow waves is equally important. Our data demonstrate that without slow waves (i.e. without the pacemaker activity of ICC-MY), spontaneous activity of smooth muscle cells emerges, but this compensatory excitability lacks organization and directional control of propagation, resulting in multiple activity fronts, annihilating collisions of activity, and highly disorganized motor patterns. In wild-type animals, slow wave pacemaker activity results in orderly contractions around the entire circumference of the small bowel. In the absence of slow waves, spontaneous LM activity was initiated at random points around the circumference, thereby creating contractions with serpentine movements. These aberrant movements may explain the abnormal intestinal movements observed in W/WV mice with video-fluoroscopy 16. The negative membrane potentials achieved in wild-type muscles during the period between slow waves may be fundamental to the organizational characteristics of slow waves because the open probability for openings of Ca2+ channels is low during these potentials, thereby preventing spontaneous muscle activity.
There are important motility behaviors that are not lost in W/WV mice and this may account for how these animals survive and even maintain approximately equal body weight with their wild-type siblings17. ICC-DMP, involved in transduction of neural inputs from excitatory and inhibitory motor neurons 34 are preserved in W/WV mice 7,8. Thus, neural reflexes involving activation of the muscularis are maintained in the small intestine of these mice, and they may be able to respond with polarized responses in a manner analogous to wild-type mice (i.e. enhanced motor activation above a stimulus and relaxation distal to the stimulus). Migrating motor complexes (MMC) are recorded from the small intestines of wild-type mice and these responses depend upon input from enteric neurons 33. Normal MMC are also recorded in W/WV mice 35. Thus, the development and propagation of organised motor patterns, such as MMC, are due to regulation by intrinsic neurons and not dependent upon ICC-MY, slow waves or the intestinal slow wave gradient. Video fluoroscopic studies showed that contractions were highly irregular in W/WV mice 16, and this form of activity may still accomplish one of the main purposes of segmentation in the small intestine: stirring of contents to facilitate digestion and absorption of chyme. The aberrant motility in W/WV tissues and intestinal loops may be functionally compensated for by the combined myogenic activity of intestinal muscles and the regulation of muscular excitability by enteric motor neurons. It is likely, however, that a more generalized loss of ICC, involving major losses in both ICC-MY and ICC-DMP, may result in a much more severe motility disorder, such as intestinal pseudo-obstruction 36,37.
The main defective motility feature lost in association with reduced ICC-MY is organization and coherent propagation of phasic contractions. This defect may be a generalized feature of GI motility disorders in which a substantial lesion in ICC-MY develops. Our data also indicate that disruption in the normal motility patterns can occur without total loss of ICC-MY or when normal muscles experience minor tonic depolarization.
Supplementary Material
Regular spread of Ca2+ transients in LM (Wild-type mouse small intestine: isolated tubular preparation).
Regular spread of Ca2+ transients in LM (Wild-type mouse small intestine: exteriorized loop preparation).
Uncoordinated Ca2+ transients in LM (W/WV mouse small intestine: isolated tubular preparation).
Fast spreading Ca2+ transients in LM (W/WV mouse small intestine: isolated tubular preparation).
Regular propagating contractions in LM (Wild-type mouse small intestine: flat-sheet preparation with surface markers).
Multiple uncoordinated contractions in LM (W/WV mouse small intestine: flat-sheet preparation with surface markers).
Acknowledgments
The authors would like to acknowledge Guizhi Song for excellent technical assistance in making the intracellular electrical recordings and Sang Don Koh for discussions about membrane potential regulation. Support from: NIH P01 DK41315 (KMS, SMW, SJW and HTL); R01 DK45713 (TKS) & COBRE P20RR018751 (GWH and NJS). Ca2+ imaging was performed in a Core laboratory supported by P20 RR-18751. Morphology images were collected using a Zeiss LSM510 confocal microscope obtained with support from NIH1 S10 RR16871.
Footnotes
Competing Interests: the authors have no competing interests.
References
- 1.Vanderwinden JM, Rumessen JJ. Interstitial cells of Cajal in human gut and gastrointestinal disease. Microsc Res Tech. 1999;47:344–360. doi: 10.1002/(SICI)1097-0029(19991201)47:5<344::AID-JEMT6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 2.Sanders KM, Ordög T, Koh SD, Torihashi S, Ward SM. Development and plasticity of interstitial cells of Cajal. Neurogastroenterol Motil. 1999;11:311–38. doi: 10.1046/j.1365-2982.1999.00164.x. [DOI] [PubMed] [Google Scholar]
- 3.Burns AJ. Disorders of interstitial cells of Cajal. J Pediatr Gastroenterol Nutr. 2007;45:103–6. doi: 10.1097/MPG.0b013e31812e65e0. [DOI] [PubMed] [Google Scholar]
- 4.Rolle U, Piaseczna-Piotrowska A, Puri P. Interstitial cells of Cajal in the normal gut and in intestinal motility disorders of childhood. Pediatr Surg Int. 2007;23:1139–52. doi: 10.1007/s00383-007-2022-7. [DOI] [PubMed] [Google Scholar]
- 5.Farrugia G. Interstitial cells of Cajal in health and disease. Neurogastroenterol Motil. 2008;20:54–63. doi: 10.1111/j.1365-2982.2008.01109.x. [DOI] [PubMed] [Google Scholar]
- 6.Maeda H, Yamagata A, Nishikawa S, et al. Requirement of c-kit for development of intestinal pacemaker system. Development. 1992;116:369–75. doi: 10.1242/dev.116.2.369. [DOI] [PubMed] [Google Scholar]
- 7.Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol. 1994;480:91–97. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huizinga JD, Thuneberg L, Klüppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–9. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- 9.Park KJ, Hennig GW, Lee HT, et al. Spatial and temporal mapping of pacemaker activity in interstitial cells of Cajal in mouse ileum in situ. Am J Physiol. 2006;290:1411–27. doi: 10.1152/ajpcell.00447.2005. [DOI] [PubMed] [Google Scholar]
- 10.Hirst GD, Edwards FR. Role of interstitial cells of Cajal in the control of gastric motility. J Pharmacol Sci. 2004;96:1–10. doi: 10.1254/jphs.crj04002x. [DOI] [PubMed] [Google Scholar]
- 11.Sanders KM, Koh SD, Ward SM. Interstitial cells of cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006;68:307–343. doi: 10.1146/annurev.physiol.68.040504.094718. [DOI] [PubMed] [Google Scholar]
- 12.Cousins HM, Edwards FR, Hickey H, Hill CE, Hirst GD. Electrical coupling between the myenteric interstitial cells of Cajal and adjacent muscle layers in the guinea-pig gastric antrum. J Physiol. 2003;550:829–44. doi: 10.1113/jphysiol.2003.042176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitsui R, Komuro T. Direct and indirect innervation of smooth muscle cells of rat stomach, with special reference to the interstitial cells of Cajal. Cell Tissue Res. 309:219–27. doi: 10.1007/s00441-002-0592-1. [DOI] [PubMed] [Google Scholar]
- 14.Boddy G, Willis A, Galante G, Daniel EE. Sodium-, chloride-, and mibefradil-sensitive calcium channels in intestinal pacing in wild-type and W/WV mice. Can J Physiol Pharmacol. 2006;84:589–99. doi: 10.1139/y06-009. [DOI] [PubMed] [Google Scholar]
- 15.Malysz J, Thuneberg L, Mikelsen HB, Huizinga JD. Action potential generation in the small intestine of W mutant mice that lack interstitial cells of Cajal. Am J Physiol. 1996;271:387–99. doi: 10.1152/ajpgi.1996.271.3.G387. [DOI] [PubMed] [Google Scholar]
- 16.Der-Silaphet T, Malysz J, Hagel S, Arsenault LA, Huizinga JD. Interstitial cells of cajal direct normal propulsive contractile activity in the mouse small intestine. Gastroenterology. 1998;114:724–36. doi: 10.1016/s0016-5085(98)70586-4. [DOI] [PubMed] [Google Scholar]
- 17.Chi MM, Powley TL. c-Kit mutant mouse behavioral phenotype: altered meal patterns and CCK sensitivity but normal daily food intake and body weight. Am J Physiol Regul Integr Comp Physiol. 2003;285:1170–1183. doi: 10.1152/ajpregu.00015.2003. [DOI] [PubMed] [Google Scholar]
- 18.Yin J, Hou X, Chen JD. Roles of interstitial cells of Cajal in intestinal transit and exogenous electrical pacing. Dig Dis Sci. 2006;51:1818–23. doi: 10.1007/s10620-006-9313-z. [DOI] [PubMed] [Google Scholar]
- 19.Hennig GW, Smith CB, O’Shea DM, Smith TK. Patterns of intracellular and intercellular Ca2+ waves in the longitudinal muscle layer of the murine large intestine in vitro. J Physiol. 2002;543:233–53. doi: 10.1113/jphysiol.2002.018986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forrest AS, Hennig GW, Jokela-Willis S, Park CD, Sanders KM. Prostaglandin regulation of gastric slow waves and peristalsis. Am J Physiol. 2009;296:1180–90. doi: 10.1152/ajpgi.90724.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ordög T, Baldo M, Danko R, Sanders KM. Plasticity of electrical pacemaking by interstitial cells of Cajal and gastric dysrhythmias in W/W mutant mice. Gastroenterology. 2002;123:2028–40. doi: 10.1053/gast.2002.37056. [DOI] [PubMed] [Google Scholar]
- 22.Daniel EE, Willis A, Cho WJ, Boddy G. Comparisons of neural and pacing activities in intestinal segments from W/W++ and W/W(V) mice. Neurogastroenterol Motil. 2005;17:355–65. doi: 10.1111/j.1365-2982.2005.00639.x. [DOI] [PubMed] [Google Scholar]
- 22.Bayliss WM, Starling EH. The movements and innervation of the small intestine. J Physiol. 1901;26:125–38. doi: 10.1113/jphysiol.1901.sp000827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ward SM, Burns AJ, Torihashi S, Harney SC, Sanders KM. Impaired development of interstitial cells and intestinal electrical rhythmicity in steel mutants. Am J Physiol. 1995;269:1577–85. doi: 10.1152/ajpcell.1995.269.6.C1577. [DOI] [PubMed] [Google Scholar]
- 24.Hou X, Yin J, Liu J, Pasricha PJ, Chen JD. In vivo gastric and intestinal slow waves in W/WV mice. Dig Dis Sci. 2005;50:1335–41. doi: 10.1007/s10620-005-2783-6. [DOI] [PubMed] [Google Scholar]
- 25.Sarna SK. Are interstitial cells of Cajal plurifunction cells in the gut? Am J Physiol. 2008;294:372–90. doi: 10.1152/ajpgi.00344.2007. [DOI] [PubMed] [Google Scholar]
- 26.Lammers WJ, Slack JR. Of slow waves and spike patches. NIPS. 2001;16:138–44. doi: 10.1152/physiologyonline.2001.16.3.138. [DOI] [PubMed] [Google Scholar]
- 27.Tomita T. Electrical activity (spikes and slow waves) in gastrointestinal smooth muscles. In: Bulbring E, Brading AF, Jones AW, Tomita T, editors. Smooth Muscle. Edward Arnold Ltd; 1981. pp. 127–156. [Google Scholar]
- 28.Cohen NM, Lederer WJ. Calcium current in isolated neonatal rat ventricular myocytes. J Physiol. 1987;391:169–91. doi: 10.1113/jphysiol.1987.sp016732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benham CD, Bolton TB, Lang RJ, Takewaki T. The mechanism of action of Ba2+ and TEA on single Ca2+-activated K+ -channels in arterial and intestinal smooth muscle cell membranes. Pflugers Arch. 1985;403:120–7. doi: 10.1007/BF00584088. [DOI] [PubMed] [Google Scholar]
- 30.Koh SD, Dick GM, Sanders KM. Small-conductance Ca(2+)-dependent K+ channels activated by ATP in murine colonic smooth muscle. Am J Physiol. 1997;273:2010–21. doi: 10.1152/ajpcell.1997.273.6.C2010. [DOI] [PubMed] [Google Scholar]
- 31.Yarom Y, Sugimori M, Llinás R. Ionic currents and firing patterns of mammalian vagal motoneurons in vitro. Neuroscience. 1985;16:719–37. doi: 10.1016/0306-4522(85)90090-9. [DOI] [PubMed] [Google Scholar]
- 32.Torihashi S, Ward SM, Nishikawa S, Nishi K, Kobayashi S, Sanders KM. c-kit-dependent development of interstitial cells and electrical activity in the murine gastrointestinal tract. Cell Tissue Res. 1995;280:97–111. doi: 10.1007/BF00304515. [DOI] [PubMed] [Google Scholar]
- 33.Bayguinov O, Ward SM, Kenyon JL, Sanders KM. Voltage-gated Ca2+ currents are necessary for slow-wave propagation in the canine gastric antrum. Am J Physiol Cell Physiol. 2007;293:1645–59. doi: 10.1152/ajpcell.00165.2007. [DOI] [PubMed] [Google Scholar]
- 34.Sanders KM, Koh SD, Ward SM. Organization and eletrophysiology of interstitial cells of Cajal and smooth muscle cells in the gastrointestinal tract. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. 4. Elsevier Press; 2006. pp. 533–76. [Google Scholar]
- 35.Spencer NJ, Sanders KM, Smith TK. Migrating motor complexes do not require electrical slow waves in the mouse small intestine. J Physiol. 2003;55:881–93. doi: 10.1113/jphysiol.2003.049700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Struijs MC, Diamond IR, Pencharz PB, et al. Absence of the interstitial cells of Cajal in a child with chronic pseudoobstruction. J Pediatr Surg. 2008;43:25–9. doi: 10.1016/j.jpedsurg.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 37.Yamataka A, Ohshiro K, Kobayashi H, et al. Abnormal distribution of intestinal pacemaker (c-KIT-positive) cells in an infant with chronic idiopathic intestinal pseudoobstruction. J Pediatr Surg. 1998;33:859–62. doi: 10.1016/s0022-3468(98)90660-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Regular spread of Ca2+ transients in LM (Wild-type mouse small intestine: isolated tubular preparation).
Regular spread of Ca2+ transients in LM (Wild-type mouse small intestine: exteriorized loop preparation).
Uncoordinated Ca2+ transients in LM (W/WV mouse small intestine: isolated tubular preparation).
Fast spreading Ca2+ transients in LM (W/WV mouse small intestine: isolated tubular preparation).
Regular propagating contractions in LM (Wild-type mouse small intestine: flat-sheet preparation with surface markers).
Multiple uncoordinated contractions in LM (W/WV mouse small intestine: flat-sheet preparation with surface markers).