Abstract
Replacement or regeneration of load bearing soft tissues has long been the impetus for the development bioactive materials. While maturing, current efforts continue to be confounded by our lack of understanding of the intricate multi-scale hierarchical arrangements and interactions typically found in native tissues. The current state of the art in biomaterial processing enables a degree of controllable microstructure that can be used for the development of model systems to deduce fundamental biological implications of matrix morphologies on cell function. Furthermore, the development of computational frameworks which allow for the simulation of experimentally derived observations represents a positive departure from what has mostly been an empirically driven field, enabling a deeper understanding of the highly complex biological mechanisms we wish to ultimately emulate. Ongoing research is actively pursuing new materials and processing methods to control material structure down to the micro-scale to sustain or improve cell viability, guide tissue growth, and provide mechanical integrity all while exhibiting the capacity to degrade in a controlled manner. The purpose of this review is not to focus solely on material processing but to assess the ability of these techniques to produce mechanically sound tissue surrogates, highlight the unique structural characteristics produced in these materials, and discuss how this translates to distinct macroscopic biomechanical behaviors.
INTRODUCTION
Advances in regenerative medicine continue in response to the ever growing need for tissue replacement options. To meet this need, a multidisciplinary approach combining biology, medicine, and engineering is required to overcome the significant challenges preventing the successful repair or replacement of biomechanically functioning tissues. Many of the reasons for poor implant performance or failure remain ill defined. Often they are a combination of inadequate or miss-matched mechanical properties and biological complexities. As such, a great deal of effort focuses on gaining a deeper insight into the structural and behavioral characteristics of native tissues to guide design criteria for the development of tissue surrogates.
Despite the multitude of challenges, many early approaches and technologies have shown promising results. For example, heart valve prostheses derived from bovine pericardium or porcine aortic valves have long been used to enhance survival and improve the quality of life of patients presenting with a variety of valvular maladies. Similarly, engineered dermal grafts have successfully been used clinically to treat severe burns or wounds that would otherwise be unable to close and heal properly. Pioneering work by Badylak et al. in applying decellularized extracellular matrix scaffolds has also shown successes in regenerating organized tissue after severe tissue loss or injury [1]. In addition to providing invaluable educational experience to guide future efforts, this incremental progress moves the field ever closer towards the ultimate goal of developing technologies for safer and more efficacious tissue repairs and replacements.
In order for an engineered tissue to perform a predominantly load bearing function and sufficiently recapitulate the mechanical behavior of native tissues, advancements in the current technologies are necessary to attain more complex biological functionality as well as biomechanical stability. It is generally accepted that both chemical and mechanical factors modulate cell biosynthesis when producing extracellular matrix [2–5]. Healthy native tissues undergo intricate, multi-scale modes of deformation which work synergistically with biochemical stimuli to determine physiologic responses. In order to mimic native tissue structure and organization it is first necessary to develop techniques to produce scaffolds in a controlled manner with characteristic lengths on a scale comparable to those observed in nature (Figure 1).
Figure 1.
Ability to produce scaffolds which mimic native tissue constituent scales. Viable tissue replacements are confounded by complex multi-scale architectures, hierarchical interactions, and modes of deformation characteristically observed in native tissues. Overcoming the limitations of current medical therapies necessitates new production methods or adaptations to current techniques to produce scaffolds in a controlled manner with characteristic lengths comparable to those observed in nature.
The ability to create engineered tissue replacements would be enhanced by a detailed command of the complex, dynamic, and reciprocal interactions which occur at the cell-matrix interface. This includes how mechanical cues from the tissue or organ level are transmitted to the cell or cellular components and elucidation of the signaling pathways responsible for the cellular processes observed experimentally. Furthermore, the use of new technologies in the production of engineered scaffolds necessitates a detailed understanding of the structure-function relationships unique to these materials. Currently, the exact microstructural characteristics of engineered scaffolds (that induce deformations experienced by the cellular inclusions) often remain ill defined and presumably will have a profound influence on cellular function. Long term efficacy of tissue replacements or regenerative therapies will rely on the critical processes of cell proliferation and differentiation, the production of organized matrix, and concurrent tissue remodeling or growth. This review will survey commonly used materials, from native extracellular matrix to a range of synthetic scaffold materials and their unique processing methods, with an emphasis on assessing these techniques to produce mechanically sound viable tissue surrogates, highlight the unique structural characteristics produced in these materials, and discuss how this translates to macroscopic mechanical function.
NATIVE ECM ISOLATION FOR THERAPEUTIC APPLICATIONS
One of the earliest implemented approaches for tissue replacement was the use of processed or preserved allograft and homograft tissues. These tissues are typically obtained from cadaveric sources and cryopreserved without chemical cross-linking to control biological function for transplantation into another individual. Cryopreserved allograft heart valves exhibit good hemodynamic profiles and require little or no chronic anticoagulation therapy but are plagued by progressive degradation characterized by leaflet distention, fibrosis, possible leaflet calcification or even valve stenosis attributed to somatic growth of the surrounding tissues. Similarly, xenograft tissues are used with proper tissue processing to overcome immunogenetic responses and stabilize the extracellular matrix. Chemical processing (i.e. cross-linking) of xenograft tissues has long been used in therapeutic applications to stabilize collagen architecture while significantly reducing the risks of an immunogenic response.
The mechanical response of native and glutaraldehyde treated porcine aortic valve leaflets and bovine pericardium have been systematically evaluated in their application to valve replacement therapies [6–11]. After chemical cross-linking, the highly mobile fiber kinematics typical of native valvular tissues is altered significantly owing to disparate mechanical behavior such as becoming less extensible [6]. Furthermore, it has been shown that cross-linked tissues for valve replacement are susceptible to fatigue induced changes which lead to compromised mechanical behavior. These injuries can present in both locations of calcific nucleation and without calcification [12–14]. Lastly, aldehyde cross-linking of tissues with glutaraldehyde, which is known to exhibit cytotoxic properties, is associated with poor host cell infiltration. This lack of viable cells precludes growth and remodeling, resulting in an inability to maintain tissue homeostasis and repair structural insults [15]. Unfortunately, the processing techniques required for storage (cryopreservation) or matrix stabilization (cross-linking) of valvular tissue replacements significantly alters tissue mechanical behavior resulting in a continuing risk for morbidity and mortality [16].
More recently, non-crosslinked terminally sterilized biologic scaffolds of naturally occurring extracellular matrix have shown promise as a therapeutic option. For example, porcine small intestinal submucosa (SIS) has been successfully used in multiple applications to treat damaged or diseased tissue in human patients (i.e. musculotendinous, dermal, cardiovascular, gastrointestinal, lower urinary tract). This approach represents a “top down” method to obtain materials for therapeutic purposes whereby undesirable constituents such as cellular materials are stripped away leaving potentially bioactive ECM. DNA and membrane proteins have been implicated in the immune response typical of transplanted tissues and must be removed while organized ECM proteins are relatively well conserved across species causing lower rates of rejection [17]. Though there are many variables affecting the viability of these materials, such as tissue source (presence of antigens), rate of scaffold degradation, and manufacturing methods, they have been shown to exhibit adequate mechanical function in many load bearing applications.
The mechanical response of these materials is dictated by their fibrous constituent arrangements and kinematics. Systematic evaluations of decellularized tissues are currently limited with the exception of small intestinal submucosa (SIS), urinary bladder matrix (UBM), and aortic valve leaflets. SIS and valvular tissue sources exhibit anisotropic mechanical behaviors owing to a preferred fiber alignment while UBM (submucosa and tunica propria) has a more isotropic fiber orientation and mechanical behavior [18]. The mechanical resiliency of an individual SIS layer is typically inadequate but the material mechanical response can be tailored to particular applications by the use of multiple layers [19]. Gilbert et al. used a multi-layer SIS device in the repair of canine Achilles tendon. The implant showed the ability to support the remodeling response of host cell infiltration and tissue ingrowth, leading to an organized, dense collagenous SIS-ECM similar to normal tendon tissue without catastrophic loss of function [1].
Despite these intriguing in-vivo remodeling events, the processing techniques required to eliminate cellular debris from these tissues can cause profound alterations in mechanical behavior. Chemical cross-linking, as mentioned above, alters the collagen fiber architecture resulting in a change in mechanical behavior. In a recent study by Liao et al., the effects of chemical decellularization on the mechanical and structural properties of the porcine aortic valve leaflet were investigated [20]. In short, an anionic detergent, enzymatic agent, and a non-ionic detergent (sodium dodecyl sulfate, Typsin, Triton X-100 respectively) were used to process porcine aortic valve leaflets prior to mechanical testing or structural examination [20]. It was determined that the processing chemicals were responsible for profound increases in tissue extensibility and reductions in flexural rigidity all while no measureable change in gross fiber orientation was observed. Other common methods for terminal sterilization of biological materials include ethylene oxide, gamma radiation, and electron beam irradiation and each have unique effects on material behavior. When SIS is terminally sterilized, each of the above sterilization methods has been shown to reduce maximum material stiffness. Both methods of irradiation have been shown to reduce maximum force at failure and maximum tangential stiffness while ethylene oxide treatment induced marked increases in extensibility without significant alterations to force at failure [21]. The specific mechanisms by which the matrix architecture is altered during manufacturing are currently ill defined and require further investigation to improve the effectiveness of these technologies.
SYNTHETIC ECM AND PROCESSING METHODS FOR SYNTHETIC SCAFFOLDS
Though native tissues represent ideal scaffolds in their in-vivo form and function, the use of native ECM based scaffolds can have significant drawbacks. As mentioned above, host recognition of antigens can induce an acute or chronic immunogenic host response compromising implant function. In addition, the processing methods required for the production of these materials can be a source of inconsistencies between specimens resulting in varied degradation behaviors and mechanical responses. The use of biodegradable synthetic scaffolds can circumvent the pathogen transmission and immune recognition concerns associated with collagen based tissues from animal or cadaver sources, albeit with local inflammation characteristics of such implants.
Designing scaffolds for tissue repair represents a “bottom up” approach whereby scaffolds are produced with desired chemical, physical, and mechanical characteristics in a controlled and reproducible manner to recapitulate native ECM structure and function. Common physical characteristics of interest include: surface texture to promote cell attachment, highly porous microstructure to allow tissue ingrowth, and an interconnected porous network to allow adequate nutrient transport all while maintaining a desired mechanical behavior (Table 1). As such, material processing techniques are implemented to produce 3D scaffolds with desired material behaviors which encourage desirable cellular responses. For the purposes of this article, scaffold morphologies will be segregated into two types: non-fibrous and fibrous. Both scaffold types have unique manufacturing processes and characteristics allowing them to function in a wide range of mechanical environments. Obtaining tissue-like tensile mechanical behaviors can be challenging with non-fibrous scaffolds as it is difficult to match the highly anisotropic and highly non-linear response characteristic of soft collagenous tissues. Fibrous scaffolds on the other hand, have the ability to bear significant tensile loads while maintained relatively low bending rigidities and can be manufactured to exhibit varying mechanical behaviors. Furthermore, long fiber composites better mimic natively observed matrix architectures with the potential to direct 3D tissue formation and organization [22].
Table 1.
Structural scaffold design criteria and their corresponding function for engineered tissues.
| Scaffold design criteria | Resulting function in engineered tissue | |
|---|---|---|
| Biologic compatibility | Non-toxic and minimal inflamatory response | |
| Non-thrombogenic | ||
| Non immunogenic | ||
| Low or zero toxicity (degradation products) | ||
| 3D matrix architecture | Physiologically relevant environment for cell function | |
| Known multi-scale architectural features mediating macro- micro transmission of force | ||
| Void space | Highly porous and interconnected pores allow cell infiltration, transport of nutrients, humoral factors, and waste products |
|
| Surface chemistry and topography | Cell attachment and cell-matrix interactions | |
| Degradation rate | Scaffold gives way to functional matrix formation | |
| Structural anisotorpy | Anisotropic mechanical behavior | |
| Influence orientation of cells and ECM deposition | ||
| Appropriate mechanical behavior | Seamless integration with surrounding tissue(s), able to | |
| withstand in-vivo forces, and avoid stress shielding | ||
Non-fibrous synthetic scaffolds
Polymer scaffold processing techniques such as phase separation, particle leaching, and high pressure gas foaming can all produce highly porous morphologies and provide a 3D environment to support a viable cell population (Figure 2). Each of these processes allows a degree of control over pore size and pore structure; both of which are critical during culture to facilitate cellular ingrowth [23]. For instance, if pores are too small or not well interconnected, cell penetration will be limited or nutritional support of interior cells will be insufficient.
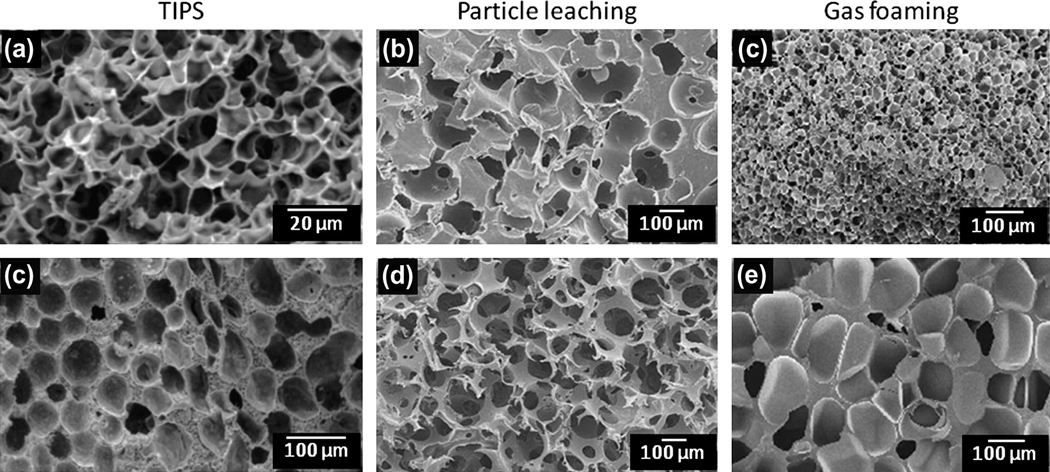
Figure 2.
Cross-sectional scanning EM micrographs of non-fibrous methods to manipulate micro-morphology. (a, d) Porous poly (L-lactic acid) prepared via TIPS at 30 °C with metastable state residence times of (a) 5 min, (b) 60 min so as to control pore size. (b, e) Polycaprolactone scaffold created by particle leaching with a poly(ethyl methacrylate) bead (200 µm diameter) porogen. Increased porosity was attained by compressing the beads in a mould prior to injecting melted polycaprolactone. (c, f) A porous poly(ester amine) sample prepared via gas foaming at 105 °C. By increasing the gas saturation pressure, pore density is increased and average pore diameter decreases. Saturation pressure equals 20 bar and 40 bar respectively. Figure adapted with permission from [175–177].
Emulsification/freeze-drying and thermally induced phase separation (TIPS) are two commonly employed phase separation approaches used in the production of non-fibrous scaffolds [24–28]. The porous media produced by phase separation techniques are capable of attaining porosities greater than 96% [29]. Moreover, TIPS is a versatile processing method allowing considerable control over scaffold morphology [30] with phase separation thermodynamics playing a crucial role in determining structure [31, 32]. The process of solvent casting and particulate leaching allows the preparation of regularly porous structures and can produce scaffolds with ~87% porosity and pore sizes greater than 100 µm.. However, the reliance on cytotoxic solvents for these techniques means that special care must be taken to remove such hazardous chemicals to ensure cell viability.
Gas foaming represents a processing technique which circumvents the use of cytotoxic organic solvents [33, 34]. The cell nucleation process during expansion is important as it dictates scaffold microstructure and in turn, material properties. Manufacturing parameters affecting scaffold morphology include: processing temperatures, degree of saturation, hydrostatic pressure, interfacial energy, and visco-elastic properties of gas/polymer mixture [34]. Some drawbacks of this process include excessive heat during compression molding, which limits the incorporation of thermo-labile materials, and difficulties obtaining highly interconnected pore structures.
From a mechanical design perspective, using these techniques must strike a balance between porosity and mechanical integrity to withstand the dynamic environment of a load bearing tissue. Increased porosity necessitates a reduction of scaffold material per unit volume, reducing mechanical strength. Furthermore, tailoring mechanical behavior to emulate tissue mechanical behavior such as anisotropy is difficult and requires an ability to controllably induce structural anisotropy. The thermally induced phase separation technique can be modified by applying thermal gradients during the separation process to induce oriented pore formation resulting in an anisotropic material response with the preferred material direction being approximately 6 fold stiffer (Figure 3 a,d) [35, 36] . Moreover, the oriented pores could potentially induce a cell micro-patterning effect to orient the cells and the matrix which they produce.
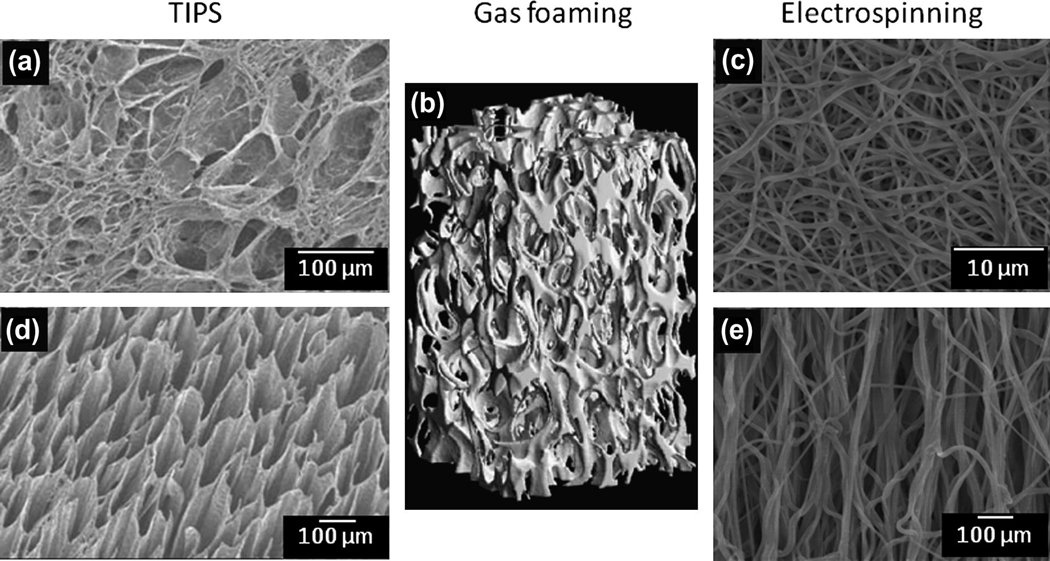
Figure 3.
Attaining structural anisotropy through material processing. (a, d) Polyurethane scaffolds produced by thermally induced phase separation with oriented pores produced by imposing a heat transfer gradient during cooling. (b) Supercritical gas foaming used to create open cell composite foams for bone tissue engineering which exhibit morphologic and mechanical anisotropy with pores oriented in the foaming direction. (c, e) Electrospinning allows controlled fiber deposition by the use of a rotating collection surface and has the potential to produce scaffolds which exhibit gross, anisotropic soft tissue-like mechanical behaviors. Figure adapted with permission from [35, 178].
Fibrous synthetic scaffolds
Synthetic scaffolds comprised of fibrous micro or nano-architectures present many advantages for tissue engineering applications. Namely, long continuous structures with diameters on the order of native ECM (50 – 500 nm) grossly approximate the local cellular environment (Figure 4). A population of fibrous structures makes them appropriate for handling tensile loads while maintaining relatively low bending rigidities. Control of the distribution of fibers during manufacturing enables the production of scaffolds exhibiting a wide array of mechanical behaviors. Furthermore, scaffolds comprised predominantly of fibrous structures provide high surface area to volume ratios and high porosity. These characteristics encourage cell contact and transport of nutrients or removal of waste products respectively.
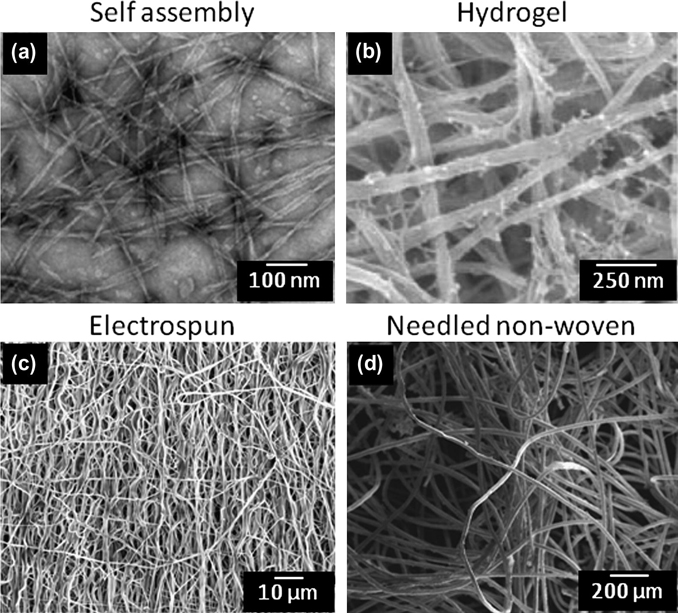
Figure 4.
Scanning electron micrographs of methods commonly employed to create 3D scaffolds exhibiting fibrous structures with diameters on the order of native ECM. (a) Nano-scale self-assembled alkylated peptide amphiphiles forming tristed ribbon morphologies [179]. (b) Biopolymer gels like those made of fibrin readily support cellular viability and can be used to investigate cellular behavior in a reasonably well controlled environment. (b) Electrospinning scaffolds for tissue engineered applications have seen widespread use owing to the inherent ability to produce a synthetic matrix with tunable fiber architectures (diameter, orientation, etc.). (c) Needled nonwoven fabrics made of biocompatible polymers have shown promise in tissue engineered efforts to produce de-novo ECM in a well defined microstructure. Figure adapted with permission from [180, 181]
Self assembly
Self assembling materials are a relatively new technology capable of producing nano-scale fibers. The process of self-assembly can be observed throughout the natural world (nucleic acid synthesis, protein synthesis, etc.) and is mediated by weak non-covalent bonds, ionic bonds, hydrophobic interactions, van der Waals interactions, and water mediated hydrogen bonds [37, 38]. Self assembly or self-organization is a spontaneous event where individual components combine to form an ordered structure with preprogrammed non-covalent bonds within and between molecules. This process can be used to produce a range of structures which can themselves self-organize into superstructures [37].
Controlling the self assembly process is a complex procedure and fabricating 3D scaffolds with reproducible microstructure and satisfactory mechanical properties poses significant challenges. Self assembled materials typically exhibit morphological characteristics on a sub-cellular scale. For instance, fiber dimensions are on the order of 5–10 nm in diameter and 1 µm in length with pore diameters ranging from 5–200 nm. These materials have many relevant applications such as the investigation of molecular interactions [39], environmental sensors, use as a delivery vehicle for drugs or cells [40], and the development of 3D tissue engineering scaffolds [41, 42]. Bioactive molecularly engineered nanofibers have also shown promising results in guiding de novo tissue regeneration processes such as angiogenesis [43, 44]. As the production methods mature, this technology may prove viable in the production of organized structures up to the macroscopic scale comparable to native tissues. Furthermore, understanding mechanisms of the self assembly process may prove valuable in attempts to guide in-vivo or in-vitro self assembly to regenerate viable tissues or organs.
Hydrogels
Hydrogels represent a state of material and are comprised of cross-linked, hydrophilic polymers allowing them to maintain large fractions of interstitial fluid (water) [45]. The properties of these materials can be designed for various applications by specifying material characteristics such as biocompatibility, permeability, mechanical and chemical stability, as well as easily controlling gross scaffold geometry and cellular distribution. Cellularized hydrogel constructs can be produced by three main methods; adhesion, matrix entrapment, and mico-encapsulation [46, 47]. Hydrogels can be rather weak, exhibiting only marginal mechanical strength. However, their mechanical properties can often be tailored to a reasonable degree by controlled alterations of their microstructure. The density of cross-links (chemical bonds, ionic interactions, hydrogen bonds, physical bonds) directly influences mechanical strength both in shear and tension [48]. Polymerization conditions can also dramatically alter the material behavior of the hydrogel produced. Alterations in reaction time, temperature, and the amount of solvent used can influence polymer structure, number of cross-links, and type of cross-linking bonds formed.
In tension, hydrogels typically do not exhibit material strengths observed in dense collagenous tissues. Instead, these materials exhibit ultimate stress levels on the order of tens to several hundred kPa. Due to their hydrogel characteristics, significant amounts of fluid (incompressible) can be retained, producing a high compressive modulus comparable with native articular cartilage. As a result, hydrogel materials are often investigated in orthopedic applications for the treatment or repair of articular cartilage displaying loss of function due to arthritis or acquired via severe trauma to the joint. Retention of their aqueous component during loading is important since fluid loss often results in reduced mechanical integrity, visco-elastic like behavior, and can even produce anisotropic material behaviors. These phenomena, though interesting and important to global mechanical function of these materials, can obscure true polymer material behaviors under loading. Since polymer chain architecture will dictate gross mechanical behavior it is important to understand how the polymer chains behave individually and how they interact with one another during loading. Currently, the understanding of hydrogel lattice microstructure during deformation is limited and requires further investigation.
A subclass of hydrogels utilizes biomacromolecules (collagen, glycosaminoglycans, fibrin, etc.) to create artificial scaffolds for tissue engineering applications (Figure 4a) [49–53]. For example, Tranquillo et al. has extensively investigated the use of fibrin gels as a base scaffold for seeding cells in attempts to develop engineered constructs for cardiovascular applications such as heart valve tissues and vascular grafts and the study of Schwann cell mechanobiology [51–56]. Collagen gels have seen similar use to characterize mechanical behavior as well as to study a variety of cell-matrix interactions and their resulting phenomena such as the effects of matrix stiffness on cellular contraction or the ability of cells to remodel the collagen matrix [57]. The naturally occurring polymers chitosan and alginate have both seen widespread use in tissue engineering applications for cartilage, liver, nerve, cardiac tissue [58–63]. Though they both exhibit minimal foreign body reactions, can be readily processed without harsh chemicals, and can be designed to exhibit controllable mechanical and degradation properties, these materials often lack mechanical integrity without additional processing to manipulate polymer crosslinks or being augmented with other materials [64, 65].
Biopolymer gels inherently exhibit good cytocompatibility, are easily formed into physiologically relevant geometries, and can be manipulated to produce constructs which exhibit a relatively large range of mechanical behaviors. Currently, it is not clear whether these scaffolds exhibit a structure-function relationship comparable to native collagenous tissues nor has there been substantial evidence demonstrating the characteristic length of these polymerized biopolymers. In a recent study by Thomopoulous et al.[66], the authors attempted to apply a structural continuum model [67–69] but found it to underestimate the level of mechanical anisotropy in aligned gels. This may be indicative that these gels do not functionally behave as long fiber composite materials. This may also lend insight to the shortcoming of these biopolymer gels of lacking mechanical integrity, rendering them unable to adequately mimic native tissues in a functional manner. Native valvular tissues on the other hand exhibit collagen fibers or fiber bundles spanning the leaflet which measure on the order of tens of mm.
Needled non-wovens
Polymer processing techniques originating in the textile industry have proven valuable in producing synthetic fiber meshes which are capable of stimulating isolated cells to regenerate tissue (Figure 4d) [70, 71]. Needled non-woven scaffolds can be manufactured quickly, at relatively low cost, and withstand sterilization processes necessary for in-vivo use. Isolated cells of a desired lineage can then be seeded and cultured in static or dynamic conditions. Since these highly porous scaffolds exhibit an open pore structure, the seeded cells can quickly and easily infiltrate the scaffold producing a construct populated with cells throughout.
Flat sheets of PGA/PLLA non-woven textile have recently been employed to recapitulate the geometry of the native pulmonary valve and trunk by Sutherland et al [72]. After seeding and culturing ovine endothelial progenitor cells on the non-woven scaffold, the constructs were implanted into the pulmonary valve position of a juvenile ovine model. After 16- to 20-weeks, the engineered valve constructs were explanted for histological evaluation. Interestingly, histological preparations of the ECM architecture resembled that of native valves remarkably well with a tri-layered structure of organized tissue. To investigate more fundamental mechanisms of tissue evolution in response to mechanical cues and their resulting effects on mechanical behavior, Engelmayr et al. developed a modeling framework for the flexural properties of these needled non-woven scaffolds [71, 73]. In short, the structural model accounts for unique fiber morphologies which arise from the fabric manufacturing process and the production of new ECM to predict the scaffold’s effective stiffness during bending which was in agreement with experimental values. Furthermore, it was determined that matrix deposition during culture resulted in increased scaffold stiffness and can be attributed to an increased number of fiber-fiber bonds. For additional information regarding the utility of these materials in elucidating aspects of tissue development see section titled “Engineered tissues as model systems”.
Electrospinning
The final fibrous scaffold production technique covered in this review is electrospinning (figure 4c). This technology was first patented at the turn of the twentieth century by J.F. Cooley and W.J. Morton but the theoretical foundation of this phenomenon was not determined until Sir Geoffrey Ingram Taylor published three seminal papers dating from 1964 to 1969 [74–76]. Scaffolds fabricated by electrospinning natural polymers, synthetic polymers or polymer blends have received widespread attention. Beyond its relative affordability and simplicity, this popularity is largely a result of a versatile manufacturing process where slight alterations during fabrication enable the production of scaffolds with a wide array of fiber morphologies (i.e. fiber diameter, porosity, packing density, orientation, etc) directly influencing bulk mechanical properties [77, 78]. Controlled structural and mechanical anisotropy are highly desirable material properties in mimicking native tissue architecture and has even been shown to approximate the highly nonlinear biaxial mechanical response of collagenous soft tissues, such as the native porcine pulmonary valve leaflet [79]. This has been accomplished for some non-fibrous constructs and one commonly employed method for controlled mechanical anisotropy during electrospinning is attained by using a rotating collection surface which induces a preferred fiber direction as the rotational speed of the collector increases (Figure 3c, e) [79–81]. Similarly, the electrospinning process has shown the ability to produce biocompatible polymer constructs which exhibit tissue-like mechanical behaviors comparable with nucleus fibrosis tissues (Figure 5a) [79, 82].
Figure 5.
Synthetic scaffold production to recapitulate native tissue mechanical behavior. (a) Homogenation model prediction of material modulus for native inner and outer annulus fibrosus lamella (IAF and OAF respectively) and annulus fibrosus cell seeded electrospun scaffolds. (b) Representative stress versus strain curves from compression testing of porous poly (lactide-co-glycolide) scaffolds and articular cartilage sourced from porcine and ovine models. (c) Planar biaxial response comparison of native porcine pulmonary valve and highly aligned electrospun poly (ester urethane) urea construct. Figure adapted with permission from [79, 82, 182].
Electrospinning produces continuous fiber scaffolds exhibiting a wide range of mechanical properties, while also providing suitable surfaces for cell proliferation and growth [82–87]. A substantial amount of work can be found in recent literature concerning the mechanical and structural characterization of electrospun scaffolds [79, 82, 88–90]. Initial attempts to produce electrospun scaffolds for tissue engineering were concerned with the production and characterization of the materials including uniaxial tensile properties, measurements of porosity, and fiber diameter. Courtney et al. were the first to characterize multiaxial mechanical behavior of electrospun fabrics via planar biaxial testing and found their behavior remarkably comparable to the gross biaxial mechanical response of the native pulmonary valve leaflet [79].
The production of continuous fibers using this process has the added benefit of creating multiple interrelated functional length scales, a characteristic observed in biological materials. However, while electrospinning can fabricate scaffolds that possess ECM-like structures, this morphology also results in pore sizes that are generally smaller (<5 µm) and more irregular than those produced by some of the non-fibrous production methods introduced above [91, 92]. While it may be possible that cells seeded on the surfaces of electrospun matrices can migrate into the interior by displacing or enzymatically degrading individual fibers, an extended culture period and appropriate signals for cell migration into thick construct interiors might also be required. Thus, while electrospinning permits fabrication of biodegradable matrices that resemble the scale, architecture, and mechanical behavior of the native ECM [93], achieving high cellular density and infiltration remains challenging. To overcome this limitation numerous potential approaches have been proposed in recent literature. These approaches range from controlling microstructure through the alteration of production parameters (e.g. fiber diameter and packing) [94, 95], inclusion of native ECM proteins [96], or the inclusion of labile porogens or fiber families [97–100]. Cell populations can also be dispersed throughout an electrospun scaffold directly as initially shown by Stankus et al. [83]. This process which employs a technique to concurrently deposit electrospun polymer and electrosprayed cells suspended in culture media is the subject of active work (see section titled “Emulating native tissue mechanical behavior”).
Rapid prototyping
Though not strictly fibrous in nature, rapid prototyping (RP) allows for the production of scaffolds with precise control over matrix architecture with a freedom of design unattainable in conventional processing techniques. RP, an additive process, starts from a computer aided design and is built up layer by layer to create 3D scaffolds with designed porosity, channel interconnections, pore distribution, and mechanical strength [101, 102]. There are numerous manufacturing techniques for RP materials which are classified into two categories. Direct fabrication, as the name implies, involves the successive build of the scaffold by melt-dissolution deposition or particle bonding techniques [102]. In contrast, indirect RP fabrication aims to create a sacrificial mold which is then used to cast the actual engineered scaffold. Efforts are currently underway to expand the repertoire of RP materials for tissue engineering to match desirable degradation rates and products, mechanical strength, and biocompatibility. As new RP materials are developed (i.e. segmented polyurethanes, PLLA, PCL) it is possible to develop soft tissue like materials from this technology [103]. RP technologies have also served as inspiration for other automated fabrication approaches to build cell integrated constructs such as organ printing [104], cell laser printing [105], and photopatterning of hydrogels [106]. These approaches overcome limitations of cell infiltration and can likely be manipulated to incorporate some degree of control over material behavior. A thorough exploration of the mechanical behavior of these constructs and their fundamental effects on cell function (cell-matrix interactions, biosynthetic activity, proliferation/apaptosis, etc.) would be a valuable addition to the field of tissue engineering.
ENGINEERED TISSUES AS MODEL SYSTEMS
Although the feasibility of many of the technologies for the production of cell-based engineered tissue constructs mentioned above have been demonstrated, the long-term function, safety, and efficacy of these tissue replacements as well as their capacity to grow and adapt remain largely unknown. The challenge with designing engineered tissues for load bearing applications requires more than matching a single mechanical parameter as many tissues exhibit complex viscoelastic, anisotropic, and highly nonlinear behaviors. Long-term function requires the ability to withstand in-vivo stresses of significant magnitude and temporal loading regimes from the time of implantation. Deficient mechanical integrity at the time of implantation will likely lead to catastrophic implant failure or adverse integration of the construct with surrounding tissue. For instance, inflammatory response associated changes could drastically alter implant mechanical behavior or render them non-functional in two disparate ways: a dramatic increase in stiffness due to fibrosis or accelerated scaffold degradation leading to a weakening. As such, the ability to control scaffold composition, 3D geometry and structure at multiple scales provides a unique opportunity to recapitulate critical mechanical behaviors of the tissue while elucidating fundamental biological phenomenon.
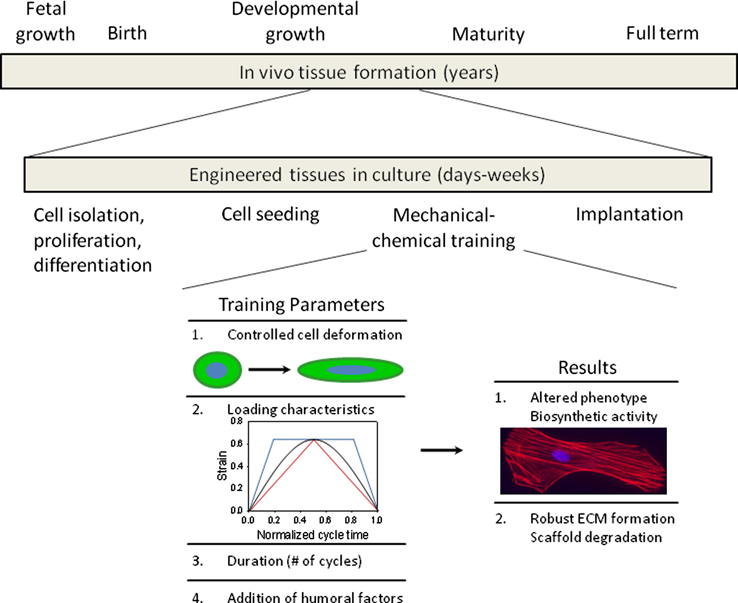
Tissue growth and maturation
Laboratory tissue culture devices such as bioreactors enable the development of engineered tissues in a controlled mechanically active environment. Mechanical stimuli ranging from cyclic tension, compression, or bending to altered hydrodynamic conditions [107] can be investigated independently or in conjunction to create complex deformation modes approximating those encountered in-vivo. It is well accepted that mechanical stimulation has a profound impact on cellular processes [108, 109]. For example, endothelial cell orientation and morphology is known to be influenced by mechanical stimulation such as strain or shear stress [110]. Cellular differentiation of mesenchymal cells can be guided through the application of compressive forces [111, 112]. Song et al. have shown that cyclic strain promotes proliferation of rat bone marrow mesenchymal stem cells [113]. Similarly, both static and cyclic modes of mechanical stimulation have been shown to alter protein synthesis and the amount and integrity of ECM proteins [114–118].
For example, needled nonwoven scaffolds have proven useful in efforts to model tissue formation as they have well defined micromechanics and allow adequate nutrient transport benefiting cell viability. Englemayr et al. recently showed that cyclic flexure coupled with laminar flow synergistically accelerates bone marrow stem cell mediated tissue formation on poly(glycolic acid)/poly (L-lactic acid) scaffolds, with a 75% increase in collagen concentration [119]. Though these scaffolds are relatively inextensible, the mechanical stability of PGA/PLLA non-woven scaffolds enables complex deformations to be decoupled allowing the establishment of a strong relationship between construct effective stiffness and collagen concentration in the absence of measurable scaffold mechanical degradation [120–123]. By implementing a composite beam model, Englemayr et al. were able to delineate the effects of constituent mass and intrinsic stiffness demonstrating that basic approaches to promote tissue formation by physical stimulation have effects beyond just the upregulation of tissue component mass production [73].
Cell responses to exogenous cues
Bioreactors aid in the maturity of cell-based constructs into engineered tissues improving construct mass, composition of ECM constituents [123–126], and cell proliferation prior to implantation (Figure 6). Moreover, such devices are ideal for the investigation of the more fundamental biological phenomenon revealing mechanisms of cell function. For instance, the controlled environment can be used to guide stem cell differentiation or other cellular processes by physical stimuli [127–130] both in the presence and absence of humoral or pharmacological factors [114, 131–134]. Despite our growing understanding of the cause-effect relationship of mechanical stimulation on cellular processes, the specific mechanisms responsible for these phenomenona continue to be poorly understood. Not only are these phenomena unclear for native tissues, it is particularly true of cells embedded within three dimensional synthetic scaffolds [88, 135].
Figure 6.
Expediting natural processes for the production of engineered tissue technologies. The time span required to produce practical options for tissue engineering is constrained significantly compared to native tissue development. As such, it is necessary to tailor culture parameters including mechanical and chemical cues in an effort to improve construct mass, composition of ECM constituents, and cell proliferation prior to implantation.
Development of engineered tissue or organ replacements would ideally be build upon a strong fundamental knowledge of cellular interactions with the local environment and how these interactions span multiple length scales to contribute to the overall function [136–138]. Cells interact with local matrix proteins via focal adhesions, a transmembrane complex of integrins and other proteins such as focal adhesion kinase, talin, and vinculin. Focal adhesions connect cytoskeletal actin fibers with ECM allowing the cell to sense or communicate with its environment [139] which then dictates cell shape, motility, and adhesion characteristics [140–142]. It is known that cell morphology profoundly affects a range of cellular functions, and that changes in the cell cytoskeleton lead to altered stress levels imparted on the nucleus, ultimately affecting cell function. For example, Thomas et al. [143] showed that gene expression and protein synthesis of primary osteogenic cells were altered by changing nuclear shape. Specifically, collagen Type I synthesis correlated directly with nuclear shape, where certain nuclear aspec ratio values promoted maximum synthesis, supporting the concept of gene expression and protein synthesis based on optimal distortion of the nucleus. Guilak et al. investigated chondrocyte nuclear deformations under compressive loads in articular cartilage in an attempt to explore how cell deformation may be a stimulus to cell metabolic activity [136]. They observed a reduction in chondrocyte volume with compressive loading, linked to mechanical transduction and signaling through mechano-sensitive channels [5].
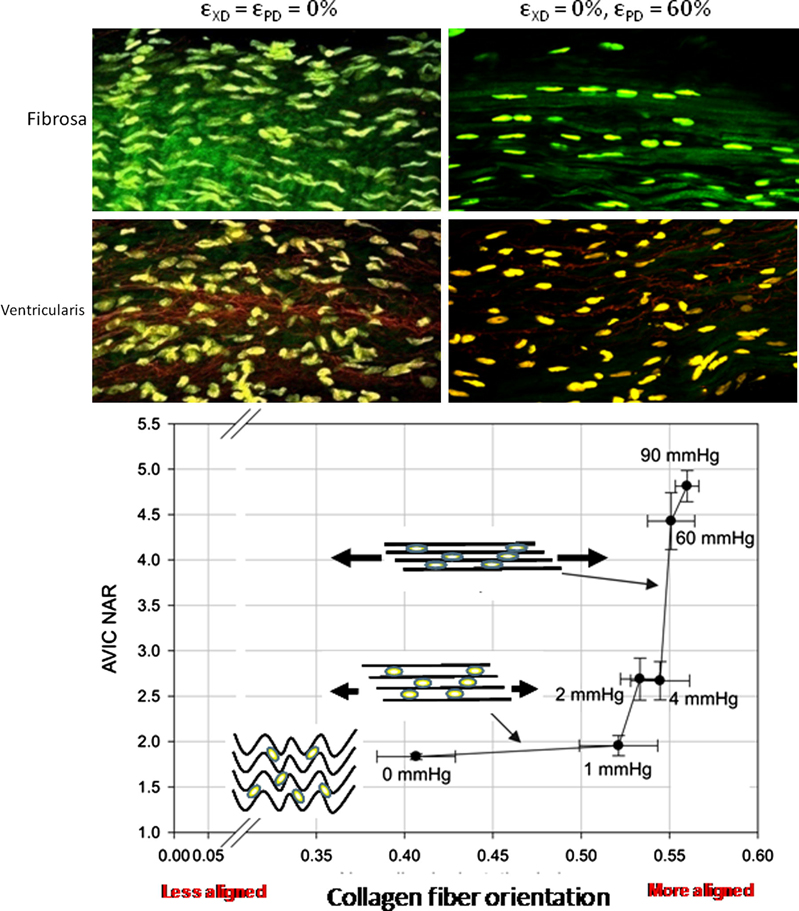
Under the hypothesis that cellular deformations which occur during tissue or organ level motion or deformation, recently conducted cell morphology studies on native valvular tissue and engineered constructs for valve replacement during deformation were performed (Figure 7–8) [88, 144]. Aortic valve interstitial cell (AVIC) deformation behavior in response to increasing transvalvular pressure has been shown to be mediated by local fiber kinematics. Specifically, a bimodal response was observed where little AVIC deformation occurs with the large amount of fiber straightening for pressures below ~1 mmHg, followed by substantial increases in AVIC nuclear aspect ratio from 4 to 90 mmHg (Figure 7) [144]. Taken as a whole, AVIC responses to tissue level stresses are modulated through complex micromechanical and fiber-compaction effects that occur under physiological stress levels.
Figure 7.
Native aortic valve cell-matrix interaction and cell deformation response to increasing transvalvular pressure. Cell deformation in native porcine aortic valve leaflets, as quantified by changes in nuclear aspect ratio, was highly dependent on local collagen fiber kinematics. Generally speaking, increasing transvalvular pressure resulted in increased cell nuclear aspect ratios but unique layer dependent responses were observed. Furthermore, a bimodal trend was observed between cell deformation and increased diastolic loads. Figure adapted with permission from [144].
Figure 8.
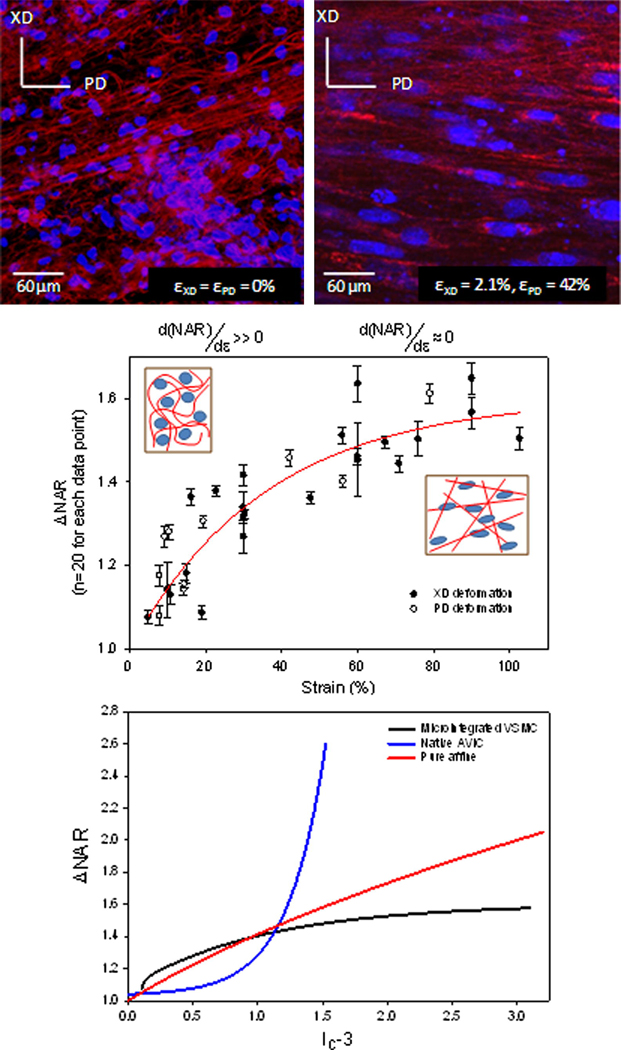
Strain induced changes in electrospun polyester micro-architecture and resulting nuclei deformation. When exposed to biaxial modes of deformation, electrospun fibers were observed to transition from a tortuous configuration in the unstrained state to an interconnected web-like architecture at high strains. A composite of all NAR measurements (mean ± s.e.m) demonstrated a rapid increase to ~60% strain, after which a plateau was observed with further strain increases, indicating that nuclei deformations are dominated by local fiber straightening. A composite cell-scaffold deformation response (bottom) is provided for native porcine aortic valve leaflet, cell integrated electrospun PEUU, and a theoretical purely affine cell deformation response to macroscopic strain. Figure reproduced with permission from [88].
In a similar manner, cell micro-integrated electrospun scaffolds exhibited micro-fiber morphologies and kinematics that were shown to directly influence local cellular deformations (Figure 8). For instance, in the unstrained configuration the polymer fibers exhibited a tortuous architecture which transitioned to a web-like network of straight, interconnected fibers at high levels of strain (Figure 8). The deformations of the micro-integrated cells were found to be primarily mediated by this phenomenon. The cell integrated electrospun constructs underwent fully recoverable large deformations akin to many native tissues. Moreover, while a non-linear relation between the tissue strain and nuclear aspect ratio (NAR) was observed for both the aortic valve and cell integrated electrospun PEUU, the underlying micro-mechanical mechanisms were clearly different. Initially, the integrated vascular smooth muscle cells exhibited a rapid increase in NAR as fibers straightened and tortuosity was reduced. Once the PEUU fibers became straightened and the architecture transitioned to an interconnected web like structure, changes in NAR were observed to plateau.
Due to the particular methods employed to investigate the deformation responses of cells in the native porcine aortic valve and cell integrated electrospun constructs, it can be difficult to interpret their respective behaviors. For comparison purposes, the measured deformation responses were cast into invariant forms of their right Cauchy-Green strain tensor (C), where C = FTF, to evaluate the deformation responses irrespective of the basis used to define the strain components during testing. The first invariant of the Cauchy-Green tensor was chosen to compare the cellular deformation response to macroscopic matrix deformation for three cases (Figure 8c). Namely, the experimentally measured deformation responses of the AVIC in response to increasing transvalvular pressure, the response of cells integrated in electrospun scaffolds under strip biaxial planar deformations, and the simulated response of a cell which convects in a pure affine manner with global scaffold planar deformation. In the simulated case a planar strip biaxial deformation, characterized by deforming the specimen on one axis to a desired level while holding its orthogonal component fixed, was imposed as it was similar to imposed deformations of the cell integrated electrospun constructs. The unique cellular deformation responses observed in the native aortic valve and cell integrated electrospun constructs were preserved in this invariant form with the AVIC exhibiting large changes in nuclear aspect ratio (Figure 8c). The simulated affine response was seen to fall between the integrated VSMC and AVIC behaviors at high strain.
Empirical results indicate a wide array of potential cellular adaptations in response to exogenous forces ranging from cell morphology and metabolic activity to cell motility or proliferation. As incremental improvements in the understanding of mechanical and chemical cues on cell function are obtained, it might be possible to exploit these mechanisms to accelerate ECM protein production to prepare engineered tissues prior to implantation. Currently, much is known about the arrangement and connectivity of load bearing elements from the ECM to cell cytoskeletal structure and further to nuclear structure. Despite this reasonably detailed knowledge, the field currently lacks a detailed mechanistic understanding of the role of force on nuclear mechanotranstuction and requires further investigation.
EMULATING NATIVE TISSUE MECHANICAL BEHAVIOR
All of the scaffold manufacturing methods cited above can be employed to produce a 3D scaffold appropriate for cell culture and will likely foster the production of new tissue. However, the fields of tissue engineering and regenerative medicine continue to struggle to adequately match the mechanical behavior of native tissues. Collagenous soft tissues and tissue surrogates exhibit a wide array of unique mechanical behaviors which can be probed in a variety of testing modes (tension, shear, bending, torsion, etc). Unfortunately, traditional engineering indices of material behavior can fall short in describing the complete response of highly nonlinear and anisotropic materials and often require more sophisticated or extensive characterization approaches (Table 2). Load bearing soft collagenous tissues are typically characterized by a bimodal stress-strain response with a compliant response or “toe region” at low strains which then transitions to a stiff response at large strain. While matching metrics related to tensile strength or tensile modulus has merit when applied appropriately, this approach gives little indication of the full material response during loading potentially leading to insufficient implant performance, undesirable tissue formation, or a lack of viable tissue production.
Table 2.
Complexities to consider when characterizing the mechanical responses of biological materials versus traditional engineering materials.
| Traditional materials | Biological materials | ||
|---|---|---|---|
| Linear (linear stress-strain relationship) | Non-linear | ||
| Elastic (fully recoverable deformation) | Inelastic | ||
| Homogeneous (postion independent) | Heterogenous | ||
| Isotropic (directionally independent) | Anisotropic | ||
| Other properties include | |||
| Infintesimal strains | Finite strains | ||
| No need to distinguish between reference and deformed configurations |
Geometrical variations between reference and deformed configurations |
||
| Can utilize infintesimal stress/strain tensors and fundamental material laws |
Necessitates special theories (and measures of stress and strain) that account for finite defromations |
||
| Time independent | Tme dependent | ||
| Constant properties | Grow and remodel | ||
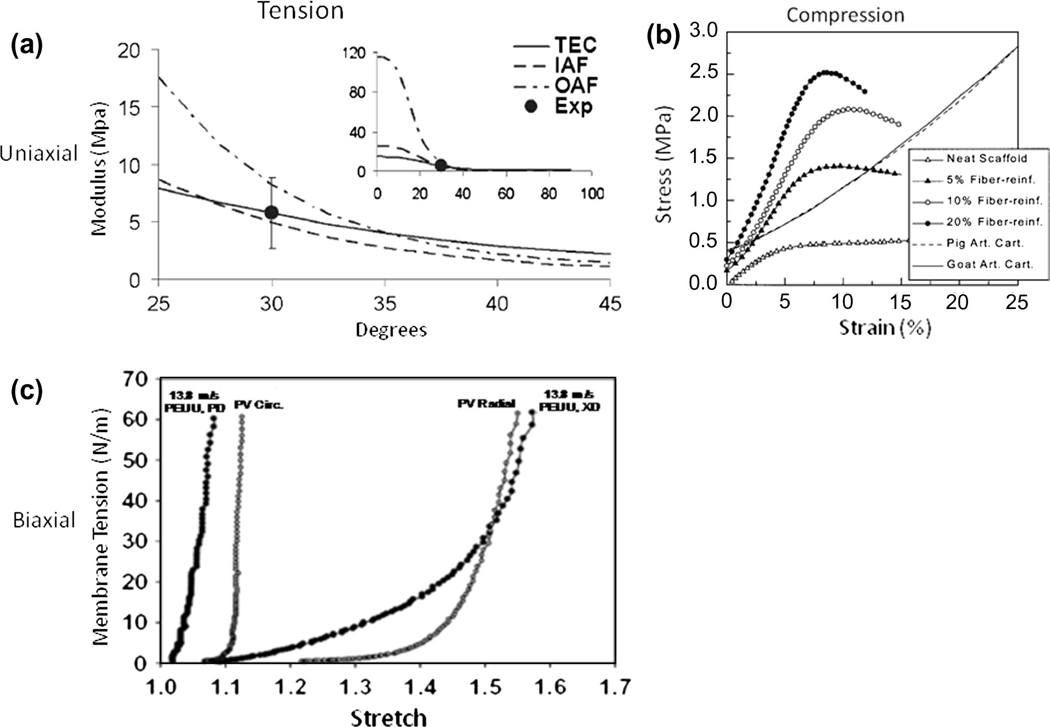
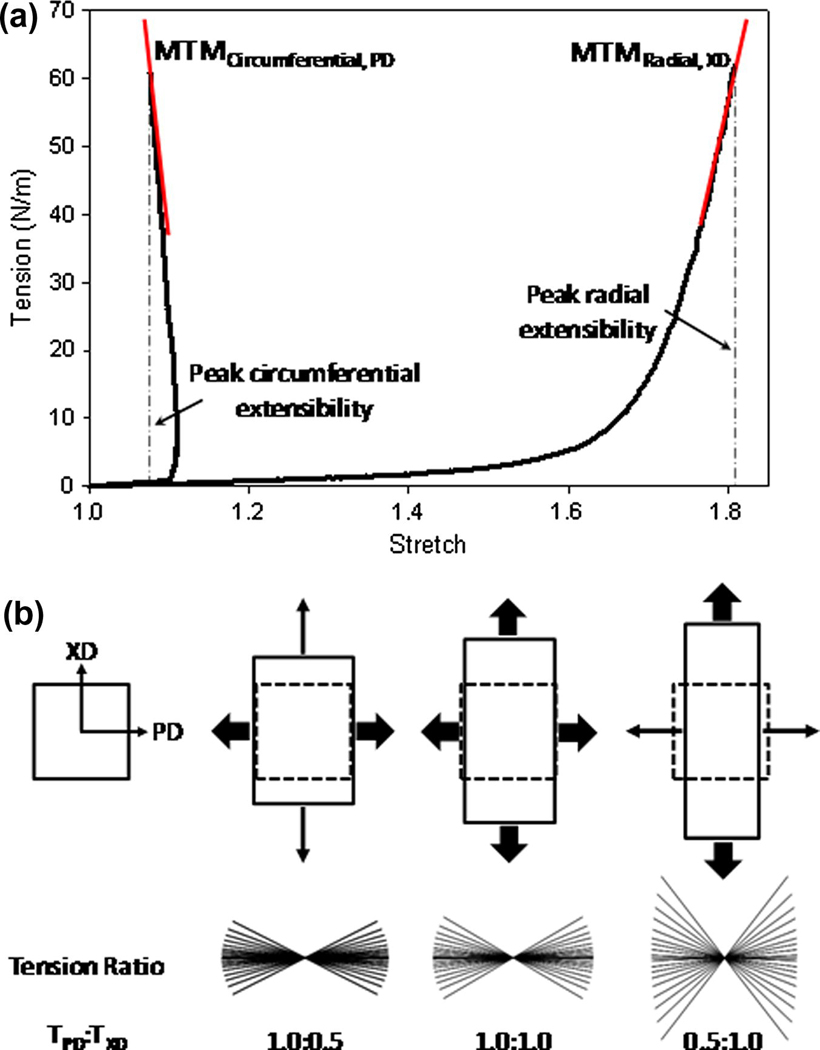
Practically speaking, the application of traditional engineering metrics of material behavior to soft biological materials can not only be difficult but potentially unsuitable. When characterizing material performance, it is important to consider the functional relevance of a chosen method of mechanical testing and its interpretation relating to tissue function. For instance, valvular tissue stiffness with their highly anisotropic and highly nonlinear behavior cannot be adequately described by singular indices of material behavior. This is apparent in the circumferential response of valvular tissues under biaxial loading where specimen contraction is observed at high stress states rendering the calculation of a maximum tangent modulus erroneous (Figure 9). The basis for this coupled, anisotropic response between material directions is the result of a reorganization of the fiber constituents (i.e. structural anisotropy). While helpful in providing potentially comparable metrics of tissue behavior, tabulated values which appear in contemporary literature can be misleading and belie the complex mechanical responses characteristically observed in soft load bearing tissues. While matching metrics related to tensile strength (i.e. ultimate tensile stress and ultimate percent elongation) or tensile modulus has merit when applied appropriately, this approach gives little indication of the full material response during loading. Again, in the case of valvular tissues, if one was to simply match peak stress-strain values without regard to the native material loading response, it would likely lead to inadequate coaptation or closing of the heart valve and result in poor function. Native tissues often exhibit highly specific, complex mechanical behaviors necessitating more sophisticated and thorough methods of characterization.
Figure 9.
Application of traditional engineering metrics of material behavior are often inadequate descriptors of the complex behaviors observed in biological materials. (a) Load bearing matrix rich biological materials, like native aortic valve leaflets, typically exhibit highly non-linear and highly anisotropic tensile behaviors. (b) The transition from low to high stiffness is attributed to a coupled fiber recruitment process capable of exhibiting lateral contraction at high stress levels (circumferential shortening). As a consequence, the determination of traditional engineering indices of material behavior, such as tensile modulus has little meaning (i.e. negative modulus). Adequate characterization of biological materials can necessitate more sophisticated methods to characterize their true mechanical behaviors.
Elastomeric scaffolds fabricated by electrospinning natural polymers, synthetic polymers or polymer blends have received widespread attention which is largely due to the ability to produce biocompatible polymer constructs which exhibit many soft tissue-like mechanical behaviors [79, 82, 85]. Electrospinning is a highly versatile process capable of creating a wide array of material behaviors through the alteration of production parameters or polymer choice. As such, it is necessary to asses these mechanical behaviors in a systematic manner. For practicality reasons, uniaxial tensile testing is often employed to obtain basic material properties such as the elastic modulus, strain at the onset of yielding, and ultimate tensile strength [93, 145–150]. From the systematic mechanical evaluation conducted by Courtney et al., several scaffolds were manufactured with varying degrees of structural anisotropy or fiber alignment which was controlled by altering the rotational velocity of the collection mandrel. Increasing rotational velocity results in an increase in fiber alignment [80–82, 87] and was experimentally measured from SEM micrographs via an image analysis algorithm. The controlled structural anisotropy produced during manufacturing directly led to more non-linear, anisotropic material responses. These structural properties form a complex 3D scaffold with tunable tissue-level mechanical behavior that can be remarkably similar to the gross biaxial mechanical response of the native pulmonary valve leaflet [79]. It has also been shown that the tensile response of some musculoskeletal tissues can be emulate well by electrospun scaffolds, namely, knee meniscus and annulus fibrosis tissues [82, 151].
Much of the work found in recent literature has focused on structural and mechanical characterization of electrospun materials with limited effort in the development of modeling frameworks to further assess our understanding of electrospun material behavior. Nerurkar et al. have adapted a homogenization modeling approach originally presented by Yin and Elliot to compare predicted mechanical behaviors of electrospun poly-ε-caprolatone and the inner and outer lamella of native annulus fibrosus tissue. Structural based modeling attempts originally developed for dense collagenous planar tissues have proven quite successful in capturing the mechanical behavior of electrospun scaffolds under biaxial modes of deformation [79]. It was noted that the model predicted a higher degree of fiber orientation than measured experimentally. This is attributed to structural characteristics of the polymer fibers measured in the image analysis algorithm but are not accounted for in the model formulation.
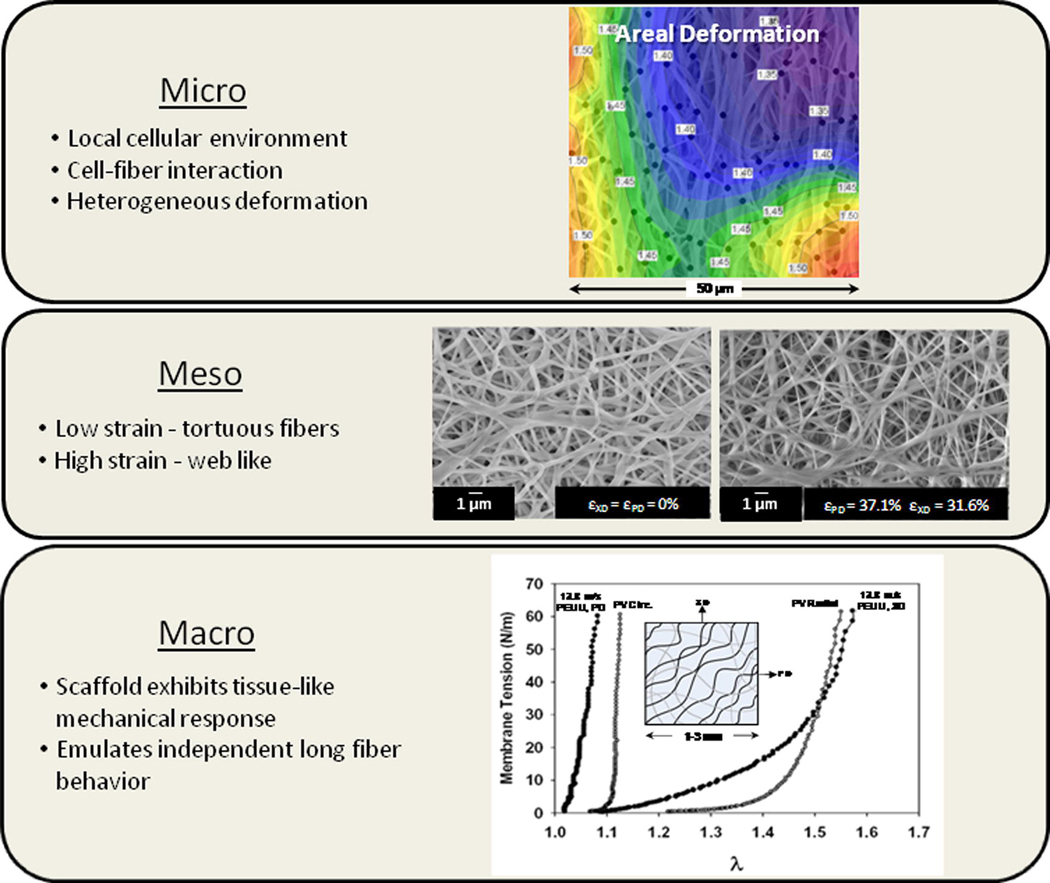
In order to improve upon these initial modeling efforts and gain a better appreciation of how these materials function across multiple length scales, recent work by Stella et al. was conducted to quantify additional structural characteristics. The electrospun scaffolds investigated in this study exhibited complex, hierarchical architectures spanning multiple length scales (Figure 10). As a result, understanding the mechanisms by which these materials deform and behave under various loading conditions is not an elementary task. Fiber tortuosity, diameter, and fiber orientation distributions were all quantified as the electrospun specimen underwent planar biaxial modes of deformation. In addition, the deformation behavior of the scaffold was investigated across multiple scales by defining three characteristic lengths (micro (1–2 µm), meso (40–50 µm), and macro (1–3 mm)). Fiber tortuosity, a measure of how much a fiber deviated from being straight, in the unloaded scaffold, was observed to be dependent on both mandrel velocity during production and orientation. As the scaffold underwent planar biaxial modes of deformation, fiber tortuosity is extinguished and substantial fiber rotational kinematics was observed contributing to an intricate fiber recruitment process [88]. Electrospun constructs were observed to follow gross affine fiber transformations and can be described in a manner similar to collagenous scaffolds [18]. Interestingly, some fibers were observed to rotate or change their direction of orientation during deformation while as a population, no net change was measured. This is likely an additional manifestation of the local heterogeneity which exists at the micro scale. With increased specimen deformation, a monotonic decrease in PEUU fiber diameter was measured for all specimens. Furthermore, it was observed that neighboring the fibers were well attached where they overlapped or intersected impeding translation of fibers with respect to one another but does not appear to inhibit rotational fiber kinamatics about these points of intersection. Johnson et al. presented a similar hypothesis for reduced fiber mobility in electrospun polymers exhibiting “point bonding” [152]. In short, polymer sintering was utilized on electrospun poly (ε-caprolatone) scaffold to invoke definite point bonds between fibers. Scale-dependent variations in deformation were observed and were attributed to the complex, spatially variant structure which results from the electrospinning process. These heterogeneous variations occur at the micro-scale which a cell might experience. The overall strain behavior tends to become increasingly more homogenous as the scale of interest approaches the tissue level.
Figure 10.
Cell integrated electrospun scaffold hierarchical structure and function. Despite exhibiting a tissue-like mechanical response at the macro scale, the scaffold exhibits vastly different micro and meso mechanical behaviors. For instance, at the micro-scale a heterogeneous deformation response is observed. In addition, fibers in the unstrained configuration exhibit an undulated or tortuous morphology which transitions to a highly interconnected web-like architecture at finite strains. At the macro scale, we observe a complex 3D scaffold with tunable tissue-level mechanical behavior that can be remarkably similar to the biaxial mechanical response of the native porcine pulmonary leaflet. Figure adapted with permission from [181].
The unique coupled matrix-cell deformation response of electrospun scaffold integrated with cells was also recently investigated (Figure 8) [88]. The scaffolds exhibited micro-fiber morphologies and kinematics that were shown to directly influence local cellular deformations. For instance, in the unstrained configuration the electrospun fibers exhibited a tortuous architecture which transitioned to a web-like network of straight, interconnected fibers at high levels of strain. The deformations of the micro-integrated cells were found to be primarily mediated by this phenomenon. The cell integrated constructs underwent fully recoverable large deformations akin to many native tissues. Initially, the integrated cells exhibited a rapid increase in NAR as fibers straightened and tortuosity was reduced. Once the PEUU fibers became straightened and the architecture transitioned to an interconnected web like structure, changes in NAR were observed to remain constant. Microintegrated cell deformation was mediated by the local reduction of tortuosity or straightening of the PEUU fibers. Thus, cell-scaffold interactions can be subtle and can bring about unique deformation behaviors. These results indicate that it may be possible to successfully emulate gross native tissue behavior without exactly replicating their highly complex micro-architectures. Attempting to delineate the individual contributions of structural fiber characteristics such as tortuosity and fiber kinematics to the constructs mechanical behavior would be quite cumbersome and better lends itself to the development of a numerical framework to explore this unique, interrelated phenomenon.
MODELING SCAFFOLD STRUCTURE-FUNCTION RELATIONSHIPS
Tissue engineering and regenerative medicine have evolved from fields which strongly rely on empirical findings to solve practical problems or drive our understanding of complex biological phenomena. Elucidating the mechanical behavior of native biological materials is further confounded by tissue specific, highly specialized multi-scale constituent arrangements which dictate their unique mechanical functions. While the development of new materials continues to be the focus of ongoing research, achieving material behavior functionally comparable to native tissue is currently limited (Figure 5).Despite advancements made in recent years, there continues to be a multitude of fundamental questions which remain unanswered inhibiting the production of truly functional tissue surrogates. While more thorough methods for the characterization of native and engineered biological tissues are necessary,answering many fundamental biological or structural questions cannot be ascertained through experimental testing alone. By combining well posed theoretical frameworks with experimentally derived observations it is possible to elucidate the complex interrelated mechanisms presented to us by nature. Not only can modeling approaches serve as valuable tools to simplify and test our understanding of complex biological systems but they can be used to guide future hypothesis based investigations.
The development of constitutive relations for soft tissues has been a rich field of study for several decades beginning with the seminal work by Fung [153]. Early constitutive models for soft tissues, though successful at capturing characteristic tissue behaviors for a spectrum of applications, were phenomenologically based. This inherently limits their ability to probe underlying mechanisms governing tissue behavior. In response, structural approaches aimed at characterizing material response in terms of the underlying tissue constituents have gained favor [67, 68, 154, 155]. These approaches extend into current trends in the biomechanics community which have focused on computational implementations of established soft tissue models or new approaches specifically aimed to investigate underlying tissue structure-function mechanisms across multiple functional scales.
Correspondingly, methods to restore, maintain, or improve tissue or whole organ function must incorporate a thorough understanding of the intricate multi-scale hierarchical arrangements typically found in nature. Engineering sustainable solutions concerned only with tissue or organ level function belies the multifaceted, coordinated function of these tissue structures and their constituents which are in turn a result of cellular or subcellular processes that reach down to the molecular scale of protein interactions and gene transcription. Surely, one model cannot incorporate this large range in scales with our current understanding of biological processes. Instead, a hierarchy of models and approaches is necessary to connect the established continuum level relationships (i.e. phenomenological [153, 156–159], structural [67, 68, 154, 160–164]) with underpinning cell and subcellular events. From a modeling point of view, a vital aspect of these models consists of the difficult task of seamlessly coupling various length scales.
For materials with regular, repeating structures, unit cell based modeling approaches can successfully relate microstructural responses to global material behavior. Stylianopoulos et al. have developed such a model for the mechanical behavior of collagen fiber networks [165–167]. Briefly, the unit cell, the representative unit of the continuum that encompasses the periodicity of the microstructural parameters, contains an idealized fiber mesh generated in-silico. A group of unit cells are then perturbed in some defined manner (i.e. uniaxial tension) and a force balance within each unit cell results in a volume-averaged macroscopic response. In related work, Zahalak et al [168] and Marquez et al. [169, 170] have developed constitutive relations to relate individual cellular contributions to macroscopic material response in an effort to elucidate active and passive cell deformation responses and material properties. The fundamental unit of this model is comprised of cells, idealized as contractile rods, within a compliant matrix. Both constituents were parametrically assigned linear elastic, isotropic material properties. Due to these assumptions, the predictive capabilities are limited since biologic materials typically exhibit nonlinear viscoelastic behaviors. However, they did show that the strain experience by the cell can be related to macroscopic strain via a scalar valued strain factor and that reasonable approximations of cell stiffness can be determined from measured tissue properties. One shortcoming of the unit cell approach is that it neglects the structural heterogeneity seen in biological tissues and the incorporation of increased structural complexity is penalized by significant computational demands. Moreover, native dense collagenous tissues are long fiber composites with fiber lengths up to the millimeter scale while the characteristic length scale of this model is much shorter as it is defined by unit cell dimensions.
Additionally, insufficient efforts have been spent on assessing appropriate material specific representative volume element (RVE) size. Morphology descriptors produced through image analysis such as material porosity, fiber density, fiber alignment distribution, fiber connectivity distribution, and fiber diameter strongly depend on the material architecture at micro-meso level. This can be demonstrated studying the evolution of these parameters over regions of interest of increasing sizes and/or repeating the image analysis over analogous regions differing in location. Morphology feature fluctuations and location dependency gradually cease as the analyzed region of interest approaches an appropriate RVE size. A direct implication is that an image analysis technique remains incomplete if it does not identify an appropriate RVE for the variable of interest. RVE size in random composites can be derived statistically, numerically or empirically studying the stabilization of the analyzed variable over RVE’s of increasing sizes [171, 172]. Thus, the importance of identifying the appropriate RVE size is twofold. Firstly, in terms of material characterization, it contributes to develop reliable tools to assess scaffold manufacturing process repeatability and secondly, in terms of mechanical modeling, the analysis performed at the RVE can provide physically meaningful data. In particular, structural deterministic models dramatically depend on rigorously defined material structural descriptions. This need has been extensively highlighted in recent literature where the model capability relies prevalently on the accuracy of the network topology [165–167, 173, 174].
Furthermore, not only is micro-architectural data extraction accuracy crucial but it is fundamental for stochastic representations of engineered scaffolds such that the error between the real and simulated structure is minimized. The potential of a structural deterministic approach in elucidating the inherently multi-scale nature of native and engineered soft tissues response seems to justify its apparent complexity [165–167, 173]. An alternative path of the deterministic modeling is to reproduce the entire scaffold area or volume without duplicating the RVE. The main benefits of this alternative solution are that the implicit error introduced by the multi-scale approach is removed and the information at the meso level is preserved. For instance, tortuosity measurements of a collagen fiber in a heart valve leaflet under loaded and unloaded configurations can be performed which would otherwise be neglected in the multi-scale approach where the fibers cannot cross element boundaries. However, these expected benefits are counterbalanced by a significant increase of computational cost.
The modeling strategies outlined above also have potential use for the rational design of future scaffold morphologies at the macro (tissue engineered construct size, shape etc.) and micro level (fiber connectivity, fiber density, fiber alignment etc.). This has the potential to profoundly impact the fields of tissue engineering and mechanobiology whereby empirically driven experimentation can be augmented or replaced with more rational design approaches. Relating macroscopic kinematic events to the cell environment and understanding the cellular responses to these cues is critical in the production of engineered tissue surrogates. It has been shown that mechanical cues modulate many cellular processes and the ability to understand and predict the events leading to healthy tissue accretion or adaptive repair/growth can guide mechanical training regimes to produce robust tissue formation. Efficacious repair or replacement of abnormal or lost tissue relies on our ability to reproducibly control cellular responses to exogenous cues.
CONCLUSIONS
The development of efficacious therapies for tissue repair, replacement, or regeneration rests in large part on our ability to employ new materials, manufacturing and processing techniques, and manage the events of cellular mechanobiology. Understanding factors responsible for tissue function and dysfunction necessitates a strong fundamental knowledge of native biological material structure and function across multiple scales. Cells continually assess their local environment through various mechanosensors (focal adhesion complexes, transmembrane proteins, stretch activated ion channels, etc.) and react accordingly by activation of signaling cascades to produce physiologic responses. Furthermore, the intricate interactions between cells and their environment dictates mechanotransduction of proteins critical for cell function and maintaining a mechanically sound, organized matrix. From a tissue engineering perspective, managing cellular processes through controlled exogenous cues has immense and widespread implications. As our understanding of the structure-function relationships governing cell mechanics expands, gaining a mechanistic understanding of how mechanical stimuli translates to protein mechanotranstuction will become feasible. This detailed understanding will then enable rational design of efficacious clinical approaches for improving health along with sound hypothesis driven examinations of new problems and even provide analytical tools to evaluate engineered implant performance.
ACKNOWLEDGEMENTS
Funding for this work was provided by the United States National Institutes of Health through grant numbers R01 HL68816, HL-089750, and R01 HL069368.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gilbert TW, et al. Degradation and remodeling of small intestinal submucosa in canine Achilles tendon repair. J Bone Joint Surg Am. 2007;89(3):621–630. doi: 10.2106/JBJS.E.00742. [DOI] [PubMed] [Google Scholar]
- 2.Fujimoto KL, et al. An elastic, biodegradable cardiac patch induces contractile smooth muscle and improves cardiac remodeling and function in subacute myocardial infarction. J Am Coll Cardiol. 2007;49(23):2292–2300. doi: 10.1016/j.jacc.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shelton JC, Bader DL, Lee DA. Mechanical conditioning influences the metabolic response of cell-seeded constructs. Cells Tissues Organs. 2003;175(3):140–150. doi: 10.1159/000074630. [DOI] [PubMed] [Google Scholar]
- 4.van der Meulen MC, Huiskes R. Why mechanobiology? A survey article. J Biomech. 2002;35(4):401–414. doi: 10.1016/s0021-9290(01)00184-1. [DOI] [PubMed] [Google Scholar]
- 5.Sarkadi B, Parker JC. Activation of ion transport pathways by changes in cell volume. Biochim Biophys Acta. 1991;1071(4):407–427. doi: 10.1016/0304-4157(91)90005-h. [DOI] [PubMed] [Google Scholar]
- 6.Billiar KL, Sacks MS. Biaxial mechanical properties of the natural and glutaraldehyde treated aortic valve cusp--Part I: Experimental results. Journal of Biomechanical Engineering. 2000a;122(1):23–30. doi: 10.1115/1.429624. [DOI] [PubMed] [Google Scholar]
- 7.Sacks MS. Biomechanics of native and engineered heart valve tissues. In: Guilak F, et al., editors. Functional Tissue Engineering. New York: Spring-Verlag; 2003. [Google Scholar]
- 8.Vesely I, Lozon A. Natural preload of aortic valve leaflet components during glutaraldehyde fixation: Effects on tissue mechanics. Journal of Biomechanics. 1993;26(2):121–131. doi: 10.1016/0021-9290(93)90043-e. [DOI] [PubMed] [Google Scholar]
- 9.Stella JA, Sacks MS. On the biaxial mechanical properties of the layers of the aortic valve leaflet. J Biomech Eng. 2007;129(5):757–766. doi: 10.1115/1.2768111. [DOI] [PubMed] [Google Scholar]
- 10.Stella JA, Liao J, Sacks MS. Time-dependent biaxial mechanical behavior of the aortic heart valve leaflet. J Biomech. 2007;40(14):3169–3177. doi: 10.1016/j.jbiomech.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sacks MS. Biaxial mechanical behavior of bovine pericardium as a bioprosthetic material. Proceedings of the 11th Conference Engineering, Engineering Mechanics Division/ASCE; Fort Lauderdale, FL. 1996. [Google Scholar]
- 12.Smith DB, et al. Fatigue-induced changes in bioprosthetic heart valve three-dimensional geometry and the relation to tissue damage. J Heart Valve Dis. 1999;8(1):25–33. [PubMed] [Google Scholar]
- 13.Sacks MS, Schoen FJ. Collagen fiber disruption occurs independent of calcification in clinically explanted bioprosthetic heart valves. J Biomed Mater Res. 2002;62(3):359–371. doi: 10.1002/jbm.10293. [DOI] [PubMed] [Google Scholar]
- 14.Vesely I, Barber JE, Ratliff NB. Tissue damage and calcification may be independent mechanisms of bioprosthetic heart valve failure. J Heart Valve Dis. 2001;10(4):471–477. [PubMed] [Google Scholar]
- 15.Sung HW, et al. In vitro evaluation of cytotoxicity of a naturally occurring cross-linking reagent for biological tissue fixation. J Biomater Sci Polym Ed. 1999;10(1):63–78. doi: 10.1163/156856299x00289. [DOI] [PubMed] [Google Scholar]
- 16.Schoen FJ. Future directions in tissue heart valves: impact of recent insights from biology and pathology. J Heart Valve Dis. 1999;8(4):350–358. [PubMed] [Google Scholar]
- 17.Zheng MH, et al. Porcine small intestine submucosa (SIS) is not an acellular collagenous matrix and contains porcine DNA: Possible implications in human implantation. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2005;73B(1):61–67. doi: 10.1002/jbm.b.30170. [DOI] [PubMed] [Google Scholar]
- 18.Gilbert TW, et al. Fiber kinematics of small intestinal submucosa under biaxial and uniaxial stretch. J Biomech Eng. 2006;128(6):890–898. doi: 10.1115/1.2354200. [DOI] [PubMed] [Google Scholar]
- 19.Badylak S. The extracellular matrix as a biologic scaffold material. Biomaterials. 2007;28:3587–3593. doi: 10.1016/j.biomaterials.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 20.Liao J, Joyce EM, Sacks MS. Effects of decellularization on the mechanical and structural properties of the porcine aortic valve leaflet. Biomaterials. 2008;29(8):1065–1074. doi: 10.1016/j.biomaterials.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donald O, Freytes RMSSFB. Uniaxial and biaxial properties of terminally sterilized porcine urinary bladder matrix scaffolds. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2008;84B(2):408–414. doi: 10.1002/jbm.b.30885. [DOI] [PubMed] [Google Scholar]
- 22.Baker BM, Mauck RL. The effect of nanofiber alignment on the maturation of engineered meniscus constructs. Biomaterials. 2007;28(11):1967–1977. doi: 10.1016/j.biomaterials.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bidanda B, Bartolo P. Virtual prototyping & bio manufacturing in medical applications. New York: Springer; 2008. p. 299. xv. [Google Scholar]
- 24.Freitas S, Merkle HP, Gander B. Microencapsulation by solvent extraction/evaporation: reviewing the state of the art of microsphere preparation process technology. Journal of Controlled Release. 2005;102(2):313–332. doi: 10.1016/j.jconrel.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 25.Aubert JH, Clough RL. Low-density, microcellular polystyrene foams. Polymer. 1985;26(13):2047–2054. [Google Scholar]
- 26.Caneba GT, Soong DS. Polymer Membrane Formation through the Thermal-Inversion Process. 1. Experimental Study of Membrane Structure Formation. Mucromolecules. 1985;18:2538–2545. [Google Scholar]
- 27.Lloyd DR, Kinzer KE, Tseng HS. Microporous membrane formation via thermally induced phase separation. I. Solid-liquid phase separation. Journal of Membrane Science. 1990;52(3):239–261. [Google Scholar]
- 28.Lloyd DR, Kim SS, Kinzer KE. Microporous membrane formation via thermally-induced phase separation. II. Liquid--liquid phase separation. Journal of Membrane Science. 1991;64(1–2):1–11. [Google Scholar]
- 29.Liu X, Ma PX. Phase separation, pore structure, and properties of nanofibrous gelatin scaffolds. Biomaterials. 2009;30(25):4094–4103. doi: 10.1016/j.biomaterials.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van de Witte P, et al. Phase transitions during membrane formation of polylactides. I. A morphological study of membranes obtained from the system polylactide-chloroform-methanol. Journal of Membrane Science. 1996;113(2):223–236. [Google Scholar]
- 31.Nam YS, Park TG. Biodegradable polymeric microcellular foams by modified thermally induced phase separation method. Biomaterials. 1999;20(19):1783–1790. doi: 10.1016/s0142-9612(99)00073-3. [DOI] [PubMed] [Google Scholar]
- 32.Ho M-H, et al. Preparation of porous scaffolds by using freeze-extraction and freeze-gelation methods. Biomaterials. 2004;25(1):129–138. doi: 10.1016/s0142-9612(03)00483-6. [DOI] [PubMed] [Google Scholar]
- 33.Leatrese DH, Byung-Soo K, David JM. Open pore biodegradable matrices formed with gas foaming. Journal of Biomedical Materials Research. 1998;42(3):396–402. doi: 10.1002/(sici)1097-4636(19981205)42:3<396::aid-jbm7>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 34.Lips P, et al. Gas foaming of segmented poly(ester amide) films. Polymer. 2005;46(22):9396–9403. [Google Scholar]
- 35.Guan J, Fujimoto KL, Wagner WR. Elastase-sensitive elastomeric scaffolds with variable anisotropy for soft tissue engineering. Pharm Res. 2008;25(10):2400–2412. doi: 10.1007/s11095-008-9628-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atkinson PM, Lloyd DR. Anisotropic flat sheet membrane formation via TIPS: atmospheric convection and polymer molecular weight effects. Journal of Membrane Science. 2000;175(2):225–238. [Google Scholar]
- 37.Barnes CP, et al. Nanofiber technology: Designing the next generation of tissue engineering scaffolds. Adv Drug Deliv Rev. 2007 doi: 10.1016/j.addr.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 38.Zhang S. Fabrication of novel biomaterials though molecular self-assembly. Nature Biotechnology. 2003;21(10):1171–1178. doi: 10.1038/nbt874. [DOI] [PubMed] [Google Scholar]
- 39.Niece KL, et al. Self-Assembly Combining Two Bioactive Peptide-Amphiphile Molecules into Nanofibers by Electrostatic Attraction. Journal of the American Chemical Society. 2003;125(24):7146–7147. doi: 10.1021/ja028215r. [DOI] [PubMed] [Google Scholar]
- 40.Kisiday J, et al. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: Implications for cartilage tissue repair. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(15):9996–10001. doi: 10.1073/pnas.142309999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Di Zio K, Tirrell DA. Mechanical Properties of Artificial Protein Matrices Engineered for Control of Cell and Tissue Behavior. Macromolecules. 2003;36(5):1553–1558. [Google Scholar]
- 42.Caplan MR, et al. Effects of systematic variation of amino acid sequence on the mechanical properties of a self-assembling, oligopeptide biomaterial. Journal of Biomaterials Science, Polymer Edition. 2002;13:225–236. doi: 10.1163/156856202320176493. [DOI] [PubMed] [Google Scholar]
- 43.Ghanaati S, et al. Dynamic in vivo biocompatibility of angiogenic peptide amphiphile nanofibers. Biomaterials. 2009;30(31):6202–6212. doi: 10.1016/j.biomaterials.2009.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rajangam K, et al. Peptide amphiphile nanostructure-heparin interactions and their relationship to bioactivity. Biomaterials. 2008;29(23):3298–3305. doi: 10.1016/j.biomaterials.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meyers RA. Molecular biology and biotechnology : a comprehensive desk reference. New York: VCH; 1995. p. 1034. xxxviii. [Google Scholar]
- 46.Nedoviâc V, Willaert R. Focus on biotechnology ; v. 8B. Dordrecht ; New York: Springer; 2005. Applications of cell immobilisation biotechnology; p. 573. vi. [Google Scholar]
- 47.Jen AC, Wake MC, Mikos AG. Review: Hydrogels for cell immobilization. Biotechnol Bioeng. 1996;50(4):357–364. doi: 10.1002/(SICI)1097-0290(19960520)50:4<357::AID-BIT2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 48.Anseth K, Bowman C, Brannon-Peppas L. Mechanical properties of hydrogels and their experimental determination. Biomaterials. 1996;17:1647–1657. doi: 10.1016/0142-9612(96)87644-7. [DOI] [PubMed] [Google Scholar]
- 49.Neidert MR, Tranquillo RT. Tissue-engineered valves with commissural alignment. Tissue Eng. 2006;12(4):891–903. doi: 10.1089/ten.2006.12.891. [DOI] [PubMed] [Google Scholar]
- 50.Thomopoulos S, Fomovsky GM, Holmes JW. The development of structural and mechanical anisotropy in fibroblast populated collagen gels. J Biomech Eng. 2005;127(5):742–750. doi: 10.1115/1.1992525. [DOI] [PubMed] [Google Scholar]
- 51.Isenberg BC, Williams C, Tranquillo RT. Endothelialization and flow conditioning of fibrin-based media-equivalents. Ann Biomed Eng. 2006;34(6):971–985. doi: 10.1007/s10439-006-9101-0. [DOI] [PubMed] [Google Scholar]
- 52.Isenberg BC, Williams C, Tranquillo RT. Small-diameter artificial arteries engineered in vitro. Circ Res. 2006;98(1):25–35. doi: 10.1161/01.RES.0000196867.12470.84. [DOI] [PubMed] [Google Scholar]
- 53.Williams C, et al. Cell sourcing and culture conditions for fibrin-based valve constructs. Tissue Eng. 2006;12(6):1489–1502. doi: 10.1089/ten.2006.12.1489. [DOI] [PubMed] [Google Scholar]
- 54.Grassl ED, Oegema TR, Tranquillo RT. A fibrin-based arterial media equivalent. J Biomed Mater Res A. 2003;66(3):550–561. doi: 10.1002/jbm.a.10589. [DOI] [PubMed] [Google Scholar]
- 55.Rosner BI, Hang T, Tranquillo RT. Schwann cell behavior in three-dimensional collagen gels: evidence for differential mechano-transduction and the influence of TGF-beta 1 in morphological polarization and differentiation. Exp Neurol. 2005;195(1):81–91. doi: 10.1016/j.expneurol.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 56.Robinson PS, et al. Functional tissue-engineered valves from cell-remodeled fibrin with commissural alignment of cell-produced collagen. Tissue Eng Part A. 2008;14(1):83–95. doi: 10.1089/ten.a.2007.0148. [DOI] [PubMed] [Google Scholar]
- 57.Karamichos D, Brown RA, Mudera V. Collagen stiffness regulates cellular contraction and matrix remodeling gene expression. J Biomed Mater Res A. 2007;83(3):887–894. doi: 10.1002/jbm.a.31423. [DOI] [PubMed] [Google Scholar]
- 58.Francis Suh JK, Matthew HWT. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: a review. Biomaterials. 2000;21(24):2589–2598. doi: 10.1016/s0142-9612(00)00126-5. [DOI] [PubMed] [Google Scholar]
- 59.Rachel G, et al. Hepatocyte behavior within three-dimensional porous alginate scaffolds. Biotechnology and Bioengineering. 2000;67(3):344–353. doi: 10.1002/(sici)1097-0290(20000205)67:3<344::aid-bit11>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 60.Koichi M, et al. A novel two-step method for the formation of tissue-engineered cartilage by mature bovine chondrocytes: The alginate-recovered-chondrocyte (ARC) method. Journal of Orthopaedic Research. 2003;21(1):139–148. doi: 10.1016/S0736-0266(02)00109-2. [DOI] [PubMed] [Google Scholar]
- 61.Mosahebi A, et al. A Novel Use of Alginate Hydrogel as Schwann Cell Matrix. Tissue Engineering. 2001;7(5):525–534. doi: 10.1089/107632701753213156. [DOI] [PubMed] [Google Scholar]
- 62.Dar A, et al. Optimization of cardiac cell seeding and distribution in 3D porous alginate scaffolds. Biotechnol Bioeng. 2002;80(3):305–312. doi: 10.1002/bit.10372. [DOI] [PubMed] [Google Scholar]
- 63.Murat E, Vivek D, Gary G. Hepatocyte Attachment on Biodegradable Modified Chitosan Membranes: In Vitro Evaluation for the Development of Liver Organoids. Artificial Organs. 1998;22(10):837–846. doi: 10.1046/j.1525-1594.1998.06182.x. [DOI] [PubMed] [Google Scholar]
- 64.Madihally SV, Matthew HWT. Porous chitosan scaffolds for tissue engineering. Biomaterials. 1999;20(12):1133–1142. doi: 10.1016/s0142-9612(99)00011-3. [DOI] [PubMed] [Google Scholar]
- 65.Chung TW, et al. Preparation of alginate/galactosylated chitosan scaffold for hepatocyte attachment. Biomaterials. 2002;23(14):2827–2834. doi: 10.1016/s0142-9612(01)00399-4. [DOI] [PubMed] [Google Scholar]
- 66.Thomopoulos S, Fomovsky, Gregory M, Chandran Preethi L, Holmes, Jeffry W. Collagen fiber alignment does not explain mechanical anisotropy in fibroblasr populated collagen gels. JBME. 2007;129(5):642–650. doi: 10.1115/1.2768104. [DOI] [PubMed] [Google Scholar]
- 67.Lanir Y. Constitutive Equations for Fibrous Connective Tissues. journal of biomechanics. 1983;16:1–12. doi: 10.1016/0021-9290(83)90041-6. [DOI] [PubMed] [Google Scholar]
- 68.Sacks MS. Incorporation of experimentally-derived fiber orientation into a structural constitutive model for planar collagenous tissues. J Biomech Eng. 2003;125(2):280–287. doi: 10.1115/1.1544508. [DOI] [PubMed] [Google Scholar]
- 69.Sacks MS. A structural constitutive model for chemically treated planar tissues under biaxial loading. Computational Mechanics. 2000;26:243–249. [Google Scholar]
- 70.Freed LE, et al. Biodegradable Polymer Scaffolds for Tissue Engineering. Nature Biotechnology. 1994;12:689–693. doi: 10.1038/nbt0794-689. [DOI] [PubMed] [Google Scholar]
- 71.Engelmayr GC, Sacks MS. A structural model for the flexural mechanics of nonwoven tissue engineering scaffolds. J Biomech Eng. 2006;128:610–622. doi: 10.1115/1.2205371. [DOI] [PubMed] [Google Scholar]
- 72.Sutherland FW, et al. From stem cells to viable autologous semilunar heart valve. Circulation. 2005;111(21):2783–2791. doi: 10.1161/CIRCULATIONAHA.104.498378. [DOI] [PubMed] [Google Scholar]
- 73.Engelmayr GC, Jr, Sacks MS. Prediction of extracellular matrix stiffness in engineered heart valve tissues based on nonwoven scaffolds. Biomech Model Mechanobiol. 2008;7(4):309–321. doi: 10.1007/s10237-007-0102-1. [DOI] [PubMed] [Google Scholar]
- 74.Taylor G. Disintegration of Water Drops in an Electric Field. Proceedings of the Royal Society of London. Series A. Mathematical and Physical Sciences. 1964;280(1382):383–397. [Google Scholar]
- 75.Taylor G. The Force Exerted by an Electric Field on a Long Cylindrical Conductor. Proceedings of the Royal Society of London. Series A. Mathematical and Physical Sciences. 1966;291(1425):145–158. [Google Scholar]
- 76.Taylor G. Electrically Driven Jets. Proceedings of the Royal Society of London. A. Mathematical and Physical Sciences. 1969;313(1515):453–475. [Google Scholar]
- 77.Venugopal J, et al. Interaction of cells and nanofiber scaffolds in tissue engineering. J Biomed Mater Res B Appl Biomater. 2007 doi: 10.1002/jbm.b.30841. [DOI] [PubMed] [Google Scholar]
- 78.Chen M, et al. Role of fiber diameter in adhesion and proliferation of NIH 3T3 fibroblast on electrospun polycaprolactone scaffolds. Tissue Eng. 2007;13(3):579–587. doi: 10.1089/ten.2006.0205. [DOI] [PubMed] [Google Scholar]
- 79.Courtney T, et al. Design and analysis of tissue engineering scaffolds that mimic soft tissue mechanical anisotropy. Biomaterials. 2006;27(19):3631–3638. doi: 10.1016/j.biomaterials.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 80.Pham QP, Sharma U, Mikos AG. Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Eng. 2006;12(5):1197–1211. doi: 10.1089/ten.2006.12.1197. [DOI] [PubMed] [Google Scholar]
- 81.Teo WE, He W, Ramakrishna S. Electrospun scaffold tailored for tissue-specific extracellular matrix. Biotechnol J. 2006;1(9):918–929. doi: 10.1002/biot.200600044. [DOI] [PubMed] [Google Scholar]
- 82.Nerurkar NL, Elliott DM, Mauck RL. Mechanics of oriented electrospun nanofibrous scaffolds for annulus fibrosus tissue engineering. J Orthop Res. 2007;25(8):1018–1028. doi: 10.1002/jor.20384. [DOI] [PubMed] [Google Scholar]
- 83.Stankus JJ, et al. Microintegrating smooth muscle cells into a biodegradable, elastomeric fiber matrix. Biomaterials. 2006;27(5):735–744. doi: 10.1016/j.biomaterials.2005.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhong S, et al. An aligned nanofibrous collagen scaffold by electrospinning and its effects on in vitro fibroblast culture. J Biomed Mater Res A. 2006;79(3):456–463. doi: 10.1002/jbm.a.30870. [DOI] [PubMed] [Google Scholar]
- 85.Yoshimoto H, et al. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials. 2003;24(12):2077–2082. doi: 10.1016/s0142-9612(02)00635-x. [DOI] [PubMed] [Google Scholar]
- 86.Tian F, et al. Quantitative analysis of cell adhesion on aligned micro- and nanofibers. J Biomed Mater Res A. 2007 doi: 10.1002/jbm.a.31304. [DOI] [PubMed] [Google Scholar]
- 87.Li WJ, et al. Engineering controllable anisotropy in electrospun biodegradable nanofibrous scaffolds for musculoskeletal tissue engineering. J Biomech. 2007;40(8):1686–1693. doi: 10.1016/j.jbiomech.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stella JA, et al. Tissue-to-cellular level deformation coupling in cell micro-integrated elastomeric scaffolds. Biomaterials. 2008;29(22):3228–3236. doi: 10.1016/j.biomaterials.2008.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Riboldi SA, et al. Electrospun degradable polyesterurethane membranes: potential scaffolds for skeletal muscle tissue engineering. Biomaterials. 2005;26(22):4606–4615. doi: 10.1016/j.biomaterials.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 90.Rockwood DN, et al. Characterization of biodegradable polyurethane microfibers for tissue engineering. J Biomater Sci Polym Ed. 2007;18(6):743–758. doi: 10.1163/156856207781034115. [DOI] [PubMed] [Google Scholar]
- 91.Wake MC, Gupta PK, Mikos AG. Fabrication of pliable biodegradable polymer foams to engineer soft tissues. Cell Transplant. 1996;5(4):465–473. doi: 10.1177/096368979600500405. [DOI] [PubMed] [Google Scholar]
- 92.Guan J, et al. Preparation and characterization of highly porous, biodegradable polyurethane scaffolds for soft tissue applications. Biomaterials. 2005;26(18):3961–3971. doi: 10.1016/j.biomaterials.2004.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stankus JJ, Guan J, Wagner WR. Fabrication of biodegradable elastomeric scaffolds with sub-micron morphologies. J Biomed Mater Res. 2004;70A(4):603–614. doi: 10.1002/jbm.a.30122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Balguid A, et al. Tailoring Fiber Diameter in Electrospun Poly(epsilon-Caprolactone) Scaffolds for Optimal Cellular Infiltration in Cardiovascular Tissue Engineering. Tissue Eng Part A. 2008 doi: 10.1089/ten.tea.2007.0294. [DOI] [PubMed] [Google Scholar]
- 95.Pham QP, Sharma U, Mikos AG. Electrospun Poly(Îμ-caprolactone) Microfiber and Multilayer Nanofiber/Microfiber Scaffolds: Characterization of Scaffolds and Measurement of Cellular Infiltration. Biomacromolecules. 2006;7(10):2796–2805. doi: 10.1021/bm060680j. [DOI] [PubMed] [Google Scholar]
- 96.Telemeco TA, et al. Regulation of cellular infiltration into tissue engineering scaffolds composed of submicron diameter fibrils produced by electrospinning. Acta Biomaterialia. 2005;1(4):377–385. doi: 10.1016/j.actbio.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 97.Nam J, et al. Improved cellular infiltration in electrospun fiber via engineered porosity. Tissue Eng. 2007;13(9):2249–2257. doi: 10.1089/ten.2006.0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Marc S, et al. Ultraporous 3D polymer meshes by low-temperature electrospinning: Use of ice crystals as a removable void template. Polymer Engineering & Science. 2007;47(12):2020–2026. [Google Scholar]
- 99.Baker BM, et al. The potential to improve cell infiltration in composite fiber-aligned electrospun scaffolds by the selective removal of sacrificial fibers. Biomaterials. 2008;29(15):2348–2358. doi: 10.1016/j.biomaterials.2008.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Frey MW, Li L. Electrospinning and porosity measurements of nylon-6/poly(ethylene oxide) blended nonwovens. Journal of Engineered Fibers and Fabrics. 2007;2(1):31–37. [Google Scholar]
- 101.Yang S, et al. The Design of Scaffolds for Use in Tissue Engineering. Part II. Rapid Prototyping Techniques. Tissue Eng. 2002;8(1):1–11. doi: 10.1089/107632702753503009. [DOI] [PubMed] [Google Scholar]
- 102.Yeong W-Y, et al. Rapid prototyping in tissue engineering: challenges and potential. Trends in Biotechnology. 2004;22(12):643–652. doi: 10.1016/j.tibtech.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 103.Wei X, et al. Rapid Prototyping of Polyurethane for the Creation of Vascular Systems. Journal of Bioactive and Compatible Polymers. 2008;23(2):103–114. [Google Scholar]
- 104.Thomas B, et al. Cell and organ printing 2: Fusion of cell aggregates in three-dimensional gels. The Anatomical Record Part A: Discoveries in Molecular, Cellular, and Evolutionary Biology. 2003;272A(2):497–502. doi: 10.1002/ar.a.10059. [DOI] [PubMed] [Google Scholar]
- 105.Ringeisen BR, et al. Laser Printing of Pluripotent Embryonal Carcinoma Cells. Tissue Eng. 2004;10(3–4):483–491. doi: 10.1089/107632704323061843. [DOI] [PubMed] [Google Scholar]
- 106.Liu VA, Bhatia SN. Three-Dimensional Photopatterning of Hydrogels Containing Living Cells. Biomedical Microdevices. 2002;4(4):257–266. [Google Scholar]
- 107.Vunjak-Novakovic G, et al. Bioreactor cultivation conditions modulate the composition and mechanical properties of tissue-engineered cartilage. J Orthop Res. 1999;17(1):130–138. doi: 10.1002/jor.1100170119. [DOI] [PubMed] [Google Scholar]
- 108.Wang JH, Thampatty BP. An introductory review of cell mechanobiology. Biomech Model Mechanobiol. 2006;5(1):1–16. doi: 10.1007/s10237-005-0012-z. [DOI] [PubMed] [Google Scholar]
- 109.Butcher JT, Simmons CA, Warnock JN. Mechanobiology of the aortic heart valve. J Heart Valve Dis. 2008;17(1):62–73. [PubMed] [Google Scholar]
- 110.Matsumoto T, et al. Mechanical strain regulates endothelial cell patterning in vitro. Tissue Eng. 2007;13(1):207–217. doi: 10.1089/ten.2006.0058. [DOI] [PubMed] [Google Scholar]
- 111.Yanagisawa M, et al. Effects of compressive force on the differentiation of pluripotent mesenchymal cells. Life Sci. 2007;81(5):405–412. doi: 10.1016/j.lfs.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 112.Terraciano V, et al. Differential response of adult and embryonic mesenchymal progenitor cells to mechanical compression in hydrogels. Stem Cells. 2007;25(11):2730–2738. doi: 10.1634/stemcells.2007-0228. [DOI] [PubMed] [Google Scholar]
- 113.Song G, et al. Mechanical stretch promotes proliferation of rat bone marrow mesenchymal stem cells. Colloids Surf B Biointerfaces. 2007;58(2):271–277. doi: 10.1016/j.colsurfb.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 114.Merryman WD, et al. Synergistic effects of cyclic tension and transforming growth factorbeta1 on the aortic valve myofibroblast. Cardiovasc Pathol. 2007;16(5):268–276. doi: 10.1016/j.carpath.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Screen HR, et al. Cyclic tensile strain upregulates collagen synthesis in isolated tendon fascicles. Biochem Biophys Res Commun. 2005;336(2):424–429. doi: 10.1016/j.bbrc.2005.08.102. [DOI] [PubMed] [Google Scholar]
- 116.MacKenna D, Summerour SR, Villarreal FJ. Role of mechanical factors in modulating cardiac fibroblast function and extracellular matrix synthesis. Cardiovasc Res. 2000;46(2):257–263. doi: 10.1016/s0008-6363(00)00030-4. [DOI] [PubMed] [Google Scholar]
- 117.Kulik TJ, Alvarado SP. Effect of stretch on growth and collagen synthesis in cultured rat and lamb pulmonary arterial smooth muscle cells. J Cell Physiol. 1993;157(3):615–624. doi: 10.1002/jcp.1041570322. [DOI] [PubMed] [Google Scholar]
- 118.Gupta V, Grande-Allen KJ. Effects of static and cyclic loading in regulating extracellular matrix synthesis by cardiovascular cells. Cardiovasc Res. 2006;72(3):375–383. doi: 10.1016/j.cardiores.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 119.Engelmayr GC, Jr., et al. Cyclic flexure and laminar flow synergistically accelerate mesenchymal stem cell-mediated engineered tissue formation: Implications for engineered heart valve tissues. Biomaterials. 2006;27(36):6083–6095. doi: 10.1016/j.biomaterials.2006.07.045. [DOI] [PubMed] [Google Scholar]
- 120.Mol A, et al. The relevance of large strains in functional tissue engineering of heart valves. Thorac Cardiovasc Surg. 2003;51(2):78–83. doi: 10.1055/s-2003-38993. [DOI] [PubMed] [Google Scholar]
- 121.Kim BS, Mooney DJ. Scaffolds for engineering smooth muscle under cyclic mechanical strain conditions. J Biomech Eng. 2000;122(3):210–215. doi: 10.1115/1.429651. [DOI] [PubMed] [Google Scholar]
- 122.Engelmayr GC, Jr., et al. The independent role of cyclic flexure in the early in vitro development of an engineered heart valve tissue. Biomaterials. 2005;26(2):175–187. doi: 10.1016/j.biomaterials.2004.02.035. [DOI] [PubMed] [Google Scholar]
- 123.Sacks MS, Schoen FJ, Mayer JE., Jr Bioengineering Challenges for Heart Valve Tissue Engineering. Annual Review of Biomedical Engineering. 2009;11 doi: 10.1146/annurev-bioeng-061008-124903. [DOI] [PubMed] [Google Scholar]
- 124.Pei M, et al. Bioreactors mediate the effectiveness of tissue engineering scaffolds. Faseb J. 2002;16(12):1691–1694. doi: 10.1096/fj.02-0083fje. [DOI] [PubMed] [Google Scholar]
- 125.Gooch KJ, et al. Effects of mixing intensity on tissue-engineered cartilage. Biotechnol Bioeng. 2001;72(4):402–407. doi: 10.1002/1097-0290(20000220)72:4<402::aid-bit1002>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 126.Bursac N, et al. Cultivation in rotating bioreactors promotes maintenance of cardiac myocyte electrophysiology and molecular properties. Tissue Eng. 2003;9(6):1243–1253. doi: 10.1089/10763270360728152. [DOI] [PubMed] [Google Scholar]
- 127.Buschmann MD, et al. Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. J Cell Sci. 1995;108(Pt 4):1497–1508. doi: 10.1242/jcs.108.4.1497. [DOI] [PubMed] [Google Scholar]
- 128.Seidel JO, et al. Long-term culture of tissue engineered cartilage in a perfused chamber with mechanical stimulation. Biorheology. 2004;41(3–4):445–458. [PubMed] [Google Scholar]
- 129.Fink C, et al. Chronic stretch of engineered heart tissue induces hypertrophy and functional improvement. Faseb J. 2000;14(5):669–679. doi: 10.1096/fasebj.14.5.669. [DOI] [PubMed] [Google Scholar]
- 130.Zimmermann WH, et al. Tissue engineering of a differentiated cardiac muscle construct. Circ Res. 2002;90(2):223–230. doi: 10.1161/hh0202.103644. [DOI] [PubMed] [Google Scholar]
- 131.Mauck RL, et al. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122(3):252–260. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 132.Kim BS, et al. Cyclic mechanical strain regulates the development of engineered smooth muscle tissue. Nat Biotechnol. 1999;17(10):979–983. doi: 10.1038/13671. [DOI] [PubMed] [Google Scholar]
- 133.Akhyari P, et al. Mechanical stretch regimen enhances the formation of bioengineered autologous cardiac muscle grafts. Circulation. 2002;106(12 Suppl 1):I137–1142. [PubMed] [Google Scholar]
- 134.Long JL, Tranquillo RT. Elastic fiber production in cardiovascular tissue-equivalents. Matrix Biol. 2003;22(4):339–350. doi: 10.1016/s0945-053x(03)00052-0. [DOI] [PubMed] [Google Scholar]
- 135.Roeder BA, et al. Local, Three-Dimensional Strain Measurements Within Largely Deformed Extracellular Matrix Constructs. Journal of Biomechanical Engineering. 2004;126(6):699–708. doi: 10.1115/1.1824127. [DOI] [PubMed] [Google Scholar]
- 136.Guilak F, Ratcliffe A, Mow VC. Chondrocyte deformation and local tissue strain in articular cartilage: a confocal microscopy study. J Orthop Res. 1995;13(3):410–421. doi: 10.1002/jor.1100130315. [DOI] [PubMed] [Google Scholar]
- 137.Ingber DE. Mechanobiology and diseases of mechanotransduction. Ann Med. 2003;35(8):564–577. doi: 10.1080/07853890310016333. [DOI] [PubMed] [Google Scholar]
- 138.Lee DA, Knight MM. Mechanical loading of chondrocytes embedded in 3D constructs: in vitro methods for assessment of morphological and metabolic response to compressive strain. Methods Mol Med. 2004;100:307–324. doi: 10.1385/1-59259-810-2:307. [DOI] [PubMed] [Google Scholar]
- 139.Sheetz MP. Cell control by membrane-cytoskeleton adhesion. Nat Rev Mol Cell Biol. 2001;2(5):392–396. doi: 10.1038/35073095. [DOI] [PubMed] [Google Scholar]
- 140.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310(5751):1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 141.Engler A, et al. Substrate compliance versus ligand density in cell on gel responses. Biophys J. 2004;86(1 Pt 1):617–628. doi: 10.1016/S0006-3495(04)74140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jiang G, et al. Rigidity Sensing at the Leading Edge through ±v23 Integrins and RPTP±. 2006;90(5):1804–1809. doi: 10.1529/biophysj.105.072462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Thomas CH, et al. Engineering gene expression and protein synthesis by modulation of nuclear shape. Proc Natl Acad Sci U S A. 2002;99(4):1972–1977. doi: 10.1073/pnas.032668799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Huang HY, Liao J, Sacks MS. In-situ deformation of the aortic valve interstitial cell nucleus under diastolic loading. J Biomech Eng. 2007;129(6):880–889. doi: 10.1115/1.2801670. [DOI] [PubMed] [Google Scholar]
- 145.Yarin AL, Zussman E. Upward needleless electrospinning of multiple nanofibers. Polymer. 2004;45(9):2977–2980. [Google Scholar]
- 146.Li WJ, et al. Fabrication and characterization of six electrospun poly(alpha-hydroxy ester)- based fibrous scaffolds for tissue engineering applications. Acta Biomater. 2006;2(4):377–385. doi: 10.1016/j.actbio.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 147.Li WJ, et al. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J Biomed Mater Res. 2002;60(4):613–621. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 148.Matthews JA, et al. Electrospinning of collagen nanofibers. Biomacromolecules. 2002;3(2):232–238. doi: 10.1021/bm015533u. [DOI] [PubMed] [Google Scholar]
- 149.Pedicini and Farris. Mechanical behavior of electrospun polyurethane. Polymer. 2003;44:6857–6862. [Google Scholar]
- 150.Bibekananda S, et al. Electrospinning of continuous aligned polymer fibers. Applied Physics Letters. 2004;84(7):1222–1224. [Google Scholar]
- 151.Mauck RL, et al. Engineering on the Straight and Narrow: The Mechanics of Nanofibrous Assemblies for Fiber-Reinforced Tissue Regeneration. Tissue Engineering Part B: Reviews. 2009;15(2):171–193. doi: 10.1089/ten.teb.2008.0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Johnson J, Ghosh A, Lannutti J. Microstructure-Property Relationships in a Tissue- Engineering Scaffold. Journal of Applied Polymer Science. 2007;104:2919–2927. [Google Scholar]
- 153.Fung YC, Fronek K, Patitucci P. Pseudoelasticity of arteries and the choice of its mathematical expression. Am J Physiol. 1979;237(5):H620–H631. doi: 10.1152/ajpheart.1979.237.5.H620. [DOI] [PubMed] [Google Scholar]
- 154.David G, et al. Regional multiaxial mechanical properties of the porcine anterior lens capsule. J Biomech Eng. 2007;129(1):97–104. doi: 10.1115/1.2401188. [DOI] [PubMed] [Google Scholar]
- 155.Driessen NJ, Bouten CV, Baaijens FP. A structural constitutive model for collagenous cardiovascular tissues incorporating the angular fiber distribution. J Biomech Eng. 2005;127(3):494–503. doi: 10.1115/1.1894373. [DOI] [PubMed] [Google Scholar]
- 156.Choi HS, Vito RP. Two-dimensional stress-strain relationship for canine pericardium. J Biomech Eng. 1990;112(2):153–159. doi: 10.1115/1.2891166. [DOI] [PubMed] [Google Scholar]
- 157.Humphrey JD, Vawter DL, Vito RP. Pseudoelasticity of Excised Visceral Pleura. Journal of Biomechanical Engineering. 1987;109:115–120. doi: 10.1115/1.3138652. [DOI] [PubMed] [Google Scholar]
- 158.Fung YC. A model of the lung structure and its validation. J Appl Physiol. 1988;64(5):2132–2141. doi: 10.1152/jappl.1988.64.5.2132. [DOI] [PubMed] [Google Scholar]
- 159.Holzapfel GA. Determination of material models for arterial walls from uniaxial extension tests and histological structure. J Theor Biol. 2006;238(2):290–302. doi: 10.1016/j.jtbi.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 160.Humphrey JD, Yin FCP. A new constitutive formulation for characterizing the mechanical behavior of soft tissues. Biophysical Journal. 1987;52:563–570. doi: 10.1016/S0006-3495(87)83245-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Holzapfel GA, Gasser TC. A new constitutive framework for arterial wall mechanics and a comparative study of material models. Journal of Elasticity. 2000;61:1–48. [Google Scholar]
- 162.Lanir Y. Plausibility of Structural Constitutive-Equations for Isotropic Soft-Tissues in Finite Static Deformations. Journal of Applied Mechanics-Transactions of the Asme. 1994;61(3):695–702. [Google Scholar]
- 163.Lanir Y. Plausibility of structural constitutive equations for swelling tissues--implications of the C-N and S-E conditions. J Biomech Eng. 1996;118(1):10–16. doi: 10.1115/1.2795935. [DOI] [PubMed] [Google Scholar]
- 164.Sacks MS. A structural constitutive model for chemically treated planar connective tissues under biaxial loading. Computational Mechanics. 2000;26(3):243–249. [Google Scholar]
- 165.Agoram B, Barocas VH. Coupled macroscopic and microscopic scale modeling of fibrillar tissues and tissue equivalents. J Biomech Eng. 2001;123(4):362–369. doi: 10.1115/1.1385843. [DOI] [PubMed] [Google Scholar]
- 166.Stylianopoulos T, Barocas VH. Volume-averaging theory for the study of the mechanics of collagen networks. Computer Methods in Applied Mechanics and Engineering. 2007;196(31–32):2981–2990. [Google Scholar]
- 167.Preethi LC, Victor HB. Deterministic Material-Based Averaging Theory Model of Collagen Gel Micromechanics. Journal of Biomechanical Engineering. 2007;129(2):137–147. doi: 10.1115/1.2472369. [DOI] [PubMed] [Google Scholar]
- 168.Zahalak GI, et al. A cell-based constitutive relation for bio-artificial tissues. Biophys J. 2000;79(5):2369–2381. doi: 10.1016/S0006-3495(00)76482-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Marquez JP, et al. Thin bio-artificial tissues in plane stress: the relationship between cell and tissue strain, and an improved constitutive model. Biophys J. 2005;88(2):765–777. doi: 10.1529/biophysj.104.040808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Marquez JP, et al. The relationship between cell and tissue strain in three-dimensional bioartificial tissues. Biophys J. 2005;88(2):778–789. doi: 10.1529/biophysj.104.041947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kanit T, Forest S, Galliet I. Determination of the size of the representative volume element for random composites: statistical and numerical approach. International Journal of Solids and Structures. 2003;40:3647–3679. [Google Scholar]
- 172.Khisaeva ZF, Ostoja-Starzewski2 aM. On the Size of RVE in Finite Elasticity of Random Composites. Journal of Elasticity. 2006;85(2):153–173. [Google Scholar]
- 173.Stylianopoulos A, et al. Computational predictions of the tensile properties of electrospun fibre meshes: Effect of fibre diameter and fibre orientation. Journal of Mechanical Behavior of Biomedical Materials. 2008;1:326–335. doi: 10.1016/j.jmbbm.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Burd HJ. A structural constitutive model for the human lens capsule. Biomech Model Mechanobiol. 2008 doi: 10.1007/s10237-008-0130-5. [DOI] [PubMed] [Google Scholar]
- 175.Pavia FC, et al. Polymeric scaffolds prepared via thermally induced phase separation: Tuning of structure and morphology. Journal of Biomedical Materials Research Part A. 2008;86A(2):459–466. doi: 10.1002/jbm.a.31621. [DOI] [PubMed] [Google Scholar]
- 176.Lebourg M, et al. Biodegradable polycaprolactone scaffold with controlled porosity obtained by modified particle-leaching technique. Journal of Materials Science: Materials in Medicine. 2008;19(5):2047–2053. doi: 10.1007/s10856-007-3282-4. [DOI] [PubMed] [Google Scholar]
- 177.Lips PAM, et al. Gas foaming of segmented poly(ester amide) films. Polymer. 2005;46(22):9396–9403. [Google Scholar]
- 178.Mathieu LM, et al. Architecture and properties of anisotropic polymer composite scaffolds for bone tissue engineering. Biomaterials. 2006;27(6):905–916. doi: 10.1016/j.biomaterials.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 179.Cui H, et al. Self-Assembly of Giant Peptide Nanobelts. Nano Letters. 2009;9(3):945–951. doi: 10.1021/nl802813f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sander EA, Barocas VH. Comparison of 2D fiber network orientation measurement methods. Journal of Biomedical Materials Research Part A. 2009;88A(2):322–331. doi: 10.1002/jbm.a.31847. [DOI] [PubMed] [Google Scholar]
- 181.Stella JA, Wagner WR, Sacks MS. Scale dependent kinematics of elastomeric electrospun scaffolds for soft tissue engineering. Journal of Biomedical Materials Research. 2009 doi: 10.1002/jbm.a.32593. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Slivka MA, et al. Porous, Resorbable, Fiber-Reinforced Scaffolds Tailored for Articular Cartilage Repair. Tissue Eng. 2001;7(6):767–780. doi: 10.1089/107632701753337717. [DOI] [PubMed] [Google Scholar]