Abstract
In the unicellular green alga Chlamydomonas reinhardtii, translation of the chloroplast-encoded psbA mRNA is regulated by the light-dependent binding of a nuclear-encoded protein complex (RB38, RB47, RB55 and RB60) to the 5′-untranslated region of the RNA. Despite the absence of any report identifying a red light photoreceptor within this alga, we show that the expression of the rb38, rb47 and rb60 genes, as well as the nuclear-encoded psbO gene that directs the synthesis of OEE1 (oxygen evolving enhancer 1), is differentially regulated by red light. Further elucidation of the signal transduction pathway shows that calmodulin is an important messenger in the signaling cascade that leads to the expression of rb38, rb60 and psbO, and that a chloroplast signal affects rb47 at the translational level. While there may be several factors involved in the cascade of events from the perception of red light to the expression of the rb and psbO genes, our data suggest the involvement of a red light photoreceptor. Future studies will elucidate this receptor and the additional components of this red light signaling expression pathway in C. reinhardtii.
Keywords: Calmodulin, Chlamydomonas reinhardtii, Nuclear encoded, Red light, RNA-binding protein, Signal transduction pathway
Introduction
Similar to higher plants, single-celled, photosynthetic organisms respond to both the quality and quantity of light they perceive within their environment. In the unicellular green alga Chlamydomonas reinhardtii, light plays a key role in motility (Schaller et al. 1997), sexual reproduction (Pan et al. 1997, Huang et al. 2002, Ermilova et al. 2004), chloroplast biogenesis (Rochaix 1995, Harris 2001, Grossman et al. 2004) and the expression of numerous genes, whose products participate in a variety of biochemical and physiological processes (Matters and Beale 1995, Rodriguez et al. 1999, Im and Beale 2000, Kropat et al. 2000, Bohne and Linden 2002, Teramoto et al. 2006). Light-regulated gene expression has been shown to involve complex signaling pathways, in which photoreceptors perceive light, and in turn activate regulatory factors that initiate the transcription of specific genes (Gyula et al. 2003, Im et al. 2006, Jiao et al. 2007). While many studies in C. reinhardtii have demonstrated white light- and blue light-induced gene expression (Matters and Beale 1995, Herman et al. 1999, Bohne and Linden 2002, Barneche et al. 2006), few have demonstrated red light-regulated control (Petridou et al. 1997, Teramoto et al. 2006). In fact, the absence of research in this area has probably hindered the identification of a red light photoreceptor in C. reinhardtii (Mittag et al. 2005, Im et al. 2006), despite the presence of sequences within the alga’s genome that suggest the existence of a phytochrome-like molecule (Grossman et al. 2004).
In plants and green algae, light-regulated chloroplast biogenesis requires the close cooperation of the nuclear and chloroplast genomes. Many of the proteins within the photosynthetic complexes are nuclear encoded, and their synthesis is driven by light-regulated transcription (Gilmartin et al. 1990, Kuhlemeier 1992, Barkan and Goldschmidt-Clermont 2000). In C. reinhardtii, the genes encoding the light harvesting chlorophyll a/b-binding proteins (LHCPs) and the oxygen evolving enhancer (OEE) complex proteins are expressed when cells are shifted from the dark into white light (Malnoë et al. 1988). Light also plays a role in the translation of chloroplast-encoded photosynthetic mRNAs (Mayfield 1995, Rochaix 1996, Sugita and Sugiura 1996, Deng and Quail 1999, Zerges 2000). Synthesis of the D1, D2, P5 and P6 proteins is greatly elevated in the light (Malnoë et al. 1988), and controlled by the interaction of nuclear-encoded protein complexes with the 5′-untranslated region (UTR) of the corresponding mRNAs (Danon and Mayfield 1991, Mayfield et al. 1994, Yohn et al. 1998a, Nickelsen et al. 1999, Ossenbuhl and Nickelsen 2000, Auchincloss et al. 2002, Barnes et al. 2004).
Translation of the chloroplast-encoded psbA mRNA, which encodes the Photosystem II (PSII)-associated D1 protein, is controlled by the light-regulated binding of a nuclear- encoded protein complex (RB38, RB47, RB55 and RB60) to the 5′ UTR of the mRNA (Danon and Mayfield 1991, Yohn et al. 1998a). These proteins bind to a stem–loop structure within the 5′ UTR to activate translation in a light-dependent manner (Danon and Mayfield 1991). The translation of the psbA mRNA is influenced by the light-regulated oxidation–reduction (redox) state of the RB60 protein (Trebitsh et al. 2000). In the light RB60 reduces RB47, which increases the affinity of RB47 for the 5′ UTR, allowing for the complex to bind and translation to occur (Kim and Mayfield 1997, Fong et al. 2000). Thus, changes in light intensity, which lead to downstream signaling events, affect the synthesis of the D1 protein.
Nuclear mutants that are deficient in the synthesis of RB47 show a loss of psbA translation. The absence of RB47 results in a complete loss of RNA–protein interaction and psbA–ribosome association (Yohn et al. 1998b). Because the nuclear-encoded RB47 protein plays a pivotal role in the translation of the psbA mRNA, our attention became focused on determining the signals that activate expression of this protein, as well as the other proteins that interact with the 5′ UTR of the psbA mRNA to regulate its translation. The dissection of the signal transduction pathway, which regulates the psbA rb (RNA-binding) genes, will allow us to define events that lead to the translational regulation of the psbA mRNA and provide important insight into the molecular mechanism(s) by which protein–RNA interactions trigger translational activation of specific chloroplast mRNAs.
In this report we show that the rb genes are regulated by light or chloroplast signals. Interestingly, we show that red light induces the expression of the rb38 and rb60 genes, and that calmodulin (CaM) is an important messenger in the signal transduction pathway. While rb47 mRNA accumulation remains unchanged in response to light treatments, translation of the mRNA appears to be affected by red light and a chloroplast signal. Our findings demonstrate that red light is an important component of the visible spectrum for signaling pathways in C. reinhardtii that lead to changes in gene expression, and strongly support the presence of a red light photoreceptor in this unicellular green alga.
Results
Light and chloroplast signals regulate the expression of the nuclear-encoded psbA RNA-binding protein genes
The nuclear-encoded RB38, RB47, RB55 and RB60 proteins are part of a complex that regulates the translation of the chloroplast-encoded psbA mRNA in a light-dependent manner. Translation of psbA occurs when the complex binds to the 5′ UTR of the mRNA, a consequence of light-activated signaling events within the chloroplast. Both reduction and phosphorylation of specific proteins in the complex must occur for binding and translation to take place (Danon and Mayfield 1994a, Danon and Mayfield 1994b, Kim and Mayfield 1997, Alergand et al. 2006). In order to understand fully the signal transduction pathway that leads to psbA translation, the cues that induce the expression of the corresponding rb genes in the nucleus must be recognized. As light plays a pivotal role in psbA translation, its effect on rb gene expression was evaluated.
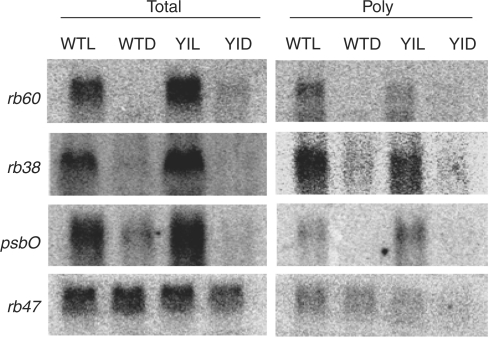
The accumulation of rb38, rb47 and rb60 total and polysomal RNA was analyzed in C. reinhardtii cells that were grown in constant dark or constant light. rb55 was not evaluated, as neither the cDNA nor the gene have been cloned. In order to distinguish between light- and chloroplast-activated signaling, wild-type (wt) and y-1 cells were used. Wt strains of C. reinhardtii possess a functional chloroplast in both the light and the dark, while the y-1 mutant mimics higher plants, displaying light-activated chloroplast development (Ohad et al. 1967a, Ohad et al. 1967b, Cahoon and Timko 2000). Northern analyses showed that rb38 and rb60 are light induced, while rb47 is regulated by a chloroplast signal. Accumulation of rb38 and rb60 total RNA was seen in light-grown wt and y-1 cells, and not in dark-grown cells (Fig. 1). A similar pattern was observed for polysomal RNA accumulation, indicating that there was an increase in translation in the light. Both total and polysomal psbO RNA accumulation was also elevated in the light (Fig. 1), and followed the same pattern as rb38 and rb60. psbO served as a control, as the light-regulated expression of this nuclear-encoded photosynthetic gene had been previously described (Mayfield et al. 1987, Malnoë et al. 1988). Interestingly, while the accumulation of rb47 total and polysomal RNA remained relatively unchanged upon a dark to light shift in wt cells, a slight decrease in total RNA accumulation, and no detectable association with polyribosomes, was observed after a dark to light shift in y-1 cells (Fig. 1). These results support the previous finding of Danon and Mayfield (1991), who suggested that a chloroplast signal regulates rb47 translation.
Fig. 1.
Accumulation of rb38, rb47, rb60 and psbO RNA in dark- or light-grown wt and y-1 cells. The steady-state levels of total (Total) and polyribosome-associated (Poly) RNA were examined by Northern blot analysis after exposure of cells to constant white light (L) or constant darkness (D).
Red light induces the accumulation of the rb38 and rb60 RNA-binding protein genes
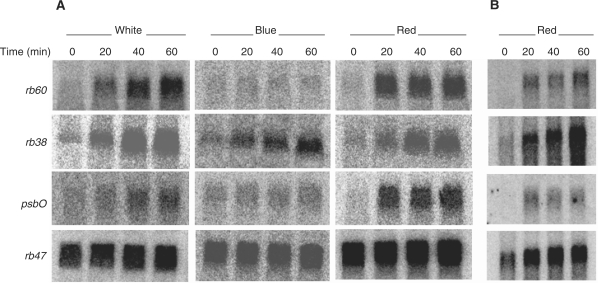
To understand further how light activates the expression of rb38, rb60 and psbO, dark-grown wt C. reinhardtii cells were exposed to white light (390–780 nm) or single-source wavelengths of blue (470 nm) or red (670 nm) light. Surprisingly, each gene was induced by red light in addition to white light (Fig. 2A). This finding is somewhat novel, as there have been few reports describing red light-induced gene expression in C. reinhardtii (Petridou et al. 1997, Teramoto et al. 2006). The accumulation of rb38 mRNA gradually increased over a 60 min period of red light exposure, while the rb60 and psbO mRNAs showed a more dramatic increase between the dark and 20 min of exposure, followed by little change thereafter. Exposure of cells to blue light resulted in an increase in only rb38 mRNA, with the pattern of accumulation similar to that seen after exposure to white and red light. The accumulation pattern observed for rb60 and psbO in response to white light was not the same as that seen after exposure to red light, indicating that other wavelengths in the visible spectrum play a role in the signaling pathway that regulates the expression of these genes. As white light did not induce rb47 in wt or y-1 cells (Fig. 1), it was not unexpected that this transcript remained constant during a dark to light shift under all three light qualities (Fig. 2A).
Fig. 2.
Accumulation of rb38, rb47, rb60 and psbO mRNA in response to different light qualities. Wt C. reinhardtii cells were grown in the dark and exposed to white, blue or red light for 0, 20, 40 or 60 min. A 15 μg aliquot of total RNA (A) or 2 μg of polysomal RNA (B) was evaluated using Northern blot analysis.
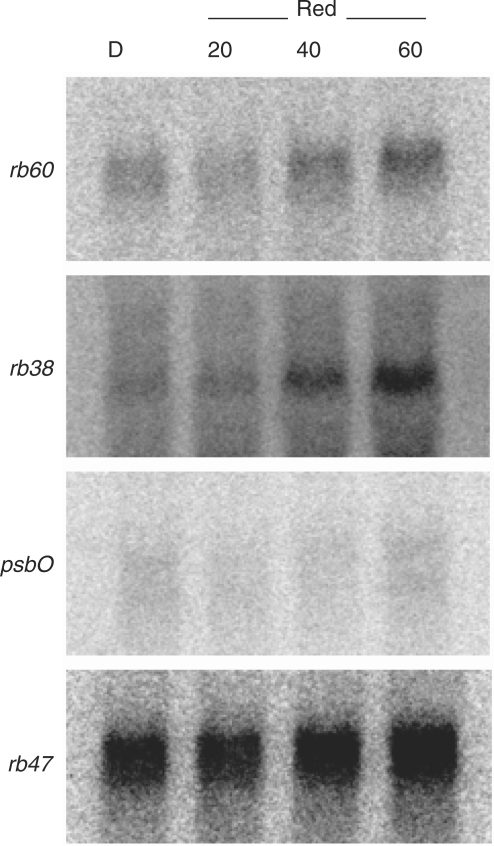
In order to evaluate the effects of photosynthesis on the red light-induced expression of the rb38, rb60 and psbO genes, mRNA accumulation was evaluated in y-1 cells. The minimal accumulation of each mRNA in the dark, and increasing accumulation between 20 and 60 min of red light exposure (Fig. 3), strongly suggests the involvement of a novel red light photoreceptor, as neither photosystem has formed in this cell line during the 60 min period of illumination. Interestingly, rb38 and rb60 mRNA accumulation was more evident than psbO accumulation, suggesting that the transcription of psbO may be somewhat influenced by the biogenesis of the chloroplast. Furthermore, the pattern of red light-induced rb60 mRNA accumulation observed in y-1 cells was different from that observed in wt cells, indicating that the photosystems may still play a role in expression of this gene. As expected, rb47 accumulation remained relatively unchanged in response to the red light treatment.
Fig. 3.
Northern blot analysis of rb38, rb47, rb60 and psbO accumulation in y-1 cells after exposure to red light for 0, 20, 40 or 60 min. A 15 μg aliquot of total RNA was assessed.
RB38 and RB60 protein accumulation in response to light does not parallel mRNA accumulation profiles despite light-regulated translation
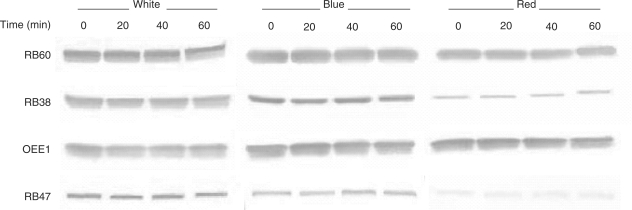
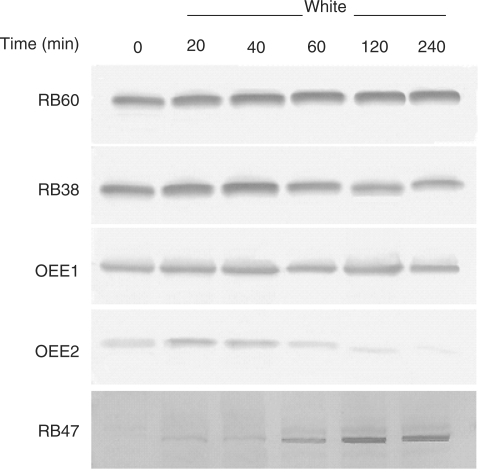
The light-induced association of rb38, rb60 and psbO mRNA with polysomes suggested that the translated proteins would accumulate in a similar pattern. However, RB38, RB60 and OEE1 protein accumulation was unchanged in wt cells that were grown in the dark and shifted to white, blue or red light (Fig. 4). RB47 protein accumulation was also unaffected after a light shift. As each blot was not developed for the same amount of time, a difference in protein accumulation can be seen from one light treatment to the next. In order to validate the constitutive accumulation patterns that were observed, the identification of a protein profile that changed upon a shift from the dark into the light was necessary. Therefore, y-1 cells were grown in constant dark and shifted into white light over a 240 min period. As seen in Fig. 5, RB38, RB60 and OEE1 protein accumulation remained unchanged after 240 min of light exposure, while RB47 showed a gradual increase in accumulation after exposure to white light. The OEE2 protein was also evaluated, and showed a decrease in accumulation between 60 and 240 min of white light exposure. Therefore, despite the increased association of the rb38, rb60 and psbO mRNAs with polysomes in the light (Fig. 1), the RB38, RB60 and OEE1 proteins do not display a light-induced pattern of accumulation (Figs. 4, 5). The increase in RB47 accumulation in y-1 cells upon a shift into the light further demonstrates that rb47 is regulated at the translational level by a chloroplast signal (Fig. 5).
Fig. 4.
Accumulation of RB38, RB47, RB60 and OEE1 protein in response to different light qualities. Wt C. reinhardtii cells were grown in the dark and exposed to white, blue or red light for 0, 20, 40 or 60 min. Equal amounts of soluble protein were separated by size using SDS–PAGE and transferred to nitrocellulose. Blots were decorated with a primary and secondary antibody, followed by alkaline phosphatase staining.
Fig. 5.
Accumulation of RB38, RB47, RB60, OEE1 and OEE2 protein in y-1 cells under white light. Cells were grown in the dark and exposed to white light for 0, 20, 40, 60, 120 or 240 min. Equal amounts of soluble protein were separated by size using SDS–PAGE and transferred to nitrocellulose. Blots were decorated with a primary and secondary antibody, followed by alkaline phosphatase staining.
As protein accumulation is not a measure of translation, polysomal RNA accumulation was further evaluated for the three rb genes and psbO after exposure to red light. As seen in Fig. 2B, little to no rb38, rb60 or psbO polysomal RNA is observed in the dark-grown cells. However, these mRNAs appear to be actively translated after exposure to red light, as seen by an increase in accumulation between 20 and 60 min of red light exposure. Interestingly, the red light-induced accumulation patterns for rb38, rb60 and psbO polysomal and total RNA parallel one another; rb38 continues to increase over the 60 min period of red light illumination, while rb60 and psbO show a more striking increase between the dark and 20 min red light treatment, with minimal change thereafter (Fig. 2A, B). This parallel was not seen for rb47. While the accumulation of rb47 total RNA remained unchanged in cells grown in the dark or red light (Fig. 2A), an increase in the accumulation of polysomal RNA was observed after a shift into red light, increasing slightly over the 60 min period (Fig. 2B). This finding suggests not only that it is a chloroplast signal controlling the translation of this mRNA (Fig. 1 and Danon and Mayfield 1991), but that the red light may play a role as well.
Calmodulin plays a role in the red light-induced expression of rb38 and rb60
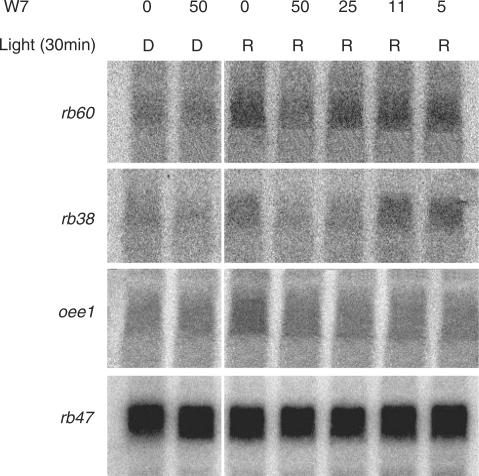
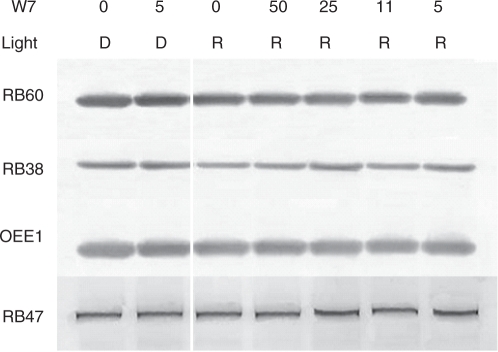
Signal transduction pathways, from the perception of light to the expression of specific genes, involve several regulatory molecules. In many pathways, Ca2+ and CaM act as secondary messengers. In order to determine if CaM plays a role in the red light induction of the rb38, rb60 and psbO genes, their expression was evaluated after CaM activity was inhibited. C. reinhardtii cells were grown in the dark for a week and then treated with varying concentrations of the CaM antagonist W7 for 30 min in the dark or under red light. As seen in Fig. 6, rb38, rb60 and psbO mRNA accumulation was unchanged in the dark, regardless of whether cells were treated with W7 (50 μM). However, a noticeable decrease in mRNA accumulation was observed in cells grown under red light and treated with the inhibitor. A decrease in rb60 mRNA accumulation occurred in cells treated with 50 μM W7, while a decrease in rb38 mRNA accumulation was seen at a concentration of 25 and 50 μM. Treatment of cells with lower concentrations of the inhibitor did not affect the accumulation of either mRNA during the 30 min treatment period. The pattern of psbO mRNA accumulation was quite different from the pattern observed for rb38 and rb60 in the presence of W7 and red light; a similar decrease was observed with each of the four concentrations of inhibitor tested (5–50 μM). The accumulation of rb47 remained constant in treated and untreated cells grown in the dark or in red light, indicating that CaM was not involved in its expression, and that the decrease in mRNA accumulation seen for rb38, rb60 and psbO in the presence of the inhibitor was not due to sample degradation. Cells were also treated with W5, the inactive analog of W7. No change in the accumulation of rb38, rb47, rb60 or psbO was observed in the presence (5, 11, 25 and 50 μM) or absence of this inhibitor under red light (data not shown), further indicating that the decreases in rb and psbO accumulation that were observed with W7 in the presence of red light are specifically the result of W7 acting on CaM. Regardless of the light regime or W7 treatment, RB38, RB47, RB60 and OEE1 accumulation remained unchanged (Fig. 7).
Fig. 6.
Accumulation of rb38, rb47, rb60 and psbO after treatment with the CaM antagonist W7. Wt C. reinhardtii cells were grown in the dark and then treated with 50, 25, 11 or 5 μM W7 for 30 min in the dark (D) or in red (R) light. Total RNA accumulation was evaluated using Northern analysis.
Fig. 7.
Accumulation of RB38, RB47, RB60 and OEE1 protein after treatment with the CaM antagonist W7. Wt C. reinhardtii cells were grown in the dark and then treated with 50, 25, 11 or 5 μM W7 for 30 min in the dark (D) or in red (R) light. Equal amounts of soluble protein were separated by size using SDS–PAGE and transferred to nitrocellulose. Blots were decorated with a primary and secondary antibody, followed by alkaline phosphatase staining.
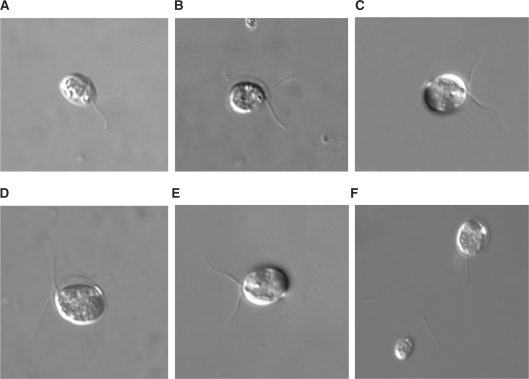
In order to verify that decreases in mRNA accumulation in response to W7 were the result of the inhibitor and not cell death, the morphology and motility of C. reinhardtii cells within each sample were closely monitored under the light microscope. As seen in Fig. 8, there are no unusual morphological defects after 30 min of treatment with any concentration of W7 used (5–50 μM). In addition, the observed motility of treated cells paralleled the motility of untreated cells. Therefore, the changes in mRNA accumulation that were observed after 30 min of W7 treatment are the result of inhibitor activity and not cell toxicity. After an extended period of 120 min, all cells treated with 5, 11 or 25 μM W7 appeared intact, while a 50 μM concentration of the drug resulted in deflagellation. This result was not unexpected as the IC50 for Ca2+/CaM-dependent phosphodiesterase and myosin light chain kinase is 28 and 51 μM, respectively.
Fig. 8.
Morphology of C. reinhardtii in the presence and absence of the CaM antagonist W7. Wt C. reinhardtii cells were exposed to red light for 30 min without (A) or with 50 μM (B), 25 μM (C), 11 μM (D) or 5 μM (E) W7. A 50 μM W7 treatment was also carried out for 120 min (F). Cells were viewed using an Olympus BX-60 optical microscope with ×60 magnification. Pictures were taken from each sample using a Hamamatsu digital camera.
Discussion
We have identified red light as an important component of the signal transduction cascade that leads to the translation of the psbA mRNA in C. reinhardtii. The nuclear-localized rb38 and rb60 genes, which encode the psbA translational activators, RB38 and RB60, were induced by white light and a single-source wavelength of red light (670 nm) over a 60 min period of exposure (Fig. 2A). Accumulation of the corresponding mRNAs occurred within 20 min of shifting the cells from the dark into the light. This pattern of red light-induced expression was also seen for the nuclear-encoded psbO gene, which directs OEE1 synthesis. Blue light (470 nm) also elicited the accumulation of rb38 mRNA, but not the accumulation of the rb60 or psbO mRNAs. Interestingly, rb47 mRNA accumulation was constitutive under all light conditions tested. It was somewhat surprising that red light had an impact on rb38, rb60 and psbO mRNA accumulation. Despite numerous examples of red light-induced gene expression in higher plants, only a limited number of published reports describe red light-induced gene expression in C. reinhardtii (Grossman et al. 2004). Recently, Im et al. (2006) showed that the mRNAs encoding glutamate-1-semialdehyde aminotransferase (GSAT) and the light-harvesting polypeptide LHCBM6 are elevated in cells exposed to low-intensity red, blue and white light. The Lhl4 gene, which encodes a distant relative of LHCP, is also induced by red light (Teramoto et al. 2006), albeit to a lesser extent than blue light. In fact, most light-induced genes in C. reinhardtii appear to be regulated by blue light signaling pathways, with cryptochromes and phototropins acting as the photoreceptor that perceive the light signal (Small et al. 1995, Huang et al. 2002, Grossman et al. 2004). Thus, our identification of red light-induced genes in C. reinhardtii is somewhat novel, and supports red light as an important regulatory signal in this green alga. As rb38 expression is also affected by blue light, we will further explore the role that this quality of light has, in concert with red light, on the signal transduction pathway that leads to the expression of the rb genes.
Not only are there few reports describing red light-induced gene expression in C. reinhardtii, but examples of red light- stimulated developmental and physiological processes are also lacking. This absence of data is interesting given the numerous examples of red light control in higher plants (Nagy and Schäfer 2002, Chen et al. 2004, Quail 2007). We were able to identify only two reports that describe red light-induced responses in C. reinhardtii. The first reveals that the circadian clock can be reset in CW15 cells with pulses of red light (660 nm) (Kondo et al. 1991). The second demonstrates that the conversion of pre-gametes to gametes, and reactivation of dark-inactivated gametes, is promoted by both red and blue light (Pan et al. 1997). While each of these responses strongly suggests the presence of a red light-absorbing photoreceptor in C. reinhardtii, neither phytochrome nor a phytochrome-like pigment has ever been conclusively identified. Early experiments that utilized higher plant phytochrome antibodies to probe for the red/ far-red photoreversible photoreceptor within C. reinhardtii suggested that a protein of similar size existed (Cordonnier et al. 1986, Ruyters et al. 1991). However, phytochrome sequences have not been identified in the alga’s genome, and no photoreversible responses have been described in the literature. We have also been unable to provide definitive evidence at this time that shows that the red light-induced expression of rb38, rb60 and psbO is reversed by far-red light. Additionally, our attempt to identify a C. reinhardtii phytochrome molecule, using the same pea phytochrome antibody (Pea-25) employed by Cordonnier et al. (1986), was not successful (data not shown).
Despite the inconclusive evidence for the existence of a red/far-red photoreversible photoreceptor in C. reinhardtii, we have identified a potential phytochrome-like sequence in the alga’s genome, and are actively working to isolate the gene and characterize the expressed protein. It seems reasonable that C. reinhardtii possesses a red light photoreceptor, as phytochromes or phytochrome-like molecules have been found in higher and lower plant species (Thummler et al. 1992, Lagarias et al. 1995, Sheehan et al. 2004), photosynthetic (Lamparter et al. 2002) and non-photosynthetic bacteria (Davis et al. 1999), and other green algae (Winands et al. 1992, Wu et al. 1997). In the cyanobacterium Synechocystis sp. PCC 6803, two phytochrome-like proteins, Cph1 and Cph2, have been identified (Hughes et al. 1997, Park et al. 2000a, Park et al. 2000b) that regulate the expression of approximately 20 other genes (Hubschmann et al. 2005). Both Cph1 and Cph2 display the typical red/far-red photoconversion of an attached bilin chromophore that is seen in higher plants. With so many evolutionary relations of C. reinhardtii possessing a phytochrome-like molecule, it is difficult to imagine that this green alga does not possess a similar photoreceptor. In addition, we have demonstrated that the red light-induced expression of the rb38, rb60 and psbO genes is not solely a consequence of photosynthesis, as each mRNA accumulates in y-1 cells grown in red light, despite the absence of PSI or PSII (Fig. 3). Thus a novel red light photoreceptor does appear to exist in this green alga.
As in all transduction pathways, the primary signal is perceived by a molecule and passed to its target through a series of secondary messengers, resulting in a specific response. In C. reinhardtii, CaM has been shown to play a role in the blue light regulatory pathways leading to deflagellation, flagellar regrowth and gene expression (Cheshire and Keller 1991, Im et al. 1996). Data also exist for the involvement of Ca2+/CaM in the red light induction of genes, e.g. rbcS in tobacco and cab in soybean (Lam et al. 1989, Zhou et al. 2001). Therefore, we recognized CaM as a prime candidate for an intermediate signaling molecule in the red light induction of the rb genes. Treatment of cells grown under red light with the CaM antagonist W7 resulted in a decrease in rb38, rb60 and psbO mRNA accumulation (Fig. 6). The drug did not affect the morphology or motility of the cells (Fig. 8), nor did it decrease the accumulation of the rb47 mRNA (Fig. 6). These results indicate that the reduction in rb38, rb60 and psbO gene expression in the presence of W7 is not the result of a stress response, RNA degradation or problems in other signaling pathways. Thus, it is reasonable to imagine that red light activates CaM through a series of other messengers, which in turn continue the cascade that leads to the induction of the rb38, rb60 and psbO genes. In most pathways, CaM acts as a sensor, detecting changes in Ca2+ levels (Sanders et al. 1999). Ca2+ is bound by CaM and the complex in turn regulates the activity of a wide range of target proteins. It is not unreasonable to imagine that changes in Ca2+ levels also affect rb expression. Therefore, we are actively pursuing studies with Ca2+ antagonists and ionophores to elucidate the rb signal transduction pathway further.
Our analyses have also revealed that several other regulatory mechanisms must exist for rb gene expression. As expected, accumulation of the rb38 and the rb60 mRNAs was greater in cells grown under white light than in cells grown in the dark (Fig. 1). Interestingly, while the association of these mRNAs with polyribosomes was also elevated in the light (Figs. 1, 2B), the RB38 and RB60 proteins accumulated in a constitutive manner under light and dark growth conditions (Fig. 4). Initially, we found these results puzzling. However, given the variable roles that each protein may carry out within this single-celled organism, coupled with the complex nature of the photosynthetic process, the steady-state accumulation of the proteins may not mirror their translation. RB60, which was initially identified in C. reinhardtii as a protein disulfide isomerase (PDI) homolog (Kim and Mayfield 1997), regulates the translation of psbA through the redox status of RB47 (Fong et al. 2000, Alergand et al. 2006). More recently, it was demonstrated that RB60 localizes to the endoplasmic reticulum (ER), and associates with the ER marker protein BiP (Levitan et al. 2005). In addition, Wagner at al. (2004) identified a circadian-expressed PDI by mass spectrometry that is homologous to RB60. Therefore, other regulatory influences aside from light may be acting on the accumulation of this protein. It is possible that RB38 also has multiple roles within the cell. To date, the protein has been identified as only a novel psbA RNA-binding protein (Barnes et al. 2004). However, the RNA-binding domains found within RB38 suggest that it too may be multifaceted. As OEE1 and the RB proteins are directly and indirectly linked to the PSII complex, it is not surprising that the associated nuclear-encoded genes are regulated by similar control mechanisms.
Interestingly, chloroplast signals also appear to play a role in rb47 expression. The rb47 gene, which encodes the RB47 psbA translational activator, was not induced by light at the transcriptional level. The mRNA accumulated in a constitutive manner in both wt and y-1 cells that were grown under dark and light conditions (Fig. 1). The association of rb47 mRNA with polyribosomes was approximately equal in light- and dark-grown wt cells, as well as light-grown y-1 cells (Fig. 1). However, little if any association was observed in dark-grown y-1 cells, which lack functional chloroplasts. In addition, accumulation of the RB47 protein was detected only after y-1 cells were shifted from the dark into the light, stimulating chloroplast biogenesis (Fig. 5). These results support the previous findings of Danon and Mayfield (1991), who showed that accumulation of RB47 is dependent upon chloroplast development. The authors also suggested that a decrease in the accumulation of RB47, which was seen in dark-grown y-1 cells, resulted in the inability of the RB complex to bind to the 5′ UTR of the psbA mRNA and activate translation. In fact, nuclear mutants that are deficient in the synthesis of RB47 show a loss of psbA translation as a result of a complete loss of RNA–protein interaction and psbA–ribosome association (Yohn et al. 1998b). Thus, a functional chloroplast appears to be necessary for translation of psbA to occur through the rb signaling pathway. The increased accumulation of rb47 polysomal RNA that is observed in wt cells after a shift from the dark into red light (Fig. 2B) also suggests that red light plays a role in translation of this mRNA.
We have shown that the regulation of the rb genes, which encode the psbA translational activators RB38, RB47 and RB60, is complex. As D1 is one of the most highly synthesized proteins in this green alga, it is not surprising that expression and activation of these three RB proteins include several levels of control. Both red light and chloroplast signal(s) are directly involved in the rb expression pathway. CaM has also been identified as a secondary messenger in the induction of rb38 and rb60, as well as psbO. Our initial characterization of the rb red light signaling pathway in C. reinhardtii strongly suggests the existence of a novel red light photoreceptor. We are actively working to clone a gene that encodes a potential phytochrome-like protein, which we recently identified in the alga’s genome. Isolation and characterization of this photoreceptor, and future RNAi (RNA interference) knock-down experiments of the molecule, will validate the importance of red light as a primary signal for changes in gene expression in C. reinhardtii.
Materials and Methods
Cell growth conditions
Wt 2137a or y-1 mutant C. reinhardtii cells were grown in constant darkness or constant light in Tris-acetate phosphate (TAP; Harris et al. 1989) medium with shaking at 22°C in an I-35L growth chamber (Percival Scientific, Perry, Iowa, USA) until a density of approximately 5 × 106 cells/ml was achieved. Exposure of dark-grown cells to white light (390–780 nm), or single-source wavelengths of blue (470 nm) or red (670 nm) light, was carried out in an I-35L or E-30LED chamber (Percival Scientific). Specifically, 500 ml of TAP was inoculated with approximately 2.5 × 104 cells/ml and grown in the dark for a week at 22°C before exposure to white (500 μmol m−2 s−1), blue (10 μmol m−2 s−1) or red (90 μmol m−2 s−1) light for 0, 20, 40 or 60 min. Cells were treated with 5, 11, 25 or 50 μM N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide (W7; Sigma-Aldrich, St Louis, MO, USA) or N-(6-aminohexyl)-1-naphthalenesulfonamide hydrochloride (W5; Enzo Life Sciences International, Inc., Plymouth Meeting, PA, USA) in the dark, or under red light, for 30 min. Cells were pelleted, frozen in liquid N2 and stored at −80°C.
RNA extraction and Northern blot analysis
Total and polysomal RNA isolation was carried out according to the procedures of Cohen et al. (1998). Northern blot analysis was performed as described by Mayfield et al. (1987, 1994). Total (15 μg per lane) and polysomal (2 μg per lane) RNA was separated on a 1.2% agarose–formaldehyde gel and blotted onto a Gene Screen Plus Hybridization Membrane (Perkin Elmer, Boston, MA, USA). Membranes were stained with methylene blue (0.04%) in 0.5% M NaOAc (pH 5.3) in order to use the rRNA bands as internal controls for equal sample loading. Accumulation of each transcript was evaluated by hybridization of the membranes with a denatured, radiolabeled probe (Cohen et al. 1998). Hybridization signals were detected using a Cyclone Storage Phosphor System (Perkin Elmer).
Protein extraction and Western blot analysis
C. reinhardtii cells were lysed using a PARR Cell Disruption Bomb (Parr Instrument Co., Moline, IL, USA) with nitrogen decompression at 2,200 p.s.i. for 5 min. The lysate was spun at 47,000 × g for 30 min at 4°C. The soluble protein fraction was removed, frozen in liquid N2, and stored at −80°C. SDS–PAGE was carried out according to Laemmli (1970). Proteins were electroblotted onto a nitrocellulose membrane (Invitrogen, Carlsbad, CA, USA), which were decorated with a primary antibody (αRB38, αRB47, αRB60, αOEE1 or αOEE2) followed by a goat anti-rabbit IgG alkaline phosphatase-conjugated secondary antibody (Sigma-Aldrich). Blots were developed using NBT and BCIP (Fisher, Pittsburgh, PA, USA).
Light microscopy images
Morphology and motility of C. reinhardtii cells were monitored with an Olympus BX-60 microscope (Olympus, San Diego, CA, USA). Slides were prepared from cells grown in the dark or under red light, and treated with or without the CaM antagonist, W7. Pictures were taken from each sample with a Hamamatsu digital camera (Hamamatsu Phonics, Bridgewater, NJ, USA).
Funding
This work was supported by the National Institute of Health [GM58808 to A.C.]; CSU Fullerton [Program for Research, Scholarship and Creative Activity grant to A.C and Junior/Senior/General Research Award to A.C.].
Acknowledgments
We thank S. P. Mayfield for the RB38, RB47, RB60, OEE1 and OEE2 antibodies and his critical reading of this manuscript.
Glossary
Abbreviations
- CaM
calmodulin
- ER
endoplasmic reticulum
- LHCP
light-harvesting chlorophyll a/b-binding protein
- OEE
oxygen evolving enhancer
- PABP
poly(A)-binding protein
- PDI
protein disulfide isomerase
- RB and rb
RNA binding
- UTR
untranslated region
- wt
wild type.
References
- Alergand T, Peled-Zehavi H, Katz Y, Danon A. The chloroplast protein disulfide isomerase RB60 reacts with a regulatory disulfide of the RNA-binding protein RB47. Plant Cell Physiol. 2006;47:540–548. doi: 10.1093/pcp/pcj023. [DOI] [PubMed] [Google Scholar]
- Auchincloss AH, Zerges W, Perron K, Girard-Bascou J, Rochaix JD. Characterization of Tbc2, a nucleus-encoded factor specifically required for translation of the chloroplast psbC mRNA in Chlamydomonas reinhardtii. J. Cell Biol. 2002;157:953–962. doi: 10.1083/jcb.200201060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan A, Goldschmidt-Clermont M. Participation of nuclear genes in chloroplast gene expression. Biochimie. 2000;82:559–572. doi: 10.1016/s0300-9084(00)00602-7. [DOI] [PubMed] [Google Scholar]
- Barneche F, Winter V, Crevecoeur M, Rochaix JD. ATAB2 is a novel factor in the signalling pathway of light-controlled synthesis of photosystem proteins. EMBO J. 2006;25:5907–5918. doi: 10.1038/sj.emboj.7601472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes D, Cohen A, Bruick RK, Kantardjieff K, Fowler S, Efuet E, et al. Identification and characterization of a novel RNA binding protein that associates with the 5′-untranslated region of the chloroplast psbA mRNA. Biochemistry. 2004;43:8541–8550. doi: 10.1021/bi035909j. [DOI] [PubMed] [Google Scholar]
- Bohne F, Linden H. Regulation of carotenoid biosynthesis genes in response to light in Chlamydomonas reinhardtii. Biochim. Biophys. Acta. 2002;1579:26–34. doi: 10.1016/s0167-4781(02)00500-6. [DOI] [PubMed] [Google Scholar]
- Cahoon AB, Timko MP. yellow-in-the-dark mutants of Chlamydomonas lack the CHLL subunit of light-independent protochlorophyllide reductase. Plant Cell. 2000;12:559–568. doi: 10.1105/tpc.12.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Chory J, Fankhauser C. Light signal transduction in higher plants. Annu. Rev. Genet. 2004;38:87–117. doi: 10.1146/annurev.genet.38.072902.092259. [DOI] [PubMed] [Google Scholar]
- Cheshire JL, Keller LR. Uncoupling of Chlamydomonas flagellar gene expression and outgrowth from flagellar excision by manipulation of Ca2+ J. Cell Biol. 1991;115:1651–1659. doi: 10.1083/jcb.115.6.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A, Yohn CB, Bruick RK, Mayfield SP. Translational of chloroplast gene expression in Chlamydomonas reinhardtii. Methods Enzymol. 1998;297:192–208. [Google Scholar]
- Cordonnier MM, Greppin H, Pratt LH. Identification of a highly conserved domain on phytochrome from angiosperms to algae. Plant Physiol. 1986;80:982–987. doi: 10.1104/pp.80.4.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danon A, Mayfield SP. Light regulated translational activators: identification of chloroplast gene specific mRNA binding proteins. EMBO J. 1991;10:3993–4001. doi: 10.1002/j.1460-2075.1991.tb04974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danon A, Mayfield SP. ADP-dependent phosphorylation regulates RNA-binding in vitro: implications in light-modulated translation. EMBO J. 1994a;13:2227–2235. doi: 10.1002/j.1460-2075.1994.tb06500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danon A, Mayfield SP. Light-regulated translation of chloroplast messenger RNAs through redox potential. Science. 1994b;266:1717–1719. doi: 10.1126/science.7992056. [DOI] [PubMed] [Google Scholar]
- Davis SJ, Vener AV, Vierstra RD. Bacteriophytochromes: phytochrome-like photoreceptors from nonphotosynthetic eubacteria. Science. 1999;286:2517–2520. doi: 10.1126/science.286.5449.2517. [DOI] [PubMed] [Google Scholar]
- Deng XW, Quail PH. Signalling in light-controlled development. Semin. Cell Dev. Biol. 1999;10:121–129. doi: 10.1006/scdb.1999.0287. [DOI] [PubMed] [Google Scholar]
- Ermilova EV, Zalutskaya ZM, Huang K, Beck CF. Phototropin plays a crucial role in controlling changes in chemotaxis during the initial phase of the sexual life cycle in Chlamydomonas. Planta. 2004;219:420–427. doi: 10.1007/s00425-004-1241-6. [DOI] [PubMed] [Google Scholar]
- Fong CL, Lentz A, Mayfield SP. Disulfide bond formation between RNA binding domains is used to regulate mRNA binding activity of the chloroplast poly(A)-binding protein. J. Biol. Chem. 2000;275:8275–8278. doi: 10.1074/jbc.275.12.8275. [DOI] [PubMed] [Google Scholar]
- Gilmartin PM, Sarokin L, Memelink J, Chua NH. Molecular light switches for plant genes. Plant Cell. 1990;2:369–378. doi: 10.1105/tpc.2.5.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman AR, Lohr M, Im CS. Chlamydomonas reinhardtii in the landscape of pigments. Annu. Rev. Genet. 2004;38:119–173. doi: 10.1146/annurev.genet.38.072902.092328. [DOI] [PubMed] [Google Scholar]
- Gyula P, Schäfer E, Nagy F. Light perception and signalling in higher plants. Curr. Opin. Plant Biol. 2003;6:446–452. doi: 10.1016/s1369-5266(03)00082-7. [DOI] [PubMed] [Google Scholar]
- Harris EH. Chlamydomonas as a model organism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001;52:363–406. doi: 10.1146/annurev.arplant.52.1.363. [DOI] [PubMed] [Google Scholar]
- Harris EH, Burkhart BD, Gillham NW, Boynton JE. Antibiotic resistance mutations in the chloroplast 16S and 23S rRNA genes of Chlamydomonas reinhardtii: correlation of genetic and physical maps of the chloroplast genome. Genetics. 1989;123:281–292. doi: 10.1093/genetics/123.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman CA, Im CS, Beale SI. Light-regulated expression of the gsa gene encoding the chlorophyll biosynthetic enzyme glutamate 1-semialdehyde aminotransferase in carotenoid-deficient Chlamydomonas reinhardtii cells. Plant Mol. Biol. 1999;39:289–297. doi: 10.1023/a:1006100822721. [DOI] [PubMed] [Google Scholar]
- Huang K, Merkle T, Beck CF. Isolation and characterization of a Chlamydomonas gene that encodes a putative blue-light photoreceptor of the phototropin family. Physiol. Plant. 2002;115:613–622. doi: 10.1034/j.1399-3054.2002.1150416.x. [DOI] [PubMed] [Google Scholar]
- Hubschmann T, Yamamoto H, Gieler T, Murata N, Borner T. Red and far-red light alter the transcript profile in the cyanobacterium Synechocystis sp. PCC 6803: impact of cyanobacterial phytochromes. FEBS Lett. 2005;579:1613–1618. doi: 10.1016/j.febslet.2005.01.075. [DOI] [PubMed] [Google Scholar]
- Hughes J, Lamparter T, Mittmann F, Hartmann E, Gartner W, Wilde A, et al. A prokaryotic phytochrome. Nature. 1997;386:663. doi: 10.1038/386663a0. [DOI] [PubMed] [Google Scholar]
- Im CS, Beale SI. Identification of possible signal transduction components mediating light induction of the Gsa gene for an early chlorophyll biosynthetic step in Chlamydomonas reinhardtii. Planta. 2000;210:999–1005. doi: 10.1007/s004250050709. [DOI] [PubMed] [Google Scholar]
- Im CS, Eberhard S, Huang K, Beck CF, Grossman AR. Phototropin involvement in the expression of genes encoding chlorophyll and carotenoid biosynthesis enzymes and LHC apoproteins in Chlamydomonas reinhardtii. Plant J. 2006;48:1–16. doi: 10.1111/j.1365-313X.2006.02852.x. [DOI] [PubMed] [Google Scholar]
- Im CS, Matters GL, Beale SI. Calcium and calmodulin are involved in blue light induction of the gsa gene for an early chlorophyll biosynthetic step in Chlamydomonas. Plant Cell. 1996;8:2245–2253. doi: 10.1105/tpc.8.12.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Lau OS, Deng XW. Light-regulated transcriptional networks in higher plants. Nat. Rev. Genet. 2007;8:217–230. doi: 10.1038/nrg2049. [DOI] [PubMed] [Google Scholar]
- Kim J, Mayfield SP. Protein disulfide isomerase as a regulator of chloroplast translational activation. Science. 1997;278:1954–1957. doi: 10.1126/science.278.5345.1954. [DOI] [PubMed] [Google Scholar]
- Kondo T, Johnson CH, Hastings JW. Action spectrum for resetting the circadian phototaxis rhythm in the CW15 strain of Chlamydomonas: I. Cells in darkness. Plant Physiol. 1991;95:197–205. doi: 10.1104/pp.95.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropat J, Oster U, Rudiger W, Beck CF. Chloroplast signalling in the light induction of nuclear HSP70 genes requires the accumulation of chlorophyll precursors and their accessibility to cytoplasm/nucleus. Plant J. 2000;24:523–531. doi: 10.1046/j.1365-313x.2000.00898.x. [DOI] [PubMed] [Google Scholar]
- Kuhlemeier C. Transcriptional and post-transcriptional regulation of gene expression in plants. Plant Mol. Biol. 1992;19:1–14. doi: 10.1007/978-94-011-2656-4_1. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lagarias DM, Wu SH, Lagarias JC. Atypical phytochrome gene structure in the green alga Mesotaenium caldariorum. Plant Mol. Biol. 1995;29:1127–1142. doi: 10.1007/BF00020457. [DOI] [PubMed] [Google Scholar]
- Lam E, Benedyk M, Chua NH. Characterization of phytochrome-regulated gene expression in a photoautotrophic cell suspension: possible role for calmodulin. Mol. Cell Biol. 1989;9:4819–4823. doi: 10.1128/mcb.9.11.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamparter T, Michael N, Mittmann F, Esteban B. Phytochrome from Agrobacterium tumefaciens has unusual spectral properties and reveals an N-terminal chromophore attachment site. Proc. Natl Acad. Sci. USA. 2002;99:11628–11633. doi: 10.1073/pnas.152263999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan A, Trebitsh T, Kiss V, Pereg Y, Dangoor I, Danon A. Dual targeting of the protein disulfide isomerase RB60 to the chloroplast and the endoplasmic reticulum. Proc. Natl Acad. Sci. USA. 2005;102:6225–6230. doi: 10.1073/pnas.0500676102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malnoë P, Mayfield SP, Rochaix JD. Comparative analysis of the biogenesis of photosystem II in the wild-type and Y-1 mutant of Chlamydomonas reinhardtii. J. Cell Biol. 1988;106:609–616. doi: 10.1083/jcb.106.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matters GL, Beale SI. Blue-light-regulated expression of genes for two early steps of chlorophyll biosynthesis in Chlamydomonas reinhardtii. Plant Physiol. 1995;109:471–479. doi: 10.1104/pp.109.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield SP, Bennoun P, Rochaix JD. Expression of the nuclear encoded OEE1 protein is required for oxygen evolution and stability of photosystem II particles in Chlamydomonas reinhardtii. EMBO J. 1987;6:313–318. doi: 10.1002/j.1460-2075.1987.tb04756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield SP, Cohen A, Danon A, Yohn CB. Translation of the psbA mRNA of Chlamydomonas reinhardtii requires a structured RNA element contained within the 5′ untranslated region. J. Cell Biol. 1994;127:1537–1545. doi: 10.1083/jcb.127.6.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield SP, Yohn CB, Cohen A, Danon A. Regulation of chloroplast gene expression. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1995;46:147–166. [Google Scholar]
- Mittag M, Kiaulehn S, Johnson CH. The circadian clock in Chlamydomonas reinhardtii. What is it for? What is it similar to? Plant Physiol. 2005;137:399–409. doi: 10.1104/pp.104.052415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy F, Schäfer E. Nuclear and cytosolic events of light-induced, phytochrome-regulated signaling in higher plants. EMBO J. 2000;19:157–163. doi: 10.1093/emboj/19.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickelsen J, Fleischmann M, Boudreau E, Rahire M, Rochaix JD. Identification of cis-acting RNA leader elements required for chloroplast psbD gene expression in Chlamydomonas. Plant Cell. 1999;11:957–970. doi: 10.1105/tpc.11.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohad I, Siekevitz P, Palade GE. Biogenesis of chloroplast membranes. I. Plastid dedifferentiation in a dark-grown algal mutant (Chlamydomonas reinhardi) J. Cell Biol. 1967a;35:521–552. doi: 10.1083/jcb.35.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohad I, Siekevitz P, Palade GE. Biogenesis of chloroplast membranes. II. Plastid differentiation during greening of a dark-grown algal mutant (Chlamydomonas reinhardi) J. Cell Biol. 1967b;35:553–584. doi: 10.1083/jcb.35.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossenbuhl F, Nickelsen J. cis- and trans-Acting determinants for translation of psbD mRNA in Chlamydomonas reinhardtii. Mol. Cell Biol. 2000;20:8134–8142. doi: 10.1128/mcb.20.21.8134-8142.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan JM, Haring MA, Beck CF. Characterization of blue light signal transduction chains that control development and maintenance of sexual competence in Chlamydomonas reinhardtii. Plant Physiol. 1997;115:1241–1249. doi: 10.1104/pp.115.3.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CM, Kim JI, Yang SS, Kang JG, Kang JH, Shim JY, et al. A second photochromic bacteriophytochrome from Synechocystis sp. PCC 6803: spectral analysis and down-regulation by light. Biochemistry. 2000a;39:10840–10847. doi: 10.1021/bi992831r. [DOI] [PubMed] [Google Scholar]
- Park CM, Shim JY, Yang SS, Kang JG, Kim JI, Luka Z, et al. Chromophore–apoprotein interactions in Synechocystis sp. PCC6803 phytochrome Cph1. Biochemistry. 2000b;39:6349–6356. doi: 10.1021/bi992916s. [DOI] [PubMed] [Google Scholar]
- Petridou S, Foster K, Kindle K. Light induces accumulation of isocitrate lyase mRNA in a carotenoid-deficient mutant of Chlamydomonas reinhardtii. Plant Mol. Biol. 1997;33:381–392. doi: 10.1023/a:1005728411921. [DOI] [PubMed] [Google Scholar]
- Quail P. Phytochrome-regulated gene expression. J. Integr. Plant Biol. 2007;49:11–20. [Google Scholar]
- Rochaix JD. Chlamydomonas reinhardtii as the photosynthetic yeast. Annu. Rev. Genet. 1995;29:209–230. doi: 10.1146/annurev.ge.29.120195.001233. [DOI] [PubMed] [Google Scholar]
- Rochaix JD. Post-transcriptional regulation of chloroplast gene expression in Chlamydomonas reinhardtii. Plant Mol. Biol. 1996;32:327–341. doi: 10.1007/BF00039389. [DOI] [PubMed] [Google Scholar]
- Rodriguez H, Haring MA, Beck CF. Molecular characterization of two light-induced, gamete-specific genes from Chlamydomonas reinhardtii that encode hydroxyproline-rich proteins. Mol. Gen. Genet. 1999;261:267–274. doi: 10.1007/s004380050966. [DOI] [PubMed] [Google Scholar]
- Ruyters G, Grotjohann N, Kowallik W. Phytochrome in Dunaliella, Chlorella and other green algae. Biochem. Physiol. Pflanzen. 1991;187:97–103. [Google Scholar]
- Sanders D, Brownlee C, Harper JF. Communicating with calcium. Plant Cell. 1999;11:691–706. doi: 10.1105/tpc.11.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller K, David R, Uhl R. How Chlamydomonas keeps track of the light once it has reached the right phototactic orientation. Biophys. J. 1997;73:1562–1572. doi: 10.1016/S0006-3495(97)78188-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan MJ, Farmer PR, Brutnell TP. Structure and expression of maize phytochrome family homeologs. Genetics. 2004;167:1395–1405. doi: 10.1534/genetics.103.026096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small GD, Min B, Lefebvre PA. Characterization of a Chlamydomonas reinhardtii gene encoding a protein of the DNA photolyase/blue light photoreceptor family. Plant. Mol. Biol. 1995;28:443–454. doi: 10.1007/BF00020393. [DOI] [PubMed] [Google Scholar]
- Sugita M, Sugiura M. Regulation of gene expression in chloroplasts of higher plants. Plant Mol. Biol. 1996;32:315–326. doi: 10.1007/BF00039388. [DOI] [PubMed] [Google Scholar]
- Teramoto H, Ishii A, Kimura Y, Hasegawa K, Nakazawa S, Nakamura T, et al. Action spectrum for expression of the high intensity light-inducible Lhc-like gene Lhl4 in the green alga Chlamydomonas reinhardtii. Plant Cell Physiol. 2006;47:419–425. doi: 10.1093/pcp/pcj009. [DOI] [PubMed] [Google Scholar]
- Thummler F, Dufner M, Kreisl P, Dittrich P. Molecular cloning of a novel phytochrome gene of the moss Ceratodon purpureus which encodes a putative light-regulated protein kinase. Plant Mol. Biol. 1992;20:1003–1017. doi: 10.1007/BF00028888. [DOI] [PubMed] [Google Scholar]
- Trebitsh T, Levitan A, Sofer A, Danon A. Translation of chloroplast psbA mRNA is modulated in the light by counteracting oxidizing and reducing activities. Mol. Cell Biol. 2000;20:1116–1123. doi: 10.1128/mcb.20.4.1116-1123.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner V, Fiedler M, Markert C, Hippler M, Mittag M. Functional proteomics of circadian expressed proteins from Chlamydomonas reinhardtii. FEBS Lett. 2004;559:129–135. doi: 10.1016/S0014-5793(04)00051-1. [DOI] [PubMed] [Google Scholar]
- Winands A, Wagner G, Marx S, Schneider-Poetsch HA. Partial nucleotide sequence of phytochrome from the zygnematophycean green alga Mougeotia. Photochem. Photobiol. 1992;56:765–770. doi: 10.1111/j.1751-1097.1992.tb02232.x. [DOI] [PubMed] [Google Scholar]
- Wu SH, McDowell MT, Lagarias JC. Phycocyanobilin is the natural precursor of the phytochrome chromophore in the green alga Mesotaenium caldariorum. J. Biol. Chem. 1997;272:25700–25705. doi: 10.1074/jbc.272.41.25700. [DOI] [PubMed] [Google Scholar]
- Yohn CB, Cohen A, Danon A, Mayfield SP. A poly(A) binding protein functions in the chloroplast as a message-specific translation factor. Proc. Natl Acad. Sci. USA. 1998a;95:2238–2243. doi: 10.1073/pnas.95.5.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yohn CB, Cohen A, Rosch C, Kuchka MR, Mayfield SP. Translation of the chloroplast psbA mRNA requires the nuclear-encoded poly(A)-binding protein, RB47. J. Cell Biol. 1998b;142:435–442. doi: 10.1083/jcb.142.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerges W. Translation in chloroplasts. Biochimie. 2000;82:583–601. doi: 10.1016/s0300-9084(00)00603-9. [DOI] [PubMed] [Google Scholar]
- Zhou J, Ma L, Zhang S, Zhu Y, Sun D. Extracellular calmodulin stimulates light-independent RbcS-GUS expression in suspension-cultured cells of transgenic tobacco. Plant Cell Physiol. 2001;42:1049–1055. doi: 10.1093/pcp/pce131. [DOI] [PubMed] [Google Scholar]