Abstract
Objectives To test the efficacy of problem-solving skill training (PST) in improving health-related quality of life (HRQOL) of children with persistent asthma from predominantly lower socioeconomic status (SES) Spanish-speaking Hispanic families. Methods Randomized controlled trial comparing standard care waitlist (SC) control, home-visiting asthma education/care coordination (CC), and combined intervention (CC + PST) at baseline, after intervention, and 6-month follow-up. The primary outcome was parent proxy-report child HRQOL (PedsQL). Results Participants (n = 252) were 83.3% Hispanic and 56.3% monolingual Spanish speakers, and 72.6% of mothers had not graduated high school. We found a significant (P = 0.05) intervention effect for parent proxy-reported child generic (but not asthma-specific) HRQOL, with CC + PST superior to SC [83.8 vs 79.8; adjusted mean difference of 4.05 points (95% confidence interval 0.63–7.4], but no difference between the CC and SC groups. Conclusions In this sample of vulnerable families of children with persistent asthma, a CC + PST intervention was efficacious in improving children’s generic HRQOL.
Keywords: asthma, problem solving, quality of life, randomized controlled trial
Introduction
Asthma, the most common childhood chronic health condition, is associated with significant morbidity and mortality (Akinbami, Moorman, Garbe, and Sondik, 2009; Bloom, Dey, and Freeman, 2006). Despite clear guidelines for optimal asthma care (National Asthma Education and Prevention Program Expert Panel Report 2, 1997; National Asthma Education and Prevention Program Expert Panel Report 3, 2007), asthma prevalence and morbidity remains high (National Center for Health Statistics, 2009). Children with asthma have a higher burden of illness (Akinbami et al., 2009) and lower health-related quality of life (HRQOL) (Varni, Burwinkle, Rapoff, Kamps, and Olson, 2004; Varni, Limbers, and Burwinkle, 2007) than do those without a chronic health condition, and children with persistent asthma from lower socioeconomic status (SES) minority families are at greater risk for both poor health care (Canino et al., 2006; Inkelas, Garro, McQuaid, & Ortega, 2008) and worse health outcomes (Getahun, Demissie, and Rhoads, 2005). Reducing the burden of illness and improving the HRQOL of children with persistent asthma, especially those from lower SES and minority families, is a key goal of asthma intervention and health disparities research.
In managing a chronic illness on a daily basis, individuals face a variety of challenges for which there are not single, standard remedies and for which they must identify and implement solutions (Strauss, Corbin, and Fagerhaugh, 1984). Problem solving is the behavior process through which individuals pursue daily activities necessary for asthma control, including accessing high-quality medical care and optimizing home self-management and adherence (National Asthma Education and Prevention Program Expert Panel Report 3, 2007). Problem-solving skill training (PST) is a psychoeducational intervention for teaching-specific strategies to resolve daily problems (D'Z;urilla, 1986; Nezu, Nezu, Felgoise, McClure, and Houts, 2003; Nezu and Perri, 1989). This is a ‘generic’ intervention that is not limited only to illness-related problems. Problem-solving interventions have been widely used with parents of children with chronic health conditions (Varni, La Greca, and Spirito, 2000) including asthma (Colland, 1993; Evans et al., 1999; Ronchetti et al., 1997), cancer (Sahler et al., 2005), traumatic brain injury (Wade, Wolfe, Brown, and Pestian, 2005), attention-deficit hyperactivity disorder (Barkley, Edwards, Laneri, Fletcher, and Metevia, 2001), and oppositional defiant disorder (Greene, Ablon, and Goring, 2003). PST has been shown to be efficacious for mothers of children with cancer in reducing negative affectivity, and especially for Spanish-speaking and younger single mothers (Sahler et al., 2005), possibly because the active problem-solving approach teaches a new set of skills and a new way of approaching problems. Problem solving may therefore be useful in helping families, especially lower SES Hispanic families, of children with persistent asthma to perform the kinds of behaviors necessary to maximize their child’s HRQOL.
Research Objectives and Hypotheses
We sought to determine whether a linguistically appropriate PST intervention with vulnerable families improves the generic HRQOL of children with persistent asthma. We chose generic HRQOL over asthma-specific HRQOL as our primary outcome because we hypothesize that PST as a generic intervention would be expected to affect child HRQOL broadly. We compared a standard care waitlist (SC) control, a home-visitor asthma education/care coordination (CC) intervention, and a CC + PST intervention (home-visitor asthma education/CC plus in-home PST). We tested the a priori hypothesis that, compared to SC or CC, CC + PST would improve parent proxy report of children’s generic health-related quality of life (HRQOL). Secondary outcomes included child self-reported generic HRQOL, asthma symptoms, and asthma-related utilization.
Methods
This single-site randomized controlled group clinical trial with repeated outcome measures (clinicaltrials.gov Identifier: NCT00250588) was approved by the Institutional Review Boards at Rady Children’s Hospital, the RAND Corporation, and Cincinnati Children’s Hospital Medical Center.
Participants
Families were recruited in San Diego, California, from Federally Qualified Health Centers (FQHCs; n = 212), a commercial HMO (n = 15), school/daycare (n = 11), local asthma initiatives (n = 3), or were self-referred (n = 11). FQHC’s are federally subsidized community clinics that generally treat the uninsured or underinsured on a sliding scale fee structure. Most patients have low income. Eligible patients were 2–14 years old with a physician diagnosis of persistent asthma (mild, moderate, or severe) and parents who spoke English or Spanish. Persistent asthma was defined determined based on NHLBI criteria using symptoms, activity level, and exacerbations. Patients with a comorbid condition that could affect care or outcomes (e.g., Down syndrome, other pulmonary disease) were ineligible. Physicians approached potentially eligible patients, informed them of the study, and faxed a study referral form to the research staff if patients were interested in learning more about the study.
Bilingual, bicultural research staff, blinded to the intervention group, administered surveys in English or Spanish in participants’ homes. When in-person measurement was not possible, the surveys were completed by telephone or by mail. Blinding success was demonstrated by the fact that measurement staff guessed the subject’s group correctly at follow up only 43% of the time-only slightly better than chance.
Participant Flow
Of 610 patients referred, 144 (23.6%) could not be located, 122 (20%) were ineligible, and 344 (56.4%) were eligible. Of the eligible participants, 252 (73.3%) enrolled and 92 (26.7%) refused. There were no differences between participants and those eligible but refused in child age or gender, referral source, or asthma severity. Participants (77%) were more likely than those eligible but refused (50%) to prefer Spanish as the interview language [X2(1) = 15.8, P = 0.001].
Participant flow is shown in Figure 1. Dropout rates were 16, 12, and 29% at T2 and 24, 20 and 32% at T3 for SC, CC, and CC + PST, respectively. T2 CC + PST dropout rate was greater than CC (X2(2) = 7.8, P = 0.02). Dropout rates did not differ significantly across condition at T3. Dropouts (27.6%) were more likely than those with at least one follow-up survey (12.9%) to prefer Spanish as the interview language (X2(1) = 7.1, P = 0.01). There were no baseline differences in HRQOL between those who dropped and those who did not. There were no baseline differences between those who completed all PST sessions and those who did not.
Figure 1.
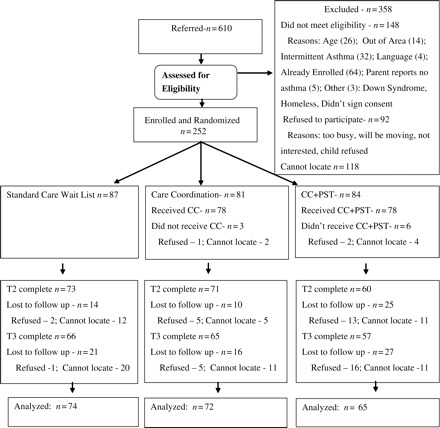
Flow of participants in randomized trial of PST
Recruitment and Baseline Data
Participants were recruited between June 11, 2004 and January 15, 2007. The final T3 follow up was completed on October 16, 2007. Most participants were Hispanic (83.3%) and 56.3% spoke only Spanish, and 72.6% of mothers (73.4% of fathers) had not completed high school. There were no demographic differences across conditions (Table I).
Table I.
Demographics of Participants
| Variable, % (n) | Overall (n = 252) | SC (n = 87) | CC (n = 81) | CC + PST (n = 84) | X2 P-value |
|---|---|---|---|---|---|
| Age mean (SD) t-test | 7.37 (3.07) | 7.26 (3.02) | 7.47 (3.13) | 7.37 (3.10) | 0.911 |
| Gender | 0.122 | ||||
| Male | 61.1 (154) | 60.9 (53) | 69.1 (56) | 53.6 (45) | |
| Ethnicity | 0.668 | ||||
| Hispanic | 83.3 (210) | 80.5 (70) | 86.4 (70) | 83.3 (70) | |
| non-Hispanic White | 4.4 (11) | 5.7 (5) | 2.5 (2) | 4.8 (4) | |
| non-Hispanic Black | 8.3 (21) | 10.3 (9) | 4.9 (4) | 9.5 (8) | |
| Other | 4.0 (10) | 3.4 (3) | 6.1 (5) | 2.4 (2) | |
| Language preference | 0.260 | ||||
| Pref Eng Bilingual | 7.9 (20) | 5.7 (5) | 13.6 (11) | 4.8 (4) | |
| Pref Spa Bilingual | 20.6 (52) | 20.7 (18) | 23.5 (19) | 17.9 (15) | |
| Pref Eng Not Bilingual | 15.1 (38) | 18.4 (16) | 12.3 (10) | 14.3 (12) | |
| Pref Spa Not Bilingual | 56.3 (142) | 55.2 (48) | 50.6 (41) | 63.1 (53) | |
| Mother's; education | 0.411 | ||||
| <6th grade | 25.8 (65) | 23.3 (20) | 26.6 (21) | 28.6 (24) | |
| 7th–9th grade | 23.0 (58) | 18.6 (16) | 26.3 (21) | 25.0 (21) | |
| 10th–12th grade | 23.8 (60) | 25.6 (22) | 17.5 (14) | 28.6 (24) | |
| High school graduate | 8.3 (21) | 9.3 (8) | 10.0 (8) | 6.0 (5) | |
| Some college | 13.1 (33) | 17.4 (15) | 11.3 (9) | 10.7 (9) | |
| College graduate | 4.8 (12) | 5.8 (5) | 7.5 (6) | 1.2 (1) | |
| Grad/Prof Degree | 0.4 (1) | 0.0 (0) | 1.3 (1) | 0.0 (0) | |
| Father's; education | 0.578 | ||||
| <6th grade | 27.5 (57) | 22.7 (15) | 27.5 (19) | 31.9 (23) | |
| 7th–9th grade | 25.1 (52) | 25.8 (17) | 24.6 (17) | 25.0 (18) | |
| 10th–12th grade | 20.8 (43) | 19.7 (13) | 20.3 (14) | 22.2 (16) | |
| High school graduate | 8.2 (17) | 12.1 (8) | 8.7 (6) | 4.2 (3) | |
| Some college | 11.1 (23) | 7.6 (5) | 11.6 (8) | 13.9 (10) | |
| College graduate | 6.8 (14) | 10.6 (7) | 7.2 (5) | 2.8 (2) | |
| Graduation/professional degree | 0.5 (1) | 1.5 (1) | 0.0 (0) | 0.0 (0) | |
| Severity on intake | 0.566 | ||||
| Mild | 27.0 (68) | 25.3 (22) | 28.4 (23) | 27.4 (23) | |
| Moderate | 40.5 (102) | 43.7 (38) | 33.3 (27) | 44.0 (38) | |
| Severe | 32.5 (82) | 31.0 (27) | 38.3 (31) | 28.6 (23) |
Note. Pref Eng, prefers English; Pref Spa, prefers Spanish.
Numbers Analyzed
Subjects with at least one follow-up measure were included in the analyses. Consequently, for most analyses, sample sizes were 74 (SC), 72 (CC), and 65 (CC + PST). For the child self-report pediatric quality of life inventory (PedsQL), sample sizes were 59 (SC), 54 (CC), and 52 (CC + PST).
Interventions
Interventions were administered in English or Spanish, based on parental preference. All intervention staff were both bilingual and bicultural, having been born in Mexico or Central America or being first-generation immigrants to the USA.
Standard Care
The SC wait list control group received ongoing asthma care from their place of care during the trial. They were offered the CC + PST intervention after the T3 follow up.
Care Coordination
A common approach to improving asthma care and outcomes is for paraprofessional home visitors to deliver in-home asthma education and CC (Asthma Health Outcomes Project, 2007). The five-session (45–60 min, weekly) CC was based on NHLBI guidelines (National Asthma Education and Prevention Program, 1997) and the Robert Wood Johnson Foundation’s Allies Against Asthma community health worker model (Friedman et al., 2006) and was delivered by two bachelor’s level bilingual, bicultural asthma home visitors. The home visitors implemented a structured set of educational interventions, with written materials in English or Spanish, on the following topics: what is asthma, asthma medications and devices, asthma action plan, how to recognize and respond to symptom onset, and how to reduce irritants and allergens in the home. Home visitors referred families, when necessary, to existing health insurance enrollment assistance, smoking cessation, and other community support services. Home visitors communicated with the primary care provider via FAX, giving summaries of interventions, updates on progress, and noting family difficulties and needs [for example, needing equipment, prescriptions, or an (updated) asthma treatment plan]. Materials are available from the first author.
Problem-Solving Skills Training
The CC + PST consisted of CC plus a six-session (45–60 min, weekly) PST intervention carried out by a bilingual, bicultural master’s level health educator. PST is a generic psychoeducational approach in which problems are normalized and participants are taught to approach problems proactively, define the problem, generate alternative solutions, choose the best solution, implement the solution, and evaluate how well that solution worked. The PST intervention was based on D’Zurilla’s (D'Z;urilla, 1986; D'Z;urilla and Goldfried, 1971) conceptualization and adapted from a comprehensive protocol used in a previous trial of PST in mothers of children with cancer (Varni et al., 1999). It was aimed at the primary caregiver, although children were encouraged to participate. The intervention targeted problems in general, rather than illness-specific problems. To facilitate learning, the actual problems discussed during the PST intervention were identified by the primary caregiver as particularly relevant.
Following Varni et al. (1999) and Sahler et al. (2002), the PST curriculum used the acronym “Bright IDEAS” as an organizational structure. Bright represents the sense of optimism (positive orientation) necessary for successful problem solving. The letters “IDEAS” stand for the five major steps of problem solving: Identify the problem, Determine options, Evaluate options and choose the best, Act, and See if it worked. Instructional material included a treatment manual, worksheets for each step, and cartoon handouts to reinforce the main ideas. PST materials are available from the first author (MS).
The six PST sessions flowed in a systematized therapeutic manner. Session 1 was devoted to rapport building, understanding the relevant social and medical situation, presenting an overview of the PST curriculum, and assigning the first homework—identifying a solvable problem. Session 2 reviewed the prior homework, introduced the idea of developing alternative solutions, and assigned homework—defining and evaluating options. Session 3 reviewed the homework, developed an action plan, and assigned homework—implementing the action plan. Sessions 4–6 depended on the outcome of the actions, focusing on alternative plans if the results of the action plan were not satisfactory to the client or on additional problems if the results were satisfactory.
Intervention Fidelity
Interventionists received 2 weeks of training, including didactic instruction, role-playing, and shadowing an experienced interventionist. All interventions were audio-taped. Weekly supervision, using audio-taped sessions, was designed to prevent interventionist drift. Interventions were responsive to family needs, but essential intervention behaviors were standardized via training manuals, standard materials, and behavioral checklists denoting specific prescribed intervention behaviors. These checklists were used by the interventionist to structure the intervention. A random 10% of audio-tapes were coded by two project personnel against the checklists (discrepancies resolved through consensus) to determine the rate at which prescribed behaviors were performed. Materials and checklists are available from the corresponding author.
Outcomes
Measurement occurred at baseline (T1), post intervention (∼3 months after baseline; T2), and at 6-month follow-up (∼9 months after baseline; T3).
Primary Outcome
Parent-reported child generic HRQOL was measured by the PedsQL 4.0 Generic Core Scales Total Scale Score (PedsQL), which has been shown to be internally consistent, valid, and responsive to indicators of clinical change for children with asthma (Chan, Mangione-Smith, Burwinkle, Rosen, and Varni, 2005; Seid et al., in press; Varni et al., 2004). The 23-item PedsQL asks respondents how often various issues have been a “problem” in the past month, yields a score of 0–100 (higher scores are better), and includes parallel child self-report (ages 5–18 years) and parent proxy report (ages 2–18 years) forms. We measured both self- and proxy report, although our a priori primary outcome was parent proxy report.
Secondary Outcome
Asthma symptoms (asthma-specific HRQOL) were assessed using the PedsQL 3.0 Asthma Module Asthma Symptoms Scale (Chan et al., 2005; Seid et al., in press; Varni et al., 2004). Higher scores indicate fewer symptoms. Example items include “It is hard to take a deep breath.”; “I feel wheezy.”; “My chest hurts or feels tight.”; “I cough.”; “I get out of breath.”
Asthma symptom frequency was measured via the number of days and nights with asthma symptoms over the past 2 weeks (Evans et al., 1999; Lozano et al., 2004; Morgan et al., 2004).
Secondary Outcome
Utilization was measured by parent recall of emergency room, inpatient, or urgent doctor’s appointments for asthma over the last 6 months (at T1), 3 months (at T2), and 6 months (at T3).
Sample Size
Sample size was based on the parent proxy-report PedsQL 4.0, which was assumed to be continuous and approximately normally distributed. The repeated measures at T2 and T3 were regarded as a vector of responses with pairwise correlations assumed to be 0.50. Sample size was based on two a priori comparisons of interest: CC + PST vs CC, and CC + PST vs SC. A parent proxy-report PedsQL difference of 4.5 points has been determined to be a minimal clinically important difference (Varni, Burwinkle, Seid, and Skarr, 2003). Based on the previous literature, we assumed a standard deviation of 12. With 80% power (alpha = 0.05, two-sided), assuming 20% attrition, 107 subjects per group (321 total) were required (Diggle, Liang, and Zeger, 1994).
Randomization
Blocked randomization, stratified by site of care (FQHC versus other) and disease severity (mild vs moderate or severe), was used. Prepared randomization lists were created by the statistician (DS) and concealed until intervention assignment. A pediatrician with asthma expertise (PG) verified eligibility prior to assignment and the project manager (LRG) carried out the assignment.
Analysis
All analyses were intent-to-treat and carried out according to a pre-established plan using SAS 9.1.3. The primary effects of interest, condition and condition by time are fixed effects. PedsQL scores were analyzed as continuous normal outcomes with mixed effects regression models, which accounts for repeated measures over time for T2 and T3 (Kleinbaum, Kupper, Nizam, and Muller, 2008). In other words, these analyses controlled for group differences at T1 and tested for difference among the groups in change in outcome over time. For all models, independent variables included baseline measure, time, asthma severity, condition, and condition by time interaction. A significant condition effect indicates differences across treatment condition in the change in outcome over time. A significant condition by time interaction indicates that this change in outcome over time is not uniform across time periods. We report the differences across groups in the adjusted mean changes over time. Since mixed effects models do not require complete data across all time periods, all subjects with data at T2 or T3 were included in the analyses. The missing data mechanism for mixed effects models is missing at random (Verbeke and Molenberghs, 2000). Akaike’s Information Criterion was used to assess model fit and to determine the best covariance structure. A number of structures were fitted and we found compound symmetry to be the best overall fit. Analyses were repeated adjusting for child’s age, race/ethnicity, language, and mother’s education.
Symptom frequency and utilization were analyzed using generalized linear mixed models (GLMM), with appropriate distribution and link functions. All analyses accounted for repeated measures and included the same terms in the model previously described. For daytime symptoms, GLMM was set up as an ordinal regression. Nighttime symptoms are a dichotomous outcome, and a logistic model was constructed. Although number of emergency room visits and unscheduled doctor visits were counting outcomes, poor fit precluded attempts to fit a Poisson model. Both variables were dichotomized. Again, a logistic model was utilized. Attempts to analyze hospital visits failed even after dichotomizing due to minimal outcome variability.
Results
Implementation of Intervention
Treatment fidelity, in terms of the percent of prescribed intervention behaviors performed, was 98.4% for CC and 97.5% for CC + PST. Intervention fidelity, as measured by percent of sessions delivered, was 91.6% for CC and 71.8% for CC + PST. The relatively low rate for CC + PST is reflected in the fact that, while most CC + PST families received at least some CC sessions, 23.8% received no PST sessions, while 52.4% received all PST sessions. It should be noted that the PST-specific intervention for the CC + PST group was administered after the five-session CC-specific intervention was delivered.
Outcomes and Estimation
All results were nearly identical after adjusting for child’s age, race/ethnicity, Spanish language, and mother’s education. Tables II–V provide descriptive statistics and results from the mixed effects and GLMM models. Table II displays unadjusted mean scores for generic- and disease-specific HRQOL. Unadjusted mean differences from T1 to T3 for the primary outcome, parent proxy-report PedsQL scores, were 4.5 for SC, 8.3 for CC, and 11.4 for CC + PST (Table II). Table IV shows the adjusted means and adjusted mean differences for the comparisons of interest for the continuous outcomes. There were no significant interactions between time and condition. Therefore, we tested main effects for condition and report the average (over T2 and T3) change in adjusted mean outcomes. There was a significant main effect for condition (P = 0.05) for parent-proxy PedsQL scores. A priori hypotheses indicated two comparisons: CC + PST vs SC and CC vs SC. Applying a Bonferroni adjustment, CC + PST was significantly higher than SC. The adjusted difference between these groups at T2 was 3.5 (82.1–78.6) and at T3 was 4.6 (85.4–80.8), for an average adjusted mean difference of 4.05 (95% CI 0.63–7.4). CC and SC did not differ significantly.
Table II.
Sample Sizes and Unadjusted Means (Standard Deviations) by Condition and Time Period for Continuous Outcome Variables
| Condition |
||||||
|---|---|---|---|---|---|---|
| SC | CC | CC + PST | ||||
| Outcomes | Sample Sizes and Means (Standard Deviations) | |||||
| PedsQL total: parent | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) |
| Baseline | 87 | 77.0 (12.4) | 81 | 75.7 (12.5) | 84 | 73.9 (16.0) |
| T2 | 73 | 80.0 (12.4) | 71 | 81.3 (13.1) | 60 | 81.4 (16.3) |
| T3 | 65 | 81.5 (13.6) | 65 | 84.0 (14.6) | 57 | 85.3 (13.3) |
| PedsQL Total: Child | ||||||
| Baseline | 68 | 73.3 (15.7) | 63 | 72.0 (15.4) | 67 | 70.0 (14.9) |
| T2 | 58 | 76.3 (15.7) | 53 | 78.5 (14.1) | 48 | 77.9 (15.1) |
| T3 | 51 | 82.0 (14.6) | 49 | 80.6 (19.3) | 46 | 81.5 (14.7) |
| PedQL Asthma: Parent | ||||||
| Baseline | 68 | 63.0 (16.4) | 64 | 64.8 (18.1) | 67 | 55.9 (20.6) |
| T2 | 58 | 76.7 (16.9) | 54 | 81.3 (17.9) | 48 | 76.9 (19.6) |
| T3 | 50 | 77.2 (20.5) | 49 | 79.2 (20.6) | 46 | 75.8 (22.1) |
| PedQL Asthma: Child | ||||||
| Baseline | 68 | 65.9 (18.6) | 64 | 67.6 (17.1) | 67 | 64.3 (19.7) |
| T2 | 58 | 75.5 (16.9) | 53 | 79.0 (15.4) | 47 | 74.4 (18.3) |
| T3 | 50 | 79.2 (18.8) | 49 | 83.8 (16.8) | 46 | 76.2 (21.6) |
Table V.
Comparisons among the Three Conditions using Generalized Linear Mixed Models Adjusting for the Baseline Level of Outcome and Baseline Severity
| Odds Ratios (95% CI) |
P-value of Effects |
||||
|---|---|---|---|---|---|
| SC | CC | CC + PST | Time by Condition | Condition | |
| Outcomes | |||||
| Day Time Symptomsa | |||||
| 1.0 | 0.73 (.39, 1.37) | 0.90 (.48, 1.71) | .32 | .55 | |
| Night Time Symptoms (>1x/wk vs <1x/wk) | |||||
| T2 | 1.0 | 0.83 (.38, 1.81) | 0.33 (.13, .82) | .011 | – |
| T3 | 1.0 | 0.99 (.38, 2.54) | 1.95 (.78, 4.83) | ||
| Visits to Emergency Room (≥1 vs 0) | |||||
| 1.0 | 1.22 (.53, 2.83) | 0.49 (.18, 1.38) | .85 | .21 | |
| Unscheduled Office Visits (≥1 vs 0) | |||||
| 1.0 | 1.03 (.56, 1.91) | 0.59 (.30, 1.14) | .94 | .19 | |
Note. P-value based on additional adjustment for age, race/ethnicity, Spanish language and mother’s education. CC: care coordination; SC: standard care wait list; CC + PST: problem solving skills training.
aOdds ratios for day time symptoms are interpreted as an increasing frequency of symptoms.
Table IV.
Adjusted Means (SE) at T2 and T2 by Condition and Differences between Groups in Average Change in Outcome Over Time (T2 to T3), using Mixed Effects Models Adjusting for the Baseline Level of Outcome and Baseline Severity
| Adjusted Means (SE) |
Differences in Average Change Over Time (95% CI) |
P-value of Effects |
|||||
|---|---|---|---|---|---|---|---|
| Outcomes | SC | CC | CC + PST | CC-SC | CC + PST-SC | Time × Condition | Condition |
| PedsQL total: parent | 3.1 (−.21, 6.4) | 4.0 (.63, 7.4) | 0.85 | 0.05 | |||
| T2 | 78.6 (1.4) | 81.2 (1.4) | 82.1 (1.5) | ||||
| T3 | 80.8 (1.5) | 84.4 (1.5) | 85.4 (1.5) | ||||
| PedsQL total: child | 1.9 (−2.8, 6.6) | 1.6 (−3.1, 6.4) | 0.54 | 0.69 | |||
| T2 | 75.4 (1.9) | 79.0 (2.0) | 78.3 (2.0) | ||||
| T3 | 81.1 (2.0) | 81.3 (2.0) | 81.6 (2.1) | ||||
| PedQL asthma: parent | 2.9 (−3.1, 7.6) | 1.4 (−4.8, 7.5) | 0.82 | 0.64 | |||
| T2 | 76.0 (2.5) | 79.8 (2.6) | 77.9 (2.7) | ||||
| T3 | 77.2 (2.6) | 78.9 (2.7) | 77.3 (2.8) | ||||
| PedQL asthma: child | 3.7 (−1.9, 9.2) | −2.6 (−8.2, 3.0) | 0.77 | 0.1 | |||
| T2 | 75.5 (2.2) | 78.7 (2.3) | 73.2 (2.4) | ||||
| T3 | 79.3 (2.4) | 83.9 (2.4) | 76.3 (2.5) | ||||
Note. P-value based on additional adjustment for age, race/ethnicity, Spanish language and mother’s education. CC: care coordination; SC: standard care wait list; CC + PST: problem solving skills training.
Table III.
N and Percentage Within Category by Condition and Time Period for Categorical Outcome
| Condition |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SC | CC | CC + PST | ||||||||||
| Daytime Symptoms | ||||||||||||
| N | <2x/wk | 3-6x/wk | Every Day | N | <2x/wk | 3-6x/wk | Every Day | N | <2x/wk | 3-6x/wk | Every Day | |
| Baseline | 87 | 50.6 | 27.6 | 21.8 | 81 | 38.3 | 30.9 | 30.9 | 84 | 39.3 | 32.1 | 28.6 |
| T2 | 72 | 65.3 | 25.0 | 9.7 | 70 | 67.1 | 27.1 | 5.7 | 60 | 75.0 | 13.3 | 11.7 |
| T3 | 64 | 73.4 | 12.5 | 14.1 | 64 | 79.7 | 12.5 | 7.8 | 55 | 69.1 | 16.4 | 14.6 |
| Nightime Symptoms | ||||||||||||
| N | <1x/wk | >1x/wk | N | <1x/wk | >1x/wk | N | <1x/wk | >1x/wk | ||||
| Baseline | 87 | 51.7 | 48.3 | 81 | 45.7 | 54.3 | 84 | 44.1 | 55.9 | |||
| T2 | 70 | 65.7 | 34.3 | 69 | 69.6 | 30.4 | 60 | 85.0 | 15.0 | |||
| T3 | 64 | 81.3 | 18.7 | 64 | 81.3 | 18.7 | 56 | 69.6 | 30.4 | |||
| Emergency Room Visits | ||||||||||||
| N | None | >One | N | None | >One | N | None | >One | ||||
| Baseline | 87 | 64.4 | 35.6 | 81 | 65.4 | 34.6 | 84 | 69.1 | 30.9 | |||
| T2 | 73 | 89.0 | 11.0 | 71 | 85.9 | 14.1 | 60 | 95.0 | 5.0 | |||
| T3 | 66 | 84.9 | 15.2 | 65 | 84.6 | 15.4 | 57 | 93.0 | 7.0 | |||
| Visits to Hospital | ||||||||||||
| N | None | >One | N | None | >One | N | None | >One | ||||
| Baseline | 87 | 88.5 | 11.5 | 81 | 90.1 | 9.9 | 84 | 94.1 | 6.0 | |||
| T2 | 73 | 95.9 | 4.1 | 71 | 97.2 | 2.8 | 60 | 100.0 | 0.0 | |||
| T3 | 66 | 92.4 | 7.6 | 65 | 93.9 | 6.2 | 57 | 98.3 | 1.7 | |||
| Unscheduled Office Visits | ||||||||||||
| N | None | >One | N | None | >One | N | None | >One | ||||
| Baseline | 87 | 20.7 | 79.3 | 81 | 29.6 | 70.4 | 84 | 26.2 | 73.8 | |||
| T2 | 73 | 68.5 | 31.5 | 71 | 69.0 | 31.0 | 60 | 80.0 | 20.0 | |||
| T3 | 66 | 65.2 | 34.9 | 65 | 69.2 | 30.8 | 57 | 77.2 | 22.8 | |||
For secondary outcomes, we observed a significant time by condition interaction for night time symptoms (P = 0.011) (Table V). Stratifying by time, we found a significant condition effect at T2. CC + PST participants had one-third the odds (OR = .33; 95% CI: 0.13–0.82) at T2 of having more than one nighttime symptom per week compared to SC. No T3 differences existed (P = 0.20). There were no significant effects for child self-report generic HRQOL, nor for asthma-specific HRQOL or daytime symptoms.
No differences were found for emergency room or unscheduled office visits. The odds ratios consistently showed that CC + PST had about half the odds of one or more visits versus SC, but sample sizes were too small to detect a significant difference.
Discussion
A primary strength of this randomized controlled clinical trial and an important contribution to the literature is the initial demonstration of a potentially efficacious intervention (PST) with respect to parent proxy-reported child generic HRQOL for vulnerable families (lower SES and primarily Spanish-speaking) with children with persistent asthma. We found a statistically significant difference in parent proxy-reported PedsQL scores (the a priori primary outcome) when comparing the CC + PST and SC conditions, although no difference was found between CC + PST and CC, nor between CC and SC groups. For our secondary outcomes, no statistically significant effects were found for child self-reported HRQOL, PedsQL asthma symptoms, or daytime symptoms. There was an effect at T2 for nighttime symptoms favoring the CC + PST condition. For utilization, while the analysis was not statistically significant, children in the CC + PST condition consistently had about half the odds of an emergency, inpatient, or unscheduled office visit.
This study has limitations. The CC and CC + PST conditions were not equivalent in terms of contact time nor the educational level of the interventionist. Therefore, it is possible that the results could have arisen from the increased contact time in the CC + PST group. As well, there is an interventionist by condition confound such that group differences could potentially be attributed to characteristics of the single health educator who delivered the PST. Substantial dropout, particularly from CC + PST, is a limitation. Our sample size was not large enough, nor was there sufficient variability in our secondary outcomes, to detect differences in health care utilization despite the odds ratios. It is not clear why the effect for self-reported PedsQL scores or symptoms were not significant. The exact mechanisms by which CC + PST affected HRQOL are not clear. We did not find group differences for primary care quality, nor the rate of prescriptions for inhaled corticosteroids (results not shown); other aspects of care might have changed or problem solving may have affected other areas such as adherence. Further research will be needed to understand the exact mechanism of effect. Subjects who dropped out suggested that there were too many intervention sessions in the CC + PST condition. The problem-solving component of CC + PST took place after the care-coordination component, so there may have been intervention burn-out even prior to the problem-solving component; it may be useful to consider integrating these two interventions. While we used an intention-to-treat analysis, effectiveness of this intervention might be lower than its efficacy: Research is needed to develop and test a brief intervention. We had no measures of problem-solving skills, so cannot be certain that the PST-specific component increased these skills. Generalizability of our findings to other groups and settings is unknown. Participants were referred by their health care providers and may therefore be systematically different from families with similar sociodemographics who were unable to access health care. The greater percentage of Hispanics in the study reflects San Diego County demographics, as well as the fact that subjects were recruited predominantly from FQHCs. We do not have information on subjects referred but not located and cannot determine whether these were systematically different from those who were eligible and located.
Despite these limitations, this study makes a contribution to the literature. Other studies have shown effects of PST on parental distress in families of children with cancer (Askins et al., 2009; Sahler et al., 2002; Sahler et al., 2005) and traumatic brain injury (Wade, Carey, and Wolfe, 2006) and on improving adherence in children with asthma (Walders et al., 2006). Sahler et al. (2005) found PST efficacy to be greater for Spanish-speaking versus English-speaking mothers. Our study extends these findings by showing evidence that CC + PST is efficacious with respect to parent proxy-reported child generic HRQOL and that it is efficacious in a sample consisting primarily of low SES Hispanic families with children with persistent asthma.
Prior work has demonstrated that the minimum clinically important difference (MCID) for the PedsQL is 4.5 points (Varni et al., 2003). Unadjusted mean PedsQL scores in the CC + PST condition increased almost 2½ times the MCID from T1 to T3. Another way to contextualize the outcomes is in terms of utilization. While there were no statistically significant differences in utilization among the conditions, odds ratios consistently showed that CC + PST was associated with about half the odds of emergency room and unscheduled office visits. In fact, the T3 incidence of emergency room visits (7%) was less than half that of both SC (15.2%) and CC (15.4%). Given that asthma control is known to reduce these kinds of health care utilization and that many researchers view unscheduled or emergency visits as a sign of lower quality asthma care, this is an encouraging finding, especially given T1 emergency room visit rates of 30–35%.
Further research is required to determine the mechanism of action of the treatment package and to test the efficacy of a brief intervention that may have greater practical utilization. This could be done by further integrating the CC and PST sessions and by training the same care manager to deliver the CC intervention with the PST built in so that the full dose of PST can be delivered. It will also be important to test the generalizability of this intervention to other populations of children with chronic conditions.
Funding
This research was supported by a grant from the Maternal and Child Health Bureau of the Health Resources and Services Administration (R40 MC01214/08044). The funder had no role in the design nor conduct of the study, in the collection, analysis, nor interpretation of the data, nor in the preparation, review, nor approval of the manuscript. The principal investigator had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of Interest: Dr Varni holds the copyright and the trademark for the PedsQL and receives financial compensation from the Mapi Research Trust, which is a non-profit research institute that charges distribution fees to for-profit companies that use the Pediatric Quality of Life Inventory™.
References
- Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980–2007. Pediatrics. 2009;123(Suppl 3):S131–S145. doi: 10.1542/peds.2008-2233C. [DOI] [PubMed] [Google Scholar]
- Askins MA, Sahler OJ, Sherman SA, Fairclough DL, Butler RW, Katz ER, et al. Report from a multi-institutional randomized clinical trial examining computer-assisted problem-solving skills training for English- and Spanish-speaking mothers of children with newly diagnosed cancer. J Pediatr Psychol. 2009;34(5):551–563. doi: 10.1093/jpepsy/jsn124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asthma Health Outcomes Project. Asthma programs with an environmental component: a review of the field and lessons for success. Ann Arbor, MI: Center for Managing Chronic Disease; 2007. [Google Scholar]
- Barkley RA, Edwards G, Laneri M, Fletcher K, Metevia L. The efficacy of problem-solving communication training alone, behavior management training alone, and their combination for parent-adolescent conflict in teenagers with ADHD and ODD. J Consult Clin Psychol. 2001;69(6):926–941. [PubMed] [Google Scholar]
- Bloom B, Dey AN, Freeman G. Summary health statistics for U.S. children: National Health Interview Survey, 2005. Vital Health Stat. 2006;10(231):1–84. [PubMed] [Google Scholar]
- Canino G, Koinis-Mitchell D, Ortega AN, McQuaid EL, Fritz GK, Alegria M. Asthma disparities in the prevalence, morbidity, and treatment of Latino children. Soc Sci Med. 2006;63(11):2926–2937. doi: 10.1016/j.socscimed.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Chan KS, Mangione-Smith R, Burwinkle TM, Rosen M, Varni JW. The PedsQL™: reliability and validity of the short-form generic core scales and asthma module. Med Care. 2005;43(3):256–265. doi: 10.1097/00005650-200503000-00008. [DOI] [PubMed] [Google Scholar]
- Colland VT. Learning to cope with asthma: a behavioural self-management program for children. Patient Educ Couns. 1993;22(3):141–152. doi: 10.1016/0738-3991(93)90094-d. [DOI] [PubMed] [Google Scholar]
- D'Z;urilla TJ. Problem-Solving Therapy: A Social Competence Approach to Clinical Intervention. New York: Springer; 1986. [Google Scholar]
- D'Z;urilla TJ, Goldfried M. Problem-solving and behavior modification. J Abnorm Psychol. 1971;78:107–126. doi: 10.1037/h0031360. [DOI] [PubMed] [Google Scholar]
- Diggle P, Liang KY, Zeger S. Analysis of Longitudinal Data. Oxford: Clarendon Press; 1994. [Google Scholar]
- Evans R, III, Gergen PJ, Mitchell H, Kattan M, Kercsmar C, Crain E, et al. A randomized clinical trial to reduce asthma morbidity among inner-city children: results of the National Cooperative Inner-City Asthma Study. J Pediatr. 1999;135(3):332–338. doi: 10.1016/s0022-3476(99)70130-7. [DOI] [PubMed] [Google Scholar]
- Friedman AR, Butterfoss FD, Krieger JW, Peterson JW, Dwyer M, Wicklund K, et al. Allies community health workers: bridging the gap. Health Promot Pract. 2006;7(2 Suppl):96S–107S. doi: 10.1177/1524839906287065. [DOI] [PubMed] [Google Scholar]
- Getahun D, Demissie K, Rhoads GG. Recent trends in asthma hospitalization and mortality in the United States. J Asthma. 2005;42(5):373–378. doi: 10.1081/JAS-62995. [DOI] [PubMed] [Google Scholar]
- Greene RW, Ablon JS, Goring JC. A transactional model of oppositional behavior: underpinnings of the Collaborative Problem Solving approach. J Psychosom Res. 2003;55(1):67–75. doi: 10.1016/s0022-3999(02)00585-8. [DOI] [PubMed] [Google Scholar]
- Inkelas M, Garro N, McQuaid EL, Ortega AN. Race/ethnicity, language, and asthma care: findings from a 4-state survey. Ann Allergy Asthma Immunol. 2008;100(2):120–127. doi: 10.1016/S1081-1206(10)60420-6. [DOI] [PubMed] [Google Scholar]
- Kleinbaum D, Kupper L, Nizam A, Muller K. Applied Regression Analysis and Other Multivariable Methods. 4th. Belmont, CA: Duxbury Press; 2008. [Google Scholar]
- Lozano P, Finkelstein JA, Carey VJ, Wagner EH, Inui TS, Fuhlbrigge AL, et al. A multisite randomized trial of the effects of physician education and organizational change in chronic-asthma care: health outcomes of the Pediatric Asthma Care Patient Outcomes Research Team II Study. Arch Pediaticr Adolesc Med. 2004;158(9):875–883. doi: 10.1001/archpedi.158.9.875. [DOI] [PubMed] [Google Scholar]
- Morgan WJ, Crain EF, Gruchalla RS, O'C;onnor GT, Kattan M, Evans R., III, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;351(11):1068–1080. doi: 10.1056/NEJMoa032097. [DOI] [PubMed] [Google Scholar]
- National Asthma Education and Prevention Program. Expert Panel Report II: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Institutes of Health; 1997. [Google Scholar]
- National Asthma Education and Prevention Program Expert Panel Report 2. Guidelines for the Diagnosis and Management of Asthma (NIH Publication No. 97-4051) Bethesda, MD: US DHHS, NIH; 1997. [Google Scholar]
- National Asthma Education and Prevention Program Expert Panel Report 3. Guidelines for the Diagnosis and Management of Asthma (NIH Publication No. 07-4051) Bethesda, MD: US DHHS, NIH; 2007. [Google Scholar]
- National Center for Health Statistics. Summary Health Statistics for U.S. Children: National Health Interview Survey, 2007. Hyattsville, MD: U.S. Department of Health and Human Services; 2009. [Google Scholar]
- Nezu AM, Nezu CM, Felgoise SH, McClure KS, Houts PS. Project Genesis: assessing the efficacy of problem-solving therapy for distressed adult cancer patients. J Consult Clin Psychol. 2003;71(6):1036–1048. doi: 10.1037/0022-006X.71.6.1036. [DOI] [PubMed] [Google Scholar]
- Nezu AM, Perri MG. Problem-solving therapy for unipolar depression: an initial dismantling study. J Consult Clin Psychol. 1989;57:408–413. [PubMed] [Google Scholar]
- Ronchetti R, Indinnimeo L, Bonci E, Corrias A, Evans D, Hindi-Alexander M, et al. Asthma self-management programmes in a population of Italian children: a multicentric study. Italian Study Group on Asthma Self-Management Programmes. Eur Respiratory J. 1997;10(6):1248–1253. doi: 10.1183/09031936.97.10061248. [DOI] [PubMed] [Google Scholar]
- Sahler OJ, Fairclough DL, Phipps S, Mulhern RK, Dolgin MJ, Noll RB, et al. Using problem-solving skills training to reduce negative affectivity in mothers of children with newly diagnosed cancer: report of a multisite randomized trial. J Consult Clin Psychol. 2005;73(2):272–283. doi: 10.1037/0022-006X.73.2.272. [DOI] [PubMed] [Google Scholar]
- Sahler OJ, Varni JW, Fairclough DL, Butler RW, Noll RB, Dolgin MJ, et al. Problem-solving skills training for mothers of children with newly diagnosed cancer: a randomized trial. J Dev Behav Pediatr. 2002;23(2):77–86. doi: 10.1097/00004703-200204000-00003. [DOI] [PubMed] [Google Scholar]
- Seid M, Limbers C, Driscoll K, Opipari-Arrigan L, Reyes Gelhard L, Varni J. Reliability, validity, and responsiveness of the Pediatric Quality of Life Inventory™ (PedsQL™) Generic Core Scales and Asthma Symptoms Scale in vulnerable children with asthma. J Asthma. doi: 10.3109/02770900903533966. [DOI] [PubMed] [Google Scholar]
- Strauss A, Corbin J, Fagerhaugh S. Chronic Illness and Quality of Life. 2nd. St. Louis: Mosby; 1984. [Google Scholar]
- Varni JW, Burwinkle TM, Rapoff MA, Kamps JL, Olson N. The PedsQL™ in pediatric asthma: Reliability and validity of the Pediatric Quality of Life Inventory™ Generic Core Scales and Asthma Module. J Behav Med. 2004;27(3):297–318. doi: 10.1023/b:jobm.0000028500.53608.2c. [DOI] [PubMed] [Google Scholar]
- Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL™ 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr. 2003;3(6):329–341. doi: 10.1367/1539-4409(2003)003<0329:tpaapp>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Varni JW, La Greca AM, Spirito A. Cognitive-behavioral interventions for children with chronic health conditions. In: Kendall PC, editor. Child and adolescent therapy: Cognitive-behavioral procedures. (2nd ed.), New York: Guilford; 2000. [Google Scholar]
- Varni JW, Limbers CA, Burwinkle TM. Impaired health-related quality of life in children and adolescents with chronic conditions: a comparative analysis of 10 disease clusters and 33 disease categories/severities utilizing the PedsQL 4.0 Generic Core Scales. Health Qual Life Outcomes. 2007;5:43. doi: 10.1186/1477-7525-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varni JW, Sahler OJ, Katz ER, Mulhern RK, Copeland DR, Noll RB, et al. Maternal problem-solving therapy in pediatric cancer. J Psychosoc Oncol. 1999;16(3/4):41–71. [Google Scholar]
- Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. New York: Springer; 2000. [Google Scholar]
- Wade SL, Carey J, Wolfe CR. An online family intervention to reduce parental distress following pediatric brain injury. J Consult Clin Psychol. 2006;74(3):445–454. doi: 10.1037/0022-006X.74.3.445. [DOI] [PubMed] [Google Scholar]
- Wade SL, Wolfe C, Brown TM, Pestian JP. Putting the pieces together: preliminary efficacy of a web-based family intervention for children with traumatic brain injury. J Pediatr Psychol. 2005;30(5):437–442. doi: 10.1093/jpepsy/jsi067. [DOI] [PubMed] [Google Scholar]
- Walders N, Kercsmar C, Schluchter M, Redline S, Kirchner HL, Drotar D. An interdisciplinary intervention for undertreated pediatric asthma. Chest. 2006;129(2):292–299. doi: 10.1378/chest.129.2.292. [DOI] [PubMed] [Google Scholar]


