Abstract
Context: The contribution of autoimmunity to the multisystem dysregulation that characterizes the frailty syndrome in older adults is unknown.
Objective: The aim of the study was to investigate the relationship between thyroid antibodies and frailty in older women.
Design, Setting, and Participants: We conducted a cross-sectional study nested within the Women’s Health and Aging Studies I and II. Thyroglobulin antibodies (TgAbs), thyroid peroxidase antibodies (TPOAbs), and antinuclear antibodies were measured in the baseline sera of 641 community-dwelling older women.
Main Outcome Measure: Frailty was defined using a validated five-component measure.
Results: The prevalence of prefrailty and frailty was lower in TgAb-positive than negative older women (37.1 vs. 47.8% and 6.7 vs.11.9%, respectively; P = 0.01 and 0.03). The prevalence of prefrailty, but not frailty, was lower in TPOAb-positive than negative women (38.9 vs. 48.0% and 10.1 vs. 11.3%; P = 0.04 and 0.34). After adjustment for covariates including serum thyroid stimulation hormone concentration and thyroid medication usage in multinomial regression models, TgAb-positive older women had lower odds of prefrailty and frailty compared with TgAb-negative women (odds ratio 0.57 and 0.30; 95% confidence interval 0.34–0.98 and 0.10–0.85, respectively). Similarly, TPOAb-positive older women had lower odds of frailty compared with TPOAb-negative women (odds ratio 0.44; 95% confidence interval 0.20–0.96). These trends were not observed with antinuclear antibodies.
Conclusion: Independent of thyroid function status, community-dwelling older women who are seropositive for TgAbs and TPOAbs are less likely to be frail than seronegative women.
Independent of thyroid function status, community-dwelling older women who are seropositive for thyroglobulin antibodies and thyroid peroxidase antibodies are less likely to be frail than seronegative women.
Frailty is a geriatric syndrome affecting approximately 7–17% of older adults older than 65 yr of age and 25–30% of those older than 85 yr (1,2,3,4). It has been characterized as a state of decreased physiological reserve, loss of physiological complexity, and accumulation of deficits (1,5,6) and is an independent risk factor for adverse outcomes in older adults (1,2,4). The emergence of operational definitions of frailty has permitted a standardized approach to the epidemiological and pathophysiologic investigations of this common geriatric syndrome (1,2,3,7). Distinguished from disability and comorbidity (8) and theorized as a clinical syndrome of energy dysregulation (1,2), frailty has been cross-sectionally associated with elevated levels of serum IL-6 and C-reactive protein (9). These observations have led some investigators to hypothesize that inflammation, above and beyond that hypothesized to accompany aging (10), plays a role in the pathogenesis of frailty through its effects on multiple physiological systems (8,9,11). Little is known regarding the relationship between autoimmunity and frailty in older adults. Because a close clinicopathological association exists between inflammation and autoimmunity (12), we hypothesized that autoimmunity, triggering or triggered by inflammation, might contribute to the development of frailty in some older adults.
Autoimmunity arises out of dysregulation in immunoregulatory mechanisms that result in the breakdown of self-tolerance and production of self-reactive autoantibodies (13). Thyroid autoimmunity is the paradigm of organ-specific autoimmunity. Thyroglobulin antibodies (TgAbs) and thyroid peroxidase antibodies (TPOAbs) are found in 11.2 and 11.9% of 30- to 39-yr-old adults, and prevalence increases with age to 18.8 and 22.3% of 70- to 79-yr-old adults in the United States (14). Individuals harboring either TgAbs or TPOAbs are more likely to have abnormal serum concentrations of TSH (15). Because of the fundamental role of the thyroid gland in regulating metabolism and energy homeostasis (16), we hypothesized that thyroid autoimmunity would be associated with frailty, either through direct pathological effects of the autoreactive T cells or autoantibodies or indirectly through primary changes in thyroid function. To test this hypothesis, we measured TgAbs and TPOAbs in a well-characterized population of community-dwelling older women in whom frailty status was rigorously measured.
Subjects and Methods
Study population
This cross-sectional study involved 641 older women who participated in the Women’s Health and Aging Studies (WHAS) I and II, two complementary prospective observational studies of women living in the community (17,18). WHAS I enrolled women aged 65 yr and older who had self-reported difficulty in two or more of four domains of physical function. WHAS II enrolled women aged 70–79 yr who had difficulty in no more than one domain. Both cohorts were sampled from the same sampling frame, the Health Care Financing Administration’s Medicare eligibility lists for Baltimore, MD. Details on the study methods and sampling design of WHAS have been published elsewhere (17,18). WHAS I enrolled 1002 women, of whom 672 participated in blood drawing. WHAS II enrolled 436 women, 93% of whom participated in blood drawing. Women who did and did not participate in blood drawing were different by age (76.3 vs. 80.8 yr, respectively; P < 0.0001) and prevalence of cardiovascular disease (51.3 vs. 61.4%, respectively; P = 0.02). For both cohorts, diagnoses of 17 major chronic diseases were adjudicated by physicians using ascertainment algorithms (17). The Johns Hopkins University’s Institutional Review Board approved all research protocols. Informed consent was obtained from all participants.
The population for the present study was derived by combining WHAS I and II and including women from WHAS I only if they were in the same age range as those in WHAS II (70–79 yr). Of the 829 eligible women, 667 had baseline serum specimens available for the detection of autoantibodies, all of whom except seven had complete data on frailty measures. Of these, 11 and eight were missing baseline IL-6 and TSH measurements, respectively. A resultant final sample size of 641 for this study consisted of 276 WHAS I and 365 WHAS II participants.
Definition and classification of frailty
We defined frailty using a five-component measure that was originally proposed in the Cardiovascular Health Study (1) and subsequently validated in WHAS (2). The five components, each a binary criterion measured using standardized questions or protocols, consist of: 1) shrinking, defined as unintentional weight loss of 10% or more since age 60 yr or body mass index less than 18.5 kg/m2; 2) exhaustion, defined as self-report of having low usual energy level (≤3 on a 0–10 scale), feeling unusually tired in the last month, or feeling unusually weak in the last month; 3) low energy expenditure, defined as being in the lowest quintile of energy expenditure measured using a six-item version of the Minnesota Leisure Time Activity Questionnaire (17); 4) slowness, defined as being in the lowest quintile of walking speed over a 4-m distance; 5) weakness, defined as being in the lowest quintile of hand grip strength measured using a Jamar handheld dynamometer (model BK-7498; Fred Sammons, Inc., Burr Ridge, IL). Participants meeting three or more of these criteria were classified as frail; those meeting one or two as prefrail; and those meeting none as nonfrail.
Laboratory measurements
Serum and plasma were separated from nonfasting venous blood samples using centrifugation and stored at −70 C until the time of analysis. Serum TgAbs and TPOAbs and antinuclear antibodies (ANAs) were measured using commercial ELISA kits (QUANTA Lite Thyroid T, TPO, and ANA, respectively; INOVA Diagnostics, San Diego, CA). Plasma IL-6 was measured using a commercial ELISA kit (Quantikine human IL-6; R&D Systems, Minneapolis, MN). Serum TSH was measured using an immunochemiluminometric assay (Ciba Corning Diagnostic Corp., Medfield, MA) by Quest Diagnostic Laboratories (Teterboro, NJ). All assays were performed while masked to frailty status.
For both TgAbs and TPOAbs, we defined a titer of 62.5 or greater and less than 100 World Health Organization (WHO) units as being moderately positive and 100 or more WHO units as strongly positive. For ANAs, we defined a titer of 20 U or greater as being moderately positive and 60 U or greater as strongly positive. These cutoffs for autoantibody positivity were specified by the manufacturer. In regression analyses, we defined autoantibody positivity as being at least moderately positive. In sensitivity analyses, we examined the robustness of our results by redefining antibody positivity as being strongly positive only.
Covariates
Age, strongly associated with both frailty (1,4) and thyroid autoimmunity (14), was represented as a continuous variable. Race and education, both associated with frailty (1,4), could serve as surrogate markers of the socioeconomic environment that could influence the development of thyroid autoimmunity (19). Race was coded as a categorical indicator variable (white, black, American Indian or Alaskan Native, or Asian or Pacific Islander). Education was coded as a categorical indicator variable (completed 0–8th grade, 9–11th grade, 12th grade or GED, or >12th grade or GED). Body mass index (BMI), associated with both thyroid autoimmunity and frailty (4,20), was coded as a continuous variable. Thyroid antibody concentration was reported to decrease on T4 replacement therapy (21). Thyroid medication usage, coded as a binary variable, included thyroid hormone preparations or antithyroid drugs; no participants used amiodarone at baseline. Smoking, associated with both frailty (4) and lower prevalence of thyroid antibodies (22), was coded as a categorical indicator variable (never smoker, former smoker who quit >1 yr ago, former smoker who quit ≤1 yr ago, or current smoker). Type 1 diabetes and thyroid autoimmunity are related by likely similar immunoregulatory and genetically susceptible mechanisms (23), and type 2 diabetes has been associated with thyroid autoimmunity (24); diabetes was coded as a binary variable. Potential associations exist between thyroid antibodies and cardiovascular disease (25), which in turn has been associated with frailty (26). Cardiovascular disease was coded as a single binary variable representing the diagnosis of angina, myocardial infarction, peripheral arterial disease, congestive heart failure, or stroke. In this study, diseases were considered present if classified as definite or probable by adjudication or as self-reported for nonadjudicated diagnoses. IL-6, a cytokine that has been observed to be elevated in frail older adults, including those in WHAS (6), and might play a role in thyroid autoimmunity (27), was coded as logarithm-transformed continuous variable.
Statistical analysis
Baseline characteristics between women with and without thyroid antibodies were compared, using Student’s t test for continuous variables and the χ2 test for categorical variables. Associations between autoantibody prevalence and frailty status were tested using the χ2 test. Multinomial logistic regression models were constructed to assess the effect of thyroid antibodies on the odds of being frail or prefrail vs. nonfrail in unadjusted and adjusted models. Potential confounders included as covariates in the regression models were age, race, education, thyroid medication usage, smoking, diabetes mellitus, cardiovascular disease, and plasma IL-6 concentration. In addition to these two models (unadjusted and adjusted), we constructed a third model in which a term for logarithm-transformed serum TSH concentration was added to the adjusted model.
Probability weights were calculated and incorporated into all descriptive and regression analyses to reference inferences to the population of community-dwelling women aged 70–79 yr (28). P < 0.05 was considered statistically significant for all analyses, which were conducted using Stata 10.0 (StataCorp, College Station, TX).
Results
Baseline characteristics
Overall, women participating in WHAS I and II with positive thyroid antibodies (TgAbs or TPOAbs, n = 175) had similar baseline characteristics to women with negative thyroid antibodies (n = 466, Table 1). Exceptions were higher percentages of women taking a thyroid medication (P < 0.001) or having abnormal serum TSH (P = 0.003) in the thyroid antibody-positive group (Table 1).
Table 1.
Baseline characteristics of the study participants in WHAS I and II (n = 641)
| Characteristic | Presence of thyroid antibodies (TgAb or TPOAb)a
|
||
|---|---|---|---|
| Positive (n = 175, 27.8%)b | Negative (n = 466, 72.2%) | P valuec | |
| Age (yr) | 73.9 ± 2.8 | 74.0 ± 2.7 | 0.88 |
| White race, n (%) | 135 (78.4) | 352 (76.4) | 0.62 |
| Thyroid medication use, n (%) | 31 (17.2) | 36 (7.6) | <0.001 |
| Serum TSH concentration (mU/liter)d | 2.7 (3.0) | 1.9 (2.0) | <0.001 |
| Abnormal, n (%)e | 34 (18.1) | 48 (9.8) | 0.003 |
| BMI (kg/m2) | 27.1 ± 5.9 | 27.7 ± 6.2 | 0.30 |
| High school education, n (%) | 103 (58.7) | 265 (58.1) | 0.89 |
| Current or former smoking, n (%) | 85 (48.0) | 245 (50.7) | 0.58 |
| Diabetes mellitus, n (%) | 20 (10.4) | 77 (15.3) | 0.11 |
| Angina pectoris, n (%) | 34 (20.5) | 102 (21.9) | 0.74 |
| Myocardial infarction, n (%) | 21 (12.6) | 52 (11.4) | 0.69 |
| Congestive heart failure, n (%) | 21 (10.8) | 76 (15.1) | 0.16 |
| Osteoarthritis, n (%) | 134 (76.6) | 333 (71.7) | 0.24 |
| Osteoporosis, n (%) | 79 (46.6) | 191 (41.0) | 0.23 |
| History of stroke, n (%) | 12 (8.0) | 38 (8.2) | 0.94 |
| Pulmonary disease, n (%) | 64 (36.9) | 188 (39.9) | 0.51 |
| Plasma IL-6 concentration (pg/ml)e | 3.2 ± 2.0 | 3.8 ± 5.8 | 0.48 |
Continuous variables are presented as means ± sd, whereas categorical variables are presented as counts and percentages.
Positivity for the presence of thyroid antibody was defined as a titer of 62.5 or more WHO units;
study-specific probability weights were used to reference the percentages in the sampling population-to-population representation;
P values were calculated with the use of t test for continuous variables and χ 2 test for binary variables;
abnormal serum TSH concentration was defined as less than 0.45 or greater than 4.5 mU/liter;
P values were calculated with the use of Mann-Whitney test because of skewed distributions.
Relationships between autoantibodies and frailty in community-dwelling older women
We determined the percentages of women who were nonfrail, prefrail, and frail according to TgAb or TPOAb seropositivity. As shown in Table 2, a significantly smaller percentage of TgAb-positive women were prefrail or frail, compared with TgAb-negative women (37.1 vs. 47.8% and 6.7 vs. 11.9%, respectively; P = 0.01 and 0.03). The prevalence of prefrailty, but not frailty, was lower in TPOAb-positive than -negative women (38.9 vs. 48.0% and 10.1 vs. 11.3%; P = 0.04 and 0.34).
Table 2.
Prevalence of frailty by status of autoantibody seropositivity in WHAS I and II
| Autoantibody | Frailty status | Presence of thyroid antibodiesa
|
P valueb | |
|---|---|---|---|---|
| Positive | Negative | |||
| TgAb | Nonfrail, n (%)c | 59 (56.2) | 204 (40.3) | |
| Prefrail, n (%) | 44 (37.1) | 258 (47.8) | 0.01 | |
| Frail, n (%) | 8 (6.7) | 68 (11.9) | 0.03 | |
| Total | 111 (100) | 530 (100) | ||
| TPOAb | Nonfrail, n (%) | 71 (51.0) | 192 (40.7) | |
| Prefrail, n (%) | 60 (38.9) | 242 (48.0) | 0.04 | |
| Frail, n (%) | 15 (10.1) | 61 (11.3) | 0.34 | |
| Total | 146 (100) | 495 (100) | ||
Positivity for thyroid antibody was defined as a titer of 62.5 or more WHO units.
P values were calculated against the nonfrail group with the use of χ 2 test.
Study-specific probability weights were used to reference the percentages in the sampling population-to-population representation.
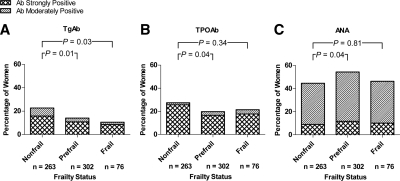
We then compared the prevalence of TgAbs and TPOAbs across women classified with incremental states of frailty, i.e. women who were nonfrail, prefrail, and frail. Figure 1A demonstrates that the prevalence of TgAbs (moderate or strong positivity) was higher in nonfrail women (22.7%) than prefrail (14.0%; P = 0.01) or frail women (10.6%; P = 0.03). The prevalence of TPOAbs in nonfrail women (27.5%) was higher than in prefrail women (19.7%; P = 0.04) but was not statistically significantly higher than in frail women (21.4%; P = 0.34; Fig. 1B).
Figure 1.
Prevalence of autoantibodies in women of incremental states of frailty in WHAS I and II. Shaded regions in bars indicate percentage of women within the respective frailty-status group who were seropositive for TgAbs (A),TPOAbs (B), and ANAs (C). Study-specific probability weights were used to derive the percentages. P values were determined by the χ2 test.
To investigate whether the lower prevalences of thyroid antibodies in prefrail and frail women when compared with nonfrail women was a generalized phenomenon affecting other autoantibodies or a unique one restricted to TgAbs and TPOAbs, we measured serum ANA titers in the same population of WHAS participants. ANA was chosen because, as a commonly measured autoantibody, it can be found to be elevated at low dilutions (1:40) in up to 50% of the population (29). Because the assay we used captures antibodies to a variety of autoantigens (chromatin, dsDNA, Sm/RNP, SS-A, SS-B, Scl-70, centromere, PCNA, Jo-1, mitochondrial M-2, and ribosomal P protein), the test served well to represent a nonthyroidal, systemic autoantibody formation. Figure 1C shows that, in contrast to thyroid antibody prevalences, ANA prevalence (moderate or strong positivity) did not decrease from nonfrail to frail states but were actually higher in prefrail (54.3%) than nonfrail women (44.6%; P = 0.04) and not statistically different between frail and nonfrail women.
Odds of being prefrail and frail in community-dwelling older women possessing thyroid antibodies
The effect of thyroid antibody presence on the odds of being prefrail or frail in older women was assessed using multinomial logistic regression models. For women who were positive for TgAbs, their likelihood of being prefrail or frail, compared with that of being nonfrail, was incrementally lower, respectively (Table 3). These lower odds persisted, even after adjusting for age, race, education, BMI, thyroid medication usage, smoking, diabetes, cardiovascular disease, and IL-6 concentration, such that the odds ratio (OR) of being prefrail was 0.56 [95% confidence interval (CI) 0.33–0.94] and being frail was 0.29 (95% CI 0.10–0.83). Because thyroid antibodies have been reported to be associated cross-sectionally and longitudinally with thyroid dysfunction (14,30), we added serum TSH concentration to the adjusted model to assess whether the observed associations between thyroid antibodies and frailty were independent of TSH concentration. As shown in Table 3, the lower odds of being prefrail and frail in women with TgAbs persisted after adjusting for TSH. Similarly, women with TPOAbs had a lower adjusted odds of being prefrail (OR 0.59; 95% CI 0.36–0.94) and an even lower adjusted odds of being frail (OR 0.45; 95% CI 0.21–0.95). In these women, similar trends in lower odds persisted after additionally adjusting for TSH, but the 95% CI for the OR relating TPOAbs to prefrailty contained the null value.
Table 3.
Association between autoantibody and frailty in WHAS I and II
| Autoantibodyc | Frailty status | Unadjusted model (n = 641)
|
Adjusted model (n = 641)a
|
Adjusted model with TSH (n = 641)b
|
|||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | ||
| TgAb | Nonfrail | 1.00 | 1.00 | 1.00 | |||
| Prefrail | 0.56 (0.35–0.89) | 0.01 | 0.56 (0.33–0.94) | 0.03 | 0.57 (0.34–0.98) | 0.04 | |
| Frail | 0.40 (0.18–0.91) | 0.03 | 0.29 (0.10–0.83) | 0.02 | 0.30 (0.10–0.85) | 0.02 | |
| TPOAb | Nonfrail | 1.00 | 1.00 | 1.00 | |||
| Prefrail | 0.65 (0.42–0.99) | 0.04 | 0.59 (0.36–0.94) | 0.03 | 0.62 (0.38–1.01) | 0.06 | |
| Frail | 0.72 (0.37–1.41) | 0.34 | 0.45 (0.21–0.95) | 0.04 | 0.44 (0.20–0.96) | 0.04 | |
| ANA | Nonfrail | 1.00 | 1.00 | 1.00 | |||
| Prefrail | 1.47 (1.03–2.12) | 0.04 | 1.46 (0.97–2.17) | 0.07 | 1.44 (0.96–2.15) | 0.08 | |
| Frail | 1.07 (0.62–1.84) | 0.81 | 1.01 (0.54–1.91) | 0.97 | 1.01 (0.53–1.91) | 0.98 | |
Adjusted for age, race, education, BMI, thyroid medication usage, smoking, diabetes mellitus, cardiovascular disease, and IL-6 concentration with the use of multinomial logistic regression modeling.
Adjusted for all of the covariates above and TSH.
Positivity for autoantibody was defined as a titer of 62.5 or more WHO units for Tg and TPO Ab and 20 U or greater for ANA.
To determine whether the lower odds of being prefrail and frail in the presence of autoantibodies were unique to thyroid antibodies, we constructed similar multinomial logistic regression models, which incorporated ANA as the predictor variable. In contrast to thyroid antibodies, seropositivity for ANA in older women was associated with higher crude and adjusted odds of being prefrail (but not when TSH was added to the model) and was not significantly associated with the odds of being frail (Table 3).
We performed sensitivity analyses to test the robustness of these results by redefining antibody positivity as being strongly positive; women whose antibody titers were moderately positive were redefined as being seronegative. In both adjusted models (without and with TSH), the magnitudes of the ORs for thyroid antibodies remained similar to analyses in which antibody positivity was defined as being moderately or strongly positive (data not shown). The P values of the association between TgAbs and frailty increased to 0.05 and 0.06 in the adjusted models without and with TSH, respectively. The P values of the association between TPOAbs and frailty decreased to 0.03 and 0.03. The relationship between ANA and frailty remained insignificant, the P values being 0.48 and 0.49 between ANA and prefrailty and 0.78 and 0.83 between ANA and frailty.
We performed further sensitivity analyses by excluding women who were taking thyroid medications (n = 80) and repeating the regression analyses above. The magnitudes of the ORs remained similar to analyses that included thyroid medication users. The associations remained significant between TgAbs and frailty but were attenuated between TgAbs and prefrailty and between TPOAbs and prefrailty and frailty (data not shown), as would be expected from a 12% decrease in sample size.
Relationship between thyroid antibody seropositivity and serum TSH concentration
Older women in the combined WHAS I and II population who were seropositive for thyroid antibodies and not taking thyroid hormones were more likely to have abnormal TSH concentrations (P < 0.001; Table 4). A direct comparison with the study sample from the National Health and Nutrition Examination Survey (NHANES) III is not possible because of the inclusion of men in age-specific data or lack of age-group separation in gender-specific data in the published tables (15,31). Nevertheless, the prevalence distribution across different TSH concentration groups among thyroid antibody-positive individuals in our study population follows a trend similar to that observed in NHANES III.
Table 4.
Prevalence of thyroid antibodies according to category of TSH concentration in WHAS I and IIa
| Thyroid antibodiesb | Serum TSH concentration (mU/liter) (n = 641)
|
P valuec | |||
|---|---|---|---|---|---|
| Less than 0.4 (n = 20, 2.9%) | 0.4–2.49 (n = 413, 71.7%) | 2.5–4.5 (n = 108, 19.6%) | Greater than 4.5 (n = 33, 5.8%) | ||
| TgAb, n (%)d | <0.001 | ||||
| Positive | 2 (1.9) | 51 (55.3) | 24 (25.1) | 16 (17.8) | |
| Negative | 18 (3.1) | 362 (74.8) | 84 (18.5) | 17 (3.5) | |
| TPOAb, n (%) | <0.001 | ||||
| Positive | 2 (1.4) | 64 (53.2) | 33 (29.0) | 15 (16.4) | |
| Negative | 18 (3.3) | 349 (76.7) | 75 (17.1) | 18 (3.0) | |
| Either TgAb or TPOAb, n (%) | <0.001 | ||||
| Positive | 2 (1.2) | 85 (57.5) | 38 (27.2) | 19 (14.1) | |
| Negative | 18 (3.5) | 328 (76.6) | 70 (16.9) | 14 (2.9) | |
Women who were taking thyroid hormones were excluded;
positivity for TgAbs and TPOAbs was defined as a titer of 62.5 or more WHO units;
P values were calculated with the use of χ 2 test;
percentage within the respective thyroid antibody serostatus group. Study-specific probability weights were used to reference the percentages in the sampling population-to-population representation.
Discussion
In this study, we measured serum TgAbs and TPOAbs in a population of community-dwelling older women well characterized in their frailty status. We found that older women seropositive for TgAbs were less likely to be prefrail and frail in a graded manner. A similar trend was noted for TPOAbs, except that its relationship with prefrailty was not statistically significant after adjustment for serum TSH. To determine whether the decreased likelihood of frailty associated with thyroid antibody presence would extend to other autoantibodies, we further measured a systemic marker of autoantibody, ANA, in the same population of older women. In contrast to thyroid antibody findings, we found that women seropositive for ANA did not have decreased odds of being frail or prefrail. To the authors’ knowledge, this is the first study that demonstrated a specific, inverse association between thyroid-specific antibodies and the frailty syndrome.
The cross-sectional nature of this study precludes inferences on the causality underlying the presently observed association between thyroid antibodies and frailty. It is unclear whether these autoantibodies are pathogenic or mere bystanders in this geriatric syndrome. Several possible explanations can be provided for this finding.
First, the decreased prevalence of thyroid antibodies in frail older women could suggest a generalized immunodeficiency globally affecting the production of antibodies. The previously reported higher prevalence of IgG antibodies against cytomegalovirus in frail older women (32) refutes this hypothesis. Alternatively, a deficiency limited to autoantibody production could be postulated. However, the lack of an association between a classical autoantibody, ANA, and frailty suggests that the lower prevalence of autoantibodies in frail older women is restricted, at least in this study, to thyroid antibodies. It is conceivable that differences in the mechanisms of thyroid and antinuclear antibody production could affect frail and nonfrail older adults differentially. In the case of TgAbs, at least, the antibody titer corresponds to thyroglobulin concentration (33). Whether frail older women might have decreased thyroglobulin concentration or thyroid mass, thereby potentially affecting thyroid antibody generation, is unknown. Although we do not believe that the data reported here reflect a generalized immunodeficiency affecting frail older women, further studies are needed to determine whether an immunologically mediated mechanism underlies the lower prevalence of thyroid antibodies in these women.
Second, a beneficial effect of thyroid antibodies on the pathogenesis of frailty could also be envisioned. The concept of beneficial autoimmunity was raised more than 3 decades ago in the context of antiidiotype antibody responses (34). Recent findings reveal that patients with rheumatoid arthritis produce a self-reactive antibody that neutralizes the proinflammatory mediator, TNF-α (35). These self-reactive antibodies are of the IgG isotype and are very specific, interacting with three determinants on the TNF-α molecule that display no cross-reactivity to any other known gene product (35). On the other hand, autoantibodies that occur in healthy individuals, referred to as natural autoantibodies, are usually of the IgM isotype and bind to several unrelated antigens with moderate affinity (36). Natural autoantibodies have a role in the development of the B cell repertoire and the homeostasis of the immune system (37). For example, rheumatoid factor has been suggested to enhance the binding of low-affinity IgG antibodies to their antigens (38), thereby potentially assisting in first-line immunological defense against foreign pathogens before the development of specific, higher-affinity antibodies. The thyroid antibodies, however, do not fit readily into either of these classifications of potentially beneficial autoantibodies. Other than their specificity for thyroglobulin or thyroid peroxidase, the two thyroid antibodies tested in this study are not known, to date, to bind to any proinflammatory or cytotoxic mediator that might have a detrimental effect on the health of individuals who harbor them. Therefore, it remains to be elucidated how thyroid antibodies might interact with the process of induction or maintenance of the frailty syndrome and whether some form of beneficial autoimmunity might be at play.
Third, confounding by differences in baseline characteristics, uncontrolled confounders, or residual confounding between participants with and without thyroid antibodies is possible. As expected, the group of WHAS participants seropositive for thyroid antibodies had a higher prevalence of abnormal serum TSH concentration. However, the inverse association between thyroid antibodies and frailty persisted after adjusting for TSH in the regression models and is therefore statistically independent of TSH concentration. In addition, the higher prevalence of thyroid medication usage in thyroid antibody-positive women might be a surrogate marker of better clinical follow-up or better social or family support and thus represents a lower frailty risk (39). However, the inverse association between thyroid antibodies and frailty persisted, even after we adjusted for thyroid medication usage in the regression models. Nevertheless, we recognize the possibility of residual or uncontrolled confounding, such as the lack of data on iodine status.
It is conceivable that the phenomenon observed here might reflect an exhaustion of thyroid autoimmunity, prevalent in older age (14), in frail older women. A possible pathway to such an exhaustion of thyroid autoimmunity would be the depletion of thyroid antigen by an ongoing autoimmune response on the thyroid gland (40). Such a hypothesis is consistent with the observation of elevated serum concentrations of inflammatory biomarkers in frail older adults (9). A lower thyroid antigenic mass and the concomitant compromise in the important role of the thyroid gland in maintaining energy homeostasis (16) could, in turn, be consistent with the conceptualization of frailty as a clinical syndrome of energy dysregulation (1,2). Therefore, more mechanistic studies are warranted to clarify the pathophysiological mechanisms driving the inverse association between TgAb prevalence and frailty reported here.
Several strengths of this study lend support to the inferences herein derived. We used a large, well-characterized population of community-dwelling older women encompassing a range of disability (17,18). The definition of frailty used in this study is a well-validated measure (1,2,8). The usage of study-specific probability weights enables extrapolation of the results to a larger population (28). Finally, among thyroid antibody-positive individuals in our study population, the prevalence distribution across different TSH concentration groups follows a trend similar to that observed in NHANES III.
In conclusion, we report for the first time a graded inverse association between seropositivity for TgAbs and TPOAbs and the likelihood of prevalent frailty in older women. Whether this finding originates from differences in immune system function and homeostasis among women classified as nonfrail, prefrail, and frail or it reflects changes in the thyroid target organ remains to be elucidated. Replication of our results in another population is essential in guiding further research.
Acknowledgments
We thank all study participants in the Women’s Health and Aging Studies I and II.
Footnotes
This research was supported by the T. Franklin Williams Research Scholars Award from the Atlantic Philanthropies, American Geriatrics Society, the John A. Hartford Foundation, and the Association of Subspecialty Professors; the American Autoimmune Related Disease Association; Grant KL2-RR025006 from the National Center for Research Resources; Grant R01-DK55670 from the National Institute of Diabetes and Digestive and Kidney Diseases; Grants R01-AG11703, R37-AG19905, and P30-AG021334 from the National Institute on Aging (NIA); NIA Contract N01-AG-1-2112; and the Johns Hopkins Hospital and Johns Hopkins Bayview Medical Center General Clinical Research Center Grants RR00722 and R01-A141956.
Disclosure Summary: The authors have nothing to declare.
First Published Online January 8, 2010
Abbreviations: ANA, Antinuclear antibody; BMI, body mass index; CI, confidence interval; NHANES, National Health and Nutrition Examination Survey; OR, odds ratio; TgAb, thyroglobulin antibody; TPOAb, thyroid peroxidase antibody; WHAS, Women’s Health and Aging Studies; WHO, World Health Organization.
References
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA, Cardiovascular Health Study Collaborative Research G 2001 Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56:M146–M156 [DOI] [PubMed] [Google Scholar]
- Bandeen-Roche K, Xue QL, Ferrucci L, Walston J, Guralnik JM, Chaves P, Zeger SL, Fried LP 2006 Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci 61:262–266 [DOI] [PubMed] [Google Scholar]
- Ensrud KE, Ewing SK, Taylor BC, Fink HA, Cawthon PM, Stone KL, Hillier TA, Cauley JA, Hochberg MC, Rodondi N, Tracy JK, Cummings SR 2008 Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med 168:382–389 [DOI] [PubMed] [Google Scholar]
- Woods NF, LaCroix AZ, Gray SL, Aragaki A, Cochrane BB, Brunner RL, Masaki K, Murray A, Newman AB, Women’s Health I 2005 Frailty: emergence and consequences in women aged 65 and older in the Women’s Health Initiative Observational Study. J Am Geriatr Soc 53:1321–1330 [DOI] [PubMed] [Google Scholar]
- Lipsitz LA 2004 Physiological complexity, aging, and the path to frailty. Sci Aging Knowledge Environ 2004:pe16 [DOI] [PubMed] [Google Scholar]
- Fried LP, Xue QL, Cappola AR, Ferrucci L, Chaves P, Varadhan R, Guralnik JM, Leng SX, Semba RD, Walston JD, Blaum CS, Bandeen-Roche K 2009 Nonlinear multisystem physiological dysregulation associated with frailty in older women: implications for etiology and treatment. J Gerontol A Biol Sci Med Sci 64:1049–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwood K, Andrew M, Mitnitski A 2007 A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci 62:738–743 [DOI] [PubMed] [Google Scholar]
- Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G 2004 Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci 59:255–263 [DOI] [PubMed] [Google Scholar]
- Walston J, McBurnie MA, Newman A, Tracy RP, Kop WJ, Hirsch CH, Gottdiener J, Fried LP, Cardiovascular Health S 2002 Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med 162:2333–2341 [DOI] [PubMed] [Google Scholar]
- Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, Cevenini E, Castellani GC, Salvioli S 2007 Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev 128:92–105 [DOI] [PubMed] [Google Scholar]
- Giunta S, Sergio G 2008 Exploring the complex relations between inflammation and aging (inflamm-aging): anti-inflamm-aging remodelling of inflamm-aging, from robustness to frailty. Inflamm Res 57:558–563 [DOI] [PubMed] [Google Scholar]
- Mackay IR, Leskovsek NV, Rose NR 2008 Cell damage and autoimmunity: a critical appraisal. J Autoimmun 30:5–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamradt T, Mitchison NA 2001 Tolerance and autoimmunity. N Engl J Med 344:655–664 [DOI] [PubMed] [Google Scholar]
- Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE 2002 Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 87:489–499 [DOI] [PubMed] [Google Scholar]
- Surks MI, Hollowell JG 2007 Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab 92:4575–4582 [DOI] [PubMed] [Google Scholar]
- Silva JE, Bianco SD 2008 Thyroid-adrenergic interactions: physiological and clinical implications. Thyroid 18:157–165 [DOI] [PubMed] [Google Scholar]
- Fried LP, Kasper JD, Guralnik JM, Simonsick EM 1995 The Women’s Health and Aging Study: An introduction. In: Guralnik JM, Fried LP, Simonsick EM, Kasper JD, Lafferty ME eds. The Women’s Health and Aging Study: Health and Social Characteristics of Old Women with Disability (NIH Publication no 95–4009). Bethesda, MD: National Institute on Aging; 1–8 [Google Scholar]
- Fried LP, Bandeen-Roche K, Chaves PH, Johnson BA 2000 Preclinical mobility disability predicts incident mobility disability in older women. J Gerontol A Biol Sci Med Sci 55:M43–52 [DOI] [PubMed] [Google Scholar]
- Kondrashova A, Viskari H, Haapala AM, Seiskari T, Kulmala P, Ilonen J, Knip M, Hyöty H 2008 Serological evidence of thyroid autoimmunity among schoolchildren in two different socioeconomic environments. J Clin Endocrinol Metab 93:729–734 [DOI] [PubMed] [Google Scholar]
- Bunevicius A, Peceliuniene J, Mickuviene N, Girdler SS, Bunevicius R 2008 The association of thyroid immunity with blood pressure and body mass index in primary care patients. Endocr Res 33:93–103 [DOI] [PubMed] [Google Scholar]
- Chiovato L, Marcocci C, Mariotti S, Mori A, Pinchera A 1986 l-Thyroxine therapy induces a fall of thyroid microsomal and thyroglobulin antibodies in idiopathic myxedema and in hypothyroid, but not in euthyroid Hashimoto’s thyroiditis. J Endocrinol Invest 9:299–305 [DOI] [PubMed] [Google Scholar]
- Belin RM, Astor BC, Powe NR, Ladenson PW 2004 Smoke exposure is associated with a lower prevalence of serum thyroid autoantibodies and thyrotropin concentration elevation and a higher prevalence of mild thyrotropin concentration suppression in the third National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 89:6077–6086 [DOI] [PubMed] [Google Scholar]
- Villano MJ, Huber AK, Greenberg DA, Golden BK, Concepcion E, Tomer Y 2009 Autoimmune thyroiditis and diabetes: dissecting the joint genetic susceptibility in a large cohort of multiplex families. J Clin Endocrinol Metab 94:1458–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matejková-Behanová M, Zamrazil V, Vondra K, Vrbiková J, Kucera P, Hill M, Andel M 2002 Autoimmune thyroiditis in non-obese subjects with initial diagnosis of type 2 diabetes mellitus. J Endocrinol Invest 25:779–784 [DOI] [PubMed] [Google Scholar]
- Aho K, Gordin A, Palosuo T, Punsar S, Valkeila E, Karvonen M, Inkovaara J, Pasternack A 1984 Thyroid autoimmunity and cardiovascular diseases. Eur Heart J 5:43–46 [DOI] [PubMed] [Google Scholar]
- Newman AB, Gottdiener JS, McBurnie MA, Hirsch CH, Kop WJ, Tracy R, Walston JD, Fried LP, Cardiovascular Health Study Research G 2001 Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci 56:M158–M166 [DOI] [PubMed] [Google Scholar]
- Kayser L, Broholm H, Francis D, Perrild H, Olsen BE, Bendtzen K, Hoyer PE 1995 Immunocytochemical localisation of interleukin-1α and interleukin-6 in thyroid tissues from patients with neoplastic or autoimmune thyroid disorders. Autoimmunity 20:75–82 [DOI] [PubMed] [Google Scholar]
- Chu A, Maffeo CE, Lo A, Morganstein D, Bandeen-Roche KJ, Kasper JD, Dwight BB 1995 Sample design, weighting and estimation procedures, and computation of sampling errors. In: Guralnik JM, Fried LP, Simonsick EM, Kasper JD, Lafferty ME, eds. The Women’s Health and Aging Study: health and social characteristics of old women with disability (NIH publication no. 95-4009). Bethesda, MD: National Institute on Aging; A1–A13 [Google Scholar]
- Marin GG, Cardiel MH, Cornejo H, Viveros ME 2009 Prevalence of antinuclear antibodies in 3 groups of healthy individuals: blood donors, hospital personnel, and relatives of patients with autoimmune diseases. J Clin Rheumatol 15:325–329 [DOI] [PubMed] [Google Scholar]
- Vanderpump MP, Tunbridge WM, French JM, Appleton D, Bates D, Clark F, Grimley Evans J, Hasan DM, Rodgers H, Tunbridge F, et al. 1995 The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol (Oxf) 43:55–68 [DOI] [PubMed] [Google Scholar]
- Spencer CA, Hollowell JG, Kazarosyan M, Braverman LE 2007 National Health and Nutrition Examination Survey III thyroid-stimulating hormone (TSH)-thyroperoxidase antibody relationships demonstrate that TSH upper reference limits may be skewed by occult thyroid dysfunction. J Clin Endocrinol Metab 92:4236–4240 [DOI] [PubMed] [Google Scholar]
- Schmaltz HN, Fried LP, Xue QL, Walston J, Leng SX, Semba RD 2005 Chronic cytomegalovirus infection and inflammation are associated with prevalent frailty in community-dwelling older women. J Am Geriatr Soc 53:747–754 [DOI] [PubMed] [Google Scholar]
- Sinclair D 2006 Clinical and laboratory aspects of thyroid autoantibodies. Ann Clin Biochem 43:173–183 [DOI] [PubMed] [Google Scholar]
- Janeway C 1982 Beneficial autoimmunity? Nature 299:396–397 [DOI] [PubMed] [Google Scholar]
- Wildbaum G, Nahir MA, Karin N 2003 Beneficial autoimmunity to proinflammatory mediators restrains the consequences of self-destructive immunity. Immunity 19:679–688 [DOI] [PubMed] [Google Scholar]
- Elkon K, Casali P 2008 Nature and functions of autoantibodies. Nat Clin Pract Rheumatol 4:491–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurasov S, Nussenzweig MC 2007 Regulation of autoreactive antibodies. Curr Opin Rheumatol 19:421–426 [DOI] [PubMed] [Google Scholar]
- Silvestris F, Anderson W, Goodwin JS, Williams Jr RC 1985 Discrepancy in the expression of autoantibodies in healthy aged individuals. Clin Immunol Immunopathol 35:234–244 [DOI] [PubMed] [Google Scholar]
- Woo J, Goggins W, Sham A, Ho SC 2005 Social determinants of frailty. Gerontology 51:402–408 [DOI] [PubMed] [Google Scholar]
- Chiovato L, Latrofa F, Braverman LE, Pacini F, Capezzone M, Masserini L, Grasso L, Pinchera A 2003 Disappearance of humoral thyroid autoimmunity after complete removal of thyroid antigens. Ann Intern Med 139:346–351 [DOI] [PubMed] [Google Scholar]