Abstract
The incidence and prognosis of acute kidney injury (AKI) developing during acute resuscitation have not been well characterized in burn patients. The recently developed Risk, Injury, Failure, Loss, and End-stage (RIFLE) classification provides a stringent stratification of AKI severity and can allow for the study of AKI after burn injury. We hypothesized that AKI frequently develops early during resuscitation and is associated with poor outcomes in severely burned patients. We conducted a retrospective review of patients enrolled in the prospective observational multicenter study “Inflammation and the Host Response to Injury.” A RIFLE score was calculated for all patients at 24 hours and throughout hospitalization. Univariate and multivariate analyses were performed to distinguish the impact of early AKI on progressive renal dysfunction, need for renal replacement therapy, and hospital mortality. A total of 221 adult burn patients were included, with a mean TBSA burn of 42%. Crystalloid resuscitation averaged 5.2 ml/kg/%TBSA, with urine output of 1.0 ± 0.6 ml/kg/hr at 24 hours. Sixty-two patients met criteria for AKI at 24 hours: 23 patients (10%) classified as risk, 32 patients (15%) as injury, and 7 (3%) as failure. After adjusting for age, TBSA, inhalation injury, and nonrenal Acute Physiology and Chronic Health Evaluation II ≥20, early AKI was associated with an adjusted odds ratio 2.9 for death (95% CI 1.1–7.5, P = .03). In this cohort of severely burned patients, 28% of patients developed AKI during acute resuscitation. AKI was not always transient, with 29% developing progressive renal deterioration by RIFLE criteria. Early AKI was associated with early multiple organ dysfunction and higher mortality risk. Better understanding of how early AKI develops and which patients are at risk for progressive renal dysfunction may lead to improved outcomes.
Acute renal failure with severe oliguria is an uncommon but ominous development during burn shock resuscitation. Transient oliguria and acute creatinine elevations, however, occur more commonly during the resuscitative phase but are of uncertain prognostic significance. Because of varying definitions, the incidence of acute renal dysfunction in burn intensive care units (ICUs) has varied widely from 15 to 40%,1,2 and few studies have evaluated the functional significance of transient renal dysfunction.
The Risk, Injury, Failure, Loss, and End-stage kidney (RIFLE) classification system (Table 1)3 was recently developed to categorize severity of renal dysfunction and has enabled investigators to examine the impact of acute kidney dysfunction in critically ill patients. RIFLE defines three grades of increasing severity of acute kidney injury (AKI): risk, injury, and failure, based on changes in either serum creatinine or urine output, and two outcome categories (loss and end-stage kidney disease). Several critical care studies have validated the use of RIFLE criteria in mixed ICU patient populations.4–6 Several recent reports in burn patients have correlated worst RIFLE score during hospitalization to outcomes and examined the effect of continuous renal replacement therapy (RRT) on AKI compared with historical controls.7,8 However, the use of RIFLE as a tool to identify AKI during burn resuscitation has not yet been evaluated.
Table 1.
Rifle classification3
| Category | GFR Criteria | UOP Criteria |
|---|---|---|
| Risk | Increased SCr × 1.5 or GFR decrease >25% | UOP <0.5 ml/kg/hr × 6 hr |
| Injury | Increased SCr × 2 or GFR decrease >50% | UOP <0.5 ml/kg/hr × 12 hr |
| Failure | Increased SCr × 3 or GFR decrease 75%, or SCr >4mg/dl | UOP <0.3 ml/kg/hr × 24hr or anuria × 12 hr |
| Loss | Persistent ARF = complete loss of kidney function >4 wk | |
| End stage | End-stage kidney disease (> 3 mo) |
Scr, serum creatinine; GFR, glomerular filtration rate; UOP, urinary output; ARF, acute renal failure.
The “Inflammation and Host Response to Injury” is a collaborative program supported by the National Institute of General Medical Sciences with primary intent to better define the proteomic and genomic response to trauma and burn injuries. As part of this study, a large clinical database of patients with extensive burn injury has been maintained. In the context of this prospective cohort study, we sought out to investigate the incidence of AKI during burn resuscitation and its impact on hospitalization outcomes, including initiation of RRT and death.
METHODS
Study Design
We conducted a retrospective cohort study to evaluate the relationship between early AKI and outcome after thermal injury in the context of a prospective multicenter observational study. The principal exposure of interest was development of AKI (defined as a RIFLE classification of risk, injury, or failure) in the first 24 hours after burn injury, and main outcomes of interest were progression of AKI, administration of RRT, and inhospital mortality.
Patients and Data Collection
Eligible subjects included all adults with complete outcome data enrolled in the burn component of the Inflammation and Host Response to Injury (Glue Grant) program as of August 15, 2008.9 There are five participating centers in the study: Loyola University Medical Center, Massachusetts General Hospital, Parkland Memorial Hospital, University of Texas-Galveston, and the University of Washington. Criteria for adult patient enrollment into the multicenter study were age ≥18 years, burn size ≥20% TBSA, no other concomitant trauma, and admission to the study center within 96 hours of injury. Patients who were not resuscitated and placed on comfort care were not eligible for enrollment. This study was conducted after approval by the Glue Grant administrative core and by the University of Washington Institutional Review Board.
Data Collection
Clinical data were prospectively collected by trained nurse abstractors and entered into a web-based data collection platform specifically adapted for this program. Data integrity was evaluated centrally and by external review, as previously described.9 For this study, we abstracted data on all enrolled adult patients with complete hospitalization entries as of August 15, 2008.
Definitions
Initiation and methods of RRT were not part of standard operating procedures and were, thus, managed at the discretion of the burn surgeons at individual centers. AKI was identified by retrospectively calculating a RIFLE score at 24 hours on all patients following the method detailed in Table 1 and categorized as “early AKI.” Subjects with identified preinjury chronic renal insufficiency were excluded from this analysis. Because baseline creatinine levels were not available in injured patients, and the initial creatinine level at hospital admission likely reflects the effects of the burn injury that occurred before hospital admission, or during the initial phase of care, baseline values were estimated by age, racial background, and sex according to the method of Bellomo et al3 in the second international consensus conference of the Acute Dialysis Quality Initiative Group using the modification of diet in renal disease equation. After reviewing each patient’s medical history, their comorbidities (Table 2) were compared with the conditions listed in Table 3 to create a Charlson score. Any patient with a Charlson score of ≥1 was described as “comorbidity present.”
Table 2.
Patient comorbidities
| Comorbidity | Total Number (%) |
Early AKI (%) |
No AKI (%) |
|---|---|---|---|
| Smoking | 95 (43) | 24 (39) | 71 (45) |
| Alcohol | 40 (18) | 13 (21) | 27 (17) |
| Hypertension | 39 (18) | 21 (34) | 18 (11) |
| Psychiatric illness | 38 (17) | 10 (16) | 28 (18) |
| COPD/asthma | 21 (10) | 8 (13) | 13 (8) |
| Diabetes | 19 (9) | 8 (13) | 11 (7) |
| TBI/seizure disorder | 15 (7) | 5 (8) | 10 (6) |
| Liver disease | 10 (5) | 5 (8) | 5 (3) |
| MI | 9 (4) | 4 (6) | 5 (3) |
| Cancer | 8 (4) | 4 (6) | 4 (3) |
| Hypercholesterolemia | 7 (3) | 3 (5) | 4 (3) |
| CAD | 6 (3) | 3 (5) | 3 (2) |
| CHF/valvular disease | 6 (3) | 4 (6) | 2 (1) |
| Rheumatologic disease | 5 (2) | 3 (5) | 2 (1) |
| PVD/AAA | 5 (2) | 2 (3) | 3 (2) |
| Dysrhythmia | 4 (2) | 2 (3) | 2 (1) |
| Hypothyroidism | 4 (2) | 3 (5) | 1 (1) |
| CVA | 4 (2) | 4 (6) | 0 (0) |
| Dementia/Parkinson’s disease |
2 (1) | 0 (0) | 2 (1) |
| PUD | 2 (1) | 1 (2) | 1 (1) |
| Hyperthyroid | 1 (<1) | 0 (0) | 1 (1) |
| Crohn disease | 1 (<1) | 0 (0) | 1 (1) |
| HIV | 1 (<1) | 0 (0) | 1 (1) |
| History of advanced cancer | 1 (<1) | 1 (2) | 0 (0) |
| Pregnancy | 1 (<1) | 0 (0) | 1 (1) |
AKI, acute kidney injury; COPD, chronic obstructive pulmonary disease; TBI, traumatic brain injury; MI, myocardial infarction; CAD, coronary artery disease; CHF, congestive heart failure; PVD, peripheral vascular disease; AAA, abdominal aortic aneurysm; CVA, cerebral vascular accident; PUD, peptic ulcer disease.
Table 3.
Charlson score
| Assigned Weights for Conditions* |
Conditions |
|---|---|
| 1 | Acute myocardial infarction |
| Congestive heart failure | |
| Peripheral vascular disease | |
| Cerebrovascular disease | |
| Dementia | |
| Chronic pulmonary disease | |
| Connective tissue disorder | |
| Peptic ulcer disease | |
| Mild liver disease | |
| Diabetes | |
| 2 | Hemiplegia |
| Moderate or severe renal disease | |
| Diabetes with end-organ damage | |
| Any tumor | |
| Leukemia | |
| Lymphoma | |
| 3 | Moderate or severe liver disease |
| 6 | Metastatic tumor |
| Acquired immunodeficiency syndrome (AIDS) |
Total equals comorbidity score.10
Data Analysis
We compared baseline patient and clinical characteristics between patients with and without AKI including age, sex, ethnicity, %TBSA, burn mechanism, presence of inhalation injury, Acute Physiology and Chronic Health Evaluation (APACHE) II score, Denver multiorgan failure score9 at 24 hours and maximal Denver score, resuscitation volume, urine output, abdominal compartment syndrome, escharotomy, and decompression laparotomy. Total fluid volume (including colloid and crystalloid) administered in the first 24 hours after injury was examined as a function of the volume predicted by the Parkland Formula (4 ml/kg/%TBSA). Acute respiratory distress syndrome (ARDS), progression of AKI defined by worsening RIFLE classification, initiation of RRT, and discharge status were recorded for each patient. Given that APACHE II and Denver scores both include a renal dysfunction component, a modified APACHE II score and Nonrenal Organ Failure (NROF) score were calculated by omitting the creatinine component from the total APACHE II and Denver scores, respectively. The NROF score assigns one point for each nonrenal organ system in dysfunction (cardiac, pulmonary, or hepatic), as recently advocated by Barrantes et al.11 Thus, a nonrenal Denver score of ≥4 from two or more organ systems (cardiac, pulmonary, or hepatic) was defined as NROF ≥2 organ systems and, thus, represent early nonrenal multiple organ dysfunction syndrome (MODS).
Categorical variables, reported as proportions, were compared with χ2 tests. Continuous variables are reported as mean ± SD. Means were compared using Student’s t-test if a normal distribution was detected. Nonparametric variables were compared with Wilcoxon rank-sum test where appropriate. Logistic regression analyses were performed to assess an odds ratio (OR) for death for each variable, including early AKI and AKI at any point during the hospitalization, as well as severity of illness variables such as NROF ≥2 and nonrenal APACHE II ≥20.
To adjust for confounding variables and assess possible effect modification, multiple logistic regression analyses were performed. Variables were included in the regression analyses if they demonstrated significant association with the outcome of interest in the bivariate analyses. Given the significant effect of early multiple organ failure, multivariate analyses were performed with and without the variable “NROF ≥2” to test the level of association between early AKI and hospital mortality. All statistical analyses were performed with the use of STATA 10 (College Station, TX), a statistical software package. Actual P values are reported.
RESULTS
Baseline Patient and Injury Characteristics
At the time of our analysis on August 15, 2008, there were 437 patients enrolled with data recorded. Children (n = 202) and adults with incomplete resuscitation data (n = 14) were excluded. Thus, the study population consisted of 221 adult subjects. Patients and their families were interviewed about their medical history and any history of preexisting renal dysfunction. When available, medical records before injury were also reviewed to learn about medical illnesses including chronic renal dysfunction. Through this process, none of the 221 patients included in this study had a known history of chronic renal dysfunction. Of these 221 patients, 62 patients (28%) met AKI criteria at 24 hours (Table 4). Twenty-three patients (10%) met risk criteria, 32 (15%) were categorized as injury, and 7 (3%) as failure. Those who developed early AKI were significantly older (50.8 vs 39.1 years, P < .01), had a higher illness severity on admission (APACHE II: 23.6 vs 19.4, P < .01), and had a greater percentage with comorbidities (41.9 vs 20.8%, P < .01; Table 4). Mean burn size and full-thickness component were similar among patients with and without AKI.
Table 4.
Patient and injury characteristics by development of AKI
| Variable | AKI (n = 62) |
No AKI (n = 159) |
P |
|---|---|---|---|
| Age | 50.8 (18.3) | 39.1 (14.0) | <.01 |
| % Male | 69.4 | 74.2 | .47 |
| BMI | 30.1 (8.8) | 27.9 (11.7) | .09 |
| % TBSA | 42.5 (17.6) | 41.8 (19.4) | .40 |
| % Full-thickness | 33.1 (21.0) | 29.9 (19.1) | .14 |
| Burn mechanism | |||
| Flame/flash | 87.1 | 91.8 | .32 |
| Scald | 3.2 | 3.8 | |
| Other | 9.7 | 4.4 | |
| Inhalation injury | 45.2 | 36.7 | .25 |
| APACHE II | 23.6 (9.0) | 19.4 (9.0) | <.01 |
| Nonrenal APACHE II ≥20 (%) | 59.7 | 50.9 | .24 |
| Comorbidity present (%) | 41.9 | 20.8 | <.01 |
Data are reported as means (±SD) or percentage, where appropriate.
AKI, acute kidney injury; BMI, body mass index; APACHE, Acute Physiology and Chronic Health Evaluation.
Resuscitation Over the First 24 Hours
Time from injury to admission to the burn unit was similar between groups (4.1 vs 4.4 hours, P = 0.36), and in that time period, both groups received fluids at a volume greater than predicted by Parkland (Table 5). Total 24-hour resuscitation volume and amount of colloid were similar between those who developed early AKI and those who did not. Seven patients received plasmapheresis. Five of those patients did not have early AKI, and two had early AKI (one risk, one injury). No patients received high-dose vitamin C. A significant difference between groups was the initial base deficit (BD) and the worst BD over the initial 24 hours (−6.5 vs −4.9, P = 0.01 and −8.0 vs −6.1, P < .01, respectively). Both groups averaged a urine output within the recommended 0.5 to 1.0 ml/kg/hr or greater; however, those who developed early AKI had a significantly lower average urine output (0.6 vs 1.16 ml/kg/hr, P < .01).
Table 5.
First 24-hr resuscitation
| Variable | AKI (n = 62) |
No AKI (n = 159) |
P |
|---|---|---|---|
| Injury to admission (hr) | 4.1 (4.9) | 4.4 (7.1) | .36 |
| Preadmission fluid (observed/expected) |
1.5 (1.2) | 2.5 (3.2) | .08 |
| Initial BD | −6.5 (5.7) | −4.9 (4.0) | .01 |
| Worst BD | −8.0 (5.5) | −6.1 (4.3) | <.01 |
| Albumin | 318.1 (451.4) | 330.6 (776.1) | .45 |
| Parkland score | 1.24 (0.50) | 1.32 (0.62) | .82 |
| Urine output (ml/kg/hr) |
0.60 (0.35) | 1.16 (0.67) | <.01 |
Data are reported as means (±SD) or percentage, where appropriate.
AKI, acute kidney injury; BD, base deficit.
Outcomes
Early AKI
Eighteen (29%) of the 62 patients with early AKI had further deterioration in renal function, such that 48% (11 of 23) of patients initially classified as risk progressed to injury or failure and 22% (7 of 32) of patients initially classified as injury progressed to failure (Figure 1). Twelve patients (19%) with early AKI eventually received RRT, and 9 of those 12 (75%) did not survive. Four of six patients (67%) with deteriorating renal function who did not receive RRT died. Two died within the first 8 days, and the other two died in the second and third months of their hospitalization. In contrast, 35 of the 44 patients (80%) who had stabilization or improvement of their early AKI were discharged alive. Thus, all early AKI patients with renal deterioration had a markedly higher mortality (72%) than those who stabilized or improved in their renal function (20%). Overall, early AKI patients had higher mortality (36%) compared with those without AKI (13%), with multiple organ failure and sepsis as the most common causes of death.
Figure 1.
Early acute kidney injury was common (28%) and associated with higher mortality, especially if it was progressive: 13 patients with renal deterioration died (72%).
Late-Onset AKI
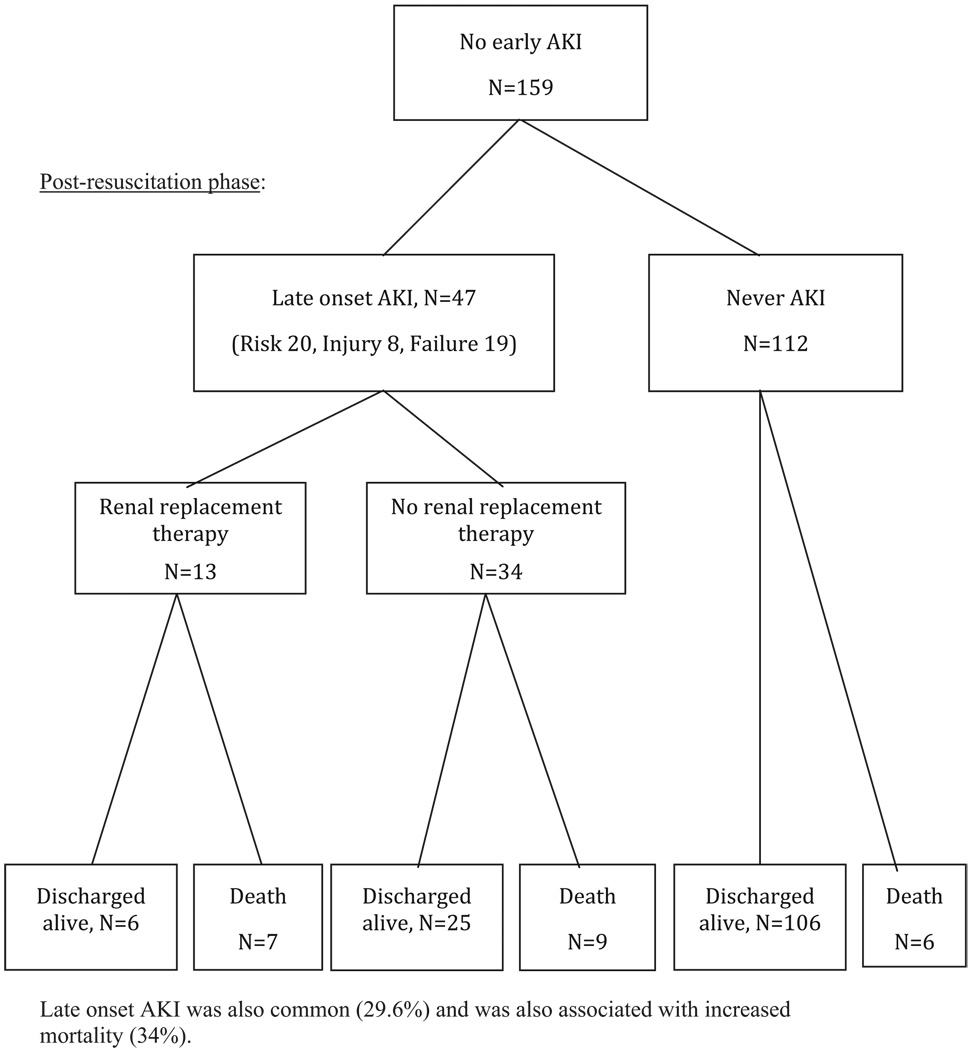
Of those who did not develop early AKI, 47 patients (30%) developed AKI later in their hospitalization (Figure 2). The most common cause of late-onset AKI was MODS (occurring in 22 patients, with 5 of the 22 also suffering from sepsis). The second most common etiology was sepsis (preceding multiple organ failure in two patients). The remaining 23 patients who developed late-onset AKI did so due to unknown causes. Thirteen patients (28%) with late-onset AKI received RRT, and 7 of those 13 (54%) did not survive. All late-onset AKI patients who received RRT had concomitant multiple organ failure. Twenty-five of the 34 patients (74%) who did not receive RRT were discharged alive. Late-onset AKI patients had 54% mortality in those receiving RRT and 26% in those without RRT. Interestingly, there was no significant difference in Baux scores on admission in those who eventually received RRT and those who did not (94 vs 92). In contrast, of the 112 patients who never developed AKI, 106 patients (95%) were discharged alive.
Figure 2.
Late onset acute kidney injury was also common (29.6%) and was also associated with increased mortality (34%).
Renal Replacement Therapy
Twenty-five patients received RRT at a mean of 19 days of hospitalization. Seven patients were started on RRT in the first week, and the remaining 18 patients had RRT initiated by a mean of 25 days. Thirteen of those patients were initially categorized as no AKI, 6 patients as risk, and 6 patients as injury. Overall, RRT was associated with a 64% mortality (16 patients) and was higher for patients with early AKI (75% mortality) compared with late-onset AKI (54% mortality). The RRT method was not protocolized among the participating centers. Thus, both continuous RRT and intermittent hemodialysis were used, and the RRT modality depended on the hemodynamic status and volume status of patients over time.
Development of Early AKI
Bivariate analysis revealed that age, presence of a comorbidity, preadmission fluid ratio, NROF ≥2, initial BD, and worst BD in the initial 24 hours were significantly associated with development of early AKI (Table 6). Therefore, a multivariate analysis was performed, which revealed age and preadmission fluid ratio to be significantly associated with development of early AKI and worst BD in the initial 24 hours to be nearly significant (Table 7).
Table 6.
Unadjusted logistic regression for development AKI
| Variable | Odds Ratio | P | 95% CI |
|---|---|---|---|
| Age | 1.05 | <.01 | 1.03–1.07 |
| % TBSA | 1.00 | .81 | 0.99–1.02 |
| Presence of a comorbidity | 2.76 | <.01 | 1.46–5.20 |
| Nonrenal APACHE II >20 | 1.42 | .24 | 0.79–2.58 |
| NROF ≥2 | 4.07 | <.01 | 1.55–10.68 |
| Injury to admission (hr) | 0.99 | .72 | 0.94–1.04 |
| Preadmission fluid ratio (observed/expected) |
0.78 | .02 | 0.64–0.96 |
| Initial BD | 0.93 | .04 | 0.87–0.99 |
| Worst BD first 24 hr | 0.92 | .02 | 0.86–0.98 |
AKI, acute kidney injury; APACHE, Acute Physiology and Chronic Health Evaluation; BD, base deficit; NROF, nonrenal organ failure.
Table 7.
Multivariate logistic regression for development of early AKI
| Variable | Odds Ratio |
P | 95% CI |
|---|---|---|---|
| Age | 1.06 | <.01 | 1.03–1.09 |
| Presence of a comorbidity | 0.87 | .76 | 0.34–2.18 |
| Preadmission fluid ratio (observed/expected) |
0.60 | <.01 | 0.43–0.82 |
| NROF ≥2 | 1.81 | .36 | 0.51–6.33 |
| Initial BD | 1.06 | .61 | 0.85–1.32 |
| Worst BD first 24 hr | 0.81 | .07 | 0.65–1.02 |
AKI, acute kidney injury; BD, base deficit; NROF, nonrenal organ failure.
Association Between Early AKI and Mortality
Mortality was higher among patients with early AKI than those without (36 vs 13%, P < .01; Table 8). Early AKI was associated with an unadjusted OR of 3.61 for death (95% CI 1.81–7.23, P < .01; Table 9). Multivariate logistic regression, adjusting for the effects of age, %TBSA, inhalation injury, nonrenal APACHE II ≥20, and presence of a comorbidity, indicated that early AKI had an adjusted OR of 2.87 for death (95% CI 1.11–7.45, P = 0.03). However, a significant proportion of patients with early AKI also developed early-onset NROF ≥2 during the resuscitation time frame. This factor was also associated with the mortality outcome by unadjusted analysis. When early-onset NROF ≥2 was factored into the regression analysis, early AKI had an adjusted OR of 2.32 for death (95% CI 0.85–6.36, P = .10), thus decreasing the strength of the association because of the greater effect of early MODS on mortality (Table 10).
Table 8.
Treatment and hospitalization outcomes, by development of Acute Kidney Injury (AKI)
| Variable | AKI (n = 62) | No AKI (n = 159) | P |
|---|---|---|---|
| 24-hr resuscitation (Parkland score) | 1.24 (0.50) | 1.32 (0.62) | .82 |
| 48-hr resuscitation (Parkland score) | 0.52 (0.33) | 0.45 (0.35) | .09 |
| 24-hr urine output (ml/kg/hr) | 0.60 (0.35) | 1.16 (0.67) | <.01 |
| 48-hr urine output (ml/kg/hr) | 0.91 (0.47) | 1.06 (0.54) | .03 |
| No. transfusions | 8.6 (8.5) | 9.3 (16.4) | .63 |
| Amount transfused (ml) | 7181 (8019) | 7172 (9601) | .50 |
| Abdominal compartment syndrome (%) | 3.2 | 3.1 | .97 |
| Escharotomies (%) | 30.7 | 33.3 | .70 |
| Total operations* | 5.9 (4.9) | 4.8 (4.4) | .05 |
| Length of stay (d)† | 52.37 (28.75) | 47.56 (41.89) | .25 |
| ICU length of stay (d)† | 37.13 (29.22) | 34.08 (41.11) | .34 |
| Ventilator days† | 17.18 (15.25) | 16.85 (25.03) | .47 |
| Early-onset NROF ≥2 (%) | 17.7 | 5.0 | <.01 |
| ARDS (%) | 37.1 | 32.1 | .48 |
| Pneumonia (%) | 50.0 | 48.4 | .83 |
| Sepsis (%) | 11.3 | 7.5 | .37 |
| Mortality (%) | 35.5 | 13.2 | <.01 |
Data are reported as means (±SD) or percentage, where appropriate.
Calculated only in patients who underwent operations.
Calculated in survivors only.
AKI, acute kidney injury; ICU, intensive care unit; NROF, nonrenal organ failure; ARDS, acute respiratory distress syndrome.
Table 9.
Unadjusted logistic regression for mortality
| Variable | Odds Ratio |
P | 95% CI |
|---|---|---|---|
| Age | 1.05 | <.01 | 1.03–1.07 |
| % TBSA | 1.06 | <.01 | 1.04–1.08 |
| % Full thickness | 1.05 | <.01 | 1.03–1.07 |
| Inhalation injury | 2.34 | .01 | 1.19–4.60 |
| Male | 1.11 | .80 | 0.52–2.36 |
| BMI | 1.00 | .97 | 0.97–1.03 |
| Early AKI | 3.61 | <.01 | 1.81–7.23 |
| AKI over hospitalization | 9.08 | <.01 | 3.64–22.62 |
| Nonrenal APACHE II ≥20 | 2.70 | .01 | 1.30–5.60 |
| Early-onset NROF ≥2 | 9.46 | <.01 | 3.45–25.90 |
| Presence of a comorbidity | 3.99 | <.01 | 1.98–8.03 |
AKI, acute kidney injury; APACHE, Acute Physiology and Chronic Health Evaluation; NROF, nonrenal organ failure.
Table 10.
Adjusted logistic regressions for mortality
| Variable | Odds Ratio |
P | 95% CI |
|---|---|---|---|
| Without early-onset NROF factor |
|||
| Early AKI | 2.87 | .03 | 1.11–7.45 |
| Age | 1.07 | <.01 | 1.04–1.11 |
| % TBSA | 1.09 | <.01 | 1.06–1.12 |
| Inhalation injury | 1.07 | .88 | 0.43–2.68 |
| Nonrenal APACHE II ≥20 | 1.39 | .51 | 0.52–3.70 |
| Presence of a comorbidity | 1.61 | .37 | 0.57–4.31 |
| With early-onset NROF factor | |||
| Early AKI | 2.32 | .10 | 0.85–6.36 |
| Age | 1.07 | <.01 | 1.04–1.12 |
| % TBSA | 1.09 | <.01 | 1.06–1.12 |
| Inhalation injury | 0.79 | .64 | 0.29–2.13 |
| Nonrenal APACHE II ≥20 | 1.11 | .83 | 0.41–3.05 |
| Presence of a comorbidity | 1.44 | .50 | 0.49–4.22 |
| Early-onset NROF ≥2 | 4.52 | .03 | 1.20–17.05 |
AKI, acute kidney injury; APACHE, Acute Physiology and Chronic Health Evaluation; NROF, nonrenal organ failure.
DISCUSSION
Resuscitation from burn shock remains one of the essential challenges of modern burn care. The severely burned patient has multiple risk factors for AKI during burn shock resuscitation. Intravascular hypovolemia, release of local and systemic cytokines, and cardiovascular dysfunction that accompany extensive tissue destruction in the acute injury phase, all create significant potential for early AKI.12–15 RIFLE is a recently described classification that allows for a more precise examination of the impact of renal dysfunction on outcome. The prognostic value of the RIFLE classification has been validated by correlating the patient’s worst RIFLE stage during hospitalization with mortality risk in a mixed ICU population and in burn patients.5,6,8 We have attempted to separate out early AKI in this cohort study to focus on AKI as a complication of burn shock.
By using RIFLE, we identified AKI as a common complication (28%) during burn resuscitation, with older and sicker patients with more comorbidities to be more likely to suffer from early AKI and found that patients with early AKI had a high incidence of deterioration of their renal function and increased mortality compared with patients without early AKI. These data indicate that episodes of oliguria and creatinine elevation during resuscitation that meet risk or injury criteria (Table 1) should be interpreted as complications of burn shock, predicting worse hospitalization outcomes. Early AKI, however, should be recognized and construed in the context of early multiple organ failure, which clearly portends a higher mortality risk. Adjusting for the presence of early nonrenal multiple organ failure in the regression model inevitably weakens the association between early AKI and mortality.
Historically, renal failure resulting from under resuscitation was a focus in burn care; however, the majority of recent studies have focused on the risks of excess resuscitation, which is associated with complications, including pneumonia, ARDS, elevated compartment syndromes, and higher risk of death.9,16,17 Average resuscitation volume in the first 24 hours in this cohort was 5.2 ml/kg/%TBSA and did not statistically differ between early AKI and nonearly AKI patients. Although higher than predicted Parkland formula-estimated volumes, this average is consistent with many other contemporary studies, which includes reported experiences of civilian and military burn centers.16,18 Given the lack of difference in resuscitation volumes between those who developed early AKI and those who did not, our data suggest that development of early AKI is multifactorial and not solely dependent on the amount of fluid received. In addition, the fact that the 24-hour average urine output for patients who developed early AKI was within the recommended guideline of 0.5 to 1.0 ml/kg/hr (0.6 ml/kg/hr) suggests that a patient may still develop early AKI despite maintaining an average urine output within 0.5 to 1.0 ml/kg/hr. Interestingly, patients who developed early AKI had a worse initial and worst BD in the first 24 hours, suggesting a greater degree of shock with its associated malperfusion. Cartotto et al19 have shown that a 24-hour BD < −6 was associated with a greater degree of systemic inflammatory response syndrome, ARDS, and MODS. Similarly, Cochran et al20 have shown BD and lactate values in the first 24 hours to be significantly worse in nonsurvivors. This is similar to other burn studies, which have shown elevated BDs to be associated with more extensive burns, inhalation injuries, higher than anticipated fluid requirements, and an increased risk of death.21–23 Thus, perhaps the greater degree of inflammation and malperfusion in these patients leads to the development of early AKI, with its associated increased mortality. However, currently available methods of monitoring resuscitation, including preload monitoring, BD, and other indices of shock, have not been proven superior to target mean arterial pressure and urinary output,23–25 and it remains unknown whether an effort to actively correct these parameters would lead to improved outcomes.
The high incidence of early AKI, coupled with the lack of difference in total resuscitation volume in the two groups, may also suggest that adjunct therapies to counter burn-associated systemic inflammation should be considered to mitigate early complications. These may include high-dose ascorbic acid, plasma exchange therapy, or early institution of RRT for AKI, as advocated by a recent report by the U.S. Army Institute of Surgical Research.26–28 Although each of these highly promising interventions is currently practiced at various centers, confirmation by well-conducted prospective randomized trials, such as the anticipated multicenter trials group investigation of early continuous RRT, is necessary before they are widely accepted by the burn community.
Our study has several limitations to consider. First, this cohort is limited to adults with burns of ≥20% TBSA and, therefore, does not address children or patients who are less severely burned, hence, may be less generalizable to children or those with smaller burns. Second, the retrospective nature of our cohort study hindered the completeness of data acquisition, such that although average urine output could be calculated, individual hourly urine output, periods of hypotension, and invasive measurements of intravascular volume status were not available. Third, baseline creatinine values before injury were not known and, thus, required estimation, given the outcome of interest of development of AKI at 24 hours. This is an inherent limitation to research in injured patients, because it is not possible to verify actual baseline (preinjury) creatinine. Although imperfect, we estimated baseline creatinine levels using reference values provided by the Acute Dialysis Quality Initiative Group, who used the modification of diet in renal disease equation, taking into account age, sex, and ethnicity. Finally, the outcomes we selected—progressive renal dysfunction, RRT, and hospital mortality—may not capture all relevant consequences of AKI. Increased resource utilization in and out of the burn ICU, subsequent development of chronic kidney disease, and need for future RRT because of successive acute, chronic, or acute and chronic injuries are all potential additional outcomes of significance. Future analysis of RRT initiation may also suggest optimal timing and intensity of renal replacement for improved outcomes.
CONCLUSION
AKI after burn injury is a common occurrence, with a quarter of patients developing early AKI and nearly half developing AKI over the acute hospitalization. Early AKI can be progressive and is more likely with larger TBSA burns. Patients with early AKI have a significantly increased mortality. Better understanding of the pathophysiology of AKI during burn shock, recognition of its significance, and improved treatment may improve future outcomes in severely burned patients.
ACKNOWLEDGMENTS
We thank the contribution of the Inflammation and the Host Response to Injury Large-Scale Collaborative Project Award 2-U54-GM062119 from the National Institute of General Medical Sciences, KL2 (1KL2RR025015-01) from the National Center for Research Resources), and the David and Nancy Auth-Washington Research Foundation Endowment.
Supported by the National Institute of General Medical Sciences.
This manuscript was prepared using a dataset obtained from the Glue Grant program and does not necessarily reflect the opinions or views of the Inflammation and the Host Response to Injury Investigators or the NIGMS.
REFERENCES
- 1.Mustonen KM, Vuola J. Acute renal failure in intensive care burn patients. J Burn Car Res. 2008;29:227–237. doi: 10.1097/BCR.0b013e31815f3196. [DOI] [PubMed] [Google Scholar]
- 2.Holm C, Horbrand F, von Donnersmarck GH, Mühlbauer W. Acute renal failure in severely burned patients. Burns. 1999;25:171–178. doi: 10.1016/s0305-4179(98)00144-2. [DOI] [PubMed] [Google Scholar]
- 3.Bellomo R, Ronocco C, Kellum JA, Mehta RL, Palevsky P. Acute Dialysis Quality Initiative Workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ricci Z, Cruz D, Ronco C. The RIFLE criteria and mortality in acute kidney injury: a systematic review. Kidney Int. 2008;73:538–546. doi: 10.1038/sj.ki.5002743. [DOI] [PubMed] [Google Scholar]
- 5.Hoste EA, Clemont G, Kersten A, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10:R73. doi: 10.1186/cc4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bagshaw SM, George C, Dinu I, Bellomo R. A multi-centre evaluation of the RIFLE criteria for early acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2008;23:1203–1210. doi: 10.1093/ndt/gfm744. [DOI] [PubMed] [Google Scholar]
- 7.Chung KK, Juncos LA, Wolf SE, et al. Continuous renal replacement therapy improves survival in severely burned military casualties with acute kidney injury. J Trauma. 2008;64:S179–S187. doi: 10.1097/TA.0b013e3181608676. discussion S185–7. [DOI] [PubMed] [Google Scholar]
- 8.Coca SG, Bauling P, Schifftner T, Howard CS, Teitelbaum I, Parikh CR. Contribution of acute kidney injury toward morbidity and mortality in burns: a contemporary analysis. Am J Kidney Dis. 2007;49:517–523. doi: 10.1053/j.ajkd.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 9.Klein MB, Hayden D, Elson C, et al. The association between fluid administration and outcome following major burn: a multicenter study. Ann Surg. 2007;245:622–628. doi: 10.1097/01.sla.0000252572.50684.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 11.Barrantes F, Tian J, Vazquez R, Amoateng-Adjepong Y, Manthous CA. Acute kidney injury criteria predict outcomes of critically ill patients. Crit Care Med. 2008;36:1397–1403. doi: 10.1097/CCM.0b013e318168fbe0. [DOI] [PubMed] [Google Scholar]
- 12.Davies MP, Evans J, McGonigle RJ. The dialysis debate: acute renal failure in burn patients. Burns. 1994;20:71–73. doi: 10.1016/0305-4179(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 13.Schiavon M, Di Landro D, Baldo M, De Silvestro G, Chiarelli A. A study of renal damage in severely burned patients. Burns. 1988;14:107–114. doi: 10.1016/0305-4179(88)90213-6. [DOI] [PubMed] [Google Scholar]
- 14.Aikawa N, Wakabayashi G, Ueda M, Shinozawa Y. Regulation of renal function in thermal injury. J Trauma. 1990;30:S174–S178. doi: 10.1097/00005373-199012001-00035. [DOI] [PubMed] [Google Scholar]
- 15.Planas M, Wachtel T, Frank H, Henderson LW. Characterization of acute renal failure in the burned patient. Arch Intern Med. 1982;142:2087–2091. [PubMed] [Google Scholar]
- 16.Cancio LC, Chavez S, Alvarado-Ortega M, et al. Predicting increased fluid requirements during the resuscitation of thermally injured patients. J Trauma. 2004;56:404–413. doi: 10.1097/01.TA.0000075341.43956.E4. discussion 413–4. [DOI] [PubMed] [Google Scholar]
- 17.Pham TN, Cancio LC, Gibran NS. American Burn Association practice guidelines burn shock resuscitation. J Burn Care Res. 2008;29:257–266. doi: 10.1097/BCR.0b013e31815f3876. [DOI] [PubMed] [Google Scholar]
- 18.Ennis JL, Chung KK, Renz EM, et al. Joint Theater Trauma System implementation of burn resuscitation guidelines improves outcomes in severely burned military casualties. J Trauma. 2008;64:S146–S151. doi: 10.1097/TA.0b013e318160b44c. [DOI] [PubMed] [Google Scholar]
- 19.Cartotto R, Choi J, Gomez M, Cooper A. A prospective study on the implications of a base deficit during fluid resuscitation. J Burn Car Rehabil. 2003;24:75–84. doi: 10.1097/01.BCR.0000054177.24411.13. [DOI] [PubMed] [Google Scholar]
- 20.Cochran A, Edelman L, Saffle J, Morris SE. The relationship of serum lactate and base deficit in burn patients to mortality. J Burn Care Res. 2007;28:231–240. doi: 10.1097/BCR.0B013E318031A1D1. [DOI] [PubMed] [Google Scholar]
- 21.Kaups KL, Davis J, Dominic WJ. Base deficit as an indicator of resuscitation needs in patients with burn injuries. J Burn Car Rehabil. 1998;19:346–348. doi: 10.1097/00004630-199807000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Wolf SE, Rose J, Desai MH, Mileski JP, Barrow RE, Herndon DN. Mortality determinants in massive pediatric burns: an analysis of 103 children with > or = 80% TBSA burns (> or = 70% full-thickness) Ann Surg. 1997;225:554–565. doi: 10.1097/00000658-199705000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeng JC, Lee K, Jablonski K, et al. Serum lactate and base deficit suggest inadequate resuscitation of patients with burn injuries: application of a point of care laboratory instrument. J Burn Car Rehabil. 1997;18:402–405. doi: 10.1097/00004630-199709000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Cancio LC, Galvez E, Jr, Turner CE, Jordan MH. Base deficit and alveolar-arterial gradient during resuscitation contribute independently but modestly to the prediction of mortality after burn injury. J Burn Care Res. 2006;27:289–296. doi: 10.1097/01.BCR.0000216457.25875.F4. discussion 296–7. [DOI] [PubMed] [Google Scholar]
- 25.Holm C, Mayr M, Tegeler J, et al. A clinical randomized study on the effects of invasive monitoring on burn shock resuscitation. Burns. 2004;30:798–807. doi: 10.1016/j.burns.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka H, Matsuda T, Miyagantani Y, Yukioka T, Matsuda H, Shimazaki S. Reduction of resuscitation fluid volumes in severely burned patients using ascorbic acid administration: a randomized, prospective study. Arch Surg. 2000;135:326–331. doi: 10.1001/archsurg.135.3.326. [DOI] [PubMed] [Google Scholar]
- 27.Chung KK, Juncos L, Wolf SE, et al. Continuous renal replacement therapy improves survival in severely burned military casualties with acute kidney injury. J Trauma. 2008;64:S179–S185. doi: 10.1097/TA.0b013e3181608676. [DOI] [PubMed] [Google Scholar]
- 28.Kravitz M, Warden G, Sullivan JJ, Saffle JR. A randomized trial of plasma exchange in the treatment of burn shock. J Burn Car Rehabil. 1989;10:17–26. doi: 10.1097/00004630-198901000-00004. [DOI] [PubMed] [Google Scholar]