Abstract
Objectives
Mixed hematopoietic chimerism is associated with islet allograft tolerance and may reverse autoimmunity. We developed low intensity regimens for the induction of mixed chimerism and examined effects on autoimmunity in pre-diabetic NOD mice.
Research Design and Methods
NOD mice received various combinations of total body irradiation, anti-CD154, anti-CD8α, anti-CD4, anti-Thy1.2 mAbs, with or without transplantation of C57BL/6 BMC and were followed for development of diabetes, chimerism and donor skin graft survival. Autoimmunity was assessed by histologic examination of salivary glands and pancreata.
Results
While conditioning alone prevented or delayed the onset of diabetes, stable mixed chimerism was required for the reversal of isletitis. Mixed chimerism and skin graft tolerance were achieved in NOD mice receiving anti-CD154 with BMT as the means of tolerizing peripheral CD4+ T cells to alloantigens. However, isletitis was not reversed in allotolerant mixed chimeras prepared with this regimen.
Conclusions
Partial depletion of peripheral autoreactive NOD CD4+ T cells is needed to achieve full reversal of isletitis by mixed chimerism induction from a protective donor strain, but is not required for induction of specific tolerance to donor alloantigens. Thus, the requirements for tolerizing alloreactive and autoreactive NOD CD4 cells are distinct.
Keywords: Tolerance, Immunology, Transplantation
INTRODUCTION
Islet transplantation can restore insulin production in patients with type I diabetes. Nevertheless, clinical islet allotransplantation only allows a minority (~10%) of patients to maintain insulin independence, possibly due to allo- and autoimmune responses plus drug toxicity (1). Tolerance induction would overcome alloresponses to islet alloantigens and could prevent recurrent autoimmunity in donor islets, while avoiding continuous immunosuppressive therapy (2).
Allogeneic hematopoietic stem cell engraftment in non-myeloablated recipients results in donor-specific tolerance, allowing acceptance of donor islet allografts, as well modulation of autoimmunity (3,4). However, clinical application of this approach is limited by the toxicity of conditioning regimens used to achieve marrow engraftment and by concerns about graft-versus-host disease (GVHD) and failure of engraftment, especially when HLA barriers are transgressed (5,6). A non-myeloablative method of achieving engraftment without these toxicities would bring this approach toward clinical application.
Mixed hematopoietic chimerism, in which multilineage hematopoietic populations of both the recipient and the donor co-exist (6,7), can be achieved by mild, non-myeloablative regimens in mice, non-human primates and humans, and induces donor-specific tolerance (6–11).
Mixed chimerism achieved using myeloablative conditioning prevented the development of diabetes in non-diabetic NOD mice. (12,13). Co-stimulatory blockade plus sublethal total body irradiation (TBI) allowed nearly full allogeneic chimerism with tolerance to donor islets in diabetic NOD mice (14). Mixed chimerism from MHC-identical donors achieved with non-myeloablative conditioning in diabetic NOD mice allowed acceptance of donor islets (15). We established mixed chimerism across extensive MHC barriers in overtly diabetic NOD mice with non-myeloablative conditioning involving a low dose of TBI (4 Gy), extensive recipients T cell depletion, and anti-CD154. These mice accepted donor islets, which cured diabetes. Despite significant T cell reconstitution by the NOD recipients, destructive autoimmunity was reversed, permitting acceptance of syngeneic islet grafts (4). We now sought to establish less lymphoablative regimens for the induction of mixed chimerism and reversal of autoimmunity in pre-diabetic NOD mice.
MATERIALS AND METHODS
Animals
Female NOD (H-2g7), BALB/c (H-2d), C57BL/6 (B6: H-2b), SJL (H-2s), and B10.RIII (H-2r) mice were purchased from Frederick Cancer Research Center, Frederick, MD or from the Jackson Laboratory, Bar Harbor, ME. Mice were maintained as previously described (4). Donors were 10–12 weeks of age.
Conditioning and BMT
Pre-diabetic NOD mice (6–14 weeks old; age-matched within each experiment) were treated with: anti-CD4 (3.5 mg per injection, mAb GK1.5 (16)), anti-CD8α.1 to deplete recipient CD8 cells (0.33 mg per injection mAb 116-13-1), anti-CD8α.2 to deplete donor CD8 cells (1.44 mg of mAb 2.43), anti-Thy1.2 (0.56mg of mAb 30-H12 (17)) and anti-CD154 mAb (2mg of mAb MR1 (18)). On Day 0, 3–4 Gy TBI was administered. Where indicated, 7 Gy thymic irradiation was given on Day 0. After completion of conditioning on Day 0, 1.5–3.0×107 B6 BMC were administered intravenously (i.v.). When indicated, ex vivo T cell depletion (TCD) of CD4+ and CD8+ T cells from the donor marrow was performed using mAbs and rabbit complement as described (19).
Flow cytometric (FCM) analysis of chimerism
Donor T- and non-T cell reconstitution was evaluated in lymphocytes, granulocytes, and monocytes in peripheral white blood cells (WBC) by two- or four-color FCM analysis on a FACSort or FACSCalibur (Becton Dickinson, Mountain View, CA) as previously described (4,20).
Skin grafting
Full thickness tail skin grafting was performed as previously described (7). Grafts were inspected on day 7, then daily for the first month and two to three times per week thereafter. Grafts were considered to be rejected at the time of complete sloughing or formation of a dry scab.
Islet scoring
Islets were graded by the amount and pattern of islet inflammation (isletitis), peri-islet inflammation, and atrophy as described (21,22) Grade 0, normal-appearing islets without isletitis or peri-islet inflammation; Grade 1, peri-islet inflammation and/or islet inflammation involving <25% of an islet; Grade 2, isletitis involving 25 to 50% of an islet; Grade 3, mononuclear inflammation involving greater than 50% of an islet; Grade 4, a small, retracted and atrophic islet, confirmed by absence of insulin staining by immunohistochemistry. All islets were graded individually in three to five sections of pancreas and a mean score determined.
Histology and immunohistochemical staining
Histology and immunohistochemical staining were performed as previously described (4). Photographs were taken with an RT Spot Camera (Diagnostic Instruments, Inc.), a Zeiss Axioskop microscope, and imported into Photoshop (Adobe).
Adoptive transfer of splenocytes to NOD-SCID mice
CD4+ and CD8+ T cells were positively selected from spleens, pancreatic and non-pancreatic lymph nodes of conditioned controls, failed chimeras or stable mixed chimeras using either positive selection with CD4 and CD8 microbeads or with a negative “untouched T cell” magnetic bead isolation kits (MACS, Miltenyi Biotech, Auburn, CA) per the manufacturer’s protocol. The purity of T cells determined by FCM was >90%. 3–4×106 lymph node cells or 2.5–20×106 splenic T cells was injected i.v. into recipient NOD-SCID mice in sterile PBS. For donor cell depletion of splenic T cells from chimeras, negative selection with biotin-conjugated anti-CD45.2 mAb and MACS beads were used. Depletion was >95% effective. Recipient NOD-SCID mice were monitored by twice-weekly blood glucose measurement and considered to be diabetic after three sequential measurements above 11mM (200mg/dl).
Statistical analysis
Statistical significance was determined using Student’s t-test. A P value of less than 0.05 was considered to be statistically significant.
RESULTS
Development of a successful non-myeloablative conditioning regimen
We evaluated non-myeloablative conditioning regimens followed by transplantation of C57BL/6 (B6) allogeneic bone marrow cells (BMC) in efforts to achieve sustained mixed hematopoietic chimerism across MHC barriers in pre-diabetic NOD mice (Table 1). Although less vigorous conditioning permits mixed chimerism induction in other mouse strains (20, 23–26), the majority were ineffective in NOD mice. Mice receiving donor-specific CD8-depleting mAb, anti-CD4, with or without anti-Thy1.2, and 3 or 4 Gy TBI with thymic irradiation, achieved only initial chimerism, which gradually disappeared (Table 1, Regimens A and B). The replacement of thymic irradiation with anti-CD154 mAb, with or without an additional anti-CD4 treatment, did not permit durable chimerism (Table I, Regimens C and D). Lasting mixed chimerism was achieved when recipient CD8+ T cell depletion was added to Regimen C (Table 1, Regimen F). The regimen included anti-CD4 (Days −6 and −1), anti-CD8α.1 (Days −1, 0, 1, 6, 7 and 8), anti-CD8α.2 (Day 0, depleting donor T cells), anti-Thy1.2 (Days −6, and −1), anti-CD154 mAbs (Day 0, 2mg/injection MR1), and 4 Gy TBI (Day 0), followed by transplantation of 30×106 B6 BMC; these mice developed lasting (>1 year post-BMT) mixed chimerism. We have previously achieved similar levels of mixed chimerism with this regimen in diabetic NOD mice (4). Conditioned mice, with or without BMT, showed no mortality, demonstrating that the regimen is non-myeloablative. There was no clinical evidence of GVHD. A regimen that was otherwise identical but included CTLA4Ig on Day 2 instead of the second course (Days 6–8) of anti-CD8 α.1 mAb (Table 1, Regimen E) did not permit durable chimerism. Therefore, exhaustive recipient CD8 depletion plays an important role in the achievement of durable mixed chimerism in NOD mice.
Table 1.
Mixed hematopoietic chimerism in pre-diabetic NOD mice receiving various non-myeloablative conditioning regimens followed by B6 bone marrow transplantation.
The percentage of donor-derived (H-2Kb+) cells was determined by flow cytometry 2, 6, 8 and 12 weeks after bone marrow transplantation.
| Regimen | Day -6 | Day -1 | Day 0 | Day 1 | Day 2 | Day 6–8 | Outcome |
|---|---|---|---|---|---|---|---|
| A | Anti-CD4 Anti-CD8α.2 |
Anti-CD4 Anti-CD8α.2 |
3Gy TBI + 7Gy TI 15 × 106 B6 BMC |
Transient chimerism 0/21* |
|||
| B | Anti-CD4 Anti-Thy1.2 Anti-CD8α.2 |
Anti-CD4 Anti-Thy1.2 Anti-CD8α.2 |
3Gy TBI + 7Gy TI 15 × 106 B6 BMC |
Transient chimerism 0/7* |
|||
| C | Anti-CD4 Anti-Thy1.2 Anti-CD8α.2 |
Anti-CD4 Anti-Thy1.2 Anti-CD8α.2 |
3 or 4 Gy TBI Anti-CD154 30 × 106 B6 BMC |
Transient chimerism 0/14* |
|||
| D | Anti-CD4 Anti-Thy1.2 Anti-CD8α.2 |
Anti-CD4 Anti-Thy1.2 Anti-CD8α.2 |
3 or 4 Gy TBI Anti-CD154 30 × 106 B6 BMC |
Anti-CD4 (only Day 7) |
Transient chimerism 0/6* |
||
| E | Anti-CD4 Anti-Thy1.2 |
Anti-CD4 Anti-Thy1.2 Anti-CD8α.1 |
4 Gy TBI Anti-CD154 Anti-CD8α.1, Anti-CD8α.2 30 × 106 B6 BMC |
Anti-CD8α.1 | CTLA4 Ig | Transient chimerism 0/10* |
|
| F | Anti-CD4 Anti-Thy1.2 |
Anti-CD4 Anti-Thy1.2 Anti-CD8α.1 |
4 Gy TBI Anti-CD154 Anti-CD8α.1, Anti-CD8α.2 30 × 106 B6 BMC |
Anti-CD8α.1 | Anti-CD8α.1 | Lasting chimerism 67/78* |
|
| G | Anti-CD4 | Anti-CD4 Anti-CD8α.1 |
4 Gy TBI Anti-CD154 Anti-CD8α.1, Anti-CD8α.2 30 × 106 B6 BMC |
Anti-CD8α.1 | Anti-CD8α.1 | Lasting chimerism 11/14* |
|
| H | Anti-CD8α.1 | 4 Gy TBI (Day-1 or Day -2) Anti-CD154 (Day-1 or Day -2) Anti-CD8α.1, Anti-CD8α.2 30 × 106 B6 BMC |
Anti-CD8α.1 | Anti-CD8α.1 | Lasting chimerism 35/74* |
The presence of chimerism was defined as at least 1% donor-derived cells.
TBI - Total Body Irradiation, TI- Thymic irradiation, BMC - bone marrow cells, Anti-CD8α.1 (116-13-1), Anti-CD8α.2 (2.43) anti-CD4 (GK1.5), anti-Thy1.2 (30-H12), anti-CD154 (MR1)
number of long-term chimeras/total number of animals.
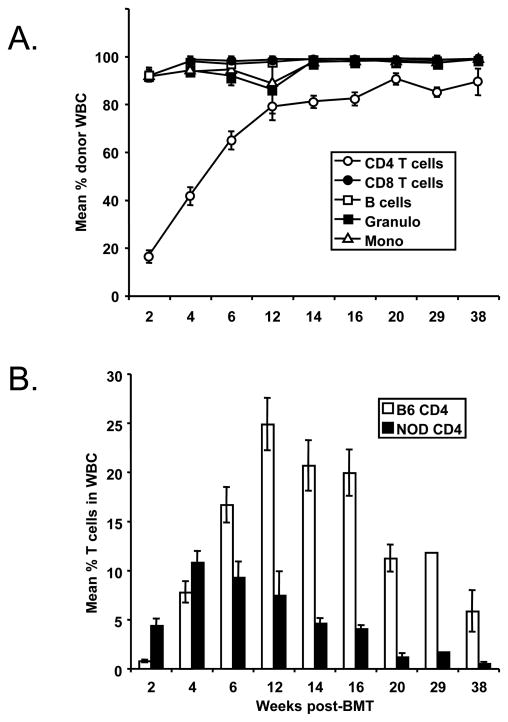
While the myeloid, B cell and CD8 cell lineages were predominantly of donor origin in mixed chimeras prepared with Regimen F, initial CD4 reconstitution was largely recipient-derived in the first month post-BMT. Donor CD4 reconstitution gradually took over, to a plateau level of approximately 90% in long-term chimeras (Figure 1).
Figure 1. Mixed allogeneic chimerism in NOD mice receiving incomplete CD4-depleting conditioning regimen.
A) Six to eight-week-old pre-diabetic female NOD mice received conditioning with anti-CD8 α.1 (Days −1,0,1,6,7,8), anti-Thy1.2 (Days −6, −1), anti-CD154 (Day 0) mAbs, and 4 Gy TBI (Day 0) followed by transplantation with 30 × 106 allogeneic C57BL/6 bone marrow cells. The mean levels of donor chimerism in NOD mouse blood were measured by FACS at various time-points post-BMT (n=9). B) CD4+ T cell recovery in B6-->NOD (diabetic) mixed allogeneic chimeras prepared with non-CD4-depleting, non-myeloablative conditioning. The mean levels of donor (white bars) and recipient CD4+ T cells (black bars) in blood of pre-diabetic B6→NOD chimeras, as measured by FACS at various time points post-BMT.
Anti-CD154 avoids the requirement for CD4 T cell depletion in other strains of mice receiving 3 Gy TBI, anti-CD8 mAb and BMT (27). We therefore evaluated the effect of omitting CD4+ T-cell depletion by anti-CD4 mAb and anti-Thy1.2 mAb (Table 1, Regimen H). Among control recipients of Regimen F, high levels of stable multi-lineage mixed chimerism were again achieved. Omission of anti-Thy1.2 resulted in similar levels of mixed chimerism in 9 of 11 recipients (Table 1, Regimen G). About 50% of NOD mice that received 4Gy TBI, anti-CD154 mAb, anti-CD8 α.2 and 30×106 B6 BM cells on Day 0, and anti-CD8 α.1 mAb on Days −1, 0, 1, 6, 7, and 8 (Table 1, Regimen H) also developed stable chimerism (Figure 1a). Overall, durable chimerism was achieved in 7 of 11 experiments, including 35 of 74 mice receiving this non-CD4-depleting protocol. Nearly full donor chimerism was observed among B cells and myeloid cells, even though host NOD CD4+ T cells persisted at significant levels (Figure 1b). Because TBI given 1 or 2 days prior to BMT rather than Day 0 improved the success rate of anti-CD154-based regimens in other strains (28), some of these experiments involved evaluation of TBI on Day −1 or −2 instead of Day 0. However, no difference in outcome was observed in recipients of TBI on the various days (data not shown).
Peripheral T cells of NOD mice resist depletion with mAbs
As NOD lymphocytes have been reported to be resistant to T cell depleting mAbs, irradiation and co-stimulatory blockade (29–33), we investigated the level of T cell depletion achieved with our successful non-myeloablative conditioning regimen, Regimen F. Lymphocytes in spleens, lymph nodes, and thymi of conditioned NOD and BALB/c mice were compared at Day 4 postconditioning. Pre-diabetic NOD mice were treated with anti-CD4, anti-CD8 α.1, anti-Thy1.2 and anti-CD154 mAb as in Regimen F and received 4 Gy TBI on Day 0. Age and sex-matched BALB/c mice received anti-CD4 (1.75 mg GK1.5 Days −6 and −1), anti-CD8 α.2 (1.44 mg 2.43 Day 0), anti-Thy1.2 (0.56 mg 30-H12 Days −6, and −1) and anti-CD154 mAb (2mg MR1 Day 0) and on Day 0 received 3 Gy TBI.
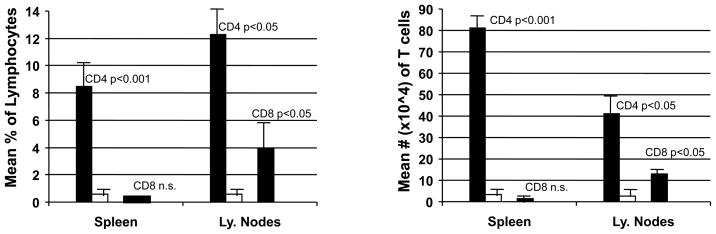
Despite the higher doses of mAbs and TBI, NOD mice achieved less depletion of CD8+ T cells in the lymph nodes (average 3.9%) than BALB/c mice, in which complete depletion (0% CD8+ T cells detectable) of lymph node CD8+ T cells was observed (p<0.02) (Figure 2). CD8+ T cell depletion in the spleens (Figure 2), WBC, and thymi (data not shown) was similar in NOD and BALB/c mice. A more striking difference in CD4+ cell depletion was observed (Figure 2). In BALB/c mice, there was nearly complete depletion of CD4+ T cells in the spleen (0.6%) and lymph nodes (0.9%). In contrast, CD4+ T cells constituted an average of 8.47% (0.8×106) of splenocytes (p<0.001 compared to the treated BALB/c spleens) and 12.3% (0.4×106) of lymph node cells of conditioned NOD mice (p<0.001). In summary, the exhaustive T cell-depleting regimen administered to NOD mice caused only partial depletion of CD4+ and CD8+ T cells, leaving large numbers of CD4+ cells in the lymphoid tissues of NOD mice. These results are consistent with previous studies (32).
Figure 2. Peripheral T cells of NOD mice resist depletion by conditioning regimen.
The level of conditioning regimen-induced T cell depletion in spleens and lymph nodes of conditioned NOD and BALB/c mice were analyzed on Day 4. Pre-diabetic NOD mice (n=4) were treated with combinations of anti-CD4 (Days −6 and −1), anti-CD8α.1 (Days −6, −5, −4, −1, 0 and 1), anti-Thy1.2 (Days −6, and −1) and anti-CD154 mAb (Day 0). Age and sex-matched BALB/c mice (n=4) received anti-CD4 (Days −6 and −1), anti-CD8α.2 (Days −6 and −1), anti-Thy1.2 (Days −6, and −1) and anti-CD154 mAb (MR1). NOD mice received 4 Gy TBI (Day 0) and Balb/c mice received 3 Gy TBI (Day 0) without donor BMT. The mean percentages (left panel) and the mean total numbers (right panel) of CD4+ and CD8+ T cells in NOD (black bars) and BALB/c (white bars) spleens and lymph nodes were measured by FACS at Day 4. P values compare NOD and BALB/c mice.
Skin graft tolerance following induction of chimerism with non-myeloablative conditioning
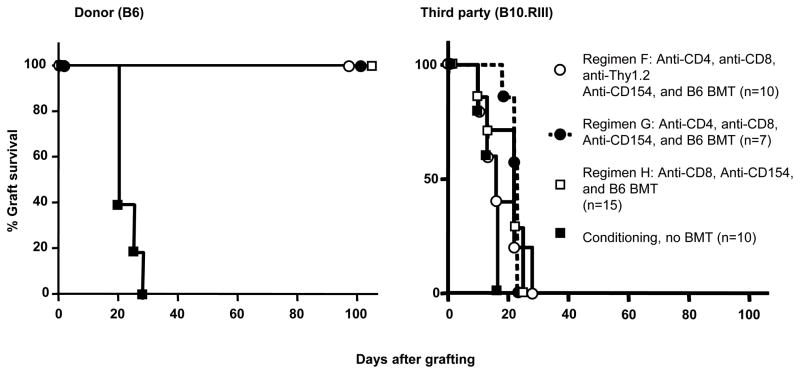
To assess tolerance, donor (B6) or third-party (B10.RIII) skin was grafted 15–20 weeks after BMT (Figure 3). All skin-grafted chimeras generated with Regimens F, G and H accepted B6 (donor) skin, while B10.RIII (third party) skin grafts were rejected within 28 days. NOD mice receiving these conditioning regimens without B6 BMT rejected both B6 and B10.RIII skin grafts within 28 days. NOD mice in groups F, G and H (Table I), which received B6 BMT but developed only transient chimerism (n=5), rapidly rejected donor skin (Median Survival Time (MST)=18 days). Thus, mixed chimerism was associated with tolerance toward donor-derived skin allografts in NOD mice.
Figure 3. Donor-specific skin graft tolerance in NOD mice receiving non-myeloablative conditioning, followed by B6 bone marrow cells.
NOD mice received non-myeloablative conditioning followed by transplantation of 30×106 allogeneic C57BL/6 (B6) bone marrow cells (BMC). Durable mixed chimerism was established in all recipients. Donor C57BL/6 and third party Balb/c skin was grafted 14 weeks post-BMT. Donor C57BL/6 skin grafts were accepted through the entire observation period (left panel). Third-party skin grafts (Balb/c) were rejected within 18 days after skin grafting (n=5 per group) (right panel).
Although conditioning alone delays/prevents the onset of diabetes, stable chimerism is required for full reversal of autoimmunity
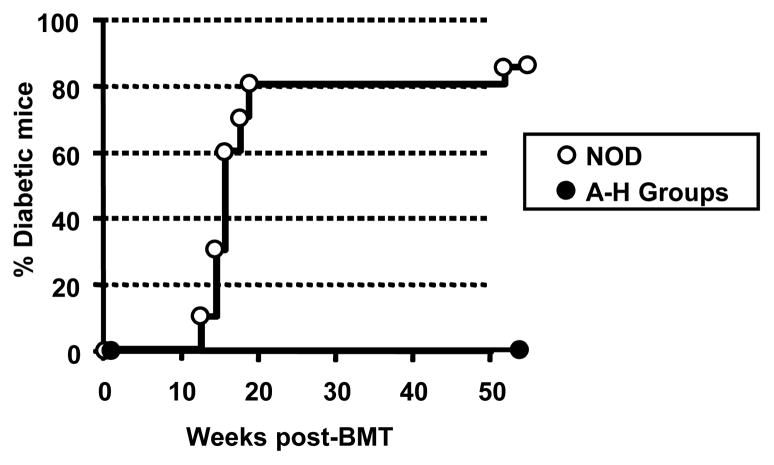
Pre-diabetic NOD mice receiving each regimen with and without BMT were compared to untreated NOD mice for diabetes development. None of the successful mixed chimeras (Regimens F–H) developed diabetes for the duration of follow-up (up to 50 weeks post BMT), while 81% of untreated concurrent control NOD mice were diabetic by 24 weeks of age (e.g. Figure 4). Furthermore, none of the NOD mice (n=19) receiving Regimens F–H without BMT developed diabetes up to 50 weeks (Figure 4). Although none of the mice from groups A–E (Table 1) developed lasting chimerism, they were also protected from diabetes throughout the observation period (> 40 weeks).
Figure 4. Conditioning with or without BMT delays the development of diabetes in NOD mice.
Six to eight week old pre-diabetic female NOD mice received no treatment (untreated, n=20) or conditioning Regimens A–H (conditioning regimen n=55). Additional animals received similar conditioning followed by transplantation of 30 × 106 allogeneic C57BL/6 bone marrow cells (n=12). Diabetes was assessed by bi-weekly measurement of blood glucose using Dextrostix and a glucometer. Animals were considered to be diabetic after three sequential glucose measurements above 200mg/dl (hyperglycemia).
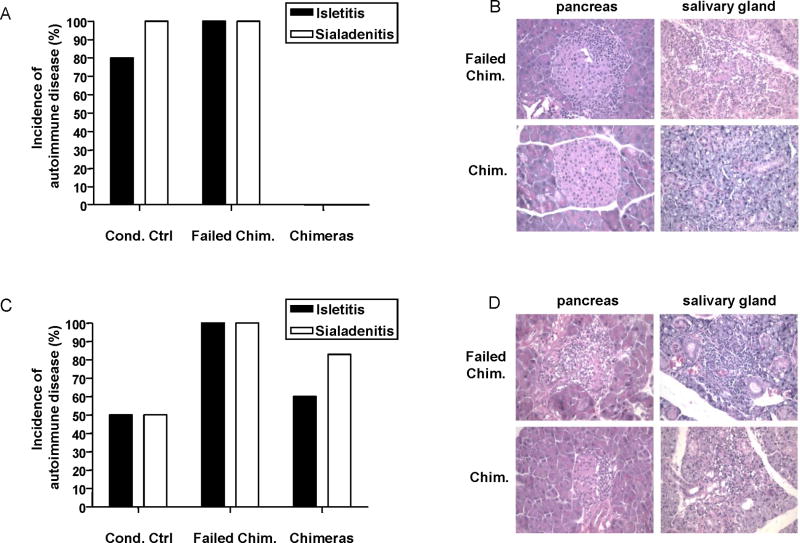
Although conditioning, with or without allogeneic bone marrow transplantation, prevented or markedly delayed the development of diabetes, histological examination of pancreata demonstrated that pre-diabetic NOD mice that received the successful conditioning Regimen F without BMT and failed chimeras (i.e. mice that received B6 BMT, but lost initial chimerism) receiving this regimen (Figure 5A and B) or Regimen H (Figure 5C and D) had islet inflammation and infiltrates at the time of sacrifice 31 weeks after conditioning. In contrast, 6 of 6 pancreata from mixed chimeras generated with Regimen F were free of isletitis (Figure 5A and B). Although analyzed somewhat earlier (15 weeks after BMT), two of two mixed chimeras prepared with regimen G showed an absence of isletitis, when one failed chimera that received this regimen showed significant isletitis (not shown). Interestingly, four of six pancreata from successful mixed chimeras induced with the non-CD4 T cell-depleting regimen (Regimen H) showed islet inflammation and infiltrates when sacrificed 38 weeks after BMT (Figure 5C and D).
Figure 5. B6 mixed chimerism induced in pre-diabetic NOD mice with partially T cell depleting conditioning reverses autoimmunity in both islets and salivary glands. Conditioning alone does not.
A, B) Pancreata and salivary glands of conditioned pre-diabetic NOD mice receiving CD4 partially-depleting conditioning regimens with or without allogeneic BMT, as well as non-treated control mice, were analyzed for the presence of inflammatory infiltrates at 31 weeks after conditioning. C, D) Pancreata and salivary glands of conditioned pre-diabetic NOD mice receiving non-CD4-depleting conditioning regimen with or without allogeneic BMT, as well as non-treated control mice, were analyzed for the presence of inflammatory infiltrates at 38 weeks after conditioning.
NOD mice typically develop sialitis by about 20 weeks of age (34), which may persist when anti-islet autoimmunity is suppressed (35). To assess reversal of sialitis, we examined the salivary glands. Failed chimeras receiving conditioning Regimen F (6 of 6, Figure 5A and B) or H (2 of 2, Figure 5C and D) and the majority of stable chimeras (5 of 6) induced with the non-CD4-depleting regimen (Regimen H, Figure 5C and D) had evidence of autoimmune sialitis (Figure 5A and B). In contrast, 9 of 9 mixed chimeras induced with Regimen F had either no infiltrates or mild residual lymphocytic infiltrates without ductitis (Figure 5A and B). Thus, although conditioning can prevent diabetes, the presence of stable chimerism is required to prevent or reverse autoimmune isletitis and sialitis. Some degree of recipient CD4 depletion is required to achieve tolerance of the pre-exisiting autoimmune cell population, as reversal of autoimmunity is incomplete in chimeras prepared with the that included anti-CD4 regimen.
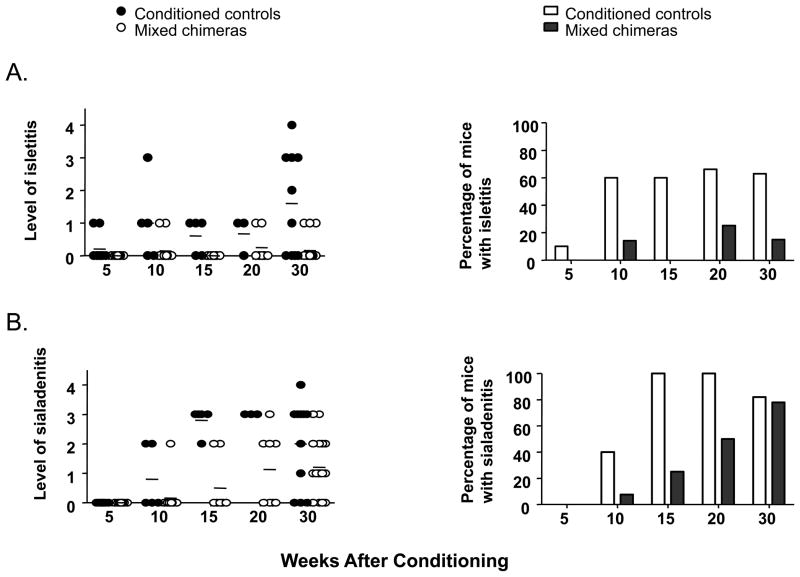
To examine the impact of conditioning and induction of chimerism with Regimen F on the development of autoimmunity in a prospective fashion, a large cohort of mice received conditioning at 6 weeks of age, with or without B6 BMT. Groups of mice were sacrificed at intervals of 5 weeks. An additional cohort was conditioned at 10 weeks of age and sacrificed 30 weeks later. Isletitis was evaluated blinded by a pathologist. As shown in Figure 6A, the incidence of isletitis increased in conditioned controls between 5 and 10 weeks post-treatment and then plateaued at later time points, with 60–70% of animals showing mild isletitis at 10 weeks and thereafter. The severity of isletitis was greater in the 40-week-old conditioned mice than in the cohorts that were treated and sacrificed at earlier ages.
Figure 6. Progression of autoimmunity in NOD mice conditioned with Regimen F, with or without B6 BMT.
Six-week-old NOD mice were conditioned with Regimen F, with or without BMT from B6 donors. At intervals of 5 weeks (5,10,15,20 weeks), 8–10 mice per group were sacrificed and analyzed for isletitis and sialadenitis. An additional cohort of mice received conditioning at 10 weeks of age and was sacrificed for histological analysis 30 weeks later. A shows the level (left) and incidence (right) of isletitis measured using the scale described in (22) and B shows the level (left) and incidence (right) of sialadenitis measured using the following scale: 0, no inflammation; 1, focal inflammation or lymphoid aggregates; 2, mild sialadenitis; 3, moderate sialadenitis; 4, severe sialadenitis.
In marked contrast to the conditioned controls in the studies in Figure 6A, mixed chimeras showed a low incidence (0–25%) of isletitis and it remained mild, even in the few animals with isletitis at 40 weeks of age (Figure 6A). Thus, both the incidence and the severity of isletitis were attenuated by the induction of mixed chimerism in recipients of Regimen F.
We also compared the above cohorts for the presence of sialoadenitis. As shown in Figure 6B, sialoadenitis developed in 40% of conditioned control mice between 5 and 10 weeks after conditioning and increased in severity and incidence (to 100% by 10 weeks) thereafter. The development of sialoadenitis was delayed but not completely prevented in mixed chimeras, which showed a gradual increase in incidence over time, and no difference was apparent between mixed chimeras and conditioned controls in the 40-week-old mice.
Adoptive transfer from B6->NOD mixed chimeric mice prepared with Regimen F to NOD-SCID mice, suggests that reversal of autoimmunity occurs by a suppressive mechanism
We further evaluated the diabetogeneic potential of T cells in chimeric and conditioned control mice using an adoptive transfer approach. 20×106 T cells isolated from spleens or 3–5×106 unfractionated pancreatic lymph node cells of conditioned control mice, failed chimeras, stable chimeras or non-diabetic age-matched controls were transferred to untreated NOD-SCID recipients, which were assessed for autoimmunity. Since similar results were obtained using lymph node cells or splenic T cells, the results are presented together.
Two of four NOD-SCID mice receiving adoptively transferred lymphocytes from pre-diabetic NOD mice (10 weeks old) developed diabetes within 10 weeks. NOD-SCID mice receiving cells from successful chimeras (n=4) or non-chimeras (n=3) conditioned with Regimen F did not develop diabetes by 41 weeks post transfer. Histological examination demonstrated that adoptive transfer of T cells from failed chimeras or conditioned controls receiving Regimen F led to severe infiltrates in islets and destruction of β cells (3 of 3) as well as sialitis in the NOD-SCID recipients (2 of 3). In contrast, adoptive transfer of T cells from mixed chimeras induced with Regimen F did not induce any inflammation in the islets (0 of 4) or salivary glands (0 of 4) of NOD-SCID recipients. In addition, adoptive transfer of cells from mixed chimeras prepared with Regimen F in established diabetic NOD recipients did not induce any autoimmunity in secondary NOD-SCID recipients. The absence of overt diabetes in NOD-scid adoptive recipients of T cells from non-chimeras, despite the clear-cut development of autoimmunity in the islets and salivary glands, may reflect the low level of NOD CD8 T cell reconstitution in conditioned mice, as both NOD CD4 and CD8 T cells have been shown to contribute to diabetes development following adoptive transfer to NOD-scid mice (36). While NOD CD4 levels in peripheral blood had recovered to a mean level of 39.2 ± 9.6 (SD)% by 14 weeks post-treatment, CD8 levels had recovered to only 0.3± 0.1% by the same time point and to 1.5± 0.8% by 16 weeks.
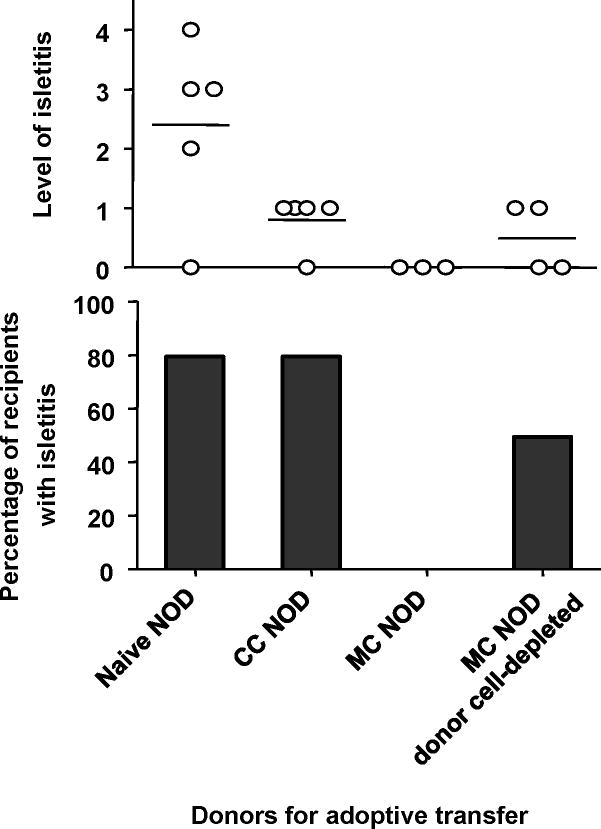
To determine whether or not a requlatory mechanism dependent on donor chimerism might contribute to the suppression of autoimmunity in mixed chimeric NOD mice receiving Regimen F, we performed additional adoptive transfer studies in which donor cells were or were not depleted from the transferred inoculum. As shown in Figure 7, adoptive transfer of splenic T cells from 12-week-old? naive NOD mice induced severe isletitis in 4 of 5 NOD-scid recipients within 6 weeks of transfer. Transfer of splenic T cells (almost all CD4+) from conditioned control mice led to milder isletitis in 4 of 5 adoptive recipients, whereas transfer of 4 times as many splenic T cells from mixed chimeras, which were 75% donor-derived, did not induce measurable isletitis in any of 3 adoptive recipients. However, when the donor cells were depleted from these inocula, 2 of 4 adoptive recipients developed isletitis within 6 weeks. These studies suggest that donor cells suppressed the ability of NOD T cells in mixed chimeras to induce isletitis. None of the groups of adoptive recipients developed marked sialadenitis within the 6-week period following T cell transfer.
Figure 7. Donor cells suppress autoimmunity in mixed chimeras prepared with Regimen F.
Six-week-old NOD mice were conditioned with Regimen F, with or without BMT from B6 donors. Six weeks later the animals were euthanized and T cells were purified from splenocytes. Two million purified splenic T cells from naive NOD mice or conditioned control (CC NOD) mice were adoptively transferred to NOD-scid recipients. Two million NOD T cells from mixed chimeras were transferred to additional groups with (Mixed Chimerism (MC) NOD donor cell-depleted) or without (in 8 million total T cells with approximately 75% donor-type T cell chimerism at the time of sacrifice) (MC NOD) depletion of donor cells. Almost all transferred T cells from conditioned NOD mice were CD4+ and CD8−.
DISCUSSION
We demonstrate here that establishment of bone marrow chimerism with non-myeloablative conditioning including partial CD4 T cell depletion reversed autoimmunity and achieved skin graft tolerance, despite a significant contribution to T cell reconstitution by the NOD recipients, especially in the first 3 months following BMT. Conditioning alone (without BMT) or achievement of durable mixed chimerism without CD4 depletion did not reverse or prevent autoimmunity, even though skin graft tolerance was achieved. Therefore, mixed bone marrow chimerism and some degree of pre-existing recipient CD4 depletion are both required to reverse autoimmunity and alloantigen tolerance can be achieved across extensive MHC barriers without reversal of autoimmunity in NOD mice. Previous studies have indicated that the lack of certain diabetes-susceptability alleles can prevent autoimmunity without overcoming the resistance of NOD mice to allograft prolongation by costimulation blockade, suggesting that alloresponses are more difficult to overcome than autoimmunity (30,31). Our data suggest, in contrast, that the requirements for overcoming CD4-mediated autoimmunity are greater than those for overcoming resistance to allograft tolerance with BMT and costimulatory blockade. Nevertheless, when recipient CD4 cells are partially depleted with anti-CD4 mAb, donor cells actively suppress the autoimmune potential of the remaining NOD CD4 cells, as demonstrated in adoptive transfer studies.
Allogeneic BMT following myeloablative TBI is known to prevent diabetes in pre-diabetic NOD mice (12,13, 37). Full donor chimerism achieved with costimulatory blockade and sublethal TBI also prevents diabetes (14). Using CD8 depletion and anti-CD154 in 6 Gy irradiated NOD mice, durable mixed chimerism, prevention of diabetes and reduced isletitis were achieved, with improved immunocompetence (38). In another study, anti-CD8 and anti-CD154 mAbs synergistically enhanced engraftment of allogeneic bone marrow with relatively high levels of irradiation (>6.5Gy) (39). In contrast to our results, preconditioning with anti-CD4 mAb impaired engraftment, resulting in a requirement for increased TBI in order to achieve engraftment (39).
We evaluated a number of non-myeloablative conditioning regimens, using lower TBI doses than those discussed above, followed by transplantation of allogeneic BMC in efforts to achieve sustained mixed hematopoietic chimerism across MHC barriers in NOD mice. Most conditioning regimens led only to initial chimerism, which gradually disappeared. Consistent with previous reports (29–33), we observed increased resistance to irradiation and mAb depletion of NOD T cells compared to those in a non-autoimmune strain. We ultimately established a non-myeloablative regimen permitting mixed chimerism induction that includes low-dose (4 Gy) TBI, anti-CD154, anti-CD4, anti-CD8 α, and anti-Thy1.2 mAbs. Durable mixed chimerism, allograft tolerance and reversal of autoimmunity occurred despite a significant contribution to CD4 T cell reconstitution by the NOD hosts. This regimen is also effective in established diabetic NOD mice, allowing cure of diabetes by transplantation of donor-type or NOD-SCID islets (4). Our studies showed clearly that both recipient CD8 depletion was essential to the achievement of durable mixed chimerism in our regimen, as regimens that omitted this component (Regimen B–D) failed to achieve durable chimerism. Anti-Thy1.2 mAb did not appear to play an essential role, as its omission (Regimen G) was not associated with a reduction in the incidence of chimerism or skin graft tolerance. Since donor CD8 cells were depleted with anti-CD8α.2 in our regimen, donor CD8 cells and facilitating cells (40) do not seem to be required to achieve durable chimerism.
CD4+ T cells are rapidly tolerized to donor antigens, allowing durable mixed chimerism and donor-specific tolerance in CD8-depleted non-autoimmune mice receiving 3 Gy TBI and anti-CD154 (25). Conditioning with low dose TBI (4 Gy) with anti-CD154 and CD8+ T cell depletion, without CD4+ T cell depletion, also permitted durable mixed chimerism induction in about 50% of NOD mice. Mixed chimerism was associated with donor-specific skin graft tolerance, indicating that anti-CD154 and BMT could successfully tolerize pre-existing alloreactive CD4 cells in the NOD strain. However, reversal of autoimmunity was incomplete in these chimeras, suggesting that partial recipient CD4 depletion is required to achieve tolerance of the pre-existing autoimmune cell population. Thus, the requirements for overcoming autoimmunity via mixed chimerism induction are distinct from and more stringent than those required to overcome strong alloimmunity. The higher level of autoimmunity detected in failed chimeras compared to conditioned controls receiving this regimen suggests that failure of chimerism may be a marker for stronger immune responses, including those leading to autoimmunity.
Chimerism without reversal of autoimmunity is consistent with other results. Conditioning with anti-lymphocyte serum, fludarabine, cyclophosphamide and rapamycin allowed MHC-matched marrow engraftment and acceptance of MHC-matched NOR islet grafts in NOD mice (15), but mononuclear cell infiltrates surrounded the donor islet grafts (15). Overtly diabetic nonobese resistant (NOR) mice were cured by lethal irradiation plus anti-CD4 and anti-NK cell antibodies followed by cotransplantation of allogeneic hematopoietic stem cells and donor islets, whereas mixed chimerism achieved with sublethal irradiation did not allow long-term donor islet graft survival (41). While BMT with cyclophosphamide, anti-CD154 mAb and rapamycin permitted islet allograft acceptance in chemically-induced diabetic B6 mice, similar chimerism did not protect islet allografts in diabetic NOD mice (42). Post-transplant donor lymphocyte infusions to low-level mixed chimeras led to increased chimerism, induced islet allograft tolerance and reversed autoimmunity (43). However, this approach would be associated with significant GVHD risk in humans.
In our experiments, BMT with non-myeloablative conditioning reversed both allo- and auto- immunity in NOD mice, despite the persistence of host CD4+ T cells following conditioning. Thus, NOD CD4+ T cells were tolerized to both allo- and autoantigens with an anti-CD154-based allogeneic BMT regimen. Peripheral alloreactive CD4+ T cells are specifically deleted in other mouse strains receiving allogeneic BMT with anti-CD154, and newly developing donor-reactive thymocytes are then centrally deleted (44). Similar mechanisms may explain the development of tolerance to alloantigens in CD4+ T cells of mixed chimeric NOD mice. While the central intrathymic deletion of newly developing T cells could prevent diabetes in pre-diabetic NOD mice, such deletion does not readily explain the reversal of pre-existing autoimmunity in diabetic NOD mice receiving BMT with this incompletely T cell-depleting regimen. Our studies suggest that donor cell populations suppress the diabetogenic potential of pre-existing residual host T cells in the NOD mice. CD4+CD25+ regulatory T cells have been implicated in the maintenance of peripheral tolerance to self-antigens and deficiency of regulatory cells and/or dendritic cells required for their activation has been implicated in the pathogenesis of type I diabetes in NOD mice (45). Mixed chimerism from diabetes-resistant donors could allow for the development of normal dendritic cells and regulatory cells that could dominantly inhibit pre-existing autoimmunity. Mixed chimerism thereby has the potential to correct other autoimmune syndromes associated with hematopoietic cell-intrinsic defects in the ability to generate and or activate regulatory cells.
In summary, non-myeloablative conditioning with low-dose TBI, anti-CD154, anti-CD8 α, anti-CD4 and anti-Thy1.2 mAbs allowed durable mixed chimerism and allograft tolerance to be achieved across extensive MHC barriers in NOD mice. Reversal of autoimmunity occurred despite a significant contribution to CD4 T cell reconstitution by pre-existing T cells in the NOD hosts. Recipient CD8 cell depletion and anti-CD154, but not anti-Thy1.2, are critical to the achievement of durable mixed chimerism and skin allograft tolerance in this regimen. Recipient CD4 depletion was not required to induce tolerance towards alloantigens, but partial CD4 depletion was essential for reversal of autoimmunity.
Acknowledgments
This work was supported in part by the Juvenile Diabetes Foundation Center for Islet Transplantation at Harvard Medical School, by the JDRF Center on Immunological Tolerance in Type 1 Diabetes, by the NIH/NHLBI R01 HL049915, by the NIH/NIDDK R03 DK067940, by The Patricia Welder Robinson Young Investigator Grant of the National Kidney Foundation (BN) and by a Post-Doctoral Fellowship for study abroad from the Uehara Memorial Foundation (TO). We thank Ms. Kelly Walsh and Ms. Gena Coleman for assistance in preparing the manuscript. We also thank Drs. Yong-Guang Yang and Thomas Fehr for helpful review of the manuscript and Ms. Guiling Zhao for technical assistance.
ABBREVIATIONS
- BMC
bone marrow cells
- BMT
bone marrow transplantation
- FCM
flow cytometry
- GVHD
graft-versus-host disease
- MAb
monoclonal antibody
- MHC
major histocompatibility complex
- NOD
Non-obese diabetic
- PBL
peripheral blood lymphocytes
- PEA
phycoerythrin-streptavidin
- PPHSC
pluripotent hematopoietic stem cells
- TCD
T cell depletion
- TCR
T cell receptor
- TI
thymic irradiation
- WBC
white blood cells
- TBI
total body irradiation
REFERENCE LIST
- 1.Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JR, Shapiro AM. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060–2069. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 2.Rother KI, Harlan DM. Challenges facing islet transplantation for the treatment of type 1 diabetes mellitus. J Clin Invest. 2004;114:877–883. doi: 10.1172/JCI23235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sykes M, Nikolic B. Treatment of severe autoimmune disease by stem-cell transplantation. Nature. 2005;435:620–627. doi: 10.1038/nature03728. [DOI] [PubMed] [Google Scholar]
- 4.Nikolic B, Takeuchi Y, Leykin I, Fudaba Y, Smith RN, Sykes M. Mixed hematopoietic chimerism allows cure of autoimmune diabetes through allogeneic tolerance and reversal of autoimmunity. Diabetes. 2004;53:376–383. doi: 10.2337/diabetes.53.2.376. [DOI] [PubMed] [Google Scholar]
- 5.Saliba RM, de Lima M, Giralt S, Andersson B, Khouri IF, Hosing C, Ghosh S, Neumann J, Hsu Y, De Jesus J, Qazilbash MH, Champlin RE, Couriel DR. Hyper-acute GVHD: risk factors, outcomes, and clinical implications. Blood. 2007;109:2751–2758. doi: 10.1182/blood-2006-07-034348. [DOI] [PubMed] [Google Scholar]
- 6.Sykes M. Mixed chimerism and transplant tolerance. Immunity. 2001;14:417–424. doi: 10.1016/s1074-7613(01)00122-4. [DOI] [PubMed] [Google Scholar]
- 7.Sharabi Y, Sachs DH. Mixed chimerism and permanent specific transplantation tolerance induced by a non-lethal preparative regimen. J Exp Med. 1989;169:493–502. doi: 10.1084/jem.169.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang CA, Fuchimoto Y, Scheier-Dolberg R, Murphy MC, Neville DM, Jr, Sachs DH. Stable mixed chimerism and tolerance using a nonmyeloablative preparative regimen in a large-animal model. J Clin Invest. 2000;105:1779–1789. doi: 10.1172/JCI7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kimikawa M, Sachs DH, Colvin RB, Bartholomew A, Kawai T, Cosimi AB. Modifications of the conditioning regimen for achieving mixed chimerism and donor-specific tolerance in cynomolgus monkeys. Transplantation. 1997;64:709–716. doi: 10.1097/00007890-199709150-00008. [DOI] [PubMed] [Google Scholar]
- 10.Fudaba Y, Spitzer TR, Shaffer JM, Kawai T, Fehr T, Delmonico FL, Preffer FI, Tolkoff-Rubin, Dey BR, Saidman SL, Kraus A, Bonnefoix T, McAfee S, Power K, Kattelman K, Colvin RB, Sachs DH, Cosimi AB, Sykes M. Myeloma responses and tolerance following combined kidney and non-myeloablative marrow transplantation in vivo and in vitro analyses. Am J Transplant. 2006;6:2121–2133. doi: 10.1111/j.1600-6143.2006.01434.x. [DOI] [PubMed] [Google Scholar]
- 11.Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman S, Shaffer J, Preffer F, Ding R, Sharma V, Fishman J, Dey BR, Ko D, Hertl M, Goes N, Wong W, Williams W, Colvin RB, Sykes M, Sachs DH. HLA-mismatched renal transplantation without maintenance immunosupression. New Engl J Med. 2008;358(4):353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ikehara S, Ohtsuki H, Good RA, Asamoto H, Nakamura T, Sekita K, Muso E, Tochino Y, Ida T, Kuzuya H, et al. Prevention of type I diabetes in nonobese diabetic mice by allogeneic bone marrow transplantation. Proc Natl Acad Sci USA. 1985;82:7743–7747. doi: 10.1073/pnas.82.22.7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LaFace DW, Peck AB. Reciprocal allogeneic bone marrow transplantation between NOD mice and diabetes-nonsusceptible mice associated with transfer and prevention of autoimmune diabetes. Diabetes. 1989;38:894–899. doi: 10.2337/diab.38.7.894. [DOI] [PubMed] [Google Scholar]
- 14.Seung E, Iwakoshi N, Woda BA, Markees TG, Mordes JP, Rossini AA, Greiner DL. Allogeneic hematopoietic chimerism in mice treated with sublethal myeloablation and anti-CD154 antibody: absence of graft-versus-host disease, induction of skin allograft tolerance, and prevention of recurrent autoimmunity in islet-allografted NOD/Lt mice. Blood. 2000;95:2175–2182. [PubMed] [Google Scholar]
- 15.Wu T, Levay-Young B, Heuss N, Sozen H, Kirchhof N, Sutherland DE, Hering B, Guo Z. Inducing tolerance to MHC-matched allogeneic islet grafts in diabetic NOD mice by simultaneous islet and bone marrow transplantation under nonirradiative and nonmyeloablative conditioning therapy. Transplantation. 2002;74:22–27. doi: 10.1097/00007890-200207150-00005. [DOI] [PubMed] [Google Scholar]
- 16.Dialynas DP, Quan ZS, Wall KA, Pierres A, Quintans J, Loken MR, Pierres M, Fitch FW. Characterization of murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to human Leu3/T4 molecule. J Immunol. 1983;131:2445–2451. [PubMed] [Google Scholar]
- 17.Ledbetter JA, Herzenberg LA. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunologic Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- 18.Noelle RJ, Roy M, Shepherd DM, Stamenkovic I, Ledbetter JA, Aruffo A. A 39-kDa protein on activated helper T cells binds CD40 and transduces the signal for cognate activation of B cells. Proc Natl Acad Sci USA. 1992;89:6550–6554. doi: 10.1073/pnas.89.14.6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sykes M, Chester CH, Sundt TM, Romick ML, Hoyles KA, Sachs DH. Effects of T cell-depletion in radiation bone marrow chimeras. III. Characterization of allogeneic bone marrow cell populations which increase allogeneic chimerism independently of GVHD in mixed marrow recipients. J Immunol. 1989;143:3503–3511. [PubMed] [Google Scholar]
- 20.Tomita Y, Sachs DH, Khan A, Sykes M. Additional mAb injections can replace thymic irradiation to allow induction of mixed chimerism and tolerance in mice receiving bone marrow transplantation after conditioning with anti-T cell mAbs and 3 Gy whole body irradiation. Transplantation. 1996;61:469–477. doi: 10.1097/00007890-199602150-00027. [DOI] [PubMed] [Google Scholar]
- 21.Jaeckel E, Klein L, Martin-Orozco N, von Boehmer H. Normal incidence of diabetes in NOD mice tolerant to glutamic acid decarboxylase. J Exp Med. 2003;197(12):1635–1644. doi: 10.1084/jem.20030215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoon JW, Yoon CS, Lim HW, Huang QQ, Kang Y, Pyun KH, Hirasawa K, Sherwin RS, Jun HS. Control of autoimmune diabetes in NOD mice by GAD expression or suppression in beta cells. Science. 1999;284 (5417):1183–1187. doi: 10.1126/science.284.5417.1183. [DOI] [PubMed] [Google Scholar]
- 23.Nikolic B, Zhao G, Swenson K, Sykes M. A novel application of cyclosporine A in nonmyeloablative pretransplant host conditioning for allogeneic BMT. Blood. 2000;96:1166–1172. [PubMed] [Google Scholar]
- 24.Wekerle T, Sayegh MH, Ito H, Hill J, Chandraker A, Pearson DA, Swenson KG, Zhao G, Sykes M. Anti-CD154 or CTLA4Ig obviates the need for thymic irradiation in a non-myeloablative conditioning regimen for the induction of mixed hematopoietic chimerism and tolerance. Transplantation. 1999;68:1348–1355. doi: 10.1097/00007890-199911150-00022. [DOI] [PubMed] [Google Scholar]
- 25.Wekerle T, Sayegh MH, Hill J, Zhao Y, Chandraker A, Swenson KG, Zhao G, Sykes M. Extrathymic T cell deletion and allogeneic stem cell engraftment induced with costimulatory blockade is followed by central T cell tolerance. J Exp Med. 1998;187:2037–2044. doi: 10.1084/jem.187.12.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wekerle T, Kurtz J, Ito H, Ronquillo JV, Dong V, Zhao G, Shaffer J, Sayegh M, Sykes M. Allogeneic bone marrow transplantation with co-stimulatory blockade induces macrochimerism and tolerance without cytoreductive host treatment. Nature Med. 2000;6:464–469. doi: 10.1038/74731. [DOI] [PubMed] [Google Scholar]
- 27.Ito H, Takeuchi Y, Shaffer J, Sykes M. Anti-CD40L monoclonal antibodies can replace anti-CD4 monoclonal antibodies for the nonmyeloablative induction of mixed xenogeneic chimerism. Transplantation. 2006;82:251–257. doi: 10.1097/01.tp.0000226147.69877.6f. [DOI] [PubMed] [Google Scholar]
- 28.Takeuchi Y, Ito H, Kurtz J, Wekerle T, Ho L, Sykes M. Earlier low-dose TBI or DST overcomes CD8+ T-cell-mediated alloresistance to allogeneic marrow in recipients of anti-CD40L. Am J Transplant. 2004;4:31–40. doi: 10.1046/j.1600-6135.2003.00272.x. [DOI] [PubMed] [Google Scholar]
- 29.Markees TG, Serreze DV, Phillips NE, Sorli CH, Gordon EJ, Shultz LD, Noelle RJ, Woda BA, Greiner DL, Mordes JP, Rossini AA. NOD mice have a generalized defect in their response to transplantation tolerance induction. Diabetes. 1999;48:967–974. doi: 10.2337/diabetes.48.5.967. [DOI] [PubMed] [Google Scholar]
- 30.Pearson T, Markees TG, Serreze DV, Pierce MA, Marron MP, Wicker LS, Peterson LB, Shultz LD, Mordes JP, Rossini AA, Greiner DL. Genetic disassociation of autoimmunity and resistance to costimulation blockade-induced transplantation tolerance in nonobese diabetic mice. J Immunol. 2003;171:185–195. doi: 10.4049/jimmunol.171.1.185. [DOI] [PubMed] [Google Scholar]
- 31.Pearson T, Markees TG, Wicker LS, Serreze DV, Peterson LB, Mordes JP, Rossini AA, Greiner DL. NOD congenic mice genetically protected from autoimmune diabetes remain resistant to transplantation tolerance induction. Diabetes. 2003;52:321–326. doi: 10.2337/diabetes.52.2.321. [DOI] [PubMed] [Google Scholar]
- 32.Mottram PL, Murray-Segal LJ, Han W, Maguire J, Stein-Oakley A, Mandel TE. Long-term survival of segmental pancreas isografts in NOD/Lt mice treated with anti-CD4 and anti-CD8 monoclonal antibodies. Diabetes. 1998;47:1399–1405. doi: 10.2337/diabetes.47.9.1399. [DOI] [PubMed] [Google Scholar]
- 33.Kaufman CL, Li H, Ildstad ST. Patterns of hemopoietic reconstitution in nonobese diabetic mice: dichotomy of allogeneic resistance versus competitive advantage of disease-resistant marrow. J Immunol. 1997;158:2435–2442. [PubMed] [Google Scholar]
- 34.Boulard O, Fluteau G, Eloy L, Damotte D, Bedossa P, Garchon HJ. Genetic analysis of autoimmune sialadenitis in nonobese diabetic mice: a major susceptibility region on chromosome 1. J Immunol. 2002;168:4192–4201. doi: 10.4049/jimmunol.168.8.4192. [DOI] [PubMed] [Google Scholar]
- 35.Gerling IC, Serreze DV, Christianson SW, Leiter EH. Intrathymic islet cell trnsplantation reduces beta-cell autoimmunity and prevents diabetes in NOD/Lt mice. Diabetes. 1992;41:1672–1676. doi: 10.2337/diab.41.12.1672. [DOI] [PubMed] [Google Scholar]
- 36.Yoon JW, Jun HS. Cellular and molecular roles of beta cell autoantigens, macrophages and T cells in the pathogenesis of autoimmune diabetes. Arch Pharm Res. 1999;22(5):437–447. doi: 10.1007/BF02979150. [DOI] [PubMed] [Google Scholar]
- 37.Mathieu C, Casteels K, Bouillon R, Waer M. Protection against autoimmune diabetes in mixed bone marrow chimeras. J Immunol. 1997;158:1453–1457. [PubMed] [Google Scholar]
- 38.Serreze DV, Osborne MA, Chen YG, Chapman HD, Pearson T, Brehm MA, Greiner DL. Partial versus full allogeneic hemopoietic chimerization is a preferential means to inhibit type 1 diabetes as the latter induces generalized immunosuppression. J Immunol. 2006;177:6675–6684. doi: 10.4049/jimmunol.177.10.6675. [DOI] [PubMed] [Google Scholar]
- 39.Ildstad ST, Chilton PM, Xu H, Domenick MA, Ray MB. Preconditioning of NOD mice with anti-CD8 mAb and costimulatory blockade enhances chimerism and tolerance and prevents diabetes, while depletion of alpha beta-TCR+ and CD4+ cells negates the effect. Blood. 2005;105:2577–2584. doi: 10.1182/blood-2004-04-1340. [DOI] [PubMed] [Google Scholar]
- 40.Fugier-Vivier J, Rezzoug F, Huang Y, Graul-Layman AJ, Schanie CL, Xu H, Chilton PM, Ildstad ST. Plasmacytoid precursor dendritic cells facilitate allogeneic hematopoietic stem cell engraftment. J Exp Med. 2005;201 (3):373–383. doi: 10.1084/jem.20041399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beilhack GF, Scheffold YC, Weissman IL, Taylor C, Jerabek L, Burge MJ, Masek MA, Shizuru JA. Purified allogeneic hematopoietic stem cell transplantation blocks diabetes pathogenesis in NOD mice. Diabetes. 2003;52:59–68. doi: 10.2337/diabetes.52.1.59. [DOI] [PubMed] [Google Scholar]
- 42.Guo Z, Wu T, Sozen H, Pan Y, Heuss N, Kalscheuer H, Sutherland DE, Blazar BR, Hering BJ. A substantial level of donor hematopoietic chimerism is required to protect donor-specific islet grafts in diabetic NOD mice. Transplantation. 2003;75:909–915. doi: 10.1097/01.TP.0000057832.92231.F5. [DOI] [PubMed] [Google Scholar]
- 43.Liu B, Hao J, Pan Y, Luo B, Westgard B, Heremans Y, Sutherland DE, Hering BJ, Guo Z. Increasing donor chimerism and inducing tolerance to islet allografts by post-transplant donor lymphocyte infusion. Am J Transplant. 2006;6:933–946. doi: 10.1111/j.1600-6143.2006.01283.x. [DOI] [PubMed] [Google Scholar]
- 44.Kurtz J, Shaffer J, Lie A, Anosova N, Benichou G, Sykes M. Mechanisms of early peripheral CD4 T-cell tolerance induction by anti-CD154 monoclonal antibody and allogeneic bone marrow transplantation: evidence for anergy and deletion but not regulatory cells. Blood. 2004;103:4336–4343. doi: 10.1182/blood-2003-08-2642. [DOI] [PubMed] [Google Scholar]
- 45.Kukreja A, Cost G, Marker J, Zhang C, Sun Z, Lin-Su K, Ten S, Sanz M, Exley M, Wilson B, Porcelli S, Maclaren N. Multiple immuno-regulatory defects in type-1 diabetes. J Clin Invest. 2002;109:131–140. doi: 10.1172/JCI13605. [DOI] [PMC free article] [PubMed] [Google Scholar]