Abstract
Cell cycle controls ensure that DNA replication (S phase) follows mitosis resulting in two precise copies of the genome. A failure of the control mechanisms can result in multiple rounds of DNA replication without cell division. In endoreplication, cells with replicated genomes bypass mitosis, then replicate their DNA again, resulting in polyploidy. Endoreplication from G2 phase lacks all hallmarks of mitosis. Using synchronized cells, we show that the c-Jun N-terminal kinase (JNK) inhibitor, SP600125, prevents the entry of cells into mitosis and leads to endoreplication of DNA from G2 phase. We show that cells proceed from G2 phase to replicate their DNA in the absence of mitosis. This effect of SP600125 is independent of its suppression of JNK activity. Instead, the inhibitory effect of SP600125 on mitotic entry predominantly occurs upstream of Aurora A kinase and Polo-like kinase 1, resulting in a failure to remove the inhibitory phosphorylation of Cdk1. Importantly, our results directly show that the inhibition of Cdk1 activity and the persistence of Cdk2 activity in G2 cells induces endoreplication without mitosis. Furthermore, endoreplication from G2 phase is independent of p53 control.
Keywords: Cdk1 activation, endoreplication, G2/M progression, JNK, polyploidy, SP600125
Introduction
Maintenance of genome ploidy is a fundamental aspect of cell division. Control mechanisms ensure that cells accomplish this goal by replicating their DNA only once per cycle and only when preceded by mitosis. Polyploidy in mammalian cells can either arise from continuous reinitiation of DNA replication within the S phase (re-replication) (Arias and Walter, 2007), or by endoreplication in which cells exit S phase, bypass mitosis, and double their DNA content again. Endoreplication from G2 phase lacks all hallmarks of mitosis such as nuclear envelope breakdown or chromosome condensation (Edgar and Orr-Weaver, 2001). Although polyploidy arising from failure of cells in mitosis is often included in the broader definition of endoreplication, the mechanisms that render cells polyploid after failure of mitosis are different (Storchova and Pellman, 2004). In this case, cells enter mitosis but fail to execute mitosis properly, resulting in subsequent entry into interphase with a doubled DNA content, which doubles again in the next S phase. With the exception of developmentally regulated polyploidy in mammalian systems, polyploidy arising in other cells leads to genomic instability (Margolis et al., 2003; Storchova and Pellman, 2004).
Several studies report DNA polyploidy on inhibition of Cdk1 (Itzhaki et al., 1997; Niculescu et al., 1998; Damiens et al., 2001; Laronne et al., 2003; Cai et al., 2006; L’Italien et al., 2006; Vassilev et al., 2006; Hochegger et al., 2007). Although previous studies has established that Cdk1 inhibition in mitosis leads to polyploidy as a result of mitotic failure (Damiens et al., 2001; Hochegger et al., 2007; Vassilev et al., 2006), it remained to be unequivocally established whether Cdk1 inhibition can lead to endoreplication from G2 phase.
We present here explicit evidence that endoreplication in human cells can occur from G2 phase when Cdk1 is inhibited. Further, we find that endoreplication directly from the G2 phase requires Cdk2 activity. Interestingly, the c-Jun N-terminal kinase (JNK) inhibitor, SP600125, prevents G2 to M phase transition leading to DNA endoreplication directly from the G2 phase, generating polyploid cells with 8N DNA content. The effect of SP600125 is independent of its suppression of JNK activity. Instead, SP600125 indirectly suppresses the activation of Cdk1.
Results
SP600125 blocks the progression of G2 cells into mitosis
To study the role of mitogen-activated protein kinase in the G2 phase to mitosis transition, HCT116 cells with wild-type p53 were synchronized at the G1/S phase boundary with thymidine and were released. After 1 h, nocodazole was added to better analyze cells for G2 to M progression (Supplementary Figure 1). The integrity of the microtubule cytoskeleton is required for mitosis but not for interphase progression (Hamilton and Snyder, 1982; Uetake and Sluder, 2007).
We used SB202190, a selective p38 inhibitor (Lee et al., 1994); U0126, a selective inhibitor of MEK1/2, the upstream activators of ERK1/ERK2 (Duncia et al., 1998); and SP600125, a selective ATP competitive inhibitor of JNK (Bennett et al., 2001; Han et al., 2001). The inhibitors were each added to cells at 1 h after release. The inhibitors effectively inactivated their known target kinases (Figure 1a), although the protein levels of JNK1/2, p38 and ERK1/2 remained unchanged during the time of treatment (data not shown, see Figure 5a and Supplementary Figure 2).
Figure 1.
Effect of MAPK inhibitors on G2 to M phase progression. (a) Verification of the effect of MAPK inhibitors. HCT116 cells released from thymidine (Thy) synchrony were treated with nocodazole (NOC) and 40 μM MEK1/2 inhibitor U0126 (U0), 10 μM p38 inhibitor SB202190 (SB), 20 μM JNK inhibitor SP600125 (SP) at 1 h after release from thymidine treatment, and dimethyl sulfoxide was added to the cells that were not treated with inhibitor. Nocodazole was added at 1 h after release. The concentrations of SB202190 (Bulavin et al., 1999; Jin et al., 2002), U0126 (Roberts et al., 2002) and SP600125 (Mingo-Sion et al., 2004) chosen for treatment of cells were based on previous studies and our preliminary experiments. The activities of ERK, p38 and JNK were determined by immunoblotting cell extracts, obtained before release (Thy) and at indicated times after release from thymidine synchrony, with antibodies recognizing phosphorylated forms of ERK1/ERK2 (Thr202 and Tyr204 in Erk1, Thr185 and Tyr187 in Erk2; substrates of MEK1/2), c-Jun (Ser63; substrate of JNK) and MAPKAP2 (substrate of p38). The slow mobility form of MAPKAP2 results from phosphorylation by p38, giving two bands. Vinculin is a loading control. (b) Cell cycle profile of HCT116 cells released from thymidine synchrony and treated with nocodazole and either 40 μM U0126 (MEK1/2 inhibitor), 10 μM SB202190 (p38 inhibitor) or 20 μM SP600125 (JNK inhibitor) at 1 h after release from thymidine block. Nocodazole was also added at 1 h after release. Cells collected before release (Thy) and at the indicated times after release from thymidine synchrony were stained with propidium iodide (PI) and with MPM2 antibody and examined by flow cytometry. Only PI data are shown. 2N indicates G1 DNA content. (c) SP600125 suppresses entry into mitosis. The percentage of 4N population staining for MPM2 (Supplementary Figure 3) from the experiment in (b) is shown. SP600125 suppresses progression of cells from G2 phase to mitosis as indicated by the lack of MPM2 staining in cells with 4N DNA content. Cells treated with SB202190 (p38 inhibitor) or U0126 (MEK1/2 inhibitor) entered mitosis almost at the same time as control cells. (d) Quantitation of the percentage of MPM2 staining cells from three different experiments carried out as in (b) including results shown in (b). Error bars represent s.d. (e) SP600125 suppresses Ser10 phosphorylation of Histone H3. Western blot analysis of extracts from cells treated with MAPK inhibitors, before release (Thy) and at the indicated times after release from thymidine synchrony, with antibodies recognizing the phosphorylated Ser10 in histone H3. The extracts were prepared from the experiment shown in (b). Only SP600125 suppresses Ser10 phosphorylation of histone H3.
Figure 5.
Effect of JNK1/2 downregulation on progression of G2 cells into mitosis and on endoreplication. (a) JNK1/2 downregulation by specific siRNAs. HCT116 cells transfected with a combination of JNK1- and JNK2-specific siRNAs (two siRNAs each for JNK1 and JNK2) or control siRNA for 48 h were synchronized with nocodazole for 6 h, released for 3 h and then synchronized with thymidine. Nocodazole (NOC) was added at 1 h after release. Extracts obtained from cells at the indicated times after release from thymidine synchrony were immunoblotted with antibodies recognizing JNK1/2 and phosphohistone H3. Similarly, immunoblotted extracts from control siRNA-transfected cells treated with 20 μM SP600125 (SP) at 4 h after release from thymidine block is shown as a control. (b) Progression of cells into mitosis after JNK1/2 downregulation. Cells from (a) were harvested at the indicated times, stained with PI and MPM2 and examined by flow cytometry. Numbers in DNA content plots are percentage of population that is 8N. Numbers in MPM2 dot plots are percentage of the 4N population that is in mitosis. Cells transfected with control siRNA and treated with SP600125 exhibited both a significant suppression of mitotic entry and an increase in endoreplication. (c) Downregulation of JNK1/2 by specific siRNAs suppresses JNK activity. Extracts obtained from HCT116 cells transfected with a combination of JNK1- and JNK2-specific siRNA or control siRNA, synchronized with thymidine and released as in (a) were immunoblotted with antibodies recognizing JNK1/2 and its phospho-c-Jun substrate. (d) SP600125 treatment of downregulated JNK1/2 cells results in the generation of 8N population. HCT116 cells transfected with a combination of JNK1- and JNK2-specific siRNA or control siRNA as in (a) were synchronized with thymidine. Cells were treated with 20 μM SP600125 at 4 h after release from thymidine block. Nocodazole was added at 1 h after release. Cells harvested at 48 h were stained with PI and examined by flow cytometry. Numbers in DNA content plots are percentage of population that is 8N.
For further analysis, cells treated with SB202190, U0126 or SP600125 were monitored at close time intervals by two-dimensional flow cytometry staining for both DNA content and for mitosis-specific phosphoepitope MPM2 (Davis et al., 1983) to distinguish 4N cells that were in mitosis from those that were still in G2 phase. At 6 h after release from thymidine, the majority of cells had progressed to G2 phase as evidenced by 4N DNA content (Figure 1b) and the lack of MPM2 staining (Figures 1c and d, Supplementary Figure 3), with few, if any, cells in the G1/S phase. By 8–10 h, control cells were entering mitosis as indicated by MPM2 staining. Cells treated with SB202190 (p38 inhibitor) and U0126 (MEK1/2 inhibitor) entered mitosis almost at the same time as control cells (Figures 1c and d). In contrast, only 10% of SP600125-treated cells stained positive for MPM2 (Figures 1c and d, 12 h). Thus, unlike SB202190 and U0126, exposure to SP600125 substantially suppresses mitotic entry. Phospho-c-Jun indicates that JNK is active in control cells released from thymidine but is inactive when cells are exposed to SP600125 (Figure 1a).
Aurora kinase B-dependent Ser10-histone H3 phosphorylation normally occurs on entry of cells into mitosis (Crosio et al., 2002), and phosphorylated H3 has been used as a specific mitotic marker (Hendzel et al., 1997). Consistent with MPM2 results, the levels of phosphorylated histone-H3 were high at 10 h after thymidine release in control HCT116 cells, but SP600125 completely prevented this phosphorylation (Figure 1e). In contrast to the result with SP600125, histone H3 phosphorylation in the presence of p38 and mitogen-activated protein kinase inhibitors, SB202190 and U0126, respectively, was similar to control cells (Figure 1e).
Cells released from thymidine synchronization were then followed for nuclear envelope breakdown, a marker of prometaphase entry (Georgatos et al., 1997). Immunofluorescent staining of nuclear envelope with lamin B1 indicated that ∼80–90% of control cells lacked lamin B1 staining at 12 h after thymidine release (Figure 2a); a result consistent with entry into mitosis as indicated by flow cytometry MPM2 staining (Figure 2b). Treatment of cells with SP600125 suppressed nuclear envelope breakdown, with >70% of SP600125-treated cells staining for lamin B1 at 12 h after thymidine release (Figure 2a). Lamin B1 dispersal occurs after chromosome condensation (Georgatos et al., 1997). Cells released from thymidine showed a near absence of condensed chromatin (data not shown), in accord with the absence of phosphorylated histone H3 and of MPM2 mitotic markers. We therefore conclude that SP600125 prevents synchronized cells from entering mitosis as assayed by nuclear envelope breakdown, MPM2 staining, Ser10 phosphorylation of histone H3 and chromosome condensation.
Figure 2.
SP600125 suppresses the progression of G2 cells to nuclear envelope breakdown. (a) HCT116 cells released from thymidine synchrony were treated with 20 μM SP600125 (SP) at 4 h after release from thymidine block. Nocodazole (NOC) was added at 1 h after release. Cells obtained before release (Thy) and at the indicated times after release from thymidine synchrony were stained for lamin B1 and DNA (Hoechst) as previously described (Lee et al., 2009). The percentage of cells (n = 250/time point) with intact lamin rings around nuclei stained with Hoechst were scored. (b) Cells collected at the indicated times in (a) were stained with PI and with MPM2 antibody and examined by flow cytometry. Numbers in MPM2 dot plots are the percentage of the 4N population in mitosis.
SP600125 induces endoreplication from G2 phase
Next we determined the fate of the cells that fail to enter mitosis on exposure to SP600125. Cells exposed to increasing concentrations of SP600125 show a concentration-dependent decrease in 4N G2 cells and an increase in polyploid (8N) DNA content (Figure 3a). Figure 3b shows that 33 ± 4% of cells become polyploid and attain a DNA content of 8N at 24 h after release from thymidine synchrony into SP600125 in three different experiments. SP600125-treated cells thus undergo endoreplication as defined by their ability to double their DNA after S phase without entering mitosis (Edgar and Orr-Weaver, 2001). Endoreplication from G2 could also be observed on SP600125 treatment of thymidine-released U2OS cells (Supplementary Figure 5). Nocodazole-treated cells enter mitosis as evidenced by MPM2 staining (Figures 1c and d), histone H3 phosphorylation (Figure 1e) and lamin B1 breakdown (Figure 2), and do not progress to 8N (Figures 1b and 3a). Nocodazole treatment alone does not arrest cells in G2 or induce endoreplication and thus serves as a control in these experiments. As further evidence that microtubule status does not influence the result, SP600125-induced endoreplication could be observed in the absence of nocodazole (Figure 3c).
Figure 3.
SP600125 leads to endoreplication directly from G2 phase. (a) SP600125 treatment of G2 cells results in a dose-dependent generation of 8N population. HCT116 cells released from thymidine synchrony were treated with different concentrations of SP600125 (SP) at 4 h after release from thymidine block. Nocodazole (NOC) was added at 1 h after release. Cells were stained with PI at 15 h and 27 h after release from thymidine synchrony and examined by flow cytometry. Numbers in PI (DNA content) plots are percentage of population that is 8N. (b) Percentage of cells with 8N DNA content at 24 h after treatment with 20 μM SP600125 from three different experiments carried out as in (a) is shown. Error bars represent s.d. (c) SP600125 treatment leads to endoreplication in the presence or absence of nocodazole. HCT116 cells released from thymidine block were treated with 20 μM SP600125 (SP) at 4 h after release. Nocodazole (NOC) was either added at 1 h after release or omitted. Cells collected at the indicated times after release from thymidine synchrony were stained with PI and with MPM2 antibody and examined by flow cytometry. Numbers in DNA content plots are percentage of population that is 8N. Numbers in MPM2 dot plots are the percentage of the total 4N population that is in mitosis. (d) SP600125 treatment of mitotic cells does not result in endoreplication. HCT116 cells released from thymidine synchrony were treated with nocodazole (NOC) 1 h after release for 15 h and then 20 μM SP600125 was added. Cells collected at the indicated times after release from thymidine synchrony were stained with PI and with MPM2 antibody and examined by flow cytometry. Numbers in DNA content plots are percentage of population that is 8N. Numbers in MPM2 dot plots are percentage of 4N population that is in mitosis.
We next ruled out the possibility that the 8N population that we observe in Figures 3a and b derives from the small fraction of thymidine-released SP600125-treated cells that enter mitosis (Figure 1c). After failure to execute mitosis properly, cells are able to exit mitosis and enter interphase with 4N DNA content, despite the presence of the mitotic spindle inhibitor nocodazole (Andreassen and Margolis, 1994). The G1 cells with 4N DNA content can become polyploid if they initiate a new cell cycle and undergo DNA synthesis. To address this, thymidine-synchronized cells were released and treated with nocodazole. SP600125 was added to the culture medium at 15 h when the cells are normally in mitosis. Treatment with SP600125 after the entry of cells into mitosis does not lead to the accumulation of cells with 8N DNA content in the continued presence of nocodazole (Figure 3d). Thus, endoreplication requires exposure of cells to SP600125 during G2 phase.
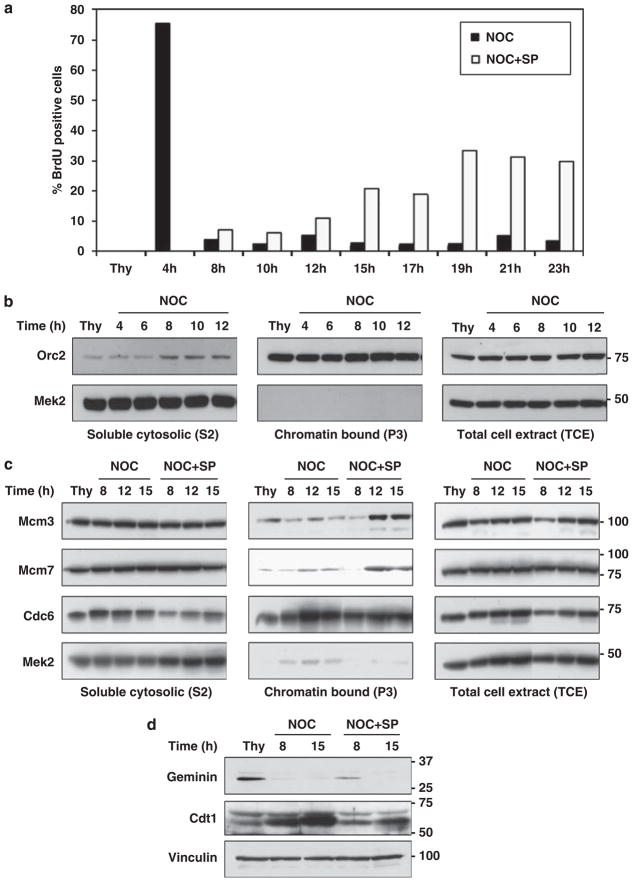
To verify that cells treated with SP600125 initiate a new round of replication, we identified cells undergoing DNA synthesis by 5-bromodeoxyuridine (BrdU) labeling. As expected, thymidine-blocked cells were BrdU negative and the cells acquired BrdU positivity on release from G1/S synchrony (Figure 4a). Afterwards, control cells released from thymidine into nocodazole entered mitosis and remained BrdU negative. In contrast, the cells treated with both nocodazole and SP600125 underwent DNA synthesis as verified by BrdU positivity (Figure 4a).
Figure 4.
Formation of pre-replication complexes containing Mcm3 and Mcm7 and the occurrence of DNA synthesis in SP600125-treated G2 cells. (a) DNA synthesis in SP600125-treated cells. HCT116 cells released from thymidine synchrony were treated with 20 μM SP600125 (SP) at 4 h after release from thymidine block. Nocodazole (NOC) was added at 1 h after release. DNA synthesis was measured by BrdU incorporation as previously described (Lee et al., 2009). Cells synchronized and treated with SP600125 were pulsed with 10 μM BrdU (Sigma) for 30 min before release (Thy) and at the indicated times after release from thymidine synchrony. The cells were stained with anti-BrdU antibody and PI. The percentage of BrdU-positive cells (n = 250/time point) was scored. There were no BrdU-positive thymidine-blocked cells. (b) Isolation of chromatin fractions. HCT116 released from thymidine synchrony were treated with nocodazole and SP600125 as in (a). Biochemical fractionation of extracts at the indicated times after release from thymidine synchrony was carried out as described in Materials and methods. Briefly, soluble cytosolic proteins (S2) from lysed cells were separated from the nuclei by low-speed centrifugation and further clarified by high-speed centrifugation. An insoluble fraction containing proteins bound to the chromatin or nuclear matrix (P3) was derived from the nuclear pellet. Total cell extract (TCE) was prepared by lysing cells directly in Laemmli buffer, followed by sonication. MEK2, a cytosolic kinase, was recovered solely in the S2 fraction, whereas origin recognition protein 2 (Orc2), a nuclear protein, was associated only with the chromatin P3 fraction, as shown previously (Mendez and Stillman, 2000). (c) Re-binding of replication initiation proteins, Mcms, to chromatin in SP600125-treated cells. Fractionated cell extracts obtained as in (b) were immunoblotted for the indicated proteins with specific antibodies. A significant fraction of Cdc6 is present on chromatin in S and G2/M (Mendez and Stillman, 2000). (d) Geminin and Cdt1 in control and SP600125-treated cells. Extracts were obtained from HCT116 cells released from thymidine synchrony and treated with nocodazole and SP600125. Cell extracts were prepared in NP40 lysis buffer. Extracts prepared before release (Thy) and at the indicated times after release from thymidine synchrony were immunoblotted with Geminin- and Cdt1-specific antibodies.
Mcm proteins are essential components of the pre-replication complex (Diffley and Labib, 2002). Mcm2–7 are excluded from chromatin in G2 phase and need to be loaded onto the DNA to license origins for a new round of DNA replication. Consistent with our observation that thymidine-released cells treated with SP600125 undergo DNA synthesis, we detected re-binding of Mcm3 and Mcm7 with chromatin (compare 8 and 12 h, Figure 4c) but not in control cells. The licensing inhibitor, Geminin, binds to and inactivates the pre-RC assembly factor Cdt1 (Diffley and Labib, 2002; Sclafani and Holzen, 2007). The degradation of Geminin both in control and SP600125-treated cells, and the presence of Cdt1 in both control and SP600125-treated cells, suggests that the early licensing of the origins in SP600125-treated cells is controlled downstream of these proteins (Figure 4d).
SP600125-mediated endoreplication is independent of JNK inhibition
SP600125 was originally reported to be a specific ATP-competitive JNK inhibitor with greater than 20-fold selectivity for JNK over other kinases tested (Bennett et al., 2001; Han et al., 2001). However, Bain et al. (2003, 2007) disputed the claim that SP600125 was a specific JNK inhibitor. We therefore tested whether the effects of SP600125 could be reproduced with JNK1 and JNK2 small interfering RNA (siRNA). Knockdown of JNK1 and JNK2 proteins in synchronized cells was near absolute (Figure 5a), yet it did not prevent progression of cells to mitosis as indicated by the presence of phosphorylated histone H3 (Figure 5a) and MPM2-positive status of the cells (Figure 5b). Down-regulation of JNK1/2 by specific siRNA was accompanied by a near-complete inhibition of JNK activity (Figure 5c). Further, when cells with downregulated JNK1/2 were treated with SP600125, these cells exhibited a significant suppression of entry into mitosis and an increase in endoreplication (Figure 5d). We therefore conclude that the effect of SP600125 on cells is independent of its ability to inhibit JNK.
SP600125 suppresses the activation of Cdk1-cyclin B upstream of Aurora A and Polo-like kinase 1 in G2 phase
The entry of cells into mitosis is controlled by the activation of Cdk1 (Minshull et al., 1989; Nurse, 1990) through dephosphorylation at Tyr15 (O’Farrell, 2001). The inhibitory phosphorylation of Cdk1 at Tyr15 decreased during the G2 to M phase transition in control cells, whereas this decrease was suppressed in cells treated with SP600125 without an affect on the abundance of Cdk1 (Figure 6a). In accord with the retention of Cdk1 phosphorylation at Tyr15, we found that cells treated with SP600125 fail to show a dramatic increase in Cdk1- or cyclin B1-associated kinase activity, comparable with that present in control cells (Figures 6b and c).
Figure 6.
SP600125 suppresses the activation of Cdk1-cyclin B1 kinase in G2 cells. (a) SP600125 suppresses de-phosphorylation of Cdk1 at Tyr15 but does not affect Cdk1 protein levels. HCT116 cells released from thymidine synchrony were treated with 20 μM SP600125 (SP) at 4 h after release from thymidine block. Nocodazole (NOC) was added at 1 h after release. Cell extracts prepared before release (Thy) and at the indicated times after release from thymidine synchrony were examined for cyclin B1, Cdk1, and phosphorylation of Cdk1 at Tyr15 by immunoblotting with specific antibodies. (b) SP600125-dependent suppression of Cdk1-associated kinase activity. Cells released from thymidine synchrony were treated with nocodazole and SP600125. Cdk1-associated kinase activity was measured in Cdk1 immunoprecipitates of cell extracts prepared at the indicated times after release from thymidine synchrony. Histone H1 (H1) was used as a substrate. (c) SP600125-dependent suppression of cyclin B1-associated kinase activity. Cells released from thymidine synchrony were treated with nocodazole and SP600125. Cyclin B-associated kinase activity was measured in cyclin B immunoprecipitates from cell extracts prepared at different times. Cyclin B-associated kinase activity increases in control cells coincident with cells entering mitosis. Histone H1 was used as a substrate. (d) SP600125 suppresses the activation of Aurora A and Polo-like kinase 1 in G2 cells. Cells released from thymidine synchrony were treated with nocodazole and SP600125 as in (a). Aurora A-associated kinase activity was measured in Aurora A immunoprecipitates of cell extracts using myelin basic protein (MBP) as a substrate. Plk1-associated kinase activity was measured in Plk1 immunoprecipitates of cell extracts using α-casein as a substrate. Western blot analysis of cell extracts prepared before release (Thy) and at the indicated times after release from thymidine synchrony with Aurora A- and Plk1-specific antibodies is shown.
Cdk1-cyclin activity is indirectly regulated by Plk1 (Glover et al., 1998; Nigg, 1998) and Aurora A (Hirota et al., 2003). The activation of Plk1 and its phosphorylation of Cdk1-activating phosphatase Cdc25 is probably an initiating event in Cdk1 activation (Kumagai and Dunphy, 1996; Glover et al., 1998; Nigg, 1998; Roshak et al., 2000; O’Farrell, 2001; Qian et al., 2001). Plk1 is in turn activated by Aurora A, whose activity increases in G2 phase (Hirota et al., 2003; Seki et al., 2008). Aurora A kinase and Plk1 activities increased in synchronized control cell extracts at 8 h after the release of cells from thymidine block, when the cells were in G2 phase (Figure 6d). In contrast, only a minor increase in Aurora A and Plk1 kinase activities was detected in cells treated with SP600125 (Figure 6d). SP600125 thus seems to suppress mitotic entry by acting upstream of Aurora A and Plk1 activation in G2 phase.
SP600125-mediated endoreplication from G2 phase after inhibition of Cdk1 activity requires Cdk2 kinase activity
DNA synthesis requires CDK activity (Sclafani and Holzen, 2007). We have shown that Cdk1 is only marginally activated in thymidine-released cells treated with SP600125 (Figure 6); cyclin E- and Cdk2-associated kinase activities, however, persist in cells treated with SP600125 (Figure 7a), thereby explaining the ability of SP600125-treated cells to undergo DNA synthesis. To corroborate the above results, siRNA-mediated down-regulation of Cdk2 (Figure 7b) in thymidine-released cells treated with SP600125 was found to prevent endoreplication (Figure 7c). Similarly, cells released from thymidine and treated with SP600125 and roscovitine, an inhibitor of Cdk1- and Cdk2-associated kinase activity (Knockaert et al., 2002), fail to proceed to 8N (Figure 7d). Thus, either Cdk1/2 inhibition with roscovitine or Cdk2 down-regulation with siRNA leads to the same outcome. To further substantiate our results that indirect targeting of Cdk1 by SP600125 leads to endoreplication from G2 phase, cells released from thymidine synchronization were treated with RO-3306, a specific inhibitor of Cdk1 activity (Vassilev et al., 2006). As with SP600125, cells treated in this manner proceeded to 8N without entering mitosis as evidenced by the lack of MPM2 staining (Figure 7e). As expected, release of thymidine-synchronized cells into roscovitine, instead of SP600125, led to an arrest in G2 phase and a failure of cells to proceed to 8N (Figure 7f). As roscovitine suppresses both Cdk1 and Cdk2 activity, our results are thus consistent with a model in which the failure of Cdk1 activation after SP600125 treatment leads to endoreplication directly from G2 phase, in a process requiring the continued presence of Cdk2 activity.
Figure 7.
Cdk2 regulates SP600125-mediated progression of G2 cells to endoreplication. (a) SP600125-treated cells retain Cdk2- and cyclin E-associated kinase activity longer than control cells. HCT116 cells released from thymidine synchrony were treated with 20 μM SP600125 (SP) at 4 h after release from thymidine block. Nocodazole (NOC) was added at 1 h after release. Cdk2- and cyclin E-associated kinase activities were measured in Cdk2 and cyclin E immunoprecipitates of cell extracts prepared before release (Thy) and at the indicated times after release from thymidine synchrony (left panels). Histone H1 was used as a substrate. Western blot analysis of cell extracts with cyclin E-specific antibodies is shown as a control (right panels). (b) Verification of Cdk2 downregulation by specific siRNA. HCT116 cells transfected with Cdk2 or control siRNA for 6 h were synchronized with thymidine, released and then treated with nocodazole and SP600125 as in (a). Extracts obtained from cells at the 24 h after release from thymidine synchrony were immunoblotted with antibodies recognizing Cdk2 and Cdk1. Cdk1 protein levels are not affected by Cdk2 siRNA. (c) Generation of 8N population after SP600125 treatment depends on Cdk2. Cells from (b) were harvested at 24 h, stained with PI and examined by flow cytometry. Numbers in DNA content plots are percentage of population that is 8N. (d) Inhibition of CDKs prevents SP600125-induced endoreplication. HCT116 cells released from thymidine synchrony were treated with 20 μM SP600125 (SP) at 4 h after release from thymidine block. Nocodazole (NOC) was added at 1 h after release. Roscovitine (50 μM), an inhibitor of Cdk1- and Cdk2-associated kinase activity, was added to the cells at 10 h after release. Roscovitine (Ros) was then present for the duration of the experiment. Cells were harvested at 24 h, stained with PI and examined by flow cytometry. Numbers in DNA content plots are percentage of population that is 8N. (e) Inhibition of Cdk1 is sufficient to induce endoreplication from G2 in the absence of SP600125. HCT116 cells released from thymidine synchrony were treated with nocodazole (NOC) at 1 h after release. RO-3306 (10 μM), an inhibitor of Cdk1-associated kinase activity, was added to the cells at 6 h after release. RO-3306 was then present for the duration of the experiment. Cells harvested at the indicated times were stained for DNA with PI and examined by flow cytometry. Numbers in DNA content plots are percentage of population that is 8N. Numbers in MPM2 dot plots are the percentage of the 4N population in mitosis. (f) Simultaneous inhibition of Cdk1- and Cdk2-associated kinase activities does not lead to endoreplication in the absence of SP600125. HCT116 cells released from thymidine synchrony were treated with nocodazole (NOC) at 1 h after release. Roscovitine (10 μM) was added to the cells at 6 h after release. Roscovitine was then present for the duration of the experiment. Cells harvested at the indicated times were stained with PI and examined by flow cytometry. Numbers in DNA content plots are the percentage of population that is 8N. Numbers in MPM2 dot plots are the percentage of the 4N population in mitosis.
Discussion
We show that DNA endoreplication can occur directly from G2 phase in the absence of Cdk1 activity. Our demonstration relies on the evidence that SP600125 prevents the progression of cells from G2 phase into mitosis by suppressing the activation of Cdk1 and cyclin B. Instead of proceeding to mitosis, cells treated with SP600125 proceed directly from G2 phase to endo-replicate and finally exhibit a polyploid DNA content as a result of SP600125 treatment. Our demonstration that S-phase synchronized SP600125-treated cells fail to enter mitosis rests on failure of nuclear envelope breakdown, on a substantial lack of MPM2 signal, and on the absence of Ser10 phosphorylation of histone H3. Despite the absence of mitosis, the cells then proceed through DNA synthesis, as monitored by the incorporation of BrdU and by the binding of origin licensing proteins to the DNA. In contrast to SP600125-treated cells, control cells enter mitosis, as evidenced by nuclear envelope breakdown and by a progressive increase in MPM2 signal and Ser10 phosphorylation of histone H3.
Although the JNK inhibitor, SP600125, leads to accumulation of cells with 4N or greater than 4N DNA content in several cell lines (Du et al., 2004; MacCorkle and Tan, 2004; Mingo-Sion et al., 2004; Miyamoto-Yamasaki et al., 2007; Wang et al., 2009), we could not observe the same effect on suppression of JNK1 and JNK2 with siRNA. Using a combination of JNK1 and JNK2 siRNA, we observed a near-complete downregulation of JNK1 and JNK2, but the down-regulation did not reproduce the phenotype of SP600125 treatment. The effect of SP600125 on cells is thus independent of its ability to inhibit JNK. Our results are in accord with the data of Schmidt et al. (2005), which show that SP600125 treatment of JNK1/2−/− double-deficient fibroblasts results in G2 accumulation, despite being devoid of JNK activity. Our study also shows that endoreplication on SP600125 treatment is independent of JNK inhibition. We conclude that SP600125 is not a specific inhibitor of JNK inhibition in accord with Bain et al. (2003, 2007).
We show that the failure of Cdk1 activation after SP600125 treatment leads to endoreplication from G2 phase. Further, the failure to activate Aurora A and Plk1 in SP600125-treated cells in G2 phase may directly result in failure to remove the inhibitory phosphorylation of Cdk1. Plk1 stimulates the Cdk1-activating phosphatase, Cdc25, and downregulates the Cdk1-inhibitory protein kinase, Wee1, through phosphorylation. During G2 to M phase progression, Plk1 is activated by phosphorylation at Thr210 in its activation loop (Barr et al., 2004) by Aurora A (Seki et al., 2008). In G2 phase, Aurora A kinase activity in turn is regulated by autophosphorylation stimulated by association with Ajuba (Marumoto et al., 2005) and by p21-activated kinases (Zhao et al., 2005) or cyclic AMP-dependent protein kinase A (Walter et al., 2000). To further substantiate our results that suppression of Cdk1 is the end result of SP600125 exposure, leading to endoreplication from G2 phase, we show that cells released from thymidine and treated with the Cdk1-specific inhibitor, RO-3306, instead of SP600125, also proceed to 8N. Although the proximal target of SP600125 relevant to G2 arrest remains unknown, the ultimate target seems to be Cdk1.
Previous studies has shown that treatment of asynchronous cells with SP600125 generates polyploid cells with 8N DNA content (MacCorkle and Tan, 2004; Mingo-Sion et al., 2004; Wang et al., 2009) (Supplementary Figure 4). However, the previous studies did not distinguish whether SP600125-treated cells passed through mitosis before re-replicating their DNA. Our approach, in distinction from previous studies, permits the conclusion that the 8N population derives from progression of SP600125-treated cells from G2 phase directly to DNA endoreplication.
It is important to contrast endoreplication from G2 phase with endoreplication resulting from a failure in mitosis, in order to understand the distinct mechanisms that can lead to polyploidy. Our evidence shows that endoreplication from G2 is independent of p53, unlike polyploidy resulting from mitotic failure, which is only observed in cells that lack p53 function (Margolis et al., 2003; Storchova and Pellman, 2004). Thus, the studies that previously showed that Cdk1 inhibition leads to polyploidy as a result of failure in mitosis (Damiens et al., 2001; Vassilev et al., 2006; Hochegger et al., 2007) all used cells that were compromised in p53 function. In contrast, the cells used in our study, HCT116 and U2OS, express wild-type p53, but nonetheless undergo endoreplication from G2 phase. Endoreplication from G2 phase is thus independent of p53 control.
Our study explicitly shows that the absence of Cdk1 activity in G2 phase, before entry into mitosis, induces endoreplication in mammalian cells. In accord with our data, a recent study has established that mitotic cyclin-dependent kinase restricts S phase to once per cell cycle in fission yeast (Kiang et al., 2009). Our cellular model system of inducing mammalian G2 cells to endoreplication in the absence of Cdk1 activity will provide a model to further study the control mechanisms that normally restrict replication to once per cell cycle.
Two additional cell cycle controls deserve mention. Emi1, an inhibitor of the ubiquitin ligase APC, couples S phase with mitosis (Di Fiore and Pines, 2007; Machida and Dutta, 2007). As Emi1 is not degraded prematurely in our experimental system, we can rule out the possibility that its absence drives the observed endo-replication. In addition, SP600125-mediated Cdk1 inhibition is not triggered by a DNA damage checkpoint, as we find that UCN-01, which inhibits Chk1 (Busby et al., 2000) and Chk2 kinases (Yu et al., 2002), does not induce SP600125-treated cells to enter mitosis nor does it prevent endoreplication (Supplementary Figure 6).
Our results show that the absence of Cdk1 activity in G2 phase, before entry into mitosis, induces endo-replication by stimulating origin licensing. Although the inactivation of Cdks is thought to establish competence to initiate DNA replication, actual initiation of replication requires CDK activity (Sclafani and Holzen, 2007). Consistent with an important role of Cdk activity in DNA replication, we find that endoreplication competence requires Cdk2 activity, and that downregulation of Cdk2 with siRNA prevents endoreplication in SP600125-treated cells. The effect is specific to Cdk2, as suppression of endoreplication does not occur on exposure to RO-3306, which specifically inhibits Cdk1.
In conclusion, our results show that the inhibitory effect of SP600125 on mitotic entry most likely occurs through inhibition of Cdk1. The failure to activate Cdk1 directs cells to proceed from G2 phase to endoreplicate in a p53-independent manner, in a process that requires Cdk2 activity.
Materials and methods
Cells and synchronization
HCT116, near-diploid human colorectal carcinoma cells, and U2OS, a human osteosarcoma, were used (Lee et al., 2009). For synchronization by thymidine, cells were treated with 2 mM thymidine for 20 h, washed twice with phosphate-buffered saline and re-plated in drug-free medium. Cells were treated with 1.6 μM nocodazole at 1 h after release.
Flow cytometry
Flow cytometry using MPM-2 as a mitotic marker and propidium iodide as a marker of DNA content was performed as previously described (Lee et al., 2009). Cells with less than 2N DNA content have not been gated out.
Cell extracts and kinase assays
Cell extracts were prepared as previously described (Lee et al., 2009). Kinase activities of cyclin B, Cdk1, Cdk2, cyclin E and Plk1 were performed as previously described (Fotedar et al., 1996; Lee et al., 2009). For Aurora A kinase assay, 500 μg of cell extract was incubated with 10 μl of packed protein A-agarose (Sigma-Aldrich, St Louis, MO, USA) that had been preincubated with anti-Aurora A antibody. After keeping overnight at 4 °C, the beads were washed four times with Plk1 immunoprecipitation buffer and once with kinase buffer (40 mM Hepes pH 7.5, 8 mM MgCl2). The kinase assays were carried out in 18 μl of kinase reaction mixture containing 166 μM ATP, 5 μCi [γ 32P]-ATP, 6 μg myelin basic protein (M1891, Sigma) and 10 μl packed protein A-agarose for 20 min at 37 °C.
RNA interference
RNA interference experiments were carried out as described (Jascur et al., 2005; Lee et al., 2009).
Chromatin extraction
Chromatin isolation in Figure 4 was performed according to published procedures (Mendez and Stillman, 2000).
Supplementary Methods include reagent and antibody information, siRNA sequences and chromatin extraction method.
Supplementary Material
Acknowledgments
We dedicate this paper in fond memory of Arun Fotedar, a colleague and friend, whose laughter infected us all. We thank Kristian Helin (European Institute of Oncology, Italy) for Cdc6 antibody, Hideo Nishitani (Kyushu University) for Cdt1 antibody and Robert J Schultz (NCI, Bethesda, MD) for UCN-01. This work was supported by grants from the National Institutes of Health to RF (CA108947 and CA101810) and to RM (GM068107 and GM088716).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
References
- Andreassen PR, Margolis RL. Microtubule dependency of p34cdc2 inactivation and mitotic exit in mammalian cells. J Cell Biol. 1994;127:789–802. doi: 10.1083/jcb.127.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias EE, Walter JC. Strength in numbers: preventing rereplication via multiple mechanisms in eukaryotic cells. Genes Dev. 2007;21:497–518. doi: 10.1101/gad.1508907. [DOI] [PubMed] [Google Scholar]
- Bain J, McLauchlan H, Elliott M, Cohen P. The specificities of protein kinase inhibitors: an update. Biochem J. 2003;371:199–204. doi: 10.1042/BJ20021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, et al. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr FA, Sillje HH, Nigg EA. Polo-like kinases and the orchestration of cell division. Nat Rev Mol Cell Biol. 2004;5:429–440. doi: 10.1038/nrm1401. [DOI] [PubMed] [Google Scholar]
- Bennett BL, Sasaki DT, Murray BW, O’Leary EC, Sakata ST, Xu W, et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci USA. 2001;98:13681–13686. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulavin DV, Saito S, Hollander MC, Sakaguchi K, Anderson CW, Appella E, et al. Phosphorylation of human p53 by p38 kinase coordinates N-terminal phosphorylation and apoptosis in response to UV radiation. EMBO J. 1999;18:6845–6854. doi: 10.1093/emboj/18.23.6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busby EC, Leistritz DF, Abraham RT, Karnitz LM, Sarkaria JN. The radiosensitizing agent 7-hydroxystaurosporine (UCN-01) inhibits the DNA damage checkpoint kinase hChk1. Cancer Res. 2000;60:2108–2112. [PubMed] [Google Scholar]
- Cai D, Latham VM, Jr, Zhang X, Shapiro GI. Combined depletion of cell cycle and transcriptional cyclin-dependent kinase activities induces apoptosis in cancer cells. Cancer Res. 2006;66:9270–9280. doi: 10.1158/0008-5472.CAN-06-1758. [DOI] [PubMed] [Google Scholar]
- Crosio C, Fimia GM, Loury R, Kimura M, Okano Y, Zhou H, et al. Mitotic phosphorylation of histone H3: spatiotemporal regulation by mammalian Aurora kinases. Mol Cell Biol. 2002;22:874–885. doi: 10.1128/MCB.22.3.874-885.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiens E, Baratte B, Marie D, Eisenbrand G, Meijer L. Anti-mitotic properties of indirubin-3′-monoxime, a CDK/GSK-3 inhibitor: induction of endoreplication following prophase arrest. Oncogene. 2001;20:3786–3797. doi: 10.1038/sj.onc.1204503. [DOI] [PubMed] [Google Scholar]
- Davis FM, Tsao TY, Fowler SK, Rao PN. Monoclonal antibodies to mitotic cells. Proc Natl Acad Sci USA. 1983;80:2926–2930. doi: 10.1073/pnas.80.10.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Fiore B, Pines J. Emi1 is needed to couple DNA replication with mitosis but does not regulate activation of the mitotic APC/C. J Cell Biol. 2007;177:425–437. doi: 10.1083/jcb.200611166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diffley JF, Labib K. The chromosome replication cycle. J Cell Sci. 2002;115:869–872. doi: 10.1242/jcs.115.5.869. [DOI] [PubMed] [Google Scholar]
- Du L, Lyle CS, Obey TB, Gaarde WA, Muir JA, Bennett BL, et al. Inhibition of cell proliferation and cell cycle progression by specific inhibition of basal JNK activity: evidence that mitotic Bcl-2 phosphorylation is JNK-independent. J Biol Chem. 2004;279:11957–11966. doi: 10.1074/jbc.M304935200. [DOI] [PubMed] [Google Scholar]
- Duncia JV, Santella JB, III, Higley CA, Pitts WJ, Wityak J, Frietze WE, et al. MEK inhibitors: the chemistry and biological activity of U0126, its analogs, and cyclization products. Bioorg Med Chem Lett. 1998;8:2839–2844. doi: 10.1016/s0960-894x(98)00522-8. [DOI] [PubMed] [Google Scholar]
- Edgar BA, Orr-Weaver TL. Endoreplication cell cycles: more for less. Cell. 2001;105:297–306. doi: 10.1016/s0092-8674(01)00334-8. [DOI] [PubMed] [Google Scholar]
- Fotedar A, Cannella D, Fitzgerald P, Rousselle T, Gupta S, Doree M, et al. Role for cyclin A dependent kinase in DNA replication in human S phase cell extracts. J Biol Chem. 1996;271:31627–31637. doi: 10.1074/jbc.271.49.31627. [DOI] [PubMed] [Google Scholar]
- Georgatos SD, Pyrpasopoulou A, Theodoropoulos PA. Nuclear envelope breakdown in mammalian cells involves stepwise lamina disassembly and microtubule-drive deformation of the nuclear membrane. J Cell Sci. 1997;110:2129–2140. doi: 10.1242/jcs.110.17.2129. [DOI] [PubMed] [Google Scholar]
- Glover DM, Hagan IM, Tavares AA. Polo-like kinases: a team that plays throughout mitosis. Genes Dev. 1998;12:3777–3787. doi: 10.1101/gad.12.24.3777. [DOI] [PubMed] [Google Scholar]
- Hamilton BT, Snyder JA. Rapid completion of mitosis and cytokinesis in PtK cells following release from nocodazole arrest. Eur J Cell Biol. 1982;28:190–194. [PubMed] [Google Scholar]
- Han Z, Boyle DL, Chang L, Bennett B, Karin M, Yang L, et al. c-Jun N-terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J Clin Invest. 2001;108:73–81. doi: 10.1172/JCI12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendzel MJ, Wei Y, Mancini MA, Van Hooser A, Ranalli T, Brinkley BR, et al. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma. 1997;106:348–360. doi: 10.1007/s004120050256. [DOI] [PubMed] [Google Scholar]
- Hirota T, Kunitoku N, Sasayama T, Marumoto T, Zhang D, Nitta M, et al. Aurora-A and an interacting activator, the LIM protein Ajuba, are required for mitotic commitment in human cells. Cell. 2003;114:585–598. doi: 10.1016/s0092-8674(03)00642-1. [DOI] [PubMed] [Google Scholar]
- Hochegger H, Dejsuphong D, Sonoda E, Saberi A, Rajendra E, Kirk J, et al. An essential role for Cdk1 in S phase control is revealed via chemical genetics in vertebrate cells. J Cell Biol. 2007;178:257–268. doi: 10.1083/jcb.200702034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhaki JE, Gilbert CS, Porter AC. Construction by gene targeting in human cells of a ‘conditional’ CDC2 mutant that rereplicates its DNA. Nat Genet. 1997;15:258–265. doi: 10.1038/ng0397-258. [DOI] [PubMed] [Google Scholar]
- Jascur T, Brickner H, Salles-Passador I, Barbier V, El Khissiin A, Smith B, et al. Regulation of p21(WAF1/CIP1) stability by WISp39, a Hsp90 binding TPR protein. Mol Cell. 2005;17:237–249. doi: 10.1016/j.molcel.2004.11.049. [DOI] [PubMed] [Google Scholar]
- Jin S, Tong T, Fan W, Fan F, Antinore MJ, Zhu X, et al. GADD45-induced cell cycle G2-M arrest associates with altered subcellular distribution of cyclin B1 and is independent of p38 kinase activity. Oncogene. 2002;21:8696–8704. doi: 10.1038/sj.onc.1206034. [DOI] [PubMed] [Google Scholar]
- Kiang L, Heichinger C, Watt S, Bahler J, Nurse P. Cyclin-dependent kinase inhibits reinitiation of a normal S-phase program during G2 in fission yeast. Mol Cell Biol. 2009;29:4025–4032. doi: 10.1128/MCB.00185-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knockaert M, Greengard P, Meijer L. Pharmacological inhibitors of cyclin-dependent kinases. Trends Pharmacol Sci. 2002;23:417–425. doi: 10.1016/s0165-6147(02)02071-0. [DOI] [PubMed] [Google Scholar]
- Kumagai A, Dunphy WG. Purification and molecular cloning of Plx1, a Cdc25-regulatory kinase from Xenopus egg extracts. Science. 1996;273:1377–1380. doi: 10.1126/science.273.5280.1377. [DOI] [PubMed] [Google Scholar]
- L’Italien L, Tanudji M, Russell L, Schebye XM. Unmasking the redundancy between Cdk1 and Cdk2 at G2 phase in human cancer cell lines. Cell Cycle. 2006;5:984–993. doi: 10.4161/cc.5.9.2721. [DOI] [PubMed] [Google Scholar]
- Laronne A, Rotkopf S, Hellman A, Gruenbaum Y, Porter AC, Brandeis M. Synchronization of interphase events depends neither on mitosis nor on cdk1. Mol Biol Cell. 2003;14:3730–3740. doi: 10.1091/mbc.E02-12-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Kim JA, Barbier V, Fotedar A, Fotedar R. DNA damage triggers p21WAF1-dependent Emi1 down-regulation that maintains G2 arrest. Mol Biol Cell. 2009;20:1891–1902. doi: 10.1091/mbc.E08-08-0818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- MacCorkle RA, Tan TH. Inhibition of JNK2 disrupts anaphase and produces aneuploidy in mammalian cells. J Biol Chem. 2004;279:40112–40121. doi: 10.1074/jbc.M405481200. [DOI] [PubMed] [Google Scholar]
- Machida YJ, Dutta A. The APC/C inhibitor, Emi1, is essential for prevention of rereplication. Genes Dev. 2007;21:184–194. doi: 10.1101/gad.1495007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis RL, Lohez OD, Andreassen PR. G1 tetraploidy checkpoint and the suppression of tumorigenesis. J Cell Biochem. 2003;88:673–683. doi: 10.1002/jcb.10411. [DOI] [PubMed] [Google Scholar]
- Marumoto T, Zhang D, Saya H. Aurora-A—a guardian of poles. Nat Rev Cancer. 2005;5:42–50. doi: 10.1038/nrc1526. [DOI] [PubMed] [Google Scholar]
- Mendez J, Stillman B. Chromatin association of human origin recognition complex, cdc6, and minichromosome maintenance proteins during the cell cycle: assembly of prereplication complexes in late mitosis. Mol Cell Biol. 2000;20:8602–8612. doi: 10.1128/mcb.20.22.8602-8612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingo-Sion AM, Marietta PM, Koller E, Wolf DM, Van Den Berg CL. Inhibition of JNK reduces G2/M transit independent of p53, leading to endoreduplication, decreased proliferation, and apoptosis in breast cancer cells. Oncogene. 2004;23:596–604. doi: 10.1038/sj.onc.1207147. [DOI] [PubMed] [Google Scholar]
- Minshull J, Blow JJ, Hunt T. Translation of cyclin mRNA is necessary for extracts of activated Xenopus eggs to enter mitosis. Cell. 1989;56:947–956. doi: 10.1016/0092-8674(89)90628-4. [DOI] [PubMed] [Google Scholar]
- Miyamoto-Yamasaki Y, Yamasaki M, Tachibana H, Yamada K. Induction of endoreduplication by a JNK inhibitor SP600125 in human lung carcinoma A 549 cells. Cell Biol Int. 2007;31:1501–1506. doi: 10.1016/j.cellbi.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Niculescu AB, III, Chen X, Smeets M, Hengst L, Prives C, Reed SI. Effects of p21(Cip1/Waf1) at both the G1/S and the G2/M cell cycle transitions: pRb is a critical determinant in blocking DNA replication and in preventing endoreduplication. Mol Cell Biol. 1998;18:629–643. doi: 10.1128/mcb.18.1.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg EA. Polo-like kinases: positive regulators of cell division from start to finish. Curr Opin Cell Biol. 1998;10:776–783. doi: 10.1016/s0955-0674(98)80121-x. [DOI] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990;344:503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- O’Farrell PH. Triggering the all-or-nothing switch into mitosis. Trends Cell Biol. 2001;11:512–519. doi: 10.1016/s0962-8924(01)02142-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian YW, Erikson E, Taieb FE, Maller JL. The polo-like kinase Plx1 is required for activation of the phosphatase Cdc25C and cyclin B-Cdc2 in Xenopus oocytes. Mol Biol Cell. 2001;12:1791–1799. doi: 10.1091/mbc.12.6.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts EC, Shapiro PS, Nahreini TS, Pages G, Pouyssegur J, Ahn NG. Distinct cell cycle timing requirements for extracellular signal-regulated kinase and phosphoinositide 3-kinase signaling pathways in somatic cell mitosis. Mol Cell Biol. 2002;22:7226–7241. doi: 10.1128/MCB.22.20.7226-7241.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshak AK, Capper EA, Imburgia C, Fornwald J, Scott G, Marshall LA. The human polo-like kinase, PLK, regulates cdc2/cyclin B through phosphorylation and activation of the cdc25C phosphatase. Cell Signal. 2000;12:405–411. doi: 10.1016/s0898-6568(00)00080-2. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Budirahardja Y, Klompmaker R, Medema RH. Ablation of the spindle assembly checkpoint by a compound targeting Mps1. EMBO Rep. 2005;6:866–872. doi: 10.1038/sj.embor.7400483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani RA, Holzen TM. Cell cycle regulation of DNA replication. Annu Rev Genet. 2007;41:237–280. doi: 10.1146/annurev.genet.41.110306.130308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki A, Coppinger JA, Du H, Jang CY, Yates III, JR, Fang G. Plk1- and beta-TrCP-dependent degradation of Bora controls mitotic progression. J Cell Biol. 2008;181:65–78. doi: 10.1083/jcb.200712027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storchova Z, Pellman D. From polyploidy to aneuploidy, genome instability and cancer. Nat Rev Mol Cell Biol. 2004;5:45–54. doi: 10.1038/nrm1276. [DOI] [PubMed] [Google Scholar]
- Uetake Y, Sluder G. Cell-cycle progression without an intact microtuble cytoskeleton. Curr Biol. 2007;17:2081–2086. doi: 10.1016/j.cub.2007.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilev LT, Tovar C, Chen S, Knezevic D, Zhao X, Sun H, et al. Selective small-molecule inhibitor reveals critical mitotic functions of human CDK1. Proc Natl Acad Sci USA. 2006;103:10660–10665. doi: 10.1073/pnas.0600447103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Atayar C, Rosati S, Bosga-Bouwer A, Kluin P, Visser L. JNK is constitutively active in mantle cell lymphoma: cell cycle deregulation and polyploidy by JNK inhibitor SP600125. J Pathol. 2009;218:95–103. doi: 10.1002/path.2521. [DOI] [PubMed] [Google Scholar]
- Walter AO, Seghezzi W, Korver W, Sheung J, Lees E. The mitotic serine/threonine kinase Aurora2/AIK is regulated by phosphorylation and degradation. Oncogene. 2000;19:4906–4916. doi: 10.1038/sj.onc.1203847. [DOI] [PubMed] [Google Scholar]
- Yu Q, La Rose J, Zhang H, Takemura H, Kohn KW, Pommier Y. UCN-01 inhibits p53 up-regulation and abrogates gamma-radiation-induced G(2)-M checkpoint independently of p53 by targeting both of the checkpoint kinases, Chk2 and Chk1. Cancer Res. 2002;62:5743–5748. [PubMed] [Google Scholar]
- Zhao ZS, Lim JP, Ng YW, Lim L, Manser E. The GIT-associated kinase PAK targets to the centrosome and regulates Aurora-A. Mol Cell. 2005;20:237–249. doi: 10.1016/j.molcel.2005.08.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.