Abstract
Background
Chronic renal disease is common, and its prevalence is rising. Its main causes are hypertension and diabetes mellitus. An abnormally low glomerular filtration rate (GFR) often escapes medical notice in the earliest, most treatable stage, so that an increasing number of patients progress to end-stage renal failure. Early recognition of low GFR would thus be an important clinical advance.
Methods
The authors selectively review the literature retrieved by a PubMed search on the topic and also present their own clinical and laboratory data.
Results
Chronic renal failure can be detected early by direct measurement of the GFR with the aid of an exogenous filtration marker. Such techniques are costly and time-consuming and are therefore indicated only for patients at special risk. Chronic renal disease can also be diagnosed early with the aid of the endogenous filtration markers creatinine and cystatin C, which serve as indicators of a low GFR. The serum levels of these two substances are not taken as measures of GFR in themselves, but are rather entered into predictive equations for the estimation of GFR. Cystatin C-based equations seem to be more sensitive indicators of low GFR than creatinine-based equations.
Conclusions
Creatinine- and cystatin C-based equations for the estimation of GFR are valuable tools for the early diagnosis of chronic renal disease and for disease staging according to the US National Kidney Foundation criteria.
Keywords: renal failure, chronic disease, nephropathy, hypertension, diabetes mellitus
Chronic renal disease (CRD) is defined as a glomerular filtration rate (GFR) of <60 (mL × min–1 per 1.73 m2 body surface area) for at least three months, whatever the cause and regardless of the presence of kidney damage (1). Patients in whom signs of damage are found on diagnostic imaging or renal biopsy and those with albuminuria also have nephropathy, even if their GFR is >60. Patients without signs of kidney damage whose GFR is >60 are highly unlikely to be nephropathic (2). CRD is classified into five stages according to the GFR (Table 1) (1). In the USA (3) approximately 10% of adults are estimated to be in an early stage of impaired renal function, of whom 40% have a GFR <60 and 60% show elevated albumin excretion (>30 mg/g creatinine) (e1). According to a European study (4), the prevalence of CRD stage 4 and 5 is 1% in hospital patients under the age of 30 years and 12% in those over 80 years of age.
Table 1. Classification of chronic renal failure, modified from (1).
| Stage | GFR | Renal disease | Measures |
| 1 | ≥90 | With normal GFR | Confirm diagnosis, inhibit progression |
| 2 | 89 to 60 | With mild functional impairment | Inhibit progression |
| 3 | 59 to 30 | With moderate failure | Confirm diagnosis, treat secondary complications |
| 4 | 29 to 15 | With severe failure | Prepare for renal replacement ‧treatment |
| 5 | <15 | With end-stage renal failure | Institute renal replacement treatment |
GFR, glomerular filtration rate; GFR in mL × min–1 × (1.73 m2)–1
Persons with impaired renal function are at greater risk of cardiovascular morbidity and mortality (5), and the prevalence is considerably higher in older people (6). The latter generally display an annual GFR decrease of <1 (7), but a yearly drop of >3, regardless of baseline GFR, has proved to be an independent risk factor for increased mortality (8).
The GFR is considered the best marker for renal function (1). The early stages of renal function impairment are clinically silent and are diagnosed only by measuring GFR by means of external filtration markers (measured GFR, mGFR) (9). Once GFR has decreased to <60, functional impairments can be detected by determining internal filtration markers and calculating the estimated GFR (eGFR) (10). The complications of CRD increase with decreasing GFR and may progress from gradual reduction in renal function to end-stage renal failure. The goal of GFR determination is to detect CRD early in order to slow its progress.
The determination of mGFR and eGFR is indicated
as an isolated measurement to assess renal function at a particular point in time, e.g., in patients with high prevalence of GFR <60;
to evaluate the progression of CRD;
to assess the efficacy of function-preserving treatment measures.
GFR can be determined using exogenous and endogenous markers of filtration (Box 1). Measurements employing exogenous filtration markers (mGFR) yield reliable results and represent the gold standard. However, they are costly, time-consuming, and labor-intensive, can be performed only in specialized laboratories, and are therefore indicated primarily in patients displaying nephrological symptoms. Simpler, but less precise, is estimation of GFR (eGFR) by means of the endogenous filtration markers creatinine and cystatin C.
Box 1. Methods for determination and estimation of GFR and their evaluation.
-
Clearance of exogenous substances
Inulin, iohexol, 51Cr-EDTA, 125I-iothalamate, 99mTc-diethylenetriaminepentaacetic acid (DTPA)
Evaluation: Precise and accurate, but costly, time-consuming, and labor-intensive
-
Clearance of endogenous blood substances
Serum creatinine
Evaluation: Insufficiently sensitive for detection of chronic renal disease (CRD)
Creatinine clearance
Evaluation: No longer recommended due to errors in urine collection
Exception: Patients with highly abnormal muscle mass or vegetarian diet (e8, e9)
Serum cystatin C
Evaluation: More sensitive than serum creatinine for detection of GFR reduction in the range 70 to 40; better than creatinine in children
-
Estimated GFR (eGFR)
Creatinine-based and use of patient-specific data
Counahan-Barratt equation
Evaluation: Only suitable for children, overestimates GFR by ca. 20% to 30%
Cockcroft-Gault equation
Evaluation: Only suitable for adults, slightly overestimates GFR, well suited for estimation of GFR changes during pharmacotherapy
MDRD (Modification of Diet in Renal Disease) equation
Evaluation: Practicable in adults with CRD; not suitable for children
Cystatin C-based eGFR
No patient-specific data required
Evaluation: In the range 70 to 40, estimates GFR more sensitively than creatinine-based equations
The aim of this review is to depict the methods used to determine GFR and—by sifting the nephrological, internal medical and clinical chemistry literature available in PubMed—ascertain their reliability in the detection and monitoring of CRD.
Measuring GFR by means of exogenous filtration markers (mGFR)
The clearance of various markers of filtration, such as inulin, 51Cr-EDTA, iohexol, iothalamate, and 99mTc-diethylenetriaminepentaacetic acid (DTPA), is determined. The GFR is either expressed in absolute terms as mL × min–1 or standardized to 1.73 m2, the body surface area of a person weighing 70 kg. The unit of measurement is: mL × min–1 × (1.73 m2)–1. Age- and gender-specific reference values for GFR can be found in Table 2 (11). The reduction in GFR correlates with the extent of functional impairment of the nephrons and thus with the degree of renal failure. A patient whose GFR falls below 15 usually requires dialysis. Nevertheless, in certain cases GFR is insensitive to the loss of functioning nephrons. In the early stage of diabetes-related kidney disease, for instance, characterized by microalbuminuria, the renal hypertrophy and hyperperfusion mean that GFR is normal or raised; thus, determination of GFR is of no value in the diagnosis of incipient diabetic nephropathy (e2). The different methods for mGFR do not show full agreement: at GFR values >80, GFRiohexol gives lower readings than GFREDTA, but below this threshold GFREDTA is lower than GFRiohexol (13).
Table 2. Reference values for GFR (11).
| Premature births | >0.5 mL × min–1 × kg–1 |
| Neonates | >10 mL × min–1 × [m2]–1 |
| Children (2 to 8 weeks) | 16.3 to 44.6 (mL × min–1 × [1.73 m2]–1) |
| Children (3 to 12 months) | >70 (mL × min–1 × [1.73 m2]–1) |
| Children/adolescents (1 to 20 years) | >80 (mL × min–1 × [1.73 m2]–1) |
| Adults (age group, years) | Men* | Women* |
| 20–29 | 77–179 | 71–165 |
| 30–39 | 70–162 | 64–149 |
| 40–49 | 63–147 | 58–135 |
| 50–59 | 56–130 | 51–120 |
| 60–69 | 49–113 | 45–104 |
| 70–79 | 42–98 | 39–90 |
| 80–89 | 35–81 | 32–75 |
* (mL × min –1 × [1,73 m2] –1)
Measuring GFR by means of endogenous filtration markers (eGFR)
Internal markers of filtration such as creatinine and cystatin C are endogenous substances that are almost completely filtered out by the glomeruli. Increasing serum levels of these parameters indicate decreasing GFR. It is recommended that whenever creatinine is determined the eGFR should be calculated and reported along with the serum value (14). Equations frequently used to ascertain eGFR based upon creatinine and cystatin C are presented in Box 2.
Box2.
| Children | |||
| Counahan-Barratt equation (e14) | Creatinine-based | ||
| eGFR (mL × min–1) = 0.43 × height (cm) × (SCr[mg/dL])–1 | |||
| Equation according to Grubb et al. (24) | Cystatin C-based | ||
| eGFR (mL × min–1 × [1.73 m2] –1) = 84.69 × (Scystatin C [mg × L–1])–1.68 × 1.384 (in children <14 years) | |||
| Adults | |||
| Cockcroft-Gault equation (19) | Creatinine-based | ||
| Ccr(mL × min–1) = (140 – age [years]) × (SCr[mg × dL–1])–1 × (BW [kg] × [72]–1) | |||
| Correction factor: for women × 0.85 | |||
| MDRD equation (10) | Creatinine-based | ||
| eGFR (mL × min–1 × [1.73 m2] –1) = 175 × (SCr standardized [mg × dL–1])–1.154 × (age [years])–0.203 | |||
| Correction factor: | for women × 0.742 | ||
| for blacks × 1.18 | |||
| Equation according to Hoek et al. (25) | Cystatin C-based | ||
| eGFR (mL × min–1 × [1.73 m2] –1) = 80.35 × (Scystatin C[mg × L–1] – 4)–1.68 | |||
Serum creatinine
Determination of creatinine in serum is the method most frequently used to evaluate renal function. Creatinine derives from the muscular metabolism of creatine and phosphocreatine. As such, the synthesis of creatinine at a daily rate of approximately 20 mg/kg body weight reflects muscle mass and varies little from day to day.
Creatinine synthesis is age-dependent. As measured by urinary excretion, it decreases with increasing age, falling from a mean 23.8 mg/kg body weight in men aged 20 to 29 years to 9.8 mg/kg body weight in men aged 90 to 99 years (e2). The essential reason is reduction in muscle mass.
When renal function is normal, creatinine is filtered out by the glomeruli and 15% of it is secreted by the tubuli (e3). There is a reciprocal non-linear relationship between GFR and serum creatinine, such that a decrease in GFR to around 40 often does not lead to an increase to above the upper limit of normal (e4). If no previously obtained values are available, a concentration within the normal range cannot be interpreted as potentially showing a decrease in GFR. In acute renal failure serum creatinine rises within 2 days as a direct result of retention within the body. In CRD the increase in serum is only 30% to 50% of what would be expected from the prevailing GFR. The reason for this is that, depending on the extent of GFR reduction, 16% to 66% of creatinine is eliminated extraglomularly (e5). Tubular secretion and intestinal elimination reach their maximum when GFR falls to =15. Noteworthy extrarenal patient-related factors that influence creatinine synthesis and thus the concentration in serum include sex, age, ethnicity, muscle mass, chronic illness, and the consumption of cooked meat. Lack of standardization of methods also impacts negatively on the validity of serum creatinine for assessment of GFR. Medications such as cimetidine and trimethoprim inhibit creatinine secretion and increase the serum concentration without affecting GFR. It must also be realized that serum creatinine is not suitable for evaluation of rapid changes in GFR: The estimated GFR is too high in swiftly decreasing renal function and too low when function recovers.
Serum cystatin C
Cystatin C is a plasma protein with a molecular weight of 13.4 kDa and belongs to the cysteine protease inhibitors. It is synthesized at a constant rate by all nucleated cells, excreted into plasma, filtered by the glomeruli, and reabsorbed and metabolized by the proximal tubule cells. In the age group from 1 to 50 years, the serum concentration is independent of muscle mass, sex, and age.
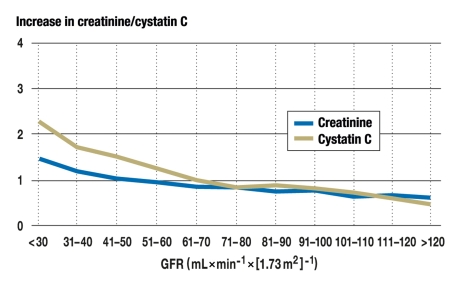
These properties show that cystatin C is a good marker for assessment of renal function. Comparably with serum creatinine, there is an inverse, non-linear relationship between GFR and serum cystatin C. In comparison with serum creatinine, the proportional increase of cystatin C is higher when GFR falls to a level between 70 and 40 (Figure) (17). Cystatin C rises age-dependently from the age of 50 years and correlates with the decrease in GFR.
Figure.
Proportional increase in serum creatinine (blue) and serum cystatin C (yellow) with decreasing glomerular filtration rate (GFR)
Cystatin is not always a reliable marker of renal function, as its synthesis is increased in smokers, patients with hyperthyroidism, and those on glucocorticoid therapy and decreased in hypothyroidism (e6). According to a meta-analysis, however, cystatin C is a more reliable parameter than creatinine for detection of CRD (18).
Creatinine-based eGFR
eGFR is determined by means of equations that take account of empirically patient-related parameters and thus permit more precise and accurate assessment of GFR. All of the equations employed for estimating GFR were developed using cross-sectional data from patient collectives. The Cockcroft-Gault equation (19) and the Modification of Diet in Renal Disease (MDRD) equation (20) are recommended (Box 2). The former incorporates age, body weight, sex, and serum creatinine concentration, while the latter considers age, ethnicity, sex, and serum creatinine concentration. The Counahan-Barratt equation is recommended for children.
Cockcroft-Gault equation
The Cockcroft-Gault equation estimates creatinine clearance in mL × min–1, but not GFR, and is not standardized to the body surface area of 1.73 m2. In relation to GFR it systematically overestimates clearance because tubular creatinine secretion is not taken into account (19, 20). Because this equation includes body weight, it is particularly recommended for the monitoring of renal function during treatment with medications that influence kidney performance.
MDRD equation
The MDRD equation includes age, sex, and ethnicity to take account of differences among population subgroups. Therefore reductions in GFR are detected earlier than with serum creatinine.
Because the MDRD equation was developed exclusively using data from patients with CRD, a GFR of >60 should be reported not as an absolute value but as eGFR >60 mL = (mL × min–1 × [1.73 m2]–1) (20, 21). More recently individuals without CRD have also been studied. The diagnostic reliability of the MDRD equation for estimation of GFR can be summarized as follows:
The eGFR can be 6% too high in CRD (11, e7– e9), and may be 29% too low in individuals without CRD (e10, e11).
In 90% of cases in the MDRD study group the eGFR was within ±30% of the mGFR (21). For a GFR of 60 (mL × min–1 × [1.73 m2]–1) this would mean a range of 42 to 78 (mL × min–1 × [1.73 m2]–1). This degree of accuracy is considered acceptable provided eGFR is determined again after 3 months (22).
The MDRD equation over-stages patients in CRD stages 2 and 3, but correctly classifies those in stages 4 and 5 (4).
This overestimation of GFR by the MDRD equation is important for the monitoring of CRD. Patients in stage 3 are expected to exhibit an annual decrease in GFR of 1.4 to 3.9. In a comparison of mGFR and eGFR, however, 41.8% of patients showed a decrease in eGFR that was less than that in mGFR by =2. Thus monitoring of CRD by eGFR must be viewed critically (23).
Any patient with eGFR <60 very probably has CRD. Young patients with eGFR as low as this may have a true GFR that is 29% higher, but will still probably have impaired renal function (22). In such cases demonstration of, for example, albuminuria is required for the diagnosis of renal damage (1).
To ensure comparability of eGFR among laboratories it is important to use kinetic methods such as the Jaffé reaction or enzymatic techniques to determine creatinine. To this end calibrators and controls of the tests carried out must be based on highly specific procedures for creatinine determination and specific reference materials (21).
Cystatin C-based eGFR
All that is needed for calculation of eGFR is the serum concentration of cystatin C. This method is particularly indicated in children (e12, e13), because the MDRD equation cannot be used in this age group (e9), and in the elderly (6). For children the equation according to Grubb (24) has proved more reliable than the Counahan-Barratt equation, and for adults the equation according to Hoek (25) is more sensitive than the MDRD equation (Box 2). In older age groups the physiological decrease in GFR from year to year is registered more sensitively with cystatin C-based eGFR than with the MDRD equation (6), and a drop of >3 is associated with a higher subsequent risk of mortality (8). Further indications for cystatin C-based determination of eGFR are listed in Box 3.
Box 3. Advantages of cystatin C-based eGFR over creatinine-based eGFR (examples).
| Patient category | Advantage |
| Children (e23) | Children have low levels of creatinine and determination is unreliable in the low er range of measurement |
| The elderly (e15) | Owing to physiological reduction in renal function and decrease in muscle mass, cystatin C correlates better than creatinine with inulin clearance |
| Myasthenics, leg amputees, paraplegics (e16) | Because of the lower muscle mass, creatinine synthesis is low and creatinine-based eGFR is late to detect renal failure |
| Diabetics (e17) | Early stages of renal failure are detected more reliably with cystatin C based than with creatinine-based eGFR |
| Liver cirrhosis (18) | Creatinine methods are slow to detect the decrease in GFR because creatinine metabolism in the liver is reduced |
| Cytostatic treatment (e19) | The nephrotoxicity of cisplatin is dose-dependent and a reduction in GFR is detected earlier by cystatin C-based than by creatinine-based eGFR |
Conclusion
Serum creatinine and establishment of eGFR with the MDRD equation are important basic investigations for the diagnosis of CRD. The determination of cystatin C and reporting of cystatin C-based eGFR offers advantages, but on grounds of cost (determination of cystatin C is 20 to 30 times more expensive than that of creatinine) it should be reserved for certain categories of patients.
Key Messages.
The diagnostic sensitivity of serum creatinine determination is too low for early detection of CRD.
In addition to measurement of serum creatinine the MDRD equation should be used to calculate eGFR, allowing early diagnosis of CRD.
In individuals without CRD the MDRD equation underestimates GFR, but in CRD the agreement is acceptable.
Reductions in GFR are detected earlier by means of cystatin C and cystatin C-based eGFR than by serum creatinine. Because of the higher costs, however, cystatin C determination should be requested only in particular indications.
When a reduction in eGFR is found, direct measurement of GFR (mGFR) should be used to establish the exact base value of GFR and assess the progression of CRD.
Acknowledgments
Translated from the original German by David Roseveare.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists according to the guidelines of the International Committee of Medical Journal Editors.
References
- 1.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002 V>39:1–286. [PubMed] [Google Scholar]
- 2.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney func -tion—measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 3.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third Health Nutrition and Examination Survey. Am J Kidney Dis. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 4.Marsik C, Endler G, Gulesserian T, Wagner O, Sunder-Plassmann G. Classification of chronic kidney disease by estimated glomerular filtration rate. Eur J Clin Invest. 2008;38:253–259. doi: 10.1111/j.1365-2362.2008.01934.x. [DOI] [PubMed] [Google Scholar]
- 5.Go A, Cherow G, Fan D, Mc Culloch CE, Hsu CY. Chronic kidney disease and the risks of death and cardiovascular events and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 6.Shlipak MG, Katz R, Kestenhbaum B, Fried LF, Newman AB, Sisco-vick DS, et al. Rate of kidney function decline in older adults: a comparison using creatinine and cystatin C. Am J Nephrol. 2009;30:171–178. doi: 10.1159/000212381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc. 1985;33:278–285. doi: 10.1111/j.1532-5415.1985.tb07117.x. [DOI] [PubMed] [Google Scholar]
- 8.Rifkin D, Shlipak MG, Katz R, et al. Rapid kidney function decline and mortality risk in older adults. Arch Intern Med. 2008;168:2212–2218. doi: 10.1001/archinte.168.20.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coresh J, Byrd-Holt D, Astor BC, et al. Chronic kidney disease awareness, prevalence, and trends among U.S. adults, 1999 to 2000. J Am Soc Nephrol. 2005;16:180–188. doi: 10.1681/ASN.2004070539. [DOI] [PubMed] [Google Scholar]
- 10.Stevens LA, Zhang Y, Schmid CH. Evaluating the performance of equations for estimating glomerular filtration rate. J Nephrol. 2008;21:797–807. [PMC free article] [PubMed] [Google Scholar]
- 11.Duarte CG, Preuss HG. Assessment of renal function—glomerular and tubular. Clin Lab Med. 1993;13:33–52. [PubMed] [Google Scholar]
- 12.Schleicher E. Pathobiochemie und Diagnostik der diabetischen Nephropathie. J Lab Med. 1999;23:199–204. [Google Scholar]
- 13.Peters AM, Bird NJ, Halsall I, Peters C, Michell AR. Evaluation of the modification of diet in renal disease equation (eGFR) against simultaneous, dual marker multi-sample measurements of glomerular filtration rate. Ann Clin Biochem. 2009;46:58–64. doi: 10.1258/acb.2008.008078. [DOI] [PubMed] [Google Scholar]
- 14.National Kidney Disease Education Program. Health Professionals: Chronic kidney disease overview. http://nkdep.nih.gov/professionals/chronic_kidney_disease.htm (last accessed August 2006)
- 15.Thomas L. Creatinin. In: Thomas L, editor. Labor und Diagnose. Frankfurt: TH-Books; 2008. pp. 535–541. [Google Scholar]
- 16.Laterza OF, Price CP, Scott MG. Cystatin C: an improved estimator of glomerular filtration rate. Clin Chem. 2002;48:699–707. [PubMed] [Google Scholar]
- 17.Newman DJ, Thakkar H, Edwards RG, et al. Serum cystatin C measured by automated immunoassay: a more sensitive marker of changes in GFR than serum creatinine for glomerular filtration rate. Kidney Int. 1995;47:312–318. doi: 10.1038/ki.1995.40. [DOI] [PubMed] [Google Scholar]
- 18.Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Amer J Kidney Dis. 2002;40:221–226. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 19.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Bosch JP, Breyer Lewis J, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 21.Myers GL, Miller WG, Coresh J, et al. Recommendations for improv -ing serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem. 2006;52:5–18. doi: 10.1373/clinchem.2005.0525144. [DOI] [PubMed] [Google Scholar]
- 22.Leyey AS, Stevens LA, Hostetter T. Automatic reporting of estimated glomerular filtration rate—just what the doctor ordered. Clin Chem. 2006;52:2188–2193. doi: 10.1373/clinchem.2006.078733. [DOI] [PubMed] [Google Scholar]
- 23.Xie D, Joffe MM, Brunelli SM, et al. A comparison of change in measured and estimated glomerular filtration rate in patients with non-diabetic kidney disease. Clin J Am Soc Nephrol. 2008;3:1332–1338. doi: 10.2215/CJN.05631207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grubb A, Nyman U, Björk J, et al. Simple cystatin C-based predic -tion equations for glomerular filtration rate compared with the modification of diet in renal disease prediction equation for adults and the Schwartz and the Counahan-Barratt prediction equations for children. Clin Chem. 2005;51:1420–1431. doi: 10.1373/clinchem.2005.051557. [DOI] [PubMed] [Google Scholar]
- 25.Hoek FJ, Kempermann FA, Krediet RT. A comparison between cystatin C, plasma creatinine and the Cockcroft-Gault formula for the estimation of glomerular filtration rate. Nephrol Dial Transplant. 2003;18:2024–2031. doi: 10.1093/ndt/gfg349. [DOI] [PubMed] [Google Scholar]
- e1.Miller WG, Bruns DE, Hortin GL, Sandberg S, Aakre KM, McQueen MJ, et al. Current issues in measurement and reporting of urinary albumin excretion. Clin Chem. 2009;55:24–38. doi: 10.1373/clinchem.2008.106567. [DOI] [PubMed] [Google Scholar]
- e2.Kampmann J, Siersbaek-Nielsen K, Kristensen M, Hansen JM. Rapid evaluation of creatinine clearance. Acta Med Scand. 1974;196:517–520. doi: 10.1111/j.0954-6820.1974.tb01053.x. [DOI] [PubMed] [Google Scholar]
- e3.Kampmann JP, Hansen JM. Glomerular filtration rate and creati -nine clearance. Br J Pharmacol. 1981;12:7–14. doi: 10.1111/j.1365-2125.1981.tb01848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e4.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffens MW, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- e5.Goldman R. Creatinine excretion in renal failure. Proc Soc Exp Biol Med. 1954;85:446–448. doi: 10.3181/00379727-85-20912. [DOI] [PubMed] [Google Scholar]
- e6.Ichihara K, Saito K, Itoh Y. Sources of variation and reference intervals for serum cystatin C in a healthy Japanese adult population. Clin Chem Lab Med. 2007;45:1232–1236. doi: 10.1515/CCLM.2007.504. [DOI] [PubMed] [Google Scholar]
- e7.Vervoort G, Willems HL, Wetzels JF. Assessment of glomerular filtration rate in healthy subjects and and normoalbuminuric diabetic patients: validity of a new (MDRD) prediction equation. Nephrol Dial Transplant. 2002;17:1909–1913. doi: 10.1093/ndt/17.11.1909. [DOI] [PubMed] [Google Scholar]
- e8.Rule AD, Gussak HM, Pond GR, Bergstralh EJ, Stegall MD, Cosio FG, et al. Measured and estimated GFR in healthy potential liver donors. Am J Kidney Dis. 2004;43:112–119. doi: 10.1053/j.ajkd.2003.09.026. [DOI] [PubMed] [Google Scholar]
- e9.Pierrat A, Gravier E, Saunders C, Caira MV, Ait-Djafer Z, Legras B, et al. Predicting GFR in children and adults: a comparison of the Cockcroft-Gault, Schwartz and Modification of Diet in Renal Disease formulas. Kidney Int. 2003;64:1425–1436. doi: 10.1046/j.1523-1755.2003.00208.x. [DOI] [PubMed] [Google Scholar]
- e10.Lin J, Knight EL, Hogan ML, Singh AK. A comparison of prediction equations for estimating glomerular filtration rate in adults without kidney disease. J Am Soc Nephrol. 2003;14:2573–2580. doi: 10.1097/01.asn.0000088721.98173.4b. [DOI] [PubMed] [Google Scholar]
- e11.Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobson SJ, Cosio FG, et al. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004;141:929–937. doi: 10.7326/0003-4819-141-12-200412210-00009. [DOI] [PubMed] [Google Scholar]
- e12.Harmon WE. Glomerular filtration rate in children with chronic kidney disease. Clin Chem. 2009;55:400–401. doi: 10.1373/clinchem.2008.123067. [DOI] [PubMed] [Google Scholar]
- e13.Miller WG. Estimating equations for glomerular filtration rate in children: laboratory considerations. Clin Chem. 2009;554:02–03. doi: 10.1373/clinchem.2008.122218. [DOI] [PubMed] [Google Scholar]
- e14.Counahan R, Chantler C, Ghazali S, Kirkwood B, Rose F, Barratt TM. Estimation of glomerular filtration rate from plasma creatinine concentration in children. Arch Dis Child. 1976;51:875–878. doi: 10.1136/adc.51.11.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e15.Fliser D, Ritz E. Serum cystatin C concentration as a marker of renal dysfunction in the elderly. Am J Kidney Dis. 2001;37:79–83. doi: 10.1053/ajkd.2001.20628. [DOI] [PubMed] [Google Scholar]
- e16.Thomassen SA, Johannesen IL, Erlandsen EJ, Abrahamsen J, Randers E. Serum cystatin C as a marker of the renal function in patients with spinal cord injury. Spinal Cord. 2002;40:524–528. doi: 10.1038/sj.sc.3101320. [DOI] [PubMed] [Google Scholar]
- e17.Christensson AG, Grubb AO, Nilsson JA, Norrgren K, Sterner G, Sundkvist G. Serum cystatin C advantageous compared with serum creatinine in the detection of mild but not severe diabetic nephropathy. J Intern Med. 2004;256:510–518. doi: 10.1111/j.1365-2796.2004.01414.x. [DOI] [PubMed] [Google Scholar]
- e18.Orlando R, Mussap M, Plebani M, Piccoli P, De Martin S, Floreani, et al. Diagnostic value of plasma cystatin C as a glomerular filtration marker in decompensated liver cirrhosis. Clin Chem. 2002;48:850–858. [PubMed] [Google Scholar]
- e19.Stabuc B, Vrhovec L, Stabuc-Silih M, Cizej TE. Improved prediction of decreased creatinine clearance by serum cystatin C: use in cancer patients before and during chemotherapy. Clin Chem. 2000;46:193–197. [PubMed] [Google Scholar]
- e20.Thomas L. Cystatin C. In: Thomas L, editor. Labor und Diagnose. Frankfurt: TH-Books; 2008. pp. 548–554. [Google Scholar]