Abstract
Chronic neuroendocrine stress usually leads to the elevation of the stress hormones and increased metabolic rate, which is frequently accompanied by oxidative damage to the CNS. In the present study we hypothesized that chronic psychosocial isolation (CPSI) of male Wistar rats, characterized by decreased serum corticosterone (CORT), unaltered catecholamines (CTs), and low blood glucose (GLU), may also promote oxidative imbalance in the CNS, by targeting antioxidant defense system. To test it, we have examined the relation between these input signals and protein expression/activity of antioxidant enzymes (AOEs): superoxide dismutases (SODs), catalase (CAT), glutathione peroxidase (GPx), and glutathione reductase (GLR) in the hippocampus (HIPPO) of CPSI animals. We found that CPSI did not affect SODs or CAT, but decreased activity of GPx and compromised GLR, an enzyme highly dependent on blood GLU for its substrate precursor. Further, we have tested whether the CPSI experience altered AOEs response to a novelty stress, and found that it attenuated peroxide-metabolizing enzymes, CAT and GPx, and decreased GLR activity, even though blood GLU was restored. The altered ratios of hippocampal AOEs in CPSI animals, which were worsened under the combined stress conditions, may lead to the accumulation of peroxide products and oxidative imbalance. The mechanism by which CPSI generate oxidative imbalance in the HIPPO is most likely based on poor systemic energy conditions set by this stress. Such conditions may cause functional decline of CNS structures, such as HIPPO, and are likely to promote state linked to onset of many mood disorders.
Keywords: Social isolation, Antioxidant enzymes, Hippocampus, Corticosterone, Catecholamines
Introduction
Chronic neuroendocrine stress has been considered as an etiological factor for the onset and exacerbation of many mood disorders and, in recent years, animal models have been developed that use chronic stress to induce neuroendocrine and CNS alterations similar to those occurring in the course of the development of these disorders (De Kloet et al. 2005). Redox imbalance has been implicated in the pathogenic mechanisms underlying many of these disorders, since the CNS exhibits great vulnerability to oxidative damage (Ng et al. 2008). At least a part of chronic neuroendocrine stress actions is thought to occur via systemic oxidative damage in general and that of the CNS in particular (Zafir and Banu 2007). Neuroendocrine stress response is triggered by the glucocorticoids (GCs) which synergize with the catecholamines (CTs) in the CNS to restore physiological and behavioral homeostasis (Kvetnansky et al. 1995). The hippocampus (HIPPO) is a part of the CNS ‘limbic system’ known to participate in the regulation of hypothalamic–pituitary–adrenal (HPA) axis homeostasis and is most susceptible to oxidative damage (Magarinos et al. 1987; Reagan et al. 1999). Redox imbalance in this structure, as well as, in other tissues, is defined as the condition arising from disproportion between generation of reactive oxygen species (ROS), such as superoxide, hydrogen peroxide, and hydroxyl radicals, and activity of the antioxidant systems. It could also occur due to decrease in the levels of antioxidants without much change in the levels of ROS. The high-energy demands of the neurons, together with their high levels of ROS production, place them at risk during conditions of prolonged neuroendocrine stress (Mattson and Liu 2002). ROS are kept at physiologically optimal levels by antioxidant defense systems, which comprises of antioxidant enzymes (AOEs), such as CuZn- and Mn superoxide dismutases (CuZn- and MnSODs), peroxidases (catalase, CAT; glutathione peroxidase, GPx), and glutathione reductase (GLR), capable of neutralizing or transforming particular ROS species and altogether creating a powerful detoxification system. Among other AOEs specificities, one of the important features of the CNS is that GPx, which catalyzes the reductive destruction of lipid hydroperoxides, is predominately localized in the microglia and less abundant in the neurons and the astrocytes (Lindenau et al. 1998), while CAT is much more important for clearance of H2O2 in the neurons (Schad et al. 2003). The reducing power of GPx is maintained via reduced glutathione, a product of sustained GLR activity which also plays an essential role in the cell defense against ROS metabolites.
Studies from several laboratories have demonstrated impaired antioxidant defense in different chronic neuroendocrine stress models, such as restraint (Zafir and Banu 2009), immobilization (Grillo et al. 2003), or cold (Kaushik and Kaur 2003). The important common characteristic of these models is significantly elevated level of serum corticosterone (CORT), which may be implicated in ROS generation via increase in the cell metabolic rate (Liu and Mori 1999). On the other side, our recent study with the chronically isolated Wistar animals demonstrated quite the opposite result, with low level of CORT in blood serum and reduced oxidative phosphorylation capacity in the HIPPO suggesting downregulation of mitochondrial respiratory capacity (Adzic et al. 2009a). Since, low CORT state and its consequent cell alterations may also be reflected in cellular redox capacity, it was of interest to characterize AOEs and define antioxidant defense in the animals exposed to the chronic social isolation. Moreover, in order to test the ability of the HIPPO to sustain adaptive stress–response regarding AOEs parameters, we have also exploited acute 30-min immobilization, which was applied either to naïve animals, or to previously isolated Wistar rats. In all animals we have followed the protein expression and activity of AOEs (CuZn- and MnSODs, CAT, GPx, and GLR), and related them with the input signal of serum CTs, CORT, and blood glucose (GLU). We investigated the hypotheses that the chronic psychosocial isolation (CPSI) may alter AOE status in the HIPPO, and that these alterations might alter/compromise its response to the subsequent novelty stress.
Methods
Animal Care and Treatment
All experiments were performed in adult (3 months old) Wistar male rats (body mass 330–400 g), housed four per standard size cage and offered food (commercial rat pellets) and water ad libitum. Light was kept on, between 07:00 a.m. and 07:00 p.m., and room temperature (RT) was kept at 20 ± 2°C. All animal procedures were approved by the Ethical Committee for the Use of Laboratory Animals of the VINCA Institute of Nuclear Sciences, according to the guidelines of the EU registered Serbian Laboratory Animal Science Association (SLASA). For the purpose of the experiment, animals were divided in four groups: group I consisted of unstressed animals (control group); group II animals were exposed to the acute immobilization for 30 min (immobilization stress was induced as described by Kvetnansky and Mikulaj 1970); group III animals were subjected to the chronic isolation stress, by housing them individually for 21 days; group IV was exposed to the chronic isolation for 21 days followed by 30 min immobilization.
Determination of Serum Catecholamines and Corticosterone Concentrations and Blood Glucose Level
Animals were killed immediately after the termination of the stress procedure by decapitation with a guillotine (Harvard-Apparatus, USA). Blood was immediately collected and serum was prepared by 15 min centrifugation at 3000 rpm. Serum CTs, noradrenaline (NA) and adrenaline (A) were assayed by the modified radioenzymatic method of Peuler and Johnson (1977). Serum CORT level was determined using the OCTEIA Corticosterone EIA kit according to manufacturer’s instructions (American Laboratory Products Co.). Absorbance at 450 nm (reference 650 nm) was determined by microplate reader (Wallac, VICTOR2 1420, PerkinElmer). CORT concentration (ng/ml) was determined using a standard curve. Blood GLU level was determined colorimetrically by Accutrend strips.
Isolation of Tissue and Preparation of Cytoplasmic, Mitochondrial, and Peroxisomal Extracts
All animals were killed between 9:00 and 11:00 a.m., i.e., immediately after stress treatment by rapid decapitation. The HIPPO was removed and immediately frozen in liquid nitrogen until further preparation. Frozen tissues were weighed and homogenized (1:2 w/v) in ice-cold 20 mM Tris–HCl (pH 7.2) buffer containing 10% glycerol, 50 mM NaCl, 1 mM EDTA, 1 mM EGTA, 2 mM DTT, and protease inhibitors (20 mM Na2MoO4, 0.15 mM spermin, 0.15 mM spermidin, 0.1 mM PMSF, 5 μg/ml antipain, 5 μg/ml leupeptin, 5 μg/ml aprotinin, 10 μg/ml trypsin inhibitor, and 3 mM benzamidine) and phosphatase inhibitors (20 mM β-glycerophosphate, 5 mM Na4P2O7·10H2O, 2 mM Na3VO4, 25 mM NaF) with 20 strokes of Potter–Elvehjem Teflon-glass homogenizer. Samples were centrifuged 10 min at 2,000×g at 4°C. Supernatant was further centrifuged at 20,000×g for 30 min to provide crude mitochondrial pellet. The crude mitochondrial pellet was washed (3 times) in 0.5 ml of homogenization buffer and centrifuged at 20,000×g for 30 min. Mitochondrial pellets were then lysed in buffer containing 50 mM Tris–HCl (pH 7.4), 5% glycerol, 1 mM EDTA, 5 mM DTT, protease inhibitors, and 0.05% Triton X-100 and incubated on ice for 1.5 h with frequent vortexing. Resulting fraction was used as final mitochondrial extract. The resulting supernatant of this centrifugation was ultracentrifuged at 105,000×g for 1 h to obtain final supernatants used as cytoplasmic fraction. Pellets from this centrifugation were washed (3 times) in 0.5 ml of homogenization buffer, and then lysed in the same buffer used for the lysation of mitochondria, incubated on ice for 1.5 h with frequent vortexing. Resulting fraction was used as final peroxisomal extract.
Western Blot Analysis of Antioxidant Enzymes in Rat Hippocampus
Protein concentration in cytoplasm, mitochondrial and peroxisomal fractions was determined by method of Markwell et al. (1978). The samples were prepared with denaturing buffer according to Laemmli (1970), boiled for 5 min at 100°C, and 30 μg of protein were subjected to electrophoresis on 10% sodium dodecyl sulfate-polyacrylamide gel (SDS-PAG). Subsequently, proteins were transferred onto PVDF membrane (Immobilon-P membrane, Millipore) using a blot system (Transblot, BioRad). The membranes were incubated in appropriate primary and secondary antibodies. Rabbit polyclonal anti-β-actin (ab8227, Abcam) and anti-mHsp70 (MA3-028, Affinity Bioreagents) were used as loading controls for cytoplasmic/peroxisomal and mitochondrial fractions, respectively. Anti-MnSOD (Stressgen), anti-CuZnSOD (Stressgen), anti-catalase (Calbiochem), anti-GPx (Santa Cruz Biotechnology), and anti-GLR (Santa Cruz Biotechnology) were used to detect MnSOD, CuZnSOD, CAT, GLR and GPx. Blots were developed with ECL Rabbit IgG, HRP-linked whole antibody and ECL Mouse IgG, HRP-linked whole antibody (Amersham). The signal was developed using enhanced chemiluminescence reagent (ECL, Pierce) and exposed to X-ray film (Agfa). Their quantification was performed by Image J PC software analysis.
Antioxidant Enzymatic Activities
Total SOD activity was determined using a commercial kit (Randox Laboratories, Crumlin, UK). Briefly, this method uses xantine and xantine oxidase to generate superoxide radicals which react with 2-(4-iodophenyl)-3-(4-nitrophenol)-5-phenyltetrazolium chloride to form a red formazan dye. One unit of SOD activity causes a 50% inhibition of the rate of reduction of 2-(4-iodophenyl)-3-(4-nitrophenol)-5-phenyltetrazolium chloride. For measuring MnSOD activity samples were incubated with 4 mM KCN for 30 min in order to block CuZnSOD. CuZnSOD activity was determined by subtracting MnSOD from total SOD activity. The SOD activity was expressed as unit per mg of protein.
Catalase activity was determined by the method of Claiborne (1985), using H2O2 as substrate. The disappearance of H2O2 was followed spectrophotometrically at 240 nm. Catalase activity was also expressed as unit per mg of protein. The activity of GPx was assayed at 340 nm, using t-butyl hydroperoxide and GSH as substrates according to Maral et al. (1977), and the activity was expressed as unit per mg of protein. Glutathione reductase activity was measured also by using a commercial kit (Randox Laboratories, Crumlin, UK), and the activity was expressed as unit per gram of protein.
Statistical Analysis of Data
Data are presented as mean ± SEM from 3 independent measurements of samples obtained from 2 separate groups each consisting of five animals (i.e., total number of animals was 10 per experimental group). For establishing significant differences data were analyzed by the one-way ANOVA followed by the Tukey post hoc test. Values were considered statistically significant if the P value was less than 0.05. In order to simplify presentation of data all statistically significant differences are given as P < 0.05, including P < 0.01 and P < 0.001.
Results
As shown in Table 1, the acute (30 min) exposure to the high intensity physical–emotional–psychosocial stress, such as immobilization, led to a significant increase in adrenaline (A) and noradrenaline (NA) serum levels, while after the chronic isolation (low intensity, but long-term psychosocial stress) both observed parameters remained unchanged. When the subsequent acute immobilization was applied to chronically stressed animals (i.e., combined stress), the levels of both A and NA were significantly increased with respect to all three groups (*P < 0.05 stress vs. control, $ P < 0.05 chronic vs. combined, % P < 0.05 acute vs. combined). The acute immobilization resulted in a significant 4.5-fold increase of serum CORT level with respect to control (*P < 0.05), which confirmed the intense GC secretion in response to this stress. On the contrary, chronic social isolation for 21 days led to significant decrease of CORT serum level (*P < 0.05) (Table 1). When the chronically stressed animals were subsequently subjected to acute immobilization, serum CORT increased to a similar level as that observed in response to the acute stress (Table 1, *P < 0.05, $ P < 0.05). The increase in CORT in animals subjected to acute or combined stress was also reflected in the increased concentration (*P < 0.05, $ P < 0.05) of blood GLU, (Table 1), whereas decreased CORT of chronically stressed animals led to low blood GLU level (*P < 0.05), since this hormone is the major regulator of gluconeogenesis (McKay and Cidlowsky 2000; Friedman et al. 1993).
Table 1.
Serum catecholamines and corticosterone concentrations and blood glucose levels in control and stressed animals
| Stress | Control | Acute | Chronic | Combined |
|---|---|---|---|---|
| Adrenaline (pg/ml) | 49.2 ± 6.5 | 714.3 ± 54.2* | 52.2 ± 7.1 | 1211.4 ± 101.2*$% |
| Noradrenaline (pg/ml) | 196.3 ± 24.1 | 554.8 ± 35.6* | 205.3 ± 12.5 | 1312.7 ± 125.5*$% |
| Corticosterone (ng/ml) | 136.8 ± 44.5 | 626.9 ± 107.1* | 64.7 ± 28.3* | 601.2 ± 89.7*$ |
| Glucose (mmol/l) | 5.7 ± 0.8 | 8.1 ± 0.7* | 3.4 ± 0.7* | 7.2 ± 0.8*$ |
* P < 0.05 stress vs. control, $ P < 0.05 chronic vs. combined, % P < 0.05 acute vs. combined
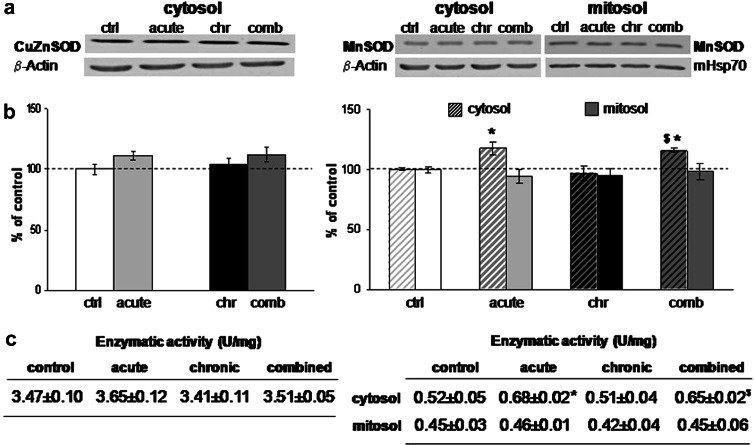
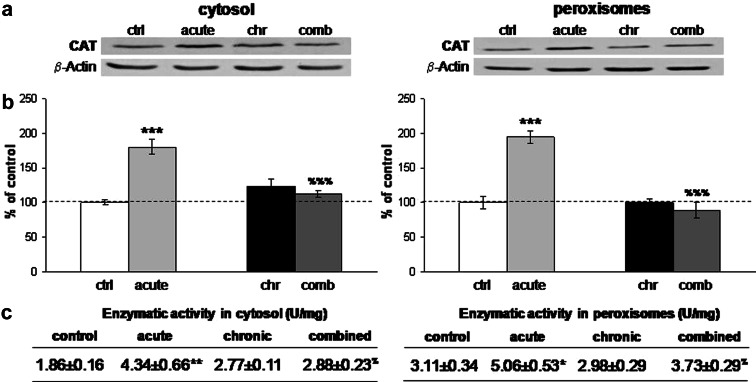
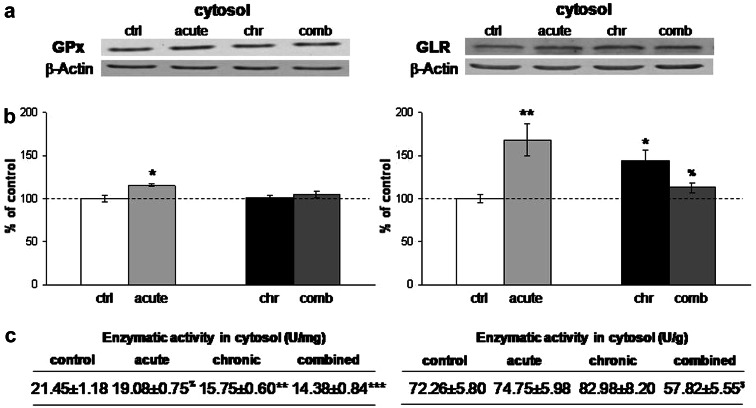
The quantification of CuZn-superoxide dismutase (CuZnSOD) did not show alterations in any of the stresses either at the level of the protein expression, or at the level of the enzymatic activity (Fig. 1b, c, left panel). Mn-superoxide dismutase (MnSOD) revealed induction of protein expression in the cytosol under acute and combined stress conditions (Fig. 1b, *P < 0.05, $ P < 0.05, right panel), while mitochondrial MnSOD remained unaffected by any of the stresses. The enzymatic activity of MnSOD (Fig. 1c, right panel) was elevated in the cytosol under acute stress with respect to the control (*P < 0.05), and under combined stress with respect to the chronic stress ($ P < 0.05). These findings were in accordance with the fact that MnSOD is an inducible form of SOD and can respond to inflammatory signaling or cytokines, while CuZnSOD is expressed constitutively (Sugiano et al. 1998). One of the two principal hydroperoxide-metabolizing enzymes, CAT, also responded to 30-min immobilization stress with increased protein expression in cytosolic and peroxisomal fraction (Fig. 2b) (***P < 0.001, %%% P < 0.001) and this increase was accompanied by the respective elevation in its enzymatic activity (Fig. 2c). Protein expression of GPx revealed induction under acute stress (Fig. 3b, left panel, *P < 0.05) but enzymatic activity of this protein remained unchanged (Fig. 3c). In terms of chronic stress conditions, protein expression was unaltered (Fig. 3b, left panel) while enzymatic activity was significantly decreased (Fig. 3c, **P < 0.01). Even though protein level of GPx remained unaltered under combined stress (Fig. 3b, left panel), its enzymatic activity was significantly decreased with respect to control, as well as, with respect to acute stress (Fig. 3c, left panel, ***P < 0.001, % P < 0.05). Protein expression of GLR was increased under the acute and chronic stress with respect to control, and was decreased in combined with respect to the acute stress (Fig. 3b, right panel, *P < 0.05, % P < 0.05). This protein showed altered enzymatic activity only in combined stress, where GLR activity was decreased with respect to chronically stressed animals (Fig. 3c, right panel, $ P < 0.05).
Fig. 1.
a Representative Western blots of CuZn-superoxide dismutases (CuZnSOD) protein expression in the cytosol (left panel) and Mn-superoxide dismutase (MnSOD) protein expression in the cytosol and in the mitochondria (right panel) in the hippocampus of stressed Wistar rats. b Relative quantification of CuZnSOD (left panel) and MnSOD (right panel) protein expression in the hippocampus obtained from 2 separate experiments each performed in triplicates. c Antioxidant enzyme activities of CuZnSOD (left panel) and MnSOD (right panel) in the hippocampus of control and stressed Wistar rats. Data are presented as mean ± SEM (as described under “Statistical analysis of data” section). * P < 0.05 stress vs. control, $P < 0.05 chronic vs. combined
Fig. 2.
a Representative Western blots of catalase (CAT) protein expression in the cytosol (left panel) and in the peroxisomes (right panel) in the hippocampus of control and stressed Wistar rats. b Relative quantification of CAT protein expression in the cytosol (left panel) and in the peroxisomes (right panel) in the hippocampus obtained from 2 separate experiments each performed in triplicates. c Antioxidant enzyme activities of CAT in the cytosol (left panel) and in the peroxisomes (right panel) in the hippocampus of control and stressed Wistar rats. Data are presented as mean ± SEM (as described under “Statistical analysis of data” section). * P < 0.05 stress vs. control, %P < 0.05 acute vs. combined
Fig. 3.
a Representative Western blots of glutathione peroxidase (GPx) protein expression in the cytosol (left panel) and glutathione reductase (GLR) protein expression in the cytosol (right panel) in the hippocampus of control and stressed Wistar rats. b Relative quantification of GPx protein expression (left panel) and GLR protein expression (right panel) in the cytosol in the hippocampus obtained from 2 separate experiments each performed in triplicates. c Antioxidant enzyme activities of GPx (left panel) and GLR (right panel) in the cytosol in the hippocampus of control and stressed Wistar rats. Data are presented as mean ± SEM (as described under “Statistical analysis of data” section). *P < 0.05 stress vs. control, %P < 0.05 acute vs. combined, $P < 0.05 chronic vs. combined
Discussion
Knowledge of the antioxidant defense system in the stress-responding structures of the CNS is of crucial importance, since oxidative damage is a phenomenon accompanying many stress-related psychiatric and neurodegenerative disorders (Barnham et al. 2004). The brain is particularly vulnerable to oxidative injury because of its high rate of oxidative metabolic activity, intense production of reactive oxygen metabolites, high content of polyunsaturated fatty acids, and relatively low antioxidant capacity (Evans 1993). In the present study, we have exploited a model of chronically isolated male Wistar rats to characterize AOEs in the HIPPO, in which we had previously documented reduced oxidative phosphorylation and compromised mitochondrial respiratory capacity (Adzic et al. 2009a). The isolated animals exhibited unaltered levels of CTs, while their CORT and blood GLU were decreased indicating compromised (poor) energy status (Table 1). At the same time, the expression levels and activities of hippocampal SODs (CuZnSOD, MnSOD), and CAT, enzymatic biomarkers of oxidative stress, remained at the control level (Figs. 1, 2), suggesting that redox balance of the HIPPO was not compromised. However, the analysis of hippocampal GPx indicated that, in spite of its unaltered expression, its enzymatic activity was diminished (Fig. 3). The great neuroprotective role of this enzyme was documented by many authors (DeHaan et al. 2003; Weisbrot-Lefkowitz et al. 1998), and its compromised activity was related with stress-induced behavioral depression in animal models (Eren et al. 2007), as well as, with the pathogenesis of the neurodegenerative diseases in some clinical studies (Power and Blumbergs 2009). Compromised activity of GPx may shift the redox balance towards the accumulation of untoxified H2O2 and •OH radicals, and peroxidized biomolecules which could further alter the activity of the enzymes sensitive to H2O2, such as GC receptor kinases, JNKs and CDKs, or nuclear factor κB (NFκB), as shown in our previous work (Adzic et al. 2009b; Djordjevic et al. 2009). In parallel with diminished GPx activity, the increase in GLR protein expression might be interpreted as an attempt to restore redox balance within the cell by increasing the pool of GSH, and by mechanisms other then those involving GPx. However, the activity of the GLR enzyme could not rise up to its full potential (even though it exhibited an increasing trend) (Fig. 3c), due to low GLU level and consequent lack of its substrate, NADPH (Suh et al. 2007). The discrepancy between the expression and the activity of the GLR might also be taken as an indicator of the underlying oxidative stress. Our findings on the GLR could also be related to the previous literature data, which showed that GLR expression level is a useful biomarker in etiology of anxiety-like behavior (Hovatta et al. 2005). In summary, our data showed that the CPSI caused significant decrease of serum CORT, and was also accompanied by the perturbations of the redox balance in the CNS, via the decreased activities of GPx and GLR, rather than through increased metabolic generation of ROS.
In the next set of experiments we have tested if the observed redox imbalance set by the CPSI, could affect the adaptive response of the HIPPO to the subsequent novelty stress. The acute 30-min immobilization was applied either to naïve or chronically isolated Wistar males. In naïve animals acute immobilization caused fourfold increases in serum CTs and CORT, and was accompanied by increase in blood GLU (Table 1). At the same time, protein expression of all AOEs showed increasing trend (Figs. 1, 2, and 3), which could be interpreted as an adaptive response to the overproduction of ROS, and may serve to combat the neuronal injury. The origin of ROS overproduction could be found in the metabolism of CTs (Smythies and Galzigna 1998) which were enhanced, or in high CORT and GLU, indicators of the higher energy status (McIntosh et al. 1998; Liu and Mori 1999). The initial signal for this upregulation could originate from the activated microglia and IL1 and TNFα signals, which are known inducers of the antioxidant defense (Bruce et al. 1996). The activity of CAT, the main scavenger of H2O2 in the neurons, was doubled in the cytoplasm, as well as, in the peroxisomes (Fig. 2), and as such, could efficiently scavenge generated H2O2. The GPx was also induced, suggesting that probably microglial cells, the major GPx source, were alerted, but GPx protein was still not fully active in 30-min time frame. Similar situation was observed in the case of GLR. Taken together, the results indicated that short-term immobilization is a strong stressor (judged by the serum level of stress hormones), and leads to redox imbalance due to increased metabolic rate. However, the increase in expression of all AOEs of HIPPO under acute immobilization, suggested that this structure would successfully manage to overcome the accompanying increase in ROS production under these conditions.
When the chronically isolated Wistar males were subjected to the subsequent acute immobilization (i.e., combined stress), we observed that CORT and GLU were increased to the same level as in acutely stressed animals, while the CTs were increased even more (Table 1). The previous CPSI experience also altered the AOEs response to a novelty stress, by attenuating the activity of peroxide-metabolizing enzymes, CAT and GPx, whose activities were almost fixed at the values set by the CPSI (Figs. 2, 3). The impaired peroxidase-metabolizing system in the brain was earlier reported in some aging studies on mice (DeHaan et al. 2003), suggesting that stress could also accelerate the aging process in the brain. Furthermore, regarding both downregulated expression and activity of GLR, chronically isolated animals responded to the acute stress in quite the opposite manner then the naïve animals (Fig. 3), even though blood GLU was restored. This could lead to GSH depletion that is known to be related with stress-induced behavioral depression and cognitive impairments (Pal and Dandiya 1994; Dean et al. 2009). Based on these data we concluded that CPSI caused permanent and irreversible changes in the antioxidant defense of HIPPO, particularly targeting peroxide-metabolizing enzymes.
In summary, our study documented that chronic social isolation of Wistar male rats compromised redox balance in the HIPPO via the decreased activities of GPx and GLR. It also compromised the response of the HIPPO to the subsequent stress particularly targeting peroxide-metabolizing enzymes, CAT, GPx, and GLR. The consequences of such alterations in antioxidant defense system of HIPPO could further lead to accumulation of peroxidizable products, especially lipids, the main constituents of neuronal membranes, and could be related with initiation of proapoptotic signaling in this CNS structure, observed in our previous study (Djordjevic et al. 2009). Although the mechanism behind the vulnerability of hippocampal neurons to the chronic neuroendocrine stress remains to be further elucidated, our findings provide important information regarding undesirable effects of prolonged stress, and also a framework for treatment of stress-related disorders.
Acknowledgments
This work was supported by the Ministry of Science and Technological Development of the Republic of Serbia, Grant No. 143042B.
References
- Adzic M, Djordjevic A, Demonacos C, Krstic-Demonacos M, Radojcic MB (2009a) The role of phosphorylated glucocorticoid receptor in mitochondrial functions and apoptotic signalling in brain tissue of stressed Wistar rats. Int J Biochem Cell Biol 41(11):2181–2188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adzic M, Djordjevic J, Djordjevic A, Niciforovic A, Demonacos C, Radojcic MB, Krstic-Demonacos M (2009b) Acute or chronic stress induce cell compartment-specific phosphorylation of glucocorticoid receptor and alter its transcriptional activity in Wistar rat brain. J Endocrinol 202(1):87–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnham KJ, Masters CL, Bush AI (2004) Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov 3(3):205–214 [DOI] [PubMed] [Google Scholar]
- Bruce AJ, Boling W, Kindy MS, Peschon J, Kraemer PJ, Carpenter MK, Holtsberg FW, Mattson MP (1996) Altered neuronal and microglial responses to excitotoxic and ischemic brain injury in mice lacking TNF receptors. Nat Med 2(7):788–794 [DOI] [PubMed] [Google Scholar]
- Claiborne A (1985) Handbook of methods for oxygene radical research. CRC Press, Boca Raton, FL, pp 283–284 [Google Scholar]
- De Kloet ER, Joels M, Holsboer F (2005) Stress and the brain: from adaptation to disease. Nat Rev Neurosci 6(6):463–475 [DOI] [PubMed] [Google Scholar]
- Dean O, Bush AI, Berk M, Copolov DL, van den Buuse M (2009) Glutathione depletion in the brain disrupts short-term spatial memory in the Y-maze in rats and mice. Behav Brain Res 198(1):258–262 [DOI] [PubMed] [Google Scholar]
- DeHaan JB, Iannello RC, Crack PJ, Hertzog P, Kola I (2003) Oxidative and free radical mechanisms in brain ageing. In: Sachdev PS (ed) The ageing brain. Psychology Press, UK, pp 187–203
- Djordjevic A, Adzic M, Djordjevic J, Radojcic MB (2009) Chronic social isolation is related to both upregulation of plasticity genes and initiation of proapoptotic signaling in Wistar rat hippocampus. J Neural Transm 116:1579–1589 [DOI] [PubMed] [Google Scholar]
- Eren I, Naziroglu M, Demirdas A, Celik O, Uguz AC, Altunbasak A, Ozmen I, Uz E (2007) Venlafaxine modulates depression-induced oxidative stress in brain and medulla of rat. Neurochem Res 32(3):497–505 [DOI] [PubMed] [Google Scholar]
- Evans PH (1993) Free radicals in brain metabolism and pathology. Br Med Bull 49(3):577–587 [DOI] [PubMed] [Google Scholar]
- Friedman JE, Yun JS, Patel YM, McGrane MM, Hanson RW (1993) Glucocorticoids regulate the induction of phosphoenolpyruvate carboxykinase (GTP) gene transcription during diabetes. J Biol Chem 268:12952–12957 [PubMed] [Google Scholar]
- Grillo CA, Piroli GG, Rosell DR, Hoskin EK, McEwen BS, Reagan LP (2003) Region specific increases in oxidative stress and superoxide dismutase in the hippocampus of diabetic rats subjected to stress. Neuroscience 121(1):133–140 [DOI] [PubMed] [Google Scholar]
- Hovatta I, Tennant RS, Helton R, Marr RA, Singer O, Redwine JM, Ellison JA, Schadt EE, Verma IM, Lockhart DJ, Barlow C (2005) Glyoxalase 1 and glutathione reductase 1 regulate anxiety in mice. Nature 438(7068):662–666 [DOI] [PubMed] [Google Scholar]
- Kaushik S, Kaur J (2003) Chronic cold exposure affects the antioxidant defense system in various rat tissues. Clin Chim Acta 333:69–77 [DOI] [PubMed] [Google Scholar]
- Kvetnansky R, Mikulaj L (1970) Adrenal and urinary catecholamines in rat during adaptation to repeated immobilization stress. Endocrinology 87:1868–1874 [DOI] [PubMed] [Google Scholar]
- Kvetnansky R, Pacak K, Fukuhara K, Viskupic E, Hiremagalur B, Nankova B, Goldstein DS, Sabban EL, Kopin IJ (1995) Sympathoadrenal system in stress. Interaction with the hypothalamic–pituitary–adrenocortical system. Ann N Y Acad Sci 771:131–158 [DOI] [PubMed] [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- Lindenau J, Noack H, Asayama K, Wolf G (1998) Enhanced cellular glutathione peroxidase immunoreactivity in activated astrocytes and in microglia during excitotoxin induced neurodegeneration. Glia 24(2):252–256 [PubMed] [Google Scholar]
- Liu J, Mori A (1999) Stress, aging, and brain oxidative damage. Neurochem Res 24(11):1479–1497 [DOI] [PubMed] [Google Scholar]
- Magarinos AM, Somoza G, De Nicola AF (1987) Glucocorticoid negative feedback and glucocorticoid receptors after hippocampectomy in rats. Horm Metab Res 19:105–109 [DOI] [PubMed] [Google Scholar]
- Maral J, Puget K, Michelson AM (1977) Comparative study of superoxide dismutase, catalase and glutathione peroxidase levels in erythrocytes of different animals. Biochem Biophys Res Commun 77:1525–1535 [DOI] [PubMed] [Google Scholar]
- Markwell MA, Haas SM, Bieber LL, Tolbert NE (1978) A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem 87(1):206–210 [DOI] [PubMed] [Google Scholar]
- Mattson MP, Liu D (2002) Energetics and oxidative stress in synaptic plasticity and neurodegenerative disorders. Neuromolecular Med 2(2):215–231 [DOI] [PubMed] [Google Scholar]
- McIntosh LJ, Hong KE, Sapolsky RM (1998) Glucocorticoids may alter antioxidant enzyme capacity in the brain: baseline studies. Brain Res 791:209–214 [DOI] [PubMed] [Google Scholar]
- McKay LI, Cidlowsky JA (2000) Corticosteroids. In: Bast Jr RC, Kufe DW, Pollock RE (eds) Cancer medicine, vol 54, 5th edn. Decker, Hamilton, pp 730–742 [Google Scholar]
- Ng F, Berk M, Dean O, Bush AI (2008) Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol 11:851–876 [DOI] [PubMed] [Google Scholar]
- Pal SN, Dandiya PC (1994) Glutathione as a cerebral substrate in depressive behavior. Pharmacol Biochem Behav 48:845–851 [DOI] [PubMed] [Google Scholar]
- Peuler JD, Johnson GA (1977) Simultaneous single isotope radioenzymatic assay of plasma norepinephrine, epinephrine and dopamine. Life Sci 21:625–636 [DOI] [PubMed] [Google Scholar]
- Power JHT, Blumbergs PC (2009) Cellular glutathione peroxidase in human brain: cellular distribution, and its potential role in the degradation of Lewy bodies in Parkinson’s disease and dementia with Lewy bodies. Acta Neuropathol 117:63–73 [DOI] [PubMed] [Google Scholar]
- Reagan LP, Magarinos AM, McEwen BS (1999) Neurological changes induced by stress in streptozotocin diabetic rats. Ann N Y Acad Sci 893:126–137 [DOI] [PubMed] [Google Scholar]
- Schad A, Fahimi HD, Völkl A, Baumgart E (2003) Expression of catalase mRNA and protein in adult rat brain: detection by nonradioactive in situ hybridization with signal amplification by catalyzed reporter deposition (ISH-CARD) and immunohistochemistry (IHC)/immunofluorescence (IF). J Histochem Cytochem 51(6):751–760 [DOI] [PubMed] [Google Scholar]
- Smythies J, Galzigna L (1998) The oxidative metabolism of catecholamines in the brain: a review. Biochim Biophys Acta 1380(2):159–162 [DOI] [PubMed] [Google Scholar]
- Sugiano N, Telleria CM, Gibori G (1998) Differential regulation of copper-zinc superoxide dismutase and manganese superoxide dismutase in the rat corpus luteum: induction of manganese superoxide dismutase messenger ribonucleic acid by inflammatory cytokines. Biol Reprod 59:208–215 [DOI] [PubMed] [Google Scholar]
- Suh SW, Gum ET, Hamby AM, Chan PH, Swanson RA (2007) Hypoglycemic neuronal death is triggered by glucose reperfusion and activation of neuronal NADPH oxidase. J Clin Invest 117:910–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisbrot-Lefkowitz M, Reuhl K, Perry B, Chan PH, Inouye M, Mirochnitchenko O (1998) Overexpression of human glutathione peroxidase protects transgenic mice against focal cerebral ischemia/reperfusion damage. Brain Res Mol Brain Res 53(1–2):333–338 [DOI] [PubMed] [Google Scholar]
- Zafir A, Banu N (2007) Antioxidant potential of fluoxetine in comparison to Curcuma longa in restraint-stressed rats. Eur J Pharmacol 572(1):23–31 [DOI] [PubMed] [Google Scholar]
- Zafir A, Banu N (2009) Induction of oxidative stress by restraint stress and corticosterone treatments in rats. Indian J Biochem Biophys 46(1):53–58 [PubMed] [Google Scholar]