Abstract
The secreted protein Sonic Hedgehog (SHH) acts in graded fashion to pattern the dorsal–ventral axis of the vertebrate neural tube. This is a dynamic process in which increasing concentrations and durations of exposure to SHH generate neurons with successively more ventral identities. Interactions between the receiving cells and the graded signal underpin the mechanism of SHH action. In particular, negative feedback, involving proteins transcriptionally induced or repressed by SHH signaling, plays an essential role in shaping the graded readout. On one hand, negative feedback controls, in a noncell-autonomous manner, the distribution of SHH across the field of receiving cells. On the other, it acts cell-autonomously to convert different concentrations of SHH into distinct durations of intracellular signal transduction. Together, these mechanisms exemplify a strategy for morphogen interpretation, which we have termed temporal adaptation that relies on the continuous processing and refinement of the cellular response to the graded signal.
Sonic hedgehog (SHH) signals pattern the neural tube's dorsal–ventral axis. Negative feedback mechanisms control the distribution and transduction of the SHH signal.
Fundamental to embryonic development is the establishment of accurate and reliable patterns of gene expression and cellular differentiation in forming tissues. This usually takes place in a progressive manner resulting in a gradual emergence of the spatial profiles of gene expression. The deployment of morphogens to direct this process raises the question of how cells respond to a graded signal to acquire the appropriate spatial and temporal identity for their position. This question is further emphasized by studies indicating that the duration of exposure as well as the concentration of the morphogen determines the cellular response (Harfe et al. 2004; Pages and Kerridge 2000), and data revealing that the accuracy of patterning often appears greater than can be accounted for by the precision of the morphogen distribution (Bergmann et al. 2007; Bergmann et al. 2008; Bollenbach et al. 2008; Gregor et al. 2007; Houchmandzadeh et al. 2002; Jaeger et al. 2004). Whether the mechanism of the response of cells to a morphogen explains the temporal features, the precision and the reliability of tissue patterning needs to be addressed.
A realization is that, in many systems, an active molecular dialogue between the graded signal and the target cells takes place. Both computational modeling and molecular genetics have suggested that this interchange is required for the interpretation of the graded signal and the spatial and temporal precision of the gene expression profiles set by morphogens (Ben-Zvi et al. 2008; Jaeger et al. 2004). Moreover, the interactions allow target cells to adjust their intracellular response to the signal and to shape the gradient itself (Ashe and Briscoe 2006; Freeman 2000; Gurdon and Bourillot 2001; Ibanes and Belmonte 2008; Jaeger et al. 2008; Pages and Kerridge 2000). In this view, the patterning action of morphogens is a dynamic process constantly refined by its interpretation. Here, we illustrate this concept using, as an example, the activity of the secreted protein sonic hedgehog (SHH) during the specification of vertebrate spinal neurons. We will discuss how negative feedback, involving proteins transcriptionally regulated by SHH, are essential for the appropriate response of cells to graded SHH signaling and to the progressive elaboration of discrete domains of neural progenitors. Moreover, by buffering the response of cells to fluctuations in the morphogen gradient, these mechanisms are likely to provide robustness and precision on pattern formation by the SHH gradient.
NEURAL TUBE PATTERNING BY GRADED SONIC HEDGEHOG SIGNALING
The vertebrate spinal cord displays a highly organized topology in which distinct neuronal subtypes are generated in characteristic order along the dorsoventral (DV) axis of the neural tube (Jessell 2000). The identity of these neurons is imposed by specific combinations of transcription factors (TFs) expressed in the progenitor cells from which the neurons derive (Guillemot 2007; Lupo et al. 2006; Zhuang and Sockanathan 2006) (Fig. 1). In the ventral spinal cord, graded SHH signaling regulates the expression of a subset of these TFs. Collectively, these define six pools of progenitors arrayed along the DV axis, termed floor plate (FP), p3, pMN, p2, p1, and p0 domains (Fig. 2A,B) (Briscoe et al. 2000; Chiang et al. 1996; Ericson et al. 1996; Lei et al. 2006; Marti et al. 1995; Pierani et al. 1999; Vokes et al. 2007; Vokes et al. 2008; Wijgerde et al. 2002). These domains emerge in a progressive manner such that the expression of TFs characteristic of increasingly more ventral progenitor populations appear sequentially at the ventral midline of the neural tube (Jeong and McMahon 2005) (Fig. 2C). The order of appearance of the TFs corresponds with their requirement for increasing concentrations and durations of SHH signaling (Briscoe et al. 2000; Dessaud et al. 2007; Ericson et al. 1997). This is apparent from ex vivo experiments using explants of chick neural tissue, which showed that two- to threefold increases in the concentration of recombinant SHH sequentially switches the identity of cells towards a more ventral cell fate (Briscoe et al. 2000; Dessaud et al. 2007; Ericson et al. 1997; Roelink et al. 1995). Similarly, increasing the length of time for which cells are exposed to SHH directs the explants toward a more ventral identity (Dessaud et al. 2007; Ericson et al. 1996). These features are consistent with a morphogen mechanism of action and raise the question of how progenitor cells interpret the quantitative information encoded by the concentration and duration of exposure to SHH. Here, we will argue that this process is tightly coupled to transcriptional targets of SHH signaling that influence the graded readout of SHH signaling.
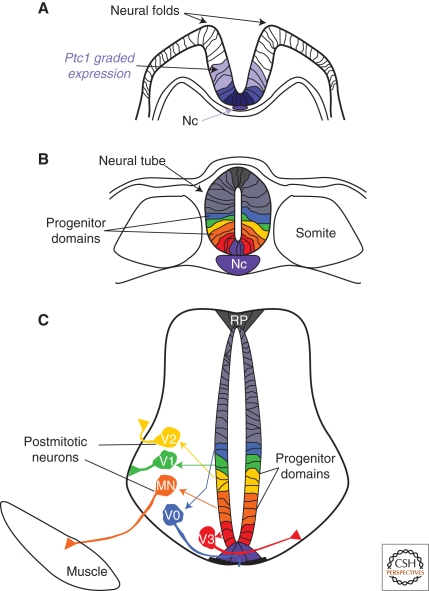
Figure 1.
Establishment of the spatial organization of neurons from ventral progenitor cells of the spinal cord. (A) Graded expression of the receptor Ptc1 (blue) in the folding neural plate, induced by SHH secreted from the Notochord. (B) After neural tube closure, six domains of progenitor cells, termed FP, p3, pMN, p2, p1, and p0 (purple, red, orange, yellow, green, blue) are precisely arranged along the dorsal–ventral (DV) axis of the neural tube. (C) Each progenitor domain generates a distinct subtype of interneurons (V0–V3) or motor neurons (MN). These postmitotic cells reach their final settling positions within the spinal cord via stereotypic migration pathways. The extension of their axons along specific routes initiates the formation of functional circuits. (Nc) Notochord, (FP) floor plate.
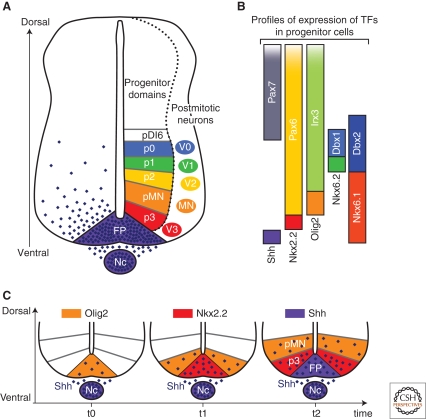
Figure 2.
Progressive emergence of SHH-regulated gene expression profiles within progenitor cells defines neuronal subtype identities in the ventral spinal cord. (A) Arrayed along the dorsal–ventral (DV) axis of the ventral neural tube are six domains of progenitor cells, FP, p3, pMN, p2, p1, and p0, which generate V0–V3 and MN neuronal subtypes. The spatial organization of the progenitor domains is established by a gradient of SHH protein (purple) secreted from the Nc and FP. (B) The restricted expression profiles of the TFs Nkx2.2, Olig2, Nkx6.1, Nkx6.2, Dbx1, Dbx2, Irx3, Pax6, and Pax7 within progenitors is regulated by graded SHH signaling. Each progenitor domain expresses a unique combination of TFs. (C) The TFs Olig2 and Nkx2.2, as well as SHH itself, distinguish the three most ventral progenitor domains (pMN, p3, and FP, respectively). The expression of each of these markers is initiated at successive developmental time points within the midline of the neural tube and extends to more dorsal positions with the appearance of the next marker at the midline. This series of gene induction events occurs in parallel to the accumulation and extension of the gradient of SHH protein in the ventral neural tube. (Nc) Notochord, (FP) floor plate.
A Temporal and Spatial Gradient of SHH Protein in the Neural Tube
Attention has focused on visualizing and measuring the distribution of SHH protein in the neural tube. SHH protein is first secreted from the notochord (Echelard et al. 1993; Roelink et al. 1995). As this structure regresses, a second center of SHH production is set up within FP cells of the neural tube (Marti et al. 1995; Roelink et al. 1995). However, this source is only established after the molecular markers of the six ventral neural progenitor domains have been induced, suggesting that ventral patterning may rely primarily on SHH derived from the notochord (Chamberlain et al. 2008; Jeong and McMahon 2005). Visualization of SHH protein in neural tissue has revealed its long range spread and indicated that most, if not all, progenitors in the ventral neural tube are exposed to SHH (Chamberlain et al. 2008; Gritli-Linde et al. 2001; Huang et al. 2007). Accordingly, inhibiting the response of cells to SHH located some distance from the ventral midline blocks their ability to generate ventral gene expression profiles and the associated differentiated cell types (Briscoe et al. 2001; Persson et al. 2002; Wijgerde et al. 2002).
The generation of a mouse SHH allele that encodes a biologically active SHH fused with the green fluorescent protein (SHH-GFP) has allowed a detailed assessment of the distribution and spread of the morphogen through the target field (Chamberlain et al. 2008). SHH-GFP molecules display punctate patterns of distribution within the neural tube. Intriguingly, these punctae seem to be enriched apically in progenitors and are found close to ciliary basal bodies. The functional significance of this association is unclear. Quantification of the distribution of this fusion protein reveals that the gradient of SHH protein decays exponentially along the DV axis (Chamberlain et al. 2008) (see Fig. 5B). As expected, the most ventral progenitor cells, the FP and p3 domain, are exposed to the highest levels of ligand. Decreased ligand levels are observed across the MN progenitor domain and the levels drop below detectability within the p2 progenitor domain.
The amplitude of the SHH-GFP gradient increases as neural tube development progresses (Chamberlain et al. 2008). As a consequence, cells close to the ventral midline are exposed to higher concentrations of SHH and for a longer period of time than more dorsal cells. Significantly, this matches the progressive induction of genes that require increasing levels or durations of SHH signaling at the ventral midline of the neural tube (Jeong and McMahon 2005; Dessaud et al. 2007) (Figs. 2C and 6A). For example, the expression of Olig2, a determinant of MN identity, is initially observed in ventral midline cells at a time when these cells have been exposed to a low concentration of SHH for a short period of time (Chamberlain et al. 2008; Jeong and McMahon 2005) (Fig. 2C). Olig2 expression expands rapidly to more dorsal positions as the gradient of SHH extends dorsally. Concomitant with this, the level of SHH protein increases at the midline, hence these cells switch off Olig2 and induce Nkx2.2, a progenitor marker located ventral to the MN progenitor domain.
A detailed analysis of SHH distribution once the TF combinations defining the ventral progenitor domains have been set up is not yet available. Nevertheless, it is apparent that it varies during time (Chamberlain et al. 2008; Danesin et al. 2006). Thus, the SHH gradient within the ventral spinal cord appears to be continually and actively modified over the course of development. The functional relevance of the dynamic nature of the SHH gradient is suggested by the close correlation between the amount and time of SHH exposure required to generate differential expression profiles in vitro and the timing and spatial patterns of gene induction in the neural tube.
The spread of SHH through the neural tube and the accuracy of its distribution are likely to be a function of the tight regulation of its secretion, diffusion, retention, and degradation by the combined action of multiple proteins (Dessaud et al. 2008; Ingham and McMahon 2001; Varjosalo and Taipale 2008). Before secretion, SHH is cholesterol-modified at the carboxyl terminus and palmitoylated at the amino terminus and then assembled into a high-molecular-weight complex (Varjosalo and Taipale 2008). The presence of lipid moieties and formation of the high-molecular- weight complex affects the diffusivity of SHH (Zeng et al. 2001). In addition, the spread of SHH is influenced by the expression of extracellular and transmembrane proteins that bind to SHH and alter its rate of diffusion or degradation within the target field. Heparan sulfate proteoglycans (HSPGs), components of the extracellular matrix, bind SHH and are likely to contribute to the rate of spread of SHH through the neural tube (Rubin et al. 2002). Similarly, the extracellular protein You/Scube2, a positive regulator of SHH signaling, might act by facilitating transport or stabilizing SHH protein within the extracellular matrix (Woods and Talbot 2005). Besides these SHH binding proteins, which are uniformly present in the neural tube, other factors affecting the spread of SHH display restricted expression patterns. The expression of several of these, including the SHH-receptor Patched 1 (Ptc1), Hedgehog interacting protein (Hhip1), and the proteins Cdo, Boc, and Gas1, is regulated by SHH signaling itself (Allen et al. 2007; Chuang et al. 2003; Goodrich et al. 1996; Goodrich et al. 1997; Jeong and McMahon 2005; Marigo and Tabin 1996; Martinelli and Fan 2007; Tenzen et al. 2006; Yao et al. 2006; Zhang et al. 2006). Thus, neural cells express many extracellular modulators of SHH and the expression of a subset of these is controlled by SHH signaling. In the following section, we will discuss the function and significance of the SHH regulated subset on the establishment and interpretation of a graded signal.
Regulation of Gli Activity by SHH Signaling is Required for the Graded Response
Two transmembrane proteins mediate the intracellular transmission of a SHH signal, Patched1 (Ptc1), which binds to SHH, and the seven-pass transmembrane protein Smoothened (Smo) (Dessaud et al. 2008; Ingham and McMahon 2001; Varjosalo and Taipale 2008) (Fig. 3). The binding of SHH to Ptc1 relieves its inhibition of Smo, allowing intracellular signal transduction. Several lines of evidence suggest that Smo activity is necessary and sufficient for transduction of a graded SHH signal in receiving cells. First, the spinal cord of Smo−/− mouse embryos lacks all cell fates normally generated ventral to the p1 progenitor domain (Wijgerde et al. 2002; Zhang et al. 2001). Second, a dominant active form of Smo is sufficient to induce, in a cell-autonomous manner, gene expression and cellular differentiation programs characteristic of the ventral neural tube (Hynes et al. 2000). Third, small molecule agonists and antagonists that directly bind and activate or inhibit Smo, independently of Ptc1 activity, are able to recapitulate the effect of a SHH concentration gradient on the expression of ventral markers in vitro (Dessaud et al. 2007). Thus, graded activation of Smo mimics the graded response produced by different concentrations of SHH, indicating that Smo activity is sufficient to explain the graded response of neural cells to SHH.
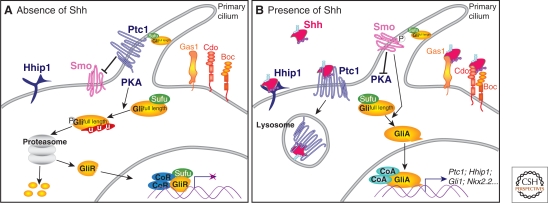
Figure 3.
SHH signal transduction. (A) In the absence of SHH, the receptor Ptc1 represses the activity of the transmembrane protein Smo and the translocation of Smo to the primary cilium of the cell. In these cells, protein kinase A (PKA) promotes the proteasome-dependent partial processing or complete degradation of the Gli transcription factors (Gli2 and Gli3). The truncated forms of these proteins (GliR) translocate to the nucleus and repress the transcription of SHH signaling targets. SuFu maintains any remaining full-length Gli proteins in an inactive state. (B) The binding of SHH to Ptc1 releases repression of Smo, allowing its translocation into the cell's cilium. The activation of Smo inhibits the proteolytic processing of Gli proteins and culminates in activated Gli proteins (GliA) translocating to the nucleus to activate target gene expression. At the cell surface, in addition to Ptc1, the SHH-binding membrane protein Hhip1 binds SHH and inhibits signaling. Conversely, the SHH-binding proteins Gas1, Cdo, and Boc enhance the response of cells to the morphogen.
The molecular cascade acting downstream of Smo activation remains poorly defined but it appears to depend on the primary cilium and the integrity of intraflagellar transport (Eggenschwiler and Anderson 2007). Ultimately, Smo activity regulates the activity of a family of zinc-finger-containing transcriptional effectors, the Gli proteins (Gli1, -2, and -3) (Matise and Joyner 1999) (Fig. 3), which are capable of acting both as activators (GliA) and repressors (GliR) of transcription. In the neural tube, the three Gli proteins together control the expression of SHH target genes (Jacob and Briscoe 2003; Vokes et al. 2007; Vokes et al. 2008).
In the absence of SHH, GliR activity is thought to dominate. This is a result of the degradation of Gli2 protein and the partial proteolytic processing of Gli3 into a truncated isoform, Gli3R, which functions as a transcriptional repressor (Aza-Blanc et al. 2000; Pan et al. 2006; Tempe et al. 2006; Wang et al. 2000) (Fig. 3A). Thus, in the neural tube, where SHH decreases below the threshold necessary to activate signaling, the transcriptional inhibitory activity of Gli3R is expected to govern responses. In line with this prediction, embryos with a mutation in Gli3 show a dorsal expansion of some markers of the ventral neural tube (Persson et al. 2002). This phenotype is rescued by an allele of Gli3 that encodes only the transcriptional repressor form of Gli3, demonstrating that the repressor activity of Gli3 is crucial for defining the dorsal extent of the ventral neural tube (Persson et al. 2002).
In cells responding to SHH, the proteolytic processing of Gli3 is inhibited, resulting in a reduction in the levels of Gli3R. Consistent with this, the inactivation in embryos, of both SHH and Gli3, leads to a partial rescue of SHH null phenotype. The rescue includes the expression of markers characteristic of the p1, p2, and pMN domains (Persson et al. 2002). A similar result is also observed in embryos that are depleted for all three Gli proteins (Bai et al. 2004; Lei et al. 2004) and in embryos lacking both Smo and Gli3 (Wijgerde et al. 2002). These data indicate that the SHH-dependent removal of Gli3 repressor activity is sufficient for the specification of p1, p2, and pMN cell identities. Nevertheless, in the compound mutants that lack SHH and Gli activity, an abnormal intermingling of the different progenitor identities is observed. This suggests that an input from the gradient of SHH is necessary for the accurate patterning and/or the positioning of these cell types.
The response to the highest levels of SHH signaling requires more than the removal of Gli3R activity. Notably, the most ventral cell identities, p3 and FP, require positive Gli transcription activity. In cells exposed to high SHH concentrations, Gli2 degradation ceases, resulting in the accumulation of the full length transcriptional activator isoform (Pan et al. 2006). In addition, the transcription of Gli3 is repressed and Gli1, which appears to act exclusively as an activator, is induced (Aza-Blanc et al. 2000; Dai et al. 1999; Lee et al. 1997; Marigo et al. 1996; Ruiz i Altaba 1998). Together, these events result in an increase in GliA activity, which might be boosted further by SHH-mediated posttranslational events that enhance the transcriptional activity of Gli1 and Gli2 (Barnfield et al. 2005; Merchant et al. 2004; Pan et al. 2006) (Fig. 3B). The loss of FP and p3 cells in mouse embryos lacking Gli2 emphasizes the importance of Gli2 in mediating the response to high levels of SHH. This phenotype is exacerbated when the Gli1 locus is also disrupted (Ding et al. 1998; Matise et al. 1998; Park et al. 2000). Together, these studies lead to the attractive and simple idea that different levels of Gli activity mediate the graded response of cells to SHH. In support of this, gain-of-function experiments indicate that changes in the transcriptional activity of GliA are sufficient to recapitulate the patterning activity of graded SHH signaling (Lei et al. 2004; Stamataki et al. 2005).
In addition to the level, the duration of Gli activity also appears to be an essential feature of how cells interpret graded SHH signaling (Dessaud et al. 2007; Stamataki et al. 2005). The length of time necessary for a dominant active form of Gli3 to induce ventral gene expression correlates with the DV position of expression of the gene in vivo (Stamataki et al. 2005). Accordingly, ventrally expressed genes take a longer time to be induced than genes expressed further away from the ventral midline (Dessaud et al. 2007; Stamataki et al. 2005). In this view, the sequential induction of more ventrally expressed genes is a consequence of a progressive increase in the duration and the level of Gli activity in response to the gradual augmentation in morphogen concentration.
A Temporal Adaptation Mechanism Integrates the Duration and Concentration of SHH Signaling
The mechanism of SHH signal transduction raises the question of how cells integrate temporal variations in SHH concentration to generate distinct quantities and durations of Gli activity. Ex vivo assays indicate that when exposed for short periods of time to either high or low concentrations of SHH, cells generate similar levels of Gli activity and express the same sets of genes (Dessaud et al. 2007) (Fig. 6A). Over time, the levels of Gli activity in cells exposed to a constant amount of SHH decrease such that the rate of decrease is inversely proportional to SHH concentration (Fig. 6A). In other words, at early time points, relatively low concentrations of SHH are sufficient to activate the highest levels of signal transduction. With increasing time, however, cells become desensitized to ongoing SHH signaling. In this way, cells convert a concentration of SHH into a proportional period of Gli activity. The progressive increase in the length of these periods in response to higher SHH concentration correlates with the sequential appearance of more ventrally expressed genes (Dessaud et al. 2007; Jeong and McMahon 2005).
These data suggest a novel mechanism—“temporal adaptation”—for how cells interpret a morphogen. In this model, extracellular concentrations of signal are converted into intracellular periods of signal transduction. One feature of this mechanism is that changing the concentration and/or the duration of SHH exposure has a similar effect on gene expression: Reducing either results in a decrease in the duration of intracellular Gli activity. Together with the evidence that increasing levels of Gli activity induce progressively more ventral responses, the data suggest that the induction of a particular DV identity requires Gli activity to be maintained above a specific level for a set period of time. This reconciles previous models of morphogen activity that have emphasized either concentration- or time-dependent mechanisms (Dessaud et al. 2008; Gurdon and Bourillot 2001; Harfe et al. 2004; Ibanes and Belmonte 2008; Pages and Kerridge 2000; Scherz et al. 2007). A temporal adaptation mechanism transforms both the duration and concentration of the extracellular ligand into corresponding durations and strength of intracellular signaling. Importantly, the way in which cells convert an extracellular concentration of SHH into different durations of intracellular Gli activity depends on negative feedback initiated by SHH-Gli signaling. We will discuss the function and implications of these regulatory loops in the next section.
NEGATIVE FEEDBACK LOOPS INVOLVING SHH BINDING PROTEINS SHAPE THE GRADED READOUT OF SHH SIGNALING
A diverse set of membrane-linked proteins influence the spread of SHH through the neural tube and the response of cells to this signal. These factors transcriptionally respond to SHH signaling and bind to SHH to either enhance or inhibit signal transduction. These proteins can be divided into two categories (Fig. 4). The first group comprises Cdo, Boc, and Gas1. These enhance signaling, but are transcriptionally down-regulated by SHH signaling (Allen et al. 2007; Martinelli and Fan 2007; Tenzen et al. 2006; Yao et al. 2006; Zhang et al. 2006). A second category acts as negative feedback regulators. It includes the receptor Ptc1 and the EGF-repeat-containing membrane-linked protein Hhip1, both of which inhibit SHH signal transduction and are transcriptionally up-regulated by SHH signaling (Chuang et al. 2003; Goodrich et al. 1996; Goodrich et al. 1997; Jeong and McMahon 2005; Marigo and Tabin 1996). Together, these two sets of proteins constitute a regulatory feedback system that, as described below, is crucial for the morphogen activity of SHH in the neural tube and is likely to provide robustness and precision to pattern formation.
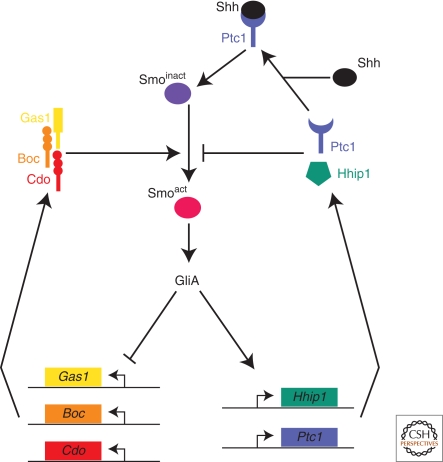
Figure 4.
Negative feedback loops in the SHH signaling pathway driven by SHH-binding proteins. The membrane proteins Gas1, Cdo, and Boc promote the activation of Smo and are transcriptionally inhibited by SHH signaling. Conversely, Ptc1 and Hhip1 are transcriptionally induced by Gli activation and inhibit the transduction of a SHH signal to Smo.
Feedback Loops Involving Gas1, Cdo, and Boc Initiate the Ventral Specification Program
The related transmembrane proteins Cdo and Boc, as well as the GPI-linked protein Gas1 enhance intracellular SHH signaling possibly by binding SHH and presenting it to Ptc1 (Allen et al. 2007; Martinelli and Fan 2007; Tenzen et al. 2006; Yao et al. 2006; Zhang et al. 2006). During neural development, Gas1 is initially expressed within ventral progenitors of the spinal cord (Allen et al. 2007). Its expression in the ventral neural tube is then down-regulated and expression is observed in intermediate and dorsal positions. Cdo and Boc are mainly expressed within the intermediate and dorsal neural tube. The expression domain of Boc extends more ventrally than that of Cdo (Tenzen et al. 2006). In addition, Cdo is transiently expressed within presumptive FP cells at the ventral midline of the neural tube at early developmental stages. The repression of each of these genes in the ventral neural tube is likely to be a consequence of their negative regulation by SHH signaling (Allen et al. 2007; Tenzen et al. 2006).
Despite the transience of Cdo and Gas1 expression in the ventral neural tube, their function is essential for the appropriate specification of cellular identities in this region. Null mutations in Cdo or Gas1 result in neural tube phenotypes reminiscent of a decrease in SHH signaling (Fig. 5A). These are further exacerbated in Shh+/− embryos (Allen et al. 2007; Martinelli and Fan 2007; Tenzen et al. 2006). Furthermore, the neural tube of Cdo−/−; Gas1−/− double mutant embryos lack FP, p3, and pMN cells (Allen et al. 2007). Consistent with a positive function of these proteins on SHH signaling, forced expression of any of the three proteins in the chick neural tube promotes the cell-autonomous induction of more ventral cell fates (Allen et al. 2007; Martinelli and Fan 2007; Tenzen et al. 2006) (Fig. 5B). Together, these studies suggest that Gas1 and Cdo contribute to the generation of an early burst of SHH signaling in the ventral neural tube, perhaps by sensitizing cells to low levels of SHH protein that are presumably present at these times (Allen et al. 2007). The SHH dependent inhibition of Gas1 and Cdo expression ensures that this initial boost in signaling is only temporary. Likewise, the dorsal expression of the three proteins might provide a mechanism to enhance sensitivity to low levels of SHH in these regions.
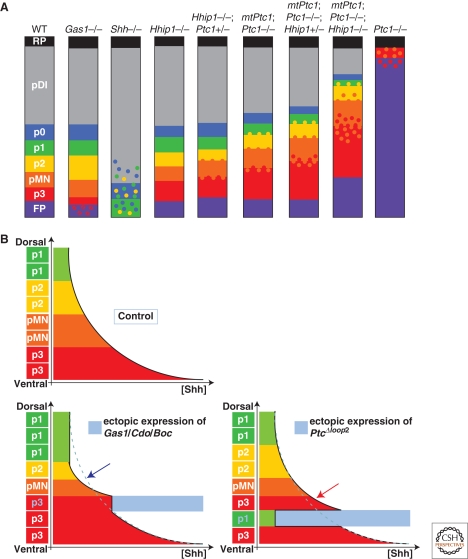
Figure 5.
Regulation of SHH-mediated pattern formation in the ventral spinal cord by the SHH-binding proteins. (A) Diagrammatic representation of the effect of mutations of SHH binding proteins on pattern formation in the neural tube. SHH−/− spinal cord displays a complete absence of molecular identities characteristic of the FP, p3, and pMN progenitor domains and severe reduction and ventral displacement of p2, p1, and p0 identities. Loss of Gas1 (Gas1−/−) results in a reduction of the number of p3 cells, reminiscent of a mild reduction in the SHH signaling activity. In contrast to Gas1 and SHH mutant embryos, compound mutant embryos for Hhip1 and Ptc1 display expansion of the ventral most progenitor domains. In the Hhip1 mutant, the p3 progenitor domain increases in size. The other ventral progenitor domains remain unaffected. More dramatically, in Ptc1 mutants, dorsal progenitor identities are absent, with the exception of the roof plate, and most progenitor cells adopt a FP identity. A few scattered cells with pMN and p3 characteristics are intermixed in the most dorsal regions. This phenotype is partially rescued by a transgene expressing Ptc1 ubiquitously at low levels (MtPtc1). Thus, in the MtPtc1;Ptc1−/−, the p3, pMN progenitor domains are expanding dorsally. Most strikingly, cells with distinct fates are mixed at the boundaries between the p3 and pMN, and the pMN and the p2 progenitor domains in these compound mutants, indicating a role for Ptc1-mediated feedback in the precision of pattern formation. The progressive loss of alleles of Hhip1 in the MtPtc1;Ptc1−/− background exacerbates these phenotypes, demonstrating that Ptc1 and Hhip1 share redundant functions in defining the position and precision of boundaries between distinct progenitor domains. (B) Schematic representation of the gradient of SHH along the DV axis of the neural tube and of the subsequent position of the ventral cell fates in control embryos or in embryos in which Gas1/Cdo/Boc or Ptc1Δloop2 have been ectopically expressed (blue shading). The expression of Gas1, Cdo, or Boc results in a local increase in the concentration of SHH protein, thus ventralizing the fate of cells. Because of the local sequestration of the ligand, SHH is reduced dorsally (blue arrow). As a consequence, the size of pMN and p1 domains are decreased in size. The expression of Ptc1Δloop2 leads to a cell-autonomous reduction of the response of cells to SHH. A consequence of this is that cells that would normally adopt a p3 identity are transformed to a more dorsal fate. Ptc1Δloop2 does not sequester SHH protein. Therefore, SHH concentration is increased in a noncell-autonomous manner, dorsal to the Ptc1Δloop2 expressing region, which leads to the generation of p3 cell fate at a position where pMN is normally generated.
Ptc1 and Hhip1 Feedback Loops Contribute at Multiple Levels to the Accurate Readout of Graded SHH Signaling
In contrast to the proteins described in the preceding section, Hhip1 and Ptc1 are strong inhibitors of SHH signaling (Coulombe et al. 2004; Goodrich et al. 1996; Goodrich et al. 1997; Jeong and McMahon 2005; Ochi et al. 2006; Olsen et al. 2004; Taipale et al. 2002). In vivo, these factors share overlapping functions to limit the extent and the level of SHH signaling in the target field. The complete elimination of Ptc1 expression results in derepression of SHH signaling throughout the neural tube and the induction of ventral identities along the entire DV axis (Goodrich et al. 1997) (Fig. 5A). This phenotype can be partially rescued by expressing ubiquitously low levels of Ptc1 using a metallothionein promoter (Jeong and McMahon 2005; Milenkovic et al. 1999) (Fig. 5A). In these mtPtc1:Ptc1−/− embryos, the progressive removal of Hhip1 alleles leads to a gradual dorsal expansion of gene expression and cell identities characteristic of the ventral neural tube (Fig. 5A). Conversely, ectopic expression of Hhip1 or Ptc1 blocks, in a cell-autonomous manner, the establishment of ventral cell types in the neural tube (Briscoe et al. 2001; Stamataki et al. 2005).
A similar activity of Ptc is observed in Drosophila tissue patterned by Hh signaling (Chen and Struhl 1996). In this case, an alteration in the patterning of the surrounding tissue is also observed. This nonautonomous effect is attributable to the ability of Ptc to bind and sequester Hh, thereby altering the local concentration of Hh and restricting its spread through the tissue (Chen and Struhl 1996). Several experiments support the idea that the activity of Ptc1 and Hhip1 and their regulation by SHH signaling play a similar role in shaping the extracellular gradient of SHH protein in the vertebrate neural tube. The forced expression of a form of Ptc1 that does not bind SHH but retains the ability to inhibit SHH signal transduction (Ptc1Δloop2) identified a noncell-autonomous role for Ptc1 in restricting the dorsal expansion of ventral cell fates (Fig. 5B). This suggested that SHH dependent up-regulation of Ptc1, or other molecules capable of binding SHH, influences the spatial distribution of the ligand (Briscoe et al. 2001; Chen and Struhl 1996). Similarly, overexpression of Hhip1 results in a noncell-autonomous induction of Pax7, a gene normally repressed by low levels of SHH signaling, in abnormal ventral positions. This is consistent with a local depletion of SHH as a consequence of its sequestration by cells overexpressing Hhip1 (Stamataki et al. 2005). Direct support for the importance of SHH signaling to limit the extent of SHH spread comes from analysis of the SHH-GFP allele. In embryos mutant for Smo, which lack the ability to respond to SHH signaling, SHH-GFP spreads twice as far as in wild-type embryos (Chamberlain et al. 2008). The decay length of the ligand gradient is also extended in the absence of Smo, consistent with a disproportionate expansion of the more ventral domains of gene expression in embryos in which Ptc1 and Hhip1 gene dosage is reduced (Jeong and McMahon 2005).
The cell-autonomous inhibition of SHH signaling by Ptc1 and Hhip1 also appears important for the cellular interpretation of SHH signaling in the neural tube. A priori, the transcriptional up-regulation of Ptc1 and Hhip1 by SHH signaling and their ability to cell-autonomously inhibit SHH signaling would result in the gradual desensitization of cells to SHH. Consistent with this, the profile of Gli activity induced by directly activating Smo using purmorphamine, a small molecule that functions downstream of Ptc1 and Hhip1, is different from that generated by activating the pathway with SHH protein (Dessaud et al. 2007). Although activation by SHH produces periods of Gli activity proportional to the SHH concentration, this is not observed with Smo agonists. This suggests that negative feedback upstream of Smo is required for the normal response of cells (Fig. 6A,B). In addition, inhibition of Ptc1 up-regulation alters the response of cells to defined concentrations of SHH, permitting a lower than normal concentration of SHH to induce more ventral gene expression (Dessaud et al. 2007). This indicates that the SHH dependent up-regulation of Ptc1 is necessary for the temporal adaptation of cells to SHH signaling. Together, the data support a model in which the negative feedback loop involving SHH induction of Ptc1 and Hhip1 plays a crucial role in establishing the normal distribution of SHH in the neural tube and converting the extracellular concentration of SHH into an appropriate period of intracellular signaling.
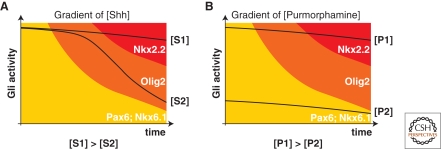
Figure 6.
Correlation between the temporal profiles of Gli activity and gene expression profiles in cells exposed to either SHH or the Smo-agonist purmorphamine. (A) Different concentrations of SHH generate distinct durations of Gli activity. Both low (S1) and high (S2) concentrations of morphogen initially induce high levels of Gli activity. Over time, in cells exposed to a low concentration of SHH, the Gli activity drops quickly, whereas at the high concentration of morphogen, Gli activity is maintained at high levels. Consequently, high concentrations of SHH induce Nkx2.2 after transiently expressing Olig2, whereas at the lower concentration, cells maintain Olig2 expression. (B) In contrast to SHH, purmorphamine, a Smo agonist, generates profiles of Gli activity that do not vary as greatly through time. A high concentration (P2) consistently generates higher levels of Gli activity at each time point than a lower concentration (P1). This suggests that the generation of distinct periods of Gli activity by SHH is mediated upstream of Smo.
The cell-autonomous and nonautonomous modes of action of Hhip1 and Ptc1 on SHH signaling and their regulation by the pathway constitute a powerful means to generate spatially and temporally restricted patterns of SHH signaling within the target field. The importance of these proteins in the interpretation of graded SHH signaling is illustrated by the progressive increase in the expansion of ventral identities as negative feedback is genetically reduced in mutant mouse embryos (Jeong and McMahon 2005) (Fig. 5A). The relative contribution that the autonomous and nonautonomous mechanisms make to these phenotypes has yet to be assessed and will require methods that provide a way to correlate the distribution of SHH ligand with ongoing measurements of the activity of the pathway. More generally, these reagents will be important for testing the model of morphogen interpretation and providing a more detailed picture of the dynamics of signaling in the neural tube.
NEGATIVE FEEDBACK AND THE TRANSCRIPTIONAL RESPONSE OF CELLS ARE NECESSARY FOR MORPHOGEN ACTIVITY
Together, the studies of graded SHH mediated patterning of the neural tube have revealed the importance of negative feedback in the establishment and interpretation of morphogen signaling. On the one hand, this system generates the spatial and temporal dynamics in the cell response to SHH signaling that are essential for the appropriate gene expression responses. On the other, the opposing roles and activity of Hhip1 and Ptc1, versus Gas1, Cdo, and Boc might act as a buffering system to enhance signaling when levels of SHH are low and reduce signaling in cells exposed to high levels of ligand. This may compensate for fluctuations in ligand availability, particularly at early stages of neural tube development, thereby rendering some robustness to gradient interpretation (Chamberlain et al. 2008; Jeong and McMahon 2005). The role of inhibitors in providing precision and robustness to morphogen interpretation have been clearly illustrated in Xenopus, where chordin, emanating from the organizer, establishes a well-defined, graded activation profile of BMP signaling (Ben-Zvi et al. 2008). It appears likely that the negative feedback initiated by SHH-binding proteins has a similar precision-enhancing function during patterning of the vertebrate neural tube. In embryos in which negative feedback is disrupted, patterning appears less precise, such that cells belonging to adjacent progenitor domains are extensively intermixed instead of sharply delimited (Allen et al. 2007; Jeong and McMahon 2005) (Fig. 5A). Moreover, just as regulatory feedback involving Chordin is important for scaling the patterns of gene expression to the size of Xenopus embryos, Ptc1- and Hhip1-mediated negative feedback appear important for producing ventral progenitor domains proportioned appropriately to the size of the spinal cord (Allen et al. 2007; Jeong and McMahon 2005; Ben-Zvi et al. 2008).
In addition to the negative feedback relayed by the SHH-binding proteins, the accurate interpretation of SHH signaling depends on further interactions between the genes that respond to the SHH. Studies are beginning to reveal the gene regulatory network (GRN) operating downstream of SHH-Gli signaling and highlight the importance of the regulatory links within the network for determining the appropriate temporal and spatial gene expression profiles (Briscoe and Ericson 2001; Hallikas et al. 2006; Lei et al. 2006; Ribes et al. 2008; Vokes et al. 2007; Vokes et al. 2008). Two features of the network are almost certainly relevant. First, the Gli proteins appear to directly regulate the expression of several TFs that constitute important functional links within the GRN (Lei et al. 2006; Ribes et al. 2008; Vokes et al. 2007; Vokes et al. 2008). Selective positive and negative cross-regulation between these proteins and other members of the GRN then contribute to the refinement and the maintenance of gene expression profiles (Bailey et al. 2006; Briscoe et al. 2000). Second, Gli proteins are likely to cooperate at the level of cis-regulatory elements with other TFs of the GRN (Hallikas et al. 2006; Lei et al. 2006; Ribes et al. 2008), some of which are asymmetrically expressed within the neural tube or activated by other localized signals. This cooperation is an efficient way to create unique combinations of TF activity at distinct locations along the DV axis of the neural tube and thereby increase the diversity of gene responses.
DYNAMIC INTERPRETATION OF MORPHOGEN GRADIENTS
Significant additional work is needed to provide a comprehensive picture of the molecular mechanisms underlying the response of cells to graded SHH signaling and the precision in pattern formation in the neural tube. Nevertheless, taken as a whole, the studies of SHH signaling suggest a novel mechanism to explain the response of cells to a morphogen. Cells appear to integrate both extracellular concentrations and durations of the signal to produce discrete durations of intracellular signal transduction. This reconciles models of morphogen activity that have emphasized concentration dependent responses with those that highlight temporal responses (Pages and Kerridge 2000; Jaeger et al. 2008). Importantly, this temporal adaptation mechanism means that positional information, as defined in the French Flag model (Wolpert 1969), cannot be equated solely with a concentration of morphogen. This might also be the case for other morphogens, in which no direct biochemical correlate of position is immediately apparent in a tissue (Jaeger et al. 2008). Instead, the process of morphogen interpretation is dynamic and one in which a cell constantly refines its response to the signal and in the process of doing so alters the signal itself.
ACKNOWLEDGMENTS
We are grateful to Anna Kicheva, Eva Kutejova, and Eric Dessaud for critical comments and discussion. V.R. is supported by an EMBO Long Term Fellowship. J.B. is supported by the MRC (UK).
Footnotes
Editors: James Briscoe, Peter Lawrence, and Jean-Paul Vincent
Additional Perspectives on Generation and Interpretation of Morphogen Gradients available at www.cshperspectives.org
REFERENCES
- Allen BL, Tenzen T, McMahon AP 2007. The Hedgehog- binding proteins Gas1 and Cdo cooperate to positively regulate SHH signaling during mouse development. Genes Dev 21:1244–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashe HL, Briscoe J 2006. The interpretation of morphogen gradients. Development 133:385–394 [DOI] [PubMed] [Google Scholar]
- Aza-Blanc P, Lin HY, Ruiz i Altaba A, Kornberg TB 2000. Expression of the vertebrate Gli proteins in Drosophila reveals a distribution of activator and repressor activities. Development 127:4293–4301 [DOI] [PubMed] [Google Scholar]
- Bai CB, Stephen D, Joyner AL 2004. All mouse ventral spinal cord patterning by hedgehog is Gli dependent and involves an activator function of Gli3. Dev Cell 6:103–115 [DOI] [PubMed] [Google Scholar]
- Bailey PJ, Klos JM, Andersson E, Karlen M, Kallstrom M, Ponjavic J, Muhr J, Lenhard B, Sandelin A, Ericson J 2006. A global genomic transcriptional code associated with CNS-expressed genes. Exp Cell Res 312:3108–3119 [DOI] [PubMed] [Google Scholar]
- Barnfield PC, Zhang X, Thanabalasingham V, Yoshida M, Hui CC 2005. Negative regulation of Gli1 and Gli2 activator function by Suppressor of fused through multiple mechanisms. Differentiation 73:397–405 [DOI] [PubMed] [Google Scholar]
- Ben-Zvi D, Shilo BZ, Fainsod A, Barkai N 2008. Scaling of the BMP activation gradient in Xenopus embryos. Nature 453:1205–1211 [DOI] [PubMed] [Google Scholar]
- Bergmann S, Sandler O, Sberro H, Shnider S, Schejter E, Shilo BZ, Barkai N 2007. Pre-steady-state decoding of the Bicoid morphogen gradient. PLoS Biol 5:e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann S, Tamari Z, Schejter E, Shilo BZ, Barkai N 2008. Re-examining the stability of the Bicoid morphogen gradient. Cell 132:15–17; author reply 17–18 [DOI] [PubMed] [Google Scholar]
- Bollenbach T, Pantazis P, Kicheva A, Bokel C, Gonzalez-Gaitan M, Julicher F 2008. Precision of the Dpp gradient. Development 135:1137–1146 [DOI] [PubMed] [Google Scholar]
- Briscoe J, Chen Y, Jessell TM, Struhl G 2001. A hedgehog- insensitive form of patched provides evidence for direct long-range morphogen activity of sonic hedgehog in the neural tube. Mol Cell 7:1279–1291 [DOI] [PubMed] [Google Scholar]
- Briscoe J, Ericson J 2001. Specification of neuronal fates in the ventral neural tube. Curr Opin Neurobiol 11:43–49 [DOI] [PubMed] [Google Scholar]
- Briscoe J, Pierani A, Jessell TM, Ericson J 2000. A homeodomain protein code specifies progenitor cell identity and neuronal fate in the ventral neural tube. Cell 101:435–445 [DOI] [PubMed] [Google Scholar]
- Chamberlain CE, Jeong J, Guo C, Allen BL, McMahon AP 2008. Notochord-derived SHH concentrates in close association with the apically positioned basal body in neural target cells and forms a dynamic gradient during neural patterning. Development 135:1097–1106 [DOI] [PubMed] [Google Scholar]
- Chen Y, Struhl G 1996. Dual roles for patched in sequestering and transducing Hedgehog. Cell 87:553–563 [DOI] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA 1996. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature 383:407–413 [DOI] [PubMed] [Google Scholar]
- Chuang PT, Kawcak T, McMahon AP 2003. Feedback control of mammalian Hedgehog signaling by the Hedgehog-binding protein, Hip1, modulates Fgf signaling during branching morphogenesis of the lung. Genes Dev 17:342–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombe J, Traiffort E, Loulier K, Faure H, Ruat M 2004. Hedgehog interacting protein in the mature brain: membrane-associated and soluble forms. Mol Cell Neurosci 25:323–333 [DOI] [PubMed] [Google Scholar]
- Dai P, Akimaru H, Tanaka Y, Maekawa T, Nakafuku M, Ishii S 1999. Sonic Hedgehog-induced activation of the Gli1 promoter is mediated by GLI3. J Biol Chem 274:8143–8152 [DOI] [PubMed] [Google Scholar]
- Danesin C, Agius E, Escalas N, Ai X, Emerson C, Cochard P, Soula C 2006. Ventral neural progenitors switch toward an oligodendroglial fate in response to increased Sonic hedgehog (SHH) activity: involvement of Sulfatase 1 in modulating SHH signaling in the ventral spinal cord. J Neurosci 26:5037–5048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessaud E, McMahon AP, Briscoe J 2008. Pattern formation in the vertebrate neural tube: a sonic hedgehog morphogen-regulated transcriptional network. Development 135:2489–2503 [DOI] [PubMed] [Google Scholar]
- Dessaud E, Yang LL, Hill K, Cox B, Ulloa F, Ribeiro A, Mynett A, Novitch BG, Briscoe J 2007. Interpretation of the sonic hedgehog morphogen gradient by a temporal adaptation mechanism. Nature 450:717–720 [DOI] [PubMed] [Google Scholar]
- Ding Q, Motoyama J, Gasca S, Mo R, Sasaki H, Rossant J, Hui CC 1998. Diminished Sonic hedgehog signaling and lack of floor plate differentiation in Gli2 mutant mice. Development 125:2533–2543 [DOI] [PubMed] [Google Scholar]
- Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP 1993. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell 75:1417–1430 [DOI] [PubMed] [Google Scholar]
- Eggenschwiler JT, Anderson KV 2007. Cilia and developmental signaling. Annu Rev Cell Dev Biol 23:345–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson J, Morton S, Kawakami A, Roelink H, Jessell TM 1996. Two critical periods of Sonic Hedgehog signaling required for the specification of motor neuron identity. Cell 87:661–673 [DOI] [PubMed] [Google Scholar]
- Ericson J, Rashbass P, Schedl A, Brenner-Morton S, Kawakami A, van Heyningen V, Jessell TM, Briscoe J 1997. Pax6 controls progenitor cell identity and neuronal fate in response to graded SHH signaling. Cell 90:169–180 [DOI] [PubMed] [Google Scholar]
- Freeman M 2000. Feedback control of intercellular signalling in development. Nature 408:313–319 [DOI] [PubMed] [Google Scholar]
- Goodrich LV, Johnson RL, Milenkovic L, McMahon JA, Scott MP 1996. Conservation of the hedgehog/patched signaling pathway from flies to mice: induction of a mouse patched gene by Hedgehog. Genes Dev 10:301–312 [DOI] [PubMed] [Google Scholar]
- Goodrich LV, Milenkovic L, Higgins KM, Scott MP 1997. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science 277:1109–1113 [DOI] [PubMed] [Google Scholar]
- Gregor T, Tank DW, Wieschaus EF, Bialek W 2007. Probing the limits to positional information. Cell 130:153–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritli-Linde A, Lewis P, McMahon AP, Linde A 2001. The whereabouts of a morphogen: direct evidence for short- and graded long-range activity of hedgehog signaling peptides. Dev Biol 236:364–386 [DOI] [PubMed] [Google Scholar]
- Guillemot F 2007. Spatial and temporal specification of neural fates by transcription factor codes. Development 134:3771–3780 [DOI] [PubMed] [Google Scholar]
- Gurdon JB, Bourillot PY 2001. Morphogen gradient interpretation. Nature 413:797–803 [DOI] [PubMed] [Google Scholar]
- Hallikas O, Palin K, Sinjushina N, Rautiainen R, Partanen J, Ukkonen E, Taipale J 2006. Genome-wide prediction of mammalian enhancers based on analysis of transcription-factor binding affinity. Cell 124:47–59 [DOI] [PubMed] [Google Scholar]
- Harfe BD, Scherz PJ, Nissim S, Tian H, McMahon AP, Tabin CJ 2004. Evidence for an expansion-based temporal SHH gradient in specifying vertebrate digit identities. Cell 118:517–528 [DOI] [PubMed] [Google Scholar]
- Houchmandzadeh B, Wieschaus E, Leibler S 2002. Establishment of developmental precision and proportions in the early Drosophila embryo. Nature 415:798–802 [DOI] [PubMed] [Google Scholar]
- Huang X, Litingtung Y, Chiang C 2007. Region-specific requirement for cholesterol modification of sonic hedgehog in patterning the telencephalon and spinal cord. Development 134:2095–2105 [DOI] [PubMed] [Google Scholar]
- Hynes M, Ye W, Wang K, Stone D, Murone M, Sauvage F, Rosenthal A 2000. The seven-transmembrane receptor smoothened cell-autonomously induces multiple ventral cell types. Nat Neurosci 3:41–46 [DOI] [PubMed] [Google Scholar]
- Ibanes M, Belmonte JC 2008. Theoretical and experimental approaches to understand morphogen gradients. Mol Syst Biol 4:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham PW, McMahon AP 2001. Hedgehog signaling in animal development: paradigms and principles. Genes Dev 15:3059–3087 [DOI] [PubMed] [Google Scholar]
- Jacob J, Briscoe J 2003. Gli proteins and the control of spinal-cord patterning. EMBO Rep 4:761–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger J, Irons D, Monk N 2008. Regulative feedback in pattern formation: towards a general relativistic theory of positional information. Development 135:3175–3183 [DOI] [PubMed] [Google Scholar]
- Jaeger J, Surkova S, Blagov M, Janssens H, Kosman D, Kozlov KN, Manu Myasnikova E, Vanario-Alonso CE, Samsonova M et al. 2004. Dynamic control of positional information in the early Drosophila embryo. Nature 430:368–371 [DOI] [PubMed] [Google Scholar]
- Jeong J, McMahon AP 2005. Growth and pattern of the mammalian neural tube are governed by partially overlapping feedback activities of the hedgehog antagonists patched 1 and Hhip1. Development 132:143–154 [DOI] [PubMed] [Google Scholar]
- Jessell TM 2000. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet 1:20–29 [DOI] [PubMed] [Google Scholar]
- Lee J, Platt KA, Censullo P, Ruiz i Altaba A 1997. Gli1 is a target of Sonic hedgehog that induces ventral neural tube development. Development 124:2537–2552 [DOI] [PubMed] [Google Scholar]
- Lei Q, Jeong Y, Misra K, Li S, Zelman AK, Epstein DJ, Matise MP 2006. Wnt signaling inhibitors regulate the transcriptional response to morphogenetic SHH-Gli signaling in the neural tube. Dev Cell 11:325–337 [DOI] [PubMed] [Google Scholar]
- Lei Q, Zelman AK, Kuang E, Li S, Matise MP 2004. Transduction of graded Hedgehog signaling by a combination of Gli2 and Gli3 activator functions in the developing spinal cord. Development 131:3593–3604 [DOI] [PubMed] [Google Scholar]
- Lupo G, Harris WA, Lewis KE 2006. Mechanisms of ventral patterning in the vertebrate nervous system. Nat Rev Neurosci 7:103–114 [DOI] [PubMed] [Google Scholar]
- Marigo V, Johnson RL, Vortkamp A, Tabin CJ 1996. Sonic hedgehog differentially regulates expression of GLI and GLI3 during limb development. Dev Biol 180:273–283 [DOI] [PubMed] [Google Scholar]
- Marigo V, Tabin CJ 1996. Regulation of patched by sonic hedgehog in the developing neural tube. Proc Natl Acad Sci 93:9346–9351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti E, Bumcrot DA, Takada R, McMahon AP 1995. Requirement of 19K form of Sonic hedgehog for induction of distinct ventral cell types in CNS explants. Nature 375:322–325 [DOI] [PubMed] [Google Scholar]
- Martinelli DC, Fan CM 2007. Gas1 extends the range of Hedgehog action by facilitating its signaling. Genes Dev 21:1231–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matise MP, Epstein DJ, Park HL, Platt KA, Joyner AL 1998. Gli2 is required for induction of floor plate and adjacent cells, but not most ventral neurons in the mouse central nervous system. Development 125:2759–2770 [DOI] [PubMed] [Google Scholar]
- Matise MP, Joyner AL 1999. Gli genes in development and cancer. Oncogene 18:7852–7859 [DOI] [PubMed] [Google Scholar]
- Merchant M, Vajdos FF, Ultsch M, Maun HR, Wendt U, Cannon J, Desmarais W, Lazarus RA, de Vos AM, de Sauvage FJ 2004. Suppressor of fused regulates Gli activity through a dual binding mechanism. Mol Cell Biol 24:8627–8641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milenkovic L, Goodrich LV, Higgins KM, Scott MP 1999. Mouse patched1 controls body size determination and limb patterning. Development 126:4431–4440 [DOI] [PubMed] [Google Scholar]
- Ochi H, Pearson BJ, Chuang PT, Hammerschmidt M, Westerfield M 2006. Hhip regulates zebrafish muscle development by both sequestering Hedgehog and modulating localization of Smoothened. Dev Biol 297:127–140 [DOI] [PubMed] [Google Scholar]
- Olsen CL, Hsu PP, Glienke J, Rubanyi GM, Brooks AR 2004. Hedgehog-interacting protein is highly expressed in endothelial cells but down-regulated during angiogenesis and in several human tumors. BMC Cancer 4:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pages F, Kerridge S 2000. Morphogen gradients. A question of time or concentration? Trends Genet 16:40–44 [DOI] [PubMed] [Google Scholar]
- Pan Y, Bai CB, Joyner AL, Wang B 2006. Sonic hedgehog signaling regulates Gli2 transcriptional activity by suppressing its processing and degradation. Mol Cell Biol 26:3365–3377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HL, Bai C, Platt KA, Matise MP, Beeghly A, Hui CC, Nakashima M, Joyner AL 2000. Mouse Gli1 mutants are viable but have defects in SHH signaling in combination with a Gli2 mutation. Development 127:1593–1605 [DOI] [PubMed] [Google Scholar]
- Persson M, Stamataki D, te Welscher P, Andersson E, Bose J, Ruther U, Ericson J, Briscoe J 2002. Dorsal-ventral patterning of the spinal cord requires Gli3 transcriptional repressor activity. Genes Dev 16:2865–2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierani A, Brenner-Morton S, Chiang C, Jessell TM 1999. A sonic hedgehog-independent, retinoid-activated pathway of neurogenesis in the ventral spinal cord. Cell 97:903–915 [DOI] [PubMed] [Google Scholar]
- Ribes V, Stutzmann F, Bianchetti L, Guillemot F, Dolle P, Le Roux I 2008. Combinatorial signalling controls Neurogenin2 expression at the onset of spinal neurogenesis. Dev Biol 321:470–481 [DOI] [PubMed] [Google Scholar]
- Roelink H, Porter JA, Chiang C, Tanabe Y, Chang DT, Beachy PA, Jessell TM 1995. Floor plate and motor neuron induction by different concentrations of the amino-terminal cleavage product of sonic hedgehog autoproteolysis. Cell 81:445–455 [DOI] [PubMed] [Google Scholar]
- Rubin JB, Choi Y, Segal RA 2002. Cerebellar proteoglycans regulate sonic hedgehog responses during development. Development 129:2223–2232 [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A 1998. Combinatorial Gli gene function in floor plate and neuronal inductions by Sonic hedgehog. Development 125:2203–2212 [DOI] [PubMed] [Google Scholar]
- Scherz PJ, McGlinn E, Nissim S, Tabin CJ 2007. Extended exposure to Sonic hedgehog is required for patterning the posterior digits of the vertebrate limb. Dev Biol 308:343–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamataki D, Ulloa F, Tsoni SV, Mynett A, Briscoe J 2005. A gradient of Gli activity mediates graded Sonic Hedgehog signaling in the neural tube. Genes Dev 19:626–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipale J, Cooper MK, Maiti T, Beachy PA 2002. Patched acts catalytically to suppress the activity of Smoothened. Nature 418:892–897 [DOI] [PubMed] [Google Scholar]
- Tempe D, Casas M, Karaz S, Blanchet-Tournier MF, Concordet JP 2006. Multisite protein kinase A and glycogen synthase kinase 3beta phosphorylation leads to Gli3 ubiquitination by SCFbetaTrCP. Mol Cell Biol 26:4316–4326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenzen T, Allen BL, Cole F, Kang JS, Krauss RS, McMahon AP 2006. The cell surface membrane proteins Cdo and Boc are components and targets of the Hedgehog signaling pathway and feedback network in mice. Dev Cell 10:647–656 [DOI] [PubMed] [Google Scholar]
- Varjosalo M, Taipale J 2008. Hedgehog: functions and mechanisms. Genes Dev 22:2454–72 [DOI] [PubMed] [Google Scholar]
- Vokes SA, Ji H, McCuine S, Tenzen T, Giles S, Zhong S, Longabaugh WJ, Davidson EH, Wong WH, McMahon AP 2007. Genomic characterization of Gli-activator targets in sonic hedgehog-mediated neural patterning. Development 134:1977–1989 [DOI] [PubMed] [Google Scholar]
- Vokes SA, Ji H, Wong WH, McMahon AP 2008. A genome-scale analysis of the cis-regulatory circuitry underlying sonic hedgehog-mediated patterning of the mammalian limb. Genes Dev 22:2651–2663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Fallon JF, Beachy PA 2000. Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell 100:423–434 [DOI] [PubMed] [Google Scholar]
- Wijgerde M, McMahon JA, Rule M, McMahon AP 2002. A direct requirement for Hedgehog signaling for normal specification of all ventral progenitor domains in the presumptive mammalian spinal cord. Genes Dev 16:2849–2864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert L 1969. Positional information and the spatial pattern of cellular differentiation. J Theor Biol 25:1–47 [DOI] [PubMed] [Google Scholar]
- Woods IG, Talbot WS 2005. The you gene encodes an EGF-CUB protein essential for Hedgehog signaling in zebrafish. PLoS Biol 3:e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao S, Lum L, Beachy P 2006. The ihog cell-surface proteins bind Hedgehog and mediate pathway activation. Cell 125:343–357 [DOI] [PubMed] [Google Scholar]
- Zeng X, Goetz JA, Suber LM, Scott WJ Jr, Schreiner CM, Robbins DJ 2001. A freely diffusible form of Sonic hedgehog mediates long-range signalling. Nature 411:716–720 [DOI] [PubMed] [Google Scholar]
- Zhang W, Kang JS, Cole F, Yi MJ, Krauss RS 2006. Cdo functions at multiple points in the Sonic Hedgehog pathway, and Cdo-deficient mice accurately model human holoprosencephaly. Dev Cell 10:657–665 [DOI] [PubMed] [Google Scholar]
- Zhang XM, Ramalho-Santos M, McMahon AP 2001. Smoothened mutants reveal redundant roles for SHH and Ihh signaling including regulation of L/R symmetry by the mouse node. Cell 106:781–792 [PubMed] [Google Scholar]
- Zhuang B, Sockanathan S 2006. Dorsal-ventral patterning: a view from the top. Curr Opin Neurobiol 16:20–24 [DOI] [PubMed] [Google Scholar]