Abstract
The localized control of second messenger levels sculpts dynamic and persistent changes in cell physiology and structure. Inositol trisphosphate [Ins(1,4,5)P 3] 3-kinases (ITPKs) phosphorylate the intracellular second messenger Ins(1,4,5)P 3. These enzymes terminate the signal to release Ca2+ from the endoplasmic reticulum and produce the messenger inositol tetrakisphosphate [Ins(1,3,4,5)P 4]. Independent of their enzymatic activity, ITPKs regulate the microstructure of the actin cytoskeleton. The immune phenotypes of ITPK knockout mice raise new questions about how ITPKs control inositol phosphate lifetimes within spatial and temporal domains during lymphocyte maturation. The intense concentration of ITPK on actin inside the dendritic spines of pyramidal neurons suggests a role in signal integration and structural plasticity in the dendrite, and mice lacking neuronal ITPK exhibit memory deficits. Thus, the molecular and anatomical features of ITPKs allow them to regulate the spatiotemporal properties of intracellular signals, leading to the formation of persistent molecular memories.
Keywords: Inositol phosphates, Intracellular Ca2+, Lymphocyte, Neutrophil, Pyramidal neuron, F-actin, Rho GTPase, Dendritic spines, Integration
Introduction
How cells translate brief signals from their environment into persistent modifications in physiology, shape, and function during development and behavior is a fundamental problem in cell biology. In the immune system and in the central nervous system, the cellular changes triggered by experience may persist for as long as the organism, creating an integrated memory, encoded by modifications in both cell structure and gene expression. This review first summarizes our current understanding of the structure, biochemistry, and regulation of the inositol trisphosphate 3-kinase (ITPK) family of enzymes, and then it focuses on recent breakthroughs in our understanding of ITPKs function at immune synapses [1] and in the dendritic spines of neurons [2], two prominent sites of spatiotemporally regulated, integrative signaling that produce cellular memory in higher animals.
Metabolism of inositol trisphosphate
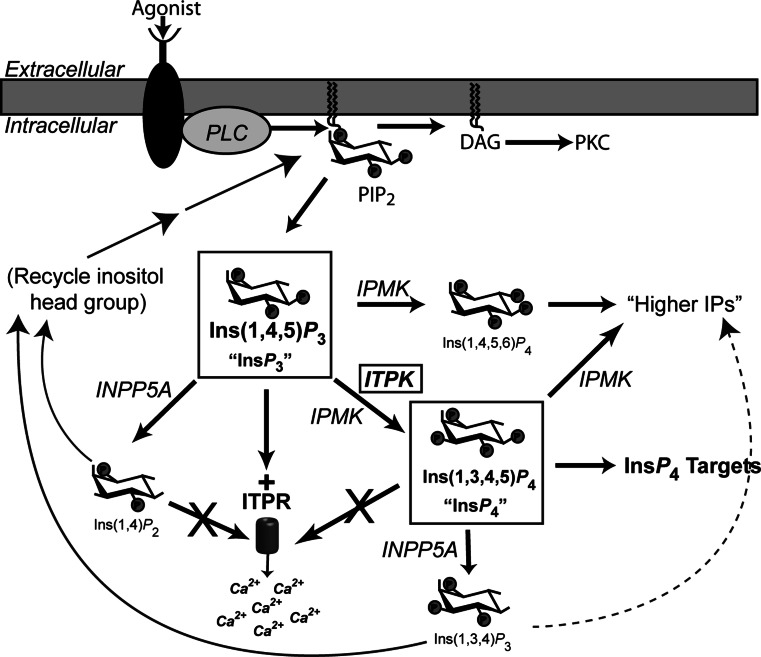
Extracellular stimuli generate intracellular second messengers, which transmit and amplify biochemical information in the temporal and spatial domains [3]. Messengers have short lifetimes inside cells, and highly regulated attenuation systems—such as cyclic nucleotide phosphodieserases, protein phosphatases, and Ca2+ sequestration/buffering/extrusion systems—help sculpt intracellular signals and create signaling microdomains [4–7]. In cells enriched in the inositol 1,4,5-trisphosphate [Ins(1,4,5)P 3] signaling cascade, inositol trisphosphate 3-kinases (ITPKs) catalyze a selective phosphorylation of the second messenger Ins(1,4,5)P 3 at the 3-OH position on the inositol ring, producing Ins(1,3,4,5)P 4 (Fig. 1) [8]. While Ins(1,4,5)P 3 triggers intracellular Ca2+ release from the endoplasmic reticulum (ER) by gating the inositol trisphosphate receptor channel (ITPR), Ins(1,3,4,5)P 4 is not a potent ITPR agonist [9]. Thus, ITPKs terminate Ins(1,4,5)P 3 signals by shortening the Ins(1,4,5)P 3 lifetime. The Ins(1,3,4,5)P 4 produced by ITPKs may itself act as third messenger by binding to protein targets in cells, and the identification of physiological targets for Ins(1,3,4,5)P 4 is an active area of current research [10–12].
Fig. 1.
Ins(1,4,5)P 3 signaling and metabolism. Activation of cell surface receptors triggers the canonical bifurcating pathway in which PLC hydrolyzes the lipid phosphatidylinositol 4,5 bisphosphate (PIP2). The soluble Ins(1,4,5)P 3 head group diffuses to the ER, where it gates the inositol trisphosphate receptor (ITPR), triggering Ca2+ release from intracellular stores. The diacylglycerol (DAG) remains in the lipid bilayer, where it activates protein kinase C (PKC). Ins(1,4,5)P 3 is subject to metabolism by cascades of phosphatases and kinases [10]. Inositol trisphosphate 3-kinases (ITPKs) phosphorylate Ins(1,4,5)P 3 at the 3-OH position to produce Ins(1,3,4,5)P 4, which does not gate ITPR channels but can bind various other protein targets in cells. Inositol polyphosphate multikinase (IPMK) phosphorylates Ins(1,4,5)P 3 twice, at the 6- and 3-positions, and these reactions govern the production of higher inositol phosphates (IPs) [34]. Both Ins(1,4,5)P 3 and Ins(1,3,4,5)P 4 are substrates for the type 1 inositol 5-phosphatase (INPP5A) [14]. If Ins(1,4,5)P 3 is the substrate, Ins(1,4,)P 2 is produced, which is dephosphorylated further and ultimately re-incorporated into new inositol lipids [17]. If Ins(1,3,4,5)P 4 is the substrate, Ins(1,3,4)P 3 is produced; the fate of Ins(1,3,4)P 3 varies among cells, and involves both kinase and phosphatase pathways [211]
Ins(1,4,5)P 3 signaling to ITPR probably occurs only in metazoans [8]. Multi-cellular animals have evolved complex spatial and temporal components to their intracellular Ca2+ response. Since ITPKs evolved soon after the appearance of ITPR, the signaling roles for Ins(1,3,4,5)P 4 are likely to be related to messenger pathways that involve Ins(1,4,5)P 3-triggered Ca2+ release. The elusive physiological Ins(1,3,4,5)P 4 receptors will occur in higher organisms only, as components of spatiotemporal elaborations on Ca2+ signaling and homeostasis. Some of the most dynamic intracellular Ca2+ signaling occurs in the immune and nervous systems, where Ins(1,4,5)P 3 signaling machinery is notably enriched. Extracts from immune or neuronal tissues exhibit 3-tenfold more ITPK activity than other tissues [13]. ITPKs are positioned at points in signaling cascades where they function as translators and integrators of intracellular stimuli, leading to persistent changes in development, physiology and structure of complex metazoan cells.
Both kinases and phosphatases control the lifetime of Ins(1,4,5)P 3 (Fig. 1) [10]. The type 1 Ins(1,4,5)P 3 5-phosphatase, called INPP5A [14], works in parallel to ITPK by catalyzing the hydrolysis of Ins(1,4,5)P 3 and Ins(1,3,4,5)P 4 at the 5 position. Depending on the substrate, INPP5A produces either Ins(1,4)P 2 or Ins(1,3,4)P 3, and neither of these Ins(1,4,5)P 3 metabolites has known signaling properties. In cells stimulated chronically with Ins(1,4,5)P 3-generating agonists, Ins(1,4)P 2 and Ins(1,3,4)P 3 are the major inositol phosphates that accumulate at steady-state in most [15] but not all [16] cells and tissues. The bulk of these inositol head groups are ultimately re-incorporated into lipid [17]. Thus, the rapid metabolism of receptor-generated Ins(1,4,5)P 3 is a metabolic duet [18] between the 3-kinase and 5-phosphatase, and complex co-metabolism can occur. For example, in some cases the Ins(1,3,4,5)P 4 generated by ITPKs can saturate the activity of INPP5A, prolonging the lifetime of Ins(1,4,5)P 3 [19, 20].
ITPKs resemble other kinases but phosphorylate Ins(1,4,5)P3 only
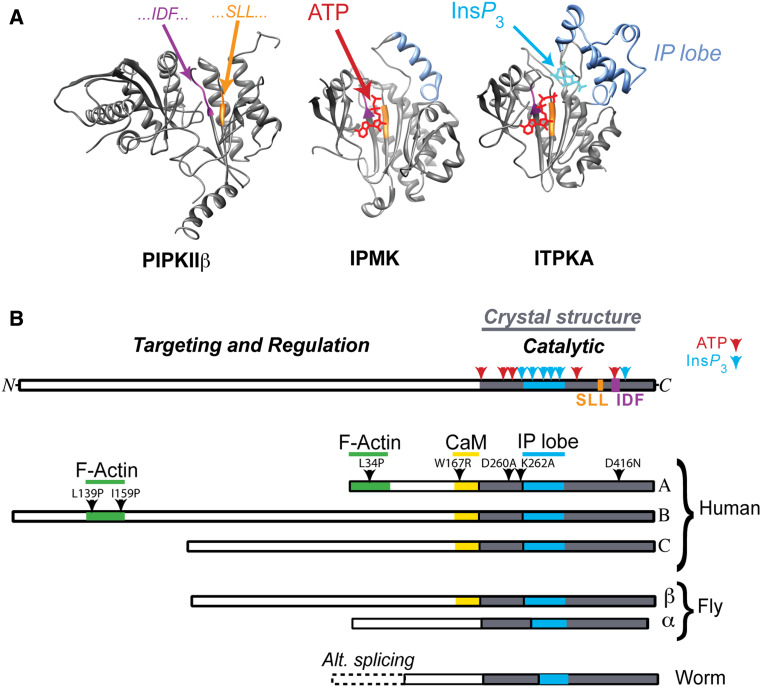
Based on the solved crystal structures of the ITPKA catalytic domain [21, 22] (Fig. 2a), ITPKs have been assigned to the lipid kinase-like branch of the group 1 protein kinase superfamily [23]. Although ITPKs are sometimes confused with the more famous phosphatidylinositol lipid 3-kinases (PI3Ks), they are not lipid kinases at all, because their structure sterically prevents them from binding substrates in the lipid membrane [21, 22]. ITPKs share structural homology with an ATP-binding pocket found in the lipid kinase phosphatidylinositol 4-phosphate 5-kinase beta (PIPKIIβ; Fig. 2a). The most conserved part of the ITPK structure is that which binds and holds the ATP substrate in an orientation such that it can donate its gamma phosphate. ITPKs belong to the inositol polyphosphate kinase (IPK) family [11, 24]. This gene family includes not only ITPKs, but also the inositol polyphosphate multikinase (IPMK, also known as IPK2 in yeast and Arabidopsis), and the InsP 6 kinases [25, 26]. For each of these enzymes, the various inositol phosphate substrates are held in orientations that facilitate phosphate transfer [27]. The structural domains that surround this binding site in three-dimensional space determine what the kinase can phosphorylate.
Fig. 2.
Structure of ITPKs. a Comparison of crystal structures of kinase superfamily members. Crystal structures of human phosphatidylinositol 4-phosphate 5-kinase beta (PIPKIIβ, left) [212], yeast inositol polyphosphate multikinase (IPMK, middle) [27], and human ITPKA (right) [21]. Structures are oriented such that one is looking into the active sites, which bind ATP (red) through a conserved motif (SLL…IDF…, colored purple/orange). Inositol phosphates (IPs), such Ins(1,4,5)P 3 (InsP 3, blue) are oriented through their interaction with the IP lobe (blue), which holds the IP so that phosphotransfer from ATP is facilitated. IPMK (middle) has a smaller IP lobe than ITPK, allowing it to accommodate multiple IP substrates; by contrast, the more elaborated IP lobe found in ITPKs (right) holds Ins(1,4,5)P 3 in an orientation that allows phosphorylation at the 3-position only. b Domain structure of the three human ITPK isoforms compared to fly and worm varieties. The catalytic domains of ITPKs are highly conserved and located in the C-terminal parts of the protein (top domain diagram). By contrast, the amino terminal domains are divergent, and govern selective targeting and regulation in different cells and tissues. All ITPK crystal structures solved thus far have been obtained from catalytic fragments (gray), which lack the N-terminal regulatory regions; the catalytic region contains all of the substrate binding sites for ATP (red) and InsP 3 (blue). In the presence of Ca2+, all three human isoforms bind calmodulin (CaM) in a Ca2+-dependent manner, via a conserved domain [45]. The three human isoforms of ITPK show diversity in their N-termini [8]. Isoforms A [177] and B [77] bind filamentous actin (F-actin), while isoform C shows a mixed cytosolic/nuclear localization [213]. Arrowheads are labeled with point mutations that can selectively destroy domain functions in ITPKA or ITPKB. The L34P mutation destroys F-actin binding in ITPKA [133], while L139P and L159P reduce F-actin binding in ITPKB [77]. The W167R mutation destroys CaM binding in ITPKA; analogous mutations behave similarly for ITPKB and C [45]. Arrowheads in the blue and gray areas point to two of the many mutations in the active site that destroy catalytic activity [21]. The fruit fly Drosophila melanogaster possesses two isoforms [42], one of which is positively regulated by calmodulin [45]. The nematode Caenorhabditis elegans posesses one ITPK gene, which is not regulated by CaM and shows diversity generated by alternative splicing at the 5′ end [39]
The substrate-binding pockets of most IPK family members are promiscuous. Thus, IPMK, which probably occurs in all eukaryotes, functions as a primitive ITPK by catalyzing either the 3-phosphorlyation of Ins(1,4,5)P 3 to produce Ins(1,3,4,5)P 4 (the same reaction catalyzed by ITPKs) or else the 6-phosphorlylation to produce Ins(1,4,5,6)P 4. Both inositol tetrakisphosphate isomers are themselves substrates for the IPMK, with both reactions producing Ins(1,3,4,5,6)P 5 (Fig 1). The human IPMK is also an Ins(1,3,4,6)P 4 5-kinase [28], as is the IPK2 of Arabidopsis [29]. If substrate concentrations are high, IPMK can catalyze the phosphorylation of phosphate groups to make inositol pyrophosphates, at least in vitro [30]. Lastly, unlike ITPK, IPMK can also act as an inositol lipid 3-kinase [31], which is distinct from the more conventional PI3Ks involved in growth-factor signaling and membrane trafficking. The physiological relevance of this reaction in cells remains uncertain. The closely related InsP 6 kinases catalyze the pyrophosphorylation of various higher inositol phosphates [32]. Again, the substrate-binding pocket is flexible enough to accommodate different inositol phosphate orientations [33], and more than one reaction can be catalyzed. The IPMK pathway to InsP6 is the major means for producing higher inositol phosphates in eukaryotes [26, 34–36]. The physiological roles of higher inositol phosphates, especially in the nucleus, are under intense study, and this topic has been reviewed elsewhere [10, 24, 37].
By contrast, ITPKs possess exquisite specificity for the 3-phosphorylation of Ins(1,4,5)P 3 only. All available evidence suggests that ITPKs, unlike other IPKs, evolved explicitly for removing the second messenger pool of Ins(1,4,5)P 3. All ITPKs have a similar domain structure, consisting of a conserved catalytic C-terminal end and a variable N-terminal end, which is involved in the targeting and regulation of the enzyme [10] (Fig 2b). The structural explanation for the exquisite ITPK enzymatic specificity lies in an elaboration of the Ins(1,4,5)P 3 binding site called the IP lobe (Fig 2a) [21]. This stretch of approximately 35 amino acids holds Ins(1,4,5)P 3 in an orientation that constrains phosphorylation to the 3-OH position only and prevents the phosphorylation of lipids. The presence of an elaborated IP lobe distinguishes ITPKs from other IPK family members (Fig 2a).
ITPKs are expressed heterogeneously in metazoan tissues
Table 1 lists some ITPK isoforms and the phenotypes associated with their over- or under-expression. The simplest biochemically validated ITPK, called LFE-2, occurs in the roundworm C. elegans [38]. These organisms possess one ITPK gene, which exhibits alternative splicing at the N-terminus [39] (Fig 2b, bottom). Besides splicing, no regulatory mechanisms are known for ITPK in C. elegans. Ca2+/calmodulin (CaM) does not regulate worm ITPK as it does in mammals [38]. Genetic analysis indicates that the worm version of ITPK regulates signal transduction triggered by insulin-like growth-factor 1 as part of an ITPR and phospholipase C (PLC)-dependent pathway involved in fertility [38]. The worm homolog of INPP5A also regulates Ins(1,4,5)P 3 removal in the same pathway [40]. Thus, from the earliest point in evolution that an ITPK can be identified with certainty, it participates in the regulation of ITPR-dependent cell signaling and works as a team with INPP5A. Although both enzymes regulate Ins(1,4,5)P 3 removal downstream of insulin-like growth factor, the phenotypes produced by under- or over-expression of ITPK or INPP5A are different [40, 41]. Even in this simple organism, fine-tuning Ins(1,4,5)P 3 metabolism produces selective phenotypes.
Table 1.
Comparison of the effects of too much or too little ITPKs in metazoans
| ITPK isoform | Expression pattern | Knockout/inactivation phenotype | Overexpression phenotype |
|---|---|---|---|
| Worm (C46H11.4; lfe-2) | Vulval precursor cells of the spermatheca | Suppresses IGF-1 receptor-dependent (let-23) sterility mutations [38] | Sterility due to attenuation of LET-23-triggered InsP3 generation [40] |
| Fly α, IP3K1 (CG4026; SCIM15) | Tubule, hindgut, brain | Decreased miniochromosome inheritance [43] | Resistance to oxidative stress [44] Attenuation of InsP3 Ca2+ response [45] |
| Fly β, IP3K2 (CG1630) | Tubule | None reported | Attenuation of InsP3 Ca2+ response [45] |
| ITPKA (human chromosome 15q15.1) | Principal neurons in forebrain, plus Purkinje neurons. Testis; expressed late in postnatal brain development | Enhanced LTP in CA1 [48]; decreased LTP in CA3, memory deficits [132] | Attenuation of InsP3 Ca2+ response [45, 76, 207–209] |
| ITPKB (human chromosome 1q42.13) | Immune system: lymphocytes and neutrophils; widespread expression in brain; detectable expression in most other tissues | Failure of T lymphoblasts to mature [51, 52]; defects in B cell selection [110, 117, 121]; hyperactive neutrophil activation [53]; enhanced proliferation of monocyte progenitors [54] | Attenuation of InsP3 Ca2+ response [45, 75, 76, 210] |
| ITPKC (human chromosome 19q13.1) | Expressed in most tissues; expression differences reported between rat [57] and human [58] | Possibly Kawasaki disease [59] | Attenuation of InsP3 Ca2+ response [45, 76] |
The fruit fly Drosophila melanoganster has two ITPK genes, IP3Kα and IP3Kβ (Table 1) [42]. P-element-based disruption of a chromosomal region that contains the gene for ITPKα (SCIM23) produces a diminution in mini-chromosome inheritance [43], while stable over-expression of ITPKα increases resistance to oxidative stress in a way genetically related to ITPR expression [44]. No phenotypes have yet been reported associated with changes in ITPKβ expression. The two ITPK isoforms in fruit flies can be distinguished by their differential regulation by Ca2+/CaM [45]. Like the worm ITPK, ITPKα is not regulated by CaM (and lacks a CaM-binding domain). A head-to-head comparison of various ITPKs in a mammalian expression system indicated that drosophila ITPKα was the most efficient ITPK tested for its ability to attenuate an agonist-generated Ins(1,4,5)P 3-dependent Ca2+ signal, despite its lack of stimulation by Ca2+ [45]. By contrast, ITPKβ in drosophila binds CaM in a Ca2+-dependent manner, increasing its activity. Thus, CaM-regulated ITPKs seem to have a lower basal activity in the absence of Ca2+/CaM than do the non-CaM-regulated varieties. This property may allow for better control of the dynamic range of the Ins(1,4,5)P 3 signal. Interestingly, honeybees don’t express the isoform that lacks CaM regulation but rather possess different alternatively spliced versions of a CaM-dependent isoform [46]. Ca2+/CaM positively regulates all known ITPKA isoforms in higher animals, creating a negative feedback loop following Ca2+ release.
Most mammals have three ITPK genes (Fig. 2b). The isoforms exhibit variable expression patterns [13], and reducing or enhancing their expression produces different phenotypes in different cells and tissues (Table 1). One consistent finding is that over-expression of ITPKs in cells produces a reduction in the Ins(1,4,5)P 3-triggered Ca2+ signal in response to agonist. Isoform A (ITPKA) is found mainly in the principal neurons of the forebrain and in the cerebellar Purkinje neurons [47]. Mice with targeted disruption of ITPKA show large changes in the degree of long-term potentiation (LTP) in the hippocampus [48, 49] (see later section). Isoform B (ITPKB) is widely expressed [50], with notably high levels in immune tissues. Mice lacking ITPKB exhibit major deficits in lymphocyte maturation [51, 52], neutrophil activation [53], and myelopoiesis [54]. The proposed molecular mechanisms underlying these deficits will be discussed in more detail in the sections below. Abundant ITPKB expression also occurs in brain, probably in glial cells [55, 56]. It should be noted, however, that microarrays from human tissue (rather than rodents) indicate substantial ITPKB expression in gray matter regions that lack ITPKA expression [52]. This suggests that a subset of neurons expresses ITPKB, at least in humans. Isoform C (ITPKC) is also widely expressed [57, 58]. Recently, genetic mutations in the human ITPKC gene have been linked to Kawasaki disease, an auto-immune disease [59]. The available evidence suggests that an overactive Ins(1,4,5)P 3 signaling pathway leading to the transcription factor NFAT may underlie the immune hyperactivity (see later section).
ITPKs are regulated by phosphorylation
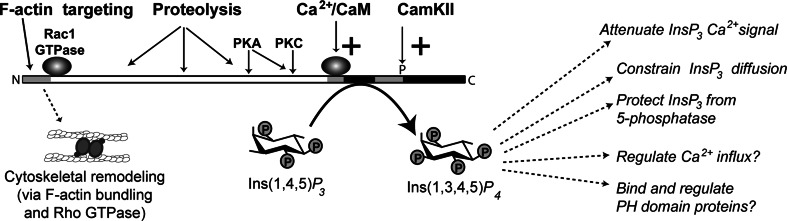
In addition to direct stimulation by Ca2+/CaM, at least three other mechanisms regulate ITPKs: phosphorylation, proteolysis, and intracellular targeting (Fig. 3). No new studies addressing the regulation of ITPKs by protein phosphorylation have been published in almost a decade, yet a surprising amount remains unclear. In cells or tissues expressing ITPKA or ITPKB, pretreatment with the protein phosphatase inhibitor okadaic acid prior to addition of agonist is the most effective means of prolonging Ins(1,3,4,5)P 4 generation [56, 60]. This may be because Ca2+/CaM-dependent protein kinase II (CaMKII) stimulates ITPKA and ITPKB and simultaneously inhibits INPP5A [61]. CamKII increases enzyme activity by increasing V max but no change in K m. Protein phosphorylation increases the affinity of ITPKA (but not ITPKB) for Ca2+/CaM. By contrast, phosphorylation causes ITPKB (but not ITPKA) to translocate from the cytosolic to the membrane fraction [56]. ITPKC lacks the CamKII phosphorylation site altogether, and its regulation by phosphorylation has been examined in much less detail than the other isoforms.
Fig. 3.
Regulation of ITPKs and its consequences. ITPK action is regulated by at least four mechanisms. All mammalian ITPKs are positively regulated by Ca2+/calmodulin; this creates a negative feedback loop on the Ins(1,4,5)P 3 signal to release Ca2+ via ITPR [45]. ITPKs are also regulated by various protein kinases, such as protein kinase C (PKC), protein kinase A (PKA), and Ca2+/CaM-activated protein kinase II (CamKII). Phosphorylation at some sites activates the enzyme, and at others inhibits it (see text). The amino terminal regions of ITPKA and ITPKB bind F-actin, which targets the enzymes near sites of Ins(1,4,5)P 3 generation. The ITPKA amino terminal-binding region [177] affects F-actin structure through a mechanism independent of ITPKA catalytic activity [179]. This mechanism has been linked to the Rho family GTPase Rac1 [132]. The F-actin remodeling involves cross-linking or bundling of the actin filaments, and controls the selective targeting of ITPKA to dendritic spines [133]. ITPKs are subject to the actions of proteases, which usually cleave between the N-terminal targeting region and the catalytic region. Protease cleavage of ITPKs changes enzymatic localization [74, 75], and can also affect the sensitivity to or substrate sites for protein kinases [70]. ITPK catalysis has many suggested functions in cells [10]. These can be subdivided into functions involving the attenuation of Ins(1,4,5)P 3 signals via restriction of those signals to spatial and temporal domains through the control of Ins(1,4,5)P 3 lifetime, and those which depend on the specific actions of the enzymatic product Ins(1,3,4,5)P4. Cells possess a variety of Ins(1,3,4,5)P 4-binding proteins. Some of these bind Ins(1,3,4,5)P 4 selectively via their pleckstrin homology (PH) domains; others are channel proteins whose gating or conductance properties change upon Ins(1,3,4,5)P 4 binding (see text)
In vitro, ITPKA is also a substrate for both cAMP-dependent protein kinase A (PKA) and protein kinase C (PKC). The effects of these protein kinases on ITPKs are difficult to generalize and may be different among the isoforms. Some studies found that PKA increased the V max of ITPKA and ITPKB about twofold [62, 63], but another study found no effect of the adenylate cyclase activator forskolin on either isoform [56]. Likewise, the effects of PKC differ among the reports in the literature, with some studies suggesting inhibition [62, 63], and others a modest stimulation [56]. The discrepancies appear to be due to the particular enzyme preparation assayed, its state of proteolysis, the time of incubation with the kinase, the isoform being studied, and/or the presence of CaM. Furthermore, combinations of protein kinases may produce effects different from each kinase alone [56, 64]. The available data suggest that multiple kinases, perhaps in conjunction with proteolysis and CaM, differentially regulate different ITPK isoforms. Further studies will be required to resolve the specifics.
Regulation by proteolysis and intracellular targeting
Early biochemical studies showed that ITPKA is highly susceptible to proteolysis during purification [65, 66]. ITPKA purified from brain runs on gels as a ladder of three to five catalytically active bands between 53 kD and about 30 kD. Inclusion of calpain inhibitors in the buffers during purification reduces the laddering [66]. When ITPKA was molecularly cloned [67], it was reported that the protein contained a PEST sequence, which is a stretch of roughly 12–20 amino acids hypothesized to cause a protein to be highly susceptible to proteolysis through Ca2+-regulated proteases (calpains), followed by rapid (1–2 h) degradation [68]. PEST stands for a proline (P), glutamine (E), serine (S), and threonine (T)-rich stretch of amino acids, and originally the PEST sequence was said to occur between residues 99 and 136 of the full-length rat sequence [67].
PEST sequences occur with high frequency in CaM-binding proteins [69]. More refined versions of the original prediction program (PESTfind) identify one very weak PEST sequence in ITPKA, now re-assigned to the middle of the IP lobe, where proteolysis would almost certainly destroy enzyme activity [70]. Since all proteolytic products isolated biochemically retain enzymatic activity [65, 66], the PEST sequence in ITPKA, if it exists, is not directing the proteolysis by Ca2+-activated proteases. Indeed, the PEST hypothesis has now been modified to exclude calpain-mediated mechanisms [68, 71]. Immunolocalization of ITPKA in brain suggests very low levels of ITPKA in the neuronal cell soma, indicating that most or all of the protein remains anchored to the synaptic cytoskeleton in vivo [72]. Consistent with this, we have tagged ITPKA with enhanced green fluorescent protein (EGFP) at the C-terminus and then expressed the fusion protein in hippocampal neurons for up to 2 weeks, yet we do not observe a significant cytosolic GFP fluorescence. Therefore, despite the obvious susceptibility of ITPKA to proteolysis during purification, the idea that ITPKA contains a PEST sequence that directs its proteolysis inside cells should be retired.
By contrast, many studies indicate the importance of proteolysis in regulating ITPKB and ITPKC. PESTfind predicts a number of strong PEST sequences in the amino terminal halves of ITPKB and ITPKC, as has been discussed in more detail elsewhere [70, 73]. No data have yet emerged to indicate that these sequences play a significant role in the regulation of ITPK proteolysis. Nevertheless, recent experimental evidence supports the general idea that ITPKB is regulated by proteolysis, with the functional consequence being that proteolysis changes the enzyme’s subcellular localization [74, 75]. When full-length ITPKB is expressed in cells, it exhibits a heterogeneous localization and occurs with different molecular weights [50, 74–76]. Depending on the cell, ITPKB is associated with the actin cytoskeleton, on intracellular membranes, or in the cytosol. The F-actin-binding domain of rat ITPKB was identified between amino acids 108 and 170 [77]. Proteolytic cleavage separates the catalytic region from its cytoskeletal-targeting domain, thereby changing the cellular location of ITPK activity (Fig. 3). A shift in the cellular location of Ins(1,4,5)P 3 phosphorylation could potentially affect the spatiotemporal dynamics of Ins(1,4,5)P 3 Ca2+ signals. In fact, we have demonstrated that cytoskeletal anchoring of ITPKA [45] and ITPKB [75] enhances the ability of the enzyme to attenuate a Ca2+ signal. Earlier studies of ITPKB that used a truncated version of the protein that lacked the F-actin-binding region localized ITPKB to the ER membrane, and it would be interesting to investigate how placing an ITPK near the ITPR instead of close to the site of Ins(1,4,5)P 3 generation would influence the spatiotemporal dynamics of the ITPR-induced Ca2+ signal.
ITPKs regulate Ins(1,4,5)P3 lifetime
The ER Ca2+ stores are contiguous [78, 79]. Cytosolic Ca2+ sequestered in one part of the cell’s ER can be released at remote sites at the other end of the cell [80]. Signals controlling the intracellular release of Ca2+ depend not only on the site(s) of Ins(1,4,5)P 3 generation, but also the spatiotemporal relationships between Ca2+ dynamics, Ins(1,4,5)P 3 diffusion/metabolism, and ITPR. In this context, where and when Ins(1,4,5)P 3 is removed may determine the local or global properties of the Ca2+ signal. ITPK-regulated signals can be subdivided into those that involve modulating of Ins(1,4,5)P 3 lifetime and those that require the rapid, selective generation of a messenger pool of Ins(1,3,4,5)P 4 [8]. Figure 3 depicts a summary of ITPK regulatory mechanisms and also indicates the major consequences of ITPK activity suggested from a variety of studies.
How ITPKs control Ins(1,4,5)P 3 lifetime and influence the spatiotemporal properties of the Ca2+ signal remains largely unknown. Ca2+ itself does not diffuse very far within a cell, owing to extrusion, sequestration, and buffering [6, 81–83]. Ins(1,4,5)P 3 and Ins(1,3,4,5)P 4 can diffuse much longer distances and may persist in cells for seconds or even minutes [83–86]. The lifetime of an Ins(1,4,5)P 3 molecule is determined chiefly by its rate of metabolism, and possibly by the presence of Ins(1,4,5)P 3 buffering proteins. The functional lifetime of an Ins(1,4,5)P 3 molecule is also subject to passive diffusion [87]. As locally generated Ins(1,4,5)P 3 equilibrates in a cell, its concentration may fall below its affinity for binding ITPR, silencing the signal. ITPKs, INPP5A, and IPMK probably all participate in Ins(1,4,5)P 3 metabolism (Fig. 1). Their relative influence in shaping the lifetime of the receptor-generated pool of Ins(1,4,5)P 3 is poorly understood, and varies among cells types and within sub-domains of single cells [10, 24, 37, 45].
In at least eight published reports, the over-expression of an ITPK isoform causes a dramatic attenuation in the ability of cells to produce a Ca2+ signal via Ins(1,4,5)P 3-generating agonists (see Table 1). In these studies, it is assumed that ITPKs shorten the lifetime of Ins(1,4,5)P 3 in the cell. ITPKs have a higher affinity for Ins(1,4,5)P 3 than does INPP5A, so they have privileged access to pools of Ins(1,4,5)P 3 produced rapidly upon receptor stimulation. By contrast, INPP5A has a much greater V max, so its influence is more on the steady-state concentration of Ins(1,4,5)P 3 in the cell. These studies are borne out by knockout and knockdown studies. ITPK knockout mice show no change in their basal Ins(1,4,5)P 3 levels [48, 51], while INPP5A knockdown cells show increased steady-state Ins(1,4,5)P 3 levels, and a lower threshold for triggering a Ca2+ response [88].
On the other hand, Ins(1,3,4,5)P 4 can also prolong the lifetime of Ins(1,4,5)P 3 by competitively inhibiting the phosphatase INPP5A [19]. In this scenario, a cell stimulus generates Ins(1,4,5)P 3 and Ins(1,3,4,5)P 4. The Ins(1,3,4,5)P 4 that lingers following the stimulus functions as a “memory trace” of recent cellular activity. The counterintuitive prolonging of the Ins(1,4,5)P 3 lifetime by ITPK occurs because the affinity of Ins(1,3,4,5)P 4 for INPP5A is greater than for Ins(1,4,5)P 3 [89]. Thus, if a second Ins(1,4,5)P 3-generating stimulus arrives before Ins(1,3,4,5)P 4 returns to basal levels, Ins(1,3,4,5)P 4 will “protect” the Ins(1,4,5)P 3 by competitively inhibiting INPP5A. This mechanism may be further reinforced if the Ca2+/CaM-dependent kinase II (CamKII) is present in the cell, since this protein kinase enhances the activity of ITPKA [60] and ITPKB [56], but inhibits INPP5A [61]. In any cell where ITPK and INPP5A collaborate to control the Ins(1,4,5)P 3 lifetime, a prior Ins(1,3,4,5)P 4 signal will likely prime the system towards enhanced Ins(1,3,4,5)P 4 production and possibly increased Ins(1,4,5)P 3 lifetime if a second Ins(1,4,5)P 3 signal arrives while Ins(1,3,4,5)P 4 levels remain elevated. This model of cellular coincidence detection has been called the “InsP4 protection racket” [20].
There is no a priori reason to assume that Ins(1,4,5)P 3 metabolism is involved at all in the generation of frequency-encoded calcium signals in cells. Ca2+ oscillations can be triggered in cells by introducing metabolically inert Ins(1,4,5)P 3 analogs [90]. A number of ITPK-independent mechanisms can account for physiological oscillations, such as Ca2+ or protein kinase feedback on surface receptors, ITPRs, or on phospholipase C [91, 92]. Nevertheless, mathematical modeling suggests that physiological conditions occur inside cells when ITPK activity affects the threshold, waveform, or frequency of Ca2+ oscillations [93–96]. Such models take into account enzyme and receptor affinities, enzyme velocities, and positive and negative feedbacks by Ca2+. The aim of these models is to predict the conditions under which cells exhibit global calcium spiking and oscillation, thereby adding frequency encoding to the Ca2+ signal. A related question is whether or not cellular levels of inositol phosphates themselves exhibit frequency encoding. The development of fluorescent biosensors has led to evidence that Ins(1,4,5)P 3 levels in cells do indeed exhibit periodic behaviors [97–99].
ITPKs as generators of Ins(1,3,4,5)P4 signals to downstream targets
The rapid and highly selective generation of Ins(1,3,4,5)P 4 in response to intracellular stimuli implies that the attenuation of a second messenger may lead to the creation of a third one. Despite more than 20 years of research, the identities of Ins(1,3,4,5)P 4-based signaling systems remain unclear [10, 100]. Following the discovery of the receptor-generated Ins(1,3,4,5)P 4 pathway in cells, receptor binding studies spearheaded the search for Ins(1,3,4,5)P 4 receptors [101–103]. The vast majority of Ins(1,3,4,5)P 4-binding proteins isolated in these studies contain pleckstrin homology (PH) domains that also bind to inositol lipids (Table 2). These include the Centaurins/p42IP4 [104, 105] and the GAP1 family of Ras GTPases, especially RASA3 [106, 107]. All have been suggested to be involved in Ins(1,3,4,5)P 4 signaling, but how is far from clear.
Table 2.
Candidate Ins(1,3,4,5)P 4 targets. Ins(1,3,4,5)P 4 targets are usually PH domains or channels. The immune system is rich in a variety of PH-domain containing proteins, and putative Ins(1,3,4,5)P 4-modulated channels
| Cell type | Molecular target | Suggested effect of InsP4 |
|---|---|---|
| Thymocyte/T cell | ITK | Enhanced binding of ITK to plasma membrane lipid [123] |
| B lymphoblast | GAP1IP4BP (Rasa3), a GAP for Ras or Rap [111] | Reduced GAP1 activity at plasma membrane [110] |
| Many cells | Centaurin/p42IP4, an Arf6 GAP | Compete with PIP3 binding at plasma membrane [105] |
| B lymphoblast | Store-operated Ca2+ channel | Inhibition of Ca2+ influx [117, 121] |
| Granulocyte/monocyte progenitors | PH domain of AKT | Reduced AKT translocation to plasma membrane [54] |
| Neutrophil | PH domain of AKT | Reduced AKT translocation to plasma membrane [53] |
| RBL mast cell | INPP5A | Saturation of INPP5A, leading to increased IP3 lifetime [19] |
| RBL mast cell | RASA3 (GAP1IP4BP) | Enhanced Ras GAP activity [109] |
| Ras-transformed NIH/3T3 fibroblast | Voltage-regulated influx channel in response to bradykinin | InsP4-enhanced channels, especially if cell is hyperpolarized [116] |
| Pyramidal neuron | Voltage-regulated Ca2+ channel(s) | Increased channel open time [118, 119] |
In the majority of cases, the downstream consequence of Ins(1,3,4,5)P 4 action is modulation of the Ras/extracellular signal-regulated protein kinase (ERK) signaling system [51, 52, 107, 108]. Among the various models suggested, Ins(1,3,4,5)P 4 has been proposed to function either as an activator of RASA3 GAP activity [107, 109], or as an inhibitor RASA3 GAP activity by removing RASA3 from the plasma membrane through competition with the lipid phosphatidylinositol (3,4,5)P 3 [110] (see Fig. 4). Differences in results may be partly explained by which isoform of Ras (or Rap; [111]) is activated. Moreover, the cellular location of Ras activity (plasma membrane versus Golgi apparatus) has emerged as a major mechanism of Ras regulation relevant to these different signaling systems, adding an additional layer of complexity to Ras activation relevant to Ins(1,3,4,5)P 4 [112, 113].
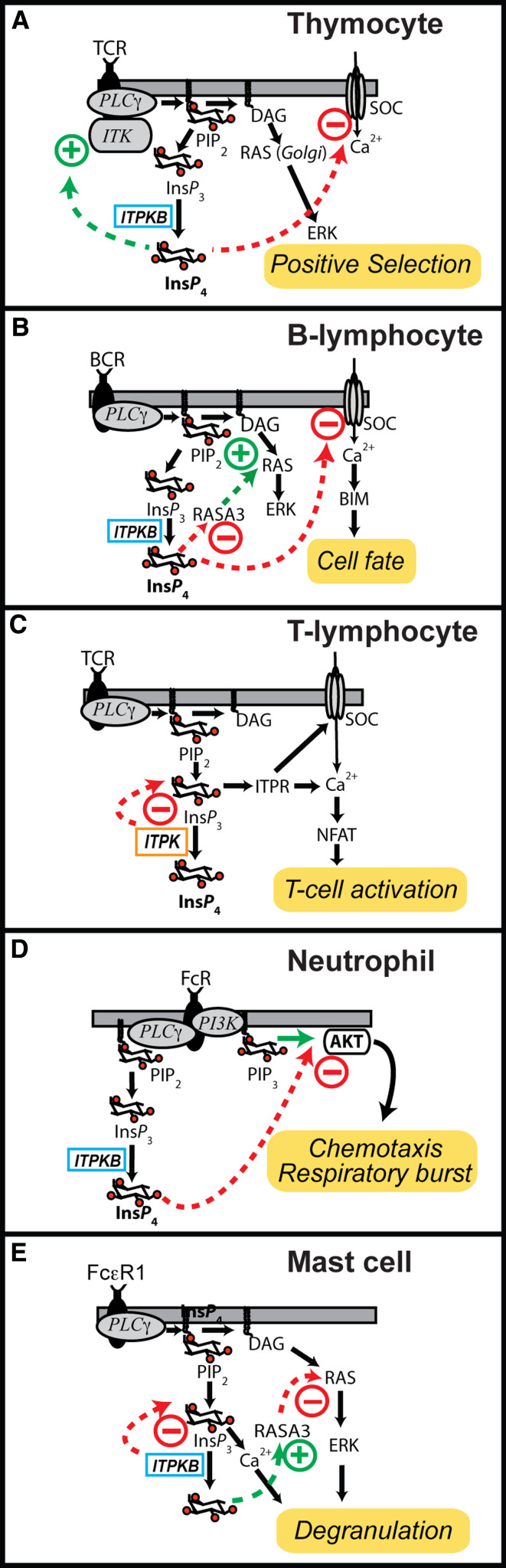
Fig. 4.
Diversity of ITPK signaling in immune cells. a Mice with targeted disruption in the gene for ITPKB exhibit major deficits in thymocyte maturation, with very few thymocytes undergoing positive selection [51, 52]. Activation of the T cell receptor in thymocytes derived from these mice results in less MAP kinase activation than in controls. At least two molecular mechanisms have been proposed to explain the phenotype. In one mechanism, Ins(1,3,4,5)P 4 triggers a feed-forward mechanism, whereby it binds and activates the interleukin-2 inducible T cell tyrosine kinase (ITK) [123] (green arrow). Downstream targets of ITK, such as phospholipase C gamma (PLCγ1), are thus hypoactive in the knockouts. A second mechanism proposes that store-operated Ca2+ channels (SOCs) are negatively regulated by Ins(1,3,4,5)P 4 (red arrow). In this model, lack of Ins(1,3,4,5)P 4 causes excessive Ca2+ influx, and this leads to deficits in Ca2+ homeostasis and its associated signaling [117]. b B lymphocytes from ITPKB knockout are anergic and undergo excessive apoptosis. Knockout B cells exhibit enhanced Ca2+ influx through SOCs, and this is attributed to a loss of normal inhibition on SOC by Ins(1,3,4,5)P 4 [117, 121] (red arrow). A second model suggests that Ins(1,3,4,5)P 4 is a negative regulator RASA3, a Ras GTPase activating protein that binds Ins(1,3,4,5)P 4 with high affinity [110] (red arrow). In this model, lack of Ins(1,3,4,5)P 4 leads to excessive GTPase activity, thus attenuating Ras and its downstream targets [110]. c Proposed role for ITPK signaling in mature, circulating T lymphocytes based on polymorphisms in the ITPKC gene linked to Kawasaki disease, an autoimmune disorder [59]. This model posits a hyperactive Ins(1,4,5)P 3-triggered Ca2+ response following activation of the T cell receptor (TCR), leading to excessive activation of the phosphatase calcineurin, causing dephosphorylation and over activation of the transcription factor NFAT. The hyperactive NFAT would result in an inappropriately large immune response in the vascular and mucosal systems, accounting for the Kawasaki disease phenotype. In contrast to the other immune models, no gain-of-function role for Ins(1,3,4,5)P 4 is invoked. d ITPKB also plays widespread roles in the innate immune system [120]. In neutrophils, Ins(1,3,4,5)P 4 generated downstream of Fc receptor (FcR) activation is proposed to compete with the head group of the lipid phosphatidylinositol (3,4,5)P3 (PIP3) for binding to the tyrosine kinase AKT. Thus, neutrophils derived from ITPKB knockouts exhibit enhanced PIP3-regulated responses, such as chemotaxis and the respiratory burst [53]. e Yet another mechanism is suggested to explain ITPKB signaling in mast cells [109]. Here, Fc receptor epsilon 1 (FcεR1) regulates degranulation via two ITPKB-dependent processes. The first is a straightforward reduction of Ca2+ release via ITPKB attenuation of Ins(1,4,5)P 3 signals (red arrow). The second, in contrast to the mechanism depicted in panel B, involves a positive regulation of RASA3 and concomitant negative effect on Ras activation status. In either mechanism shown in panel e, lack of ITPKB would produce hyperactive degranulation
The second major class of Ins(1,3,4,5)P 4 receptors are channel proteins located in the plasma membrane. Channels were one of earliest suggested identities for Ins(1,3,4,5)P 4 effectors [100, 114]. For example, one series of studies has demonstrated Ins(1,3,4,5)P 4 enhancement of channels in a tyrosine kinase-triggered PLCγ signaling pathway that used ras-transformed NIH/3T3 fibroblasts [115]. This pathway required tyrosine kinase activation [116] and was hypothesized to require the GAP1 protein RASA3 [115]. This channel system resembles one characterized in immune cells [117]. Other channels implicated as Ins(1,3,4,5)P 4 targets include a Ca2+-dependent Cl-channel [114]. In the nervous system, the available data indicate that Ins(1,3,4,5)P 4 can enhance the activity of voltage-gated Ca2+ channels in pyramidal neurons located in the CA1 layer of the hippocampus [118, 119].
Widespread roles for ITPKs in the immune response
Some of the most compelling recent data supporting a signaling role for Ins(1,3,4,5)P 4 have been obtained from studies in the immune system. ITPKB knockout mice exhibit deficits throughout both the acquired and innate branches of the system (Fig. 4). Deficits in innate immunity include overactive neutrophils [53, 54] and mast cells [109]. The suggested molecular mechanisms involve competition of Ins(1,3,4,5)P 4 with the lipid PIP3, to bind to and regulate the function of proteins with PH domains, notably the serine/threonine kinase AKT [54] and RASA3 [109]. Some of the roles of ITPKs in innate immunity are depicted in Fig. 4d, e, and this topic was recently reviewed [120].
ITPKB plays a critical role in T cell development [51, 52]. Mice with targeted disruption in ITPKB exhibit massive defects in T cell maturation in the thymus. Specifically, T cells undergo negative selection normally but not positive selection. Lack of ITPKB during T cell selection causes a 90% reduction in the number of T cells circulating in peripheral lymphoid organs [51]. ITPKB also plays a crucial role in B cell survival and selection [110, 117, 121]. Mice with ITPKB knockout have tolerant B lymphocytes, which undergo inappropriate apoptosis. Recent data suggest that the B cell anergy observed in ITPKB knockout mouse lymphocytes is linked to enhancement of Ca2+ entry [121]. Whether the effect of ITPKB involves a direct Ins(1,3,4,5)P 4 inhibition of Ca2+ stores-operated channels (SOCs), or rather an indirect effect via enhanced Ca2+ stores depletion (or both) remains unclear.
The molecular mechanisms underlying the defects in thymocyte maturation are intriguing because they provide the strongest evidence for ITPK-generated, Ins(1,3,4,5)P 4-selective signal transduction [12, 122]. The intracellular Ca2+ responses measured in thymocytes lacking ITPKB appear to be normal following signaling through the T cell receptor [51]. However, the thymocytes exhibited reduced activation of the Ras–ERK pathway following TCR stimulation [51, 52]. The molecular mechanisms explaining these phenotypes remain incompletely defined. In one study [123], Ins(1,3,4,5)P 4 is suggested to function in a feed-forward mechanism in which it binds to the interleukin-2 inducible T cell tyrosine kinase (ITK) and enhances its phosphorylation of downstream targets (Fig. 4a). However, other studies in lymphocytes suggest a different molecular mechanism that involves Ins(1,3,4,5)P 4 modulation of the Ras pathway, possibly via its action on a GTPase activating protein of the GAP1 family (Fig 4a, b) [111]. The situation in B cells is also hypothesized to involve GAP1 modulation of the Ras pathway, leading defects in B cell selection to the control of the pro-apoptotic gene Bim following BCR stimulation (Fig 4b) [110]. In addition, ITPKB knockout B cells exhibit increased capacitative Ca2+ influx via SOCs following activation the BCR [121]. The proposed mechanism is that Ins(1,3,4,5)P 4 negatively regulates SOC (Fig. 4b), but it is not clear if Ins(1,3,4,5)P 4 binds directly to the channel or requires a transducer.
A summary of the immune data obtained from knockout studies (Fig. 4) suggests that Ins(1,3,4,5)P 4 is capable of acting on different or multiple targets in different cells, and surprisingly, most of the ITPKB-dependent mechanisms do not seem to involve the attenuation of Ins(1,4,5)P 3 Ca2+ signals. As further data emerge, the situation is likely to turn out to be less absolute because the interplay among Ca2+ release, Ca2+ entry, and Ras regulation is coordinated both spatially and temporally [124]. The spatiotemporal control of Ca2+ responses is critical for proper lymphocyte maturation and activation [125, 126]. The Ins(1,4,5)P 3-triggered Ca2+ response and subsequent sustained Ca2+ influx through stores operated channels (SOCs) regulate a range of T cell responses on different spatiotemporal scales [125]. The proper coordination of local and global Ca2+ signals is essential for eliciting appropriate immune signals emanating from the immunological synapse and determines both transcriptional profile and developmental fate [1].
Many immune responses require that the signal produced by a surface receptor be tuned to “just right,” meaning that either too much or too little activation can lead to disease [125, 127–129]. Ca2+ spikes and oscillations occur following TCR or BCR activation over time scales from seconds to hours. For example, the intracellular polarity and frequency of the Ca2+ signals are crucial to developing T cells in the thymus [130]. Here, they regulate positive selection by prolonging thymocyte interactions with the stroma [131]. Furthermore, the degree of activation of the Ras/ERK pathway depends both on the frequency coding of Ca2+ signal [124] and on the intracellular membrane locus of Ras [112, 113]. The localized, Ca2+-dependent generation of Ins(1,3,4,5)P 4 from Ins(1,4,5)P 3 can be imagined to participate in many of these processes, and investigations into ITPKB functions in immune development, tolerance, selection, and memory will remain fruitful areas of research for the foreseeable future [12, 120, 122].
Independent of ITPKB control of Ca2+ signals and Ins(1,3,4,5)P 4 levels, the phenotypes of ITPKB knockout mice require reconsideration in the context of the recent discovery that the amino terminus of the brain isoform ITPKA functions as a scaffold for Rho GTPases and associated F-actin machinery [132]. It is sobering to consider that the amino terminal regulatory region of ITPKB is about four times as long as that of ITPKA. It interacts with F-actin, shows structural homology to the actin-binding domain of ITPKA [77, 133], and remains largely uncharacterized. Since Rho GTPases are crucial regulators of lymphocyte development, polarity, and function [134], it is interesting to speculate that some of the phenotypes observed in the immune system of ITPKB knockouts are explained by the loss of a non-enzymatic function of ITPKB, such as control of the cytoskeleton.
ITPKC polymorphisms linked with Kawasaki disease
Because thymocytes in ITPKB knockout mice fail to mature normally, the knockout studies tell us little about the roles for ITPKs in mature, circulating T cells during activation of TCR. One recent hint of ITPK function in T cells comes from a suggested link between ITPKC and the auto-immune disorder Kawasaki disease—an acute, systemic vasculitis, which affects mainly infants and children [59]. In Kawasaki disease, an unidentified infectious agent triggers hyper-activation and inflammation of the vascular and mucosal systems in genetically susceptible children [135]. Kawasaki disease can cause coronary aneurisms, and this is the most common cause of acquired heart disease in children in Japan and the US [136].
Single-nucleotide polymorphisms in ITPKC suggest that reduced ITPC activity produces a hyper-activated T cell responsible for inappropriate immune activation [59]. In this model, increased Ins(1,4,5)P 3 lifetimes in ITPKC-hypoactive patients increases the release of intracellular Ca2+. The Ca2+/CaM-activated protein phosphatase calcineurin becomes hyper-activated, and the transcription factor NFAT becomes dephosphorylated, activating inappropriate transcription in the T cell [128]. This model is depicted in Fig. 4c, and it is distinguished from other cell signaling systems depicted in the figure because there is no explicit requirement for Ins(1,3,4,5)P 4.
Cellular and developmental co-expression of ITPR1 and ITPKA in brain
The sequestration and release of intracellular Ca2+ contributes substantially to the broad palette of spatiotemporal signaling in neurons, and this topic is comprehensively reviewed elsewhere [7, 137, 138]. The goal here is to highlight those systems most likely to be regulated by ITPKA. Brain is more enriched in the intracellular Ca2+ signaling “toolkit” than other tissues [3, 9, 139]. ITPR1, which is the main neuronal isoform of the intracellular Ca2+ release channel, is notably concentrated within large spiny neurons, such as pyramidal neurons in the hippocampus and cerebral cortex, and cerebellar Purkinje cells [140]. ITPKA is concentrated in most of these same cells, but the levels of co-expression with ITPR1 are not absolutely correlated [141, 142]. For example, the expression of ITPKA in CA1 pyramidal neurons is greater than it is in Purkinje cells, despite the much higher expression of ITPR1 in Purkinje neurons. By contrast, Purkinje neurons have much higher levels of the Ins(1,4,5)P 3 phosphatase INPP5A than do pyramidal neurons [143]. Thalamic areas have moderate ITPR1 expression, but virtually no ITPKA [142].
ITPR and ITPKA are also co-expressed ontologically. The intracellular Ca2+ signaling system arises late in development, appearing during the first postnatal week and reaching its full expression only after 4 weeks in rodents [18, 143–145]. In hippocampus, ITPR-related systems increase during and following periods of robust synaptogenesis (postnatal weeks 2 and 3 in rodents). ITPR and ITPKA expression in neurons both appear to be controlled by an endogenous developmental clock, because the increase in expression between postnatal weeks 1 and 3 is retained when neonatal cells are grown in dissociated culture, separated from anatomical constraints of their normal synaptic inputs [146, 147].
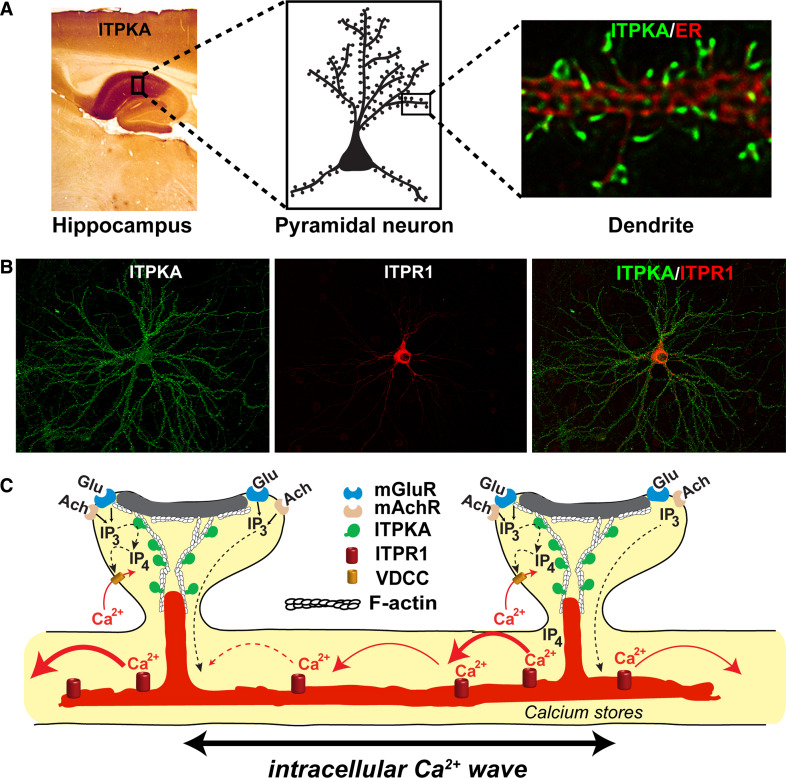
There are probably no ITPKA-positive/ITPR1-negative neurons, yet certain higher brain regions are notable for their extreme ITPKA levels relative to ITPR1 [141, 142]. The pyramidal neurons in the CA1 region of hippocampus express more ITPKA than anywhere else in the body (Fig. 5a, left). Within the cerebral cortex, ITPKA protein levels are especially high in the synapse-rich layers 1 and 3 [72], while IPTR1 localizes in soma-rich layers 2 and 5 [140]. The frontal cortex, amygdala, nucleus accumbens, septum, and striatum are all rich in ITPKA. It is in these brain regions where ITPKA and Ins(1,3,4,5)P 4-dependent signaling systems are most likely to occur. ITPKA gene expression in hippocampus increases and remains elevated for days following the training of rodents in a spatial memory task, suggesting a role for ITPKA in long-term consolidation and storage of recent synaptic activity [49].
Fig. 5.
ITPKA-regulated signaling in dendritic spines. a Regional and cellular localization of ITPKA in brain. Antibody staining against ITPKA (left) shows that the highest levels of ITPKA protein occur in the synapse-rich neuropil of the CA1 region of hippocampus (left, dark brown stain), while much lower expression occurs in the adjacent CA3 region. ITPKA is enriched in large pyramidal neurons, in dendritic spines (cartoon, middle). Deconvolved microscopic images (right) of a dendrite from a hipocampal neuron, co-labeled for ITPKA (green) and the endoplasmic reticulum (ER, red). Note how ITPKA is localized to Y-shaped structures, which lie inside dendritic spines and are situated between the synapse and the ER [147]. b Comparison of the localization of ITPKA and ITPR1 in a cultured hippocampal neuron. The image on the left (green) depicts a neuron transfected with ITPKA, which is localized toward the distal dendritic processes, in postsynaptic zones. The middle image (red) depicts transfected ITPR1, which is localized in the proximal ER and envelops the nucleus. The overlay (right) illustrates the inverse gradients of ITPK, located at distal (synaptic) sites, and ITPR1, located more proximally. c Cartoon depicting the spatial distribution of Ca2+ signaling systems regulated by ITPKA. Ins(1,4,5)P 3 is generated in spines chiefly through the activation of class 1 metabotropic glutamate (Glu) receptors (mGluR), which couple to phospholipase C beta (PLCβ). In the ITPKA-rich CA1 region of hippocampus, the mGluR subtype is mGluR5. Muscarinic acetylcholine (Ach) receptor (mAchR) subtypes M1 and M3 also occur in pyramidal neurons, and they constitute a second prominent Ins(1,4,5)P 3-generating system. Ins(1,4,5)P 3 generated inside dendritic spines diffuses to reach ITPR1, located in ER in or near the spine. ITPKA, which is intensely concentrated on bundles of filamentous actin (F-actin) inside spines [133], lies between sites of Ins(1,4,5)P 3 generation and action. Thus, the enzymatic activity of ITPKA is positioned as a molecular gatekeeper for synaptic Ins(1,4,5)P 3 signals. The Ca2+ released from intracellular stores can trigger an intracellular Ca2+ wave, which is driven by Ca2+-triggered Ca2+ release and also by Ins(1,4,5)P 3 produced in adjacent spines and propagates bi-directionally inside the main dendrite [137, 165]. Ins(1,3,4,5)P 4, the enzymatic product, has a direct effect on Ca2+ influx across the spine plasma membrane through its ability to enhance the activity of voltage-dependent Ca2+ channels (VDCCs) [118, 119]
Seminal early studies using cerebral cortical slices showed that stimulation of muscarinic acetylcholine receptors with the agonist carbachol triggered a rapid and transient production of Ins(1,3,4,5)P 4 [148]. Subsequent studies demonstrated that depolarization of brain slices by increasing [K+] from 5 to 20 mM is also an effective means of producing Ins(1,3,4,5)P 4 in cortical slices [149]. Moreover, a modest elevation in [K+] synergizes with muscarinic activation to enhance production of Ins(1,3,4,5)P 4 [150]. By far the best glutamatergic agonist for stimulating Ins(1,3,4,5)P 4 production in cortical slices is quisqualate, which possesses dual agonist activity at AMPA (amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptors and class 1 metabotropic glutamate receptors, but has little direct effect on NMDA receptors. Quisqualate-stimulated Ins(1,3,4,5)P 4 effects were mimicked by the combination of the class 1 metabotropic glutamate agonist ACPD (1-aminocyclopentane-trans-1,3-dicarboxylic acid) and AMPA, but not by carbachol and AMPA under similar conditions [151]. These experiments suggest that Ins(1,4,5)P 3 and Ins(1,3,4,5)P 4 pools mobilized by muscarinic versus metabotropic glutamatergic agonists are not identical.
Exposure of cortical brain slices to concentrations of N-methyl-d-aspartate (NMDA) between 1 and 10 μM enhanced Ins(1,3,4,5)P 4 generation up to fourfold, but higher concentrations were inhibitory; no Ins(1,3,4,5)P 4 was produced at 100 μM NMDA or higher [152]. In numerous studies, kainic acid has been shown to block inositol phosphate generation triggered by carbachol [153]. In summary, a modest Ca2+ influx is necessary to elicit significant Ins(1,3,4,5)P 4 production, but larger Ca2+ influxes are inhibitory to ITPKA. The stimulation by Ca2+ is likely explained by ITPKA being positively regulated by Ca2+/CaM, CaMKII, and also by the stimulation of phospholipase C via Ca2+. The inhibition by Ca2+ may occur because, in biochemical experiments, the Ca2+ effect on ITPKA shows an inverted U shape, with inhibition of enzyme activity occurring at Ca2+ concentrations above 1 mM [154]. A second contributing factor for the Ca2+ inhibition is that large Ca2+ influxes drive the ITPKA-decorated F-actin out of dendritic spines, away from sites of Ins(1,4,5)P 3 generation [147].
ITPKA may participate in ITPR-dependent signal integration
Neurons rich in Ins(1,4,5)P 3-dependent signaling machinery specialize in synaptic integration—the spatiotemporal reception of hundreds or thousands of synaptic inputs to compute a binary output [155, 156]. For example, the cerebellar Purkinje neurons, which perform astounding feats of integration, express more ITPR than any other cell type [144]. Compelling experimental evidence supports the idea that Ins(1,4,5)P 3 signaling participates in synaptic integration in Purkinje neurons [157–159] and in the CA1 pyramidal neurons of hippocampus [160, 161]. ITPRs themselves are subject to numerous molecular inputs, which impart integrative properties at the level of individual channels [162].
The ER Ca2+ stores of neurons, as in other cells, are contiguous [78]. This property positions the ER to perform integrative functions at electrical, biochemical, and transcriptional levels [163, 164]. The neuronal ER is excitable because it can produce regenerative intracellular Ca2+ waves (Fig. 5c) [137, 165–169]. The fill state of the Ca2+ stores is regulated by the extent of recent synaptic activity, which imparts the ER with a “memory” of recent action potentials [170–173]. The disposition of Ca2+ in the ER may also regulate biochemical changes relevant to neuronal plasticity—such as protein translation, and especially transcription in the nucleus (since the nuclear envelope is contiguous with the ER). Indeed, the rise in nuclear Ca2+ required to activate transcription is triggered preferentially through the Ins(1,4,5)P 3-sensitive intracellular stores, compared to other Ca2+ sources [163, 166–168, 173].
The role of ITPKA in neuronal integrative systems remains largely unexplored. In this context, however, the spatial relationship between ITPR and IPKA within neurons appears relevant. Although ITPR1-labeled ER can extend to distal dendritic processes, it is most concentrated proximally, within the somatodendritic regions of neurons (Fig. 5b) [174, 175]. By contrast, ITPKA is concentrated in distal regions of the dendrite, in dendritic spines, near sites of Ins(1,4,5)P 3 generation at synapses. Figure 5b depicts a hippocampal pyramidal neuron grown in culture, which is co-expressing a red-tagged ITPR1 and a green-tagged ITPKA. They form an inverse gradient, where ITPK predominates distally and ITPR1 predominates in the large apical dendrites at summation zones [176] and ITPR1 “hotspots” [169] located at dendritic branch conversion points near the cell soma.
Thus, ITPKA may be involved in spatially based integration by establishing an inositol phosphate gradient, with high Ins(1,3,4,5)P 4 levels predominating near distal synapses, in contrast to much higher densities of ITPR near the soma and nucleus. This may in turn affect the location and intracellular Ca2+ wave trigger zones, and the directionality or geometry of wave propagation, and this arrangement could in turn influence the Ca2+ signal in and near the nucleus. At the level of individual synapses, ITPKA is positioned in the neuron as a “metabolic gatekeeper” or “firewall” situated between sites of synaptic Ins(1,4,5)P 3 generation in dendritic spines, and sites of Ins(1,4,5)P 3 action at ITPRs, located more proximally on the ER in the dendritic shaft (Fig. 5c).
ITPKA possesses a number of properties that make it attractive as a molecular coincidence detector. ITPKA is directly activated by Ca2+/CaM as part of a classical negative feedback loop on Ins(1,4,5)P 3 levels following intracellular Ca2+ release. ITPKA is also positively regulated by the “memory kinase” CamKII [60], which is highly enriched at synapses [2] near ITPKA [177]. By contrast, the Ins(1,4,5)P 3 phosphatase INPP5A becomes inhibited by 90% upon phosphorylation by CamKII [61]. Thus, synaptic activity will produce a Ca2+/CaM-dependent “memory trace” that affects the routes of Ins(1,4,5)P 3 metabolism [10]. Recent synaptic activity will drive metabolic pathways towards more Ins(1,3,4,5)P 4 production in a dendrite, and lead to longer Ins(1,3,4,5)P 4 lifetimes in and near dendritic spines. The consequence of this remains unclear. The simplest consequence of positive regulation of ITPKA by Ca2+/CaM is that Ins(1,4,5)P 3 signals will be more rapidly attenuated, allowing activation of protein kinase C without triggering Ca2+ release. However, the ability of Ins(1,3,4,5)P 4 to protect Ins(1,4,5)P 3 from destruction by INPP5A [19] (which itself may have also become inactivated by CaMKII) implies that Ins(1,3,4,5)P 4 levels may remain elevated for seconds (or longer) following synaptic activity. If Ins(1,4,5)P 3 escapes ITPKA metabolism in the spine, it may diffuse to the dendritic shaft or cell soma, where its lifetime and steady-state concentration will be determined more by INPP5A. If INPP5A has been inhibited by a prior burst of Ins(1,3,4,5)P 4, the concentration of Ins(1,4,5)P 3 may rise along with Ins(1,3,4,5)P 4. The effects of elevated Ins(1,3,4,5)P 4 on its putative molecular targets in neurons are largely unexplored, but the currently available data suggest an enhancement of Ca2+ channel openings in the plasma membrane and/or a modulation of signaling that involves small G-proteins such as Ras (see below).
Regulation of F-actin by ITPKA in dendritic spines
The microstructure of the actin cytoskeleton in neurons is the focus of intense study because it participates in most kinds of structural plasticity [178]. Independent of its enzymatic activity, ITPKA interacts with actin filaments and may function as a persistent regulator of activity-dependent changes in postsynaptic F-actin microstructure [132, 133, 147, 179]. Early electron microscope studies localized ITPKA in brain and observed that it was concentrated in the dendritic spines of pyramidal neurons in cerebral cortex and hippocampus, and in cerebellar Purkinje neurons [47, 72, 180, 181]. These studies also noted that adjacent spines from the same cortical pyramidal neuron sometimes showed very different levels of ITPKA content, ranging from very high to not detected [47]. The disposition of ITPKA as a synaptic enzyme seemed at odds with many biochemical studies showing that ITPK activity occurred predominantly in the cytosolic fraction from brain [65, 66].
The morphological and biochemical data were reconciled when we demonstrated that ITPKA is enriched in dendritic spines because its 66 most amino terminal amino acids bind F-actin [177]. ITPKA binds a labile pool of synaptic actin [147] with micromolar affinity [133]. When ITPKA is isolated from brain homogenates (usually done in sucrose buffers containing no salt), the F-actin becomes depolymerized, and ITPKA dissociates from its cytoskeletal anchoring. We recently demonstrated that the F-actin binding is the sole means by which ITPKA is targeted to spines. A single point mutation at residue L34, located in a putative alpha helical region in the F-actin-binding N-terminus of rat ITPKA (Fig. 2), was sufficient to render the enzyme cytosolic [133].
Owing to the delicate and labile state of F-actin located beneath the posynaptic density in dendritic spines, much remains unclear about the structure and function of postsynaptic actin. If brain tissue is processed using standard electron microscopy techniques, ITPKA appears localized on “cytosolic matrix” [47] and “fluffy material” [72] associated with the plasma membrane and with the spine ER. Better methods have been developed, which prevent the destruction of F-actin during processing for EM [182]. However, ITPKA has not yet been localized ultrastructurally under these conditions. In recent studies where F-actin microstructure in spines has been properly preserved [182], actin filaments occur as bundles that, in part, span between the spine ER lamellae and the plasma membrane (Fig. 5c) [183]. Our studies using deconvolution light microscopy localized ITPKA to bundles of actin that formed Y-shaped projections between the ER and the PM [147], consistent with the observations of Campani et al. [183] (Fig. 5).
Recently, we demonstrated that the N-terminus of rat ITPKA bundles F-actin via a mechanism that involves multimerization of the predicted alpha helix centered near amino acid L34 [133]. The bundling of filaments contributes to the targeting of ITPKA to dendrites and away from axons, and it may also affect spine actin microstructure by increasing the length of the spine neck. Since we previously demonstrated that IPKA-decorated F-actin in dendritic spines is dynamic, and that it moves in and out of dendritic spines in a Ca2+ regulated manner [147], these observations suggest a complex interplay between the targeting of ITPKA to synaptic actin and the regulation of F-actin microstructure during and after synaptic activity. This may in turn affect the ability of ITPKA to modulate and compartmentalize synaptic Ins(1,4,5)P 3. The idea that ITPK regulates F-actin structure in spines recently gained further support with the discovery that it functions as a scaffold for the Rho family GTPase Rac1 [132]. During synaptic activity, ITPKA recruits Rac1 and associated signaling machinery to dendritic spine actin, and this regulates the shape of the spine. These studies open up a new and exciting line of research investigating how synaptic signals in spines lead to a rapid yet persistent change in spine shape and internal microstructure. It is worth noting that the ITPKA interaction with F-actin is unique to mammals (including marsupials), and some birds. Inspection of the recently published genome of the platypus [184] indicates that the N-terminal region of ITPKA from this “pre-mammal” is unlikely to interact with F-actin (MJS, unpublished). This implies that the targeting of ITPKA to synaptic actin is a very recent elaboration in animal evolution, which may contribute to a selective advantage for big-brained animals.
Does ITPKA regulate Ins(1,4,5)P3 lifetime in dendritic spines?
The positioning of ITPKA on the F-actin inside dendritic spines—between sites of Ins(1,4,5)P 3 generation and sites of Ins(1,4,5)P 3 action—implies that it regulates Ins(1,4,5)P 3 concentrations in the spatial and/or temporal domains during synaptic activity. However, very few studies have addressed this question experimentally.
Although ITPKA is incredibly enriched in the dendritic spines in the CA1 region, where it would be expected to have privileged access to Ins(1,4,5)P 3 generated following activation of metabotropic glutamate receptors and muscarinic acetylcholine receptors, clear evidence that ITPKA regulates Ins(1,4,5)P 3 lifetime at synapses is lacking. One recent study examined Ins(1,4,5)P 3 lifetime in CA1 pyramidal neurons using hippocampal slices and Ins(1,4,5)P 3 released by photolysis [87]. They concluded that the major determinant of the timing window for Ins(1,4,5)P 3-regulated coincidence detection with action potentials was simply Ins(1,4,5)P 3 diffusion, rather than metabolism. Even if localized metabolism of Ins(1,4,5)P 3 in spines does occur, the functional consequence of ITPKA activity, and Ins(1,3,4,5)P 4 synthesis, remains unclear.
The physiological mechanism for Ins(1,4,5)P 3 production in neurons has been delineated best in the cerebellum. In Purkinje spines, mGluR1 and Ca2+ influx through AMPA receptors and/or voltage-gated receptors synergize to activate Ca2+sensitive phospholipase C, producing Ins(1,4,5)P 3 as part of a Ca2+-dependent feed-forward mechanism that controls long-term depression, a physiological system of coincidence detection between different synaptic inputs onto Purkinje neurons [157, 185, 186]. In this system, Ca2+ release from intracellular stores in and near dendritic spines reports the coincidence of synaptic inputs originating from climbing and parallel fibers [158]. The role, if any, for ITPKA in this system is not known. One recent study that examined Ins(1,4,5)P 3 diffusion in Purkinje cell dendrites concluded that both trapping of Ins(1,4,5)P 3 in spines and Ins(1,4,5)P 3 metabolism contributed to anomalous diffusion of Ins(1,4,5)P 3 [83]. Another recent study released Ins(1,4,5)P 3 via photolysis in and near Purkinje cell spines from cerebellar slices and estimated that about 20% of the Ins(1,4,5)P 3 generated in a spine gets degraded in the spine before passing through the spine neck [86]. The authors then pursued the spatiotemporal relationship between climbing fiber activation and Ins(1,4,5)P 3 release via flash photolysis, and suggested that the predominant integrative role for Ins(1,4,5)P 3 metabolism in Purkinje cells is spatial, not temporal [159]. Since Purkinje spines contain substantial levels of both ITPKA and INSP5A, the metabolic pathway(s) operating are unclear.
The hippocampal CA1 region is another prominent site of ITPKA expression, but surprisingly little is known about how ITPKA regulates Ins(1,4,5)P 3 lifetime in a CA1 neuron. The mechanisms of Ca2+ release operating in Purkinje cells may not be the same as those in CA1 pyramidal neurons. Compared to Purkinje neurons, CA1 pyramidal neurons have more ITPKA, less ITPR, and less INPP5A, and they express NMDA receptors abundantly (while adult Purkinje cells have none). All dendritic spines of Purkinje neurons contain ITPR-rich ER, but only the largest spines in CA1 neurons contain ER called the spine apparatus [187, 188]. Whether the spine apparatus of CA1 neurons even contains ITPR1 is controversial [174].
Studies using ITPR1 and ITPKA knockout mice have not led to clear conclusions about the roles of the Ins(1,4,5)P 3 signaling system in hippocampal synaptic plasticity. Mice with targeted disruption of ITPR1 exhibit enhanced LTP, suggesting that ITPR1 functions as a negative regulator [189]. Yet a number of studies have demonstrated that Ins(1,4,5)P 3 production following activation of metabotropic glutamate receptors “primes” neurons, lowering the threshold of stimulus required to trigger LTP [190]. Mice with targeted disruption of ITPKA exhibit a large increase in the magnitude of LTP in the CA1 region, but normal spatial learning in the Morris water maze [48]. Basal levels of Ins(1,4,5)P 3 are not different from control animals, but levels of Ins(1,3,4,5)P 4 were reduced by about 75%. The ITPK knockout study also examined global Ca2+ responses following perfusion of glutamate into neuronal cultures derived from knockout mice, and they found responses to be similar to controls [48]. However, it should be noted that these measurements were taken from neurons cultured for between 7 and 10 days, which was not old enough to allow significant ITPKA expression in the controls.
Recently, new physiological and behavioral phenotypes were reported in ITPKA knockout mice [132]. In contrast to the case in the CA1 region, LTP in the CA3 region is dramatically reduced. Furthermore, deficits in spatial learning were uncovered using the behavioral tests of novel object recognition and the radial arm maze [132]. This study provides considerable new evidence that ITPKA is indeed critical for some types of learning and memory storage in brain. However, it remains unclear if the phenotypes are a consequence of modified Ins(1,4,5)P 3 metabolism, modified F-actin functions in dendritic spines, or both.
Finally, Ins(1,4,5)P 3 generated by muscarinic receptors was recently shown to produce a long-lasting enhancement of synaptic transmission in CA1 pyramidal neurons [191]. The Ins(1,4,5)P 3 generated through cholinergic pathways may function differently than when it arises from stimulation of class 1 metabotropic glutamate receptors. For example, in the amygdala, the two pools of receptor-generated Ins(1,4,5)P 3 may work cooperatively to regulate levels of Ca2+ in the cell soma and nucleus [168].
Does Ins(1,3,4,5)P4 bind protein targets in spines?
The most attractive candidate downstream targets for Ins(1,3,4,5)P 4 in dendritic spines are the same as those found in lymphocytes: Ca2+ channels and modulators of the small G-proteins Ras and Rap. The evidence is strongest for Ins(1,3,4,5)P 4 regulation of voltage-dependent Ca2+ channels, located postsynaptically in hippocampal neurons. These channels become more active following a stroke [118]. In one study, Ins(1,3,4,5)P 4 was applied to the cytoplasmic face of excised patches of CA1 pyramidal neurons [118]. At a holding potential of −60 mV, a small increase in channel opening was observed in the presence of 7.5 μM Ins(1,3,4,5)P 4 (see Fig. 1 of that study). However, if animals had undergone an ischemic stroke 24–36 h before the patch-clamp experiment, the open probability of the Ins(1,3,4,5)P 4-modulated channels increased dramatically. The single channel conductance of these channels was 7.5 pS, and they were permeable to cations (Ca2+, Ba2+, and Sr2+)—but not chloride. Pharmacological tests indicated that the channels operated at negative holding potentials and were blocked by ω-conotoxin GVIA, but not ω-agatoxin IVA or nifedipine.
A different study of CA1 pyramidal neurons used intracellular applications of Ins(1,3,4,5)P 4 in the whole cell configuration [119]. If 100 μM Ins(1,3,4,5)P 4 was infused into the cell prior to (but not after) tetanization to induce long-term potentiation (LTP), the degree of potentiation was enhanced. When the experiment was repeated, substituting 2,3-dideoxy Ins(1,4,5)P 3, a non-metabolizable Ins(1,4,5)P 3 analog, no potentiation was observed, showing that the potentiation was selective for Ins(1,3,4,5)P 4. Similar to the other study, the Ins(1,3,4,5)P 4 effect was blocked by ω-conotoxin GVIA. However, the Ins(1,3,4,5)P 4 effect was also dependent on release of Ca2+ from intracellular stores through ITPR because co-application of the ITPR-blocker heparin (but not the ryanodine receptor blocker ryanodine) during the tetanus occluded the Ins(1,3,4,5)P 4-dependent component of the potentiation. Thus, this study suggested that Ins(1,3,4,5)P 4 participates in the complex interplay between voltage-dependent Ca2+ entry and Ins(1,4,5)P 3-dependent Ca2+ release that occurs in the dendrites of CA1 pyramidal neurons during synaptic activity. These studies are difficult to reconcile with the phenotypes of ITPKA knockout mice, which exhibit enhanced LTP in CA1, decreased LTP in CA3, but apparently normal Ca2+ responses [48, 132].
Direct evidence for neurons using ITPK-regulated signaling systems analogous to those found lymphocytes does not yet exist, but circumstantial evidence makes the possibility worthy of further investigation. The regulation of small G-protein signaling by Ins(1,3,4,5)P 4 has not been reported in hippocampal neurons, but the analogy with Ins(1,3,4,5)P 4 signaling in the immune system makes this an attractive possibility. The likely downstream targets for Ins(1,3,4,5)P 4 in neurons are signaling proteins that control the activation state of Ras and Rap (see Fig. 4). These participate in the biochemical integration of signals in spines (reviewed in [2]). In some cases, the regulation appears to occur via modulation of the GAP1 family GTPase activating protein RASA3, also known as GAP1IP4BP. This protein was originally purified from platelet membranes and cloned based on its high affinity binding for Ins(1,3,4,5)P 4 [107], and it is widely expressed in lymphocytes and in neurons [192].
Ras and Rap signaling are prominent in CA1 pyramidal neurons, especially in and near dendritic spines, where they regulate spine morphology through Ca2+-dependent mechanisms [193–197]. In lymphocytes, Ras and Rap operate downstream of tyrosine kinase pathways coupled to phospolipase C γ to control cell fate [113], and activation of the Map kinase cascade is the usual downstream effect. PLCγ is also expressed abundantly in pyramidal neurons, where its activation via EphrinB regulates dendritic spine morphology through control of the actin cytoskeleton [198]. In neurons, the generation of Ins(1,4,5)P 3 through PLCγ activation downstream of growth factors or “growth-factor like” stimuli is much less studied compared to the more classical PLCβ pathways downstream of G-proteins. The understandably intense focus on Ins(1,4,5)P 3 generation via metabotropic and muscarinic-driven PLCβ pathways for release of Ca2+ may have focused attentions away from PLCγ-dependent Ins(1,4,5)P 3/Ins(1,3,4,5)P 4-generating systems more similar to those operating in lymphocytes.
The potential of ITPK-based therapies
The high expression of ITPK isoforms in the immune and nervous systems suggests that small molecules targeted to ITPK isoforms have potential as drugs and as tools for research [122]. ITPK-targeted drugs would function in a way analogous to the pharmacologically useful phosphodiesterase inhibitors, which can control cellular levels of cyclic nucleotides. The pharmaceutical aim would be to produce a drug to enhance or inhibit ITPK activity selectively in the immune system or in the brain. The suggested medical aims for such therapies vary from immunosuppression or immuno-activation, cognition enhancement and/or neuroprotection. Because of the universal importance of Ins(1,4,5)P 3-based Ca2+ signaling in all animals and the controversial mechanisms of Ins(1,3,4,5)P 4 signaling, it remains unclear whether the desired effect would be to enhance or inhibit ITPK. The critical role for ITPKB during thymocyte maturation and the late expression of ITPKA in neurons during and after synaptogenesis suggests that the age of the organism or patient given an ITPK modulator drug would be an important consideration if such compounds are developed as therapy. While no ITPK-targeted drug is near clinical use, the example of phosphodiesterase inhibitors as therapeutic agents points to the promise of modulating second messenger systems as therapy.
All ITPK inhibitors described so far interact with either the Ins(1,4,5)P 3 or ATP recognition sites in the catalytic domain [199–201]. Early attempts to identify inhibitory lead compounds employed exhaustive screens of inositol phosphate isomers [199, 202]. The results of these screens emphasize the incredibly high selectivity of the ITPK catalytic site for Ins(1,4,5)P 3, as no other isomer is binds with similar or higher affinity. The selectivity of ITPK for Ins(1,4,5)P 3 even exceeds that of ITPR [203]. If effective drugs acting at this site are to be developed, they must be strict structural mimics of Ins(1,4,5)P 3 in the ITPK-binding pocket. Screens of purine-based inhibitors acting at the ATP site have identified possible lead compounds for ITPK inhibition, but the likely problem here is lack of selectivity over other types of kinases, especially other members of the IPK family [203, 204]. Indeed, a promising purine-based lead compound for ITPKA inhibition [200] was later shown to be a significantly more potent inhibitor of InsP6 kinase [205]. The structural similarity of the ITPK ATP-binding site with an ancient kinase family emphasizes the difficulties of obtaining selectivity at the ATP site [23].
Creating isoform-selective ITPK inhibitors may prove even more difficult because of the very high structural homology among the catalytic sites of ITPKA [21, 22], ITPKB [206], ITPKC (PDB accession 2a98), and IPMK [27]. Developing isoform-selective compounds acting at these sites poses a significant challenge for medicinal chemistry. A recent compound library screen for ITPK inhibitors found the isoform selectivity to be poor among the highest affinity inhibitors identified [201]. Moreover, molecules acting at the active sites are likely to be positively charged, and highly charged drugs may prove difficult to target to cells and tissues inside organisms. One unexplored possibility is allosteric modulation, which could circumvent problems of cellular and/or tissue penetration encountered with charged molecules. The potential of allosterically modulating ITPKs is unproven. The hypothetical allosteric ITPKA modulator might interact with the calmodulin-binding site, mimic the effects on activity produced when ITPKA becomes phosphorylated, inhibit ITPKA dimerization, or affect ITPKA localization.
Conclusions
ITPKs are conserved among metazoans, where they function as “off” signals for the second messenger Ins(1,4,5)P 3. In doing so they produce Ins(1,3,4,5)P 4, a putative “third messenger.” While Ins(1,4,5)P 3 signals can be attenuated by both kinase and phosphatase pathways, ITPKs are notably expressed in immune and neuronal tissues, and their predominant roles in these tissues are borne out by the phenotypes of knockout mice. Compared to the Ins(1,4,5)P 3 phosphatase INPP5A, ITPKAs have higher affinity and selectivity for Ins(1,4,5)P 3. They are regulated by extensive cross-talk with other signaling systems, and they exhibit targeting to subcellular compartments. These properties suggest a prominent role for ITPKs in controlling Ca2+ signals within the spatiotemporal domains of cells via control of Ins(1,4,5)P 3 lifetimes. The status of Ins(1,3,4,5)P 4 as a messenger is bolstered by phenotypes of immune cells and neurons lacking ITPK isoforms. In some of these cases, the Ins(1,4,5)P 3-dependent Ca2+ signals measured appeared normal, suggesting that it was a loss of Ins(1,3,4,5)P 4 function that accounted for the cellular phenotype. The two types of likely Ins(1,3,4,5)P 4 targets are channels residing in the plasma membrane and various regulators of the small G proteins Ras and Rap. The intense concentration of ITPKA on actin filaments in association with Rho GTPases inside dendritic spines of hippocampal neurons suggests a role in signal integration in the dendrite via control of biochemical, structural, and transcriptional plasticity. Further studies are required to define this role precisely. The selective expression of ITPKA isoforms in cells of the immune and nervous systems suggests that small molecule modulators of ITPKs have potential as therapeutic agents. However, conservation among the catalytic sites of ITPK isoforms and the highly charged nature of the natural substrates imply that ITPKA modulators pose a significant challenge for drug development.
Acknowledgments
Thanks to Pavel Gusev for critical reading and Gudrun Ihrke for assistance with the figures. The author is supported by USU grants RO75NX and RO75PJ, and a Research Starter grant from the PhRMA Foundation. The opinions or assertions contained herein are the private ones of the author and are not to be construed as official or reflecting the view of the Department of Defense or the Uniformed Services University.
Abbreviations
- ACPD
1-Aminocyclopentane-trans-1, 3-dicarboxylic acid
- AMPA
Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- BCR
B cell receptor
- CaM
Calmodulin
- CamKII
Ca2+/calmodulin-dependent kinase II
- ERK
Extracellular signal-regulated kinase
- ER
Endoplasmic reticulum
- IP
Inositol phosphate
- InsP3
Inositol trisphosphate [Ins(1,4,5)P 3]
- InsP4
Inositol tetrakisphosphate [Ins(1,3,4,5)P 4]
- IPK
Inositol polyphosphate kinase
- F-actin
Filamentous actin
- INPP5A
Inositol polyphosphate 5-kinase type 1
- IPMK
Inositol polyphosphate multikinase
- ITK
Interleukin-2 inducible T cell tyrosine kinase
- ITPK
Inositol trisphosphate 3-kinase
- ITPR
Inositol trisphosphate receptor
- LTP
Long term potentiation
- NMDA
N-methyl-d-aspartate
- PH
Pleckstrin homology
- PI3K
Phosphatidylinositol lipid 3-kinase
- SOC
Stores operated channel
- TCR
T cell receptor
References
- 1.Kummerow C, Junker C, Kruse K, Rieger H, Quintana A, Hoth M. The immunological synapse controls local and global calcium signals in T lymphocytes. Immunol Rev. 2009;231:132–147. doi: 10.1111/j.1600-065X.2009.00811.x. [DOI] [PubMed] [Google Scholar]
- 2.Kennedy MB, Beale HC, Carlisle HJ, Washburn LR. Integration of biochemical signalling in spines. Nat Rev Neurosci. 2005;6:423–434. doi: 10.1038/nrn1685. [DOI] [PubMed] [Google Scholar]
- 3.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 4.Neves SR, Tsokas P, Sarkar A, Grace EA, Rangamani P, Taubenfeld SM, Alberini CM, Schaff JC, Blitzer RD, Moraru II, Iyengar R. Cell shape and negative links in regulatory motifs together control spatial information flow in signaling networks. Cell. 2008;133:666–680. doi: 10.1016/j.cell.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willoughby D, Cooper DM. Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol Rev. 2007;87:965–1010. doi: 10.1152/physrev.00049.2006. [DOI] [PubMed] [Google Scholar]
- 6.Sabatini BL, Oertner TG, Svoboda K. The life cycle of Ca2+ ions in dendritic spines. Neuron. 2002;33:439–452. doi: 10.1016/s0896-6273(02)00573-1. [DOI] [PubMed] [Google Scholar]
- 7.Augustine GJ, Santamaria F, Tanaka K. Local calcium signaling in neurons. Neuron. 2003;40:331–346. doi: 10.1016/s0896-6273(03)00639-1. [DOI] [PubMed] [Google Scholar]
- 8.Irvine RF, Lloyd-Burton SM, Yu JC, Letcher AJ, Schell MJ. The regulation and function of inositol 1,4,5-trisphosphate 3-kinases. Adv Enzyme Regul. 2006;46:314–323. doi: 10.1016/j.advenzreg.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Worley PF, Baraban JM, Supattapone S, Wilson VS, Snyder SH. Characterization of inositol trisphosphate receptor binding in brain. Regulation by pH and calcium. J Biol Chem. 1987;262:12132–12136. [PubMed] [Google Scholar]
- 10.Irvine RF, Schell MJ. Back in the water: the return of the inositol phosphates. Nat Rev Mol Cell Biol. 2001;2:327–338. doi: 10.1038/35073015. [DOI] [PubMed] [Google Scholar]
- 11.Seeds AM and York JD (2007) Inositol polyphosphate kinases: regulators of nuclear function. Biochem Soc Symp 183–197 [DOI] [PubMed]
- 12.Miller AT, Chamberlain PP, Cooke MP. Beyond IP3: roles for higher order inositol phosphates in immune cell signaling. Cell Cycle. 2008;7:463–467. doi: 10.4161/cc.7.4.5518. [DOI] [PubMed] [Google Scholar]
- 13.Vanweyenberg V, Communi D, D’Santos CS, Erneux C. Tissue- and cell-specific expression of Ins(1,4,5)P3 3-kinase isoenzymes. Biochem J. 1995;306:429–435. doi: 10.1042/bj3060429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erneux C, Govaerts C, Communi D, Pesesse X. The diversity and possible functions of the inositol polyphosphate 5-phosphatases. Biochim Biophys Acta. 1998;1436:185–199. doi: 10.1016/s0005-2760(98)00132-5. [DOI] [PubMed] [Google Scholar]
- 15.Batty IH, Letcher AJ, Nahorski SR. Accumulation of inositol polyphosphate isomers in agonist-stimulated cerebral-cortex slices. Comparison with metabolic profiles in cell-free preparations. Biochem J. 1989;258:23–32. doi: 10.1042/bj2580023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zilberman Y, Howe LR, Moore JP, Hesketh TR, Metcalfe JC. Calcium regulates inositol 1,3,4,5-tetrakisphosphate production in lysed thymocytes and in intact cells stimulated with concanavalin A. EMBO J. 1987;6:957–962. doi: 10.1002/j.1460-2075.1987.tb04845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Irvine RF, Moor RM, Pollock WK, Smith PM, Wreggett KA. Inositol phosphates: proliferation, metabolism and function. Philos Trans R Soc Lond B Biol Sci. 1988;320:281–298. doi: 10.1098/rstb.1988.0077. [DOI] [PubMed] [Google Scholar]
- 18.Moon KH, Lee SY, Rhee SG. Developmental changes in the activities of phospholipase C, 3-kinase, and 5-phosphatase in rat brain. Biochem Biophys Res Commun. 1989;164:370–374. doi: 10.1016/0006-291x(89)91728-2. [DOI] [PubMed] [Google Scholar]
- 19.Hermosura MC, Takeuchi H, Fleig A, Riley AM, Potter BV, Hirata M, Penner R. InsP4 facilitates store-operated calcium influx by inhibition of InsP3 5-phosphatase. Nature. 2000;408:735–740. doi: 10.1038/35047115. [DOI] [PubMed] [Google Scholar]
- 20.Irvine R. Inositol phosphates: does IP(4) run a protection racket? Curr Biol. 2001;11:R172–R174. doi: 10.1016/s0960-9822(01)00086-0. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez B, Schell MJ, Letcher AJ, Veprintsev DB, Irvine RF, Williams RL. Structure of a human inositol 1,4,5-trisphosphate 3-kinase: substrate binding reveals why it is not a phosphoinositide 3-kinase. Mol Cell. 2004;15:689–701. doi: 10.1016/j.molcel.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Miller GJ, Hurley JH. Crystal structure of the catalytic core of inositol 1,4,5-trisphosphate 3-kinase. Mol Cell. 2004;15:703–711. doi: 10.1016/j.molcel.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Cheek S, Ginalski K, Zhang H, Grishin NV. A comprehensive update of the sequence and structure classification of kinases. BMC Struct Biol. 2005;5:6. doi: 10.1186/1472-6807-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Resnick AC, Saiardi A. Inositol polyphosphate multikinase: metabolic architect of nuclear inositides. Front Biosci. 2008;13:856–866. doi: 10.2741/2726. [DOI] [PubMed] [Google Scholar]
- 25.Saiardi A, Erdjument-Bromage H, Snowman AM, Tempst P, Snyder SH. Synthesis of diphosphoinositol pentakisphosphate by a newly identified family of higher inositol polyphosphate kinases. Curr Biol. 1999;9:1323–1326. doi: 10.1016/s0960-9822(00)80055-x. [DOI] [PubMed] [Google Scholar]
- 26.York JD, Odom AR, Murphy R, Ives EB, Wente SR. A phospholipase C-dependent inositol polyphosphate kinase pathway required for efficient messenger RNA export. Science. 1999;285:96–100. doi: 10.1126/science.285.5424.96. [DOI] [PubMed] [Google Scholar]
- 27.Holmes W, Jogl G. Crystal structure of inositol phosphate multikinase 2 and implications for substrate specificity. J Biol Chem. 2006;281:38109–38116. doi: 10.1074/jbc.M606883200. [DOI] [PubMed] [Google Scholar]
- 28.Chang SC, Miller AL, Feng Y, Wente SR, Majerus PW. The human homolog of the rat inositol phosphate multikinase is an inositol 1,3,4,6-tetrakisphosphate 5-kinase. J Biol Chem. 2002;277:43836–43843. doi: 10.1074/jbc.M206134200. [DOI] [PubMed] [Google Scholar]
- 29.Stevenson-Paulik J, Bastidas RJ, Chiou ST, Frye RA, York JD. Generation of phytate-free seeds in Arabidopsis through disruption of inositol polyphosphate kinases. Proc Natl Acad Sci USA. 2005;102:12612–12617. doi: 10.1073/pnas.0504172102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang T, Caffrey JJ, Shears SB. The transcriptional regulator, Arg82, is a hybrid kinase with both monophosphoinositol and diphosphoinositol polyphosphate synthase activity. FEBS Lett. 2001;494:208–212. doi: 10.1016/s0014-5793(01)02351-1. [DOI] [PubMed] [Google Scholar]
- 31.Resnick AC, Snowman AM, Kang BN, Hurt KJ, Snyder SH, Saiardi A. Inositol polyphosphate multikinase is a nuclear PI3-kinase with transcriptional regulatory activity. Proc Natl Acad Sci USA. 2005;102:12783–12788. doi: 10.1073/pnas.0506184102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bennett M, Onnebo SM, Azevedo C, Saiardi A. Inositol pyrophosphates: metabolism and signaling. Cell Mol Life Sci. 2006;63:552–564. doi: 10.1007/s00018-005-5446-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saiardi A, Caffrey JJ, Snyder SH, Shears SB. The inositol hexakisphosphate kinase family. Catalytic flexibility and function in yeast vacuole biogenesis. J Biol Chem. 2000;275:24686–24692. doi: 10.1074/jbc.M002750200. [DOI] [PubMed] [Google Scholar]
- 34.Fujii M, York JD. A role for rat inositol polyphosphate kinases rIPK2 and rIPK1 in inositol pentakisphosphate and inositol hexakisphosphate production in rat-1 cells. J Biol Chem. 2005;280:1156–1164. doi: 10.1074/jbc.M412006200. [DOI] [PubMed] [Google Scholar]
- 35.Seeds AM, Bastidas RJ, York JD. Molecular definition of a novel inositol polyphosphate metabolic pathway initiated by inositol 1,4,5-trisphosphate 3-kinase activity in Saccharomyces cerevisiae . J Biol Chem. 2005;280:27654–27661. doi: 10.1074/jbc.M505089200. [DOI] [PubMed] [Google Scholar]
- 36.Leyman A, Pouillon V, Bostan A, Schurmans S, Erneux C, Pesesse X. The absence of expression of the three isoenzymes of the inositol 1,4,5-trisphosphate 3-kinase does not prevent the formation of inositol pentakisphosphate and hexakisphosphate in mouse embryonic fibroblasts. Cell Signal. 2007;19:1497–1504. doi: 10.1016/j.cellsig.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 37.Seeds AM, Frederick JP, Tsui MM, York JD. Roles for inositol polyphosphate kinases in the regulation of nuclear processes and developmental biology. Adv Enzyme Regul. 2007;47:10–25. doi: 10.1016/j.advenzreg.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clandinin TR, DeModena JA, Sternberg PW. Inositol trisphosphate mediates a RAS-independent response to LET-23 receptor tyrosine kinase activation in C. elegans . Cell. 1998;92:523–533. doi: 10.1016/s0092-8674(00)80945-9. [DOI] [PubMed] [Google Scholar]
- 39.Kashyap L, Tabish M, Ganesh G, Dubey D. Computational and molecular characterization of multiple isoforms of lfe-2 gene in nematode C. elegans . Bioinformation. 2007;2:17–21. doi: 10.6026/97320630002017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bui YK, Sternberg PW. Caenorhabditis elegans inositol 5-phosphatase homolog negatively regulates inositol 1,4,5-triphosphate signaling in ovulation. Mol Biol Cell. 2002;13:1641–1651. doi: 10.1091/mbc.02-01-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pilipiuk J, Lefebvre C, Wiesenfahrt T, Legouis R, Bossinger O. Increased IP3/Ca2+ signaling compensates depletion of LET-413/DLG-1 in C. elegans epithelial junction assembly. Dev Biol. 2008;327:34–47. doi: 10.1016/j.ydbio.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 42.Seeds AM, Sandquist JC, Spana EP, York JD. A molecular basis for inositol polyphosphate synthesis in Drosophila melanogaster . J Biol Chem. 2004;279:47222–47232. doi: 10.1074/jbc.M408295200. [DOI] [PubMed] [Google Scholar]
- 43.Dobie KW, Kennedy CD, Velasco VM, McGrath TL, Weko J, Patterson RW, Karpen GH. Identification of chromosome inheritance modifiers in Drosophila melanogaster . Genetics. 2001;157:1623–1637. doi: 10.1093/genetics/157.4.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monnier V, Girardot F, Audin W, Tricoire H. Control of oxidative stress resistance by IP3 kinase in Drosophila melanogaster . Free Radic Biol Med. 2002;33:1250–1259. doi: 10.1016/s0891-5849(02)01019-5. [DOI] [PubMed] [Google Scholar]
- 45.Lloyd-Burton SM, Yu JC, Irvine RF, Schell MJ. Regulation of inositol 1,4,5-trisphosphate 3-kinases by calcium and localization in cells. J Biol Chem. 2007;282:9526–9535. doi: 10.1074/jbc.M610253200. [DOI] [PubMed] [Google Scholar]
- 46.Kucharski R, Maleszka R. Molecular profiling of behavioural development: differential expression of mRNAs for inositol 1,4,5-trisphosphate 3-kinase isoforms in naive and experienced honeybees (Apis mellifera) Brain Res Mol Brain Res. 2002;99:92–101. doi: 10.1016/s0169-328x(01)00325-4. [DOI] [PubMed] [Google Scholar]
- 47.Go M, Uchida T, Takazawa K, Endo T, Erneux C, Mailleux P, Onaya T. Inositol 1,4,5-trisphosphate 3-kinase highest levels in the dendritic spines of cerebellar Purkinje cells and hippocampal CA1 pyramidal cells. A pre- and post-embedding immunoelectron microscopic study. Neurosci Lett. 1993;158:135–138. doi: 10.1016/0304-3940(93)90247-i. [DOI] [PubMed] [Google Scholar]
- 48.Jun K, Choi G, Yang SG, Choi KY, Kim H, Chan GC, Storm DR, Albert C, Mayr GW, Lee CJ, Shin HS. Enhanced hippocampal CA1 LTP but normal spatial learning in inositol 1,4,5-trisphosphate 3-kinase(A)-deficient mice. Learn Mem. 1998;5:317–330. [PMC free article] [PubMed] [Google Scholar]
- 49.Kim IH, Park SK, Sun W, Kang Y, Kim HT, Kim H. Spatial learning enhances the expression of inositol 1,4,5-trisphosphate 3-kinase A in the hippocampal formation of rat. Brain Res Mol Brain Res. 2004;124:12–19. doi: 10.1016/j.molbrainres.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 50.Hascakova-Bartova R, Pouillon V, Dewaste V, Moreau C, Jacques C, Banting G, Schurmans S, Erneux C. Identification and subcellular distribution of endogenous Ins(1,4,5)P3 3-kinase B in mouse tissues. Biochem Biophys Res Commun. 2004;323:920–925. doi: 10.1016/j.bbrc.2004.08.152. [DOI] [PubMed] [Google Scholar]
- 51.Pouillon V, Hascakova-Bartova R, Pajak B, Adam E, Bex F, Dewaste V, Van Lint C, Leo O, Erneux C, Schurmans S. Inositol 1,3,4,5-tetrakisphosphate is essential for T lymphocyte development. Nat Immunol. 2003;4:1136–1143. doi: 10.1038/ni980. [DOI] [PubMed] [Google Scholar]
- 52.Wen BG, Pletcher MT, Warashina M, Choe SH, Ziaee N, Wiltshire T, Sauer K, Cooke MP. Inositol (1,4,5) trisphosphate 3 kinase B controls positive selection of T cells and modulates Erk activity. Proc Natl Acad Sci USA. 2004;101:5604–5609. doi: 10.1073/pnas.0306907101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jia Y, Subramanian KK, Erneux C, Pouillon V, Hattori H, Jo H, You J, Zhu D, Schurmans S, Luo HR. Inositol 1,3,4,5-tetrakisphosphate negatively regulates phosphatidylinositol-3,4,5-trisphosphate signaling in neutrophils. Immunity. 2007;27:453–467. doi: 10.1016/j.immuni.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jia Y, Loison F, Hattori H, Li Y, Erneux C, Park SY, Gao C, Chai L, Silberstein LE, Schurmans S, Luo HR. Inositol trisphosphate 3-kinase B (InsP3KB) as a physiological modulator of myelopoiesis. Proc Natl Acad Sci USA. 2008;105:4739–4744. doi: 10.1073/pnas.0800218105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mailleux P, Takazawa K, Albala N, Erneux C, Vanderhaeghen JJ. Astrocytic localization of the messenger RNA encoding the isoenzyme B of inositol (1,4,5) 3-kinase in the human brain. Neurosci Lett. 1992;148:177–180. doi: 10.1016/0304-3940(92)90833-s. [DOI] [PubMed] [Google Scholar]
- 56.Communi D, Dewaste V, Erneux C. Calcium-calmodulin-dependent protein kinase II and protein kinase C-mediated phosphorylation and activation of D-myo-inositol 1,4,5-trisphosphate 3-kinase B in astrocytes. J Biol Chem. 1999;274:14734–14742. doi: 10.1074/jbc.274.21.14734. [DOI] [PubMed] [Google Scholar]
- 57.Dewaste V, Pouillon V, Moreau C, Shears S, Takazawa K, Erneux C. Cloning and expression of a cDNA encoding human inositol 1,4,5-trisphosphate 3-kinase C. Biochem J. 2000;352(Pt 2):343–351. [PMC free article] [PubMed] [Google Scholar]
- 58.Nalaskowski MM, Bertsch U, Fanick W, Stockebrand MC, Schmale H, Mayr GW. Rat inositol 1,4,5-trisphosphate 3-kinase C is enzymatically specialized for basal cellular inositol trisphosphate phosphorylation and shuttles actively between nucleus and cytoplasm. J Biol Chem. 2003;278:19765–19776. doi: 10.1074/jbc.M211059200. [DOI] [PubMed] [Google Scholar]
- 59.Onouchi Y, Gunji T, Burns JC, Shimizu C, Newburger JW, Yashiro M, Nakamura Y, Yanagawa H, Wakui K, Fukushima Y, Kishi F, Hamamoto K, Terai M, Sato Y, Ouchi K, Saji T, Nariai A, Kaburagi Y, Yoshikawa T, Suzuki K, Tanaka T, Nagai T, Cho H, Fujino A, Sekine A, Nakamichi R, Tsunoda T, Kawasaki T, Nakamura Y, Hata A. ITPKC functional polymorphism associated with Kawasaki disease susceptibility and formation of coronary artery aneurysms. Nat Genet. 2008;40:35–42. doi: 10.1038/ng.2007.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Communi D, Vanweyenberg V, Erneux C. D-myo-inositol 1,4,5-trisphosphate 3-kinase A is activated by receptor activation through a calcium:calmodulin-dependent protein kinase II phosphorylation mechanism. EMBO J. 1997;16:1943–1952. doi: 10.1093/emboj/16.8.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Communi D, Gevaert K, Demol H, Vandekerckhove J, Erneux C. A novel receptor-mediated regulation mechanism of type I inositol polyphosphate 5-phosphatase by calcium/calmodulin-dependent protein kinase II phosphorylation. J Biol Chem. 2001;276:38738–38747. doi: 10.1074/jbc.M105640200. [DOI] [PubMed] [Google Scholar]
- 62.Sim SS, Kim JW, Rhee SG. Regulation of D-myo-inositol 1,4,5-trisphosphate 3-kinase by cAMP-dependent protein kinase and protein kinase C. J Biol Chem. 1990;265:10367–10372. [PubMed] [Google Scholar]
- 63.Woodring PJ, Garrison JC. Expression, purification, and regulation of two isoforms of the inositol 1,4,5-trisphosphate 3-kinase. J Biol Chem. 1997;272:30447–30454. doi: 10.1074/jbc.272.48.30447. [DOI] [PubMed] [Google Scholar]
- 64.Biden TJ, Altin JG, Karjalainen A, Bygrave FL. Stimulation of hepatic inositol 1,4,5-trisphosphate kinase activity by Ca2+-dependent and -independent mechanisms. Biochem J. 1988;256:697–701. doi: 10.1042/bj2560697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takazawa K, Passareiro H, Dumont JE, Erneux C. Purification of bovine brain inositol 1,4,5-trisphosphate 3-kinase. Identification of the enzyme by sodium dodecyl sulphate/polyacrylamide-gel electrophoresis. Biochem J. 1989;261:483–488. doi: 10.1042/bj2610483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee SY, Sim SS, Kim JW, Moon KH, Kim JH, Rhee SG. Purification and properties of D-myo-inositol 1,4,5-trisphosphate 3-kinase from rat brain. Susceptibility to calpain. J Biol Chem. 1990;265:9434–9440. [PubMed] [Google Scholar]
- 67.Choi KY, Kim HK, Lee SY, Moon KH, Sim SS, Kim JW, Chung HK, Rhee SG. Molecular cloning and expression of a complementary DNA for inositol 1,4,5-trisphosphate 3-kinase. Science. 1990;248:64–66. doi: 10.1126/science.2157285. [DOI] [PubMed] [Google Scholar]
- 68.Rechsteiner M, Rogers SW. PEST sequences and regulation by proteolysis. Trends Biochem Sci. 1996;21:267–271. [PubMed] [Google Scholar]
- 69.Barnes JA, Gomes AV. PEST sequences in calmodulin-binding proteins. Mol Cell Biochem. 1995;149–150:17–27. doi: 10.1007/BF01076559. [DOI] [PubMed] [Google Scholar]
- 70.Pattni K, Banting G. Ins(1,4,5)P3 metabolism and the family of IP3–3 Kinases. Cell Signal. 2004;16:643–654. doi: 10.1016/j.cellsig.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 71.Molinari M, Anagli J, Carafoli E. PEST sequences do not influence substrate susceptibility to calpain proteolysis. J Biol Chem. 1995;270:2032–2035. doi: 10.1074/jbc.270.5.2032. [DOI] [PubMed] [Google Scholar]
- 72.Yamada M, Kakita A, Mizuguchi M, Rhee SG, Kim SU, Ikuta F. Specific expression of inositol 1,4,5-trisphosphate 3-kinase in dendritic spines. Brain Res. 1993;606:335–340. doi: 10.1016/0006-8993(93)91004-c. [DOI] [PubMed] [Google Scholar]
- 73.Dewaste V, Roymans D, Moreau C, Erneux C. Cloning and expression of a full-length cDNA encoding human inositol 1,4,5-trisphosphate 3-kinase B. Biochem Biophys Res Commun. 2002;291:400–405. doi: 10.1006/bbrc.2002.6456. [DOI] [PubMed] [Google Scholar]
- 74.Pattni K, Millard TH, Banting G. Calpain cleavage of the B isoform of Ins(1,4,5)P3 3-kinase separates the catalytic domain from the membrane anchoring domain. Biochem J. 2003;375:643–651. doi: 10.1042/BJ20030505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu JC, Lloyd-Burton SM, Irvine RF, Schell MJ. Regulation of the localization and activity of inositol 1,4,5-trisphosphate 3-kinase B in intact cells by proteolysis. Biochem J. 2005;392:435–441. doi: 10.1042/BJ20050829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dewaste V, Moreau C, De Smedt F, Bex F, De Smedt H, Wuytack F, Missiaen L, Erneux C. The three isoenzymes of human inositol-1,4,5-trisphosphate 3-kinase show specific intracellular localization but comparable Ca2+ responses on transfection in COS-7 cells. Biochem J. 2003;374:41–49. doi: 10.1042/BJ20021963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brehm MA, Schreiber I, Bertsch U, Wegner A, Mayr GW. Identification of the actin-binding domain of Ins(1,4,5)P3 3-kinase isoform B (IP3K-B) Biochem J. 2004;382:353–362. doi: 10.1042/BJ20031751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Terasaki M, Slater NT, Fein A, Schmidek A, Reese TS. Continuous network of endoplasmic reticulum in cerebellar Purkinje neurons. Proc Natl Acad Sci USA. 1994;91:7510–7514. doi: 10.1073/pnas.91.16.7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Park MK, Petersen OH, Tepikin AV. The endoplasmic reticulum as one continuous Ca2+ pool: visualization of rapid Ca2+ movements and equilibration. EMBO J. 2000;19:5729–5739. doi: 10.1093/emboj/19.21.5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mogami H, Nakano K, Tepikin AV, Petersen OH. Ca2+ flow via tunnels in polarized cells: recharging of apical Ca2+ stores by focal Ca2+ entry through basal membrane patch. Cell. 1997;88:49–55. doi: 10.1016/s0092-8674(00)81857-7. [DOI] [PubMed] [Google Scholar]
- 81.Dargan SL, Schwaller B, Parker I. Spatiotemporal patterning of IP3-mediated Ca2+ signals in Xenopus oocytes by Ca2+-binding proteins. J Physiol. 2004;556:447–461. doi: 10.1113/jphysiol.2003.059204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Korkotian E, Segal M. Spatially confined diffusion of calcium in dendrites of hippocampal neurons revealed by flash photolysis of caged calcium. Cell Calcium. 2006;40:441–449. doi: 10.1016/j.ceca.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 83.Santamaria F, Wils S, De Schutter E, Augustine GJ. Anomalous diffusion in Purkinje cell dendrites caused by spines. Neuron. 2006;52:635–648. doi: 10.1016/j.neuron.2006.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang SS, Alousi AA, Thompson SH. The lifetime of inositol 1,4,5-trisphosphate in single cells. J Gen Physiol. 1995;105:149–171. doi: 10.1085/jgp.105.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sims CE, Allbritton NL. Metabolism of inositol 1,4,5-trisphosphate and inositol 1,3,4,5-tetrakisphosphate by the oocytes of Xenopus laevis . J Biol Chem. 1998;273:4052–4058. doi: 10.1074/jbc.273.7.4052. [DOI] [PubMed] [Google Scholar]
- 86.Sarkisov DV, Gelber SE, Walker JW, Wang SS. Synapse specificity of calcium release probed by chemical two-photon uncaging of inositol 1,4,5-trisphosphate. J Biol Chem. 2007;282:25517–25526. doi: 10.1074/jbc.M609672200. [DOI] [PubMed] [Google Scholar]
- 87.Manita S and Ross WN (2009) IP3 mobilization and diffusion determine the timing window of Ca2+ release by synaptic stimulation and a spike in rat CA1 pyramidal cells. Hippocampus [DOI] [PMC free article] [PubMed]
- 88.Speed CJ, Neylon CB, Little PJ, Mitchell CA. Underexpression of the 43 kDa inositol polyphosphate 5-phosphatase is associated with spontaneous calcium oscillations and enhanced calcium responses following endothelin-1 stimulation. J Cell Sci. 1999;112:669–679. doi: 10.1242/jcs.112.5.669. [DOI] [PubMed] [Google Scholar]
- 89.Connolly TM, Bansal VS, Bross TE, Irvine RF, Majerus PW. The metabolism of tris- and tetraphosphates of inositol by 5-phosphomonoesterase and 3-kinase enzymes. J Biol Chem. 1987;262:2146–2149. [PubMed] [Google Scholar]
- 90.Petersen CC, Toescu EC, Potter BV, Petersen OH. Inositol triphosphate produces different patterns of cytoplasmic Ca2+ spiking depending on its concentration. FEBS Lett. 1991;293:179–182. doi: 10.1016/0014-5793(91)81181-7. [DOI] [PubMed] [Google Scholar]
- 91.Taylor CW, Thorn P. Calcium signalling: IP3 rises again and again. Curr Biol. 2001;11:R352–R355. doi: 10.1016/s0960-9822(01)00192-0. [DOI] [PubMed] [Google Scholar]
- 92.Nash MS, Schell MJ, Atkinson PJ, Johnston NR, Nahorski SR, Challiss RA. Determinants of metabotropic glutamate receptor-5-mediated Ca2+ and inositol 1,4,5-trisphosphate oscillation frequency. Receptor density versus agonist concentration. J Biol Chem. 2002;277:35947–35960. doi: 10.1074/jbc.M205622200. [DOI] [PubMed] [Google Scholar]
- 93.Dupont G, Erneux C. Simulations of the effects of inositol 1,4,5-trisphosphate 3-kinase and 5-phosphatase activities on Ca2+ oscillations. Cell Calcium. 1997;22:321–331. doi: 10.1016/s0143-4160(97)90017-8. [DOI] [PubMed] [Google Scholar]
- 94.Mishra J, Bhalla US. Simulations of inositol phosphate metabolism and its interaction with InsP3-mediated calcium release. Biophys J. 2002;83:1298–1316. doi: 10.1016/S0006-3495(02)73901-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Politi A, Gaspers LD, Thomas AP, Hofer T. Models of IP3 and Ca2+ oscillations: frequency encoding and identification of underlying feedbacks. Biophys J. 2006;90:3120–3133. doi: 10.1529/biophysj.105.072249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dupont G, Combettes L, Leybaert L. Calcium dynamics: spatio-temporal organization from the subcellular to the organ level. Int Rev Cytol. 2007;261:193–245. doi: 10.1016/S0074-7696(07)61005-5. [DOI] [PubMed] [Google Scholar]
- 97.Hirose K, Kadowaki S, Tanabe M, Takeshima H, Iino M. Spatiotemporal dynamics of inositol 1,4,5-trisphosphate that underlies complex Ca2+ mobilization patterns. Science. 1999;284:1527–1530. doi: 10.1126/science.284.5419.1527. [DOI] [PubMed] [Google Scholar]
- 98.Nash MS, Young KW, Challiss RA, Nahorski SR. Intracellular signalling. Receptor-specific messenger oscillations. Nature. 2001;413:381–382. doi: 10.1038/35096643. [DOI] [PubMed] [Google Scholar]
- 99.Thore S, Dyachok O, Tengholm A. Oscillations of phospholipase C activity triggered by depolarization and Ca2+ influx in insulin-secreting cells. J Biol Chem. 2004;279:19396–19400. doi: 10.1074/jbc.C400088200. [DOI] [PubMed] [Google Scholar]
- 100.Irvine RF. Is inositol tetrakisphosphate the second messenger that controls Ca2+ entry into cells? Adv Second Messenger Phosphoprotein Res. 1992;26:161–185. [PubMed] [Google Scholar]
- 101.Theibert AB, Estevez VA, Mourey RJ, Marecek JF, Barrow RK, Prestwich GD, Snyder SH. Photoaffinity labeling and characterization of isolated inositol 1,3,4,5-tetrakisphosphate- and inositol hexakisphosphate-binding proteins. J Biol Chem. 1992;267:9071–9079. [PubMed] [Google Scholar]
- 102.Donie F, Reiser G. Purification of a high-affinity inositol 1,3,4,5-tetrakisphosphate receptor from brain. Biochem J. 1991;275(Pt 2):453–457. doi: 10.1042/bj2750453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cullen PJ, Irvine RF. Inositol 1,3,4,5-tetrakisphosphate binding sites in neuronal and non-neuronal tissues. Properties, comparisons and potential physiological significance. Biochem J. 1992;288:149–154. doi: 10.1042/bj2880149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hammonds-Odie LP, Jackson TR, Profit AA, Blader IJ, Turck CW, Prestwich GD, Theibert AB. Identification and cloning of centaurin-alpha. A novel phosphatidylinositol 3,4,5-trisphosphate-binding protein from rat brain. J Biol Chem. 1996;271:18859–18868. doi: 10.1074/jbc.271.31.18859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stricker R, Adelt S, Vogel G, Reiser G. Translocation between membranes and cytosol of p42IP4, a specific inositol 1,3,4,5-tetrakisphosphate/phosphatidylinositol 3,4,5-trisphosphate-receptor protein from brain, is induced by inositol 1,3,4, 5-tetrakisphosphate and regulated by a membrane-associated 5-phosphatase. Eur J Biochem. 1999;265:815–824. doi: 10.1046/j.1432-1327.1999.00795.x. [DOI] [PubMed] [Google Scholar]
- 106.Cozier GE, Lockyer PJ, Reynolds JS, Kupzig S, Bottomley JR, Millard TH, Banting G, Cullen PJ. GAP1IP4BP contains a novel group I pleckstrin homology domain that directs constitutive plasma membrane association. J Biol Chem. 2000;275:28261–28268. doi: 10.1074/jbc.M000469200. [DOI] [PubMed] [Google Scholar]
- 107.Cullen PJ, Hsuan JJ, Truong O, Letcher AJ, Jackson TR, Dawson AP, Irvine RF. Identification of a specific Ins(1,3,4,5)P4-binding protein as a member of the GAP1 family. Nature. 1995;376:527–530. doi: 10.1038/376527a0. [DOI] [PubMed] [Google Scholar]
- 108.Loomis-Husselbee JW, Walker CD, Bottomley JR, Cullen PJ, Irvine RF, Dawson AP. Modulation of Ins(2,4,5)P3-stimulated Ca2 + mobilization by ins(1,3,4,5)P4: enhancement by activated G-proteins, and evidence for the involvement of a GAP1 protein, a putative Ins(1,3,4,5)P4 receptor. Biochem J. 1998;331:947–952. doi: 10.1042/bj3310947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stokes AJ, Shimoda LM, Lee JW, Rillero C, Chang YT, Turner H. Fcepsilon RI control of Ras via inositol (1,4,5) trisphosphate 3-kinase and inositol tetrakisphosphate. Cell Signal. 2006;18:640–651. doi: 10.1016/j.cellsig.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 110.Marechal Y, Pesesse X, Jia Y, Pouillon V, Perez-Morga D, Daniel J, Izui S, Cullen PJ, Leo O, Luo HR, Erneux C, Schurmans S. Inositol 1,3,4,5-tetrakisphosphate controls proapoptotic Bim gene expression and survival in B cells. Proc Natl Acad Sci USA. 2007;104:13978–13983. doi: 10.1073/pnas.0704312104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kupzig S, Deaconescu D, Bouyoucef D, Walker SA, Liu Q, Polte CL, Daumke O, Ishizaki T, Lockyer PJ, Wittinghofer A, Cullen PJ. GAP1 family members constitute bifunctional Ras and Rap GTPase-activating proteins. J Biol Chem. 2006;281:9891–9900. doi: 10.1074/jbc.M512802200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bivona TG, Perez De Castro I, Ahearn IM, Grana TM, Chiu VK, Lockyer PJ, Cullen PJ, Pellicer A, Cox AD, Philips MR. Phospholipase Cgamma activates Ras on the Golgi apparatus by means of RasGRP1. Nature. 2003;424:694–698. doi: 10.1038/nature01806. [DOI] [PubMed] [Google Scholar]
- 113.Yasuda T, Kurosaki T. Regulation of lymphocyte fate by Ras/ERK signals. Cell Cycle. 2008;7:3634–3640. doi: 10.4161/cc.7.23.7103. [DOI] [PubMed] [Google Scholar]
- 114.Morris AP, Gallacher DV, Irvine RF, Petersen OH. Synergism of inositol trisphosphate and tetrakisphosphate in activating Ca2+-dependent K+ channels. Nature. 1987;330:653–655. doi: 10.1038/330653a0. [DOI] [PubMed] [Google Scholar]
- 115.Higashida H, Taketo M, Takahashi H, Yokoyama S, Hashii M. Potential mechanism for bradykinin-activated and inositol tetrakisphosphate-dependent Ca2+ influx by Ras and GAP1 in fibroblast cells. Immunopharmacology. 1999;45:7–11. doi: 10.1016/s0162-3109(99)00051-x. [DOI] [PubMed] [Google Scholar]
- 116.Hashii M, Nakashima S, Yokoyama S, Enomoto K, Minabe Y, Nozawa Y, Higashida H. Bradykinin B2 receptor-induced and inositol tetrakisphosphate-evoked Ca2+ entry is sensitive to a protein tyrosine phosphorylation inhibitor in ras-transformed NIH/3T3 fibroblasts. Biochem J. 1996;319:649–656. doi: 10.1042/bj3190649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Miller AT, Sandberg M, Huang YH, Young M, Sutton S, Sauer K, Cooke MP. Production of Ins(1,3,4,5)P4 mediated by the kinase Itpkb inhibits store-operated calcium channels and regulates B cell selection and activation. Nat Immunol. 2007;8:514–521. doi: 10.1038/ni1458. [DOI] [PubMed] [Google Scholar]
- 118.Tsubokawa H, Oguro K, Robinson HP, Masuzawa T, Kawai N. Intracellular inositol 1,3,4,5-tetrakisphosphate enhances the calcium current in hippocampal CA1 neurones of the gerbil after ischaemia. J Physiol. 1996;497:67–78. doi: 10.1113/jphysiol.1996.sp021750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Szinyei C, Behnisch T, Reiser G, Reymann KG. Inositol 1,3,4,5-tetrakisphosphate enhances long-term potentiation by regulating Ca2 + entry in rat hippocampus. J Physiol. 1999;516:855–868. doi: 10.1111/j.1469-7793.1999.0855u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jia Y, Schurmans S, Luo HR. Regulation of innate immunity by inositol 1,3,4,5-tetrakisphosphate. Cell Cycle. 2008;7:2803–2808. doi: 10.4161/cc.7.18.6688. [DOI] [PubMed] [Google Scholar]
- 121.Miller AT, Beisner DR, Liu D, Cooke MP. Inositol 1,4,5-trisphosphate 3-kinase B is a negative regulator of BCR signaling that controls B cell selection and tolerance induction. J Immunol. 2009;182:4696–4704. doi: 10.4049/jimmunol.0802850. [DOI] [PubMed] [Google Scholar]
- 122.Huang YH, Hoebe K, Sauer K. New therapeutic targets in immune disorders: ItpkB, Orai1 and UNC93B. Expert Opin Ther Targets. 2008;12:391–413. doi: 10.1517/14728222.12.4.391. [DOI] [PubMed] [Google Scholar]
- 123.Huang YH, Grasis JA, Miller AT, Xu R, Soonthornvacharin S, Andreotti AH, Tsoukas CD, Cooke MP, Sauer K. Positive regulation of Itk PH domain function by soluble IP4 . Science. 2007;316:886–889. doi: 10.1126/science.1138684. [DOI] [PubMed] [Google Scholar]
- 124.Kupzig S, Walker SA, Cullen PJ. The frequencies of calcium oscillations are optimized for efficient calcium-mediated activation of Ras and the ERK/MAPK cascade. Proc Natl Acad Sci USA. 2005;102:7577–7582. doi: 10.1073/pnas.0409611102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Feske S. Calcium signalling in lymphocyte activation and disease. Nat Rev Immunol. 2007;7:690–702. doi: 10.1038/nri2152. [DOI] [PubMed] [Google Scholar]
- 126.Scharenberg AM, Humphries LA, Rawlings DJ. Calcium signalling and cell-fate choice in B cells. Nat Rev Immunol. 2007;7:778–789. doi: 10.1038/nri2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Engels N, Engelke M, Wienands J. Conformational plasticity and navigation of signaling proteins in antigen-activated B lymphocytes. Adv Immunol. 2008;97:251–281. doi: 10.1016/S0065-2776(08)00005-9. [DOI] [PubMed] [Google Scholar]
- 128.Rao A. Signaling to gene expression: calcium, calcineurin and NFAT. Nat Immunol. 2009;10:3–5. doi: 10.1038/ni0109-3. [DOI] [PubMed] [Google Scholar]
- 129.Muller MR, Sasaki Y, Stevanovic I, Lamperti ED, Ghosh S, Sharma S, Gelinas C, Rossi DJ, Pipkin ME, Rajewsky K, Hogan PG, Rao A. Requirement for balanced Ca/NFAT signaling in hematopoietic and embryonic development. Proc Natl Acad Sci USA. 2009;106:7034–7039. doi: 10.1073/pnas.0813296106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bhakta NR, Oh DY, Lewis RS. Calcium oscillations regulate thymocyte motility during positive selection in the three-dimensional thymic environment. Nat Immunol. 2005;6:143–151. doi: 10.1038/ni1161. [DOI] [PubMed] [Google Scholar]
- 131.Witt CM, Raychaudhuri S, Schaefer B, Chakraborty AK, Robey EA. Directed migration of positively selected thymocytes visualized in real time. PLoS Biol. 2005;3:e160. doi: 10.1371/journal.pbio.0030160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kim IH, Park SK, Hong ST, Jo YS, Kim EJ, Park EH, Han SB, Shin HS, Sun W, Kim HT, Soderling SH, Kim H. Inositol 1,4,5-trisphosphate 3-kinase a functions as a scaffold for synaptic Rac signaling. J Neurosci. 2009;29:14039–14049. doi: 10.1523/JNEUROSCI.2483-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Johnson HW, Schell MJ. Neuronal IP3 3-Kinase Is an F-actin Bundling Protein: Role in Dendritic Targeting and Regulation of Spine Morphology. Mol Biol Cell. 2009;20:5166–5180. doi: 10.1091/mbc.E09-01-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tybulewicz VL, Henderson RB. Rho family GTPases and their regulators in lymphocytes. Nat Rev Immunol. 2009;9:630–644. doi: 10.1038/nri2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Burgner D, Davila S, Breunis WB, Ng SB, Li Y, Bonnard C, Ling L, Wright VJ, Thalamuthu A, Odam M, Shimizu C, Burns JC, Levin M, Kuijpers TW, Hibberd ML. A genome-wide association study identifies novel and functionally related susceptibility Loci for Kawasaki disease. PLoS Genet. 2009;5:e1000319. doi: 10.1371/journal.pgen.1000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rowley AH, Baker SC, Orenstein JM, Shulman ST. Searching for the cause of Kawasaki disease—cytoplasmic inclusion bodies provide new insight. Nat Rev Microbiol. 2008;6:394–401. doi: 10.1038/nrmicro1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- 138.Higley MJ, Sabatini BL. Calcium signaling in dendrites and spines: practical and functional considerations. Neuron. 2008;59:902–913. doi: 10.1016/j.neuron.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 139.Fisher SK, Agranoff BW. Receptor activation and inositol lipid hydrolysis in neural tissues. J Neurochem. 1987;48:999–1017. doi: 10.1111/j.1471-4159.1987.tb05618.x. [DOI] [PubMed] [Google Scholar]
- 140.Sharp AH, Dawson TM, Ross CA, Fotuhi M, Mourey RJ, Snyder SH. Inositol 1,4,5-trisphosphate receptors: immunohistochemical localization to discrete areas of rat central nervous system. Neuroscience. 1993;53:927–942. doi: 10.1016/0306-4522(93)90478-x. [DOI] [PubMed] [Google Scholar]
- 141.Mailleux P, Takazawa K, Erneux C, Vanderhaeghen JJ. Inositol 1,4,5-trisphosphate 3-kinase distribution in the rat brain. High levels in the hippocampal CA1 pyramidal and cerebellar Purkinje cells suggest its involvement in some memory processes. Brain Res. 1991;539:203–210. doi: 10.1016/0006-8993(91)91622-8. [DOI] [PubMed] [Google Scholar]
- 142.Mailleux P, Takazawa K, Albala N, Erneux C, Vanderhaeghen JJ. Comparison of neuronal inositol 1,4,5-trisphosphate 3-kinase and receptor mRNA distributions in the human brain using in situ hybridization histochemistry. Neurosci Lett. 1992;137:69–71. doi: 10.1016/0304-3940(92)90300-v. [DOI] [PubMed] [Google Scholar]
- 143.Heacock AM, Seguin EB, Agranoff BW. Developmental and regional studies of the metabolism of inositol 1,4,5-trisphosphate in rat brain. J Neurochem. 1990;54:1405–1411. doi: 10.1111/j.1471-4159.1990.tb01976.x. [DOI] [PubMed] [Google Scholar]
- 144.Worley PF, Baraban JM, Snyder SH. Inositol 1,4,5-trisphosphate receptor binding: autoradiographic localization in rat brain. J Neurosci. 1989;9:339–346. doi: 10.1523/JNEUROSCI.09-01-00339.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sharp AH, Nucifora FC, Jr, Blondel O, Sheppard CA, Zhang C, Snyder SH, Russell JT, Ryugo DK, Ross CA. Differential cellular expression of isoforms of inositol 1,4,5-triphosphate receptors in neurons and glia in brain. J Comp Neurol. 1999;406:207–220. [PubMed] [Google Scholar]
- 146.Mizuguchi M, Yamada M, Rhee SG, Kim SU. Development of inositol 1,4,5-trisphosphate 3-kinase immunoreactivity in cerebellar Purkinje cells in vivo and in vitro. Brain Res. 1992;573:157–160. doi: 10.1016/0006-8993(92)90126-t. [DOI] [PubMed] [Google Scholar]
- 147.Schell MJ, Irvine RF. Calcium-triggered exit of F-actin and IP3 3-kinase A from dendritic spines is rapid and reversible. Eur J Neurosci. 2006;24:2491–2503. doi: 10.1111/j.1460-9568.2006.05125.x. [DOI] [PubMed] [Google Scholar]
- 148.Batty IR, Nahorski SR, Irvine RF. Rapid formation of inositol 1,3,4,5-tetrakisphosphate following muscarinic receptor stimulation of rat cerebral cortical slices. Biochem J. 1985;232:211–215. doi: 10.1042/bj2320211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Challiss RA, Nahorski SR. Neurotransmitter and depolarization-stimulated accumulation of inositol 1,3,4,5-tetrakisphosphate mass in rat cerebral cortex slices. J Neurochem. 1990;54:2138–2141. doi: 10.1111/j.1471-4159.1990.tb04920.x. [DOI] [PubMed] [Google Scholar]
- 150.Baird JG, Nahorski SR. Increased intracellular calcium stimulates 3H-inositol polyphosphate accumulation in rat cerebral cortical slices. J Neurochem. 1990;54:555–561. doi: 10.1111/j.1471-4159.1990.tb01907.x. [DOI] [PubMed] [Google Scholar]
- 151.Baird JG, Challiss RA, Nahorski SR. Role for ionotropic and metabotropic receptors in quisqualate-stimulated inositol polyphosphate accumulation in rat cerebral cortex. Mol Pharmacol. 1991;39:745–753. [PubMed] [Google Scholar]
- 152.Baird JG, Nahorski SR. Stimulatory and inhibitory effects of N-methyl-D-aspartate on 3H-inositol polyphosphate accumulation in rat cortical slices. J Neurochem. 1991;57:629–635. doi: 10.1111/j.1471-4159.1991.tb03794.x. [DOI] [PubMed] [Google Scholar]
- 153.Schmidt BH, Weiss S, Sebben M, Kemp DE, Bockaert J, Sladeczek F. Dual action of excitatory amino acids on the metabolism of inositol phosphates in striatal neurons. Mol Pharmacol. 1987;32:364–368. [PubMed] [Google Scholar]
- 154.Takazawa K, Lemos M, Delvaux A, Lejeune C, Dumont JE, Erneux C. Rat brain inositol 1,4,5-trisphosphate 3-kinase. Ca2 + -sensitivity, purification and antibody production. Biochem J. 1990;268:213–217. doi: 10.1042/bj2680213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang SS, Major G. Integrating over time with dendritic wave-fronts. Nat Neurosci. 2003;6:906–908. doi: 10.1038/nn0903-906. [DOI] [PubMed] [Google Scholar]
- 156.Spruston N. Pyramidal neurons: dendritic structure and synaptic integration. Nat Rev Neurosci. 2008;9:206–221. doi: 10.1038/nrn2286. [DOI] [PubMed] [Google Scholar]
- 157.Finch EA, Augustine GJ. Local calcium signalling by inositol-1,4,5-trisphosphate in Purkinje cell dendrites. Nature. 1998;396:753–756. doi: 10.1038/25541. [DOI] [PubMed] [Google Scholar]
- 158.Wang SS, Denk W, Hausser M. Coincidence detection in single dendritic spines mediated by calcium release. Nat Neurosci. 2000;3:1266–1273. doi: 10.1038/81792. [DOI] [PubMed] [Google Scholar]
- 159.Sarkisov DV, Wang SS. Order-dependent coincidence detection in cerebellar Purkinje neurons at the inositol trisphosphate receptor. J Neurosci. 2008;28:133–142. doi: 10.1523/JNEUROSCI.1729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Raymond CR, Redman SJ. Spatial segregation of neuronal calcium signals encodes different forms of LTP in rat hippocampus. J Physiol. 2006;570:97–111. doi: 10.1113/jphysiol.2005.098947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dudman JT, Tsay D, Siegelbaum SA. A role for synaptic inputs at distal dendrites: instructive signals for hippocampal long-term plasticity. Neuron. 2007;56:866–879. doi: 10.1016/j.neuron.2007.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Patterson RL, Boehning D, Snyder SH. Inositol 1,4,5-trisphosphate receptors as signal integrators. Annu Rev Biochem. 2004;73:437–465. doi: 10.1146/annurev.biochem.73.071403.161303. [DOI] [PubMed] [Google Scholar]
- 163.Hardingham GE, Arnold FJ, Bading H. Nuclear calcium signaling controls CREB-mediated gene expression triggered by synaptic activity. Nat Neurosci. 2001;4:261–267. doi: 10.1038/85109. [DOI] [PubMed] [Google Scholar]
- 164.Park MK, Choi YM, Kang YK, Petersen OH. The endoplasmic reticulum as an integrator of multiple dendritic events. Neuroscientist. 2008;14:68–77. doi: 10.1177/1073858407305691. [DOI] [PubMed] [Google Scholar]
- 165.Nakamura T, Lasser-Ross N, Nakamura K, Ross WN. Spatial segregation and interaction of calcium signalling mechanisms in rat hippocampal CA1 pyramidal neurons. J Physiol. 2002;543:465–480. doi: 10.1113/jphysiol.2002.020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Power JM, Sah P. Nuclear calcium signaling evoked by cholinergic stimulation in hippocampal CA1 pyramidal neurons. J Neurosci. 2002;22:3454–3462. doi: 10.1523/JNEUROSCI.22-09-03454.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Stutzmann GE, LaFerla FM, Parker I. Ca2+ signaling in mouse cortical neurons studied by two-photon imaging and photoreleased inositol triphosphate. J Neurosci. 2003;23:758–765. doi: 10.1523/JNEUROSCI.23-03-00758.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Power JM, Sah P. Distribution of IP3-mediated calcium responses and their role in nuclear signalling in rat basolateral amygdala neurons. J Physiol. 2007;580:835–857. doi: 10.1113/jphysiol.2006.125062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Fitzpatrick JS, Hagenston AM, Hertle DN, Gipson KE, Bertetto-D’Angelo L, Yeckel MF. Inositol-1,4,5-trisphosphate receptor-mediated Ca2+ waves in pyramidal neuron dendrites propagate through hot spots and cold spots. J Physiol. 2009;587:1439–1459. doi: 10.1113/jphysiol.2009.168930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Irving AJ, Collingridge GL. A characterization of muscarinic receptor-mediated intracellular Ca2+ mobilization in cultured rat hippocampal neurones. J Physiol. 1998;511:747–759. doi: 10.1111/j.1469-7793.1998.747bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Power JM, Sah P. Intracellular calcium store filling by an L-type calcium current in the basolateral amygdala at subthreshold membrane potentials. J Physiol. 2005;562:439–453. doi: 10.1113/jphysiol.2004.076711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hong M, Ross WN. Priming of intracellular calcium stores in rat CA1 pyramidal neurons. J Physiol. 2007;584:75–87. doi: 10.1113/jphysiol.2007.137661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hagenston AM, Fitzpatrick JS, Yeckel MF. MGluR-mediated calcium waves that invade the soma regulate firing in layer V medial prefrontal cortical pyramidal neurons. Cereb Cortex. 2008;18:407–423. doi: 10.1093/cercor/bhm075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sharp AH, McPherson PS, Dawson TM, Aoki C, Campbell KP, Snyder SH. Differential immunohistochemical localization of inositol 1,4,5-trisphosphate- and ryanodine-sensitive Ca2+ release channels in rat brain. J Neurosci. 1993;13:3051–3063. doi: 10.1523/JNEUROSCI.13-07-03051.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Jacob SN, Choe CU, Uhlen P, DeGray B, Yeckel MF, Ehrlich BE. Signaling microdomains regulate inositol 1,4,5-trisphosphate-mediated intracellular calcium transients in cultured neurons. J Neurosci. 2005;25:2853–2864. doi: 10.1523/JNEUROSCI.4313-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Manita S, Ross WN. Synaptic activation and membrane potential changes modulate the frequency of spontaneous elementary Ca2+ release events in the dendrites of pyramidal neurons. J Neurosci. 2009;29:7833–7845. doi: 10.1523/JNEUROSCI.0573-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Schell MJ, Erneux C, Irvine RF. Inositol 1,4,5-trisphosphate 3-kinase A associates with F-actin and dendritic spines via its N terminus. J Biol Chem. 2001;276:37537–37546. doi: 10.1074/jbc.M104101200. [DOI] [PubMed] [Google Scholar]
- 178.Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- 179.Windhorst S, Blechner C, Lin HY, Elling C, Nalaskowski M, Kirchberger T, Guse AH, Mayr GW. Ins(1,4,5)P3 3-kinase-A overexpression induces cytoskeletal reorganization via a kinase-independent mechanism. Biochem J. 2008;414:407–417. doi: 10.1042/BJ20080630. [DOI] [PubMed] [Google Scholar]
- 180.Yamada M, Kakita A, Mizuguchi M, Rhee SG, Kim SU, Ikuta F. Ultrastructural localization of inositol 1,4,5-trisphosphate 3-kinase in rat cerebellar cortex. Brain Res. 1992;578:41–48. doi: 10.1016/0006-8993(92)90227-z. [DOI] [PubMed] [Google Scholar]
- 181.Yamada M, Kakita A, Mizuguchi M, Rhee SG, Kim SU, Ikuta F. Developmental profile of inositol 1,4,5-trisphosphate 3-kinase in rat cerebellar cortex: light and electron microscopic immunohistochemical studies. Brain Res Dev Brain Res. 1993;71:137–145. doi: 10.1016/0165-3806(93)90114-p. [DOI] [PubMed] [Google Scholar]
- 182.Capani F, Deerinck TJ, Ellisman MH, Bushong E, Bobik M, Martone ME. Phalloidin-eosin followed by photo-oxidation: a novel method for localizing F-actin at the light and electron microscopic levels. J Histochem Cytochem. 2001;49:1351–1361. doi: 10.1177/002215540104901103. [DOI] [PubMed] [Google Scholar]
- 183.Capani F, Martone ME, Deerinck TJ, Ellisman MH. Selective localization of high concentrations of F-actin in subpopulations of dendritic spines in rat central nervous system: a three-dimensional electron microscopic study. J Comp Neurol. 2001;435:156–170. doi: 10.1002/cne.1199. [DOI] [PubMed] [Google Scholar]
- 184.O’Brien SJ. The platypus genome unraveled. Cell. 2008;133:953–955. doi: 10.1016/j.cell.2008.05.038. [DOI] [PubMed] [Google Scholar]
- 185.Eilers J, Takechi H, Finch EA, Augustine GJ, Konnerth A. Local dendritic Ca2+ signaling induces cerebellar long-term depression. Learn Mem. 1997;4:159–168. doi: 10.1101/lm.4.1.159. [DOI] [PubMed] [Google Scholar]
- 186.Miyata M, Finch EA, Khiroug L, Hashimoto K, Hayasaka S, Oda SI, Inouye M, Takagishi Y, Augustine GJ, Kano M. Local calcium release in dendritic spines required for long-term synaptic depression. Neuron. 2000;28:233–244. doi: 10.1016/s0896-6273(00)00099-4. [DOI] [PubMed] [Google Scholar]
- 187.Spacek J, Harris KM. Three-dimensional organization of smooth endoplasmic reticulum in hippocampal CA1 dendrites and dendritic spines of the immature and mature rat. J Neurosci. 1997;17:190–203. doi: 10.1523/JNEUROSCI.17-01-00190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Holbro N, Grunditz A, Oertner TG. Differential distribution of endoplasmic reticulum controls metabotropic signaling and plasticity at hippocampal synapses. Proc Natl Acad Sci USA. 2009;106:15055–15060. doi: 10.1073/pnas.0905110106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Nagase T, Ito KI, Kato K, Kaneko K, Kohda K, Matsumoto M, Hoshino A, Inoue T, Fujii S, Kato H, Mikoshiba K. Long-term potentiation and long-term depression in hippocampal CA1 neurons of mice lacking the IP3 type 1 receptor. Neuroscience. 2003;117:821–830. doi: 10.1016/s0306-4522(02)00803-5. [DOI] [PubMed] [Google Scholar]
- 190.Wilsch VW, Behnisch T, Jager T, Reymann KG, Balschun D. When are class I metabotropic glutamate receptors necessary for long-term potentiation? J Neurosci. 1998;18:6071–6080. doi: 10.1523/JNEUROSCI.18-16-06071.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Fernandez de Sevilla D, Nunez A, Borde M, Malinow R, Buno W. Cholinergic-mediated IP3-receptor activation induces long-lasting synaptic enhancement in CA1 pyramidal neurons. J Neurosci. 2008;28:1469–1478. doi: 10.1523/JNEUROSCI.2723-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Baba H, Fuss B, Urano J, Poullet P, Watson JB, Tamanoi F, Macklin WB. GapIII, a new brain-enriched member of the GTPase-activating protein family. J Neurosci Res. 1995;41:846–858. doi: 10.1002/jnr.490410615. [DOI] [PubMed] [Google Scholar]
- 193.Vazquez LE, Chen HJ, Sokolova I, Knuesel I, Kennedy MB. SynGAP regulates spine formation. J Neurosci. 2004;24:8862–8872. doi: 10.1523/JNEUROSCI.3213-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Carlisle HJ, Manzerra P, Marcora E, Kennedy MB. SynGAP regulates steady-state and activity-dependent phosphorylation of cofilin. J Neurosci. 2008;28:13673–13683. doi: 10.1523/JNEUROSCI.4695-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yasuda R, Harvey CD, Zhong H, Sobczyk A, van Aelst L, Svoboda K. Supersensitive Ras activation in dendrites and spines revealed by two-photon fluorescence lifetime imaging. Nat Neurosci. 2006;9:283–291. doi: 10.1038/nn1635. [DOI] [PubMed] [Google Scholar]
- 196.Harvey CD, Yasuda R, Zhong H, Svoboda K. The spread of Ras activity triggered by activation of a single dendritic spine. Science. 2008;321:136–140. doi: 10.1126/science.1159675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ryu J, Futai K, Feliu M, Weinberg R, Sheng M. Constitutively active Rap2 transgenic mice display fewer dendritic spines, reduced extracellular signal-regulated kinase signaling, enhanced long-term depression, and impaired spatial learning and fear extinction. J Neurosci. 2008;28:8178–8188. doi: 10.1523/JNEUROSCI.1944-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhou L, Martinez SJ, Haber M, Jones EV, Bouvier D, Doucet G, Corera AT, Fon EA, Zisch AH, Murai KK. EphA4 signaling regulates phospholipase Cgamma1 activation, cofilin membrane association, and dendritic spine morphology. J Neurosci. 2007;27:5127–5138. doi: 10.1523/JNEUROSCI.1170-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Choi G, Chang YT, Chung SK, Choi KY. Molecular interactions of all possible regioisomers of synthetic myo-inositol phosphates with inositol 1,4,5-trisphosphate 3-kinase. Bioorg Med Chem Lett. 1997;7:2709–2714. [Google Scholar]
- 200.Chang YT, Choi G, Bae YS, Burdett M, Moon HS, Lee JW, Gray NS, Schultz PG, Meijer L, Chung SK, Choi KY, Suh PG, Ryu SH. Purine-based inhibitors of inositol-1,4,5-trisphosphate-3-kinase. Chembiochem. 2002;3:897–901. doi: 10.1002/1439-7633(20020902)3:9<897::AID-CBIC897>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 201.Mayr GW, Windhorst S, Hillemeier K. Antiproliferative plant and synthetic polyphenolics are specific inhibitors of vertebrate inositol-1,4,5-trisphosphate 3-kinases and inositol polyphosphate multikinase. J Biol Chem. 2005;280:13229–13240. doi: 10.1074/jbc.M500545200. [DOI] [PubMed] [Google Scholar]
- 202.Kwon YU, Im J, Choi G, Kim YS, Choi KY, Chung SK. Synthesis of three enantiomeric pairs of scyllo-inositol phosphate and molecular interactions between all possible regioisomers of scyllo-inositol phosphate and inositol 1,4,5-trisphosphate 3-kinase. Bioorg Med Chem Lett. 2003;13:2981–2984. doi: 10.1016/s0960-894x(03)00629-2. [DOI] [PubMed] [Google Scholar]
- 203.Suh BC, Kim MJ, Choi G, Choi KY, Han JK, Chung SK, Kim KT. Differential stereoselectivity of D- and L-myo-inositol 1,2,4,5-tetrakisphosphate binding to the inositol 1,4,5-trisphosphate receptor and 3-kinase. Neurochem Int. 2000;37:47–52. doi: 10.1016/s0197-0186(00)00004-8. [DOI] [PubMed] [Google Scholar]
- 204.Legraverend M, Grierson DS. The purines: potent and versatile small molecule inhibitors and modulators of key biological targets. Bioorg Med Chem. 2006;14:3987–4006. doi: 10.1016/j.bmc.2005.12.060. [DOI] [PubMed] [Google Scholar]
- 205.Padmanabhan U, Dollins DE, Fridy PC, York JD, Downes CP. Characterization of a selective inhibitor of inositol hexakisphosphate kinases: use in defining biological roles and metabolic relationships of inositol pyrophosphates. J Biol Chem. 2009;284:10571–10582. doi: 10.1074/jbc.M900752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Chamberlain PP, Sandberg ML, Sauer K, Cooke MP, Lesley SA, Spraggon G. Structural insights into enzyme regulation for inositol 1,4,5-trisphosphate 3-kinase B. Biochemistry. 2005;44:14486–14493. doi: 10.1021/bi051256q. [DOI] [PubMed] [Google Scholar]
- 207.Balla T, Sim SS, Iida T, Choi KY, Catt KJ, Rhee SG. Agonist-induced calcium signaling is impaired in fibroblasts overproducing inositol 1,3,4,5-tetrakisphosphate. J Biol Chem. 1991;266:24719–24726. [PubMed] [Google Scholar]
- 208.Balla T, Sim SS, Baukal AJ, Rhee SG, Catt KJ. Inositol polyphosphates are not increased by overexpression of Ins(1,4,5)P3 3-kinase but show cell-cycle dependent changes in growth factor-stimulated fibroblasts. Mol Biol Cell. 1994;5:17–27. doi: 10.1091/mbc.5.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Verjans B, Petersen CC, Berridge MJ. Overexpression of inositol 1,4,5-trisphosphate 3-kinase in Xenopus oocytes inhibits agonist-evoked capacitative calcium entry. Biochem J. 1994;304:679–682. doi: 10.1042/bj3040679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Millard TH, Cullen PJ, Banting G. Effects of elevated expression of inositol 1,4,5-trisphosphate 3-kinase B on Ca2+ homoeostasis in HeLa cells. Biochem J. 2000;352:709–715. [PMC free article] [PubMed] [Google Scholar]
- 211.Shears SB. Molecular basis for the integration of inositol phosphate signaling pathways via human ITPK1. Adv Enzyme Regul. 2009;49:87–96. doi: 10.1016/j.advenzreg.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Rao VD, Misra S, Boronenkov IV, Anderson RA, Hurley JH. Structure of type IIbeta phosphatidylinositol phosphate kinase: a protein kinase fold flattened for interfacial phosphorylation. Cell. 1998;94:829–839. doi: 10.1016/s0092-8674(00)81741-9. [DOI] [PubMed] [Google Scholar]
- 213.Nalaskowski MM, Windhorst S, Stockebrand MC, Mayr GW. Subcellular localisation of human inositol 1,4,5-trisphosphate 3-kinase C: species-specific use of alternative export sites for nucleo-cytoplasmic shuttling indicates divergent roles of the catalytic and N-terminal domains. Biol Chem. 2006;387:583–593. doi: 10.1515/BC.2006.075. [DOI] [PubMed] [Google Scholar]