Abstract
Maintenance of intestinal epithelial barrier function is of vital importance in preventing uncontrolled influx of antigens and the potentially ensuing inflammatory disorders. Intestinal intraepithelial lymphocytes (IEL) are in intimate contact with epithelial cells and may critically regulate the epithelial barrier integrity. While a preserving impact has been ascribed to the T-cell receptor (TCR)-γδ subset of IEL, IEL have also been shown to attenuate the barrier function. The present study sought to clarify the effects of IEL by specifically investigating the influence of the TCR-αβ CD8αβ and TCR-αβ CD8αα subsets of IEL on the intestinal epithelial barrier integrity. To this end, an in vitro coculture system of the murine intestinal crypt-derived cell-line mICcl2 and syngeneic ex vivo isolated IEL was employed. Epithelial integrity was assessed by analysis of transepithelial resistance (TER) and paracellular flux of fluorescein isothiocyanate-conjugated (FITC-) dextran. The TCR-αβ CD8αα IEL and resting TCR-αβ CD8αβ IEL did not affect TER of mICcl2 or flux of FITC-dextran. In contrast, activated TCR-αβ CD8αβ IEL clearly disrupted the integrity of the mICcl2 monolayer. No disrupting effect was seen with activated TCR-αβ CD8αβ IEL from interferon-γ knockout mice. These findings demonstrate that secretion of interferon-γ by activated TCR-αβ CD8αβ IEL is strictly required and also sufficient for disrupting the intestinal epithelial barrier function.
Keywords: cell interactions, cytokines, epithelial cells, mucosal immunity, T cell
Introduction
In the intestinal tract, a single layer of epithelial cells separates the inner from the outer antigen-rich environment. This vast epithelial interface has to permit the absorption of nutrients while presenting an effective barrier to microbes and potentially noxious antigens. A dysfunctional or disrupted epithelial barrier may present a significant contributary and even aetiological factor in the pathogenesis of intestinal inflammatory disorders.1,2
The semi-permeable barrier function of the intestinal epithelium is mainly regulated at the basis of an intercellular junctional complex of proteins, which is located towards the apical end of enterocytes and comprises tight junctions, adherens junctions and desmosomes.3 While adherens junction proteins, such as E-cadherin, participate in cell recognition and adhesion, proteins of the tight junctions, including claudin family members and occludins, regulate the paracellular flux of water and ions and effectively restrict the access of proteins and micro-organisms.3
Far from presenting a static innate immune barrier, the intestinal epithelium actively participates in the induction and regulation of adaptive immune responses. As a result, epithelial cells can attract lymphocytes by the secretion of chemokines,4 are capable of acting as antigen-presenting cells,5 and further drive B-cell immunoglobulin A (IgA) class switch recombination by bacteria-induced production of a proliferation-inducing ligand (APRIL) and B-cell-activating factor (BAFF).6,7 Secreted IgA, in return, is transported by the polymeric IgA receptor of epithelial cells to the luminal side to exert its protective functions.
Importantly, the intestinal epithelium itself is also home to a large number of intestinal intraepithelial lymphocytes (IEL), which comprise a mostly CD8+ yet heterogeneous population of T cells. The IEL are classified into two major subgroups based on phenotypical and functional characteristics.8 T-cell receptor (TCR)-αβ CD8αβ IEL, termed type a IEL, are derived from conventional CD8 T cells that have entered the intestinal epithelium upon antigen-induced activation and participate in local host defence.8,9 In contrast, unconventional type b IEL, express a CD8αα coreceptor in conjunction with a TCR-αβ or a TCR-γδ and are considered to be permanently resident cells with potential regulatory functions.8,9 All IEL express the integrin αEβ7, which anchors IEL to the E-cadherin of the epithelial adherens junctions.10 This αEβ7 expression may be induced and maintained by epithelially derived transforming growth factor-β11 and epithelial cells can provide additional cytokines required for IEL differentiation and survival.12–14 With the intimate physical association of IEL with epithelial cells, their interactions are probably mutual. Yet, fairly limited and partially conflicting information is available regarding the effects of IEL on the intestinal epithelium. The TCR-γδ subset of IEL may actively contribute to and regenerate the epithelial barrier function by the secretion of keratinocyte growth factor or transforming growth factor-β,15–17 because a deficiency in TCR-γδ T cells has been associated with a more severe phenotype in mouse models of intestinal inflammation.16,18 On the other hand, several in vitro studies have demonstrated a loss of epithelial integrity upon coculture of epithelial cells with IEL or IEL-derived supernatants.19–21 These models, however, largely relied on the use of IEL-derived cell lines, which may represent mixed subtypes that no longer retain the characteristics of bona fide IEL. Moreover, while type a IEL are able to secrete interferon-γ (IFN-γ),22 and the deleterious effects of exogenous IFN-γ on the integrity of epithelial monolayers in vitro are well acknowledged,23–29 no conclusive evidence regarding the responsible subsets and barrier-disrupting mechanism of IEL has so far been presented.
With the already well-documented preserving role of the TCR-γδ subset of IEL, the present study specifically sought to investigate the effects of purified, bona fide TCR-αβ CD8αβ and TCR-αβ CD8αα IEL on the intestinal epithelial barrier integrity. Using an in vitro coculture system of the murine small intestinal crypt-derived cell-line mICcl2 and syngeneic ex vivo isolated IEL subsets, we clearly demonstrate that activated TCR-αβ CD8αβ, but not TCR-αβ CD8αα, IEL are capable of mediating epithelial barrier disruption by a mechanism strictly and solely dependent on the secretion of IFN-γ.
Materials and methods
Mice
C57BL/6J (B6) mice were originally purchased from Harlan (Horst, the Netherlands). B6 mice transgenic for the lymphocytic choriomeningitis virus (LCMV) gp33-specific P14 TCR (line 318)30 were kindly provided by H. P. Pircher and H. Hengartner (University Hospital Zurich, Zurich, Switzerland). The IFN-γ knockout (IFN-γ−/−, B6.129S7-Ifngtms1Ts) mice were provided by A. Ochsenbein and M. Matter (Department of Clinical Research, Inselspital, University of Bern, Switzerland). Mice were kept in individually ventilated cages in the central animal facility of the Medical School, University of Bern, Switzerland and used in experiments at 12–20 weeks of age. Experiments were performed in compliance with national and cantonal regulations on animal experimentation.
Cell lines and culture conditions
The murine B6-derived intestinal crypt-like cell line mICcl231 was cultured in Dulbecco’s modified Eagle medium/Ham’s F-12 12 g/l (1 : 1 vol/vol; Gibco/Invitrogen Corporation, Carlsbad, CA), NaHCO3 2·438 g/l (Fluka, Deisenhofen, Germany), 10 ng/ml mouse epidermal growth factor (Calbiochem/Merck, Darmstadt, Germany), 5 μg/ml insulin (Sigma, St Louis, MO), 60 nm sodium selenite (Sigma), 5 μg/ml human Apo-transferrin (Sigma), 1 nm triiodo-thyronin (Calbiochem/Merck, Germany), 30 mm HEPES (Fluka, Germany), 2% fetal calf serum (FCS; Sigma), 2 mm l-alanyl-l-glutamine (Sigma) and 40 U/ml penicillin/streptomycin (Gibco/Invitrogen Corporation) at 37° in 5% CO2 atmosphere. Cells of passage 7–12 were used for experiments.
The L929 fibroblast cell line was cultured in Dulbecco’s medium supplemented with 5% FCS and 40 U/ml penicillin/streptomycin at 37° in 5% CO2 atmosphere. The CMT93 cell line, derived from a mouse rectum carcinoma,32 and kindly provided by H. C. Reineker (Massachusetts General Hospital, Boston, MA) was maintained in Iscove’s modified Dulbecco’s medium, 5% FCS, 2 mm l-alanyl-l-glutamine and 40 U/ml penicillin/streptomycin at 37° in 5% CO2 atmosphere.
Cell isolation and purification
Small intestinal IEL were isolated as described previously.33 Briefly, tissues were placed in Ca2+ and Mg2+-free Hanks’ balanced salt solution (HBSS) containing 10 mm HEPES and 2% horse serum. Small pieces of intestinal tissue were stirred in 50 ml HBSS containing 2 mm dithiothreitol and 0·5 mm ethylenediamine tetraacetic acid (EDTA) at 37° for 30 min. Isolated cells were passed through a 40-μm pore size nylon cell strainer (BD Biosciences, San Diego, CA) and kept on ice while a second and a third round of isolation of intestinal tissue was performed in HBSS medium containing 0·5 mm EDTA. The IEL were then purified by elutriation centrifugation using a Beckmann induction drive centrifuge with a JE-6B Rotor containing a 40-ml elutriation chamber and a Masterflex L/S digital Pump (Cole-Parmer Instrument Co, Vernon Hills, IL). When all the cells were loaded in the elutriation chamber, flow rate was increased to elute the IEL fraction. To obtain pure populations of CD8αα CD4− and CD8αβ CD4− TCR-αβ IEL, elutriated IEL were sorted with a FACS DiVa Cell Sorter (Becton Dickinson, San Jose, CA). The following monoclonal antibodies or conjugates were used: anti-mouse TCR-αβ-allophycocyanin (H57-597), Vα2-biotin (B20.1) for specific detection of the P14 TCR, CD8α-phycoerythrin Cy5 (53-6.7), CD8β-phycoerythrin (53-5.8), CD4-fluorescein isothiocyanate (FITC; GK1.5) and streptavidin-allophycocyanin (all purchased from eBioscience, San Diego, CA). In some experiments, IEL were sorted based on their forward and side scatter characteristics only, without further discrimination of subsets. CD8+ splenocytes were obtained following a regular magnetic antibody cell sorting separation protocol using MS columns and rat anti-mouse CD8α paramagnetic beads (Miltenyi Biotech, Bergisch Gladbach, Germany).
Cocultures of intestinal epithelial cells and IEL, measurement of transepithelial resistance (TER) and paracellular tracer flux
Cell lines were grown on rat tail collagen type I-coated (450 ng/cm2; Sigma) permeable polycarbonate filters (0·33 cm2) with 3-μm pore size (Costar, Cambridge, MA). Seventy-two hours after seeding, mICcl2 and CMT93 formed a high-resistance monolayer that remained stable for at least four additional days. At this time-point (72 hr post seeding), 105 IEL or splenic T cells were added to the apical side of the epithelial cell monolayers in medium supplemented with mouse recombinant interleukin-2 (IL-2), IL-3, IL-4 (all used at final concentration of 1 ng/ml), IL-7 (2 ng/ml) and IL-15 (50 ng/ml). In some conditions, IFN-γ or tumour necrosis factor (TNF-α; 5–10 ng/ml) (all purchased from PeproTech, London, UK) were added to the culture medium. For T-cell stimulation, coculture medium was supplemented with 1 μg/μl anti-CD3 (145-2c11) and anti-CD28 (37.51) (eBioscience). Stimulation of P14 TCR-derived cells was performed with 1 μg/ml of the irrelevant, adenovirus-derived peptide Adn5 or with the specific LCMV-derived gp33 peptide (kindly provided by H.P. Pircher, University of Freiburg, Freiburg, Germany).
Monolayer tight junction formation was monitored daily using the EVOM™ Endohm®-System (World Precision Instruments, Sarasota, FL) to measure TER. Cell culture medium was changed before measuring TER to adjust the pH and temperature in the culture wells and the endohm chamber. The mICcl2 or CMT93 cells cultured in medium containing the cytokine cocktail in the absence of IEL were used as controls. Values of TER obtained at 72 hr culture in the presence of IEL or recombinant cytokines were then normalized to and expressed as percentage of the TER of controls (set to 100%) of the respective experiment. The paracellular tracer flux assay was performed with 10 000 molecular weight FITC-dextran (Sigma). The FITC-dextran was dissolved in P buffer (10 mm HEPES, 1 mm sodium pyruvate, 10 mm glucose, 3 mm CaCl2 and 145 mm NaCl) at 1 mg/ml and added to the apical compartment of the cocultures while the medium of the lower compartment was also replaced with P buffer. Permeability of monolayers was evaluated 3 hr post incubation with FITC-dextran at 37° by measuring the amount of FITC-dextran present in the basolateral compartment with a fluorometer (excitation 485 nm, emission 535 nm; Perkin-Elmer Fluorescence Spectrometer 3000).
Statistical analyses
The unpaired Student’s t-test was used to calculate two-tailed P-values. P-values < 0·05 were considered statistically significant.
Results
Characterization of mICcl2 monolayers
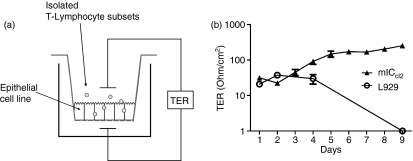
The present study aimed to establish a robust in vitro coculture system of intestinal epithelial cells and IEL that would reflect the in vivo situation as closely as possible. To this end, the murine small intestinal crypt-derived cell line mICcl2 was employed, which allows for a coculture with syngeneic (H-2b) C57BL/6 (B6) -derived ex vivo isolated IEL. The mICcl2 exhibit characteristic features of small intestinal epithelial cells, including a cuboidal shape with short apical microvilli and a basolateral expression of the pIgR.31 Importantly, these cells form tight junctions and are able to build up a stable polarized monolayer.31 Growth of mICcl2 on collagen-coated transwell inserts permits coculture with IEL and further allows for analysis of TER as a functional parameter for the monolayer integrity (Fig. 1a). As shown in Fig. 1(b), cells not expressing tight junction proteins, such as the fibroblast cell line L929, failed to build up TER. In contrast, mICcl2 showed an increase in TER starting at 72 hr post seeding and a relatively constant TER of around 100 Ohm/cm2 over a prolonged period of time, indicative of the formation of a stable cell monolayer (Fig. 1b). Consequently, in the following experiments, IEL were added to mICcl2 at 72 hr and TER was assessed after an additional 72 hr of coculture. The IEL effectively homed into the mICcl2 monolayer also when added at the apical side of mICcl2 (as determined by confocal microscopy; data not shown) so this application route was subsequently employed for coculture of IEL with mICcl2 (Fig. 1a).
Figure 1.
Schematic representation of the epithelial cell–intraepithelial lymphocytes (IEL) coculture system and formation of transepithelial resistance (TER) by the mouse intestinal crypt-like cell line mlCcl2. (a) Epithelial monolayers were grown on polycarbonate filters for 3 days. At this time, IEL were added to the apical side for subsequent cocultures. Monolayer integrity was assessed by measurement of TER in the absence or presence of IEL. (b) Formation of stable monolayer integrity by mlCcl2 but not by the fibroblast cell line L929 as assessed by measurement of TER. Mean of n = 3 cocultures ± SEM is shown.
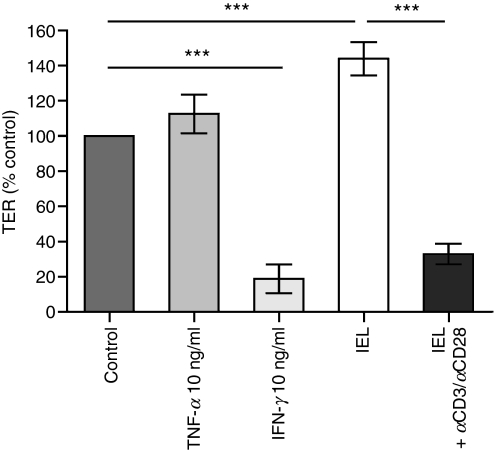
Recombinant IFN-γ and activated IEL reduce TER in mICcl2
Initial experiments compared the effects of syngeneic ex vivo isolated, unfractionated, resting versus activated IEL on the mICcl2 monolayer integrity. Recombinant IFN-γ was used as an internal control because of its established capacity to reduce the TER of various intestinal cell lines.23–29 Indeed, addition of exogenous IFN-γ, but not TNF-α, significantly reduced the TER of mICcl2 (Fig. 2). Coculture of mICcl2 with unfractionated resting IEL consistently resulted in an increase in TER (Fig. 2). In contrast, stimulation of IEL with monoclonal antibodies against αCD3 and αCD28 during coculture with mICcl2 led to a significant loss of TER by mICcl2. Very similar effects were observed when IEL were added to the basolateral side of the mICcl2 monolayer (data not shown).
Figure 2.
Recombinant interferon-γ (IFN-γ) or activated intraepithelial lymphocytes (IEL) reduce the epithelial monolayer integrity. mICcl2 cells were cocultured with IEL in the presence or absence of monoclonal antibodies against CD3/CD28 or treated with recombinant IFN-γ or tumour necrosis factor-α (TNF-α). Transepithelial resistance (TER) was measured after 72 hr of culture with IEL or recombinant cytokines and is expressed as the percentage of TER of mICcl2 in the respective control cell culture medium. Mean values ± SEM of n = 10 to n = 13 cocultures with IEL and n = 5 cocultures with recombinant cytokines from a total of five independent experiments are shown. ***P < 0·001.
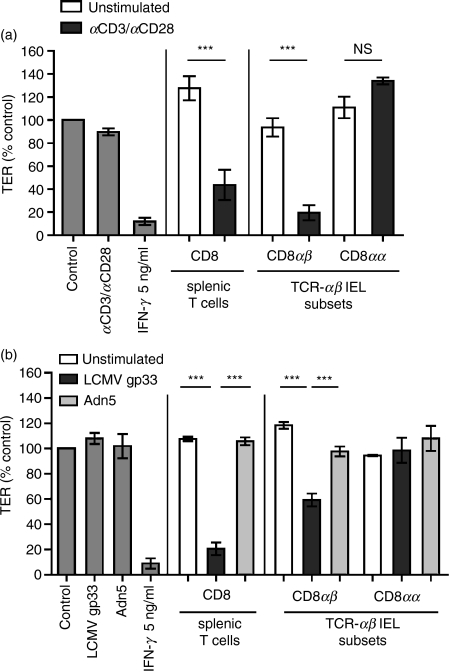
Activated TCR-αβ CD8αβ IEL, but not TCR-αβ CD8αα IEL, are responsible for impairing the mICcl2 monolayer integrity
The IEL are a highly heterogeneous population. While TCR-γδ IEL have been described as having a preserving and healing impact on the intestinal epithelium,16,18 the direct effects of TCR-αβ IEL on the intestinal barrier function are unclear. To determine which subset of TCR-αβ IEL was capable of reducing TER in mICcl2, IEL were purified into TCR-αβ CD8αβ and TCR-αβ CD8αα subsets by high-speed cell sorting. The TCR-αβ CD8αα IEL did not substantially affect TER even after potent polyclonal activation (Fig. 3a). In contrast, the presence of activated TCR-αβ CD8αβ IEL significantly reduced the TER of mICcl2 to almost the same extent as a saturating concentration of IFN-γ (Fig. 3a). A disrupting effect on the epithelial barrier function was also seen with activated CD8+ splenic T cells, which were used as an additional control (Fig. 3a). To assess whether an activation of TCR-αβ CD8αβ IEL upon recognition of their cognate antigen in situ was sufficient to mediate a reduction in TER, mice transgenic for the TCR specific for the LCMV-derived peptide gp33 were used as donors of IEL. Compared with control wells, no reduction of TER was observed after the addition of the irrelevant peptide adn5 to the cocultures or after activation of TCR-αβ CD8αα IEL with their cognate antigen gp33 (Fig. 3b). In contrast, antigen-specific activation of splenic CD8 T cells and TCR-αβ CD8αβ IEL with the gp33 peptide resulted in a significantly reduced TER. Hence, mICcl2 were able to function as antigen-presenting cells and activate CD8 T cells in a major histocompatibility complex class I-dependent manner, confirming the results from Fig. 2 in a highly physiological setting (Fig. 3b).
Figure 3.
Activated T-cell receptor (TCR)-αβ CD8αβ T cells, but not activated TCR-αβ CD8αα intraepithelial lymphocytes (IEL) are responsible for the reduction of transepithelial resistance (TER) in mICcl2. (a) Purified CD8αα and CD8αβ TCR-αβ IEL or splenic CD8 T cells were cocultured with mICcl2 cells in the absence or presence of monoclonal antibodies against CD3/CD28. (b) Purified T-cell subsets derived from P14 TCR transgenic mice (line 318) were cultured with mICcl2 in the presence of their specific peptide gp33 or the irrelevant control peptide adn5, respectively. (a, b) TER was assessed after 72 hr of coculture and is expressed as the percentage of TER of mICcl2 in the respective control cell culture medium. Mean values ± SEM of n = 5 to n = 7 cocultures with IFN-γ, CD3/CD28 or peptides alone, n = 6 to n = 7 cocultures with CD8αβ splenic T cells or CD8αβ IEL, and n = 2 to n = 4 cocultures with CD8αα IEL from a total of three (a) and two (b) independent experiments are shown. ***P < 0·001; NS, non-significant.
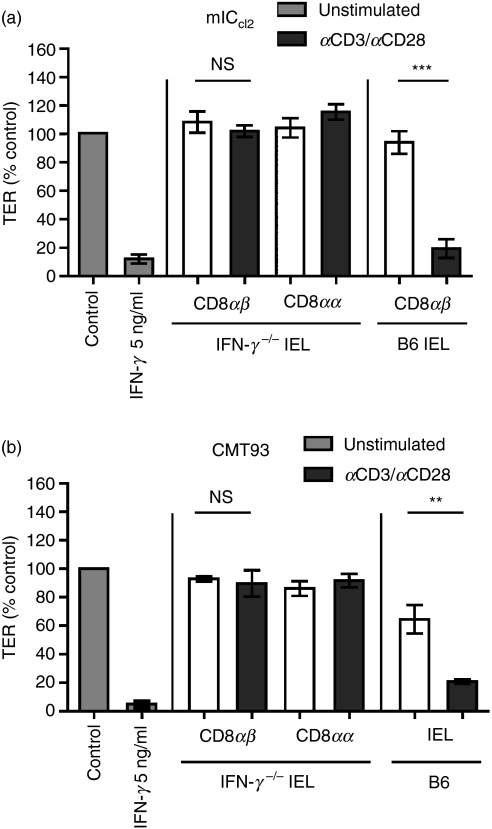
IFN-γ is required and sufficient for the TCR-αβ CD8αβ IEL-mediated reduction in TER
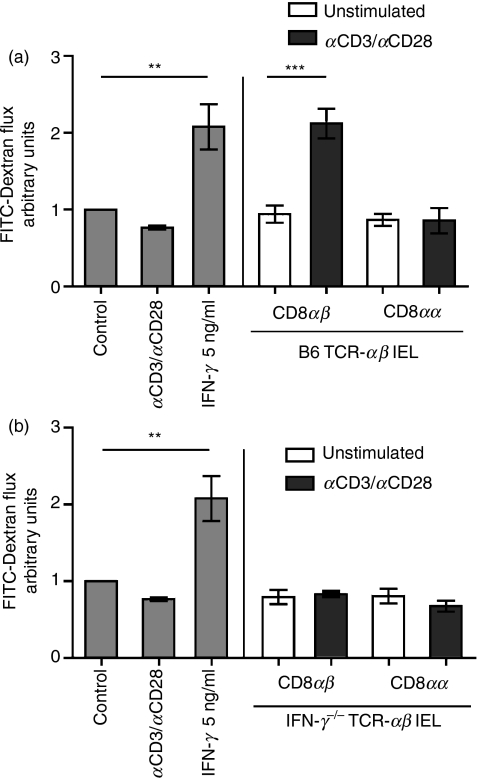
The IFN-γ alone effectively reduced TER and stimulation of IEL was evidently required for their disrupting effect on the mICcl2 monolayer so a role for IFN-γ secretion by activated IEL was clearly indicated. However, previous studies, which analysed the presence of IFN-γ or used neutralizing antibodies in cocultures of intestinal cell lines with IEL, have failed to demonstrate an exclusive function for IEL-produced IFN-γ in intestinal barrier disruption.19–21,29 Therefore, to unequivocally assess the impact of IFN-γ secretion by IEL on the mICcl2 monolayer integrity, IEL from IFN-γ−/− mice were used for subsequent co-cultures. In contrast to their wild-type (B6) counterparts, activated TCR-αβ CD8αβ IEL from IFN-γ−/− mice completely failed to reduce TER of mICcl2 as the TER values remained in the range of those observed for control mICcl2 and mICcl2 cocultured with TCR-αβ CD8αα or resting TCR-αβ CD8αβ IEL (Fig. 4a). The same results were obtained when coculturing IFN-γ−/− TCR-αβ CD8αβ IEL with the mouse rectal carcinoma cell line CMT93 (Fig. 4b). To determine whether measurement of paracellular flux of macromolecules revealed greater sensitivity with respect to the potential effects of IFN-γ−/− TCR-αβ CD8αβ IEL on the mICcl2 monolayer integrity, FITC-dextran was added to the apical side of cocultures of mICcl2 with distinct IEL subsets from wild-type (B6) and IFN-γ−/− mice and the presence of FITC-dextran in the basolateral chamber was subsequently determined. As anticipated, wild-type TCR-αβ CD8αα and resting TCR-αβ CD8αβ IEL did not increase the flux of FITC-dextran across mICcl2 monolayers compared with mICcl2 cultured in the absence of IEL (Fig. 5a). In contrast, the presence of activated wild-type TCR-αβ CD8αβ IEL or recombinant IFN-γ resulted in a significantly augmented flux of FITC-dextran (Fig. 5a). The TCR-αβ CD8αβ IEL from IFN-γ−/− mice, however, failed to induce a flux of FITC-dextran above control levels (Fig. 5b). Taken together, these results demonstrate that secretion of IFN-γ by activated TCR-αβ CD8αβ IEL was essential and sufficient for disrupting the monolayer integrity of intestinal epithelial cells.
Figure 4.
Disruption of epithelial monolayer integrity by activated T-cell receptor (TCR)-αβ CD8αβ strictly and exclusively depends on their secretion of interferon-γ (IFN-γ). (a) mICcl2 cells were cocultured with sorted intraepithelial lymphocyte (IEL) subsets from IFN-γ−/− or wild-type (B6) mice in the presence or absence of monoclonal antibodies against CD3/CD28. Mean values ± SEM of n = 6 cocultures with IFN-γ, n = 6 cocultures with CD8αβ IEL, and n = 4 cocultures with CD8αα IEL from a total of three independent experiments are shown. (b) Experiments were performed as described in (a) but using CMT93 cells (a mouse colon cancer cell line) and unfractionated IEL from wild-type (B6) mice. Bars represent mean values ± SEM of n = 5 cocultures with IFN-γ, n = 4 cocultures with IFN-γ−/− CD8αβ IEL, n = 6 cocultures with B6 IEL, and n = 4 cocultures with CD8αα IEL from a total of two independent experiments. **P < 0·01; ***P < 0·001.
Figure 5.
Activated T-cell receptor (TCR)-αβ CD8αβ intraepithelial lymphocytes (IEL) increase fluorescein isothiocyanate (FITC)-dextran flux across mICcl2 monolayers in an interferon-γ (IFN-γ)-dependent manner. mICcl2 were cocultured with purified IEL subsets derived from either wild-type (B6) mice (a) or IFN-γ−/− mice (b) in the absence or presence of monoclonal antibodies against CD3/CD28. After 72 hr, FITC-dextran was added to the apical side of the mICcl2 monolayer and paracellular permeability was assessed. FITC-dextran flux is expressed as the fold increase of permeability of mICcl2 in control cell culture medium. Mean values ± SEM of n = 6 to n = 7 cocultures with IFN-γ or CD3/CD28, n = 6 cocultures with CD8αβ IEL, and n = 4 cocultures with CD8αα IEL from a total of three individual experiments are shown. **P < 0·01; ***P < 0·001.
Discussion
The intimate physical association of IEL with intestinal epithelial cells strongly suggests an impact on the integrity of the intestinal barrier. Here, we demonstrate that activated TCR-αβ CD8αβ IEL, but not TCR-αβ CD8αα IEL, are capable of reducing the TER of mICcl2 in a strictly IFN-γ-dependent manner. These findings could further be ascertained with the analysis of FITC-dextran flux as additional functional read-out for the loss of epithelial integrity and with CMT93 cells as an intestinal cell line of an advanced differentiation stage.
In our coculture system, IEL were added to the apical side of enterocytes, which may seem at odds with the positioning of IEL in vivo and results from in vitro studies that have described a strictly polarized migration of IEL (IEL-derived cell lines) into epithelial monolayers from their basolateral, but not apical sides.34 However, our preliminary studies employing confocal microscopy and GFP+ IEL demonstrated that IEL also re-locate into monolayers when added to the apical side of enterocytes (data not shown). Because this set-up did not require any unnecessary, potentially delicate culturing of the intestinal cell lines on inverted cell culture inserts, the apical application route was subsequently employed for all experiments.
In support of the relative physiological significance of our model and consistent with the reported antigen-presenting capacity of intestinal epithelial cells,5 activation of P14 TCR transgenic CD8αβ T cells in cocultures with their cognate LCMV-derived peptide gp33, similar to polyclonal activation αCD3/CD28, also led to a loss of TER by mICcl2. However, compared with their splenic counterparts, antigen-specifically activated CD8αβ IEL appeared less potent in disrupting mICcl2 monolayer integrity. This discrepancy is probably explained by the higher frequency of LCMV-gp33 TCR transgenic T cells among CD8αβ T cells in the spleen versus the IEL compartment. Accordingly, the transgenic TCR in P14 mice is carried by ∼50% of splenic CD8 T cells, but only by ∼20% of CD8αβ IEL (data not shown).
The capacity of activated, but not resting, TCR-αβ CD8αβ IEL to reduce intestinal epithelial monolayer integrity is certainly consistent with the (re-)acquisition of effector functions and strongly argues against any potentially unspecifically damaging effects, such as bacterial contamination of our IEL preparations, on the epithelial cells. Most importantly, the use of CD8 T cells from IFN-γ−/− mice allowed us to unambiguously demonstrate that secretion of IFN-γ is not only required for but is also sufficient for IEL to disrupt mICcl2 and CMT93 monolayers. While CD8αβ IEL can exert cytolytic effector functions, the contribution of such a mechanism seems highly unlikely as IFN-γ−/− T cells still retain CTL activity.35 The clear pre-requisite for the production of IFN-γ by IEL is further in keeping with the already well-documented detrimental effects of recombinant IFN-γ on the integrity of intestinal epithelial cell monolayers in vitro,21,23–28 which we also observed for mICcl2 and CMT93. Nonetheless, our results somewhat contrast the findings of previous investigations on the disrupting impact of IEL (IEL-derived cell lines) on intestinal epithelial monolayers, which did define a role for soluble factors, but failed to demonstrate the presence of IFN-γ in coculture supernatants or any clear abrogating effects of IFN-γ neutralizing monoclonal antibodies.19–21 Possibly, these differences can be attributed to the use of T-cell lines as opposed to bona fide IEL, technical limitations, or indeed a role for additional soluble factors in those systems. In their model of Eimeria vermiformis infection, Inagaki-Ohara et al.29 demonstrated secretion of both IFN-γ and TNF-α by IEL of infected mice, and a combination of blocking monoclonal antibodies against these two cytokines abrogated the reduction in TER induced by the IEL-derived supernatant in an epithelial monolayer in vitro. The authors, however, did not determine the relative individual contribution of IEL-derived IFN-γ and TNF-α in disrupting the epithelial barrier integrity. In our study, we observed no reduction in TER of mICcl2 and CMT93 upon addition of recombinant TNF-α, which is consistent with the findings from others that TNF-α alone is ineffective in damaging the integrity of intestinal epithelial monolayers.25,27 Although synergistic effects of recombinant TNF-α with IFN-γ in reducing TER of intestinal epithelial cell lines have been described,25,27 our results based on the use of IFN-γ−/− IEL clearly argue against an additional requirement for IEL-derived TNF-α or other cytokines.
With the present study, we have been able to assign disrupting effects of IEL on intestinal epithelial monolayers to the TCR-αβ CD8αβ subset of IEL. The fact that TCR-αβ CD8αα IEL did not reduce TER of mICcl2 and CMT93, even after potent activation in vitro, agrees with the absence of a pro-inflammatory gene expression profile and confirms our previous results in vivo.36,37 We observed no signs for autoreactivity in mice that were double-transgenic for the LCMV-derived gp33 and a gp33-specific TCR even after activation of resident self-specific TCR-αβ CD8αα IEL by infection of mice with LCMV.37
Accumulating evidence from experimental models and clinical data indicate that IFN-γ may also be an important mediator of intestinal epithelial barrier disruption in vivo. Exposing mice to a repetitive stress regimen resulted in increased colonic IFN-γ and augmented epithelial permeability; these effects were not observed in IFN-γ−/− mice.38 Moreover, in dextran sodium sulphate-induced colitis, where disruption of the intestinal epithelium is considered a primary cause of inflammation, IFN-γ−/− mice were reported to be protected from disease.39 Neutralization of IFN-γ in the CD4 T-cell transfer model of colitis also prevented onset of disease for up to 12 weeks.40 In patients with inflammatory bowel disease (IBD), expression of IFN-γ is up-regulated in the inflamed intestinal mucosa,41–43 and consistent with the capacity of IFN-γ to alter tight junction protein composition of intestinal cell lines in vitro, an altered expression pattern of tight junction proteins was indeed seen in inflamed, but not in uninflamed, tissues of patients with IBD.44
The potential role of IEL and IEL-derived IFN-γ in experimental colitis and IBD still remains poorly defined. Increased numbers of activated, perforin- or granzyme-expressing CD4 and CD8 T cells have been identified not only in the lamina propria but also in the IEL compartment of patients with ulcerative colitis and Crohn’s disease compared with normal controls.45 Based on their cytotoxic effector phenotype it may be speculated that these IEL also secrete considerable amounts of IFN-γ and substantially contribute to an early loss of the epithelial barrier integrity.
Although the impact of IEL subsets on epithelial integrity and loss of epithelial barrier function in vivo clearly demands investigation, our robust in vitro culture system may prove a valuable model to dissect the individual roles of the different IEL subsets in regulating the epithelial barrier integrity and to characterize the likely complex interactions operative also in vivo.
Acknowledgments
The present work was supported by grant 98-05 of the 3R Foundation and Grant 3100A0-105598 of the Swiss National Science foundation to C.M. The authors thank Bernadette Wider for excellent assistance with the cell sorting.
Glossary
Abbreviations:
- IEL
intestinal intraepithelial lymphocytes
- TER
transepithelial resistance
Disclosures
The authors state no conflicting financial interests.
References
- 1.Laukoetter MG, Nava P, Nusrat A. Role of the intestinal barrier in inflammatory bowel disease. World J Gastroenterol. 2008;14:401–7. doi: 10.3748/wjg.14.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGuckin MA, Eri R, Simms LA, Florin TH, Radford-Smith G. Intestinal barrier dysfunction in inflammatory bowel diseases. Inflamm Bowel Dis. 2009;15:100–13. doi: 10.1002/ibd.20539. [DOI] [PubMed] [Google Scholar]
- 3.Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286:C1213–28. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- 4.Williams IR. Chemokine receptors and leukocyte trafficking in the mucosal immune system. Immunol Res. 2004;3:283–92. doi: 10.1385/IR:29:1-3:283. [DOI] [PubMed] [Google Scholar]
- 5.Hershberg RM, Mayer LF. Antigen processing and presentation by intestinal epithelial cells – polarity and complexity. Immunol Today. 2000;21:123–8. doi: 10.1016/s0167-5699(99)01575-3. [DOI] [PubMed] [Google Scholar]
- 6.He B, Xu W, Santini PA, et al. Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity. 2007;26:812–26. doi: 10.1016/j.immuni.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 7.Xu W, He B, Chiu A, et al. Epithelial cells trigger frontline immunoglobulin class switching through a pathway regulated by the inhibitor SLPI. Nat Immunol. 2007;8:294–303. doi: 10.1038/ni1434. [DOI] [PubMed] [Google Scholar]
- 8.Hayday A, Theodoridis E, Ramsburg E, Shires J. Intraepithelial lymphocytes: exploring the Third Way in immunology. Nat Immunol. 2001;2:997–1003. doi: 10.1038/ni1101-997. [DOI] [PubMed] [Google Scholar]
- 9.Cheroutre H. IELs: enforcing law and order in the court of the intestinal epithelium. Immunol Rev. 2005;206:114–31. doi: 10.1111/j.0105-2896.2005.00284.x. [DOI] [PubMed] [Google Scholar]
- 10.Cepek KL, Shaw SK, Parker CM, Russell GJ, Morrow JS, Rimm DL, Brenner MB. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature. 1994;372:190–3. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- 11.Rihs S, Walker C, Virchow JC, Jr, Boer C, Kroegel C, Giri SN, Braun RK. Differential expression of alpha E beta 7 integrins on bronchoalveolar lavage T lymphocyte subsets: regulation by alpha 4 beta 1-integrin crosslinking and TGF-beta. Am J Respir Cell Mol Biol. 1996;15:600–10. doi: 10.1165/ajrcmb.15.5.8918367. [DOI] [PubMed] [Google Scholar]
- 12.Inagaki-Ohara K, Nishimura H, Mitani A, Yoshikai Y. Interleukin-15 preferentially promotes the growth of intestinal intraepithelial lymphocytes bearing gamma delta T cell receptor in mice. Eur J Immunol. 1997;27:2885–91. doi: 10.1002/eji.1830271121. [DOI] [PubMed] [Google Scholar]
- 13.Laky K, Lefrancois L, Lingenheld EG, et al. Enterocyte expression of interleukin 7 induces development of gammadelta T cells and Peyer’s patches. J Exp Med. 2000;191:1569–80. doi: 10.1084/jem.191.9.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–32. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 15.Boismenu R, Havran WL. Modulation of epithelial cell growth by intraepithelial gamma delta T cells. Science. 1994;266:1253–5. doi: 10.1126/science.7973709. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Chou K, Fuchs E, Havran WL, Boismenu R. Protection of the intestinal mucosa by intraepithelial gamma delta T cells. Proc Natl Acad Sci USA. 2002;99:14338–43. doi: 10.1073/pnas.212290499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhagat G, Naiyer AJ, Shah JG, Harper J, Jabri B, Wang TC, Green PH, Manavalan JS. Small intestinal CD8+TCRgammadelta+NKG2A+ intraepithelial lymphocytes have attributes of regulatory cells in patients with celiac disease. J Clin Invest. 2008;118:281–93. doi: 10.1172/JCI30989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dalton JE, Cruickshank SM, Egan CE, et al. Intraepithelial gammadelta+ lymphocytes maintain the integrity of intestinal epithelial tight junctions in response to infection. Gastroenterology. 2006;131:818–29. doi: 10.1053/j.gastro.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Kaoutzani P, Colgan SP, Cepek KL, Burkard PG, Carlson S, Delp-Archer C, Brenner MB, Madara JL. Reconstitution of cultured intestinal epithelial monolayers with a mucosal-derived T lymphocyte cell line. Modulation of epithelial phenotype dependent on lymphocyte–basolateral membrane apposition. J Clin Invest. 1994;94:788–96. doi: 10.1172/JCI117398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor CT, Murphy A, Kelleher D, Baird AW. Changes in barrier function of a model intestinal epithelium by intraepithelial lymphocytes require new protein synthesis by epithelial cells. Gut. 1997;40:634–40. doi: 10.1136/gut.40.5.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shibahara T, Miyazaki K, Sato D, Matsui H, Yanaka A, Nakahara A, Tanaka N. Alteration of intestinal epithelial function by intraepithelial lymphocyte homing. J Gastroenterol. 2005;40:878–86. doi: 10.1007/s00535-005-1631-y. [DOI] [PubMed] [Google Scholar]
- 22.Wang HC, Zhou Q, Dragoo J, Klein JR. Most murine CD8+ intestinal intraepithelial lymphocytes are partially but not fully activated T cells. J Immunol. 2002;169:4717–22. doi: 10.4049/jimmunol.169.9.4717. [DOI] [PubMed] [Google Scholar]
- 23.Madara JL, Stafford J. Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers. J Clin Invest. 1989;83:724–7. doi: 10.1172/JCI113938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Planchon SM, Martins CA, Guerrant RL, Roche JK. Regulation of intestinal epithelial barrier function by TGF-beta 1. Evidence for its role in abrogating the effect of a T cell cytokine. J Immunol. 1994;153:5730–9. [PubMed] [Google Scholar]
- 25.Fish SM, Proujansky R, Reenstra WW. Synergistic effects of interferon gamma and tumour necrosis factor alpha on T84 cell function. Gut. 1999;45:191–8. doi: 10.1136/gut.45.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Youakim A, Ahdieh M. Interferon-gamma decreases barrier function in T84 cells by reducing ZO-1 levels and disrupting apical actin. Am J Physiol. 1999;276:G1279–88. doi: 10.1152/ajpgi.1999.276.5.G1279. [DOI] [PubMed] [Google Scholar]
- 27.Bruewer M, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM, Nusrat A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol. 2003;171:6164–72. doi: 10.4049/jimmunol.171.11.6164. [DOI] [PubMed] [Google Scholar]
- 28.Willemsen LE, Hoetjes JP, van Deventer SJ, van Tol EA. Abrogation of IFN-gamma mediated epithelial barrier disruption by serine protease inhibition. Clin Exp Immunol. 2005;142:275–84. doi: 10.1111/j.1365-2249.2005.02906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inagaki-Ohara K, Dewi FN, Hisaeda H, et al. Intestinal intraepithelial lymphocytes sustain the epithelial barrier function against Eimeria vermiformis infection. Infect Immun. 2006;74:5292–301. doi: 10.1128/IAI.02024-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pircher H, Mak TW, Lang R, Ballhausen W, Ruedi E, Hengartner H, Zinkernagel RM, Burki K. T cell tolerance to Mlsa encoded antigens in T cell receptor V beta 8.1 chain transgenic mice. EMBO J. 1989;8:719–27. doi: 10.1002/j.1460-2075.1989.tb03431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bens M, Bogdanova A, Cluzeaud F, et al. Transimmortalized mouse intestinal cells (m-ICc12) that maintain a crypt phenotype. Am J Physiol. 1996;1:C1666–74. doi: 10.1152/ajpcell.1996.270.6.C1666. [DOI] [PubMed] [Google Scholar]
- 32.Franks LM, Hemmings VJ. A cell line from an induced carcinoma of mouse rectum. J Pathol. 1978;124:35–8. doi: 10.1002/path.1711240108. [DOI] [PubMed] [Google Scholar]
- 33.Binda E, Erhart D, Schenk M, Zufferey C, Renzulli P, Mueller C. Quantitative isolation of mouse and human intestinal intraepithelial lymphocytes by elutriation centrifugation. J Immunol Methods. 2009 doi: 10.1016/j.jim.2009.02.006. doi 10.1016/j.jim.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Shaw SK, Hermanowski-Vosatka A, Shibahara T, et al. Migration of intestinal intraepithelial lymphocytes into a polarized epithelial monolayer. Am J Physiol. 1998;1:G584–91. doi: 10.1152/ajpgi.1998.275.3.G584. [DOI] [PubMed] [Google Scholar]
- 35.Graham MB, Dalton DK, Giltinan D, Braciale VL, Stewart TA, Braciale TJ. Response to influenza infection in mice with a targeted disruption in the interferon gamma gene. J Exp Med. 1993;178:1725–32. doi: 10.1084/jem.178.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Denning TL, Granger SW, Mucida D, et al. Mouse TCRalphabeta+CD8alphaalpha intraepithelial lymphocytes express genes that down-regulate their antigen reactivity and suppress immune responses. J Immunol. 2007;178:4230–9. doi: 10.4049/jimmunol.178.7.4230. [DOI] [PubMed] [Google Scholar]
- 37.Saurer L, Seibold I, Rihs S, Vallan C, Dumrese T, Mueller C. Virus-induced activation of self-specific TCR alpha beta CD8 alpha alpha intraepithelial lymphocytes does not abolish their self-tolerance in the intestine. J Immunol. 2004;172:4176–83. doi: 10.4049/jimmunol.172.7.4176. [DOI] [PubMed] [Google Scholar]
- 38.Ferrier L, Mazelin L, Cenac N, et al. Stress-induced disruption of colonic epithelial barrier: role of interferon-gamma and myosin light chain kinase in mice. Gastroenterology. 2003;125:795–804. doi: 10.1016/s0016-5085(03)01057-6. [DOI] [PubMed] [Google Scholar]
- 39.Ito R, Shin-Ya M, Kishida T, et al. Interferon-gamma is causatively involved in experimental inflammatory bowel disease in mice. Clin Exp Immunol. 2006;146:330–8. doi: 10.1111/j.1365-2249.2006.03214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–62. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 41.Stallmach A, Giese T, Schmidt C, Ludwig B, Mueller-Molaian I, Meuer SC. Cytokine/chemokine transcript profiles reflect mucosal inflammation in Crohn’s disease. Int J Colorectal Dis. 2004;19:308–15. doi: 10.1007/s00384-003-0554-4. [DOI] [PubMed] [Google Scholar]
- 42.Niessner M, Volk BA. Altered Th1/Th2 cytokine profiles in the intestinal mucosa of patients with inflammatory bowel disease as assessed by quantitative reversed transcribed polymerase chain reaction (RT-PCR) Clin Exp Immunol. 1995;101:428–35. doi: 10.1111/j.1365-2249.1995.tb03130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Camoglio L, Te Velde AA, Tigges AJ, Das PK, Van Deventer SJ. Altered expression of interferon-gamma and interleukin-4 in inflammatory bowel disease. Inflamm Bowel Dis. 1998;4:285–90. doi: 10.1002/ibd.3780040406. [DOI] [PubMed] [Google Scholar]
- 44.Gassler N, Rohr C, Schneider A, Kartenbeck J, Bach A, Obermuller N, Otto HF, Autschbach F. Inflammatory bowel disease is associated with changes of enterocytic junctions. Am J Physiol Gastrointest Liver Physiol. 2001;281:G216–28. doi: 10.1152/ajpgi.2001.281.1.G216. [DOI] [PubMed] [Google Scholar]
- 45.Muller S, Lory J, Corazza N, Griffiths GM, Z’Graggen K, Mazzucchelli L, Kappeler A, Mueller C. Activated CD4+ and CD8+ cytotoxic cells are present in increased numbers in the intestinal mucosa from patients with active inflammatory bowel disease. Am J Pathol. 1998;152:261–8. [PMC free article] [PubMed] [Google Scholar]