Abstract
Bivalent ligands—compounds incorporating two receptor-interacting moieties linked by a flexible chain—often exhibit profoundly enhanced binding affinity compared to their monovalent components, implying concurrent binding to multiple sites on the target protein. It is generally assumed that neurotransmitter sodium symporter (NSS) proteins, such as the dopamine transporter (DAT), contain a single domain responsible for recognition of substrate molecules. In this report, we show that molecules possessing two substrate-like phenylalkylamine moieties linked by a progressively longer aliphatic spacer act as progressively more potent DAT inhibitors (rather than substrates). One compound bearing two dopamine-like pharmacophoric ‘heads’ separated by an 8-carbon linker achieved an 82-fold gain in inhibition of [3H]CFT binding compared to dopamine itself; bivalent compounds with a 6-carbon linker and heterologous combinations of dopamine-, amphetamine- and β-phenethylamine-like heads all resulted in considerable and comparable gains in DAT affinity. A series of short-chain bivalent-like compounds with a single N-linkage was also identified, the most potent of which displayed a 74-fold gain in binding affinity. Computational modeling of the DAT protein and docking of the two most potent bivalent (-like) ligands suggested simultaneous occupancy of two discrete substrate-binding domains. Assays with the DAT mutants W84L and D313N—previously employed by our lab to probe conformation-specific binding of different structural classes of DAT inhibitors—indicated a bias of the bivalent ligands for inward-facing transporters. Our results strongly indicate the existence of multiple DAT substrate-interaction sites, implying that it is possible to design novel types of DAT inhibitors based upon the ‘multivalent ligand’ strategy.
Keywords: bivalent ligands, dopamine transporter, phenethylamines, secondary substrate-binding site
Introduction
The dopamine transporter protein (DAT) is a key regulator of dopaminergic signaling in the brain. DATs control the concentration of extraneuronal dopamine by actively shuttling released dopamine molecules back across the plasma membrane for later reuse or catabolism. Dopaminergic signaling is involved in many aspects of brain function, most notably cognition, motor function, affect, motivation and reward (Greengard 2001), with the DAT itself implicated in a number of neuropsychiatric disorders: ADHD, Parkinson’s disease, anhedonia/depression and addiction (Gainetdinov and Caron 2003; Mash 2008; Kurian et al. 2009). The DAT is also of significant pharmacological interest. The clinically used psychostimulants methylphenidate, d-amphetamine, modafinil and the antidepressant bupropion are notable DAT ligands (Rothman and Baumann 2003; Zolkowska et al. 2009). Moreover, interaction with the DAT is thought to underlie the potent reinforcing effects of two of the most prominent drugs of addiction—the psychostimulants cocaine and d-methamphetamine (Mortensen and Amara 2003; Thomsen et al. 2009). While established pharmacological agents exist for treatment of addiction to certain drugs (opioids, for example), no approved or reliable therapeutics are currently available for the treatment of addiction to cocaine or methamphetamine. Better understanding of DAT function—particularly the biophysical mechanism of substrate translocation and inhibitor binding—will enable development of novel and improved therapeutics for neuropsychiatric disorders and psychostimulant addiction.
Like other members of the neurotransmitter/sodium symporter (NSS) protein family, the DAT employs potential energy inherent to the inwardly directed Na+ electrochemical gradient to facilitate the thermodynamically unfavorable movement of substrate molecules against their concentration gradient (Gether et al. 2006; Forrest et al. 2008). The ‘alternating access’ model—originally proposed decades ago (Jardetzky 1966)—is generally considered to be the most accurate description of the substrate translocation mechanism in NSS proteins. According to this model, the transporter protein cycles between ‘outward-facing’ and ‘inward-facing’ conformations, during which a buried substrate/ion interaction site is successively exposed to the extracellular space (‘picking up’ symported ion(s) and substrate) and the intracellular space (releasing them into the cytosol), respectively. No direct structural information regarding the nature of substrate interaction with NSS proteins was available until Yamashita et al. (2005) published the crystal structure of the leucine transporter LeuTAa—a prokaryotic NSS family member from Aquifex aeolicus. In line with the alternating access model, the substrate leucine was found to occupy a binding pocket in the center of the plasma membrane, formed by partially unwound regions of TMs 1 and 6, as well as certain residues of TMs 3 and 8 (Yamashita et al. 2005). While providing tremendous structural insight, a crystallographic structure represents—by definition—a discrete geometric ‘snapshot’ of a molecular machine that must undergo continuous structural change in order to achieve its function. Hence, the question of ‘how’ substrate binding catalyzes the transport cycle remains unanswered.
Recently, Shi and colleagues (2008) found evidence supporting a novel variant of the classic alternating access mechanism in LeuT. Combining kinetic analysis of substrate interaction with in silico simulation of substrate translocation, the authors proposed that binding of a second leucine molecule to an allosteric secondary site (termed S2)—located ≈11 Å above the classical (primary) substrate site—triggers cytosolic release of substrate and Na+ from the primary site (S1). By causing a conformational shift from an outward facing to an inward-facing state, substrate binding at S2 serves as an integral symport-cycle effector (Shi et al. 2008). These findings raise the question of whether eukaryotic NSS proteins (like the DAT) possess proximal recognition sites for their respective substrates. If so, then one would expect a bivalent ligand, incorporating two substrate-like pharmacophores linked by a flexible spacer, to exhibit substantially greater binding potency than its monovalent parent. By virtue of cooperativity, the affinity of such a compound would theoretically be equivalent to the product of the binding contribution of both putative sites (Portoghese 1989; Kramer and Karpen 1998). This ‘multivalent’ strategy has been previously employed in designing potent and selective opioid, serotonin and muscarinic receptor ligands, with bivalent compounds often displaying an order of magnitude (or greater) jump in affinity over their monovalent counterparts (see e.g. Chrisopoulous et al. 2001; Daniels et al. 2005a; Steinfeld et al. 2007).
We have designed and synthesized a series of bivalent DAT ligands based upon the substrates dopamine (DA), amphetamine (AMP) and β-phenethylamine (β-PEA), each bearing two substrate-like “head” moieties linked by a flexible polymethylene spacer (see Fig. 1 for chemical structures). Spacer-linked bivalent ligands based upon DAT inhibitor pharmacophores (in particular, the phenyltropane class of cocaine-like inhibitors) have been previously explored with some success. The Kozikowski lab was the first to demonstrate the feasibility of the bivalent approach for monoamine transporters, finding that certain bivalent phenylpiperidine inhibitors bind to the DAT with far greater affinity than monovalent equivalents (Tamiz et al. 2001); for example, conversion of one of their lowest-affinity monovalent ligands into a pentamethylene spacer-linked bivalent compound yielded a 2300-fold jump in inhibitory activity (Tamiz et al. 2000). Later work by Frandrick et al. (2003) and Meltzer et al. (2008) subsequently showed bivalent molecules comprised of two phenyltropane moieties linked by a 6–8 carbon spacer to be potent DAT inhibitors. While bivalent phenyltropanes investigated in the latter two studies did not exhibit significantly greater DAT affinity than their respective monovalent analogues, their preserved high-affinity binding did imply that the cavity connecting the central S1 site with the extracellular face is large enough to house relatively bulky spacer-linked molecules. Most recently, Nielsen et al. (2009) reported a modest 5-fold gain in DAT affinity (over the parent monomer) with a bivalent phenyltropane molecule possessing a 10-atom alkyl-triazole linker (which the authors estimated to be ≈13 Å in length). However, to our knowledge, the present work is the first investigation of bivalent substrate-like compounds as DAT ligands.
Figure 1.
Chemical structures of the substrate-like DAT ligands investigated in the present study. (A) Classical monovalent phenethylamine substrates and monovalent analogues bearing an N-alkyl substituent. (B) Bivalent spacer-linked diphenethylamines, bivalent ligands possessing two independent substrate moieties tethered by a variable length N,N’-polymethylene chain. (C) Bivalent-like monoamines, short-chain bivalent compounds bearing two aromatic substrate-like “heads” linked at a shared nitrogen atom.
In this article, we compare these novel ligands and their parent monovalent components in radioligand binding assays and inhibition of [3H]dopamine uptake. We show that the binding affinity of certain bivalent compounds is increased by as much as 80-fold over respective monovalent DAT substrates. By mapping the structure-activity relationships (SAR) of systematic variation to (1) the length of the aliphatic linker separating the two substrate-like aromatic ‘heads’ and (2) the identity of the respective heads, we were able to employ these bivalent ligands as pharmacological probes of potential secondary substrate-binding domains on the DAT protein. In addition, we investigate the binding profile of the bivalent ligands at two DAT mutants (W84L and D313N). We have previously shown these mutants to have a disrupted conformational equilibrium between outward- and inward-facing states, increasing the probability that DAT substrate-binding site(s) will be exposed to the extracellular space (Chen et al. 2004; Schmitt et al. 2008; also see Loland et al. 2008 for detailed evaluation of a DAT mutant with opposing effects on transporter conformational equilibrium). Thus, they present us with an opportunity to compare potential conformation-specific binding properties of these bivalent compounds with those of other characterized DAT ligands. Finally, in order to obtain a clearer picture of how bivalent ligands might interact with the transporter, we docked the most potent compounds into a DAT homology model. Together, the data presented here support of the idea that NSS proteins contain more than a single domain for recognition of a substrate molecule and these domains can be simultaneously targeted by a multivalent ligand.
Methods and Materials
Generation and maintenance of wild-type and mutant hDAT cells
The pCIN4 vector and the wild-type human DAT construct employed herein (pCIN4-hDAT) were kindly provided by Dr. Jonathan Javitch (Columbia University; New York). Mutant hDAT plasmids and stable DAT-expressing cell lines were generated as previously reported (Chen et al. 2001). In summary, site-directed mutagenesis in the pCIN4-hDAT construct was performed using the QuickChange Kit (Stratagene; La Jolla, CA) and verified by PCR and restriction enzyme mapping. Human embryonic kidney cells (HEK293) were subsequently transfected with using Lipofectamine (Invitrogen; Carlsbad, CA) and desired clones were selected with geneticin. Cells were maintained as previously outlined (Chen et al. 2001).
[3H]CFT binding inhibition and [3H]dopamine uptake assays
For both binding and uptake assays, suspensions of intact HEK-hDAT were prepared according to the method outlined previously (Chen et al. 2001). This cell slurry was then incubated for 1 hr at 21°C and centrifuged at 800g; the subsequent pellet was washed and gently resuspended in 6 mL modified Krebs/Ringer/HEPES (KRH) buffer solution in preparation for assay (Chen et al. 2004). All assays were conducted in 96-well plates at ≈21°C, with all determinations performed in triplicate wells. Binding or uptake was initiated by addition of 50 μL cell suspension to buffer containing the respective radioligand and varying concentrations of the test DAT ligand, for a final per-well reaction volume of 200 μL. For binding, cells were incubated with 2-4 nM [3H]CFT (85.9 Ci/mmol, Perkin-Elmer; Boston, MA) and test compounds for 15 min (which is sufficient for equilibration, see Reith et al. 1996). The level of nonspecific binding was determined with 1 μM non-radiolabeled CFT. This nonspecific definition gives the same results as vector-transfected cells, correcting for low-affinity [3H]CFT binding to components other than the DAT (Reith et al. 1996). Binding was terminated by vacuum filtration onto a Wallac A filtermat and washing with ice-cold saline (0.9% w/v) using a Tomtec cell harvester (Tomtec; Orange, CT). The binding assay reflects primarily surface DAT (see Chen et al. 2004). For uptake assays, cells were incubated with 7–10 nM [3H]DA (40.0 Ci/mmol, Perkin-Elmer) and test compounds for 5 min. Nonspecific uptake was defined as remnant uptake in the presence of 100 μM cocaine. Termination of uptake was achieved by vacuum filtration onto a Wallac B filtermat, which was washed with ice-cold saline using a Brandel manual cell harvester (Brandel; Gaithersburg, MD). Tritium accumulation was quantified using a Wallac Microbeta 1405 liquid scintillation counter.
Data analysis and statistics
The equilibrium dissociation constant of radioligand binding (KD) and the half-saturation constant of uptake (Km) were determined by competition analysis with non-radiolabeled β-CFT or DA, using Kell RADLIG (Biosoft; Cambridge, UK). Half-effective concentrations (IC50) for inhibition of [3H]CFT binding and [3H]DA uptake were calculated with Origin 7.5 (OriginLab Software; Northamptom, MA). Constants reported are the mean of at least three independent assays ± SEM. Binding IC50 values were converted into relative inhibition constants (Ki) using the Cheng-Prusoff equation (Cheng and Prusoff 1973). In uptake studies, IC50 values were converted to apparent affinity constants (Kapp) using the same conversion factor used to calculate Ki values. For statistical tests, all affinity constants were log- transformed for normal distribution and the threshold of significance was set at p < 0.05.
The term Kapp was used to express relative uptake inhibition constants (as opposed to Ki) because the kinetic model describing the uptake process is more complex than that of binding: whereas inhibition of [3H]DA uptake by a non-transported DAT ligand (an inhibitor) simply reflects the ligand’s dissociation rate constant (KD), inhibition of uptake by another substrate reflects the rate constant of the entire ligand translocation cycle (Km) (Zimányi et al. 1989; Bönisch 1998). That is, as unlabeled substrate competes with [3H]DA for uptake, it too is translocated, requiring the DAT to shift from an outward-facing to an inward-facing conformation. The DAT protein is unable to bind another substrate molecule until it undergoes ‘reorientation’ back to the outward-facing state. Reorientation is the rate-limiting step for translocation and is far slower than the initial step of substrate binding to the outward-facing conformation (Erreger et al. 2008). In contrast, the relative potency (Ki) of any DAT ligand—be it substrate or inhibitor to—inhibit binding of a non-transported radioligand (such as [3H]CFT) only reflects the initial binding kinetics (KD) of the ligand (Bönisch 1998). Thus, the Ki value of a ligand behaving as a substrate would be expected to be significantly greater than its Kapp value: a substrate will saturate the transporter’s ability to take up [3H]DA at a far lower concentration than is needed to saturate all of the available [3H]CFT binding sites (Zimányi et al. 1989).
Chemistry
All chemicals, reagents and solvents were purchased from commercial suppliers and used as received unless otherwise noted. Dopexamine was a gift provided by Hospira Pharmaceuticals (Lake Forest IL, USA). Detailed synthetic procedures and analytic data for the novel compounds D-363 (N-hexyldopamine), D-278 (hydroxydobutamine), D-279 (dopamphetamine), D-310 (hydroxydopentamine) and the diphenethylamines D-307, D-308, D-309, D-347, D-361, D-362, D-370 and D-371 (Fig. 1B) are available online in the Supplementary Materials (elemental analyses are given in Supplementary Table S1).
Computational methods: homology modeling and docking
A comprehensive description of the procedures used for in silico modeling of the DAT and ligand docking can be found online, as part of the Supplementary Materials. In brief, the DAT model was based upon the crystal structure of the bacterial leucine transporter LeuTAa bound to the ligands leucine and the tricyclic antidepressant (TCA) desipramine (Zhou et al. 2007; PDB index 2QJU). The homology model was constructed according to the NSS-family protein amino acid sequence alignment proposed by Beuming et al. (2006). The DAT model was generated using the MODELLER algorithm and the resultant lowest energy structure was imported into the Molecular Operating Environment (MOE) program (Chemical Computing Group; Montreal, Canada). A Ramachandran plot of the model DAT protein is shown in Supplementary Fig. S1. Ligand binding sites in the DAT model were identified in MOE—for docking, ligands were converted to their protonated forms. Following preliminary docking, selected poses were further refined by minimization of the protein/ligand complex. In refinement rounds, the protein backbone atoms were loosely tethered, with the side-chain and ligand atoms unconstrained to allow for flexibility.
Results
Ligand Design and Classification
The substrate-like DAT ligands described in the present work can be separated into three distinct structural families: monovalent phenethylamines (Fig. 1A), bivalent spacer-linked diphenethylamines (Fig. 1B) and bivalent-like monoamines (Fig. 1C). The well-characterized DAT substrates DA, AMP and β-PEA were chosen as pharmacophores for bivalent design. Members of the spacer-linked diphenethylamine series incorporate two complete, independent substrate moieties—in either a homobivalent (DA–(CH2)n–DA) or heterobivalent (e.g. dopexamine, β-PEA–(CH2)6–DA)—configuration with the respective amines tethered by a variable-length polymethylene spacer (Fig. 1B). An aliphatic polymethylene linker was employed to ensure flexibility of the two pharmacophores with minimal steric hindrance imparted by the spacer. Spacer length ranges from three methylene units (D-307, D-309) to a total of eight (D-362). Importantly, two matching monovalent substrate analogues—one with a short N-alkyl substituent (N-propylamphetamine) and another bearing a longer chain (N-hexyldopamine, D-363)—were included as control compounds, in an effort to rule out the possibility that the spacer itself is responsible for the activity of the spacer-linked ligands.
The bivalent-like monoamine family is comprised of hybridized compounds in which the two substituted phenylalkylamine head moieties are not fully independent, but instead overlap at a mutual nitrogen atom (Fig. 1C). Structurally, members of this series diverge slightly from what might be considered “classical” bivalent design: they incorporate two aromatic fragments, but their single-nitrogen configuration makes it difficult to assign them a discrete ‘spacer length’ value. The shortest series member—dopamphetamine (D-279)—can be regarded as a direct fusion of two phenylalkylamine structures (in this case, DA and AMP), giving it an effective ‘spacer length’ of zero. Extending the alkyl chain of one of these two fused phenylalkylamine heads by a single methylene unit, we arrive at dobutamine and its catechol analogue hydroxydobutamine (D-278); and further alkyl chain extension (to a total of two additional methylene units) gives the longest series member, hydroxydopentamine (D-310).
All of the ligands were tested for their ability to displace [3H]CFT binding to the DAT in whole (membrane-intact) cells and to inhibit [3H]DA uptake. Binding assays were performed with WT, W84L and D313N mutant transporters; uptake, however, was only assessed in WT hDAT cells, as the mutants exhibited prohibitively slow DA uptake kinetics (data not shown). It is important to point out that the bivalent ligands presented herein were designed to target proximal substrate-interaction domains on a single DAT protein. This is in contrast to the “bivalent approach” that has been historically applied in the design of G-protein coupled receptor ligands—such as the bivalent opioid receptor ligands of Portoghese and colleagues, which are thought to bridge two externally located ligand-recognition sites of a dimerized receptor complex (Daniels et al. 2005a). There is robust evidence to suggest that DATs form oligomers at the plasma membrane, with transporter homodimers unequivocally demonstrated by Förster resonance energy transfer (FRET) microscopy (Sorkina et al. 2003) and higher-order (multimer-of-dimers) arrays theorized to exist (Sitte et al. 2004). However, compared to the aforementioned bivalent opioids, which have spacer regions ranging from 19.1 to 25.4 Å in length (Daniels et al. 2005a), our longest compound (D-362) has an octamethylene spacer with a maximum length of 11.3 Å (distance between the two nitrogens following energy minimization of the undocked ligand molecule by itself). As indicated by our DAT homology model, the diameter of a single transporter protein is ≈45–51 Å, making it extremely unlikely that the bivalent ligands explored in this work are capable of spanning multiple DAT protomers in an oligomeric array (the zoomed-out images of the final DAT/ligand complexes presented in Supplementary Fig. S2 offer a scaled perspective)
Monovalent phenethylamine substrates and their N-substituted analogues
As Table 1 shows, dopamine itself inhibited [3H]CFT binding to wild-type hDAT with a Ki of approximately 4.3 μM and a corresponding Kapp for inhibition of [3H]DA uptake of 430 nM—the 10-fold separation between binding and uptake values is highly consistent with our previous findings for this substrate in intact HEK-hDAT cells (Chen et al. 2004). Attachment of a bulky N-hexyl substituent to dopamine (yielding D-363) gave a moderate 2.5-fold increase in DAT binding affinity, but a dramatic (>7-fold) loss of activity as an inhibitor of [3H]DA uptake. Of the non-catechol substrates, β-PEA was slightly more potent in binding but slightly weaker in uptake inhibition than dopamine. Both enantiomers of amphetamine were far more potent than β-PEA in binding and uptake, with (d)-amphetamine possessing an advantage over the (l)-enantiomer in each parameter. Our observation that dextroamphetamine is roughly 4-fold more effective than levoamphetamine in inhibiting [3H]DA uptake is in agreement with prior findings of stereoselective DAT interaction both in rat striatal tissue slices (Heikkila et al. 1975) and heterologous hDAT-expressing cells (Eshleman et al. 1999). In binding assays, (d)-methamphetamine showed a slight advantage compared to the unsubstituted parent (d)-amphetamine, but the two were equipotent as uptake inhibitors. Extension of the N-alkyl substituent (to propylamphetamine) caused only a small decrease in binding affinity, but did result in a substantial drop in potency for inhibition of uptake (Table 1). Of the monovalent compounds tested, all except propylamphetamine and D-363 displayed far greater potency as inhibitors of [3H]DA uptake than as inhibitors of [3H]CFT binding (that is, exhibited a Kapp / Ki ratio < 1.0) at WT hDAT. Propylamphetamine and D-363 were each approximately two-fold more active at inhibiting binding than uptake (Kapp / Ki ratio ≈ 1.8–2.5), hinting that N-substituted phenethylamines bearing a “steric heft” of three or more methylene units behave more like non-translocated DAT inhibitors than true substrates (see below for a more detailed discussion of the Kapp / Ki ratio).
Table 1.
Potencies of investigated substrate-like DAT ligands, assessed by displacement of intact-cell [3H]CFT binding to WT or mutant hDAT and inhibition of [3H]DA uptake
| Compound | hDAT Binding Ki (nM) | Uptake Kapp (nM) | ||
|---|---|---|---|---|
| WT | W84L | D313N | WT | |
| Classical DAT Inhibitors (Reference) | ||||
| β-CFT | 15.4 ± 2.1a | 4.44 ± 0.69a | 6.14 ± 0.29a | 30.0 ± 4.0b |
| cocaine | 163.6 ± 1.20a | 46.7 ± 4.52a | 51.5 ± 5.06a | 246.0 ± 25.0b |
| benztropine | 75.3 ± 7.40a | 189.5 ± 6.82a | 181.4 ± 30.3a | 375.5 ± 6.35 |
| bupropion | 319.5 ± 24.9a | 745.9 ± 14.0a | 353.9 ± 16.7a | 576.5 ± 29.1 |
| Monovalent Substrate-like Series | ||||
| dopamine | 4287 ± 46.5 | 3357 ± 372 | 13560 ± 900* | 429.9 ± 64.7§ |
| β-phenethylamine | 3261 ± 21.5 | 6230 ± 181* | 14144 ± 1476* | 1103 ± 206§ |
| (d)-amphetamine | 592.8 ± 38.4 | 1486 ± 148* | 7534 ± 756* | 163.5 ± 4.57§ |
| (l)-amphetamine | 1096 ± 89.9 | 3078 ± 397* | 4797 ± 296* | 664.6 ± 60.4§ |
| (d)-methamphetamine | 340.7 ± 42.7 | 1152 ± 183* | 1455 ± 156* | 141.7 ± 18.8§ |
| (dl)-propylamphetamine | 988.2 ± 67.6 | 892.2 ± 157 | 845.2 ± 142 | 2497 ± 138 |
| N-hexyldopamine (D-363) | 1742 ± 191 | 1781 ± 126 | 2036 ± 345 | 3075 ± 223 |
| Bivalent Spacer-linked Diphenethylamine Series | ||||
| DA (CH2)3 DA (D-307) | 875.4 ± 71.4 | 644.2 ± 48.9* | 401.8 ± 1.51* | 1691 ± 197 |
| DA (CH2)4 DA (D-308) | 487.4 ± 26.3 | 487.3 ± 60.6 | 406.2 ± 17.5 | 840.5 ± 72.0 |
| DA (CH2)5 DA (D-347) | 83.33 ± 8.74 | 221.6 ± 15.1* | 240.9 ± 38.1* | 171.5 ± 1.60 |
| DA (CH2)6 DA (D-361) | 69.68 ± 9.54 | 113.3 ± 11.9* | 190.5 ± 16.2* | 134.9 ± 17.5 |
| DA (CH2)8 DA (D-362) | 51.91 ± 4.54 | 41.97 ± 5.49 | 139.0 ± 6.24* | 105.7 ± 2.98 |
| AMP (CH2)3 DA (D-309) | 322.4 ± 15.3 | 586.7 ± 88.5* | 299.4 ± 8.64 | 740.8 ± 30.1 |
| β-PEA (CH2)6 DA (dopexamine) | 96.95 ± 13.7 | 146.6 ± 5.09* | 192.2 ± 20.6* | 187.5 ± 19.2 |
| β-PEA (CH2)6 AMP (D-370) | 97.76 ± 10.0 | 174.3 ± 22.2* | 161.2 ± 6.38* | 204.7 ± 9.75 |
| AMP (CH2)6 DA (D-371) | 100.8 ± 13.4 | 214.2 ± 24.7* | 210.4 ± 36.6* | 244.5 ± 21.5 |
| Bivalent-like Monoamine Series | ||||
| (dl)-dobutamine | 196.5 ± 14.3 | 237.0 ± 23.9 | 257.0 ± 26.5 | 329.4 ± 16.5 |
| hydroxydobutamine (D-278) | 58.03 ± 2.90 | 79.79 ± 16.97 | 94.96 ± 4.86* | 187.5 ± 30.2 |
| hydroxydopentamine (D-310) | 91.36 ± 12.5 | 140.3 ± 28.4 | 66.37 ± 7.74 | 155.3 ± 18.6 |
| (dl)-dopamphetamine (D-279) | 341.8 ± 5.38 | 474.9 ± 40.5* | 771.9 ± 83.7* | 605.3 ± 46.7 |
Data are means ± SEM for 3-6 assays, each performed in triplicate.
Indicates significant difference versus WT binding value (p < 0.05; t test, two tailed).
Indicates WT uptake Kapp value significantly smaller than WT binding Ki (p < 0.05; t test, one tailed).
Data from Schmitt et al. (2008) and Chen et al. (2004), respectively, are included for comparison with the compounds tested in this study.
Bivalent spacer-linked diphenethylamines: two heads are better than one
All of the spacer-linked diamine ligands (Fig. 1B) were more potent inhibitors of [3H]CFT binding than their respective monovalent parents at WT hDAT (Table 1). Binding affinity increased steadily as the number of methylene units in the spacer was incremented. Of the homobivalent ligands, the tri-and tetramethylene-linked compounds D-307 and D-308 both showed only modest (5- and 10-fold, respectively) increases in binding activity over monovalent DA. Extension of the spacer beyond 4 methylene units resulted in enormous affinity gains, with the pentamethylene (D-347) and hexamethylene (D-361) ligands exhibiting similar (51- and 61-fold) respective increases over DA (Ki = 69.7–83.3 nM). The longest compound, the octamethylene-linked D-362, was the most potent diphenethylamine ligand tested, showing an 82-fold increase in affinity compared to monovalent DA (Ki = 51.9 nM, compared with dopamine’s 4.28 μM affinity constant). Amongst the heterobivalent diamine ligands, a longer aliphatic spacer also proved advantageous. The trimethylene-linked DA/AMP hybrid D-309 was nearly two-fold more potent at inhibiting [3H]CFT binding than monovalent (d)-amphetamine (and 13-fold more potent than DA). Elongation of the spacer to 6 methylene units (yielding the DA/AMP hybrid D-371) resulted in a subsequent three-fold jump in affinity, representing an overall 6-fold increase compared to (d)-amphetamine and a 42-fold increase over DA (Table 1). However, there was no appreciable difference between various heterobivalent ligands of the same length: the hexamethylene compounds dopexamine (DA/β-PEA), D-370 (AMP/β-PEA) and D-371 (DA/AMP) were all equipotent as inhibitors of binding (Ki ≈ 100 nM). While each appeared slightly less potent than the comparable homobivalent compound (D-361), a post-hoc ANOVA (Dunnett’s correction) did not reveal a significant affinity difference between D-361 and any of the other hexamethylene-linked diamines. Finally, the monovalent ligands propylamphetamine and N-hexyldopamine (D-363) displayed far weaker binding affinities than their respective bivalent siblings (D-309 and D-361). While N-hexyldopamine did show a mild increase in affinity compared to DA itself, it was still 25-fold weaker than the bivalent D-361. This strongly suggests that the presence of the spacer itself is not responsible for the behavior of the bivalent spacer-linked compounds.
Given the structural similarities between traditional DAT substrates—such as DA, β-PEA and AMP—and our spacer-linked compounds, we questioned if the bivalent ligands might themselves act as substrates. For ligands acting as bona fide substrates, the IC50 value for inhibition of the uptake process will be markedly lower than the IC50 value for displacement of a bound radiolabeled inhibitor (Nelson and Rudnick 1979; Schömig et al. 1988). As mentioned in the Methods section, this is because inhibition of substrate uptake by a non-transported ligand depends solely on that particular ligand’s dissociation rate constant at the interaction site (Schömig et al. 1988). Inhibition of uptake by another substrate, however, reflects the rate constant of the entire translocation cycle—including the rate-limiting step of transporter reorientation following intracellular substrate release (Zimányi et al. 1989; Erreger et al. 2008). Therefore, any bivalent ligand that is itself translocated would be expected to have an apparent uptake / binding inhibition constant ratio (Kapp / Ki) that is significantly less than 1.0 (Kapp < Ki). However, for non-translocated ligands, one would expect the Kapp / Ki ratio to be equal to or greater than 1.0 (Kapp≥ Ki). As shown in Table 1, all of the spacer-linked bivalent ligands were more potent inhibitors of [3H]CFT binding to WT DAT than [3H]DA uptake, having Kapp / Ki ratios ranging from 1.72 to 2.42. By contrast, the only compounds found to be more potent inhibitors of [3H]DA uptake than [3H]CFT binding (significantly lower Kapp than Ki value, p < 0.05) were the monovalent phenethylamines DA, β-PEA, (d)-AMP, (l)-AMP and (d)-methamphetamine—all previously confirmed to act as substrates (Rothman and Baumann 2006; Blough 2008). These data indicate that despite their substrate-like design, the spacer-linked bivalent ligands are not translocated by the DAT, but instead act as inhibitors.
Dobutamine and other bivalent-like monoamines: potent short-chain inhibitors
Early in our investigation of various bivalent ligands, we observed that the adrenoreceptor agonist dobutamine was a relatively potent inhibitor of [3H]CFT binding (Ki≈ 200 nM at wild-type DAT), showing a surprising 21-fold improvement over DA. Compared to the diphenethylamines, dobutamine is a much shorter molecule, with two overlapping pharmacophoric heads converging at a shared nitrogen atom. These unique structural features led us to synthesize analogous bivalent-like monoamines with different aromatic head configurations. Changing the tyramine-like phenol moiety of dobutamine into a second catechol ring gives hydroxydobutamine (D-278). This analogue displayed a 4-fold increase in affinity compared to dobutamine, making it the most potent of the series. Moreover, with a Ki value of roughly 58 nM, hydroxydobutamine was equipotent with the diphenethylamines D-361 and D-362, achieving a 74-fold gain in affinity over DA. Incrementing the number of methylene units to yield hydroxydopentamine (D-310) did not result in further binding gains; but this compound was still more potent than dobutamine, suggesting that for this series of ligands, pharmacophore type (i.e. catechol versus non-catechol heads) is of more importance than chain length. Finally, the shortest compound, dopamphetamine (D-279), showed only a moderate (2-fold) increase in binding over (d)-amphetamine (note that the racemate (dl)-dopamphetamine was tested, so direct comparison to single-enantiomer parent monomers was not possible). As with the spacer-linked ligands, all of the bivalent-like monoamines acted as non-translocated inhibitors, being more potent inhibitors of [3H]CFT binding than [3H]DA uptake.
Mutations enhancing affinity of cocaine-like ligands negatively impact binding of bivalent substrate-like compounds
Different classes of DAT inhibitors have been shown to stabilize the transporter protein in distinct conformational states upon binding; moreover, interaction with a specific conformation has been posited to affect the reinforcing strength (“addictiveness”) of a given inhibitor (Loland et al. 2008). For example, studies have suggested that cocaine and structural analogues preferentially stabilize the DAT in an outward-facing conformation (Reith et al. 2001; Loland et al. 2002), whereas atypical inhibitors lacking cocaine-like potential for addiction—such as benztropine, GBR12909 and bupropion—stabilize the inward-facing conformation (Loland et al. 2008; Schmitt et al. 2008). Since the bivalent compounds uniformly behaved as non-translocated inhibitors, we wished to compare their binding profiles and potential conformational binding preferences to those of other DAT inhibitors. We have previously characterized two hDAT mutations (W84L and D313N) that disrupt the conformational transition between outward- and inward-facing states, increasing the likelihood that the transporter will adopt an outward-facing conformational state (Chen et al. 2001; Chen et al. 2004). These mutations considerably increase the affinity of cocaine-like inhibitors as measured by inhibition of [3H]CFT binding, but have negligible or opposing effects on the affinity of atypical inhibitors like benztropine and bupropion (Schmitt et al. 2008). Thus, a given DAT ligand’s affinity ratio at mutant versus WT transporters can offer insight into whether the ligand preferentially interacts with the outward- or the inward-facing conformational state. In general, the bivalent compounds behaved similarly to the atypical DAT inhibitors benztropine and bupropion rather than cocaine and its phenyltropane analogue β-CFT with respect to mutation. That is, the bivalent ligands showed either unchanged or significantly decreased [3H]CFT binding affinity (increased Ki value) at both the W84L and D313N mutants compared to WT DAT (Table 1). The only bivalent compound that did not follow this trend was D-307, which had a greater binding affinity (significantly lower Ki value) at both the W84L and D313N mutants than at WT. The N-substituted monovalent ligands propylamphetamine and N-hexyldopamine showed no significant difference in affinity between WT and either of the mutants, a binding profile shared by the bivalent D-308.
Flexible docking of ligands at a homology model of the DAT
In the hopes of providing some qualitative structural insight into the binding of these novel bivalent (-like) ligands, we developed a human DAT homology model and docked the highest-affinity compounds (D-362 and hydroxydobutamine)—as well as DA itself—with flexible docking algorithms (Supplementary Figs. S2A–C). The hDAT model was based upon the structure of LeuT co-crystallized with the TCA desipramine (Zhou et al. 2007) and the NSS-family alignment developed by Beuming et al. (2006). Two ligand-binding pockets identified in the hDAT model were used for docking. The first binding pocket was embedded near the protein midpoint, analogous to the S1 site occupied by leucine in the LeuT crystal. The second binding pocket was located higher up in the substrate permeation pore (towards the extracellular vestibule), enveloping the putative S2 site of LeuT—or at least the region of S2 occupied by co-crystallized TCAs (like desipramine). Superposition of the final dopamine-bound hDAT model and the crystal structure gave an RMSD value of 1.24 Å for backbone Cα atoms. Comparison of the two structures indicated a high degree of spatial overlap in the TM regions, with lowest congruence found in extracellular loop regions (Supplementary Fig. S2D).
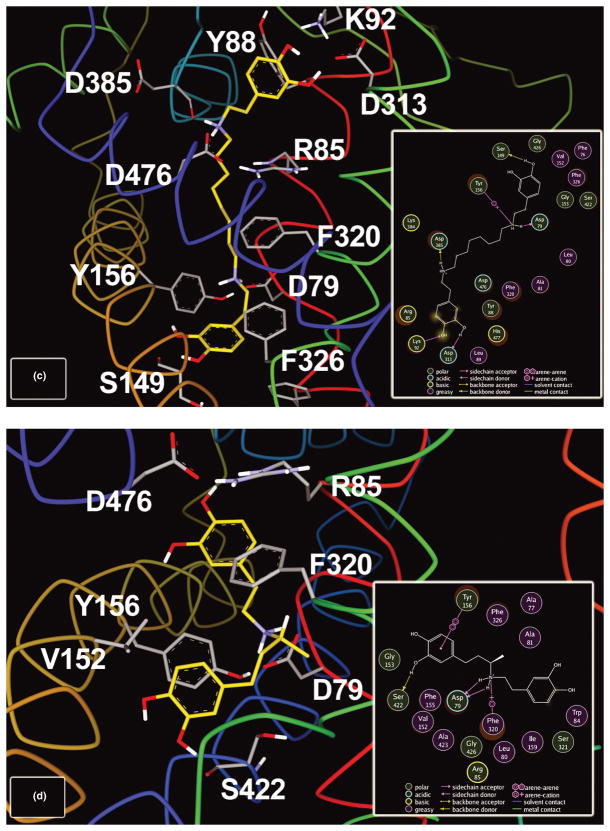
The majority of poses generated during the docking procedure suggested interactions between all of the ligands and residues of TMs 1, 3, 6, 8 and 10, as well as Lys384 and Asp385 in extracellular loop 4 (linking TMs 7 and 8). We found that many of the binding pocket residues were common to all of the docked ligands, which is not surprising given their shared structural moieties. For DA docked at the S1 site (Fig. 2A), the catechol hydroxyl groups formed hydrogen bonds with Ser149 and Ser422, the phenyl ring engaged in an aromatic π-stacking interaction with Tyr156 and the protonated amine was anchored by a combination of hydrogen bonds with Asp79 and Phe320 and a cation-π interaction with the phenyl ring of Phe320 (see Fig. 2A). These binding interactions for DA at the S1 site are similar to those proposed in prior docking and dynamic molecular simulation studies (Huang and Zhan 2007; Indarte et al. 2008; Beuming et al. 2008). At the S2 site (Fig. 2B), the catechol hydroxyls of DA formed hydrogen bonds with Lys92 and Asp313 and the protonated amine moiety displayed a combination of hydrogen bonding with Lys384 and Asp476 and a cation-π interaction with the phenyl ring of Tyr88. The docking model of DA at the S2 site also suggested a cation-π interaction between the aromatic ring of DA and His477, which was predicted to exist in the singly-protonated state.
Figure 2.
Representative energy-minimized poses of model DAT/ligand complex following docking of dopamine (A, B) and selected bivalent (-like) ligands (C, D). Selected DAT residues from respective ligand-binding pockets are labeled and rendered as sticks (transmembrane helices lacking interacting residues are hidden for visual clarity). The ligand molecules are highlighted with yellow-colored carbon atoms. For each pose, the inset panel depicts all residues located within 4.5 Å of the ligand; the strongest DAT/ligand interactions are indicated with dotted lines and a symbol depicting the chemistry of the interaction. (A, B) DA docked at both the S1 and S2 sites. At each site, the protonated amine of DA is anchored by a combination of hydrogen bonding and cation-π interactions and the ring hydroxyl moieties participate in hydrogen bonding. (C) Docking configuration of the most potent spacer-linked diphenethylamine ligand, D-362. In this representative low-energy pose, the catecholamine pharmacophores are oriented such that they interact with the transporter at both the S1 and S2 sites simultaneously. A number of major molecular interactions are similar to those found for DA (Asp79, Tyr156 and Ser149 at the S1 site and Lys92 and Asp313 at the S2 site). Cooperative binding to these two sites may underlie the dramatically higher binding affinity (82-fold) of D-362 compared to monovalent DA. (D) Docking configuration of D-278 (hydroxydobutamine), the most potent of the bivalent-like monoamine ligand series.
The bivalent diphenethylamine D-362 (Fig. 2C) made a number of the same connections as the two respective DA molecules when docked: hydrogen bonds involving ring hydroxyl groups and residues Ser149, Lys92 and Asp313, as well as an interaction between one of the two protonated amines and Asp79. One notable difference in binding modes between DA and D-362 was in the nature of the interaction with Tyr156. Compared to DA, the S1-localized head of D-362 was twisted somewhat, skewing the alignment angle needed for an aromatic π-stacking interaction; instead, Tyr156 established a cation-π interaction with the amine nitrogen, similar to the interaction between DA and Phe320 (compare Figs. 2A and 2C). Hydroxydobutamine (D-278) was situated such that the longer (3,4-dihydroxyphenylpropyl) end of the molecule—as opposed to the dopamine-like fragment—displayed a number of residue interactions in common with DA at the S1 site (Fig. 2D). These interactions include hydrogen bonds with Asp79 and Ser422, a cation-π interaction with Phe320 and aromatic π-stacking with the ring of Tyr156. This docking orientation suggests that hydrogen bonding between the catechol meta-hydroxyl group and Ser422 underlies the increased potency of hydroxydobutamine compared to the parent dobutamine.
Discussion
Multivalent design is increasingly being investigated as a strategy for development of novel small-molecule ligands. The motivation for this approach is clear: for target proteins with multiple ligand-interaction domains, a bivalent ligand can exhibit dramatically greater binding affinity and target selectivity than a parent monovalent ligand (Steinfeld et al. 2007; Kim et al. 2008). The superior affinity of a bivalent ligand (versus two molecules of a monovalent ligand) stems from the thermodynamic advantage inherent to a cooperative binding mechanism. That is, the total entropy of the ligand/protein complex is substantially lowered by having the second (tethered) pharmacophoric unit automatically localized within its binding pocket upon binding of the first unit (Portoghese 1989; Haviv et al. 2007). Bivalent compounds based upon the inhibitor-like phenylpiperidine and phenyltropane frameworks have been found to be potent DAT and SERT ligands (Tamiz et al. 2001; Frandrick et al. 2003); with certain compounds showing an upward shift in affinity that is characteristic of cooperative binding to multiple sites (Tamiz et al. 2000; Nielsen et al. 2009). Moreover, Shi and colleagues’ (2008) demonstration that the NSS-family member LeuT can simultaneously bind two molecules of substrate challenged the notion that symporter proteins contain a solitary substrate-interaction domain.
Our present findings suggest that the DAT protein also possesses more than one substrate-interaction site. We synthesized and tested a number of bivalent substrate-like DAT ligands based upon the phenylalkylamine pharmacophore. These novel compounds fell into two distinct structural classes: (1) bivalent N,N’-spacer-linked diphenethylamines and (2) bivalent-like monoamines (Fig. 1). Both bivalent series yielded highly potent DAT inhibitors. Relative to monovalent DA, the most potent members of the two classes showed 82- and 74-fold gains in binding affinity, respectively. Importantly, monovalent substrate analogues incorporating the same type of spacer as the diphenethylamines, yet missing a second pharmacophore, did not show these profound gains. The finding that removal of the second substrate moiety eliminates these increases in potency is an indication that both moieties contribute to the binding process. Amongst the diphenethylamines, binding activity increased as the spacer was lengthened, with a chain of 6–8 methylene units affording the highest affinity ligands. Spacer length is perhaps the most critical parameter in bivalent ligand design, as maximization of the thermodynamic favorability of cooperative binding requires that the spacer match the distance between binding sites. In LeuT, molecular simulations have indicated that the S2 site overlaps with the known TCA binding site, suggesting a distance of 11–13 Å between the S1 and S2 sites (Shi et al. 2008; Quick et al. 2009). In our DAT homology model, DA docked at two discrete sites, separated by a distance of 12.8–17 Å (measured as the distance between the amines and aromatic ring centroids, respectively). One of the docked DA molecules showed considerable spatial overlap with leucine bound at the S1 locus of LeuT, whereas the second molecule of DA bound at a position slightly higher in the extracellular vestibule than desipramine at S2 (Supplementary Fig. S2D). When D-362 was docked, the second DA-like pharmacophore was situated at a similar position in the S2 site. The two amines were separated by a 10.2 Å distance and the two catechol groups were 17 Å apart (measured as the distance between ring centroids). Hence, the model of D-362/DAT interaction agrees with the hypothesis that the large increase in affinity displayed by this bivalent DA analogue is due to simultaneous occupancy of two discrete DA-binding domains. For further discussion of this issue, please refer to the Supplementary Materials, under the subheading Binding assays: Hill numbers.
Binding assays performed with the two conformation-biasing DAT mutants (W84L and D313N) suggested that the majority of the bivalent ligands (most notably, diphenethylamines with a pentamethylene or longer spacer) preferentially interact with the inward-facing transporter. Compared to WT DAT, the W84L and D313N mutants are more likely to adopt an outward-facing state, resulting in a characteristic increase in affinity for cocaine-like ligands. However, atypical DAT inhibitors (such as benztropine and bupropion) and substrates (such as DA and AMP) show a decrease in binding affinity at one or both of the mutants. The longer diamine ligands and the monoamine hydroxydobutamine share this “atypical” binding profile with respect to the mutants. Our modeling and docking data are also consistent with the idea that D-362 and hydroxydobutamine interact with the DAT in a different manner than cocaine. Recently, Beuming et al. (2008) showed that the distance between residues Asp79 and Tyr156 may indicate the type of interaction a given compound has with the DAT: cocaine-like compounds disrupt formation of a hydrogen bond between the side chains of Asp79 and Tyr156 whereas substrates and atypical inhibitors allow for this bond. In their molecular simulations, the authors found that docking of β-CFT and cocaine resulted in Asp79-Tyr156 distances (measured as the minimum distance between the respective side chain oxygen atoms) of ≈5.5 Å and ≈7.5 Å, respectively—far greater than the 3.5 Å maximum for hydrogen bonding. By contrast, simulations of DA, (d)-AMP, MDMA and benztropine binding showed preserved hydrogen bonding, with an Asp79-Tyr156 distance <3.5 Å in each case (Beuming et al. 2008). Our docking models were in accordance with this finding: the three ligands (DA, D-362 and hydroxydobutamine) yielded highly similar Asp79-Tyr156 distances, ranging from 2.28–2.32 Å (see Supplementary Figs. S3A-C). In order to reasonably compare this modeling result with those of Beuming et al., we also docked β-CFT at the S1 site of our model DAT (employing the procedures outlined in the Supplementary Materials). As opposed to the three substrate-like ligands, docking of β-CFT resulted in a minimum Asp79-Tyr156 distance of 4.64 Å, indicative of a disrupted hydrogen bond.
It should be noted that static in silico procedures (modeling and flexible docking) do not permit specific conclusions regarding the structural basis of protein/ligand interaction in nature. A computational approach involving all-atom simulated molecular dynamics would allow for superior inferences, offering a four-dimensional mapping of the ‘conformational space;’ but this approach comes at the expense of vastly increased computational complexity. That said, the presence of a distal S2 site in the DAT is consistent with the sharp jump in activity that we observed when increasing alkyl spacer length from a trimethylene (D-307) to a hexamethylene (D-361) chain (a 12.5-fold increase in DAT affinity). As one approaches a correct ‘critical distance’ between the two pharmacophores, binding becomes more cooperative, dramatically increasing affinity. Length-dependent improvement in activity was also observed for the heterobivalent compounds: for DA/AMP dimers, going from a trimethylene (D-309) to a hexamethylene linker (D-371) resulted in a 3-fold gain in binding affinity. Notably, there was no difference in DAT affinity amongst the longer heterobivalent compounds—dopexamine (DA/βPEA), D-370 (AMP/β-PEA) and D-371 (DA/AMP)—despite clear differences between their monovalent parents. This finding may reflect the promiscuity of the S1 and S2 sites. In LeuT, for example, the S1 site will accommodate a number of different amino acids (Singh et al. 2008) and the S2 site appears to bind compounds from a variety of structural classes, including amino acid substrates (Shi et al. 2008), TCAs (Zhou et al. 2007; Singh et al. 2007) and alkyl-glucoside detergents (Quick et al. 2009). The finding may also reflect the inadequacies of traditional DAT-inhibitor radioligands, like [3H]CFT and other phenyltropanes, to faithfully capture subtle differences in substrate preference that may exist between the S1 and S2 sites. While tropanes have traditionally been considered to be selective for the S1 site, they may actually be capable of interacting with either site. Indeed, a recent molecular simulation by Huang et al. (2009) offers structural insight into how cocaine may inhibit DAT activity by binding to the putative S2 site. In this study, the authors point out that various in silico modeling and site-directed mutagenesis assays have yielded data consistent with both binding mechanisms.
One observation not adequately explained by the notion of cooperative binding at both the S1 and LeuT-like S2 site is the high potency of short-chain bivalent-like monoamines, such as hydroxydobutamine (D-278). This ligand is incapable of spanning the distance required for the two catechol moieties to reach the respective S1 and S2 sites, as shown in the docking model (Figs. 2D and S2B). The distance between the catechol rings of D-278 is only 7.9 Å (measured centroid-to-centroid), which places the second pharmacophore just below the side-chains of Arg85 and Asp476—two residues thought to be a part of an ‘extracellular gate’ that closes upon binding of substrate at S1 (Huang and Zhan 2007). In contrast, in the LeuT/TCA structure, the S2 site is localized just above the equivalent conserved amino acid pair (Arg30 and Asp404), matching the docking location of the second molecule of DA and the second DA-like head of D-362 (see Supplementary Figs. S3D-F).
Therefore, our data are also consistent with a more continuous model of DAT/substrate interaction. In a continuous model, the substrate permeation pathway is considered to be a pore lined with a series of transiently occupied interaction sites. These ephemeral sites are differentially exposed and occluded during the translocation process, providing a piecewise conduit for the substrate. Evidence supporting such a model was recently demonstrated in LeuT: crystallization of LeuT bound to the substrate-like inhibitor tryptophan revealed a transiently occupied secondary tryptophan site, located roughly 4 Å above the S1 site (Singh et al. 2008). Much like our bivalent DAT ligands, tryptophan is a substrate-like LeuT ligand that is too large to be translocated, essentially stalling the transporter in a given conformation upon binding. It could be that bivalent substrate-like ligands, by interacting with two transient, low-affinity pore sites, are ‘freezing’ the DAT in a particular conformational state encountered during the translocation process. However, in the case of the smaller, dobutamine-like ligands, it is also conceivable that two independent ligand molecules interact with the DAT: one at the S1 site and another at the S2 site, above the Arg85-Asp476 gate. This type of mechanism cannot necessarily be excluded, as DAT radioligand mainstays ([3H]CFT and other phenyltropanes) may be less selective for the S1 site than previously assumed. Further studies with other bivalent ligands—perhaps even compounds incorporating both substrate-like and inhibitor-like pharmacophores, if synthetically feasible will—help to elucidate the molecular mechanism of substrate/transporter interaction.
Supplementary Material
Acknowledgments
This work was supported by NIH Grant DA013261, DA019676 and MH083840. The authors have no competing financial interests to disclose. We thank Nathan Karpowich for his assistance with the MODELLER algorithm and valuable discussions concerning computational procedures.
Abbreviations
- ADHD
attention deficit hyperactivity disorder
- CFT
2β-carbomethoxy-3β-(4-fluorophenyl)-tropane
- AMP
amphetamine
- PEA
phenethylamine
- MDMA
3,4-methylenedioxy-N-methylamphetamine
- DAT
dopamine transporter
- NSS
neurotransmitter sodium symporter
- TM
transmembrane domain
- WT
wild type
References
- Beuming T, Kniazeff J, Bergmann ML, et al. The binding sites for cocaine and dopamine in the dopamine transporter overlap. Nat Neurosci. 2008;11:780–789. doi: 10.1038/nn.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuming T, Shi L, Javitch JA, Weinstein H. A comprehensive structure-based alignment of prokaryotic and eukaryotic neurotransmitter/Na+ symporters (NSS) aids in the use of the LeuT structure to probe NSS structure and function. Mol Pharmacol. 2006;70:1630–1642. doi: 10.1124/mol.106.026120. [DOI] [PubMed] [Google Scholar]
- Blough BE. Dopamine-releasing agents. In: Trudell ML, Izenwasser S, editors. Dopamine Transporters: Chemistry, Biology and Pharmacology. Wiley: Hoboken, NJ; 2008. pp. 305–320. [Google Scholar]
- Bönisch H. Transport and drug binding kinetics in membrane vesicle preparations. In: Amara S, editor. Methods of Enzymology. Vol. 296. Academic Press; London, UK: 1998. pp. 259–290. [Google Scholar]
- Chen N, Vaughan RA, Reith MEA. The role of conserved tryptophan and acidic residues in the human dopamine transporter as characterized by site-directed mutagenesis. J Neurochem. 2001;77:1116–1127. doi: 10.1046/j.1471-4159.2001.00312.x. [DOI] [PubMed] [Google Scholar]
- Chen N, Zhen J, Reith MEA. Mutation of Trp84 and Asp313 of the dopamine transporter reveals similar mode of binding interaction for GBR12909 and benztropine as opposed to cocaine. J Neurochem. 2004;89:853–864. doi: 10.1111/j.1471-4159.2004.02386.x. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50% inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Christopoulos A, Grant MK, Ayoubzadeh N, et al. Synthesis and pharmacological evaluation of dimeric muscarinic acetylcholine receptor agonists. J Pharmacol Exp Ther. 2001;298:1260–1268. [PubMed] [Google Scholar]
- Daniels DJ, Lenard NR, Etienne CL, Law PY, Roerig SC, Portoghese PS. Opioid-induced tolerance and dependence in mice is modulated by the distance between pharmacophores in a bivalent ligand series. Proc Natl Acad Sci. 2005a;102:19208–19213. doi: 10.1073/pnas.0506627102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erreger K, Grewer C, Javitch JA, Galli A. Currents in response to rapid concentration jumps of amphetamine uncover novel aspects of human dopamine transporter function. J Neurosci. 2008;28:976–989. doi: 10.1523/JNEUROSCI.2796-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshleman AJ, Carmolli M, Cumbay M, Martens CR, Neve KA, Janowsky A. Characteristics of drug interactions with recombinant biogenic amine transporters expressed in the same cell type. J Pharmacol Exp Ther. 1999;289:877–885. [PubMed] [Google Scholar]
- Fandrick K, Feng X, Janowsky A, Johnson R, Cashman JR. Bivalent biogenic amine reuptake inhibitors. Bioorg Med Chem. 2003;13:2151–2154. doi: 10.1016/s0960-894x(03)00386-x. [DOI] [PubMed] [Google Scholar]
- Forrest LR, Zhang Y, Jacobs MT, et al. Mechanism for alternating access in neurotransmitter transporters. Proc Natl Acad Sci. 2008;105:10338–10343. doi: 10.1073/pnas.0804659105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainetdinov RR, Caron MG. Monoamine Transporters: From Genes to Behavior. Annu Rev Pharmacol Toxicol. 2003;43:261–284. doi: 10.1146/annurev.pharmtox.43.050802.112309. [DOI] [PubMed] [Google Scholar]
- Gether U, Andersen PH, Larsson OM, Schousboe A. Neurotransmitter transporters: molecular function of important drug targets. Trends Pharmacol Sci. 2006;27:375–383. doi: 10.1016/j.tips.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Greengard P. The neurobiology of slow synaptic transmission. Science. 2001;294:1024–1030. doi: 10.1126/science.294.5544.1024. [DOI] [PubMed] [Google Scholar]
- Haviv H, Wong DM, Silman I, Sussman JL. Bivalent ligands derived from Huperzine A as acetylcholinesterase inhibitors. Curr Topics Med Chem. 2007;7:375–387. doi: 10.2174/156802607779941215. [DOI] [PubMed] [Google Scholar]
- Heikkila RE, Orlansky H, Mytilineou C, Cohen G. Amphetamine: evaluation of d-and l-isomers as releasing agents and uptake inhibitors for 3H-dopamine and 3H-norepinephrine in slices of rat neostriatum and cerebral cortex. J Pharmacol Exp Ther. 1975;194:47–56. [PubMed] [Google Scholar]
- Huang X, Zhan CG. How dopamine transporter interacts with dopamine: insights from molecular modeling and simulation. Biophys J. 2007;93:3627–3639. doi: 10.1529/biophysj.107.110924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Gu HH, Zhan CG. Mechanism for cocaine blocking the transport of dopamine: Insights from molecular modeling and dynamics simulation. J Phys Chem B. 2009;113:15057–15066. doi: 10.1021/jp900963n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indarte M, Madura JD, Surratt CK. Dopamine transporter comparative molecular modeling and binding site prediction using the LeuTAa leucine transporter as a template. Proteins. 2008;70:1033–1046. doi: 10.1002/prot.21598. [DOI] [PubMed] [Google Scholar]
- Jardetzky O. Simple allosteric model for membrane pumps. Nature. 1966;211:969–970. doi: 10.1038/211969a0. [DOI] [PubMed] [Google Scholar]
- Kim Y, Cao Z, Tan W. Molecular assembly for high-performance bivalent nucleic acid inhibitor. Proc Natl Acad Sci. 2008;105:5664–5669. doi: 10.1073/pnas.0711803105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer RH, Karpen JW. Spanning binding sites on allosteric proteins with polymer-linked ligand dimers. Nature. 1998;395:710–713. doi: 10.1038/27227. [DOI] [PubMed] [Google Scholar]
- Kurian M, Zhen J, Cheng Y, et al. Homozygous loss-of-function mutations in the gene encoding the dopamine transporter are associated with infantile parkinsonism-dystonia. J Clin Invest. 2009;119:1595–1603. doi: 10.1172/JCI39060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loland CJ, Desai RI, Zou MF, et al. Relationship between Conformational Changes in the Dopamine Transporter and Cocaine-Like Subjective Effects of Uptake Inhibitors. Mol Pharmacol. 2008;73:813–823. doi: 10.1124/mol.107.039800. [DOI] [PubMed] [Google Scholar]
- Loland CJ, Norregaard L, Litman T, Gether U. Generation of an activating Zn2+ switch in the dopamine transporter: mutation of an intracellular tyrosine constitutively alters the conformational equilibrium of the transport cycle. Proc Natl Acad Sci. 2002;99:1683–1688. doi: 10.1073/pnas.032386299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mash DC. Dopamine transporter, disease states and pathology. In: Trudell ML, Izenwasser S, editors. Dopamine Transporters: Chemistry, Biology and Pharmacology. Wiley; Hoboken, NJ: 2008. pp. 29–47. [Google Scholar]
- Meltzer PC, Kryatova O, Pham-Huu DP, Donovan P, Janowsky A. The synthesis of bivalent 2beta-carbomethoxy-3beta-(3,4-dichlorophenyl)-8-heterobicyclo[3.2.1]octanes as probes for proximal binding sites on the dopamine and serotonin transporters. Bioorg Med Chem. 2008;16:1832–1841. doi: 10.1016/j.bmc.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen O, Amara S. Dynamic regulation of the dopamine transporter. Eur J Pharmacol. 2003;479:159–170. doi: 10.1016/j.ejphar.2003.08.066. [DOI] [PubMed] [Google Scholar]
- Nelson PJ, Rudnick G. Coupling between platelet 5-hydroxytryptamine and potassium transport. J Biol Chem. 1979;254:10084–10089. [PubMed] [Google Scholar]
- Nielsen S, Pedersen CM, Hansen SG, et al. An extended study of dimeric phenyltropanes. Bioorg Med Chem. 2009;17:4900–4909. doi: 10.1016/j.bmc.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Portoghese PS. Bivalent ligands and the message-address concept in the design of selective opioid receptor antagonists. Trends in Pharmacol Sci. 1989;10:230–235. doi: 10.1016/0165-6147(89)90267-8. [DOI] [PubMed] [Google Scholar]
- Quick M, Winther AM, Shi L, Nissen P, Weinstein H, Javitch JA. Binding of an octylglucoside detergent molecule in the second substrate (S2) site of LeuT establishes an inhibitor-bound conformation. Proc Natl Acad Sci. 2009;106:5563–5568. doi: 10.1073/pnas.0811322106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith MEA, Xu C, Zhang L, Coffey LL. Translocation of dopamine and binding of WIN35428 measured under identical conditions in cells expressing the cloned human dopamine transporter. Naunyn-Schmiedeberg’s Arch Pharmacol. 1996;354:295–304. doi: 10.1007/BF00171060. [DOI] [PubMed] [Google Scholar]
- Reith MEA, Berfield JL, Wang LC, Ferrer JV, Javitch JA. The uptake inhibitors cocaine and benztropine differentially alter the conformation of the human dopamine transporter. J Biol Chem. 2001;276:29012–29018. doi: 10.1074/jbc.M011785200. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Monoamine transporters and psychostimulant drugs. Eur J Pharmacol. 2003;479:23–40. doi: 10.1016/j.ejphar.2003.08.054. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Therapeutic potential of monoamine transporter substrates. Curr Topics Med Chem. 2006;6:1845–1859. doi: 10.2174/156802606778249766. [DOI] [PubMed] [Google Scholar]
- Schmitt KC, Zhen J, Kharkar P, Mishra M, Chen N, Dutta AK, Reith MEA. Interaction of cocaine-, benztropine- and GBR12909-like compounds with wild-type and mutant human dopamine transporters: molecular features that differentially determine antagonist-binding properties. J Neurochem. 2008;107:928–940. doi: 10.1111/j.1471-4159.2008.05667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schömig E, Körber M, Bönisch H. Kinetic evidence for a common binding site for substrates and inhibitors of the neuronal noradrenaline carrier. Naunyn Schmiedebergs Arch Pharmacol. 1988;337:626–632. doi: 10.1007/BF00175787. [DOI] [PubMed] [Google Scholar]
- Shi L, Quick M, Zhao Y, Weinstein H, Javitch JA. The mechanism of a neurotransmitter sodium symporter—inward release of Na+ and substrate is triggered by substrate in a second binding site. Mol Cell. 2008;30:667–677. doi: 10.1016/j.molcel.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Piscitelli CL, Yamashita A, Gouaux E. A Competitive Inhibitor Traps LeuT in an Open-to-Out Conformation. Science. 2008;322:1655–1661. doi: 10.1126/science.1166777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Yamashita A, Gouaux E. Antidepressant binding site in a bacterial homologue of neurotransmitter transporters. Nature. 2007;448:952–956. doi: 10.1038/nature06038. [DOI] [PubMed] [Google Scholar]
- Sitte HH, Farhan H, Javitch JA. Sodium-dependent neurotransmitter transporters: oligomerization as a determinant of transporter function and trafficking. Mol Interv. 2004;4:38–47. doi: 10.1124/mi.4.1.38. [DOI] [PubMed] [Google Scholar]
- Sorkina T, Doolen S, Galperin E, Zahniser NR, Sorkin A. Oligomerization of dopamine transporters visualized in living cells by fluorescence resonance energy transfer microscopy. J Biol Chem. 2003;278:28274–28283. doi: 10.1074/jbc.M210652200. [DOI] [PubMed] [Google Scholar]
- Steinfeld T, Mammen M, Smith JAM, Wilson RD, Jasper JR. A novel multivalent ligand that bridges the allosteric and orthosteric binding sites of the M2 muscarinic receptor. Mol Pharmacol. 2007;72:291–302. doi: 10.1124/mol.106.033746. [DOI] [PubMed] [Google Scholar]
- Tamiz AP, Bandyopadhyay BC, Zhang J, et al. Pharmacological and behavioral analysis of the effects of some bivalent ligand-based monoamine reuptake inhibitors. J Med Chem. 2001;44:1615–1622. doi: 10.1021/jm000552s. [DOI] [PubMed] [Google Scholar]
- Tamiz AP, Zhang J, Zhang M, Wang CZ, Johnson KM, Kozikowski AP. Application of the bivalent ligand approach to the design of novel dimeric serotonin reuptake inhibitors. J Am Chem Soc. 2000;122:5393–5394. [Google Scholar]
- Thomsen M, Hall FS, Uhl GR, Caine SB. Dramatically decreased cocaine self-administration in dopamine but not serotonin transporter knock-out mice. J Neurosci. 2009;29:1087–1092. doi: 10.1523/JNEUROSCI.4037-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl−-dependent neurotransmitter transporters. Nature. 2005;437:215–223. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Zhen J, Karpowich NK, Goetz RM, Law CJ, Reith MEA, Wang DN. LeuT-desipramine structure reveals how antidepressants block neurotransmitter reuptake. Science. 2007;317:1390–1394. doi: 10.1126/science.1147614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimányi I, Lajtha A, Reith MEA. Comparison of characteristics of dopamine uptake and mazindol binding in mouse striatum. Naunyn-Schmiedeberg’s Arch Pharmacol. 1989;340:626–632. doi: 10.1007/BF00717737. [DOI] [PubMed] [Google Scholar]
- Zolkowska D, Jain R, Rothman RB, et al. Evidence for the Involvement of Dopamine Transporters in Behavioral Stimulant Effects of Modafinil. J Pharmacol Exp Ther. 2009;329:738–746. doi: 10.1124/jpet.108.146142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.