Abstract
For over a century, scientists have strived to understand the mechanisms that govern the accurate segregation of chromosomes during mitosis. The most intriguing feature of this process, which is particularly prominent in higher eukaryotes, is the complex behaviour exhibited by the chromosomes. This behaviour is based on specific and highly regulated interactions between the chromosomes and spindle microtubules. Recent discoveries, enabled by high-resolution imaging combined with the various genetic, molecular, cell biological and chemical tools, support the idea that establishing and controlling the dynamic interaction between chromosomes and microtubules is a major factor in genomic fidelity.
Mitosis is the shortest but most visually stunning phase of the cell cycle, particularly in metazoan cells, in which the mitotic apparatus and chromosomes can be followed directly by high-resolution microscopy. In these cells, the mitotic spindle — the macromolecular machine responsible for chromosome segregation — comprises over 800 proteins1. Structurally, the spindle is built from highly dynamic microtubules (BOX 1). Most of the less dynamic microtubule minus ends reside near the poles of the spindle, whereas the more dynamic plus ends extend towards the spindle equator and the cortex of the cell (FIG. 1). As a result, microtubules between the spindle poles are organized into an antiparallel array, and microtubules outside of the spindle body form two radial asters that converge on the spindle poles.
Box 1 | Microtubule dynamics in the spindle.
Microtubules are dynamic polymers that are assembled from tubulin heterodimers, which are organized such that the microtubules have an intrinsic polarity (see the figure, part a). Microtubules undergo periods of polymerization and depolymerization and interconvert randomly between these states, a property known as dynamic instability. Although microtubules exhibit dynamic instability at both ends of the microtubule, the plus ends are more dynamic than the minus ends. Microtubules in the spindle also exhibit dynamic instability (see the figure, part b), which occurs primarily at the plus ends of the microtubules as the minus ends are often capped at the centrosome. However, spindle microtubules and kinetochore (k)-fibres exhibit an additional dynamic property, known as microtubule flux, in which there is a net addition of tubulin heterodimers at the plus ends near the kinetochores and a net loss of tubulin subunits at the minus ends near the centrosomes (see the figure, part c).
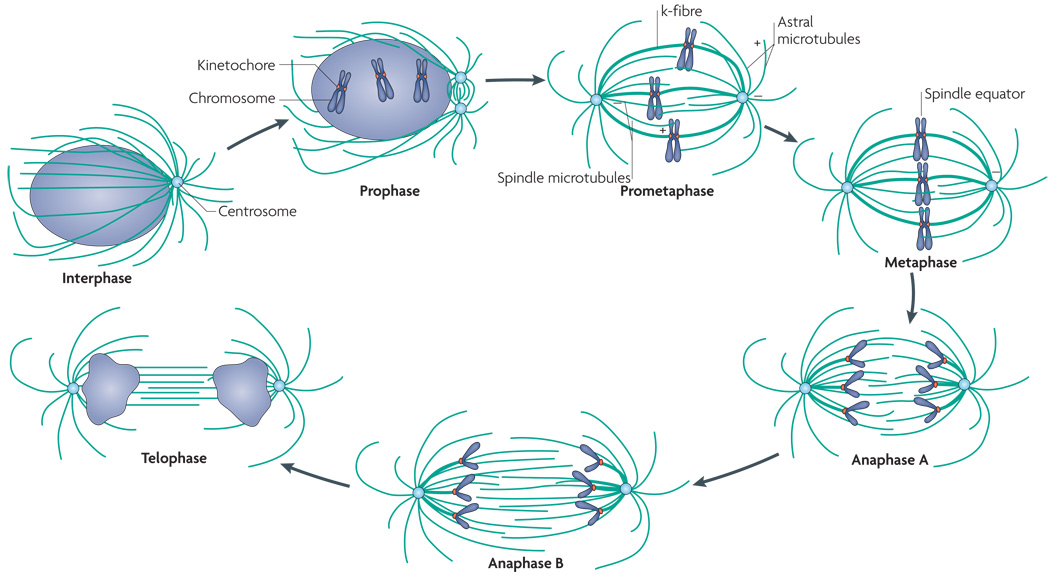
Figure 1. Structure of the mitotic spindle.
Mitosis can be staged into individual phases. In interphase, most of the chromatin is decondensed in the nucleus so that individual chromosomes cannot be seen, and the microtubules are organized in a radial array from the centrosome. During prophase, the chromosomes become highly condensed, and the centrosomes begin to separate. Nuclear envelope breakdown manifests the transition between prophase and prometaphase so that the individual chromosomes are no longer constrained in the nucleus. During prometaphase, kinetochore (k)-fibres (bundles of stabilized microtubules) connect the spindle microtubules and the kinetochores on the chromosomes, such that the chromosome can align at the spindle equator, which defines metaphase. The microtubules are uniformly oriented with their minus ends at the centrosome and their plus ends extending towards the spindle equator, where they often overlap. The astral microtubules emanate from the centrosomes and extend their plus ends towards the cell cortex. The movement of the chromosomes towards the poles occurs during anaphase A, and the two spindle poles separate during anaphase B. The nuclear envelope begins to reform and the DNA begins to decondense during telophase. An organized central spindle bundle of microtubules is also present. Cytokinesis divides the cytoplasm of the cell so that the two daughter nuclei are segregated into individual cells, which enter interphase and begin the process again.
The key interaction between chromosomes and microtubules occurs at the centromere of the chromosome, which contains two identical macromolecular complexes termed kinetochores. Kinetochores attach the chromosome to a subset of spindle microtubules called the kinetochore microtubules, which are organized into bundles known as kinetochore fibres (k-fibres). Challenges for a cell are ensuring that the spindle microtubules attach properly to each of the many chromosomes (46 in humans), positioning them at the spindle equator and ultimately driving the poleward movement of sister chromatids to the opposite poles of the spindle. The segregation of multiple chromosomes in a cell must be executed in perfect synchrony, which implies that the state of every chromosome is monitored by the spindle and that mitotic exit is delayed until all chromosomes are attached. This monitoring is achieved through a pathway termed the spindle assembly checkpoint2. Finally, given the complexity of the system, sporadic errors in chromosome attachment to the spindle microtubules are inevitable. Failure to correct these errors results in chromosome missegregation, a condition that underlies chromosomal instability, which is a hallmark of aggressive malignancies3,4. How the spindle integrates all of its tasks remains unclear. However, significant progress has been made recently in understanding the mechanisms that drive the complicated movements of chromosomes on the spindle.
The process of mitosis is divided into distinct phases that are defined largely by the organization and behaviour of the chromosomes (FIG. 1; see Supplementary information S1 (movie)). During prophase the chromosomes become progressively condensed inside the nucleus. In parallel, microtubule nucleation at centrosomes increases fivefold from the level seen during interphase5 and microtubules become more dynamic6. Nuclear envelope breakdown marks the transition between prophase and prometaphase, during which the attachment of the microtubules to the chromosomes begins. During prometaphase, chromosomes exhibit a complex pattern of movement that is often described as ‘the dance of chromosomes’ in classic cytology literature. Some chromosomes move poleward while other chromosomes move away from the spindle poles and others remain relatively motionless. Over time, these seemingly chaotic movements result in the congression of chromosomes to the spindle equator, such that more and more chromosomes become aligned between the separated spindle poles. Congression of the last chromosome marks the transition to the next stage of mitosis, metaphase — the stage at which all chromosomes are aligned at the spindle equator. Shortly after metaphase alignment, the cohesion between sister chromatids is broken, and the cell enters anaphase. At this stage, new daughter chromosomes move poleward (anaphase A) and the poles separate from each other (anaphase B). During the next stage, telophase, the chromosomes decondense as the nuclear envelopes reform around the two daughter nuclei. The cell is divided in two by cytokinesis, but the sister cells remain connected by a thin bridge termed the midbody. Finally, abscission of the midbody results in the complete separation of the two daughter cells.
Although the goal of mitosis — equal segregation of chromosomes — is achieved during anaphase, it is becoming increasingly clear that the proper attachment and alignment of the chromosomes, which occurs during prometaphase, is the defining aspect of mitosis that holds the key to understanding the fidelity of genome inheritance. In this Review, we combine a historical perspective with current ideas regarding mechanisms of chromosome behaviour during mitosis. Our focus is not on the detailed molecular mechanisms of spindle assembly, kinetochore architecture or checkpoint signalling, which have been covered extensively in several excellent recent reviews2,7–9. Instead, we present key findings that guide current thinking in the field and provide our perspective of the challenges that remain for the future. It should be noted that although we focus primarily on mitosis in metazoa, most of the principle components of the molecular machinery that drives cell division are common among different phyla.
Chromosome attachments on the spindle
The crucial region on chromosomes for interaction with the mitotic spindle is at the kinetochores. Proper chromosome segregation requires that both kinetochores on each chromosome attach to spindle micro tubules. Furthermore, chromosomes must be bi-oriented, with sister kinetochores connected to the opposite spindle poles. This highlights the need for a cell to monitor how attachments are made, and to sense whether attachments are made correctly and correct any that are not.
Kinetochores mediate chromosome attachment
The architecture of the kinetochore has been characterized in detail by classic electron microscopy in chemically fixed samples. These studies first defined the kinetochore as a trilaminar plate-like structure with electron-opaque outer and inner plates separated by an electron-translucent middle layer10 (FIG. 2a). On the outer plate of an unattached kinetochore is a fibrous corona, which is proposed to form the major site for microtubule capture by the kinetochore11,12. More recent analyses of the kinetochore structure, which employed high-pressure freezing instead of chemical fixation, revealed that the microtubules terminate in a mat of lightly stained material at the centromeric heterochromatin rather than in a distinct trilaminar plate, suggesting that the plate-like structure may be a consequence of chemical fixation13. In this mat of material, individual connections to the microtubule ends seem to be distinct in attached versus unattached kinetochores14, suggesting that attachments of microtubules are correlated with structural changes at the kinetochore.
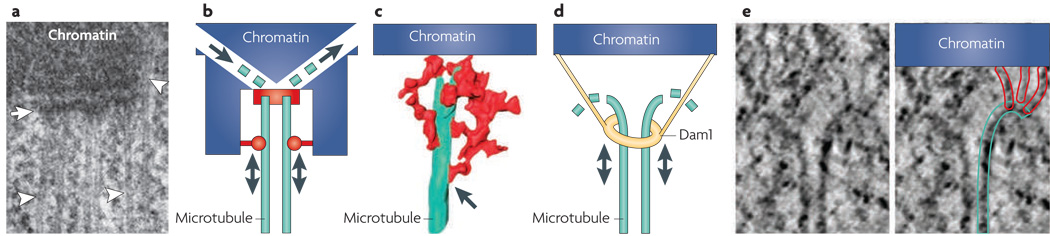
Figure 2. Organization of the kinetochore–microtubule interface.
a | An electron microscopy section (100 nm) of a chemically fixed PtK1 cell. The kinetochore appears as a trilaminar plate (arrow) adjacent to the chromatin. Several microtubules (arrowheads) form a prominent bundle that terminates in the kinetochore outer plate. The ends of some microtubules penetrate deeper into the chromatin (right arrowhead). b | In the ‘Hill sleeve’ model31 the microtubule–kinetochore interaction occurs by two distinct attachments: the links responsible for force generation connect the kinetochore with the wall of the microtubules (double arrows), and the complexes responsible for the regulation of microtubule dynamics interact directly with the tip of the microtubule (single arrows). c | An electron microscopy tomography reconstruction of the kinetochores in mammalian cells also indicates that there are two distinct attachment points: the tip of the microtubule is embedded in a radial mesh in the kinetochore outer plate (red) and a set of dense fibres extends out to bind the microtubule walls (arrow). d | In this view, a ring formed by oligomerization of Dam1 complexes encircles the end of a microtubule. The ring is capable of limited movement along the microtubule lattice but cannot easily detach from the tip of the microtubule. e | An electron microscopy tomography reconstruction of the microtubule end at the kinetochore interface, depicting a set of fibrils connecting the kinetochore to the inner surface of microtubule protofilaments. One wall of the microtubule (cyan) and the fibrils (red) are colour-traced for clarity. Image in part c is modified, with permission, from Nature Cell Biology REF. 14 © (2007) Macmillan Publishers Ltd. All rights reserved. Image in part e is reproduced, with permission, from Cell REF. 42 © (2008) Elsevier.
The past 10 years have seen a big increase in our understanding of the molecular identity of the key proteins that direct kinetochore assembly. The kinetochore contains several highly conserved protein complexes, such as the MIS12, KNL and NDC80 complexes, that all have a distinct role in the hierarchy of kinetochore assembly8,9. Despite substantial variations among the DNA sequence of centromeres in different organisms15, the protein complexes that assemble on this centromeric heterochromatin are remarkably conserved throughout evolution. This suggests that a unique fundamental architecture exists, which is advantageous for maintaining a strong but dynamic linkage between microtubules and kinetochores.
Geometry of kinetochore–microtubule attachments
To be distributed equally between the daughter cells, chromosomes must establish proper ‘amphitelic’ attachments to spindle microtubules, whereby one kinetochore attaches to microtubules emanating from one spindle pole, while the sister kinetochore attaches to the microtubules from the opposite spindle pole (BOX 2). Achieving amphitelic attachments usually involves intermediate steps. Initially, kinetochores associate laterally with microtubules so that the kinetochore outer plate is parallel to the microtubule lattice. This lateral association is subsequently replaced with a proper end-on microtubule attachment. Owing to a lack of coordination between the attachments of sister kinetochores, many chromosomes undergo a transient ‘monotelic’ state, in which only one of the sister kinetochores interacts with microtubules in an end-on manner. There are also two types of erroneous kinetochore attachments that intermittently occur during spindle assembly. In one, the ‘syntelic’ state, both sister kinetochores interact with microtubules that emanate from the same spindle pole. In the second, the ‘merotelic’ state, a single kinetochore simultaneously connects to both spindle poles. Syntelic and merotelic attachments must be resolved by special correction mechanisms or they will result in chromosome missegregation16–19.
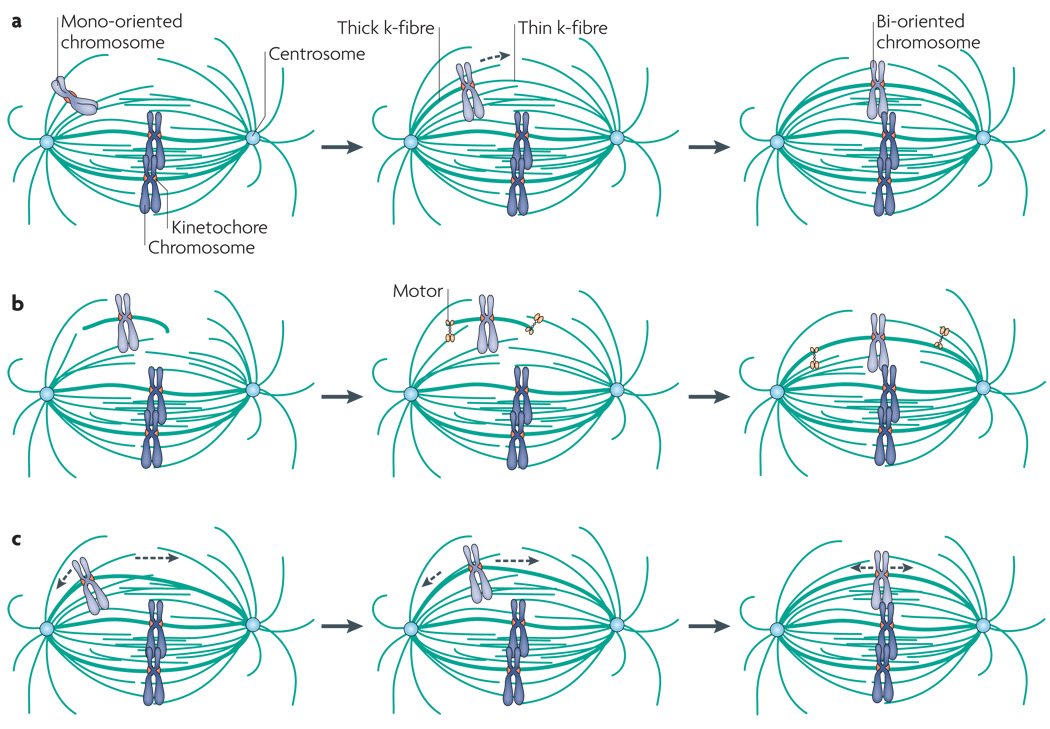
Box 2 | Kinetochore–microtubule attachments.
During mitosis, chromosomes are attached by their kinetochores to the microtubules of the spindle. In an ‘amphitelic’ attachment, the kinetochore (k)-fibre bundles of microtubules attach each sister kinetochore to opposite spindle poles (see part a in the figure). Establishing amphitelic attachments and bi-orientation of all chromosomes is the goal of mitotic spindle assembly. Mono-oriented chromosomes can either be attached in an end-on manner to a single spindle pole (‘monotelic’; see part b in the figure, left chromosome) or be laterally attached to a microtubule (see part b in the figure, right chromosome). A ‘syntelic’ attachment is a state in which both sister kinetochores are erroneously attached to a single spindle pole (see part c in the figure). If not corrected, a syntelic attachment will result in the segregation of both sister chromatids to a single daughter cell. In a ‘merotelic’ attachment, a single kinetochore is attached to microtubules extending from both spindle poles (see part d in the figure). At anaphase onset, a merotelic attachment will lead to lagging chromatids being left behind at the spindle equator and can lead to aneuploidy.
It was originally proposed that kinases were involved in the error correction process because treatment of cells with kinase inhibitors perturbed chromosome movements, resulting in an inaccurate chromosome distribution20. The correction mechanisms are now known to involve the Aurora B kinase, which regulates the activity of multiple key players that help mediate proper microtubule attachment. It has been postulated that phosphorylation of NDC80 regulates the plus end dynamics of kinetochore microtubules21,22, which allows Aurora B to modulate the strength of the microtubule attachment. In addition, Aurora B phosphorylation of mitotic centromere-associated kinesin (MCAK; also known as KIF2C) regulates the association of MCAK with kinetochores and centromeres, and inhibits the microtubule depolymerization activity of MCAK23–25. The ability of Aurora B to phosphorylate kinetochore proteins depends on the geometry of the centromere. In an amphitelic configuration sister kinetochores are pulled towards the opposite spindle poles, which spatially separates Aurora B from its substrates26. In contrast, erroneous attachments do not stretch the centromere, allowing Aurora B to destabilize k-fibre microtubules. These recent studies are important because they allow us to put some of the classic micro-manipulation experiments looking at the role of tension at the centromere and error correction into a molecular perspective27. There are also additional mechanisms that regulate microtubule stability, such as the presence of a gradient of Aurora B kinase28. Together, these mechanisms ensure that improper attachments are swiftly corrected, while proper attachments are maintained.
Kinetochores attach to dynamic microtubules
Although k-fibres are less dynamic than astral microtubules, kinetochore-associated microtubule ends still undergo cycles of polymerization and depolymerization (BOX 1). Even slight deviations in k-fibre microtubule dynamics result in chromosomal instability29,30. Thus, kinetochores have to maintain stable attachments to microtubule ends that are loose enough to be able to adjust to the changing state of the microtubule ends. Conceptually, this apparent controversy was solved by Hill, who postulated that individual microtubules in the kinetochore are encircled by ‘sleeves’ with multiple dynamic linkages between the kinetochore and the microtubule31. In this organization, force-generating tethers connect the sleeve with the wall of the microtubule, leaving the microtubule tip accessible for the complexes responsible for the regulation of micro tubule dynamics (FIG. 2b). A recent high-resolution electron microscopy tomography study confirmed the existence of two distinct fibrous connections between the kinetochore and k-fibre microtubules14. One set of fibres directly encircles the tip of the microtubule and another set of fibres attach to the microtubule wall (FIG. 2c). Intriguingly, the two types of connections do not seem to form a discrete micro tubule-binding site. Instead, the organization of the outer plate of the kinetochore resembles a spider’s web, which allows the kinetochore to interact with micro tubules at various angles.
The exact molecular nature of the tethers between kinetochores and the microtubule lattice has not been established. Originally, it was hypothesized that the tethers were formed by motor proteins, which couple the energy of ATP hydrolysis to force production32,33. More recently, the NDC80 complex emerged as a candidate for establishing the kinetochore–microtubule interface because it has both microtubule- and kinetochore-binding sites34–37. The NDC80–microtubule interactions seem to be sufficiently strong to maintain connections with microtubule ends under the weight of an associated chromosome. In addition, NDC80 was shown to processively track the depolymerizing microtubule ends38, providing a potential molecular basis for Hill’s sleeve model, in which the dynamic NDC80 linkage slides the kinetochore along a microtubule. One interesting correlation is that the size and the angle of the fibres that approach the microtubule lattice in the electron microscopy tomography reconstruction of the k-fibre14 match the size and the angle of the NDC80 hooks that decorate microtubules in vitro37,39, providing evidence that NDC80 might be the primary microtubule linker at the kinetochore.
An alternative view of dynamic kinetochore– microtubule attachments emerged from studies of the Saccharomyces cerevisiae Dam1 (also known as DASH) complex40. In vitro assemblies of the Dam1 complex form a ring-like structure that surrounds the microtubule and stays associated with dynamic microtubule ends41 (FIG. 2d). This is an enticing model because it eliminates the need for force-generating connections from the Hill model. Instead, depolymerizing microtubules provide the energy that is necessary to move the chromosome33,42 and the Dam1 ring acts as a ratchet, allowing the kinetochore to track the plus end of the microtubule. Compatible with this idea, it has been shown that minus end-directed molecular motors are not essential for chromosome movements in S. cerevisiae43. Furthermore, the Dam1 complex is not essential when minus end-directed molecular motors are present44,45. It should be noted that Dam1 is poorly conserved in higher eukaryotes, and disruption of putative mammalian orthologues (spindle and kinetochore associated 1 (SKA1), SKA2 and SKA3 (also known as RAMA1)) has relatively mild effects on chromosome segregation46–48. Thus, it is likely that Dam1 represents an adaptation for the unique S. cerevisiae situation, in which each kinetochore attaches to a single microtubule49,50. This type of attachment probably requires a specialized type of linkage in addition to that provided by the NDC80 complex and/or by other conserved kinetochore components. Finally, it is noteworthy that so far no ring-like structures have been visualized in kinetochores in situ, even in S. cerevisiae. Thus, it is likely that non-encircling fibres of Dam1 oligomers, instead of complete rings, are responsible for the coupling between microtubules and kinetochores51. Indeed, a recent study identified thin fibrils that extend from the kinetochore to the inner lattice of microtubule ends52. It will be interesting to establish the chemical nature of these fibrils, as they highlight the fact that the link between kinetochores and micro tubule ends is likely to be more complex than had originally been envisioned (FIG. 2e).
In vertebrate cells, a typical k-fibre comprises 20–25 microtubules53, which means that transient loss of connection between the kinetochore and each individual microtubule would not result in the detachment of the entire k-fibre. However, the multiplicity of microtubules in the k-fibre also implies the existence of a mechanism that coordinates polymerization and depolymerization of these bundled microtubules. The nature of this mechanism remains unclear. Somewhat surprisingly, a recent structural study found a mixture of microtubule end conformations that were thought to represent polymerizing and depolymerizing microtubules in individual k-fibres54. This result suggests that the dynamics of individual microtubules in a k-fibre are not strictly coordinated. However, an alternative explanation is that, in contrast to the in vitro situation, the structural conformation of the microtubule plus end in the k-fibre does not correlate with the polymerization or depolymerization state.
Forces at chromosomes and kinetochores
The multiplicity of interactions between chromosomes and microtubules correlates with the various forces that drive chromosome movements during mitosis. Some of these forces act on chromosome arms but most are generated at the kinetochore. Our current knowledge regarding the nature of these forces comes from live cell observations and from in vitro chromosome motility assays, in which the direct measurement of force is more readily achievable. Mathematical modelling has also provided additional insight into how forces at chromosomes and at kinetochores contribute to chromosome motility. Below, we briefly outline the forces that act on chromosomes before delving into their underlying molecular mechanisms.
Forces associated with chromosome arms
Mazia once compared the role of chromosome arms during mitosis with that of a corpse during a funeral: “They provide the reason for the proceedings but do not take an active part in them.”55 However, this elegant comparison was an overstatement as recent data suggest that forces acting on chromosome arms strongly influence chromosome behaviour. In vertebrates, any fragment of a chromosome that lacks a kinetochore is inevitably expelled from the spindle by the action of a force called the polar wind56. The molecular nature of this force remains largely unknown (see discussion below); however, laser microsurgery experiments show that it is sufficiently strong to deliver a chromosome arm from the vicinity of the spindle pole to the spindle equator57. Furthermore, the pattern of the centromere movements probably changes when the arms are severed58,59.
Forces in kinetochore–microtubule dynamics
Kinetochore activities are essential for the poleward movement of chromosomes. High-resolution differential interference contrast analyses reveal that the kinetochore residing on the front of the moving chromosome, called the leading kinetochore, is often stretched, as if the chromosome is pulled by the kinetochore60. Consistent with this observation, severing the centromere between the sister kinetochores with a laser beam results in the leading kinetochore continuing to move poleward while the trailing kinetochore stops moving57.
Intriguingly, the force generated at the kinetochore does not seem to depend on the number of microtubules in a k-fibre bundle. In fact, the number of microtubules attached to the leading kinetochore can be smaller or larger than the number on the trailing kinetochore53. Thus, directionality of the chromosome movement is determined by complex mechanisms that regulate the coupling of kinetochores to dynamic microtubules. It is also likely that the forces associated with chromosome arms (that is, the polar wind) either directly influence chromosome behaviour or indirectly influence the activity of kinetochores61.
Forces associated with laterally attached kinetochores
Although establishing an end-on microtubule–kinetochore attachment is traditionally viewed as a prerequisite for proper chromosome behaviour, it has become clear that many types of chromosome movements can be supported by lateral interactions between kinetochores and microtubules. One movement that specifically requires kinetochores to become laterally associated with astral microtubules is the rapid poleward gliding exhibited by some chromosomes during prometaphase62. Lateral interactions can also move chromosomes towards the spindle equator during congression63,64. This mode of congression is predominant during meiosis65,66. Of relevance here is that lateral kinetochore interactions support both poleward (towards microtubule minus ends) and anti-poleward (towards microtubule plus ends) movements. In both cases, the force is likely to be generated by molecular motors that are bound to the kinetochore64,67.
The multiplicity of forces that act on a chromosome raises the important question of what dictates the particular pathway of movement for any given chromosome. A related question is how chromosomes integrate different mechanisms during a particular type of movement; for example, during chromosome congression. More definitive answers to these questions lie in the development of better markers and imaging systems to track the motility of kinetochores, as well as more sophisticated means of monitoring the behaviour of individual chromosomes and kinetochores.
Mechanisms of chromosome congression
Although the exact trajectory of each chromosome in the process of spindle formation during prometaphase is unique, these complex behaviours ultimately result in chromosome congression — the alignment of chromosomes at the spindle equator. Here, chromosomes remain stably ‘bi-oriented’ (simultaneously connected to both spindle poles) until anaphase. Thus, congression involves both establishing proper amphitelic kinetochore attachments and the alignment of chromosomes at the spindle equator. Unfortunately, chromosome congression is also a perfect example of a ‘chicken and egg’ scenario, with no clear indication of whether bi-orientation is necessary for congression or if chromosome congression promotes bi-orientation. Several models have been proposed to explain the transition from monotelic mono-oriented to amphitelic bi-oriented chromosome attachments.
Search and capture model
In the classic search and capture model68 (FIG. 3a), monotelic chromosomes are expected to remain near one spindle pole until a micro-tubule emanating from the opposite spindle pole makes an attachment to the opposite sister kinetochore. The now bi-oriented chromosome moves towards the spindle equator by the action of microtubule depolymerization at the leading kinetochore, perhaps coupled to the action of kinetochore-associated microtubule motors such as centromere-associated protein E (CENPE)69,70 and cytoplasmic dynein67. A key feature of this model is that an amphitelic attachment is essential to the congression process. Although the model is very intuitive, computer simulations suggest that the probability of the unattached kinetochore on a mono-oriented chromosome becoming bi-oriented is extremely low. Thus, congression of the 46 human chromosomes would require several hours71. However, in vivo, chromosomes become fully congressed in 10–15 minutes, which implies that other pathways contribute to the process.
Figure 3. Congression models for chromosome bi-orientation.
a | A mono-oriented chromosome becomes bi-oriented when a microtubule from the opposite pole is captured by a kinetochore. As the chromosome begins to congress towards the spindle equator in the direction of the dashed arrow, the leading kinetochore is associated with a thinner kinetochore (k)-fibre, whereas the trailing kinetochore is associated with a thicker k-fibre. The chromosome then becomes aligned at the spindle equator. b | Microtubules are nucleated from the k-fibre (left). These k-fibre microtubules get incorporated into the spindle proper through the action of minus end-directed motors that slide the k-fibre along spindle microtubules. c | In the traction-fibre model, the position of the chromosome is dictated by the amount of force exerted on each k-fibre (indicated by the relative sizes of the dashed arrows). The forces on each sister kinetochore are balanced at the spindle equator.
Kinetochore-mediated k-fibre formation
Many classic cytologists believed that k-fibres originate directly at kinetochores72 rather than through the capture of centrosomal microtubules (FIG. 3b). In support of this, microtubules are seen at the chromosomes in vitro73 or after washout of microtubule depolymerizing drugs in vivo74,75. More recently, kinetochore-mediated formation of k-fibres and their subsequent incorporation into the rest of the mitotic spindle was seen in live cells76,77. It is likely that k-fibre formation is initiated when a kinetochore captures short, non-centrosomal microtubules that are nucleated in the vicinity of the centromere owing to the activity of the protein RAN78. Once captured, the plus ends of these microtubules begin to steadily polymerize inside the kinetochore, which pushes the naturally stable microtubule minus ends outwards. These minus ends eventually encounter other spindle microtubules and are transported poleward by a dynein-mediated mechanism. This transport of the k-fibre repositions the chromosome at the spindle equator. Thus, similar to the search and capture model, attachment of both kinetochores to k-fibres is a prerequisite for congression. However, kinetochore-mediated k-fibre formation seems to be more universal as it readily explains how k-fibres can be generated in cells lacking centrosomes, such as those in female meiosis in flies or in plant cells79,80.
A major question in either the search and capture model or the k-fibre nucleation model is to understand how and why chromosomes align at the spindle equator. One of the earliest models for chromosome congression, the traction fibre model, was put forward by Ostergren81, who postulated that k-fibres generate a poleward force that is proportional to their length. The forces of sister k-fibres should come to balance when the chromosome is positioned at the spindle equator (FIG. 3c). Although this idea has gained some experimental support in plants and invertebrates, there is little data to support the traction-fibre model in vertebrates82,83.
Kinetochore motors contribute to congression
Molecular motor-mediated gliding of unattached kinetochores alongside microtubule bundles provides a radical alternative to the congression models described above. Both plus end- and minus end-directed motors, such as CENPE and cytoplasmic dynein, associate with kinetochores, suggesting that motors could drive the movement of chromosomes either towards or away from the spindle equator84–86 (FIG. 4a,b). Indeed, in vitro reconstitution experiments showed that isolated chromosomes could move along in vitro polymerized microtubules, and the direction of movement seemed to be regulated by phosphorylation of unidentified proteins87. The finding that loss of the plus end-directed kinesin CENPE was associated with a failure in chromosome congression provided the first real evidence that motors drive chromosome motility69,70,86. More recently, CENPE was directly implicated in the movement of mono-oriented chromosomes to the spindle equator63,64. During this movement, the leading kinetochore is not attached to microtubules in the classic end-on manner but seems to be attached laterally, highlighting the importance of the dynamic linkage of kinetochores to microtubule ends. This study also shows that chromosome congression can precede the establishment of the amphitelic attachment.
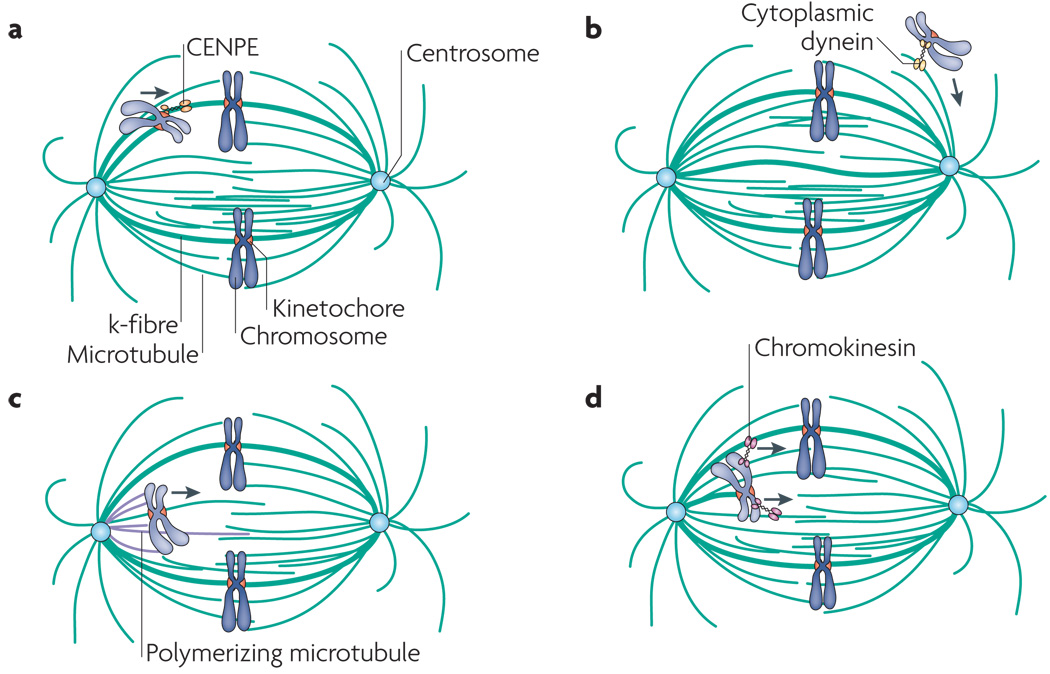
Figure 4. Congression models involving microtubule- and motor-based forces.
a | Plus end-directed kine to chore-associated motors, such as centromere-associated protein E (CENPE), have been implicated in chromosome movement towards the spindle equator. b | Cytoplasmic dynein, a minus end-directed motor, contributes to the movement of laterally associated kinetochores towards spindle poles during early mitosis. Although only laterally attached kinetochores are depicted, dynein can also contribute to the polewards movement of chromosomes, the microtubules of which are attached in an end-on manner. c | Forces associated with polymerizing microtubules could push a chromosome towards the spindle equator during congression. d | Motors, such as KID (also known as KIF22) or other chromokinesins, associated with chromosome arms could drag a chromosome towards the spindle equator.
The polar wind model
A complementary, but not necessarily mutually exclusive, model is one in which the polar wind contributes spatial cues that allow chromosomes to find the equator. An initial version of this idea was proposed in 1937 by Darlington, who suggested that repulsive forces generated by the spindle poles can push the chromosomes to the spindle equator88 (FIG. 4c). This was an incredibly insightful idea, given that it took nearly 50 years before the existence of a polar wind was directly shown by laser microsurgery experiments56. The molecular nature of this force remains unknown today. One possibility is that the polar wind is generated by the plus ends of astral microtubules mechanically pushing against the chromosomes. In agreement with this, depolymerization of astral microtubules by nocodazole or colcemid prevents expulsion of the severed chromosome arms from the spindle, whereas stabilization of microtubules by taxol drives chromosomes to the periphery of the astral array of microtubules89.
Another possibility is that the polar wind is derived from chromosome arm-associated motor proteins (FIG. 4d). The kinesin 10 family member, KID (also known as KIF22), is a plus end-directed motor that dynamically localizes on the chromosome arms90,91, where it can position the arms at the spindle equator92,93. whereas the effects on chromosome alignment on inhibition of KID are somewhat subtle in bipolar spindles91,94,95, loss of KID in mono-astral spindles results in an accumulation of chromosomes in the aster centre, suggesting that KID might be particularly important in cells with chromosomes that are not bi-oriented94. The Xenopus laevis kinesin 4 member kinesin-like protein 1 (KLP1; also known as KIF4) is also important for chromosome alignment96, but it is unclear if the failure to congress after KLP1 perturbation is owing to the need for KLP1 to interact with microtubules near the chromosomes96,97, the activity of KLP1 in the regulation of plus end microtubule dynamics98 or the role of KLP1 in chromosome condensation99. Finally, the observation that disruption of the polar wind by severing the chromosome arms alters kinetochore motility59 highlights the growing consensus that congression is not a singular event but rather an integration of numerous forces and activities that cooperate to carry out the important task of aligning chromosomes at the spindle equator.
Factors governing chromosome movement
The dramatic movements of chromosomes on the spindle are often characterized by the rates and direction of motility. We are just beginning to understand the complex activities that modulate chromosome behaviour.
Velocity of chromosome movement
Intuitively, it seems that quantitative descriptions of the velocity and directionality of chromosome motion should provide insights into the mechanisms underlying this process. However, kinetic analyses of chromosome behaviour have so far failed to reveal interrelationships between different activities of kinetochores, dynamic microtubules and chromosome arms. The velocity of both poleward and anti-poleward chromosome movements is remarkably constant for attached chromosomes, at ~ 2 µm min−1. This rate of motion is much slower than the rates of polymerization of microtubules in the spindle (11–13 µm min−1)100,101, suggesting that kinetochore attachment somehow governs the rate of chromosome motility. Likewise, microtubules depolymerize at ~17 µm min−1, faster than the rates of chromosome motility.
The velocities of chromosome motility are more consistent with the rates of motor proteins associated with kinetochores. For example, CENPE moves microtubules in vitro at ~1.8 µm min−1 (REF. 102), which is close to the measured values for chromosome motion towards the spindle equator (FIG. 4a). Similarly, cytoplasmic dynein is a fast microtubule minus end-directed motor, consistent with the observation that disruption of dynein at kinetochores leads to a loss of the initial rapid movements of laterally associated kinetochores towards spindle poles in early mitosis67 (FIG. 4b). Despite these observations, the motility of chromosomes cannot be due solely to these factors as knockdown of CENPE still allows the majority of chromosomes to align on the spindle during mitosis with apparently normal motility properties64,103. In addition, knockout of kinetochore dynein does not have much of an effect on the rates of chromosome motility during congression67. Together, these studies support the idea that chromosome motility during congression is a complicated mix of interactions between both micro tubule- and motor-based forces.
Chromosome directional instability
One remarkable aspect of chromosome motility is that chromosomes exhibit an oscillatory behaviour, whereby they move both towards and away from the spindle equator throughout all stages of mitosis. This oscillatory behaviour, termed directional instability60, was initially thought to result from coordinated polymerization dynamics on the two sister kinetochores, with the switch mechanism controlled by tension at each sister kinetochore60,104. However, mono-oriented chromosomes also exhibit oscillatory behaviour105, implying that oscillations can be supported by a single attached kinetochore.
Support for the idea that kinetochore microtubule dynamics contribute to kinetochore oscillations comes from several observations. Treatment of cells with low levels of the microtubule stabilizing agent taxol dampens chromosome oscillations89. In addition, knockdown and overexpression studies in human cells revealed that the kinesin 8 KIF18A contributes to the suppression of the amplitude of kinetochore oscillations by increasing the frequency by which kinetochores change direction106. These data are consistent with the idea that KIF18A acts to regulate the dynamics of the plus ends of kinetochore microtubules107,108. In addition, kinetochore-mediated attachments probably have at least some role in the oscillatory behaviour as disruption of the end-on attachments of microtubules results in diffusive chromosome motility109. Together, these studies support the notion that both microtubule attachments and dynamics at the kinetochore do indeed contribute to sister kinetochore oscillations.
However, not all forces for directional switching are associated with kinetochores. Compton and colleagues made the initially surprising observation that inhibition of the chromosomal arm-associated kinesin KID resulted in a suppression of chromosome oscillations94. In support of this, mathematical models predict a major contribution from forces associated with chromosome arms in directional instability110. The observation that perturbation of the polar ejection force by severing a chromosome arm results in an increased amplitude of the remaining kinetochores59 suggests that the forces on chromosome arms actually lead to tension at the leading kinetochore, which in turn affects the oscillatory behaviour61,111–113.
It is not currently clear whether the coordination of the two sister kinetochores is an important part of this behaviour. If regulated oscillations contribute to the process of congression the cell would undergo a tug-of-war between the kinetochores if their behaviour were not somehow coordinated. One potential player is the microtubule depolymerizing kinesin MCAK, which is important for sister kinetochore coordination during chromosome oscillations in prometaphase114. MCAK associates with centromeres of mitotic cells115, with higher levels of MCAK associated with the leading kinetochore114,116. Depletion of centromeric MCAK disrupted the coordinated behaviour of sister kinetochores114. This phenotype might be due to an increase in the stability of the k-fibre114,117, which prevents the plus ends of microtubules being optimally dynamic. However, chromosomes with unpaired kinetochores118 and individual kinetochores not attached to chromosome arms can efficiently congress onto the spindle equator119,120, suggesting that a tug-of-war between sister kinetochores is not a prerequisite for congression.
Mechanisms of chromosome segregation
Mitosis culminates in anaphase — the stage that affects segregation of the genetic material between the two daughter cells. Two types of characteristic movements occur during this stage. The sister chromatids disjoin and synchronously move poleward (anaphase A) and the spindle poles move away from one another (ana-phase B) (FIG. 1b). The timing and extent of the contributions of anaphase A and anaphase B to chromatid separation vary among different organisms. In most organisms, anaphase A and anaphase B are coincidental, although in some cells these phases can occur sequentially.
During anaphase A, chromosome movement must be synchronized with the depolymerization of k-fibre microtubules. In mammals, the bulk of this depolymerization occurs at the kinetochore121; although, in other organisms microtubule depolymerization at the poles can be significant122–124. Thus, mammalian kinetochores resemble the once popular video-game character Pac-Man, who chewed through the maze to get to the target. This property implies that kinetochores possess two precisely coordinated activities that are responsible for walking along the microtubule wall and simultaneously depolymerizing the microtubule plus end. It was shown early on that the motor for poleward chromosome motion was at the kinetochore125, and the best candidate motor is cytoplasmic dynein, which is associated with kinetochores84,85. However, trying to show a direct link between cytoplasmic dynein and anaphase A chromosome movements has been hampered by the fact that dynein functions in multiple aspects of mitosis, including centrosome separation, focusing of microtubule minus ends and in the mitotic checkpoint. Thus, global inhibition of dynein causes multiple defects in spindle assembly that can indirectly affect the rates of chromosome movement in anaphase67,126–129.
It has long been hypothesized that a microtubule depolymerase at the kinetochore would be associated with anaphase A microtubule depolymerization at the kinetochore. Identification of MCAK at centromeres115 first gave credence to this idea; however, disruption of MCAK causes defects in chromosome segregation that are probably due to improper attachment of chromosomes rather than to defects in the motility of chromo somes116,130. In Drosophila melanogaster embryos, inhibition of the kinesin 13 KLP59C correlates with a reduction in chromatid-to-pole movement131. However, a functional equivalent has yet to be identified in verte brate cells. Like kinetochore micro tubule depolymerization, spindle pole microtubule depolymerization has been associated with members of the kinesin 13 family131–133. In particular, it has been postulated that KLP10A in D. melanogaster and KIF2A in vertebrates are responsible for minus end depolymerization and anaphase flux131,133. How these activities are controlled in metazoa remains unclear.
One interesting question is whether the same activities that drive chromosome movement during congression also act during anaphase. It is important to note that although the bulk of chromosome movement during anaphase is poleward, the chromosomes do exhibit transient oscillatory movements, suggesting that some anti-poleward forces are still active60. Another interesting possibility is that proteins, such as those of the NDC80 complex, may act to couple kinetochores to depolymerizing microtubules, an idea that is yet to be explored. It is clear that both cytoplasmic dynein and multiple members of the kinesin 13 family act during both congression and anaphase. It will therefore be crucial to dissect which defects are due to a specific requirement of the protein in anaphase and which anaphase defects arise as a consequence of earlier defects in prometaphase. In addition, it will be important to determine whether microtubule depolymerization drives chromosome movement, and motors are used as couplers to stay attached to the depolymerizing microtubules, or whether the motors have a more direct role in the poleward movement of the chromosomes.
Conclusions and future directions
The studies reviewed here reveal the complex nature of chromosome movements. Traditionally, much attention was paid to chromosome segregation during anaphase, but now the mechanisms that ensure proper kinetochore attachments and chromosome congression have moved to the forefront of research in mitosis. It has become clear that chromosome congression is an extremely complex process that serves multiple purposes in cells. Congression is needed to align chromosomes at the spindle equator and to promote bi-orientation of chromosomes to the two spindle poles. Like many aspects of spindle function, congression seems to be a highly redundant process comprised of multiple independent mechanisms that contribute to the alignment of chromosomes. This redundancy is necessary because defects in the alignment of chromosomes lead to segregation errors that contribute to genomic instability.
One emerging theme is that although multiple mole cules have been implicated in the process of congression, not all congression defects are equivalent. For example, knockout of many proteins causes a defect in chromosome alignment, and the severity of the defect varies tremendously, implying that certain proteins are more central to the congression process than others. In addition, defects in the organization of the spindle itself contribute to defects in chromosome motility134. This suggests that it will be necessary to dissect which proteins have a direct role in the attachment and movement of the chromosomes, which proteins modulate the interaction or regulation of these proteins and which proteins are more likely to have an indirect role by altering mitotic spindle structure.
Such a complex problem will require a multidisciplinary approach to provide new insight. Clearly, the continued identification of important proteins and defining the mechanisms by which they work will provide fundamentally important information. However, there needs to be an even more sophisticated development of imaging technologies and analyses to be able to dissect chromosome and kinetochore behaviour in a more quantitative manner and without any user bias. A resurgence of biophysical approaches combined with the increased sophistication in technology will allow scientists to measure forces in both normal and perturbed systems. When combined with powerful biochemical reconstitution experiments, it may be possible to recapitulate more complex behaviours of microtubules and artificial kinetochores to understand force generation. Finally, the identification of additional players will allow for an increased complexity in the computational approaches that can be applied to uncover new ideas for force generation in the spindle, which can in turn be tested experimentally by the multiple approaches discussed above. One outcome can be guaranteed from these studies — mitosis will continue to fascinate biologists for the next century as it has done for the past 100 years.
Supplementary Material
Acknowledgements
The authors would like to thank all members of their laboratories for insightful discussions on chromosome motility. In particular, we are grateful to L. Weaver and J. Powers for their comments on the manuscript. Our research is supported by funding from the National Institutes of Health (GM59618 to C.E.W and GM59363 to A.K).
Glossary
- Centromere
A specialized thin region, often referred to as the ‘primary constriction’, on a chromosome. It is composed of highly condensed heterochromatin that serves as the platform for the assembly of kinetochores.
- Kinetochore
A structure that assembles on the mitotic chromosome to attach to microtubules of the spindle.
- K-fibre
A bundle of microtubules that connects to a kinetochore on a mitotic chromosome.
- Segregation
The process by which sister chromatids are distributed to two daughter cells.
- Spindle assembly checkpoint
A surveillance mechanism that monitors the attachment state of individual chromosomes. The checkpoint blocks anaphase onset until the kinetochores are attached to microtubules of the spindle.
- Centrosome
A specialized organelle at the spindle pole that is the site of microtubule nucleation.
- Congression
The process by which chromosomes align at the spindle equator.
- Bi-oriented
Here, used to describe the attachment of a chromosome to microtubules emanating from opposite spindle poles.
- Centromeric heterochromatin
Distinct regions of chromatin at the centromere of a chromosome that are highly condensed and contribute to specifying the location of kinetochore assembly.
- Astral microtubule
A microtubule that emanates from the spindle pole to the cell cortex.
- Mono-oriented
Here, used to describe the attachment of a chromosome to microtubules coming from a single spindle pole.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
DATABASES
UniProtK B: http://www.uniprot.org
Aurora B | CENPE | KID | KIF2A | KIF18A | KLP1 | KLP10A | KLP59C | MCAK | NDC80 | RAN | SKA1 | SKA2 | SKA3
FURTHER INFORMATION
Claire E. Walczak’s homepage: http://medsci.indiana.edu/medsci/research/walczak/walczaklab.htm
Alexey Khodjakov’s homepage: http://www.wadsworth.org/resnres/bios/khodjakov.htm
Mitosis world: http://www.bio.unc.edu/faculty/salmon/lab/mitosis/index.html
MitoCheck: http://www.mitocheck.org
ASCB image and video library: http://cellimages.ascb.org
References
- 1.Sauer G, et al. Proteome analysis of the human mitotic spindle. Mol. Cell. Proteomics. 2005;4:35–43. doi: 10.1074/mcp.M400158-MCP200. [DOI] [PubMed] [Google Scholar]
- 2.Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nature Rev. Mol. Cell Biol. 2007;8:379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- 3.Holland AJ, Cleveland DW. Boveri revisited: chromosomal instability, aneuploidy and tumorigenesis. Nature Rev. Mol. Cell Biol. 2009;10:478–487. doi: 10.1038/nrm2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rajagopalan H, Lengauer C. Aneuploidy and cancer. Nature. 2004;432:338–341. doi: 10.1038/nature03099. [DOI] [PubMed] [Google Scholar]
- 5.Kuriyama R, Borisy GG. Microtubule-nucleating activity of centrosomes in chinese hamster ovary cells is independent of the centriole cycle but coupled to the mitotic cycle. J. Cell Biol. 1981;91:822–826. doi: 10.1083/jcb.91.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saxton WM, et al. Tubulin dynamics in cultured mammalian cells. J. Cell Biol. 1984;99:2175–2186. doi: 10.1083/jcb.99.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walczak CE, Heald R. Mechanisms of mitotic spindle assembly and function. Int. Rev. Cytol. 2008;265:111–158. doi: 10.1016/S0074-7696(07)65003-7. [DOI] [PubMed] [Google Scholar]
- 8.Cheeseman IM, Desai A. Molecular architecture of the kinetochore-microtubule interface. Nature Rev. Mol. Cell Biol. 2008;9:33–46. doi: 10.1038/nrm2310. [DOI] [PubMed] [Google Scholar]
- 9.Santaguida S, Musacchio A. The life and miracles of kinetochores. EMBO J. 2009;28:2511–2531. doi: 10.1038/emboj.2009.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brinkley BR, Stubblefield E. The fine structure of the kinetochore of a mammalian cell in vitro. Chromosoma. 1966;19:28–43. doi: 10.1007/BF00332792. [DOI] [PubMed] [Google Scholar]
- 11.Jokelainen PT. The ultrastructure and spatial organization of the metaphase kinetochore in mitotic rat cells. J. Ultrastruct. Res. 1967;19:19–44. doi: 10.1016/s0022-5320(67)80058-3. [DOI] [PubMed] [Google Scholar]
- 12.Ris H, Witt PL. Structure of the mammalian kinetochore. Chromosoma. 1981;82:153–170. doi: 10.1007/BF00286101. [DOI] [PubMed] [Google Scholar]
- 13.McEwen BF, Hsieh CE, Mattheyses AL, Rieder CL. A new look at kinetochore structure in vertebrate somatic cells using high-pressure freezing and freeze substitution. Chromosoma. 1998;107:366–375. doi: 10.1007/s004120050320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dong Y, Vanden Beldt KJ, Meng X, Khodjakov A, McEwen BF. The outer plate in vertebrate kinetochores is a flexible network with multiple microtubule interactions. Nature Cell Biol. 2007;9:516–522. doi: 10.1038/ncb1576.. A high-resolution look at the ultrastructure of the kinetochore.
- 15.Vagnarelli P, Ribeiro SA, Earnshaw WC. Centromeres: old tales and new tools. FEBS Lett. 2008;582:1950–1959. doi: 10.1016/j.febslet.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 16.Cimini D, Moree B, Canman JC, Salmon ED. Merotelic kinetochore orientation occurs frequently during early mitosis in mammalian tissue cells and error correction is achieved by two different mechanisms. J. Cell Sci. 2003;116:4213–4225. doi: 10.1242/jcs.00716. [DOI] [PubMed] [Google Scholar]
- 17.Ganem NJ, Godinho SA, Pellman D. A mechanism linking extra centrosomes to chromosomal instability. Nature. 2009;460:278–282. doi: 10.1038/nature08136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Silkworth WT, Nardi IK, Scholl LM, Cimini D. Multipolar spindle pole coalescence is a major source of kinetochore mis-attachment and chromosome mis-segregation in cancer cells. PLoS One. 2009;4:e6564. doi: 10.1371/journal.pone.0006564.. References 17 and 18 make the important discovery that multipolarity leads to improper kinetochore attachments, which are the ultimate source of chromosome instability.
- 19.Thompson SL, Compton DA. Examining the link between chromosomal instability and aneuploidy in human cells. J. Cell Biol. 2008;180:665–672. doi: 10.1083/jcb.200712029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicklas RB, Krawitz LE, Ward SC. Odd chromosome movement and inaccurate chromosome distribution in mitosis and meiosis after treatment with protein kinase inhibitors. J. Cell Sci. 1993;104:961–973. doi: 10.1242/jcs.104.4.961. [DOI] [PubMed] [Google Scholar]
- 21.Cimini D, Wan X, Hirel CB, Salmon ED. Aurora kinase promotes turnover of kinetochore microtubules to reduce chromosome segregation errors. Curr. Biol. 2006;16:1711–1718. doi: 10.1016/j.cub.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 22.DeLuca JG, et al. Kinetochore microtubule dynamics and attachment stability are regulated by Hec1. Cell. 2006;127:969–982. doi: 10.1016/j.cell.2006.09.047. [DOI] [PubMed] [Google Scholar]
- 23.Andrews PD, et al. Aurora B regulates MCAK at the mitotic centromere. Dev. Cell. 2004;6:253–268. doi: 10.1016/s1534-5807(04)00025-5. [DOI] [PubMed] [Google Scholar]
- 24.Lan W, et al. Aurora B phosphorylates centromeric MCAK and regulates its localization and microtubule depolymerization activity. Curr. Biol. 2004;14:273–286. doi: 10.1016/j.cub.2004.01.055. [DOI] [PubMed] [Google Scholar]
- 25.Ohi R, Sapra T, Howard J, Mitchison TJ. Differentiation of cytoplasmic and meiotic spindle assembly MCAK functions by Aurora B-dependent phosphorylation. Mol. Biol. Cell. 2004;15:2895–2906. doi: 10.1091/mbc.E04-02-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu D, Vader G, Vromans MJ, Lampson MA, Lens SM. Sensing chromosome bi-orientation by spatial separation of Aurora B kinase from kinetochore substrates. Science. 2009;323:1350–1353. doi: 10.1126/science.1167000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicklas RB. How cells get the right chromosomes. Science. 1997;275:632–637. doi: 10.1126/science.275.5300.632. [DOI] [PubMed] [Google Scholar]
- 28.Fuller BG, et al. Midzone activation of Aurora B in anaphase produces an intracellular phosphorylation gradient. Nature. 2008;453:1132–1136. doi: 10.1038/nature06923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bakhoum SF, Genovese G, Compton DA. Deviant kinetochore microtubule dynamics underlie chromosomal instability. Curr. Biol. 2009;19:1937–1942. doi: 10.1016/j.cub.2009.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bakhoum SF, Thompson SL, Manning AL, Compton DA. Genome stability is ensured by temporal control of kinetochore-microtubule dynamics. Nature Cell Biol. 2009;11:27–35. doi: 10.1038/ncb1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hill TL. Theoretical problems related to the attachment of microtubules to kinetochores. Proc. Natl. Acad. Sci. USA. 1985;82:4404–4408. doi: 10.1073/pnas.82.13.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lombillo VA, Nislow C, Yen TJ, Gelfand VI, McIntosh JR. Antibodies to the kinesin motor domain and CENPE inhibit microtubule depolymerization-dependent motion of chromosomes in vitro. J. Cell Biol. 1995;128:107–115. doi: 10.1083/jcb.128.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lombillo VA, Stewart RJ, McIntosh JR. Minus-end-directed motion of kinesin-coated microspheres driven by microtubule depolymerization. Nature. 1995;373:161–164. doi: 10.1038/373161a0.. An elegant demonstration of the insight provided by in vitro reconstitution experiments.
- 34.Martin-Lluesma S, Stucke VM, Nigg EA. Role of Hec1 in spindle checkpoint signaling and kinetochore recruitment of Mad1/Mad2. Science. 2002;297:2267–2270. doi: 10.1126/science.1075596. [DOI] [PubMed] [Google Scholar]
- 35.DeLuca JG, et al. Nuf2 and Hec1 are required for retention of the checkpoint proteins Mad1 and Mad2 to kinetochores. Curr. Biol. 2003;13:2103–2109. doi: 10.1016/j.cub.2003.10.056. [DOI] [PubMed] [Google Scholar]
- 36.McCleland ML, et al. The highly conserved Ndc80 complex is required for kinetochore assembly, chromosome congression, and spindle checkpoint activity. Genes Dev. 2003;17:101–114. doi: 10.1101/gad.1040903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheeseman IM, Chappie JS, Wilson-Kubalek EM, Desai A. The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell. 2006;127:983–997. doi: 10.1016/j.cell.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 38.Powers AF, et al. The Ndc80 kinetochore complex forms load-bearing attachments to dynamic microtubule tips via biased diffusion. Cell. 2009;136:865–875. doi: 10.1016/j.cell.2008.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson-Kubalek EM, Cheeseman IM, Yoshioka C, Desai A, Milligan RA. Orientation and structure of the Ndc80 complex on the microtubule lattice. J. Cell Biol. 2008;182:1055–1061. doi: 10.1083/jcb.200804170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Westermann S, et al. Formation of a dynamic kinetochore–microtubule interface through assembly of the Dam1 ring complex. Mol. Cell. 2005;17:277–290. doi: 10.1016/j.molcel.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 41.Westermann S, et al. The Dam1 kinetochore ring complex moves processively on depolymerizing microtubule ends. Nature. 2006;440:565–569. doi: 10.1038/nature04409. [DOI] [PubMed] [Google Scholar]
- 42.Grishchuk EL, Molodtsov MI, Ataullakhanov FI, McIntosh JR. Force production by disassembling microtubules. Nature. 2005;438:384–388. doi: 10.1038/nature04132. [DOI] [PubMed] [Google Scholar]
- 43.Grishchuk EL, Spiridonov IS, McIntosh JR. Mitotic chromosome biorientation in fission yeast is enhanced by dynein and a minusenddirected, kinesin-like protein. Mol. Biol. Cell. 2007;18:2216–2225. doi: 10.1091/mbc.E06-11-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daum JR, et al. Ska3 Is required for spindle checkpoint silencing and the maintenance of chromosome cohesion in mitosis. Curr. Biol. 2009;19:1467–1472. doi: 10.1016/j.cub.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gaitanos TN, et al. Stable kinetochore-microtubule interactions depend on the Ska complex and its new component Ska3/C13Orf3. EMBO J. 2009;28:1442–1452. doi: 10.1038/emboj.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raaijmakers JA, Tanenbaum ME, Maia AF, Medema RH. RAMA1 is a novel kinetochore protein involved in kinetochore-microtubule attachment. J. Cell Sci. 2009;122:2436–2445. doi: 10.1242/jcs.051912. [DOI] [PubMed] [Google Scholar]
- 47.Liu X, McLeod I, Anderson S, Yates JR, 3rd, He X. Molecular analysis of kinetochore architecture in fission yeast. EMBO J. 2005;24:2919–2930. doi: 10.1038/sj.emboj.7600762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanchez-Perez I, et al. The DASH complex and Klp5/Klp6 kinesin coordinate bipolar chromosome attachment in fission yeast. EMBO J. 2005;24:2931–2943. doi: 10.1038/sj.emboj.7600761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peterson JB, Ris H. Electron-microscopic study of the spindle and chromosome movement in the yeast Saccharomyces cerevisiae. J. Cell Sci. 1976;22:219–242. doi: 10.1242/jcs.22.2.219. [DOI] [PubMed] [Google Scholar]
- 50.Winey M, et al. Three-dimensional ultrastructural analysis of the Saccharomyces cerevisiae mitotic spindle. J. Cell Biol. 1995;129:1601–1615. doi: 10.1083/jcb.129.6.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grishchuk EL, et al. The Dam1 ring binds microtubules strongly enough to be a processive as well as energy-efficient coupler for chromosome motion. Proc. Natl. Acad. Sci. USA. 2008;105:15423–15428. doi: 10.1073/pnas.0807859105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McIntosh JR, et al. Fibrils connect microtubule tips with kinetochores: a mechanism to couple tubulin dynamics to chromosome motion. Cell. 2008;135:322–333. doi: 10.1016/j.cell.2008.08.038.. A high-resolution look at the ultrastructure of the kinetochore–microtubule linkage.
- 53.McEwen BF, Heagle AB, Cassels GO, Buttle KF, Rieder CL. Kinetochore fiber maturation in PtK1 cells and its implications for the mechanisms of chromosome congression and anaphase onset. J. Cell Biol. 1997;137:1567–1580. doi: 10.1083/jcb.137.7.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. VandenBeldt KJ, et al. Kinetochores use a novel mechanism for coordinating the dynamics of individual microtubules. Curr. Biol. 2006;16:1217–1223. doi: 10.1016/j.cub.2006.04.046.. This paper reports the surprising finding that the distribution of microtubule-end states at the kinetochore are mixed on both leading and lagging kinetochores.
- 55.Mazia Din. In: The Cell. Brachet J, Mirsky AE, editors. Vol. 3. New York: Academic Press, Inc.; 1961. pp. 77–412. [Google Scholar]
- 56.Rieder CL, Davison EA, Jensen LCW, Cassimeris L, Salmon ED. Oscillatory movements of monooriented chromosomes and their position relative to the spindle pole result from the ejection properties of the aster and the half-spindle. J. Cell Biol. 1986;103:581–591. doi: 10.1083/jcb.103.2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Khodjakov A, Rieder CL. Kinetochores moving away from their associated pole do not exert a significant pushing force on the chromosome. J. Cell Biol. 1996;135:315–327. doi: 10.1083/jcb.135.2.315.. A classic cytology paper defining forces on kinetochores.
- 58.Rieder CL, Salmon ED. Motile kinetochores and polar ejection forces dictate chromosome position on the vertebrate mitotic spindle. J. Cell Biol. 1994;124:223–233. doi: 10.1083/jcb.124.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ke K, Cheng J, Hunt AJ. The distribution of polar ejection forces determines the amplitude of chromosome directional instability. Curr. Biol. 2009;19:807–815. doi: 10.1016/j.cub.2009.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Skibbens R, Skeen VP, Salmon ED. Directional instability of kinetochore motility during chromosome congression and segregation in mitotic newt lung cells: a push-pull mechanism. J. Cell Biol. 1993;122:859–875. doi: 10.1083/jcb.122.4.859.. The first paper to define the features of kinetochore directional instability.
- 61.Kapoor TM, Compton DA. Searching for the middle ground: mechanisms of chromosome alignment during mitosis. J. Cell Biol. 2002;157:551–556. doi: 10.1083/jcb.200202073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rieder CL, Alexander S. Kinetochores are transported polewards along a single astral microtubule during chromosome attachment to the spindle in newt lung cells. J. Cell Biol. 1990;110:81–95. doi: 10.1083/jcb.110.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cai S, O’Connell CB, Khodjakov A, Walczak CE. Chromosome congression in the absence of kinetochore fibres. Nature Cell Biol. 2009;11:832–838. doi: 10.1038/ncb1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kapoor TM, et al. Chromosomes can congress to the metaphase plate before biorientation. Science. 2006;311:388–391. doi: 10.1126/science.1122142.. This seminal paper redefined ideas on how chromosomes congress to the spindle equator.
- 65.Wignall SM, Villeneuve AM. Lateral microtubule bundles promote chromosome alignment during acentrosomal oocyte meiosis. Nature Cell Biol. 2009;11:839–844. doi: 10.1038/ncb1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brunet S, et al. Kinetochore fibers are not involved in the formation of the first meiotic spindle in mouse oocytes, but control the exit from the first meiotic M phase. J. Cell Biol. 1999;146:1–12. doi: 10.1083/jcb.146.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang Z, Tulu US, Wadsworth P, Rieder CL. Kinetochore dynein is required for chromosome motion and congression independent of the spindle checkpoint. Curr. Biol. 2007;17:973–980. doi: 10.1016/j.cub.2007.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kirschner MW, Mitchison TJ. Beyond self assembly: from microtubules to morphogenesis. Cell. 1986;45:329–342. doi: 10.1016/0092-8674(86)90318-1. [DOI] [PubMed] [Google Scholar]
- 69.Schaar BT, Chan GKT, Maddox P, Salmon ED, Yen TJ. CENPE function at kinetochores is essential for chromosome alignment. J. Cell Biols. 1997;139:1373–1382. doi: 10.1083/jcb.139.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wood KW, Sakowicz R, Goldstein LSB, Cleveland DW. CENPE is a plus end-directed kinetochore motor required for metaphase chromosome alignment. Cell. 1997;91:357–366. doi: 10.1016/s0092-8674(00)80419-5. [DOI] [PubMed] [Google Scholar]
- 71. Wollman R, et al. Efficient chromosome capture requires a bias in the ‘search-and-capture’ process during mitotic-spindle assembly. Curr. Biol. 2005;15:828–832. doi: 10.1016/j.cub.2005.03.019.. An elegant example of how mathematical modelling can contribute to our understanding of chromosome motility.
- 72.Schrader F. Mitosis. Columbia University Press; 1953. [Google Scholar]
- 73.Telzer BR, Moses MJ, Rosenbaum JL. Assembly of microtubules onto kinetochores of isolated mitotic chromosomes of HeLa cells. Proc. Natl. Acad. Sci. USA. 1975;72:4023–7402. doi: 10.1073/pnas.72.10.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Debrabander M, et al. Microtubule dynamics during the cell cycle: the effects of taxol and nocodazole on the microtubule system of PtK2 cells at different stages of the mitotic cycle. Int. Rev. Cytology. 1986;101:215–273. doi: 10.1016/s0074-7696(08)60250-8. [DOI] [PubMed] [Google Scholar]
- 75.Witt PL, Ris H, Borisy GG. Origin of kinetochore microtubules in Chinese hamster ovary cells. Chromosoma. 1980;81:483–505. doi: 10.1007/BF00368158. [DOI] [PubMed] [Google Scholar]
- 76. Khodjakov A, Copenagle L, Gordon MB, Compton DA, Kapoor TM. Minus-end capture of preformed kinetochore fibers contributes to spindle morphogenesis. J. Cell Biol. 2003;160:671–683. doi: 10.1083/jcb.200208143.. This paper was the first to truly challenge the search and capture model of chromosome congression.
- 77.Maiato H, Rieder CL, Khodjakov A. Kinetochore-driven formation of kinetochore fibers contributes to spindle assembly during animal mitosis. J. Cell Biol. 2004;167:831–840. doi: 10.1083/jcb.200407090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tulu US, Fagerstrom C, Ferenz NP, Wadsworth P. Molecular requirements for kinetochore-associated microtubule formation in mammalian cells. Curr. Biol. 2006;16:536–541. doi: 10.1016/j.cub.2006.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lambert AM, Lloyd CWin. In: Microtubules. Hyams JS, Lloyd CW, editors. New York: Wiley-Liss; 1994. pp. 325–342. [Google Scholar]
- 80.McKim KS, Hawley RS. Chromosomal control of meiotic cell division. Science. 1995;270:1595–1601. doi: 10.1126/science.270.5242.1595. [DOI] [PubMed] [Google Scholar]
- 81.Ostergren G. The mechanism of coordination in bivalents and multivalents. The theory of orientation by pulling. Hereditas. 1951;37:85–156. [Google Scholar]
- 82.Hays T, Salmon ED. Poleward force at the kinetochore in metaphase depends on the number of kinetochore microtubules. J. Cell Biol. 1990;110:391–404. doi: 10.1083/jcb.110.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hays TS, Wise D, Salmon ED. Traction force on a kinetochore at metaphase acts as a linear function of kinetochore fiber length. J. Cell Biol. 1982;93:374–389. doi: 10.1083/jcb.93.2.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pfarr CM, et al. Cytoplasmic dynein is localized to kinetochores during mitosis. Nature. 1990;345:263–265. doi: 10.1038/345263a0. [DOI] [PubMed] [Google Scholar]
- 85.Steuer ER, Wordeman L, Schroer TA, Sheetz MP. Localization of cytoplasmic dynein to mitotic spindles and kinetochores. Nature. 1990;345:266–268. doi: 10.1038/345266a0. [DOI] [PubMed] [Google Scholar]
- 86.Yen TJ, et al. CENPE, a novel human centromere-associated protein required for progression from metaphase to anaphase. EMBO J. 1991;10:1245–1254. doi: 10.1002/j.1460-2075.1991.tb08066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hyman AA, Mitchison TJ. Tw o different microtubule-based motor activities with opposite polarites in kinetochores. Nature. 1991;351:206–211. doi: 10.1038/351206a0. [DOI] [PubMed] [Google Scholar]
- 88.Darlington C. Recent Advances in Cytology. London: Churchill: 1937. [Google Scholar]
- 89. Ault JG, DeMarco AJ, Salmon ED, Rieder CL. Studies on the ejection properties of asters: astral microtubule turnover influences the oscillatory behavior and positioning of mono-oriented chromosomes. J. Cell Sci. 1991;99:701–710. doi: 10.1242/jcs.99.4.701.. The first paper to clearly show the polar ejection force.
- 90.Tokai N, et al. Kid, a novel kinesin-like DNA binding protein, is localized to chromosomes and the mitotic spindle. EMBO J. 1996;15:457–467. [PMC free article] [PubMed] [Google Scholar]
- 91.Yajima J, et al. The human chromokinesin Kid is a plus end-directed microtubule-based motor. EMBO J. 2003;22:1067–1074. doi: 10.1093/emboj/cdg102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Antonio C, et al. Xkid, a chromokinesin required for chromosome alignment on the metaphase plate. Cell. 2000;102:425–435. doi: 10.1016/s0092-8674(00)00048-9. [DOI] [PubMed] [Google Scholar]
- 93.Funabiki H, Murray AW. The Xenopus chromokinesin Xkid is essential for metaphase chromosome alignment and must be degraded to allow anaphase chromosome movement. Cell. 2000;102:411–424. doi: 10.1016/s0092-8674(00)00047-7. [DOI] [PubMed] [Google Scholar]
- 94.Levesque AA, Compton DA. The chromokinesin Kid is necessary for chromosome arm orientation and oscillation, but not congression, on mitotic spindles. J. Cell Biol. 2001;154:1135–1146. doi: 10.1083/jcb.200106093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tokai-Nishizumi N, Ohsugi M, Suzuki E, Yamamoto T. The chromokinesin Kid is required for maintenance of proper metaphase spindle size. Mol. Biol. Cell. 2005;16:5455–5463. doi: 10.1091/mbc.E05-03-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vernos I, et al. Xklp1, a chromosomal Xenopus kinesin-like protein essential for spindle organization and chromosome positioning. Cell. 1995;81:117–127. doi: 10.1016/0092-8674(95)90376-3. [DOI] [PubMed] [Google Scholar]
- 97.Walczak CE, Vernos I, Mitchison TJ, Karsenti E, Heald R. A model for the proposed roles of different microtubule based motor proteins in establishing spindle bipolarity. Curr. Biol. 1998;8:903–913. doi: 10.1016/s0960-9822(07)00370-3. [DOI] [PubMed] [Google Scholar]
- 98.Bringmann H, et al. A kinesin-like motor inhibits microtubule dynamic instability. Science. 2004;303:1519–1522. doi: 10.1126/science.1094838. [DOI] [PubMed] [Google Scholar]
- 99.Mazumdar M, Sundareshan S, Misteli T. Human chromokinesin KIF4A functions in chromosome condensation and segregation. J. Cell Biol. 2004;166:613–620. doi: 10.1083/jcb.200401142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Piehl M, Cassimeris L. Organization and dynamics of growing microtubule plus ends during early mitosis. Mol. Biol. Cell. 2003;14:916–925. doi: 10.1091/mbc.E02-09-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rusan NM, Fagerstrom CJ, Yvon AM, Wadsworth P. Cell cycle-dependent changes in microtubule dynamics in living cells expressing green fluorescent protein-α tubulin. Mol. Biol. Cell. 2001;12:971–980. doi: 10.1091/mbc.12.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim Y, Heuser JE, Waterman CM, Cleveland DW. CENPE combines a slow, processive motor and a flexible coiled coil to produce an essential motile kinetochore tether. J. Cell Biol. 2008;181:411–419. doi: 10.1083/jcb.200802189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McEwen BF, et al. CENPE is essential for reliable bioriented spindle attachment, but chromosome alignment can be achieved via redundant mechanisms in mammalian cells. Mol. Biol. Cell. 2001;12:2289–2776. doi: 10.1091/mbc.12.9.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Skibbens R, Rieder CL, Salmon ED. Kinetochore motility after severing between sister chromosomes using laser microsurgery: evidence that kinetochore directional instability and position is regulated by tension. J. Cell Sci. 1995;108:2537–2548. doi: 10.1242/jcs.108.7.2537. [DOI] [PubMed] [Google Scholar]
- 105.Rieder CL, Davison EA, Jensen LC, Cassimeris L, Salmon ED. Oscillatory movements of monooriented chromosomes and their position relative to the spindle pole result from the ejection properties of the aster and half-spindle. J. Cell Biol. 1986;103:581–591. doi: 10.1083/jcb.103.2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stumpff J, von Dassow G, Wagenbach M, Asbury C, Wordeman L. The kinesin-8 motor Kif18A suppresses kinetochore movements to control mitotic chromosome alignment. Dev. Cell. 2008;14:252–262. doi: 10.1016/j.devcel.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gupta ML, Jr, Carvalho P, Roof DM, Pellman D. Plus end-specific depolymerase activity of Kip3, a kinesin8 protein, explains its role in positioning the yeast mitotic spindle. Nature Cell Biol. 2006;8:913–923. doi: 10.1038/ncb1457. [DOI] [PubMed] [Google Scholar]
- 108.Varga V, et al. Yeast kinesin-8 depolymerizes microtubules in a length-dependent manner. Nature Cell Biol. 2006;8:957–962. doi: 10.1038/ncb1462. [DOI] [PubMed] [Google Scholar]
- 109.DeLuca JG, et al. Hec1 and Nuf2 are core components of the kinetochore outer plate essential for organizing microtubule attachment sites. Mol. Biol. Cell. 2005;16:519–531. doi: 10.1091/mbc.E04-09-0852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Joglekar AP, Hunt AJ. A simple, mechanistic model for directional instability during mitotic chromosome movements. Biophys. J. 2002;83:42–58. doi: 10.1016/S0006-3495(02)75148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu J, Desai A, Onuchic JN, Hwa T. An integrated mechanobiochemical feedback mechanism describes chromosome motility from prometaphase to anaphase in mitosis. Proc. Natl. Acad. Sci. USA. 2008;105:13752–13757. doi: 10.1073/pnas.0807007105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Civelekoglu-Scholey G, Sharp DJ, Mogilner A, Scholey JM. Model of chromosome motility in Drosophila embryos: adaptation of a general mechanism for rapid mitosis. Biophys. J. 2006;90:3966–3982. doi: 10.1529/biophysj.105.078691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mogilner A, Wollman R, Civelekoglu-Scholey G, Scholey J. Modeling mitosis. Trends Cell Biol. 2006;16:88–96. doi: 10.1016/j.tcb.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 114.Wordeman L, Wagenbach M, von Dassow G. MCAK facilitates chromosome movement by promoting kinetochore microtubule turnover. J. Cell Biol. 2007;179:789–869. doi: 10.1083/jcb.200707120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wordeman L, Mitchison TJ. Identification and partial characterization of mitotic centromere-associated kinesin, a kinesin-related protein that associates with centromeres during mitosis. J. Cell Biol. 1995;128:95–104. doi: 10.1083/jcb.128.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kline-Smith SL, Khodjakov A, Hergert P, Walczak CE. Depletion of centromeric MCAK leads to chromosome congression and segregation defects due to improper kinetochore attachments. Mol. Biol. Cell. 2004;15:1146–1159. doi: 10.1091/mbc.E03-08-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rizk RS, et al. MCAK and paclitaxel have differential effects on spindle microtubule organization and dynamics. Mol. Biol. Cell. 2009;20 doi: 10.1091/mbc.E08-09-0985. 1639-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Khodjakov A, Cole RW, McEwen BF, Buttle KF, Rieder CL. Chromosome fragments possessing only one kinetochore can congress to the spindle equator. J. Cell Biol. 1997;136:229–240. doi: 10.1083/jcb.136.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.O’Connell CB, Loncarek J, Kalab P, Khodjakov A. Relative contributions of chromatin and kinetochores to mitotic spindle assembly. J. Cell Biol. 2009;187:43–51. doi: 10.1083/jcb.200903076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wise DA, Brinkley BR. Mitosis in cells with unreplicated genomes (MUGs): spindle assembly and behavior of centromere fragments. Cell. Motil. Cytoskeleton. 1997;36:291–302. doi: 10.1002/(SICI)1097-0169(1997)36:3<291::AID-CM9>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 121.Gorbsky GJ, Sammak PJ, Borisy GG. Chromosomes move poleward in anaphase along stationary microtubules that coordinately disassemble from their kinetochore ends. J. Cell Biol. 1987;104:9–18. doi: 10.1083/jcb.104.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Brust-Mascher I, Scholey JM. Microtubule flux and sliding in mitotic spindles of Drosophila embryos. Mol. Biol. Cell. 2002;13:3967–3975. doi: 10.1091/mbc.02-05-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Desai A, Maddox PS, Mitchison TJ, Salmon ED. Anaphase A chromosome movement and poleward spindle microtubule flux occur at similar rates in Xenopus extract spindles. J. Cell Biol. 1998;141:703–713. doi: 10.1083/jcb.141.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Maddox P, Desai A, Oegema K, Mitchison TJ, Salmon ED. Poleward microtubule flux is a major component of spindle dynamics and anaphase a in mitotic Drosophila embryos. Curr. Biol. 2002;12:1670–1674. doi: 10.1016/s0960-9822(02)01183-1. [DOI] [PubMed] [Google Scholar]
- 125.Nicklas RB. The motor for poleward chromosome movement in anaphase is in or near the kinetochore. J. Cell Biol. 1989;109:2245–2255. doi: 10.1083/jcb.109.5.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Echeverri CJ, Paschal BM, Vaughan KT, Vallee RB. Molecular characterization of the 50kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J. Cell Biol. 1996;132:617–633. doi: 10.1083/jcb.132.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Goshima G, Nedelec F, Vale RD. Mechanisms for focusing mitotic spindle poles by minus end-directed motor proteins. J. Cell Biol. 2005;171:229–240. doi: 10.1083/jcb.200505107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sharp DJ, Rogers GC, Scholey JM. Cytoplasmic dynein is required for poleward chromosome movement during mitosis in Drosophila embryos. Nature Cell Biol. 2000;2:922–930. doi: 10.1038/35046574. [DOI] [PubMed] [Google Scholar]
- 129.Vorozhko VV, Emanuele MJ, Kallio MJ, Stukenberg PT, Gorbsky GJ. Multiple mechanisms of chromosome movement in vertebrate cells mediated through the Ndc80 complex and dynein/dynactin. Chromosoma. 2008;117:169–179. doi: 10.1007/s00412-007-0135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Maney T, Hunter AW, Wagenbach M, Wordeman L. Mitotic centromere-associated kinesin is important for anaphase chromosome segregation. J. Cell Biol. 1998;142:787–801. doi: 10.1083/jcb.142.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rogers GC, et al. Two mitotic kinesins cooperate to drive sister chromatid separation during anaphase. Nature. 2004;427:364–370. doi: 10.1038/nature02256. [DOI] [PubMed] [Google Scholar]
- 132.Buster DW, Zhang D, Sharp DJ. Poleward tubulin flux in spindles: regulation and function in mitotic cells. Mol. Biol. Cell. 2007;18:3094–3104. doi: 10.1091/mbc.E06-11-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ganem NJ, Upton K, Compton DA. Efficient mitosis in human cells lacking poleward microtubule flux. Curr. Biol. 2005;15:1827–1832. doi: 10.1016/j.cub.2005.08.065. [DOI] [PubMed] [Google Scholar]
- 134.Gordon MB, Howard L, Compton DA. Chromosome movement in mitosis requires microtubule anchorage at spindle poles. J. Cell Biol. 2001;152:425–434. doi: 10.1083/jcb.152.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.