Abstract
p21-activated protein kinases (Paks) are cytosolic serine/threonine protein kinases that act as effectors for small (p21) GTPases of the Cdc42 and Rac families. It has long been established that Paks play a major role in a host of vital cellular functions such as proliferation, survival and motility, and abnormal Pak function is associated with a number of human diseases. Here, we discuss emerging evidence that these enzymes also play a major role in the entry, replication and spread of many important pathogenic human viruses, including HIV. Careful assessment of the potential role of Paks in antiviral immunity will be pivotal to evaluate thoroughly the potential of agents that inhibit Pak as a new class of anti-viral therapeutics.
Paks – multitalented effectors of signaling by small GTPases
For a pathogenic human virus to infect cells, replicate and disseminate, it must exploit the signaling machinery of the host. The first step, virus entry and uncoating, often involves the subversion of a host cell-surface protein as a virus receptor, with subsequent internalization mediated by virus-induced changes in the actin and tubulin cytoskeleton of the infected cell [1]. Many viruses also find ways to activate cell survival pathways and to alter host transcriptional programs to prevent premature apoptosis of infected cells. Following replication and reassembly of virus particles, viruses again often borrow the actin and/or tubulin polymerization machinery from the host to ensure successful exit from the cell. Whereas a large and diverse set of host signaling proteins is involved in these processes, emerging evidence indicates that Pak serine/threonine kinases are central to the life cycles of several important pathogenic viruses.
Paks are a family of serine/threonine-specific protein kinases that act as downstream effectors of the small GTPases Cdc42 and Rac, mediating an important subset of the signaling activities of these enzymes, and aberrant Pak signaling has long been associated with a number of human diseases (Box 1). The Pak family comprises six proteins that can be conceptually divided into two subfamilies based on their distinctive structures and biochemical features: group I (Pak1, −2 and −3) and group II (Pak4, −5 and −6) (Figure 1). Tissue distribution varies significantly between different Pak family members. Pak1 is expressed in brain, spleen and muscle, Pak2 and Pak4 are ubiquitously expressed, Pak3 and Pak5 are mainly expressed in the brain [2,3], and Pak6 is expressed predominantly in the testis, prostate, placenta, brain and kidney [4].
Box 1. Group I Paks and human diseases.
Aberrant Pak function is associated with a number of diseases in man, in particular cancer and mental retardation. Whereas the genes encoding Paks are rarely mutated in cancer, elevated Pak1 levels are commonly seen in cancers of the breast, ovary, brain, and bladder, usually in association with amplification of the 11q13.5 chromosomal locus [103]. Inactivating mutations in the PAK3 gene are the cause of a human non-syndromal X-linked mental retardation syndrome, and Pak dysfunction might also contribute to Fragile X syndrome and Alzheimer disease [104]. There is also increasing evidence that Paks play an important role in particular subsets of leukocytes. For example, mast cells from Pak1-null mice mature normally but have severe motility and degranulation defects, and Pak1-null macrophages have migration defects [12,13].
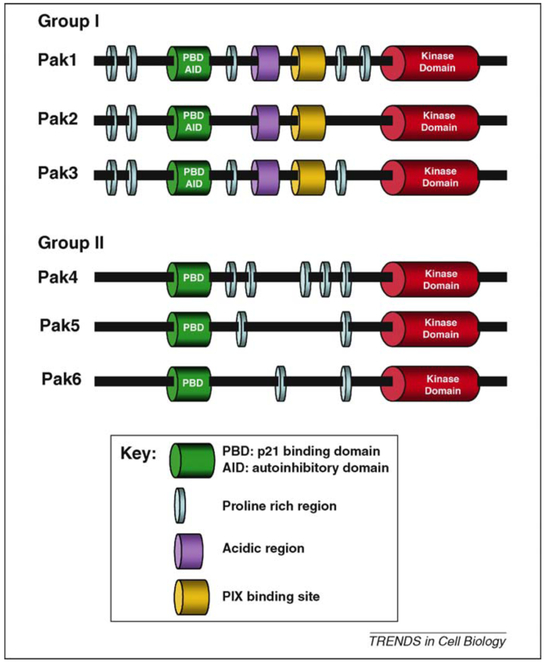
Figure 1.
Structural comparison of group I and group II Paks. Group I Pak members share a high degree of homology. The N-terminal part constitutes the regulatory region and contains the p21-binding domain that overlaps with the autoinhibitory domain. The C terminal part has a kinase domain that is more than 90% conserved among Pak I members. Group II Paks have the same domains, but they lack the autoinhibitory domain. The kinase domain of group II Paks is less conserved than that of group I Pak members.
Of the two subfamilies, group I Paks are better understood both in terms of regulation and function. Major cellular functions regulated by group I Paks include proliferation, survival and motility. All three of these functions are likely to impact upon various aspects of virus–host interactions. Less is known about the functions of group II Paks. In general, these enzymes are thought to play similar roles to group I Paks, but their actions are probably mediated by different substrates [5–7]. Paks are best known for their effects on the cytoskeleton, playing a key role in the dynamic reorganization of F-actin and tubulin associated with cell polarization and movement and, importantly with respect to virus entry, in macropinocytosis, a specialized form of endocytosis in which invaginations of the plasma membrane capture extracellular fluid and molecules [8]. Pak activation leads to loss of stress fibers and focal adhesion complexes, and, depending on the cell type, induces the formation of polarized lamellipodia, weakening of cell–cell adhesion, and increased cell motility [9–11]. Conversely, loss of Pak1 leads to defects in lamellipodia formation and stability, and decreased motility [12,13]. These effects result from Pak-mediated phosphorylation of several key regulators of the cytoskeleton – these include guanine nucleotide exchange factors (GEFs), GTPase activating proteins (GAPs) and GDP dissociation inhibitors (GDIs), proteins that directly affect actin filament polymerization or severing, as well as myosin light chain kinase and regulators of microtubule dynamics [9,14,15].
Apoptosis represents an important normal Pak function that may be subverted by various pathogenic viruses. Pak1 increases cell survival by directly phosphorylating the pro-apoptotic Bad or by phosphorylating Raf1 and disrupting the binding between Raf1 and Bad [15]. Pak1 also interacts with and phosphorylates dynein light chain 1 and BimL, triggering their degradation and blocking the pro-apoptotic signal of BimL [16]. The relationship between Pak2 activity and cell survival is more complicated. On the one hand, activation of Pak2, as with Pak1, promotes cell survival by phosphorylating Bad and Bcl-2 (Ref. [17]). On the other hand, apoptotic stimuli such as DNA damage lead to caspase-mediated cleavage of Pak2, generating a p34 fragment (Figure 2). This highly active form of Pak2 leads to extensive membrane blebbing, cytoplasmic shrinkage, and apoptosis [18–20]. The mechanisms underlying these effects are not completely understood, but Pak2-p34 activates Jnk and inactivates Mnk, leading to inactivation of suppressors of the mitochondrial-dependent death pathway and reduced translation, respectively [19,21].
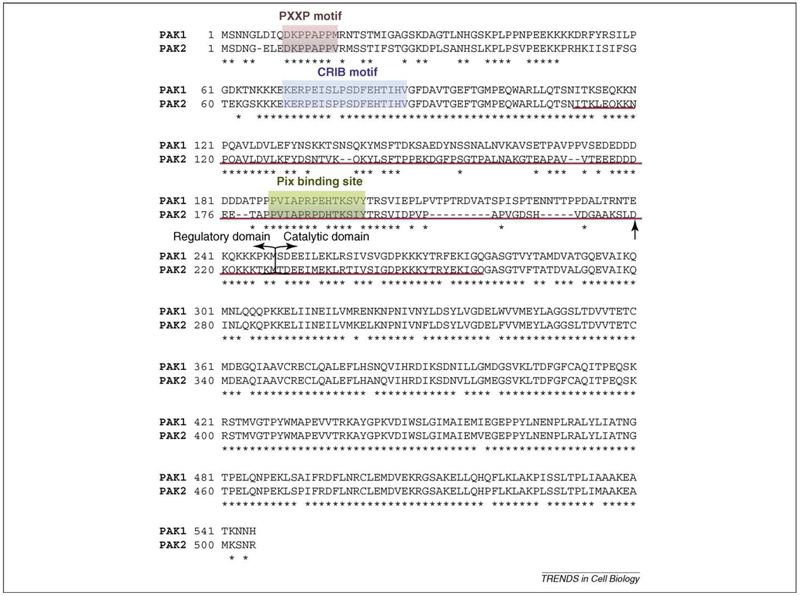
Figure 2.
Pak1 and Pak2 amino acid sequence alignment. Conserved residues are marked by stars. The PxxP motif is responsible for binding of the adaptor protein Nck, whereas the CRIB motif is responsible for binding Cdc42. Delimitation between the regulatory and the catalytic region is shown by brackets. The arrow indicates the aspartic acid at position 212 that identifies the caspase cleavage site in Pak2, but not in Pak1. Binding of Nef to Pak is not dependent on the presence of the PxxP motif, Pix binding site, CRIB motif or the caspase cleavage site within Pak2. The Pak sequence important for Nef binding is underlined in red.
The role(s) of Pak in cell cycle progression are also complex. In most cell types, group I Paks are pro-proliferative, and overexpression of wild-type or activated mutants of these kinases, in particular Pak1, is associated with tumorigenicity both in vitro and in vivo. There are likely to be many signaling pathways that contribute to these effects. In the G1 phase of the cell cycle, Pak1-mediated activation of Erk (via activating phosphorylation of c-Raf and Mek) and NF-κB lead to increased transcription of cyclin D1. Later in the cell cycle Pak1 is recruited to the centrosome where it is activated, leading to phosphorylation of polo-like kinase I and Aurora A kinase, two important proteins that regulate mitosis through their effects on centrosome maturation, mitotic entry and cytokinesis [22]. Pak1 also plays a role in the early phases of mitosis, when it phosphorylates histone H3 and promotes condensation of the chromosomes [23]. During mitosis, Pak1 is phosphorylated by cyclin-B1–Cdc2 and loses binding partners that have functions in cell division [24,25]. However, as in cell survival signaling, Pak2 could play a contrary role to Pak1. Overexpression of Pak2 is cytostatic when overexpressed in COS7 and 293T cells, mediated at least in part by phosphorylation of eIF4 g that inhibits cap-dependent translation [26].
Because Paks regulate many of the same cell biological processes that are fundamental to virus entry, reproduction, and egress, it is perhaps not surprising that several viruses have been reported to co-opt Pak functions to gain access to cells and to influence the behavior of infected cells. It is becoming apparent, however, that different viruses exploit different aspects of Pak function to achieve these ends, and that individual Pak isoforms might play unique roles in virus biology. In this review, we will discuss how a variety of important human pathogenic viruses interact with Paks, how these interactions contribute to infectivity, and why understanding these processes might yield therapeutic benefits.
Virus interactions with Paks
Increasing evidence indicates that members of several evolutionarily distinct virus families interact with Paks at one or multiple stages of their replication cycle. The vast majority of these interactions promote virus replication, spread, and/or immune evasion, although there is also evidence for an antiviral role of Paks. The following subsections will give a comprehensive overview of our current knowledge on interactions of different viruses with Paks, and how they appear to benefit the virus or the host.
Human immunodeficiency virus (HIV)
The Nef (negative factor) protein of HIV is a key factor in the pathogenesis of AIDS [27,28]. Nef is a multifunctional protein that is expressed very early upon infection of a host cell and is thought to influence virus replication, spread, and immune evasion through association with host signaling proteins.
In 1994, Sawai and coworkers found that, in T-lymphocytes, the Nef protein of HIV-1 associates with a cellular serine kinase, termed Nef-associated kinase (NAK) [29]. Soon thereafter, evidence from several researchers aiming to identify NAK pointed to p21-activated kinases, although it remained unclear which Pak isoform(s) was/were involved [28,30,31]. Currently, the general view is that Nef can associate with different Pak isoforms, with Pak2 being the typical isoform (Box 2) (Refs [32,33]).
Box 2. Nef, NAK, and Pak.
Nef is a protein that is crucial to HIV pathogenesis. Nef associates with a ~65 kDa serine/threonine protein kinase, operationally termed NAK, that has been identified by various groups as either Pak1 or Pak2. Which one is it, and does it matter? The answer seems to be ‘both, depending on context’, and ‘probably yes’. Using specific antisera against different Pak isoforms, Renkema and colleagues identified NAK as being Pak2 and confirmed these results by demonstrating NAK cleavage by caspase 3, a property unique to Pak2 among the Pak family [105]. Arora and coworkers further confirmed these results by demonstrating that Nef mediates robust activation of Pak2 but not Pak1 [106]. One study, however, identified NAK as being Pak1, based on an N-terminal antibody that preferentially recognizes Pak1 and by use of a Pak1 inhibitory peptide, although the authors later discovered that this peptide might also inhibit Pak2 [33,107]. Further stirring the controversy, knock-down of different endogenous Paks identified Pak1 as the main Pak isoform involved in HIV infection in one study, with Pak3, but not Pak2, also being involved, whereas another study identified Pak2 as the main Pak isoform activated by Nef using a similar approach [32,33]. The controversy about the Pak isoform is probably due to extensive homology between both isoforms (Figure 2) and possibly to Nef allele specificity, and is an important issue given the different signaling properties of these two kinases.
Thus, the particular type of Nef, or the cellular context, might determine whether it binds Pak1 or Pak2. Because Pak1 and Pak2 have different roles in cell motility, survival, and proliferation, the recruitment of Pak1 versus Pak2 might have consequences for HIV pathobiology.
The mechanism by which Nef activates Pak2 is poorly understood. Association of Nef with Pak2 is conserved in HIV and simian immunodeficiency virus (SIV), but Pak2 activation is less well conserved [34–36]. Nef is thought to activate Pak2 through a multiprotein complex – a 1 MDa signalosome [33]. Assembly of this complex occurs at cellular membranes, more specifically in detergent-resistant membrane microdomains known as lipid rafts [35,37].
In addition to Nef and Pak2, the complex contains phosphoinositide 3-kinase (PI3K), the small Rho-GTPases Cdc42 and Rac1, as well as a factor providing GEF activity for Rac1/Cdc42 (Refs [30,31,38]). The nature of this GEF has also been a matter of debate. Three different GEFs have been suggested to be associated with Nef: Vav, DOCK2/ELMO and β-PIX [39–41]. A recent study, using a variety of approaches, suggests that Vav is the GEF that is involved in the functional Nef–Pak2 interaction (Figure 3) and that β-PIX and DOCK2/ELMO are dispensible for Nef-mediated Pak2 activation [33].
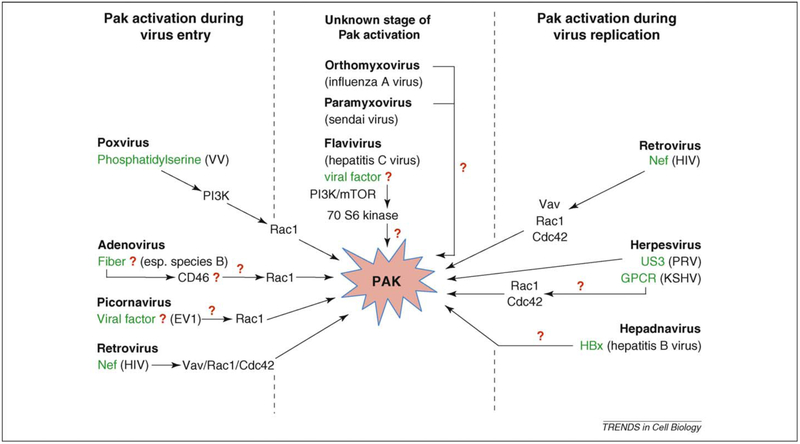
Figure 3.
Virus-mediated activation of Pak. The figure shows the different virus families, and if known the virus protein and downstream signaling cascades, that lead to Pak activation at some point during the virus replication cycle. The best-studied viral activation of Paks takes place in HIV infection where Paks are activated by the virus Nef protein via a Nef–Vav–Rac1–Cdc42 complex. Pak, p21-activated kinase; PI3K, phosphoinositide 3-kinase; Hbx, Hepatitis B virus X protein; GPCR, G-protein-coupled receptor; VV, vaccinia virus; EV1, echovirus 1; HIV, human immunodeficiency virus; PRV, pseudorabies virus; KSHV, Kaposi’s sarcoma-associated herpesvirus.
Nef protein has been implicated in a plethora of functions, and some could be attributed directly to the Nef-mediated activation of Paks (Figure 4). It seems reasonable to assume that there is a significant biological role for Pak2 in Nef-mediated functions because the Nef–Pak2 interaction is conserved among different groups of lentiviruses [28,42,43]. However, there is some dispute about the role of the Nef–Pak2 association in AIDS pathogenesis. In favor of a functional role in HIV-related disease, infection of rhesus macaques with a SIV expressing a Nef protein that is unable to activate Pak was found to lead to strong selective pressure in favor of wild-type virus [28,44]. However, other studies found that the association of Nef with Paks is not an absolute prerequisite for the development of AIDS [42,45,46].
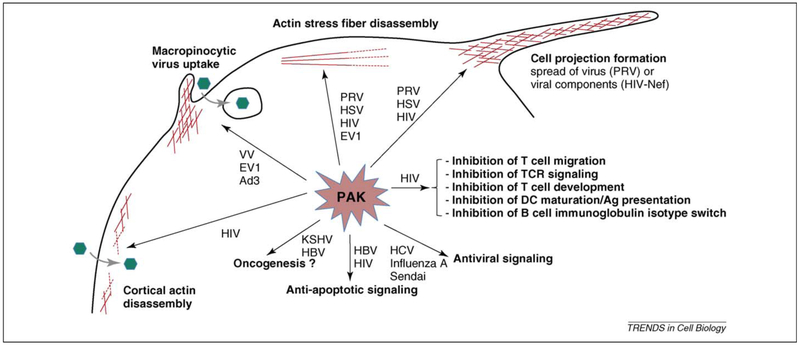
Figure 4.
Consequences of virus-mediated activation of Pak. Activation of Pak by a wide variety of viruses may lead to (i) cytoskeletal rearrangements such as cortical actin disassembly, macropinocytotic virus uptake, actin stress fiber disassembly and projection formation, (ii) anti-apoptotic signaling, (iii) oncogenesis, (iv) immune evasion and (v) antiviral signaling. Pak, p21-activated kinase; PRV, pseudorabies virus; HSV, herpes simplex virus; HIV, human immunodeficiency virus; EV1, echovirus 1; HCV, hepatitis C virus; KSHV, Kaposi’s sarcoma-associated herpesvirus; HBV, hepatitis B virus; VV, vaccinia virus; Ad3, adenovirus 3; DC, dendritic cell.
In 1996, Nef-mediated activation of Pak2 was found to increase HIV replication through an unresolved mechanism [31]. One explanation for its stimulatory effect could be that Nef enhances virus entry by allowing the virus to cross the cortical actin barrier of a host cell, a process that is thought to take place through Pak2-driven changes in the actin cytoskeleton [39,47]. Activation of Pak2 by Nef also has consequences for virus replication by interfering with apoptotic cell death. The Nef–Pak–PI3K complex was found to phosphorylate the proapoptotic Bad protein, leading to its inactivation, resulting in a block of apoptosis and a remarkable increase in virus particle release [48].
Besides its direct effect on virus replication, the Nef–Pak2 interaction appears to be involved in many aspects of the complex interplay between HIV and T lymphocytes, affecting T cell development, activity, T cell receptor (TCR)-mediated signaling and T cell motility, all of which might affect HIV pathogenesis and immune evasion. For example, interaction with Pak2 was found to be required for Nef-mediated interference with T cell development, one of the key elements involved in HIV-mediated T cell depletion during the development of AIDS [49].
The Nef–PAK2 interaction has also been suggested to be involved in increasing T cell activation, perhaps by Nef–Pak2-mediated phosphorylation of the cytoskeletal tumor-suppressor protein Merlin [50]. At the same time, Nef–Pak2 seems to be of predominant importance in reducing the ability of T cells to react to exogenous stimulation [51,52]. Nef reduces actin polymerization, maturation of stimulatory T cell contacts, and cell spread by modulation of Pak2 and N-WASP activity in T-lymphocytes upon stimulation of the T cell receptor (TCR) [52]. Moreover, Pak2 activation by Nef is involved in immunological synapse (IS) modulation by reducing signal transduction through actin remodeling and Lck recruitment to the IS [51,53,54]. It is thought that the combined effects of Nef on T cell activity and signaling is probably involved in establishing a balance that allows sufficient T cell activation necessary for optimal virus replication, but at the same time preventing excessive antiviral T cell activation, ultimately leading to an increase in virus replication and spread [55].
Pak activity can induce phosphorylation and activation of LIM kinase and subsequent phosphorylation of cofilin, a crucial regulator of actin polymerization [56]. Likewise, the Nef–Pak2 interaction has recently been shown to result in phosphorylation of cofilin, thereby impairing chemoattractant-triggered T cell motility that might disturb the antiviral response [57].
The Nef–Pak2 interaction also affects other immune cells in addition to T lymphocytes. Recently, Nef-mediated signaling in HIV-infected macrophages was shown to induce actin remodeling that resulted in the formation of cellular projections or nanotubes that contacted non-infected B-cells. Nef protein was found to shuttle via these projections from the macrophages to the B-cells, subsequently impairing immunoglobulin class-switching by interfering with CD40 signaling in B-cells [58,59]. This is in line with the finding that Pak2 also interferes with CD40 signaling in Nef-expressing dendritic cells (DCs), thereby inhibiting DC maturation and antigen presentation [58,60].
Overall, the Nef–Pak2 interaction appears to be a crucial element of several aspects of HIV biology, not only directly affecting virus replication but also indirectly promoting virus spread and persistence via disturbance of key players in the antiviral immune response.
Adenoviruses
Activation of Paks by specific types of adenoviruses is involved in virus uptake. Over a decade ago, Li and colleagues showed that entry of adenovirus 2 (Ad2), a species C adenovirus, was associated with PI3K-mediated activation of Rac1 and Cdc42 and subsequent internalization of the virus [61]. Although experiments using inhibitors and dominant-negative constructs clearly showed that the actin-regulating properties of Rac1 and Cdc42 were required for Ad2 entry, based on in-gel kinase assays, the authors did not find evidence for Pak activation during virus entry.
However, in 2008, Amstutz and colleagues showed that Ad2 and Ad5 (another species C adenovirus) do indeed activate Pak1, albeit at very low levels [62]. Ad3 by contrast, a species B adenovirus, induced robust Rac1-mediated activation of Pak1 and subsequent Pak1-dependent macropinocytic uptake of the virus. These and other reports unveiled a crucial role for Pak1 in macropinocytosis [8,63–65]. Ad3-mediated activation of Pak1 resulted in phosphorylation of CtBP1 (C-terminal binding protein 1 of E1A), a transcriptional co-repressor involved in tumorigenesis that turned out to be required for macropinocytosis based on siRNA assays [64]. Interestingly, CtBP1 phosphorylation resulted in a shift of the protein from the nucleoplasm to the cytoplasm, most notably at the periphery of Ad3-bearing endosomes, suggesting that the physical presence of CtBP1 is needed at the site of virus uptake.
The reason for the difference in Pak1-dependency between species B and C adenoviruses is not completely clear, although there are indications that this might be due to differences in receptor usage. Species B adenoviruses use CD46 as the initial receptor (Figure 3), whereas all other adenoviruses use the coxsackievirus and adenovirus receptor (CAR) [66].
Poxviruses
The first strong evidence that Paks are involved in poxvirus entry was obtained on myxomavirus, a rabbit poxvirus. It had been shown previously that specific subsets of mouse 3T3 fibroblasts were permissive for this virus whereas others were restrictive, and this appeared to depend on differences in the ability of these subsets of cells to induce signaling cascades during the early stages of infection [67]. In 2003, Johnston and colleagues showed that infection of permissive 3T3 cells resulted in activation of group I Pak proteins, that inhibition of Paks in these cells using a peptide inhibitor of Pak resulted in reduced infection, and that expression of a constitutively active recombinant of Pak1 increased replication of the virus in the restrictive 3T3 cells, indicating that Paks play a central role during the early stages of myxomavirus infection [68].
Recently, Mercer and Helenius showed that siRNA-mediated Pak1-knockdown abrogated infection by vaccinia virus, the most widely studied poxvirus [63]. As for Ad3, they found that vaccinia virus enters cells through Pak1-dependent macropinocytosis. Surface-exposed phosphatidylserine on vaccinia virus particles triggered Rac1 and Pak1 activation and subsequent virus uptake – hence these viruses were suggested to mimic the uptake of apoptotic bodies [63,69]. However, a subsequent study showed that also other phospholipids, not known to be involved in apoptosis, can function similarly as phosphatidylserine in virus uptake, thereby questioning the viral mimicry model [70].
Picornaviruses
Pak activation is also involved in successful picornavirus entry. Within minutes after attachment to a host cell, echovirus 1 (EV1) induces activation of group I Paks, accumulation of Pak1 in the nucleus, disassembly of actin stress fibers, and subsequent group I Pak-dependent uptake of the virus [71]. The mechanism of Pak activation by echovirus 1 remains elusive. As for adenoviruses and poxviruses, Pak-dependent EV1 uptake displayed several features of macropinocytosis, although it is intriguing to note that internalized virus was sorted to caveosomes. Until then, caveosomes were solely reported to be involved in caveolae-mediated internalization. Hence, the data on EV1 point to a novel Pak-driven sorting pathway to caveosomes.
Pak-dependent uptake of EV1 was confirmed to be via macropinocytosis in another study that also showed that Pak1 leads to phosphorylation of CtBP1, just as is observed for entry of Ad3 (Refs [62,64]). Importantly, this study showed that CtBP1 phosphorylation was required for fission of macropinosomes, probably explaining the physical presence of CtBP1 at endosomes during Ad3 entry [62]. It will be interesting to dissect further whether Pak1-dependent fission of macropinosomes involves the direct activity of CtBP1 or whether CtBP1 regulates the assembly of a fission machinery complex. In any case, these results show that, in addition to their established important roles in actin polymerization and the formation of membrane ruffles that are required for macropinocytic cup formation, group I Paks are also of pivotal importance during the later stages of macropinocytosis.
Herpesviruses
For herpesviruses there is increasing evidence that Pak activation could be involved in efficient intercellular virus spread (for alphaherpesviruses) and tumorigenesis (for gammaherpesviruses). The US3 serine/threonine kinase is conserved among all alphaherpesviruses, the largest subfamily of the herpesviruses, and is an important virulence factor for different alphaherpesviruses [72,73]. Transfection of the gene encoding herpes simplex virus 2 (HSV-2) US3 was found to disrupt actin stress fibers by affecting Rac1/Cdc42 signaling pathways downstream of the small Rho GTPases, probably at the level of group I Paks. HSV2 US3 was also found to display some limited homology to group I Paks [74]. Because Paks are known to undergo autophosphorylation during activation [5], US3 homology with Paks could suggest US3-mediated phosphorylation of Paks and/or other Pak substrates.
Subsequently, US3 orthologs of other alphaherpesviruses – pseudorabies virus (PRV) and Marek’s disease virus (MDV) – were shown to disrupt actin stress fibers and, in the case of PRV and HSV-2, to induce the formation of long cellular nanotube-like projections [75–80]. US3-mediated actin reorganization was associated with increased intercellular virus spread [77]. Van den Broeke and colleagues showed that US3-mediated actin changes are dependent on group I Pak activity [81]. US3 of PRV was found to be able to directly activate and phosphorylate both Pak1 and Pak2 [81]. Interestingly, study of Pak1 and Pak2 knockout cells revealed that the two isoforms have distinct activities, with Pak2 being required for US3-mediated actin stress-fiber disassembly and Pak1 being mainly involved in the formation of nanotube-like cell projections. These data add to a growing body of literature indicating that there might be important differences in the biological activities of the group I Pak isoforms [5,82].
Kaposi’s sarcoma (KS)-associated herpesvirus (KSHV), a gammaherpesvirus, also activates Pak1 through its G-protein-coupled receptor (GPCR), a viral oncogene implicated in KSHV-mediated malignancies [83,84]. The mechanism of action probably consists of KSHV-GPCR-mediated activation of PI3K, that in turn activates Rac1/Cdc42 and hence Paks. Because HIV–KSHV co-infection is a frequent occurrence, and could lead to Kaposi’s sarcoma in AIDS patients, it has been speculated that the ability of both HIV Nef and KSHV-GPCR to activate Paks might be an important factor in several aspects of KS disorders [84].
Hepadnaviruses
Indications for the involvement of Paks in virus-mediated tumorigenesis have also been found for the hepadnavirus Hepatitis B virus (HBV). The X protein (HBx) of HBV is a viral oncogene that plays a crucial role in the pathogenesis of HBV-induced hepatocellular carcinoma [85]. HBx displays several functions, including the regulation of a series of cell signaling cascades, of which Ras- and Raf-induced MAP kinase pathways appear to be the most prominent [86]. Expression of HBx has been reported to result in translocation of Raf to mitochondria [87]. This translocation was dependent on the activity of group I Pak kinases and Src kinases, confirming other reports that Pak/Src-mediated phosphorylation of Raf results in mitochondrial localization of this protein [15]. Mitochondrially located Raf kinase activity protects cells from apoptosis, thereby possibly contributing to HBx-mediated oncogenesis [87]. Whether HBx directly activates Pak (and/or Src) remains unknown at this stage.
Paks in antiviral signaling
There is conflicting evidence that group I Paks are involved in activating innate intracellular antiviral signaling pathways. Viral stimuli such as dsRNA are known to activate IRF-3, a key transcription factor in the expression of IFN-beta, a potent antiviral cytokine [88,89]. Experiments using wild-type and dominant-negative Pak1 indicated that Pak1 activity is necessary, though not sufficient, for IRF-3 activation during infection of MDCK cells with three RNA viruses – a human H1N1 and an avian H7N7 influenza A virus and Sendai virus [90]. Pak1 activation relied on Rac1 and, importantly, expression of dominant-negative Rac1 constructs resulted in increased virus titers, supporting the idea of Rac1/Pak1-mediated involvement in antiviral interferon signaling. However, expression of the same dominant negative Pak1 construct was found more recently not to affect the expression of genes regulated by IRF-3 or the induction of an antiviral state during infection of A549 cells with Sendai virus [91]. In line with the latter finding, infection of Huh7 cells with Sendai virus did not result in activation of group I Paks, and siRNA knockdown of Pak1 did not affect Sendai virus induced activation of IRF-3 (Ref. [92]), arguing against a role for Paks in virus-induced expression of interferon-beta and the establishment of an interferon-mediated antiviral state.
However, these results do not exclude a role for Paks in antiviral signaling. In support of this, knockdown of Pak1 using siRNA has been reported to increase replication of Hepatitis C virus (HCV) replicons and infectious virus, and to cause a 2-fold increase in extracellular released virus, again without affecting IRF-3 activation [92]. HCV is an RNA virus belonging to the flaviviruses and is a worldwide important cause of chronic hepatitis, cirrhosis, and hepatocellular carcinoma. Interestingly, the HCV data point to a novel mode of Pak activation via the phosphatidyl inositol 3-kinase, the mTOR translation factor, and its downstream effector p70 S6 kinase [92]. Hence, although the underlying mechanism is unclear at this stage, Paks appear to be involved in antiviral signaling against specific viruses, and this certainly merits further research.
Theurapeutic possibilities
Given the role of Pak in the life cycles of so many pathogenic viruses, it is possible that Pak inhibitors might prove useful as antiviral agents, assuming that toxicities are not limiting. In this regard, it is worth noting that loss of Pak1 and Pak3 is well-tolerated in mice, and deletion of either of these genes causes only mild phenotypes, whereas deletion of the gene encoding Pak2 results in early embryonic lethality [93]. In vitro, many cells tolerate simultaneous siRNA-mediated knock-down of Pak1 and Pak2 [82], suggesting that combined signaling from group A Paks is not required for cell viability. Whereas no clinically usable Pak drugs yet exist, a number of early-stage, specific small-molecule inhibitors have been recently described [94–97]. The most selective Pak inhibitor described to date is IPA-3, an allosteric small-molecule inhibitor of group I Paks [96]. This molecule has been shown to interfere with Pak-driven signaling events, including activation of the Erk pathway and formation of membrane ruffles in response to phorbol esters [94,96]. It will be interesting and instructive to test the effect of this compound and its successors on virus infection.
However, a considerable obstacle in the assessment of Pak-targeting drugs as potential antivirals is the poorly understood involvement of Paks in antiviral signaling and in the innate immune response. If this potential antiviral role of Paks is substantiated, targeting Paks could unintentionally result in augmented virus growth. Better insight into these processes will therefore be pivotal for a thorough evaluation of Pak inhibitors as antivirals, and could reveal whether targeting Pak-inhibiting drugs to specific tissues or cells might circumvent these problems. In this respect, the construction and use of tissue-specific knock-out or knock-down Pak laboratory animals will be invaluable.
Summary and Perspectives
For several viruses, increasing evidence points to Paks as central molecules in different aspects of their replication cycle, including virus entry (members of the adenoviruses, poxviruses, retroviruses, and picornaviruses), virus spread (members of the herpesviruses and retroviruses), tumorigenesis (herpesviruses and hepadnaviruses), and the complex interplay between virus and immune cells (retroviruses) (Figure 4).
Based on this increasing volume of literature, several potential lines of future research can be proposed (Future Questions are outlined in Box 3). For example, it will be interesting to dissect further the role of Pak-driven macro-pinocytosis-like processes in virus uptake because these are widely used gateways of virus entry into a host cell [98]. Such exploration will further elucidate the role of Paks during the earliest and crucial phase of virus infection, and could also reveal novel insights in this important endocytic pathway and highlight aspects of this broadly used entry pathway that could serve as novel antiviral targets.
Box 3. Outstanding questions.
What are the underlying mechanisms of virus-mediated activation of Paks, and are some of these mechanisms conserved over different virus families? This information is essential to design strategies to interfere with viral Pak activation, and at the same time might also increase our understanding of cellular pathways of Pak activation.
Why does PAK or Rac1 knockdown result in increased virus replication for some specific viruses such as HCV, Sendai, and influenza A viruses? Is this restricted to specific types of viruses (e.g. RNA viruses) or types of host cells, and what is the underlying mechanism – because interferon signaling does not appear to be involved, does this effect rely on novel antiviral signaling pathways?
What roles do Paks play in adaptive immune responses? Currently, relatively little is known about the relationship between Paks and the adaptive immune system. Development of laboratory animals with tissue- and cell-specific knock-out or knockdown of Paks in immune tissues and cells will allow these issues to be addressed.
Increasing evidence points to important differences in the cell biology of different Pak isoforms, for example with respect to tumor cell invasion [82]. Because different virus proteins such as PRV US3 have been reported to have Pak isoform-dependent activities [81], viruses and specific virus proteins could be used to further dissect these differences in Pak biology.
Research on HIV has indicated that the Nef–Pak2 pathway suppresses T cell development, immunological synapse formation and motility, and also Ig class-switching in B-lymphocytes and DC maturation and antigen presentation, suggesting a crucial but poorly understood role for Pak-mediated signaling in several central aspects of the immune response. In addition, for some viruses such as Sendai virus, influenza virus and HCV, there are indications that Paks are involved in innate antiviral signaling through a currently unknown mechanism, underscoring the importance of deciphering the effects of Paks, and the viral activation of Paks, on the innate and adaptive antiviral immune system.
Viruses such as HIV, PRV, and HSV also use Pak-mediated signaling for the induction of membrane nanotubes that contact neighboring cells [58,80,81], a process that can facilitate the spreading of infection and/or individual virus proteins. Increasing evidence points to membrane nanotubes as novel routes of intercellular communication, for example between immune cells, and studying these effects of viral interactions upon Paks could increase our knowledge on the general properties of these nanotubes and their importance in health and (infectious) disease [99–102].
Unraveling the interplay between a wide range of viruses and Paks at different stages of infection, and discovering whether different viruses have evolved similar interactions with Paks, could further result in identifying new therapeutic opportunities. In vivo studies, based on (tissue-specific) knock-out or knock-down of individual Pak isoforms, will be pivotal for the careful analysis of the importance of Paks in invasion, replication, spread, and persistence of viruses on the one hand, and antiviral signaling and immune response on the other, enabling the design of strategies to tip the balance in favor of the host.
References
- 1.Radtke K et al. (2006) Viral interactions with the cytoskeleton: a hitchhiker’s guide to the cell. Cell Microbiol. 8, 387–400 [DOI] [PubMed] [Google Scholar]
- 2.Manser E et al. (1994) A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature 367, 40–46 [DOI] [PubMed] [Google Scholar]
- 3.Pandey A et al. (2002) Cloning and characterization of PAK5, a novel member of mammalian p21-activated kinase-II subfamily that is predominantly expressed in brain. Oncogene 21, 3939–3948 [DOI] [PubMed] [Google Scholar]
- 4.Jaffer ZM and Chernoff J (2002) p21-activated kinases: three more join the Pak. Int J. Biochem. Cell Biol. 34, 713–717 [DOI] [PubMed] [Google Scholar]
- 5.Arias-Romero LE and Chernoff J (2008) A tale of two Paks. Biol Cell 100, 97–108 [DOI] [PubMed] [Google Scholar]
- 6.Cammarano MS et al. (2005) Pak4 induces premature senescence via a pathway requiring p16INK4/p19ARF and mitogen-activated protein kinase signaling. Mol. Cell Biol. 25, 9532–9542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dan C et al. (2001) Cytoskeletal changes regulated by the PAK4 serine/threonine kinase are mediated by LIM kinase 1 and cofilin. J. Biol. Chem. 276, 32115–32121 [DOI] [PubMed] [Google Scholar]
- 8.Dharmawardhane S et al. (2000) Regulation of macropinocytosis by p21-activated kinase-1. Mol. Biol. Cell 11, 3341–3352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J et al. (1999) Reciprocal signaling between heterotrimeric G proteins and the p21-stimulated protein kinase. J. Biol. Chem. 274, 31641–31647 [DOI] [PubMed] [Google Scholar]
- 10.Manser E et al. (1997) Expression of constitutively active alpha-PAK reveals effects of the kinase on actin and focal complexes. Mol. Cell Biol. 17, 1129–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sells MA et al. (1998) Characterization of Pak2p, a pleckstrin homology domain-containing, p21-activated protein kinase from fission yeast. J. Biol. Chem. 273, 18490–18498 [DOI] [PubMed] [Google Scholar]
- 12.Smith SD et al. (2008) PAK1-mediated activation of ERK½ regulates lamellipodial dynamics. J. Cell Sci. 121, 3729–3736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen JD et al. (2009) p21-activated kinase regulates mast cell degranulation via effects on calcium mobilization and cytoskeletal dynamics. Blood 113, 2695–2705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DerMardirossian C et al. (2004) Phosphorylation of RhoGDI by Pak1 mediates dissociation of Rac GTPase. Mol. Cell 15, 117–127 [DOI] [PubMed] [Google Scholar]
- 15.Jin S et al. (2005) p21-activated Kinase 1 (Pak1)-dependent phosphorylation of Raf-1 regulates its mitochondrial localization, phosphorylation of BAD, and Bcl-2 association. J. Biol. Chem. 280, 24698–24705 [DOI] [PubMed] [Google Scholar]
- 16.Vadlamudi RK et al. (2004) Dynein light chain 1, a p21-activated kinase 1-interacting substrate, promotes cancerous phenotypes. Cancer Cell 5, 575–585 [DOI] [PubMed] [Google Scholar]
- 17.Jakobi R et al. (2001) p21-activated protein kinase gamma-PAK suppresses programmed cell death of BALB3T3 fibroblasts. J. Biol. Chem. 276, 16624–16634 [DOI] [PubMed] [Google Scholar]
- 18.Rudel T and Bokoch GM (1997) Membrane and morphological changes in apoptotic cells regulated by caspase-mediated activation of PAK2. Science 276, 1571–1574 [DOI] [PubMed] [Google Scholar]
- 19.Liu J and Lin A (2005) Role of JNK activation in apoptosis: a double-edged sword. Cell Res. 15, 36–42 [DOI] [PubMed] [Google Scholar]
- 20.Jakobi R et al. (2003) Caspase-activated PAK-2 is regulated by subcellular targeting and proteasomal degradation. J. Biol. Chem. 278, 38675–38685 [DOI] [PubMed] [Google Scholar]
- 21.Orton KC et al. (2004) Phosphorylation of Mnk1 by caspase-activated Pak2/gamma-PAK inhibits phosphorylation and interaction of eIF4G with Mnk. J. Biol. Chem. 279, 38649–38657 [DOI] [PubMed] [Google Scholar]
- 22.Zhao ZS and Manser E (2005) PAK and other Rho-associated kinases – effectors with surprisingly diverse mechanisms of regulation. Biochem. J. 386, 201–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li F et al. (2002) p21-activated kinase 1 interacts with and phosphorylates histone H3 in breast cancer cells. EMBO Rep. 3, 767–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thiel DA et al. (2002) Cell cycle-regulated phosphorylation of p21-activated kinase 1. Curr. Biol. 12, 1227–1232 [DOI] [PubMed] [Google Scholar]
- 25.Banerjee M et al. (2002) Pak1 phosphorylation on t212 affects microtubules in cells undergoing mitosis. Curr. Biol. 12, 1233–1239 [DOI] [PubMed] [Google Scholar]
- 26.Ling J et al. (2005) Inhibition of cap-dependent translation via phosphorylation of eIF4G by protein kinase Pak2. EMBO J. 24, 4094–4105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foster JL and Garcia JV (2008) HIV-1 Nef: at the crossroads. Retrovirology 5, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sawai ET et al. (1996) Activation of PAK by HIV and SIV Nef: importance for AIDS in rhesus macaques. Curr. Biol. 6, 1519–1527 [DOI] [PubMed] [Google Scholar]
- 29.Sawai ET et al. (1994) Human immunodeficiency virus type 1 Nef associates with a cellular serine kinase in T lymphocytes. Proc. Natl. Acad. Sci. U. S. A. 91, 1539–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nunn MF and Marsh JW (1996) Human immunodeficiency virus type 1 Nef associates with a member of the p21-activated kinase family. J. Virol. 70, 6157–6161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu X et al. (1996) CDC42 and Rac1 are implicated in the activation of the Nef-associated kinase and replication of HIV-1. Curr. Biol. 6, 1677–1684 [DOI] [PubMed] [Google Scholar]
- 32.Nguyen DG et al. (2006) “UnPAKing” human immunodeficiency virus (HIV) replication: using small interfering RNA screening to identify novel cofactors and elucidate the role of group I PAKs in HIV infection. J. Virol. 80, 130–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rauch S et al. (2008) Human immunodeficiency virus type 1 Nef recruits the guanine exchange factor Vav1 via an unexpected interface into plasma membrane microdomains for association with p21-activated kinase 2 activity. J. Virol. 82, 2918–2929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agopian K et al. (2007) CD4 and MHC-I downregulation are conserved in primary HIV-1 Nef alleles from brain and lymphoid tissues, but Pak2 activation is highly variable. Virology 358, 119–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pulkkinen K et al. (2004) Nef associates with p21-activated kinase 2 in a p21-GTPase-dependent dynamic activation complex within lipid rafts. J. Virol. 78, 12773–12780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sawai ET et al. (1995) A conserved domain and membrane targeting of Nef from HIV and SIV are required for association with a cellular serine kinase activity. J. Biol. Chem. 270, 15307–15314 [DOI] [PubMed] [Google Scholar]
- 37.Krautkramer E et al. (2004) Human immunodeficiency virus type 1 Nef activates p21-activated kinase via recruitment into lipid rafts. J. Virol. 78, 4085–4097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Linnemann T et al. (2002) Interaction between Nef and phosphatidylinositol-3-kinase leads to activation of p21-activated kinase and increased production of HIV. Virology 294, 246–255 [DOI] [PubMed] [Google Scholar]
- 39.Fackler OT et al. (1999) Activation of Vav by Nef induces cytoskeletal rearrangements and downstream effector functions. Mol. Cell 3, 729–739 [DOI] [PubMed] [Google Scholar]
- 40.Brown A et al. (1999) Activation of the PAK-related kinase by human immunodeficiency virus type 1 Nef in primary human peripheral blood lymphocytes and macrophages leads to phosphorylation of a PIX-p95 complex. J. Virol. 73, 9899–9907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janardhan A et al. (2004) HIV-1 Nef binds the DOCK2-ELMO1 complex to activate rac and inhibit lymphocyte chemotaxis. PLoS Biol. 2, E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lang SM et al. (1997) Association of simian immunodeficiency virus Nef with cellular serine/threonine kinases is dispensable for the development of AIDS in rhesus macaques. Nat. Med. 3, 860–865 [DOI] [PubMed] [Google Scholar]
- 43.Kirchhoff F et al. (2004) Nef proteins from simian immunodeficiency virus-infected chimpanzees interact with p21-activated kinase 2 and modulate cell surface expression of various human receptors. J. Virol. 78, 6864–6874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khan IH et al. (1998) Role of the SH3-ligand domain of simian immunodeficiency virus Nef in interaction with Nef-associated kinase and simian AIDS in rhesus macaques. J. Virol 72, 5820–5830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schindler M et al. (2007) Association of Nef with p21-activated kinase 2 is dispensable for efficient human immunodeficiency virus type 1 replication and cytopathicity in ex vivo-infected human lymphoid tissue. J. Virol. 81, 13005–13014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carl S et al. (2000) Simian immunodeficiency virus containing mutations in N-terminal tyrosine residues and in the PxxP motif in Nef replicates efficiently in rhesus macaques. J. Virol 74, 4155–4164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Campbell EM et al. (2004) Disruption of the actin cytoskeleton can complement the ability of Nef to enhance human immunodeficiency virus type 1 infectivity. J. Virol. 78, 5745–5755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolf D et al. (2001) HIV-1 Nef associated PAK and PI3-kinases stimulate Akt-independent Bad-phosphorylation to induce anti-apoptotic signals. Nat. Med. 7, 1217–1224 [DOI] [PubMed] [Google Scholar]
- 49.Stove V et al. (2003) Signaling but not trafficking function of HIV-1 protein Nefis essential for Nef-induced defects in human intrathymic T-cell development. Blood 102, 2925–2932 [DOI] [PubMed] [Google Scholar]
- 50.Wei BL et al. (2005) Activation of p21-activated kinase 2 by human immunodeficiency virus type 1 Nefinduces merlin phosphorylation. J. Virol. 79, 14976–14980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haller C et al. (2007) HIV-1 Nef employs two distinct mechanisms to modulate Lck subcellular localization and TCR induced actin remodeling. PLoS One 2, e1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haller C et al. (2006) The HIV-1 pathogenicity factor Nef interferes with maturation of stimulatory T-lymphocyte contacts by modulation of N-Wasp activity. J. Biol. Chem. 281, 19618–19630 [DOI] [PubMed] [Google Scholar]
- 53.Rudolph JM et al. (2009) Inhibition of T cell receptor induced actin remodeling and relocalization of Lck are evolutionarily conserved activities of lentiviral Nef proteins. J. Virol. 83, 11528–11539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thoulouze MI et al. (2006) Human immunodeficiency virus type-1 infection impairs the formation of the immunological synapse. Immunity 24, 547–561 [DOI] [PubMed] [Google Scholar]
- 55.Fackler OT et al. (2007) Modulation of the immunological synapse: a key to HIV-1 pathogenesis? Nat. Rev. Immunol. 7, 310–317 [DOI] [PubMed] [Google Scholar]
- 56.Edwards DC et al. (1999) Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat. Cell Biol. 1, 253–259 [DOI] [PubMed] [Google Scholar]
- 57.Stolp B et al. (2009) HIV-1 Nef interferes with host cell motility by deregulation of Cofilin. Cell Host Microbe 6, 174–186 [DOI] [PubMed] [Google Scholar]
- 58.Xu W et al. (2009) HIV-1 evades virus-specific IgG2 and IgA responses by targeting systemic and intestinal B cells via long-range intercellular conduits. Nat. Immunol. 10, 1008–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rudnicka D and Schwartz O (2009) Intrusive HIV-1-infected cells. Nat. Immunol. 10, 933–934 [DOI] [PubMed] [Google Scholar]
- 60.Mann J et al. (2005) Functional analysis of HIV type 1 Nef reveals a role for PAK2 as a regulator of cell phenotype and function in the murine dendritic cell line, DC2.4. J. Immunol 175, 6560–6569 [DOI] [PubMed] [Google Scholar]
- 61.Li E et al. (1998) Adenovirus endocytosis requires actin cytoskeleton reorganization mediated by Rho family GTPases. J. Virol 72, 8806–8812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Amstutz B et al. (2008) Subversion of CtBP1-controlled macropinocytosis by human adenovirus serotype 3. EMBO J. 27, 956–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mercer J and Helenius A (2008) Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science 320, 531–535 [DOI] [PubMed] [Google Scholar]
- 64.Liberali P et al. (2008) The closure of Pak1-dependent macropinosomes requires the phosphorylation of CtBP1/BARS. EMBO J. 27, 970–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Puto LA et al. (2003) p21-activated kinase 1 (PAK1) interacts with the Grb2 adapter protein to couple to growth factor signaling. J. Biol. Chem. 278, 9388–9393 [DOI] [PubMed] [Google Scholar]
- 66.Sharma A et al. (2009) Adenovirus receptors and their implications in gene delivery. Virus Res. 143, 184–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Masters J et al. (2001) Poxvirus infection rapidly activates tyrosine kinase signal transduction. J. Biol. Chem. 276, 48371–48375 [DOI] [PubMed] [Google Scholar]
- 68.Johnston JB et al. (2003) Role of the serine-threonine kinase PAK-1 in myxoma virus replication. J. Virol. 77, 5877–5888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hoffmann PR et al. (2001) Phosphatidylserine (PS) induces PS receptor-mediated macropinocytosis and promotes clearance of apoptotic cells. J. Cell Biol. 155, 649–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Laliberte JP and Moss B (2009) Appraising the apoptotic mimicry model and the role of phospholipids for poxvirus entry. Proc. Natl. Acad. Sci. U. S. A. 106, 17517–17521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Karjalainen M et al. (2008) A Raft-derived, Pak1-regulated entry participates in alpha2beta1 integrin-dependent sorting to caveosomes. Mol. Biol. Cell 19, 2857–2869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Inagaki-Ohara K et al. (2001) Effect of the deletion of US2 and US3 from herpes simplex virus type 2 on immune responses in the murine vagina following intravaginal infection. Vaccine 20, 98–104 [DOI] [PubMed] [Google Scholar]
- 73.Kimman TG et al. (1994) Inactivation of glycoprotein gE and thymidine kinase or the US3-encoded protein kinase synergistically decreases in vivo replication of pseudorabies virus and the induction of protective immunity. Virology 205, 511–518 [DOI] [PubMed] [Google Scholar]
- 74.Murata T et al. (2000) Expression of herpes simplex virus type 2 US3 affects the Cdc42/Rac pathway and attenuates c-Jun N-terminal kinase activation. Genes Cells 5, 1017–1027 [DOI] [PubMed] [Google Scholar]
- 75.Van den Broeke C et al. (2009) The kinase activity of pseudorabies virus US3 is required for modulation of the actin cytoskeleton. Virology 385, 155–160 [DOI] [PubMed] [Google Scholar]
- 76.Schumacher D et al. (2005) The protein encoded by the US3 orthologue of Marek’s disease virus is required for efficient deenvelopment of perinuclear virions and involved in actin stress fiber breakdown. J. Virol 79, 3987–3997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Favoreel HW et al. (2005) Cytoskeletal rearrangements and cell extensions induced by the US3 kinase of an alphaherpesvirus are associated with enhanced spread. Proc. Natl. Acad. Sci. U. S. A. 102, 8990–8995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Calton CM et al. (2004) The pseudorabies virus serine/threonine kinase Us3 contains mitochondrial, nuclear and membrane localization signals. Virus Genes 29, 131–145 [DOI] [PubMed] [Google Scholar]
- 79.Van Minnebruggen G et al. (2003) Pseudorabies virus US3 protein kinase mediates actin stress fiber breakdown. J. Virol. 77, 9074–9080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Finnen RL et al. (2010) Analysis of filamentous process induction and nuclear localization properties of the HSV-2 serine/threonine kinase Us3. Virology 397, 23–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Van den Broeke C et al. (2009) Alphaherpesvirus US3-mediated reorganization of the actin cytoskeleton is mediated by group A p21-activated kinases. Proc. Natl. Acad. Sci. U. S. A. 106, 8707–8712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Coniglio SJ et al. (2008) Pak1 and Pak2 mediate tumor cell invasion through distinct signaling mechanisms. Mol. Cell Biol. 28, 4162–4172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cannon M (2007) The KSHV and other human herpesviral G protein-coupled receptors. Curr. Top. Microbiol. Immunol. 312, 137–156 [DOI] [PubMed] [Google Scholar]
- 84.Dadke D et al. (2003) Activation of p21-activated kinase 1-nuclear factor kappaB signaling by Kaposi’s sarcoma-associated herpes virus G protein-coupled receptor during cellular transformation. Cancer Res. 63, 8837–8847 [PubMed] [Google Scholar]
- 85.Lupberger J and Hildt E (2007) Hepatitis B virus-induced oncogenesis. World J. Gastroenterol. 13, 74–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bouchard MJ and Schneider RJ (2004) The enigmatic X gene of hepatitis B virus. J. Virol. 78, 12725–12734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen J and Siddiqui A (2007) Hepatitis B virus X protein stimulates the mitochondrial translocation of Raf-1 via oxidative stress. J. Virol. 81, 6757–6760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yoneyama M et al. (1998) Direct triggering of the type I interferon system by virus infection: activation of a transcription factor complex containing IRF-3 and CBP/p300. EMBO J. 17, 1087–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lin R et al. (1998) Virus-dependent phosphorylation of the IRF-3 transcription factor regulates nuclear translocation, transactivation potential, and proteasome-mediated degradation. Mol. Cell Biol. 18, 2986–2996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ehrhardt C et al. (2004) Rac1 and PAK1 are upstream of IKK-epsilon and TBK-1 in the viral activation of interferon regulatory factor-3. FEBS Lett. 567, 230–238 [DOI] [PubMed] [Google Scholar]
- 91.Noyce RS et al. (2006) Identification of a novel pathway essential for the immediate-early, interferon-independent antiviral response to enveloped virions. J. Virol. 80, 226–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ishida H et al. (2007) p21-activated kinase 1 is activated through the mammalian target of rapamycin/p70 S6 kinase pathway and regulates the replication of hepatitis C virus in human hepatoma cells. J. Biol. Chem. 282, 11836–11848 [DOI] [PubMed] [Google Scholar]
- 93.Hofmann C et al. (2004) The genetics of Pak. J. Cell Sci 117, 4343–4354 [DOI] [PubMed] [Google Scholar]
- 94.Viaud J and Peterson JR (2009) An allosteric kinase inhibitor binds the p21-activated kinase autoregulatory domain covalently. Mol. Cancer Ther. 8, 2559–2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Maksimoska J et al. (2008) Targeting large kinase active site with rigid, bulky octahedral ruthenium complexes. J. Am. Chem. Soc. 130, 15764–15765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Deacon SW et al. (2008) An isoform-selective, small-molecule inhibitor targets the autoregulatory mechanism of p21-activated kinase. Chem. Biol. 15, 322–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Porchia LM et al. (2007) 2-amino-N-{4-[5-(2-phenanthrenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]-phe nyl} acetamide (OSU-03012), a celecoxib derivative, directly targets p21-activated kinase. Mol. Pharmacol. 72, 1124–1131 [DOI] [PubMed] [Google Scholar]
- 98.Mercer J and Helenius A (2009) Virus entry by macropinocytosis. Nat. Cell Biol. 11, 510–520 [DOI] [PubMed] [Google Scholar]
- 99.Davis DM and Sowinski S (2008) Membrane nanotubes: dynamic long-distance connections between animal cells. Nat. Rev. Mol. Cell Biol. 9, 431–436 [DOI] [PubMed] [Google Scholar]
- 100.Gerdes HH and Carvalho RN (2008) Intercellular transfer mediated by tunneling nanotubes. Curr. Opin. Cell Biol. 20, 470–475 [DOI] [PubMed] [Google Scholar]
- 101.Gerdes HH (2009) Prions tunnel between cells. Nat. Cell Biol. 11, 235–236 [DOI] [PubMed] [Google Scholar]
- 102.Sherer NM et al. (2007) Retroviruses can establish filopodial bridges for efficient cell-to-cell transmission. Nat. Cell Biol. 9, 310–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dummler B et al. (2009) Pak protein kinases and their role in cancer. Cancer Metastasis Rev. 28, 51–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kreis P and Barnier JV (2009) PAK signalling in neuronal physiology. Cell. Signal. 21, 384–393 [DOI] [PubMed] [Google Scholar]
- 105.Renkema GH et al. (1999) Identification of the Nef-associated kinase as p21-activated kinase 2. Curr. Biol. 9, 1407–1410 [DOI] [PubMed] [Google Scholar]
- 106.Arora VK et al. (2000) Lentivirus Nef specifically activates Pak2. J. Virol. 74, 11081–11087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fackler OT et al. (2000) p21-activated kinase 1 plays a critical role in cellular activation by Nef. Mol. Cell Biol. 20, 2619–2627 [DOI] [PMC free article] [PubMed] [Google Scholar]