Abstract
We evaluated real-time myoelectric pattern recognition control of a virtual arm by transradial amputees. Five unilateral patients performed 10 wrist and hand movements using their amputated and intact arms. In order to demonstrate the value of information from intrinsic hand muscles, this data was included in EMG recordings from the intact arm. With both arms, motions were selected in approximately 0.2 s on average, and completed in less than 1.25 s. Approximately 99% of wrist movements were completed using either arm; however, the completion rate of hand movements was significantly lower for the amputated arm (53.9% ± 14.2%) than for the intact arm (69.4% ± 13.1%). For the amputated arm, average classification accuracy for only 6 movements—including a single hand grasp—was 93.1% ± 4.1%, compared to 84.4% ± 7.2% for all 10 movements. Use of 6 optimally-placed electrodes only reduced this accuracy to. These results suggest that muscles in the residual forearm produce sufficient myoelectric information for real-time wrist control, but not for performing multiple hand grasps. The outcomes of this study could aid the development of a practical multifunctional myoelectric prosthesis for transradial amputees, and suggest that increased EMG information—such as made available through targeted muscle reinnervation—could improve control of these prostheses.
Index Terms: Electromyography, multifunctional prosthesis, pattern recognition, real-time control, transradial amputation
I. Introduction
People who undergo a transradial amputation are impaired in their ability to perform many daily activities, and conventional transradial prostheses do not adequately restore the lost function [1]–[3]. Currently, most powered transradial prostheses use the amplitudes of surface electromyography (EMG) signals from the forearm flexors and extensors to control hand opening and closing. If wrist rotation is desired, amputees must co-contract their forearm muscles to switch into this mode; the same signals are then used to control the wrist rotator [3], [4]. Switching to different modes is slow and cumbersome. Furthermore, it is not intuitive to use the same muscle contractions to control two different functions.
A control approach using pattern recognition of EMG signals may yield a significant improvement in control over the conventional myoelectric control strategy [4]–[21]. It is grounded on the assumption that the patterns of EMG signals in the forearm contain information about many desired movements of the hand and wrist [6]. Using a pattern classification technique, the distinguishing characteristics of EMG patterns can be used to identify a variety of different intended movements. Once a pattern has been classified, a command is sent to a prosthesis controller to implement the movement. This control scheme is also intuitive, as the intended movement matches the prosthesis function. Many studies have been conducted with able-bodied subjects in order to assess the feasibility and performance of pattern recognition algorithms using EMG signals from forearm muscles [4]–[21]. In each case, several bipolar electrodes (from 4 to 16) were placed on the circumference of the midportion of the forearm. Using various pattern classification techniques—such as linear discriminant analysis (LDA) [6], [17], artificial neural networks [17], [19], and fuzzy logic [12], [18]—high accuracies (> 93%) for classification of 6–10 wrist and hand movements were consistently achieved. This suggests that a variety of pattern recognition algorithms can be used to predict the user’s intended movements with high accuracy. However, it remains unclear whether transradial amputees can achieve similar performance, as limited work has been done with this population. A recent study included two transradial amputees (one with a trauma-induced unilateral transradial amputation and another with a congenital unilateral transradial limb deficiency) and used 3 electrodes to collect surface EMG signals on the residual forearm [18]. Classification accuracies for 3 wrist classes (wrist flexion/extension and either wrist pronation or supination) ranged from 74% to 99%. Another study involved six subjects with transradial amputations (five transradial amputees and one congenital below-elbow failure of formation) and used 8 electrodes placed on the residual forearm for EMG recordings [20]. This study showed a low average accuracy (approximately 70%) for classification of 10 arm classes (wrist flexion/extension plus 8 hand grasps) with an artificial neural network–based classifier.
It is important to note that almost all of the previous studies used classification accuracy to evaluate the performance of pattern recognition algorithms. Classification accuracy is the ability of the algorithm to appropriately recognize the desired movements during each time window (usually 100–200 ms) while the subject holds different movements for several seconds [4]–[21]. This accuracy is calculated by post-processing EMG recordings and is not a true measure of real-time function; a pilot study revealed a low correlation between classification accuracy and real-time performance [22]. Thus, it remains unclear whether the residual muscles of the forearm following amputation can provide stable EMG information for accurate real-time control of multifunctional transradial prostheses. Furthermore, real-time performance metrics are required to examine the clinical robustness and accuracy of pattern recognition control.
We have developed an experimental protocol to assess realtime pattern recognition control of multifunctional myoelectric prostheses [23]. In this study, pattern recognition control was used by transradial amputees to control a computer-generated virtual prosthesis with four wrist functions, a hand open command, and five different hand-grasp patterns. Three realtime control performance metrics (motion-selection time, motion-completion time and motion-completion rate) were quantified. In addition, we performed a pilot analysis of the electrode configuration, an important clinical issue in the development of multifunctional myoelectric prostheses. The outcomes of this study could aid the future development of practical multifunctional myoelectric prostheses for transradial amputees.
II. Methods
A. Subject Information and Testing Overview
Five people with unilateral transradial (TR) amputations participated in the study (Table I). Their ages ranged from 28 to 77 years and their post-amputation times varied from 3 months to 21 years. Three subjects used a myoelectric prosthesis, one subject used a body-powered prosthesis and one subject (only 3 months post-amputation) had not yet received a prosthesis. The protocol of this study was approved by the Northwestern University Institutional Review Board. All subjects gave written informed consent and provided permission for publication of photographs for scientific and educational purposes.
TABLE I.
Demographic Data of the Five Transradial Subjects
| Subject | Age | Amputated Arm | Time since Amputation | Residual Forearm Length | Type of Prosthesis Useda |
|---|---|---|---|---|---|
| TR1 | 28 | Right | 6 years | 19 cm | Myo |
| TR2 | 39 | Right | 7 months | 15 cm | BP |
| TR3 | 41 | Left | 2 years | 20 cm | Myo |
| TR4 | 51 | Left | 3 months | 26 cm | None |
| TR5 | 77 | Left | 21 years | 19 cm | Myo |
Myo = Myoelectric, BP = Body-Powered.
Three trials were performed on each subject. Each of the trials was composed of two consecutive sessions: 1) EMG data acquisition to train the pattern classification algorithm and 2) virtual prosthesis manipulation to quantify pattern recognition control. The first trial was performed with the amputated arm. The second trial was performed with the intact arm; this trial was intended to demonstrate how well a subject could do with a richer EMG signal set from both forearm and intrinsic hand muscles, and provided additional training. The third trial was performed with the amputated arm again to quantify possible performance improvement. The time between two consecutive trials ranged from a few days to approximately three months, depending on subject availability. All five subjects completed the three testing trials in one to three months.
B. EMG Data Acquisition and Pattern Classification
For every subject, 12 self-adhesive Ag/AgCl snap bipolar electrodes with a 1.25-cm-diameter circular contact (Noraxon USA, Inc.) were placed on the forearm with a center-to-center distance of 2 cm. For amputated arms, 8 of the 12 electrodes were uniformly placed around the proximal portion of the forearm over the apex of the muscle bulge (2–3 cm distal to the elbow crease), and the other 4 electrodes were positioned on the distal end, as illustrated in Fig. 1(a). The distance between electrode rings varied with the length of the residual limb. For intact arms, the 12 electrodes were placed on the proximal forearm (6 around the apex of the muscle bulge, 2–3 cm distal to the elbow crease), the wrist (3 around, 2–3 cm proximal to the wrist crease), and the hand (the thenar, first dorsal interosseous, and hypothenar muscles), as illustrated in Fig. 1(b). These electrodes recorded EMG signals from muscles physiologically related to wrist and hand movements in order to demonstrate the performance that could be achieved with enhanced EMG information. The distance between electrode rings on the intact arm varied with the forearm length of the subjects. A large circular electrode was placed on the elbow of the tested arm for a ground. The 12 electrodes were connected to preamplifiers (Liberating Technologies, Inc., Hollister, MA) and then custom amplifiers. The EMG signals were amplified and band-pass filtered (5–400 Hz), sampled at a frequency of 1 kHz, and acquired with a Matlab-based custom data acquisition and processing system [23].
Fig. 1.
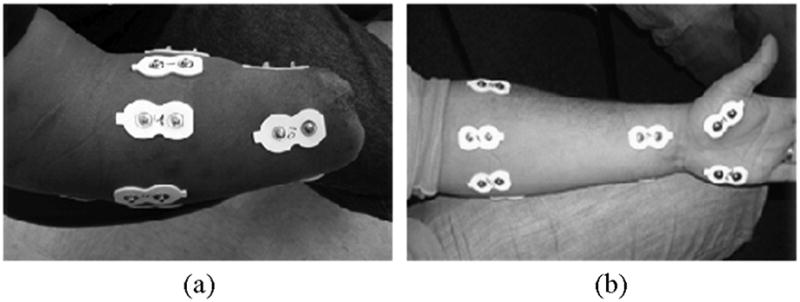
Placement of 12 bipolar electrodes for EMG recordings on (a) an amputated arm and (b) an intact arm.
Ten classes of wrist and hand motions plus a no movement class were included in the study. The 10 motion classes were wrist flexion and extension, wrist pronation and supination, hand open, and 5 hand-grasp patterns including chuck grip, key grip, power grip, fine pinch grip, and tool grip. EMG data were first acquired to train the pattern recognition classifier and test classification accuracy. Subjects were instructed to follow demonstrations of each movement displayed in random order on a computer screen and to perform the movements with a comfortable and consistent level of effort. EMG data were collected in eight consecutive trials. In each trial, all 11 motion classes were repeated twice and held for 4 s, producing 8 s of EMG recordings per class. There was a 3 s interval between consecutive movements in the four even-numbered trials, and a variable time interval (3 s, 2 s, 0 s, and 1 s, in turn) in the four odd-numbered trials in order to enhance the classifier’s robustness. To avoid muscle and mental fatigue, subjects were allowed to rest for 1–5 min between trials.
EMG data from the four odd-numbered trials were concatenated, producing 32 s of EMG recordings per movement, and used as a training set to build a classifier; data from the four even-numbered trials were similarly combined, producing 32 s of EMG recordings per movement, and used as a test set to evaluate the classification accuracy. EMG recordings were segmented into a series of 150 ms analysis windows with 50 ms of overlap. Four time-domain features (mean absolute value, number of zero crossings, waveform length, and number of slope sign changes) [6] were extracted from each analysis window as a representation of the EMG signal patterns [24], [25]. For each analysis window, a feature set was extracted on each of the 12 channels, producing a four-dimensional feature vector (four time-domain features). After concatenating the feature sets of all 12 channels, the entire 4 × 12 feature set vector was provided to the classifier. EMG features from the training set were used to train a linear discriminant analysis (LDA) [25] classifier for the 11 motion classes, and EMG features from the test set were used to test the classifier’s accuracy. The performance of the trained classifier in identifying a movement was measured by the classification accuracy, which is defined as
The classification accuracies were averaged over all 11 movements to calculate the overall classification accuracy.
The LDA classifier was then used to classify features extracted from EMG signals in real time. Again, 150 ms analysis windows were used with 50 ms of overlap, producing a new prediction of the motion class every 100 ms. The real-time classification decisions were used to control a virtual reality arm. Computational time for each analysis window was less than 3 ms.
C. Virtual Prosthesis Control and Performance Metrics
Experiments with the virtual prosthesis were performed immediately following classifier training. Subjects were instructed to follow visual prompts for each movement. A virtual arm which responded to the class decisions allowed subjects to observe the real-time results of their movement commands (Fig. 2). Subjects were asked to maintain each muscle contraction until the virtual arm completed the movement.
Fig. 2.

The graphical user interface used for real-time testing. The prompted movement is shown on the right and the virtual arm is shown on the left.
In each trial, subjects were asked to sequentially perform a series of motions. Each of the ten motions was randomly presented three times in a trial, and trials were repeated six times for a total of 180 movements—72 wrist movements and 108 hand movements. Subjects rested for 3 s between consecutive movements and for 2–5 min between trials. Dynamic data were recorded and used to quantitatively evaluate the speed and consistency of pattern recognition control.
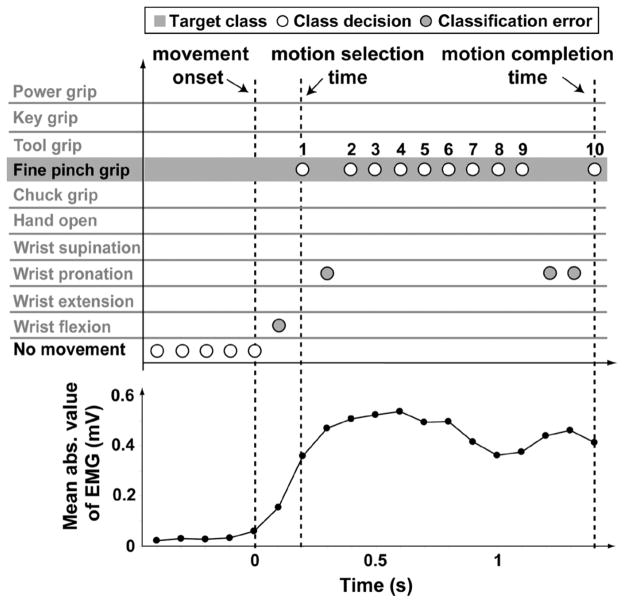
Three metrics were used to quantify real-time prosthesis control performance [23]. The motion-selection time was defined as the time taken to correctly select a target movement. This quantity represented how quickly motor command information (here represented with myoelectric signals) could be translated into the correct motion predictions. It was measured as the time from the onset of movement to the first correct prediction of the movement, as illustrated in Fig. 3. The onset of movement was identified as the time of the last no movement classification; this corresponded to approximately a 5% increase in the mean absolute value of the baseline EMG signals. All onset times were visually checked to avoid miscalculations. The motion-completion time was the time taken to successfully complete a movement through the full range of motion. It was measured as the time from the onset of movement to the completion of the intended movement, calculated as the time of the tenth correct classification. Ten accumulated correct classifications were required for a motion completion. The minimum possible time to complete any motion was normalized to 1 s, corresponding to ten consecutive correct classifications with a new classification occurring every 100 ms (each incorrect decision added 100 ms to the completion time). The update rate of the prosthesis control system was chosen to be 100 ms to insure a continuous decision stream in real time, allowing for the delays caused by EMG and pattern recognition processing (up to 25 ms) and virtual reality rendering (approximately 50 ms). This has been found to be within the range of acceptable prosthesis controller delays [26]. The motion-completion rate was defined as the percentage of successfully completed motions. This metric was a measure of performance reliability. A motion trial was considered completed if it was successfully performed through the full range of motion within the designated time limit. The time limit was chosen as 5 s based on clinical experience: after approximately this amount of time, prosthesis operation would be too slow and users may become frustrated and cease attempting a movement. If the target movement was not completed within the 5 s time limit, the movement was considered a failure and the completion time was not counted.
Fig. 3.
Calculation of real-time performance metrics. Movement onset, motion-selection time, and motion-completion time were measured with respect to classifier decisions. Movement onset was also related to the magnitude of the mean absolute (abs.) value of the recorded EMG signals. Each target movement started from a state of rest (no movement). The classifier made a motion prediction every 100 ms.
D. EMG Channel Reduction
Although increasing the number of electrodes increases the number of myoelectric signals captured, it simultaneously adds more complexity, weight, and cost to a prosthesis. A pilot analysis was performed to investigate the feasibility of using a reduced number of electrodes without compromising classification accuracy. In a previous experiment in which the total number of electrodes was greater than 100, we used the sequential forward selection (SFS) method to select the suboptimal electrode combination [27]. With a total of 12 electrodes, it is possible to use the straightforward exhaustive search algorithm to determine the ideal number of EMG electrodes based on the 12-channel EMG recordings from all five subjects. All possible electrode combinations for a reduced number of channels (ranging from 1 to 11) were evaluated by classification accuracy for the 10 movement classes (the no movement class was not considered in this analysis). The EMG recordings from the channels in each combination were selected from the 12-channel training set and used to train individual LDA classifiers. The EMG recordings from the same channels were selected from the 12-channel testing set and used to estimate each classifier’s accuracy. The channel combinations that produced the highest classification accuracies for each number of channels were considered the optimal channel configurations.
E. Statistical Analysis
Average classification accuracy and motion-completion rate are reported with mean and standard deviation (±SD). Motion-selection and motion-completion time distributions are skewed; therefore, we report the average and standard deviation of the median values from all subjects. Motion-selection time and motion-completion time are also represented with histograms with time bins of 0.1 s and 0.5 s, respectively. The paired t-test was used to assess the statistical difference between the means of compared data.
III. Results
A. Classification Accuracy
Classification accuracy over all 11 motion classes was calculated for all three trials with the five subjects [Fig. 4(a)]. The average classification accuracy for the five subjects was approximately 5% higher in the second amputated arm trial than in the first, but this difference was not significant (p > 0.05); therefore, further analyses focused on the second trial on the amputated arm (trial 3). Testing on amputated arms produced significantly lower classification accuracies than testing on intact arms (p < 0.02). The average accuracy over all five subjects was 79% ± 11% for the amputated arm and 94% ± 3% for the intact arm. Hand-grasp classification accuracies for the amputated arm (69% ± 18%) were significantly lower (p < 0.05) than wrist-movement classification accuracies (89% ± 7%), and were more variable [Fig. 4(b)].
Fig. 4.
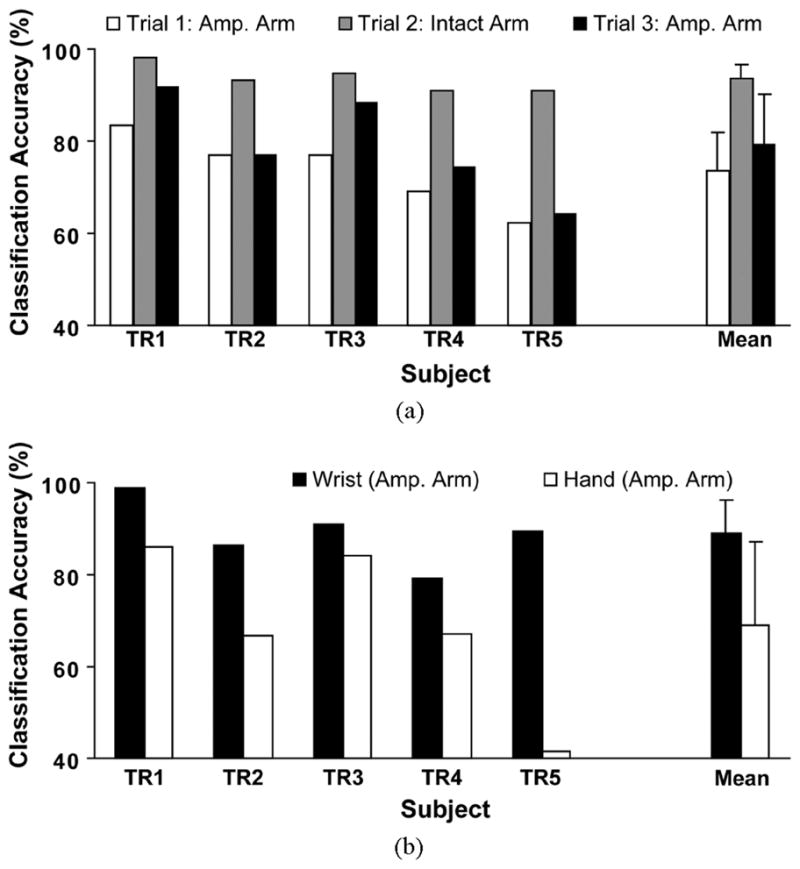
Classification accuracy for 11 movement classes. (a) The classification accuracies for five transradial subjects from three trials. (b) Classification accuracies for wrist and hand movements for the amputated (amp.) arm (trial 3). Error bars denote standard deviation.
B. Virtual Prosthesis Control Performance
1) Learning Trend
Real-time performance metrics from the two trials on the amputated arm (trials 1 and 3) were compared. Although there was a trend towards improved performance in the later trial, performance differences were small and not statistically significant. Subsequent analyses used the amputated arm data collected in trial 3.
2) Motion-Selection Time
The motion-selection time of a movement was counted if the movement was successfully completed within 5 s (Table II). The average time to select a movement was not significantly different (p =0.7) for the amputated or intact arms (0.19 ± 0.07 s and 0.18 ± 0.08 s, respectively). Although the average motion-selection time for the hand was higher for the intact arm than for the amputated arm (Table II), this difference was not significant. The histogram of motion-selection times for the hand shows that, in fact, a similar percentage of hand movements were selected quickly with the amputated and intact arms [Fig. 5(a)]. Thus, if the subjects were able to perform a given hand grasp with their amputated arm, they were able to select it just as quickly as with their intact limb. The histogram of wrist motion-selection times for the amputated arm was also similar to that for the intact arm [Fig. 5(a)]. These histograms demonstrate that subjects could select a correct motion class using their amputated arms as quickly as they could with their intact arms. There was no significant difference between wrist and hand motion-selection times on either arm.
TABLE II.
Real-Time Performance Metrics for Virtual Prosthesis Control With a 5 s Time Limit
| Motion-Selection Time (SD) | Motion-Completion Time (SD) | Motion-Completion Rate (SD) | ||||
|---|---|---|---|---|---|---|
| Amputated | Intact | Amputated | Intact | Amputated | Intact | |
| Wrist & Hand (n=180) | 0.19 s (0.07 s) | 0.18 s (0.08 s) | 1.24 s (0.27 s) | 1.14 s (0.17 s) | 72.1% (8.8%) | 81.2% (8.1%) |
| Wrist (n=72) | 0.21 s (0.09 s) | 0.18 s (0.08 s) | 1.16 s (0.19 s) | 1.12 s (0.11 s) | 99.4% (1.2%) | 98.9% (1.8%) |
| Hand (n=108) | 0.16 s (0.05 s) | 0.33 s (0.28 s) | 1.45 s (0.35 s) | 1.54 s (0.65 s) | 53.9% (14.2%) | 69.4% (13.0%) |
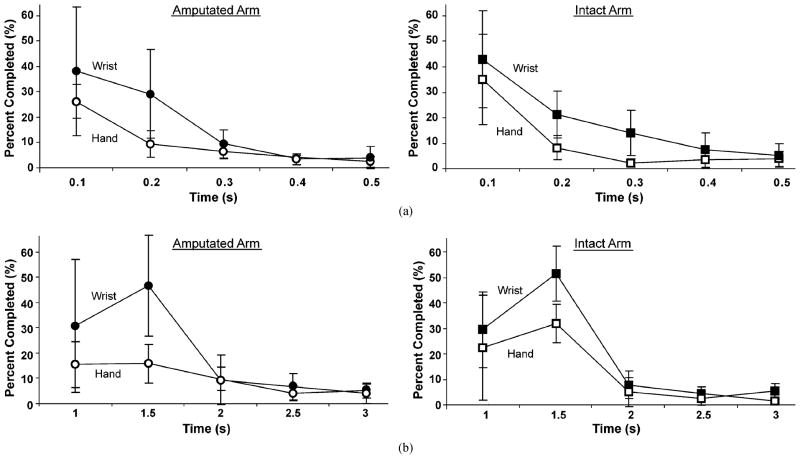
Fig. 5.
Time histograms for (a) motion-selection time and (b) motion-completion time for the amputated arms (left panels) and intact arms (right panels). The vertical axes represent the percentage of attempted movements selected or completed within times bins of 0.1 s and 0.5 s, respectively.
3) Motion-Completion Time
The motion-completion times were only counted for those movements completed in 5 s or less. The average time to complete an arm movement was 1.24 ± 0.27 s for amputated arms and 1.14 ± 0.17 for intact arms (p = 0.2) (Table II). On average, wrist movements were completed 20% faster than hand movements with the amputated arm and 38% faster with the intact arm, though only the former difference was statistically significant (p = 0.04 and p = 0.2, respectively). Subjects had perfect motion-completion times (1 s) in less than 30% of the attempts; however, the majority of intended movements were completed in 2 s [Fig. 5(b)].
4) Motion-Completion Rate
At 5 s, the motion-completion rate for the amputated arm (72.1% ± 8.8%) was significantly lower than that for the intact arm (81.2% ± 8.1%) (p = 0.04). The motion-completion rates for wrist movements for both the amputated and intact arms were near 100% at 5 s. Motion-completion rates for hand grasps were much lower for both arms. For the amputated arm, the motion-completion rate for the hand was nearly half that of the wrist [Fig. 6(b)], and for the intact arm, the average motion-completion rate for the hand was 29.5% lower than that for the wrist. These differences were statistically significant (p ≤ 0.01).
Fig. 6.
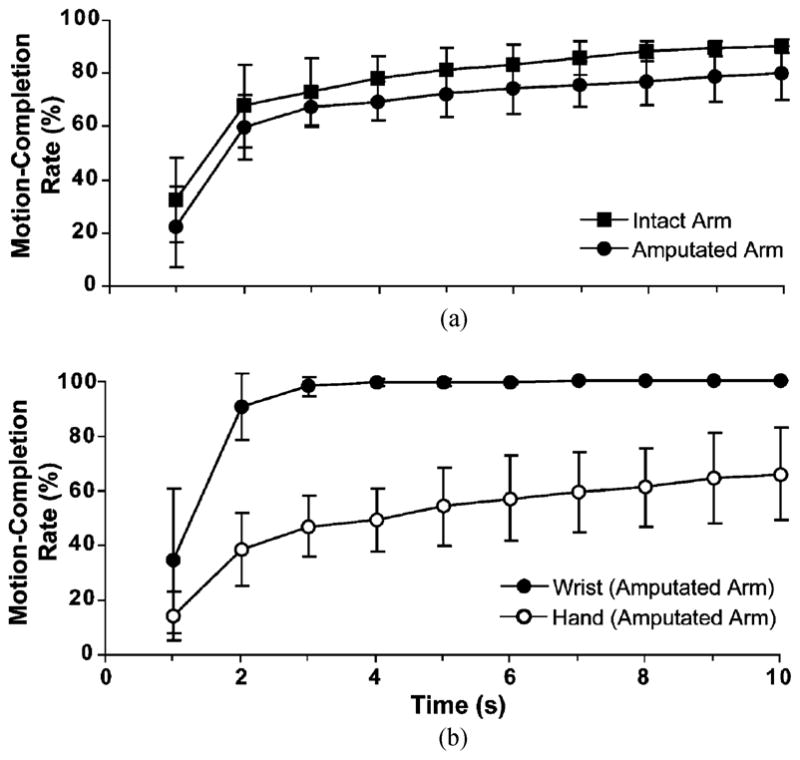
Motion-completion rate versus time for (a) amputated and intact arms and (b) wrist and hand of amputated arms.
Further analysis was performed on the number of attempts needed to successfully select a grasp. There was no significant difference between the amputated and intact arms for trials successfully completed by the 5 s limit; 81.4% of hand grasps were selected on the first attempt, 18% were selected on the second attempt, and 0.5% were selected on the third attempt.
C. Channel Reduction
Use of 6–8 optimally placed bipolar electrodes produced comparable accuracy to the use of all 12 electrodes for classification of the 10 motion classes (Fig. 7). Reducing EMG channels from 12 to 8 only decreased classification accuracy by 1%–3% in the subjects. When the motion classes were reduced to six basic motion classes (wrist flexion/extension, wrist pronation/supination, hand open, and power grip), the classification accuracy plateaued at 4–6 electrodes. Using 4 or 6 optimally selected electrodes with 6 motion classes produced an average accuracy of 88.5% ± .6% and 91.5% ± 4.9%, respectively, compared to 93.1% ± 4.1% with all 12 electrodes. Furthermore, optimal placement was not necessary to achieve relatively high accuracy. For the six motion classes, 4 suboptimal channels (4 electrodes spaced evenly around the proximal forearm) produced a classification accuracy of 83% ± 7%. Adding a fifth electrode on either side of the distal forearm increased accuracy by 2%–3%.
Fig. 7.
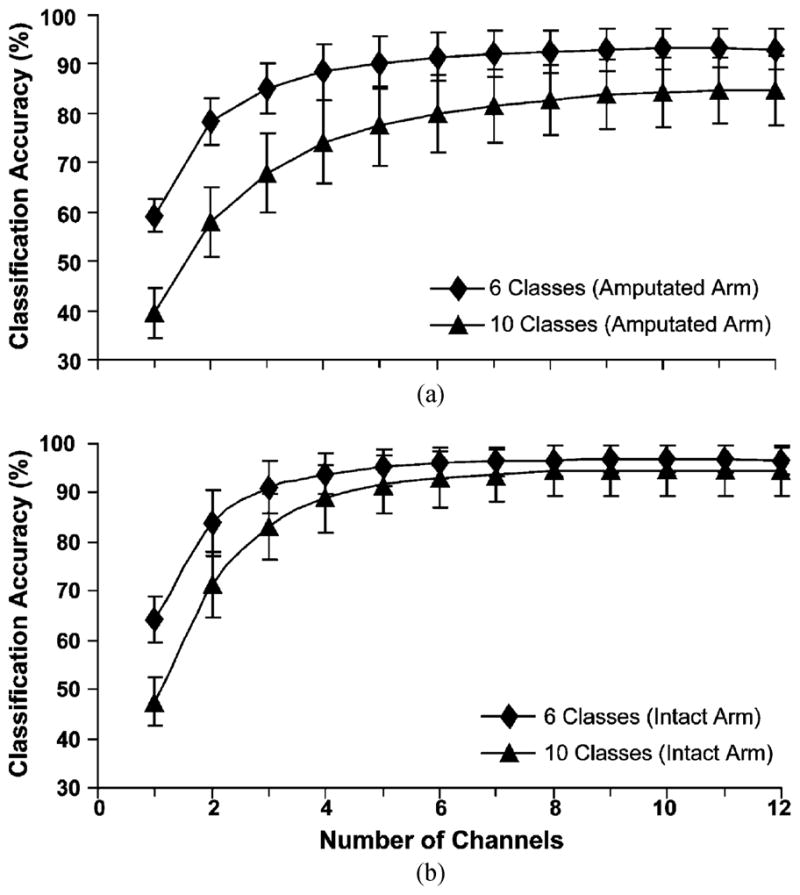
Classification accuracy versus number of surface electrodes for 6 or 10 movement classes as measured on (a) the amputated arms and (b) the intact arms.
IV. Discussion
It is a challenge to evaluate the performance of upper-limb prostheses. Historically, investigators have used able-bodied subjects to quantify EMG–pattern recognition performance with the simple goal of comparing the classification accuracy of different pattern recognition algorithms. We found high pattern recognition accuracies on the intact limbs—in which EMG data was collected from both forearm and intrinsic hand muscles—and significantly lower accuracies on the amputated limb. These results were comparable to accuracies found in other investigations using EMG pattern recognition for classification of wrist and hand movements in able-bodied and transradial subjects [4]–[21]. However, classification accuracy is calculated offline and is not a reliable measure of real-time performance. We have developed real-time performance measures to assess important control parameters and gain insight into the feasibility of clinically implementing EMG–pattern recognition–based controllers for transradial amputees. These metrics could also be used for comparing conventional myoelectric control with any neural–machine control systems developed in the future.
Two of the real-time performance metrics (motion-selection time and motion-completion time) were associated with speed, and the third (motion-completion rate) measured the robustness of prosthesis use, offering different means of assessing a user’s functional ability. Comparison of motion-selection and motion-completion times revealed that wrist movements could be selected and completed quickly with both the amputated and intact limbs, with no difference between arms. Similarly, the motion-completion rates for wrist movements with both arms were close to 100%. Interestingly, there was also no difference in motion-selection or motion-completion times for hand grasp patterns between the amputated and intact arms. However, the motion-completion rates for hand grasps were significantly higher on the intact arm. Therefore, when hand grasps were successfully performed in 5 s or less with the amputated arm, they were selected and completed just as quickly as with the intact arm, but fewer hand grasps were successfully performed with the amputated limb. The most probable cause of this difference was the EMG data from intrinsic hand muscles collected on the intact arm. In light of these findings, it appears that motion-completion rate was the most telling performance metric. A high completion rate is needed for adequate prosthesis function and to prevent user frustration. Although the motion-completion rates were high for wrist movements, the motion-completion rates for hand grasps were much lower for the amputated and intact arms (54% and 71%, respectively). These values are perhaps lower than might be expected based on the pattern recognition accuracies of 79% and 94% calculated for the amputated and intact arms, respectively. Clearly the subjects had to struggle as they tried to perform the various hand grasps.
It is noteworthy that the pattern recognition accuracy for the transradial arms was 79%, a lower rate than was found for transhumeral and shoulder disarticulation amputees with targeted muscle reinnervation (88%) [23]. This may be due to the reinnervation of the target muscles by the motoneurons of the intrinsic hand muscles, which takes place following targeted reinnervation and provides important EMG data about grasping patterns. No EMG data from intrinsic hand muscles was available on the amputated arms of the transradial amputees to contribute to the control of hand-grasp patterns. Thus, performing targeted reinnervation with the residual nerves of transradial amputees may increase the EMG information content and result in more robust control of multifunctional prosthetic hands. It is also noteworthy that the targeted reinnervation patients who participated in the previous study had more laboratory experience with pattern recognition control than the transradial patients tested in this study; therefore, learning may have played a part in the relative performance of the two groups.
Additional practice with the system is one way in which transradial patients might improve hand-grasp control. In this study, an initial trial with the amputated arm was carried out in order to familiarize the subjects with the experimental routine and with pattern recognition control. The results from the three experiments demonstrated that performance improved from the first to the third experiments and that the amputated arm did not perform as well as the intact arm. However, this is far from a comprehensive analysis of important learning parameters such as user experience, user education, learning within a session, issues of how to best train both the pattern recognition classifier and the patient, and recall from day to day or week to week. Additional research is required to quantify learning with pattern recognition control.
Using fewer hand grasp patterns would also improve pattern recognition of the remaining hand grasps. In this study we saw that reducing the number of hand grasps from five to one greatly increased the pattern recognition accuracy for the amputated arm. The number of hand grasp patterns a transradial amputee might be able to effectively control remains to be studied.
In general, pattern recognition control would benefit from better EMG interfaces. In particular, intramuscular EMG systems offer the possibility of more stable EMG recording and opportunities to refine the selectivity of each EMG channel. There is also a need to develop more robust pattern recognition algorithms to improve prosthesis control.
To be clinically relevant, the number of EMG channels used to provide robust pattern recognition control must be kept to a minimum. In this study, 6 to 8 electrode channels were able to achieve comparable levels of classification accuracy for the 10 classes of wrist and hand movements as 12 electrode channels [Fig. 7(a)]. We also performed an analysis that excluded variations in hand grasp movements, as the performance metrics for multiple hand grasps were low and as no multifunctional hands are currently available. We found that only 4 electrode channels were needed to provide good pattern recognition classification accuracy for the 6 basic movements (wrist flexion/extension, wrist pronation/supination, and hand open/close).
A virtual arm was used in these experiments instead of a physical prosthesis, since prosthetic hands with many hand grasp capabilities are not yet available commercially or to our lab. There are many differences between using a virtual arm and a physical device that should be considered, including differences in visual feedback, the effect of the weight of a physical prosthesis on the EMG recording interface, and the system dynamics of a physical prosthesis. However, a virtual arm allows us to perform real-time experiments with visual feedback and measure new and relevant performance parameters, and mitigates the challenge of keeping complex multifunctional prostheses in an operable condition. Use of a virtual arm also reduces the testing time required of the subjects, as a physical prosthesis would require casting and fitting of an appropriate socket, and any additional time needed to address mechanical issues arising during testing.
The clinical implementation of a pattern recognition control system with wrist movements and one hand grasp looks promising for people with transradial amputations, based on the results of this study. Such a system could provide the ability to control powered wrist flexion in addition to wrist rotation and control of the terminal device. Furthermore, pattern recognition control would be more intuitive and faster than conventional switching techniques.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) National Institute of Child and Human Development under Grant R01 HD043137-01 and Grant R01 HD058000-01.
The authors would like to thank K. Stubblefield and E. Corbett for their assistance in the experiments.
Biographies

Guanglin Li (M’01–SM’06) received the B.S. degree and the M.S. degree in electrical engineering from Shandong University, Jinan, China, in 1983 and 1988, respectively, and the Ph.D. degree in biomedical engineering from Zhejiang University, Hangzhou, China, in 1997.
He joined the Department of Automatic Control Engineering, Shandong University, where he became an Associate Professor in 1998. From 1999 to 2002 he was a Research Fellow, and later a Postdoctoral Research Associate, in the Department of Bioengineering, University of Illinois at Chicago. From 2002 to 2006 he was a senior Research Scientist at BioTechPlex Corporation. From 2006 to 2008 he was a Senior Research Scientist in the Neural Engineering Center for Artificial Limbs at the Rehabilitation Institute of Chicago, and also a Research Assistant Professor in the Department of Physical Medicine and Rehabilitation at Northwestern University. Since 2008, he has been with Shenzhen Institute of Advanced Technology (SIAT), Chinese Academy of Sciences, Shenzhen, China, where he is currently a Professor in the Research Centre for Neural Engineering at the Institute of Biomedical and Health Engineering. His current research interests include neuroprostheses, neural–machine interfaces, biomedical signal analysis, and computational biomedical engineering.

Aimee E. Schultz received the B.S. degree in engineering from Swarthmore College, Swarthmore, PA, in 2003, and the M.S. degree in mechanical engineering from Northwestern University, Evanston, IL, in 2007.
She is currently a Research Engineer and Technical Writer/Editor in the Neural Engineering Center for Artificial Limbs at the Rehabilitation Institute of Chicago, Chicago, IL.

Todd A. Kuiken (M’99–SM’07) received the B.S. degree in biomedical engineering from Duke University, Durham, NC, in 1983, the Ph.D. degree in biomedical engineering from Northwestern University, Evanston, IL, in 1989, and the M.D. degree from the Feinberg School of Medicine, Northwestern University Medical School, Chicago, IL, in 1990.
He completed his residency in physical medicine and rehabilitation at the Rehabilitation Institute of Chicago and Northwestern University Medical School, Chicago, IL, in 1995. In 1995 he was with the Rehabilitation Institute of Chicago and the Northwestern University Medical School as a Residency Trainee in physical medicine and rehabilitation. He is currently the Director of the Neural Engineering Center for Artificial Limbs and of Amputee Services at the Rehabilitation Institute of Chicago, Chicago, IL. He is also an Associate Professor in the Department of Physical Medicine and Rehabilitation (PM&R) and Biomedical Engineering, Northwestern University. Evanston, IL. He is also the Associate Dean for Academic Affairs at the Feinberg School of Medicine, Northwestern University Medical School, Chicago, IL.
Contributor Information
Guanglin Li, Email: gl.li@sub.siat.ac.cn, Neural Engineering Center for Artificial Limbs, Rehabilitation Institute of Chicago, Chicago, IL 60611 USA.
Aimee E. Schultz, Email: aes@northwestern.edu, Neural Engineering Center for Artificial Limbs, Rehabilitation Institute of Chicago, Chicago, IL 60611 USA
Todd A. Kuiken, Email: tkuiken@northwestern.edu, Neural Engineering Center for Artificial Limbs, Rehabilitation Institute of Chicago, Chicago, IL 60611 USA, and with the Department of Physical Medicine and Rehabilitation, Feinberg School of Medicine, Northwestern University, Chicago, IL 60611 USA, and also with the Biomedical Engineering Department, Northwestern University, Evanston, IL 60208 USA.
References
- 1.Kay H, Newman J. Relative incidence of new amputations. Orthotics Prosthetics. 1975;29:3–16. [Google Scholar]
- 2.Dillingham TR, Pezzin LE, MacKenzie EJ. Limb amputation and limb deficiency: Epidemiology and recent trends in the United States. South Med J. 2002 Aug;95:875–883. doi: 10.1097/00007611-200208000-00018. [DOI] [PubMed] [Google Scholar]
- 3.Sears HH. Atlas of Limb Prosthetics. St. Louis, MO: Mosby; 1992. Trends in upper-extremity prosthetic development. [Google Scholar]
- 4.Parker PA, Scott RN. Myoelectric control of prostheses. Crit Rev Biomed Eng. 1986;13:283–310. [PubMed] [Google Scholar]
- 5.Saridis GN, Gootee TP. EMG pattern analysis and classification for a prosthetic arm. IEEE Trans Biomed Eng. 1982 Jun;29(6):403–412. doi: 10.1109/TBME.1982.324954. [DOI] [PubMed] [Google Scholar]
- 6.Hudgins B, Parker P, Scott R. A new strategy for multifunction myoelectric control. IEEE Trans Biomed Eng. 1993 Jan;40(1):82–94. doi: 10.1109/10.204774. [DOI] [PubMed] [Google Scholar]
- 7.Kang WJ, Shiu JR, Cheng CK, Lai JS, Tsao HW, Kuo TS. The application of cepstral coefficients and maximum likelihood method in EMG pattern recognition. IEEE Trans Biomed Eng. 1995 Aug;42(8):777–785. doi: 10.1109/10.398638. [DOI] [PubMed] [Google Scholar]
- 8.Park SH, Lee SP. EMG pattern recognition based on artificial intelligence techniques. IEEE Trans Rehab Eng. 1998 Dec;6(4):400–405. doi: 10.1109/86.736154. [DOI] [PubMed] [Google Scholar]
- 9.Gallant PJ, Morin EL, Peppard LE. Feature-based classification of myoelectric signals using artificial neural networks. Med Biol Eng Comp. 1998 Jul;36:485–489. doi: 10.1007/BF02523219. [DOI] [PubMed] [Google Scholar]
- 10.Englehart K, Hudgins B, Parker PA, Stevenson M. Classification of the myoelectric signal using time-frequency based representations. Med Eng Phys. 1999 Jul.–Sep;21:431–438. doi: 10.1016/s1350-4533(99)00066-1. [DOI] [PubMed] [Google Scholar]
- 11.Micera S, Sabatini AM, Dario P, Rossi B. A hybrid approach to EMG pattern analysis for classification of arm movements using statistical and fuzzy techniques. Med Eng Phys. 1999 Jun;21:303–311. doi: 10.1016/s1350-4533(99)00055-7. [DOI] [PubMed] [Google Scholar]
- 12.Chan FHY, Yang YS, Lam FK, Zhang YT, Parker PA. Fuzzy EMG classification for prosthesis control. IEEE Trans Rehab Eng. 2000 Sep;8(3):305–311. doi: 10.1109/86.867872. [DOI] [PubMed] [Google Scholar]
- 13.Englehart K, Hudgins B. A robust, real-time control scheme for multifunction myoelectric control. IEEE Trans Biomed Eng. 2003 Jul;50(7):848–854. doi: 10.1109/TBME.2003.813539. [DOI] [PubMed] [Google Scholar]
- 14.Chan AD, Englehart KB. Continuous myoelectric control for powered prostheses using hidden Markov models. IEEE Trans Biomed Eng. 2005 Jan;52(1):121–124. doi: 10.1109/TBME.2004.836492. [DOI] [PubMed] [Google Scholar]
- 15.Huang YH, Englehart KB, Hudgins B, Chan ADC. A gaussian mixture model based classification scheme for myoelectric control of powered upper limb prostheses. IEEE Trans Biomed Eng. 2005 Nov;52(12):1801–1811. doi: 10.1109/TBME.2005.856295. [DOI] [PubMed] [Google Scholar]
- 16.Chu JU, Moon I, Mun MS. A real-time EMG pattern recognition system based on linear-nonlinear feature projection for a multifunction myoelectric hand. IEEE Trans Biomed Eng. 2006 Nov;53(11):2232–39. doi: 10.1109/TBME.2006.883695. [DOI] [PubMed] [Google Scholar]
- 17.Hargrove L, Englehart K, Hudgins B. A comparison of surface and intramuscular myoelectric signal classification. IEEE Trans Biomed Eng. 2007 May;54(5):847–853. doi: 10.1109/TBME.2006.889192. [DOI] [PubMed] [Google Scholar]
- 18.Ajiboye AB, Weir RF. A heuristic fuzzy logic approach to EMG pattern recognition for multifunctional prosthesis control. IEEE Trans Neural Syst Rehab Eng. 2005 Sep;13(3):280–291. doi: 10.1109/TNSRE.2005.847357. [DOI] [PubMed] [Google Scholar]
- 19.Tenore F, Ramos A, Fahmy A, Acharya S, Etienne-Cummings R, Thakor NV. Towards the control of individual fingers of a prosthetic hand using surface EMG signals. Proc IEEE Eng Med Biol Soc. 2007;2007:6146–6149. doi: 10.1109/IEMBS.2007.4353752. [DOI] [PubMed] [Google Scholar]
- 20.Sebelius FC, Rosen BN, Lundborg GN. Refined myoelectric control in below-elbow amputees using artificial neural networks and a data glove. J Hand Surg Am. 2005 Jul;30:780–789. doi: 10.1016/j.jhsa.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Momen K, Krishnan S, Chau T. Real-time classification of forearm electromyographic signals corresponding to user-selected intentional movements for multifunction prosthesis control. IEEE Trans Neural Syst Rehab Eng. 2007 Dec;15(4):535–542. doi: 10.1109/TNSRE.2007.908376. [DOI] [PubMed] [Google Scholar]
- 22.Lock BA, Englehart K, Hudgins B. MyoElectric Controls Symp. New Brunswick; Canada: 2005. Real-time myoelectric control in a virtual environment to relate usability vs. accuracy; pp. 122–127. [Google Scholar]
- 23.Kuiken TA, Li G, Lock BA, Lipschutz RD, Miller LA, Stubblefield KA, Englehart KB. Targeted muscle reinnervation for real-time myoelectric control of multifunction artificial arms. JAMA. 2009 Feb;301:619–628. doi: 10.1001/jama.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou P, Lowery M, Englehart K, Huang H, Li G, Hargrove L, Dewald JPA, Kuiken TA. Decoding a new neural-machine interface for control of artificial limbs. J Neurophysiol. 2007;98:2974–2982. doi: 10.1152/jn.00178.2007. [DOI] [PubMed] [Google Scholar]
- 25.Huang H, Kuiken T, Lipschutz R. A strategy for identifying locomotion modes using surface electromyography. IEEE Trans Biomed Eng. 2009 Jan;51(1):65–73. doi: 10.1109/TBME.2008.2003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farrell TR, Weir RF. The optimal controller delay for myoelectric prostheses. IEEE Trans Neural Syst Rehab Eng. 2007 Mar;15(1):111–118. doi: 10.1109/TNSRE.2007.891391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang H, Zhou P, Li G, Kuiken TA. An analysis of EMG electrode configuration for targeted muscle reinnervation based neural machine interface. IEEE Trans Neural Syst Rehab Eng. 2008 Feb;16(1):37–45. doi: 10.1109/TNSRE.2007.910282. [DOI] [PMC free article] [PubMed] [Google Scholar]