Abstract
One of the challenges in epidemiology is to account for the complex morphological structure of hosts such as plant roots, crop fields, farms, cells, animal habitats and social networks, when the transmission of infection occurs between contiguous hosts. Morphological complexity brings an inherent heterogeneity in populations and affects the dynamics of pathogen spread in such systems. We have analysed the influence of realistically complex host morphology on the threshold for invasion and epidemic outbreak in an SIR (susceptible–infected–recovered) epidemiological model. We show that disorder expressed in the host morphology and anisotropy reduces the probability of epidemic outbreak and thus makes the system more resistant to epidemic outbreaks. We obtain general analytical estimates for minimally safe bounds for an invasion threshold and then illustrate their validity by considering an example of host data for branching hosts (salamander retinal ganglion cells). Several spatial arrangements of hosts with different degrees of heterogeneity have been considered in order to separately analyse the role of shape complexity and anisotropy in the host population. The estimates for invasion threshold are linked to morphological characteristics of the hosts that can be used for determining the threshold for invasion in practical applications.
Keywords: epidemics, heterogeneity, percolation
1. Introduction
One of the main questions in epidemiology regards the outbreak of epidemics, i.e. whether an infectious disease can spread throughout a given ensemble of hosts or not. Many systems display a threshold for epidemics which divides the parameter space into regions where an outbreak may occur from regions where the disease cannot spread (Marro & Dickman 1999; Murray 2002). Identification of the factors that determine such a threshold is of great importance in devising robust strategies to control the spread of disease through a population of susceptible hosts. Successful control deflects the system into the non-invasive region of parameter space so that a pathogen fails to invade. This simple concept can be applied to the spread of infection and disease, at a range of scales from the cellular, in which the host comprises a single cell, through populations of plants and animals in which a host equates with an individual organism, up to larger-scale systems, in which the unit of interest may be an individual field or farm for crop and livestock disease, a school, a village or other natural clustering for human disease. The challenge at each scale lies in dealing in a quantitative manner with factors such as stochasticity and heterogeneity, so that thresholds used to identify strategies for control are robust to these uncertainties. Stochasticity in disease spread is linked to the fact that susceptible hosts become infected only with a certain probability when challenged by inoculum from infected hosts under otherwise identical circumstances. Heterogeneity, on the other hand, is associated with a range of factors that may differ among hosts. These include disorder in characteristics such as pathogen infectivity and host susceptibility, and the spatial arrangement of hosts. For certain types of hosts, heterogeneity also includes the inherent morphological complexity (i.e. irregularity in shape) of the host. Morphological complexity is especially important in epidemiological spread where it affects the contact rate between contiguous hosts. Examples of morphologically complex hosts include dendritic cells and plants, crop fields and farms, and individual clusters in social networks (Boender et al. 2007; Davis et al. 2008; Eisinger & Thulke 2008; González et al. 2008; Soriano et al. 2008).
While the problem of disease invasion has been extensively studied, both experimentally and theoretically, most attention has been focused first on deterministic systems and increasingly on stochastic models (Murray 2002; Truscott & Gilligan 2003; Gibson et al. 2004). Heterogeneity has traditionally received less attention although there are some remarkable exceptions (e.g. Levin & Durrett 1996; Newman 2002; Sander et al. 2002, 2003; Cook et al. 2007; Boender et al. 2007; Kenah & Robins 2007; Miller 2007; Eisinger & Thulke 2008; Volz 2008). However, none of the previous work has established a link between heterogeneity and morphological features of the system leading to such a heterogeneity. In particular, the heterogeneity associated with the host morphology and the effects it may have on the features of the epidemics remain to be understood.
In systems where the pathogen is transmitted between hosts owing to their proximity, one can identify three main factors that determine the invasion threshold (see figure 1): (i) spatial arrangement of the hosts in the population, (ii) morphology, i.e. the shape of the hosts, and (iii) the infection efficiency resulting from the net effect of the interplay between the pathogen infectivity and the host susceptibility upon contact.
Figure 1.
Example of a system formed by complex hosts represented, for concreteness, by planar neurons corresponding to salamander retinal ganglion cells placed on the nodes of a triangular lattice with lattice spacing a in such a way that the somata coincide with the lattice nodes. The pathogen infests the surroundings (shaded area) of infected (I) hosts and, eventually, reaches the neighbouring susceptible hosts (e.g. amber susceptible (S) neuron on the right from I). The probability of infection of a susceptible host and the probability of a global epidemic outbreak depend on overlaps, J, between the infested region and susceptible hosts, and infection efficiency, k. The overlaps are dictated by the host morphology and spatial arrangement of hosts. The infection efficiency determines the effectiveness of the contact in terms of transmission of infection. Besides these factors, the inset shows that J and thus the invasion threshold depend, in general, on the local orientation of the hosts, ϕ.
Several models have been proposed for the dynamics of epidemics spreading by contacts between hosts (Liggett 1985; Marro & Dickman 1999; Hinrichsen 2000; Murray 2002; Ódor 2004). In such models, the hosts can be in different states, e.g. susceptible (S), infected (I) and recovered (or removed) (R) in the prototype SIR epidemiological model. The state of each host may change according to certain model-dependent rules. For instance, the SIR model assumes that infected hosts can infect others that are susceptible and then become recovered and fully immune to further pathogen attacks. In such models, the stochasticity and heterogeneity for the spreading process are treated in a simplistic way by using phenomenological probability densities for relevant parameters and thus not linking the host morphology and invasion threshold.
In this paper, we establish a quantitative link between host morphology and the invasion threshold in an ensemble of hosts with realistically complex morphology. By analysing the conditions for epidemic outbreak in several systems with different degrees of configurational heterogeneity, we conclude that the invasion threshold is mainly determined by (i) the average overlap between neighbouring hosts, (ii) the morphological complexity of hosts, and (iii) the host shape anisotropy. In particular, we demonstrate analytically and numerically that the resilience of the system to invasion increases with morphological complexity and anisotropy of hosts. This result is valid under very general conditions and is therefore applicable to a wide range of host ensembles. We show that irrespective of the degree of the host anisotropy, the spreading process can be described in terms of a mean-field system, so that analytical estimates for the invasion threshold can be obtained. In addition, we complete our analysis by identifying several morphological characteristics that can be used for determining the threshold for invasion resulting in an epidemic outbreak.
2. Methods
We consider a set of N morphologically different branching structures, 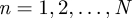 , placed on all nodes
, placed on all nodes 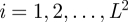 of an
of an  regular lattice with spacing a and nearest-neighbour links only (see table 1 for a summary of the notation used in the text). In particular, we deal with a triangular lattice (see figure 1) because this arrangement corresponds to the organization of hosts with the highest risk of epidemic spread, in the sense that any other two-dimensional regular lattices with the same lattice spacing is more resistant to epidemic invasion (Isichenko 1992; Stauffer & Aharony 1994). An additional advantage of the triangular lattice is that the next-nearest-neighbour links (ignored in our approach) are less likely in such a lattice as compared with other two-dimensional lattices. A certain spatial configuration of generally anisotropic branching structures is fully defined in terms of the set of hosts placed on the lattice nodes,
regular lattice with spacing a and nearest-neighbour links only (see table 1 for a summary of the notation used in the text). In particular, we deal with a triangular lattice (see figure 1) because this arrangement corresponds to the organization of hosts with the highest risk of epidemic spread, in the sense that any other two-dimensional regular lattices with the same lattice spacing is more resistant to epidemic invasion (Isichenko 1992; Stauffer & Aharony 1994). An additional advantage of the triangular lattice is that the next-nearest-neighbour links (ignored in our approach) are less likely in such a lattice as compared with other two-dimensional lattices. A certain spatial configuration of generally anisotropic branching structures is fully defined in terms of the set of hosts placed on the lattice nodes,  (where
(where  is the host number placed on node i), and their orientations,
is the host number placed on node i), and their orientations,  . The system of hosts can be either homogeneous, if morphologically identical hosts,
. The system of hosts can be either homogeneous, if morphologically identical hosts,  , of the same orientation,
, of the same orientation,  , are placed on the lattice, or heterogeneous if the hosts
, are placed on the lattice, or heterogeneous if the hosts  and/or their orientations
and/or their orientations  are chosen, e.g. at random. Specifically, we consider three types of arrangements with different degrees of heterogeneity which allow the various factors contributing to the invasion threshold to be analysed separately.
are chosen, e.g. at random. Specifically, we consider three types of arrangements with different degrees of heterogeneity which allow the various factors contributing to the invasion threshold to be analysed separately.
— Arrangements 1. Both
 and
and  are distributed according to uniform distributions so that all possible hosts and orientations are equally probable. These are highly disordered configurations.
are distributed according to uniform distributions so that all possible hosts and orientations are equally probable. These are highly disordered configurations.— Arrangements 2. The same host is placed on all the nodes with the same orientation (i.e.
 and
and  for all the nodes i). Such ordered arrangements highlight the role of the host anisotropy leading to different overlaps along different lattice directions.
for all the nodes i). Such ordered arrangements highlight the role of the host anisotropy leading to different overlaps along different lattice directions.— Arrangements 3. The same host is placed on all the nodes and its orientation is drawn from a uniform distribution of width
 and mean value
and mean value  . Such arrangements allow both the morphological complexity and anisotropy of hosts to be analysed in a comparative manner and they contain arrangements of type 2 as particular cases with
. Such arrangements allow both the morphological complexity and anisotropy of hosts to be analysed in a comparative manner and they contain arrangements of type 2 as particular cases with  and
and  .
.
Table 1.
Symbols and definitions used in the text. Indexes i and j span the L × L nodes in the (triangular) lattice. Index α spans the three main lattice directions, i.e. α = 1, 2, 3. Hosts are labelled by an index n = 1, 2, … , N. The symbol pc stands for the bond-percolation threshold.
| host characteristics, arrangement and overlaps | |
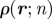 |
host density |
| ϕ | host orientation |
| a | lattice spacing |
 |
overlap between hosts at nodes i and j |
 |
set of overlaps between all the pair of hosts |
 |
dispersion of 𝒥 associated with the shape complexity |
 |
dispersion of 𝒥 associated with the shape anisotropy |
| disease transmission | |
| k | infection efficiency |
 |
recovery time |
 |
transmission rate |
 |
transmissibility |
| invasion of infection | |
 |
probability of invasion |
 |
invasion threshold in terms of the lattice spacing |
 |
invasion threshold in terms of the infection efficiency |
 |
invasion threshold in a mean-field system with overlaps 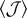
|
 |
non-mean-field contribution to kc originated by the shape complexity |
 |
non-mean-field contribution to kc originated by the shape anisotropy |
| morphological characteristics relevant to the invasion threshold | |
 |
radius of effective circular hosts in the mean-field approximation |
 , ( , ( ) ) |
lattice-adapted diameters |
| SLAD = d2 | second lattice-adapted diameter |
In order to investigate the spread of epidemics in such systems, we apply the dynamical rules of the SIR formalism. Infection and hence disease can be transmitted between infected and susceptible hosts with transmission rate β, and infected hosts recover after a fixed time τ. We assume that the value of τ is time independent, identical for all the hosts, and thus can be chosen as the time scale of the problem,  . Such homogeneity in τ provides a minimally safe bound for the invasion threshold (Kuulasmaa 1982; Cox & Durrett 1988; see brief explanation in the electronic supplementary material).
. Such homogeneity in τ provides a minimally safe bound for the invasion threshold (Kuulasmaa 1982; Cox & Durrett 1988; see brief explanation in the electronic supplementary material).
The transmission of infection from an infected host,  at node i, to a susceptible nearest neighbour,
at node i, to a susceptible nearest neighbour,  at node j, separated by a unit-cell vector a, is a Poisson process with a transmission rate
at node j, separated by a unit-cell vector a, is a Poisson process with a transmission rate  . The value of
. The value of  is assumed to be proportional to the overlap
is assumed to be proportional to the overlap 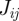 between hosts i and j, i.e.
between hosts i and j, i.e.  , where k is the infection efficiency which accounts for the effectiveness of the overlap for transmission of infection. We shall set k to be identical for all the pairs of nearest neighbours. Possible variability in k can be easily incorporated into the model but it does not change the main results qualitatively.
, where k is the infection efficiency which accounts for the effectiveness of the overlap for transmission of infection. We shall set k to be identical for all the pairs of nearest neighbours. Possible variability in k can be easily incorporated into the model but it does not change the main results qualitatively.
The overlap between hosts is defined as
| 2.1 |
in terms of the host density,
| 2.2 |
where the position vector p scans all the points in the host structure  .
.
One of the main quantities involved in the SIR process is the transmissibility  defined as the probability that the pathogen is transmitted from an infected host at node i to infect a susceptible host located at node j during the lifetime,
defined as the probability that the pathogen is transmitted from an infected host at node i to infect a susceptible host located at node j during the lifetime,  , of the infected node. For a Poisson process, the transmissibility is given by the following expression (Grassberger 1983):
, of the infected node. For a Poisson process, the transmissibility is given by the following expression (Grassberger 1983):
| 2.3 |
Therefore, the value of  depends on the lattice spacing, a, which determines the overlap,
depends on the lattice spacing, a, which determines the overlap,  , and the infection efficiency, k.
, and the infection efficiency, k.
In a finite-size population, the SIR process lasts for finite time and after its termination two types of hosts, R and S, can be found in the system. The region of the recovered hosts can be mapped onto one of the clusters in the bond-percolation problem by mapping the transmissibilities  to the bond probabilities (Grassberger 1983). According to this mapping, the probability of invasion of disease starting from a single host,
to the bond probabilities (Grassberger 1983). According to this mapping, the probability of invasion of disease starting from a single host,  , is identified with the probability that this first infected host belongs to the infinite cluster of connected sites in the bond-percolation problem. The invasion threshold is formally defined as the boundary between the invasive and the non-invasive regimes characterized by
, is identified with the probability that this first infected host belongs to the infinite cluster of connected sites in the bond-percolation problem. The invasion threshold is formally defined as the boundary between the invasive and the non-invasive regimes characterized by  and
and  , respectively. This condition introduces a separatrix in the parameter space which can be given in terms of several (control) parameters of the system, such as, for example, the infection efficiency and lattice spacing. In terms of the infection efficiency, the invasion threshold is the value
, respectively. This condition introduces a separatrix in the parameter space which can be given in terms of several (control) parameters of the system, such as, for example, the infection efficiency and lattice spacing. In terms of the infection efficiency, the invasion threshold is the value  such that relatively small values of
such that relatively small values of  correspond to the non-invasive regime (i.e.
correspond to the non-invasive regime (i.e.  ), while larger values,
), while larger values,  , describe the invasive domain (i.e.
, describe the invasive domain (i.e.  ). Similarly, the critical lattice spacing,
). Similarly, the critical lattice spacing,  , splits the range of lattice spacings into two regions:
, splits the range of lattice spacings into two regions:  and
and  corresponding to invasive and non-invasive regimes, respectively. The host morphology affects implicitly (through the overlaps between hosts
corresponding to invasive and non-invasive regimes, respectively. The host morphology affects implicitly (through the overlaps between hosts  ) both
) both  and
and  . The morphological variability of the hosts together with the heterogeneity in their arrangement makes the set
. The morphological variability of the hosts together with the heterogeneity in their arrangement makes the set  disperse in general. The overlap can then be regarded as a random variable 𝒥 taking the values
disperse in general. The overlap can then be regarded as a random variable 𝒥 taking the values  . We will describe 𝒥 in terms of its average, 〈𝒥〉, and deviations from 〈𝒥〉 originated by its dispersion. Consequently, it is convenient to split the expression for invasion threshold into two contributions
. We will describe 𝒥 in terms of its average, 〈𝒥〉, and deviations from 〈𝒥〉 originated by its dispersion. Consequently, it is convenient to split the expression for invasion threshold into two contributions
| 2.4 |
where kc0 and Δk are associated with the average and the dispersion of 𝒥, respectively. For the spatial arrangements of branching hosts considered below, the source of dispersion in the overlaps is twofold: (i) V1, which is due to variability arising from complexity in the shapes of different hosts for arrangements of type 1 and (ii) V2, which is due to variability in shape anisotropy for arrangements of type 2. Correspondingly, the value of Δk can be split into two components, i.e.  , where
, where  and
and  . Both complexity and anisotropy contribute to dispersion of the overlaps for arrangements of type 3.
. Both complexity and anisotropy contribute to dispersion of the overlaps for arrangements of type 3.
For numerical illustration and concreteness, in this paper we use a set of N ( ) neurons (figure 1) corresponding to salamander retinal ganglion cells (Ascoli 2006), which are mostly planar, as typical representatives of complex branching structures. In this case, the vector p introduced in equation (2.2) scans all the pixels defining the digital image of each neuron. Technically, the δ-functions in equation (2.2) are replaced by Gaussians of width comparable with the pixel size. This broadening mimics the (diffusive) spreading of the pathogen around the host. While we are not aware of documented examples of pathogen transmission in these structures, they are representative of a broad class of structures that are known to transmit virus infections (e.g. Lavail et al. 1997; Ehrengruber et al. 2002; Chen et al. 2007; Samuel et al. 2007). The importance here is to use the published data on complex morphology to test the general methods introduced below.
) neurons (figure 1) corresponding to salamander retinal ganglion cells (Ascoli 2006), which are mostly planar, as typical representatives of complex branching structures. In this case, the vector p introduced in equation (2.2) scans all the pixels defining the digital image of each neuron. Technically, the δ-functions in equation (2.2) are replaced by Gaussians of width comparable with the pixel size. This broadening mimics the (diffusive) spreading of the pathogen around the host. While we are not aware of documented examples of pathogen transmission in these structures, they are representative of a broad class of structures that are known to transmit virus infections (e.g. Lavail et al. 1997; Ehrengruber et al. 2002; Chen et al. 2007; Samuel et al. 2007). The importance here is to use the published data on complex morphology to test the general methods introduced below.
3. Results
In this section, we analyse the invasion threshold resulting in each of the spatial arrangements listed above and propose morphological characteristics for description of the invasion threshold.
3.1. Arrangements 1. ni and ϕi random
The invasion probability in host arrangements of type 1 is presented in figure 2. In particular, the inset shows the dependence of the probability of invasion on the infection efficiency. The value of  is zero at small values of k and becomes positive above the threshold,
is zero at small values of k and becomes positive above the threshold,  . As expected,
. As expected, 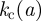 increases with the distance between hosts (cf. the curves marked by different symbols in the inset), a, as the infection mechanism between neighbouring hosts should be more efficient in order to invade the system with larger lattice spacing. However, the curves for
increases with the distance between hosts (cf. the curves marked by different symbols in the inset), a, as the infection mechanism between neighbouring hosts should be more efficient in order to invade the system with larger lattice spacing. However, the curves for  at different a collapse onto a single master curve (figure 2), if plotted as a function of an alternative control parameter, the average transmissibility, 〈𝒯 〉, which plays the same role as bond probability in the percolation problem (Sander et al.
2002, 2003). This statement was first demonstrated heuristically by Sander et al. (2002) for SIR processes with heterogeneous transmissibility. Indeed, it is easy to go beyond heuristic arguments and prove that the statement is valid if the transmissibilities
at different a collapse onto a single master curve (figure 2), if plotted as a function of an alternative control parameter, the average transmissibility, 〈𝒯 〉, which plays the same role as bond probability in the percolation problem (Sander et al.
2002, 2003). This statement was first demonstrated heuristically by Sander et al. (2002) for SIR processes with heterogeneous transmissibility. Indeed, it is easy to go beyond heuristic arguments and prove that the statement is valid if the transmissibilities  are independent and self-averaging quantities (see demonstration in the electronic supplementary material, and Kuulasmaa 1982; Kenah & Robins 2007; Miller 2007, for cases in which the transmissibilities are not independent and such description is not appropriate). The collapse in figure 2 suggests that transmissibilities in arrangements of type 1 satisfy these conditions. In fact, the master curve defined by the collapse coincides with that obtained for the effective homogeneous ‘mean-field’ system with transmissibility 〈𝒯〉 between all the nearest neighbours (see the dashed curve in figure 2 which is identical for all lattice spacings). This means that the heterogeneous system is equivalent to a homogeneous mean-field one for which the invasion threshold can be determined in an efficient manner by solving the following equation:
are independent and self-averaging quantities (see demonstration in the electronic supplementary material, and Kuulasmaa 1982; Kenah & Robins 2007; Miller 2007, for cases in which the transmissibilities are not independent and such description is not appropriate). The collapse in figure 2 suggests that transmissibilities in arrangements of type 1 satisfy these conditions. In fact, the master curve defined by the collapse coincides with that obtained for the effective homogeneous ‘mean-field’ system with transmissibility 〈𝒯〉 between all the nearest neighbours (see the dashed curve in figure 2 which is identical for all lattice spacings). This means that the heterogeneous system is equivalent to a homogeneous mean-field one for which the invasion threshold can be determined in an efficient manner by solving the following equation:
| 3.1 |
where  is the bond-percolation threshold in an infinite (i.e.
is the bond-percolation threshold in an infinite (i.e.  ) triangular lattice (Isichenko 1992; Stauffer & Aharony 1994). The value of
) triangular lattice (Isichenko 1992; Stauffer & Aharony 1994). The value of  provides the minimally safe bound for invasion threshold in other two-dimensional lattices (Isichenko 1992; Stauffer & Aharony 1994). As the average 〈𝒯〉 converges very fast with L to its limiting value for
provides the minimally safe bound for invasion threshold in other two-dimensional lattices (Isichenko 1992; Stauffer & Aharony 1994). As the average 〈𝒯〉 converges very fast with L to its limiting value for  , the value of
, the value of 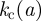 estimated from equation (3.1) is representative for macroscopic systems. The dependence of infection efficiency on lattice spacing defines the phase boundary in the (k,a) plane (see solid circles in figure 3a) between the invasive and non-invasive regimes. The threshold
estimated from equation (3.1) is representative for macroscopic systems. The dependence of infection efficiency on lattice spacing defines the phase boundary in the (k,a) plane (see solid circles in figure 3a) between the invasive and non-invasive regimes. The threshold  provides the same separatrix.
provides the same separatrix.
Figure 2.
Probability of invasion, Pinv, for morphologically complex hosts in disordered arrangements of type 1 on a lattice of size L × L = 200 × 200. The main plot displays Pinv as a function of the average transmissibility,  , for heterogeneous systems with different lattice spacings a (marked by different symbols) and for a mean-field system with homogeneous transmissibility
, for heterogeneous systems with different lattice spacings a (marked by different symbols) and for a mean-field system with homogeneous transmissibility  (dashed line). The bond-percolation critical probability, pc, marked by the arrow gives the invasion threshold in the thermodynamic limit. The inset shows the invasion probability as a function of the infection efficiency k for different values of lattice spacing marked by the same symbols as in the main figure. Circle, a = 20; asterisk, a = 40; square, a = 60; triangle, a = 80; diamond, a = 100.
(dashed line). The bond-percolation critical probability, pc, marked by the arrow gives the invasion threshold in the thermodynamic limit. The inset shows the invasion probability as a function of the infection efficiency k for different values of lattice spacing marked by the same symbols as in the main figure. Circle, a = 20; asterisk, a = 40; square, a = 60; triangle, a = 80; diamond, a = 100.
Figure 3.
Invasion threshold for the system of branching hosts. (a) Representation in terms of the infection efficiency, k, and the lattice spacing, a. The threshold kc(a) for arrangements of type 1 is shown by circles. The shaded region corresponds to the statistically possible values for the estimation of kc(a) in terms of effective circular hosts with homogeneous overlaps defined in equation (3.10). Diamonds indicate the average thresholds  (solid symbols) and
(solid symbols) and  (open symbols) for arrangements of type 3. (b) Invasion threshold kc as a function of the average overlap
(open symbols) for arrangements of type 3. (b) Invasion threshold kc as a function of the average overlap 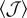 for arrangements of type 1 (circles), type 2 with orientation ϕ = 0 (diamonds) and type 3 with mean orientation
for arrangements of type 1 (circles), type 2 with orientation ϕ = 0 (diamonds) and type 3 with mean orientation  and two widths of uniform distribution,
and two widths of uniform distribution,  (solid squares) and
(solid squares) and  (open squares). The branching host used as a motif for arrangements of type 2 and 3 is displayed in the figure. The dashed line represents the dependence of kc0 versus
(open squares). The branching host used as a motif for arrangements of type 2 and 3 is displayed in the figure. The dashed line represents the dependence of kc0 versus 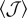 given by equation (3.2). The insets show the dispersions V1 and V2 of the overlaps associated with the disorder and anisotropy, respectively, corresponding to the same arrangements as in the main figure (the symbol code is the same as in the main figure).
given by equation (3.2). The insets show the dispersions V1 and V2 of the overlaps associated with the disorder and anisotropy, respectively, corresponding to the same arrangements as in the main figure (the symbol code is the same as in the main figure).
The influence of the host morphology on the invasion threshold can be better understood by analysing the dependence of  on the overlaps 𝒥. The first term in equation (2.4) can be found by solving equation (3.1) for the homogeneous system in which all the overlaps are replaced by its mean value, 〈𝒥〉, i.e.
on the overlaps 𝒥. The first term in equation (2.4) can be found by solving equation (3.1) for the homogeneous system in which all the overlaps are replaced by its mean value, 〈𝒥〉, i.e.
| 3.2 |
It can be rigorously shown that the value of  underestimates the threshold, i.e.
underestimates the threshold, i.e.  (cf. circles and dashed line in figure 3b). To prove this inequality, we write the expression (3.1) in terms of the overlap as
(cf. circles and dashed line in figure 3b). To prove this inequality, we write the expression (3.1) in terms of the overlap as  . Applying then the general inequality
. Applying then the general inequality  to the above relation, we obtain
to the above relation, we obtain  which reduces to
which reduces to  after using the expression (3.2) for
after using the expression (3.2) for  . This inequality implies that the contribution Δk to
. This inequality implies that the contribution Δk to  associated with the dispersion of the overlaps makes systems more resilient to epidemic invasion. An approximate solution of equation (3.1) obtained by keeping the first correction to
associated with the dispersion of the overlaps makes systems more resilient to epidemic invasion. An approximate solution of equation (3.1) obtained by keeping the first correction to  gives the low-bound estimate
gives the low-bound estimate  of Δk, i.e.
of Δk, i.e.
 |
3.3 |
which is proportional to the variance of the overlaps, 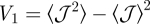 , with other central moments of higher order being dropped (see the derivation of equation (3.3) in the electronic supplementary material). The upper inset in figure 3 shows that the value of V1 is non-zero, as expected from the comparison of
, with other central moments of higher order being dropped (see the derivation of equation (3.3) in the electronic supplementary material). The upper inset in figure 3 shows that the value of V1 is non-zero, as expected from the comparison of  and
and  plotted in the main figure.
plotted in the main figure.
The low-bound estimate of Δk given by equation (3.3) provides a safe threshold, 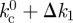 , for the actual system. We expect this to be the case for a wide class of morphologically complex hosts so that describing 𝒥 in terms of its average and standard deviation provides a minimally safe bound to the invasion threshold in heterogeneous arrangements.
, for the actual system. We expect this to be the case for a wide class of morphologically complex hosts so that describing 𝒥 in terms of its average and standard deviation provides a minimally safe bound to the invasion threshold in heterogeneous arrangements.
3.2. Arrangements 2. Identical ni and ϕi at all the nodes
In ordered arrangements of type 2, all the hosts are identical branching structures with the same orientation. The host overlaps along the main lattice directions,  (figure 4a), depend on the lattice spacing a, the host used as a motif, n, and its orientation, ϕ. For a given n, the set of all possible overlaps obtained by varying a and ϕ within their respective domains define the host overlap locus. Figure 4b shows the overlap locus corresponding to a typical branching host displayed in figure 4a. Each configuration with given a and ϕ is represented by a point
(figure 4a), depend on the lattice spacing a, the host used as a motif, n, and its orientation, ϕ. For a given n, the set of all possible overlaps obtained by varying a and ϕ within their respective domains define the host overlap locus. Figure 4b shows the overlap locus corresponding to a typical branching host displayed in figure 4a. Each configuration with given a and ϕ is represented by a point  belonging to the overlap locus. The inherent anisotropy of the host is reflected in the dispersion of the overlaps,
belonging to the overlap locus. The inherent anisotropy of the host is reflected in the dispersion of the overlaps,  , and gives rise to a significant deviation of the overlap locus (surface in blue) from the straight line (in black) corresponding to the locus for isotropic host (
, and gives rise to a significant deviation of the overlap locus (surface in blue) from the straight line (in black) corresponding to the locus for isotropic host ( ).
).
Figure 4.
Arrangements of type 2 (an identical host with the same orientation placed at all the nodes). (a) Unit cell of two different configurations constructed by using the same branching host. The orientation, ϕ, and spacing, a, corresponding to each case are indicated in the schematic reference frames displayed at the top. As shown in the left frame, there are three different values of the overlaps, {J1, J2, J3}, corresponding to each of the main directions in the lattice. (b) Space of overlaps, (J1, J2, J3). Each configuration with different a and ϕ is mapped into a point in this space. The set of overlaps corresponding to all the possible configurations obtained for a given host defines its overlap locus (surface in blue). The deviation of the blue surface from the straight black line representing overlaps between isotropic hosts (J1 = J2 = J3) shows the degree of anisotropy in the overlaps for a typical branching host (shown in (a)). High values of the overlaps correspond to small lattice spacings a. In particular, the points labelled as C1 and C2 correspond to the configurations shown in (a). The critical surface defined by g(J1, J2, J3, k) = 0 (see equation (3.5)) is shown in red for two values of the infection efficiency: k = 0.02 and k = 0.008. For a given value of k, the points in the overlap locus below/above the critical surface correspond to safe/vulnerable configurations. The intersection of the overlap locus with the critical surfaces parametrized by k determines the critical threshold  . The critical infection efficiency
. The critical infection efficiency  corresponding to a configuration with lattice spacing a and orientation ϕ is given by the value of k related to the critical surface containing the configuration point
corresponding to a configuration with lattice spacing a and orientation ϕ is given by the value of k related to the critical surface containing the configuration point 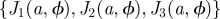 .
.
Similarly to arrangements of type 1, the spread of disease in ordered arrangements can be mapped onto the bond-percolation problem but now with anisotropic bond probabilities corresponding to the values of transmissibilities,  , along the main lattice directions defined as
, along the main lattice directions defined as  for
for 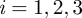 . From this mapping, the invasion threshold is defined by the following condition (Sykes & Essam 1964):
. From this mapping, the invasion threshold is defined by the following condition (Sykes & Essam 1964):
| 3.4 |
This condition can be recast in terms of the infection efficiency and overlaps, as  , where
, where
 |
3.5 |
For given value of k, the condition  defines a critical surface (in red in figure 4b) in the overlap space. Therefore, the intersection of the critical surface with the overlap locus (in blue) defines the invasion threshold,
defines a critical surface (in red in figure 4b) in the overlap space. Therefore, the intersection of the critical surface with the overlap locus (in blue) defines the invasion threshold,  . On the other hand, for a given value of a and ϕ, the critical infection efficiency
. On the other hand, for a given value of a and ϕ, the critical infection efficiency 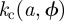 is given by the value of k which generates a critical surface containing the point
is given by the value of k which generates a critical surface containing the point  .
.
In practice, it is useful to consider the minimally resilient thresholds 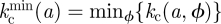 or
or  which ensure that the system is safe for any orientation if
which ensure that the system is safe for any orientation if 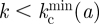 or
or 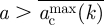 , respectively. The invasion thresholds are host-dependent and define different separatrices in the (a, k) plane for each host. Figure 3a shows the average thresholds
, respectively. The invasion thresholds are host-dependent and define different separatrices in the (a, k) plane for each host. Figure 3a shows the average thresholds 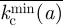 and
and  over all the branching hosts {n}. The phase boundary,
over all the branching hosts {n}. The phase boundary,  , gives the safest estimate for the invasion threshold, which is a consequence of the multi-valued nature of
, gives the safest estimate for the invasion threshold, which is a consequence of the multi-valued nature of  in contrast to single-valued function
in contrast to single-valued function 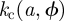 (see the electronic supplementary material for more detail).
(see the electronic supplementary material for more detail).
The effects of the host anisotropy on the invasion threshold can be analysed in a similar way as for configurations of type 1 by investigating the dependence of  on the overlaps. Similarly, the critical infection efficiency is given by equation (2.4) with the mean-field value
on the overlaps. Similarly, the critical infection efficiency is given by equation (2.4) with the mean-field value  evaluated for the system with mean overlap
evaluated for the system with mean overlap  . The dispersion in the overlaps results in the following approximate expression for Δk (see detailed derivation in the electronic supplementary material):
. The dispersion in the overlaps results in the following approximate expression for Δk (see detailed derivation in the electronic supplementary material):
 |
3.6 |
where the quantity V2 = (1/3(1 + pc))∑α=13(Jα − 〈𝒥〉)2 accounts for the anisotropy of the overlaps. The value of 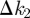 is non-negative meaning that
is non-negative meaning that  , i.e. the anisotropy in host shape makes the system more resilient as compared with the system of isotropic hosts with the same mean overlap.
, i.e. the anisotropy in host shape makes the system more resilient as compared with the system of isotropic hosts with the same mean overlap.
As an example, figure 3b shows that  in the system of branching hosts, in agreement with the predicted behaviour (cf. diamonds and the dashed line). The inset shows the corresponding dispersion V2. Given that the deviation of
in the system of branching hosts, in agreement with the predicted behaviour (cf. diamonds and the dashed line). The inset shows the corresponding dispersion V2. Given that the deviation of  from
from  is small in the system of branching hosts considered, the correction
is small in the system of branching hosts considered, the correction  is in fact a good approximation to the actual deviation Δk. This is the expected behaviour for systems of hosts with moderate anisotropy.
is in fact a good approximation to the actual deviation Δk. This is the expected behaviour for systems of hosts with moderate anisotropy.
3.3. Arrangements 3. Identical ni and random ϕi
In the two previous sections, it has been shown that both the disorder and anisotropy of the hosts make systems of branching hosts more resilient against epidemics. The arrangements of types 1 and 2 are extreme cases in the sense that the first type highlights the morphological complexity ( and
and  ) whereas the second type highlights the effects of the anisotropy of the hosts (
) whereas the second type highlights the effects of the anisotropy of the hosts ( and
and  ). In generic arrangements, such as those of type 3 defined above, the two effects are present. By assuming that the transmissibilities between different pairs of hosts are independent, it is possible to show that the behaviour of the actual heterogeneous system is equivalent to that of a mean-field homogeneous system with anisotropic transmissibilities
). In generic arrangements, such as those of type 3 defined above, the two effects are present. By assuming that the transmissibilities between different pairs of hosts are independent, it is possible to show that the behaviour of the actual heterogeneous system is equivalent to that of a mean-field homogeneous system with anisotropic transmissibilities  , where
, where  is the average of the transmissibility along the direction α in the lattice. A proof for this statement, which represents a generalization to systems with anisotropic transmissibilities of the mean-field description suggested in Sander et al. (2002, 2003), is given in the electronic supplementary material. The equation for the invasion threshold in this case is
is the average of the transmissibility along the direction α in the lattice. A proof for this statement, which represents a generalization to systems with anisotropic transmissibilities of the mean-field description suggested in Sander et al. (2002, 2003), is given in the electronic supplementary material. The equation for the invasion threshold in this case is
| 3.7 |
which generalizes the formulae (3.1) and (3.4) in such a way that equation (3.1) corresponds to the particular case of equation (3.7) when  is the same along all the directions and equation (3.4) emerges when there is no disorder in the anisotropic transmissibilities.
is the same along all the directions and equation (3.4) emerges when there is no disorder in the anisotropic transmissibilities.
As for the previous arrangements, the critical infection efficiency obeys equation (2.4) with both heterogeneity and anisotropy contributing to Δk. The lowest-order approximation to the lower bound for Δk is given by the relation  (see details in the electronic supplementary material) where
(see details in the electronic supplementary material) where  and
and  are defined in equations (3.3) and (3.6), respectively, with the dispersions terms generalized to
are defined in equations (3.3) and (3.6), respectively, with the dispersions terms generalized to
 |
3.8 |
and
 |
3.9 |
Figure 3b shows that the increase in orientational variability  brings additional heterogeneity in the system and thus results in a decrease of V2 but this does not necessarily induce a decrease in the value of critical infection efficiency as the contribution V1 may increase. This illustrates the interplay between the role of disorder and host anisotropy.
brings additional heterogeneity in the system and thus results in a decrease of V2 but this does not necessarily induce a decrease in the value of critical infection efficiency as the contribution V1 may increase. This illustrates the interplay between the role of disorder and host anisotropy.
3.4. Description of the invasion threshold in terms of morphological characteristics
In the previous sections, we have established a link between the invasion threshold and host overlaps 𝒥 characterized by the first moment,  , and the deviations from the mean, V1 and V2. The missing link between the host morphology and invasion threshold can be recovered by studying how the morphology affects overlaps. Here, we show that both
, and the deviations from the mean, V1 and V2. The missing link between the host morphology and invasion threshold can be recovered by studying how the morphology affects overlaps. Here, we show that both  and the anisotropy of 𝒥 (i.e. V2) can be well described in terms of a reduced number of morphological characteristics. In contrast, a proper description of the disorder-induced contribution from
and the anisotropy of 𝒥 (i.e. V2) can be well described in terms of a reduced number of morphological characteristics. In contrast, a proper description of the disorder-induced contribution from  in terms of a reasonably small set of morphological characteristics is hardly possible and requires instead knowledge of the spatial host density,
in terms of a reasonably small set of morphological characteristics is hardly possible and requires instead knowledge of the spatial host density,  (see equation (2.2)). However, we can ignore the disorder-induced contributions from
(see equation (2.2)). However, we can ignore the disorder-induced contributions from  in order to obtain a safe lower bound for critical infection efficiency and thus connect this quantity with morphological characteristics of hosts.
in order to obtain a safe lower bound for critical infection efficiency and thus connect this quantity with morphological characteristics of hosts.
We start the analysis by considering arrangements of type 1. The overlaps 𝒥 are statistically isotropic (i.e.  , figure 3b) so that the invasion threshold can be described in terms of a mean-field system with homogeneous and isotropic overlaps,
, figure 3b) so that the invasion threshold can be described in terms of a mean-field system with homogeneous and isotropic overlaps,  , and corresponding transmissibilities 〈𝒯 〉. The mean-field system consists of effective circular hosts of radius
, and corresponding transmissibilities 〈𝒯 〉. The mean-field system consists of effective circular hosts of radius  with overlap
with overlap  . These effective circles are fully described by the radius,
. These effective circles are fully described by the radius,  , and density,
, and density,  , which is positive for
, which is positive for  and is zero otherwise. The functional form of
and is zero otherwise. The functional form of  is the same as of real branching hosts in [0, rmf], i.e.
is the same as of real branching hosts in [0, rmf], i.e.  where
where  stands for the fractal dimension (see the electronic supplementary material for more detail). The radius of the effective circles is defined as
stands for the fractal dimension (see the electronic supplementary material for more detail). The radius of the effective circles is defined as 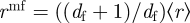 to ensure that the average radius of the effective circles coincides with the average radius of the actual hosts,
to ensure that the average radius of the effective circles coincides with the average radius of the actual hosts,  (see the electronic supplementary material). Under these assumptions, the expression for the overlap between neighbouring effective circles is given by
(see the electronic supplementary material). Under these assumptions, the expression for the overlap between neighbouring effective circles is given by
 |
3.10 |
valid for small overlaps when  . The parameter M is a normalization constant (see the electronic supplementary material). The substitution of this expression for
. The parameter M is a normalization constant (see the electronic supplementary material). The substitution of this expression for  into equation (3.2) gives the required link between the invasion threshold and the effective radius,
into equation (3.2) gives the required link between the invasion threshold and the effective radius,  . In fact, for the particular system of branching hosts studied here, this is a very good estimate for the value of the critical infection efficiency,
. In fact, for the particular system of branching hosts studied here, this is a very good estimate for the value of the critical infection efficiency,  , as demonstrated in figure 3 (the solid circled line falls inside the shaded grey area representing the set of possible values for
, as demonstrated in figure 3 (the solid circled line falls inside the shaded grey area representing the set of possible values for  within statistical errors). This means that for the arrangements of type 1, the mean-field homogeneous system of effective circles can be reliably used for estimating the phase boundaries.
within statistical errors). This means that for the arrangements of type 1, the mean-field homogeneous system of effective circles can be reliably used for estimating the phase boundaries.
In the case of ordered (type 2) and partially ordered (type 3) arrangements, the overlaps along distinct lattice directions can be significantly different and thus crucial for evaluation of the invasion threshold in contrast to the mean overlap which is important for disordered (mean-field-like) arrangements of type 1. Therefore, instead of a single characteristic such as mean radius of the effective circles for disordered arrangements, we introduce an ordered set of linear sizes of branching hosts along the lattice directions, i.e. the set of lattice-adapted diameters (LADs),  (
( ; see illustration in figure 5). The second lattice-adapted diameter (SLAD),
; see illustration in figure 5). The second lattice-adapted diameter (SLAD),  , plays the most important role for finding the critical value of, for example, lattice spacing, and thus estimating the invasion threshold. This is due to the fact that equations (3.4) and (3.7) have a solution for the critical threshold only if the overlaps at least along two directions are finite. The overlaps become greater than zero if the LADs are comparable or greater than the lattice spacing and thus solution of equations (3.4) and (3.7) exists if
, plays the most important role for finding the critical value of, for example, lattice spacing, and thus estimating the invasion threshold. This is due to the fact that equations (3.4) and (3.7) have a solution for the critical threshold only if the overlaps at least along two directions are finite. The overlaps become greater than zero if the LADs are comparable or greater than the lattice spacing and thus solution of equations (3.4) and (3.7) exists if  . This qualitative analysis suggests the existence of strong correlations between the SLAD and
. This qualitative analysis suggests the existence of strong correlations between the SLAD and 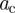 . Indeed, we have observed such correlations, i.e.
. Indeed, we have observed such correlations, i.e.  , for ordered arrangements of type 2 (figure 5). The description of
, for ordered arrangements of type 2 (figure 5). The description of  in terms of SLAD gets worse for small values of k (see the black circles in figure 5) when the strong overlaps between hosts should be achieved at criticality and thus the interior density of the hosts, rather than the diameter only, becomes important.
in terms of SLAD gets worse for small values of k (see the black circles in figure 5) when the strong overlaps between hosts should be achieved at criticality and thus the interior density of the hosts, rather than the diameter only, becomes important.
Figure 5.
Dependence of the critical lattice spacing,  , on the SLAD, d2, for all the branching hosts in arrangements of type 2. Different symbols refer to different values of k and each point for a particular symbol corresponds to an individual branching host. The solid lines represent the linear regression fit for each value of k (e.g. ac(k) = 14.1 + 0.94d2 with correlation coefficient ≃ 0.97 for k = 1). The inset defines graphically the LADs {d1, d2, d3}. Circle, k = 0.1; square, k = 1; triangle, k = 10.
, on the SLAD, d2, for all the branching hosts in arrangements of type 2. Different symbols refer to different values of k and each point for a particular symbol corresponds to an individual branching host. The solid lines represent the linear regression fit for each value of k (e.g. ac(k) = 14.1 + 0.94d2 with correlation coefficient ≃ 0.97 for k = 1). The inset defines graphically the LADs {d1, d2, d3}. Circle, k = 0.1; square, k = 1; triangle, k = 10.
4. Discussion and conclusions
Using the framework of an SIR epidemiological model, we have investigated the transmission of infection and spread of disease in systems of hosts with realistically complex morphology. Our main finding is that the greater the irregularity in the host morphology, the more resilient is the population to epidemic invasion under otherwise identical conditions (for instance, identical spatial arrangement of hosts). We derive a safe lower bound for the invasion threshold, which has been obtained for branching hosts with independent transmission rates placed on a triangular lattice. We have shown mathematically that this bound holds for all other two-dimensional topological arrangements of hosts with nearest-neighbour transmission and even in the case when the transmission rates between different hosts are correlated with each other. In particular, irregularity in the host positions, i.e. small random displacements of hosts from lattice nodes, brings correlations in transmissibilities and thus makes the system more resilient. Of course, for some real systems, the assumption about nearest-neighbour transmission may be violated by the presence of short-cuts between remote nodes owing, for example, to wind or animal motion. In this case, it is known that the system becomes less resilient to epidemic invasion (Sander et al. 2002) and the bounds given above are no longer valid.
We have used a set of planar neurons to illustrate the effects of complex branching hosts on the spread of infection. We have identified two sources of heterogeneity in the systems considered: (i) morphological complexity of hosts and (ii) the host shape anisotropy. Both contribute to the resilience of the system against epidemic invasion and can be described by means of two morphological characteristics of hosts, i.e. by the mean effective radius and the SLAD. Such characterization is not exact in general but it provides a safe bound to the invasion threshold. The main conclusions are generic and remain valid for any type of morphologically complex hosts. In particular, the methodology introduced here and the bounds for resilience to invasion apply to the transmission of infection in other two-dimensional systems with analogous disorder expressed in the host morphology and anisotropy. Examples include the spread of plant disease through contacts between adjacent plants in a field, orchard or forest in which the host plants frequently occur on a two-dimensional lattice. Here, the contact structures between nearest neighbours are determined by overlap of shoots (for aerial pathogens) or roots (for soil-borne pathogens; Gilligan 2008) in which the three-dimensional structure of the plant can be collapsed onto a two-dimensional framework when considering transmission of infection between nearest neighbours. In principle, a similar analysis could be performed for ensembles of morphologically complex three-dimensional hosts arranged on a two-dimensional lattice that takes explicit account of the three-dimensional host structure. Expressions for the invasion threshold are not known analytically in this case, and the precise definition of quantities directly linked to host morphologies such as fractal dimensions or average radius is system-dependent that requires further study. Other potential applications of the methods include analysis of the transmission of infection via the ‘morphology’ of contacts between clusters of susceptible hosts in social networks, in which the clusters can be approximated by a two-dimensional lattice (cf. recent work on percolation models for the spread of plague through gerbil populations in lattices of interconnecting burrows; Davis et al. 2008).
The present work opens several possible directions of further research for practical applications in considering disease control strategies and for basic understanding of epidemic spread involving heterogeneous transmission of infection. For instance, our analysis suggests new ways for control of epidemics in real systems where host morphology is inherently complex. For example, in a system where an epidemic is active, a treatment enhancing the anisotropy in the transmission rates would be more efficient as compared with reduction of transmission rates in all directions, i.e. isotropically. Such might arise with the deployment of biological control agents to restrict the spread of infection of soil-borne pathogens in plant populations (Gibson et al. 1999). Biological control agents often exhibit marked variability in performance (Gibson et al. 1999), failing to provide isotropic control of infection on targeted hosts, for example, owing to uneven colonization of roots by microbial antagonists deployed as biological control agents. Our results suggest that increasing the degree of anisotropy owing to these organisms may yet contribute to success in controlling invasion. Anisotropy may also be fostered in social or animal systems by preferential treatment of some, rather than all, connections between clusters. The findings in the current paper are also important for better understanding virus tracing of neurons (e.g. Loewy 1998), which is a biological staining method where virus propagation from neuron to neuron is used as a means to histologically mark the interconnections, so that they become visible to the microscope. More specifically, our results imply that virus tracing might be not so effective in the case of anisotropic or not so complex neuronal cells, which could therefore be overlooked by this type of marking. Analogue effects can also be important in transneuronal spreading of virus in order to deliver gene therapy (Oztas 2003).
Finally, the approach presented here is relevant to epidemics for which an SIR model is suitable and a mapping to ordinary percolation exists. While many diseases can be described by the SIR framework, others cannot. It would be interesting to analyse the effect of host morphology within the framework of a different family of epidemiological models with final state not immune to the disease (such as the susceptible–infected–susceptible model). Such an extension is challenging as the mapping to ordinary percolation is no longer possible requiring tools such as directed percolation (Marro & Dickman 1999; Hinrichsen 2000) from non-equilibrium physics.
Acknowledgements
F.J.P.R., S.N.T., F.M.N. and C.A.G. thank BBSRC for funding (grant no. RG46853). C.A.G. also acknowledges support of a BBSRC professorial fellowship. The authors are grateful to Prof. David Schubert (Salk Institute) for comments on disease spreading among neurons. L.D.F.C. thanks CNPq (308231/03-1) and FAPESP (05/00587-5) for sponsorship. Part of this work was performed during a Visiting Scholarship of L.D.F.C. to St Catharine's College, University of Cambridge.
References
- Ascoli G. A. 2006. Mobilizing the base of neuroscience data: the case of neuronal morphologies. Nat. Rev. Neurosci. 7, 318–324. ( 10.1038/nrn1885) [DOI] [PubMed] [Google Scholar]
- Boender G. J., Hagenaars T. J., Bouma A., Nodelijk G., Elbers A. R. W., de Jong M. C. M., van Boven M. 2007. Risk maps for the spread of highly pathogenic avian influenza in poultry. PLoS Comput. Biol. 3, e71 ( 10.1371/journal.pcbi.0030071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.-S., Yao Y.-C., Lin S.-C., Lee Y.-P., Wang Y.-F., Wang J.-R., Liu C.-C., Lei H.-Y., Yu C.-K. 2007. Retrograde axonal transport: a major transmission route of enterovirus 71 in mice. J. Virol. 81, 8996–9003. ( 10.1128/JVI.00236-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook A. R., Otten W., Marion G., Gibson G. J., Gilligan C. A. 2007. Estimation of multiple transmission rates for epidemics in heterogeneous populations. Proc. Natl Acad. Sci. USA 104, 20 392–20 397. ( 10.1073/pnas.0706461104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J. T., Durrett R. 1988. Limit theorems for the spread of epidemics and forest fires. Stoch. Proc. Appl. 30, 171–191. ( 10.1016/0304-4149(88)90083-X) [DOI] [Google Scholar]
- Davis S., Trapman P., Leirs H., Begon M., Heesterbeek J. 2008. The abundance threshold for plague as a critical percolation phenomenon. Nature 454, 634–637. ( 10.1038/nature07053) [DOI] [PubMed] [Google Scholar]
- Ehrengruber M. U., Ehler E., Billeter M. A., Naim H. Y. 2002. Measles virus spreads in rat hippocampal neurons by cell-to-cell contact and in a polarized fashion. J. Virol. 76, 5720–5728. ( 10.1128/JVI.76.11.5720-5728.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisinger D., Thulke H.-H. 2008. Spatial pattern formation facilitates eradication of infectious diseases. J. Appl. Ecol. 45, 415–423. ( 10.1111/j.1365-2664.2007.01439.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G. J., Gilligan C. A., Kleczkowski A. 1999. Predicting variability in biological control of a plant–pathogen system using stochastic models. Proc. R. Soc. Lond. B 266, 1743–1753. ( 10.1098/rspb.1999.0841) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G. J., Kleczkowski A., Gilligan C. A. 2004. Bayesian analysis of botanical epidemics using stochastic compartmental models. Proc. Natl Acad. Sci. USA 101, 12 120–12 124. ( 10.1073/pnas.0400829101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilligan C. A. 2008. Sustainable agriculture and plant diseases: an epidemiological perspective. Phil. Trans. R. Soc. B 363, 741–759. ( 10.1098/rstb.2007.2181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- González M. C., Hidalgo C. A., Barabási A.-L. 2008. Understanding individual human mobility patterns. Nature 453, 779–782. ( 10.1038/nature06958) [DOI] [PubMed] [Google Scholar]
- Grassberger P. 1983. On the critical behavior of the general epidemic process and dynamical percolation. Math. Biosci. 63, 157–172. ( 10.1016/0025-5564(82)90036-0) [DOI] [Google Scholar]
- Hinrichsen H. 2000. Non-equilibrium critical phenomena and phase transitions into absorbing states. Adv. Phys. 49, 815–958. ( 10.1080/00018730050198152) [DOI] [Google Scholar]
- Isichenko M. B. 1992. Percolation, statistical topography, and transport in random media. Rev. Mod. Phys. 64, 961–1043. ( 10.1103/RevModPhys.64.961) [DOI] [Google Scholar]
- Kenah E., Robins J. M. 2007. Second look at the spread of epidemics on networks. Phys. Rev. E 76, 036113 ( 10.1103/PhysRevE.76.036113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuulasmaa K. 1982. The spatial general epidemic and locality dependent random graphs. J. Appl. Prob. 19, 745–758. ( 10.2307/3213827) [DOI] [Google Scholar]
- LaVail J. H., Topp K. S., Giblin P. A., Garner J. A. 1997. Factors that contribute to the transneuronal spread of herpes simplex virus. J. Neurosci. Res. 49, 485–496. ( 10.1002/(SICI)1097-4547(19970815)49:4%3C485::AID-JNR9%3E3.0.CO;2-4) [DOI] [PubMed] [Google Scholar]
- Levin S. A., Durrett R. 1996. From individuals to epidemics. Phil. Trans. R. Soc. Lond. B 351, 1615–1621. ( 10.1098/rstb.1996.0145) [DOI] [PubMed] [Google Scholar]
- Liggett T. M. 1985. Interacting particle systems. New York, NY: Springer. [Google Scholar]
- Loewy A. D. 1998. Viruses as transneuronal tracers for defining neural circuits. Neurosci. Biobehav. Rev. 22, 679–684. ( 10.1016/S0149-7634(98)00006-2) [DOI] [PubMed] [Google Scholar]
- Marro J., Dickman R. 1999. Nonequilibrium phase transitions in lattice models. Cambridge, MA: Cambridge University Press. [Google Scholar]
- Miller J. C. 2007. Epidemic size and probability in populations with heterogeneous infectivity and susceptibility. Phys. Rev. E 76, 010101 ( 10.1103/PhysRevE.76.010101) [DOI] [PubMed] [Google Scholar]
- Murray J. D. 2002. Mathematical biology. I. An introduction, 3rd edn. Berlin, Germany: Springer. [Google Scholar]
- Newman M. E. J. 2002. Spread of epidemic disease on networks. Phys. Rev. E 66, 016128 ( 10.1103/PhysRevE.66.016128) [DOI] [PubMed] [Google Scholar]
- Ódor G. 2004. Universality classes in nonequilibrium lattice systems. Rev. Mod. Phys. 76, 663–724. ( 10.1103/RevModPhys.76.663) [DOI] [Google Scholar]
- Oztas E. 2003. Neuronal tracing. Neuroanatomy 2, 2–5. [Google Scholar]
- Samuel M. A., Wang H., Siddharthan V., Morrey J. D., Diamond M. S. 2007. Axonal transport mediates West Nile virus entry into the central nervous system and induces acute flaccid paralysis. Proc. Natl Acad. Sci. USA 104, 17 140–17 145. ( 10.1073/pnas.0705837104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander L., Warren C. P., Sokolov I. M., Simon C., Koopman J. 2002. Percolation on heterogeneous networks as a model for epidemics. Math. Biosci. 180, 293–305. ( 10.1016/S0025-5564(02)00117-7) [DOI] [PubMed] [Google Scholar]
- Sander L., Warren C. P., Sokolov I. M. 2003. Epidemics, disorder, and percolation. Physica A 325, 1–8. ( 10.1016/S0378-4371(03)00176-6) [DOI] [Google Scholar]
- Soriano J., Martínez M. R., Tlusty T., Moses E. 2008. Development of input connections in neural cultures. Proc. Natl Acad. Sci. USA 105, 13 758–13 763. ( 10.1073/pnas.0707492105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer J., Aharony A. 1994. Introduction to percolation theory, 2nd edn. Washington, DC: Taylor & Francis. [Google Scholar]
- Sykes M. F., Essam J. W. 1964. Exact critical percolation probabilities for site and bond problems in two dimensions. J. Math. Phys. 5, 1117–1127. ( 10.1063/1.1704215) [DOI] [Google Scholar]
- Truscott J. E., Gilligan C. A. 2003. Response of a deterministic epidemiological system to a stochastically varying environment. Proc. Natl Acad. Sci. USA 100, 9067–9072. ( 10.1073/pnas.1436273100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz E. 2008. Susceptible–infected–recovered epidemics in populations with heterogeneous contact rates. Eur. Phys. J. B 63, 381–386. ( 10.1140/epjb/e2008-00131-0) [DOI] [Google Scholar]







