Abstract
Mixtures of fast-acting and long-acting insulins were administered nasally to anesthetized, hyperglycemic rats in the presence and absence of tetradecyl-β-D-maltoside (TDM). The fast-acting analogs, aspart insulin, lispro insulin, and glulisine insulin, were all rapidly absorbed from the nose when applied individually with 0.125% TDM (Tmax = 15 minutes). One long-acting insulin analog, glargine insulin, was also absorbed from the nose when applied individually in the presence of 0.125% TDM (Tmax = 60 minutes). The other long-acting insulin analog, detemir insulin, was not soluble when formulated with 0.125% TDM. A series of mixtures (1:1) of the three rapid-acting insulins and long-acting glargine insulin were formulated with 0.125% TDM and applied nasally. The pharmacokinetic and pharmacodynamic profiles of the insulin mixtures reflected the additive contributions of both the rapid-acting and the long-acting insulin. These results support the possibility of formulating certain insulin mixtures in tandem to provide nasal insulin products that match the needs of patients with diabetes mellitus better than those currently available.
Keywords: Absorption Enhancer, Nasal Absorption, Surfactants, Insulin, Diabetes, Drug Delivery
1. Introduction
Patients with diabetes mellitus require precise and timely administration of insulin to maintain normal glycemic control. Continuous subcutaneous insulin infusion (CSII) pumps provide a steady flow of insulin, but require the purchase of expensive equipment and vigilant patient oversight. CSII is further complicated by the short and sometimes variable length of time a device will remain patent after insertion. Occlusion of CII lines then creates a hazardous situation in which patients are not receiving adequate insulin delivery. Subcutaneous injections of insulin remain the most widely used approach for insulin delivery. Fast-acting and long-acting analogs of insulin have allowed many patients to achieve improved glycemic control, with fast-acting insulin injections before each meal to cover food intake and one or possibly two injections of long acting insulin to cover insulin requirements between meals and overnight. The currently available fast– acting insulins (lispro insulin, aspart insulin and glulisine insulin) have distinctly quicker onsets of action compared to regular insulins used previously. Unfortunately, many patients still encounter great difficulty in successfully utilizing injections of these insulins to maintain a glycemic status near normal. Additionally, many Type 2 diabetic patients are averse to beginning insulin therapy because of a reluctance or fear of performing subcutaneous injections. The fact that the fast-acting and long-acting insulin analogs currently available cannot be mixed, and injected together, mandates more injections per day, and this creates a still higher barrier for patients with Type 1 or Type 2 diabetes to overcome.
To circumvent the problems associated with subcutaneous injections or CSII, alternate routes of insulin administration have been explored, but to date these have been found to present barriers to insulin absorption of varying intensities. The pulmonary route of delivery can be utilized without the aid of any absorption-enhancing agent, albeit with a modest bioavailability of approximately 10% of the insulin administered. An inhaled insulin product was developed, tested and shown to be effective. Unfortunately, issues with patient acceptance and pulmonary health, variability of insulin absorption among smokers, and a requirement for monitoring pulmonary function, all contributed to the decision to withdraw this product. While this was certainly a disappointing episode, it provided the proof-of-concept that, with improved technology and refined formulations, an insulin product could be administered by a non-invasive route. This realization is further evidenced by the fact that another inhaled insulin product is in development. Other routes of insulin delivery under consideration include oral, buccal, transdermal and nasal delivery.
The nasal route of delivery, like all alternate routes, offers some potential advantages and disadvantages. The primary challenge to any nasal insulin product is to achieve substantial and reproducible bioavailability from the nasal cavity, a system that is naturally designed to warm and moisten air, and to remove particulate matter, not designed to absorb drugs. Despite the nearly absolute impermeability of the nasal passage to insulin under basal conditions, there is a remarkable increase in insulin absorption when a very small concentration of an alkylglycoside surfactant agent, with the appropriate size and hydrophilic/hydrophobic balance, is added to the formulation. Alkylglycoside surfactants that consist of an alkyl chain 12-14 carbons in length, and a disaccharide, such as dodecyl-β-D-maltoside (DDM), tetradecyl-β-D-maltoside (TDM) or dodecylsucrose, produce a transient increase in the nasal permeability to a wide variety of both peptide (Pillion et al., 1998; Pillion et al., 2002; Arnold et al., 2004) and non-peptide (Arnold et al., 2002) drugs. It is important to note that alkylglycosides with side chains shorter than 10 carbons in length, or those linked to a monosaccharide, fail to have this effect on the nasal cavity. Studies with cultured epithelial cells and with intact animals provide evidence that the alkylglycosides increase both paracellular and transcellular insulin absorption (Arnold et al., 2004; Ahsan et al., 2003).
The effects of alkylglycosides on the permeability of the nasal cavity in vivo are transient, with maximal effectiveness observed immediately after application and gradual reversal over the next 2-4 hours. From a clinical perspective, this pattern of transient loosening of the permeability barrier of the nose overlaps with the most likely pattern of use for a nasal fast-acting insulin product, i.e. the nasal insulin would be taken just before meals and produce an immediate hypoglycemic response. Animal experiments have shown that the pharmacokinetic profiles of regular insulin and the fast-acting analog lispro insulin were similar when applied nasally in the presence of TDM, with very rapid absorption of insulin (Tmax = 15 minutes) and return to baseline insulin values within 1-2 hours (Pillion et al., 1998). The long-acting insulin analog, glargine insulin, gave different results when applied nasally in the presence of alkyglycosides (Arnold et al., 2009). Glargine insulin showed a slower onset (Tmax = 60 minutes) and an extended duration of absorption and action.
This study is designed to address the following two questions:
Can a fast-acting insulin analog be mixed with a long-acting insulin analog and be formulated in the presence of an alkylglycoside?
If so, can the mixture be applied nasally and absorbed into the circulation to produce a blended pharmacokinetic/pharmacodynamic profile that would be suitable for treating a patient with diabetes mellitus?
2. Materials and Methods
2.1 Animals
Studies were performed in Sprague-Dawley male rats (200-450 g) obtained from Charles River Laboratories (Charlotte, NC). Standard laboratory food and tap water were available ad libitum.
The animal study was conducted according to the principles outlined in the “Guide for the Care and Use of Laboratory Animals,” Institute of Laboratory Animal Resources, National Research Council.
2.2 Materials
Glargine insulin (Lantus®) and glulisine insulin (Apidra®) were obtained from Sanofi/Aventis (Bridgewater, NJ). Detemir insulin (Levemir®,) aspart insulin (Novolog®), NPH insulin (Novolin N®), and regular insulin (Novolin R®) were all obtained from Novo Nordisk Pharmaceuticals, Inc. (Princeton, NJ). Lispro insulin (Humalog ®) was obtained from Eli. Lilly Corp. (Indianapolis, IN.) Tetradecyl-B-D-maltoside (TDM) was purchased from Anatrace, Inc. (Maumee, OH). Xylazine HCl (100 mg/ml) was obtained from Vedco Inc. (St. Joseph, MO). Ketamine HCl (100 mg/ml) was obtained from Lloyd Laboratories (Shenandoah, IA). Isoflurane was obtained from Minrad Inc. (Bethlehem, PA). Heparin (1000 U/ml) was obtained from Elinks-Sin Inc, (Cherry Hill, NJ). A human insulin specific radioimmunoassay kit was obtained from Millipore Corp. (St. Louis, MO).
2.3 Analytic Procedures
Rats were anesthetized with 4% isoflurane, initially at a flow rate of 3-4 L/min, and then anesthesia was maintained, when necessary, with 1-2% isoflurane at a flow rate of 1-2 L/min. Rats were then removed from isoflurane and injected with ketamine (100 mg/kg) and xylazine (10 mg/kg) intramuscularly to induce hyperglycemia. Anesthesia was maintained with additional ketamine/xylazine as needed throughout the experiment. Sixty minutes after the first administration of ketamine/xylazine, blood was collected from the tail for the determination of basal glucose and insulin levels. Nose drops (0.02 ml) containing 1 Unit of insulin were administered to the right nares of anesthetized rats in the supine position using a pipettor with a disposable plastic tip. Rats then received a second 0.02 ml dose of the nasal formulation containing 1 Unit of insulin applied to the right nares 2 minutes later. The rats were turned over to the prone position 2 minutes later. This experimental protocol has been utilized to prevent airway obstruction that can occur if nose drops are applied to both nares at the same time and to eliminate leakage of the formulation from the nose. Nose drops were formulated by mixing one volume of insulin (U-100) with one volume of either 0.9% NaCl or with 0.25% TDM in 0.9% NaCl. In some experiments, phosphate buffered saline was used in place of 0.9% NaCl. No differences were observed in the experiments utilizing phosphate buffered saline. Glucose levels were measured in drops of blood taken from the tip of the rat tail using a glucose meter (Glucometer Elite™, Bayer Corp., Elkhart, IN) at various times after the administration of insulin. The upper limit of the glucose meter was 600 mg/dL. Insulin levels were measured using a commercially available human insulin RIA kit. Plasma samples were prepared by collecting rat blood from the tip of the tail in tubes containing 5 units of heparin.
2.4 Statistical Analysis
In nasal drug delivery studies, the maximal concentration (Cmax) of insulin and the time to maximal concentration (Tmax) were determined directly from the pharmacokinetic profile. The area under the curve (AUC0-180) was determined via the linear trapezoidal rule. Results are presented as mean +/- standard error. Statistical significance was determined with Student's T-test (SigmaStat Software, SPSS, Inc., Chicago, IL). Differences with a p value of less than 0.05 were considered significant.
3. Results
Previous studies have shown that rats anesthetized with ketamine/xylazine become hyperglycemic and hypoinsulinemic within 60 minutes (Pillion et al., 1994; Saha et al., 2005; Saha et al., 2006). This experimental system allows an exquisitely sensitive platform in which to study the absorption of exogenous human insulin delivered nasally. In this study, seven different forms of insulin were compared. Nasal administration of three different fast-acting insulin analogs, in the presence of 0.125% TDM, caused a rapid and significant increase in plasma insulin levels (Figure 1A). Maximal insulin levels were obtained within 15 minutes for all 3 insulin analogs and insulin levels returned to baseline within 120 minutes. Two of the three fast-acting insulin analogs, lispro and aspart, were absorbed more effectively (Cmax = 907 +/- 65 uU/ml and 1073 +/-45 uU/ml respectively) than glulisine (Cmax = 505 +/- 99 uU/ml). None of the three fast-acting insulin analogs were absorbed when formulated without TDM. Three other forms of insulin (regular, NPH and glargine insulins) were also absorbed from the nasal cavity when formulated with 0.125% TDM (Figure 1B). Like the fast-acting insulins shown in Fig 1A, none of these three forms of insulin were absorbed in the absence of TDM. The Tmax for NPH insulin (30 minutes) and glargine insulin (60 minutes) were different than the Tmax for regular insulin and the fast-acting insulin analogs (15 minutes). Glargine insulin applied nasally in the presence of 0.125% TDM caused a much greater increase in plasma insulin levels 180 minutes after administration than the other forms of insulin tested
Figure 1.
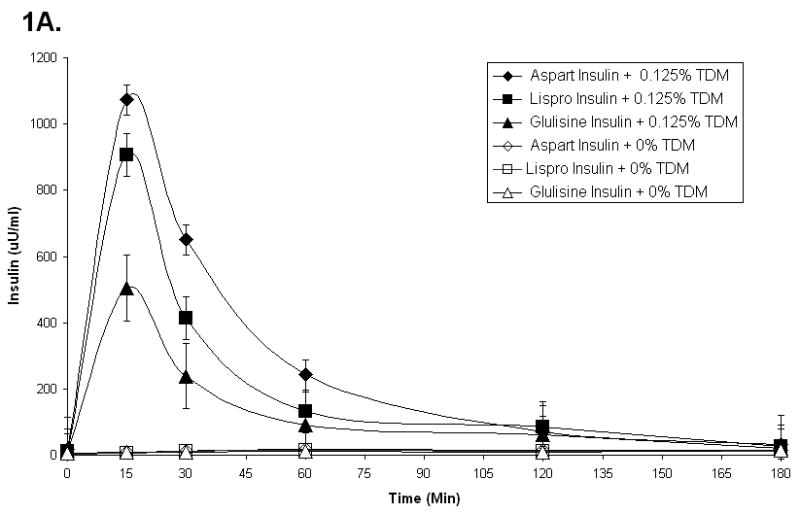

Plasma insulin levels in rats that received 2 units of various fast-acting insulins (A) or regular or long-acting insulins (B) with or without 0.125% TDM. Each data point represents the mean +/- SEM of 3-6 animals.
The other long-acting form of insulin, detemir insulin, formed a cloudy mixture when formulated with 0.125% TDM. When this cloudy mixture was applied to rats nasally, under the same conditions as those described above for the other six insulin products, no measurable absorption of insulin was detected.
The data in Figure 1 have been used to determine the total amount of insulin absorbed from the nasal cavity (AUC0-180) for each of the insulin analogs (Table 1). It should be noted that glargine insulin levels had not returned to baseline values 180 minutes after nasal administration. Hence, the values reported for glargine insulin absorption in Table 1 under-represent total glargine insulin absorption when compared to each of the other forms of insulin tested. The total absorption of glulisine insulin (AUC 0-180) and the maximal concentration of glulisine insulin (Cmax) were consistently less than those observed for the other insulin analogs. Nasal delivery of aspart insulin generated the highest concentration of insulin (Cmax) immediately following administration, while nasal delivery of glargine insulin produced the greatest bioavailability of insulin (AUC 0-180) (Table 1).
Table I.
Effect of Tetradecyl-β-D-maltoside (TDM) on the Nasal Absorption of Various Insulins
| Conditions | Plasma Insulin | |||
|---|---|---|---|---|
| 0.125% TDM (+/-) |
Type of Insulin | AUC 0-180 (uU/ml × minutes) |
C-max (uU/ml) |
T-max (minutes) |
| (-) | Regular Insulin * | N/D | N/D | N/D |
| (+) | Regular Insulin | 33398 +/- 2500 a | 874 +/- 56 a | 15 |
| (+) | Lispro Insulin | 41820 +/- 3300 a | 907 +/- 62 a | 15 |
| (+) | Aspart Insulin | 55238 +/- 4600 a,b | 1073 +/- 45 a, b | 15 |
| (+) | Glulisine Insulin | 25335 +/- 1200 a,b | 505 +/- 99 a, b | 15 |
| (+) | NPH Insulin | 41790 +/- 3900 a | 653 +/- 48 a, b | 30 |
| (+) | Glargine Insulin | 67170 +/- 4800 a,b | 500 +/- 52 a, b | 60 |
Rats received nose drops containing 2 units of insulin with or without 0.125% TDM. Plasma insulin levels were determined at various times up to 180 minutes after nasal insulin administration. The AUC values were measured by the trapezoidal rule and compared to the AUC values observed with Regular Insulin. Data represent mean +/-SEM (n = 3-6.)
Similar results were obtained when other insulins were tested in the presence of 0% TDM.
Significantly different than the same insulin formulated with 0% TDM (p < 0.05.)
Significantly different than Regular insulin formulated with 0.125% TDM (p < 0.05
N/D = Not detectable
Blood glucose levels were also measured in parallel in the experiments described above to determine the bioavailability of the various insulin analogs, to determine the time-course of insulin action following nasal delivery, and to confirm that intact biologically active insulin, rather than a partially degraded but immunologically competent fragment was delivered to the circulation. All three of the fast-acting insulin analogs, formulated with 0.125% TDM and applied nasally, produced a rapid and substantial decrease in blood glucose concentrations compared to the same formulations lacking 0.125% TDM (Figure 2A.) Of the three fast-acting insulin analogs formulated with 0.125% TDM, glulisine insulin produced the smallest hypoglycemic response, consistent with the insulin absorption data presented in Fig. 1A and Table 1. The effects of all three fast-acting insulin analogs on the glycemic levels of the rats diminished after 180 minutes, again consistent with insulin absorption data presented in Fig. 1A. In Fig. 2B, the effects of regular insulin, NPH insulin, and glargine insulin nose drops formulated with and without 0.125% TDM are presented. Unlike the other 5 forms of insulin tested, long-acting glargine insulin produced a reduction in blood glucose concentration that was still robust after 180 minutes. Hence, the total hypoglycemic response to glargine insulin is under-represented when the AUC 0-180 data are compared to the other insulins. Additional experiments are required to define the duration of this effect. When the total hypoglycemic responses to the six forms of insulin were compared directly, glulisine insulin produced the smallest response when applied nasally, whereas regular insulin and glargine insulin produced the largest responses (Table 2.)
Figure 2.


Blood glucose levels in rats that received 2 units of various fast acting insulins (A) or regular or long-acting insulins (B) with or without 0.125% TDM. Each data point represents the mean +/- SEM of 3-6 animals.
Table II.
Effect of TDM on the Hypoglyemic Response to Various Insulins
| Conditions | Blood Glucose | ||
|---|---|---|---|
| 0.125% TDM (+/-) | Type of Insulin | AUC 0-180 (mg/dL × minutes) | Blood Glucose at 60 minutes after Insulin administration (mg/dL) |
| (-) | Regular Insulin * | 93728 +/- 2600 | 600 +/- 10 ** |
| (+) | Regular Insulin | 32745 +/- 2400 a | 135 +/- 22 a |
| (+) | Lispro Insulin | 39863 +/- 3200 a | 160 +/- 27 a |
| (+) | Aspart Insulin | 43470 +/- 4300 a,b | 205 +/- 13 a,b |
| (+) | Glulisine Insulin | 55335 +/- 4000 a,b | 242 +/- 15 a,b |
| (+) | NPH Insulin | 41730 +/- 3100 a,b | 174 +/- 19 a |
| (+) | Glargine Insulin | 36345 +/- 3600 a | 183 +/- 26 a |
Rats received nose drops containing 2 units of insulin with or without 0.125% TDM. The blood glucose levels were measured at various times up to 180 minutes after nasal insulin administration. The AUC values were measured by the trapezoidal rule and compared to the AUC values observed with Regular Insulin. Basal glucose levels ranged from 303 to 393mg/dl. Data represent mean +/- SEM (n=3-6.)
Significantly different than the same insulin formulated with 0% TDM (p < 0.05).
Significantly different than Regular insulin formulated with 0.125% TDM (p <0.05).
Similar results were obtained when other insulins were tested in the presence of 0% TDM.
Upper limit of glucometer = 600mg/dL
As described above, detemir insulin formed a cloudy mixture when formulated with 0.125% TDM. In order to test the impact of TDM on the nasal absorption of detemir insulin, a different experimental protocol was required. In these experiments, the nasal passages were pre-treated with or without 0.125% TDM. Nose drops containing 2 Units of detemir insulin formulated in 0.9% NaCl were administered 15 minutes later. For comparison, two other forms of insulin, fast-acting aspart insulin, and long-acting glargine insulin, were run in parallel. Under these experimental conditions, all three forms of insulin were absorbed from the nasal cavity when applied 15 minutes after 0.125% TDM, but not when applied after 0.9% NaCl (Figure 3). However, nasal delivery of aspart insulin produced a robust increase in plasma insulin concentrations, whereas glargine insulin and detemir insulin produced progressively smaller responses. Plasma insulin concentrations remained elevated for a longer duration when glargine insulin was applied nasally 15 minutes after 0.125% TDM. Blood glucose concentrations were also obtained in these experiments to determine the time course of insulin action following nasal delivery and to confirm that a biologically active form of insulin had been delivered to the circulation (Figure 4). Fast-acting aspart insulin produced a rapid and more substantial decrease in blood glucose when applied 15 minutes after 0.125% TDM than the long-acting insulin analogs, detemir and glargine. All of these changes in blood glucose concentrations were consistent with the insulin absorption data in Fig. 4.
Figure 3.

Plasma insulin levels in rats that received 2 units of various insulins 15 minutes after the administration of 0% or 0.125% TDM. Each data point represents the mean +/-SEM of 3-6 animals.
Figure 4.

Blood glucose levels in rats that received 2 units of various insulins 15 minutes after the administration of 0% or 0.125% TDM. Each data point represents the mean +/- SEM of 3-6 animals.
To determine if mixtures of fast-acting and long-acting insulins could be prepared with TDM and used successfully in nasal insulin delivery studies, long-acting glargine insulin (1 Unit) was mixed with either aspart insulin, lispro insulin or glulisine insulin (1 Unit) and applied to rats nasally in the presence or absence of 0.125% TDM (Fig. 5). All three formulations were clear and remained clear for several weeks when stored at 4° C. Absorption of insulin was observed with all three mixed insulin formulations that contained TDM, but not with formulations that lacked TDM. All three mixed insulin formulations containing TDM produced rapid insulin absorption from the nasal cavity (T max = 15 minutes), but insulin concentrations remained elevated for more than 180 minutes after administration, a pattern that reflected the combined uptake profiles of fast-acting and long-acting insulins. Of note, the absorption of aspart insulin and lispro insulin were not greater than the absorption of glulisine insulin when each fast-acting analog of insulin was mixed with glargine insulin, a result that was different from the results of experiments depicted in Fig.1A, where the short-acting insulins were tested individually. Importantly, at extended time points after administration of the insulin mixtures (T = 60, 120 and 180 minutes), insulin was present in the plasma at levels far above baseline (>100 uU/ml). No such residual insulin was observed in the experimental data presented in Fig. 1A when short-acting insulin analogs were tested individually, but it was observed in Fig. 1B when glargine insulin was tested individually. Blood glucose data from these experimental animals is presented in Fig. 6. The data are consistent with the insulin absorption results shown in Fig. 5. Animals displayed a rapid fall in blood glucose and a sustained hypoglycemic effect when any of the three mixtures were applied nasally in the presence of TDM.
Figure 5.
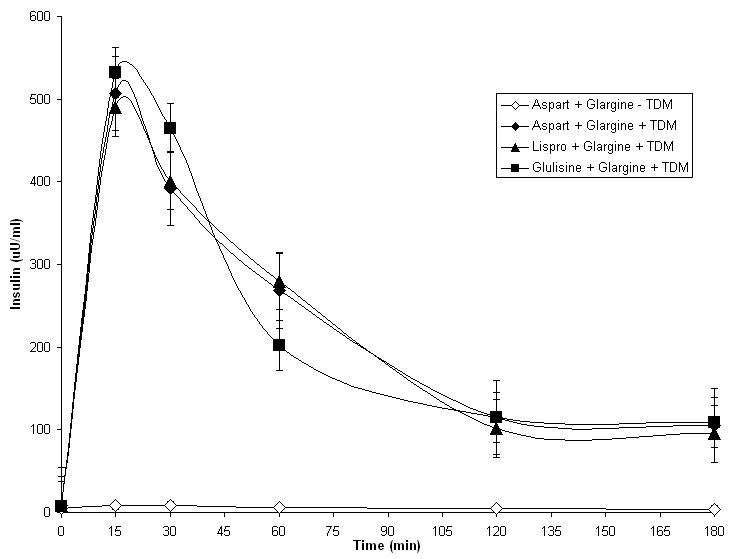
Plasma insulin levels in rats that received 2 units of a mixed insulin formulation with 0.125% TDM. Each data point represents the mean +/- SEM of 3-6 animals.
Figure 6.
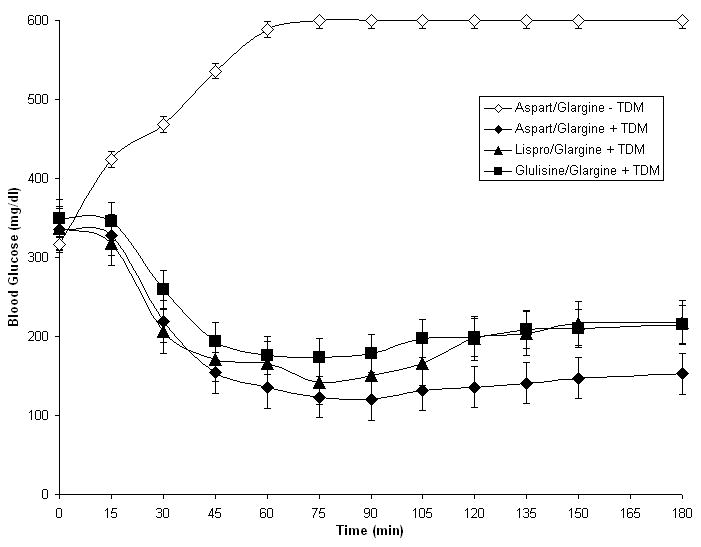
Blood glucose levels in rats that received 2 units of a mixed insulin formulation with 0.125% TDM. Each data point represents the mean +/- SEM of 3-6 animals.
4. Discussion
The results described above provide two seminal pieces of information:
All three forms of fast-acting insulin can be mixed with one long-acting form of insulin, glargine insulin, and formulated with TDM. The other long-acting form of insulin, detemir insulin, could not.
All three of the mixed insulin formulations, containing a fast-acting insulin analog and glargine insulin plus TDM, produced a blend of rapid and extended insulin absorption and corresponding hypoglycemic effects when applied nasally.
The nasal insulin absorption data obtained in the experiments utilizing mixtures of a fast-acting insulin analog and glargine insulin formulated with TDM are most directly interpreted as follows: the blended response to the insulin mixtures described in Figs. 5 and 6 include two overlapping and additive events. The initial peak of insulin absorption (Tmax = 15 minutes) is provided primarily by the absorption of the fast-acting insulin analog. This initial peak of fast-acting insulin absorption is followed by a prolonged period of glargine insulin absorption. This interpretation is consistent with the results obtained when the fast-acting forms of insulin and the long-acting glargine insulin were tested individually (Figs. 1 and 2).
Previous studies have shown that the concentration of TDM used in these studies, i.e. 0.125%, provided a robust increase in regular human insulin absorption with minimal toxicity to the cells that line the nasal cavity (Arnold et al., 2004). At a concentration of 0.25% or 0.50% TDM, slightly more insulin absorption was observed, while at a concentration of 0.06% TDM, incrementally less insulin absorption was observed. Nasal administration of regular human insulin in the presence of 0.06% and 0.125% TDM resulted in 44% and 55% relative bioavailability respectively when compared to subcutaneous administration of regular insulin (Arnold et al., 2004). By comparison, nasal administration of regular insulin in the presence of 0.25% and 0.50% TDM resulted in 64% and 77% relative bioavailability when compared to subcutaneous administration of regular insulin. At a concentration of 0.50% TDM, changes in the appearance of the cells that line the nasal cavity became more evident than the minor changes observed at a concentration of 0.125% TDM (Arnold et al., 2004).
In all of the nasal insulin delivery experiments described in this report, nasal insulin was administered in the form of liquid nose drops, applied from a stock solution by a pipettor. It is anticipated that nasal delivery of insulin formulations using a spray device, to instill small droplets of solution throughout the nasal cavity, will increase the surface area contacted by the insulin and increase the overall amount of insulin absorbed.
The results obtained in cell culture experiments and whole animal experiments are consistent with the hypothesis that the alkylglycoside causes a temporary perturbation of the nasal barrier to drug absorption. Earlier studies have shown that alkylglycosides increase both paracellular transport and transcellular transport of insulin (Ahsan et al., 2003; Arnold et al., 2004). Substitution of DDM for TDM provided essentially identical results.
While it is possible that an interaction takes place between molecules of the alkylglycoside surfactant and insulin in the nasal formulation prior to administration, direct evidence that this type of interaction directly alters insulin absorption is lacking. Formulations containing 0.125% TDM plus a mixture of glargine insulin with any of the three fast-acting insulins remained clear even after storage for several weeks. Alkylglycosides such as TDM and DDM can stabilize protein formulations and increase their effective shelf-life (Maggio, 2006). Hence, in these experiments, TDM may serve a dual role, to both stabilize the mixtures of short-acting and long-acting insulins, and also to serve as an absorption-enhancing agent. This remarkable duality of actions provides an important and unique role for TDM and DDM in the development of an effective stable nasal insulin formulation, whether it contains a single form of insulin or a mixture of fast-acting and long-acting insulins.
Several new questions are raised as a result of these findings. In the case of nasal delivery of glargine insulin, the precise mechanism involved in the delayed onset and prolonged duration of glargine insulin absorption observed in these experiments is not known. The nasal cavity has a large surface area and is bathed in fluid with a slightly acidic pH. This acidic environment could certainly present an opportunity for localized foci of glargine insulin precipitation to occur. It is not known for certain if long-acting glargine insulin remains in the nose in a depot environment and gradually is released from the depot to be absorbed slowly, as is generally considered to be the case following subcutaneous injections of glargine insulin.
The time course of the reversal of TDM action on nasal permeability to insulin becomes very important when considering how, and if, a patient with diabetes mellitus could utilize nasal formulations that contained both fast-acting and long-acting forms of insulin. Optimally, a patient could take a mixed nasal insulin formulation containing TDM at mealtime. Immediate absorption of fact-acting insulin would occur, to provide appropriate coverage for the glycemic burden consumed at the meal. Thereafter, the effect of TDM on the permeability of the nasal cavity would extend for several hours and allow long-acting insulin to be absorbed and provide extended duration of insulin coverage for at least three hours after the meal. Additional experimentation will be required to demonstrate that this same scenario holds true in humans and to better define the optimal insulin formulation composition to use (fast-acting and long-acting insulin concentrations and alkylglycoside concentrations can be varied to obtain optimal pharmacodynamic responses).
The rate and extent of absorption of all insulin analogs would be expected to be limited by several factors, including mucociliary clearance of unabsorbed insulin and TDM from the nose, impermeability of the nasal cavity cell surfaces that did not receive an effective dose of TDM because of how and where it was administered, the limited surface area available for absorption, and enzymatic destruction of insulin analogs and TDM, both in the nasal cavity and/or following absorption into the epithelial cells that line the nasal cavity (Newman et al., 1994; Illum L. 2003; Pillion et al., 2007). The distinct chemical composition of the insulin analogs may cause one or more of them to behave differently than the others during one or more of the several different stages of the nasal absorptive process.
It has been reported that mixtures of glargine insulin and either lispro insulin or aspart insulin became cloudy, but were still effective, when injected subcutaneously in humans (Kaplan et al., 2004). No increase in pain or adverse reactions was observed in patients receiving the cloudy insulin mixtures. Detemir insulin was not clear when mixed with 0.125% TDM and it could not be absorbed from the nose into the systemic circulation when mixed with TDM. This form of insulin was the only one of seven insulins tested that was not absorbed from the nose in the presence of TDM. NPH insulin was also cloudy, with or without TDM addition, but NPH insulin was absorbed from the nose when mixed with TDM. In some experiments, animals received nose drops with TDM alone and then the insulins were applied 15 minutes later. Under these conditions, detemir insulin, like aspart insulin and glargine insulin, was absorbed from the nose. Detemir insulin displayed a prolonged duration of action, much like glargine insulin. This type of approach is useful to demonstrate an aspect of TDM action on the nasal cavity, but it is impractical to develop a commercially successful nasal formulation containing detemir insulin if it requires the administration of an alkylglycoside absorption enhancer minutes before a separate application of the insulin. No such requirement exists for a formulation containing glargine insulin.
Recently it was reported that nasal administration of insulin formulated without an absorption enhancing agent caused a transient increase in insulin levels in the brain, without affecting plasma insulin levels or blood glucose levels (Reger et al., 2008). Remarkably, this treatment produced a significant improvement in short-term learning performance in patients with early signs of Alzheimer's disease (Benedict et al., 2007; Reger et al., 2008). This observation opens to door to increased scrutiny of how insulin can be absorbed from the nasal cavity by one route into the peripheral circulation, and by another route into the central nervous system. Extensive characterization of the optimal insulin formulations to be used in a nasal insulin product for patients with diabetes mellitus and alternate formulations for patients with Alzheimer's disease awaits further studies.
In summary, three fast acting insulin analogs (aspart, glulisine, and lispro insulins), were absorbed rapidly following nasal delivery in a formulation containing 0.125% TDM. The long-acting insulin analog, glargine insulin, demonstrated a slower rate of absorption and an extended hypoglycemic effect. Mixtures of long-acting glargine insulin with each of the three different short-acting insulins plus 0.125% TDM were successfully absorbed from the nasal cavity. All three mixtures displayed a pharmacokinetic profile that reflected absorption of both the fast-acting and the long-acting forms of insulin.
Acknowledgments
We would like to acknowledge the efforts of Ms. Libby Wilson, Dr. John Arnold, John Carstens, and Nich Kelley. Dr. Pillion is a member of the University of Alabama at Birmingham Diabetes Research and Training Center supported by the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahsan F, Arnold JJ, Yang T, Meezan E, Schwiebert EM, Pillion DJ. Effects of the permeability enhancers, tetradecylmaltoside and dimethyl-B-cyclodextrin, on insulin movement across human bronchial epithelial cells 16HBE 14o. Eur J Pharm Sci. 2003;20:27–34. doi: 10.1016/s0928-0987(03)00163-5. [DOI] [PubMed] [Google Scholar]
- 2.Arnold JJ. Doctoral Dissertation. University of Alabama at Birmingham; 2004. Systemic Delivery of Peptides and Macromolecules in Nasal Formulations Containing an Alkylglycoside. [Google Scholar]
- 3.Arnold JJ, Ahsan F, Meezan E, Pillion DJ. Nasal administration of low molecular weight heparin. J Pharm Sci. 2002;91(7):1707–14. doi: 10.1002/jps.10171. [DOI] [PubMed] [Google Scholar]
- 4.Arnold JJ, Ahsan F, Meezan E, Pillion DJ. Correlation of tetradecylmaltoside induced increase in nasal peptide drug delivery with morphological changes in nasal epithelial cells. J Pharmaceut Sci. 2004;93:2205–2213. doi: 10.1002/jps.20123. [DOI] [PubMed] [Google Scholar]
- 5.Arnold JJ, Fyrberg MD, Meezan E, Pillion DJ. Reestablishment of the Nasal Permeability Barrier to Several Peptides Following Exposure to the Absorption Enhancer Tetradecyl-β-D-Maltoside. J Pharmaceut Sci. 2009 doi: 10.1002/jps.21977. in press. [DOI] [PubMed] [Google Scholar]
- 6.Benedict C, Hallschmid M, Schmitz K, Schultes B, Ratter F, Fehm HL, Born J, Kern W. Intranasal insulin improves memory in humans: superiority of insulin aspart. Neuropsychopharmacology. 2007;32:239–243. doi: 10.1038/sj.npp.1301193. [DOI] [PubMed] [Google Scholar]
- 7.Illum L. Nasal drug delivery-possibilities, problems and solutions. J Controlled Release. 2003;87:187–98. doi: 10.1016/s0168-3659(02)00363-2. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan W, Rodriguez L, Smith OE, Haymond MW, Heptulla RA. Effects of Mixing Glargine and Short-Acting Insulin Analogs on Glucose Control. Diabetes Care. 2004;27:2739–40. doi: 10.2337/diacare.27.11.2739. [DOI] [PubMed] [Google Scholar]
- 9.Maggio ET. Intravail™: highly effective intranasal delivery of peptide and protein drugs. Expert Opinion on Drug Delivery. 2006;3:529–539. doi: 10.1517/17425247.3.4.529. [DOI] [PubMed] [Google Scholar]
- 10.Newman SP, Steed KP, Hardy JG, Wilding IR, Hooper G, Sparrow RA. The distribution of an intranasal insulin formulation in healthy volunteers: effect of different administration techniques. J Pharm Pharmacol. 1994;46:657–660. doi: 10.1111/j.2042-7158.1994.tb03877.x. [DOI] [PubMed] [Google Scholar]
- 11.Pillion DJ, Ahsan F, Arnold JJ, Balusubramanian BM, Piraner O, Meezan E. Synthetic long-chain alkyl maltosides and alkyl sucrose esters as enhancers of nasal insulin absorption. J Pharm Sci. 2002;91:1456–62. doi: 10.1002/jps.10150. [DOI] [PubMed] [Google Scholar]
- 12.Pillion DJ, Arnold JJ, Meezan E. Nasal delivery of peptide drugs. In: Touitou E, Boyd BW, editors. Enhancement in Drug Delivery. Chap. 20. 2007. pp. 373–392. [Google Scholar]
- 13.Pillion DJ, Atchison J, Garguilo C, Wang RX, Wang P, Meezan E. Insulin delivery in nosedrops: new formulations containing alkylglycosides. Endocrinology. 1994;135:2386–91. doi: 10.1210/endo.135.6.7988421. [DOI] [PubMed] [Google Scholar]
- 14.Pillion DJ, Hosmer S, Meezan E. Dodecylmaltoside-mediated nasal and ocular absorption of lyspro-insulin: independence of surfactant action from multimer dissociation. Pharmaceut Res. 1998;15:1637–1639. doi: 10.1023/a:1011975721569. [DOI] [PubMed] [Google Scholar]
- 15.Reger MA, Watson GS, Green PS, Wilkinson CW, Baker LD, Cholerton B, Fishel MA, Rlymate SR, Beitner JCS, DeGroodt W, Mehta P, Craft S. Intranasal insulin improves cognition and modulates β-amyloid in early AD. Neurology. 2008;70:440–448. doi: 10.1212/01.WNL.0000265401.62434.36. [DOI] [PubMed] [Google Scholar]
- 16.Saha JK, Xia J, Engle SK, Chen Y, Glaesner W, Jaubowski JS. A model of controlled acute hyperglycemia in rats: Effects of insulin and glucagon-like peptide-1 analog. J Pharm Exp Res. 2006;316:1159–1164. doi: 10.1124/jpet.105.093534. [DOI] [PubMed] [Google Scholar]
- 17.Saha JK, Xia J, Grondin J, Engle SK, Jaubowski JS. Acute hyperglycemia induced by ketamine/xylazine anesthesia in rats: Mechanisms and implications for preclinical models. Exp Biol Med. 2005;230:777–784. doi: 10.1177/153537020523001012. [DOI] [PubMed] [Google Scholar]


