Abstract
In the metabolically versatile bacterium Pseudomonas aeruginosa, the RNA-binding protein Crc is involved in catabolite repression of a range of degradative genes, such as amiE (encoding aliphatic amidase). We found that a CA-rich sequence (termed CA motif) in the amiE translation initiation region was important for Crc binding. The small RNA CrcZ (407 nt) containing 5 CA motifs was able to bind the Crc protein with high affinity and to remove it from amiE mRNA in vitro. Overexpression of crcZ relieved catabolite repression in vivo, whereas a crcZ mutation pleiotropically prevented the utilization of several carbon sources. The sigma factor RpoN and the CbrA/CbrB two-component system, which is known to maintain a healthy carbon–nitrogen balance, were necessary for crcZ expression. During growth on succinate, a preferred carbon source, CrcZ expression was low, resulting in catabolite repression of amiE and other genes under Crc control. By contrast, during growth on mannitol, a poor carbon source, elevated CrcZ levels correlated with relief of catabolite repression. During growth on glucose, an intermediate carbon source, CrcZ levels and amiE expression were intermediate between those observed in succinate and mannitol media. Thus, the CbrA–CbrB–CrcZ–Crc system allows the bacterium to adapt differentially to various carbon sources. This cascade also regulated the expression of the xylS (benR) gene, which encodes a transcriptional regulator involved in benzoate degradation, in an analogous way, confirming this cascade's global role.
Keywords: CbrA/CbrB two-component system, Crc, CrcZ, σ54, amidase
Microorganisms living in nutrient-rich environments choose energetically favorable, balanced diets. A paradigm of how bacteria select preferred nutrients was established by Monod (1). He observed that Escherichia coli and Bacillus subtilis, when given two different carbon sources at the same time, in some cases degrade the substrate allowing faster growth (such as glucose) first, resulting in biphasic (“diauxic”) growth. Two mechanisms essentially establish diauxie in these bacteria: inducer exclusion and catabolite repression. Inducer exclusion signifies that the uptake of the less-preferred substrate is inhibited by the preferred substrate. Catabolite repression is a mechanism preventing transcriptional expression of genes required for the degradation of the less-preferred substrate in the presence of the preferred substrate. In E. coli, growth on less-preferred substrates correlates with high levels cAMP, which is needed to activate the cAMP receptor protein, a positive transcriptional regulator of pathways used for the degradation of less-favorable sugars, such as lactose or arabinose (2, 3).
In the metabolically versatile bacterium Pseudomonas aeruginosa an initial observation of catabolite repression was made by Liu (4), who found that the addition of succinate or citrate blocked glucose degradation. Subsequent studies showed that, in this bacterium and other fluorescent pseudomonads, intermediates of the tricarboxylic acid cycle generally cause catabolite repression of degradative pathways for sugars, amino acids, and other carbon sources (5–7). A crc mutant of P. aeruginosa is pleiotropically defective for catabolite repression of several catabolic pathways by carboxylic acids (8, 9). Similarly, a crc mutant of Pseudomonas putida has lost catabolite repression of alkane and benzoate degradation (10, 11). Neither cAMP nor transcriptional control of degradative pathways via a cAMP-binding protein seem to be responsible for catabolite repression in fluorescent pseudomonads (12, 13). Rather, the Crc protein binds RNA and acts as a translational repressor. Its recognition specificity, however, has not been determined (10, 11, 14, 15).
In secondary metabolism of fluorescent pseudomonads and other γ-proteobacteria, RNA-binding proteins termed RsmA or CsrA function as translational repressors by binding to target mRNAs at or near the ribosome binding sites. Repression is relieved when small RNAs (sRNAs) having high affinity for RsmA sequester these proteins. The conserved two-component system GacS/GacA drives the expression of these titrating sRNAs in response to secreted chemical signals (16–19). We hypothesized that a similar mechanism might operate in catabolite repression of P. aeruginosa. We have identified an sRNA termed CrcZ that sequesters the Crc protein with high affinity, and we find that CrcZ is expressed under the control of a carbon source-sensitive two-component system, CbrA/CbrB. This regulatory system ensures carbon–nitrogen balance in P. aeruginosa (20, 21) and in Pseudomonas fluorescens (22). The CbrB response regulator has a predicted σ54 activation domain and belongs to the NtrC family of transcription factors (20). As a Crc target we have used the amiE gene, which encodes aliphatic amidase and is subject to Crc-mediated catabolite repression; inducer exclusion has previously been ruled out as a mechanism in this regulation (5, 6). Remarkably, the CbrA/CbrB system gradually adjusts CrcZ levels in response to different carbon sources. The resulting variable CrcZ/Crc ratios allow the bacterium to establish a gradual rather than a diauxic mode of catabolite repression operating at a posttranscriptional level.
Results
Expression of the amiE Gene Is Repressed by Crc at a Posttranscriptional Level.
In the model system chosen, the amiEBCRS operon of P. aeruginosa, the amiE gene is preceded by the 100-bp untranslated leader amiL (Fig. 1). When the AmiR antiterminator protein binds to the ρ-independent terminator located at the 3′ end of amiL, this causes read-through transcription and expression of the ami operon. In the absence of inducing aliphatic amides, the AmiC regulatory protein sequesters AmiR, resulting in transcription termination. Inducing amides bind to AmiC and thereby prevent the formation of the AmiC-AmiR complex, allowing AmiR to act as an antiterminator and to allow transcription of the ami operon (23). In the following experiments we routinely included the inducer lactamide in the culture media. Amidase specific activity was low when cells grew in minimal medium containing succinate (a preferred substrate causing strong catabolite repression). Amidase levels were high with mannitol (a substrate not causing catabolite repression) and intermediate with glucose (a substrate causing mild catabolite repression) (Fig. 2A). We constructed a transcriptional lacZ fusion to the ami promoter; β-galactosidase expression from this construct (pME9656; Fig. 1) was not influenced by a crc deletion mutation (in strain PAO6673) and was not affected by the carbon source (Fig. 2B). This result indicates that the ami promoter functions constitutively. By contrast, the expression of a translational amiE′-′lacZ fusion (pME9655; Fig. 1) depended on the carbon source (Fig. 2C). In the crc mutant growing with succinate or glucose, amiE′-′lacZ expression was significantly derepressed, by comparison with the wild type (Fig. 2C). This finding establishes that Crc-mediated catabolite repression of amiE involves a posttranscriptional mechanism. When cells were cultivated in mannitol minimal medium, the crc mutation had no influence on the constitutively high expression of the amiE′-′lacZ fusion (Fig. 2C), confirming that mannitol does not exert catabolite repression.
Fig. 1.
Genetic organization of the amiL-amiE region in the PAO1 chromosome. The amiL nucleotide sequence, the -35 and -10 promoter elements, and the amiE start codon (32) are shown in boldface. Convergent arrows and a stem-loop symbol indicate the ρ-independent terminator of amiL. Vertical arrows indicate the sites of lacZ fusions, followed by designations of the resulting plasmid constructs. The CA motif and the corresponding mutated sequence in pME9657 (shown above the CA motif) are boxed.
Fig. 2.
Posttranscriptional catabolite repression of aliphatic amidase. Bacteria were grown to OD600 ≈ 1.5 in minimal medium amended with succinate (gray bars), glucose (white bars), or mannitol (black bars). Media also contained the inducer lactamide. (A) Catabolite repression of amidase was measured by determining specific enzyme activities (in micromoles of acetylhydroxamate formed per minute and OD600) in the wild-type PAO1 using an acetyltransferase assay (33). (B) Crc-dependent catabolite repression of amiE does not involve the ami promoter. β-Galactosidase activities of a transcriptional ami-lacZ fusion carried by pME9656 were similar in the wild-type PAO1 and in the crc mutant PAO6673. (C) Crc-dependent catabolite repression of amiE occurs at the translational level. β-Galactosidase activities of a translational amiE′-′lacZ fusion carried by pME9655 varied in parallel to amidase activities. (D) Mutation of the CA motif preceding amiE (in pME9657) results in partial loss of catabolite repression, by comparison with wild-type expression (in pME9655). β-Galactosidase activity of the mutated amiE′-′lacZ fusion was measured in the wild-type PAO1/pME9657 (white bar) and compared with that of the parental amiE′-′lacZ fusion in PAO1/pME9655 (dotted bar) and in PAO6673/pME9655 (hatched bar), after growth in succinate minimal medium to an OD600 ≈ 1.5.
We verified that the crc mutation could be complemented in trans [supporting information (SI) Fig. S1]; for complementation we used the wild-type crc gene fused at its 3′ end to a His6 tag and expressed under the control of the tac promoter. This experiment also shows that the tag does not interfere with the biological function of Crc.
The CA Motif, a Hallmark of Regulation by Crc.
Not all catabolite repression phenomena disappear when the crc gene is mutated in fluorescent pseudomonads. However, an involvement of Crc in catabolite repression has been documented for approximately 10 Pseudomonas genes encoding catabolic regulators or enzymes (6, 7, 11, 24). We hypothesized that these genes might have common Crc recognition sequences at or near the ribosome binding sites. An alignment of five well-characterized, Crc-controlled genes including amiE of P. aeruginosa and benR of P. putida revealed a common AACAACAA motif (Fig. S2), to which we will refer as the CA motif (acronym for catabolite activity) hereafter. In amiE, this motif is fully conserved and overlaps the Shine-Dalgarno sequence (Fig. 1). We replaced the CA motif in the translational amiE′-′lacZ fusion by random nucleotides (Fig. 1). In the wild-type PAO1 growing in succinate or glucose minimal medium, this mutant construct (pME9657) gave derepressed expression, by comparison with the parental amiE′-′lacZ fusion (pME9655). The level of derepression was similar to that found for the crc mutant PAO6673 carrying pME9655 (Fig. 2D). This result indicates that the CA motif is important for catabolite repression. However, some Crc-dependent regulation persisted in the mutant construct (Fig. 2D), and this observation will be discussed later.
Carbon Source Does Not Seem to Influence crc Expression.
Crc levels vary in P. putida, depending mainly on the growth phase (25). Thus, catabolite repression could be brought about by higher Crc amounts under repressing conditions (e.g., in succinate or glucose minimal medium) than under nonrepressing conditions (e.g., in mannitol medium). However, we found that in P. aeruginosa the expression of a translational crc′-′lacZ fusion was similar in all three media during exponential growth (Fig. S3). Therefore, we postulated that the activity, rather than the amount of Crc, would be relevant for catabolite repression of amiE under our experimental conditions.
Discovery of CrcZ, an sRNA Having Multiple CA Motifs.
We hypothesized that the activity of Crc as a translational repressor could be countered by one or perhaps several sRNAs having high affinity for this protein, by analogy with RsmA (CsrA), whose regulatory activity is antagonized by a family of sRNAs in γ-proteobacteria (19). We therefore searched for intergenic regions of P. aeruginosa that contained one or several CA motifs and were large enough to specify an sRNA. In the 900-bp intergenic region located between the cbrB and pcnB genes, five CA motifs are present, three of which are concentrated in the 5′ region (Fig. 3). Previously Livny et al. (26) reported an sRNA, termed P30, to be transcribed from this region. However, the published orientation of P30 would result in an sRNA having antisense CA motifs. We therefore reinvestigated the location and orientation of this sRNA gene using both double- and single-stranded probes (Fig. 4 A and B, lanes 1). This analysis revealed a major transcript of ≈407 nt named CrcZ, which is transcribed in inverse orientation to that reported for P30. A 160-bp deletion of the promoter region and the 5′ part of the crcZ gene (in strain PAO6679) completely abolished the expression of this sRNA (Fig. 4 A and B, lanes 3). The crcZ transcription start site can be predicted accurately from the fact that a well-conserved σ54 (RpoN) recognition sequence occurs upstream of the CA motifs (Fig. 3) and that crcZ expression depends entirely on the alternative sigma factor σ54 (pertinent data will be shown below). A crcZ-internal deletion of 193 bp (in PAO6677; Fig. 3) resulted in the expected shortening of the major transcript to ≈210 nt (Fig. 4 A and B, lanes 2). The crcZ gene also produced several transcripts that were smaller than 407 nt (Fig. 4 A and B), owing either to premature transcription termination or to degradation of the full-length transcript, whose sequence and proposed secondary structure are shown in Fig. S4. Interestingly, all five CA motifs occur in unpaired regions of CrcZ.
Fig. 3.
Location of the crcZ gene (open arrow) between the cbrB and pcnB genes (gray arrows) in P. aeruginosa. The existence of an inversely oriented sRNA gene, P30 (open arrow with dashed lines), has been published (26). ρ-independent terminators are indicated by stem–loop structures. The deletions of the putative P30 sequence (in strain PAO6677) and of the crcZ promoter region (in PAO6679) are indicated by underlining the nucleotide sequence. The nucleotide sequence of crcZ is shown in boldface, and its σ54-promoter (-12 and -24 boxes) is shown in boldface and boxed. The five CA motifs are boxed. A possible ρ-independent terminator of P30 is indicated by convergent arrows placed above the sequence. The 3′ end of the in vitro transcribed CrcZ′-RNA used for the band shift experiment (Fig. 6 and Fig. S6) is indicated by an asterisk.
Fig. 4.
Evidence for CrcZ sRNA. Northern blots of the wild-type PAO1 (lane 1), the crcZ-internal deletion mutant PAO6677 (lane 2), and the crcZ promoter mutant PAO6679 (lane 3) were obtained from cells grown in succinate minimal medium to OD600 ≈ 2. Ten micrograms of total RNA was loaded onto a 12% polyacrylamide gel, and CrcZ sRNA and fragments thereof were detected with a digoxigenin (DIG)-labeled double-stranded probe (A) or a strand-specific single-stranded probe (B), respectively. The signal of 5S rRNA was used as a loading control.
Carbon Source Regulates crcZ Expression.
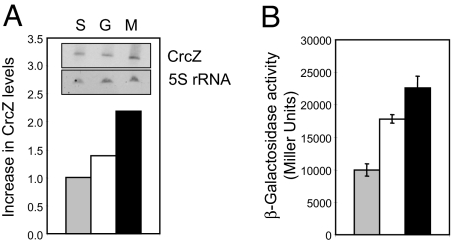
Because Crc-mediated catabolite repression is most manifest in succinate medium, weaker in glucose medium, and absent from mannitol medium (Fig. 2 A and C), the concentration of CrcZ is expected to go in the opposite direction. This was found to be the case (Fig. 5A) and was confirmed by a transcriptional crcZ-lacZ fusion, which was expressed at a low level in succinate medium, at an intermediate level in glucose medium, and at a high level in mannitol medium (Fig. 5B). These data indicate that, under the growth conditions chosen, it is the concentration of the CrcZ sRNA that varies and not that of the Crc protein.
Fig. 5.
The levels of CrcZ expression vary according to the carbon sources used. (A) Ten micrograms of total RNA purified from the wild-type PAO1, grown either in minimal medium with succinate (S), glucose (G), or mannitol (M) to OD600 ≈ 1.5, was used for an estimation of CrcZ levels in a Northern blot experiment, which was performed with a DIG-labeled double-stranded probe. 5S rRNA was used as a loading control. The normalized signals of CrcZ are shown in the graph. (B) β-Galactosidase activities of a chromosomally encoded crcZ-lacZ fusion were measured in strain PAO1 grown to OD600 ≈ 1.5 in minimal medium with succinate (gray bar), glucose (white bar), or mannitol (black bar), respectively.
A crcZ Mutant Shows Repressed amiE Levels and Has Pleiotropic Defects.
The crcZ mutant PAO6679 expressed the amiE′-′lacZ fusion at a very low basal level throughout growth in succinate medium and did not grow on acetamide as the only C source (Fig. S5 A and B). Complementation of this mutant by the crcZ+ plasmid pME9669 restored amiE expression roughly to the wild-type level and enabled growth on acetamide. In the wild type, the crcZ+ plasmid caused amiE′-′lacZ overexpression (Fig. S5 A and B). These results are in agreement with the model according to which the CrcZ sRNA is an antagonist of the Crc protein and thereby relieves catabolite repression.
In addition to its inability to use acetamide, the crcZ mutant was pleiotropically defective in the utilization of a number of C and N sources, as revealed by a qualitative test in the Biolog system. The phenotypes of the crcZ mutant resembled those of a cbrB mutant (Table S1), suggesting that the CrcZ sRNA mediates a substantial part of the output of the CbrA/CbrB two-component system. The phenotypes observed for the cbrB mutant confirm the results of previous studies conducted in P. aeruginosa and P. fluorescens (20, 22).
Competitive Binding of Crc to amiE mRNA and CrcZ sRNA.
In the Gac/Rsm signal transduction pathway of fluorescent pseudomonads, the relative concentrations and affinities of three elements—the RNA-binding protein RsmA, the antagonistic sRNAs, and the target mRNA—determine to which extent secondary metabolism is switched on (19). Following up the idea that Crc-dependent catabolite repression could be based on a similar principle, we first established that the Crc protein binds to amiE mRNA at a site including the CA motif. A 5′-labeled 154-nt amiE′ run-off transcript was seen to form two complexes with Crc protein in a gel shift experiment, whereas an amiE′ transcript with a randomly substituted CA motif gave only one complex (Fig. S6A). This result demonstrates the importance of the CA motif for Crc binding and also suggests that a second Crc binding site exists in the amiE translation initiation region, explaining why amiE′-′lacZ expression was only partially derepressed in the ΔCA motif mutant (Fig. 2D). We did not explore the nature of the second binding site; we suspect that it might involve the A-rich sequence preceding the CA motif (Fig. 1).
Crc protein also effectively bound to CrcZ sRNA. In this experiment we used a 151-nt CrcZ′ transcript that lacked the 3′-terminal part, but still contained the three proximal CA motifs (Fig. 3). Crc formed at least two complexes with CrcZ′ (Fig. S6B). More work will be needed to establish the Crc:CrcZ′ stoichiometry. When Crc was incubated with amiE′ mRNA, CrcZ′ titrated the protein away from the target mRNA. By contrast, the unrelated sRNA RsmZ (27) had no effect (Fig. 6). These in vitro results establish the mode of action of Crc: this protein interacts with a sequence encompassing the CA motif, which is located near the Shine-Dalgarno sequence of amiE, and CrcZ sRNA competitively inhibits this interaction.
Fig. 6.
CrcZ′ prevents the Crc protein from binding to amiE′ mRNA. Different amounts of Crc were added to radioactively labeled amiE′ mRNA (from left, first four lanes). To an incubation mixture containing a 50-fold molar excess of Crc over amiE′, nonlabeled CrcZ′ was added at the concentrations indicated (next three lanes), showing that CrcZ sequesters Crc from amiE′ mRNA. The same experiment was performed with nonlabeled RsmZ sRNA, where this titrating effect was not observed (last three lanes).
The CbrA/CbrB Two-Component System and RpoN Positively Regulate crcZ Expression.
The crcZ gene lies downstream of the genes encoding the CbrA/CbrB two-component system in P. aeruginosa (Fig. 3). This gene arrangement is conserved in fluorescent pseudomonads and related γ-proteobacteria, such as Saccharophagus degradans (28). The CbrB response regulator therefore seemed to be a likely candidate for transcriptional activation of the crcZ gene. We monitored the expression of a transcriptional crcZ-lacZ fusion, which was inserted as a single copy into the chromosome of the wild-type PAO1, the cbrB mutant PAO6711, and the rpoN mutant PAO6358. In both mutants, expression was virtually abolished, whereas the wild type gave high expression when growing in L broth (Fig. S7). In succinate minimal medium, the rpoN mutant did not grow, because it is auxotrophic for glutamine (29). However, under these conditions crcZ expression was also very strongly downregulated in the cbrB mutant, by comparison with the wild type. These results put the CbrA/CbrB two-component system upstream of the CrcZ/Crc regulatory system and lead to the establishment of the novel signal transduction pathway shown in Fig. 7.
Fig. 7.
Model of CrcZ as an antagonist of Crc in catabolite repression. The concentration of CrcZ changes according to the carbon source. In the presence of a preferred carbon source (e.g., succinate), the level of CrcZ is low and Crc binds to catabolite repression-sensitive mRNAs such as amiE mRNA and thereby blocks ribosome binding. When a nonpreferred substrate source such as mannitol is the sole carbon source, the expression of CrcZ sRNA increases under the control of the CbrA/CbrB two-component system. This results in sequestration of Crc protein by CrcZ and allows ribosome binding and translation of the target mRNAs. With glucose as the sole carbon source, an intermediate amount of CrcZ allows partial sequestration of Crc protein, leading to moderate expression of target mRNAs.
Global Function of the Cbr/Crc Signal Transduction Pathway.
The CA motif was deduced initially from a sequence comparison of Crc-regulated genes of P. aeruginosa (Fig. S2). In P. putida, benR is an example of a Crc-regulated gene (11). The homologue of P. putida benR is dubbed xylS in P. aeruginosa (http://www.pseudomonas.com). The 5′ xylS leader contains a conserved CA motif (Fig. S2). To show that xylS is regulated by the Cbr/Crc cascade in P. aeruginosa, we constructed a translational xylS′-′lacZ fusion (in pME9671) and measured its β-galactosidase activity in strain PAO1 and in PAO1 mutants deleted for cbrB, crcZ, or crc. Cells were grown to OD600 ≈ 1.5 in minimal medium containing 40 mM succinate and 2 mM toluate. The expression of this fusion was low in the cbrB mutant (957 ± 60 Miller units) and in the crcZ mutant (1,034 ± 2 Miller units) and was increased in the crc mutant (4,316 ± 257 Miller units), by comparison with the wild-type strain (2,645 ± 317 Miller units). This result confirms that the Cbr/Crc system regulates the expression of the xylS and amiE genes similarly and suggests a global regulatory role of this system in catabolite repression of Pseudomonas spp.
Discussion
This work shows that catabolite repression exerted by the Crc protein in P. aeruginosa is regulated via the antagonistic sRNA CrcZ, whose expression is controlled in turn by the CbrA/CbrB two-component system and RpoN. Depending on the C source, expression of both crcZ and a target mRNA (amiE) varied significantly. Expression was low in the presence of the preferred C source succinate, intermediate with glucose, and high in the presence of the less-favorable substrate mannitol. These findings lead to a model of catabolite repression in which Crc protein and CrcZ sRNA play a central role. Recent work in P. putida has established that Crc is an RNA-binding protein (10, 11), and in the present study we have not only confirmed this property but have also shown that a CA-rich sequence is involved in Crc binding. The organization of the Cbr/Crc signal transduction pathway (Fig. 7), which regulates primary metabolism in Pseudomonas spp., has striking similarity with the organization of the Gac/Rsm pathway, which regulates secondary metabolism in many γ-proteobacteria (19): the output of a two-component system regulates the expression of one or several sRNAs, which prevent RNA-binding proteins from repressing the translational expression of target mRNAs.
We suspect that the model shown in Fig. 7 represents just the backbone of a regulatory pathway and that future research might reveal additional control elements and control circuits. There is circumstantial evidence for this. For instance, a crcZ null mutation affects some catabolic pathways that have been reported not to be under Crc control (e.g., the histidine utilization pathway) (Table S1) (6). Furthermore and importantly, a crc null mutant showed residual catabolite repression control of amiE′-′lacZ in succinate and glucose media (Fig. 2C). This finding may explain why in the wild type the amplitude of catabolite repression as measured by amidase expression is larger than the 2.5-fold variation of CrcZ levels (Fig. 5). Thus, CrcZ and Crc might not be the only catabolite-responsive elements in amidase regulation, and the existence of a second Crc-like protein is not excluded. The crc gene has sequence homology with a family of nucleases, in particular with the xthA gene encoding exonuclease III in E. coli (9). However, Crc does not bind DNA and does not exhibit DNase activity in P. aeruginosa (6). Genes with similarity to crc of pseudomonads and xthA of enteric bacteria can be found in a large variety of bacteria. At present, there is no information on functionally important amino acid residues of Crc, and therefore it is technically difficult to predict the function of crc- and xthA-like genes in bacteria.
The size and orientation of the crcZ gene deserve some comment. In a previous study (26), the intergenic region between cbrB and pcnB (encoding polyA polymerase) was found to contain an sRNA gene termed P30 whose orientation was given as opposite to that of crcZ. The reason for this discrepancy is not known. However, an intrinsic annotation difficulty lies in the fact that the crcZ gene does not have a typical ρ-independent terminator. It is possible that termination of crcZ transcription might depend on ρ factor. Considering the size of CrcZ, this sRNA might well have regulatory functions in addition to those required for Crc binding. For instance, CrcZ might undergo base-pairing interactions with some mRNAs.
The significance of this work extends beyond the pseudomonads. For instance, we found evidence for a crcZ homologue in the marine γ-proteobacterium S. degradans. This versatile microorganism has a multitude of cellulases, glucanases, and other carbohydrate-degrading enzymes and has biotechnological potential in the context of biofuel production from plant polymers (30). A major advantage of the posttranscriptional regulatory mechanism relying on the CrcZ/Crc ratio lies in the fact that the expression of catabolic genes can be adjusted gradually in response to different carbon sources.
Materials and Methods
Bacterial Strains, Plasmids, and Culture Conditions.
Construction of plasmids, mutants, and oligonucleotides used (Table S2) are described in SI Materials and Methods. Growth and β-galactosidase experiments were performed in a minimal medium (BSM) containing 30.8 mM K2HPO4, 19.3 mM KH2PO4, 15 mM (NH4)2SO4, 1 mM MgCl2, and 2 μM FeSO4 supplemented with either 40 mM succinate, 40 mM glucose, 40 mM mannitol, or 40 mM acetamide; 40 mM lactamide was always added to induce amiE transcription. Unless indicated otherwise, cells were grown to OD600 ≈ 1.5 (corresponding to approximately 1.5 × 109 cells per milliliter. When required, antibiotics were added to media at the following concentrations: 100 μg·mL−1 ampicillin, 25 μg·mL−1 tetracycline, and 25 μg·mL−1 kanamycin for Escherichia coli and 50 μg·mL−1 gentamicin, 100 μg·mL−1 tetracycline, and 250 μg·mL−1 carbenicillin for P. aeruginosa.
β-Galactosidase Assays.
These were performed as previously described (31) with 20-mL cultures of P. aeruginosa strains grown in BSM medium supplemented with different carbon sources. Data are mean values of three independent samples ± SD.
RNA Techniques and Purification of Crc Protein.
Standard methods were used; they are detailed in SI Materials and Methods.
Acknowledgments.
We thank Karine Lapouge for advice on enzyme assays and Cornelia Reimmann for critically reading this manuscript. This work was supported by Swiss National Foundation Project 3100A0-120365 and Erwin-Schrödinger Research Fellowship J2663_B03 (to E.S.).
Note Added in Proof.
Moreno et al. (34) recently found that the Crc protein binds to a short, unpaired A-rich RNA sequence with a consensus AA(C/U)AA(C/U)AA in P. putida.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910308106/DCSupplemental.
References
- 1.Monod J. Recherches sur la croissance des cultures bactériennes. Paris: Hermann; 1942. [Google Scholar]
- 2.Ullmann A. Catabolite repression: A story without end. Res Microbiol. 1996;147:455–458. doi: 10.1016/0923-2508(96)83999-4. [DOI] [PubMed] [Google Scholar]
- 3.Görke B, Stülke J. Carbon catabolite repression in bacteria: Many ways to make the most out of nutrients. Nat Rev Microbiol. 2008;6:613–624. doi: 10.1038/nrmicro1932. [DOI] [PubMed] [Google Scholar]
- 4.Liu P. Utilization of carbohydrates by Pseudomonas aeruginosa. J Bacteriol. 1952;64:773–781. doi: 10.1128/jb.64.6.773-781.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smyth PF, Clarke PH. Catabolite repression of Pseudomonas aeruginosa amidase: The effect of carbon source on amidase synthesis. J Gen Microbiol. 1975;90:81–90. doi: 10.1099/00221287-90-1-81. [DOI] [PubMed] [Google Scholar]
- 6.Collier DN, Hager PW, Phibbs PV. Catabolite repression control in the pseudomonads. Res Microbiol. 1996;147:551–561. doi: 10.1016/0923-2508(96)84011-3. [DOI] [PubMed] [Google Scholar]
- 7.Rojo F, Dinamarca MA. Catabolite repression and physiological control. In: Ramos JL, editor. Pseudomonas: Virulence and Gene Regulation. Vol 2. New York: Kluwer Academic/Plenum; 2004. pp. 365–387. [Google Scholar]
- 8.Wolff JA, MacGregor CH, Eisenberg RC, Phibbs PV. Isolation and characterization of catabolite repression control mutants of Pseudomonas aeruginosa PAO. J Bacteriol. 1991;173:4700–4706. doi: 10.1128/jb.173.15.4700-4706.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacGregor CH, Arora SK, Hager PW, Dail MB, Phibbs PV. The nucleotide sequence of the Pseudomonas aeruginosa pyrE-crc-rph region and the purification of the crc gene product. J Bacteriol. 1996;178:5627–5635. doi: 10.1128/jb.178.19.5627-5635.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moreno R, Ruiz-Manzano A, Yuste L, Rojo F. The Pseudomonas putida Crc global regulator is an RNA binding protein that inhibits translation of the AlkS transcriptional regulator. Mol Microbiol. 2007;64:665–675. doi: 10.1111/j.1365-2958.2007.05685.x. [DOI] [PubMed] [Google Scholar]
- 11.Moreno R, Rojo F. The target for the Pseudomonas putida Crc global regulator in the benzoate degradation pathway is the BenR transcriptional regulator. J Bacteriol. 2008;190:1539–1545. doi: 10.1128/JB.01604-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siegel LS, Hylemon PB, Phibbs PV. Cyclic adenosine 3′,5′-monophosphate levels and activities of adenylate cyclase and cyclic adenosine 3′,5′-monophosphate phosphodiesterase in Pseudomonas and Bacteroides. J Bacteriol. 1977;129:87–96. doi: 10.1128/jb.129.1.87-96.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suh SJ, et al. Effect of vfr mutation on global gene expression and catabolite repression control of Pseudomonas aeruginosa. Microbiology. 2002;148:1561–1569. doi: 10.1099/00221287-148-5-1561. [DOI] [PubMed] [Google Scholar]
- 14.Hester KL, et al. Crc is involved in catabolite repression control of the bkd operons of Pseudomonas putida and Pseudomonas aeruginosa. J Bacteriol. 2000;182:1144–1149. doi: 10.1128/jb.182.4.1144-1149.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moreno R, Martínez-Gomariz M, Yuste L, Gil C, Rojo F. The Pseudomonas putida Crc global regulator controls the hierarchical assimilation of amino acids in a complete medium: Evidence from proteomic and genomic analyses. Proteomics. 2009;9:2910–2928. doi: 10.1002/pmic.200800918. [DOI] [PubMed] [Google Scholar]
- 16.Laville J, et al. Global control in Pseudomonas fluorescens mediating antibiotic synthesis and suppression of black root rot of tobacco. Proc Natl Acad Sci USA. 1992;89:1562–1566. doi: 10.1073/pnas.89.5.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blumer C, Heeb S, Pessi G, Haas D. Global GacA-steered control of cyanide and exoprotease production in Pseudomonas fluorescens involves specific ribosome binding sites. Proc Natl Acad Sci USA. 1999;96:14073–14078. doi: 10.1073/pnas.96.24.14073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kay E, Dubuis C, Haas D. Three small RNAs jointly ensure secondary metabolism and biocontrol in Pseudomonas fluorescens CHA0. Proc Natl Acad Sci USA. 2005;102:17136–17141. doi: 10.1073/pnas.0505673102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lapouge K, Schubert M, Allain FH, Haas D. Gac/Rsm signal transduction pathway of γ-proteobacteria: From RNA recognition to regulation of social behaviour. Mol Microbiol. 2008;67:241–253. doi: 10.1111/j.1365-2958.2007.06042.x. [DOI] [PubMed] [Google Scholar]
- 20.Nishijyo T, Haas D, Itoh Y. The CbrA-CbrB two-component regulatory system controls the utilization of multiple carbon and nitrogen sources in Pseudomonas aeruginosa. Mol Microbiol. 2001;40:917–931. doi: 10.1046/j.1365-2958.2001.02435.x. [DOI] [PubMed] [Google Scholar]
- 21.Li W, Lu CD. Regulation of carbon and nitrogen utilization by CbrAB and NtrBC two-component systems in Pseudomonas aeruginosa. J Bacteriol. 2007;189:5413–5420. doi: 10.1128/JB.00432-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang XX, Rainey PB. Dual involvement of CbrAB and NtrBC in the regulation of histidine utilization in Pseudomonas fluorescens SBW25. Genetics. 2008;178:185–195. doi: 10.1534/genetics.107.081984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drew R, Haq M. Lessons from the ami operon. In: Ramos JL, editor. Pseudomonas: Virulence and Gene Regulation. Vol 2. New York, NY: Kluwer Academic/Plenum; 2004. pp. 425–449. [Google Scholar]
- 24.Aranda-Olmedo I, Ramos JL, Marqués S. Integration of signals through Crc and PtsN in catabolite repression of Pseudomonas putida TOL plasmid pWW0. Appl Environ Microbiol. 2005;71:4191–4198. doi: 10.1128/AEM.71.8.4191-4198.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruiz-Manzano A, Yuste L, Rojo F. Levels and activity of the Pseudomonas putida global regulatory protein Crc vary according to growth conditions. J Bacteriol. 2005;187:3678–3686. doi: 10.1128/JB.187.11.3678-3686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livny J, Brencic A, Lory S, Waldor MK. Identification of 17 Pseudomonas aeruginosa sRNAs and prediction of sRNA-encoding genes in 10 diverse pathogens using the bioinformatic tool sRNAPredict2. Nucleic Acids Res. 2006;34:3484–3493. doi: 10.1093/nar/gkl453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heurlier K, et al. Positive control of swarming, rhamnolipid synthesis, and lipase production by the posttranscriptional RsmA/RsmZ system in Pseudomonas aeruginosa PAO1. J Bacteriol. 2004;186:2936–2945. doi: 10.1128/JB.186.10.2936-2945.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiner RM, et al. Complete genome sequence of the complex carbohydrate-degrading marine bacterium, Saccharophagus degradans strain 2–40 T. PLoS Genet. 2008;4:e1000087. doi: 10.1371/journal.pgen.1000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heurlier K, Dénervaud V, Pessi G, Reimmann C, Haas D. Negative control of quorum sensing by RpoN (σ54) in Pseudomonas aeruginosa PAO1. J Bacteriol. 2003;185:2227–2235. doi: 10.1128/JB.185.7.2227-2235.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor LE, et al. Complete cellulase system in the marine bacterium Saccharophagus degradans strain 2–40T. J Bacteriol. 2006;188:3849–3861. doi: 10.1128/JB.01348-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller JH. Experiments in Molecular Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 32.Drew R, Lowe N. Positive control of Pseudomonas aeruginosa amidase synthesis is mediated by a transcription anti-termination mechanism. J Gen Microbiol. 1989;135:817–823. doi: 10.1099/00221287-135-4-817. [DOI] [PubMed] [Google Scholar]
- 33.Brammar WJ, Clarke PH. Induction and repression of Pseudomonas aeruginosa amidase. J Gen Microbiol. 1964;37:307–319. doi: 10.1099/00221287-37-3-307. [DOI] [PubMed] [Google Scholar]
- 34.Moreno R, Marzi S, Romby P, Rojo F. The Crc global regulator binds to an unpaired A-rich motif at the Pseudomonas putida alkS mRNA coding sequence and inhibits translation initiation. Nucleic Acids Res. 2009 doi: 10.1093/nar/gkp825. doi: 10.1093/nar/gkp825. [DOI] [PMC free article] [PubMed] [Google Scholar]