Abstract
When cultured human keratinocytes reach confluence, they undergo a program of changes replicating features of differentiation in vivo, including exit from the proliferative pool, increased cell size and expression of specialized differentiation marker proteins. Previously, we showed that insulin is required for some of these steps and that arsenite, a human carcinogen in skin and other epithelia, opposes the differentiation process. In present work, we show that insulin signaling, probably through the IGF-I receptor, is required for the increase in cell size accompanying differentiation and that this is opposed by arsenite. We further examine the impact of insulin and arsenite on PKCδ, a known key regulator of keratinocyte differentiation, and show that insulin increases the amount, tyrosine phosphorylation and membrane localization of PKCδ. All these effects are prevented by exposure of cells to arsenite or to inhibitors of downstream effectors of insulin (phosphotidylinositol 3-kinase and mammalian target of rapamycin). Retrovirally-mediated expression of activated PKCδ resulted in increased loss of proliferative potential after confluence and greatly increased formation of cross-linked envelopes, a marker of keratinocyte terminal differentiation. These effects were prevented by removal of insulin, but not by arsenite addition. We further demonstrate a role for src family kinases in regulation of PKCδ. Finally, inhibiting epidermal growth factor receptor kinase activity diminished the ability of arsenite to prevent cell enlargement and to suppress insulin-dependent PKCδ amount and tyrosine 311 phosphorylation. Thus suppression of PKCδ signaling is a critical feature of arsenite action in preventing keratinocyte differentiation and maintaining proliferative capability.
Keywords: Cell size, EGF receptor, IGF-I receptor, mTOR, PI3 kinase
INTRODUCTION
Understanding the molecular effects of carcinogens can help elucidate normal cell function as well as pathological effects on target tissues. The salient molecular effects of inorganic arsenic remain enigmatic despite the variety of mechanisms that have been proposed by which it and its methylated metabolites act [1]. Arsenic targets a variety of tissues, eliciting adverse effects including carcinogenesis in skin, bladder, lung and possibly liver [2], developmental defects in the lung [3], atherosclerosis [4] and type 2 diabetes [5]. Arsenic and its methylated metabolites have been proposed to act by a variety of mechanisms [1] that may differ among tissue targets. A promising mouse model in which to examine such mechanisms displays carcinogenic effects in bladder, lung and liver but not epidermis as a result of exposure in utero [6]. However, models have been developed with arsenite acting in the skin as a copromoter with phorbol ester in Tg.AC mice [7] or as a co-carcinogen with UV light [8].
To complement mouse models, investigation in cultured human keratinocytes has proceeded as an imperfect but close approximation to arsenic action in the epidermis. With 3T3 feeder layer support, cultured keratinocytes proliferate rapidly until confluence, when they stratify, enlarge, accumulate proteins characteristic of the superficial layers of living epidermis and can develop cross-linked envelopes, a specific feature of the terminal stage of keratinocyte differentiation [9]. The differentiation process is accompanied by a gradual loss of proliferative potential, measured as a decrease in colony forming efficiency after trypsinization and replating. In such cultures and in epidermis, cell size correlates positively with expression of the differentiation marker involucrin [10] and negatively with proliferative potential [11].
Little additional information about signaling pathways regulating keratinocyte differentiation-dependent increase in size has accumulated in the intervening decades, other than a requirement for oxygen near ambient atmospheric levels [12], but recent observations in other systems suggest involvement of insulin and/or insulin-like growth factor-I (IGF-I). The insulin and IGF-I receptors are both strong activators of PI3-kinase and its downstream effector, mTOR. In a complex with raptor, mTOR is a major regulator of protein synthesis through its effectors, S6 kinase and the initiation factor binding protein 4EBP, and thus has been demonstrated to play an important role in the general control of cell size [13]. Genetic disruption of components of this pathway leads to alterations in cell and organism size. The role of this signaling pathway in keratinocyte cell enlargement during differentiation is unknown.
Following initial observations that inorganic arsenic suppresses expression of keratinocyte differentiation markers in culture [14], treated cells also were found to retain their proliferative potential after confluence [15]. Judging by the higher levels of β1-integrin and β-catenin and the higher fraction of cells that adhered rapidly to collagen-coated substrate, features demonstrated to be markers of keratinocyte stems cells [16], arsenite exposure enhanced maintenance of stem cell properties. Subsequent work has shown these effects to require active epidermal growth factor (EGF) receptor. Added to the culture medium to stimulate growth to confluence, insulin induces EGF receptor loss after confluence, an effect antagonized by arsenite, which thereby suppresses differentiation and preserves proliferative potential [17]. Paralleling observations on the ability of arsenite to preserve stem cell character in cultured keratinocytes, the enhanced tumorigenicity due to arsenic exposure of Tg.AC mice was attributed in part to increased stem cell number as a target for carcinogenesis [18].
Our observation that arsenic preserves keratinocyte stem cell character helps rationalize skin cancer as a consequence of exposure, but key signaling events underlying these phenomena remain to be elucidated. Previous results indicated that phorbol ester treatment could overcome certain effects of arsenite in keratinocytes, suggesting a role for protein kinase C [19]. Findings that constitutive expression of the delta isoform (PKCδ) can prevent skin tumor promotion in mice [20], that PKCδ is consistently lost in human skin carcinomas [21] and that PKCδ is downstream of insulin signaling [22] raised the possibility this kinase is a target of arsenic action. Present experiments further characterize arsenite interference with insulin signaling and demonstrate a role for modulating PKCδ, also a component of apoptotic response in keratinocytes [23,24].
MATERIALS AND METHODS
Cell Culture
Spontaneously immortalized keratinocytes (SIK) [25] or normal human epidermal cells derived from foreskin (passages 20–24 and 3–6, respectively) were propagated in DMEM/F12 (2:1) medium supplemented with fetal bovine serum (5%), hydrocortisone (0.4 µg/ml), adenine (0.18 mM), insulin (5 µg/ml, except where noted), transferrin (5 µg/ml) and triiodothyronine (20 pM) using a feeder layer of lethally irradiated 3T3 cells [26]. Medium was further supplemented with cholera toxin (10 ng/ml) at inoculation, which was replaced with EGF (10 ng/ml) starting at the first medium change. Cells were grown until just before confluence with medium changes at 3 to 4 day intervals, at which time they were treated with 2 µM sodium arsenite, 5 µM potassium antimony tartrate, 10 µM LY29003, 5 nM rapamycin (LC Laboratories, Waltham, MA), 5 nM wortmannin (Calbiochem/EMD Chemicals, La Jolla, CA) or switched to medium without insulin. In certain instances, cells were first pre-treated for 1 h with 1 µM AG1478 (LC Laboratories) or with 3 µM PP2 (Alexis Biochemicals).
Retroviral and Lentiviral Infection of SIK
The cDNA encoding PKCδ A147D activated pseudosubstrate mutant was subcloned into the retroviral vector pBabe-puro. A pBabe-puro vector only, used as control, or a PKCδ construct was transfected into the retroviral packaging cell line Phoenix Ampho (kindly provided by Dr. Garry Nolan), and the retrovirus-containing medium was then used to infect SIK cultures in the presence of 4 µg/ml polybrene at 32°C overnight. Infected cells were selected using 1 µg/ml puromycin. Immunoblotting showed the cells had approximately twice the relative amount of membrane-bound PKCδ immunoreactivity. Lentiviral constructs encoding PKCδ shRNAs were purchased from Sigma Chemical Co. (St. Louis, MO) and packaged using the lentiviral packaging system from Invitrogen (Carlsbad, CA). Cells were infected and selected as above.
Colony Forming Efficiency (CFE)
CFE was determined by inoculating 103 or 104 trypsinized cells in 6 cm dishes. Grown with feeder layer support until most were 2–5 mm in diameter, typically 10–14 days, the colonies were fixed and visualized by staining with Rhodanile blue [27]. CFE values (%) are presented as the number of colonies times 100 divided by the number of cells inoculated.
Cell Size
The size distribution of trypsinized cultures was determined using a Beckman Coulter Multisizer3 which calculates approximate diameters from the measured cell volumes assuming spherical shapes. As previously observed [12], the calculated size distribution obtained in this way with normal epidermal cells is similar to that derived microscopically.
Spontaneous Envelope Formation
In suspension experiments, cultures were pre-treated for 24 hours with 2 µM arsenite before suspension in medium containing 1.4% methylcellulose and arsenite. After 4 days cells were recovered by low speed centrifugation, rinsed 3 times with isotonic phosphate buffered saline, counted and resuspended in buffer containing 2% sodium dodecyl sulfate (SDS) and 20 mM dithiothreitol. After >30 min at room temperature, envelopes were counted by phase contrast optics in a hemocytometer. Envelope formation (%) is presented as the number of envelopes divided by the number of cells and multiplied by 100. Envelope formation in retrovirally infected surface cultures was measured by rinsing the cells with phosphate buffered isotonic saline and dissolving them in buffer with 2% SDS and 20 mM dithiothreitol. After 30 min at room temperature, the cells were passed through a 25 gauge needle. Envelopes were centrifuged, rinsed in 0.1% SDS, resuspended in 1 ml of 0.1% SDS, examined microscopically, and relative amounts measured spectrophotometrically (A340) by light scattering [28].
Immunoblotting
For most experiments, cells were scraped directly into buffer containing 2% SDS, 62.5 mM Tris (pH 6.8) and 10% glycerol. Protein was measured with bicinchoninic acid [29] from Pierce before addition of dithiothreitol to 50 mM and boiling for 3 min. In a given experiment, equal amounts (20 µg) of protein were submitted to SDS polyacrylamide gel electrophoresis, transferred to polyvinyldifluoride membranes, blocked with 5% dry milk in Tris buffered saline, 0.05% Tween 20, incubated with the indicated antibodies and detected using ECL Plus chemiluminescence detection reagent (GE Healthcare, Piscataway, NJ). Antibodies were obtained from the following sources: PKCδ (rabbit polyclonal), phosphotyrosine (mouse monoclonal), phosphotyrosine 311 PKCδ (rabbit polyclonal) from Santa Cruz Biotechnology (Santa Cruz, CA), actin (mouse monoclonal) from Sigma (St. Louis, MO) and involucrin (rabbit polyclonal) [28].
Immunoprecipitation
Cells were scraped into ice-cold phosphate buffered isotonic saline and lysed in buffer containing 50 mM Tris-HCl, 300 mM NaCl, 1 mM ethylenediamine tetraacetic acid (EDTA), 1% Nonidet P-40, 1 mM β-glycerophosphate, 0.2 mM sodium vanadate, and EDTA-free protease inhibitor cocktail (Roche). Samples were disrupted by brief sonication and clarified by microfuge centrifugation. Protein concentration was determined using Coomassie G-250 [30] from Biorad (Richmond, CA). Protein lysates were precleared by incubation of 500 µg of protein for 1 hour with 20 µl of protein A/G PLUS-agarose beads (Santa Cruz Biotechnology) at 4°C with rotation. The preparations were then centrifuged at 1000×g for 1 minute. Supernatants were incubated with 4 µg of PKCδ rabbit polyclonal antibody overnight at 4°C with rotation followed by incubation with 20 µl protein A/G PLUS-agarose beads for 1 hour. The complexes were recovered by centrifugation at 1000×g for 1 min followed by three washes with lysis buffer, diluted in 2% SDS, 62.5 mM Tris (pH 6.8), 50 mM dithiothreitol and 10% glycerol and subjected to immunoblot analysis.
Cell Fractionation
Cultures were rinsed with ice-cold phosphate buffered isotonic saline and scraped into buffer containing 20 mM Tris HCl (pH 7.4), 5 mM EDTA, 1 mM sodium vanadate, 1 mM phenylmethylsulfonyl fluoride, and EDTA-free protease inhibitor cocktail (Roche). Cells were disrupted by sonication, centrifuged at 100,000 × g for 1 hour at 4°C, and supernatants were saved as a soluble fraction. Pellets were dissolved in 2% SDS, 62.5 mM Tris (pH 6.8) and 10% glycerol buffer and used as particulate fraction. Protein content was determined with bicinchononic acid (Pierce Chemical, Rockford, IL) before addition of dithiothreitol, and samples were subjected to immunoblot analysis.
Real-Time Reverse Transcription PCR
Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA). Contaminating genomic DNA was removed by pretreatment with DNase (DNA-free kit, Ambion, Austin, TX). c-DNA synthesis was performed using a High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). The cDNA served as a template in quantitative real-time PCR utilizing TaqMan Fast Universal PCR Master Mix and TaqMan Gene Expression assay probes for involucrin, keratins 1 and 10, TGM1 protein and housekeeping genes GusB or β-actin (Applied Biosystems). The assays were performed in an ABI 7500 Fast Sequence Detection System. mRNA expression normalized to endogenous 18S RNA is presented relative to untreated control cultures in the presence of insulin (set to 1.0). Normalization to one housekeeping gene, GusB or β-actin, gave equivalent results.
Statistical Analysis
Except as noted, one way ANOVA statistical testing (with Bonferroni corrections) was performed using Stata/SE9.2 software for Windows and three way ANOVA by SAS. In certain cases, as noted, values from independent experiments were subjected to 2-tailed Student’s t-testing using Excel for calculation of p values.
RESULTS
Arsenite and Insulin Have Opposing Effects on Keratinocyte Differentiation and Size
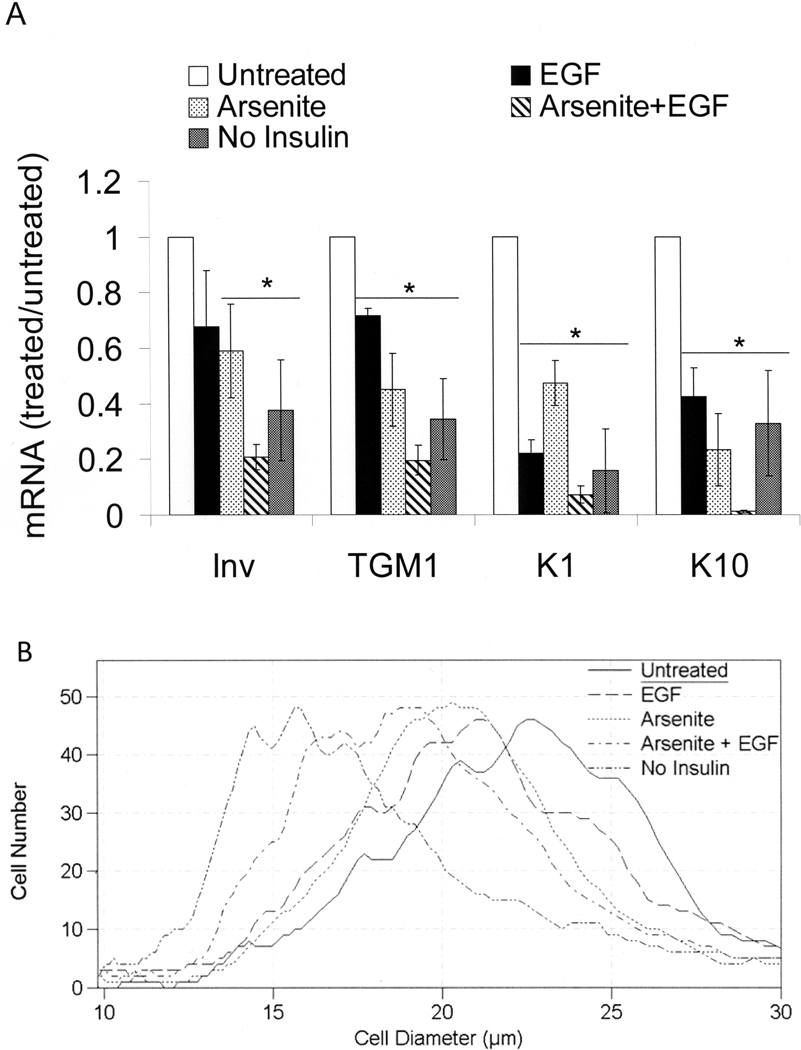
Previous work demonstrated opposing effects of arsenite and insulin on proliferative potential in the SIK line of keratinocytes (Patterson and Rice, 2007) and in normal human epidermal cells (Patterson et al, 2005 and Supplemental Tables 1 and 2). Since arsenite also suppresses differentiation marker expression [14], we examined the possibility that removal of insulin, ordinarily added to stimulate cell growth, would have a similar effect. Like cells treated with arsenite, those cultured after confluence in the absence of insulin exhibited reduced levels of mRNAs encoding the differentiation markers keratin 1, keratin 10, involucrin and TGM1 (Figure 1A). Long known to suppress such markers alone [31,32], EGF resulted in further suppression when added to arsenite-treated cultures.
Figure 1.
Keratinocyte size and differentiation after treatment with arsenite and EGF. Starting at confluence, cultures were treated in medium supplemented with the indicated combinations of 2 µM arsenite and 10 ng/ml EGF. Unless otherwise indicated, the medium contained 5 µg/ml insulin. After 7 days of treatment, cells were trypsinized and mRNA levels of involucrin, keratin 1, keratin 10, and TGM1 (A) or cell sizes (B) were measured. In (A), values are given relative to untreated cultures in the presence of insulin, normalized to 1. Data were compiled from three independent experiments. Significant differences from untreated (p < 0.01) are indicated by an asterisk (p = 0.01 for involucrin, p < 0.01 for TGM1, p < 0.001 for KRT1 and p = 0.001 for KRT10).
Similar to normal human epidermal cultures (Jones and Watt, 1993), SIK cells can be fractionated based on their adhesive properties. The cells that adhere most quickly to collagen-coated dishes have the highest proliferative potential (Patterson et al, 2005) and smallest size (Supplemental Figure 1). Consistent with the higher proliferative potential of small cells, cell size was decreased by treatment with arsenite or culture after confluence in the absence of insulin (Figure 1B). Arsenite and EGF together were more effective in preserving smaller cells than either alone. Insulin likely increased cell size due to its interaction with the IGF-I receptor since IGF-I was an order of magnitude more potent than insulin (Supplemental Figure 2). Although several other metals and metalloids suppress differentiation marker expression (Rea et al, 2003), only the chemically similar compounds, arsenite and antimonite, preserved proliferative potential (Patterson et al, 2005) and small cell size (Supplemental Figure 3).
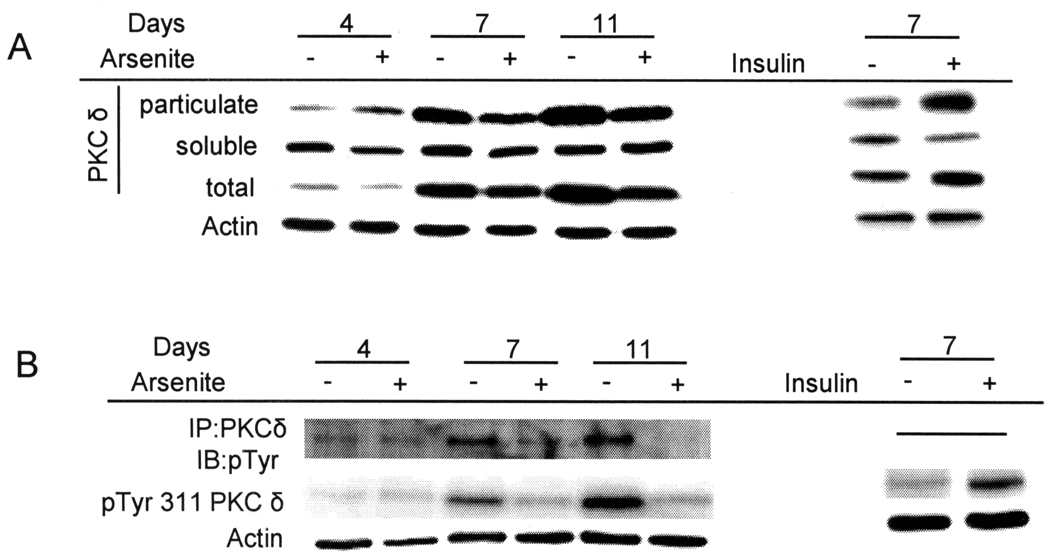
Arsenite and Insulin Modulate PKCδ Status
When the keratinocyte cultures were held at confluence, total and particulate PKCδ increased (Figure 2A). Addition of arsenite (left panel) or removal of insulin (right panel) attenuated such increases. PKCδ tyrosine phosphorylation was examined by immunoprecipitation from culture extracts. In parallel, total protein samples were probed with an antibody that recognizes phosphorylation of tyrosine 311 (Tyr 311), a major src family kinase site associated with modification of PKCδ activity, substrate specificity, and stability [33–35]. Total tyrosine phosphorylation of PKCδ, as well as phosphorylation on Tyr 311, increased in differentiating keratinocytes after confluence in the presence of insulin, while removal of insulin or addition of arsenite prevented both of these effects (Figure 2B). By contrast, neither potassium chromate, sodium vanadate nor cadmium chloride, at concentrations that suppressed insulin-dependent differentiation marker expression, reduced PKCδ amount or phosphorylation (Supplemental Figure 4).
Figure 2.
Regulation of PKCδ level and phosphorylation. SIK cultures were treated with 2 µM arsenite for the indicated number of days or switched to medium without insulin for 7 days. (A) Levels of PKCδ were measured in soluble or particulate fractions, or in unfractionated cell lysates (total). (B) PKCδ was immunoprecipitated and examined for total tyrosine phosphorylation in precipitates. In parallel, PKCδ Tyr 311 phosphorylation (pTyr 311) was measured in total cell lysates. Data are representative of four (A) or two (B) independent experiments. β-Actin was used as a loading control.
PKCδ Overexpression Induces Premature Keratinocyte Differentiation and Augments Accompanying Loss of Proliferative Potential
To explore the role of PKCδ in keratinocyte differentiation and on proliferative potential, a constitutively active pseudosubstrate domain mutant [36,37] was overexpressed in keratinocytes using retroviral infection. As a control, keratinocytes were infected with the empty vector, pBabe-puro. Differentiation marker mRNAs were measured by real time PCR of RNA harvested from pre-confluent cultures, which normally express only low levels of these mRNAs. Overexpression of PKCδ was confirmed by an increase in its mRNA of nearly two fold compared to the vector control. This was accompanied by a 4.5 fold increase in involucrin mRNA compared to the matched vector control (4.5 ± 1.1 in 3 independent experiments, p = 0.03 by two-tailed, paired t test). In two experiments, TGM1, keratin 10 and filaggrin mRNAs also increased from two to 15 fold (not shown).
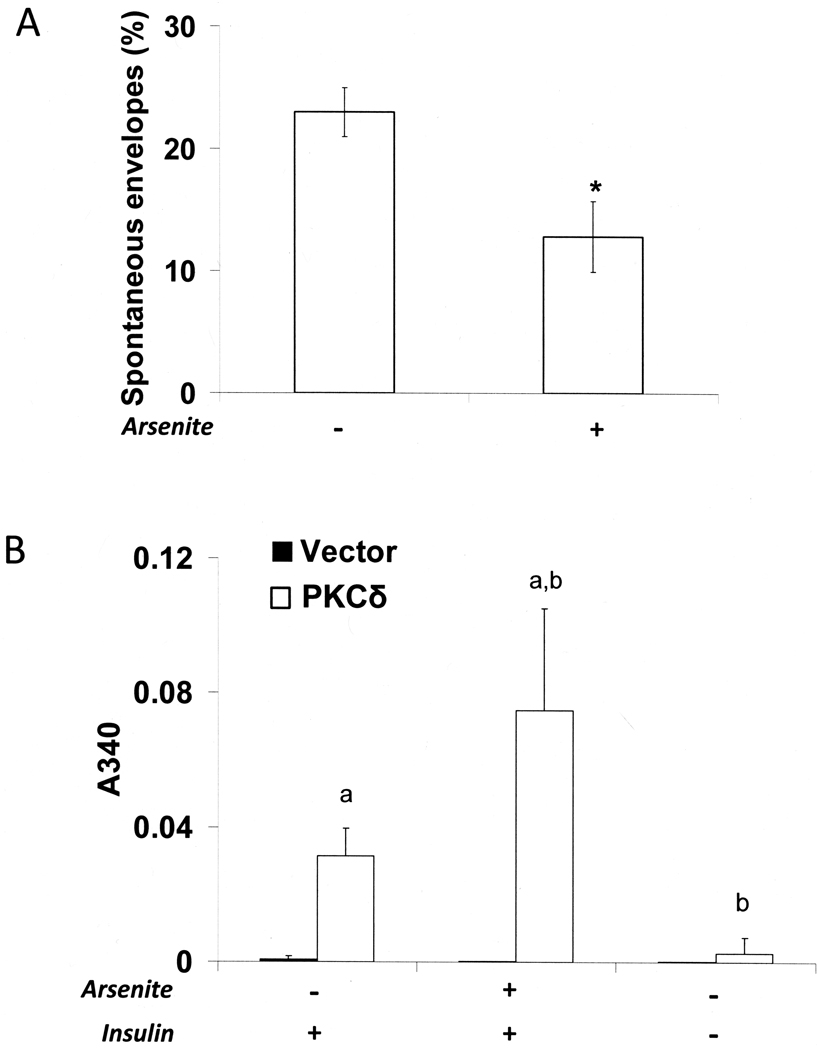
Envelope formation is an important final step of differentiation that occurs in culture after confluence or when cells are held in suspension. Unlike normal epidermal cells [38], SIK cultures form few envelopes spontaneously after confluence, but a substantial fraction (20–25%) do so in suspension culture. As shown in Figure 3A, arsenite treatment decreased spontaneous envelope formation in suspension nearly by half compared to untreated cultures. In surface culture, PKCδ overexpression greatly elevated the fraction of cells that spontaneously formed envelopes, a phenomenon that was not prevented by arsenite. In contrast, little spontaneous envelope formation induced by PKCδ occurred in the absence of insulin (Figure 3B). Spontaneous envelopes were reduced by an average of 14.8 + 1.7 fold when cells were infected with a lentiviral vector expressing any of four different PKCδ shRNAs (p < 0.01 by t-test).
Figure 3.
Spontaneous envelope formation after PKCδ overexpression. (A) SIK cultures near confluence were pre-treated with 2 µM arsenite for one day before suspension and with continued treatment for 4 days in suspension culture before cells were recovered and envelopes were counted. (B) Surface cultures of SIK, retrovirally infected with vector only control (pBabe) or with activated PKCδ construct in pBABE, were treated near confluence with 2 µM arsenite or switched to medium without insulin and, after 8 days, envelopes were quantitated by light scattering (A340). Data were compiled from three independent experiments. In panel A, values of arsenite-treated cultures were significantly different from untreated (*p < 0.05 by 2 tailed Student’s t-test). In panel B, the values for cultures expressing constitutively active PKCδ and treated with arsenite and insulin were significantly greater than that from the PKCδ expressing cultures treated with insulin alone (a, p = 0.02) and not treated with either insulin or arsenite (b, *p < 0.001). Envelope formation in cultures infected with the control pBABE vector (A340 ≈ 0.002) was negligible (0.4% of the cells in two independent experiments).
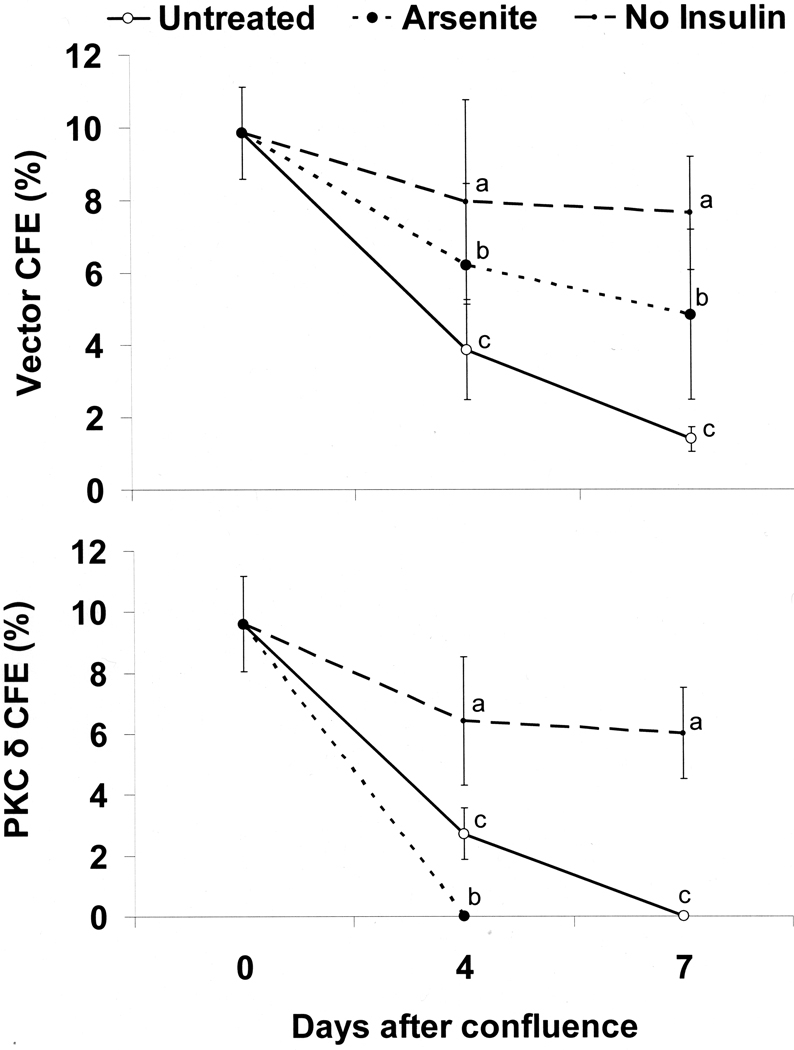
As previously reported [17], the proliferative potential, measured by CFE, of control post-confluent keratinocytes maintained in standard medium containing insulin progressively decreased with time after confluence. Exposure to arsenite or removal of insulin prevented much of the decrease in colony forming ability (Figure 4A). In contrast, over-expression of PKCδ further decreased keratinocyte proliferative potential by a week after confluence compared to the vector control (from 1.4 + 0.4% in control to 0 in PKCδ overexpressing cells in four experiments, p < 0.01 by t-test). Arsenite addition did not prevent the additional loss of proliferative potential in cells overexpressing PKCδ, and the combination was lethal within 4 days after confluence. In contrast, insulin removal after confluence protected keratinocytes from the additional decrease in CFE mediated by PKCδ overexpression.
Figure 4.
Suppression of CFE by PKCδ overexpression. Nearly confluent SIK cultures were treated with 2 µM arsenite or switched to medium without insulin for the indicated number of days. CFE values were determined in cultures retrovirally infected with either the vector only (pBabe) (A) or a constitutively active PKCδ (B) expression construct. Data (mean ± SD) are shown for three independent experiments. In each case, the values for untreated (c), arsenite-treated (b) and no insulin (a) cultures were significantly different (p< 0.05) by 3 way ANOVA.
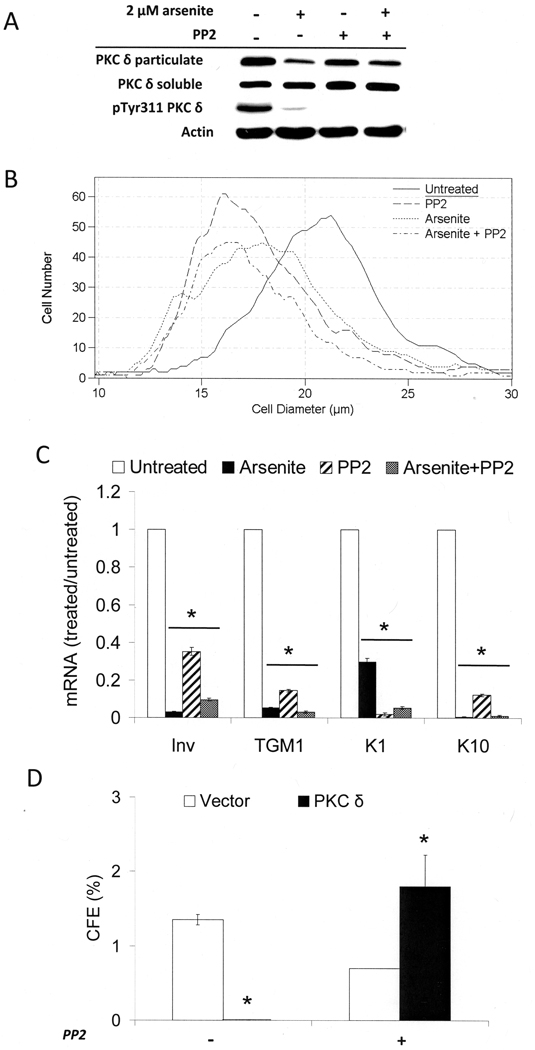
Src Family Kinase Activity Is Required for PKCδ Effects
When cultures were treated in the presence of insulin with PP2, an inhibitor of Src family kinases, phosphorylation of PKCδ on Tyr 311 was prevented (Figure 5A), although this had little effect on distribution between particulate and soluble fractions. In addition the low level of Tyr 311 phosphorylation in the presence of arsenite was further decreased by PP2. Like arsenite, PP2 reduced cell size. Cultures treated with arsenite, PP2 or arsenite and PP2 (modal diameters 16–18 µm) all were noticeably smaller than untreated cultures (21 µm) (Figure 5B). PP2 also suppressed differentiation marker mRNA expression, and co-treatment with arsenite was even more effective (Figure 5C). Finally, PP2 treatment dramatically reversed the loss of colony forming ability after confluence of cells that overexpressed active PKCδ (Figure 5D). These results suggest that, in the presence of insulin, a Src family kinase activity leads to phosphorylation on Tyr 311 of PKCδ and promotes exit from the germinative pool and differentiation marker expression.
Figure 5.
Alteration of proliferative potential, cell size and differentiation by inhibition of Src family kinases. SIK cultures were treated near confluence with 2 µM arsenite and/or 3 µM PP2, an inhibitor of Src family kinases. (A) After 4 days of treatment, protein levels of PKCδ in particulate and soluble fractions and phosphorylated Tyr 311 PKCδ (pTyr 311 PKCδ) levels in total cell lysates were assessed by immunoblotting. The data are representative of two independent experiments for PKCδ in particulate and soluble fractions and a single experiment for pTyr311 PKCδ. (B) SIK cultures were treated with the indicated agents for 8 days, then trypsinized for determination of cell size distribution. The graph is representative of three independent experiments. (C) Expression of keratin 1 and 10, involucrin, and TGM1 mRNAs measured by real-time PCR after 8 days of treatment. Values are given relative to untreated cultures, set at 1. For each gene, the values in treated cultures were significantly different from untreated (*p < 0.001). (D) CFE of cultures infected with activated PKCδ in pBABE or vector control with and without PP2 treatment for 7 days. The difference in values for PKCδ-expressing cultures in the presence and absence of PP2 was significantly different (*p < 0.03) by Student’s two tailed t-test.
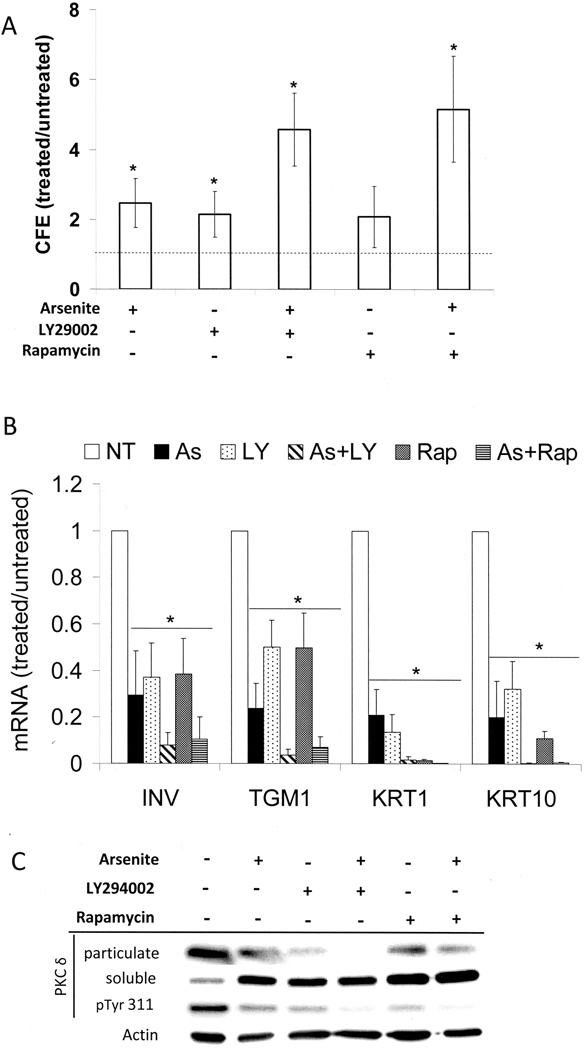
Inhibitors of Phosphatidylinositol 3-Kinase (PI3-Kinase) and mTOR Suppress Insulin Effects on Proliferative Potential and Differentiation
Since the PI3-kinase signaling pathway is strongly activated by insulin and IGF-I, we asked whether PI3-kinase and the downstream effector mTOR are required for insulin effects in keratinocytes. Inhibition of PI3-kinase with LY294002 (Figure 6A) or wortmanin (not shown) approximately doubled the colony forming efficiency in treated cultures, nearly to the same extent as arsenite treatment. The mTOR inhibitor, rapamycin, was similarly effective. Either inhibitor in concert with arsenite produced a 4 fold increase in colony forming efficiency. A similar cooperative effect was observed on cell size (Supplemental Figure 5). Cultures treated with LY294002 had a modal size of 18 µm, similar to those treated with arsenite and noticeably smaller than the untreated control (21 µm). Cultures treated with both arsenite and LY294002 had a modal cell size of 14 µm, paralleling the increased colony forming efficiency of the combined treatment. Arsenite and rapamycin together also reduced cell size further than either agent alone.
Figure 6.
Increased proliferative potential and decreased differentiation in the presence of PI 3-kinase or mTOR inhibitors. Starting near confluence, SIK cultures were treated with 2 µM arsenite, 10 µM LY294002, 5 nM rapamycin or co-treated with arsenite and LY294002 or rapamycin. (A) Fold increases in CFE are given after 7 days relative to those of untreated cultures (absolute values 1.9 + 0.9%), indicated by the dotted line. The results (mean ± SD) are representative of five independent experiments. Except for cultures treated with rapamycin alone, values are significantly different from untreated (*p < 0.02). (B) mRNA expression of involucrin, keratin 1, keratin 10, and TGM1 was measured using real-time PCR after 3 days of treatment and is presented relative to untreated, set as 1. Averages are given of 3–7 independent experiments, where significant differences from untreated for each differentiation marker are indicated by the asterisk (p < 0.001 for each marker). (C) PKCδ levels in particulate and soluble fractions were determined after 7 days of treatment. In parallel, Tyr 311 phosphorylation (pTyr 311) in total cell lysates was measured. β-Actin is used as a loading control. Data are representative of three independent experiments.
LY294002 and rapamycin each produced a substantial reduction in differentiation marker expression, similar to that produced by arsenite, and each in combination with arsenite was more effective (Figure 6B). In addition, LY294002 and rapamycin each decreased membrane localization of PKCδ as well as its Tyr 311 phosphorylation, and each was more effective in combination with arsenite (Figure 6C).
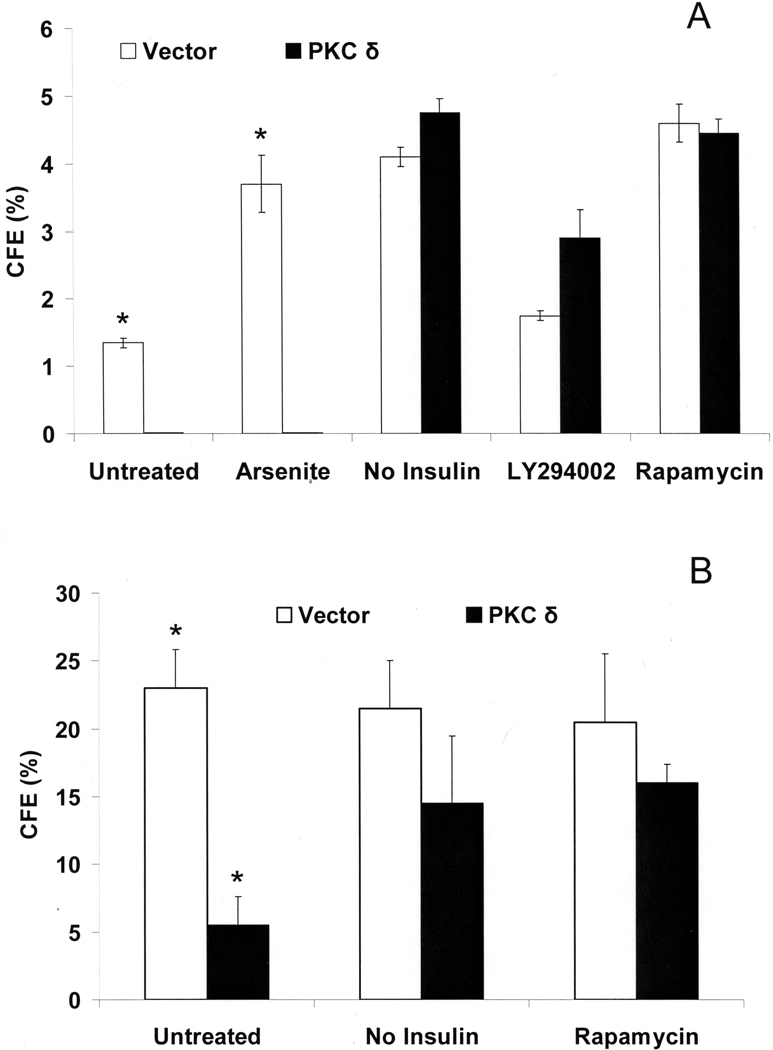
Inhibiting insulin signaling pathways also was found to have profound effects in cells overexpressing PKCδ. Inhibition of PI3-kinase or mTOR (but not arsenite treatment) reversed the loss of proliferative potential elicited by expression of activated PKCδ (Figure 7A). This effect was tested as well in a human squamous cell carcinoma culture (SCC9) in which PKCδ was overexpressed (Figure 7B). In both cases (but more dramatically in SIK cultures), overexpression of PKCδ decreased CFE. Insulin removal or treatment with rapamycin, an inhibitor of the downstream effector, mTOR/raptor complex, largely prevented the observed decrease in CFE.
Figure 7.
Effects of overexpressed PKCδ on colony forming ability: influence of inhibiting insulin action. (A) Starting at confluence, vector only (pBabe) or PKCδ overexpressing SIK cultures were treated or changed to medium without insulin for 7 days before measuring CFE. The differences between cultures expressing PKCδ or not were significant only for the untreated and arsenite-treated conditions (p < 0.01 in each case by Student’s t-test). (B) PKCδ was overexpressed in SCC9, and CFE was measured after 7 days in cultures with or without insulin, or with insulin and 5 nM rapamycin. Treatments began just before confluence. Without arsenite treatment, the values for cultures expressing and not expressing PKCδ were significantly different by Student’s t-test (*p < 0.02).
EGF Receptor Activity is Required for Arsenite Regulation of Differentiation Marker Expression and Cell Size
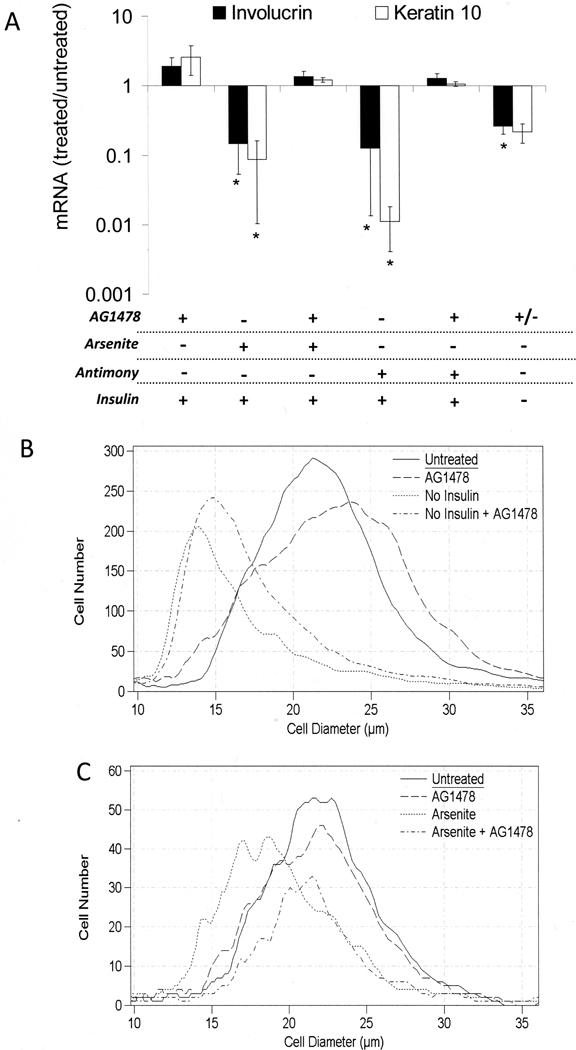
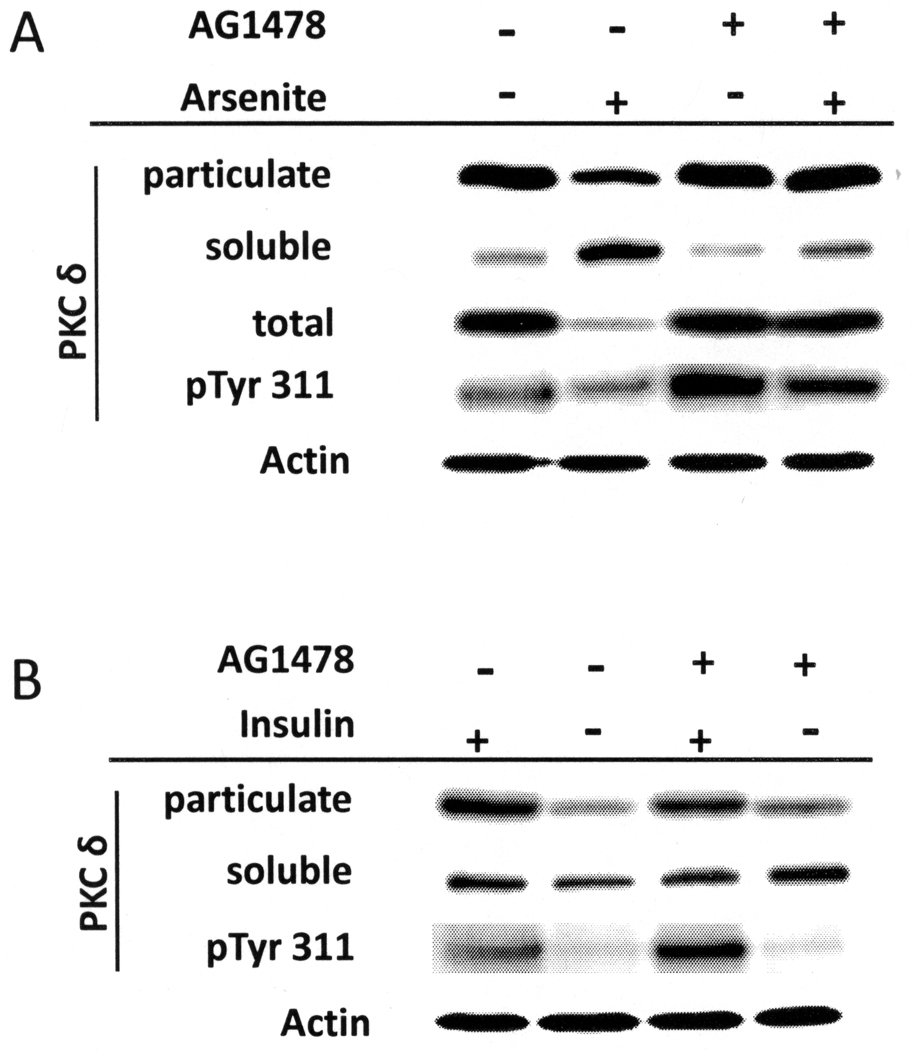
Inhibition of EGF receptor kinase activity has been shown to block the ability of arsenite to preserve proliferative potential of keratinocytes after confluence [15]. To determine whether other effects of arsenite require EGF receptor kinase action, cells were treated with the inhibitor AG1478. As shown in Figure 8A, the inhibitor prevented arsenite and antimonite from suppressing differentiation markers in the presence of insulin, but had no effect on the reduced differentiation marker expression in cells grown without insulin. In parallel experiments, the inhibitor had a small effect on cell size and did not affect the dramatic increase in diameter induced by insulin (Figure 8B). However, it did prevent arsenite (Figure 8C) and antimonite (Supplemental Figure 6) from maintaining a smaller cell size in the presence of insulin. Cells treated with arsenite or antimonite exhibited a modal diameter of 18 µm, whereas cells in the untreated cultures were considerably larger (modal diameter 22 µm). Addition of AG1478 to cultures treated with arsenite or antimonite resulted in a cell size distribution indistinguishable from the untreated control culture.
Figure 8.
Requirement for EGFR function in regulation of differentiation. SIK cultures were pre-treated starting near confluence with AG1478 or solvent for 1 hr prior to treatment with 2 µM arsenite, 5 µM antimonite or switched to medium without insulin for 7 days. (A) Expression of involucrin and keratin 10 mRNAs were measured using real-time PCR; values are given relative to untreated cultures in the presence of insulin, set as 1. Averaged from 4 independent experiments, values significantly different from untreated are indicated (*p < 0.02). (B) Starting at confluence, cultures were grown in the presence or absence of insulin and AG1478 as indicated and analyzed for size after 7 days. (C) Starting near confluence, cultures in the presence of insulin were treated with AG1478 or solvent for 1 hr prior to treatment with 2 µM arsenite as indicated and sizes analyzed after 7 days. Graphs are representative of three (B) and four (C) independent experiments.
Finally, pre-treatment of cultures with AG1478 reversed the suppressive effect of arsenite on total and membrane-localized PKCδ levels as well as on Tyr 311 phosphorylation (Figure 9A). In contrast, AG1478 had little effect on the lack of the membrane-localized PKCδ seen in cells cultivated without insulin (Figure 9B). In addition, even though AG1478 alone slightly increased pTyr 311 levels of PKCδ in the presence of insulin, it did not lead to an increase in Tyr 311 phosphorylation in cultures without insulin. Effective only in the presence of insulin, EGF receptor activity appears targeted to preventing the insulin/IGF-I signaling required for differentiation.
Figure 9.
Effect of AG1478 on PKCδ processing. Cultures were harvested for total protein lysates or fractionated into soluble and particulate fractions and subjected to immunoblot analysis. PKCδ levels in particulate, soluble fractions and total cell lysates as well as phosphorylated Tyr 311 (pTyr 311) PKCδ in total cell lysates were measured. β-Actin was used as a loading control for total lysates. Data are representative of three (A) and two (B) independent experiments.
DISCUSSION
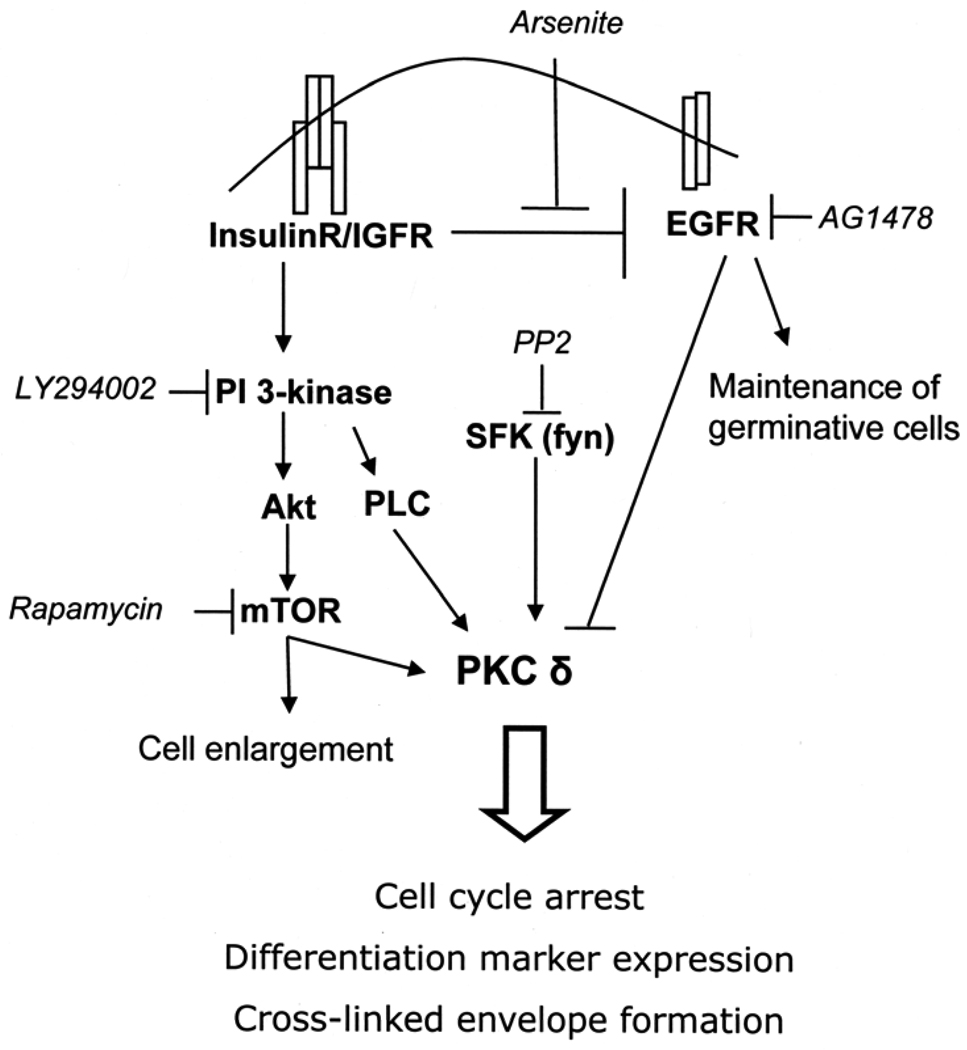
Perturbation of cell behavior often facilitates deeper understanding of normal function. Thus, perturbation by arsenic has revealed the importance in keratinocytes of signaling through insulin or IGF-1 receptors. These receptors share elements of their signaling pathways but display differences in some downstream effects [39]. As shown by gene ablation studies in mice, signaling through the insulin receptor and, more profoundly, IGF-1R are important for the proper development and function of epidermis [40,41]. In post-confluent human keratinocyte cultures, high concentrations of insulin permit normal differentiation, resulting in loss of germinative cells and decreased EGF receptor levels [17], as well as the increased cell size and expression of differentiation markers shown here (Figure 10). That all these features were prevented by removal of insulin after confluence or addition of arsenite to medium containing insulin indicates arsenite interferes with insulin/IGF-I signaling. Cell enlargement during differentiation evidently requires mTOR, since cell size is greatly diminished by rapamycin, a highly specific mTOR inhibitor.
Figure 10.
Model illustrating interrelations of insulin, PKCδ and arsenite. Activation of the insulin/IGF receptor pathway appears to suppress EGFR signaling, an action prevented by arsenite. Insulin/IGFR signaling also stimulates the PI3-kinase pathway, including mTOR signaling. These pathways converge on PKCδ, a critical determinant of cell state. Arsenite and PI3-kinase or mTOR inhibitor cotreatment thus has an additive response in maintaining proliferative potential and yields PKCδ levels equivalent to insulin deprivation. Arrows indicate activation, and bars indicate suppression. Chemical inhibitors are in italics.
The total amount of PKCδ and its membrane translocation, as well as levels of tyrosine phosphorylation, increase during keratinocyte differentiation, effects now demonstrated to require insulin and to be prevented by arsenite. Decreased PKCδ activity is associated with suppression of differentiation [42,43]. On the other hand, overexpression of PKCδ or η is sufficient to induce normal keratinocyte differentiation [44,45]. Building on these findings, present results show that increased membrane localized PKCδ parallels increased cell size and differentiation marker expression and decreased proliferative potential. More directly demonstrating the important role of PKCδ in differentiation, over-expression of an activated pseudosubstrate domain PKCδ mutant increased involucrin mRNA and cross-linked envelopes. The latter is a marker of the final state of terminal differentiation, a process that exhibits features in common with apoptosis [46]. In two experiments, PKCδ over-expression in pre-confluent keratinocytes also resulted in the appearance of a population of very large cells (>30 µm diameter) which were not present in pre-confluent uninfected cells or in the vector control (unpublished data).
PKCδ over-expression also accelerated the loss of proliferative potential that occurs after confluence in cultured keratinocytes. Proliferative potential declined in the keratinocytes infected with vector alone and in uninfected keratinocytes, perhaps because they also express substantial levels of PKCδ. PKCδ likely is not the sole factor resulting in loss of colony-forming efficiency, but it certainly contributes as evident by the augmentation induced by over-expression.
Over-expression of PKCδ has been associated with induction of apoptosis in keratinocytes (Li 1999; Denning 2002). In experiments reported here, western blotting of SIK cultures after retroviral infection with a virus encoding activated PKCδ showed increased differentiation marker expression, but no activated caspases, using antibodies to cleaved caspases 3, 7, 8 or 9 (unpublished). As a control for antibody reactivity, SIK cultures treated with staurosporine to induce apoptosis showed clear bands for cleaved caspases 3, 7 and 8. It is possible that transient caspase activation and subsequent apoptosis did occur in PKCδ-expressing cells during puromycin selection, but was undetected later in the selected cells that were re-plated for analysis. Nevertheless, PKCδ was increased in the selected cells, accompanied by increased differentiation marker expression. The outcome of PKCδ over-expression, either differentiation or apoptosis, could depend on the amount of over-expression. Those cells expressing amounts that trigger apoptosis may have been lost during the drug selection, while cells induced to differentiate may be retained long enough to be re-plated and assayed.
Insulin signaling is known to activate PKC in several cell types [47]. An increase in PKCδ activity after insulin stimulation is associated with altered tyrosine phosphorylation, mediated through src family kinases (SFK), and is PI3-kinase independent [48]. Direct interaction of PKCδ with the insulin receptor results in activation of PKCδ as well as an increase in insulin receptor tyrosine phosphorylation, internalization and signaling [49]. In addition, insulin can increase PKCδ mRNA and protein levels in skeletal muscle [50]. Recent studies in murine keratinocytes indicate that PKCδ is activated by insulin [22,39]. Present results, showing increased membrane localization in the presence of insulin, emphasize the importance of this interaction for differentiation marker expression. The insulin requirement for over-expressed PKCδ to accelerate loss of proliferative potential and envelope formation supports the proposition that PKCδ is a key mediator of insulin-facilitated differentiation and/or apoptosis.
Human PKCδ contains 20 tyrosine residues, several of which have been shown to be phosphorylated, leading either to increased or decreased activity [33]. Of these, Tyr 311, is in the hinge region and conforms to an SFK phosphorylation site. While the cell type and conditions can be critical, it is noteworthy that of several SFK members, Fyn, but not Syk, Src or Btk, associates with PKCδ in activated platelets [51]. Furthermore, activation of platelets results in increased Tyr 311 phosphorylation, likely by Fyn itself since SFK can directly phosphorylate PKCδ in vitro [34]. In platelets, membrane recruitment was required for Tyr 311 phosphorylation and was separately controlled by phospholipase C [52].
In human keratinocytes, Tyr 311 phosphorylation was shown to be critical for PKCδ-dependent activation of involucrin transcription [53]. Although the Tyr 311 kinase was not identified in that report, Fyn is plausibly responsible, since it has been identified as the kinase responsible for the phosphorylation that occurs after calcium stimulation of murine keratinocyte differentiation [54]. Fyn activity is increased in differentiating keratinocytes, leading to growth suppression, reduced EGF receptor signaling and increased differentiation marker expression [55,56]. Furthermore, Fyn is required for proper epidermal function as knockout mice display abnormal differentiation [55]. In the presence of insulin, the general SFK inhibitor PP2 blocked the differentiation-dependent increase in cell size and differentiation marker expression. Clearly, it blocks a critical step in regulation, since it also prevented the effects of over-expressed PKCδ. Although arsenite suppressed Tyr 311 phosphorylation, it did not prevent loss of proliferative potential or envelope formation as a result of PKCδ overexpression. This finding could reflect the elevated level of the PKCδ construct which, unlike the endogenous enzyme, was not suppressed by arsenite.
Present findings suggest the following model of cell signaling during keratinocyte growth and differentiation (Figure 10). At low cell density, EGF receptor signaling is dominant and combines with insulin/IGF-I signaling to stimulate cell proliferation and prevent differentiation. When the cells reach confluence, insulin/IGF-I signaling acts as a pro-differentiation signal, perhaps the result of additional, as yet unknown, signaling generated at confluence. A critical consequence is loss of EGF receptor, removing a block to differentiation, a step prevented by arsenite exposure. Insulin/IGF-I signaling leads to activation of PI3-kinase and one of its downstream effectors, mTOR. Both kinases increase PKCδ activity by increasing phosphorylation of priming sites in the activation loop (Thr505) and hydrophobic domain (Ser662). PDK1, which is activated by binding to phosphotidylinositol-3,4,5-triphosphate, the product of PI3-K, has been identified as the kinase responsible for Thr505 phosphorylation [57,58] and mTOR as the hydrophobic domain kinase [59]. Insulin also induces the membrane translocation of PKCδ, perhaps the result of phospholipase C activation. Membrane targeting brings PKCδ into proximity with SFK which phosphorylate PKCδ at Tyr311, likely further increasing its activity [53]. This phosphorylation, abolished by the SFK inhibitor PP2, may be attributable to Fyn, which has been tied to keratinocyte differentiation [55] and is itself activated by another PKC family member, PKCη. The latter is not known to be regulated by insulin/IGF-I signaling, but rather is dependent upon cholesterol sulfate, enriched in differentiating, suprabasal cells [60,61]. Increased Tyr 311 phosphorylation of PKCδ mediated by insulin is likely due to increased membrane translocation where it co-localizes with activated SFK. Since envelope formation depends upon elevated intracellular calcium, how PKCδ activation impacts the function of the various calcium channels that have been identified in keratinocytes [62,63] merits exploration. In addition, the potential impact of PKCδ on activation of the skin tumor suppressor Notch1, also reduced by arsenic [64], will be of interest.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by grants P42 ES04699 and T32 ES07059 from the U.S. Public Health Service. We thank Dr. Peter J. Parker for kindly providing the mutant PKCδ construct, Drs. S. Reza Jafarzadeh and David M. Rocke (Superfund Biostatistics Core) for assistance in one way ANOVA calculations and Dr. Neil Willits (Statistics Department, University of California, Davis) for performing three way ANOVA calculations.
Abbreviations
- IGF-I
insulin-like growth factor-I
- mTOR
mammalian target of rapamycin
- PI3-kinase
phosphatidylinositol 3-kinase
- PKC
protein kinase C
- SDS
sodium dodecyl sulfate
- SFK
src family kinases
- SIK
spontaneously immortalized keratinocytes
- TGM1
keratinocyte transglutaminase
REFERENCES
- 1.Kitchin KT. Recent advances in arsenic carcinogenesis: Modes of action, animal model systems and methylated arsenic metabolites. Toxicol Sci. 2001;172:249–261. doi: 10.1006/taap.2001.9157. [DOI] [PubMed] [Google Scholar]
- 2.Council NR. Arsenic in Drinking Water. Washington: National Academy Press; 2001. [Google Scholar]
- 3.Smith AH, Marshall G, Yuan Y, et al. Increased mortality from lung cancer and bronchiectasis in young adults after exposure to arsenic in utero and in early childhood. Environ Hlth Perspect. 2006;114:1293–1296. doi: 10.1289/ehp.8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tseng C-H. Cardiovascular disease in arsenic-exposed subjects living in the arseniasis-hyperendemic areas in Taiwan. Atherosclerosis. 2008;199:12–18. doi: 10.1016/j.atherosclerosis.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Coronado-González JA, Del Razo LM, García-Vargas G, Sanmiguel-Salazar F, Escobedo-de la Peña J. Inorganic arsenic exposure and type 2 diabetes mellitus in Mexico. Environ Res. 2007;104:383–389. doi: 10.1016/j.envres.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Waalkes MP, Liu J, Diwan BA. Transplacental arsenic carcinogenesis in mice. Toxicol Appl Pharmacol. 2007;222:271–280. doi: 10.1016/j.taap.2006.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Germolec DR, Spalding J, Boorman GA, et al. Arsenic can mediate skin neoplasia by chronic stimulation of keratinocyte-derived growth factors. Mutation Res. 1997;386:209–218. doi: 10.1016/s1383-5742(97)00006-9. [DOI] [PubMed] [Google Scholar]
- 8.Rossman TG, Uddin AN, Burns FJ. Evidence that arsenite acts as a cocarcinogen in skin cancer. Toxicol Appl Pharmacol. 2004;198:394–404. doi: 10.1016/j.taap.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 9.Green H. The keratinocyte as differentiated cell type. Harvey Lect. 1979;74:101–138. [PubMed] [Google Scholar]
- 10.Watt FM, Green H. Involucrin synthesis is correlated with cell size in human epidermal cultures. J Cell Biol. 1981;90:738–742. doi: 10.1083/jcb.90.3.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barrandon Y, Green H. Cell size as a determinant of the clone-forming ability of human keratinocytes. Proc Natl Acad Sci USA. 1985;82:5390–5394. doi: 10.1073/pnas.82.16.5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ngo MA, Sinitsyna NN, Qin Q, Rice RH. Oxygen dependent differentiation of human keratinocytes. J Invest Dermatol. 2007;126:2507–2515. doi: 10.1038/sj.jid.5700522. [DOI] [PubMed] [Google Scholar]
- 13.Fingar DC, Salama S, Tsou C, Harlow E, Blenis J. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Develop. 2002;16:1472–1487. doi: 10.1101/gad.995802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kachinskas DJ, Qin Q, Phillips MA, Rice RH. Arsenate suppression of human keratinocyte programming. Mutation Res. 1997;386:253–261. doi: 10.1016/s1383-5742(97)00015-x. [DOI] [PubMed] [Google Scholar]
- 15.Patterson TJ, Reznikova TV, Phillips MA, Rice RH. Arsenite maintains germinative state in cultured human epidermal cells. Toxicol Appl Pharmacol. 2005;207:69–77. doi: 10.1016/j.taap.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 16.Jones PH, Watt FM. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell. 1993;73:713–724. doi: 10.1016/0092-8674(93)90251-k. [DOI] [PubMed] [Google Scholar]
- 17.Patterson TJ, Rice RH. Arsenite and insulin exhibit opposing effects on epidermal growth factor receptor and keratinocyte proliferative potential. Toxicol Appl Pharmacol. 2007;221:119–128. doi: 10.1016/j.taap.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waalkes MP, Liu J, Germolec DR, et al. Arsenic exposure in utero exacerbates skin cancer response in adulthood with contemporaneous distortion of tumor stem cell dynamics. Cancer Res. 2008;68:8278–8285. doi: 10.1158/0008-5472.CAN-08-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jessen BA, Qin Q, Phillips MA, Phillips DL, Rice RH. Keratinocyte differentiation marker suppression by arsenic: Mediation by AP1 response elements and antagonism by tetradecanoylphorbol acetate. Toxicol Appl Pharmacol. 2001;174:302–311. doi: 10.1006/taap.2001.9227. [DOI] [PubMed] [Google Scholar]
- 20.Reddig PJ, Dreckschimdt N, Ahrens H, et al. Transgenic mice overexpressing protein kinase Cδ in the epidermis are resistant to skin tumor promotion by 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1999;59:5710–5718. [PubMed] [Google Scholar]
- 21.D'Costa AM, Robinson JK, Maududi T, Chaturvedi V, Nickoloff BJ, Denning MF. The proaptotic tumor suppressor protein kinase Cδ is lost in human squamous cell carcinoma. Oncogene. 2006;25:378–386. doi: 10.1038/sj.onc.1209065. [DOI] [PubMed] [Google Scholar]
- 22.Gartsbein M, Alt A, Hashimoto K, Nakajima K, Kuroki T, Tennenbaum T. The role of protein kinase C delta activation and STAT3 Ser727 phosphorylation in insulin-induced keratinocyte proliferation. J Cell Sci. 2006;119:470–481. doi: 10.1242/jcs.02744. [DOI] [PubMed] [Google Scholar]
- 23.Li L, Lorenzo PS, Bogi K, Blumberg PM, Yuspa SH. Protein kinase Cdelta targets mitochondria, alters mitochondrial membrane potential, and induces apoptosis in normal and neoplastic keratinocytes when overexpressed by an adenoviral vector. Molec Cell Biol. 1999;19:8547–8558. doi: 10.1128/mcb.19.12.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Denning MF, Wang Y, Tibudan S, Alkan S, Nickoloff BJ, Qin JZ. Caspase activation and disruption of mitochondrial membrane potential during UV radiation-induced apoptosis of human keratinocytes requires activation of protein kinase C. Cell Death Differen. 2002;9:40–52. doi: 10.1038/sj.cdd.4400929. [DOI] [PubMed] [Google Scholar]
- 25.Rice RH, Steinmann KE, deGraffenried LA, Qin Q, Taylor N, Schlegel R. Elevation of cell cycle control proteins during spontaneous immortalization of human keratinocytes. Molec Biol Cell. 1993;4:185–194. doi: 10.1091/mbc.4.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allen-Hoffmann BL, Rheinwald JG. Polycyclic aromatic hydrocarbon mutagenesis of human epidermal keratinocytes in culture. Proc Natl Acad Sci USA. 1984;81:7802–7806. doi: 10.1073/pnas.81.24.7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6:331–344. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 28.Rice RH, Green H. Presence in human epidermal cells of a soluble protein precursor of the cross-linked envelope: Activation of the cross-linking by calcium ions. Cell. 1979;18:681–694. doi: 10.1016/0092-8674(79)90123-5. [DOI] [PubMed] [Google Scholar]
- 29.Smith PK, Krohn RI, Hermanson GT, et al. Measurement of protein using bicinchoninic acid. Analyt Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 30.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analyt Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 31.Poumay Y, Pittelkow MR. Cell density and culture factors regulate keratinocyte commitment to differentiation and expression of suprabasal K1/K10 keratins. J Invest Dermatol. 1995;104:271–276. doi: 10.1111/1523-1747.ep12612810. [DOI] [PubMed] [Google Scholar]
- 32.Monzon RI, McWilliams N, Hudson LG. Suppression of cornified envelope formation and type 1 transglutaminase by epidermal growth factor in neoplastic keratinocytes. Endocrinol. 1996;137:1727–1734. doi: 10.1210/endo.137.5.8612508. [DOI] [PubMed] [Google Scholar]
- 33.Steinberg SF. Distinctive activation mechanisms and functions for protein kinase Cdelta. Biochem J. 2004;384:449–459. doi: 10.1042/BJ20040704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rybin VO, Guo J, Sabri A, Elouardighi H, Schaefer E, Steinberg SF. Stimulus-specific differences in protein kinase C delta localization and activation mechanisms in cardiomyocytes. J Biol Chem. 2004;279:19350–19361. doi: 10.1074/jbc.M311096200. [DOI] [PubMed] [Google Scholar]
- 35.Blake RA, Garcia-Paramio P, Parker PJ, Courtneidge SA. Src promotes PKCdelta degradation. Cell Growth Differ. 1999;10:231–241. [PubMed] [Google Scholar]
- 36.Mellor H, Parker PJ. The extended protein kinase C superfamily. Biochem J. 1998;332(pt 2):281–292. doi: 10.1042/bj3320281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pears CJ, Kour G, House C, Kemp BE, Parker PJ. Mutagenesis of the pseudosubstrate site of protein kinase C leads to activation. Eur J Biochem. 1990;194:89–94. doi: 10.1111/j.1432-1033.1990.tb19431.x. [DOI] [PubMed] [Google Scholar]
- 38.Sun T-T, Green H. Differentiation of the epidermal keratinocyte in cell culture: Formation of the cornified envelope. Cell. 1976;9:511–521. doi: 10.1016/0092-8674(76)90033-7. [DOI] [PubMed] [Google Scholar]
- 39.Shen S, Alt A, Wertheimer E, et al. A divergence point in the signaling of insulin and IGF-1-induced proliferation of skin keratinocytes. Diabetes. 2001;50:255–264. doi: 10.2337/diabetes.50.2.255. [DOI] [PubMed] [Google Scholar]
- 40.Liu J-P, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- 41.Sadagurski M, Yakar S, Weingarten G, et al. Insulin-like growth factor 1 receptor signaling regulates skin development and inhibits skin keratinocyte differentiation. Molec Cell Biol. 2006;26:2675–2687. doi: 10.1128/MCB.26.7.2675-2687.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Denning MF, Dlugosz AA, Howett MK, Yuspa SH. Expression of an oncogenic rasHa gene in murine keratinocytes induces tyrosine phosphorylation and reduced activity of protein kinase Cδ. J Biol Chem. 1993;268:26079–26081. [PubMed] [Google Scholar]
- 43.Deucher A, Efimova T, Eckert RL. Calcium-dependent involucrin expression is inversely regulated by protein kinase C (PKC)α and PKCδ. J Biol Chem. 2002;277:17032–17040. doi: 10.1074/jbc.M109076200. [DOI] [PubMed] [Google Scholar]
- 44.Ohba M, Ishino K, Kashiwagi M, et al. Induction of differentiation in normal human keratinocytes by adenovirus-mediated introduction of η and δ isoforms of protein kinase C. Molec Cell Biol. 1998;18:5199–5207. doi: 10.1128/mcb.18.9.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Papp H, Czifra G, Bodo E, et al. Opposite roles of protein kinase C isoforms in proliferation, differentiation, apoptosis, and tumorigenicity of human HaCaT keratinocytes. Cellular and Molecular Life Sciences. 2004;61:1095–1105. doi: 10.1007/s00018-004-4014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tschachler E. Apoptotic pathways in the epidermis. Exp Dermatol. 2004;14:790–791. doi: 10.1111/j.1600-0625.2005.0355h.x. [DOI] [PubMed] [Google Scholar]
- 47.Sampson SR, Cooper DR. Specific protein kinase C isoforms as transducers and modulators of insulin signaling. Mol Genet Metab. 2006;89:32–47. doi: 10.1016/j.ymgme.2006.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosenzweig T, Aga-Mizrachi S, Bak A, Sampson SR. Src tyrosine kinase regulates insulin-induced activation of protein kinase C (PKC) delta in skeletal muscle. Cell Signaling. 2004;16:1299–1308. doi: 10.1016/j.cellsig.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 49.Braiman L, Alt A, Kuroki T, et al. Insulin induces specific interaction between insulin receptor and protein kinase C delta in primary cultured skeletal muscle. Molec Endocrinol. 2001;15:565–574. doi: 10.1210/mend.15.4.0612. [DOI] [PubMed] [Google Scholar]
- 50.Horovitz-Fried M, Cooper DR, Patel NA, et al. Insulin rapidly upregulates protein kinase Cdelta gene expression in skeletal muscle. Cell Signaling. 2006;18:183–193. doi: 10.1016/j.cellsig.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 51.Crosby D, Poole AW. Physical and functional interaction between protein kinase c δ and fyn tyrosine kinase in human platelets. J Biol Chem. 2003;278:24533–24541. doi: 10.1074/jbc.M301847200. [DOI] [PubMed] [Google Scholar]
- 52.Hall KJ, Jones ML, Poole AW. Coincident regulation of PKCdelta in human platelets by phosphorylation of Tyr311 and Tyr565 and phospholipase C signalling. Biochem J. 2007;406:501–509. doi: 10.1042/BJ20070244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu L, Brodie C, Balasubramanian S, Eckert RL. Multiple PKCδ tyrosine residues are required for PKCδ-deopendent activation of involucrin expression - a key role of PKCδ-Y311. J Invest Dermatol. 2008;128:833–845. doi: 10.1038/sj.jid.5701103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Denning MF, Dlugosz AA, Cheng C, et al. Cross-talk between epidermal growth factor receptor and protein kinase C during calcium-induced differentiation of keratinocytes. Exp Dermatol. 2000;9:192–199. doi: 10.1034/j.1600-0625.2000.009003192.x. [DOI] [PubMed] [Google Scholar]
- 55.Calautti E, Missero C, Stein PL, Ezzell RM, Dotto GP. Fyn tyrosine kinase is involved in keratinocyte differentiation control. Genes Develop. 1995;9:2279–2291. doi: 10.1101/gad.9.18.2279. [DOI] [PubMed] [Google Scholar]
- 56.Cabodi S, Calautti E, Talora C, Kuroki T, Stein PL, Dotto GP. A PKC-eta/Fyn-dependent pathway leading to keratinocyte growth arrest and differentiation. Molec Cell. 2000;6:1121–1129. doi: 10.1016/s1097-2765(00)00110-6. [DOI] [PubMed] [Google Scholar]
- 57.Le Good JA, Ziegler WH, Parekh DB, Alessi DR, Cohen P, Parker PJ. Protein kinase C isotypes controlled by phosphoiniositide 3-kinase through the protein kinase PDK1. Science. 1998;281:2041–2045. doi: 10.1126/science.281.5385.2042. [DOI] [PubMed] [Google Scholar]
- 58.Chou MC, Hou W, Johnson J, et al. Regulation of protein kinase C ζ by PI 3-kinase and PDK-1. Curr Biol. 1998;8:1069–1077. doi: 10.1016/s0960-9822(98)70444-0. [DOI] [PubMed] [Google Scholar]
- 59.Parekh D, Ziegler W, Yonezawa K, Hara K, Parker PJ. Mammalian TOR contropls one of two kinase pathwatys acting upon nPKCδ and nPKCε. J Biol Chem. 1999;274:34758–34764. doi: 10.1074/jbc.274.49.34758. [DOI] [PubMed] [Google Scholar]
- 60.Denning MF, Kazanietz MG, Blumberg PM, Yuspa SH. Cholesterol sulfate activates multiple protein kinase C isozymes and induces granular cell differentiation in cultured murine keratinocytes. Cell Growth Differ. 1995;6:1619–1626. [PubMed] [Google Scholar]
- 61.Ikuta T, Chida K, Tajima O, et al. Cholesterol sulfate, a novel activator for eta isoform of protein kinase C. Cell Growth Differ. 1994;5:943–947. [PubMed] [Google Scholar]
- 62.Tu C-L, Chang W, Bikle DD. The extracellular calcium-sensing receptor is required for calcium-induced differentiation in human kerartinocytes. J Biol Chem. 2001;276:41079–41085. doi: 10.1074/jbc.M107122200. [DOI] [PubMed] [Google Scholar]
- 63.Tu C-L, Chang W, Bikle DD. Phospholiupase Cγ1 is required for activation of store-operated channels in human keratinocytes. J Invest Dermatol. 2004;124:187–197. doi: 10.1111/j.0022-202X.2004.23544.x. [DOI] [PubMed] [Google Scholar]
- 64.Reznikova TV, Phillips MA, Rice RH. Arsenite suppresses Notch1 signaling in human keratinocytes. J Invest Dermatol. 2009;129:155–161. doi: 10.1038/jid.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rea MA, Gregg JP, Qin Q, Phillips MA, Rice RH. Global alteration of gene expression in human keratinocytes by inorganic arsenic. Carcinogenesis. 2003;24:747–756. doi: 10.1093/carcin/bgg010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.