Abstract
Single nucleotide polymorphisms (SNPs) in the 3′untranslated region (3′UTR) of human pregnane X receptor (PXR) gene may contribute to interindividual variability in cytochrome P450 3A (CYP3A) activity.
Genotype-phenotype associations involving PXR-3′UTR SNPs were investigated through in vitro (53 human livers from primarily white donors) and in vivo (26 white or African-American volunteers) studies using midazolam 1′-hydroxylation and midazolam apparent oral clearance (CL/F), respectively, as CYP3A-specific probes.
PXR-3′UTR resequencing identified 12 SNPs, including 2 that were novel. Although none of the SNPs evaluated were associated with altered midazolam 1′-hydroxylation in the liver bank, both rs3732359 homozygotes and rs3732360 carriers showed 80% higher (P<0.05) CL/F compared with homozygous reference individuals. These differences in CL/F were even larger (100 and 120% higher, respectively; P<0.01) when only African-American subjects (n=14) were considered.
Five major haplotypes were identified containing the PXR-3′UTR SNPs and previously identified intron SNPs. Although CL/F differences were not statistically significant within the entire study cohort, African-American carriers of Haplotype-1 (which includes both rs3732359 and rs3732360 variants) exhibited 70% higher median CL/F compared with African-American non-carriers (P=0.036).
Our results identify rs3732359 and rs3732360 as PXR-3′UTR SNPs associated with higher CYP3A activity in vivo in African-Americans.
Keywords: Pregnane X receptor (PXR), single nucleotide polymorphism (SNP), 3′untranslated region (UTR), haplotype, cytochrome P450 (CYP) 3A, CYP3A
Introduction
The cytochrome P450 3A (CYP3A) subfamily enzymes are responsible for metabolizing roughly half of the currently marketed xenobiotics, as well as endogenous compounds including steroids (Wrighton et al. 1996). CYP3A expression and activity, however, exhibits substantial interindividual variability. In vivo studies have demonstrated a greater than 10-fold variability in the metabolism of the CYP3A substrates (Guengerich 1999; Thummel and Wilkinson 1998; Westlind-Johnsson et al. 2003; Westlind et al. 1999; Wilkinson 1996). Since variations in CYP3A activity can lead to altered drug levels resulting in either adverse drug reactions or subtherapeutic levels, it important to understand the factors affecting CYP3A-mediated drug metabolism.
It has been estimated as much as 70-90% of CYP3A interindividual variability may be explained by intrinsic influences, including pharmacogenetics (Greenblatt et al. 2008; Özdemir et al. 2000). Currently, 40 CYP3A4 alleles have been described (http://www.cypalleles.ki.se/cyp3a4.htm; Accessed: October 12, 2009). To date, however, no common SNPs have been discovered that account for this wide variability. Consequently, polymorphisms in other genes known to regulate CYP3A activity and expression are currently being explored.
The pregnane X receptor (PXR), a nuclear hormone receptor (Bertilsson et al. 1998; Blumberg et al. 1998; Lehmann et al. 1998), is known to induce CYP3A gene expression through heterodimerization with the retinoid X receptor α (RXR) upon ligand binding (Lehmann et al. 1998). PXR also regulates many other Phase I enzymes, Phase II enzymes (ex. UGT, SULT), and transporters (ex. MDR1) (Meijerman et al. 2006). Since PXR is one of the major regulators of drug clearance in the body, any variations in its expression or activity would likely result in significant effects on drug metabolism. The identification of novel functional polymorphisms in the PXR gene could thereby help explain the substantial variability seen in the induction of CYP3A.
The PXR (NR1I2) gene spans 3.5 kb which contains 9 exons and maps to chromosome 3q11-13 (Zhang et al. 2001). The coding region consists of exon 2 through exon 9, spanning 434 amino acids (Zhang et al. 2001). Although there are hundreds of SNPs located within the PXR gene that have been reported in the dbSNP NCBI database, only 66 PXR gene variants have been discussed in published literature (Bosch et al. 2006; Hustert et al. 2001; King et al. 2007; Koyano et al. 2002; Koyano et al. 2004; Lamba et al. 2005; Zhang et al. 2001). Of those described, Zhang et al. (Zhang et al. 2001) reported 38, including 6 SNPs within the coding region. Three of those coding region SNPs were non-synonymous, creating 3 new PXR alleles (PXR*2, P27S, g.79C to T; PXR*3, G36R, g.106G to A; and PXR*4, R122Q, g.4321G to A). All of these alleles displayed racial differences and exhibited altered basal and/or rifampin-induced activation of PXR using cell-based reporter assay (Hustert et al. 2001; Zhang et al. 2001). Additionally, several mRNA splice variants have been reported with differing 5′ and 3′ amino acid terminus sequences (Fukuen et al. 2002; Lamba et al. 2005; Lamba et al. 2004). Recently, we investigated polymorphisms in exons 2-5 of the PXR gene, along with alternative splicing, and found 3 linked variants in intron 2 (rs1464603 and rs1464602) and intron 4 (rs3732357g>a) that were associated with oral midazolam (MDZ) clearance (CL/F) measured in a group of pharmacokinetic study subjects (n=26; primarily whites and African-Americans) (He et al. 2006a). However, it is not clear whether any of these SNPs directly influence PXR function or whether they are linked to other functional SNPs.
Most studies investigating PXR polymorphisms have focused on the effect of SNPs located in the upstream enhancer, promoter, and coding regions, leaving studies of the effect of SNPs in the mRNA untranslated region (UTR) underrepresented. Similar to the enhancer, the 5′- and 3′-UTRs may contain regulatory motifs that can affect genetic regulation in a number of different ways including: mRNA nuclear export, cytoplasmic localization, translational efficiency and stability (Hughes 2006). Additionally, the UTRs also may contain binding sites of small interfering RNAs (siRNA), including microRNAs (miRNA) (Olsen and Ambros 1999). Mutations in the UTRs may affect the expression of one gene or multiple genes. For example, a transcribed mutated 3′UTR can have a dominant negative effect through sequestering trans-acting transport or regulatory protein, thereby affecting multiple genes (Conne et al. 2000). Thus, the UTR is a very important regulatory region involved in gene expression and activity.
In the present study, we investigated the association of genetic polymorphisms and mRNA levels of PXR-3′UTR and CYP3A4-3′-UTR with CYP3A activity using both in vitro (53 livers) and in vivo (26 volunteers) samples with MDZ 1′-hydroxylation (MDZ-1′OH) and MDZ CL/F as CYP3A-specific measures, respectively. The objective was to identify known and novel SNPs in the 3′UTR of the PXR gene and determine whether any of these variants could contribute to the observed high interindividual variability in CYP3A metabolism.
2. Materials and Methods
2.1. Human liver bank samples
Liver samples from human donors with no known liver disease were previously provided from either the National Disease Research Interchange (Philadelphia, PA) or the Liver Tissue Procurement and Distribution System (University of Minnesota, Minneapolis, MN). All liver samples were either intended for transplantation but had failed to tissue match, were normal tissue adjacent to surgical biopsies, or were autopsy specimens. Donors were primarily white (n=47) but also included four African-Americans and two Hispanics. Additional demographic details have been described previously (Hesse et al. 2004). Use of these tissues was approved by the Tufts University Institutional Review Board. Human liver microsomes of each liver had already been created (Hesse et al. 2004) and MDZ-1′OH activity measured (He et al. 2006b).
2.2. Pharmacokinetic study subjects
Twenty six healthy, nonsmoking subjects aged 21 to 47 years (median, 36 years) and weighing 50 to 100 kg (median, 74.5 kg) were studied as previously described (He et al. 2005). Fourteen of the subjects were African-American, 9 were white, two were Asian, and one was Hispanic. All 4 women in the study were African-American. The screening procedures used for this study have been described elsewhere (He et al. 2005). The clinical study was conducted in accordance with the ethical standards put forth by the Helsinki Declaration of 1975 (revised in 1983). The study protocol and consent form were reviewed and approved by the institutional review boards at ProMedica Clinical Research Center, Tufts University School of Medicine, and Tufts Medical Center, Boston, MA. All subjects provided written informed consent prior to their participation in the study. Our laboratory had previously determined MDZ CL/F (mL/min/kg) of each individual (He et al. 2005) and this served as an in vivo CYP3A phenotype in the genotype-phenotype analysis.
2.3. PXR-3′UTR Genotyping
The NCBI GenBank (NIH, Bethesda, MD) database sequence that we used as reference sequence in this study was AF364606.1 (PXR gene). Nucleotide positions are given relative to the first nucleotide of the start codon (CTG) in exon 2, which is nucleotide 70390 in AF364606.1. Genomic DNA was previously isolated from human liver bank samples and from pharmacokinetic study subject whole blood samples as described by He et al. (He et al. 2005). Primer pairs designed to amplify PXR exon 9 and 3′UTR included P1 (spanning from g.9779-g.10315), P2 (spanning g.10204-g.10791) and P3 (spanning g.10731-g.11305) (primer sequences given in Suppl. Table 1). PCR amplification was carried out in a total reaction volume of 25 μL, containing Platinum Taq PCR SuperMix (Invitrogen, Carlsbad, CA), 0.2 μM of each primer, and 50 ng of genomic DNA template. PCR conditions were as follows: 95 °C for 10 min followed by 30 cycles of 95 °C for 15 sec, a stepwise decrease in temperature from 60 °C to 45 °C for 30 sec and 72 ° C for one min. The samples underwent 15 cycles of 95 °C for 15 sec, 45 °C for 15 sec and 72 °C for 1 min. The reaction was concluded by 70 °C for 1 min and 4 °C for 1 min. Samples were then run on a 1.5% agarose gel to visualize and confirm the proper PCR product size. The PCR products were treated with ExoSap-IT reagent following the manufacturer instructions (USB, Cleveland, OH) and sequenced by the Tufts University Core Facility (Boston, MA).
Sequence variants were identified by alignment of each sequence to the reference PXR sequence (AF364606.1) and confirmed by visualization of the DNA sequence chromatogram. SNP genotype frequencies were evaluated for consistency with Hardy-Weinberg equilibrium using the Chi-squared test.
2.4. Quantitative real-time PCR of PXR mRNA
Message RNA levels of PXR-3′UTR, as well as 18S rRNA (control) were quantified in the human liver bank by real-time PCR after reverse transcription (7300 Real-Time PCR System, Applied Biosystems, Foster City, CA). Briefly, cDNA from each liver sample was generated from 1 μg of DNAse treated RNA using a random hexamer primer (0.1 μg), diluted 10-fold in TE buffer and 10 μL of this reaction was used for the real-time PCR amplification. 18S rRNA was quantified in a 25μL reaction volume using SYBR green (SYBR green 2X master mix, Applied Biosystems) with 0.2 μM each of primer pair P7 (Suppl. Table 1). PXR mRNA was quantified in a 25μL reaction volume using the Taqman 5′-nuclease method (Universal 2X master mix, Applied Biosystems, Foster City, CA) with 0.2 μM of primer pair P4 and 0.2 μM of a Taqman® probe P5 (IDT, Coralville, IA) (Suppl. Table 1). The amount of PXR amplification product was calculated as 1.8−Ct, where the Ct is the cycle threshold and 1.8 is the average amplification efficiency. The amount of PXR mRNA was normalized to 18S rRNA content and expressed relative to the liver with the lowest PXR mRNA level. Assays were performed in triplicate and the results were averaged.
2.5. Cloning of PXR-3′UTR into pMIR-DEST reporter
Three human liver bank DNA samples were identified based on their PXR-3′UTR haplotype (Haplotype-1, Haplotype-2, Ancestral haplotype) (Table 1). PCR amplification of the entire PXR-3′UTR was carried out with PfuUltra Hotstart DNA Polymerase (Stratagene, Cedar Creek, TX) using primer pair P6 (Suppl. Table 1), and 50 ng of genomic DNA template and subsequently cloned into pENTR/D-TOPO vector (Invitrogen, Carlsbad CA). pENTR inserts with the correct sequence were transferred to the pMIR-DEST vector using Gateway LR Clonase II Enzyme (Invitrogen). The pMIR-DEST vector was constructed from the pMIR-REPORT vector (Ambion, Austin TX) using the Gateway Vector Conversion System (Invitrogen).
Table 1.
Single nucleotide polymorphisms identified through resequencing of the PXR-3′UTR using DNA from human liver samples (n = 53 primarily white) and pharmacokinetic study subjects (n = 26 mixed race). Shown are the SNP minor allele frequencies from this study in comparison with experimentally derived frequency data available in the NCBI dbSNP database (http://www.ncbi.nlm.nih.gov/projects/SNP/).
| NCBI dbSNP database |
Liver Samples |
Human Volunteer Samples |
|||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Positio na |
Identifier | Sequenc e |
African- America n |
white s |
Entire Grou p |
white s |
Entire Grou p |
African- America n |
white s |
| (n=44- 46) |
(n=48 ) |
(n=53 ) |
(n=47 ) |
(n=26 ) |
(n=14) | (n=9) | |||
| 10330 | rs3732359 | aggat [G/A] ggcca |
0.44 | 0.79 | 0.75 | 0.77 | 0.40 | 0.18 | 0.83 |
| 10460 | rs1051139 5 |
ggctc [C/A] aggcc |
0.08 | 0.21 | 0.14 | 0.13 | 0.04 | 0.07 | 0.00 |
| 10477 | rs6176036 4 |
tcatc [G/T] gcagg |
N.D. | N.D. | 0.01 | 0.01 | 0.02 | 0.00 | 0 |
| 10483 | rs3732360 | gcagg [C/T] gcatg |
0.44 | 0.77 | 0.73 | 0.74 | 0.40 | 0.18 | 0.83 |
| 10620 | rs1054190 | agcac [C/T] gataa |
0.04 | 0.21 | 0.12 | 0.11 | 0 | 0 | 0 |
| 10719 | rs6438550 | caaac [A/G] atttg |
0.13 | 0.02 | 0.07 | 0.05 | 0.12 | 0.14 | 0.04 |
| 10799 | rs1054191 | atggc [G/A] ggcac |
0.05 | 0.23 | 0.13 | 0.11 | 0.04 | 0 | 0.08 |
| 10875 | ? | gagtg [C/T] gtgtg |
? | ? | 0.02 | 0.02 | 0 | 0 | 0 |
| 11113 | ? | ttttt [G/T] cattt |
? | ? | 0.09 | 0.10 | 0 | 0 | 0 |
| 11125 | rs1272161 5 |
cacaa [A/G] ttata |
0 | 0.02 | 0.01 | 0.01 | 0.02 | 0.06 | 0 |
| 11155 | rs3814957 | accta [A/C] gaact |
0.41 | 0.19 | 0.17 | 0.16 | 0.46 | 0.62 | 0.17 |
| 11193 | rs3814058 | taatg [T/C] caaat |
0.41 | 0.19 | 0.17 | 0.16 | 0.46 | 0.62 | 0.17 |
Position of SNP in GenBank sequence AF364606.1 with +1 being the first nucleotide of the start codon (CTG) in exon 2 (nucleotide 70390). ? - SNP could not be found in the dbSNP database. N.D. – not determined
2.6. Cell culture and transfections
HEK293T, COS-7, and LS180 cells were originally obtained from ATCC (Manassas, VA). The HEK293T and COS-7 cells were maintained in Dulbecco’s Modified Eagle’s Medium with high glucose and L-glutamine (Gibco/Invitrogen, Carlsband CA) supplemented with 10 % fetal bovine serum (FBS, HyClone Laboratories, Logan, UT), and penicillin/streptomycin (Gibco/Invitrogen). The LS180 cells were maintained in minimum essential medium (Gibco/Invitrogen) supplemented with 10 % FBS (HyClone), penicillin/streptomycin, non-essential amino acids, and sodium pyruvate (Gibco/Invitrogen). All cells were maintained in a humidified atmosphere of 5% CO2 at 37 °C.
The day before the transfection, 2 × 105 cells per well were seeded onto a 24-well plate. Cells were transfected with 0.5 μg of the pMIR-DEST plasmids and 0.336 μg of the β-Gal plasmid (Ambion) with Lipofectamine 2000 reagent (Invitrogen). Four to 6 hr post transfection, the media was changed to the proper growth media with 10% FBS. Forty-eight hours after transfection, cells were lysed following the β-galactosidase assay protocol (Promega; Madison, WI) and assayed for both β-galactosidase and luciferase activities.
2.7. Luciferase and β-galactosidase assays
For luciferase assays, 2.5 μL of the cell lysates was added to 250 μL of a d-luciferin substrate mixture (1 mM D-luciferin salt, 2 mM ATP disodium salt, 15 mM MnSO4, 3 mM HEPES pH 7.8, and dH2O) and luminescence measured in triplicate and results averaged for each sample using a Lumat LB 9501 luminometer (Berthold Technologies, Oak Ridge, TN). β-galactosidase activity was quantified using an assay kit from Promega (Madison, WI) following the manufacturer’s instructions. Absorbance values were determined in duplicate and averaged using a Labsystems Multiskan RC spectrophotometer (Fisher Scientific, Waltham, MA) at a wavelength of 405 nm.
2.8. Data and statistical analyses
Statistical analyses were conducted using SigmaStat (Version 3.0, SPSS Inc, Chicago, IL) with a P value of less than 0.05 considered statistically significant. Data were initially evaluated for normality of distribution and equal variance, and in cases where either of these tests failed the data were rank transformed prior to further analysis. For each individual SNP, the influence of genotype (homozygous reference; heterozygous; homozygous variant) on MDZ-1′OH rate (nmole/min/mg protein) or MDZ CL/F (mL/min/kg) was evaluated by a one-way ANOVA (if there were 3 groups), or an unpaired t-test (if there were 2 groups). In instances where the ANOVA indicated a significant difference between the group means of each group, a Student Newman Keuls (SNK) multiple comparison method was used to identify which groups were significantly different from each other.
Linkage disequilibrium analysis was used to determine which SNPs were significantly linked to one another using the LdPlotter Software (https://www.pharmgat.org/Tools/pbtoldplotform). The degree of linkage disequilibrium was assessed using r2 values calculated using an iterative Expectation Maximization (EM) method (Hill 1974). Haplotypes were reconstructed with the PHASE version 2.0.1 program (Stephens et al. 2001) using all PXR-3′UTR SNPs that had a minor allele frequency greater than 10% and also the three linked intron 2 and 4 SNPs previously reported by us to be associated with altered CYP3A activity (rs1464603, rs1464602, and rs3732357) (He et al. 2006a). The effect of carrying a particular haplotype on MDZ CL/F or MDZ-1′OH rate was evaluated by Mann Whitney Rank Sum test. The effect of each SNP and haplotype on PXR-3′UTR mRNA secondary structure and free energy was evaluated using GeneBee RNA secondary structure prediction software (Brodsky 1995) (http://www.genebee.msu.su/services/rna2_reduced.html).
For cell culture experiments, one-way ANOVA with post-hoc Dunnett’s test to control (pMIR-DEST with no 3′-UTR) was performed to assess the effect of three PXR-3′UTR haplotypes on pMIR-DEST gene reporter luciferase activity.
3. Results
3.1. SNP identification, genotype and allele frequencies
Sequencing of the PXR-3′UTR in the human liver bank (n=53 primarily white) and pharmacokinetic study subjects (n=26 primarily white and African-Americans) yielded 12 SNPs, two of which (g.10875c>t and g.11113g>t) appear to be novel in that they were not listed in the dbSNP database (Table 1). A graphical representation of the PXR gene with SNPs identified in this study is shown in Fig. 1. The genotype frequency distributions for each SNP were consistent with Hardy-Weinberg equilibrium in the entire study population (Chi-squared test, P>0.05). The minor allele frequencies of these SNPs in the entire human liver bank and the human pharmacokinetic study subjects are given in Table 1 in comparison with the SNP frequencies available in the NCBI dbSNP database (http://www.ncbi.nlm.nih.gov/projects/SNP/) for African-American and white subjects. In general, minor allele frequencies derived in this study were similar to those currently listed in the NCBI SNP database. Several consistent trends in minor allele frequencies related to DNA donor race were noted for all populations evaluated, including lower frequencies of rs3732359, rs3732360, and rs1054190 and higher frequencies of rs6438550, rs3814957, and rs3814058 in African-Americans compared with whites. Both of the novel SNPs were identified only in white subjects from the human liver bank as heterozygous carriers (5 out of 47 and one out of 47 individuals for g.10875c>t and g.11113g>t, respectively).
Figure 1.
Graphical representation of the structure of human PXR gene including locations of all SNPs evaluated in this study. Also shown are the PXR DNA binding domain (DBD) and ligand binding domain (LBD), as well as an expanded 3′-UTR region.
Association of PXR-3′UTR genotype with CYP3A phenotype in vitro and in vivo
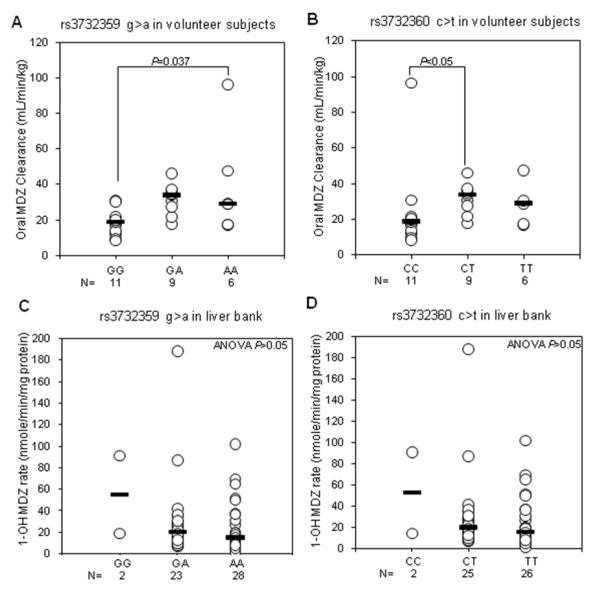
No significant associations could be demonstrated between any of the PXR-3′UTR genotypes and MDZ-1′OH rates measured in the entire set of human liver bank samples (n=53) or within the white (n=47) subgroup (Suppl. Table 2). However, in the human pharmacokinetic study subjects (n=26) two PXR-3′-UTR SNPs were identified that were significantly associated with higher oral MDZ CL/F, including rs3732359 (P = 0.034, Kruskal-Wallis ANOVA on ranks) and rs3732360 (P = 0.03, Kruskal-Wallis ANOVA on ranks) (Table 2). As shown in Fig. 2A, rs3732359 aa genotype individuals exhibited about 80% higher median MDZ CL/F compared with rs3732359 gg genotype subjects (P = 0.037, SNK test). Similarly in Fig. 2B, rs3732360 ct genotype subjects showed 80% higher median MDZ CL/F than rs3732360 cc genotype subjects (P < 0.05, SNK test). In contrast, neither rs3732359 (Fig. 2C) nor rs3732360 (Fig. 2C) were associated with altered MDZ-1′OH activity in the human liver bank samples.
Table 2.
Analysis of effects of PXR-3′UTR genotype on MDZ CL/F (mL/min/kg) in 26 healthy pharmacokinetic study subjects.
| CL/F (mL/min/kg) |
||||
|---|---|---|---|---|
| Genotype | N | Median | 25%-75% | P valuea |
| rs3732359 gg | 11 | 18.8 | 12.5-21.2 | 0.034 |
| ga | 9 | 33.9 | 26.1-36.3 | |
| aa | 6 | 29.0c | 17.5-47.6 | |
| rs10511395 cc | 24 | 21.6 | 17.3-32.9 | 0.53 |
| ca | 2 | 30.7 | 27.6-33.9 | |
| aa | 0 | -- | -- | |
| rs56147004 gg | 26 | 27.5 | 17.7-34.1 | 0.35 |
| gt | 1 | 17.0 | 17.0-17.0 | |
| tt | 0 | -- | -- | |
| rs3732360 cc | 11 | 18.8 | 12.5-21.1 | 0.030b |
| ct | 9 | 33.9c | 26.1-36.3 | |
| tt | 6 | 29.0 | 17.5-30.3 | |
| rs6438550 aa | 21 | 28.8 | 17.7-34.4 | 0.63 |
| ag | 4 | 18.6 | 15.4-27.5 | |
| gg | 1 | 21.5 | 21.5-21.5 | |
| rs1054191 gg | 23 | 21.6 | 17.6-34.6 | 0.89 |
| ga | 2 | 23.9 | 10.0-30.8 | |
| aa | 0 | -- | -- | |
| rs12721615 aa | 24 | 24.6 | 17.3-34.4 | 0.84 |
| ag | 1 | 20.1 | 20.1-20.1 | |
| gg | 0 | -- | -- | |
| rs3814957 aa | 7 | 28.8 | 18.8-45.0 | 0.26 |
| ac | 13 | 19.8 | 17.0-28.4 | |
| cc | 5 | 30.3 | 24.2-34.4 | |
| rs3814058 tt | 7 | 28.8 | 18.8-45.0 | 0.26 |
| tc | 13 | 19.8 | 17.0-28.4 | |
| cc | 5 | 30.3 | 24.2-34.4 | |
Data were analyzed by unpaired t-test (2 groups) or one-way ANOVA (3 groups) with Student-Newman-Kuels multiple comparisons testing.
Data were rank transformed since distribution was non-normal.
P<0.05 versus homozygous reference sequence.
Figure 2.
MDZ CL/F (mL/min/kg) in 26 healthy pharmacokinetic study subjects (panels A and B) and 1′OH MDZ formation rate (nmole/min/mg protein) in 53 human livers (panels C and D) grouped by PXR-3′UTR genotype for rs3732359g>a and rs3732360c>t. Bars indicate the median value for each group. Also shown on each plot are the results of statistical analyses using ANOVA followed by Student Newman Keuls test (A), Kruskal-Wallis ANOVA on ranks test followed by Dunn’s multiple comparisons test (B), or Kruskal-Wallis ANOVA on ranks (C and D).
Analyses of these SNPs were then repeated in the African-American (n=14) and white (n=9) subgroups of the pharmacokinetic study subject subjects. Larger genotype effects were observed in the African-American subgroup in that those carrying the variant allele for rs3732359 (Fig. 3A) and rs3732360 (Fig. 3C) exhibited over 100% and 120% (respectively) higher median oral CL/F than those with the homozygous reference genotype (P = 0.008, Mann-Whitney Rank Sum test for rs3732359; P < 0.001 Kruskal-Wallis ANOVA for rs3732360 and P = 0.002 for rs3732360 ct versus cc genotypes with SNK test). However, as shown in Fig. 3B and 3D, there was no obvious genotype-phenotype association in the somewhat smaller white subgroup (n=9) for either rs3732359 or rs3732360 (P > 0.05, Mann-Whitney rank-sum test and Kruskal-Wallis ANOVA on ranks test, respectively). Furthermore, there were no other statistically significant associations for any of the other PXR-3′UTR SNPs with MDZ CL/F in the pharmacokinetic study (Table 2).
Figure 3.
MDZ CL/F (mL/min/kg) in 26 pharmacokinetic study subjects grouped by self-identified race (African-American or white) and PXR genotype for variants rs3732359g>a and rs3732360c>t. Bars indicate the median value for each group. Also shown on each plot are the results of statistical analyses using Mann Whitney Rank Sum test (A, B), Kruskal-Wallis ANOVA on ranks test followed by Dunn’s multiple comparisons test (C), or Kruskal-Wallis ANOVA on ranks (D).
3.2. PXR-3′UTR linkage disequilibrium and haplotype reconstruction
Linkage disequilibrium analysis was performed using genotype data for the eight PXR-3′UTR SNPs with variant allele frequencies greater than 10% from this study as well as the three previously identified linked PXR intron 2 and 4 SNPs (rs1464603, rs1464602, rs3732357) (He et al. 2006a) (Fig. 4). Analyses were performed in separate African-American and white subgroups extracted from the combined study groups (human liver bank donors and pharmacokinetic study subjects) to evaluate possible race/ethnicity dependent linkage patterns. As shown in Fig. 4A and 4B, there was complete linkage disequilibrium (r2 = 1.0) between the rs3814057 and rs3814058 variants in both African-American and white subjects. There was also complete linkage between rs3732359 and rs3732360, and between rs10511395 and rs1054191, in the African-Americans (Fig. 4A) and tight linkage (r2 = 0.9) between these same SNP pairs in the white subjects (Fig. 4B). Both rs3732359 and rs3732360 were also tightly linked (r2 = 0.8) to rs3814057 and rs3814058 in the white subjects (Fig. 4B) but only poorly in the African-Americans (Fig. 4A). Finally, of the three intron SNPs, only the intron 2 SNP rs1464603 in African Americans showed tight linkage (r2 = 0.8) to any of the 3′-UTR SNPs, including both rs3732359 and rs3732360.
Figure 4.

Linkage disequilibrium matrix plot showing pair-wise relationships between SNPs located in the PXR gene intron 2 (rs1464603 and rs1464602), intron 4 (rs3732357) and 3′UTR regions identified in African-American (n = 18) and white (n= 56) individuals. Genotype data from human liver bank samples and pharmacokinetic study subjects were combined for this analysis. The plot was created using the LDPlotter software available at https://www.pharmgat.org/Tools/pbtoldplotform. Colored boxes represent the approximate regression value (r2) as shown in the legend for the pair of SNPs being compared.
Haplotypes were inferred for the entire study population with PHASE 2.0 software (Stephens et al. 2001) using genotype data from eight PXR-3′UTR SNPs and the three intron SNPs. Table 3 shows the five major haplotypes that were identified with an allele frequency of at least 5% in either the human liver bank samples or the pharmacokinetic study subjects. Interestingly, one of the liver bank donors (a white individual) and two of the pharmacokinetic study subjects (both African-Americans) were heterozygous for a relatively rare haplotype that had the PXR gene “ancestral” sequence at all variant positions. The ancestral allele is provided in the human dbSNP database based upon the chimpanzee genome sequence (Spencer et al. 2006). However, none of the identified haplotypes had a DNA sequence that was identical to the “reference” sequence (AF364606.1) that was used to determine nucleotide positions of each SNP in this study.
Table 3.
Five major haplotypes identified in a human liver bank (n=53) and MDZ pharmacokinetic study subjects (n=26) incorporating PXR-3′UTR SNPs and PXR intron 2 and 4 SNPs.
|
Intron 2
|
Intron 4 |
3′UTR
|
Frequency |
N
chromosomes |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| refSNP# | rs1464603a | rs1464602a | rs3732357a | rs3732359 | rs10511395 | rs3732360 | rs6438550 | rs1054191 | rs3814057 | rs3814058 | Liver Bank |
Human Subjects |
Liver Bank |
Human Subjects |
| Alleles | a/g | a/g | g/a | g/a | c/a | c/t | a/g | g/a | a/c | t/c | ||||
| Ancestralb | g | g | g | g | c | c | a | g | a | t | 0.01 | 0.04 | 1 | 2 |
| Referencec | [a] | [a] | g | g | c | c | [g] | g | a | t | 0.00 | 0.00 | 0 | 0 |
| Haplotype-1 | [a] | [a] | g | [a] | c | [t] | a | g | a | t | 0.46 | 0.13 | 49 | 7 |
| Haplotype-2 | g | g | g | g | c | c | a | g | [c] | [c] | 0.11 | 0.25 | 12 | 13 |
| Haplotype-3 | [a] | [a] | [a] | [a] | c | [t] | a | g | a | t | 0.01 | 0.21 | 1 | 11 |
| Haplotype-4 | [a] | [a] | g | [a] | [a] | [t] | a | [a] | a | t | 0.09 | 0.00 | 10 | 0 |
| Haplotype-5 | g | g | g | g | c | c | [g] | g | a | t | 0.02 | 0.10 | 2 | 5 |
|
| ||||||||||||||
| Other minor haplotypes: | 0.29 | 0.27 | 31 | 14 | ||||||||||
Genotypes for these SNPs in these samples were reported previously by He at al (He et al. 2005).
Contains all of the ancestral alleles according to dbSNP (http://www.ncbi.nlm.nih.gov/projects/SNP/).
Based on the GenBank sequence AF364606.1 (none of the samples analyzed had this reference sequence).
PXR-3′UTR haplotype association with CYP3A phenotypes
The five major identified haplotypes were subsequently evaluated for association with CYP3A activity phenotypes (MDZ CL/F or MDZ-1′OH rate). Similar to the genotype-phenotype analyses performed using individual SNP genotype data, there were no apparent associations between any of the PXR haplotypes and MDZ-1′OH rate in the human liver samples (Suppl. Table 3). Pharmacokinetic study participants that carried Haplotype-2 exhibited 50% lower median MDZ CL/F than non-carriers (Fig. 5A). Haplotype-2 consists of variant alleles for the g.252, g.275, rs3814057, and rs3814058 SNPs and reference alleles for the rs3732359 and rs3732360 SNPs (Table 1). Consequently this trend for lower clearance with Haplotype-2 may reflect an effect of the reference alleles for the rs3732359 and rs3732360 SNPs, which were previously associated with lower MDZ CL/F (Fig. 2). Upon stratifying by race, the carriers of Haplotype-2 in the African-American subpopulation also had lower median oral CL than non carriers (53% lower median MDZ CL/F than non-carriers; Fig. 5B), although again the difference was not statistically significant (P>0.05, Mann Whitney Rank Sum test). In contrast to Haplotype-2, carriers of the most frequent haplotype, Haplotype-1, exhibited a 30% higher median MDZ CL/F than non-carriers (Fig. 5A). This haplotype consists of the reference sequence at g.252 and g.275 and the variant sequences for the rs3732359 and rs3732360 SNPs. Carriers of the variant allele for all four of these SNPs were previously shown to be associated with significantly higher MDZ CL/F (Fig. 2 and (He et al. 2006a)). In the African-American subgroup, carriers of Haplotype-1 also had significantly higher MDZ CL/F (by about 56%) compared with non-carriers (Mann Whitney Rank Sum test, P=0.036; Fig. 5B), whereas there were no significant haplotype-phenotype associations with the whites only subgroup (Fig. 5C).
Figure 5.
Association of PXR haplotype carrier status with MDZ CL/F in pharmacokinetic study subjects. Shown are results for all 26 subjects (A), the 14 African-Americans (B) and the 9 whites (C). Haplotypes (shown in Table 3) were inferred using PHASE 2.0 software (Stephens et al. 2001) with genotype data from eight PXR-3′UTR SNPs and three upstream intron 2 and 4 SNPs may predict MDZ CL/F in pharmacokinetic study subjects. Bars indicate the median values for each group. Also shown are the P values of statistical comparisons between carrier and non-carrier groups using Mann Whitney Rank Sum test. N/A denotes that the statistical test was not performed since there were no carrier individuals. None of the pharmacokinetic study subjects carried Haplotype-4.
3.3. Influence of SNPs on PXR-3′UTR mRNA levels, predicted structure, and free energy
In addition to examining the influence of each SNP on CYP3A phenotype, we also investigated the effect of the SNPs on PXR-3′-UTR mRNA levels in the human liver bank measured using real time-PCR. As shown in Fig. 6A, there was high variability in PXR mRNA levels, with a median level of expression of 188 (relative to the liver with the lowest level) and an interquartile range of 51 to 414 (relative to the liver with the lowest level set at 1.0). However, there was no apparent relationship between the PXR-3′UTR mRNA levels and CYP3A activity (Spearman correlation, Rs= 0.184, P>0.05). The two completely linked PXR-3′UTR SNPs rs3814057 and rs3814058 were associated with significantly higher PXR-3′UTR mRNA levels (Fig. 6B, Table 4) with median mRNA levels that were 140% higher in variant carrier livers compared with homozygous reference livers (P=0.048, Mann Whitney Rank Sum test).
Figure 6.
Relationship between total 3′UTR PXR mRNA levels, MDZ-1′OH rate, and PXR genotype. (A). Lack of correlation between total 3′UTR mRNA PXR levels and 1-OH formation MDZ rate (Spearman rank correlation, Rs=0.184, P>0.05). (B). Liver samples carrying the rs3814057a>c and rs3810458t>c genotype have 140% greater median total 3′UTR mRNA levels than those homozygous reference (Mann Whitney Rank Sum test, P=0.048). Bar indicates the median values for each group. qPCR results are presented as relative to the lowest liver expression, which was assigned a value of 1.
Table 4.
Analysis of the effects PXR genotype on PXR-3′UTR mRNA levels in the human liver bank (relative to the liver with the lowest value).
| mRNA amount (relative to lowest) |
||||
|---|---|---|---|---|
| Genotype | N | Median | 25%-75% | P valuea |
| rs3732359 gg | 1 | 27.7 | 27.7-27.7 | 0.17 |
| ga | 18 | 237.8 | 145.2-693.4 | |
| aa | 25 | 100.7 | 38.0-341.4 | |
| rs10511395 cc | 33 | 207.4 | 51.3-414.1 | 0.18 |
| ca | 10 | 83.2 | 16.9-205.3 | |
| aa | 1 | 1004.8 | 1004.8-1004.8 | |
| rs3732360 cc | 2 | 34.9 | 27.7-42.2 | 0.082 |
| ct | 20 | 237.8 | 128.1-724.3 | |
| tt | 22 | 34.9 | 27.7-42.2 | |
| rs1054190 cc | 34 | 197.5 | 54.4-409.1 | 0.57 |
| ct | 10 | 125.7 | 16.9-755.2 | |
| tt | 0 | -- | -- | |
| rs6438550 aa | 39 | 205.3 | 55.4-620.9 | 0.48 |
| ag | 4 | 165.3 | 87.9-196.5 | |
| gg | 1 | 27.7 | 27.7-27.7 | |
| rs1054191 gg | 34 | 169.2 | 54.4-318.8 | 0.33 |
| ga | 9 | 185.5 | 14.0-800.0 | |
| aa | 1 | 1004.8 | 1004.8-1004.8 | |
| g.10875 cc | 42 | 186.5 | 42.2-429.2 | 0.89 |
| ct | 2 | 160.6 | 63.8-257.3 | |
| tt | 0 | -- | -- | |
| g.11113 gg | 36 | 206.3 | 48.3-561.3 | 0.71 |
| gt | 6 | 89.9 | 16.9-270.5 | |
| tt | 1 | 111.1 | 111.1-111.1 | |
| rs12721615 aa | 41 | 185.5 | 39.3-478.0 | 0.66 |
| ag | 1 | 429.2 | 429.2-429.2 | |
| gg | 0 | -- | -- | |
| rs3814957 aa | 30 | 111.2 | 30.7-257.3 | 0.045b |
| ac | 14 | 278.5 | 150.8-755.2 | |
| cc | 0 | -- | -- | |
| rs3814058 tt | 30 | 111.2 | 30.7-257.3 | 0.045b |
| tc | 14 | 278.5 | 150.8-755.2 | |
| cc | 0 | -- | -- | |
Data were analyzed by unpaired t-test (2 groups) or one-way ANOVA (3 groups) with Student-Newman-Kuels multiple comparisons testing.
Data were rank transformed since data distribution was non-normal.
Since it is possible that these linked variants could be exerting an effect through changes in mRNA stability, we next investigated the effect of each variant and haplotype on the predicted PXR-3′UTR mRNA secondary structure and free energy of the secondary structure using the bioinformatics computer program (GeneBee; (Brodsky 1995)). Comparisons of each 3′-UTR SNP and haplotype were made to the Ancestral haplotype (defined above) which is presumed to represent the ancestral state of each SNP (Table 5). Although most of the SNPs had only a minor effect on the 3′-UTR mRNA free energy, the rs3814058 SNP, as well as Haplotype-2 (which contains rs3814058) displayed more negative mRNA free energy (ΔG) (greater stability) relative to the Ancestral haplotype (−259.1 and −259.1 versus −252.6 kcal/mol, respectively). This effect of decreased free energy can be seen in the predicted secondary structure pictures (Fig. 7). Haplotype-2, which is influenced by the variants of rs3814057 and rs3814058, showed an alteration at the center of the predicted RNA secondary structure resulting in a longer stem structure via pairwise bonding (circled in Fig. 7C), in comparison with the predicted structure of the Ancestral haplotype (Fig. 7A). Although a more substantial effect on structure was observed for Haplotype-1 (circled in Fig. 7B), this change did not substantially affect the predicted free energy.
Table 5.
Calculated free energy of the predicted PXR-3′UTR mRNA secondary structure using the GeneBee RNA secondary structure prediction software (Brodsky 1995).
| Allele/haplotype | Free Energy (kcal/mol) |
|---|---|
| rs3732359 g>a | −250.7 |
| rs10511395 c>a | −251.3 |
| rs61760364 g>t | −246.8 |
| rs3732360 c>t | −252.9 |
| rs1054190 c>t | −257.7 |
| rs6438550 a>g | −252.6 |
| rs1054191 g>a | −247.8 |
| g.10875 c>t | −246.6 |
| g.11113 g>t | −252.6 |
| rs12721615 a>g | −250.0 |
| rs3814957 a>c | −252.6 |
| rs3814058 t>c | −259.1 |
|
| |
| Ancestral haplotype | −252.6 |
| Haplotype-1 | −252.0 |
| Haplotype-2 | −259.1 |
| aHaplotype-3 | −252.0 |
| Haplotype-4 | −251.8 |
| bHaplotype-5 | −252.6 |
Same 3′UTR sequence as Haplotype-1;
Same sequence as rs6438550 a>g
Figure 7.
Predicted PXR-3′UTR mRNA secondary structures and calculated free energies for the Ancestral haplotype (A), Haplotype-1 (B), and Haplotype-2 (C). Images were generated using the RNA GeneBee secondary structure predictor software (Brodsky 1995). The circled areas indicate secondary structure differences in Haplotype-1 and Haplotype-2 compared with the Ancestral haplotype.
3.4. Effect of PXR-3′UTR haplotype on 3′UTR luciferase reporter activity
The mechanism by which these PXR-3′UTR haplotypes might affect CYP3A activity was explored further by creating plasmid reporter constructs containing the luciferase coding sequence ahead of each of the PXR-3′UTR haplotype sequences associated with altered MDZ CL/F (Haplotype-1 and Haplotype-2) as well as the Ancestral haplotype. R esults were normalized to a reporter luciferase construct (pMIR-DEST) that lacked any PXR-3′UTR. As shown in Fig. 8, there were no significant differences in luciferase activities between the reference PXR-3′UTR and the constructs containing either of the two haplotypes. However, of note is that each of the PXR-3′UTR reporter constructs had over 100% higher luciferase activity than the pMIR-DEST plasmid alone when transfected in COS-7 and LS180 cells (P<0.05 by 1-way ANOVA with post-hoc Dunnett’s test) but not when transfected into HEK293T cells.
Figure 8.
HEK293T (A), COS-7 (B), and LS180 (C) cell transfection experiments comparing the effect of the PXR-3′UTR of the Ancestral haplotype, Haplotype-1, and Haplotype-2 on normalized luciferase activity (Luciferase activity/ β-Galactosidase activity) relative to the pMIR-DEST activity. Columns represent the mean of 4 experiments performed in triplicate and error bars denote standard error of the mean.
4. Discussion
In this study we have investigated the association of SNPs in the 3′UTR of PXR gene with CYP3A activity measured in vivo and in vitro in an effort to explain the known high interindividual variability of this enzyme. SNPs in the PXR-3′UTR could have potential effects on a number of different aspects of genetic regulation including: mRNA nuclear export, cytoplasmic localization, translational efficiency and stability (Hughes 2006), as well as microRNA binding sites (Olsen and Ambros 1999).
Of the 12 SNPs evaluated, only two were significantly associated with altered CYP3A activity. Specifically, those subjects who were homozygous variant for the rs3732359 SNP and heterozygous for the rs3732360 SNP exhibited higher median MDZ CL/F compared with those who were homozygous reference for these SNPs (Figs. 1 and 2). However, these significant genotype-phenotype associations were only observed for the in vivo pharmacokinetic study and were not found in the in vitro human liver bank study. This disparity between in vivo and in vitro studies could be the result of loss of PXR-mediated induction of CYP3A activity in human livers associated with conditions occurring immediately prior to tissue procurement. Moreover, the majority of our pharmacokinetic study subjects were African-Americans, whereas the human liver bank samples were primarily from white donors. Upon analyzing this genotype-phenotype relationship within each racial group, carriers of each variant allele still exhibited significantly higher MDZ CL within the African-American subpopulation. In contrast, this association was not seen in the white subpopulation (Fig. 3), but the sample size of this subgroup was too small to draw any firm conclusions regarding possible racial differences in genotype-phenotype association. However it should be pointed out that at least one other research group has observed CYP3A phenotype-genotype associations in African population, but not in other subpopulations (Hustert et al., 2001). It could be speculated that such racial differences in genotype-phenotype associations might arise from novel population-specific functional SNPs that are linked to the assayed SNPs.
In addition to genotype-phenotype studies, linkage disequilibrium analysis and haplotype reconstruction were performed to determine if the PXR-3′UTR SNPs were linked to the upstream intron2 and 4 SNPs (rs1464603, rs1464602, rs3732357) and/or linked to one another. Both intron 2 SNPs (rs1464603 and rs1464602) were highly linked to the rs3732359 and rs3732360 variants. Moreover, rs3814057 and rs3814058 were completely linked in all the populations studied. Through haplotype-phenotype analysis, it was apparent that the effect of each of these variants on CYP3A activity is relatively subtle. Namely, those that carried Haplotype-1 trended towards higher oral CL, whereas those that carried Haplotype-2 trended towards lower oral CL (Fig. 5A). Upon separating the data by race, a significant association was seen between carriers and non-carriers of Haplotype-1 within the African-American subpopulation (Fig. 5B). Haplotype-1 is influenced by reference alleles for both g.252 and g.275 and variant alleles for rs3732359 and rs3732360 (Table 1). Previous work in our laboratory noted that those homozygous reference for g.252 and g.275 have higher MDZ CL/F than carriers of the variant allele (P<0.05) (He et al. 2006a). In this study we have shown that carriers of the variant rs3732359 allele or homozygous variant for rs3732360 SNP also have higher MDZ CL/F (P<0.05) (Figs. 1 and 2).
Although Haplotype-1 was clearly associated with higher MDZ CL/F in the African-American volunteers, the frequency of this allele was only 13% indicating that it may only have limited impact on overall variability in CYP3A function in this population. Furthermore, the relatively small size of each sample (n = 53 livers and n = 26 pharmacokinetic study subjects) limits the statistical power needed to discern a more subtle genotype effect and to detect effects of rarer (<10% frequency) but functionally important variants. Consequently, future work is needed to confirm and extend upon the findings of the present study.
Previous research in our laboratory indicated a significant relationship between PXR mRNA levels and 1-hydroxy MDZ formation rate (He et al. 2006a). However, we did not see a correlation between 1-hydroxy MDZ formation rate and PXR-3′UTR mRNA levels (Rs=0.184; Fig. 6A). But it is important to note that the earlier work only measured normally spliced exon 4-5 PXR mRNA transcripts and the PXR-3′UTR PXR mRNA levels measured in this study probably represents multiple splice variants that contain the 3′-UTR region. Moreover, studies have noted that PXR splicing variation can influence CYP3A activity in human livers (He et al. 2006a; Hustert et al. 2001; Zhang et al. 2001). Recently, Takagi et al. (2008) investigated PXR and CYP3A4 mRNA levels in 25 human liver samples. They found a rather weak correlation between both PXR protein and CYP3A4 mRNA levels (Spearman rank coefficient, Rs=0.47, P<0.05) and a moderate correlation between CYP3A4 protein and PXR protein levels (Rs=0.67, P<0.001). However, the researchers did not specify which, if any, splice variants were detected through their mRNA quantitation technique (Takagi 2008). Despite this lack of correlation in our samples, we did see a significant association between those heterozygous for the rs3814057 and rs3814058 variants and higher total mRNA PXR-3′UTR levels suggesting that these variants might in some way regulate mRNA levels (Fig. 6B).
In contrast to Haplotype-1, Haplotype-2 is composed of variants g.252 and g.275 alleles and reference rs3732359 and rs3732360 alleles (Table 1). As seen in Fig. 5, carriers of Haplotype-2 trended towards lower oral MDZ CL. Haplotype-2 also contains the variant rs3814058 SNP. Both Haplotype-2 and the rs3814058 SNP had a lower (more negative) free energies for the predicted mRNA structure in comparison to the free energy of the reference PXR-3′UTR (Fig. 7), potentially indicating an increase in mRNA stability. This result supports our in vitro real-time PCR findings (discussed above) which indicated that carriers of the rs3814058 SNP have higher PXR-3′UTR mRNA levels (Fig. 6B), but contrasts with our in vivo findings that Haplotype-2 (containing the rs3814058 SNP) associates with lower oral MDZ CL.
The possible mechanism by which genetic variation in the PXR-3′UTR region might directly influence gene expression was initially explored by evaluating effects of the identified SNPs and associated haplotypes on predicted mRNA structure and associated free energy. The results showed more negative predicted free energy for Haplotype 2 compared with Haplotype 1, which appeared to predominantly result from effects of the rs3814058 t>c SNP. More negative free energy should lead to a more stable structure with less mRNA degradation and higher levels of Haplotype 2 mRNA compared with Haplotype 1 mRNA, as has been previously reported for SNPs in several other genes (Capasso et al. 2009; Wang et al. 2005; Wang and Sadee 2006). In support of this contention we observed higher PXR-3′UTR mRNA levels in those individuals that carried the rs3814058 SNP (including Haplotype 2). However this effect is the opposite of what would be expected in that Haplotype 2 was associated with lower rather than higher midazolam CL/F compared with Haplotype 1.
Compared with other haplotypes, Haplotype 1 was associated with more substantial changes in predicted mRNA structure (Figure 7), which could affect accessibility of regulatory proteins or miRNA. As a first step in exploring this possibility experimentally, we created luciferase-3′UTR reporter constructs containing PXR-3′UTR from either of the 2 most common haplotypes (1 and 2) or the Ancestral haplotype. We hypothesized that the luciferase activity of Haplotype-1 should be higher than that of Haplotype-2 and the Ancestral haplotype, since Haplotype-1 was associated with higher midazolam CL/F presumably as a result of higher PXR-mediated CYP3A expression. However, we did not observe any difference between these haplotypes (Fig. 7) suggesting either that the SNPs do not affect mRNA stability or translation efficiency, or perhaps the cell lines we utilized (HEK-293T, COS-7, and LS180 cells) might lack certain factors such as miRNAs that are required for differential expression of the 3′-UTR SNP effects and are normally expressed in human liver tissue. Future studies should utilize human liver cell derived lines or primary human hepatocytes. However it is noteworthy that all three of the 3′UTR containing plasmids exhibited over 100% higher luciferase activities compared with the control plasmid (pMIR-DEST) in COS-7 and LS180 cells but not in HEK293-T cells. This result suggests that COS-7 and LS180 cells (but not HEK-293T cells) express certain factors that enhance the PXR-3′UTR stability and/or translation efficiency.
The focus of this work was to assess the association of PXR polymorphisms with CYP3A in vitro and in vivo activity. However, in addition to regulating CYP3A gene expression, PXR also regulates the expression of a multitude of other genes encoding oxidative and synthetic drug metabolizing enzymes, as well as drug transporters (Meijerman et al. 2006). Consequently, the findings here have the potential to impact the metabolism and disposition of many drugs other than those metabolized by CYP3A.
In conclusion, our results suggest that polymorphisms in the 3′UTR of the PXR gene may play a role in determining interindividual variability in CYP3A metabolism. Specifically, we observed associations between CYP3A in vivo activity and common haplotypes incorporating SNPs in the PXR 3′-UTR region, particularly within African-American subjects. Although in silico analyses suggested that some of these SNPs might alter mRNA stability and/or translation effficiency, we were unable to confirm this using 3′UTR plasmid reporter constructs. Consequently, more work is needed to elucidate the molecular mechanism underlying this association.
Supplementary Material
Acknowledgments
This publication was made possible by Grant Number F31-DA023861 from the National Institute on Drug Abuse (NIDA), National Institutes of Health (Bethesda, MD) to L.O. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIDA, or the National Institutes of Health. Other support was provided by National Institutes of Health (Bethesda, MD) grant R01-GM061834 to M.H.C and grants R01-AG17880, R01-GM061834, R01-AI58784 to D.J.G. The authors also acknowledge the contributions of Dr Ping He who generated some of the published data (including MDZ CL/F and liver bank 1′-OH-MDZ formation rates) used in this study.
Footnotes
Declaration of interest: The authors report no conflict of interest. The authors alone are responsible for the content and writing of this paper.
6. References
- Bertilsson G, Heidrich J, Svensson K, Asman M, Jendeberg L, Sydow-Backman M, Ohlsson R, Postlind H, Blomquist P, Berkenstam A. Identification of a human nuclear receptor defines a new signaling pathway for CYP3A induction. Proc Natl Acad Sci U S A. 1998;95:12208–12213. doi: 10.1073/pnas.95.21.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg B, Sabbagh W, Jr., Juguilon H, Bolado J, Jr., van Meter CM, Ong ES, Evans RM. SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev. 1998;12:3195–3205. doi: 10.1101/gad.12.20.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch TM, Deenen M, Pruntel R, Smits PH, Schellens JH, Beijnen JH, Meijerman I. Screening for polymorphisms in the PXR gene in a Dutch population. Eur J Clin Pharmacol. 2006;62:395–399. doi: 10.1007/s00228-006-0108-0. [DOI] [PubMed] [Google Scholar]
- Brodsky L, Ivanov VV, Kalaidzidis YL, Leontovich AM, Nikolaev VK, Feranchuk SI, Drachev VA. GeneBee-NET:Internet-based server for analyzing biopolymers structure. Biochemistry. 1995;60:923–928. [PubMed] [Google Scholar]
- Capasso M, Ayala F, Russo R, Avvisati RA, Asci R, Iolascon A. A predicted functional single-nucleotide polymorphism of bone morphogenetic protein-4 gene affects mRNA expression and shows a significant association with cutaneous melanoma in Southern Italian population. J Cancer Res Clin Oncol. 2009;135:1799–1807. doi: 10.1007/s00432-009-0628-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conne B, Stutz A, Vassalli JD. The 3′ untranslated region of messenger RNA: A molecular ‘hotspot’ for pathology? Nat Med. 2000;6:637–641. doi: 10.1038/76211. [DOI] [PubMed] [Google Scholar]
- Fukuen S, Fukuda T, Matsuda H, Sumida A, Yamamoto I, Inaba T, Azuma J. Identification of the novel splicing variants for the hPXR in human livers. Biochem Biophys Res Commun. 2002;298:433–438. doi: 10.1016/s0006-291x(02)02469-5. [DOI] [PubMed] [Google Scholar]
- Greenblatt DJ, He P, von Moltke LL, Court MH. The CYP3 Family. In: Ioannides C, editor. Cytochrome P450: Role in the Metabolism and Toxicology of Drugs and other Xenobiotics: Royal Society of Chemistry. 2008. pp. 354–383. [Google Scholar]
- Guengerich FP. Cytochrome P-450 3A4: regulation and role in drug metabolism. Annu Rev Pharmacol Toxicol. 1999;39:1–17. doi: 10.1146/annurev.pharmtox.39.1.1. [DOI] [PubMed] [Google Scholar]
- He P, Court M, Greenblatt D, von Moltke L. Human pregnane X receptor: genetic polymorphisms, alternative mRNA splice variants, and cytochrome P450 3A metabolic activity. J Clin Pharmacol. 2006a;46:1356–1369. doi: 10.1177/0091270006292125. [DOI] [PubMed] [Google Scholar]
- He P, Court MH, Greenblatt DJ, von Moltke LL. Genotype-phenotype associations of cytochrome P450 3A4 and 3A5 polymorphism with midazolam clearance in vivo. Clin Pharmacol Ther. 2005;77:373–387. doi: 10.1016/j.clpt.2004.11.112. [DOI] [PubMed] [Google Scholar]
- He P, Court MH, Greenblatt DJ, von Moltke LL. Factors influencing midazolam hydroxylation activity in human liver microsomes. Drug Metab Dispos. 2006b;34:1198–1207. doi: 10.1124/dmd.105.008904. [DOI] [PubMed] [Google Scholar]
- Hesse LM, He P, Krishnaswamy S, Hao Q, Hogan K, von Moltke LL, Greenblatt DJ, Court MH. Pharmacogenetic determinants of interindividual variability in bupropion hydroxylation by cytochrome P450 2B6 in human liver microsomes. Pharmacogenetics. 2004;14:225–238. doi: 10.1097/00008571-200404000-00002. [DOI] [PubMed] [Google Scholar]
- Hill WG. Estimation of linkage disequilibrium in randomly mating populations. Heredity. 1974;33:229–239. doi: 10.1038/hdy.1974.89. [DOI] [PubMed] [Google Scholar]
- Hughes TA. Regulation of gene expression by alternative untranslated regions. Trends Genet. 2006;22:119–122. doi: 10.1016/j.tig.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Hustert E, Zibat A, Presecan-Siedel E, Eiselt R, Mueller R, Fuss C, Brehm I, Brinkmann U, Eichelbaum M, Wojnowski L. Natural protein variants of pregnane X receptor with altered transactivation activity toward CYP3A4. Drug Metab Dispos. 2001;29:1454–1459. others. [PubMed] [Google Scholar]
- King CR, Xiao M, Yu J, Minton MR, Addleman NJ, Van Booven DJ, Kwok PY, McLeod HL, Marsh S. Identification of NR1I2 genetic variation using resequencing. Eur J Clin Pharmacol. 2007;63:547–554. doi: 10.1007/s00228-007-0295-3. [DOI] [PubMed] [Google Scholar]
- Koyano S, Kurose K, Ozawa S, Saeki M, Nakajima Y, Hasegawa R, Komamura K, Ueno K, Kamakura S, Nakajima T. Eleven novel single nucleotide polymorphisms in the NR1I2 (PXR) gene, four of which induce non-synonymous amino acid alterations. Drug Metab Pharmacokinet. 2002;17:561–565. doi: 10.2133/dmpk.17.561. others. [DOI] [PubMed] [Google Scholar]
- Koyano S, Kurose K, Saito Y, Ozawa S, Hasegawa R, Komamura K, Ueno K, Kamakura S, Kitakaze M, Nakajima T. Functional characterization of four naturally occurring variants of human pregnane X receptor (PXR): one variant causes dramatic loss of both DNA binding activity and the transactivation of the CYP3A4 promoter/enhancer region. Drug Metab Dispos. 2004;32:149–154. doi: 10.1124/dmd.32.1.149. others. [DOI] [PubMed] [Google Scholar]
- Lamba J, Lamba V, Schuetz E. Genetic variants of PXR (NR1I2) and CAR (NR1I3) and their implications in drug metabolism and pharmacogenetics. Curr Drug Metab. 2005;6:369–383. doi: 10.2174/1389200054633880. [DOI] [PubMed] [Google Scholar]
- Lamba V, Yasuda K, Lamba JK, Assem M, Davila J, Strom S, Schuetz EG. PXR (NR1I2): splice variants in human tissues, including brain, and identification of neurosteroids and nicotine as PXR activators. Toxicol Appl Pharmacol. 2004;199:251–265. doi: 10.1016/j.taap.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Lehmann JM, McKee DD, Watson MA, Willson TM, Moore JT, Kliewer SA. The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J Clin Invest. 1998;102:1016–1023. doi: 10.1172/JCI3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijerman I, Beijnen JH, Schellens JH. Herb-drug interactions in oncology: focus on mechanisms of induction. Oncologist. 2006;11:742–752. doi: 10.1634/theoncologist.11-7-742. [DOI] [PubMed] [Google Scholar]
- Olsen PH, Ambros V. The lin-4 regulatory RNA controls developmental timing in Caenorhabditis elegans by blocking LIN-14 protein synthesis after the initiation of translation. Dev Biol. 1999;216:671–680. doi: 10.1006/dbio.1999.9523. [DOI] [PubMed] [Google Scholar]
- Özdemir V, Kalowa WA, Tang BK, Paterson AD, Walker SE, Endrenyi L, Kashuba AD. Evaluation of the genetic component of variability in CYP3A4 activity: a repeated drug administration method. Pharmacogenetics. 2000;10:373–388. doi: 10.1097/00008571-200007000-00001. [DOI] [PubMed] [Google Scholar]
- Spencer CC, Deloukas P, Hunt S, Mullikin J, Myers S, Silverman B, Donnelly P, Bentley D, McVean G. The influence of recombination on human genetic diversity. PLoS Genet. 2006;2:e148. doi: 10.1371/journal.pgen.0020148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001;68:978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi S, Nakajima M, Mohri T, Yokoi T. Post-transcriptional regulation of human pregnane X receptor by micro-RNA affects the expression of cytochrome P450 3A4. The Journal of Biological Chemistry. 2008;283:9674–9680. doi: 10.1074/jbc.M709382200. [DOI] [PubMed] [Google Scholar]
- Thummel KE, Wilkinson GR. In vitro and in vivo drug interactions involving human CYP3A. Annu Rev Pharmacol Toxicol. 1998;38:389–430. doi: 10.1146/annurev.pharmtox.38.1.389. [DOI] [PubMed] [Google Scholar]
- Wang D, Johnson AD, Papp AC, Kroetz DL, Sadee W. Multidrug resistance polypeptide 1 (MDR1, ABCB1) variant 3435C>T affects mRNA stability. Pharmacogenet Genomics. 2005;15:693–704. [PubMed] [Google Scholar]
- Wang D, Sadee W. Searching for polymorphisms that affect gene expression and mRNA processing: example ABCB1 (MDR1) AAPS J. 2006;8:E515–520. doi: 10.1208/aapsj080361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlind-Johnsson A, Malmebo S, Johansson A, Otter C, Andersson TB, Johansson I, Edwards RJ, Boobis AR, Ingelman-Sundberg M. Comparative analysis of CYP3A expression in human liver suggests only a minor role for CYP3A5 in drug metabolism. Drug Metab Dispos. 2003;31:755–761. doi: 10.1124/dmd.31.6.755. [DOI] [PubMed] [Google Scholar]
- Westlind A, Löfberg L, Tindberg N, Andersson TB, Ingelman-Sundberg M. Interindividual differences in hepatic expression of CYP3A4: relationship to genetic polymorphism in the 5′-upstream regulatory region. Biochem Biophys Res Commun. 1999;259:201–205. doi: 10.1006/bbrc.1999.0752. [DOI] [PubMed] [Google Scholar]
- Wilkinson GR. Cytochrome P4503A (CYP3A) metabolism: prediction of in vivo activity in humans. J Pharmacokinet Biopharm. 1996;24:475–490. doi: 10.1007/BF02353475. [DOI] [PubMed] [Google Scholar]
- Wrighton SA, VandenBranden M, Ring BJ. The human drug metabolizing cytochromes P450. J Pharmacokinet Biopharm. 1996;24:461–473. doi: 10.1007/BF02353474. [DOI] [PubMed] [Google Scholar]
- Zhang J, Kuehl P, Green ED, Touchman JW, Watkins PB, Daly A, Hall SD, Maurel P, Relling M, Brimer C. The human pregnane X receptor: genomic structure and identification and functional characterization of natural allelic variants. Pharmacogenetics. 2001;11:555–572. doi: 10.1097/00008571-200110000-00003. others. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.