Abstract
Background and purpose:
The aim of the current study was to investigate the role of arachidonic acid (AA) metabolism via cyclooxygenase (COX) in the endothelial dysfunction of penile arteries from pre-diabetic, obese Zucker rats (OZR).
Experimental approach:
Penile arteries from OZR and from lean Zucker rats (LZR) were mounted in microvascular myographs to assess vascular function and COX expression was determined by immunohistochemistry.
Key results:
Acetylcholine (ACh) and AA elicited relaxations that were impaired in arteries from OZR. Inhibition of both COX-1 and COX-2 reduced the relaxant effects of ACh and AA in LZR but not in OZR. Inhibitors of COX-1 and of the TXA2/PGH2 (TP) receptor enhanced the relaxations induced by AA in both LZR and OZR, whereas COX-2 inhibition enhanced these responses only in OZR. TP receptor blockade did not restore ACh relaxant responses in arteries from OZR. Inhibition of COX-1 increased basal tension in OZR and this contraction was blunted by TP receptor blockade. The vasoconstrictor responses to noradrenaline were augmented by indomethacin and by COX-2 inhibition in LZR but not in OZR. Immunohistochemical staining showed that both COX-1 and COX-2 are expressed in the endothelium of penile arteries from both LZR and OZR.
Conclusions and implications:
Vasoactive prostanoids were formed via constitutively active COX-1 and COX-2 pathways in normal rat penile arteries. Under conditions of insulin resistance, the release and/or effects of vasodilator prostanoids were impaired, contributing to the blunted endothelium-dependent vasodilatation and to the enhanced vasoconstriction.
Keywords: insulin resistance, penile arteries, endothelial dysfunction, arachidonic acid, COX-1, constitutive COX-2, obese Zucker rat
Introduction
Erectile dysfunction (ED) is a highly prevalent condition in men with cardiovascular risk factors and is now considered to be an early sign of systemic endothelial dysfunction and subclinical vascular disease (Billups, 2005). The metabolic syndrome, a cluster of metabolic abnormalities associated with obesity and including insulin resistance, hyperglycemia and hyperlipidemia, has become a major clinical and public health problem and a large group of patients are at increased risk for developing diabetes and cardiovascular disease. Approximately 35% to 75% of men with diabetes mellitus suffer ED (Vickers and Wright, 2004). Some aspects of the metabolic syndrome have also been associated with ED and an increased prevalence has been demonstrated in men with metabolic syndrome (Espósito et al., 2005; Fonseca and Jawa, 2005). On the other hand, the prevalence of obesity and associated risk factors in men reporting symptoms of ED is remarkably high and strong epidemiological evidence links the risk of ED to diabetes type 2 (Vickers and Wright, 2004; Espósito et al., 2005; Fonseca and Jawa, 2005).
Penile erection is a complex neurovascular process primarily achieved by the relaxation of penile smooth muscle and further expansion and filling of the cavernous sinusoids, due to nerve- and endothelial-derived nitric oxide (NO), released upon sexual stimulation (Prieto, 2008). Studies in diabetic animal models and in human cavernosal tissue from diabetic men have pointed to the multifactorial mechanisms of the diabetes-associated ED and both endothelial and neural NO-mediated relaxant responses have been shown to be impaired (Saenz de Tejada et al., 1989; Keegan et al., 1999; Angulo et al., 2003). Metabolic disorders associated with diabetes include hyperglycemia, excess free fatty acids and insulin resistance, components of the metabolic syndrome that are risk factors for ED and also characterized by abnormal endothelial function (Espósito et al., 2005; Fonseca and Jawa, 2005). Although diabetic endothelial dysfunction has primarily been considered to reflect a deficiency of NO, the mechanisms responsible for the impaired endothelium-dependent vasodilatation in diabetes and metabolic syndrome still need to be fully elucidated (De Vriese et al., 2000; Kuboki et al., 2000; Okon et al., 2005; Vanhoutte et al., 2009).
An imbalance between the production of vasodilator and vasoconstrictor prostanoids has recently been shown to underlie endothelial dysfunction and abnormal vascular smooth muscle reactivity in arteries from rodent models of both type 1 and type 2 diabetes (Matsumoto et al., 2007; Shi and Vanhoutte, 2008) and metabolic syndrome (Goodwill et al., 2008; Xiang et al., 2008). In healthy blood vessels, most prostanoids are formed by the constitutive isoform of cyclooxygenase 1 (COX-1). However, these mediators may also be synthesized by the inducible COX isoform COX-2 that is usually expressed at undetectable levels in vascular cells but can be up-regulated by inflammatory, mitogenic and physical stimuli (Seibert et al., 1994; Herranz et al., 2004; Warner and Mitchell, 2004). Moreover, in the last years, it has become evident that prostanoid production from constitutively expressed COX-2 is also involved in the modulation of vascular responses (Henrion et al., 1997; Baber et al., 2003; Qui et al., 2006).
We have recently demonstrated profound alterations in vascular structure that correlate with endothelial dysfunction in penile arteries from the obese Zucker rat (OZR) (Villalba et al., 2009), a well-established genetic model of insulin resistance and metabolic syndrome caused by a dysfunctional gene of the leptin receptor (Guerre-Millo, 1997). The purpose of the present study was to further elucidate the mechanisms underlying penile endothelial dysfunction and to assess whether there is a specific role of altered vascular prostanoid metabolism in the impaired dilatation and abnormal vasoconstriction of penile arteries from OZR.
Methods
Animal model
All animal care and experimental protocols conformed to the European Union Guidelines for the Care and the Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the Madrid Complutense University. Male OZR (fa/fa, n= 63) and their control strain, lean Zucker rats (LZR) (fa/-, n= 60) were purchased from Charles River Laboratories (Barcelona, Spain) at 8–10 weeks of age. Animals were housed at the Pharmacy School animal care facility and maintained on standard chow and water ad libitum, until they were used for study, at 17–18 weeks of age.
Dissection of microvessels, mounting and force measurement
Rats were killed by cervical dislocation and exanguination. The penile arteries, first- or second-order branches of the rat dorsal penile artery from LZR and OZR rats were carefully dissected by removing the connective and fat tissue, as described previously (Villalba et al., 2009). Segments of dorsal penile arteries were mounted in parallel in double microvascular myographs (Danish Myotechnology, Denmark) by inserting two 40 µm tungsten wires into the vessel lumen. After mounting, the arteries were equilibrated for 30 min in Krebs solution of the following composition (mM): NaCl 119, NaHCO3 25, KCl 4.7, KH2PO4 1.17, MgSO4 1.18, CaCl2 1.5, EDTA 0.027 and glucose 11, mantained at 37°C and continuously gassed with a mixture of 5% CO2/95% O2 to maintain pH at 7.4. The relationship between passive wall tension and internal circumference was determined for each individual artery and from this, the internal diameter, l1, that yielded a circumference equivalent to 90% of that given by an internal pressure of 100 mmHg was calculated.
Experimental procedures for the functional experiments
At the beginning of each experiment, arteries were challenged twice with 120 mM K+ (KPSS) in order to test vessel viability. The vasoactive effects of acetylcholine (ACh) (Sigma Chemical Co., St Louis, MO, USA) and of the prostanoid precursor, arachidonic acid (AA) (Sigma Chemical), were evaluated by adding cumulative concentrations of these agents on arteries precontracted with 1 µM phenylephrine (Sigma Chemical). The effects of the NOS inhibitor Nω-nitro-L-arginine (L-NNA, 100 µM) (Sigma Chemical) and of the non-selective COX inhibitor indomethacin (1 µM) (Sigma Chemical) were initially tested on the ACh-induced relaxations. The responses to exogenous ACh, AA and noradrenaline (Sigma Chemical) were further obtained in the absence and presence of specific inhibitors of COX-1 (SC-560, 1 µM) (Sigma Chemical), COX-2 (NS-398, 1 µM) (Tocris Cookson, Bristol, UK) and the antagonist of the TXA2/PGH2 (TP) receptor, (ICI-192, 1 µM) (Tocris). The effect of the TXA2 agonist U46619 was also assessed in penile arteries of LZR and OZR, in the absence and presence of ICI-192. The drugs were added to the myograph chamber 30 min before the construction of a second concentration–response curve for the corresponding agonist. The role of the vascular endothelium was examined in arteries were the endothelium was mechanically removed by inserting a human hair in the vessel lumen and guiding it back and forwards several times. The absence of functional endothelium was confirmed by the lack of relaxation to ACh (10 µM).
Immunohistochemistry
Tissue samples from the penis containing the dorsal penile artery were inmersion-fixed in 4% paraformaldehyde in 0.1 M sodium phosphate-buffer (PB), cryoprotected in 30% sucrose in PB and snap-frozen in liquid nitrogen and stored at −80°C. Tissue sections were processed following the avidin-biotin-peroxidase complex (ABC) method (Hsu et al., 1981). Sections were first immersed in a mixture of 1% H2O2 and 90% methanol in distilled water for 30 min, washed in PB and the preincubated in 10% normal goat serum in PB containing 0.3% Triton-X-100 for 2–3 h. Then, sections were incubated with either a mouse monoclonal anti-COX-1 (Cayman Chemical, Ann Arbor, MI, USA), diluted at 1:100 or a rabbit anti COX-2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) diluted 1:200 for 48 h, washed and reacted with a biotinylated goat secondary serum (Chemicon International Inc) (anti-mouse for the COX-1 and anti-rabbit for the COX-2) diluted 1:400 for 2h at room temperature, followed by incubation with avidin-biotin-complex (ABC, Vector) 1:100 dilution for 90 min at room temperature. The immunocomplex was visualized with 0.05% 3.3 diaminobenzidine and 0.001% in H2O2 in PB. No immunoreactivity could be detected in sections incubated in the absence of the primary antisera. Pre-adsorption with COX-1 and COX-2 protein showed no cross-reactivity for the antibodies.
Data presentation and statistical analysis
Results are expressed as either tension (as N·m−1) or as a percent of the response to either phenylephrine or KPSS in each artery, as means ± SEM of six to eight arteries (one to two from each animal). The sensitivity of the arteries to the relaxant and vasoconstrictor agonists is expressed in terms of pEC50 values, where pEC50 is −logEC50. EC50 is the concentration of the agonist required to producing 50% of the response and was calculated by non-linear curve fitting of the concentration–response curves for the inhibitor to the classical Hill Equation by using a standart computer software (Prism 5.0, GraphPad, San Diego, CA, USA). The EC50 value for each individual curve was first obtained and thereafter the average value for a given set of experiments was calculated. The statistical differences between means were analysed by using one-way anova, paired or unpaired Student's t-test when appropiate. Probability levels smaller than 5% were considered significant.
Drug and molecular target nomenclature follows Alexander et al. (2008).
Results
General parameters
At the time of the experiment (17–18 weeks of age), OZR were significantly heavier than LZR (483 ± 5 g vs. 375 ± 5 g, P < 0.001, n= 51). We have recently reported that animals from the OZR group exhibit mild hyperglycemia, hyperinsulinemia and dyslipidemia with elevated total cholesterol and triglyceride levels (Villalba et al., 2009). The normalized internal lumen diameters, l1, were significantly smaller in penile arteries from OZR (135 ± 3 µm) compared with LZR (150 ± 3 µm, P < 0.01, n= 51). The contractions to KPSS were reduced in the OZR group (1.8 ± 0.1 N·m−1) compared with LZR (2.4 ± 0.1 N·m−1, P < 0.01; n= 51), indicating reduced contractility of arterial smooth muscle. Although maximal contractile responses to noradrenaline were reduced, sensitivity was augmented in OZR (pEC50 6.96 ± 0.11 vs. 6.48 ± 0.08, P < 0.0001, n= 27, in LZR and OZR respectively). The endothelium-dependent responses to ACh were markedly reduced in penile arteries from OZR compared with LZR (27 ± 5% vs. 60 ± 4%, P < 0.0001, n= 24), thus indicating endothelial dysfunction.
Effect of COX inhibitors on ACh relaxant responses
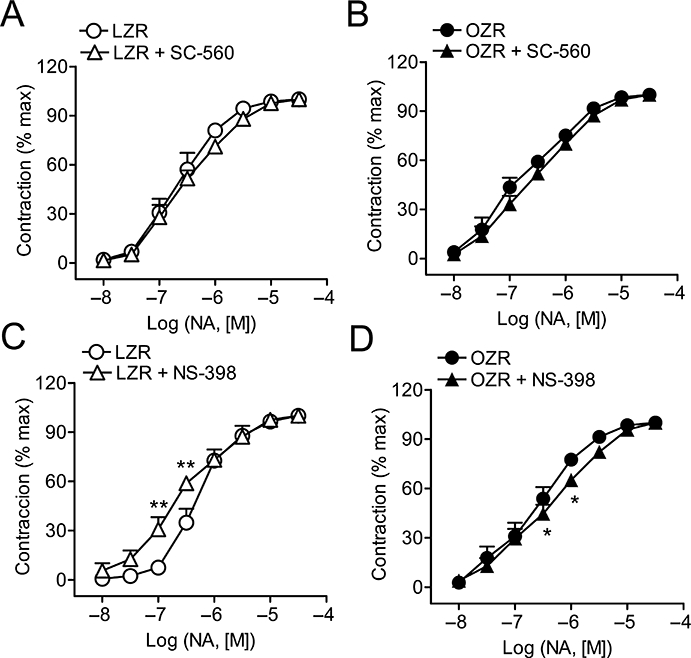
In order to assess whether changes in the AA metabolism via COX may be involved in the endothelial dysfunction observed in penile arteries from OZR, the effect of the non-selective COX inhibitor, indomethacin (1 µM) and of the selective COX-1 and COX-2 blockers SC-560 and NS-398, were examined on the relaxant responses to ACh. Indomethacin reduced the relaxations elicited by ACh in arteries from LZR and to a lesser degree in those from OZR (Figure 1, Table 1). Treatment with L-NNA (100 µM) markedly reduced the relaxations to ACh in both LZR and OZR, this inhibition being larger than that produced by indomethacin in LZR (Figure 1). Combined blockade of COX and NOS abolished the relaxant responses to ACh in both LZR and OZR (Figure 1), suggesting the involvement of both NO and prostanoids. Treatment with 1 µM of the selective COX-1 antagonist SC-560 inhibited the relaxations induced by ACh in penile arteries from LZR (Figure 2A, Table 1) but not from OZR (Figure 2B, Table 1). Furthermore, selective inhibition of COX-2 with NS-398 (1 µM) also reduced the relaxations to ACh in LZR but did not affect those in OZR (Figure 2C,D, Table 1). These results suggest that the endothelium-dependent vasodilator responses to ACh involve both COX-1- and COX-2-derived vasodilator prostanoids in LZR which are impaired in OZR.
Figure 1.

Effect of the inhibitor of COX, indomethacin (indo) (1 µM) and the inhibitor of the NO synthesis, L-NNA (100 µM) on the relaxant responses to acetylcholine (ACh) in penile arteries from LZR (A) and OZR (B). Results are expressed as percentage of the pre-contraction induced by phenylephrine (Phe). Data are shown as the means ± SEM of 6–13 arteries. *P < 0.05, **P < 0.01, ***P < 0.001 versus control before treatment. †P < 0.01; ††P < 0.001 versus indomethacin-treated. COX, cyclooxygenase; L-NNA, Nω-nitro-L-arginine; LZR, lean Zucker rats; NO, nitric oxide; OZR, obese Zucker rats.
Table 1.
Effects of indomethacin (indo) (1 µM), of the selective COX-1 inhibitor SC-560 (1 µM) and of the COX-2 inhibitor NS-398 (1 µM) on the vasodilator responses to acetylcholine (ACh) of penile arteries from LZR and OZR
|
ACh |
||||||
|---|---|---|---|---|---|---|
|
LZR |
OZR |
|||||
| pEC50 | Emax (%) | n | pEC50 | Emax (%) | n | |
| ACh | 5.77 ± 0.06 | 73.1 ± 7.3 | 7 | 5.50 ± 0.18 | 37.6 ± 9.7 | 6 |
| +Indo | 5.57 ± 0.15 | 40.3 ± 3.8** | 7 | – | 14.9 ± 6.0* | 6 |
| ACh | 6.55 ± 0.05 | 60.0 ± 7.6 | 8 | 5.64 ± 0.43 | 24.6 ± 4.3 | 8 |
| +SC-560 | 6.51 ± 0.27 | 38.4 ± 6.1* | 8 | – | 15.6 ± 5.0* | 8 |
| ACh | 6.10 ± 0.09 | 62.4 ± 7.2 | 8 | 6.02 ± 0.25 | 33.4 ± 9.9 | 10 |
| +NS-398 | 5.89 ± 0.08* | 52.1 ± 6.9 | 8 | 6.11 ± 0.17 | 26.6 ± 5.2 | 10 |
Values represent mean ± SE of the number n of individual arteries. pEC50 is −logEC50, being the concentration of agonist giving half maximal response (Emax). Significant differences from controls were analysed by a paired Student's t-test.
P < 0.05;
P < 0.01.
COX, cyclooxygenase; LZR, lean Zucker rats; OZR, obese Zucker rats.
Figure 2.

Effects of the selective COX-1 inhibitor SC-560 (1 µM) (A, B) and the selective COX-2 inhibitor NS-398 (1 µM) (C, D) on the average relaxant responses to acetylcholine (ACh) in penile arteries from LZR (A, C) and OZR (B, D). Results are expressed as percentage of the pre-contraction induced by phenylephrine (Phe). Data are shown as the means ± SEM of 8–10 arteries. *P < 0.05, **P < 0.01 versus control before treatment. COX, cyclooxygenase; LZR, lean Zucker rats; OZR, obese Zucker rats.
Effect of COX inhibitors and TP receptor antagonism on responses to AA
Exogenously administered AA (0.1–10 µM) induced concentration-dependent relaxations in penile arteries from LZR precontracted with phenylephrine, which were impaired in arteries from OZR (Figure 3).
Figure 3.

Relaxant responses to AA in penile arteries from LZR and OZR. (A, B) Representative traces showing the AA-induced relaxations in penile arteries from (A) LZR and (B) OZR. (C) Average concentration–response curves for the relaxation to AA. Data are shown as the means ± SEM of 37 and 39 arteries (one to two per animal). **P < 0.01, ***P < 0.001 versus LZR. AA, arachidonic acid; LZR, lean Zucker rats; OZR, obese Zucker rats.
In order to investigate the metabolism of AA by COX under basal conditions, the effects of the selective inhibitors of COX-1 and COX-2 and TP receptor were investigated. The COX-1 inhibitor SC-560 reduced the relaxations to the lower doses of AA (1 µM) in arteries from LZR and enhanced those at higher doses used (10 µM) in arteries from both LZR and OZR (Figure 4A,B). These results suggest that constrictor prostanoids are produced from AA via COX-1 in penile arteries from LZR and OZR. The selective COX-2 inhibitor NS-398 (1 µM) reduced the relaxations to AA in LZR (Figure 4C), but increased these relaxations in OZR (Figure 4D). These results indicate that there is a constitutive production of relaxant prostanoids via COX-2 in LZR that is changed to the formation of vasoconstrictor prostanoids in OZR.
Figure 4.

Effect of the COX-1 inhibitor SC-560 (1 µM) (A, B), the COX-2 inhibitor NS-398 (1 µM) (C, D) and the TXA2/PGH2 receptor antagonist ICI-192 (1 µM) (E, F) on the average concentration–response curves for the relaxation to AA. Results are expressed as percentage of the precontraction induced by phenylephrine (Phe). Data are shown as means ± SEM of 10 arteries (A, B) and 11 arteries (C, D) and 8 arteries (E, F). *P < 0.05, **P < 0.01, ***P < 0.001 versus controls in the absence of treatment. COX, cyclooxygenase.
The selective PGH2/TXA2 receptor antagonist IC-192 (1 µM) enhanced the relaxant effects of AA in penile arteries from both LZR and OZR, suggesting an endogenous basal production of PGH2/TXA2 that usually counterbalances the relaxant effects of AA (Figure 4E,F).
Effects of COX inhibitors and TP receptor antagonist on basal tone
Treatment with SC-560 (1 µM) significantly enhanced basal tone in arteries from OZR but not from LZR and this constriction was abolished by blockade of the TP receptor with ICI-192 (Figure 5A). However, no significant changes in baseline tension of penile arteries from either LZR or OZR were observed after blockade of COX-2 with NS-398 (Figure 5B). These results suggest that blockade of COX-1 unmasks an increased basal production of PGH2/TXA2 by the COX-2 pathway in penile arteries from OZR.
Figure 5.

Effect of the COX-1 inhibitor SC-560 (1 µM) (A) and of the COX-2 inhibitor NS-398 (1 µM) (B) alone or in the presence of the TXA2/PGH2 receptor antagonist ICI-192 (1 µM) on the basal tone of penile arteries from LZR and OZR. Results are shown as the increase in baseline tension (ΔBL), expressed as % of the contraction to 120 mM K+ (% of KPSS), after addition of the antagonists. Data are shown as the means ± SEM of 10 arteries (A, B). ††P < 0.01 versus OZR. ***P < 0.001 versus controls in the absence of treatment. COX, cyclooxygenase; LZR, lean Zucker rats; OZR, obese Zucker rats.
Effect of TP receptor antagonism on U46619 and ACh responses
The TXA2 mimetic U46619 induced concentration-dependent contractions that were enhanced in penile arteries from OZR, pD2 values for U46619 being 7.21 ± 0.09 (n= 7) in LZR, and 7.46 ± 0.05 (P < 0.01, n= 10) in OZR. The contractile effect of the TXA2 analogue was inhibited by the TP receptor antagonist, ICI-192 (1 µM) (Figure 6A,B). In order to assess whether an increased TP receptor-mediated vasoconstriction could be involved in the blunted ACh relaxant responses of penile arteries from OZR, the effect of ICI-192 (1 µM) was tested. Treatment with ICI-192 did not alter the ACh-induced relaxation in arteries form either LZR or OZR (Figure 6C,D).
Figure 6.

Effect of the TXA2/PGH2 receptor with ICI-192 (1 µM) on the contractile effects of the of TXA2 analogue U-46619 [A, B; expressed as % of the contraction to 120 mM K+ (% of KPSS)] and on the relaxant responses to acetylcholine (ACh) (C, D) in penile arteries from LZR (A, C) and OZR (B, D). Data are shown as the means ± SEM of 10 arteries (A, B), 8 arteries (C) and 6 arteries (D). *P < 0.05, ***P < 0.001 versus controls in the absence of treatment. LZR, lean Zucker rats; OZR, obese Zucker rats.
Effects of COX inhibitors on responses to noradrenaline
Treatment with indomethacin augmented noradrenaline-induced contractions in penile arteries from LZR and to a lesser extent those from OZR (Table 2). The contractile responses elicited by noradrenaline were unaltered by treatment with the COX-1 inhibitor SC-560 in penile arteries from either LZR or OZR (Figure 7A,B, Table 2). However, they were significantly enhanced by the COX-2 inhibitor NS-398 in penile arteries from LZR but not from OZR (Figure 7C,D, Table 2), indicating that there is a basal production of a vasodilator prostanoid via COX-2 in LZR rats which is absent in OZR.
Table 2.
Effects of indomethacin (indo) (1 µM), of the selective COX-1 inhibitor SC-560 (1 µM) and of the COX-2 inhibitor NS-398 (1 µM) on the vasoconstrictor responses to noradrenaline (NA) of penile arteries from LZR and OZR
|
NA |
||||||
|---|---|---|---|---|---|---|
|
LZR |
OZR |
|||||
| pEC50 | Emax (Nm−1) | n | pEC50 | Emax (Nm−1) | n | |
| NA | 6.26 ± 0.12 | 3.0 ± 0.5 | 9 | 6.58 ± 0.01 | 2.1 ± 0.2 | 10 |
| +Indo | 6.57 ± 0.14** | 2.9 ± 0.5 | 9 | 6.78 ± 0.15* | 1.9 ± 0.2 | 10 |
| NA | 6.80 ± 0.10 | 2.8 ± 0.5 | 7 | 7.16 ± 0.18 | 2.2 ± 0.5 | 8 |
| +SC-560 | 6.57 ± 0.11 | 2.9 ± 0.3 | 7 | 6.98 ± 0.40 | 2.1 ± 0.4 | 8 |
| NA | 6.51 ± 0.13 | 3.5 ± 0.3 | 12 | 7.00 ± 0.17 | 2.6 ± 0.3 | 9 |
| +NS-398 | 6.69 ± 0.12** | 3.5 ± 0.4 | 12 | 6.49 ± 0.17* | 2.4 ± 0.3* | 9 |
Values represent mean ± SE of the number n of individual arteries. pEC50 is −logEC50, being the concentration of agonist giving half maximal response (Emax). Significant differences from controls were analysed by a paired Student's t-test.
P < 0.05;
P < 0.01.
COX, cyclooxygenase; LZR, lean Zucker rats; OZR, obese Zucker rats.
Figure 7.
Effects of the selective COX-1 inhibitor SC-560 (1 µM) (A, B) and of the selective COX-2 inhibitor NS-398 (1 µM) (C, D) on the average contractile responses to noradrenaline (NA) in penile arteries from LZR (A, C) and OZR (B, D). Results are expressed as percentage of the maximum contraction induced by NA in each artery. Data are shown as the means ± SEM of seven to eight arteries (A, B) and 9–12 arteries (C, D). *P < 0.05, **P < 0.01 versus control before treatment. COX, cyclooxygenase; LZR, lean Zucker rats; OZR, obese Zucker rats.
Immunohistochemistry of COX-1 and COX-2
Staining of arterial sections with a monoclonal antibody against COX-1 revealed that this constitutive COX isoform was widely and uniformly distributed throughout the endothelial lining of penile arteries, being absent in the smooth muscle layer. No apparent differences in either the distribution or density of COX-1 immunolabeling were observed between LZR and OZR (Figure 8B). By using a polyclonal antibody against COX-2, this COX isoform was also found to be primarily expressed in the penile endothelium of both LZR and OZR, although a diffuse COX-2 immunostaining, more intense in penile arteries from OZR, was also observed in the smooth muscle layer (Figure 8C). The endothelial COX-2 immunolabeling was sparser and less uniform compared with that for COX-1 and it was restricted to small foci of endothelial cells (marked with arrows in Figure 8C). Furthermore, additional but less intense immunostaining for both COX-1 and COX-2 isoforms was also found in the adventitia and in the surrounding trabecular tissue in the case of COX-1, probably associated with macrophages and mast cell-like cells.
Figure 8.

Immunohistochemical staining of COX-1 and COX-2 in the endothelium of penile arteries from LZR and OZR. (A) Haematoxylin and eosin-stained cross sections showing the vascular structure of penile arteries from LZR and OZR. Inmunohistochemical demonstration of COX-1 (B) and COX-2 (C) in LZR and OZR. (B) COX-1 isoform was widely distributed throughout the endothelial lining of penile arteries and absent in the smooth muscle layer. Additional COX-1 immunostaining was also found in the trabecular tissue, probably associated with macrophages and mast cell-like cells (asterisk). (C) COX-2 immunolabeling was sparse and restricted to small foci of endothelial cells marked with arrows. A diffuse COX-2 immunostaining was also observed in the smooth muscle layer. COX, cyclooxygenase; LZR, lean Zucker rats; OZR, obese Zucker rats.
Discussion
In the present study we have investigated the role of AA metabolism through COX in the pathogenesis of the endothelial dysfunction of penile arteries in an animal model of the metabolic syndrome. Our results first demonstrated that both COX-1 and COX-2 were constitutively expressed in the endothelium of penile arteries and primarily produced relaxant prostanoids in healthy animals. Under conditions of insulin resistance or the metabolic syndrome, there is an impairment of the COX-1- and COX-2-mediated vasodilator effects that contributes to the blunted ACh endothelium-dependent relaxations and to the enhanced vasoconstriction induced by noradrenaline. An increased basal COX-2-mediated production of vasoconstrictor prostaglandins was also found in arteries from OZR. The present study therefore demonstrates that alterations in the AA metabolism through the COX pathway are involved in the vascular dysfunction of penile arteries from insulin-resistant OZR.
Penile erectile tissues synthesize and locally metabolize several relaxant and contractile prostanoids. In the corpus cavernosum and penile veins, there is a predominant production of contractile prostanoids such as PGF2α, TXA2 and PGE2 modulated by O2 tension and reduced by hypoxia (Christ et al., 1990; Azadzoi et al., 1992; Daley et al., 1996; Martínez et al., 2005). In penile resistance arteries, relaxant prostanoids modulate arterial spontaneous tone and they can contribute to the ACh-induced endothelium-dependent responses in some species and humans (Prieto et al., 1998; Simonsen et al., 2001; Angulo et al., 2002; Ruiz Rubio et al., 2004). The present study demonstrated that the ACh-induced endothelium-dependent relaxations of penile arteries from control LZR involved both NO and vasodilator prostanoids. Although COX-1 is constitutively expressed in vascular tissues and platelets and COX-2 is regarded as an inducible isoform up-regulated by inflammatory stimuli, our results further demonstrate that the formation of vasoactive prostanoids occurs via constitutively active COX-1 and COX-2 pathways in normal rat penile arteries. Thus, the immunohistochemical study showed the expression of both COX isoforms primarily in the penile arterial endothelium and non-selective (indomethacin) and selective COX-1 and COX-2 inhibitors significantly reduced the relaxations elicited by both ACh and AA in arteries from LZR. The functional experiments of the present study are consistent with the predominant localization of both COX isoforms in the endothelium of normal penile arteries. Thus, the COX-1 inhibitor SC-560 and the COX-2 inhibitor NS-398 reduced the ACh-elicited relaxations in LZR, thus suggesting the release of both COX-1- and COX-2-derived endothelial vasodilator prostanoids upon agonist stimulation. COX-2 constitutive expression in the vascular system has earlier been reported in a few organs such as the kidney (Qui et al., 2006) and the lung (Ermert et al., 1998; Baber et al., 2003; 2005;). In our study, COX-2 was found to be mainly distributed in the penile arterial endothelium of LZR, although a diffuse distribution in the smooth muscle layer was also found, which is in agreement with that reported in the lung where COX-2 was localized in both the endothelium and smooth muscle of small pulmonary arteries (Ermert et al., 1998; Baber et al., 2003).
As regards the functional experiments, our results further demonstrate a basal release of COX-2-derived relaxant prostanoids involved in the spontaneous tone of rat penile arteries from LZR. Thus, AA elicited a net relaxant effect that was reduced by the COX-2 inhibitor NS-398. Furthermore, both non-selective (indomethacin) and selective COX-2 inhibition enhanced noradrenaline-induced contraction, suggesting a basal release of COX-2-derived relaxant prostanoids that counterbalance vasoconstriction. The predominant formation of vasodilator prostaglandins via COX-2 in arteries from healthy animals would be in agreement with previous studies in the rat showing that selective COX-2 inhibitors reduced systemic vasodilator responses to AA with no effect of TXA2 receptor blockade (Baber et al., 2003). COX-2 has been demonstrated to preferentially couple to the cellular synthesis of PGI2 and PGE2 (Brock et al., 1999) and to be up-regulated by steady laminar shear stress, hence being proposed as a major isoform responsible for the production of PGI2 under physiological conditions (Topper et al., 1996; Henrion et al., 1997; Okahara et al., 1998). The results of the present study further extend the concept that constitutive COX-2 may functionally contribute to the formation of vasodilator prostanoids in the vascular system (Baber et al., 2003), although its role in penile erection remains to be elucidated. Interestingly, recent studies have demonstrated that COX-2 is highly expressed in penile corpus cavernosum and COX-2-derived contractile prostanoids are involved in the generation of spontaneous contractions. However, in contrast to the primary location of COX-2 in the endothelium of penile arteries, COX-2 was found in a population of interstitial-like cells in the corpus cavernosum and proposed to contribute to the neural regulation of corporal smooth muscle tone (Hashitani et al., 2005).
On the other hand, the present study also demonstrated the release of COX-1-derived vasoconstrictor prostaglandins that contributed to the spontaneous tone of penile arteries from healthy animals, as shown by the enhancing effect of both the COX-1 blocker SC-560 and of the selective TP receptor antagonist ICI-192, on the relaxations induced by high doses of AA. These results are consistent with studies showing an extensive co-distribution of COX-1 with both TXA2 and PGI2 synthases in the aortic endothelium (Kawka et al., 2007), along with a marked reduction of PGI2 and TXA2 metabolites by COX-1 inhibitors (Qui et al., 2006; Kawka et al., 2007), thus indicating the major functional contribution of endothelial COX-1 to the basal production of both PGI2 and TXA2 in the normal arterial wall.
Cyclooxygenase 2 is up-regulated by inflammatory stimuli and is involved in the abnormal vascular reactivity of arteries from type 2 diabetic mice (Bagi et al., 2005; Guo et al., 2005), although recent studies have shown that both COX-1 and COX-2 may contribute to the endothelial dysfunction (Matsumoto et al., 2007) and to the hypersensitivity of vascular smooth muscle (Shi and Vanhoutte, 2008) in arteries from type 2 and type 1 diabetic rats respectively. We have recently demonstrated that penile arteries from animals with the metabolic syndrome exhibit endothelial dysfunction, as shown by the reduced ACh-induced relaxations and the impaired NO basal activity (Villalba et al., 2009). In the present study, we confirmed this dysfunction and further demonstrated that alterations in the AA metabolism via both COX-1 and COX-2 were involved in the loss of the predominant basal and endothelial agonist-induced release of vasodilator prostaglandins in arteries from OZR, thereby contributing to the endothelial dysfunction in these animals. Thus, COX-1 and COX-2 inhibitors failed to inhibit the relaxations induced by both ACh and AA in penile arteries from OZR, suggesting a blunted formation and/or impaired effects of relaxant prostanoids. Moreover, COX-2 inhibition no longer enhanced noradrenaline vasoconstriction.
The present results showing that the relaxant effects of AA are impaired in penile arteries from OZR suggest a shift in the AA metabolism towards an enhanced formation of vasoconstrictor prostanoids. The specific involvement of COX-2 is suggested by two findings. First, both the COX-2 inhibitor NS-398 and the TP receptor antagonist ICI-192 enhanced the relaxant effect of high concentrations of AA in OZR and restored them to levels similar to those in LZR. Second, COX-1 inhibitors unmasked a marked increase in basal tension in penile arteries from OZR, which was abolished by TP receptor blockade, indicating an enhanced COX-2-mediated basal production of vasoconstrictor prostanoids that seems to be balanced by an increased basal production of COX-1-derived relaxant prostaglandins. The present results would be consistent with those reported for skeletal muscle arteries from OZR (Xiang et al., 2008) and from type 2 diabetic mice (Bagi et al., 2005), where the impaired AA relaxant responses were improved and the increased arteriolar basal tone was inhibited by TP receptor antagonism. However, measurements of TXA2 metabolites in pooled skeletal muscle arteries from OZR showed no significant changes in either basal or AA-stimulated TXA2 levels (Goodwill et al., 2008; Hodnett et al., 2009). In the present study, we have found that the responses to the exogenous TXA2 analogue U46619 were augmented in penile arteries from OZR, suggesting that an enhanced TP receptor activity might be in part responsible for the augmented basal vasoconstrictor activity in these arteries.
Penile arteries from OZR exhibit an impaired basal release of NO despite unaltered eNOS expression (Villalba et al., 2009). In arteries from healthy LZR, both NOS and COX contribute to the maintenance of penile arterial tone through the production of NO and predominantly vasodilator prostanoids. In several blood vessels, NO is responsible for a permanent feed-back inhibition which restricts the release of constrictor prostanoids, and reduced NO bioavailability has been shown to increase this constrictor activity through the enhanced formation of reactive oxygen species (Yang et al., 2002; Laemmel et al., 2003; Vanhoutte et al., 2009). Therefore, an impaired endothelial NOS activity, as occurs in penile arteries from OZR under conditions of insulin resistance (Villalba et al., 2009), could favour the synthesis/release of vasoconstrictor prostanoids and thus contribute to the enhanced COX-2-mediated basal constrictor activity observed in the present study.
In human corpus cavernosum from diabetic patients, enhanced TP receptor-mediated contractile responses have been shown to underlie the impaired endothelium-dependent relaxations to ACh (Angulo et al., 2006). However, TP receptor antagonism could not restore the blunted ACh-elicited relaxations in penile arteries from OZR, unlike its enhancing effect on the AA-induced relaxations, and in contrast to that reported for diabetic penile corporal tissue. Neither selective nor selective inhibition of COX could significantly enhance either ACh relaxant responses in penile arteries unlike that observed in mesenteric arteries from type 2 diabetic rats where an augmented production of TXA2 and PGE2 impairs ACh-induced relaxations and treatment with indomethacin improves these responses (Matsumoto et al., 2007). Although an enhanced formation of vasoconstrictor prostaglandins other than TXA2 cannot be ruled out (Vanhoutte et al., 2009), the present data suggest that other factors might contribute to the blunted prostanoid-mediated endothelium-dependent relaxations of penile arteries in insulin-resistant OZR. Recent studies have demonstrated an attenuated production of PGI2 associated to the increased nitration of tyrosine residues of the PGI2 synthase in skeletal muscle arterioles from OZR (Goodwill et al., 2008; Hodnett et al., 2009). Increased mitochondrial fatty acid oxidation with the subsequently augmented superoxide production is responsible for enhanced tyrosine nitration and inactivation of PGI2 synthase in the aortic endothelium of OZR (Du et al., 2006). We have recently demonstrated that acute antioxidant treatment partially restored the enhanced vasoconstriction of penile and coronary resistance arteries from OZR (Villalba et al., 2009). Therefore, enhanced oxidative stress is likely to contribute to the impaired relaxant prostanoid formation and thus to be involved in the endothelial/vascular dysfunction of penile arteries of insulin-resistant OZR, as reported for arteries from diabetic and hypertensive animals (Álvarez et al., 2007; Matsumoto et al., 2007; Shi and Vanhoutte, 2008; Vanhoutte et al., 2009).
In summary, the present results demonstrate that constitutively active COX-1 and COX-2 pathways contribute to the regulation of vascular tone in normal rat penile arteries through the predominant formation of vasodilator prostanoids. Although endothelial dysfunction in penile vasculature from diabetic animals has primarily been ascribed to deficiencies in the NO pathway, here we demonstrate that impaired AA metabolism with reduced release/effects of vasodilator prostaglandins from both COX-1 and COX-2 pathways, plays a key role in the pathogenesis of both endothelial dysfunction and augmented vasoconstriction in penile arteries under conditions of insulin resistance and the metabolic syndrome.
Acknowledgments
These studies were supported by grants SAF2006-09191 and SAF 2006-02376 from the Spanish Ministry of Science and Innovation. We thank Francisco Puente and Manuel Perales for expert technical assistance.
Glossary
Abbreviations:
- AA
arachidonic acid
- ED
erectile dysfunction
- l-NNA
Nω-nitro-L-arginine
- LZR
lean Zucker rat
- OZR
obese Zucker rat
Conflicts of interest
None.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edition (2008 revision) Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Álvarez Y, Pérez-Giron JV, Hernanz R, Briones AM, García-Redondo A, Beltrán A, et al. Losartan reduces the increased participation of cyclooxygenase-2-derived products in vascular responses of hypertensive rats. J Pharmacol Exp Ther. 2007;321(1):381–388. doi: 10.1124/jpet.106.115287. [DOI] [PubMed] [Google Scholar]
- Angulo J, Cuevas P, Fernández A, Allona A, Moncada I, Martín-Morales A, et al. Enhanced thromboxane receptor-mediated responses and impaired endothelium-dependent relaxation in human corpus cavernosum from diabetic impotent men: role of protein kinase C activity. J Pharmacol Exp Ther. 2006;319(2):783–789. doi: 10.1124/jpet.106.108597. [DOI] [PubMed] [Google Scholar]
- Angulo J, Cuevas P, Fernández A, Gabancho S, Allona A, Martín-Morales A, et al. Diabetes impairs endothelium-dependent relaxation of human penile vascular tissues mediated by NO and EDHF. Biochem Biophys Res Commun. 2003;312(4):1202–1208. doi: 10.1016/j.bbrc.2003.11.034. [DOI] [PubMed] [Google Scholar]
- Angulo J, Cuevas P, La Fuente JM, Pomerol JM, Ruiz-Castane E, Puigvert A, et al. Regulation of human penile smooth muscle tone by prostanoid receptors. Br J Pharmacol. 2002;136:23–30. doi: 10.1038/sj.bjp.0704675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azadzoi KM, Kim N, Brown ML, Goldstein I, Cohen RA, Saenz de Tejada I. Endothelium-derived nitric oxide and cyclooxygenase products modulate corpus cavernosum smooth muscle tone. J Urol. 1992;147:220–225. doi: 10.1016/s0022-5347(17)37201-4. [DOI] [PubMed] [Google Scholar]
- Baber RS, Hunter CC, Trinity JB, Hyman AL, Kadowitz PJ. Role of Cyclooxygenase-2 in the generation of vasoactive prostanoids in the rat pulmonary and systemic vascular beds. Circulation. 2003;108:896–901. doi: 10.1161/01.CIR.0000084536.87322.BB. [DOI] [PubMed] [Google Scholar]
- Baber SR, Deng W, Rodriguez J, Master RG, Bivalacqua TJ, Hyman AL, et al. Vasoactive prostanoids are generated from arachidonic acid by COX-1 and COX-2 in the mouse. Am J Physiol Heart Circ Physiol. 2005;289(4):H1476–H1487. doi: 10.1152/ajpheart.00195.2005. [DOI] [PubMed] [Google Scholar]
- Bagi Z, Erdei N, Toth A, Li W, Hintze TH, Koller A, et al. Type 2 diabetic mice have increased arteriolar tone and blood pressure: enhanced release of COX-2-derived constrictor prostaglandins. Arterioscler Thromb Vasc Biol. 2005;25(8):1610–1616. doi: 10.1161/01.ATV.0000172688.26838.9f. [DOI] [PubMed] [Google Scholar]
- Billups KL. Erectile dusfunction as an early sign of cardiovascular disease. Int J Impot Res. 2005;1:S19–24. doi: 10.1038/sj.ijir.3901425. [DOI] [PubMed] [Google Scholar]
- Brock TG, McNish RW, Peters-Golden M. Arachidonic acid is preferentially metabolized by cyclooxygenase-2 to prostacyclin and prostaglandin E2. J Biol Chem. 1999;274(17):11660–11666. doi: 10.1074/jbc.274.17.11660. [DOI] [PubMed] [Google Scholar]
- Christ GJ, Maayani S, Valcic M, Melman A. Pharmacological studies of human erectile tissue: characteristics of spontaneous contractions and alterations in alpha-adrenoceptor responsiveness with age and disease in isolated tissues. Br J Pharmacol. 1990;101(2):375–381. doi: 10.1111/j.1476-5381.1990.tb12717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley JT, Brown ML, Watkins T, Traish AM, Huang YH, Moreland RB, et al. Prostanoid production in rabbit corpus cavernosum: I. Regulation by oxygen tension. J Urol. 1996;155:1482–1487. [PubMed] [Google Scholar]
- De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130(5):963–974. doi: 10.1038/sj.bjp.0703393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X, Edelstein D, Obici S, Higham N, Zou MH, Brownlee M. Insulin resistance reduces arterial prostacyclin synthase and eNOS activities by increasing endothelial fatty acid oxidation. J Clin Invest. 2006;116(4):1071–1080. doi: 10.1172/JCI23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermert L, Ermert M, Althoff A, Merkle M, Grimminger F, Seeger W. Vasoregulatory prostanoid generation proceeds via cyclooxygenase-2 in noninflamed rat lungs. J Pharmacol Exp Ther. 1998;286(3):1309–1314. [PubMed] [Google Scholar]
- Espósito K, Giugliano F, Martedì E, Feola G, Marfella R, D'Armiento M, et al. High proportions of erectile dysfunction in men with the metabolic syndrome. Diabetes Care. 2005;28(5):1201–1203. doi: 10.2337/diacare.28.5.1201. [DOI] [PubMed] [Google Scholar]
- Fonseca VA, Jawa A. Endothelial and erectile dysfunction, diabetes mellitus, and the metabolic syndrome: common pathways and treatments? Am J Cardiol. 2005;96(12B):13M–18M. doi: 10.1016/j.amjcard.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Goodwill AG, James ME, Frisbee JC. Increased vascular thromboxane generation impairs dilation of skeletal muscle arterioles of obese Zucker rats with reduced oxygen tension. Am J Physiol Heart Circ Physiol. 2008;295(4):H1522–H1528. doi: 10.1152/ajpheart.00596.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerre-Millo M. Regulation of ob gene overexpression in obesity. Biomed Pharmacother. 1997;51(8):318–323. doi: 10.1016/S0753-3322(97)88048-1. [DOI] [PubMed] [Google Scholar]
- Guo Z, Su W, Allen S, Pang H, Daugherty A, Smart E, et al. COX-2 up-regulation and vascular smooth muscle contractile hyperreactivity in spontaneous diabetic db/db mice. Cardiovasc Res. 2005;67(4):723–735. doi: 10.1016/j.cardiores.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Hashitani H, Yanai Y, Shirasawa N, Soji T, Tomita A, Kohri K, et al. Interaction between spontaneous and neurally mediated regulation of smooth muscle tone in the rabbit corpus cavernosum. J Physiol. 2005;569:723–735. doi: 10.1113/jphysiol.2005.099309. Pt 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrion D, Dechaux E, Dowell FJ, Maclour J, Samuel JL, Lévy BI, et al. Alteration of flow-induced dilatation in mesenteric resistance arteries of L-NAME treated rats and its partial association with induction of cyclo-oxygenase-2. Br J Pharmacol. 1997;121(1):83–90. doi: 10.1038/sj.bjp.0701109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herranz R, Briones AM, Alonso MJ, Vila E, Salaices M. Hypertension alters role of iNOS, COX-2, and oxidative stress in bradykinin relaxation impairment after LPS in rat cerebral arteries. Am J Physiol Heart Circ Physiol. 2004;287(1):H225–H234. doi: 10.1152/ajpheart.00548.2003. [DOI] [PubMed] [Google Scholar]
- Hodnett BL, Dearman JA, Carter CB, Hester RL. Attenuated PGI2 synthesis in Obese Zucker Rats. Am J Physiol Regul Integr Comp Physiol. 2009;296(3):R715–R721. doi: 10.1152/ajpregu.90330.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Kawka DW, Ouellet M, Hétu PO, Signer II, Riendeau D. Double-label expression studies of prostacyclin synthase, thromboxane synthase and COX isoforms in normal aortic endothelium. Biochim et Biophys Acta. 2007;1771:45–54. doi: 10.1016/j.bbalip.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Keegan A, Cotter MA, Cameron NE. Effects of diabetes and treatment with the antioxidant alpha-lipoic acid on endothelial and neurogenic responses of corpus cavernosum in rats. Diabetologia. 1999;42:343–350. doi: 10.1007/s001250051161. [DOI] [PubMed] [Google Scholar]
- Kuboki K, Jiang ZY, Takahara N, Ha SW, Igarashi M, Yamauchi T, et al. Regulation of endothelial constitutive nitric oxide synthase gene expression in endothelial cells and in vivo: a specific vascular action of insulin. Circulation. 2000;101(6):676–681. doi: 10.1161/01.cir.101.6.676. [DOI] [PubMed] [Google Scholar]
- Laemmel E, Bonnardel-Phu E, Hou X, Seror J, Vicaut E. Interaction between nitric oxide and prostanoids in arterioles of rat cremaster muscle in vivo. Am J Physiol Heart Circ Physiol. 2003;285(3):H1254–H1260. doi: 10.1152/ajpheart.00839.2002. [DOI] [PubMed] [Google Scholar]
- Martínez AC, Prieto D, Hernández M, Rivera L, Recio P, García-Sacristán A, et al. Endothelial mechanisms underlying responses to ACh in the horse deep dorsal penile vein. Eur J Pharmacol. 2005;515:150–159. doi: 10.1016/j.ejphar.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Kakami M, Noguchi E, Kobayashi T, Kamata K. Imbalance between endothelium-derived relaxing and contracting factors in mesenteric arteries from aged OLETF rats, a model of Type 2 diabetes. Am J Physiol Heart Circ Physiol. 2007;293(3):H1480–H1490. doi: 10.1152/ajpheart.00229.2007. [DOI] [PubMed] [Google Scholar]
- Okahara K, Sun B, Kambayashi J. Upregulation of prostacyclin synthesis-related gene expression by shear stress in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 1998;18(12):1922–1926. doi: 10.1161/01.atv.18.12.1922. [DOI] [PubMed] [Google Scholar]
- Okon EB, Chung AW, Rauniyar P, Padilla E, Tejerina T, McManus BM, et al. Compromised arterial function in human type 2 diabetic patiens. Diabetes. 2005;54(8):2415–2423. doi: 10.2337/diabetes.54.8.2415. [DOI] [PubMed] [Google Scholar]
- Prieto D. Physiological regulation of penile arteries and veins. Int J Impot Res. 2008;20:17–29. doi: 10.1038/sj.ijir.3901581. [DOI] [PubMed] [Google Scholar]
- Prieto D, Simonsen U, Hernández M, García-Sacristán A. Contribution of K+ channels and ouabain-sensitive mechanisms to the endothelium-dependent relaxations of horse penile small arteries. Br J Pharmacol. 1998;123:1609–1620. doi: 10.1038/sj.bjp.0701780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qui Z, Cai H, Morrow JD, Breyer MD. Differentiation of cyclooxygenase 1- and 2-derived prostanoids in mouse kidney and aorta. Hypertension. 2006;48(2):323–328. doi: 10.1161/01.HYP.0000231934.67549.b7. [DOI] [PubMed] [Google Scholar]
- Ruiz Rubio JL, Hernández M, Rivera de los Arcos L, Martínez AC, García-Sacristán A, Prieto D. Mechanisms of prostaglandin E1-induced relaxation in penile resistance arteries. J Urol. 2004;171:968–973. doi: 10.1097/01.ju.0000097496.27675.c4. [DOI] [PubMed] [Google Scholar]
- Saenz de Tejada I, Goldstein I, Azadzoi K, Krane RJ, Cohen RA. Impaired neurogenic and endothelium-mediated relaxation of penile smooth muscle from diabetic men with impotence. N Engl J Med. 1989;320(16):1025–1030. doi: 10.1056/NEJM198904203201601. [DOI] [PubMed] [Google Scholar]
- Shi Y, Vanhoutte PM. Oxidative stress and COX cause hyper-responsiveness in vascular smooth muscle of the femoral artery from diabetic rats. Br J Pharmacol. 2008;154(3):639–651. doi: 10.1038/bjp.2008.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen U, Contreras J, García-Sacristán A, Martínez AC. Effect of sildenafil on non-adrenergic non-cholinergic neurotransmision in bovine penile small arteries. Eur J Pharmacol. 2001;412(2):155–169. doi: 10.1016/s0014-2999(01)00726-9. [DOI] [PubMed] [Google Scholar]
- Seibert K, Zhang Y, Leahy K, Hauser S, Masferrer J, Perkins W, et al. Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc Natl Acad Sci USA. 1994;91(25):12013–12017. doi: 10.1073/pnas.91.25.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topper JN, Cai J, Falb D, Gimbrone MA., Jr Identification of vascular endothelial genes differentially responsive to fluid mechanical stimuli: cyclooxygenase-2, manganese superoxide dismutase, and endothelial cell nitric oxide synthase are selectively up-regulated by steady laminar shear stress. Proc Natl Acad Sci USA. 1996;93(19):10417–10422. doi: 10.1073/pnas.93.19.10417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoutte PM, Shimokawa H, Tang EH, Feletou M. Endothelial dysfunction and vascular disease. Acta Physiol (Oxf) 2009;196(2):193–222. doi: 10.1111/j.1748-1716.2009.01964.x. [DOI] [PubMed] [Google Scholar]
- Vickers MA, Wright EA. Erectile dysfunction in patient with diabetes mellitus. Am J Manag Care. 2004;10(Suppl. 1):S3–S11. [PubMed] [Google Scholar]
- Villalba N, Martínez MP, Briones AM, Sánchez A, Salaíces M, García-Sacristán A, et al. Differential structural and functional changes of penile and coronary arteries from obese Zucker rats. Am J Physiol Heart Circ Physiol. 2009;297(2):H696–H707. doi: 10.1152/ajpheart.01308.2008. [DOI] [PubMed] [Google Scholar]
- Warner TD, Mitchell JA. Cyclooxygenases: new forms, new inhibitors, and lessons from the clinic. FASEB J. 2004;18(7):790–804. doi: 10.1096/fj.03-0645rev. [DOI] [PubMed] [Google Scholar]
- Xiang L, Dearman J, Abram SR, Carter C, Hester RL. Insulin resistance and impaired functional vasodilation in obese Zucker rats. Am J Physiol Heart Circ Physiol. 2008;294(4):H1658–H1666. doi: 10.1152/ajpheart.01206.2007. [DOI] [PubMed] [Google Scholar]
- Yang D, Félétou M, Boulanger CM, Wu HF, Levens N, Zhang JN, et al. Oxygen-derived free radicals mediate endothelium-dependent contractions to acetylcholine in aortas from spontaneously hypertensive rats. Br J Pharmacol. 2002;136(1):104–110. doi: 10.1038/sj.bjp.0704669. [DOI] [PMC free article] [PubMed] [Google Scholar]


