Abstract
Phenotypic plasticity in general and polyphenic development in particular are thought to play important roles in organismal diversification and evolutionary innovation. Focusing on the evolutionary developmental biology of insects, and specifically that of horned beetles, I explore the avenues by which phenotypic plasticity and polyphenic development have mediated the origins of novelty and diversity. Specifically, I argue that phenotypic plasticity generates novel targets for evolutionary processes to act on, as well as brings about trade-offs during development and evolution, thereby diversifying evolutionary trajectories available to natural populations. Lastly, I examine the notion that in those cases in which phenotypic plasticity is underlain by modularity in gene expression, it results in a fundamental trade-off between degree of plasticity and mutation accumulation. On one hand, this trade-off limits the extent of plasticity that can be accommodated by modularity of gene expression. On the other hand, it causes genes whose expression is specific to rare environments to accumulate greater variation within species, providing the opportunity for faster divergence and diversification between species, compared with genes expressed across environments. Phenotypic plasticity therefore contributes to organismal diversification on a variety of levels of biological organization, thereby facilitating the evolution of novel traits, new species and complex life cycles.
Keywords: modularity, mutation accumulation, Onthophagus, relaxed selection
1. Introduction
(a). Phenotypic plasticity—ubiquitous yet limited
Virtually all organisms as well as biological processes exhibit some degree of plasticity, that is, their function is affected by external conditions (West-Eberhard 2003). On one extreme, such effects may arise from the biochemical and biophysical dependencies of biological processes, such as the temperature dependency of enzymatic reactions or the pH-dependent folding of proteins (Schlichting & Pigliucci 1995). As such, plasticity may be viewed as a by-product, an ability that organisms did not have to evolve but that came for free as an emergent property of life given how life, in its simplest terms, works. On the other extreme are highly choreographed responses to environmental changes such as acquired immunity, learning, nutrition-dependent modification of growth and reproduction or seasonal migration (Nijhout 1999, 2003; West-Eberhard 2003). Here, plasticity represents a complex, evolved response to deal with important environmental changes, allowing organisms to maintain high fitness in the face of environmental variability (Schlichting & Pigliucci 1998). Importantly, such responses involve adjustments on not just one but many levels of biological organization. For instance, the nutrition-dependent expression of different castes in social insects involves differences in gene expression, endocrine physiology, regulation of appendage growth and development, development of reproductive organs and behavioural repertoires (Wilson 1976; Wheeler & Nijhout 1983, 1984; Evans & Wheeler 1999, 2001a,b; Abouheif & Wray 2002). What is less obvious is that much plasticity also underlies many homeostatic responses and canalization in development (Scharloo 1991). From producing constant blood sugar levels in the face of nutritional variation to the generation of proper scaling relationships of body parts during growth, organisms flexibly adjust a vast array of plastic processes on some levels of biological organization to ensure phenotypic constancy on others (Scharloo 1991; Dworkin 2005).
Despite the obvious adaptive significance of plasticity, it is clearly not without limits and apparent constraints. Organisms are not infinitely plastic and instead limited in the range of environments they can respond to. Even in the most extreme cases such as polyphenisms, expression of alternative phenotypes is almost always restricted to two morphs rather than five or eight, even though one could probably make a case that such hypothetical alternatives should do well in at least some environments (West-Eberhard 2003). The notable exceptions are social castes in the Hymenoptera (Hölldobler & Wilson 1990), a case we will later on return to. Lastly, it is worth noting that plasticity, central as it is to life on this planet including our own, also plays a central role in many important human diseases. From autoimmune diseases to obesity, many of our ‘modern’ diseases involve plasticity gone wrong, bringing about inappropriate responses to external changes (Gilbert & Epel 2009).
In this review, I will explore how plasticity contributes to organismal diversification as well as the costs and limits of this contribution. Drawing from the insect literature, and specifically from recent work on horned beetles, I will highlight avenues by which plasticity and its developmental and genetic underpinnings diversify evolutionary trajectories available to evolving lineages. I will begin by introducing my focal taxa, the insects, and within them, the horned beetles, as particularly illuminating macro- and microcosms for understanding the causes, mechanisms and consequences of plasticity.
(b). Insect biology and the range and power of plasticity
If an alien life form would visit planet Earth with the mission to identify the group of multicellular organisms that has diversified most successfully, it would select, without doubt, the insects. Insects represent over one million of the roughly 1.4 million species named thus far. Insects have evolved every imaginable feeding habit and colonized nearly every imaginable ecological niche with the remarkable exception of marine habitats (Grimaldi & Engel 2005). Much of this diversity is contributed by holometabolous insects, represented by nine insect orders that feature complete metamorphosis and a pupal stage intercalated between larvae and adults (Yang 2001). All superdiverse insect orders (Lepidoptera, Diptera, Hymenoptera, Coleoptera) are holometabolous. Combined, these four orders alone account for over 750 000 species and thus about three-quarters of all named insects and more than half of all living species. Among them, the beetles stand out the most, accounting for every fourth named species on this planet (Grimaldi & Engel 2005).
Evolution of holometabolous development and metamorphosis decoupled larval and adult stages and permitted larvae and adult versions of the same animal to specialize in often very different niches, feeding modes and habitats (Jacobs & Renner 1988). The life-stage diversity mediated by holometabolous development is truly spectacular and a remarkable testament to the phenotypic diversity and disparity, including morphological, physiological and behavioural traits, which can be accommodated by a single genome. More importantly, it is also testament to the incredible plasticity of development, which if given the proper genetic and environmental cues can transform a maggot into a fly, a caterpillar into a moth and a grub into a beetle.
Insects also stand out as exemplars of cases in which alternative phenotypes are not expressed sequentially, as in larval and adult stages, but simultaneously, as in the context of polyphenisms (Nijhout 2003). Some of the most spectacular cases of polyphenic alternatives are found among insects, including social castes in the Hymenoptera, termites and aphids (Lüscher 1960; Wheeler & Nijhout 1983; Stern & Foster 1996), seasonal polyphenisms in butterflies (Shapiro 1976), dispersal polyphenisms (Zera & Denno 1997), alternative asexual and sexual reproductive phases in aphids (Moran 1991) and alternative male morphologies in thrips (Crespi 1988) and beetles (Emlen 1994). Here again, a single genome displays tremendous ability to accommodate a wide range of often highly disparate phenotypes in response to changes in external conditions. Furthermore, in many cases, phenotypic differences between alternatives mirror major macro-evolutionary transitions such as the alternation between asexual and sexual stages in aphids or wingless and winged stages in social insects (West-Eberhard 2003).
Insects thus emerge as a taxon that combines tremendous diversity and species richness with remarkable plasticity in development. This leads to the main question that this review hopes to address: did this exuberant species richness and diversity evolve independently of, in spite of, or possibly because of the level of plasticity observed within the insects? Before addressing this question, I would briefly like to introduce a group of insects that is beginning to emerge as a particularly useful microcosm for studying the interplay between plasticity, development and evolution, and for evaluating the creative potential of phenotypic plasticity: the horned beetles.
(c). Horned beetles: a microcosm for understanding the causes, mechanisms and consequences of plasticity
Horned beetles are not a taxonomic entity. Instead, they exist as a group simply because of biologists' interests in, and at times obsessions for, beetles with exaggerated and often bizarre secondary sexual traits (figure 1). Beetle species belonging to at least six families have evolved horn-like structures (reviewed in Snell-Rood & Moczek in press), though the most extreme and diverse cases all exist within one of the most species-rich families of beetles, the Scarabaeidae (figure 1). This family is home to the spectacular subfamily Dynastinae, including the southeast Asian genus Chalcosoma that enchanted Darwin, and the subfamily Scarabaeinae, home to all true dung beetles (Darwin 1871; Arrow 1951; Balthasar 1963).
Figure 1.
Examples of horned beetles illustrating diversity and magnitude of horn expression in adult beetles: (a) Phanaeus imperator, (b) Eupatorus gracilicornis, (c) Onthophagus watanabei, (d) Golofa claviger and (e) Trypoxylus (Allomyrina) dichotoma.
Horned beetles have fascinated biologists for a number of reasons (reviewed in Moczek 2005). First, beetle horns are spectacular traits, enormous in size and often bizarre in shape. Furthermore, beetle horns attract attention because of the fantastic diversity that exists between and within species. Between species, horns differ in size, location, number and shape. Intriguingly, all of these types of variation can also be found within species, in the context of sexual dimorphisms (females commonly lack or have greatly reduced horns) as well as male dimorphism. This latter level of variation is especially noteworthy as it reflects diversity in male phenotypes produced in response to variation in larval nutrition rather than allelic differences between males. In extreme cases, the resulting variation is discrete, causing males to fall into distinct alternative morphs. In such cases, alternative male morphs often not only differ in their relative investment into horns, but also display alternative reproductive behaviours (Moczek & Emlen 2000), differential genitalic investment (Simmons & Emlen 2006; Parzer & Moczek 2008), thermoregulation (Shepherd et al. 2008), differences in brain protein expression (Y. Yerushalmi & A. Moczek 2009, unpublished data), etc. Lastly, beetle horns lack clear homology to other structures in beetles or in other insect orders. Horns are not merely modified legs or antennae; instead, they exist alongside these structures and can therefore be interpreted as an example of an evolutionary novelty, invented at some point during beetle history (Moczek et al. 2006). Hence, beetle horns have become the focus of studies interested in the early stages of organismal innovation, and the genetic, developmental and ecological mechanisms that mediate the origin of novelty from within the confines of ancestral homology.
In all these aspects, one genus of horned beetles stands out: Onthophagus. This genus is, with an estimated 2400 extant species, the most speciose genus in the animal kingdom (Balthasar 1963; Matthews 1972; Howden & Young 1981). It combines incredible species richness with dramatic diversity in the expression of horns, and as we will see in §2 important components of this diversity are linked one way or another to phenotypic plasticity. We thus arrive again at the central question that this review is trying to address: did the amazing species richness and diversity seen in horned beetles in general, and the genus Onthophagus in particular, evolve independently of, in spite of, or because of the level of plasticity observed in these taxa?
2. Phenotypic plasticity and organismal diversification
Phenotypic plasticity has been postulated to be both an inhibitor and a facilitator of phenotypic diversification (Schlichting & Pigliucci 1998; Pigliucci 2001). Conflicting viewpoints on the issue arise for a number of reasons, for instance, whether organismal diversity is equivalent to species richness, or whether other proxies might be more appropriate. For example, plasticity permits organisms to adjust to a range of environmental conditions, and as such may eliminate population subdivision arising from environmental heterogeneity, which otherwise might facilitate the evolution of specialist species. In this case, plasticity may be viewed as reducing organismal diversity because it prevents speciation. However, if species richness is not used as a proxy for organismal diversity, the same argument can actually be used to support the opposite claim. By permitting the evolution of environment-specific responses in phenotype expression, phenotypic plasticity permits organismal diversification within species, such as alternative morphs, without having to couple it to speciation. Here, phenotypic plasticity permits an increase in phenotypic diversity without a commensurate increase in species richness.
Another general, and related, argument for and against a contribution of plasticity to organismal diversification emphasizes its role in population extinction. Here, conflicting viewpoints arise primarily owing to differences in the time frame over which plasticity's contribution is evaluated. Both start with the observation that plasticity reduces the likelihood of extinctions in the face of environmental heterogeneity. If environmental changes, such as global climate change or the appearance of habitat bridges, cause new niches to become available for colonization, phenotypic plasticity may predispose plastic species to be successful colonizers, reducing the likelihood of new, specialized species to invade or evolve. Over this short time frame, plasticity would permit habitat expansion but without leading to a net increase in species diversity. At the same time, by buffering populations against extinctions owing to environmental fluctuations, plasticity can be viewed as maintaining phenotypic diversity despite exposure to new conditions with novel environmental challenges. By preventing population extinctions, plasticity now dramatically extends the opportunity for phenotypic differentiation to evolve subsequently, including speciation (West-Eberhard 2003).
In the remainder of this review, I argue that even though these general considerations all capture important aspects of plasticity's role in evolution, plasticity's contribution to organismal diversification, including speciation, goes well beyond. Instead, rather than merely increasing phenotypic diversity within species or preserving the opportunity for diversification to occur by preventing extinctions, I argue below that plasticity itself opens up opportunities for diversification, including speciation, that would not exist otherwise.
(a). Phenotypic plasticity provides novel targets for evolutionary processes
Adaptive phenotypic plasticity, as in the case of polyphenisms, involves the acquisition of information on environmental conditions and the corresponding adjustment of genetic, developmental, physiological and/or behavioural phenotypes. Responses to environmental changes may range from gradual, with one ‘unit’ of environmental change soliciting a more or less corresponding unit of phenotypic adjustment, to discrete, with a threshold separating phenotypes produced under one range of conditions from those produced under another. Numerous studies have now shown that both nature (e.g. gradual versus discrete) and magnitude (e.g. small or large changes across environments) of plastic responses can evolve independently of other aspects of the phenotype. For example, lacewings and pitcher plant mosquitoes (Tauber & Tauber 1972; Bradshaw et al. 2003) exhibit heritable variation for the photoperiodic response threshold underlying the facultative expression of diapause, families of cabbage-white butterflies show heritable variation for the ability to adjust host-plant preferences (Snell-Rood & Papaj 2009), and species of ants and termites differ, at times dramatically, in the relative size cutoffs for different nutrition-determined castes that coexist in a colony (Wilson 1953; Hölldobler & Wilson 1990). Nature and magnitude of responses to environmental variation therefore contribute to the trait repertoire of a population that is subject to evolutionary processes, and that can contribute to evolutionary diversification between populations and species.
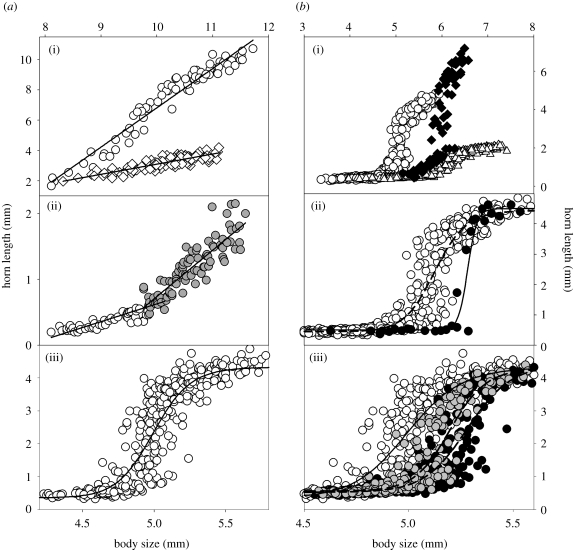
Studies on horned beetles have provided additional insights into the dynamics and consequences of plasticity evolution. Male horned beetles in the genus Onthophagus often exhibit species-specific differences in the exact scaling relationship between body size and horn length (reviewed in Emlen 2000; Moczek 2005). In all Onthophagus species, adult body size is heavily influenced by nutritional conditions experienced during larval development, and the relative sizes of other traits, such as horns, are adjusted accordingly: larvae with access to good feeding conditions will metamorphose into large adults with relatively large horns, whereas larvae with access to suboptimal conditions will give rise to small adults with relatively small horns. While this holds true for all Onthophagus species studied to date, the exact relationship between nutrition-determined body size and the corresponding horn size exhibits remarkable interspecific differences, causing body size–horn length scaling relationships to range from linear to ‘broken’ to sigmoidal (figure 2a). In all these cases, species adjust horn expression relative to nutritional variation, but the exact nature and magnitude of adjustments has undergone dramatic diversification independently of horn expression per se. Studies in the field and laboratory on one particular species now provide the first insights into the mechanisms, dynamics and consequences of plasticity evolution in nature.
Figure 2.
Examples of (a) complex-scaling relationships and (b) divergences in scaling relationships in horned beetles. (a) Examples of horn length–body size scaling relationships in the beetle genus Onthophagus: (i) linear scaling relationship of paired head horns in O. watanabei (males: open circles; females: open diamonds); (ii) broken scaling relationship of pronotal horn length in male O. binodis; (iii) S-shaped (sigmoidal) scaling relationship of paired head horns in male O. taurus (modified after Moczek 2009). (b) Examples of divergences in scaling relationships in the genus Onthophagus over a range of phylogenetic distances: (i) three Onthophagus species, which diverged approximately 20–38 Myr ago (O. taurus: open circles; O. nigriventris: solid diamonds; O. gazella: open triangles); (ii) divergences in scaling relationships between two sister species, O. taurus (open circles) and O. illyricus (solid circles), which diverged approximately 10 000 years ago (Pizzo et al. 2008); (iii) divergences in scaling relationships between three allopatric O. taurus populations established less than 40 years ago from a common Mediterranean ancestor (Eastern USA: open circles; Eastern Australia: grey circles; Western Australia: solid circles; modified after Moczek 2003).
Onthophagus taurus is a species native to the Mediterranean, which became introduced to the Eastern USA as well as to Eastern and Western Australia in the early 1970s (Moczek 2003). Males in this species exhibit a pronounced sigmoidal scaling relationship between body size and horn length, causing males below a certain size threshold to remain largely hornless and males above to express a pair of long, curved head horns. Comparisons of archival museum collections with present-day populations documented that introduced populations diverged in less than 40 years from their Mediterranean ancestors in the body size threshold that separates alternative male morphs to a degree normally only observed between species (figure 2b(ii)(iii); Moczek & Nijhout 2003). A combination of field and laboratory studies provided evidence consistent with the hypothesis that these divergences were driven by selection acting on horn length–body size thresholds, adjusting them to the local competitive conditions in which horned (fighting) and hornless (sneaking) males perform (Moczek 2003). Divergences are maintained over generations under a common garden regime, suggesting that the observed divergences indeed reflect heritable changes in the developmental machinery underlying nutrition-dependent morph expression (Moczek et al. 2002). In this example, plasticity in horn development has opened up traits, such as the body size threshold, for selection to act on, allowing populations to substantially diverge in threshold values in an extraordinarily short time period without diverging in the average phenotypes of horned and hornless male morphs, which instead are largely indistinguishable across populations (Pizzo et al. 2008).
Findings such as these illustrate that the genetic and developmental basis of plasticity in trait expression must at least in part be separate from, and independent of, the genetic and developmental basis of trait expression per se. For instance, earlier work on the endocrine regulation of horn expression in O. taurus showed that a common insect hormone, juvenile hormone (JH), appears to regulate horn expression in male beetles. Males fated to develop into hornless males will express horns if treated with certain dosages of JH analogues during a certain sensitive period (Emlen & Nijhout 1999). Follow-up studies on the divergent O. taurus populations introduced above then showed that threshold divergences appeared to have been made possible developmentally by evolved changes in the degree and timing of sensitivity to JH. The Western Australian population, which induce horn expression only at very large male body sizes, required substantially higher JH dosages during later larval development to induce horns in presumptive hornless males compared with Eastern USA males (Moczek & Nijhout 2002). Clearly, evolutionary changes in JH-mediated threshold determination can occur independently of evolutionary changes—or stasis—in other aspects of horn expression.
That said, the same studies also showed that divergences in the JH-mediated underpinnings of body size thresholds did not come entirely for free; instead, they appeared to have been accompanied by correlated changes in other traits unrelated to horn expression, in particular the length of larval development and the timing of metamorphosis. Specifically, researchers observed that populations that limit horn expression to only the largest males also exhibited an increase in the length of the last larval instar (but not earlier instars) and the timing of the larval-to-pupal moult (but not other moults), and hypothesized that these correlated differences may have a common root in JH's role in the regulation of moulting and metamorphosis, which constitutes one of its most basic, and ancestral, functions during the development of holometabolous insects (Moczek & Nijhout 2002). Specifically, the presence or absence of JH during critical periods preceding each moult determines whether moults maintain the same identity (JH present), as in a larval-to-larval moult, or change identity (JH absent), as in a larval-to-pupal or pupal-to-adult moult (Nijhout 1994). JH therefore has to be cleared from the haemolymph of late O. taurus larvae in order for them to transit to the pupal stage, which may be in conflict with the relatively high JH titers required immediately prior to this period to induce horns in large males. Current models therefore postulate that raising body size threshold to larger body sizes via delaying the sensitive period for JH and increasing JH titres required for horn induction should delay the complete removal of JH from larval haemolymph, and therefore bring about an extension of the last larval instar and a corresponding delay of the larval–pupal moult (Moczek & Nijhout 2002). While this scenario remains to be verified experimentally, these observations nevertheless begin to introduce an important new aspect in plasticity's role in organismal diversification: not only does plasticity provide new targets for evolutionary processes to act on that would otherwise not exist, but plasticity evolution may also bring about correlated changes in other traits that may not be possible in different contexts. Correlated changes in other traits are important in this context because they constitute a critical source of trade-offs in development and evolution. As we will see in the next section, evolutionary changes in components of plasticity can result in particularly interesting, and often non-intuitive, trade-offs with other traits, with the ability to bias evolutionary trajectories and patterns of phenotypic diversification in descendant lineages.
(b). Phenotypic plasticity creates novel trade-offs
At the most basic level, trade-offs during development may arise when two or more structures or developmental processes compete for a shared and limited pool of resources to sustain their growth or operation (Klingenberg & Nijhout 1998; Nijhout & Emlen 1998). Increased investment into one structure or process may only then be possible through decreased investment in another. The resulting resource allocation trade-offs not only have the ability to alter developmental outcomes but may also bias evolutionary trajectories available to descendant lineages. Resource allocation trade-offs are probably ubiquitous in the Metazoa, but appear particularly important in the development of holometabolous insects. Here, most growth of adult structures is restricted to a time period during which pupae represent a closed system, i.e. are no longer taking in nutrients and instead fuel most of metamorphosis using a finite pool of stored resources.
Resource allocation trade-offs can of course occur independently of the degree of plasticity associated with a given set of traits, i.e. should not be restricted to plastic traits, or processes underlying aspects of plasticity. However, recent work suggests that plastic trait expression can contribute trade-offs that would otherwise not occur. For instance, cabbage-white butterflies differ heritably in their ability to learn novel oviposition host plants (Snell-Rood & Papaj 2009). While learning is adaptive under at least some environmental conditions, it carries costs with it. Interestingly, some of these costs, such as investment in early mushroom-body development and overall brain size, are fixed, i.e. are incurred during development regardless of whether learning in life occurs or not. In contrast, other costs such as mushroom-body differentiation later in adult life are facultative and are only incurred if learning actually takes place (Snell-Rood et al. 2009). Importantly, learning-associated investment into brain development traded off with reproductive investment measured as the number of mature eggs at emergence (Snell-Rood et al. in press b). Simmons & Emlen (2006) reported a similar trade-off in Onthophagus beetles, where they documented a negative correlation between the degree of plasticity in horn expression and investment into testis; however, the mechanisms underlying this trade-off remain obscure.
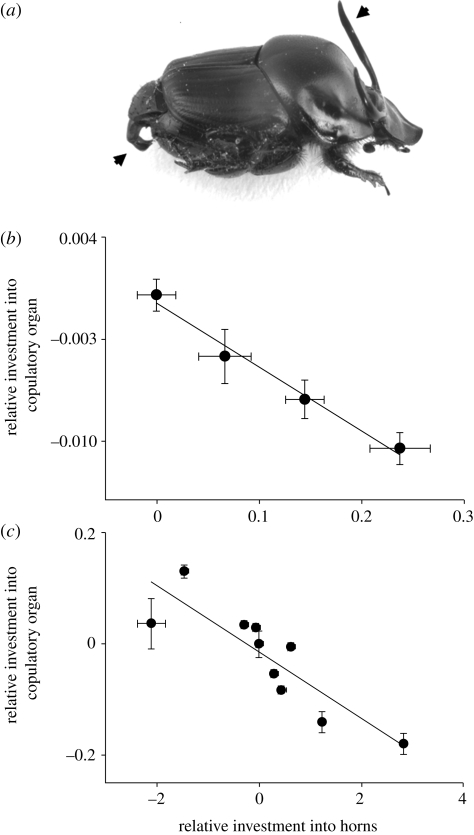
Aspects of plastic trait expression other than just the range of plasticity may also participate in trade-offs with other traits. A recent study on 10 Onthophagus species and four divergent O. taurus populations illustrates that evolutionary changes in threshold body sizes that separate alternative horned and hornless male morphs are associated with substantial trade-offs in the expression of other traits (Parzer & Moczek 2008). Earlier experimental work had shown that males that had their genital precursor cells removed during larval development, and thus were unable to develop copulatory organs, expressed significantly larger horns than untreated or sham-treated males (Moczek & Nijhout 2004). By showing that structures which develop at the opposite ends of an animal can engage in significant trade-offs, this study contradicted a common notion at the time that trade-off intensity should decay with distance. More importantly, however, it sets the stage for a series of comparative studies investigating whether corresponding trade-off signatures also exist in nature. Investigating three exotic (figure 2b(iii)) and one native populations of O. taurus, Parzer & Moczek (2008) showed that inducing horn expression at relatively smaller body sizes (as in Eastern USA males) correlates with reduced investment into male copulatory organs (figure 3a,b). Delaying horn expression to relatively larger body sizes (as in Western Australian males) had the opposite effect and resulted in increased investment into copulatory organs. Recall that threshold divergences between exotic populations evolved in less than 40 years since introduction from a common ancestor, and were apparently driven by selection acting on horn length–body size thresholds (Moczek 2003). If correct, this suggests that evolutionary changes in copulatory organ size occurred as a secondary by-product of selection operating on aspects of plasticity in secondary sexual trait expression. Replicating this approach across 10 Onthophagus species that have diverged from each other between 10 000 years and 38 Myr ago revealed a similarly strong trade-off signature (figure 3c; Parzer & Moczek 2008). Combined, these findings suggest that trade-offs between aspects of horn polyphenisms and copulatory organ development already characterize interpopulational divergences but continue to shape morphological diversification well after speciation is complete. The implication of copulatory organ size in this trade-off is of particular significance because evolutionary changes in male copulatory organs are thought to play a major role in the early evolution of reproductive isolation in insects (Eberhard 1985; Eberhard et al. 1998). It is typical for cryptic or recent insect species to be only distinguishable by copulatory organ morphology, suggesting that whatever mechanism is able to influence copulatory organ expression in a given population may have immediate consequences for that population's ability to interbreed with others. Given the importance of copulatory organ morphology for reproductive isolation, the findings outlined above begin to raise the possibility that horn plasticity evolution may have the ability to promote speciation as a by-product. If trade-offs between components of horn plasticity and male copulatory organs are indeed driving speciation in Onthophagus, this would help explain how onthophagine beetles succeeded to evolve into a genus famous for both exuberant diversity in horn expression and remarkable species richness (Arrow 1951; Matthews 1972).
Figure 3.
Trade-offs between investment into polyphenic expression of horns and canalized investment into copulatory organs in populations and species of Onthophagus beetles. (a) Horned male Onthophagus taurus. Arrows highlight horns and copulatory organ. (b) Relative investment into copulatory organ size as a function of relative investment into polyphenic horn expression in four different populations of O. taurus. Error bars represent one standard error. (c) Relative investment into copulatory organ size as a function of relative investment into horn size in 10 different Onthophagus species. Data are corrected for differences in body size. Modified after Parzer & Moczek (2008).
More generally, the examples listed above illustrate that just as plasticity can contribute novel targets for evolutionary processes to act on, it can help generate novel types of trade-offs that influence morphological, physiological or behavioural trait expression elsewhere in the same individual. On one hand, plasticity may therefore constrain and limit evolutionary trajectories available to lineages. On the other hand, such constraints may force lineages to explore phenotypic, and corresponding ecological, space that otherwise may remain unexplored. Similarly, trade-offs may cause populations and species to diverge in many more traits than just those selection may be targeting. As such, plasticity-associated trade-offs represent both constraints on and opportunities for organismal diversification. We will see the same two themes re-emerge in the next section, when we explore some of the genetic mechanisms of plasticity, and their emergent properties for plasticity evolution.
(c). Phenotypic plasticity increases genetic variation and divergence—the mutation-modularity trade-off hypothesis
The nature of the genetic basis of plasticity has been the subject of an intense debate (Via et al. 1995). A growing number of studies have now shown that plasticity, including polyphenic development, is often underlain by modularity in gene expression, i.e. different environmental conditions and phenotypes are associated with the expression of very different suites of genes (Hymenoptera: Evans & Wheeler 2001a,b; Pereboom et al. 2005; Judice et al. 2006; Donnell & Strand 2006; Isoptera: Scharf et al. 2003; Hojo et al. 2005; Hoffman & Goodisman 2007; Hemiptera: Kutsukake et al. 2004; Onthophagus: Snell-Rood et al. in press a). In such cases, the frequency by which a given suite of genes, or module, becomes expressed in a population within a given generation, and thus visible to selection, becomes a function of the frequency of the inducing environmental conditions. Rare conditions affecting gene expression either only in every couple of generations or only in a subset of individuals within a population cause genes specific to them to be hidden from selection and thus free to accumulate a larger number of mutations relative to genes expressed in every individual in every generation. Recent empirical and theoretical work (Van Dyken & Wade, in press; Snell-Rood et al. in press a) suggests that relaxed selection resulting from modularity in gene expression drives a fundamental trade-off between mutation accumulation and the degree of modularity in gene expression underlying plastic responses to environmental changes. If correct, this trade-off has far-reaching implications for defining the costs, limits and evolutionary consequences of plasticity.
The notion that certain restrictions in gene expression can result in relaxed selection permitting mutation accumulation has been proposed and examined previously in contexts outside phenotypic plasticity, such as the evolution of senescence (Charlesworth 1994) and the biology of niche conservativism (Holt 1996, see Snell-Rood et al. in press a for additional examples). In each case, genes whose expression is restricted to a subset of individuals in each generation are predicted to experience relaxed selection and accumulate mutations more quickly relative to similar genes expressed in every individual. Work on maternal effect genes has now brought the concept closer to developmental genetics and modularity in gene networks. Moreover, it has provided a first critical juxtaposition of empirical data with predictions from population-genetic models. Maternal effect genes are genes transcribed by mothers, which then incorporate transcripts or their protein products into their eggs. Strict maternal effect genes are only expressed by females and only function during early embryogenesis. Even though fathers possess the corresponding genes, they are not expressed during their life time. As such, only half of a given population expresses a given strict maternal effect gene, assuming a 1 : 1 sex ratio, reducing the efficiency of selection for such genes relative to those expressed in every individual, or the so-called zygotic genes. Population genetic theory predicts that given the halving in the number of expressed copies, maternal effect genes should accumulate twice the mutation load within populations compared with similar zygotic genes (Wade 1998). Theory furthermore predicts that provided nucleotide substitutions have at least mildly deleterious fitness effects, maternal effect genes have the potential to diverge many times faster between species than corresponding zygotic genes (Demuth & Wade 2007). Both predictions are now matched by empirical data (Barker et al. 2005; Demuth & Wade 2007; Cruickshank & Wade 2008). In the most extensive study, Cruickshank & Wade (2008) examined sequence variation within and between species of Drosophila of 39 genes instrumental for early embryonic development including 9 strict maternal effect genes and 30 zygotically expressed genes. As predicted, they found sequence variation within species (D. melanogaster) to be two- to threefold higher for maternal-effect genes than any other gene class, and sequence divergences between species (D. melanogaster and D. simulans) to be two- to fourfold higher in maternal effect genes than any other gene class. Combined, these data provide compelling support for the hypothesis that relaxed selection acting on maternal-effect genes causes increased sequence variation within species, in turn fuelling more rapid divergences between species.
This body of work focused primarily on modularity of gene expression as it arises during early embryonic development via the partitioning of gene expression into maternal effect versus zygotic genes. However, its theoretical foundation and predictions can easily be applied to modularity in gene expression arising through plasticity: genes induced in individuals experiencing rare environments should exhibit reduced selection, permitting accumulation of greater sequence diversity within species and creating the potential for more rapid divergence between species relative to similar genes expressed in every individual in every generation (Snell-Rood et al. in press a). Recent work on bacterial quorum-sensing genes, which are induced only in generations exposed to certain population densities, provides support for both predictions (Van Dyken & Wade, in press). Current studies on horned beetles are now seeking to apply the same framework to polyphenic insects. Specifically, the recent development of genomic resources for Onthophagus beetles has permitted the identification of genes whose expression is specific to horned or hornless male morphs, or shared between morphs or sexes. Surveys of sequence variation within and between species are now under way to determine whether morph-specific genes harbour greater levels of within-species nucleotide diversity and diverge faster between species than similar morph-shared genes. Preliminary data on a small number of gene pairs are consistent with these predictions, but it remains to be seen whether this will emerge as a general pattern (E. C. Snell-Rood & A. Moczek 2009, unpublished data). If so, accelerated evolution in modular plasticity genes relative to constitutively expressed genes would have far-reaching consequences for our understanding of the costs, limits and consequences of modular plasticity.
For example, by virtue of accelerating mutation accumulation and divergence, genes underlying modular plasticity may be more likely to evolve new functions compared with similar non-plastic genes. If correct, plasticity genes may be predisposed to contribute disproportionally to sub- and neo-functionalization events during organismal evolution (Demuth & Wade 2007; Cruickshank & Wade 2008). By the same argument, however, accelerated mutation accumulation may render modular plasticity genes more prone to acquire deleterious mutations and evolve into pseudogenes. This should be more pronounced in modules that are expressed rarely when compared with more commonly induced modules, and as such may place an upper limit on the range of plasticity that can be accommodated through modular plasticity: modules whose expression occurs too infrequently may simply suffer too many mutations to be functionally maintainable within populations (Snell-Rood et al. in press a). It is interesting to speculate whether it is due to relaxed selection and mutation accumulation that most polyphenisms are limited to the expression of two alternative morphs, with the conspicuous exception of castes in social insects. Here, colony-level selection, a level of selection absent in non-social insect polyphenisms, may provide an effective mechanism limiting mutation accumulation in caste-specific genes, permitting the maintenance of more than two discrete castes (Snell-Rood et al. in press a). Lastly, even though relaxed selection may impose constraints on the range of plasticity that can be accommodated through modularity in gene expression, it may at the same time create opportunities for alternative genetic underpinnings of plasticity to evolve, such as integrated networks where the same suites of genes, but via altered types of interactions, contribute to the expression of different phenotypes in different environments. While many of these implications, though fundamental, are clearly speculative at this point, recent methodological and theoretical advances promise that critical empirical evaluations will follow soon.
3. Conclusions
Phenotypic plasticity and polyphenic development contribute to organismal diversification on a variety of levels of biological organization. On one level, phenotypic plasticity contributes traits such as developmental thresholds and switches for evolutionary processes to act on. Evolutionary changes in such traits may contribute to organismal diversification, including speciation, without changes in trait expression per se. Similarly, evolutionary changes in polyphenic development can bring about correlated changes in other traits, in turn increasing the possibility for trade-offs to shape subsequent patterns of diversification. Lastly, the mutation-modularity trade-off hypothesis provides a plausible mechanism explaining how modularity in gene expression underlying plasticity can result in relaxed selection and increased mutation accumulation in highly modular genes, with the potential to contribute to both the evolutionary range and limits of modular phenotypic plasticity.
Acknowledgements
Tami Cruickshank and Emilie Snell-Rood provided helpful comments on earlier drafts of this chapter. Research presented here was supported by National Science Foundation grants IOS 0445661 and IOS 0718522.
Footnotes
One contribution of 12 to a Theme Issue ‘From polyphenism to complex metazoan life cycles’.
References
- Abouheif E., Wray G. A.2002Evolution of the gene network underlying wing polyphenism in ants. Science 297, 249–252 (doi:10.1126/science.1071468) [DOI] [PubMed] [Google Scholar]
- Arrow G. H.1951Horned beetles The Hague, The Netherlands: Junk [Google Scholar]
- Balthasar V.1963Monographie der Scarabaeidae und Aphodiidae der palaearktischen und orientalischen Region (Coleoptera: Lamellicornia). Band 2, Coprinae. Prag: Verlag der tschechoslowakischen Akademie der Wissenschaften [Google Scholar]
- Barker M. S., Demuth J. P., Wade M. J.2005Maternal expression relaxes constraint on innovation of the anterior determinant, bicoid. PloS Genet. 1, 527–530 (doi:10.1371/journal.pgen.0010057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw W. E., Quebodeaux M. C., Holzapfel C. M.2003Circadian rhythmicity and photoperiodism in the pitcher-plant mosquito: adaptive rsponse to the photic environment or correlated response to climatic adaptation? Am. Nat. 161, 735–748 (doi:10.1086/374344) [DOI] [PubMed] [Google Scholar]
- Charlesworth B.1994Evolution in age-structured populations. Camb. Stud. Math. Biol. 13, 1–306 [Google Scholar]
- Crespi B. J.1988Adaptation, compromise and constraint: the development, morphometrics, and behavioral basis of a fighter-flier polymorphism in male Hoplothrips karni (Insecta: Thysanoptera). Behav. Ecol. Sociobiol. 23, 93–104 (doi:10.1007/BF00299892) [Google Scholar]
- Cruickshank T., Wade M. J.2008Microevolutionary support for a developmental hourglass: gene expression patterns shape sequence variation and divergence in Drosophila. Evol. Dev. 10, 583–590 (doi:10.1111/j.1525-142X.2008.00273.x) [DOI] [PubMed] [Google Scholar]
- Darwin C.1871The descent of man and selection in relation to sex. London, UK: John Murray [Google Scholar]
- Demuth J. P., Wade M. J.2007Maternal expression increases the rate of bicoid evolution by relaxing selective constraint. Genetica 129, 37–43 (doi:10.1007/s10709-006-0031-4) [DOI] [PubMed] [Google Scholar]
- Donnell D. M., Strand M. R.2006Caste-based differences in gene expression in the polyembryonic wasp Copidosoma floridanum. Ins. Biochem. Mol. Biol. 36, 141–153 (doi:10.1016/j.ibmb.2005.11.009) [DOI] [PubMed] [Google Scholar]
- Dworkin I.2005Canalization, cryptic variation, and developmental buffering: a critical examination and analytical perspective. In Variation—a central concept in biology (eds Hallgrímsson B., Hall B. K.), pp. 131–158 Burlington, USA: Elsevier/Academic Press [Google Scholar]
- Eberhard W. G.1985Sexual selection and animal genitalia. Cambridge, MA: Harvard University Press [Google Scholar]
- Eberhard W. G., Huber B. A., Rodriguez R. L., Briceno R. D., Rodriguez V.1998One size fits all? Relationships between the size and degree of variation in genitalia and other body parts in twenty species of insects and spiders. Evolution 52, 415–431 (doi:10.2307/2411078) [DOI] [PubMed] [Google Scholar]
- Emlen D. J.1994Environmental control of horn length dimorphism in the beetle Onthophagus acuminatus (Coleoptera: Scarabaeidae). Proc. R. Soc. Lond. B 256, 131–136 (doi:10.1098/rspb.1994.0060) [Google Scholar]
- Emlen D. J.2000Integrating development with evolution: a case study with beetle horns. Bioscience 50, 403–418 (doi:10.1641/0006-3568(2000)050[0403:IDWEAC]2.0.CO;2) [Google Scholar]
- Emlen D. J., Nijhout H. F.1999Hormonal control of male horn length dimorphism in the dung beetle Onthophagus taurus (Coleoptera: Scarabaeidae). J. Ins. Physiol. 45, 45–53 (doi:10.1016/S0022-1910(98)00096-1) [DOI] [PubMed] [Google Scholar]
- Evans J. D., Wheeler D. E.1999Differential gene expression between developing queens and workers in the honey bee. Apis mellifera. Proc. Natl Acad. Sci. USA 96, 5575–5580 (doi:10.1073/pnas.96.10.5575) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J. D., Wheeler D. E.2001aExpression profiles during honeybee caste determination. Genome Biol. 2, research0001.1-0001.6. (doi:10.1186/gb-2000-2-1-research0001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J. D., Wheeler D. E.2001bGene expression and the evolution of insect polyphenisms. Bioessays 23, 62–68 (doi:10.1002/1521-1878(200101)23:1<62::AID-BIES1008>3.0.CO;2-7) [DOI] [PubMed] [Google Scholar]
- Gilbert S. F., Epel D.2009Ecological developmental biology: integrating epigenetics, medicine, and evolution. Sunderland, MA: Sinauer [Google Scholar]
- Grimaldi D., Engel M. S.2005Evolution of the insects. Cambridge, UK: Cambridge University Press [Google Scholar]
- Hoffman E. A., Goodisman M. A. D.2007Gene expression and the evolution of phenotypic diversity in social wasps. BMC Biol. 5, 23 (doi:10.1186/1741-7007-5-23) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojo M., Koshikawa S., Cornette R., Matsumoto T., Miura T.2005Identification of soldier-specific genes in the nasute termite Nasutitermes takasagoensis (Isoptera: Termitidae). Ent. Sci. 8, 379–387 (doi:10.1111/j.1479-8298.2005.00138.x) [Google Scholar]
- Hölldobler B., Wilson E. O.1990The ants. Berlin, Germany: Springer [Google Scholar]
- Holt R. D.1996Demographic constraints in evolution: towards unifying the evolutionary theories of senescence and niche conservatism. Evol. Ecol. 6, 433–447 (doi:10.1007/BF02270702) [Google Scholar]
- Howden H. F., Young O. P.1981Panamanian Scarabaeinae: taxonomy, distribution, and habits (Coleoptera: Scarabaeidae). Contr. Am. Ent. Inst. 18, 1–204 [Google Scholar]
- Jacobs W., Renner M.1988Biologie und Ökologie der Insekten Stuttgart, Germany: Gustav Fischer [Google Scholar]
- Judice C. C., Carazzole M. F., Festa F., Sogayar M. C., Hartfelder K., Pereira G. A. G.2006Gene expression profiles underlying alternative caste phenotypes in a highly eusocial bee, Melipona quadrifasciata. Ins. Mol. Biol. 15, 33–44 (doi:10.1111/j.1365-2583.2005.00605.x) [DOI] [PubMed] [Google Scholar]
- Klingenberg C. P., Nijhout H. F.1998Competition among growing organs and developmental control of morphological asymmetry. Proc. R. Soc. Lond. B 265, 1135–1139 (doi:10.1098/rspb.1998.0409) [Google Scholar]
- Kutsukake M., Shibao H., Nikoh N., Morioka M., Tamura T., Hoshino T., Ohgiya S., Fukatsu T.2004Venomous protease of aphid soldier for colony defense. Proc. Natl Acad. Sci. USA 101, 11 338–11 343 (doi:10.1073/pnas.0402462101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher M.1960Hormonal control of caste differentiation in termites. Ann. N. Y. Acad. Sci. 89, 549–563 (doi:10.1111/j.1749-6632.1960.tb27577.x) [Google Scholar]
- Matthews E. G.1972A revision of the scarabaeinae dung beetles of Australia. I. Tribe Onthophagini. Austr. J. Zool. Suppl. Ser. 9, 1–330 [Google Scholar]
- Moczek A. P.2003The behavioral ecology of threshold evolution in a polyphenic beetle. Behav. Ecol. 14, 831–854 (doi:10.1093/beheco/arg062) [Google Scholar]
- Moczek A. P.2005The evolution and development of novel traits, or how beetles got their horns. BioScience 11, 935–951 [Google Scholar]
- Moczek A. P.2008On the origin of novelty in development and evolution. Bioessays 5, 432–447 (doi:10.1002/bies.20754) [DOI] [PubMed] [Google Scholar]
- Moczek A. P.2009Developmental plasticity and the origins of diversity: a case study on horned beetles. In Phenotypic plasticity in insects: mechanisms and consequences (Ananthakrishnan T. N., Whitman D.), pp. 81–134 Plymouth, UK: Science Publishers [Google Scholar]
- Moczek A. P., Emlen D. J.2000Male horn dimorphism in the scarab beetle Onthophagus taurus: do alternative reproductive tactics favor alternative phenotypes? Anim. Behav. 59, 459–466 (doi:10.1006/anbe.1999.1342) [DOI] [PubMed] [Google Scholar]
- Moczek A. P., Nijhout H. F.2002Developmental mechanisms of threshold evolution in a polyphenic beetle. Evol. Dev. 4, 252–264 (doi:10.1046/j.1525-142X.2002.02014.x) [DOI] [PubMed] [Google Scholar]
- Moczek A. P., Nijhout H. F.2003Rapid evolution of a polyphenic threshold. Evol. Dev. 5, 259–268 (doi:10.1046/j.1525-142X.2003.03033.x) [DOI] [PubMed] [Google Scholar]
- Moczek A. P., Nijhout H. F.2004Trade-offs during the development of primary and secondary sexual traits in a horn dimorphic beetle. Am. Nat. 163, 184–191 (doi:10.1086/381741) [DOI] [PubMed] [Google Scholar]
- Moczek A. P., Hunt J., Emlen D. J., Simmons L. W.2002Evolution of a developmental threshold in exotic populations of a polyphenic beetle. Evol. Ecol. Rev. 4, 587–601 [Google Scholar]
- Moczek A. P., Cruickshank T. E., Shelby J. A.2006When ontogeny reveals what phylogeny hides: gain and loss of horns during development and evolution of horned beetles. Evolution 60, 2329–2341 [PubMed] [Google Scholar]
- Moran N. A.1991Phenotype fixation and genotypic diversity in the complex life cycle of the aphid Pemphigus betae. Evolution 45, 957–970 (doi:10.2307/2409702) [DOI] [PubMed] [Google Scholar]
- Nijhout H. F.1994Insect hormones Princeton, NJ: Princeton University Press [Google Scholar]
- Nijhout H. F.1999Control mechanisms of polyphenic development in insects. BioScience 49, 181–192 (doi:10.2307/1313508) [Google Scholar]
- Nijhout H. F.2003Development and evolution of adaptive polyphenisms. Evol. Dev. 5, 9–18 (doi:10.1046/j.1525-142X.2003.03003.x) [DOI] [PubMed] [Google Scholar]
- Nijhout H. F., Emlen D. J.1998Competition among body parts in the development and evolution of insect morphology. Proc. Natl Acad. Sci. USA 95, 3685–3689 (doi:10.1073/pnas.95.7.3685) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parzer H. F., Moczek A. P.2008Rapid antagonistic coevolution between primary and secondary sexual characters in horned beetles. Evolution 62, 2423–2428 (doi:10.1111/j.1558-5646.2008.00448.x) [DOI] [PubMed] [Google Scholar]
- Pereboom J. J. M., Jordan W. C., Sumner S., Hammond R. L., Bourke A. G. F.2005Differential gene expression in queen-worker caste determination in bumble-bees. Proc. R. Soc. B 272, 1145–1152 (doi:10.1098/rspb.2005.3060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigliucci M.2001Phenotypic plasticity Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- Pizzo A., Roggero A., Palestrini C., Moczek A. P., Rolando A.2008Rapid shape divergences between natural and introduced populations of a horned beetle partly mirror divergences between species. Evol. Dev. 10, 166–175 [DOI] [PubMed] [Google Scholar]
- Scharf M. E., Wu-Scharf D., Pittendrigh B. R., Bennett G. W.2003Caste- and development-associated gene expression in a lower termite. Genome Biol. 4, R62 (doi:10.1186/gb-2003-4-10-r62) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharloo W.1991Canalization: genetic and developmental aspects. Ann. Rev. Ecol. Syst. 22, 65–93 (doi:10.1146/annurev.es.22.110191.000433) [Google Scholar]
- Schlichting C. D., Pigliucci M.1995Gene regulation, quantitative genetics, and the evolution of reaction norms. Evol. Ecol. 9, 154–168 (doi:10.1007/BF01237754) [Google Scholar]
- Schlichting C. D., Pigliucci M.1998Phenotypic evolution: a reaction norm perspective. Sunderland, MA: Sinauer [Google Scholar]
- Shapiro A. M.1976Seasonal polyphenism. Evol. Biol. 9, 259–333 [Google Scholar]
- Shepherd B. L., Prange H. D., Moczek A. P.2008Some like it hot: body and weapon size affect thermoregulation in horned beetles. J. Ins. Physiol. 54, 604–611 (doi:10.1016/j.jinsphys.2007.12.007) [DOI] [PubMed] [Google Scholar]
- Simmons L. W., Emlen D. J.2006Evolutionary trade-off between weapons and testes. Proc. Natl Acad. Sci. USA 103, 16 346–16 351 (doi:10.1073/pnas.0603474103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell-Rood E. C., Moczek A. P.In press Horns, hormones, and hox genes: the role of development in the evolution of beetle contests. In Animal contests (eds Hardy I. C. W., Briffa M.), Cambridge, UK: Cambridge University Press [Google Scholar]
- Snell-Rood E. C., Papaj D. R.2009Patterns of phenotypic plasticity in common and rare environments: a study of host use and color learning in the cabbage white butterfly Pieris rapae. Am. Nat. 173, 615–631 (doi:10.1086/597609) [DOI] [PubMed] [Google Scholar]
- Snell-Rood E. C., Papaj D. R., Gronenberg W.2009Brain size: a global or induced cost of learning? Brain Behav. Evol. 73, 111–128 (doi:10.1159/000213647) [DOI] [PubMed] [Google Scholar]
- Snell-Rood E. C., Van Dyken J. D., Cruickshank T. E., Wade M. J., Moczek A. P.In press a. Toward a population genetic framework of developmental evolution: costs, limits, and consequences of phenotypic plasticity. Bioessays [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell-Rood E. C., Davidowitz G., Papaj D. R.In press b. Reproductive delays are linked to learning ability in a butterfly. Behav. Ecol. [Google Scholar]
- Stern D. L., Foster W. A.1996The evolution of soldiers in aphids. Biol. Rev. 71, 27–79 (doi:10.1111/j.1469-185X.1996.tb00741.x) [DOI] [PubMed] [Google Scholar]
- Tauber M. J., Tauber C. A.1972Geographic variation in critical photoperiod and in diapause intensity of Chrysopa carnea (Neuroptera). J. Ins. Physiol. 18, 25–29 (doi:10.1016/0022-1910(72)90061-3) [Google Scholar]
- Van Dyken J. D., Wade M. J.In press On the evolutionary genetics of conditionally expressed genes. Genetics [Google Scholar]
- Via S., Gomulkiewicz R., de Jong G., Scheiner S. M., Schlichting C. D., Van Tienderen P.1995Adaptive phenotypic plasticity: consensus and controversy. Trends Ecol. Evol. 10, 212–217 (doi:10.1016/S0169-5347(00)89061-8) [DOI] [PubMed] [Google Scholar]
- Wade M. J.1998The evolutionary genetics of maternal effects. In Maternal effects as adaptations (eds Mousseau T. A., Fox C. W.), pp. 5–21 Oxford, UK: Oxford University Press [Google Scholar]
- West-Eberhard M. J.2003Developmental plasticity and evolution New York, NY: Oxford University Press [Google Scholar]
- Wheeler D. E., Nijhout H. F.1983Soldier determination in Pheidole bicarinata: effect of methophrene on caste and size within castes. J. Ins. Physiol. 29, 847–854 (doi:10.1016/0022-1910(83)90151-8) [Google Scholar]
- Wheeler D. E., Nijhout H. F.1984Soldier determination in the ant Pheidole bicarinata: inhibition by adult soldiers. J. Ins. Physiol. 30, 127–135 (doi:10.1016/0022-1910(84)90116-1) [Google Scholar]
- Wilson E. O.1953The origin and evolution of polymorphism in ants. Quart. Rev. Biol. 28, 136–156 (doi:10.1086/399512) [DOI] [PubMed] [Google Scholar]
- Wilson E. O.1976Behavioral discretization and the number of castes in an ant species. Behav. Ecol. Sociobiol. 1, 141–154 (doi:10.1007/BF00299195) [Google Scholar]
- Yang A. S.2001Modularity, evolvability, and adaptive radiations: a comparison of the hemi- and holometabolous insects. Evol. Dev. 2, 59–72 (doi:10.1046/j.1525-142x.2001.003002059.x) [DOI] [PubMed] [Google Scholar]
- Zera A. J., Denno R. F.1997Physiology and ecology of dispersal polymorphism in insects. Ann. Rev. Entomol. 42, 207–231 (doi:10.1146/annurev.ento.42.1.207) [DOI] [PubMed] [Google Scholar]