Abstract
Objectives
We investigated whether the recovery of cultured human islets is improved through the addition of a p38α-selective mitogen activated protein kinase inhibitor, SD-282, to clinically used serum-free culture medium.
Methods
Immediately after isolation, islets were cultured for 24 hours in medium alone (control) or medium containing DMSO, 0.1 μM or 0.3 μM SD-282. Cytokine expression, apoptotic β cell percentage, and islet function were assessed post-culture.
Results
Expression of p38 and phosphorylated p38 in islets increased during culture. IL-6 mRNA expression in cultured islets, as well as IL-6, IL-8 and GM-CSF released into the medium were significantly reduced by adding SD-282. The apoptotic β cell percentage was significantly lower in islets cultured with 0.1 μM SD-282, but not 0.3 μM as compared to the control. Stimulation indices measured in vitro were higher, but without significance (p=0.06), and function of transplanted islets in diabetic NODscid mice was also better in 0.1 μM SD-282 group as compared to control.
Conclusions
Better islet function was obtained by adding 0.1 μM SD-282 to the serum-free culture medium. This improvement was associated with suppression of cytokine production and prevention of β cell apoptosis. However, this beneficial effect was diminished at a higher concentration.
Keywords: Human islet culture, p38α MAPK inhibitor, islet survival, SD-282
INTRODUCTION
Pancreatic islet transplantation has been proven to be an effective treatment for type 1 diabetes1–3. Clinical success of islet transplantation is influenced by the quality and quantity of transplanted islets. Therefore, it is necessary to preserve islet mass and function throughout donor pancreas procurement, preservation, islet isolation, culture, and transplantation. The objective of this study was to improve islet culture. After isolation, islets are cultured for one to two days prior to transplantation. This provides a period for islet quality assessment, selection of the best suited recipient, and initiation of the immunosuppression regimen. However, during culture pro-inflammatory cytokines are released, increasing the number of apoptotic islet cells, leading to significant islet loss. It is not uncommon for low post-culture islet recovery to lead to the cancellation of the transplantation. Development of a culture method that prevents islet loss and preserves β cell function is vital for transplant success 4, 5.
Pro-inflammatory cytokines and chemokines released from activated macrophages and monocytes are known to damage β cells 6. Brain death of organ donors induces macrophage infiltration into various tissues, including the pancreas and islets 7. These macrophages activate the pro-inflammatory signaling pathway and up-regulate cytokine gene expression, such as tumor necrosis factor alpha (TNF-α), interleukin (IL)-1β, and IL-6 8. Other possible sources of inflammatory molecules in the pancreas are endothelial, acinar, and even islet cells themselves. Activated vascular endothelial cells can produce IL-1β, IL-6, and IL-8 9. Pancreatic acinar cells can express TNF-α, IL-6, chemokines, monocyte chemotactic protein-1 (MCP-1), and macrophage inflammatory protein-2 10. Islet cells are known to produce IL-1β and TNFα 11. Suppression of cytokine release would not only prevent islet damage and maintain an islet mass, but also reduce inflammatory responses following transplantation 12–14.
P38 mitogen activated protein kinase (MAPK) participates in a signaling cascade controlling cellular responses to cytokines and stress15. There are four isoforms of p38 MAPK: α, β, γ, and δ14. P38α, which regulates inflammatory gene expression, is strongly expressed in monocytes, macrophages, CD4+ T cells, neutrophils, and endothelial cells 16, 17. Inhibitors of p38 MAPK are known to suppress cytokine production, such as lipopolysaccharide (LPS)-mediated IL-1β and TNF-α production by macrophages, leading to apoptosis of activated macrophages and neutrophiles 18, 19. In β cells, p38 MAPK is activated by its upstream MAPK kinase (MKK3/6) and p38 autophosphorylation, leading to β cell death 20. IL-1-induced nitric oxide synthesis in β cells is also associated with p38 MAPK activation and cell death 21. Pro-inflammatory cytokines released from endothelial or acinar cells are expected to cause β cell apoptosis and activate resident macrophages in the islet. The release of cytokines and chemokines by endothelial and acinar cells can be suppressed by a p38 MAPK inhibitor 10, 22. Since a variety of environmental stresses activate p38 MAPKs, they may also be activated by cold-preservation of the pancreata and warm digestion of the tissue during islet isolation. Exposure of purified islets to cytokines also activate p38 MAPKs 23, 24.
We have previously shown that the p38 inhibitor, SB203580 suppresses IL-1β and TNF-α production in human islets stimulated by LPS and inflammatory cytokines in vitro and treatment of islets with SB203580 prior to transplantation prevents primary graft non-function 11. Our series of investigations with SB203580 have shown that intra-ductal infusion into canine pancreata reduces β cell apoptosis after cold preservation and during islet isolation, significantly increasing islet yield25. The addition of SD-282, a selective p38α MAPK inhibitor, to cryopreservation solution resulted in significant improvement of quality and quantity of cryopreserved human islets after thawing 26.
The present study is a continuation of this series of investigations to test the effectiveness of a p38α MAPK inhibitor on cultured islets. We added SD-282 to the serum-free culture medium routinely used for transplanted islets. SD-282 suppressed the pro-inflammatory cytokine release and improved islet recovery. However, this effect was dose dependent and the optimal concentration must be used.
MATERIALS AND METHODS
Chemical description of SD-282
SD-282 is an indole-5-carboxamide ATP-competitive inhibitor of p38α MAPK (a gift from Scios Inc., Mountain View, CA) and was designed as a potent, selective p38α inhibitor of acceptable pharmaceutical properties 27.
Human islet culture and counting
Research human islets were supplied immediately after isolation by Southern California Islet Cell Resources Center, Beckman Research Institute of the City of Hope through the approval of Administrative and Bioinformatics Coordinating Center (ABCC, Duarte, CA). The use of human islets in this study was approved by the Institutional Review Committee of the City of Hope. Islet preparations of greater than 70% purity were used. Immediately after isolation, islets were cultured for 24 hours in serum-free CMRL-based medium 28 (Mediatech Inc., Holly Hill, FL) containing 2.5% human serum albumin. SD-282 was dissolved in dimethyl sulfoxide (DMSO, Sigma, St. Louis, MO) and added to the culture medium. Four different culture medium conditions were used: 1) culture medium alone (control); 2) culture medium supplemented with DMSO vehicle which contained DMSO in the amount equivalent to that contained in 0.3 μM SD-282; 3) culture medium supplemented with 0.1 μM SD-282; and 4) culture medium supplemented with 0.3 μM SD-282. To assess islet recovery, an aliquot of 10,000 islet equivalents (IEQ), the number of islets equivalent to an average of 150 μm in diameter, per group were prepared and cultured in respective culture media for 24 hours at 37°C in 5% CO2 and air. At the end of culture, islets were collected and suspended in 15 mL medium and 100 μL of sample, in triplicate, was taken from each suspension to count IEQ by diphenylthiocarbazone (Dithizone, Sigma) staining (n=3).
Western blot
Pre-culture, 2 hour- and 24 hour-cultured islets from each group (500 IEQ/sample) were harvested, washed twice with ice cold phosphate buffered saline (PBS) and stored at −80°C until use. Islet cell lysis and western blot was performed as previously described26. Antibodies directed against p38 MAPK, phospho-p38, phospho-heat shock protein (HSP) 27, and β actin were obtained from Cell Signaling Technology (Beverly, MA).
Assessment of cytokine release in culture medium during 24-hour islet culture
Culture medium supernatant from islets cultured at 500 IEQ/mL (n=7/group) was collected after 24 hours, frozen, and stored at −20°C until the day of the assay. The release of cytokines (IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, Interferon (IFN)-γ, TNF-α, and GM-CSF) in culture medium was measured using the Luminex™ assay kit, Human Cytokine10-plex (Biosource International, Camarillo, CA) using Bio-plex (Luminex ™ 100, Bio-Rad, Hercules, CA). The release of MCP-1 was measured with BD OptEIA™ Human MCP-1 Enzyme-Linked Immunosorbent Assay (ELISA) Kit (BD Biosciences, San Diego, CA).
mRNA expression assessed by quantitative reverse transcription polymerase chain reaction (RT-PCR)
RNA was extracted from pre-culture and 24-hour cultured islets using TRI REAGENT (Molecular Research Center, Inc., Cincinnati, OH). A total of 2.0 μg RNA from each sample was reverse-transcribed into first-strand cDNA using the SuperScript III First-Strand Synthesis System (Invitrogen, Carlsbad, CA). The final cDNA products were diluted by adding 60 μL H2O. The relative expression of human IL-1β, TNF-α, IL-6, IL-8, GM-CSF, MCP-1, CD14, MAC-1 (CD11b), intercellular adhesion molecule-1 (ICAM-1), platelet/endothelial cell adhesion molecule-1 (PECAM-1), and insulin mRNA in pre- and post-cultured islets (n=6/group) was measured by RT-PCR using TaqMan® Gene Expression Assays (Applied Biosystems, Foster City, CA). Assays were done in 50 μL duplicate reactions containing TaqMan® Universal PCR Master Mix, 20X TaqMan® Gene Expression Assay Mix and 2 μL cDNA according to the manufacturer’s instructions. All PCR reactions were performed in a Real Time PCR 7300 system and results were analyzed with the Sequence Detection Software version 1.3 of the system (Applied Biosystems, Foster City, CA) using the 2−ΔΔCT method. Human β-actin was used as an endogenous control gene to correct for variations in input RNA and cDNA amplification of different samples.
Insulin release assay in vitro using a perifusion system
Islet aliquots containing approximately 150 IEQ were cultured with either medium alone (control) or medium containing 0.1 μM SD-282 for 24 hours (n=6). In some experiments, 150 IEQ were handpicked and cultured for 24 hrs in each condition (n=3). After culture, islets were incubated overnight in RPMI 1640 medium containing 3 mM glucose supplemented with 10% fetal bovine serum at 37°C in 5% CO2 plus air. Insulin release in response to high glucose stimulation was tested using a perifusion system following the standard operating procedure of the Southern California Islet Cell Resources Center. Islets were placed in a small column between Cytodex™ beads (Sigma) and perifused at 37°C using basal and stimulation buffers. The basal buffer was Krebs-Ringer’s buffer containing 1% human serum albumin and 3 mM glucose. Stimulation buffer contained 16.8 mM glucose. Buffers were perfused at a rate of 0.7 mL/min. After a 30 minutes wash with basal buffer, the effluent was collected in 1 minute fractions for the next 10 minutes before switching to stimulation buffer. The effluent from the stimulation buffer was collected for 20 minutes at 1 minute intervals and then the buffer was changed again to basal buffer. Insulin content of each effluent was measured using an ELISA kit for human insulin (ALPCO, Salem, NH). The stimulation index was calculated by dividing the total insulin amount released in stimulation buffer by the total insulin amount released in basal buffer for the same time period (n=6/group).
Analysis of β cell apoptosis by Laser Scanning Cytometry (LSC)
Islets obtained before and after 24-hour culture were fixed in 10% formalin, embedded in paraffin, and processed into 5 μm sections by the City of Hope Anatomical Pathology Core facility. These paraffin sections were stained for terminal uridine deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) using the Apop Tag plus Fluorescein In Situ Apoptosis Detection Kit (Chemicon, Temecula, CA) followed by immunostaining for insulin using a guinea pig anti-human insulin primary antibody (DAKO Carpinteria, CA) and a Cy5-conjugated secondary antibody (Jackson Immuno-Research, West Grove, PA). All sections were counterstained for DNA with 4′-6-Diamidino-2-phenylindole (DAPI, Sigma). To evaluate β cell apoptosis, duplicate slides (n=5/group) were scanned through a 40X objective at a 0.5 μm step using the iCys laser scanning cytometer and the iCys 3.2.5 software (Compucyte, Cambridge, MA). A minimum 5303 and maximum 30000 of nuclei were counted per sections. The insulin-positive cells were detected using the 633 nm laser. TUNEL nuclear staining was recorded using a 488 nm laser. Cells co-staining for insulin and TUNEL were designated as apoptotic β cells and their percentage was obtained from the histogram. The percentage of apoptotic β cells was calculated by dividing the insulin and TUNEL double-positive cell number by the total insulin-positive cell number.
Assessment of in vivo islet function in diabetic NODscid mice
To assess the effect of SD-282 on islet function in vivo, human islets cultured with SD-282 were transplanted into diabetic mice. Ten- to twelve-week old male NODscid mice obtained from the Animal Resources Center of Beckman Research Institute of the City of Hope were used as islet recipients. Mice were rendered diabetic by intraperitoneal injections of 50 mg/kg streptozotocin (Sigma) on three consecutive days and those that exhibited hyperglycemia (>350 mg/dL) for two consecutive days were used as recipients. In these mice, 1200 IEQ/mouse reversed diabetes at a 50 % success rate and was considered a marginal islet number in our hand. Freshly isolated human islets were cultured in medium with or without 0.1 μM SD-282. After 24 hours, islets were collected and a marginal number of islets was transplanted under the left kidney capsule of a diabetic mouse (total n=6 each in control and 0.1 μM SD-282 groups from two different islet preparations). Thereafter, blood glucose levels were measured 2–3 times weekly. Recipient mice that maintained a blood glucose <200 mg/dL were considered to have reversed diabetes. Twenty-seven days after transplantation an intraperitoneal glucose tolerance test was performed by injecting 2 g/kg glucose after the mice were fasted overnight. At the completion of the test, left nephrectomy was performed to remove the graft and confirm graft dependence. Animal procedures followed protocols approved by the Research Animal Care Committee of the City of Hope/Beckman Research Institute.
Statistical analysis
All results are expressed as mean ± standard error of the mean. Differences between the two groups were compared using a paired, two-tailed Student’s t-test. p<0.05 was considered statistically significant.
RESULTS
p38 expression increased during islet culture and p38 substrate, phospho-HSP27, was reduced by addition of SD-282 in culture
Activation of p38MAPK in pre-culture, 2 hour- and 24 hour-culture islets were assessed by western blot. Expression of p38 normalized by β actin increased after 2 hours of control culture as compared to pre-culture (0.42±0.51 pre-culture vs. 1.51±0.30 2 hours control culture). Similarly, phospho-p38 expression also increased during 2 hours culture (0.11±0.05 pre-culture vs. 1.69±0.28 2 hours culture control) (Fig. 1A). However, the ratio of p38 phosphorylation to total p38 did not change between pre-culture and control, post-culture islets. Furthermore, the addition of SD-282 to the culture medium did not significantly change phosphorylation levels of p38 (Fig. 1B). These results were consistent with our previous observation that phosphorylation of p38 was not changed by SD-282 26. However, the attenuation of p38 pathway activation by SD-282 was demonstrated through the diminished phosphorylation of the p38 substrate, HSP27 (Fig. 1C). The ratio of p-HSP27 to total p38 was significantly lower in 24 hour cultures containing SD282 as compared to culture medium alone or medium containing DMSO.
Fig. 1. p38 expression increased during islet culture, as confirmed by Western blot.
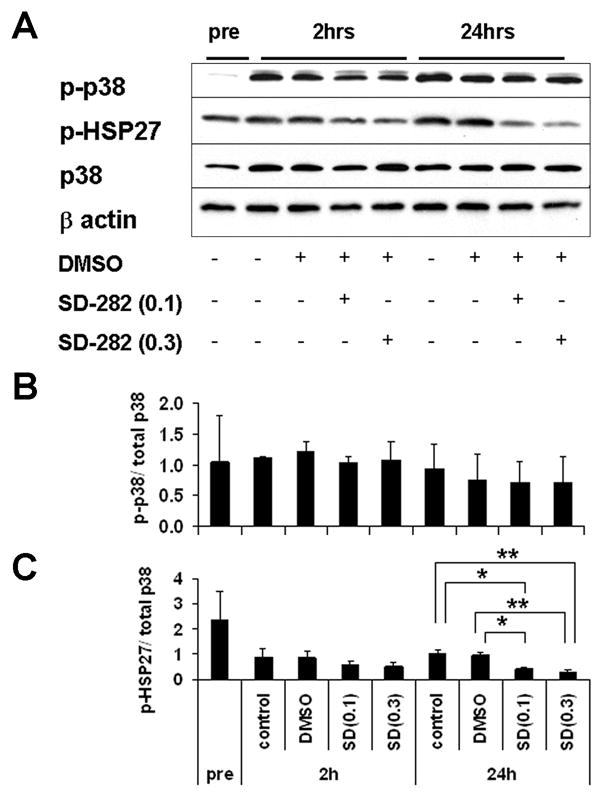
Western blot analysis of p38MAPK activation in pre-culture, 2h-, 24h-culture islets with or without SD-282. A: total p38, phosphorylated p38, phosphorylated HSP-27 and β actin in pre- and post-cultured islets. The blot is representative of 3 tests using different islet batches; B: An average level of phophorylated p38 normalized by total p38 in each culture condition at each time points (n=3); C: An average level of p38 substrate, phosphorylated HSP-27, in each culture condition at each time points (n=3, *p<0.01 and **p<0.005).
SD-282 suppressed IL-6, IL-8, and GM-CSF production during islet culture
Culture medium samples were analyzed to determine the effect of SD-282 on cytokine and chemokine production during 24 hour islet culture. SD-282 at both 0.1 μM and 0.3 μM concentrations significantly reduced the release of IL-6, IL-8, and GM-CSF. SD-282 treatment might also inhibit IL-1β release, but the measured levels were too low to withdraw a reliable conclusion. IL-2, IL-4, IL-5, IL-10, IFN-γ, and TNF-α were not detected in any culture medium samples. MCP-1 was detected in all islet culture media, but SD-282 had no effect (Table 1).
Table 1.
Effect of p38 inhibitor on IL-6, IL-8, GM-CSF, IL-1β, and MCP-1 release by human islets in culture medium
| Controls |
SD-282 |
|||
|---|---|---|---|---|
| (pg/mL) | Medium | DMSO | 0.1 μM | 0.3 μM |
| IL-6 | 1003±196 | 750±181 | 192±42b, d | 131±29b, d |
| IL-8 | 3602±701 | 2998±745 | 1824±558 | 1722±604a, c |
| GM-CSF | 61±10 | 49±8 | 24±4a, c | 20±4b, d |
| IL-1β | 53±11 | 43±9 | 30±9 | 24±8 |
| MCP-1 | 1970±361 | 2020±301 | 2343±315 | 2360±324 |
Cytokines produced during islet culture were measured by Luminex™ assay and ELISA. Data are shown as mean ± standard error for seven different preparations.
p<0.05 and
p<0.001 vs. Medium,
p<0.05 and
p<0.01 vs. DMSO (vehicle)
SD-282 suppressed IL-6 and IL-8 mRNA expression in cultured islets
The expression of several pro-inflammatory cytokine genes was analyzed in pre- and post-cultured islets by quantitative RT-PCR. In Table 2, mRNA expression levels in cultured islets were normalized to the mRNA level of the same gene in corresponding pre-culture islets. The expression of IL-1β and TNF-α was low in all groups with no significant differences between the SD-282-treated and untreated groups. IL-6 expression was up regulated in post-culture control islets, showing a 16.05±6.05 fold increase over the pre-culture level. This IL-6 up-regulation was significantly suppressed by the addition of SD-282 (3.43±1.18 fold increase with 0.1 μM SD-282 and 3.00±0.89 fold increase with 0.3 μM SD-282, SD-282 vs. control: p<0.05). IL-8 expression also increased in control islets during culture, reaching 10.32±3.69 fold of the pre-culture level (p<0.05). The increase of IL-8 expression in post-culture SD-282 groups were approximately 5-fold of the pre-culture, but no statistical significance was observed between the control and SD-282 treated islets after 24 hour-culture. Expression of GM-CSF increased 7.60± 1.65 fold after culture in all islets with or without SD-282. We also measured the expression of macrophage markers, CD14 and MAC-1, in an attempt to determine the effect of SD-282 on these cells. The expression of CD14 was similar in pre- and post-cultured islets, while the MAC-1 expression significantly decreased during culture in the DMSO and SD-282 groups (vs. pre-culture). This decrease was not noticed in the post-culture control islets. There was no effect of SD-282 on MCP-1. The expression of insulin mRNA significantly decreased in all the groups (pre-culture vs. control, p<0.001; vs. 0.1μM SD-282, p<0.05 and vs. 0.3 μM SD-282, p<0.01).
Table 2.
Expression of mRNA in cultured islets relative to pre-culture level
| Controls |
SD-282 |
|||
|---|---|---|---|---|
| Medium | DMSO | 0.1μM | 0.3μM | |
| IL-6 | 16.05±6.05a | 13.54±4.38b | 3.43±1.18a, d | 3.00±0.89a, d |
| IL-8 | 10.32±3.69a | 10.96±5.16 | 5.56±2.75 | 5.10±2.50 |
| IL-1β | 0.42±0.12b | 0.43±0.12b | 0.41±0.13b | 0.34±0.10c |
| TNF-α | 0.20±0.04c | 0.22±0.05c | 0.20±0.05c | 0.25±0.07c |
| GM-CSF | 7.60±1.65b | 5.43±1.85a | 6.30±1.60b | 5.09±1.72a |
| MCP-1 | 1.25±0.68 | 1.24±0.55 | 0.93±0.28 | 0.96±0.43 |
| CD14 | 0.80±0.40 | 0.64±0.32 | 0.79±0.40 | 0.66±0.30 |
| MAC-1 | 0.65±0.29 | 0.37±0.06c | 0.56±0.15b | 0.57±0.19a |
| ICAM-1 | 1.80±0.86 | 1.57±0.80 | 1.35±0.56 | 1.51±0.63 |
| PECAM-1 | 1.06±0.19 | 1.75±0.77 | 1.59±0.45 | 1.51±0.74 |
| Insulin | 0.61±0.06c | 0.60±0.08c | 0.68±0.10a | 0.68±0.08b |
Expressions of cytokine genes in pre- and post-cultured islets from six different preparations were analyzed by quantitative RT-PCR. Data are shown as mean ± standard error.
p<0.05,
p<0.01 and
p<0.001 vs. pre-culture.
p<0.05 vs. medium and DMSO.
0.1 μM SD-282 culture maintained higher number of islets for 24 hours
Cultures started with 10,576±505 IEQ/group (n=3) of freshly isolated islets. After 24 hours in culture, islets from each group were collected separately and the recovered islet number was counted. The islet number did not significantly decrease in the control group (9955±1308 IEQ, 95±14% recovery as compared to pre-culture), DMSO-group (10352±1240 IEQ, 99±14% recovery), or the 0.1 μM SD-282 group (11139±649 IEQ, 114±4% recovery). There was no statistically significant difference between the control, DMSO and 0.1 μM SD-282 groups after 24 hours culture. In contrast, the islet number in the 0.3 μM SD-282 group was significantly lower, recovering only 82% of the initial number (8730±971 IEQ, 82±5% recovery, p<0.05 vs. pre culture). This indicates a toxic effect of SD-282 at this concentration.
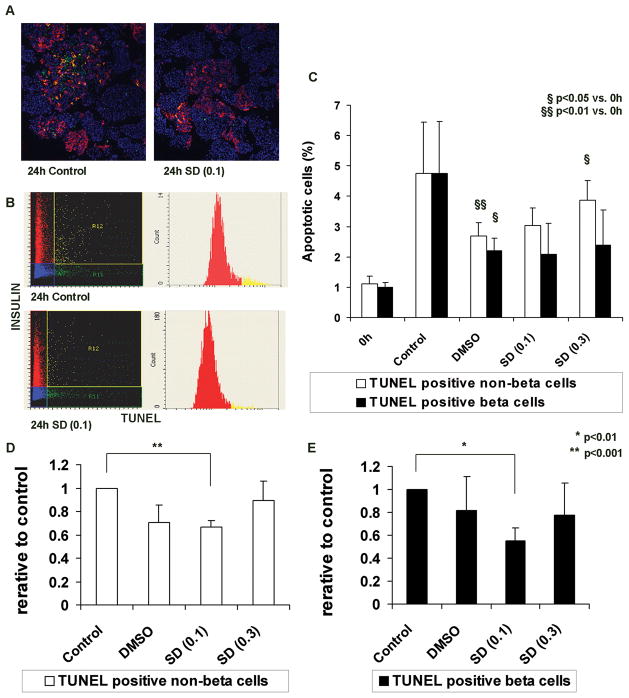
0.1 μM SD-282 protected cultured β cells from apoptosis
To detect apoptotic islet cells, islets from paraffin sections were stained for TUNEL and insulin, and analyzed by LSC. In figures 2A and 2B, cells double-stained with TUNEL and insulin represent β cells undergoing apoptosis, while cells stained for TUNEL but not insulin represent apoptotic, non-β cells. β cell and non-β cell apoptosis in the post-cultured islet preparations were compared to that of pre-culture. The mean apoptotic β cell percentage in pre-culture samples was 1.0±0.3%, which increased significantly after culture in the medium alone control (4.7±1.9%, p<0.05). However, the increase of apoptotic β cell percentage was less in DMSO cultures as compared to post-culture samples (2.2±0.4%, p=0.18 vs. control post culture). Addition of SD282 decreased β cell apoptosis, but at a level similar to the DMSO post-culture group (2.1±0.6% with 0.1 μM SD-282; 2.4±0.7% with 0.3 μM SD-282) (Figure 2C). The quality of islets was highly variable between islet batches, which was shown by the large standard deviation of percent apoptosis in 24 hour-cultured control islets. To reduce the influence of islet quality and demonstrate the effect of SD-282, the apoptotic β cell percentage of the SD-282 group after culture was divided by that of the corresponding post-culture control (Fig 2D and E). This analysis showed that SD-282 at a 0.1 μM concentration significantly decreased apoptosis of β cells (0.55±0.11, p<0.01) as well as non-β cells (0.66±0.05, p<0.001) as compared to the control. However, this protective effect was not observed at a concentration of 0.3 μM.
Fig. 2. β cell apoptosis in cultured human islets is prevented by supplementing the medium with 0.1 μM SD-282 as determined by Laser Scanning Cytometry.
Islet sections were labeled by TUNEL to identify apoptotic cells, immunostained for insulin to identify β cells, and quantified using laser scanning cytometry. A: Representative merged images of islets cultured for 24 hours (left – medium alone (control), right – medium supplemented with 0.1 μM SD-282). Green – TUNEL-positive, red – insulin-positive, yellow – TUNEL-insulin double-positive, and blue – DAPI DNA-staining; B: Scattergrams and histograms obtained by scanning the adjacent slides; C: Percentages of apoptotic cells apoptotic β cell percentages were calculated by dividing the TUNEL-insulin double-positive cell number by the total insulin-positive cell number in each section. TUNEL-positive/insulin-negative cells represent apoptotic non-β cells. Percentages of non-β cells were calculated by dividing the TUNEL-positive/insulin-negative cell number by the total number of non-β cells. After 24 hours culture, the mean apoptotic non-β cells (%) significantly increased in the DMSO (p<0.01) and 0.3 μM SD-282 (p<0.05) groups as compared to pre (0h). The mean apoptotic β cells (%) significantly increased in the DMSO (p<0.05) group as compared to pre (0h). To determine SD-282 effects, the relative ratio was obtained by dividing the post-culture apoptotic cell percentage by that of the corresponding medium alone control group; D: Relative ratios of apoptotic non-β cells – Culture medium supplemented with 0.1 μM SD-282 significantly prevented non-β cell apoptosis as compared to control (p<0.001); E: Relative ratios of apoptotic β cells – β cell apoptosis was significantly decreased with 0.1 μM SD-282 in the medium (p<0.01). This SD-282 effect was not observed at a concentration of 0.3 μM (n=5).
The above results indicate that the solvent, DMSO, possesses an islet protective effect. Since islets that will be transplanted to patients are cultured in a serum free medium (not containing DMSO), it is reasonable to compare the islets in the 0.1μM SD-282 group with the islets in control (culture medium alone) group rather than the DMSO group. This comparison will determine whether the current culture condition can be improved through the use of 0.1μM SD-282. Therefore, 0.1 μM SD-282 group was compared to the control group in the following in vitro and in vivo study.
Islets cultured with 0.1 μM SD-282 were more responsive to high glucose stimulation in vitro than control islets
The effect of SD-282 on islet function was assessed in vitro by insulin-release in response to high glucose stimulation in a perifusion system. Islet aliquots of >70% purity cultured with 0.1 μM SD-282 responded with a higher stimulation index (4.7±0.7) than the medium alone controls, however, this difference was not statistically significant (2.9± 0.2, p=0.06, n=6 each) (Fig. 3A, B). When islets were hand-picked and cultured, the stimulation index of 0.1 μM SD-282 islets (3.5±0.5) was similar to that of the control islets (3.9±0.9, n=3), indicating that contaminating cells, such as acinar cells, had a cytotoxic effect on islets cultured without SD-282. Insulin release of the 0.3 μM SD-282 islets was similar to or lower than the control culture islets (data not shown).
Fig. 3. Human islets cultured with 0.1 μM SD-282 respond to glucose stimulation in vitro with higher stimulation indices than controls.
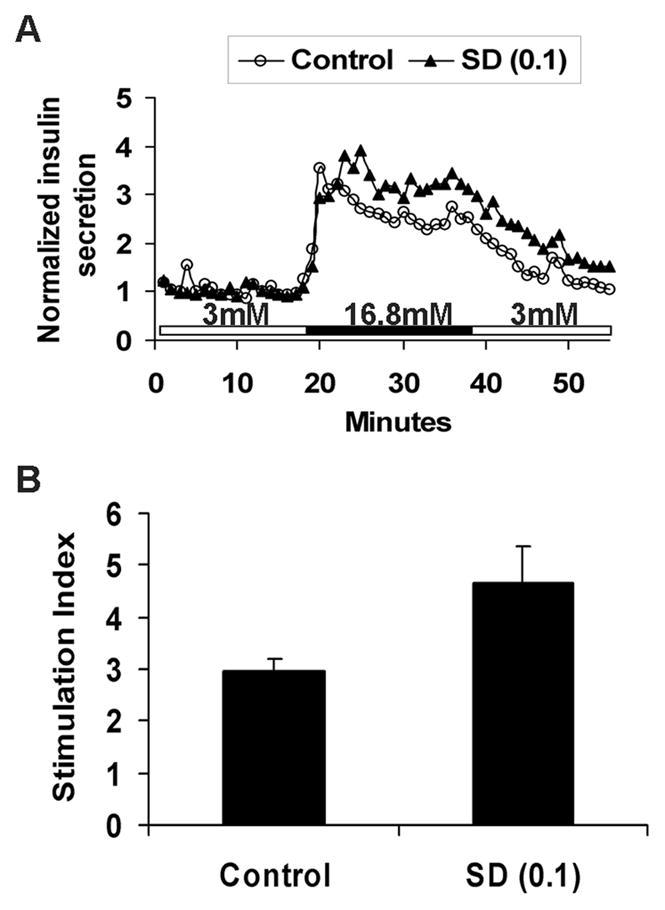
The effect of SD-282 on islet glucose responsiveness was assessed in vitro using an insulin release assay in a perifusion system. Insulin release profiles obtained from 150 IEQ cultured for 24 hours with medium alone or medium containing 0.1 μM SD-282. A: representative insulin release curve; B: Stimulation indices of control and SD-282-treated islets. SD-282-treated islets responded to high glucose with higher stimulation indices than untreated controls, but no statistical significance was obtained (p=0.06, n=6).
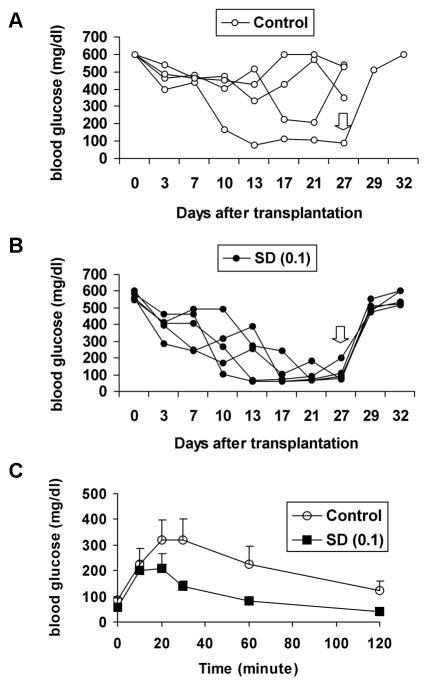
Human islets cultured with 0.1 μM SD-282 demonstrated improved function in vivo as compared to control islets
To assess in vivo islet function, a marginal number (1200 IEQ) of 24-hour cultured islets was transplanted into diabetic NODscid mice. All five mice receiving islets cultured with 0.1 μM SD-282 and only one of four mice receiving control culture islets reversed diabetes (Fig. 4A, B). One mouse that received control islets died one day after transplantation and was excluded from the experiment. The glucose disappearance curves obtained by the intraperitoneal glucose tolerance test of mice receiving 0.1 μM SD-282-treated islets were significantly better than mice receiving control islets (Fig. 4C). Removal of the islet grafts caused recurrence of diabetes in all mice.
Fig. 4. Human islets cultured with 0.1 μM SD-282 function better than controls in vivo after transplantation into diabetic NODscid mice.
Freshly isolated islets were cultured at 37°C either with or without SD-282. After 24 hours, a marginal number of islets (1200 IEQ) were transplanted under the renal capsule of a diabetic NODscid mouse. A: Blood glucose (mg/dl) of each animal in the control group after transplantation. B: Blood glucose (mg/dl) of each animal in the 0.1 μM SD-282 treated group. – All five mice receiving islets cultured with 0.1 μM SD-282 reversed diabetes by week 3 of transplantation, whereas only one of four mice receiving control islets reversed diabetes. Removal of the islet grafts (⇩) at twenty-seven days after transplantation caused recurrence of diabetes in all mice. C: Glucose tolerance curves obtained by intraperitoneal glucose tolerance tests on week 4 – The mice which received islets treated with 0.1μM SD-282 showed significantly better response to glucose challenge than the mice receiving control islets (n=4 for control group and n=5 for SD-282 group).
DISCUSSION
Transplantation of a large mass of high quality islets is essential for achieving insulin-independence in type 1 diabetic patients. The islet mass of a donor pancreas decreases throughout the islet preparation process and during culture. In this study, we attempted to modify the method of islet culture to improve outcome. For this purpose, the effectiveness of a p38α selective MAPK inhibitor, SD-282, was tested by supplementing the culture medium. The results showed that the addition of 0.1 μM SD-282 to the islet culture medium protects β cells from apoptosis and improves β cell function in vivo.
Inflammatory factors such as IL-6, IL-8, MCP-1, and GM-CSF are released from islets during culture 12, 29, 30 and MAPKs, in particular p38α, are known to play a significant role in the production of those cytokines. The p38α inhibitor, SD-282, has approximately a 14-fold selectivity for the α-isoform of p38 MAPK and a higher potency for p38α (IC50 =1.6nM) than the commonly used SB203580 (IC50 =56nM). SD-282 has been shown to regulate inflammatory gene expression in monocytes and macrophages, as well as suppress the production of TNF-α, IL-1β, IL-6, IL-8, and GM-CSF by human leukocytes in a dose-dependent manner 31, 32. SD-282 is specific as such even at a 50 μM concentration. SD-282 does not inhibit other MAPKs, extracellular signal-regulated kinases, or c-Jun N-terminal kinase activities 27. In our study, the release of IL-6, IL-8, and GM-CSF was detected in the standard islet culture medium. This cytokine release was significantly reduced by SD-282 at both 0.1 μM and 0.3 μM. In our study the affected cell population was not identified, however, Bottino et al.29 reported the possible involvement of β cells in IL-6 and MCP-1 production and the involvement of non-β islet cells in IL-8 production. Ehses et al.30 showed the co-localization of IL-8 and glucagon in isolated human islets, suggesting that α cells are also involved in cytokine production. With the speculation that macrophages and endothelial cells might also be the IL-8 source in the islets, we measured the mRNA levels of cell adhesion molecules ICAM-1 and PECAM-1 which are expressed by activated endothelial cells in a p38 MAPK-dependent manner 33, 34. However, their expression levels were similar between pre- and post-cultured islets and were not affected by SD-282. The expression of CD14 and MAC-1 mRNA was also similar between the control and SD-282 groups. Such negative results may be attributed to inappropriate test methods or timing and not necessarily support the absence of contaminating cells or their involvement in cytokine production.
The selective p38α MAPK inhibitor SD-282, at a concentration of 0.1 μM, prevented cytokine/chemokine production and reduced β cell apoptosis. However, the effect of 0.1 μM SD-282 itself on β cell apoptosis was shown to be marginal since no significant difference was observed between the 0.1 μM SD-282 and the DMSO alone group. DMSO itself had an effect on reducing β cell apoptosis, and therefore, the overall β cell protection by 0.1 μM SD-282 appears to be synergistic between DMSO and SD-282. Although DMSO has been used extensively as a commercial solvent and pharmacological agent, it is also known to have anti-inflammatory and antioxidant effects 35–38. DMSO has also been reported to inhibit NFκB activation 39, 40. The effect of DMSO on islet function has not yet been made clear. In our study, the concentration of DMSO used in DMSO group was 0.003%, which was less than 1/300 of concentration used in the reported study, but the effect of DMSO was still detectable in cultured islets.
SD-282 at a concentration of 0.1 μM maintained better β cell function than control islets in vitro. SD-282 cultured islets also showed a higher amount of insulin release in response to high glucose stimulation. In the experiment that used hand-picked islets, insulin released in response to glucose stimulation was similar between the SD-282 and control cultured islets. This suggests that the cytokines contributing to β cell destruction are released primarily by contaminating non-islet cells such as acinar cells. In vivo, diabetes was reversed in all five NODscid mice transplanted with a marginal number (1200 IEQ) of human islets cultured with 0.1 μM SD-282, whereas one of four mice with control islets. These in vivo results indicate that SD-282 not only prevents β cell apoptosis, but also supports islet function and thus SD-282 at this concentration has no adverse effect on islets. Despite the suppression of pro-inflammatory cytokine production at both 0.1 μM and 0.3 μM concentrations, 0.3 μM SD-282 was found to be harmful to human islets as indicated by the decreased insulin release in vitro and a failure to reverse diabetes in vivo (data not shown). Although p38 inhibitors have profound suppressive effects on inflammatory responses, p38 MAPK activation is also involved in other biological functions to maintain physiological cell activities and viability 41. Therefore, a use of p38 inhibitor must be carefully evaluated based on the cell type and determination of the optimal concentration and exposure time is required to maximize the anti-inflammatory effect while maintaining normal cell functions.
In summary, the addition of 0.1 μM SD-282 to the medium prevents β cell apoptosis and improves β cell function. Our data suggest the potential of this agent to improve human islet culture for clinical transplantation.
Acknowledgments
FUNDING SOURCES
This work was supported by a grant from the Nora Eccles Treadwell Foundation and NIH (U42 RR16607).
We gratefully acknowledge Sofia Loera and Tina Montgomery of the Pathology Laboratory and Dr. Michael Karos and Dr. Shu Mi of the Clinical Immunobiology Correlative Studies Laboratory (CICSL) at the City of Hope for their technical support. We also thank Dr. Kevin Ferreri at the City of Hope for his scientific advice.
ABBREVIATIONS
- DAPI
DNA with 4′-6-Diamidino-2-phenylindole
- ELISA
Enzyme-linked immunosorbent assay
- HSP27
Heat shock protein 27
- ICAM-1
Intercellular adhesion molecule-1
- IL
Interleukin
- INF
Interferon
- IVGTT
Intravenous glucose tolerance test
- JNK
c-Jun NH2-terminal kinase
- MAPK
Mitogen activated protein kinase
- LPS
Lipopolysaccharide
- LSC
Laser scanning cytometry
- MCP-1
Monocyte chemoattractant protein-1
- PECAM-1
Platelet/endothelial cell adhesion molecule-1
- RT-PCR
Real-time reverse transcription polymerase chain reaction
- TNF-α
Tumor necrosis factor-α
- TUNEL
Terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling
References
- 1.Marzorati S, Pileggi A, Ricordi C. Allogeneic islet transplantation. Expert Opin Biol Ther Nov. 2007;7(11):1627–1645. doi: 10.1517/14712598.7.11.1627. [DOI] [PubMed] [Google Scholar]
- 2.Ryan EA, Paty BW, Senior PA, et al. Five-year follow-up after clinical islet transplantation. Diabetes Jul. 2005;54(7):2060–2069. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro AM, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006 Sep 28;355(13):1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 4.Ihm SH, Matsumoto I, Zhang HJ, Ansite JD, Hering BJ. Effect of short-term culture on functional and stress-related parameters in isolated human islets. Transpl Int. 2008 Oct 13; doi: 10.1111/j.1432-2277.2008.00769.x. [DOI] [PubMed] [Google Scholar]
- 5.Berney T. Islet culture and counter-culture. Transpl Int. 2008 Nov 1; doi: 10.1111/j.1432-2277.2008.00794.x. [DOI] [PubMed] [Google Scholar]
- 6.Arnush M, Heitmeier MR, Scarim AL, Marino MH, Manning PT, Corbett JA. IL-1 produced and released endogenously within human islets inhibits beta cell function. J Clin Invest. 1998 Aug 1;102(3):516–526. doi: 10.1172/JCI844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toyama H, Takada M, Suzuki Y, Kuroda Y. Activation of macrophage-associated molecules after brain death in islets. Cell Transplant. 2003;12(1):27–32. doi: 10.3727/000000003783985205. [DOI] [PubMed] [Google Scholar]
- 8.Contreras JL, Eckstein C, Smyth CA, et al. Brain death significantly reduces isolated pancreatic islet yields and functionality in vitro and in vivo after transplantation in rats. Diabetes Dec. 2003;52(12):2935–2942. doi: 10.2337/diabetes.52.12.2935. [DOI] [PubMed] [Google Scholar]
- 9.Mantovani A, Bussolino F, Dejana E. Cytokine regulation of endothelial cell function. Faseb J May. 1992;6(8):2591–2599. doi: 10.1096/fasebj.6.8.1592209. [DOI] [PubMed] [Google Scholar]
- 10.Blinman TA, Gukovsky I, Mouria M, et al. Activation of pancreatic acinar cells on isolation from tissue: cytokine upregulation via p38 MAP kinase. Am J Physiol Cell Physiol Dec. 2000;279(6):C1993–2003. doi: 10.1152/ajpcell.2000.279.6.C1993. [DOI] [PubMed] [Google Scholar]
- 11.Matsuda T, Omori K, Vuong T, et al. Inhibition of p38 pathway suppresses human islet production of pro-inflammatory cytokines and improves islet graft function. Am J Transplant Mar. 2005;5(3):484–493. doi: 10.1046/j.1600-6143.2004.00716.x. [DOI] [PubMed] [Google Scholar]
- 12.Barbe-Tuana FM, Klein D, Ichii H, et al. CD40-CD40 ligand interaction activates proinflammatory pathways in pancreatic islets. Diabetes Sep. 2006;55(9):2437–2445. doi: 10.2337/db05-1673. [DOI] [PubMed] [Google Scholar]
- 13.Johansson U, Olsson A, Gabrielsson S, Nilsson B, Korsgren O. Inflammatory mediators expressed in human islets of Langerhans: implications for islet transplantation. Biochem Biophys Res Commun. 2003 Aug 29;308(3):474–479. doi: 10.1016/s0006-291x(03)01392-5. [DOI] [PubMed] [Google Scholar]
- 14.Ozasa T, Newton MR, Dallman MJ, Shimizu S, Gray DW, Morris PJ. Cytokine gene expression in pancreatic islet grafts in the rat. Transplantation. 1997 Oct 27;64(8):1152–1159. doi: 10.1097/00007890-199710270-00013. [DOI] [PubMed] [Google Scholar]
- 15.Raingeaud J, Gupta S, Rogers JS, et al. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995 Mar 31;270(13):7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- 16.Hale KK, Trollinger D, Rihanek M, Manthey CL. Differential expression and activation of p38 mitogen-activated protein kinase alpha, beta, gamma, and delta in inflammatory cell lineages. J Immunol. 1999 Apr 1;162(7):4246–4252. [PubMed] [Google Scholar]
- 17.Saklatvala J. The p38 MAP kinase pathway as a therapeutic target in inflammatory disease. Curr Opin Pharmacol Aug. 2004;4(4):372–377. doi: 10.1016/j.coph.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Park JM, Greten FR, Li ZW, Karin M. Macrophage apoptosis by anthrax lethal factor through p38 MAP kinase inhibition. Science. 2002 Sep 20;297(5589):2048–2051. doi: 10.1126/science.1073163. [DOI] [PubMed] [Google Scholar]
- 19.Park JM, Greten FR, Wong A, et al. Signaling pathways and genes that inhibit pathogen-induced macrophage apoptosis--CREB and NF-kappaB as key regulators. Immunity Sep. 2005;23(3):319–329. doi: 10.1016/j.immuni.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Makeeva N, Myers JW, Welsh N. Role of MKK3 and p38 MAPK in cytokine-induced death of insulin-producing cells. Biochem J. 2006 Jan 1;393(Pt 1):129–139. doi: 10.1042/BJ20050814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larsen CM, Wadt KA, Juhl LF, et al. Interleukin-1beta-induced rat pancreatic islet nitric oxide synthesis requires both the p38 and extracellular signal-regulated kinase 1/2 mitogen-activated protein kinases. J Biol Chem. 1998 Jun 12;273(24):15294–15300. doi: 10.1074/jbc.273.24.15294. [DOI] [PubMed] [Google Scholar]
- 22.Hashimoto S, Gon Y, Matsumoto K, et al. Selective inhibitor of p38 mitogen-activated protein kinase inhibits lipopolysaccharide-induced interleukin-8 expression in human pulmonary vascular endothelial cells. J Pharmacol Exp Ther May. 2000;293(2):370–375. [PubMed] [Google Scholar]
- 23.Abdelli S, Ansite J, Roduit R, et al. Intracellular stress signaling pathways activated during human islet preparation and following acute cytokine exposure. Diabetes Nov. 2004;53(11):2815–2823. doi: 10.2337/diabetes.53.11.2815. [DOI] [PubMed] [Google Scholar]
- 24.Paraskevas S, Aikin R, Maysinger D, et al. Activation and expression of ERK, JNK, and p38 MAP-kinases in isolated islets of Langerhans: implications for cultured islet survival. FEBS Lett. 1999 Jul 23;455(3):203–208. doi: 10.1016/s0014-5793(99)00882-0. [DOI] [PubMed] [Google Scholar]
- 25.Ito T, Omori K, Rawson J, et al. Improvement of canine islet yield by donor pancreas infusion with a p38 MAPK inhibitor. Transplantation. 2008 Jul 27;86(2):321–329. doi: 10.1097/TP.0b013e31817ef6c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Omori K, Valiente L, Orr C, et al. Improvement of human islet cryopreservation by a p38 MAPK inhibitor. Am J Transplant May. 2007;7(5):1224–1232. doi: 10.1111/j.1600-6143.2007.01741.x. [DOI] [PubMed] [Google Scholar]
- 27.Kapoun AM, Gaspar NJ, Wang Y, et al. Transforming growth factor-beta receptor type 1 (TGFbetaRI) kinase activity but not p38 activation is required for TGFbetaRI-induced myofibroblast differentiation and profibrotic gene expression. Mol Pharmacol Aug. 2006;70(2):518–531. doi: 10.1124/mol.105.021600. [DOI] [PubMed] [Google Scholar]
- 28.Bucher P, Mathe Z, Morel P, et al. Assessment of a novel two-component enzyme preparation for human islet isolation and transplantation. Transplantation. 2005 Jan 15;79(1):91–97. doi: 10.1097/01.tp.0000147344.73915.c8. [DOI] [PubMed] [Google Scholar]
- 29.Bottino R, Balamurugan AN, Tse H, et al. Response of human islets to isolation stress and the effect of antioxidant treatment. Diabetes Oct. 2004;53(10):2559–2568. doi: 10.2337/diabetes.53.10.2559. [DOI] [PubMed] [Google Scholar]
- 30.Ehses JA, Perren A, Eppler E, et al. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes Sep. 2007;56(9):2356–2370. doi: 10.2337/db06-1650. [DOI] [PubMed] [Google Scholar]
- 31.Koppelman B, Webb HK, Medicherla S, et al. Pharmacological properties of SD-282 - an alpha-isoform selective inhibitor for p38 MAP kinase. Pharmacology. 2008;81(3):204–220. doi: 10.1159/000112865. [DOI] [PubMed] [Google Scholar]
- 32.Smith SJ, Fenwick PS, Nicholson AG, et al. Inhibitory effect of p38 mitogen-activated protein kinase inhibitors on cytokine release from human macrophages. Br J Pharmacol Oct. 2006;149(4):393–404. doi: 10.1038/sj.bjp.0706885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsakadze NL, Sen U, Zhao Z, Sithu SD, English WR, D’Souza SE. Signals mediating cleavage of intercellular adhesion molecule-1. Am J Physiol Cell Physiol Jul. 2004;287(1):C55–63. doi: 10.1152/ajpcell.00585.2003. [DOI] [PubMed] [Google Scholar]
- 34.Zhang JJ, Kelm RJ, Biswas P, Kashgarian M, Madri JA. PECAM-1 modulates thrombin-induced tissue factor expression on endothelial cells. J Cell Physiol Feb. 2007;210(2):527–537. doi: 10.1002/jcp.20908. [DOI] [PubMed] [Google Scholar]
- 35.Colucci M, Maione F, Bonito MC, Piscopo A, Di Giannuario A, Pieretti S. New insights of dimethyl sulphoxide effects (DMSO) on experimental in vivo models of nociception and inflammation. Pharmacol Res Jun. 2008;57(6):419–425. doi: 10.1016/j.phrs.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 36.DeForge LE, Fantone JC, Kenney JS, Remick DG. Oxygen radical scavengers selectively inhibit interleukin 8 production in human whole blood. J Clin Invest Nov. 1992;90(5):2123–2129. doi: 10.1172/JCI116097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Repine JE, Pfenninger OW, Talmage DW, Berger EM, Pettijohn DE. Dimethyl sulfoxide prevents DNA nicking mediated by ionizing radiation or iron/hydrogen peroxide-generated hydroxyl radical. Proc Natl Acad Sci U S A Feb. 1981;78(2):1001–1003. doi: 10.1073/pnas.78.2.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xing L, Remick DG. Mechanisms of dimethyl sulfoxide augmentation of IL-1 beta production. J Immunol. 2005 May 15;174(10):6195–6202. doi: 10.4049/jimmunol.174.10.6195. [DOI] [PubMed] [Google Scholar]
- 39.Chang CK, Albarillo MV, Schumer W. Therapeutic effect of dimethyl sulfoxide on ICAM-1 gene expression and activation of NF-kappaB and AP-1 in septic rats. J Surg Res Feb. 2001;95(2):181–187. doi: 10.1006/jsre.2000.6033. [DOI] [PubMed] [Google Scholar]
- 40.Kelly KA, Hill MR, Youkhana K, Wanker F, Gimble JM. Dimethyl sulfoxide modulates NF-kappa B and cytokine activation in lipopolysaccharide-treated murine macrophages. Infect Immun Aug. 1994;62(8):3122–3128. doi: 10.1128/iai.62.8.3122-3128.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dambach DM. Potential adverse effects associated with inhibition of p38alpha/beta MAP kinases. Curr Top Med Chem. 2005;5(10):929–939. doi: 10.2174/1568026054985911. [DOI] [PubMed] [Google Scholar]