Abstract
Preoperative neoadjuvant chemotherapy (NAC) can significantly reduce tumour burden in patients with primarily unresectable chemosensitive tumours, allowing a more complete cytoreduction during debulking surgery and facilitating evaluation of tumour chemosensitivity, identification of appropriate treatment options and improvement of intervention protocols. In this study, we investigate, using immunohistochemistry, the impact of platinum/taxane-based NAC (NAC) on tumour-infiltrating lymphocytes (TILs) in advanced epithelial ovarian cancer (EOC) and their relationship with clinical outcome. All patients had clinical response, as shown by ascites volume and CA125 levels compared to pre-treatment findings. NAC intervention significantly increased CD4+, CD8+ and granzyme B+ infiltration while Foxp3+ accumulation remained unaffected. TILs were prognostically neutral for both progression-free survival (PFS) and overall survival (OS) before NAC. In contrast, after NAC, elevated granzyme B+ infiltration displayed a tendency for improved PFS (log-rank 0.064). Further, low Foxp3+ cell density was associated with longer PFS, as compared with strong Foxp3+ infiltration (median 20.94 vs. 11.24 months; log-rank 0.0001) and with improved OS (median 30.75 vs. 16.04 months, respectively; log-rank 0.056), demonstrating clear prognostic significance for PFS. In addition, high granzyme B+/Foxp3+ ratio post-NAC strongly correlated with improved PFS compared to low granzyme B+/Foxp3+ cell ratio (median 17.88 vs. 11.24 months, respectively), and showed to be a favourable prognostic factor for PFS (log-rank 0.014). Our findings indicate that NAC elicited an immunologic profile in which low immunosuppressive Foxp3+ infiltration and elevated numbers of activated granzyme B+ cells were significantly associated with EOC-specific PFS, suggesting a contribution of immunologic effects to improved clinical outcome.
Keywords: Neoadjuvant chemotherapy, Ovarian cancer, TILs, CD8/CD4 ratio, Foxp3, Granzyme B
Introduction
In industrialized countries, epithelial ovarian cancer (EOC) is the main cause of death in women with gynaecologic malignancies. Its bleak outcome is due to the fact that more than 70% of patients present with advanced-stage disease FIGO III/IV (International Federation of Gynaecology and Obstetrics). Although 70% of them respond well to standard management, primary radical tumour debulking followed by platinum/taxane-based chemotherapy, the majority of patients relapse and die of progressive disease [1]. Patients with FIGO stage IIIC and IV disease have an unfavourable prognosis with 5-year survival rates varying from 19 to 33% depending on resectability and response to chemotherapy [2], the extent of cytoreductive surgery and the amount of postoperative residual tumour being among the most significant prognostic factors impacting on survival [3]. However, complete tumour resection cannot be achieved in a substantial number of EOC patients. In patients with primarily unresectable chemosensitive tumours, neoadjuvant chemotherapy (NAC)—i.e. primary chemotherapy followed by tumour debulking—can considerably reduce tumour load, allowing a higher degree of cytoreduction by interval laparotomy [4–7]. In addition, NAC enables, by measuring residual disease in surgical specimens assessment of tumour chemosensitivity, definition of appropriate treatment options and improvement of intervention protocols.
Over the years, administration of systemic chemotherapy for the treatment of human malignancies has been believed to exert deleterious effects on the host’s defense system, particularly in advanced-stage patients who may as well be immune compromised. However, more recent data suggest that certain cytotoxic drugs can promote antitumour immune responses that contribute to the therapeutic effects of conventional therapy [8–10]. In this respect, recent work has provided evidence for synergistic immuno-adjuvant effects of taxane-based chemotherapy combined with tumour vaccines in experimental models and cancer patients [10]. It is by now a well-established notion that lymphocyte infiltration is a crucial regulation mechanism in oncogenesis and an indication of the host antitumour response [11, 12]. Although histopathologic alterations following NAC treatment of advanced-stage EOC have already been reported [13], and the prognostic significance of T cell infiltration has lately received considerable attention [14–20], the effect of NAC on tumour/host interactions in situ has, despite the increasing appreciation of this regime in the management of EOC, not been studied so far. Based on these premises, we conjectured that NAC-induced cytotoxicity may impact—by exerting local tissue disruption and/or release of pro-inflammatory factors by tumour cells themselves or by recruited immune cells—upon influx, phenotype or functional state of T cell infiltrates, and that these alterations may affect disease outcome and can thus be exploited therapeutically.
Therefore, we aimed at measuring immunohistochemically the extent of tumour-infiltrating CD4+, CD8+, granzyme B+ and Foxp3+ T cells as well as the ratio of CD8+/CD4+, CD4+/Foxp3+, CD8+/Foxp3+ and granzyme B+/Foxp3+ T cells in archival formalin-fixed paraffin-embedded biopsy specimens of 30 EOC patients prior to and after NAC treatment. Immune infiltrates were further analysed with regard to clinicopathologic variables known to impact on disease prognosis, including histological grade and presence of residual disease. This study is, to our knowledge, the first to demonstrate significant association between Foxp3 and granzyme B expression levels with clinical outcome in NAC-treated EOC patients. Our findings are particularly relevant in view of recent reports indicating that chemotherapeutic intervention and/or depletion or functional inactivation of Foxp3+ regulatory T cells (Tregs) may significantly enhance the efficacy of cancer vaccination protocols [9, 21].
Materials and methods
Patients and clinical parameters
From March 2003 to November 2007, a total of 93 patients with histologically confirmed EOC at FIGO IIIC and IV and ascites volume ≥500 mL were included into a prospective multicenter phase II study (Primovar) [7]. Trial objectives were evaluation of response to NAC and analysis of surgical outcome. Treatment consisted of either two or three of six cycles of i.v. administered carboplatin (AUC 5) and docetaxel (75 mg/m2) prior to cytoreductive surgery. Cytoreductive surgery was done within 4 weeks after the last scheduled chemotherapy cycle. All patients were regularly followed at 3-month intervals for the first 2 years and at 6-month intervals thereafter. The study received approval from the local institutional review board and all patients gave written informed consent.
The analysis of lymphocytic tumour infiltration and clinical follow-up was done on samples obtained from 30 out of 35 patients enrolled at our institution having adequate formalin-fixed, paraffin-embedded tumour specimens for immunohistochemistry, as well as complete clinicopathologic data. Intraperitoneal biopsies were collected by laparoscopy or laparotomy prior to chemotherapy and at debulking laparotomy at the Department of Gynaecology and Obstetrics of the Bonn University Medical Centre.
Immunohistochemistry of tissue specimens
Tissue samples were fixed in formalin and embedded in paraffin by standard procedures. For immunostaining, 2-μm sections mounted on polylysine-coated slides were deparaffinized, rehydrated, subjected to a heat-induced epitope retrieval step in a high-pressure cooker (30 min in 10 mM sodium citrate buffer, pH 6.0) and rinsed in Tris-buffered saline pH 7.4 for 20 min at room temperature. Endogenous peroxidase activity was blocked by 7.5-min incubation in Dako REAL™ Peroxidase Blocking Solution. Immunostaining was performed by incubating the slides at 4°C over night with mouse monoclonal antibodies against CD4 (clone 1F6, 1:120; Novocastra, Newcastle Upon Tyne, UK), CD8 (clone C8/144B, 1:25; DakoCytomation, Hamburg, Germany), Foxp3 (clone 236A/E7, 1:50; ebioscience, San Diego, USA) and granzyme B (clone 11F1, 1:20, Axxora, Lörrach, Germany). Labelling was detected using the Dako REAL™ Detection System, Streptavidin Peroxidase/AEC according to the manufacturer’s instructions. For Foxp3, tonsillar tissue with follicular hyperplasia showing T cells in the interfollicular area with nuclear antigen expression served as positive control. Negative controls were done by omitting the primary antibody. The stained sections were then counterstained with hematoxylin, rinsed with water and mounted with glycerol gelatine.
Quantitative assessment of tumour-infiltrating lymphocytes (TILs)
Stained TILs were assessed on representative, independent high power magnification-scanned (400×) areas of serial sections with the highest TIL density. These zones were digitally photographed, and to minimize the heterogeneous distribution of positive cells, a minimum of four high power fields (1 hpf = 0.237 mm2) for each patient sample was selected, counted manually at least three times by two different observers blinded for the patients’ clinical data (EB, NF) and the averaged counts in each case was statistically evaluated. Only viable tissue was included in the area of analysis, and only stained cell infiltrates in tumour cell nests—but not in the stromal compartment—were considered for evaluation. Median values were used as cut-offs to define subgroups with high or low infiltration for all immunohistochemical markers.
Statistical analysis
Statistical analyses were performed with the SPSS and SAS software programs. Cumulative survival time was estimated using the Kaplan–Meier method and the survival difference was detected by the two-sided log-rank test for univariate analysis. The level of significance was set at P < 0.05. Data were censored at the last follow-up for patients who were disease-free or alive at the time of analysis. Comparisons of TIL counts in pre- and post-chemotherapeutic specimens were carried out using the t test and P < 0.05 was considered significant.
Results
Clinical patient profile
The clinical and histological characteristics of the patients included in this study are summarized in Table 1. The median age of the study population at the time of diagnosis was 62 years (range 39–80). Of 30 patients, 29 were diagnosed as FIGO IIIC and 1 as FIGO IV. Histological subtypes of the tumour comprised 29 cases of serous/serous-papillary and 1 case of endometrioid carcinoma. All patients were classified as responders to initial chemotherapy, as shown by ascites volume and CA 125 levels compared to pre-treatment findings. All patients received a total of six cycles of chemotherapy. Towards the end of the study, 22 (73%) patients had developed recurrent disease, 16 (53%) succumbed to their disease, and 14 (47%) were still alive. The mean follow-up period was 24 months (range 7–65). The median CA 125 levels pre- and post-NAC treatment were 1,412 U/ml (range 43–9,030) and 59 U/ml (range 8–1,872), respectively. The median progression-free (PFS) and overall survival (OS) for all patients examined were 13.3 and 33.1 months (range 3.8–50.7 and 3.8–65 months), respectively.
Table 1.
Patient characteristics
| Total | |
|---|---|
| Number of patients | 30 |
| Age (years) | |
| Median | 62 |
| Range | 39–80 |
| PFS (months) | |
| Median | 13.3 |
| Range | 3.8–50.7 |
| OS (months) | |
| Median | 33.1 |
| Range | 3.8–65 |
| CA 125 (U/ml) | |
| Median | 1,412 |
| Range | 43–9,030 |
| Histological type (no.) | |
| Serous/serous-papillary | 29 |
| Endometrioid | 1 |
| Histological grade (no.) | |
| G2 | 12 |
| G3 | 18 |
| Stage (no.) | |
| III C | 29 |
| IV | 1 |
| Number of chemotherapy cycles before cytoreductive surgery (no.) | |
| 2 | 17 |
| 3 | 13 |
| Residual tumour (no.) | |
| >1 cm | 4 |
| ≤1 cm | 17 |
| No macroscopic tumour | 9 |
| Mortality and recurrence status (no.) | |
| Recurrent disease | 22 |
| Dead | 16 |
Analysis of T cell infiltration before and after NAC intervention
Antitumour immune response to chemotherapy was analysed by immunohistochemical staining of intratumoral CD4+, CD8+, granzyme B+ and Foxp3+ T cells in ovarian epithelium surgical specimens as well as by assessing the ratio of CD8+/CD4+, CD4+/Foxp3+, CD8+/Foxp3+ and granzyme B+/Foxp3+ T cells prior to and after NAC. Immune cells infiltrated tumour tissue in a disseminated manner as scattered solitary cells, as shown by hematoxylin staining, and displayed low level of homogeneity and broad inter-individual differences for stained cell density, the number of CD4+, CD8+, granzyme B+ and Foxp3+ cells varying significantly among samples. While CD4 and CD8 displayed cell membrane staining and were vigorously expressed, a weak accumulation of granzyme B+ and Foxp3+ cell infiltrates was observed (Fig. 1). Foxp3 showed, in line with its function as a transcription factor distinct nuclear localization. Granzyme B, a granule-associated protein crucial for cytolytic function, is constitutively expressed by NK cells and by activated but not by naïve CD8+ CTLs [22]. Here, we identified only cells with sparsely granulated cytoplasmatic pattern as stimulated cytotoxic T lymphocytes (CTLs), while densely positive natural killer (NK) cells were disregarded. Several reports have demonstrated the prognostic significance of intraepithelial—in contrast to stromal—CD8+ T cell infiltration in patients with ovarian [15, 18–20], endometrial [23], cervical [24], colorectal [25] and hepatocellular cancer [26]. In this report, an explicit analysis of stromal-infiltrating lymphocytes was not performed, as the stromal component might be caused not just by the tumour itself (epithelial-to-mesenchymal transition, EMT) but also by scar tissue following therapy-induced necrosis. This NAC effect may, therefore, not be properly distinguished from tumour-induced EMT, so that stromal lymphocytes in this neoadjuvant setting might reflect not only an immune response to tumour stroma but also an unspecific immune response within scar tissue. Accordingly, we assessed here only lymphocyte infiltrates in the tumour epithelium itself and those immediately contacting it. Representative images of lymphocyte infiltrates showing antigen expression levels prior to and after NAC as well as descriptive statistics of immunohistochemical variables are shown in Fig. 1 and Table 2, respectively.
Fig. 1.
Immunohistochemical staining pattern of intratumoral CD4+, CD8+, granzyme B+ and Foxp3+ T cells in representative specimens of EOC patients (serous type, FIGO IIIc, grade G3, ×400 magnification) prior to (a) and after preoperative neoadjuvant chemotherapy (b). Scale bar 100 μm
Table 2.
Descriptive statistics of tumour infiltrating lymphocytes
| CD4A | CD4P | CD8A | CD8P | GrBA | GrBP | Foxp3A | Foxp3P | CD8A/CD4A | CD8P/CD4P | CD4A/Foxp3A | CD4P/Foxp3P | CD8A/Foxp3A | CD8P/Foxp3P | GrBA/Foxp3A | GrBP/Foxp3P | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rangea | 0–25.5 | 0–75 | 0–51 | 6.5–139 | 0–8.5 | 0–18 | 0–19.5 | 0–11 | 0–13 | 0.34–5 | 0.77–20.0 | 0–57.0 | 0.66–36 | 2–101 | 0–2.5 | 0–9 |
| Meana | 8.35 | 20.95 | 14.46 | 37.01 | 1.98 | 4.96 | 3.55 | 3.61 | 1.86 | 2.33 | 3.34 | 9.35 | 7.29 | 19.14 | 0.75 | 1.82 |
| Std Dev | 7.53 | 19.63 | 12.39 | 27.89 | 2.31 | 4.08 | 4.85 | 3.29 | 2.49 | 1.35 | 4.45 | 12.7 | 9.72 | 24.95 | 0.87 | 2.22 |
| Mediana | 8.00 | 14.25 | 11.25 | 26.25 | 1.5 | 4.50 | 1.5 | 3.75 | 1.38 | 1.96 | 2.33 | 3.11 | 5.5 | 7.57 | 0.3 | 1.15 |
| Cases (n) | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 24b | 29b | 19b | 25b | 19b | 25b | 19b | 25b |
A ante-NAC, P post-NAC, GrB granzyme B
aNumber of TILs per high power field before (A) and after (P) preoperative neoadjuvant chemotherapy (NAC)
bThe discrepancy in the case numbers is due to the division by zero in a quotient. The denominator (FOXP3+ cells) in the corresponding samples was zero, meaning that we did not observe FOXP3+ infiltrating cells in those specimens
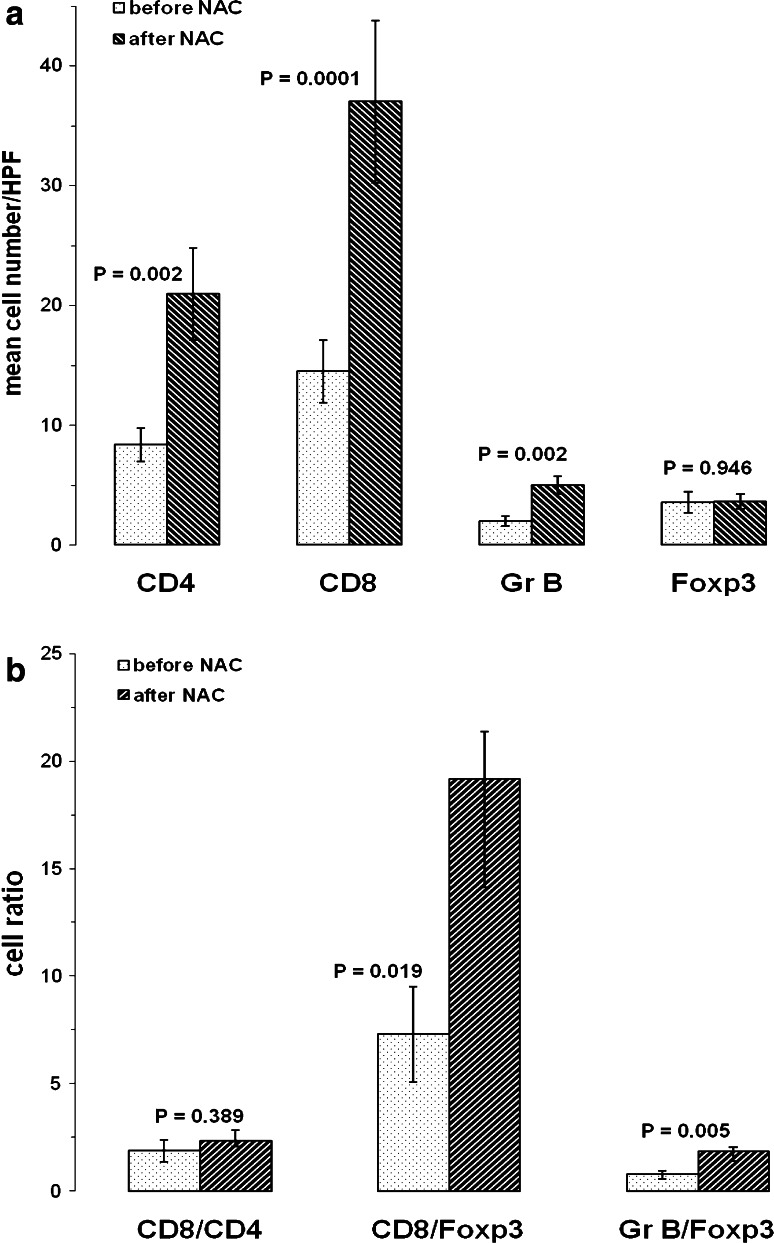
The mean number of infiltrating CD4+, CD8+ and granzyme B+ cells was significantly enhanced after NAC, as compared to cell accumulation prior to NAC (CD4: 2.5-fold increase, CD8: 2.5-fold; granzyme B: 2.5-fold) whereas Foxp3+ cell numbers were equivalent in pre- and post-NAC samples (Fig. 2a). These findings argue for a specific differential effect of NAC on recruitment or expansion of conventional and activated T cells on one side and Tregs on the other side. In addition, CD8+/Foxp3+ and granzyme B+/Foxp3+ cell ratios were significantly elevated after NAC (2.6-fold and 2.4-fold increase, respectively), whereas CD8+/CD4+ remained unaffected by chemotherapy (Fig. 2b).
Fig. 2.
Comparison of immune cell infiltrates in specimens of EOC patients prior to and after neoadjuvant chemotherapy. Columns represent the mean number of cells per high power field. Statistical analysis was done using the t test
Correlation of clinicopathologic and immunohistochemical variables with clinical outcome
On Kaplan–Meier analysis, clinical variables such as histological grade or number of preoperatively administered chemotherapeutic cycles did not show any prognostic significance for either PFS or OS (Table 3). In patients with no macroscopic evidence of residual disease after cytoreductive surgery, which has been considered the most significant predictor of EOC survival [3], longer OS than in those with tumour load was demonstrated, although without attaining full statistical significance (median 65.06 vs. 33.17 months, respectively; log-rank 0.075). To assess the influence of high versus low levels of infiltrating cells on disease prognosis, we used the corresponding median as cut-off for each lymphocyte population (Tables 2, 3). None of the T cell infiltrates examined were associated with improved clinical outcome in pre-chemotherapeutic specimens. In contrast, after NAC, patients with high granzyme B cell infiltration (>4.5) showed, as compared to those with low cell density (<4.5), a tendency towards an extended PFS (median 14.53 vs. 11.87 months, respectively; log-rank 0.064), whereas no association was found with OS (Table 3). In addition, the group of patients with low intratumoral Treg density after NAC (<3.75) exhibited longer PFS and OS (median 20.94 and 30.75 months, respectively) than did that with prevalent Foxp3+ (>3.75) cell infiltration after NAC (median 11.24 and 16.04 months, respectively), and disclosed significant association with improved PFS (log-rank 0.0001 and 0.056 for PFS and OS, respectively, Table 3). The development of Foxp3+ cell infiltrates in all EOC patients prior to and after NAC intervention is illustrated in Fig. 3. However, none of these factors reached significant independent prognostic influence in a multivariate analysis (data not shown).
Table 3.
Univariate analysis of clinicohistochemical variables and survival in neoadjuvant chemotherapy-treated EOC patients
| Variables | PFS | OS | ||||
|---|---|---|---|---|---|---|
| Mediana | 95% CIa | Log-rank | Mediana | 95% CIa | Log-rank | |
| Histological grade | ||||||
| G2 = 12 | 13.31 | 6.25–15.22 | 0.164 | 33.17 | 10.19–65.06 | 0.617 |
| G3 = 18 | 13.28 | 11.05–32.91 | 35.96 | 15.45–62.10 | ||
| NAC cycles | ||||||
| 2 = 17 | 14.53 | 10.36–24.00 | 0.984 | 33.17 | 16.04–n.e. | 0.860 |
| 3 = 13 | 13.28 | 6.25–32.91 | 35.96 | 10.19–65.06 | ||
| Residual tumour status | ||||||
| Yes = 21 | 12.62 | 10.36–14.53 | 0.189 | 33.17 | 14.83–36.75 | 0.075 |
| No = 9 | 15.22 | 8.61–n.e. | 65.06 | 16.04–65.06 | ||
| CD4A | ||||||
| >8.0 | 14.53 | 10.85–n.e. | 0.474 | 36.75 | 14.60–n.e. | 0.660 |
| <8.0 | 13.28 | 8.61–15.22 | 20.87 | 14.83–62.10 | ||
| CD4P | ||||||
| >14.25 | 12.62 | 8.61–13.87 | 0.192 | 20.74 | 14.66–36.75 | 0.133 |
| <14.25 | 17.88 | 10.36–n.e. | 35.96 | 14.83–65.06 | ||
| CD8A | ||||||
| >11.25 | 13.28 | 8.61–n.e. | 0.859 | 20.74 | 14.66–62.09 | 0.831 |
| <11.25 | 13.87 | 10.36–17.88 | 33.17 | 15.02–65.06 | ||
| CD8P | ||||||
| >26.25 | 12.62 | 8.61–15.22 | 0.547 | 20.87 | 16.04–65.06 | 0.897 |
| <26.25 | 13.58 | 10.36–32.91 | 35.96 | 14.83–n.e. | ||
| GrBA | ||||||
| >1.5 | 15.22 | 10.36–n.e. | 0.217 | 65.06 | 14.63–65.06 | 0.089 |
| <1.5 | 12.62 | 8.61–17.88 | 20.87 | 15.45–36.75 | ||
| GrBP | ||||||
| >4.5 | 14.53 | 10.85–n.e. | 0.064 | – | – | 0.474 |
| <4.5 | 11.87 | 8.32–15.22 | 33.17 | 14.83–62.10 | ||
| Foxp3A | ||||||
| >1.5 | 12.62 | 6.67–15.22 | 0.233 | 33.17 | 14.45–65.06 | 0.743 |
| <1.5 | 14.53 | 11.24–32.91 | 35.91 | 14.83–62.10 | ||
| Foxp3P | ||||||
| >3.75 | 11.24 | 8.32–12.75 | 0.0001 | 16.04 | 14.63–33.07 | 0.056 |
| <3.75 | 20.94 | 11.87–n.e. | 30.75 | 15.02–65.05 | ||
| CD8A/CD4A | ||||||
| >1.38 | 11.41 | 8.32–32.91 | 0.742 | 33.17 | 14.63–62.10 | 0.650 |
| <1.38 | 13.28 | 11.05–24.00 | 36.75 | 15.02–n.e. | ||
| CD8P/CD4P | ||||||
| >1.96 | 15.22 | 8.32–n.e. | 0.206 | 62.10 | 16.04–62.06 | 0.032 |
| <1.96 | 12.75 | 10.36–14.53 | 20.74 | 14.83–35.96 | ||
| CD4A/Foxp3A | ||||||
| >5 | 14.53 | 10.36–26.17 | 0.741 | 20.87 | 14.66–36.75 | 0.620 |
| <5 | 12.75 | 8.32–15.22 | 33.17 | 14.63–65.06 | ||
| CD4P/Foxp3P | ||||||
| >1.15 | 14.53 | 10.36–26.17 | 0.620 | 35.96 | 14.83–65.06 | 0.734 |
| <1.15 | 13.28 | 8.32–24.00 | 33.17 | 14.63–62.10 | ||
| CD8A/Foxp3A | ||||||
| >5.5 | 14.53 | 10.36–32.91 | 0.835 | 20.87 | 14.66–62.10 | 0.415 |
| <5.5 | 13.28 | 8.32–24.00 | 33.17 | 15.45–35.96 | ||
| CD8P/Foxp3P | ||||||
| >7.57 | 15.22 | 10.36–32.91 | 0.119 | 35.96 | 14.83–65.06 | 0.528 |
| <7.57 | 12.08 | 8.32–14.53 | 16.50 | 14.63–62.10 | ||
| GrBA/Foxp3A | ||||||
| >0.30 | 17.88 | 11.24–n.e. | 0.102 | 36.75 | 14.66–65.06 | 0.215 |
| <0.30 | 10.85 | 6.25–13.28 | 20.74 | 10.19–33.16 | ||
| GrBP/Foxp3P | ||||||
| >1.15 | 17.88 | 10.85–32.91 | 0.014 | 35.96 | 16.04–65.06 | 0.137 |
| <1.15 | 11.24 | 6.67–13.28 | 33.17 | 14.63–62.10 | ||
PFS progression-free survival, OS overall survival, NAC neoadjuvant chemotherapy, A ante-NAC, P post-NAC, GrB granzyme B, n.e. not estimable
a Values in months
Fig. 3.
Development of Foxp3+ cell infiltrates in EOC prior to and after NAC intervention
Accruing reports have recently portended that the specific type, functional state, balance and distribution of infiltrating immune cells rather than their absolute numbers critically determine their prognostic significance [15, 26, 27]. Accordingly, to examine the role of T cell subsets with ancillary or antagonistic functions, we assessed the impact of CD8+/CD4+, CD4+/Foxp3+, CD8+/Foxp3+ and granzyme B+/Foxp3+ cell ratio on patient survival, using as aforementioned median value as cut-off (Tables 2, 3). High intratumoral CD8+/Foxp3+ cell ratio, which has been consistently reported as a significant predictor of clinical outcome in ovarian [15], cervix [24], breast [28] and colorectal carcinoma [29], was not associated with improved survival of EOC patients, neither prior to nor after chemotherapy (Table 3). In addition, whereas CD8+/CD4+ T cell ratio prior to NAC had no influence on either PFS or OS, high CD8+/CD4+ T cell ratio after NAC (>1.96) was predictive of improved OS (median 62.10 vs. 20.74 months, respectively; log-rank 0.032) (Table 3). Significantly, and reinforcing these findings, high granzyme B+/Foxp3+ cell density after NAC (>1.15) correlated significantly with extended PFS, as compared to that of patients with a low (<1.15) granzyme B+/Foxp3+ cell ratio (median 17.88 vs. 11.24 months, respectively), and proved clearly as a prognostic parameter (log-rank 0.014, Table 3).
Taken together, these data indicate that preoperative NAC specifically facilitated increased intratumoral accumulation of CD4+, CD8+ and effector granzyme B+ cells, whereas Foxp3+ cell infiltration remained unaffected. In addition, while Foxp3 infiltration prior to NAC treatment was not predictive of survival, weak Foxp3+ cell influx following NAC was associated with prolonged OS and PFS and conversely, a higher Treg recruitment post-NAC was a significant poor predictor of EOC. Accordingly, low numbers of FOXP3+ cells in combination with enhanced infiltration of activated granzyme B+ CTLs after NAC proved to be a positive prognostic factor associated with increased PFS but not with improved OS.
Discussion
A large body of evidence has documented that high T cell infiltration augurs a favourable outcome in a variety of human cancers [11, 12]. Endorsing this paradigm, an early study of EOC showed that the presence of intratumoral T cells correlated independently with improved PFS and OS, and was associated with enhanced expression of IFN-γ, IL-2 and lymphocyte-attracting chemokines within the tumour, suggesting activation of antitumour immunity [14]. Ensuing data demonstrated that active accumulation of CD4+CD25+Foxp3+ regulatory T cells in EOC was associated with suppression of tumour-specific cytotoxicity and cytokine production by CD8+ T cells, growth of human tumours in vivo, high death hazard and reduced patient survival [17]. In line with these findings, Foxp3 mRNA expression in EOC was shown to be an independent adverse prognostic factor for PFS and OS [16], indicating that high Foxp3 expression levels may represent a surrogate marker for an immunosuppressive milieu fostering tumour immune escape. Tregs—either natural or inducible—mediate peripheral self-tolerance and preclude protective antitumour immunity by suppressing effector T cell proliferation and cytokine production through soluble- and/or contact-dependent factors [30, 31]. Foxp3, a member of the forkhead/winged helix family of DNA transcription factors, is essential for Treg development and function and represents, thus far, the most selective Treg marker [32]. Yet, more comprehensive analyses have recently indicated that the specific T cell subsets and their mutual interactions rather than the absolute numbers control the host-versus-tumour reaction and predict disease outcome [15, 26, 27]. Accordingly, only intraepithelial CD8+ T cell recruitment and high CD8+/CD4+ and CD8+/CD25+FoxpP3+ ratio were associated with favourable prognosis in EOC, Foxp3+ cells attenuating the protective effects of CD8+ effector cells [15].
In this report, we show that platinum/taxane-based neoadjuvant intervention significantly increased the frequencies of CD4+, CD8+ and granzyme B+ cells in ovarian surgical specimens as compared to NAC naïve samples, whereas Foxp3+ accumulation remained unaffected. Furthermore, while the CD8+/CD4+ cell ratio was not influenced by chemotherapy, CD8+/Foxp3+ and granzyme B+/Foxp3+ were notably enhanced after NAC. These findings suggest either differential expansion or recruitment of conventional T cells and Tregs as a result of chemotherapy, arguing for a specific treatment-associated antitumour immune response. Indeed, in accordance with this notion, previous reports have demonstrated that cytotoxic agents may modulate T cell infiltration, improving thereby clinical outcome. In this respect, a study of cervical carcinoma patients pre-surgically treated with chemotherapy or chemoradiation revealed increased incidence of activated T cells and a concomitant reduction of naïve T cells in uninvolved lymph nodes (LN), whereas the high levels and suppressive function of CD4+CD25highCD152+ Tregs were unaffected. Of note, LNs from patients with an objective response contained significantly higher numbers of activated T and NK cells and diminished frequencies of Tregs, indicating an enhanced treatment-induced capacity of the local immune response to mediate antitumour effects [33]. In addition, a study of B cell chronic lymphocytic leukaemia (CLL) demonstrated significant stage-dependent increase of CD4+CD25+Foxp3+ Tregs in peripheral blood of individuals with extended disease, but reduced frequencies and decreased or abrogated Treg inhibitory function after therapy with fludarabine [34]. Most importantly, carboplatin/paclitaxel-based chemotherapy of EOC patients displaying high CA 125 levels and compromised T cell function was shown to affect significantly decreased CA 125 levels and restore potent CD8+ T cell responses in patients at remission, pointing at an association between chemotherapy, T cell function and improved clinical outcome [35].
Several studies have identified prevalence of CD8+ T cells and high CD8+/CD4+ or CD8+/Treg ratio as significant predictors of survival in EOC [15, 18, 20], cervical [24], breast [28], colorectal [25] and hepatocellular cancer [26]. In contrast, in the current report, only an elevated CD8+/CD4+ cell ratio was found to have a positive prognostic influence on OS on Kaplan–Meier analyses, whereas neither CD8+ cell infiltration nor CD8+/Treg ratio was predictive of outcome. Consistent with our findings, no association of CD8+ TILs with improved prognosis has been documented in some other malignancies including lung cancer [36], anal squamous cell and hepatocellular carcinoma [26, 37], indicating that the effects of CD8+ cell infiltration may vary in different tumour types. Further aspects contributing to these conflicting results may include use of different detecting antibodies, preceding treatment modalities, as well as disease stage and immune status of the patients under study.
The most significant results of this study are the correlation of enhanced granzyme B+ cell density following NAC with improved PFS as well as the finding that treatment-associated decreased Foxp3+ cell infiltration is a statistically significant favourable prognostic factor for PFS, whereas patients with higher Treg numbers reveal poorer survival. In accordance with our results, abundance of Foxp3+ infiltrates has been linked to adverse outcome in various types of solid tumours including EOC [16, 17], endometrial [38], breast [39, 40], pancreatic [41] and hepatocellular cancer [42, 43]. By contrast, low Foxp3+ cell density has been shown to represent an independent prognostic factor negatively influencing survival in Hodgkin’s lymphoma [44], and high Treg numbers predicted improved survival in follicular lymphoma [45] and colorectal cancer [46], suggesting that, analogous to CD8+ cells, Tregs may not play equivalent roles in different neoplasms. Our findings are further reinforced by the observation that increased influx of activated granzyme B+ T cells counteracting the adverse prognostic effects of Treg infiltration is a significant predictor of decreased risk of recurrence, arguing for local NAC-induced immune surveillance. Indeed, recent data strongly indicate that chemotherapy-induced accumulation of apoptotic cells may foster tumour immunogenicity by providing tumour antigens for cross-presentation by dendritic cells (DC) to T lymphocytes [8]. Remarkably, one study of advanced breast cancer showed that neoadjuvant paclitaxel chemotherapy induced intratumoral apoptotic response that correlated with TIL recruitment in 67% of patients developing complete clinical response with pathological residual disease [47].
Our data indicate further that not the extent of CD8+ cell infiltration, but the activation state is critical for its prognostic significance. In this respect, the presence of high intratumoral granzyme B+ effector CTLs and low Treg levels was shown to be an independent prognostic factor for improved outcome in hepatocellular carcinoma [26]. Most importantly, pathological complete response to a preoperative anthracycline/taxane-based NAC in patients with breast carcinoma has been associated with an immunologic profile combining high levels of infiltrating CD8+ and cytotoxic granzyme B+ cells and absent or decreased Foxp3+ cell infiltrates [28].
In summary, the current study is, to our knowledge, the first to analyse the impact of carboplatin/taxane-based NAC on the lymphocyte composition of surgical specimens of patients with EOC. Systemic chemotherapy for the management of cancer has previously been perceived to exert deleterious effects on the host’s defense system, particularly in advanced-stage patients who may as well be immune compromised. Accordingly, patients undergoing cytotoxic treatment within 2 months before study entry have been excluded thus far from most immunotherapeutic trials. Although the limited number of patients studied does not allow definitive conclusions, our results underscore Foxp3+ cell infiltration as adverse prognostic factor and present evidence of a treatment-dependent increase of activated granzyme B+ T cells exerting a beneficial effect on disease outcome. Although the mechanisms underlying such an effect have not elucidated here, we conjecture that chemotherapy-induced apoptosis may induce, via cross-presentation of tumour antigens by DCs, increased levels of primed granzyme B+ effector cells which might hamper tumour progression and contribute to improved survival. Recent studies have reported that Treg depletion substantially enhances vaccine-mediated antigen-specific effector T cell immunity resulting in occasional therapeutic responses in several human malignancies [48, 49]. Based on the findings presented here, as well as previous data from our laboratory demonstrating the efficacy of a DC-based immunotherapy for relapsed metastatic EOC [50], we propose that administration of platinum/taxane-based NAC prior to tumour vaccination may, by inactivation or depletion of infiltrating Foxp3+ cells in association with increased accumulation of activated granzyme B+ cells, stimulate antitumour immune responses impacting favourably on clinical outcome of EOC patients.
Acknowledgments
This is a single institution analysis of clinical data collected within a multicenter phase 2 trial, PRIMOVAR, ClinicalTrials.gov Identifier: NCT00551577, sponsored by Sanofi-Aventis Deutschland GmbH. We thank Christiane Esch for technical assistance with immunohistochemistry.
Conflict of interest statement
Walther Kuhn (principal investigator) received research funding from Sanofi-Aventis. All other authors indicated no potential conflict of interest.
Footnotes
M. Pölcher and M. Braun contributed equally to this work.
References
- 1.Tummala MK, McGuire WP. Recurrent ovarian cancer. Clin Adv Hematol Oncol. 2005;3:723–736. [PubMed] [Google Scholar]
- 2.Heintz AP, Odicino F, Maisonneuve P, Quinn MA, Benedet JL, Creasman WT, Ngan HY, Pecorelli S, Beller U. Carcinoma of the ovary. FIGO 6th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95(Suppl 1):S161–S192. doi: 10.1016/S0020-7292(06)60033-7. [DOI] [PubMed] [Google Scholar]
- 3.Chi DS, Liao JB, Leon LF, Venkatraman ES, Hensley ML, Bhaskaran D, Hoskins WJ. Identification of prognostic factors in advanced epithelial ovarian carcinoma. Gynecol Oncol. 2001;82:532–537. doi: 10.1006/gyno.2001.6328. [DOI] [PubMed] [Google Scholar]
- 4.Kuhn W, Rutke S, Späthe K, Schmalfeldt B, Florack G, von Hundelshausen B, Pachyn D, Ulm K, Graeff H. Neoadjuvant chemotherapy followed by tumor debulking prolongs survival for patients with poor prognosis in International Federation of Gynecology and Obstetrics Stage IIIC ovarian carcinoma. Cancer. 2001;92:2585–2591. doi: 10.1002/1097-0142(20011115)92:10<2585::AID-CNCR1611>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 5.Lee SJ, Kim BG, Lee JW, Park CS, Lee JH, Bae DS. Preliminary results of neoadjuvant chemotherapy with paclitaxel and cisplatin in patients with advanced epithelial ovarian cancer who are inadequate for optimum primary surgery. J Obstet Gynaecol Res. 2006;32:99–106. doi: 10.1111/j.1447-0756.2006.00359.x. [DOI] [PubMed] [Google Scholar]
- 6.Park TW, Kuhn WC. Neoadjuvant chemotherapy in ovarian cancer. Expert Rev Anticancer Ther. 2004;4:639–647. doi: 10.1586/14737140.4.4.639. [DOI] [PubMed] [Google Scholar]
- 7.Pölcher M, Mahner S, Ortmann O, Hilfrich J, Diedrich K, Breitbach GP, Hoss C, Leutner C, Braun M, Mobus V, Karbe I, Stimmler P, Rudlowski C, Schwarz J, Kuhn W. Neoadjuvant chemotherapy with carboplatin and docetaxel in advanced ovarian cancer—a prospective multicenter phase II trial (PRIMOVAR) Oncol Rep. 2009;22:605–613. doi: 10.3892/or_00000479. [DOI] [PubMed] [Google Scholar]
- 8.van der Most RG, Currie A, Robinson BW, Lake RA. Cranking the immunologic engine with chemotherapy: using context to drive tumor antigen cross-presentation towards useful antitumor immunity. Cancer Res. 2006;66:601–604. doi: 10.1158/0008-5472.CAN-05-2967. [DOI] [PubMed] [Google Scholar]
- 9.Emens LA. Chemotherapy and tumor immunity: an unexpected collaboration. Front Biosci. 2008;13:249–257. doi: 10.2741/2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- 11.Yu P, Fu YX. Tumor-infiltrating T lymphocytes: friends or foes? Lab Invest. 2006;86:231–245. doi: 10.1038/labinvest.3700389. [DOI] [PubMed] [Google Scholar]
- 12.Talmadge JE, Donkor M, Scholar E. Inflammatory cell infiltration of tumors: Jekyll or Hyde. Cancer Metastasis Rev. 2007;26:373–400. doi: 10.1007/s10555-007-9072-0. [DOI] [PubMed] [Google Scholar]
- 13.Sassen S, Schmalfeldt B, Avril N, Kuhn W, Busch R, Hofler H, Fend F, Nahrig J. Histopathologic assessment of tumor regression after neoadjuvant chemotherapy in advanced-stage ovarian cancer. Hum Pathol. 2007;38:926–934. doi: 10.1016/j.humpath.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 15.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolf D, Wolf AM, Rumpold H, Fiegl H, Zeimet AG, Muller-Holzner E, Deibl M, Gastl G, Gunsilius E, Marth C. The expression of the regulatory T cell-specific forkhead box transcription factor FoxP3 is associated with poor prognosis in ovarian cancer. Clin Cancer Res. 2005;11:8326–8331. doi: 10.1158/1078-0432.CCR-05-1244. [DOI] [PubMed] [Google Scholar]
- 17.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 18.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N, Honjo T, Fujii S. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci USA. 2007;104:3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomsova M, Melichar B, Sedlakova I, Steiner I. Prognostic significance of CD3+ tumor-infiltrating lymphocytes in ovarian carcinoma. Gynecol Oncol. 2008;108:415–420. doi: 10.1016/j.ygyno.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 20.Han LY, Fletcher MS, Urbauer DL, Mueller P, Landen CN, Kamat AA, Lin YG, Merritt WM, Spannuth WA, Deavers MT, De Geest K, Gershenson DM, Lutgendorf SK, Ferrone S, Sood AK. HLA class I antigen processing machinery component expression and intratumoral T-Cell infiltrate as independent prognostic markers in ovarian carcinoma. Clin Cancer Res. 2008;14:3372–3379. doi: 10.1158/1078-0432.CCR-07-4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curiel TJ. Regulatory T cells and treatment of cancer. Curr Opin Immunol. 2008;20:241–246. doi: 10.1016/j.coi.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russell JH, Ley TJ. Lymphocyte-mediated cytotoxicity. Ann Rev Immunol. 2002;20:323–370. doi: 10.1146/annurev.immunol.20.100201.131730. [DOI] [PubMed] [Google Scholar]
- 23.Kondratiev S, Sabo E, Yakirevich E, Lavie O, Resnick MB. Intratumoral CD8+ T lymphocytes as a prognostic factor of survival in endometrial carcinoma. Clin Cancer Res. 2004;10:4450–4456. doi: 10.1158/1078-0432.CCR-0732-3. [DOI] [PubMed] [Google Scholar]
- 24.Piersma SJ, Jordanova ES, van Poelgeest MI, Kwappenberg KM, van der Hulst JM, Drijfhout JW, Melief CJ, Kenter GG, Fleuren GJ, Offringa R, van der Burg SH. High number of intraepithelial CD8+ tumor-infiltrating lymphocytes is associated with the absence of lymph node metastases in patients with large early-stage cervical cancer. Cancer Res. 2007;67:354–361. doi: 10.1158/0008-5472.CAN-06-3388. [DOI] [PubMed] [Google Scholar]
- 25.Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, Ohtani H. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58:3491–3494. [PubMed] [Google Scholar]
- 26.Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, Xu Y, Li YW, Tang ZY. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586–2593. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 27.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 28.Ladoire S, Arnould L, Apetoh L, Coudert B, Martin F, Chauffert B, Fumoleau P, Ghiringhelli F. Pathologic complete response to neoadjuvant chemotherapy of breast carcinoma is associated with the disappearance of tumor-infiltrating foxp3+ regulatory T cells. Clin Cancer Res. 2008;14:2413–2420. doi: 10.1158/1078-0432.CCR-07-4491. [DOI] [PubMed] [Google Scholar]
- 29.Ohtani H. Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in human colorectal cancer. Cancer Immun. 2007;7:4. [PMC free article] [PubMed] [Google Scholar]
- 30.Miyara M, Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends Mol Med. 2007;13:108–116. doi: 10.1016/j.molmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Zhou G, Levitsky HI. Natural regulatory T cells and de novo-induced regulatory T cells contribute independently to tumor-specific tolerance. J Immunol. 2007;178:2155–2162. doi: 10.4049/jimmunol.178.4.2155. [DOI] [PubMed] [Google Scholar]
- 32.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 33.Fattorossi A, Battaglia A, Ferrandina G, Coronetta F, Legge F, Salutari V, Scambia G. Neoadjuvant therapy changes the lymphocyte composition of tumor-draining lymph nodes in cervical carcinoma. Cancer. 2004;100:1418–1428. doi: 10.1002/cncr.20130. [DOI] [PubMed] [Google Scholar]
- 34.Beyer M, Kochanek M, Darabi K, Popov A, Jensen M, Endl E, Knolle PA, Thomas RK, von Bergwelt-Baildon M, Debey S, Hallek M, Schultze JL. Reduced frequencies and suppressive function of CD4+ CD25hi regulatory T cells in patients with chronic lymphocytic leukemia after therapy with fludarabine. Blood. 2005;106:2018–2025. doi: 10.1182/blood-2005-02-0642. [DOI] [PubMed] [Google Scholar]
- 35.Coleman S, Clayton A, Mason MD, Jasani B, Adams M, Tabi Z. Recovery of CD8+ T-cell function during systemic chemotherapy in advanced ovarian cancer. Cancer Res. 2005;65:7000–7006. doi: 10.1158/0008-5472.CAN-04-3792. [DOI] [PubMed] [Google Scholar]
- 36.Ikeda S, Funakoshi N, Inagaki M, Shibata T. Clinicopathologic roles of tumor-infiltrating lymphocytes and CD8-positive lymphocytes in lung cancer imprint smears in squamous cell carcinoma and adenocarcinoma. Acta Cytol. 2006;50:423–429. doi: 10.1159/000325986. [DOI] [PubMed] [Google Scholar]
- 37.Grabenbauer GG, Lahmer G, Distel L, Niedobitek G. Tumor-infiltrating cytotoxic T cells but not regulatory T cells predict outcome in anal squamous cell carcinoma. Clin Cancer Res. 2006;12:3355–3360. doi: 10.1158/1078-0432.CCR-05-2434. [DOI] [PubMed] [Google Scholar]
- 38.Giatromanolaki A, Bates GJ, Koukourakis MI, Sivridis E, Gatter KC, Harris AL, Banham AH. The presence of tumor-infiltrating FOXP3(+) lymphocytes correlates with intratumoral angiogenesis in endometrial cancer. Gynecol Oncol. 2008;110:216–221. doi: 10.1016/j.ygyno.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 39.Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, Banham AH. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24:5373–5380. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 40.Ghebeh H, Barhoush E, Tulbah A, Elkum N, Al-Tweigeri T, Dermime S. FOXP3+ Tregs and B7-H1+/PD-1+ T lymphocytes co-infiltrate the tumor tissues of high-risk breast cancer patients: Implication for immunotherapy. BMC Cancer. 2008;8:57. doi: 10.1186/1471-2407-8-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res. 2006;12:5423–5434. doi: 10.1158/1078-0432.CCR-06-0369. [DOI] [PubMed] [Google Scholar]
- 42.Kobayashi N, Hiraoka N, Yamagami W, Ojima H, Kanai Y, Kosuge T, Nakajima A, Hirohashi S. FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res. 2007;13:902–911. doi: 10.1158/1078-0432.CCR-06-2363. [DOI] [PubMed] [Google Scholar]
- 43.Sasaki A, Tanaka F, Mimori K, Inoue H, Kai S, Shibata K, Ohta M, Kitano S, Mori M. Prognostic value of tumor-infiltrating FOXP3+ regulatory T cells in patients with hepatocellular carcinoma. Eur J Surg Oncol. 2008;34:173–179. doi: 10.1016/j.ejso.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 44.Alvaro T, Lejeune M, Salvado MT, Bosch R, Garcia JF, Jaen J, Banham AH, Roncador G, Montalban C, Piris MA. Outcome in Hodgkin’s lymphoma can be predicted from the presence of accompanying cytotoxic and regulatory T cells. Clin Cancer Res. 2005;11:1467–1473. doi: 10.1158/1078-0432.CCR-04-1869. [DOI] [PubMed] [Google Scholar]
- 45.Carreras J, Lopez-Guillermo A, Fox BC, Colomo L, Martinez A, Roncador G, Montserrat E, Campo E, Banham AH. High numbers of tumor-infiltrating FOXP3-positive regulatory T cells are associated with improved overall survival in follicular lymphoma. Blood. 2006;108:2957–2964. doi: 10.1182/blood-2006-04-018218. [DOI] [PubMed] [Google Scholar]
- 46.Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, Platell C, Iacopetta B. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27:186–192. doi: 10.1200/JCO.2008.18.7229. [DOI] [PubMed] [Google Scholar]
- 47.Demaria S, Volm MD, Shapiro RL, Yee HT, Oratz R, Formenti SC, Muggia F, Symmans WF. Development of tumor-infiltrating lymphocytes in breast cancer after neoadjuvant paclitaxel chemotherapy. Clin Cancer Res. 2001;7:3025–3030. [PubMed] [Google Scholar]
- 48.Wong BY, Gregory SA, Dang NH. Denileukin diftitox as novel targeted therapy for lymphoid malignancies. Cancer Invest. 2007;25:495–501. doi: 10.1080/07357900701360096. [DOI] [PubMed] [Google Scholar]
- 49.Rasku MA, Clem AL, Telang S, Taft B, Gettings K, Gragg H, Cramer D, Lear SC, McMasters KM, Miller DM, Chesney J. Transient T cell depletion causes regression of melanoma metastases. J Transl Med. 2008;6:12. doi: 10.1186/1479-5876-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hernando JJ, Park TW, Fischer HP, Zivanovic O, Braun M, Pölcher M, Grünn U, Leutner C, Pötzsch B, Kuhn W. Vaccination with dendritic cells transfected with mRNA-encoded folate-receptor-alpha for relapsed metastatic ovarian cancer. Lancet Oncol. 2007;8:451–454. doi: 10.1016/S1470-2045(07)70142-0. [DOI] [PubMed] [Google Scholar]