Abstract
Background
Aspiration pneumonitis is a syndrome resulting from the inhalation of gastric contents. The incidence in obstetric anaesthesia has fallen, largely due to improved anaesthetic techniques and the increased use of regional anaesthesia at caesarean section. However, aspiration pneumonitis is still a cause of maternal morbidity and mortality, and it is important to use effective prophylaxis.
Objectives
To determine whether interventions given prior to caesarean section reduce the risk of aspiration pneumonitis in women with an uncomplicated pregnancy.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register (April 2009).
Selection criteria
Randomised controlled trials were included. Quasi-randomised trials were excluded.
Data collection and analysis
Authors independently assessed the studies for inclusion, assessed risk of bias and carried out data extraction. Data entry was checked.
Main results
Twenty-two studies, involving 2658 women, are included, all having a caesarean section under general anaesthesia. The studies covered a number of comparisons, but were mostly small and of unclear or poor quality.
When compared to no treatment or placebo, there was a significant reduction in the risk of intragastric pH < 2.5 with antacids (risk ratio (RR) 0.17, 95% confidence interval (CI) 0.09 to 0.32, two studies, 108 women), H2 antagonists (RR 0.09, 95% CI 0.05 to 0.18, two studies, 170 women) and proton pump antagonists (RR 0.26, 95% CI 0.14 to 0.46, one study 80 women). H2 antagonists were associated with a reduced the risk of intragastric pH < 2.5 at intubation when compared with proton pump antagonists (RR 0.39, 95% CI 0.16 to 0.97, one study, 120 women), but compared with antacids the findings were unclear. The combined use of ’antacids plus H2 antagonists’ was associated with a significant reduction in the risk of intragastric pH < 2.5 at intubation when compared with placebo (RR 0.02, 95% CI 0.00 to 0.15, one study, 89 women) or compared with antacids alone (RR 0.12, 95% CI 0.02 to 0.92, one study, 119 women).
Authors’ conclusions
The quality of the evidence was poor, but the findings suggest that the combination of antacids plus H2 antagonists was more effective than no intervention, and superior to antacids alone in preventing low gastric pH. However, none of the studies assessed potential adverse effects or substantive clinical outcomes. These findings are relevant for all women undergoing caesarean section under general anaesthesia.
Medical Subject Headings (MeSH): *Cesarean Section; Anesthesia, General [adverse effects]; Anesthesia, Obstetrical [adverse effects]; Antacids [therapeutic use]; Antiemetics [therapeutic use]; Drug Therapy, Combination [methods]; Histamine H2 Antagonists [therapeutic use]; Metoclopramide [therapeutic use]; Pneumonia, Aspiration [*prevention & control]; Proton Pump Inhibitors [therapeutic use]; Randomized Controlled Trials as Topic
MeSH check words: Female, Humans, Pregnancy
BACKGROUND
Description of the condition
Aspiration pneumonitis was first described by Mendelson in the 1940s (Mendelson 1946). It occurs when gastric acid gains access to the lungs in the absence of a cough reflex. Although rare, during anaesthesia for caesarean section, aspiration pneumonitis is still a cause of maternal mortality even in well-resourced countries such as the United Kingdom (CEMD 2001). Aspiration pneumonitis is largely associated with general anaesthesia, with passive regurgitation of gastric contents being the main risk factor. In contrast, vomiting is an active process and is not necessary for aspiration to occur. As regional anaesthesia is now used more frequently for caesarean section, the incidence of aspiration pneumonitis is very rare. However, prophylaxis against acid aspiration (also known as gastric aspiration) and aspiration pneumonitis is still important as there will be situations that require general anaesthesia for caesarean section (for example, emergency caesarean section or where regional anaesthesia has failed or is contraindicated). Restricting food and fluids in labour is another intervention aimed at reducing the risk of aspiration pneumonitis; however, evidence on the effectiveness of this is covered in another Cochrane review (Singata 2002).
Description of the intervention
Several different types of drugs have been used to reduce the risks and effects of acid aspiration. These include antacids, H2 receptor antagonists, proton pump inhibitors and prokinetic drugs, either alone or in combination. This wide range may reflect the absence of an ideal regimen (Grieff 1994; Sweeney 1986; Tordoff 1990).
How the intervention might work
Antacids
Antacids (such as sodium citrate) are alkaline agents used to directly neutralise gastric acid. Antacids are often given just prior to induction of general anaesthesia, and while they increase intragastric pH, they also increase intragastric volume, and may cause more harm than benefit (Bond 1979). It is possible that aspiration of antacid solutions may also cause lung damage. Non-particulate antacids (such as sodium citrate) are thought to be less likely to increase the risk of severe pneumonitis compared to particulate antacids (magnesium trisilicate) should aspiration occur (Gibbs 1979).
H2 receptor antagonists/inhibitors
H2 receptor antagonists (such as ranitidine) act by inhibiting the secretion of acid into the stomach which reduces both the volume and acidity of the stomach contents (Thwaites 1999).
Proton pump antagonists
Proton pump antagonists (such as omeprazole) act by blocking the production of stomach acid by interfering with the pump which secretes protons (acid) into the stomach (Browne 1993).
Prokinetic drugs
Prokinetic drugs increase gastric motility and therefore accelerate gastric emptying and reduce gastric volume. The most commonly used prokinetic drug is metoclopramide which may also act as an anti-emetic (Cohen 1984).
Nasogastric tube aspiration
Nasogastric aspiration or suction is the process of physically draining the stomach’s contents using a nasogastric tube, to remove gastric secretions and swallowed air. It can be used in preparation for surgery and to extract gastric liquid for research purposes.
Assessing effectiveness
As aspiration pneumonitis is a rare event, it is difficult to conduct a randomised controlled trial large enough to demonstrate the effectiveness of an intervention to reduce risk. For this reason, clinical trials on prophylactic drugs have focused on the surrogate measures of gastric pH and volume. This is a disadvantage because there is no guarantee that a change in the surrogate measure will reflect a difference in outcome of interest (i.e. aspiration pneumonitis). The pathophysiology of aspiration pneumonitis relates to both the volume and the acidity of the fluid aspirated. An intragastric pH lower than 2.5 and a volume greater than 0.4 ml/kg are the traditionally described criteria for increased risk of severe lung injury and mortality. These criteria were originally described by Mendelson (Mendelson 1946), and were derived from animal experiments (Roberts 1974). However, the evidence that these surrogate measures increase the risk of aspiration pneumonitis in pregnant women undergoing caesarean section under general anaesthesia is absent. Failure to adequately raise intragastric pH and lower intragastric volume may not be due to the specific drug, but due to other factors, such as the time interval between administration and surgery, or to interaction with other drugs. Opioids in particular slow down gastric emptying and can reduce the effectiveness of prophylactic drugs used. Measurements are usually taken just after induction of anaesthesia and just before extubation (removal of the endotracheal tube) to reflect the intragastric conditions at the time of greatest aspiration risk (Ewart 1990; Gin 1990; Moore 1989; Tripathi 1995).
Why it is important to do this review
The administration of an antacid and H2 receptor antagonist, and sometimes a prokinetic and antiemetic drug (such as metoclopramide, a phenothiazine-like drug aimed at accelerating gastric emptying and reducing nausea, vomiting and aspiration pneumonitis), has been standard practice prior to caesarean section in maternity units in the United Kingdom (Thomas 2001). However, clinical practice has varied across the world. Some countries including the UK also routinely administer drugs (such as ranitidine) to all women in labour with the aim of reducing the risk of aspiration pneumonitis should anaesthesia be required for caesarean section, even though the evidence for such practice is poor (Gyte 2006). Any pharmacological intervention may produce side effects or serious complications, including anaphylaxis. Pharmacological antiemetics are associated with a number of side effects such as excessive sedation, restlessness, dystonic reactions (abnormal muscle tone) and extra pyramidal symptoms (Numazaki 2000).
There is a need to review the evidence of effectiveness of pharmacological drugs to reduce aspiration pneumonitis for women who have caesarean sections. The evidence of effectiveness of pharmacological and non-pharmacological interventions to prevent nausea and vomiting for women who have caesarean sections will be considered in a separate review on ’Interventions for reducing nausea and vomiting at caesarean section’.
OBJECTIVES
To determine whether interventions given prior to caesarean section reduce the risk of aspiration pneumonitis in women with an uncomplicated pregnancy (i.e. women who had no medical complications other than the obstetric reason for caesarean section).
METHODS
Criteria for considering studies for this review
Types of studies
All published or unpublished randomised controlled trials (RCTs), including cluster-randomised trials. We excluded quasi-RCTs.
Types of participants
Pregnant women undergoing elective or emergency caesarean section under general or regional anaesthesia.
Types of interventions
Any pharmacological or non-pharmacological intervention given specifically to prevent aspiration pneumonitis at caesarean section.
Particulate or non-particulate antacids
H2 antagonists (e.g. ranitidine)
Proton pump antagonists (e.g. omeprazole)
Prokinetic drugs (e.g. metoclopramide)
Non-pharmacological interventions
Comparisons were any of the above interventions versus any other, placebo or no intervention.
Types of outcome measures
Primary outcomes
Incidence of mortality due to aspiration pneumonitis
Incidence of morbidity due to aspiration pneumonitis
Low intragastric pH below 2.5, measured after induction of anaesthesia
Increase of intragastric volume to more than 0.4 ml/kg, measured after induction of anaesthesia
Secondary outcomes
Women’s satisfaction
Incidence of nausea during caesarean section or the postoperative period
Incidence of vomiting during caesarean section or the postoperative period
Side effects - including sedation, restlessness, dystonic reactions and extrapyramidal symptoms.
Adverse event - episodes of hypotension, blood loss, atonic uterus
Neonatal morbidity - cord blood pH, Apgar scores, neonatal assessment scores and admission to neonatal intensive care unit
Breastfeeding rates - initiation of breastfeeding and duration of breastfeeding
Raised intragastric pH above 2.5, measured prior to extubation at the end of anaesthesia
Reducing of intragastric volume to less than 0.4 ml/kg, measured prior to extubation at the end of anaesthesia
In order to try to avoid outcome reporting bias in the review, studies will be included whether or not they have assessed these specific outcomes listed here. Where included studies have not reported any of our pre-specified outcomes, we have included them in the review and information can be found in the ’Characteristics of included studies’.
Many trials measured ’at risk of aspiration’ as the number of individuals who had both low gastric pH (less than 2.5) and increased gastric volume (greater than 0.4ml/kg). Although this combined measure was not one of our pre-specified outcomes we have included it in this review.
We looked for individual components of ’side effects’ and ’adverse events’. To date there are limited data for these outcomes. If more data become available in the future, we will analyse these as composite outcomes.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co-ordinator (April 2009).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co-ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL and MEDLINE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ’Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above were each assigned to a review topic (or topics). The Trials Search Co-ordinator searches the register for each review using the topic list rather than keywords.
We did not apply any language restrictions.
Data collection and analysis
Selection of studies
We obtained the full text of all potentially relevant studies and all review authors independently applied the inclusion criteria. Four authors (S Paranjothy, H Broughton, J Griffiths and G Gyte) assessed the trials for eligibility and methodological quality without consideration of the results.
Data extraction and management
We designed a data extraction form. Review authors (S Paranjothy, J Griffiths, H Broughton and G Gyte) independently extracted data, two for each included study using the forms, and then compared the results for discrepancies and process as described in Higgins 2008. Reasons for excluding any trial were given.
Trials were not assessed blindly, as we knew the author’s name, institution and the source of publication. We resolved any disagreement by discussion until a consensus was reached. We contacted authors for each included trial for further information, as necessary.
We performed statistical analysis using the Review Manager software (RevMan 2008).
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). We resolved any disagreement by discussion.
(1) Sequence generation (checking for possible selection bias)
We described for each included study the methods used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the methods as:
adequate (e.g. random number table; computer random number generator);
inadequate (e.g. odd or even date of birth; hospital or clinic record number); or
unclear.
2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal the allocation sequence in sufficient detail and determine whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
adequate (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
inadequate (e.g. open random allocation; unsealed or non-opaque envelopes, alternation; date of birth);
unclear.
(3) Blinding (checking for possible performance bias)
We described for each included study all the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We also provided any information relating to whether the intended blinding was effective. We noted where there was partial blinding (e.g. where it has not been possible to blind participants but where outcome assessment was carried out without knowledge of group assignment). Where blinding was not possible, we assessed whether the lack of blinding was likely to have introduced bias.
We assessed the methods as:
adequate, inadequate or unclear for participants;
adequate, inadequate or unclear for personnel;
adequate inadequate or unclear for outcome assessors.
where ’adequate’ was when there was blinding or where we assessed that the outcome or the outcome measurement is not likely to have been influenced by lack of blinding.
4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We described for each included study the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and any re-inclusions in analyses which we undertook.
We assessed the methods as:
adequate (e.g. where there were no missing data or where reasons for missing data were balanced across groups);
inadequate (e.g. where missing data are likely to be related to outcomes or are not balanced across groups, or where high levels of missing data are likely to introduce serious bias or make the interpretation of results difficult;
unclear (e.g. where there is insufficient reporting of attrition or exclusions to permit a judgement to me made).
5) Selective reporting bias
We described for each included study how the possibility of selective outcome reporting bias was examined by us and what we found.
We assessed the methods as:
adequate (where it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
inadequate (where not all the study’s prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear.
6) Other sources of bias
We described for each included study any important concerns we have about other possible sources of bias. For example, was there a potential source of bias related to the specific study design? Was the trial stopped early due to some data-dependent process? Was there extreme baseline imbalance? Has the study been claimed to be fraudulent?
We assessed whether each study was free of other problems that could put it at risk of bias:
yes;
no;
unclear.
7) Overall risk of bias
We made explicit judgements about risk of bias for important outcomes both within and across studies. With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We intended to explore the impact of the level of bias through undertaking sensitivity analyses (see ’Sensitivity analysis’); however, this was not possible given the limited amount of data. We will explore this in future updates should more data become available for analysis.
Measures of treatment effect
We carried out statistical analysis using the Review Manager software (RevMan 2008). We used fixed-effect meta-analyses for combining data in the absence of significant heterogeneity. Where there was heterogeneity, where results were being pooled from studies examining different interventions, or where it is not clear that the same outcome was being measured in all studies, we used random-effects meta-analyses.
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes were measured in the same way between trials. We used the standardised mean difference to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
Cluster-randomised trials
We did not identify any cluster-randomised trials; however, if these are identified in future updates we will include these in the analyses along with individually-randomised trials, using the methods described in Gates 2009.
Dealing with missing data
For included studies, levels of attrition were noted. The impact of including studies with high levels of missing data in the overall assessment of treatment effect was to be explored by using sensitivity analysis. However, we felt there were insufficient data within any one comparison to undertake sensitivity analyses by levels of missing data.
Intention-to-treat analysis
For all outcomes, analyses were carried out, as far as possible, on an intention-to-treat basis, i.e. we analysed data on all participants with available data in the group to which they are allocated, regardless of whether or not they received the allocated intervention. If in the original reports participants were not analysed in the group to which they were randomised, and there was sufficient information in the trial report, we attempted to restore them to the correct group. We attempted to include all participants randomised to each group in the analyses.
The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We have assessed statistical heterogeneity in each meta-analysis using the T2 (tau-squared), I2 and Chi2 statistics. We have regarded heterogeneity as substantial if T2 was greater than zero and either I2 was greater than 30% or there was a low p-value (less than 0.10) in the Chi2 test for heterogeneity. Where we found heterogeneity and random effects was used, we have reported the average risk risk, or average mean difference or average standard mean difference.
Assessment of reporting biases
Had there been 10 or more studies in a meta-analysis we would have investigated reporting biases (such as publication bias) using funnel plots. We would have assessed funnel plot asymmetry visually, and would have used formal tests for funnel plot asymmetry. For continuous outcomes we would have used the test proposed by Egger 1997, and for dichotomous outcomes we would have used the tests proposed by Harbord 2006. If asymmetry had been detected by any of these tests or been suggested by a visual assessment, we would have performed exploratory analyses to investigate it.
Where we suspected reporting bias (see ’Selective reporting bias’ above), we attempted to contact study authors asking them to provide missing outcome data. Where this was not possible, and the missing data were thought to introduce serious bias, the impact of including such studies in the overall assessment of results was explored by a sensitivity analysis.
Where we suspected publication bias (e.g. where only statistically significant results are reported), this was explored using visual assessment of funnel plots (Higgins 2008).
Data synthesis
We have carried out statistical analysis using the Review Manager software (RevMan 2008). We have used fixed-effect meta-analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we have used random-effects analysis to produce an overall summary, if this was considered clinically meaningful. If an average treatment effect across trials was not clinically meaningful we have not combined heterogeneous trials. If we used random-effects analyses, the results have been presented as the average treatment effect and its 95% confidence interval, the 95% prediction interval for the underlying treatment effect, and the estimates of T2 and I2.
We combined results of trials using drugs that have the same mechanism of action if the treatment regimens are assessed to be compatible. For example, studies comparing ranitidine versus placebo and famotidine versus placebo will be combined to assess the effectiveness of the H2 antagonist class of drugs. We also combined routes of administration, for example, studies comparing intravenous ranitidine and oral ranitidine were combined.
Subgroup analysis and investigation of heterogeneity
We undertook subgroup analyses for all the prespecified primary and secondary outcomes where the data were available. No data-driven post hoc analyses were undertaken. Had there been sufficient data we would have conducted planned subgroup analyses classifying whole trials by interaction tests as described by Deeks 2001.
We had planned to carry out the following subgroup analyses: elective versus emergency caesarean section. However, there were insufficient data to carry out these subgroup analyses.
Sensitivity analysis
We had planned to carry out sensitivity analyses to explore the effect of trial quality for important outcomes in the review. However, as the majority of studies were of poor quality and there were little data for each comparison, this was not feasible. We will, however, consider doing this in future updates, as more data are accumulated from published randomised controlled trials.
RESULTS
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification.
Results of the search
One hundred and thirty-nine studies were identified in the search which covered interventions for reducing nausea, vomiting and aspiration pneumonitis at caesarean section. Of these, 23 studies were identified that related to interventions for reducing aspiration pneumonitis and 66 were assigned to the review on nausea and vomiting (Griffiths 2009).
Included studies
Of the 23 studies that were identified relating to the reduction of aspiration pneumonitis, 22 were included involving 2658 women (Dewan 1985; Elhakim 2005; Ewart 1990; Frank 1984;Hong 2004; Husemeyer 1980; Iqbal 2000; Jasson 1989; Lin 1996; Ormezzano 1990; Orr 1993; Ostheimer 1982; Ozkan 2000;Pickering 1980; Rocke 1994; Rout 1993; Tripathi 1995; Tryba 1983; Wig 1987; Yau 1992; Zoroglu 1999; Zue 1999). One study was put in section on ’Awaiting classification’ whilst we try to contact authors (Karamanlioglu 1995).
Excluded studies
The excluded studies are listed in the reference section under excluded studies and the table ’Characteristics of excluded studies’ states the reasons for exclusion from this review. Most of the studies that were excluded assessed interventions for reducing nausea and vomiting at caesarean section rather than reducing the risk of aspiration pneumonitis, though the search strategy included both these circumstances in line with the original protocol. The studies looking at nausea and vomiting are included in the review of interventions for reducing nausea and vomiting at caesarean section (Griffiths 2009).
Risk of bias in included studies
Overall, the quality of studies was difficult to assess. We did not assess any trial protocols so we were unable to assess possible selective reporting bias. In addition, most trials reported only a few outcomes; therefore it is unclear whether or not there is selective reporting bias. Only one study had both adequate sequence generation and concealment allocation (Rout 1993), and the remainder were unclear with two studies having inadequate allocation concealment (Dewan 1985; Jasson 1989). The assessment of incomplete data showed half the studies having low risk of bias here and in the other half it was unclear. Only one study met the criteria of low risk of bias on all the assessment criteria (Rout 1993) (Figure 1).
Figure 1 . Methodological quality summary: review authors’ judgements about each methodological quality item for each included study.
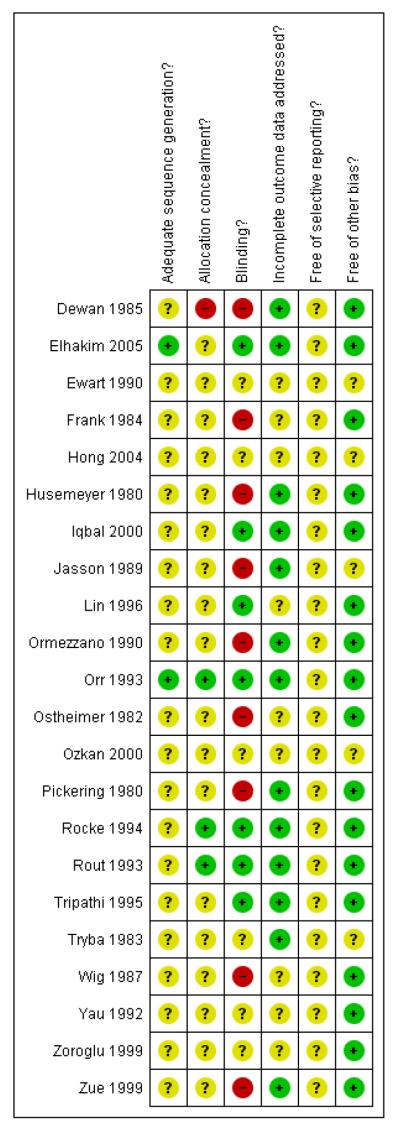
Allocation
The random sequence generation was adequate in only two studies (Elhakim 2005; Rout 1993), with the remainder of the studies being unclear. Concealment allocation was adequate in only two studies (Rocke 1994; Rout 1993); in two studies it was inadequate (Dewan 1985; Jasson 1989); and in the rest it was unclear. Thus, there was only one study (Rout 1993) where both generation and concealment were adequate.
Blinding
Blinding was assessed as adequate in six studies (Elhakim 2005; Iqbal 2000; Lin 1996; Rocke 1994; Rout 1993; Tripathi 1995), inadequate in nine (Dewan 1985; Frank 1984; Husemeyer 1980;Jasson 1989; Ormezzano 1990; Ostheimer 1982; Pickering 1980; Wig 1987; Zue 1999) and unclear in the rest of the studies. This is disappointing for studies assessing drug administration.
Incomplete outcome data
Eleven studies were assessed as having adequate reporting of outcome data (Dewan 1985; Elhakim 2005; Husemeyer 1980; Iqbal 2000; Ormezzano 1990; Pickering 1980; Rocke 1994; Rout 1993;Tripathi 1995; Tryba 1983; Zue 1999) with the remainder being assessed as unclear.
Selective reporting
We did not assess the trial protocol so it is unclear if there is any selective reporting bias. Although we did not specifically identify any selective reporting bias, we were unable to exclude the possibility.
Other potential sources of bias
All the studies appeared free from other biases with the exception of four studies where it was assessed as unclear (Ewart 1990; Hong 2004; Tryba 1983; Zoroglu 1999).
Effects of interventions
This review includes 22 studies and 16 meta-analyses, involving 2658 women.
(1) Antacids versus placebo/no treatment (three studies, 168 women)
Three studies compared antacids with placebo (Dewan 1985;Ormezzano 1990, Wig 1987).
The studies were of doubtful quality with sequence generation being unclear in all three studies, and allocation concealment being either unclear in two (Ormezzano 1990; Wig 1987) or inadequate in one (Dewan 1985). Data collection appeared complete in two studies (Dewan 1985; Ormezzano 1990) and unclear in one study (Wig 1987). There appeared to be no other sources of bias apparent in any of the studies.
Primary outcomes
Antacid, compared to placebo, was associated with a statistically significant reduction in:
intragastric pH less than 2.5 at intubation (risk ratio (RR) 0.17, 95% confidence interval (CI) 0.09 to 0.32, two studies, 108 women, Analysis 1.3).
Other primary outcomes were not assessed.
Secondary outcomes
Antacid, compared with placebo, was also associated with a statistically significant reduction in:
intragastric pH less than 2.5 at extubation (RR 0.21, 95% CI 0.09 to 0.48, one study, 86 women, Analysis 1.21).
Other secondary outcomes were not assessed.
Outcomes not pre-specified
There was no statistically significant difference identified for:
’risk of aspiration’ (RR 0.07, 95% CI 0.00 to 1.04, one study, 22 women, Analysis 1.25).
For other non-prespecified outcomes, see Analyses 1.23 to 1.27.
(2) H2 antagonists versus placebo/no treatment (six studies, 385 women)
Six studies compared H2 antagonists with placebo/no treatment (Iqbal 2000; Lin 1996; Ozkan 2000; Tryba 1983; Zoroglu 1999;Zue 1999).
The studies were of doubtful quality with unclear sequence generation and concealment allocation in the six studies. Blinding was adequate in two studies (Iqbal 2000; Lin 1996), inadequate in one study (Zue 1999) and unclear in the remainder. Data collection appeared complete in three studies (Iqbal 2000; Tryba 1983; Zue 1999) and unclear in the remainder of studies. The studies seemed to be free of other sources of bias, although this was difficult to assess due to lack of information in some.
Primary outcomes
In women undergoing elective caesarean section, H2 antagonists compared to placebo showed a statistically significant reduction in:
intragastric pH less than 2.5 at intubation (RR 0.09, 95% CI 0.05 to 0.18, two studies, 170 women, Analysis 2.3);
intragastric volume greater than 0.4 mg/kg at intubation (average RR 0.08, 95% CI 0.01 to 0.86, two studies, 170 women, random effects [T2 = 1.96, Chi2 p = 0.10, I2 = 63%], Analysis 2.4).
Other primary outcomes were not assessed.
Secondary outcomes
One study reported on intragastric pH at extubation and found a statistically significant reduction in:
risk of pH less than 2.5 (RR 0.08, 95% CI 0.01 to 0.56, one study, 30 women, Analysis 2.21).
Other secondary outcomes were not assessed.
Outcomes not pre-specified
H2 antagonists were associated with a statistically significant reduction in:
’Risk of aspiration’ at intubation (average RR 0.07, 95% CI 0.01 to 0.33, 4 studies, 255 women, random effects [T2 = 1.35, Chi2 p = 0.11, I2 = 51%], Analysis 2.25)
Risk of aspiration’ at extubation (RR 0.17, 95% CI 0.01 to 4.03, 2 studies, 125 women [although only one study of 75 women was estimable], Analysis 2.26).
For other non-prespecified outcomes see Analyses 2.23 to 2.28.
(3) Proton pump antagonists versus placebo/no treatment (2 studies, 130 women)
Two studies (Lin 1996; Ozkan 2000) compared proton pump antagonists with placebo or no treatment. Both of these studies were of doubtful quality as allocation sequence generation, allocation concealment, blinding and incomplete data assessment were unclear. The study by Lin et al reported that the trial was conducted in a “double blind manner” but it was unclear who was blinded or how this was done (Lin 1996). Both studies were judged to be free of other biases although there was limited information on which to judge this.
Primary outcomes
Proton pump antagonists, when compared with placebo, were associated with a statistically significant reduction in:
intragastric pH less than 2.5 at intubation (RR 0.26 95% CI 0.14, 0.46, one study, 80 women, Analysis 3.3);
Intragastric volume greater than 0.4 ml/kg (RR 0.46, 95% CI 0.19, 1.09, one study, 80 women, Analysis 3.4).
Other primary outcomes were not assessed.
Secondary outcomes
Neither of these studies reported on our pre-specified secondary outcomes.
Outcomes not pre-specified
Proton pump antagonists were associated with a significant reduction in:
’risk of aspiration’ compared with placebo (average RR 0.14, 95% CI 0.03 to 0.74, two studies, 130 women, random effects [T2 = 0.60, Chi2 p = 0.22, I2 = 34%], Analysis 3.24).
For other non-prespecified outcomes, see Analyses 3.23 to 3.27.
(4) Prokinetic drugs versus placebo/no treatment (one study, 50 women)
One study compared a prokinetic drug (metoclopramide) to no treatment (Ozkan 2000). The quality of this study was doubtful as allocation sequence generation, allocation concealment, blinding and incomplete data assessment were unclear. The study was judged to be free of other biases although there was limited information on which to judge this.
Primary outcomes
This study did not measure any of the primary outcomes that were pre-specified in this review.
Secondary outcomes
This study did not measure any of the secondary outcomes that were pre-specified in this review.
Outcomes not pre-specified
When prokinetic drugs were compared with no treatment, there was no statistically significant difference identified in:
’risk of aspiration’ (RR 0.67, 95% CI 0.33 to 1.35, one study, 50 women, Analysis 4.23). Though it is possible the study was too small to identify a difference.
(5) Non-pharmacological interventions versus placebo/no treatment (one study, 40 women)
One study compared the use of intravenous 5% dextrose solution to Normal Saline solution prior to induction of anaesthesia in 40 women undergoing elective caesarean section in South Korea (Hong 2004). This study was of doubtful quality as there was no information given to assess adequacy of sequence generation, allocation concealment, blinding, incomplete outcome data assessment. It was not clear if the study was free of any other bias, due to the lack of detail available.
Primary outcomes
This study did not report on any of the primary outcomes prespecified in this review.
Secondary outcomes
This study did not report on any of the secondary outcomes pre-specified in this review.
Outcomes not pre-specified
There was no statistically significant difference identified in:
’risk of aspiration’ (not clearly defined) (RR 0.50, 95% CI 0.18 to 1.40, one study, 40 women, Analysis 5.23).
(6) Antacids + H2 antagonists versus placebo/no treatment (one study, 89 women)
One study compared the use of antacids and H2 antagonists (in combination) with no treatment (Ormezzano 1990). This study was of doubtful quality as sequence generation and allocation concealment were unclear. Neither the participants nor the outcome assessors were blinded. However, data completeness were adequately assessed and this study was judged to be free of selective reporting bias or any other type of bias.
Primary outcomes
The combination of an ’antacid plus an H2 receptor antagonist’ compared with placebo showed:
a statistically significant reduction in risk of gastric pH less than 2.5 at intubation (RR 0.02, 95% CI 0.00 to 0.15, one study, 89 women, Analysis 6.3).
Other primary outcomes were not assessed.
Secondary outcomes
The combination also showed a statistically significant reduction in:
risk of gastric pH less than 2.5 at extubation (RR 0.03, 95% CI 0.00 to 0.24, one study, 89 women, Analysis 6.21).
Other secondary outcomes were not assessed.
Outcomes not pre-specified
For non-prespecified outcomes, see Analyses 6.23 to 6.24.
(7) H2 antagonists + prokinetic drugs versus placebo/no treatment (one study, 50 women)
One study compared the use of H2 antagonists plus prokinetic drugs (in combination) with no treatment (Iqbal 2000). This study was of doubtful quality as sequence generation and allocation concealment were unclear. Participants and clinicians were reported to be blinded. There was no description of data completeness and it was unclear if the study was free from selective reporting bias or any other types of bias.
Primary outcomes
The combination of ’H2 antagonists plus prokinetic drugs’ were associated with:
a statistically significant reduction gastric pH less than 2.5 after induction (RR 0.03, 95% CI 0.00, 0.48, one study, 50 women, Analysis 7.3).
Secondary outcomes
This study did not report on any of the secondary outcomes pre-specified in this review.
Outcomes not pre-specified
The was a statistically significant reduction in women:
at ’risk of aspiration’ (RR 0.03, 95% CI 0.00, 0.51, one study, 50 women, Analysis 7.23).
For non-prespecified outcomes, see Analyses 7.24 to 7.26.
(8) Antacids versus H2 antagonists (four studies, 175 women)
Four studies (Frank 1984; Husemeyer 1980; Ostheimer 1982;Pickering 1980) compared antacids versus H2 antagonists.
All of these studies were of doubtful quality as allocation sequence generation and allocation concealment were unclear and blinding was not done. Two studies (Husemeyer 1980; Pickering 1980) were judged to have adequate incomplete data assessment; while there was not enough information to assess this in the other two studies (Frank 1984; Ostheimer 1982). All four studies were considered to be free of selective reporting or other bias.
Primary outcomes
Compared with H2 receptor antagonists, antacid use was associated with:
a statistically significant reduction in risk of pH less than 2.5 at intubation (RR 0.07, 95% CI 0.01 to 0.52, two studies, 135 women, Analysis 8.3).
One study (Frank 1984) also examined the effect of this comparison in women undergoing emergency caesarean section but did not observe any events (pH less than 2.5 at intubation) in either group. Other primary outcomes were not assessed.
Secondary outcomes
None of the prespecified secondary outcomes in this review were reported in these studies.
Outcomes not pre-specified
There was no significant difference identified in:
’risk of aspiration’ between the two groups (RR 1.00, 95% CI 0.18, 5.46, one study, 16 women, Analysis 8.23). At risk of aspiration was defined as pH less than 2.5 and gastric volume of at least 25 ml.
For other non-prespecified outcomes, see Analyses 8.24 to 8.26. In contrast to the above finding, these data showed a benefit for H2 receptor antagonists, for the outcome of gastric volume measured as a continuous variable, as expected due to the nature of the treatments. However, one small study (24 women) evaluated gastric pH as a continuous variable and showed a benefit for H2 receptor antagonists (Ostheimer 1982).
(9) Antacids versus prokinetic drugs (no studies)
There were no studies that assessed this comparison.
(10) H2 antagonists versus proton pump antagonists (four studies, 332 women)
Four studies (Ewart 1990; Lin 1996; Tripathi 1995; Yau 1992) compared H2 antagonists with proton pump antagonists.
All of these studies were of doubtful quality as allocation sequence generation and allocation concealment were unclear. Blinding was done only in two studies (Lin 1996; Tripathi 1995). Only one study (Tripathi 1995) was judged to have adequate incomplete data assessment. All four studies were considered to be free of selective reporting bias or other bias, except for Ewart 1990 where there was not enough information to assess this.
Primary outcomes
Compared with proton pump inhibitors, H2 receptor antagonists showed a statistically significant reduction in:
risk of pH less than 2.5 for women undergoing elective caesarean (RR 0.39, 95% CI 0.16 to 0.97, one study, 120 women, Analysis 10.3).
Secondary outcomes
None of these studies reported on the secondary outcomes that were pre-specified in this review
Outcomes not pre-specified
All four studies (Ewart 1990; Lin 1996; Tripathi 1995; Yau 1992) on women undergoing elective and emergency caesarean section reported on ’at risk of aspiration’.
There was no statistically significant difference in risk identified (average RR 0.93, 95% CI 0.20 to 4.37, four studies, 323 women, random effects [T2 = 0.80, Chi2 p = 0.22, I2 = 32%], Analysis 10.23).
For other non-prespecified outcomes, see Analyses 10.24 to 10.27.
(11) Antacids + H2 antagonists versus antacids (two studies, 714 women)
Two studies compared the combined use of antacids and H2 antagonists versus antacids (Ormezzano 1990; Rout 1993). One study (Rout 1993) was judged to be of good quality with an adequate score on each of the domains that were used to assess risk of bias. In the other study (Ormezzano 1990), sequence generation and allocation were unclear and there was no blinding. However, incomplete outcome data were addressed and the study was judged to be free of selective reporting or other bias.
Primary outcomes
In women undergoing both emergency and elective caesarean section, a combination of antacid plus H2 receptor antagonists compared with antacids alone showed:
a significant reduction in risk of pH less than 2.5 at intubation (RR 0.12, 95% CI 0.02 to 0.92, one study, 119 women, Analysis 11.3).
Other primary outcomes were not assessed.
Secondary outcomes
None of the secondary outcomes pre-specified in this review were reported by these studies.
Outcomes not pre-specified
There was a significant reduction in risk of acid aspiration for women undergoing emergency caesarean section with a combination of antacid plus H2 receptor antagonists compared with antacids alone
(RR 0.10, 95 % CI 0.02 to 0.45, one study, 595 women, Analysis 11.27).
For other non-prespecified outcomes, see Analyses 11.23 to 11.26.
(12) H2 antagonists + prokinetic drugs versus antacids (no studies)
There were no studies that assessed this comparison.
(13) Proton pump agonists + prokinetics versus proton pump agonists (no studies)
There were no studies that assessed this comparison.
(14) H2 antagonist versus Tramadol (one study, 60 women)
One study compared H2 receptor antagonists with Tramadol (both by intramuscular injection) in 60 women undergoing elective caesarean section (Elhakim 2005). Although most aspects of the assessment of risk of bias were assessed as low risk, allocation concealment was uncertain and this gives an overall uncertain level of risk of bias for the study.
Primary outcomes
Compared with tramadol, H2 antagonists showed a statistically significant increase in:
risk of intragastric volume greater than 0.4mg/kg at intubation (RR 5.00, 95% CI 1.03 to 24.28, one study, 90 women, Analysis 14.4).
Other primary outcomes were not assessed.
Secondary outcomes
There was no statistically significant difference identified in:
nausea (RR 1.38, 95% CI 0.64 to 2.93, one study, 60 women, Analysis 14.5). Other secondary outcomes were not assessed.
Outcomes not pre-specified
This study also included ’at risk of aspiration’ defined as gastric volume greater than 0.4 ml/kg and pH less than 2.5, but there were no events observed in either group for this outcome.
(15) Antacids + H2 antagonists versus proton pump antagonists (one study, 109 women)
One study compared antacids plus H2 receptor antagonists with proton pump antagonists in women undergoing emergency caesarean section (Yau 1992). The study was of unclear quality with insufficient information to assess the main aspects of risk of bias.
Primary outcomes
This study did not measure any of the primary outcomes that were pre-specified in this review.
Secondary outcomes
This study did not measure any of the secondary outcomes that were pre-specified in this review.
Outcomes not pre-specified
Compared with proton pump antagonists, H2 receptor antagonists showed a statistically significant reduction in:
’risk of gastric aspiration’ (RR 0.12, 95% CI 0.02 to 0.91, one study, 108 women, Analysis 15.23).
(16) Proton pump antagonist + antacid versus proton pump antagonist (one study, 113 women)
One study assessed proton pump antagonists plus antacids with proton pump antagonist alone in women undergoing emergency caesarean section (Yau 1992). The study was of unclear quality with insufficient information to assess the main aspects of risk of bias.
Primary outcomes
This study did not measure any of the primary outcomes that were pre-specified in this review.
Secondary outcomes
This study did not measure any of the secondary outcomes that were pre-specified in this review.
Outcomes not pre-specified
There was no statistically significant difference identified in:
risk of gastric aspiration between the two interventions (RR 0.33. 95% CI 0.10 to 1.15, one study, 113 women, graph 16.23).
(17) H2 antagonist + prokinetic versus H2 antagonist (one study, 50 women)
One study assessed a combination of H2 receptor antagonists plus prokinetic versus H2 receptor antagonists alone in women undergoing elective caesarean section (Iqbal 2000). The study was unclear regarding the randomisation sequence generation and allocation concealment and was thus of unclear quality.
Primary outcomes
This study did not measure any of the primary outcomes that were pre-specified in this review.
Secondary outcomes
This study did not measure any of the secondary outcomes that were pre-specified in this review.
Outcomes not pre-specified
There was no significant difference in risk of gastric aspiration between the two interventions (Analysis 17.23).
For non prespecified outcomes, see Analyses 17.24, 17.26 and 17.27.
DISCUSSION
Summary of main results
Although the studies were generally of poor quality, the findings from this review have shown that:
compared to no treatment or placebo, antacids, H2 antagonists and proton pump antagonists each reduce the risk of intragastric pH less than 2.5 at intubation. The studies on prokinetic drugs and non-pharmacological interventions did not assess this outcome and, in addition, were probably too small to be able identify any differences;
when antacids were compared with H2 antagonists, the findings were unclear as to which drug might be more effective for increasing gastric pH, although antacid use was associated with increase in gastric volume;
H2 antagonists were associated with a reduced risk of intragastric pH less than 2.5 at intubation when compared with proton pump antagonists;
the combination of ’antacids plus H2 antagonists’ or ’prokinetic drugs plus H2 antagonists’ also reduced the risk of intragastric pH less than 2.5 at intubation, when compared to placebo or no treatment;
when compared to antacid use only, the combination of ’antacids plus H2 antagonists’ was associated with a reduction in the risk of intragastric pH less than 2.5 at intubation.
Overall completeness and applicability of evidence
Aspiration pneumonitis is a rare outcome and therefore the primary outcome measure in the studies that we identified were surrogate measures, i.e. intragastric pH and intragastric volume. The validity of these surrogate markers, however, is uncertain as it is based on work on animal experiments from 1974 (Roberts 1974). Many studies have defined and reported high risk of aspiration as a combination of low intragastric pH (less than 2.5) and raised intragastric volume (greater than 25 ml). This combined measure was not a pre-specified outcome in our review but we have included and presented the data on this for completeness. The studies did not answer the broader question of whether the surrogate markers (of pH and gastric volume) actually correlate with clinical outcome in the context of aspiration pneumonitis.
All but two of the studies that we identified in this review included women who had caesarean section (CS) under general anaesthesia. One study (Lin 1996) studied women who had CS under spinal anaesthesia and the type of anaesthesia used was unclear in one study (Zue 1999). The majority of studies included women who had elective CS (N = 16), five studies included women who had emergency CS, hence we are unable to draw conclusions about the differences between elective and non-elective CS. The findings of this review are generally applicable for women having CS under general anaesthesia (or those who convert from regional to general anaesthesia). Aspiration under regional anaesthesia is exceptionally rare, but may occur in the presence of other serious clinical problems such as seizures and life threatening haemorrhage.
Quality of the evidence
The quality of studies included in this review was generally poor. Only one study was assessed to have adequate sequence generation (Rout 1993), but this study did not report on any of the pre- specified outcomes of this review. It was unclear whether or not randomisation sequence generation and allocation concealment were adequate in the majority of studies. The majority of studies were not blinded, although this could have been feasibly done.
Potential biases in the review process
The possibility of introducing bias was present at every stage of reviewing process. We attempted to minimise bias in a number of ways; two review authors assessed eligibility for inclusion, carried out data extraction and assessed risk of bias. Each worked independently. Nevertheless, the process of assessing risk of bias, for example, is not an exact science and includes many personal judgements.
Agreements and disagreements with other studies or reviews
Current practice in the UK mostly includes the administration of the combination of antacids and H2 antagonists prior to CS (Thomas 2001). However, this is not routine practice in many centres worldwide. The findings from this review suggest that the combined use of antacids and H2 antagonists have a role in reducing intragastric pH less than 2.5, and hence possibly in reducing the risk of aspiration pneumonitis during CS, particularly under general anaesthesia.
AUTHORS’ CONCLUSIONS
Implications for practice
In summary, the quality of the evidence was poor, but the findings suggest that the combination of antacids plus H2 antagonists was shown to be more effective than no intervention, and superior to antacids alone in increasing gastric pH. When a single agent is used, antacids alone are superior to H2 antagonists, which are superior to proton pump inhibitors for increasing gastric pH. The effects of treatments on gastric volume are less consistently reported. These findings are relevant for all women undergoing caesarean section, particularly those under general anaesthesia. Whether women undergoing caesarean section under regional anaesthesia should receive aspiration prophylaxis is a clinical judgement; however, since the treatments are relatively well tolerated, and inexpensive, their use should be strongly considered in view of the potential of benefit, particularly as aspiration still is a cause of maternal mortality.
Implications for research
This review confirms the efficacy of many of the commonly used aspiration prophylaxis regimens compared to placebo in reducing gastric pH and volume. However, many studies, particularly those examining combinations of different modalities of prophylaxis, are small and of generally poor quality. Large well-designed studies that include women having emergency and elective caesarean section under regional and general anaesthesia are required to confirm the conclusions of this review.
Further work is required to validate the suitability of surrogate markers (of pH and gastric volume) for clinical outcomes in the context of aspiration pneumonitis.
PLAIN LANGUAGE SUMMARY.
Interventions at caesarean section for reducing the risk of lung damage from inhaling stomach contents during anaesthesia
Stomach contents can regurgitate up the gullet into the wind pipe and enter the lungs when there is no cough reflex, e.g. during general anaesthesia. Solid food can block airways and cause breathing difficulties. The acidic liquid from the stomach can damage the lungs. This is called aspiration pneumonitis or Mendelsohn’s syndrome. It can lead to serious illness or even death. Many caesarean sections now are undertaken using epidural or spinal anaesthesia, and here the risk is much lower because the woman stays awake and the cough reflex remains intact. A breathing tube, which provides a seal, is normally placed in the windpipe when setting up a general anaesthetic to try to prevent this problem. However, aspiration can still occur before the tube is inserted and when it is removed. It is thought that both the acidity and amount of fluid inhaled contribute to how much damage occurs in the lungs in the event of inhalation of the fluid into the lungs and how sick people become.
This review found 22 studies involving 2658 women looking at interventions given prior to caesarean section for reducing the risk of aspiration. There were several different drugs and drug combinations being considered and the studies were generally of poor or questionable quality. Antacids (like sodium citrate), H2 receptor antagonists (like ranitidine), proton pump antagonists (like omeprazole), all reduced the acidity of the stomach contents. An antacid plus an H2 receptor antagonist also reduced acidity. In theory, a combination like this, where the antacid acts quickly and the H2 receptor antagonists takes a little longer, should protect at periods of greatest risk, i.e. the beginning and end of the procedure (i.e. intubation and extubation). More research is needed to identify the best combination of drugs and to check for possible adverse effects.
ACKNOWLEDGEMENTS
Eugene HC Liu, Department of Anaesthesia, National University Hospital, Singapore, Singapore.
Zoe Roberts, Department of Primary Care and Public Health, School of Medicine, Cardiff University.
Alison Ledward, Sara Roden-Scott, Gillian Kenyon, Edgardo Abalos, Almira Opardija, Julie Arbon and Alex Balistreri for translating articles relevant to this review.
As part of the pre-publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group’s international panel of consumers and the Group’s Statistical Adviser.
SOURCES OF SUPPORT
Internal sources
The University of Liverpool, UK.
External sources
National Instituite for Health Research, UK.
NIHR NHS Cochrane Collaboration Programme Grant Scheme award for NHS-prioritised centrally-managed, pregnancy and childbirth systematic reviews: CPGS02
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | Randomised women to 3 groups. | |
| Participants | Healthy women at term scheduled for elective CS under GA. N = 32. |
|
| Interventions |
|
|
| Outcomes | Gastric volume and pH postintubation. | |
| Notes | We excluded data from the group that was given antacid > 60min pre-op as the optimum effectiveness for giving antacids is within 60 min of the operation | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | “…randomly assigned”. |
| Allocation concealment? | No | Not described. |
| Blinding? All outcomes |
No | No mention of blinding. |
| Incomplete outcome data addressed? All outcomes |
Yes | No loss to follow up. |
| Free of selective reporting? | Unclear | We did not assess the trial protocol. |
| Free of other bias? | Yes | No evidence of other bias. |
| Methods | RCT. | |
| Participants | Pregnant women ASA 1 undergoing elective CS. N = 60. |
|
| Interventions |
|
|
| Outcomes | Gastric pH and volume. Apgar scores. Frequency and severity of nausea. Pain scores. Blood gases - umbilical, venous and arterial blood gases |
|
| Notes | Some of the data have been presented as median and range and therefore not used | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Computer-generated random number. |
| Allocation concealment? | Unclear | Not described. |
| Blinding? All outcomes |
Yes | Paediatrician and anaesthetist. |
| Incomplete outcome data addressed? All outcomes |
Yes | No loss of participants. |
| Free of selective reporting? | Unclear | We did not assess the trial protocol. |
| Free of other bias? | Yes | No evidence for other bias. |
| Methods | RCT. | |
| Participants | Scheduled CSs under general anaesthesia in healthy uncomplicated pregnancies of at least 36 weeks N = 70. |
|
| Interventions |
|
|
| Outcomes | Gastric volume and ph measured after in the induction of anaesthesia and on completion of surgery Apgar scores. |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Not described. |
| Allocation concealment? | Unclear | Not described. |
| Blinding? All outcomes |
Unclear | Not described. |
| Incomplete outcome data addressed? All outcomes |
Unclear | 70 recruited, 5 withdrawn. |
| Free of selective reporting? | Unclear | We did not assess the trial protocol. |
| Free of other bias? | Unclear | Unclear how many women were approached but did not want to take part in the trial - this may contribute to selection bias |
| Methods | RCT. | |
| Participants | Pregnant women (ASA 1 & 2) at term having either a emergency or elective CS under general anaesthesia N = 42. |
|
| Interventions |
Elective CS interventions
Emergency CS interventions
|
|
| Outcomes | Gastric pH and gastric volume, neonatal Apgar scores. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Not described. |
| Allocation concealment? | Unclear | Not described. |
| Blinding? All outcomes |
No | No information - unlikely due to the type of interventions involved |
| Incomplete outcome data addressed? All outcomes |
Unclear | Participant loss and exclusion not described. |
| Free of selective reporting? | Unclear | We did not assess the trial protocol. |
| Free of other bias? | Yes | No evidence of other bias. |
| Methods | RCT. | |
| Participants | Pregnant women undergoing elective CS. N = 40. |
|
| Interventions |
|
|
| Outcomes | Gastric volume and ph reported as “at risk of aspiration”. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Not described. |
| Allocation concealment? | Unclear | Not described. |
| Blinding? All outcomes |
Unclear | No details given. |
| Incomplete outcome data addressed? All outcomes |
Unclear | Insufficient detail to assess. |
| Free of selective reporting? | Unclear | We did not assess the trial protocol. |
| Free of other bias? | Unclear | Insufficient detail to assess. |
| Methods | RCT. | |
| Participants | Elective caesarean section of 37 weeks or more gestation for non acute obstetric indications N = 62. |
|
| Interventions |
|
|
| Outcomes | Maternal gastric pH and volume (postinduction of anaesthesia) | |
| Notes | pH data not used as median and range given. Magnesium trisilicate is a particulate antacid and mostly not to be used nowadays |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Not described. |
| Allocation concealment? | Unclear | Not described. |
| Blinding? All outcomes |
No | No details of blinding. |
| Incomplete outcome data addressed? All outcomes |
Yes | All participants accounted for. |
| Free of selective reporting? | Unclear | We did not assess the trial protocol. |
| Free of other bias? | Yes | No evidence of other bias. |
| Methods | RCT. | |
| Participants | ASA 1-2 elective CS. N = 75. |
|
| Interventions |
|
|
| Outcomes | Gastric volume and pH. Risk of aspiration. | |
| Notes | Unclear what dose of ranitidine was given to women. | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | “…randomised”. |
| Allocation concealment? | Unclear | Not described. |
| Blinding? All outcomes |
Yes | Double-blind manner. |
| Incomplete outcome data addressed? All outcomes |
Yes | Assume ITT. |
| Free of selective reporting? | Unclear | We did not assess the trial protocol. |
| Free of other bias? | Yes | No evidence for other bias. |
| Methods | RCT. | |
| Participants | Women undergoing an elective CS under GA. N = 52. |
|
| Interventions |
|
|
| Outcomes | Gastric pH and gastric volume. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Method of allocation not stated. |
| Allocation concealment? | Unclear | Method of allocation not stated. |
| Blinding? All outcomes |
No | Not blinded. |
| Incomplete outcome data addressed? All outcomes |
Yes | No outcome data on 1 patient due to pyloric reflux. |
| Free of selective reporting? | Unclear | We did not assess the trial protocol. |
| Free of other bias? | Unclear | Difficult to assess as limited information. |
| Methods | RCT. | |
| Participants | Pregnant women ASA 1-2, aged 21-43 years old scheduled for elective CS under regional anaesthesia N = 160. |
|
| Interventions |
|
|
| Outcomes | GastricVolume and pH. Percentage of women at risk of aspiration pH < 2.5 and volume > 0.4 ml/kg | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Not described. |
| Allocation concealment? | Unclear | Not described. |
| Blinding? All outcomes |
Yes | “…double blind”. |
| Incomplete outcome data addressed? All outcomes |
Unclear | 5 women excluded as time from premed to CS < 3 hours - unclear which groups these women were excluded from |
| Free of selective reporting? | Unclear | We did not assess the trial protocol. |
| Free of other bias? | Yes | No evidence of other bias. |
| Methods | RCT. | |
| Participants | Pregnant ASA 1 & 2 women undergoing emergency and elective CS under general anaesthesia N = 147. |
|
| Interventions |
|
|
| Outcomes | Gastric pH. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Not described. |
| Allocation concealment? | Unclear | Not described. |
| Blinding? All outcomes |
No | No details of blinding. |
| Incomplete outcome data addressed? All outcomes |
Yes | All women accounted for. |
| Free of selective reporting? | Unclear | We did not assess the trial protocol. |
| Free of other bias? | Yes | No evidence of other bias. |
| Methods | RCT. | |
| Participants | Healthy pregnant women with an uncomplicated pregnancy of at least 36 weeks to be delivered by elective CS under general anaesthesia N = 94. |
|
| Interventions |
|
|
| Outcomes | Postintubation and pre-extubation gastric pH and volume, Apgar scores, neuro behavioural and adaptive scoring system (NACS), plasma and amniotic fluid omeprazole levels and risk of aspiration (pH < 2.5 and gastric volume ≥ 25 ml) | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Computer-generated schedule. |
| Allocation concealment? | Yes | Pre-packed packs with matching placebo capsules and injections |
| Blinding? All outcomes |
Yes | Women, clinicians and outcome assessors were blinded. |
| Incomplete outcome data addressed? All outcomes |
Yes | No postrandomisation exclusions; analysis was by intention to treat |
| Free of selective reporting? | Unclear | We did not assess the trial protocol. |
| Free of other bias? | Yes | No evidence of other bias. |
| Methods | RCT. | |
| Participants | Pregnant women undergoing elective CS under general anaesthesia N = 24. |
|
| Interventions |
|
|
| Outcomes | Gastric volume and pH. Intrapartum and postpartum complications. Apgar scores 1 and 5 min after birth. Neonatal gastric volumes and pH within 10 min of birth. Brazelton neonatal assessment scale | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Not described. |
| Allocation concealment? | Unclear | Not described. |
| Blinding? All outcomes |
No | No details of blinding. |
| Incomplete outcome data addressed? All outcomes |
Unclear | Not enough data to assess. |
| Free of selective reporting? | Unclear | We did not assess the trial protocol. |
| Free of other bias? | Yes | No evidence of other bias. |
| Methods | RCT. | |
| Participants | Women with singleton pregnancies undergoing elective CS under general anaesthesia N = 150. |
|
| Interventions |
|
|
| Outcomes | At risk of aspiration defined as gastric residual volume > 0.4 ml and gastric pH < 2.5, postintubation and pre-extubation | |
| Notes | Also included continuous outcomes of gastric pH and gastric residual volume but unclear if data presented are standard deviations or standard errors. We will write to the authors to clarify. In the mean time we have not entered continuous data | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Not described. |
| Allocation concealment? | Unclear | Not described. |
| Blinding? All outcomes |
Unclear | Not described. |
| Incomplete outcome data addressed? All outcomes |
Unclear | Not enough data to assess. |
| Free of selective reporting? | Unclear | We did not assess the trial protocol. |
| Free of other bias? | Unclear | Difficult to assess as limited information given. |
| Methods | RCT. | |
| Participants | Healthy pregnant women undergoing elective CS under general anaesthesia N = 17. |
|
| Interventions |
|
|
| Outcomes | Gastric pH and volume (intraoperative dilution technique) and ’at risk’ pH < 2.5 and volume > 25 ml | |
| Notes | Also measured intraoperative gastric volume using dilution technique | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Not described. |
| Allocation concealment? | Unclear | Not described. |
| Blinding? All outcomes |
No | No blinding reported. |
| Incomplete outcome data addressed? All outcomes |
Yes | All women accounted for. |
| Free of selective reporting? | Unclear | We did not assess the trial protocol. |
| Free of other bias? | Yes | No evidence for other bias. |
| Methods | RCT. | |
| Participants | Term singleton pregnancies requiring emergency CS under general anaesthesia N = 541. |
|
| Interventions |
NB: Both groups received 10mg IV metoclopramide + 0.3 M sodium citrate 30 ml orally |
|
| Outcomes | Volume and pH of stomach contents postintubation and pre extubation. Risk of aspiration | |
| Notes | Apgar score and gastric aspirate data is presented as medians and range and therefore not input | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Sequence generation not described. |
| Allocation concealment? | Yes | Pre randomised identical numbered vials. |
| Blinding? All outcomes |
Yes | Women, clinician and outcome assessor. |
| Incomplete outcome data addressed? All outcomes |
Yes | 722 recruited and randomised, 181 withdrawn as per exclusion criteria |
| Free of selective reporting? | Unclear | Data analysed in groups that were assigned but did not measure outcome in 3 controls and 6 in the study group - unable to get enough aspirate. Also we did not assess the trial protocol |
| Free of other bias? | Yes | No evidence of other bias. |
| Methods | RCT | |
| Participants | Women with term singleton pregnancy, emergency CS under general anaesthesia N = 595. |
|
| Interventions |
|
|
| Outcomes | Gastric pH and gastric volume - postintubation and pre-extubation. Risk of aspiration | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Sequence generation not described. |
| Allocation concealment? | Yes | Pre randomised identical numbered ampoules. |
| Blinding? All outcomes |
Yes | Double blind, identical preparation pre-prepared. |
| Incomplete outcome data addressed? All outcomes |
Yes | 100 women excluded postrandomisation - fully accounted for but no outcome data |
| Free of selective reporting? | Unclear | We did not assess the trial protocol. |
| Free of other bias? | Yes | No evidence for other bias. |
| Methods | RCT. | |
| Participants | Healthy women, uncomplicated singleton pregnancies at term requiring emergency CS under general anaesthesia N = 80. |
|
| Interventions |
|
|
| Outcomes | Gastric volume and pH. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors℉ judgement | Description |
| Adequate sequence generation? | Unclear | Not described - “randomly assignedℍ. |
| Allocation concealment? | Unclear | Not described. |
| Blinding? All outcomes |
Yes | Double-blind manner. |
| Incomplete outcome data addressed? All outcomes |
Yes | All women accounted for. |
| Free of selective reporting? | Unclear | We did not assess the trial protocol. |
| Free of other bias? | Yes | No evidence of other bias. |
| Methods | RCT. | |
| Participants | Pregnant women presenting for elective CS with no history of gastric problems N = 30. |
|
| Interventions |
|
|
| Outcomes | Gastric volume and gastric pH. Adverse events. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Not stated. |
| Allocation concealment? | Unclear | Not stated. |
| Blinding? All outcomes |
Unclear | The translator reports blinding; however it is unclear who was blind to the treatment |
| Incomplete outcome data addressed? All outcomes |
Yes | The translator reports ’0 dropouts’. |
| Free of selective reporting? | Unclear | We did not assess the trial protocol. |
| Free of other bias? | Unclear | No evidence of other bias but uncertain due to translation. |
| Methods | RCT. | |
| Participants | Pregnant women undergoing emergency CS under general anaesthesia N = 90. |
|
| Interventions |
|
|
| Outcomes | Gastric pH measured pre-administration of Rx, postintubation and postextubation | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Not described ’randomly allocated’. |
| Allocation concealment? | Unclear | Not described. |
| Blinding? All outcomes |
No | Not described. |
| Incomplete outcome data addressed? All outcomes |
Unclear | States randomised 30 to each group but N in each group for results not given |
| Free of selective reporting? | Unclear | We did not assess the trial protocol. |
| Free of other bias? | Yes | No evidence of other bias. |
| Methods | RCT. | |
| Participants | Chinese women undergoing emergency CS. N = 162. |
|
| Interventions |
|
|
| Outcomes | Gastric volume and gastric pH. At risk of aspiration: ph < 2.5 and volume > 25 ml | |
| Notes | Continuous data presented in graphs for gastric pH and volume; means and SD not given | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Randomised on admission to labour ward. |
| Allocation concealment? | Unclear | Not described. |
| Blinding? All outcomes |
Unclear | Not described. |
| Incomplete outcome data addressed? All outcomes |
Unclear | Not described. |
| Free of selective reporting? | Unclear | We did not assess the trial protocol. |
| Free of other bias? | Yes | No evidence of other bias. |
| Methods | RCT. | |
| Participants | Women undergoing CS under general anaesthesia. N = 75. |
|
| Interventions |
|
|
| Outcomes | Postintubation and pre-extubation gastric pH and gastric volume. Arterial and umbilical blood gases. Apgar scores and 1 and 5 min | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Not described. |
| Allocation concealment? | Unclear | Not described. |
| Blinding? All outcomes |
Unclear | Not described. |
| Incomplete outcome data addressed? All outcomes |
Unclear | Not enough data to assess. |
| Free of selective reporting? | Unclear | We did not assess the trial protocol. |
| Free of other bias? | Yes | No evidence for other bias. |
| Methods | RCT. | |
| Participants | Pregnant women presenting for emergency CS for acute fetal distress, pre-eclampsia and failed trial of labour N = 60. |
|
| Interventions |
|
|
| Outcomes | Gastric pH- measured with pH meter immediately postintubation and pre extubation | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Not described. |
| Allocation concealment? | Unclear | Not described. |
| Blinding? All outcomes |
No | No reports of blinding. |
| Incomplete outcome data addressed? All outcomes |
Yes | No loss of participants reported. |
| Free of selective reporting? | Unclear | Due to translation of paper and we did not assess the trial protocol |
| Free of other bias? | Yes | No evidence of other bias. |
CS: caesarean section
hr: hour
GA: gestational age
IM: intramuscular
IV: intravenous
ITT: intention to treat
M: molar
min: minute(s)
po: by mouth
pre-op: pre-operative
RCT: randomised controlled trial
SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abboud 1984 | Study compares two different antacids with each other, sodium citrate versus Gelusil (aluminium hydroxide and magnesium hydroxide) |
| Abouleish 1999 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Ackerman 1987 | Study assessed drugs given for analgesia, not for reducing aspiration pneumonitis |
| Ackerman 1988 | Study assessed drugs given for analgesia, not for reducing aspiration pneumonitis |
| Ackerman 1989 | Study assessed drugs given for analgesia, not for reducing aspiration pneumonitis |
| Apiliogullari 2008 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Atkinson 1980 | Not an RCT |
| Avramovic 1979 | Investigated effect of post-CS intervention on abdominal distension |
| Ayorinde 2000 | Study assessed drugs given for blood pressure control, not for reducing aspiration pneumonitis |
| Ayorinde 2001 | Study assessed drugs given for blood pressure control, not for reducing aspiration pneumonitis |
| Belzarena 1993 | Study assessed drugs given for blood pressure control, not for reducing aspiration pneumonitis |
| Bifarini 1990 | Following translation, no useable data were presented. |
| Bifarini 1992 | Could not use data as the standard deviations were not presented for the mean values |
| Birnbach 1993 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Biswas 2003 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Biwas 2002 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Bonhomme 2002 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Boone 2002 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Boschi 1984 | Not an RCT; women were divided into groups. |
| Brock-Utne 1989 | Not an RCT; women were allocated to groups. |
| Brody 2008 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Bylsma-Howell 1983 | Not ITT. |
| Caba 1997 | Outcomes assessed in the nausea and vomiting review. |
| Chan 1992 | Study was a quasi-RCT. |
| Chan 1997 | Study assessed drugs given for blood pressure control, not for reducing aspiration pneumonitis |
| Charuluxananan 2003 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Chaudhuri 2004 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Chen 2005 | Study assessed acupressure for reducing nausea and vomiting, anxiety and pain in women post-CS, not for reducing aspiration pneumonitis |
| Cherian 2001 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Chestnut 1987 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Chestnut 1989 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Chung 1998 | Study assessed postcaesarean analgesia. |
| Cohen 1983 | No information on how women were allocated to groups. |
| Colman 1988 | No information on how women were allocated to groups. |
| Connelly 1997 | Study assessed drugs for reducing side effects of intrathecal opioids at CS |
| Cooper 2002 | Study assessed drugs given for blood pressure control, not for reducing aspiration pneumonitis |
| Cowan 2002 | Study assessed drugs given for analgesia, not for reducing aspiration pneumonitis |
| Dahlgren 1997 | Study assessed drugs given for analgesia, not for reducing aspiration pneumonitis |
| Dailey 1985 | Study assessed lignocaine given for analgesia, not for reducing aspiration pneumonitis |
| Dailey 1988 | Study assessed lignocaine concentrations in the blood and no clinical outcomes assessed |
| Datta 1982 | No information on how women were allocated to groups. |
| Dewan 1982 | Not described as an RCT; women were assigned to groups. |
| Duggal 1998 | Intervention looks at reducing nausea and vomiting not aspiration pneumonitis |
| Dundee 1979 | No information on how women were allocated to groups. |
| Fan 1994 | Studying the effect of different doses of bupivacaine on anaesthesia |
| Flynn 1989a | Study of effect of 400 mg cimetidine or 150 mg ranitidine or placebo on plasma levels of bupivacaine at CS |
| Flynn 1989b | Study of effect of 200 mg cimetidine plasma levels of lignocaine at CS |
| Flynn 1989c | Study of effect of 400 mg cimetidine or 150 mg ranitidine or placebo on plasma levels of lidocaine at CS |
| Fogarty 1992 | Insufficient information provided. We wrote to authors but have not received a response |
| Freeman 1999 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Fujii 1998a | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Fujii 1998b | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Fujii 1999 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Fujii 2002 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Fujii 2004 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Gaiser 2002 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Ghods 2005 | Study to test efficacy of postoperative supplemental oxygen in reducing the incidence of postoperative nausea and vomiting |
| Gutsche 1976 | Assessing ephedrine for reducing hypotension of spinal anaesthesia |
| Habib 2006 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Harmon 2000 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Hildyard 2000 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Ho 1996 | Study assessing P-6 acupressure on nausea and vomiting for post-CS pain relief |
| Ho 2006 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Hodgkinson 1983 | Not ITT analysis for primary outcome and poor randomisation exclusion rates |
| Holdsworth 1974 | Not an RCT |
| Holdsworth 1978 | Quasi-RCT. |
| Holdsworth 1980 | Not an RCT. Assessing women’s positions for GA. |
| Huang 1992 | Not relevant for outcomes of this review. |
| Hunt 1989 | Assessed fentanyl added to bupivacaine in spinal anaesthesia for effective anaesthesia but assessed Apgar scores |
| Imbeloni 1986 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Ishiyama 2001 | Analgaesia study assessing effects of fentanyl versus flurbiprofen on nausea and vomiting |
| Kang 1982 | Hypotension study assessing continuous infusion versus bolus injection of ephedrine - effect on nausea and vomiting |
| Kangas-Saarela 1990 | Study assessed drugs given for blood pressure control, not for reducing aspiration pneumonitis |
| Kasodekar 2006 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| King 1998 | Study assessed drugs given for blood pressure control not for reducing aspiration pneumonitis |
| Kjaer 2006 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Kocamanoglu 2005 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Kotelko 1989 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Lim 1991 | Not randomised. |
| Lim 2001a | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Lim 2001b | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Loughrey 2002 | Study assessed drugs given for blood pressure control not for reducing aspiration pneumonitis |
| Lussos 1992 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Mandell 1986 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Mandell 1992 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Manullang 2000 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Maranhao 1988 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| McCaughey 1981 | Not a randomised study. |
| Mebazaa 2003 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Mukherjee 2006 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Murphy 1984 | Study assessed the effect of IV metoclopramide on gastric emptying on women having elective CSs and those having emergency CSs studying both women given narcotics and those not given narcotics. Outcomes in this review not assessed |
| Ngan 2000 | Study assessed drugs given for blood pressure control, not for reducing aspiration pneumonitis |
| Ngan 2001 | Study assessed drugs given for blood pressure control, not for reducing aspiration pneumonitis |
| Ngan 2004a | Study assessed drugs given for blood pressure control, not for reducing aspiration pneumonitis |
| Ngan 2004b | Study assessed drugs given for blood pressure control, not for reducing aspiration pneumonitis |
| Nortcliffe 2003 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Numazaki 2000 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Numazaki 2003 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| O’Sullivan 1985 | Data are presented as medians and the range and therefore cannot be used |
| O’Sullivan 1988 | Study assessed the effect of H2 receptor antagonists on bupivacaine clearance in women undergoing elective CS under epidural anaesthesia |
| Olsen 1994 | Study assessed drugs given for blood pressure control, not for reducing aspiration pneumonitis |
| Osman 1995 | Wrote to authors asking for number of participants in each group so that the data could be used. No response |
| Ouyang 2002 | Study on fentanyl for analgesia. |
| Owczarzak 1997 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Palmer 1991 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Palmer 1995 | Study assessing fentanyl for pain relief. |
| Pan 1996 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Pan 2001 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Pan 2003 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Peixoto 2006 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Pellegrini 2001 | Study assessing the analgesic effects of opioid antagonist - not included in protocol |
| Phillips 2007 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Prakash 2006 | Study looking at analgesia. |
| Quiney 1995 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Qvist 1983 | Not randomised. |
| Qvist 1985 | Studied effect of placental transfer of cimetidine given prior to induction of anaesthesia |
| Ramanathan 1983 | Study looking at preloading for blood pressure. |
| Ramin 1994 | Study assessed drugs given for blood pressure control, not for reducing aspiration pneumonitis |
| Rout 1992 | Study assessed drugs given for blood pressure control, not for reducing aspiration pneumonitis |
| Rudra 2004a | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Rudra 2004b | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Sanansilp 1998 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Santos 1984 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Sen 2001 | Study of analgesics for postoperative pain relief after CS. |
| Seyedhejazi 2007 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Shende 1998 | Study on intrathecal fentanyl in subarachnoid block for CS. |
| Siddik-Sayyid 2002 | Study on fentanyl in subarachnoid block for CS. |
| Stein 1997 | Intervention looks at reducing nausea and vomiting during CS, not aspiration pneumonitis |
| Stuart 1996 | Analysis not by intention to treat. |
| Tarhan 2007 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Taylor 1966 | Study not thought to be an RCT as it does not state how women were allocated to groups |
| Tettambel 1983 | Quasi-RCT. |
| Tzeng 2000 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Ure 1999 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Vercauteren 2000 | Study assessed drugs given for blood pressure control not for reducing aspiration pneumonitis |
| von Braun 1994 | We couldn’t use the data as we were not given the number of participants that were included in each treatment arm |
| Wang 2001 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Weiss 1995 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
| Yazigi 2002 | Study assessed interventions for reducing nausea and vomiting at CS, not for reducing aspiration pneumonitis |
CS: caesarean section
GA: gestational age
ITT: intention to treat
IV: intravenous
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | RCT. |
| Participants | Pregnant women. |
| Interventions |
|
| Outcomes | Maternal gastric pH and volume and neonatal gastric pH and volume |
| Notes | This study has been translated but there was insufficient information to allow data extraction. We are contacting authors for clarification |
RCT: randomised controlled trial
DATA AND ANALYSES
Comparison 1. Antacids versus placebo/no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality due to aspiration pneumonitis | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2 Morbidity due to aspiration pneumonitis | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3 Intragastric pH < 2.5 at intubation | 2 | 108 | Risk Ratio (M-H, Fixed, 95% CI) | 0.17 [0.09, 0.32] |
| 3.1 Elective CS | 1 | 22 | Risk Ratio (M-H, Fixed, 95% CI) | 0.13 [0.03, 0.59] |
| 3.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3.3 Elective or emergency CS not specified | 1 | 86 | Risk Ratio (M-H, Fixed, 95% CI) | 0.18 [0.09, 0.36] |
| 4 Intragastric volume > 0.4ml/kg at intubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5 Nausea | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6 Vomiting | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7 Sedation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8 Restlessness | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9 Dystonic reactions | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10 Extrapyramidal symptoms | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11 Hypotension | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 12 Blood loss | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 13 Atonic uterus | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 14 Women’s satisfaction | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15 Cord blood pH | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.1 Elective CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.2 Emergency CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.3 Elective or emergency CS not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 16 Apgar < 7 at 5 minutes | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 17 Neonatal assessment scores | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 18 Admission to neonatal intensive care unitNICU | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19 Initiation of breastfeeding | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 20 Duration of breastfeeding | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.1 Elective CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.2 Emergency CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.3 Elective or emergency CS not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 21 Intragastric pH < 2.5 at extubation | 1 | 86 | Risk Ratio (M-H, Fixed, 95% CI) | 0.21 [0.09, 0.48] |
| 21.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.3 Elective or emergency CS not specified | 1 | 86 | Risk Ratio (M-H, Fixed, 95% CI) | 0.21 [0.09, 0.48] |
| 22 Intragastric volume > 0.4ml/kg at extubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 23 Gastric volume post intubation (not pre-specified) | 2 | 74 | Mean Difference (IV, Random, 95% CI) | 30.33 [−3.97, 64.62 |
| 23.1 Elective CS | 2 | 74 | Mean Difference (IV, Random, 95% CI) | 30.33 [−3.97, 64.62 |
| 23.2 Emergency CS | 0 | 0 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 23.3 Elective or Emergency CS not specified | 0 | 0 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 24 Gastric pH post intubation (not pre-specified) | 4 | 220 | Mean Difference (IV, Fixed, 95% CI) | 2.63 [2.29, 2.96] |
| 24.1 Elective CS | 2 | 74 | Mean Difference (IV, Fixed, 95% CI) | 2.91 [2.36, 3.45] |
| 24.2 Emergency CS | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 2.74 [2.16, 3.32] |
| 24.3 Elective or Emergnecy CS not specified | 1 | 86 | Mean Difference (IV, Fixed, 95% CI) | 2.13 [1.51, 2.75] |
| 25 At risk of aspiration (not pre-specified) | 1 | 22 | Risk Ratio (M-H, Fixed, 95% CI) | 0.07 [0.00, 1.04] |
| 25.1 Elective CS | 1 | 22 | Risk Ratio (M-H, Fixed, 95% CI) | 0.07 [0.00, 1.04] |
| 25.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 25.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 26 Gastric pH at extubation (not pre-specified) | 2 | 146 | Mean Difference (IV, Fixed, 95% CI) | 2.04 [1.66, 2.42] |
| 26.1 Elective CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 26.2 Emergency CS | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 2.15 [1.71, 2.59] |
| 26.3 Elective or Emergency CS not specified | 1 | 86 | Mean Difference (IV, Fixed, 95% CI) | 1.74 [1.02, 2.46] |
| 27 Gastric volume post intubation >25ml (not pre-specified) | 1 | 22 | Risk Ratio (M-H, Fixed, 95% CI) | 1.43 [0.88, 2.32] |
| 27.1 Elective CS | 1 | 22 | Risk Ratio (M-H, Fixed, 95% CI) | 1.43 [0.88, 2.32] |
| 27.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 27.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
Comparison 2. H2 antagonists versus placebo/no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality due to aspiration pneumonitis | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2 Morbidity due to aspiration pneumonitis | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3 Intragastric pH < 2.5 at intubation | 2 | 170 | Risk Ratio (M-H, Fixed, 95% CI) | 0.09 [0.05, 0.18] |
| 3.1 Elective CS | 2 | 170 | Risk Ratio (M-H, Fixed, 95% CI) | 0.09 [0.05, 0.18] |
| 3.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4 Intragastric volume > 0.4ml/kg at intubation | 2 | 170 | Risk Ratio (M-H, Random, 95% CI) | 0.08 [0.01, 0.86] |
| 4.1 Elective CS | 2 | 170 | Risk Ratio (M-H, Random, 95% CI) | 0.08 [0.01, 0.86] |
| 4.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | Not estimable |
| 4.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | Not estimable |
| 5 Nausea | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6 Vomiting | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7 Sedation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8 Restlessness | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9 Dystonic reactions | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10 Extrapyramidal symptoms | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11 Hypotension | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 12 Blood loss | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 13 Atonic uterus | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 14 Women’s satisfaction | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15 Cord blood pH | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.1 Elective CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.2 Emergency CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.3 Elective or emergency CS not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 16 Apgar < 7 at 5 minutes | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 17 Neonatal assessment scores | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 18 Admission to NICU | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19 Initiation of breastfeeding | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 20 Duration of breastfeeding | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 21 Intragastric pH < 2.5 at extubation | 1 | 30 | Risk Ratio (M-H, Fixed, 95% CI) | 0.08 [0.01, 0.56] |
| 21.1 Elective CS | 1 | 30 | Risk Ratio (M-H, Fixed, 95% CI) | 0.08 [0.01, 0.56] |
| 21.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22 Intragastric volume > 20ml at extubation (not pre-specifed) | 1 | 30 | Risk Ratio (M-H, Fixed, 95% CI) | 0.33 [0.11, 0.99] |
| 22.1 Elective CS | 1 | 30 | Risk Ratio (M-H, Fixed, 95% CI) | 0.33 [0.11, 0.99] |
| 22.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 23 Gastric pH at intubation (not pre-specified) | 3 | 160 | Mean Difference (IV, Random, 95% CI) | 3.31 [1.82, 4.81] |
| 23.1 Elective CS | 2 | 100 | Mean Difference (IV, Random, 95% CI) | 2.59 [2.04, 3.14] |
| 23.2 Emergency CS | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 4.43 [3.97, 4.89] |
| 23.3 Elective or Emergency CS not specified | 0 | 0 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 24 Gastric pH at extubation (not pre-specified) | 2 | 110 | Mean Difference (IV, Random, 95% CI) | 3.56 [2.25, 4.87] |
| 24.1 Elective CS | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 2.86 [2.13, 3.59] |
| 24.2 Emergency CS | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 4.20 [3.70, 4.70] |
| 24.3 Elective or emergency CS not specified | 0 | 0 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 25 At risk of aspiration post intubation (not pre-specified) | 4 | 255 | Risk Ratio (M-H, Random, 95% CI) | 0.07 [0.01, 0.33] |
| 25.1 Elective CS | 4 | 255 | Risk Ratio (M-H, Random, 95% CI) | 0.07 [0.01, 0.33] |
| 25.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | Not estimable |
| 25.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | Not estimable |
| 26 At risk of aspiration pre extubation (not pre-specified) | 2 | 125 | Risk Ratio (M-H, Fixed, 95% CI) | 0.17 [0.01, 4.03] |
| 26.1 Elective CS | 2 | 125 | Risk Ratio (M-H, Fixed, 95% CI) | 0.17 [0.01, 4.03] |
| 26.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 26.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 27 Gastric volume post intubation (not pre-specified) | 2 | 100 | Mean Difference (IV, Fixed, 95% CI) | −14.06 [−18.68, −9.45] |
| 27.1 Elective CS | 2 | 100 | Mean Difference (IV, Fixed, 95% CI) | −14.06 [−18.68, −9.45] |
| 27.2 Emergency CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 27.3 Emergency and elective CS not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 28 Gastric volume pre-extubation (not pre-specified) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | −3.70 [−6.17,−1.23] |
| 28.1 Elective CS | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | −3.70 [−6.17,−1.23] |
| 28.2 Emergency CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 28.3 Emergency and elective CS not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
Comparison 3. Proton pump antagonists versus placebo/no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality due to aspiration pneumonitis | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2 Morbidity due to aspiration pneumonitis | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3 Intragastric pH < 2.5 at intubation | 1 | 80 | Risk Ratio (M-H, Fixed, 95% CI) | 0.26 [0.14, 0.46] |
| 3.1 Elective CS | 1 | 80 | Risk Ratio (M-H, Fixed, 95% CI) | 0.26 [0.14, 0.46] |
| 3.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4 Intragastric volume > 0.4ml/kg 1 at intubation | 80 | Risk Ratio (M-H, Fixed, 95% CI) | 0.46 [0.19, 1.09] | |
| 4.1 Elective CS | 1 | 80 | Risk Ratio (M-H, Fixed, 95% CI) | 0.46 [0.19, 1.09] |
| 4.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5 Nausea | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6 Vomiting | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7 Sedation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8 Restlessness | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9 Dystonic reactions | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10 Extrapyramidal symptoms | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11 Hypotension | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 12 Blood loss | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 13 Atonic uterus | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 14 Women’s satisfaction | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15 Cord blood pH | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.1 Elective CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.2 Emergency CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.3 Elective or emergency CS not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 16 Apgar < 7 at 5 minutes | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 17 Neonatal assessment scores | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 18 Admission to NICU | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19 Initiation of breastfeeding | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 20 Duration of breastfeeding | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 21 Intragastric pH > 2.5 at extubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22 Intragastric volume < 0.4ml/kg at extubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 23 Gastric pH at intubation (not pre-specified) | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 3.27 [2.82, 3.7 |
| 23.1 Elective CS | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 3.27 [2.82, 3.7 |
| 23.2 Emergency CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 23.3 Elective or emergency CS not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 24 At risk of aspiration (not pre-specified) | 2 | 130 | Risk Ratio (M-H, Random, 95% CI) | 0.14 [0.03, 0.7 |
| 24.1 Elective CS | 2 | 130 | Risk Ratio (M-H, Random, 95% CI) | 0.14 [0.03, 0.7 |
| 24.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | Not estimable |
| 24.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | Not estimable |
| 25 Gastric volume at intubation (not pre-specified) | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | −0.25 [−0.30, −0.20] |
| 25.1 Elective CS | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | −0.25 [−0.30, −0.20] |
| 25.2 Emergency CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 25.3 Emergency and elective CS not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 26 Gastric pH pre extubation (not pre-specified) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 26.1 Elective CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 26.2 Emergency CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 26.3 Emergency and elective CS not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 27 Gastric volume pre extubation (not pre-specified) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 27.1 Elective CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 27.2 Emergency CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 27.3 Emergency and elective CS not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
Comparison 4. Prokinetic drugs versus placebo/no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality due to aspiration pneumonitis | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2 Morbidity due to aspiration pneumonitis | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3 Intragastric pH > 2.5 at intubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4 Intragastric volume < 0.4ml/kg at intubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5 Nausea | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6 Vomiting | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7 Sedation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8 Restlessness | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9 Dystonic reactions | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10 Extrapyramidal symptoms | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11 Hypotension | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 12 Blood loss | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 13 Atonic uterus | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 14 Women’s satisfaction | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15 Cord blood pH | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.1 Elective CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.2 Emergency CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.3 Elective or emergency CS not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 16 Apgar < 7 at 5 minutes | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 17 Neonatal assessment scores | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 18 Admission to NICU | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19 Initiation of breastfeeding | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 20 Duration of breastfeeding | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 21 Intragastric pH > 2.5 at extubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22 Intragastric volume < 0.4ml/kg at extubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 23 At risk of aspiration post intubation (not pre-specified) | 1 | 50 | Risk Ratio (M-H, Fixed, 95% CI) | 0.67 [0.33, 1.35] |
| 23.1 Elective CS | 1 | 50 | Risk Ratio (M-H, Fixed, 95% CI) | 0.67 [0.33, 1.35] |
| 23.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 23.3 Elective or emergency not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 24 At risk of aspiration pre-extubation (not pre-specified) | 1 | 50 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 24.1 Elective CS | 1 | 50 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 24.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 24.3 Elective or emergency nor specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
Comparison 5. Non-pharmacological interventions versus placebo/no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality due to aspiration pneumonitis | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2 Morbidity due to aspiration pneumonitis | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3 Intragastric pH < 2.5 at intubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4 Intragastric volume < 0.4ml/kg at intubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5 Nausea | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6 Vomiting | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7 Sedation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8 Restlessness | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9 Dystonic reactions | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10 Extrapyramidal symptoms | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11 Hypotension | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 12 Blood loss | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 13 Atonic uterus | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 14 Women’s satisfaction | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15 Cord blood pH | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.1 Elective CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.2 Emergency CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.3 Elective or emergency CS not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 16 Apgar < 7 at 5 minutes | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 17 Neonatal assessment scores | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 18 Admission to NICU | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19 Initiation of breastfeeding | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 20 Duration of breastfeeding | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 21 Intragastric pH < 2.5 at extubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22 Intragastric volume < 0.4ml/kg at extubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 23 At risk of aspiration (not pre-specified) | 1 | 40 | Risk Ratio (M-H, Fixed, 95% CI) | 0.5 [0.18, 1.40] |
| 23.1 Elective CS | 1 | 40 | Risk Ratio (M-H, Fixed, 95% CI) | 0.5 [0.18, 1.40] |
| 23.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 23.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
Comparison 6. Antacids + H2 antagonists versus placebo/no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality due to aspiration pneumonitis | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2 Morbidity due to aspiration pneumonitis | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3 Intragastric pH < 2.5 at intubation | 1 | 89 | Risk Ratio (M-H, Fixed, 95% CI) | 0.02 [0.00, 0.15] |
| 3.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3.3 Elective or emergency CS not specified | 1 | 89 | Risk Ratio (M-H, Fixed, 95% CI) | 0.02 [0.00, 0.15] |
| 4 Intragastric volume < 0.4ml/kg at intubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5 Nausea | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6 Vomiting | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7 Sedation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8 Restlessness | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9 Dystonic reactions | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10 Extrapyramidal symptoms | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11 Hypotension | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 12 Blood loss | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 13 Atonic uterus | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 14 Women’s satisfaction | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15 Cord blood pH | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.1 Elective CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.2 Emergency CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.3 Elective or emergency CS not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 16 Apgar < 7 at 5 minutes | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 17 Neonatal assessment scores | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 18 Admission to NICU | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19 Initiation of breastfeeding | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 20 Duration of breastfeeding | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 21 Intragastric pH < 2.5 at extubation | 1 | 89 | Risk Ratio (M-H, Fixed, 95% CI) | 0.03 [0.00, 0.24] |
| 21.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.3 Elective or emergency CS not specified | 1 | 89 | Risk Ratio (M-H, Fixed, 95% CI) | 0.03 [0.00, 0.24] |
| 22 Intragastric volume < 0.4ml/kg at extubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 23 Intragastric pH post intubation (not pre-specified) | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 2.82 [2.25, 3.39] |
| 23.1 Elective CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 23.2 Emergency CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 23.3 Elective or emergency CS not specified | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 2.82 [2.25, 3.39] |
| 24 Intragastric pH at extubation (not pre-specified) | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 2.54 [1.85, 3.23] |
| 24.1 Emergency CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 24.2 Elective CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 24.3 Emeegency or elective CS not specified | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 2.54 [1.85, 3.23] |
Comparison 7. H2 antagonists + prokinetic drugs versus placebo/no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality due to aspiration pneumonitis | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2 Morbidity due to aspiration pneumonitis | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3 Intragastric pH < 2.5 at intubation | 1 | 50 | Risk Ratio (M-H, Fixed, 95% CI) | 0.03 [0.00, 0.48] |
| 3.1 Elective CS | 1 | 50 | Risk Ratio (M-H, Fixed, 95% CI) | 0.03 [0.00, 0.48] |
| 3.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4 Intragastric volume < 0.4ml/kg at intubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5 Nausea | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6 Vomiting | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7 Sedation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8 Restlessness | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9 Dystonic reactions | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10 Extrapyramidal symptoms | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11 Hypotension | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 12 Blood loss | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 13 Atonic uterus | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 14 Women’s satisfaction | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15 Cord blood pH | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.1 Elective CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.2 Emergency CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.3 Elective or emergency CS not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 16 Apgar < 7 at 5 minutes | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 17 Neonatal assessment scores | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 18 Admission to NICU | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19 Initiation of breastfeeding | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 20 Duration of breastfeeding | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 21 Intragastric pH > 2.5 at extubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22 Intragastric volume < 0.4ml/kg at extubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 23 Risk of aspiration (not pre-specified) | 1 | 50 | Risk Ratio (M-H, Fixed, 95% CI) | 0.03 [0.00, 0.51] |
| 23.1 Elective CS | 1 | 50 | Risk Ratio (M-H, Fixed, 95% CI) | 0.03 [0.00, 0.51] |
| 23.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 23.3 Emergency and elective cs not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 24 Gastric pH post intubation (not pre-specified) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 3.17 [2.57, 3.77] |
| 24.1 Elective CS | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 3.17 [2.57, 3.77] |
| 24.2 Emergency CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 24.3 Emergency and elective CS not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 25 Gastric volume post intubation (not pre-specified) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | −14.20 [−20.92, −7. 48] |
| 25.1 Elective CS | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | −14.20 [−20.92, −7.48] |
| 25.2 Emergency CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 25.3 Emergency and elective CS not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 26 Gastric volume <25ml after induction | 1 | 50 | Odds Ratio (M-H, Fixed, 95% CI) | 5.63 [1.65, 19.23] |
| 26.1 Elective CS | 1 | 50 | Odds Ratio (M-H, Fixed, 95% CI) | 5.63 [1.65, 19.23] |
| 26.2 Emergency CS | 0 | 0 | Odds Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 26.3 Elective or emergency CS not specified | 0 | 0 | Odds Ratio (M-H, Fixed, 95% CI) | Not estimable |
Comparison 8. Antacids versus H2 antagonists.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality due to aspiration pneumonitis | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2 Morbidity due to aspiration pneumonitis | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3 Intragastric pH < 2.5 at intubation | 2 | 135 | Risk Ratio (M-H, Fixed, 95% CI) | 0.07 [0.01, 0.52] |
| 3.1 Elective CS | 2 | 104 | Risk Ratio (M-H, Fixed, 95% CI) | 0.07 [0.01, 0.52] |
| 3.2 Emergency CS | 1 | 31 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4 Intragastric volume < 0.4ml/kg 0 at intubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5 Nausea | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6 Vomiting | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7 Sedation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8 Restlessness | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9 Dystonic reactions | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10 Extrapyramidal symptoms | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11 Hypotension | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 12 Blood loss | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 13 Atonic uterus | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 14 Women’s satisfaction | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15 Cord blood pH | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.1 Elective CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.2 Emergency CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.3 Elective or emergency CS not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 16 Apgar < 7 at 5 minutes | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 17 Neonatal assessment scores | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 18 Admission to NICU | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19 Initiation of breastfeeding | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 20 Duration of breastfeeding | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 21 Intragastric pH > 2.5 at extubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22 Intragastric volume < 0.4ml/kg at extubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 23 At risk of aspiration (not pre-specified) | 1 | 16 | Risk Ratio (M-H, Fixed, 95% CI) | 1.0 [0.18, 5.46] |
| 23.1 Elective CS | 1 | 16 | Risk Ratio (M-H, Fixed, 95% CI) | 1.0 [0.18, 5.46] |
| 23.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 23.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 24 Gastric volume at intubation (not pre-specified) | 3 | 102 | Std. Mean Difference (IV, Random, 95% CI) | 0.75 [0.34, 1.16] |
| 24.1 Elective CS | 3 | 102 | Std. Mean Difference (IV, Random, 95% CI) | 0.75 [0.34, 1.16] |
| 24.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | Not estimable |
| 24.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | Not estimable |
| 25 Gastric pH at intubation (not pre-specified) | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | −3.02 [−4.52, −1.52] |
| 25.1 Elective CS | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | −3.02 [−4.52, −1.52] |
| 25.2 Emergency CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 25.3 Elective or emergency CS not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 26 Gastric pH at extubation (not pre-specified) | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | −2.32 [−4.00, −0.64] |
| 26.1 Elective CS | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | −2.32 [−4.00, −0.64] |
| 26.2 Emergenct CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 26.3 Elective or emergency CS not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
Comparison 9. Antacids versus prokinetic drugs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality due to aspiration pneumonitis | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2 Morbidity due to aspiration pneumonitis | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3 Intragastric pH > 2.5 at intubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4 Intragastric volume < 0.4ml/kg at intubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5 Nausea | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6 Vomiting | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7 Sedation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8 Restlessness | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9 Dystonic reactions | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10 Extrapyramidal symptoms | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11 Hypotension | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 12 Blood loss | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 13 Atonic uterus | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 14 Women’s satisfaction | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15 Cord blood pH | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.1 Elective CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.2 Emergency CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.3 Elective or emergency CS not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 16 Apgar < 7 at 5 minutes | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 17 Neonatal assessment scores | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 18 Admission to NICU | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19 Initiation of breastfeeding | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 20 Duration of breastfeeding | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 21 Intragastric pH > 2.5 at extubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22 Intragastric volume < 0.4ml/kg at extubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
Comparison 10. H2 antagonists versus proton pump antagonists.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality due to aspiration pneumonitis | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2 Morbidity due to aspiration pneumonitis | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3 Intragastric pH < 2.5 at intubation | 1 | 120 | Risk Ratio (M-H, Fixed, 95% CI) | 0.39 [0.16, 0.97] |
| 3.1 Elective CS | 1 | 120 | Risk Ratio (M-H, Fixed, 95% CI) | 0.39 [0.16, 0.97] |
| 3.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4 Intragastric volume < 0.4ml/kg at intubation | 1 | 120 | Risk Ratio (M-H, Fixed, 95% CI) | 1.18 [1.03, 1.35] |
| 4.1 Elective CS | 1 | 120 | Risk Ratio (M-H, Fixed, 95% CI) | 1.18 [1.03, 1.35] |
| 4.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5 Nausea | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6 Vomiting | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7 Sedation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8 Restlessness | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9 Dystonic reactions | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10 Extrapyramidal symptoms | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11 Hypotension | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 12 Blood loss | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 13 Atonic uterus | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 14 Women’s satisfaction | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15 Cord blood pH | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.1 Elective CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.2 Emergency CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.3 Elective or emergency CS not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 16 Apgar < 7 at 5 minutes | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 17 Neonatal assessment scores | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 18 Admission to NICU | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19 Initiation of breastfeeding | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 20 Duration of breastfeeding | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 21 Intragastric pH > 2.5 at extubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22 Intragastric volume < 0.4ml/kg at extubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 23 At risk of aspiration (not pre-specified) | 4 | 323 | Risk Ratio (M-H, Random, 95% CI) | 0.93 [0.20, 4.37] |
| 23.1 Elective CS | 2 | 141 | Risk Ratio (M-H, Random, 95% CI) | 0.79 [0.03, 23.67] |
| 23.2 Emergency CS | 2 | 182 | Risk Ratio (M-H, Random, 95% CI) | 1.04 [0.13, 8.32] |
| 23.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | Not estimable |
| 24 Gastric pH post intubation (not pre-specified) | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | −0.68 [−1.28, −0.08] |
| 24.1 Elective CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 24.2 Emergency CS | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | −0.68 [−1.28, −0.08] |
| 24.3 Emergency and elective CS not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 25 Gastric volume post intubation (not pre-specified) | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 2.35 [−0.79, 5.49] |
| 25.1 Elective CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 25.2 Emergency CS | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 2.35 [−0.79, 5.49] |
| 25.3 Emergency and elective CS not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 26 Gastric pH pre extubation (not pre-specified) | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | −0.65 [−1.22, −0.08] |
| 26.1 Elective CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 26.2 Emergency CS | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | −0.65 [−1.22, −0.08] |
| 26.3 Emergency and elective CS not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 27 Gastric volume post extubation (not pre-specified) | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | −1.08 [−2.47, 0.31] |
| 27.1 Elective CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 27.2 Emergency CS | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | −1.08 [−2.47, 0.31] |
| 27.3 Emergency and elective CS not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
Comparison 11. Antacids + H2 antagonists versus antacids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality due to aspiration pneumonitis | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2 Morbidity due to aspiration pneumonitis | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3 Intragastric pH < 2.5 at intubation | 1 | 119 | Risk Ratio (M-H, Fixed, 95% CI) | 0.12 [0.02, 0.92] |
| 3.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3.3 Elective or emergency CS not specified | 1 | 119 | Risk Ratio (M-H, Fixed, 95% CI) | 0.12 [0.02, 0.92] |
| 4 Intragastric volume < 0.4ml/kg at intubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5 Nausea | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6 Vomiting | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7 Sedation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8 Restlessness | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9 Dystonic reactions | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10 Extrapyramidal symptoms | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11 Hypotension | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 12 Blood loss | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 13 Atonic uterus | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 14 Women’s satisfaction | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15 Cord blood pH | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.1 Elective CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.2 Emergency CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.3 Elective or emergency CS not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 16 Apgar < 7 at 5 minutes | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 17 Neonatal assessment scores | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 18 Admission to NICU | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19 Initiation of breastfeeding | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 20 Duration of breastfeeding | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 21 Intragastric pH < 2.5 at extubation | 1 | 119 | Risk Ratio (M-H, Fixed, 95% CI) | 0.16 [0.02, 1.28] |
| 21.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.3 Elective or emergency CS not specified | 1 | 119 | Risk Ratio (M-H, Fixed, 95% CI) | 0.16 [0.02, 1.28] |
| 22 Intragastric volume < 0.4ml/kg at extubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 23 Post Intubation pH (not pre-specified) | 2 | 672 | Mean Difference (IV, Random, 95% CI) | 0.43 [0.07, 0.79] |
| 23.1 Elective CS | 0 | 0 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 23.2 Emergency CS | 1 | 553 | Mean Difference (IV, Random, 95% CI) | 0.30 [0.14, 0.46] |
| 23.3 Emergency or Elective CS non specified | 1 | 119 | Mean Difference (IV, Random, 95% CI) | 0.69 [0.22, 1.16] |
| 24 Pre extubation pH (not pre-specified) | 2 | 597 | Mean Difference (IV, Fixed, 95% CI) | 0.71 [0.53, 0.89] |
| 24.1 Elective CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 24.2 Emergency CS | 1 | 478 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [0.51, 0.89] |
| 24.3 Elective or emergency CS non specified | 1 | 119 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [0.29, 1.31] |
| 25 Post intubation gastric volume (not pre-specified) | 1 | 586 | Mean Difference (IV, Fixed, 95% CI) | −1.0 [−8.75, 6.75] |
| 25.1 Elective CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 25.2 Emergency CS | 1 | 586 | Mean Difference (IV, Fixed, 95% CI) | −1.0 [−8.75, 6.75] |
| 25.3 Elective or emergency CS not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 26 Pre-extubation gastric volume (not pre-specified) | 1 | 568 | Mean Difference (IV, Fixed, 95% CI) | −2.0 [−5.21, 1.21] |
| 26.1 Elective CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 26.2 Emergency CS | 1 | 568 | Mean Difference (IV, Fixed, 95% CI) | −2.0 [−5.21, 1.21] |
| 26.3 Elective or emergency CS not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 27 At risk of aspiration (not pre-specified) | 1 | 595 | Odds Ratio (M-H, Fixed, 95% CI) | 0.10 [0.02, 0.45] |
| 27.1 Elective CS | 0 | 0 | Odds Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 27.2 Emergency CS | 1 | 595 | Odds Ratio (M-H, Fixed, 95% CI) | 0.10 [0.02, 0.45] |
| 27.3 Elective or emergency CS not specified | 0 | 0 | Odds Ratio (M-H, Fixed, 95% CI) | Not estimable |
Comparison 12. H2 antagonists + prokinetic drugs versus antacids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality due to aspiration pneumonitis | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2 Morbidity due to aspiration pneumonitis | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3 Intragastric pH > 2.5 at intubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4 Intragastric volume < 0.4ml/kg at intubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5 Nausea | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6 Vomiting | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7 Sedation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8 Restlessness | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9 Dystonic reactions | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10 Extrapyramidal symptoms | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11 Hypotension | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 12 Blood loss | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 13 Atonic uterus | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 14 Women’s satisfaction | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15 Cord blood pH | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.1 Elective CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.2 Emergency CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.3 Elective or emergency CS not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 16 Apgar < 7 at 5 minutes | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 17 Neonatal assessment scores | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 18 Admission to NICU | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19 Initiation of breastfeeding | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 20 Duration of breastfeeding | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 21 Intragastric pH > 2.5 at extubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22 Intragastric volume < 0.4ml/kg at extubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
Comparison 13. Proton pump agonists + prokinetics versus proton pump agonists.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality due to aspiration pneumonitis | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2 Morbidity due to aspiration pneumonitis | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3 Intragastric pH > 2.5 at intubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4 Intragastric volume < 0.4ml/kg at intubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5 Nausea | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6 Vomiting | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7 Sedation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8 Restlessness | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9 Dystonic reactions | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10 Extrapyramidal symptoms | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11 Hypotension | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 12 Blood loss | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 13 Atonic uterus | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 14 Women’s satisfaction | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15 Cord blood pH | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.1 Elective CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.2 Emergency CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.3 Elective or emergency CS not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 16 Apgar < 7 at 5 minutes | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 17 Neonatal assessment scores | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 18 Admission to NICU | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19 Initiation of breastfeeding | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 20 Duration of breastfeeding | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 21 Intragastric pH > 2.5 at extubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22 Intragastric volume < 0.4ml/kg at extubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 23 At risk of aspiration post intubation (not pre-specified) | 1 | 97 | Odds Ratio (M-H, Fixed, 95% CI) | 0.44 [0.11, 1.66] |
| 23.1 Elective CS | 1 | 97 | Odds Ratio (M-H, Fixed, 95% CI) | 0.44 [0.11, 1.66] |
| 23.2 Emergency CS | 0 | 0 | Odds Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 23.3 Elective or emergency CS not specified | 0 | 0 | Odds Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 24 At risk of aspiration pre extubation (not pre specified) | 1 | 94 | Odds Ratio (M-H, Fixed, 95% CI) | 0.66 [0.03, 16.70] |
| 24.1 Elective CS | 1 | 94 | Odds Ratio (M-H, Fixed, 95% CI) | 0.66 [0.03, 16.70] |
| 24.2 Emergency CS | 0 | 0 | Odds Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 24.3 Elective and emergency CS not specified | 0 | 0 | Odds Ratio (M-H, Fixed, 95% CI) | Not estimable |
Comparison 14. H2 antagonist versus Tramadol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality due to aspiration pneumonitis | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2 Morbidity due to aspiration pneumonitis | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3 Intragastric pH < 2.5 at intubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4 Intragastric volume > 0.4ml/kg at intubation | 1 | 90 | Risk Ratio (M-H, Fixed, 95% CI) | 5.0 [1.03, 24.28] |
| 4.1 Elective CS | 1 | 90 | Risk Ratio (M-H, Fixed, 95% CI) | 5.0 [1.03, 24.28] |
| 4.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5 Nausea | 1 | 60 | Risk Ratio (M-H, Fixed, 95% CI) | 1.38 [0.64, 2.93] |
| 5.1 Elective CS | 1 | 60 | Risk Ratio (M-H, Fixed, 95% CI) | 1.38 [0.64, 2.93] |
| 5.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6 Vomiting | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.3 Emergency and elective CS non specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7 Sedation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8 Restlessness | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9 Dystonic reactions | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10 Extrapyramidal symptoms | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11 Hypotension | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 12 Blood loss | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 13 Atonic uterus | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 14 Women’s satisfaction | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15 Cord blood pH | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.1 Elective CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.2 Emergency CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.3 Elective or emergency CS not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 16 Apgar score < 7 at 5 mins | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.1 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.2 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.3 Emergency and elective CS non specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 17 Neonatal assessment scores | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 18 Admission to NICU | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19 Initiation of breastfeeding | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 20 Duration of breastfeeding | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 21 Intragastric pH < 2.5 at extubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22 Intragastric volume > 0.4ml/kg at extubation | 1 | 60 | Risk Ratio (M-H, Fixed, 95% CI) | 3.0 [0.33, 27.23] |
| 22.1 Elective CS | 1 | 60 | Risk Ratio (M-H, Fixed, 95% CI) | 3.0 [0.33, 27.23] |
| 22.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 23 At risk post intubation (not pre-specified) | 1 | 60 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 23.1 Elective CS | 1 | 60 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 23.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 23.3 Emergency and elective CS non specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 24 At risk pre extubation (not pre-specified) | 1 | 60 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 24.1 Elective CS | 1 | 60 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 24.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 24.3 Emergency or elective CS non specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 25 Nausea 24hours post op (not pre-specified) | 1 | 60 | Risk Ratio (M-H, Fixed, 95% CI) | 1.43 [0.63, 3.25] |
| 25.1 Elective CS | 1 | 60 | Risk Ratio (M-H, Fixed, 95% CI) | 1.43 [0.63, 3.25] |
| 25.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 25.3 Emergency and elective CS non specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
Comparison 15. Antacids + H2 antagonists versus proton pump antagonists.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality due to aspiration pneumonitis | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2 Morbidity due to aspiration pneumonitis | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3 Intragastric pH < 2.5 at intubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4 Intragastric volume > 0.4ml/kg at intubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5 Nausea | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6 Vomiting | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.3 Emergency and elective CS non specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7 Sedation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8 Restlessness | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9 Dystonic reactions | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10 Extrapyramidal symptoms | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11 Hypotension | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 12 Blood loss | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 13 Atonic uterus | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 14 Women’s satisfaction | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15 Cord blood pH | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.1 Elective CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.2 Emergency CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.3 Elective or emergency CS not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 16 Apgar score < 7 at 5 mins | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.1 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.2 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.3 Emergency and elective CS non specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 17 Neonatal assessment scores | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 18 Admission to NICU | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19 Initiation of breastfeeding | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 20 Duration of breastfeeding | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 21 Intragastric pH < 2.5 at extubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22 Intragastric volume > 0.4ml/kg at extubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 23 At risk of aspiration (not pre-specified) | 1 | 108 | Risk Ratio (M-H, Fixed, 95% CI) | 0.12 [0.02, 0.91] |
| 23.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 23.2 Emergency CS | 1 | 108 | Risk Ratio (M-H, Fixed, 95% CI) | 0.12 [0.02, 0.91] |
| 23.3 Emergency and elective CS non specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
Comparison 16. Proton pump antagonist + antacid versus proton pump antagonist.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality due to aspiration pneumonitis | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2 Morbidity due to aspiration pneumonitis | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3 Intragastric pH < 2.5 at intubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4 Intragastric volume > 0.4ml/kg at intubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5 Nausea | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6 Vomiting | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.3 Emergency and elective CS non specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7 Sedation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.3 Elective or emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8 Restlessness not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9 Dystonic reactions | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10 Extrapyramidal symptoms | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11 Hypotension | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 12 Blood loss | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 13 Atonic uterus | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 14 Women’s satisfaction | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15 Cord blood pH | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.1 Elective CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.2 Emergency CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.3 Elective or emergency CS not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 16 Apgar score < 7 at 5 mins | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.1 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.2 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.3 Emergency and elective CS non specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 17 Neonatal assessment scores | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 18 Admission to NICU | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19 Initiation of breastfeeding | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 20 Duration of breastfeeding | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 21 Intragastric pH < 2.5 at extubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22 Intragastric volume > 0.4ml/kg at extubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 23 Risk of aspiration (not pre-specified) | 1 | 112 | Risk Ratio (M-H, Fixed, 95% CI) | 0.33 [0.10, 1.15] |
| 23.1 Emergency CS | 1 | 112 | Risk Ratio (M-H, Fixed, 95% CI) | 0.33 [0.10, 1.15] |
| 23.2 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 23.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
Comparison 17. H2 antagonist + prokinetic versus H2 antagonist.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality due to aspiration pneumonitis | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 1.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2 Morbidity due to aspiration pneumonitis | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3 Intragastric pH < 2.5 at intubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4 Intragastric volume > 0.4ml/kg at intubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5 Nausea | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6 Vomiting | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.3 Emergency and elective CS non specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7 Sedation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8 Restlessness | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9 Dystonic reactions | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10 Extrapyramidal symptoms | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11 Hypotension | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 12 Blood loss | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 12.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 13 Atonic uterus | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 14 Women’s satisfaction | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 14.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15 Cord blood pH | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.1 Elective CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.2 Emergency CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 15.3 Elective or emergency CS not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 16 Apgar score < 7 at 5 mins | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.1 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.2 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.3 Emergency and elective CS non specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 17 Neonatal assessment scores | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 17.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 18 Admission to NICU | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19 Initiation of breastfeeding | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 20 Duration of breastfeeding | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.1 Elective CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.2 Emergency CS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 20.3 Elective or emergency CS not specified | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 21 Intragastric pH < 2.5 at extubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22 Intragastric volume > 0.4ml/kg at extubation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.1 Elective CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22.3 Elective or emergency CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 23 Intragastric pH > 2.5 post intubation (not pre-specified) | 1 | 50 | Risk Ratio (M-H, Fixed, 95% CI) | 1.04 [0.93, 1.16] |
| 23.1 Elective CS | 1 | 50 | Risk Ratio (M-H, Fixed, 95% CI) | 1.04 [0.93, 1.16] |
| 23.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 23.3 Emergency and elective CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 24 Intragastric volume <0.4 ml/kg post intubation (not pre-specified) | 1 | 50 | Risk Ratio (M-H, Fixed, 95% CI) | 0.86 [0.66, 1.12] |
| 24.1 Elective CS | 1 | 50 | Risk Ratio (M-H, Fixed, 95% CI) | 0.86 [0.66, 1.12] |
| 24.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 24.3 Emergency and elective CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 25 Risk of aspiration (not pre-specified) | 1 | 50 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 25.1 Elective CS | 1 | 50 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 25.2 Emergency CS | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 25.3 Emergency and elective CS not specified | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 26 Gastric volume post intubation (not pre-specified) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [−3.17, 5.77] |
| 26.1 Elective CS | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [−3.17, 5.77] |
| 26.2 Emergency CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 26.3 Emergency and elective CS not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 27 Gastric pH post intubation (not pre-specified) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.59 [−0.14, 1.32] |
| 27.1 Elective CS | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.59 [−0.14, 1.32] |
| 27.2 Emergency CS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 27.3 Emergency and elective CS not specified | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
Analysis 1.3. Comparison 1 Antacids versus placebo/no treatment, Outcome 3 Intragastric pH < 2.5 at intubation.
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 1 Antacids versus placebo/no treatment
Outcome: 3 Intragastric pH < 2.5 at intubation

|
Analysis 1.21. Comparison 1 Antacids versus placebo/no treatment, Outcome 21 Intragastric pH < 2.5 at extubation.
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 1 Antacids versus placebo/no treatment
Outcome: 21 Intragastric pH < 2.5 at extubation

|
Analysis 1.23. Comparison 1 Antacids versus placebo/no treatment, Outcome 23 Gastric volume post intubation (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 1 Antacids versus placebo/no treatment
Outcome: 23 Gastric volume post intubation (not pre-specified)

|
Analysis 1.24. Comparison 1 Antacids versus placebo/no treatment, Outcome 24 Gastric pH post intubation (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 1 Antacids versus placebo/no treatment
Outcome: 24 Gastric pH post intubation (not pre-specified)

|
Analysis 1.25. Comparison 1 Antacids versus placebo/no treatment, Outcome 25 At risk of aspiration (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 1 Antacids versus placebo/no treatment
Outcome: 25 At risk of aspiration (not pre-specified)

|
Analysis 1.26. Comparison 1 Antacids versus placebo/no treatment, Outcome 26 Gastric pH at extubation (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 1 Antacids versus placebo/no treatment
Outcome: 26 Gastric pH at extubation (not pre-specified)

|
Analysis 1.27. Comparison 1 Antacids versus placebo/no treatment, Outcome 27 Gastric volume post intubation >25ml (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 1 Antacids versus placebo/no treatment
Outcome: 27 Gastric volume post intubation >25ml (not pre-specified)

|
Analysis 2.3. Comparison 2 H2 antagonists versus placebo/no treatment, Outcome 3 Intragastric pH < 2.5 at intubation.
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 2 H2 antagonists versus placebo/no treatment
Outcome: 3 Intragastric pH < 2.5 at intubation

|
Analysis 2.4. Comparison 2 H2 antagonists versus placebo/no treatment, Outcome 4 Intragastric volume > 0.4ml/kg at intubation.
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 2 H2 antagonists versus placebo/no treatment
Outcome: 4 Intragastric volume > 0.4ml/kg at intubation

|
Analysis 2.21. Comparison 2 H2 antagonists versus placebo/no treatment, Outcome 21 Intragastric pH < 2.5 at extubation.
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 2 H2 antagonists versus placebo/no treatment
Outcome: 21 Intragastric pH < 2.5 at extubation

|
Analysis 2.22. Comparison 2 H2 antagonists versus placebo/no treatment, Outcome 22 Intragastric volume > 20ml at extubation (not pre-specifed).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 2 H2 antagonists versus placebo/no treatment
Outcome: 22 Intragastric volume > 20ml at extubation (not pre-specifed)

|
Analysis 2.23. Comparison 2 H2 antagonists versus placebo/no treatment, Outcome 23 Gastric pH at intubation (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 2 H2 antagonists versus placebo/no treatment
Outcome: 23 Gastric pH at intubation (not pre-specified)

|
Analysis 2.24. Comparison 2 H2 antagonists versus placebo/no treatment, Outcome 24 Gastric pH at extubation (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 2 H2 antagonists versus placebo/no treatment
Outcome: 24 Gastric pH at extubation (not pre-specified)

|
Analysis 2.25. Comparison 2 H2 antagonists versus placebo/no treatment, Outcome 25 At risk of aspiration post intubation (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 2 H2 antagonists versus placebo/no treatment
Outcome: 25 At risk of aspiration post intubation (not pre-specified)

|
Analysis 2.26. Comparison 2 H2 antagonists versus placebo/no treatment, Outcome 26 At risk of aspiration pre extubation (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 2 H2 antagonists versus placebo/no treatment
Outcome: 26 At risk of aspiration pre extubation (not pre-specified)

|
Analysis 2.27. Comparison 2 H2 antagonists versus placebo/no treatment, Outcome 27 Gastric volume post intubation (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 2 H2 antagonists versus placebo/no treatment
Outcome: 27 Gastric volume post intubation (not pre-specified)

|
Analysis 2.28. Comparison 2 H2 antagonists versus placebo/no treatment, Outcome 28 Gastric volume preextubation (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 2 H2 antagonists versus placebo/no treatment
Outcome: 28 Gastric volume pre-extubation (not pre-specified)

|
Analysis 3.3. Comparison 3 Proton pump antagonists versus placebo/no treatment, Outcome 3 Intragastric pH < 2.5 at intubation.
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 3 Proton pump antagonists versus placebo/no treatment
Outcome: 3 Intragastric pH < 2.5 at intubation

|
Analysis 3.4. Comparison 3 Proton pump antagonists versus placebo/no treatment, Outcome 4 Intragastric volume > 0.4ml/kg at intubation.
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 3 Proton pump antagonists versus placebo/no treatment
Outcome: 4 Intragastric volume > 0.4ml/kg at intubation

|
Analysis 3.23. Comparison 3 Proton pump antagonists versus placebo/no treatment, Outcome 23 Gastric pH at intubation (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 3 Proton pump antagonists versus placebo/no treatment
Outcome: 23 Gastric pH at intubation (not pre-specified)

|
Analysis 3.24. Comparison 3 Proton pump antagonists versus placebo/no treatment, Outcome 24 At risk of aspiration (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 3 Proton pump antagonists versus placebo/no treatment
Outcome: 24 At risk of aspiration (not pre-specified)

|
Analysis 3.25. Comparison 3 Proton pump antagonists versus placebo/no treatment, Outcome 25 Gastric volume at intubation (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 3 Proton pump antagonists versus placebo/no treatment
Outcome: 25 Gastric volume at intubation (not pre-specified)

|
Analysis 4.23. Comparison 4 Prokinetic drugs versus placebo/no treatment, Outcome 23 At risk of aspiration post intubation (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 4 Prokinetic drugs versus placebo/no treatment
Outcome: 23 At risk of aspiration post intubation (not pre-specified)

|
Analysis 4.24. Comparison 4 Prokinetic drugs versus placebo/no treatment, Outcome 24 At risk of aspiration pre-extubation (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 4 Prokinetic drugs versus placebo/no treatment
Outcome: 24 At risk of aspiration pre-extubation (not pre-specified)

|
Analysis 5.23. Comparison 5 Non-pharmacological interventions versus placebo/no treatment, Outcome 23 At risk of aspiration (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 5 Non-pharmacological interventions versus placebo/no treatment
Outcome: 23 At risk of aspiration (not pre-specified)

|
Analysis 6.3. Comparison 6 Antacids + H2 antagonists versus placebo/no treatment, Outcome 3 Intragastric pH < 2.5 at intubation.
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 6 Antacids + H2 antagonists versus placebo/no treatment
Outcome: 3 Intragastric pH < 2.5 at intubation

|
Analysis 6.21. Comparison 6 Antacids + H2 antagonists versus placebo/no treatment, Outcome 21 Intragastric pH < 2.5 at extubation.
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 6 Antacids + H2 antagonists versus placebo/no treatment
Outcome: 21 Intragastric pH < 2.5 at extubation

|
Analysis 6.23. Comparison 6 Antacids + H2 antagonists versus placebo/no treatment, Outcome 23 Intragastric pH post intubation (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 6 Antacids + H2 antagonists versus placebo/no treatment
Outcome: 23 Intragastric pH post intubation (not pre-specified)

|
Analysis 6.24. Comparison 6 Antacids + H2 antagonists versus placebo/no treatment, Outcome 24 Intragastric pH at extubation (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 6 Antacids + H2 antagonists versus placebo/no treatment
Outcome: 24 Intragastric pH at extubation (not pre-specified)

|
Analysis 7.3. Comparison 7 H2 antagonists + prokinetic drugs versus placebo/no treatment, Outcome 3 Intragastric pH < 2.5 at intubation.
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 7 H2 antagonists + prokinetic drugs versus placebo/no treatment
Outcome: 3 Intragastric pH < 2.5 at intubation

|
Analysis 7.23. Comparison 7 H2 antagonists + prokinetic drugs versus placebo/no treatment, Outcome 23 Risk of aspiration (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 7 H2 antagonists + prokinetic drugs versus placebo/no treatment
Outcome: 23 Risk of aspiration (not pre-specified)
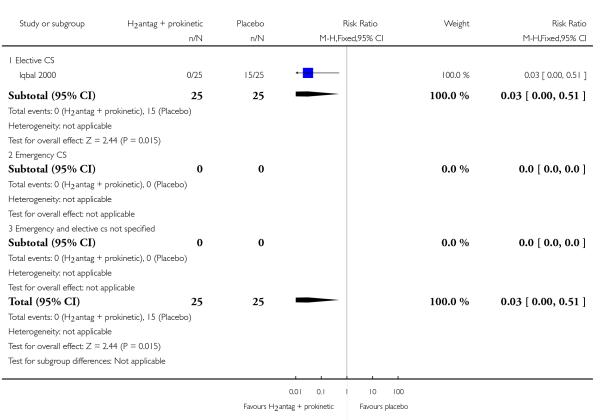
|
Analysis 7.24. Comparison 7 H2 antagonists + prokinetic drugs versus placebo/no treatment, Outcome 24 Gastric pH post intubation (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 7 H2 antagonists + prokinetic drugs versus placebo/no treatment
Outcome: 24 Gastric pH post intubation (not pre-specified)

|
Analysis 7.25. Comparison 7 H2 antagonists + prokinetic drugs versus placebo/no treatment, Outcome 25 Gastric volume post intubation (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 7 H2 antagonists + prokinetic drugs versus placebo/no treatment
Outcome: 25 Gastric volume post intubation (not pre-specified)

|
Analysis 7.26. Comparison 7 H2 antagonists + prokinetic drugs versus placebo/no treatment, Outcome 26 Gastric volume <25ml after induction.
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 7 H2 antagonists + prokinetic drugs versus placebo/no treatment
Outcome: 26 Gastric volume <25ml after induction

|
Analysis 8.3. Comparison 8 Antacids versus H2 antagonists, Outcome 3 Intragastric pH < 2.5 at intubation.
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 8 Antacids versus H2 antagonists
Outcome: 3 Intragastric pH < 2.5 at intubation
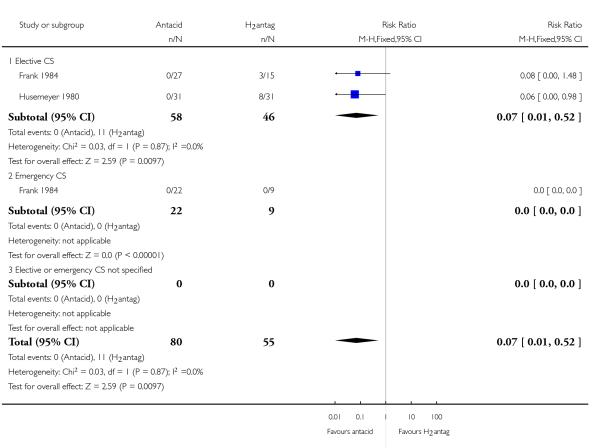
|
Analysis 8.23. Comparison 8 Antacids versus H2 antagonists, Outcome 23 At risk of aspiration (not prespecified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 8 Antacids versus H2 antagonists
Outcome: 23 At risk of aspiration (not pre-specified)

|
Analysis 8.24. Comparison 8 Antacids versus H2 antagonists, Outcome 24 Gastric volume at intubation (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 8 Antacids versus H2 antagonists
Outcome: 24 Gastric volume at intubation (not pre-specified)

|
Analysis 8.25. Comparison 8 Antacids versus H2 antagonists, Outcome 25 Gastric pH at intubation (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 8 Antacids versus H2 antagonists
Outcome: 25 Gastric pH at intubation (not pre-specified)

|
Analysis 8.26. Comparison 8 Antacids versus H2 antagonists, Outcome 26 Gastric pH at extubation (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 8 Antacids versus H2 antagonists
Outcome: 26 Gastric pH at extubation (not pre-specified)

|
Analysis 10.3. Comparison 10 H2 antagonists versus proton pump antagonists, Outcome 3 Intragastric pH < 2.5 at intubation.
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 10 H2 antagonists versus proton pump antagonists
Outcome: 3 Intragastric pH < 2.5 at intubation

|
Analysis 10.4. Comparison 10 H2 antagonists versus proton pump antagonists, Outcome 4 Intragastric volume < 0.4ml/kg at intubation.
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 10 H2 antagonists versus proton pump antagonists
Outcome: 4 Intragastric volume < 0.4ml/kg at intubation

|
Analysis 10.23. Comparison 10 H2 antagonists versus proton pump antagonists, Outcome 23 At risk of aspiration (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 10 H2 antagonists versus proton pump antagonists
Outcome: 23 At risk of aspiration (not pre-specified)

|
Analysis 10.24. Comparison 10 H2 antagonists versus proton pump antagonists, Outcome 24 Gastric pH post intubation (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 10 H2 antagonists versus proton pump antagonists
Outcome: 24 Gastric pH post intubation (not pre-specified)
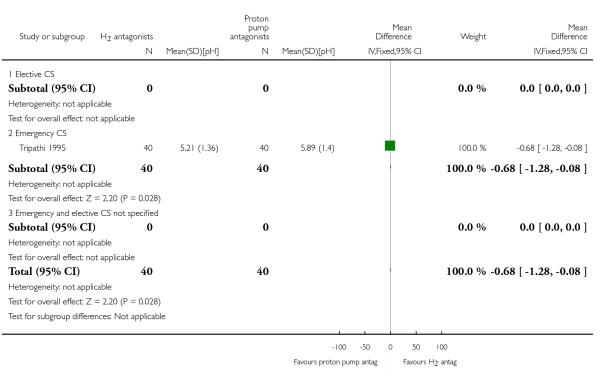
|
Analysis 10.25. Comparison 10 H2 antagonists versus proton pump antagonists, Outcome 25 Gastric volume post intubation (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 10 H2 antagonists versus proton pump antagonists
Outcome: 25 Gastric volume post intubation (not pre-specified)

|
Analysis 10.26. Comparison 10 H2 antagonists versus proton pump antagonists, Outcome 26 Gastric pH pre extubation (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 10 H2 antagonists versus proton pump antagonists
Outcome: 26 Gastric pH pre extubation (not pre-specified)

|
Analysis 10.27. Comparison 10 H2 antagonists versus proton pump antagonists, Outcome 27 Gastric volume post extubation (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 10 H2 antagonists versus proton pump antagonists
Outcome: 27 Gastric volume post extubation (not pre-specified)

|
Analysis 11.3. Comparison 11 Antacids + H2 antagonists versus antacids, Outcome 3 Intragastric pH < 2.5 at intubation.
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 11 Antacids + H2 antagonists versus antacids
Outcome: 3 Intragastric pH < 2.5 at intubation

|
Analysis 11.21. Comparison 11 Antacids + H2 antagonists versus antacids, Outcome 21 Intragastric pH < 2.5 at extubation.
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 11 Antacids + H2 antagonists versus antacids
Outcome: 21 Intragastric pH < 2.5 at extubation

|
Analysis 11.23. Comparison 11 Antacids + H2 antagonists versus antacids, Outcome 23 Post Intubation pH (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 11 Antacids + H2 antagonists versus antacids
Outcome: 23 Post Intubation pH (not pre-specified)

|
Analysis 11.24. Comparison 11 Antacids + H2 antagonists versus antacids, Outcome 24 Pre extubation pH (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 11 Antacids + H2 antagonists versus antacids
Outcome: 24 Pre extubation pH (not pre-specified)

|
Analysis 11.25. Comparison 11 Antacids + H2 antagonists versus antacids, Outcome 25 Post intubation gastric volume (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 11 Antacids + H2 antagonists versus antacids
Outcome: 25 Post intubation gastric volume (not pre-specified)

|
Analysis 11.26. Comparison 11 Antacids + H2 antagonists versus antacids, Outcome 26 Pre-extubation gastric volume (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 11 Antacids + H2 antagonists versus antacids
Outcome: 26 Pre-extubation gastric volume (not pre-specified)

|
Analysis 11.27. Comparison 11 Antacids + H2 antagonists versus antacids, Outcome 27 At risk of aspiration (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 11 Antacids + H2 antagonists versus antacids
Outcome: 27 At risk of aspiration (not pre-specified)

|
Analysis 13.23. Comparison 13 Proton pump agonists + prokinetics versus proton pump agonists, Outcome 23 At risk of aspiration post intubation (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 13 Proton pump agonists + prokinetics versus proton pump agonists
Outcome: 23 At risk of aspiration post intubation (not pre-specified)

|
Analysis 13.24. Comparison 13 Proton pump agonists + prokinetics versus proton pump agonists, Outcome 24 At risk of aspiration pre extubation (not pre specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 13 Proton pump agonists + prokinetics versus proton pump agonists
Outcome: 24 At risk of aspiration pre extubation (not pre specified)

|
Analysis 14.4. Comparison 14 H2 antagonist versus Tramadol, Outcome 4 Intragastric volume > 0.4ml/kg at intubation.
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 14 H2 antagonist versus Tramadol
Outcome: 4 Intragastric volume > 0.4ml/kg at intubation

|
Analysis 14.5. Comparison 14 H2 antagonist versus Tramadol, Outcome 5 Nausea.
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 14 H2 antagonist versus Tramadol
Outcome: 5 Nausea

|
Analysis 14.22. Comparison 14 H2 antagonist versus Tramadol, Outcome 22 Intragastric volume > 0.4ml/kg at extubation.
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 14 H2 antagonist versus Tramadol
Outcome: 22 Intragastric volume > 0.4ml/kg at extubation

|
Analysis 14.23. Comparison 14 H2 antagonist versus Tramadol, Outcome 23 At risk post intubation (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 14 H2 antagonist versus Tramadol
Outcome: 23 At risk post intubation (not pre-specified)

|
Analysis 14.24. Comparison 14 H2 antagonist versus Tramadol, Outcome 24 At risk pre extubation (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 14 H2 antagonist versus Tramadol
Outcome: 24 At risk pre extubation (not pre-specified)
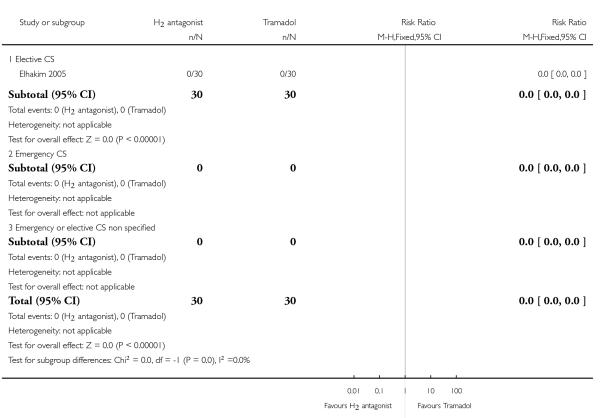
|
Analysis 14.25. Comparison 14 H2 antagonist versus Tramadol, Outcome 25 Nausea 24hours post op (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 14 H2 antagonist versus Tramadol
Outcome: 25 Nausea 24hours post op (not pre-specified)

|
Analysis 15.23. Comparison 15 Antacids + H2 antagonists versus proton pump antagonists, Outcome 23 At risk of aspiration (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 15 Antacids + H2 antagonists versus proton pump antagonists
Outcome: 23 At risk of aspiration (not pre-specified)

|
Analysis 16.23. Comparison 16 Proton pump antagonist + antacid versus proton pump antagonist, Outcome 23 Risk of aspiration (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 16 Proton pump antagonist + antacid versus proton pump antagonist
Outcome: 23 Risk of aspiration (not pre-specified)

|
Analysis 17.23. Comparison 17 H2 antagonist + prokinetic versus H2 antagonist, Outcome 23 Intragastric pH > 2.5 post intubation (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 17 H2 antagonist + prokinetic versus H2 antagonist
Outcome: 23 Intragastric pH > 2.5 post intubation (not pre-specified)
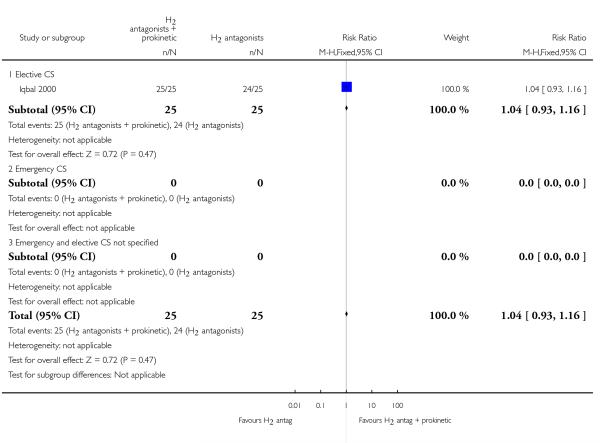
|
Analysis 17.24. Comparison 17 H2 antagonist + prokinetic versus H2 antagonist, Outcome 24 Intragastric volume <0.4 ml/kg post intubation (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 17 H2 antagonist + prokinetic versus H2 antagonist
Outcome: 24 Intragastric volume <0.4 ml/kg post intubation (not pre-specified)
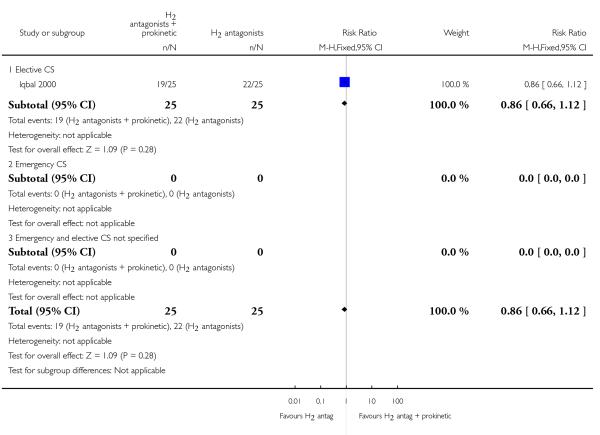
|
Analysis 17.25. Comparison 17 H2 antagonist + prokinetic versus H2 antagonist, Outcome 25 Risk of aspiration (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 17 H2 antagonist + prokinetic versus H2 antagonist
Outcome: 25 Risk of aspiration (not pre-specified)

|
Analysis 17.26. Comparison 17 H2 antagonist + prokinetic versus H2 antagonist, Outcome 26 Gastric volume post intubation (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 17 H2 antagonist + prokinetic versus H2 antagonist
Outcome: 26 Gastric volume post intubation (not pre-specified)

|
Analysis 17.27. Comparison 17 H2 antagonist + prokinetic versus H2 antagonist, Outcome 27 Gastric pH post intubation (not pre-specified).
Review: Interventions at caesarean section for reducing the risk of aspiration pneumonitis
Comparison: 17 H2 antagonist + prokinetic versus H2 antagonist
Outcome: 27 Gastric pH post intubation (not pre-specified)

|
HISTORY
| Date | Event | Description |
|---|---|---|
| 11 April 2008 | New citation required and major changes | An editorial decision was taken to split the review into two: those interventions that could be given before surgery to reduce aspiration pneumonitis and those given during or after caesarean section (CS) to reduce nausea and vomiting. We have, therefore, updated the title and the scope of the previously published protocol (Paranjothy 2004) to reflect this decision. |
| 18 February 2008 | Amended | Converted to new review format. |
DIFFERENCES BETWEEN PROTOCOL AND REVIEW
The title was changed from ’Drugs at caesarean section for preventing nausea, vomiting and aspiration pneumonitis’ to ’Interventions at caesarean section for reducing aspiration pneumonitis’ because we wished to include complimentary medicines and mechanical methods (however, we found no studies). In addition, it was felt that the interventions to reduce nausea and vomiting during caesarean section should be a separate review. This second review will look at the effect of ’Interventions given during caesarean section to reduce nausea and vomiting’.
We modified the ’Types of participants’ from ’Healthy pregnant women with an uncomplicated singleton pregnancy at term undergoing elective or emergency caesarean section under general or regional anaesthesia’ to ’Pregnant women undergoing elective or emergency caesarean section under general or regional anaesthesia’ because we felt the interventions needed should be applicable to a wider range of women.
We removed reference to route of administration in the inclusion criteria. Relevent studies will be included regardless of the route of administration of the trial medication. A comparison of the efficacy of different routes of administration will be included in subgroup analysis if sufficient data exist.
We removed the pharmacological and non-pharmacological subgroups because we felt the interventions should be considered more individually. We added elective caesarean section and emergency caesarean section subgroups because we felt the outcomes may be different in these two differing situations.
Several papers reported gastric volume and pH as continuous outcomes, and ’at risk of aspiration’. These were not pre-specified in our protocol but we have included them in the review as they are informative. In the protocol we had prespecified dichotomous outcomes for gastric pH as greater than 2.5, however we changed this to pH less than 2.5 as this is what the majority of papers have reported, and is intuitively easier to interpret.
We have taken out general versus regional anaesthesia as a sub-group comparison as all the RCTs that investigate this outcome were in women who had general anaesthesia.
We have modified the wording in the methods sections for ’Assessment of heterogeneity’, ’Assessment of reporting bias’ and ’Data synthesis’ to update them with the new methods being used by the group, developed in conjunction with the group’s statistician, Simon Gates, and Richard Riley. We have used these new methods in the review.
Footnotes
DECLARATIONS OF INTEREST
None known.
References to studies included in this review
* Indicates the major publication for the study
- Dewan 1985 {published data only} .Dewan DM, Floyd HM, Thistlewood JM, Bogard TD. Sodium citrate premedication for elective cesarean section. Anesthesia and Analgesia. 1984;63:205. [PubMed] [Google Scholar]; *; Dewan DM, Floyd HM, Thistlewood JM, Bogard TD, Spielman FJ. Sodium citrate pretreatment in elective cesarean section patients. Anesthesia and Analgesia. 1985;64:34–7. [PubMed] [Google Scholar]
- Elhakim 2005 {published data only} .Elhakim M, El-Megid WA, Metry A, El-hennawy A, El-Queseny K. Analgesic and antacid properties of i.m. tramadol given before caesarean section under general anaesthesia. British Journal of Anaesthesia. 2005;95(6):811–5. doi: 10.1093/bja/aei260. [DOI] [PubMed] [Google Scholar]
- Ewart 1990 {published data only} .Ewart MC, Yau G, Gin T, Kotur CF, Oh TE. A comparison of oral omeprazole and ranitidine as premedication for elective caesarean section; Proceedings of Spring Meeting of Obstetric Anaesthetists Association; ; London, UK. 1990; p. 33. 1990. [Google Scholar]; *; Ewart MC, Yau G, Gin T, Kotur CF, Oh TE. A comparison of the effects of omeprazole and ranitidine on gastric secretion in women undergoing elective caesarean section. Anaesthesia. 1990;45:527–30. doi: 10.1111/j.1365-2044.1990.tb14823.x. [DOI] [PubMed] [Google Scholar]
- Frank 1984 {published data only} .Frank M, Evans M, Flynn P, Aun C. Comparison of the prophylactic use of magnesium trisilicate mixture BPC,sodium citrate mixture or cimetidine in obstetrics. British Journal of Anaesthesia. 1984;56:355–61. doi: 10.1093/bja/56.4.355. [DOI] [PubMed] [Google Scholar]
- Hong 2004 {published data only} .Hong JY. Effect of dextrose on preoperative gastric content and serum gastrin [abstract] Canadian Journal of Anesthesia. 2004;51(Suppl 1):A57. [Google Scholar]
- Husemeyer 1980 {published data only} .Husemeyer RP, Davenport HT. Prophylaxis for Mendelson’s syndrome before elective caesarean section. A comparison of cimetidine and magnesium trisilicate mixture regimens. British Journal of Obstetrics and Gynaecology. 1980;87:565–70. doi: 10.1111/j.1471-0528.1980.tb05004.x. [DOI] [PubMed] [Google Scholar]
- Iqbal 2000 {published data only} .Iqbal MS, Ashfaque M, Akram M. Gastric fluid volume and pH: a comparison of effects of ranitidine alone with combination of ranitidine and metoclopramide in patients undergoing elective caesarean section. Annals of King Edward Medical College. 2000;6(2):189–91. [Google Scholar]
- Jasson 1989 {published data only} .Jasson J, Lefevre G, Talaffre ML, Legagneux F, Guerin JP, Vassilieff N, et al. Immediate pre-anesthetic administration of sodium citrate in cesarean interventions [abstract] [Administration immediate pre-anesthesique de citrate de sodium pour intervention cesarienne programmee] Annales Francaises d’Anesthesie et de Reanimation. 1987;6:R155. [Google Scholar]; *; Jasson J, Lefevre G, Tallet F, Talafre ML, Legagneux F, Conseiller C. Oral sodium citrate before general anaesthesia for elective caesarean section Effects of pH and volume of gastric content [Ingestion de citrate de sodium avant l’anesthesie generale pour cesarienne programmee. Effet sur le pH et le volume du contenu gastrique] Annales Francaises d Anesthesie et de Reanimation. 1989;8:12–8. doi: 10.1016/s0750-7658(89)80136-4. [DOI] [PubMed] [Google Scholar]
- Lin 1996 {published data only} .Lin CJ, Huang CL, Hsu HW, Chen TL. Prophylaxis against acid aspiration in regional anesthesia for elective cesarean section: a comparison between oral single-dose ranitidine, famotidine and omeprazole assessed with fiberoptic gastric aspiration. Acta Anaesthesiologica Sinica. 1996;34(4):179–84. [PubMed] [Google Scholar]
- Ormezzano 1990 {published data only} .Ormezzano X, Francois TP, Viaud JY, Bukowski JG, Bourgeonneau MC, Cottron D, et al. Aspiration pneumonitis prophylaxis in obstetric anaesthesia: comparison of effervescent cimetidine-sodium citrate mixture and sodium citrate. British Journal of Anaesthesia. 1990;64:503–6. doi: 10.1093/bja/64.4.503. [DOI] [PubMed] [Google Scholar]
- Orr 1993 {published data only} .Orr DA, Bill KM, Gillon KRW, Wilson CM, Fogarty DJ, Moore J. Effects of omeprazole, with and without metoclopramide, in elective obstetric anaesthesia. Anaesthesia. 1993;48:114–9. doi: 10.1111/j.1365-2044.1993.tb06847.x. [DOI] [PubMed] [Google Scholar]
- Ostheimer 1982 {published data only} .Ostheimer GW, Morrison JA, Lavoie C, Sepkoski C, Hoffman J, Datta S. The effect of cimetidine on the mother, newborn and neonatal neurobehavior. Anesthesiology. 1982;57:A405. [Google Scholar]
- Ozkan 2000 {published data only} .Ozkan T, Senturk M, Yavru A, Celebi S, Pembeci K. Does preoperative fluid fasting have a benefit on aspiration prophylaxis in obstetric anaesthesia [Obstetrik anestezide preoperatif oral sivi alimi aspirasyon pnomonisi profilaksisine katki saglar mi?] Turk Anesteziyoloji Ve Reanimasyon. 2000;28:425–9. [Google Scholar]
- Pickering 1980 {published data only} .Pickering BG, Palahniuk RJ, Cumming M. Cimetidine premedication in elective caesarean section. Canadian Anaesthetists Society Journal. 1980;27:33–5. doi: 10.1007/BF03006845. [DOI] [PubMed] [Google Scholar]
- Rocke 1994 {published data only} .Rocke DA, Rout CC, Gouws E. Intravenous administration of the proton pump inhibitor omeprazole reduces the risk of acid aspiration at emergency cesarean section. Anesthesia & Analgesia. 1994;78:1093–8. doi: 10.1213/00000539-199406000-00010. [DOI] [PubMed] [Google Scholar]
- Rout 1993 {published data only} .Rout CC, Rocke DA, Gouws E. Intravenous ranitidine reduces the risk of acid aspiration of gastric contents at emergency cesarean section. Anesthesia & Analgesia. 1993;76:156–61. doi: 10.1213/00000539-199301000-00026. [DOI] [PubMed] [Google Scholar]
- Tripathi 1995 {published data only} .Tripathi A, Somwanshi M, Singh B, Bajaj P. A comparison of intravenous ranitidine and omeprazole on gastric volume and ph in women undergoing emergency caesarean section. Canadian Journal of Anaesthesia. 1995;42(9):797–800. doi: 10.1007/BF03011180. [DOI] [PubMed] [Google Scholar]
- Tryba 1983 {published data only} .Tryba M, Burkert W, Husch M, Zenz M. Effectiveness of cimetidine in the prevention of aspiration pneumonia in obstetrics [Wirksamkeit von Cimetidin zur Prophylaxe der Aspirationspneumonie in der Geburtshilfe] Fortschritte der Medizin. 1983;101:1757–61. [PubMed] [Google Scholar]
- Wig 1987 {published data only} .Wig J, Biswas GC, Malhotra SK, Gupta AN. Comparison of sodium citrate with magnesium trisilicate as pre-anaesthetic antacid in emergency caesarean sections. Indian Journal of Medical Research. 1987;85:306–10. [PubMed] [Google Scholar]
- Yau 1992 {published data only} .Yau G, Kan AF, Gin T, Oh TE. A comparison of omeprazole and ranitidine for prophylaxis against aspiration pneumonitis in emergency caesarean section. Anaesthesia. 1992;47:101–4. doi: 10.1111/j.1365-2044.1992.tb02002.x. [DOI] [PubMed] [Google Scholar]
- Zoroglu 1999 {published data only} .Zoroglu F, Karamanlioglu B, Pamukcu Z. The value of nizatidine and famotidine in the prophylaxis of acid aspiration pneumonia in cesarean operation [Elektif sezaryen ameliyatlarinda nizatidin ve famotidinin asit aspirasyon pnomonisi profilaksisindeki degeri] Turk Anesteziyoloji Ve Reanimasyon. 2000;28(10):521–6. [Google Scholar]; *; Zoroglu F, Karamanlioglu B, Unal C, Pamukcu Z. The value of nizatidine and famotidine in prophylaxis of aspiration pneumonia in caesarean section. British Journal of Anaesthesia. 1999;82(Suppl):159–60. [Google Scholar]
- Zue 1999 {published data only} .Zue AS, Meye JF, Nguema PN, Nsafu DN, Milama EN. Effervescent ranitidine before urgent caesarean section:effect on gastric pH [Ranitidine effervescente avant cesarienne en urgence: effet sur le pH gastrique] Cahiers d Anesthesiologie. 1999;47:1–3. [Google Scholar]
References to studies excluded from this review
* Indicates the major publication for the study
- Abboud 1984 {published data only} .Abboud TK, Curtis J, Earl S, Henriksen EH, Hughes SC, Levinson G, et al. Efficacy of clear antacid prophylaxis in obstetrics. Acta Anaesthesiologica Scandinavica. 1984;28:301–4. doi: 10.1111/j.1399-6576.1984.tb02065.x. [DOI] [PubMed] [Google Scholar]
- Abouleish 1999 {published data only} .Abouleish EI, Rashid S, Haque S, Giezentanner A, Joynton P, Chuang AZ. Ondansetron versus placebo for the control of nausea and vomiting during caesarean section under spinal anaesthesia. Anaesthesia. 1999;54:479–82. doi: 10.1046/j.1365-2044.1999.00798.x. [DOI] [PubMed] [Google Scholar]
- Ackerman 1987 {published data only} .Ackerman WE, Colclough GW, Guiler JM, Guilder DS, Akin JM. Epidural lipophilic opioids administered prophylactically for control of nausea, vomiting and pain during cesarean section; Proceedings of 19th Annual Meeting of Society for Obstetric Anesthesia and Perinatology; 1987; Halifax, Nova Scotia, Canada: May 20-23, p. 143. 1987. [Google Scholar]
- Ackerman 1988 {published data only} .Ackerman WE, Juneja MM, Colclough GW, Kaczorowski DM. Epidural fentanyl significantly decreases nausea and vomiting during uterine manipulation in awake patients undergoing cesarean section. Anesthesiology. 1988;69:A679. [Google Scholar]
- Ackerman 1989 {published data only} .Ackerman WE, Juneja MM, Colclough GW, Kaczorowski DM. Epidural lipophilic opioids significantly decrease nausea and emesis during uterine manipulation in awake patients undergoing cesarean section. Regional Anesthesia. 1989;14(2):23. [Google Scholar]
- Apiliogullari 2008 {published data only} .Apiliogullari S, Gok F, Canpolat A, Soysal S, Toy H. Comparison of prophylactic dimenhydrinate and metoclopramide for prevention of nausea-vomiting caused by intrathecal opioids in parturients undergoing caesarean delivery. Regional Anesthesia and Pain Medicine. 2008;33(5 Suppl 1):131. [Google Scholar]
- Atkinson 1980 {published data only} .Atkinson RE, Thomas T, Nicholas ADG, Anderson I. An antacid comparison trial. Anaesthesia. 1980;35:1126–30. [Google Scholar]
- Avramovic 1979 {published data only} .Avramovic D, Sulovic V, Lazarevic B, Cvetkovic M, Milacic D. Prevention of postoperative abdominal discomfort and gastrointestinal distension after cesarean section by use of simeticon. Jugoslavenska Ginekologija I Opstetricija. 1979;19(5-6):307–11. [PubMed] [Google Scholar]
- Ayorinde 2000 {published data only} .Ayorinde B, Brown J, Buczkowski P, Shah J, Buggy DJ. Prevention of spinal anaesthesia-induced hypotension during caesarean section: comparative sudy of pre-emptive vasopressors. British Journal of Anaesthesia. 2000;84(2):277P–288P. doi: 10.1093/bja/86.3.372. [DOI] [PubMed] [Google Scholar]
- Ayorinde 2001 {published data only} .Ayorinde BT, Buczkowski P, Brown J, Shah J, Buggy DJ. Evaluation of pre-emptive intramuscular phenylephrine and ephedrine for reduction of spinal anaesthesia-induced hypotension during caesarean section. British Journal of Anaesthesia. 2001;86(3):372–6. doi: 10.1093/bja/86.3.372. [DOI] [PubMed] [Google Scholar]
- Belzarena 1993 {published data only} .Belzarena SD. Addition of epinephrine to a combination of bupivacaine and fentanyl in spinal anesthesia for cesarean section. Regional Anesthesia. 1993;18(1 Suppl 1):15. [Google Scholar]
- Bifarini 1990 {published data only} .Bifarini G, Trabalza N, Falconi S, Mala L, Favetta P. Effects on newborn of pharmacological prophylaxis for Mendelson’s syndrome. Minerva Anestesiologica. 1990;56:1447–50. [PubMed] [Google Scholar]
- Bifarini 1992 {published data only} .Bifarini G, Favetta P, Ciammiti B, Cirulli P, Del Sindaco F, Pasqualucci A. Pharmacological prevention of Mendelson’s syndrome: controlled clinical trial. Minerva Anestesiologica. 1992;58(3):95–9. [PubMed] [Google Scholar]
- Birnbach 1993 {published data only} .Birnbach DJ, Stein DJ, Saunders TA, Grunebaum A, Kitain EM. Prophylactic use of acupressure for the prevention of nausea and vomiting during spinal anesthesia for cesarean section. Anesthesiology. 1993;79:A1020. [Google Scholar]
- Biswas 2003 {published data only} .Biswas BN, Rudra A, Das SK, Nath S, Biswas SC. A comparative study of glycopyrrolate, dexamethasone and metoclopramide in control of post-operative nausea and vomiting after spinal anaesthesia for caesarean delivery. Indian Journal of Anaesthesia. 2003;47(3):198–200. [Google Scholar]
- Biwas 2002 {published data only} .Biwas SC, Biswas BN, Rudra A, Pan A. A comparative study of metoclopramide, glycopyrrolate and combination of metoclopramide and glycopyrrolate in control of intraoperative and postoperative nausea and vomiting after spinal anaethesia for caesarean section. Journal of Obstetrics and Gynecology of India. 2002;52(3):73–5. [Google Scholar]
- Bonhomme 2002 {published data only} .Bonhomme V, Brichant JF, Wuilmart M, Dewandre PY, Hans P. Droperidol reduces nausea after caesarean section but alters the neurological status of the breastfed infants [abstract] Anesthesiology. 2002;96:A1044. [Google Scholar]
- Boone 2002 {published data only} .Boone JB, Gao GG, Clark CP, Chaudhuri S, Chaudhuri K. Dolasetron for prevention of perioperative nausea and vomiting during cesarean section under regional anesthesia [abstract] Anesthesiology. 2002;96(Suppl):A1069. [Google Scholar]
- Boschi 1984 {published data only} .Boschi S, Di MMG, Pigna A, Rossi R. The effect of ranitidine on gastric ph and volume in patients undergoing cesarean section: Possible relationship to Mendelson’s syndrome. Current Therapeutic Research, Clinical and Experimental. 1984;35(4):654–62. [Google Scholar]
- Brock-Utne 1989 {published data only} .Brock-Utne JG, Rout C, Moodley J, Mayat N. Influence of preoperative gastric aspiration on the volume and pH of gastric contents in obstetric patients undergoing caesarean section. British Journal of Anaesthesia. 1989;62:397–401. doi: 10.1093/bja/62.4.397. [DOI] [PubMed] [Google Scholar]
- Brody 2008 {published data only} .Brody R. [accessed 20 February 2008];Study of the effect of intramuscular ephedrine on the incidence of nausea and vomiting during elective cesarean section. 2008 ClinicalTrials.gov ( http://clinicaltrials.gov/)
- Bylsma-Howell 1983 {published data only} .Bylsma-Howell M, McMorland GH, Rurak DW, McErlane B, Axelson JE. Placental transport of metoclopramide: assessment of maternal and neonatal effects. Canadian Anaesthetists Society Journal. 1983;30:487–92. doi: 10.1007/BF03007082. [DOI] [PubMed] [Google Scholar]
- Caba 1997 {published data only} .Caba F, Echevarria M, Bernal-Davalos L, Pallares-Gonzalez JA, Rodriguez-Rodriguez R. Prophylaxis for intraoperative nausea and vomiting with nonhypnotic doses of propofol during intradural anesthesia for cesarean delivery [Profilaxis de las nauseas y los vomitos intraoperatorios con dosis subhipnoticas de propofol durante la anestesia intradural en cesareas] Revista Espanola De Anestesiologia y Reanimacion. 1997;44:262–6. [PubMed] [Google Scholar]
- Chan 1992 {published data only} .Chan YK. A comparison of sodium citrate and sodium citrate/ranitidine combination for acid aspiration prophylaxis. Medical Journal of Malaysia. 1992;47(1):27–30. [PubMed] [Google Scholar]
- Chan 1997 {published data only} .Chan WS, Irwin MG, Tong WN, Lam YH. Prevention of hypotension during spinal anaesthesia for caesarean section: ephedrine infusion versus fluid preload. Anaesthesia. 1997;52(9):908–13. doi: 10.1111/j.1365-2044.1997.190-az0323.x. [DOI] [PubMed] [Google Scholar]
- Charuluxananan 2003 {published data only} .Charuluxananan S, Kyokong O, Somboonviboon W, Narasethakamol A, Promlok P. Nalbuphine versus ondansetron for prevention of intrathecal morphine-induced pruritus after cesarean delivery. Anesthesia and Analgesia. 2003;96(6):1789–93. doi: 10.1213/01.ANE.0000066015.21364.7D. [DOI] [PubMed] [Google Scholar]
- Chaudhuri 2004 {published data only} .Chaudhuri K, Chaudhuri S, Lampe C, Rivera J, Solis-Estrada J. Does addition of dolasetron to metoclopramide alter nausea and vomiting in cesarean section patients under regional anesthesia [abstract] Anesthesiology. 2004;101(Suppl):A1234. [Google Scholar]
- Chen 2005 {published data only} .Chen HM, Chang FY, Hsu CT. Effect of acupressure on nausea, vomiting, anxiety and pain among post-cesarean section women in Taiwan. Kaohsiung Journal of Medical Sciences. 2005;21(8):341–50. doi: 10.1016/S1607-551X(09)70132-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherian 2001 {published data only} .Cherian VT, Smith I. Prophylactic ondansetron does not improve patient satisfaction in women using pca after caesarean section. British Journal of Anaesthesia. 2001;87(3):502–4. doi: 10.1093/bja/87.3.502. [DOI] [PubMed] [Google Scholar]
- Chestnut 1987 {published data only} .Chestnut DH, Bates JN, Choi WW, Vandewalker GE. Administration of metoclopramide for prevention of nausea and vomiting during regional anesthesia for cesarean section: a randomized, double blind, placebo controlled study. Anesthesiology. 1986;65:390. doi: 10.1097/00000542-198704000-00022. [DOI] [PubMed] [Google Scholar]; *; Chestnut DH, Vandewalker GE, Owen CL, Bates JN, Choi WW. Administration of metoclopramide for prevention of nausea and vomiting during epidural anesthesia for elective cesarean section. Anesthesiology. 1987;66:563–6. doi: 10.1097/00000542-198704000-00022. [DOI] [PubMed] [Google Scholar]
- Chestnut 1989 {published data only} .*; Chestnut DH, Owen CL, Geiger M, Bates JN, Choi WW, Ostman PL. Metoclopramide versus droperidol for prevention of nausea and vomiting during epidural anesthesia for cesarean section. Southern Medical Journal. 1989;82(10):1224–7. doi: 10.1097/00007611-198910000-00006. [DOI] [PubMed] [Google Scholar]; Chestnut DH, Owen CL, Ostman LG, Bates JN, Choi WW. A comparison of metoclopramide and droperidol for prevention of nausea and vomiting during and after elective cesarean section with epidural anesthesia; Proceedings of 19th Annual Meeting of Society for Obstetric Anesthesia and Perinatology; Halifax, Nova Scotia, Canada. 1987; May 20-23, p. 118. 1987. [Google Scholar]
- Chung 1998 {published data only} .Chung CJ, Kim JS, Park HS, Chin YJ. The efficacy of intrathecal neostigmine, intrathecal morphine, and their combination for post-cesarean section analgesia. Anesthesia and Analgesia. 1998;87(2):341–6. doi: 10.1097/00000539-199808000-00020. [DOI] [PubMed] [Google Scholar]
- Cohen 1983 {published data only} .Cohen SE, Barrier G. Does metoclopramide decrease gastric volume in cesarean section patients? Anesthesiology. 1983;59:A403. doi: 10.1097/00000542-198411000-00025. [DOI] [PubMed] [Google Scholar]; *; Cohen SE, Jasson J, Talafre ML, Chauvelot-Moachon L, Barrier G. Does metoclopramide decrease the volume of gastric contents in patients undergoing cesarean section? Anesthesiology. 1984;61:604–7. doi: 10.1097/00000542-198411000-00025. [DOI] [PubMed] [Google Scholar]
- Colman 1988 {published data only} .Colman RD, Frank M, Loughnan BA, Cohen DG, Cattermole R. Use of i.m. ranitidine for the prophylaxis of aspiration pneumonitis in obstetrics. British Journal of Anaesthesia. 1988;61:720–9. doi: 10.1093/bja/61.6.720. [DOI] [PubMed] [Google Scholar]
- Connelly 1997 {published data only} .Connelly NR, Rahimi A, Parker RK. Nalmefene or naloxone for preventing intrathecal opioid mediated side effects in cesarean delivery patients. International Journal of Obstetric Anesthesia. 1997;6:231–4. doi: 10.1016/s0959-289x(97)80028-3. [DOI] [PubMed] [Google Scholar]
- Cooper 2002 {published data only} .Cooper DW, Carpenter M, Mowbray P, Desira WR, Ryall DM, Kokri MS. Fetal and maternal effects of phenylephrine and ephedrine during spinal anesthesia for cesarean section. Anesthesiology. 2002;97:1582–90. doi: 10.1097/00000542-200212000-00034. [DOI] [PubMed] [Google Scholar]
- Cowan 2002 {published data only} .Cowan CM, Kendall JB, Barclay PM, Wilkes RG. Comparison of intrathecal fentanyl and diamorphine in addition to bupivacaine for caesarean section under spinalanaesthesia. British Journal of Anaesthesia. 2002;89(3):452–8. [PubMed] [Google Scholar]
- Dahlgren 1997 {published data only} .Dahlgren G, Hultstrand C, Jakobsson J, Norman M, Eriksson EW, Martin H. Intrathecal sufentanil, fentanyl, or placebo added to bupivacaine for cesarean section. Anesthesia & Analgesia. 1997;85(6):1288–93. doi: 10.1097/00000539-199712000-00020. [DOI] [PubMed] [Google Scholar]
- Dailey 1985 {published data only} .Dailey PA, Hughes SC, Rosen MA, Healy K, Cheek DBC, Pytka S, et al. Lidocaine levels during cesarean section after pretreatment with ranitidine or cimetidine. Anesthesiology. 1985;63:444. [Google Scholar]
- Dailey 1988 {published data only} .Dailey PA, Hughes SC, Rosen MA, Healy K, Cheek DBC, Shnider SM. Effect of cimetidine and ranitidine on lidocaine concentrations during epidural anesthesia for cesarean section. Anesthesiology. 1988;69:1013–7. doi: 10.1097/00000542-198812000-00045. [DOI] [PubMed] [Google Scholar]
- Datta 1982 {published data only} .Datta S, Alper MH, Ostheimer GW, Weiss JB. Method of ephedrine administration and nausea and hypotension during spinal anesthesia for cesarean section. Anesthesiology. 1982;56:68–70. doi: 10.1097/00000542-198201000-00019. [DOI] [PubMed] [Google Scholar]
- Dewan 1982 {published data only} .Dewan DM, Wheeler AS, James FM, III, Floyd HM, Rhyne L. Antacid anticholinergic regimens in patients undergoing elective caesarean section. Canadian Anaesthetists Society Journal. 1982;29:27–30. doi: 10.1007/BF03007944. [DOI] [PubMed] [Google Scholar]
- Duggal 1998 {published data only} .Duggal KN, Douglas MJ, Peter EA, Merrick PM. Acupressure for intrathecal narcotic-induced nausea and vomiting after caesarean section. International Journal of Obstetric Anesthesia. 1998;7(4):231–6. doi: 10.1016/s0959-289x(98)80044-7. [DOI] [PubMed] [Google Scholar]
- Dundee 1979 {published data only} .Dundee JW, Howe JP, Moore J, McCaughey W. Effect of cimetidine on gastric pH in women undergoing elective caesarean section [proceedings] British Journal of Clinical Pharmacology. 1979;8:391P–392P. doi: 10.1111/j.1365-2125.1979.tb04738.x. [DOI] [PubMed] [Google Scholar]
- Fan 1994 {published data only} .Fan SZ, Susetio L, Wang YP, Cheng YJ, Liu CC. Low dose of intrathecal hyperbaric bupivacaine combined with epidural lidocaine for cesarean section - a balance block technique. Anesthesia and Analgesia. 1994;78:474–7. doi: 10.1213/00000539-199403000-00009. [DOI] [PubMed] [Google Scholar]
- Flynn 1989a {published data only} .Flynn RJ, Moore J, Collier PS, McClean E. Does pretreatment with cimetidine and ranitidine affect the disposition of bupivacaine? British Journal of Anaesthesia. 1989;62:87–91. doi: 10.1093/bja/62.1.87. [DOI] [PubMed] [Google Scholar]
- Flynn 1989b {published data only} .Flynn RJ, Moore J. Does cimetidine effect plasma lidocaine levels in the parturient? Anesthesia & Analgesia. 1989;68:S87. doi: 10.1111/j.1399-6576.1989.tb02973.x. [DOI] [PubMed] [Google Scholar]; *; Flynn RJ, Moore J, Collier PS, Howard PJ. Effect of intravenous cimetidine on lignocaine disposition during extradural caesarean section. Anaesthesia. 1989;44:739–41. doi: 10.1111/j.1365-2044.1989.tb09259.x. [DOI] [PubMed] [Google Scholar]
- Flynn 1989c {published data only} .Flynn RJ, Moore J. Effect of H2-receptor antagonists on plasma lignocaine concentrations in the obstetric patient. British Journal of Anaesthesia. 1988;61:512P. [Google Scholar]; Flynn RJ, Moore J. Lack of effect of cimetidine and ranitidine on lidocaine disposition in the parturient. Anesthesiology. 1988;69:A656. [Google Scholar]; *; Flynn RJ, Moore J, Collier PS, Howard PJ. Single dose oral H2-antagonists do not affect plasma lidocaine levels in the parturient. Acta Anaesthesiologica Scandinavica. 1989;33:593–6. doi: 10.1111/j.1399-6576.1989.tb02973.x. [DOI] [PubMed] [Google Scholar]
- Fogarty 1992 {published data only} .Fogarty DJ, Moore J. Further investigations of omeprazole in obstetric patients. International Journal of Obstetric Anesthesia. 1992;1:180. [Google Scholar]
- Freeman 1999 {published data only} .Freeman J, Lafreniere L, Watkins E, Dresner M. Does sodium citrate increase sickness associated with spinal anaesthesia for caesarean section? British Journal of Anaesthesia. 1999;82(Suppl):160. [Google Scholar]
- Fujii 1998a {published data only} .Fujii Y, Tanaka H, Toyooka H. Granisetron prevents nausea and vomiting during spinal anaesthesia for caesarean section. Acta Anaesthesiologica Scandinavica. 1998;42(3):312–5. doi: 10.1111/j.1399-6576.1998.tb04922.x. [DOI] [PubMed] [Google Scholar]
- Fujii 1998b {published data only} .Fujii Y, Tanaka H, Toyooka H. Prevention of nausea and vomiting with granisetron, droperidol and metoclopramide during and after spinal anaesthesia for caesarean section: a randomized, double-blind, placebo-controlled trial. Acta Anaesthesiologica Scandinavica. 1998;42(8):921–5. doi: 10.1111/j.1399-6576.1998.tb05350.x. [DOI] [PubMed] [Google Scholar]
- Fujii 1999 {published data only} .Fujii Y, Saitoh Y, Tanaka H, Toyooka H. Granisetron/ dexamethasone combination for reducing nausea and vomiting during and after spinal anesthesia for cesarean section. Anesthesia & Analgesia. 1999;88(6):1346–50. doi: 10.1097/00000539-199906000-00028. [DOI] [PubMed] [Google Scholar]
- Fujii 2002 {published data only} .Fujii Y, Numazaki M. Dose-range effects of propofol for reducing emetic symptoms during cesarean delivery. Obstetrics & Gynecology. 2002;99:75–9. doi: 10.1016/s0029-7844(01)01641-6. [DOI] [PubMed] [Google Scholar]
- Fujii 2004 {published data only} .Fujii Y, Numazaki M. Randomized, double-blind comparison of subhypnotic-dose propofol alone and combined with dexamethasone for emesis in parturients undergoing cesarean delivery. Clinical Therapeutics. 2004;26(8):1286–91. doi: 10.1016/s0149-2918(04)80129-2. [DOI] [PubMed] [Google Scholar]
- Gaiser 2002 {published data only} .Gaiser RR, Dong Y, Cheek TG, Gutsche BB. Does increased intravenous hydration decrease the incidence of nausea/vomiting following cesarean section? [abstract] Anesthesiology. 2002;96(Suppl 1):P83. [Google Scholar]
- Ghods 2005 {published data only} .Ghods AA, Soleimani M, Narimani M. Effect of postoperative supplemental oxygen on nausea and vomiting after cesarean birth. Journal of Perianesthesia Nursing. 2005;20(3):200–5. doi: 10.1016/j.jopan.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Gutsche 1976 {published data only} .Gutsche BB. Prophylactic ephedrine preceding spinal analgesia for cesarean section. Anesthesiology. 1976;45:462–5. doi: 10.1097/00000542-197610000-00022. [DOI] [PubMed] [Google Scholar]
- Habib 2006 {published data only} .Habib AS, Itchon-Ramos N, Phillips-Bute BG, Gan TJ. the Duke Women’s Anesthesia (DWA) Research Group. Transcutaneous acupoint electrical stimulation with the ReliefBand for the prevention of nausea and vomiting during and after cesarean delivery under spinal anesthesia. Anesthesia & Analgesia. 2006;102(2):581–4. doi: 10.1213/01.ane.0000189217.19600.5c. [DOI] [PubMed] [Google Scholar]
- Harmon 2000 {published data only} .*; Harmon D, Ryan M, Kelly A, Bowen M. Acupressure and prevention of nausea and vomiting during and after spinal anaesthesia for caesarean section. British Journal of Anaesthesia. 2000;84(4):463–7. doi: 10.1093/oxfordjournals.bja.a013471. [DOI] [PubMed] [Google Scholar]; Harmon D, Ryan M, Kelly A, Bowen M. Acupressure and the prevention of nausea and vomiting during and after spinal anaesthesia for caesarean section. British Journal of Anaesthesia. 1999;82(Suppl):161. doi: 10.1093/oxfordjournals.bja.a013471. [DOI] [PubMed] [Google Scholar]
- Hildyard 2000 {published data only} .*; Hildyard C, Freeman J, Dresner M. Reducing the incidence of postoperative nausea and vomiting following elective caesarean section under spinal anaesthesia [abstract] International Journal of Obstetric Anesthesia. 2000;9:199. [Google Scholar]; Hildyard CL, Quinn A, Dresner M, Freeman J. Cyclizine reduces the incidence of nausea and vomiting after elective caesarean section with intrathecal diamorphine [abstract] British Journal of Anaesthesia. 2001;87(4):672P. [Google Scholar]
- Ho 1996 {published data only} .Ho CM, Hseu SS, Tsai SK, Lee TY. Effect of P-6 acupressure on prevention of nausea and vomiting after epidural morphine for post-Cesarean section pain relief. Acta Anaesthesiologica Scandinavica. 1996;40:372–5. doi: 10.1111/j.1399-6576.1996.tb04448.x. [DOI] [PubMed] [Google Scholar]
- Ho 2006 {published data only} .*; Ho CM, Tsai HJ, Chan KH, Tsai SK. P6 acupressure does not prevent emesis during spinal anesthesia for cesarean delivery. Anesthesia & Analgesia. 2006;102(3):900–3. doi: 10.1213/01.ane.0000195553.82409.00. [DOI] [PubMed] [Google Scholar]; Ho CM, Tsai SK. P-6 acupressure do not prevent emesis during cesarean section [abstract] Canadian Journal of Anesthesia. 2002;49(6 Suppl):A51. [Google Scholar]
- Hodgkinson 1983 {published data only} .Hodgkinson R, Glassenberg R, Joyce TH, Coombs DW, Ostheimer GW, Gibbs CP. Comparison of cimetidine (Tagamet) with antacid for safety and effectiveness in reducing gastric acidity before elective cesarean section. Anesthesiology. 1983;59:86–90. doi: 10.1097/00000542-198308000-00003. [DOI] [PubMed] [Google Scholar]
- Holdsworth 1974 {published data only} .Holdsworth JD, Furness RMB, Roulston RG. A comparison of apomorphine and stomach tubes for emptying the stomach before general anaesthesia in obstetrics. British Journal of Anaesthesia. 1974;46:526–9. doi: 10.1093/bja/46.7.526. [DOI] [PubMed] [Google Scholar]
- Holdsworth 1978 {published data only} .Holdsworth JD. The place of apomorphine prior to obstetric analgesia. Journal of International Medical Research. 1978;6:26–32. [PubMed] [Google Scholar]
- Holdsworth 1980 {published data only} .Holdsworth JD, Johnson K, Mascall G, Gwynne Roulston R, Tomlinson PA. Mixing of antacids with stomach contents. Anaesthesia. 1980;35:641–50. doi: 10.1111/j.1365-2044.1980.tb03877.x. [DOI] [PubMed] [Google Scholar]
- Huang 1992 {published data only} .Huang WP. Clinical observation of reliefing traction responses during cesarean section. Chinese Journal of Anesthesiology. 1992;12(1):33. [Google Scholar]
- Hunt 1989 {published data only} .Hunt CO, Naulty JS, Bader AM, Hauch MA, Vartikar JV, Datta S, et al. Perioperative analgesia with subarachnoid fentanyl-bupivacaine for cesarean delivery. Anesthesiology. 1989;71:535–40. doi: 10.1097/00000542-198910000-00009. [DOI] [PubMed] [Google Scholar]
- Imbeloni 1986 {published data only} .Imbeloni LE, Santos JMM, Santos MM. Prophylactic use of metoclopramide for control of nausea and vomiting during epidural anesthesia for cesarean section [Uso profilatico da metoclopramida no controle de nauseas e vomitos durante anestesia peridural para cesariana] Revista Brasileira de Anestesiologia. 1986;36(4):295–8. [Google Scholar]
- Ishiyama 2001 {published data only} .Ishiyama T, Yamaguchi T, Kashimoto S, Kumazawa T. Effects of epidural fentanyl and intravenous flurbiprofen for visceral pain during cesarean section under spinal anesthesia. Journal of Anesthesia. 2001;15:69–73. doi: 10.1007/s005400170029. [DOI] [PubMed] [Google Scholar]
- Kang 1982 {published data only} .Kang YG, Abouleish E, Caritis S. Prophylactic intravenous ephedrine infusion during spinal anesthesia for cesarean section. Anesthesia & Analgesia. 1982;61:839–42. [PubMed] [Google Scholar]
- Kangas-Saarela 1990 {published data only} .Kangas-Saarela T, Hollmen AI, Tolonen U, Eskelinen P, Alahuhta S, Jouppila R, et al. Does ephedrine influence newborn neurobehavioural responses and spectral EEG when used to prevent maternal hypotension during caesarean section? Acta Anaesthesiologica Scandinavica. 1990;34:8–16. doi: 10.1111/j.1399-6576.1990.tb03033.x. [DOI] [PubMed] [Google Scholar]
- Kasodekar 2006 {published data only} .Kasodekar S, Dhumne S, Balki M, Carvalho J. Prophylactic granisetron does not prevent nausea and vomiting during elective cesarean section under spinal anesthesia [abstract] Anesthesiology. 2006;104(Suppl 1):9. doi: 10.1213/01.ane.0000253036.06307.5c. [DOI] [PubMed] [Google Scholar]
- King 1998 {published data only} .King SW, Rosen MA. Prophylactic ephedrine and hypotension associated with spinal anesthesia for cesarean delivery. International Journal of Obstetric Anesthesia. 1998;7:18–22. doi: 10.1016/s0959-289x(98)80023-x. [DOI] [PubMed] [Google Scholar]
- Kjaer 2006 {published data only} .Kjaer K, Comerford M, Kondilis L, DiMaria L, Abramovitz S, Kiselev M, et al. Oral sodium citrate increases nausea amongst elective cesarean delivery patients. Canadian Journal of Anaesthesia. 2006;53(8):776–80. doi: 10.1007/BF03022794. [DOI] [PubMed] [Google Scholar]
- Kocamanoglu 2005 {published data only} .Kocamanoglu IS, Baris S, Karakaya D, Sener B, Tur A, Cetinkaya M. Effects of granisetron with droperidol or dexamethasone on prevention of postoperative nausea and vomiting after general anesthesia for cesarean section. Methods and Findings in Experimental and Clinical Pharmacology. 2005;27(7):489–93. doi: 10.1358/mf.2005.27.7.920930. [DOI] [PubMed] [Google Scholar]
- Kotelko 1989 {published data only} .*; Kotelko DM, Rottman RL, Wright WC, Stone JJ, Yamashiro AY, Rosenblatt RM. Transdermal scopolamine decreases nausea and vomiting following cesarean section in patients receiving epidural morphine. Anesthesiology. 1989;71:675–8. doi: 10.1097/00000542-198911000-00009. [DOI] [PubMed] [Google Scholar]; Kotelko DM, Rottman RL, Wright WC, Stone JJ, Yamashiro AY, Rosenblatt RM. Transderm SCOP decreases post-cesarean nausea and vomiting in patients receiving epidural morphine. Anesthesiology. 1988;69:A666. doi: 10.1097/00000542-198911000-00009. [DOI] [PubMed] [Google Scholar]
- Lim 1991 {published data only} .Lim SK, Elegbe EO. The use of single dose of sodium citrate as a prophylaxis against acid aspiration syndrome in obstetric patients undergoing caesarean section. Medical Journal of Malaysia. 1991;46(4):349–55. [PubMed] [Google Scholar]
- Lim 2001a {published data only} .Lim TJ, Chiu JW, Tan HM. Transcutaneous acupoint electrical stimulation for prevention of perioperative nausea and vomiting during cesarean section with spinal anesthesia [abstract] Anesthesiology. 2001;95(Suppl):A1078. [Google Scholar]
- Lim 2001b {published data only} .Lim TJ, Lim E, Chiu JW, Tan HM. Perioperative antiemetic efficacy of dexamethasone 4mg in cesarean section under spinal anaesthesia [abstract] Anesthesiology. 2001;95(Suppl):A466. [Google Scholar]
- Loughrey 2002 {published data only} .Loughrey JP, Walsh F, Gardiner J. Prophylactic intravenous bolus ephedrine for elective caesarean section under spinal anaesthesia. European Journal of Anaesthesiology. 2002;19(1):63–8. doi: 10.1017/s0265021502000108. [DOI] [PubMed] [Google Scholar]
- Lussos 1992 {published data only} .Lussos S, Bader A, Thornhill M, Datta S. The antiemetic efficacy of prophylactic metoclopramide for cesarean delivery. Anesthesiology. 1991;75:A1081. [PubMed] [Google Scholar]; *; Lussos SA, Bader AM, Thornhill ML, Datta S. The antiemetic efficacy and safety of prophylactic metoclopramide for elective cesarean delivery during spinal anesthesia. Regional Anesthesia. 1992;17:126–30. [PubMed] [Google Scholar]
- Mandell 1986 {published data only} .Mandell G, Dewan DM, Howard G, Floyd HM. The effectiveness of low dose droperidol in controlling nausea and vomiting during epidural anesthesia for cesarean section. Anesthesiology. 1986;65:391. doi: 10.1016/0959-289x(92)90003-m. [DOI] [PubMed] [Google Scholar]
- Mandell 1992 {published data only} .Mandell GL, Dewan DM, Howard G, Floyd HM. The effectiveness of low dose droperidol in controlling nausea and vomiting during epidural anesthesia for cesarean section. International Journal of Obstetric Anesthesia. 1992;1:65–8. doi: 10.1016/0959-289x(92)90003-m. [DOI] [PubMed] [Google Scholar]
- Manullang 2000 {published data only} .Manullang TR, Viscomi CM, Pace NL. Intrathecal fentanyl is superior to intravenous ondansetron for the prevention of perioperative nausea during cesarean delivery with spinal anesthesia. Anesthesia and Analgesia. 2000;90:1162–6. doi: 10.1097/00000539-200005000-00030. [DOI] [PubMed] [Google Scholar]
- Maranhao 1988 {published data only} .Maranhao MVM, Coelho VV, Ivo CMA, Maranhao MHC, Amaral EB. A comparative study between metoclopramide and droperidol in the control of nausea and vomit in patients subjected to spinal blockade during cesarean section [Estudo comparativo entre a metoclopramida e o droperidol no controle de nauseas e vomitos na operacao cesariana em gestantes submetidas a bloqueio subaracnoideo] Revista Brasileira de Anestesiologia. 1988;38(4):245–9. [Google Scholar]
- McCaughey 1981 {published data only} .McCaughey W, Howe JP, Moore J, Dundee JW. Cimetidine in elective caesarean section. Effect on gastric acidity. Anaesthesia. 1981;36:167–72. doi: 10.1111/j.1365-2044.1981.tb08718.x. [DOI] [PubMed] [Google Scholar]
- Mebazaa 2003 {published data only} .Mebazaa MS, Meftah RB, Abassi M, Miraoui W, Bessa Z, Ammar MSB. Post-operative nausea and vomiting after spinal anesthesia for cesarean section [abstract] Anesthesiology. 2003;99:A1201. [Google Scholar]
- Mukherjee 2006 {published data only} .Mukherjee S, Rudra A, Mandal M, Kumar P. Optimum dose of propofol for prevention of emetic episodes during caesarean delivery: an evaluation. Journal of Anaesthesiology Clinical Pharmacology. 2006;22(2):139–43. [Google Scholar]
- Murphy 1984 {published data only} .Murphy DF, Nally B, Gardiner J, Unwin A. Effect of metoclopramide on gastric emptying before elective and emergency caesarean section. British Journal of Anaesthesia. 1984;56:1113–6. doi: 10.1093/bja/56.10.1113. [DOI] [PubMed] [Google Scholar]
- Ngan 2000 {published data only} .Ngan Kee WD, Khaw KS, Lee BB, Lau TK, Gin T. A dose- response study of prophylactic intravenous ephedrine for the prevention of hypotension during spinal anesthesia for cesarean delivery. Anesthesia and Analgesia. 2000;90:1390–5. doi: 10.1097/00000539-200006000-00024. [DOI] [PubMed] [Google Scholar]
- Ngan 2001 {published data only} .Ngan Kee WD, Lau TK, Khaw KS, Lee BB. Comparison of metaraminol and ephedrine infusions for maintaining arterial pressure during spinal anesthesia for elective cesarean section. Anesthesiology. 2001;95:307–13. doi: 10.1097/00000542-200108000-00009. [DOI] [PubMed] [Google Scholar]
- Ngan 2004a {published data only} .Ngan Kee WD, Khaw KS, Ng FF. Comparison of phenylephrine infusion regimens for maintaining maternal blood pressure during spinal anaesthesia for caesarean section. British Journal of Anaesthesia. 2004;92(4):469–74. doi: 10.1093/bja/aeh088. [DOI] [PubMed] [Google Scholar]
- Ngan 2004b {published data only} .Ngan Kee WD, Khaw KS, Ng FF, Lee BB. Prophylactic phenylephedrine infusion for preventing hypotension during spinal anesthesia for cesarean delivery. Anesthesia and Analgesia. 2004;98:815–21. doi: 10.1213/01.ane.0000099782.78002.30. [DOI] [PubMed] [Google Scholar]
- Nortcliffe 2003 {published data only} .Nortcliffe SA, Buggy DJ. Prophylaxis of nausea and vomiting after spinal morphine analgesia for caesarean section: comparison between cyclizine, dexamethasone, and placebo [abstract] British Journal of Anaesthesia. 2001;87(4):657P. doi: 10.1093/bja/aeg120. [DOI] [PubMed] [Google Scholar]; *; Nortcliffe SA, Shah J, Buggy DJ. Prevention of postoperative nausea and vomiting after spinal morphine for caesarean section: comparison of cyclizine, dexamethasone and placebo. British Journal of Anaesthesia. 2003;90(5):665–70. doi: 10.1093/bja/aeg120. [DOI] [PubMed] [Google Scholar]
- Numazaki 2000 {published data only} .Numazaki M, Fujii Y. Subhypnotic dose of propofol for the prevention of nausea and vomiting during spinal anaesthesia for caesarean section. Anaesthesia and Intensive Care. 2000;28(3):262–5. [PubMed] [Google Scholar]
- Numazaki 2003 {published data only} .Numazaki M, Fujii Y. Reduction of emetic symptoms during cesarean delivery with antiemetics: propofol at subhypnotic dose versus traditional antiemetics. Journal of Clinical Anesthesia. 2003;15:423–7. doi: 10.1016/s0952-8180(03)00086-2. [DOI] [PubMed] [Google Scholar]
- O’Sullivan 1985 {published data only} .O’Sullivan G, Sear JW, Bullingham RE, Carrie LE. The effect of magnesium trisilicate mixture, metoclopramide and ranitidine on gastric ph, volume and serum gastrin. Anaesthesia. 1985;40(3):246–53. doi: 10.1111/j.1365-2044.1985.tb10750.x. [DOI] [PubMed] [Google Scholar]
- O’Sullivan 1988 {published data only} .O’Sullivan GM, Smith M, Morgan B, Brighouse D, Reynolds F. H2 antagonists and bupivacaine clearance. Anaesthesia. 1988;43:93–5. doi: 10.1111/j.1365-2044.1988.tb05471.x. [DOI] [PubMed] [Google Scholar]
- Olsen 1994 {published data only} .Olsen KS, Feilberg VL, Hansen CL, Rudkjobing O, Pedersen T, Kyst A. Prevention of hypotension during spinal anaesthesia for caesarean section. International Journal of Obstetric Anesthesia. 1994;3:20–4. doi: 10.1016/0959-289x(94)90208-9. [DOI] [PubMed] [Google Scholar]
- Osman 1995 {published data only} .Osman H. Ranitidine versus cimetidine prior to emergency obstetric anesthesia. Middle East Journal of Anesthesiology. 1995;13(2):205–11. [PubMed] [Google Scholar]
- Ouyang 2002 {published data only} .Ouyang MW, Gu MN, Lin CS. Inhibiting effect of intrathecal fentanyl on intraoperative vomiting during cesarean delivery under epidural anesthesia. Journal of the First Military Medical University (Di Yi Junyi Daxue Xuebao) 2002;22(11):1037–8. [PubMed] [Google Scholar]
- Owczarzak 1997 {published data only} .Owczarzak D, Ghellar MR, de Oliveira GR, Semeghini MM. Ondansetron and epidural morphine-induced pruritus and emesis after caesarean section: comparison with metoclopramide [abstract] British Journal of Anaesthesia. 1997;78(Suppl 1):110. [Google Scholar]
- Palmer 1991 {published data only} .Palmer AW, Waugaman WR, Conklin KA, Kotelko DM. Does the administration of oral bicitra before elective cesarean section affect the incidence of nausea and vomiting in the parturient? Nurse Anesthesia. 1991;2(3):126–33. [PubMed] [Google Scholar]
- Palmer 1995 {published data only} .Palmer CM, Voulgaropoulos D, Alves D. Subarachnoid fentanyl augments lidocaine spinal anesthesia for cesarean delivery. Regional Anesthesia. 1995;20(5):389–94. [PubMed] [Google Scholar]
- Pan 1996 {published data only} .Pan PH, Moore CH. Intraoperative antiemetic efficacy of prophylactic ondansetron versus droperidol for cesarean section patients under epidural anesthesia. Anesthesia & Analgesia. 1996;83(5):982–6. doi: 10.1097/00000539-199611000-00014. [DOI] [PubMed] [Google Scholar]
- Pan 2001 {published data only} .Pan P, Moore C, Fragneto R, Ross V, Justis G. Is antiemetic prophylaxis cost-effective for cesarean section patients [abstract] Anesthesiology. 2000;93(3A):A1092. [Google Scholar]; Pan PH, Moore C, Fragneto R, Ross V, Justis G. Efficacy and cost-effectiveness of prophylactic ondansetron versus metoclopramide for cesarean section patients under epidural anesthesia [abstract] Anesthesiology. 2000;92(Suppl):A40. [Google Scholar]; *; Pan PH, Moore CH. Comparing the efficacy of prophylactic metoclopramide, ondansetron, and placebo in cesarean section patients given epidural anesthesia. Journal of Clinical Anesthesia. 2001;13(6):430–5. doi: 10.1016/s0952-8180(01)00294-x. [DOI] [PubMed] [Google Scholar]; Pan PH, Moore CM. Efficacy and cost-effectiveness of prophylactic ondansetron versus metoclopramide for cesarean section patients under epidural anesthesia [abstract] Anesthesia and Analgesia. 2000;90(Suppl):S295. doi: 10.1097/00000539-199611000-00014. [DOI] [PubMed] [Google Scholar]
- Pan 2003 {published data only} .Pan AK, Rudra A. Prophylactic single dose intravenous administration of ondansetron in the prevention of postoperative emetic symptoms during spinal anaesthesia for caesarean delivery. Indian Journal of Anaesthesia. 2003;47(3):178–80. [Google Scholar]
- Peixoto 2006 {published data only} .Peixoto AJ, Celich MF, Zardo L, Peixoto Filho AJ. Ondansetron or droperidol for prophylaxis of nausea and vomiting after intrathecal morphine. European Journal of Anaesthesiology. 2006;23(8):670–5. doi: 10.1017/S0265021506000482. [DOI] [PubMed] [Google Scholar]
- Pellegrini 2001 {published data only} .Pellegrini JE, Bailey SL, Graves J, Paice JA, Shott S, Faut-Callahan M. The impact of nalmefene on side effects due to intrathecal morphine at cesarean section. AANA Journal. 2001;69(3):199–205. [PubMed] [Google Scholar]
- Phillips 2007 {published data only} .*; Phillips TW, Jr, Broussard DM, Sumrall WD, III, Hart SR. Intraoperative oxygen administration does not reduce the incidence or severity of nausea or vomiting associated with neuraxial anesthesia for cesarean delivery. Anesthesia and Analgesia. 2007;105(4):1113–7. doi: 10.1213/01.ane.0000278626.54116.0e. [DOI] [PubMed] [Google Scholar]; Phillips TW, Hart SR, Broussard D, Sumrall WD. Effects of supplemental oxygen administration on the incidence of nausea/vomiting during regional anesthesia for cesarean section [abstract] Anesthesiology. 2005;102(Suppl 1):28. [Google Scholar]
- Prakash 2006 {published data only} .Prakash S, Joshi N, Gogia AR, Prakash S, Singh R. Analgesic efficacy of two doses of intrathecal midazolam with bupivacaine in patients undergoing cesarean delivery. Regional Anesthesia and Pain Medicine. 2006;31(3):221–6. doi: 10.1016/j.rapm.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Quiney 1995 {published data only} .Quiney NF, Murphy PG. The effect of pretreatment with glycopyrrolate on emetic and hypotensive problems during caesarean section conducted under spinal anaesthesia [abstract] International Journal of Obstetric Anesthesia. 1995;4:66–7. [Google Scholar]
- Qvist 1983 {published data only} .Qvist N, Storm K. Cimethidine pre-anesthetic - a prophylactic method against Mendelson’s syndrome in cesarean section. Acta Obstetricia et Gynecologica Scandinavica. 1983;62:157–9. doi: 10.3109/00016348309155782. [DOI] [PubMed] [Google Scholar]
- Qvist 1985 {published data only} .Qvist N, Storm K, Holmskov A. Cimetidine as pre-anesthetic agent for cesarean section: perinatal effects on the infant, the placental transfer of cimetidine and its elimination in the infants. Journal of Perinatal Medicine. 1985;13:179–83. doi: 10.1515/jpme.1985.13.4.179. [DOI] [PubMed] [Google Scholar]
- Ramanathan 1983 {published data only} .Ramanathan S, Masih A, Rock I, Chalon J, Turndorf H. Maternal and fetal effects of prophylactic hydration with crystalloids or colloids before epidural anesthesia. Anesthesia and Analgesia. 1983;62:673–8. [PubMed] [Google Scholar]
- Ramin 1994 {published data only} .Ramin SM, Ramin KD, Cox K, Magness RR, Shearer VE, Grant NF. Comparison of prophylactic angiotensin II versus ephedrine infusion for prevention of maternal hypotension during spinal anesthesia. American Journal of Obstetrics and Gynecology. 1994;171:734–9. doi: 10.1016/0002-9378(94)90090-6. [DOI] [PubMed] [Google Scholar]
- Rout 1992 {published data only} .Rout CC, Rocke DA, Brijball R, Koovarjee RV. Prophylactic intramuscular ephedrine prior to caesarean section. Anaesthesia and Intensive Care. 1992;20:448–52. doi: 10.1177/0310057X9202000408. [DOI] [PubMed] [Google Scholar]
- Rudra 2004a {published data only} .Rudra A, Halder R, Sen A, Kundu S. Efficacy of low dose propofol for control of emetic episodes during caesarean delivery with spinal anaesthesia. Indian Journal of Anaesthesia. 2004;48(1):31–4. [Google Scholar]
- Rudra 2004b {published data only} .Rudra P, Rudra A. Comparison of intrathecal fentanyl and midazolam for prevention of nausea-vomiting during caesaraean delivery under spinal anaesthesia. Indian Journal of Anaesthesia. 2004;48(6):461–4. [Google Scholar]
- Sanansilp 1998 {published data only} .Sanansilp V, Areewatana S, Tonsukchai N. Droperidol and the side effects of epidural morphine after cesarean section. Anesthesia and Analgesia. 1998;86(3):532–7. doi: 10.1097/00000539-199803000-00016. [DOI] [PubMed] [Google Scholar]
- Santos 1984 {published data only} .Santos A, Datta S. Prophylactic use of droperidol for control of nausea and vomiting during spinal anesthesia for cesarean section. Anesthesia and Analgesia. 1984;63:85–7. [PubMed] [Google Scholar]
- Sen 2001 {published data only} .Sen A, Rudra A, Sarkar SK, Biswas B. Intrathecal midazolam for postoperative pain relief in caesarean section delivery. Journal of the Indian Medical Association. 2001;99(12):683–4. 686. [PubMed] [Google Scholar]
- Seyedhejazi 2007 {published data only} .Seyedhejazi M, Madarek E. The effect of small dose bupivacaine-fentanyl in spinal anesthesia on hemodynamic nausea and vomiting in cesarean section. Pakistan Journal of Medical Sciences. 2007;23(5):747–50. [Google Scholar]
- Shende 1998 {published data only} .Shende D, Cooper GM, Bowden MI. The influence of intrathecal fentanyl on the characteristics of subarachnoid block for caesarean section. Anaesthesia. 1998;53:702–10. doi: 10.1046/j.1365-2044.1998.329-az0482.x. [DOI] [PubMed] [Google Scholar]
- Siddik-Sayyid 2002 {published data only} .Siddik-Sayyid SM, Aouad MT, Jalbout MI, Zalaket MI, Berzina CE, Baraka AS. Intrathecal versus intravenous fentanyl for supplementation of subarachnoid block during cesarean section. Anesthesia and Analgesia. 2002;95:209–13. doi: 10.1097/00000539-200207000-00037. [DOI] [PubMed] [Google Scholar]
- Stein 1997 {published data only} .Stein DJ, Birnbach DJ, Danzer BI, Kuroda MM, Grunebaum A, Thys DM. Acupressure versus intravenous metoclopramide to prevent nausea and vomiting during spinal anesthesia for cesarean section. Anesthesia and Analgesia. 1997;84(2):342–5. doi: 10.1097/00000539-199702000-00018. [DOI] [PubMed] [Google Scholar]
- Stuart 1996 {published data only} .Gin T, Stuart JC, Kan AF, Rowbottom SJ, Yau G. IV ranitidine or omeprazole for emergency caesarean section. Anaesthesia and Intensive Care. 1994;22:483. [Google Scholar]; *; Stuart JC, Kan AF, Rowbottom SJ, Yau G, Gin T. Acid aspiration prophylaxis for emergency caesarean section. Anaesthesia. 1996;51(5):415–21. doi: 10.1111/j.1365-2044.1996.tb07782.x. [DOI] [PubMed] [Google Scholar]
- Tarhan 2007 {published data only} .Tarhan O, Canbay O, Celebi N, Uzun S, Sahin A, Coskun F, et al. Subhypnotic doses of midazolam prevent nausea and vomiting during spinal anesthesia for cesarean section. Minerva Anestesiologica. 2007;73(12):629–33. [PubMed] [Google Scholar]
- Taylor 1966 {published data only} .Taylor G, Pryse-Davies J. The prophylactic use of antacids in the prevention of the acid-pulmonary-aspiration syndrome (Mendelson’s syndrome) Lancet. 1966;1:288–91. doi: 10.1016/s0140-6736(66)90640-4. [DOI] [PubMed] [Google Scholar]
- Tettambel 1983 {published data only} .Tettambel MA. Preoperative use of antacids to prevent Mendelson’s syndrome in cesarean section: a pilot study. Journal of the American Osteopathic Association. 1983;82:858–60. [PubMed] [Google Scholar]
- Tzeng 2000 {published data only} .Tzeng JI, Wang JJ, Ho ST, Tang CS, Liu YC, Lee SC. Dexamethasone for prophylaxis of nausea and vomiting after epidural morphine for post-caesarean section analgesia: comparison of droperidol and saline. British Journal of Anaesthesia. 2000;85:865–8. doi: 10.1093/bja/85.6.865. [DOI] [PubMed] [Google Scholar]
- Ure 1999 {published data only} .Ure D, James KS, McNeill M, Booth JV. Glycopyrrolate reduces nausea during spinal anaesthesia for caesarean section without affecting neonatal outcome. British Journal of Anaesthesia. 1999;82(2):277–9. doi: 10.1093/bja/82.2.277. [DOI] [PubMed] [Google Scholar]
- Vercauteren 2000 {published data only} .Vercauteren MP, Coppejans HC, Hoffmann VH, Mertens E, Adriaensen HA. Prevention of hypotension by a single 5- mg dose of ephedrine during small-dose spinal anesthesia in prehydrated cesarean delivery patients. Anesthesia and Analgesia. 2000;90:324–7. doi: 10.1097/00000539-200002000-00016. [DOI] [PubMed] [Google Scholar]
- von Braun 1994 {published data only} .von Braun GG, Koppert W, Martus P, Schywalsky M, Schmitt H, Mogendorf F, et al. Drug induced prevention of the acid aspiration syndrome in non-elective cesarean section. Anaesthesiologie und Reanimation. 1994;19:37–42. [PubMed] [Google Scholar]
- Wang 2001 {published data only} .Wang JJ, Ho ST, Wong CS, Tzeng JI, Liu HS, Ger LP. Dexamethasone prophylaxis of nausea and vomiting after epidural morphine for post-cesarean analgesia. Canadian Journal of Anaesthesia. 2001;48(2):185–90. doi: 10.1007/BF03019733. [DOI] [PubMed] [Google Scholar]
- Weiss 1995 {published data only} .Weiss MD, Diaz F, Zuberi F, Milman B, Pasricha S. Subhypnotic doses of propofol for prevention of postoperative pruritus and nausea in cesarean section patients given spinal opioids. Anesthesiology. 1995;83(3A):A960. [Google Scholar]
- Yazigi 2002 {published data only} .Yazigi A, Chalhoub V, Madi-Jebara S, Haddad F, Hayek G. Prophylactic ondansetron is effective in the treatment of nausea and vomiting but not on pruritis after cesarean delivery with intrathecal sufentanil-morphine. Journal of Clinical Anesthesia. 2002;14:183–6. doi: 10.1016/s0952-8180(01)00381-6. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
- Karamanlioglu 1995 {published data only} .Karamanlioglu B, Canogullari M, Arslan G, Alagol A, Sengonul O. The value of omeprazole and h2 receptor blockers in the prophylaxis of aspiration in cesarean operation [Sezaryen ameliyatlarinda omeprazol ve h2 reseptor blokerlerinin aspirasyon pnomonisi profilaksisindeki degeri] Turk Anesteziyoloji Ve Reanimasyon. 1995;23(3):338–42. [Google Scholar]
Additional references
- Bond 1979 .Bond VK, Stoelting MD, Gupta CD. Pulmonary aspiration syndrome after inhalation of gastric fluid containing antacids. Anesthesiology. 1979;51:452–3. doi: 10.1097/00000542-197911000-00015. [DOI] [PubMed] [Google Scholar]
- Browne 1993 .Browne D, Powell H. Update on some earlier controversies . In: Morgan B, editor. Controversies in obstetric anaesthesia. Vol. 2. Edward Arnold; London: 1993. [Google Scholar]
- CEMD 2001 .Confidential Enquiry into Maternal Deaths . Confidential Enquiries into Maternal Deaths. Why mothers die 1997-1999: the fifth report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. 5. RCOG Press; Regents Park, London: 2001. [Google Scholar]
- Cohen 1984 .Cohen SE, Jasson J, Talafre ML, Chauvelot-Moachon L, Barrier G. Does metoclopramide decrease the volume of gastric contents in patients undergoing caesarean section? Anesthesiology. 1984;61(5):604–7. doi: 10.1097/00000542-198411000-00025. [DOI] [PubMed] [Google Scholar]
- Deeks 2001 .Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Davey Smith G, Altman DG, editors. Systematic reviews in health care: meta-analysis in context. BMJ Books; London: 2001. [Google Scholar]
- Egger 1997 .Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewart 1990 .Ewart MC, Yau G, Gin T, Kotur CF, Oh TE. A comparison of the effects of omeprazole and ranitidine on gastric secretion in women undergoing elective caesarean section. Anaesthesia. 1990;45:527–30. doi: 10.1111/j.1365-2044.1990.tb14823.x. [DOI] [PubMed] [Google Scholar]
- Gates 2009 .Gates S. The Editorial Team. Pregnancy and Childbirth Group. About the Cochrane Collaboration (Collaborative Review Groups (CRGs) 3 2009. Methodological Guidelines. [Google Scholar]
- Gibbs 1979 .Gibbs CP, Schwartz DJ, Wynne JW, Hood CI, Kuck EJ. Antacid pulmonary aspiration in the dog. Anesthesiology. 1979;51:380–5. doi: 10.1097/00000542-197911000-00003. [DOI] [PubMed] [Google Scholar]
- Gin 1990 .Gin T, Ewart MC, Yau G, Oh TE. Effect of oral omeprazole on intragastric pH and volume in women undergoing elective caesarean section. British Journal of Anaesthesia. 1990;65:616–9. doi: 10.1093/bja/65.5.616. [DOI] [PubMed] [Google Scholar]
- Grieff 1994 .Grieff JMC, Tordoff SG, Griffiths R, May AE. Acid aspiration chemoprophylaxis in 202 obstetric anaesthetic units in the UK. International Journal of Obstetric Anesthesia. 1994;3:137–42. doi: 10.1016/0959-289x(94)90225-9. [DOI] [PubMed] [Google Scholar]
- Griffiths 2009 .Griffiths JD, Gyte GML, Paranjothy S, Brown HC, Broughton HK, Thomas J. Interventions for reducing nausea and vomiting at caesarean section. Cochrane Database of Systematic Reviews. 2009;(1) DOI: 10.1002/14651858.CD007579. [Google Scholar]
- Gyte 2006 .Gyte GML, Richens Y. Routine prophylactic drugs in normal labour for reducing gastric aspiration and its effects. Cochrane Database of Systematic Reviews. 2006;(3) doi: 10.1002/14651858.CD005298.pub2. DOI: 10.1002/14651858.CD005298.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbord 2006 .Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Statistics in Medicine. 2006;25:3443–57. doi: 10.1002/sim.2380. [DOI] [PubMed] [Google Scholar]
- Higgins 2008 .Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 5.0.1. 2008 [updated September 2008] Available from www.cochrane-handbook.org.
- Mendelson 1946 .Mendelson CL. Aspiration of stomach contents into the lungs during obstetric anesthesia. American Journal of Obstetrics and Gynecology. 1946;52:191–203. doi: 10.1016/s0002-9378(16)39829-5. [DOI] [PubMed] [Google Scholar]
- Moore 1989 .Moore J, Flynn RJ, Sampaio M, Wilson CM, Gillon KR. Effect of single-dose omeprazole on intragastric acidity and volume during obstetric anaesthesia. Anaesthesia. 1989;44(7):459–62. doi: 10.1111/j.1365-2044.1989.tb11441.x. [DOI] [PubMed] [Google Scholar]
- Numazaki 2000 .Numazaki M, Fuji Y. Subhypnotic dose of propofol for the prevention of nausea and vomiting during spinal anaesthesia for caesarean section. Anaesthesia and Intensive Care. 2000;28(3):262–5. [PubMed] [Google Scholar]
- RevMan 2008 .The Cochrane Collaboration . Review Manager (RevMan). 5.0. The Cochrane Collaboration; Copenhagen, The Nordic Cochrane Centre: 2008. [Google Scholar]
- Roberts 1974 .Roberts RB, Shirley MA. Reducing the risk of acid aspiration during cesarean section. Anesthesia and Analgesia. 1974;53:859–68. doi: 10.1213/00000539-197453060-00010. [DOI] [PubMed] [Google Scholar]
- Singata 2002 .Singata M, Tranmer JE. Restricting oral fluid and food intake during labour. Cochrane Database of Systematic Reviews. 2002;(4) doi: 10.1002/14651858.CD003930.pub3. DOI: 10.1002/14651858.CD003930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney 1986 .Sweeney B, Wright I. The use of antacids as a prophylaxis against Mendelson’s syndrome in the United Kingdom. A survey. Anaesthesia. 1986;41:419–22. doi: 10.1111/j.1365-2044.1986.tb13231.x. [DOI] [PubMed] [Google Scholar]
- Thomas 2001 .Thomas J, Paranjothy S. The national sentinel caesarean section audit report. RCOG Press; London: 2001. Royal College of Obstetricians and Gynaecologists: Clinical Effectiveness Support Unit. [Google Scholar]
- Thwaites 1999 .Thwaites AJ, Riche CP, Smith I. Rapid sequence induction: a questionnaire survey of its routine conduct and continued management during a failed intubation. Anaesthesia. 1999;54:372–92. doi: 10.1046/j.1365-2044.1999.00738.x. [DOI] [PubMed] [Google Scholar]
- Tordoff 1990 .Tordoff SG, Sweeney BP. Acid aspiration prophylaxis in 288 obstetric anaesthetic departments in the United Kingdom. Anaesthesia. 1990;45:776–80. doi: 10.1111/j.1365-2044.1990.tb14455.x. [DOI] [PubMed] [Google Scholar]


