Abstract
Background
We have recently shown that native murine ventricular fibroblasts express both Cx43 and Cx45, and that the level of Cx43 expression influences intercellular coupling and cell proliferation. Relatively little is known, however, about how myocardial infarction (MI) influences expression of Cx43, or how altered Cx43 expression may affect fibroblast function post-MI. Fibroblasts are critical for infarct healing and post-infarct ventricular remodeling. They can couple electrically with cardiac myocytes and influence myocardial activation patterns. Thus, Cx43 remodeling and the level of intercellular communication in fibroblasts expressed in the infarcted heart were the subject of the present investigation.
Methods
Fibroblasts were isolated from both infarct scar and remote, noninfarcted regions of murine hearts 6 d after coronary ligation. Expression levels of Cx43, α-smooth muscle actin and N-cadherin were quantified by immunoblotting. Gap junctional intercellular communication was quantified by Lucifer yellow dye transfer.
Results and Conclusions
Fibroblasts isolated from infarcted hearts exhibited marked upregulation of Cx43 protein expression and enhanced intercellular coupling. Exogenous administration of TGF-β to fibroblast cultures from normal, non-operated hearts produced comparable upregulation of Cx43, suggesting that increased intercellular communication between fibroblasts in infarct and peri-infarct regions may be secondary to activation of a TGF-β pathway. Unlike cardiac myocytes that downregulate Cx43, presumably to limit intercellular transmission of biochemical mediators of ischemic injury, fibroblasts may upregulate Cx43 to maintain electrical and metabolic coupling at a time when intercellular communication is compromised.
Keywords: Myofibroblasts, connexin43, Cx43, intercellular coupling, TGF-β
INTRODUCTION
Fibroblasts form a 3-dimensional cellular network surrounding myocytes [1], and contribute to myocardial structure by maintaining and remodeling the extracellular matrix in healthy and diseased myocardium [2,3]. After injury, such as myocardial infarction (MI), fibroblasts play a critical role in ventricular remodeling. They migrate into damaged tissue, proliferate rapidly and produce collagen in response to cytokines such as transforming growth factor-β (TGF-β) [4]. Fibroblasts are arguably the most important component of the “living scar” responsible for myocardial repair, infarct healing and scar formation [5–8]. Several studies have shown that specific phenotypic alterations in post-MI or failing fibroblasts are maintained in vitro through several passages [9–11]; thus, post-MI remodeled fibroblasts may be grown in culture to quantify alterations in intercellular communication and to study mechanisms underlying those alterations.
Gap junctions are responsible for cell-to-cell coupling, intercellular propagation of electrical signals and exchange of small signaling molecules throughout the heart. Four different connexins, including connexin43 (Cx43), Cx40, Cx45 and Cx37, are expressed in heart tissue [12,13]. Recently, we reported that native murine ventricular fibroblasts express both Cx43 and Cx45, and that the level of Cx43 expression influences intercellular coupling and cell proliferation [14]. Because fibroblasts can couple electrically with cardiac myocytes [15,16] and may contribute to abnormal activation patterns [17], particularly as a result of increased proliferation and infarct remodeling, assessing the contribution of fibroblast intercellular communication in the infarcted heart is important. Indeed, increased expression of Cx43 has been shown to reduce arrhythmogenic potential in cardiac cell-based therapy [18]. Therefore, the present study was performed to test the hypothesis that Cx43 and Cx45 are remodeled in fibroblasts following MI and to explore the mechanism responsible for altered connexin expression and intercellular coupling post-MI.
MATERIALS AND METHODS
Animals were handled in accordance with the NIH Guide for the Care and Use of Laboratory Animals; all protocols were approved by the Washington University Animal Studies Committee.
Myocardial infarction and sham-operated controls
Survival surgery was performed on adult male or female mice (C57Bl6) in the Mouse Cardiovascular Phenotyping Core at Washington University School of Medicine as described previously [19]. Briefly, mice were anesthetized with a mixture of ketamine and xylazine (87 and 13 mg/kg, respectively), intubated and artificially ventilated. A left thoracotomy was performed and the left anterior descending coronary artery was ligated. The chest was closed and mice were allowed to recover for 6 days. Mice that were subjected to the same surgical procedure without ligation of the coronary artery served as sham-operated controls.
Adult murine cardiac fibroblast cultures
Fibroblasts were prepared from ventricles of noninfarcted and infarct regions as described previously [14] with minor modifications. Briefly, hearts were removed from anesthetized (2.5% Avertin) mice and perfused with 0.1% collagenase (Worthington Biochemical, Lakewood, NJ) in Earle's balanced salt solution. The right ventricle was removed, and the left ventricle was divided into noninfarcted and infarct regions by direct visualization. Although we attempted to carefully segregate infarcted from noninfarcted myocardium, it is possible that border zone could have been included in either or both groups. Myocytes from the noninfarcted tissue were pelleted at 50 × g for 5 min. The infarct region was incubated further in 0.1% collagenase solution for an additional 10 min, and spun down at 50 × g for 5 min to pellet the myocytes. The supernatant was subsequently spun down at 400 × g for 5 min. The cell pellet was resuspended in Dulbecco's modified Eagle medium (Sigma) containing 10% fetal bovine serum, penicillin, and streptomycin, and plated overnight in culture flasks. The next day, nonadherent cells were removed during a medium change. Flasks containing fibroblasts were kept in culture for 4 days until they reached confluency. Fibroblasts were then removed with trypsin and plated either at a density of 1.5 ×105 cells per well in 6-well plates that had been coated with collagen (type IV, 200 μg/ml, Sigma) for biochemistry, or at a density of 4 ×104 cells per chamber on 4-chamber slides that had been coated with collagen for dye transfer studies.
In addition, fibroblasts were prepared from normal (non-surgical) adult mouse ventricles using the same protocol described above. After final culturing for 3 days, fibroblasts were exposed to serum-free medium for 1 hr, then incubated for 24 hr with TGF-β1 (10 ng/ml, Sigma) in serum-free medium.
Dye-Transfer Assay
Dye transfer was measured using a “scrape-loading” protocol [20] as described previously [14]. Briefly, cells grown on 4-chamber slides were exposed to Lucifer yellow dye (2%, Sigma) after a small circular glass-cutting tool was used to make a linear cut across the fibroblast monolayer to permit the membrane-impermeant dye to enter the damaged cells on either side of the scrape line. After incubation for 2 min at 37°C, the monolayers were rinsed, fixed in 4% paraformaldehyde, mounted, and photographed. Digital images were analyzed offline using ImageJ software (NIH, http://rsb.info.nih.gov/ij/). The area of fluorescence on one side of the scrape line was divided by the length of the field to get an average distance of dye transfer, expressed in micrometers. Two to five fields from each chamber were averaged.
Immunoblot Analysis
Cell extracts were prepared by homogenization and sonication as described previously [14]. Aorta, intestine and lung samples were rinsed in phosphate-buffered saline, frozen, pulverized and homogenized as described previously [14]. Proteins were resolved by sodium dodecyl sulfate-polyacrylamide (10%) gel electrophoresis and transferred to nitrocellulose membranes for incubation with rabbit anti-Cx43 (1:5000, Zymed), protein A-purified rabbit anti-Cx45 (1:4000, generous gift from T. Steinberg, Washington University), mouse anti-pan cadherin (1:2000, Sigma), goat anti-CD-31 (PECAM-1, 1:200, Santa Cruz Biotechnology), mouse anti-smoothelin (1:200, Millipore/Chemicon), goat anti-vimentin (1:1000, Sigma), or goat anti-actin (1:1000, Santa Cruz) primary antibodies. Goat anti-rabbit (1:10,000, Jackson), sheep anti-mouse (1:10,000, GE Healthcare/Amersham) or donkey anti-goat (1:20,000, Santa Cruz) were used as secondary antibodies. No secondary antibodies were used on membranes for incubation with mouse anti-α-smooth muscle actin (α-SMA) with horseradish peroxidase (HRP) (1:150, Dako). Protein bands were visualized using Western Lightning Chemiluminescence Reagent Plus (PerkinElmer, Boston, MA). Immunoblots were quantified by densitometric analysis using Adobe Photoshop. Connexin and N-cadherin band densities were normalized to actin blots to control for protein loading.
Immunocytochemistry
Fibroblast cultures were fixed in 4% paraformaldehyde and blocked in phosphate-buffered saline containing 0.1% Triton X-100 and 3% normal goat serum (Jackson ImmunoResearch Laboratories, West Grove, PA) as described previously [14]. Cells were exposed to anti-α-SMA antibody with HRP (used neat, Dako) followed by tyramide signal amplification (TSA, NEN Life Science) as described previously [14]. Briefly, slides were incubated in Biotinyl Tyramide Amplification Reagent (1:300; NEN) for 10 min followed by Cy2-conjugated streptavidin (1:500; Jackson) for 30 min. Nuclei were stained with Hoechst nuclear stain (1:10,000, Cambrex) for 10 min for fluorescence visualization.
Statistical Analysis
All values are expressed as mean ± SD. Two group comparisons were made using Student's t test for grouped data. ANOVA and post-hoc multiple comparisons test were used for comparing 3 groups of data. Sample n's refer to the number of different hearts used in analyses. A value of p<0.05 was considered significant.
RESULTS
Phenotypic characterization of fibroblasts isolated from infarcted hearts
Injury associated with MI induces transformation of fibroblasts to myofibroblasts, which are responsible for collagen deposition critical for scar formation and infarct healing. To determine whether activation of myofibroblasts occurred equally throughout the myocardium post-MI, we examined fibroblasts isolated from both noninfarcted and infarcted regions, and measured α-SMA expression by immunofluorescence (Figure 1, top) and immunoblotting (Figure 1, bottom). We did not observe any differences in gross morphology of fibroblasts isolated from infarcts, noninfarcted areas or sham-operated hearts. Although α-SMA immunoreactive signal was clearly more robust in fibroblasts from infarcted compared to sham-operated hearts (Figure 1, top), immunoblot analysis revealed that the tendency toward increased α-SMA protein expression (over sham, 1.05 ± 0.39 relative density units, n = 4) did not reach statistical significance (p = 0.111). Furthermore, α-SMA expression in fibroblasts from the noninfarcted region (1.66 ± 0.51 density units, n=4) was nearly identical to that measured from the infarct area (1.66 ± 0.31 density units, n=3). These data suggest that a generalized response to infarction is mounted throughout the ventricular myocardium, both in the infarcted and remote regions, where activation of myofibroblasts is likely to be involved in both infarct healing and post-infarct remodeling.
Figure 1.
Top: α-SMA immunoreactive signal demonstrating increased myofibroblasts in a culture of fibroblasts isolated from the infarct region of a heart subjected to coronary ligation (right) compared to cells isolated from a sham-operated heart (left). Bar, 100 μm. Bottom: Representative immunoblots of α-SMA and actin protein expression in fibroblasts isolated from sham-operated hearts and from noninfarcted and infarct regions of hearts subjected to MI showing increased α-SMA protein expression in the fibroblasts isolated from infarcted hearts.
To positively identify the cells populating our primary cultures as cardiac fibroblasts, we performed immunoblot analysis using antibodies directed against CD-31 and smoothelin to rule out contamination by endothelial and smooth muscle cells, respectively. As shown in Figure 2, fibroblasts isolated from infarcts, noninfarcted regions and sham-operated hearts did not exhibit detectable CD-31 or smoothelin. Fibroblasts did express vimentin (Figure 2).
Figure 2.
Representative immunoblots of CD-31, smoothelin, vimentin and actin protein expression in cultured fibroblasts isolated from sham-operated hearts (lanes 1 and 2), noninfarcted (lanes 3 and 4) and infarct (lanes 5 and 6) regions of hearts subjected to MI, and tissue homogenates of mouse aorta (ao, lane 7), intestine (int, lane 8) and lung (lane 9) demonstrating that our fibroblast cultures (lanes 1–6) are devoid of measurable endothelial and smooth muscle cell contamination. The endothelial cell marker CD-31, or PECAM-1, is expressed in lung tissue; the smooth muscle cell marker smoothelin is present in aorta and intestine. The nonspecific marker, vimentin, is seen in all lanes.
Cx43 expression is upregulated in cardiac fibroblasts isolated from infarcted hearts
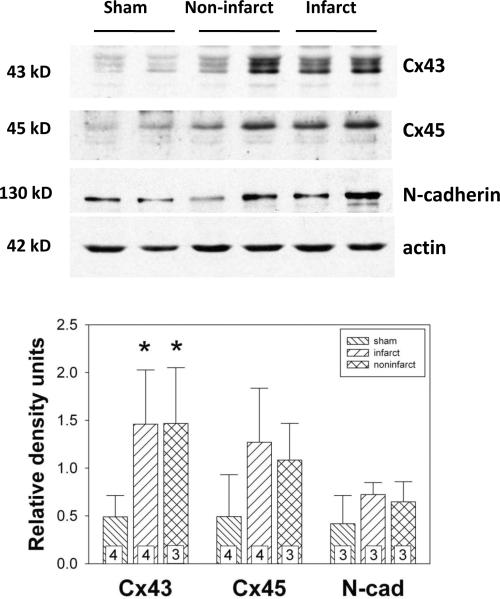
One of the most consistent ventricular remodeling responses to cardiac disease involves cardiac connexin remodeling. Specifically, hypertrophy, infarction and heart failure each results in downregulation of Cx43 [12]. Previously, we reported that cardiac fibroblasts express both Cx43 and Cx45, and that the expression level of Cx43 is a determinant of fibroblast function [14]. To determine whether Cx43 and Cx45 are remodeled in cardiac fibroblasts following MI, we isolated cardiac fibroblasts from noninfarcted and infarcted regions 6 d post-MI and quantified Cx43 and Cx45 protein expression by immunoblotting. Cx43 expression was increased significantly (p < 0.05) in fibroblasts from both noninfarcted (1.47 ± 0.58 density units, n = 3) and infarcted (1.46 ± 0.57 density units, n = 4) regions compared to Cx43 levels in cells isolated from sham-operated hearts (0.49 ± 0.22 density units, n = 4) as shown in Figure 3. Upregulation of Cx43 occurred to the same degree in fibroblasts isolated from noninfarcted versus infarct areas post-MI. Cx45 expression tended to increase in fibroblasts from both noninfarcted (1.08 ± 0.39 density units, n = 3) and infarcted (1.27 ± 0.57 density units, n = 4) regions compared to that isolated from sham-operated hearts (0.49 ± 0.44 density units, n = 4), but this difference did not reach statistical significance (p = 0.118). Finally, N-cadherin was measured by immunoblotting to assess whether this mechanical junction protein was altered in cardiac fibroblasts isolated from infarcted hearts. As shown in Figure 3, N-cadherin did not change (p = 0.290) in fibroblasts cultured from either noninfarcted (0.65 ± 0.21 density units, n= 3) or infarcted (0.72 ± 0.13 density units, n = 3) regions compared to that from sham-operated hearts (0.42 ± 0.30 density units, n = 3).
Figure 3.
Top: Representative immunoblots of Cx43, Cx45, N-cadherin (probed with an anti-pan cadherin antibody), and actin expression in fibroblasts isolated from sham-operated hearts and from noninfarcted and infarcted regions of hearts subjected to coronary ligation. Equal protein (30 μg) was loaded in each lane. Cx43, Cx45 and N-cadherin band densities were normalized to actin band densities to control for protein loading. Bottom: Histograms of summarized immunoblot data showing that Cx43 expression was increased significantly in fibroblasts from both noninfarcted and infarct regions compared to those isolated from sham-operated hearts. *, p < 0.05.
Fibroblasts isolated from infarcted hearts exhibit increased intercellular coupling
To investigate whether upregulated Cx43 expression in fibroblasts post-MI would affect cell-to-cell communication, intercellular coupling was assessed by Lucifer yellow dye transfer. Dye transfer was increased significantly (p < 0.001) in fibroblasts from both noninfarcted (92.1 ± 4.2 μm, n = 3) and infarcted (101.5 ± 7.2 μm, n = 3) regions compared to that from sham-operated hearts (58.0 ± 3.2 μm, n = 3), demonstrating that fibroblasts isolated from infarcted hearts exhibit increased gap junctional intercellular communication. There was no difference in dye transfer between fibroblasts isolated from noninfarcted versus infarcted regions (Figure 4).
Figure 4.
Top: Fluorescence images showing Lucifer yellow dye transfer after scrape-loading in fibroblasts isolated from a sham-operated heart (left) and from the infarct region of a heart subjected to MI (right). Outlines beside each fluorescence image represent the areas of dye transfer on one side of the scrape line obtained using ImageJ software. The total area of dye transfer was divided by the length of the microscopic field analyzed (top to bottom) to calculate the mean distance of dye transfer in μm. Bar, 100 μm. Bottom: Histograms of summarized data showing that the distance of dye transfer in fibroblasts from both noninfarcted and infarct regions was significantly greater than that in fibroblasts isolated from sham-operated hearts. *, p < 0.05.
TGF-β upregulates Cx43 expression in cardiac fibroblasts
TGF-β is a profibrotic cytokine that is upregulated in experimental models of MI [21–23], and plays a key role in ventricular remodeling post-MI [24]. Activation of TGF-β-mediated signaling pathways results in increased production of collagen, fibronectin and α-SMA [4]. To determine whether TGF-β can upregulate Cx43 protein expression directly in cardiac fibroblasts, fibroblast cultures were exposed to exogenous TGF-β (10 ng/ml) for 24 hr. As shown in Figure 5, immunoblot analysis demonstrated that TGF-β significantly (p = 0.016) increased Cx43 expression in treated fibroblasts (1.24 ± 0.48 density units, n = 5) compared to that in vehicle-treated control cells (0.61± 0.19 density units, n = 5), by an average of 103%. TGF-β also significantly (p = 0.005) increased α-SMA expression in fibroblasts (1.43 ± 0.19 density units, n = 5) compared to that in vehicle-treated cells (0.69 ± 0.23 density units, n = 5) as shown in Figure 5. In contrast, TGF-β failed to upregulate N-cadherin (0.16 ± 0.12 density units, control, n = 7 vs. 0.22 ± 0.19 density units, TGF-β-treated, n = 7; p = 0.488) in fibroblast cultures (Figure 5).
Figure 5.
Top: Representative immunoblots showing increased expression of Cx43 and α-SMA in cultured fibroblasts isolated from normal (non-operated) hearts and exposed to exogenous TGF-β (10 ng/ml). Equal protein (30 μg) was loaded in each lane. Cx43 and α-SMA band densities were normalized to actin band densities to control for protein loading. Bottom: Histograms of summarized immunoblot data showing that both Cx43 and α-SMA protein expression were increased significantly in fibroblasts treated with TGF-β, whereas N-cadherin (N-cad) was unchanged. *, p < 0.05.
DISCUSSION
Cx43 and Cx45 are expressed in the scar of healing infarcts in sheep [25], in which the population of cells making up the “living” infarct is primarily fibroblasts [7]. Fibroblasts play a critical role in ventricular remodeling after MI [5–8]. We recently reported that native murine ventricular fibroblasts express both Cx43 and Cx45, as has been reported by others in rat ventricular fibroblasts [15,16]. Another group found that murine cardiac fibroblasts express Cx43 and Cx40, but not Cx45 [26]. We have been able to detect only trace amounts of Cx40 in fibroblast homogenates when immunoblots are overexposed (not shown). Nevertheless, all reports agree that Cx43 is a major connexin expressed by cardiac fibroblasts. Furthermore, we have shown that the level of Cx43 expression influences intercellular communication in coupled fibroblasts in vitro [14]. The present study demonstrates that cardiac fibroblasts isolated from infarcted hearts exhibit upregulated Cx43 expression and increased intercellular dye transfer, and that these effects are observed in culture through at least one passage of the fibroblasts. Our data suggest that Cx43 remodeling in cardiac fibroblasts post-MI plays an important role in modulating fibroblast coupling and likely contributes to post-infarct ventricular remodeling.
Cx43 is the predominant connexin expressed in the heart and is responsible for electrical coupling of working ventricular myocytes in atria and ventricles. Cx43 is both downregulated and redistributed in cardiac myocytes after MI [12]. Because nearly complete ablation of Cx43 is required before arrhythmias and sudden death are observed in Cx43-deficient hearts [27,28], it appears likely that redistribution and/or internalization [12,29–32] are/is more important in inducing conduction abnormalities that underlie malignant ventricular arrhythmias in infarcted hearts. In contrast to Cx43 downregulation that has been reported in infarcted ventricular myocytes [12,33], we found that cardiac fibroblasts upregulate Cx43 protein and intercellular coupling following MI. Recent preliminary reports from Morley and colleagues have found the same effect [34,35]. One potential mechanism for the upregulation of Cx43 observed in fibroblasts isolated from infarcted hearts likely involves increased TGF-β signaling as discussed below.
MI injury induces cardiac fibroblasts to undergo a phenotypic switch to myofibroblasts which are central players in the profibrotic post-MI repair process [7,16,36]. A trend toward increased α-SMA expression indicative of transformation of fibroblasts to myofibroblasts was observed in the present study in both noninfarcted and infarcted regions as expected, and is consistent with a previous report [9]. Activation of myofibroblasts occurs via a TGF-β-mediated pathway [4]. TGF-β is important during ventricular remodeling following MI for induction of fibroblasts to myofibroblasts, as well as for other profibrotic processes [4,24]. We and others have shown that TGF-β upregulates Cx43 expression in myocytes subjected to pulsatile stretch [37,38] and in epithelial cells [39]. Our present data show that TGF-β significantly increases Cx43 and α-SMA expression in cardiac fibroblasts, suggesting that increased Cx43 expression observed in fibroblasts isolated from infarcted hearts may be due to the increased levels of TGF-β that are produced post-MI [21–23]. Our data are consistent with different regulatory mechanisms underlying electrical and mechanical junctions in both myocytes and fibroblasts, as we observed no change in cadherin expression in fibroblasts isolated from infarcted hearts.
We have observed three major differences in Cx43 remodeling in infarcted fibroblasts that are distinct from remodeling in infarcted myocytes: 1) Cx43 is dramatically upregulated in fibroblasts rather than reduced as in myocytes, 2) upregulation of Cx43 and increased dye coupling do not involve concomitant alteration in cell adhesion junctions, and 3) Cx43 is increased in both infarcted and in noninfarcted regions of the heart. The influence of upregulated Cx43 in fibroblasts in the infarcted heart is likely to encompass not only scar healing and infarct expansion, but also post-infarct remodeling, ventricular dysfunction and the potential for abnormal electrical coupling [15–17] and arrhythmogenesis.
Intercellular junctions are composed of both electrical (gap) and mechanical junctions. The latter include adherens junctions which provide mechanical stability for those membrane sites which house the critically important gap junctions [40] and which contain adhesion proteins that are required for proper assembly, maintenance and function of gap junctions at intercalated disks [41,42]. Cx43 gap junctions are not properly assembled in myocytes of cardiac cadherin-deficient mice. To our knowledge, although cadherins are expressed by fibroblasts and are important mechanotransducers that provide intercellular adhesion strength [43,44], it is not known whether they are required for surface expression of gap junctions in myofibroblasts. However, there are distinct signaling pathways that regulate expression of cardiac electrical versus mechanical junction proteins in cardiac myocytes in response to stretch [38]. Furthermore, unlike our previous study in which stretch-induced upregulation of both Cx43 and N-cadherin was observed in cardiac myocytes [45], our present data indicate that fibroblasts upregulate Cx43 in infarcted hearts without increasing N-cadherin expression. Additional studies are required to determine the extent to which fibroblast electrical junctions are dependent on mechanical junctional stability and the extent to which increased coupling may enhance junctional conductance or modulate activation in infarcted tissue.
ACKNOWLEDGMENTS
The authors would like to thank Carla J. Weinheimer in the Mouse Cardiovascular Phenotyping Core for expert skill in performing all survival surgeries; the Mouse Genetics Core for mouse husbandry support; and Dr. Robert Heuckeroth for use of his fluorescence microscope and camera system. Mice were housed in a facility supported by NCRR grant C06 RR015502. This work was supported by an American Heart Association Fellowship to Y. Zhang and NIH/NHLBI Grant HL066350 to K.A. Yamada.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Goldsmith EC, Hoffman A, Morales MO, Potts JD, Price RL, McFadden A, Rice M, Borg TK. Organization of fibroblasts in the heart. Dev Dyn. 2004;230:787–794. doi: 10.1002/dvdy.20095. [DOI] [PubMed] [Google Scholar]
- 2.Camelliti P, Borg TK, Kohl P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc Res. 2005;65:40–51. doi: 10.1016/j.cardiores.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 3.Banerjee I, Yekkala K, Borg TK, Baudino TA. Dynamic interactions between myocytes, fibroblasts, and extracellular matrix. Ann NY Acad Sci. 2006;1080:76–84. doi: 10.1196/annals.1380.007. [DOI] [PubMed] [Google Scholar]
- 4.Lijnen PJ, Petrov VV, Fagard RH. Induction of cardiac fibrosis by transforming growth factor-β1. Mol Genet Metab. 2000;71:418–435. doi: 10.1006/mgme.2000.3032. [DOI] [PubMed] [Google Scholar]
- 5.Manabe I, Shindo T, Nagai R. Gene expression in fibroblasts and fibrosis: involvement in cardiac hypertrophy. Circ Res. 2002;91:1103–1113. doi: 10.1161/01.res.0000046452.67724.b8. [DOI] [PubMed] [Google Scholar]
- 6.Brown RD, Ambler SK, Mitchell MD, Long CS. The cardiac fibroblast: therapeutic target in myocardial remodeling and failure. Annu Rev Pharmacol Toxicol. 2005;45:657–687. doi: 10.1146/annurev.pharmtox.45.120403.095802. [DOI] [PubMed] [Google Scholar]
- 7.Sun Y, Kiani MF, Postlethwaite AE, Weber KT. Infarct scar as living tissue. Basic Res Cardiol. 2002;97:343–347. doi: 10.1007/s00395-002-0365-8. [DOI] [PubMed] [Google Scholar]
- 8.Baudino TA, Carver W, Giles W, Borg TK. Cardiac fibroblasts: friend or foe? Am J Physiol Heart Circ Physiol. 2006;291:H1015–H1026. doi: 10.1152/ajpheart.00023.2006. [DOI] [PubMed] [Google Scholar]
- 9.Squires CE, Escobar GP, Payne JF, Leonardi RA, Goshorn DK, Sheats NJ, Mains IM, Mingoia JT, Flack EC, Lindsey ML. Altered fibroblast function following myocardial infarction. J Mol Cell Cardiol. 2005;39:699–707. doi: 10.1016/j.yjmcc.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Flack EC, Lindsey ML, Squires CE, Kaplan BS, Stroud RE, Clark LL, Escobar PG, Yarbrough WM, Spinale FG. Alterations in cultured myocardial fibroblast function following the development of left ventricular failure. J Mol Cell Cardiol. 2006;40:474–483. doi: 10.1016/j.yjmcc.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 11.Jarvis MD, Rademaker MT, Ellmers LJ, Currie MJ, McKenzie JL, Palmer BR, Frampton CM, Richards AM, Cameron VA. Comparison of infarct-derived and control ovine cardiac myofibroblasts in culture: response to cytokines and natriuretic peptide receptor expression profiles. Am J Physiol Heart Circ Physiol. 2006;291:H1952–H1958. doi: 10.1152/ajpheart.00764.2005. [DOI] [PubMed] [Google Scholar]
- 12.Severs NJ, Bruce AF, Dupont E, Rothery S. Remodelling of gap junctions and connexin expression in diseased myocardium. Cardiovasc Res. 2008;80:9–19. doi: 10.1093/cvr/cvn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chanson M, Kwak BR. Connexin37: a potential modifier gene of inflammatory disease. J Mol Med. 2007;85:787–795. doi: 10.1007/s00109-007-0169-2. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Kanter EM, Laing JG, Aprhys C, Johns DC, Kardami E, Yamada KA. Connexin43 expression levels influence intercellular coupling and cell proliferation of native murine cardiac fibroblasts. Cell Commun Adhes. 2008;15:289–303. doi: 10.1080/15419060802198736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaudesius G, Miragoli M, Thomas SP, Rohr S. Coupling of cardiac electrical activity over extended distances by fibroblasts of cardiac origin. Circ Res. 2003;93:421–428. doi: 10.1161/01.RES.0000089258.40661.0C. [DOI] [PubMed] [Google Scholar]
- 16.Miragoli M, Gaudesius G, Rohr S. Electronic modulation of cardiac impulse conduction by myofibroblasts. Circ Res. 2006;98:801–810. doi: 10.1161/01.RES.0000214537.44195.a3. [DOI] [PubMed] [Google Scholar]
- 17.Miragoli M, Salvarani N, Rohr S. Myofibroblasts induce ectopic activity in cardiac tissue. Circ Res. 2007;101:755–758. doi: 10.1161/CIRCRESAHA.107.160549. [DOI] [PubMed] [Google Scholar]
- 18.Roell W, Lewalter T, Sasse P, Tallini YN, Choi B-R, Breitbach M, Doran R, Becher UM, Hwang S-M, Bostani T, von Maltzahn J, Hofmann A, Reining S, Eiberger B, Gabris B, Pfeifer A, Welz A, Willecke K, Salama G, Schrickel JW, Kotlikoff MI, Fleischmann BK. Engraftment of connexin 43-expressing cells prevents post-infarct arrhythmia. Nature. 2007;450:819–824. doi: 10.1038/nature06321. [DOI] [PubMed] [Google Scholar]
- 19.Lavine KJ, Kovacs A, Ornitz DM. Hedgehog signaling is critical for maintenance of the adult coronary vasculature in mice. J Clin Invest. 2008;118:2404–2414. doi: 10.1172/JCI34561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Fouly MH, Trosko JE, Chang C-C. Scrape-loading and dye transfer: a rapid and simple technique to study gap junctional intercellular communication. Exp Cell Res. 1987;168:422–430. doi: 10.1016/0014-4827(87)90014-0. [DOI] [PubMed] [Google Scholar]
- 21.Deten A, Hölzl A, Leicht M, Barth W, Zimmer H-G. Changes in extracellular matrix and in transforming growth factor beta isoforms after coronary artery ligation in rats. J Mol Cell Cardiol. 2001;33:1191–1207. doi: 10.1006/jmcc.2001.1383. [DOI] [PubMed] [Google Scholar]
- 22.Dewald O, Ren G, Duerr GD, Zoerlein M, Klemm C, Gersch C, Tincey S, Michael LH, Entman ML, Frangogiannis NG. Of mice and dogs: species-specific differences in the inflammatory response following myocardial infarction. Am J Pathol. 2004;164:665–677. doi: 10.1016/S0002-9440(10)63154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dean RG, Balding LC, Candido R, Burns WC, Cao Z, Twigg SM, Burrell LM. Connective tissue growth factor and cardiac fibrosis after myocardial infarction. J Histochem Cytochem. 2005;53:1245–1256. doi: 10.1369/jhc.4A6560.2005. [DOI] [PubMed] [Google Scholar]
- 24.Bujak M, Frangogiannis NG. The role of TGF-β signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res. 2007;74:184–195. doi: 10.1016/j.cardiores.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Camelliti P, Devlin GP, Matthews KG, Kohl P, Green CR. Spatially and temporally distinct expression of fibroblast connexins after sheep ventricular infarction. Cardiovasc Res. 2004;62:415–425. doi: 10.1016/j.cardiores.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 26.Louault C, Benamer N, Faivre J-F, Potreau D, Bescond J. Implication of connexins 40 and 43 in functional coupling between mouse cardiac fibroblasts in primary culture. Biochim Biophys Acta. 2008;1778:2097–2104. doi: 10.1016/j.bbamem.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Gutstein DE, Morley GE, Tamaddon H, Vaidya D, Schneider MD, Chen J, Chien KR, Stuhlmann H, Fishman GI. Conduction slowing and sudden arrhythmic death in mice with cardiac-restricted inactivation of connexin43. Circ Res. 2001;88:333–339. doi: 10.1161/01.res.88.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Rijen HVM, Eckardt D, Degen J, Theis M, Ott T, Willecke K, Jongsma HJ, Opthof T, de Bakker JMT. Slow conduction and enhanced anisotropy increase the propensity for ventricular tachyarrhythmias in adult mice with induced deletion of connexin43. Circulation. 2004;109:1048–1055. doi: 10.1161/01.CIR.0000117402.70689.75. [DOI] [PubMed] [Google Scholar]
- 29.Peters NS, Coromilas J, Severs NJ, Wit AL. Disturbed connexin43 gap junction distribution correlates with the location of reentrant circuits in the epicardial border zone of healing canine infarcts that cause ventricular tachycardia. Circulation. 1997;95:988–996. doi: 10.1161/01.cir.95.4.988. [DOI] [PubMed] [Google Scholar]
- 30.Peters NS, Wit AL. Myocardial architecture and ventricular arrhythmogenesis. Circulation. 1998;97:1746–1754. doi: 10.1161/01.cir.97.17.1746. [DOI] [PubMed] [Google Scholar]
- 31.Betsuyaku T, Kanno S, Lerner DL, Schuessler RB, Saffitz JE, Yamada KA. Spontaneous and inducible ventricular arrhythmias after myocardial infarction in mice. Cardiovasc Pathol. 2004;13:156–164. doi: 10.1016/S1054-8807(03)00152-2. [DOI] [PubMed] [Google Scholar]
- 32.Kieken F, Mutsaers N, Dolmatova E, Virgil K, Wit AL, Kellezi A, Hirst-Jensen BJ, Duffy HS, Sorgen PL. Structural and molecular mechanisms of gap junction remodeling in epicardial border zone myocytes following myocardial infarction. Circ Res. 2009;104:1103–1112. doi: 10.1161/CIRCRESAHA.108.190454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peters NS, Green CR, Poole-Wilson PA, Severs NJ. Reduced content of connexin43 gap junctions in ventricular myocardium from hypertrophied and ischemic human hearts. Circulation. 1993;88:864–875. doi: 10.1161/01.cir.88.3.864. [DOI] [PubMed] [Google Scholar]
- 34.Vasquez C, Feig JE, Mohandas P, Fisher EA, Morley GE. Arrhythmogenic potential of activated fibroblasts. Biophys J. 2009;96(Suppl1):562a–563a. [Google Scholar]
- 35.Vasquez C, Feig J, Mohandas P, Fisher EA, Morley GE. Arrhythmogenic potential of activated fibroblasts. Heart Rhythm. 2009;6:S458. [Google Scholar]
- 36.Willems IE, Havenith MG, De Mey JG, Daemen MJ. The α-smooth muscle actin-positive cells in healing human myocardial scars. Am J Pathol. 1994;145:868–875. [PMC free article] [PubMed] [Google Scholar]
- 37.Pimentel RC, Yamada KA, Kléber AG, Saffitz JE. Autocrine regulation of myocyte Cx43 expression by VEGF. Circ Res. 2002;90:671–677. doi: 10.1161/01.res.0000014823.75393.4d. [DOI] [PubMed] [Google Scholar]
- 38.Yamada K, Green KG, Samarel AM, Saffitz JE. Distinct pathways regulate expression of cardiac electrical and mechanical junction proteins in response to stretch. Circ Res. 2005;97:346–353. doi: 10.1161/01.RES.0000178788.76568.8a. [DOI] [PubMed] [Google Scholar]
- 39.Tacheau C, Fontaine J, Loy J, Mauviel A, Verrecchia F. TGF-β induces connexin43 gene expression in normal murine mammary gland epithelial cells via activation of p38 and PI3K/AKT signaling pathways. J Cell Physiol. 2008;217:759–768. doi: 10.1002/jcp.21551. [DOI] [PubMed] [Google Scholar]
- 40.Saffitz JE. Adhesion molecules: why they are important to the electrophysiologist. J Cardiovasc Electrophysiol. 2006;17:225–229. doi: 10.1111/j.1540-8167.2006.00365.x. [DOI] [PubMed] [Google Scholar]
- 41.Li J, Patel VV, Kostetskii I, Xiong Y, Chu AF, Jacobson JT, Yu C, Morley GE, Molkentin JD, Radice GL. Cardiac-specific loss of N-cadherin leads to alteration in connexins with conduction slowing and arrhythmogenesis. Circ Res. 2005;97:474–481. doi: 10.1161/01.RES.0000181132.11393.18. [DOI] [PubMed] [Google Scholar]
- 42.Li J, Levin MD, Xiong Y, Petrenko N, Patel VV, Radice GL. N-cadherin haploinsufficiency affects cardiac gap junctions and arrhythmic susceptibility. J Mol Cell Cardiol. 2008;44:597–606. doi: 10.1016/j.yjmcc.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ko KS, Arora PD, McCulloch CAG. Cadherins mediate intercellular mechanical signaling in fibroblasts by activation of stretch-sensitive calcium-permeable channels. J Biol Chem. 2001;276:35967–35977. doi: 10.1074/jbc.M104106200. [DOI] [PubMed] [Google Scholar]
- 44.Chan MWC, El Sayegh TY, Arora PD, Laschinger CA, Overall CM, Morrison C, McCulloch CAG. Regulation of intercellular adhesion strength in fibroblasts. J Biol Chem. 2004;279:41047–41057. doi: 10.1074/jbc.M406631200. [DOI] [PubMed] [Google Scholar]
- 45.Zhuang J, Yamada KA, Saffitz JE, Kléber AG. Pulsatile stretch remodels cell-to-cell communication in cultured myocytes. Circ Res. 2000;87:316–322. doi: 10.1161/01.res.87.4.316. [DOI] [PubMed] [Google Scholar]