Abstract
Protein kinase C delta (PKC-δ) protein levels are frequently low in chemically and UV-induced mouse skin tumors as well as in human cutaneous squamous cell carcinomas (SCCs). Furthermore, overexpression of PKC-δ in human SCC lines and mouse epidermis is sufficient to induce apoptosis and suppress tumorigenicity, making PKC-δ a potential tumor suppressor gene for SCCs. Here we report that PKC-δ is lost in human SCCs at the transcriptional level. We used laser capture microdissection to isolate cells from three normal human epidermis and 14 human SCCs with low PKC-δ protein. Analysis by quantitative reverse transcription-PCR revealed that PKC-δ RNA was reduced an average of 90% in the SCCs tested, consistent with PKC-δ down-regulation at the protein level. Analysis of DNA from nine of the same tumors revealed that PKC-δ gene was deleted in only one tumor. In addition, Ras-transformed human keratinocytes, which have selective down-regulation of PKC-δ at both protein and mRNA levels, had significantly repressed human PKC-δ promoter activity. Together, these results indicate that PKC-δ gene expression is suppressed in human SCCs, probably via transcription repression. Our results have implications for the development of topical therapeutic strategies to trigger the re-expression of pro-apoptotic PKC-δ to induce apoptosis in SCCs.
Squamous cell carcinoma is a very common form of skin cancer, affecting more than 200,000 Americans each year.1 Protein kinase C delta (PKC-δ) has been implicated as a tumor suppressor in both human and mouse SCCs as protein levels are significantly reduced or undetectable in tumor tissue, and ectopic expression of PKC-δ in transformed human keratinocytes or mouse epidermis can dramatically inhibit tumor formation.2,3 PKC-δ is activated by diacylglycerol/phorbol esters in a Ca2+-independent manner and induces cell-cycle arrest and differentiation.4–7 An alternate mechanism of PKC-δ activation in response to apoptotic stimuli is via caspase-3 mediated cleavage, releasing the constitutively active catalytic fragment. This activation mechanism has been shown to be necessary and sufficient for UV-induced apoptosis in keratinocytes.8,9 The elimination of keratinocytes by UV-induced apoptosis is an important tumor suppressor mechanism, and the loss/inactivation of PKC-δ promotes survival of keratinocytes exposed to UV, the main etiological agent of SCC. In addition, the pro-apoptotic properties of PKC-δ have been reported in both normal primary keratinocytes and immortalized HaCaT cells containing mutant p53, indicating that PKC-δ induced apoptosis is p53-independent.9–11
Previous studies have found that PKC-δ protein is reduced in ∼30% of human SCCs, as well as the majority of chemically and UV-induced mouse skin tumors.2,3,12 Activation of Ras oncogenes are found in 58% of human SCCs and >90% of chemically induced mouse skin tumors. In addition, human SCCs with low PKC-δ had evidence of Ras pathway activation.2 Expression of oncogenic Ras or epidermal growth factor receptor (EGFR) activation can down-regulate PKC-δ protein and steady-state mRNA levels in HaCaT cells, an immortalized, nontransformed human keratinocyte cell line.2,13 PKC-δ can also be negatively regulated by Ras at the protein levels by inhibitory tyrosine phosphorylation and/or degradation in mouse keratinocytes.14,15 Thus, the mechanism of human PKC-δ down-regulation/inactivation in human SCCs may involve regulation at multiple levels. In this study, we identified transcriptional repression, not gene deletion as is common for many tumor suppressor genes, as the mechanism of PKC-δ loss in human cutaneous SCCs.
Materials and Methods
Immunohistochemistry
Tissue samples were fixed in 10% neutral buffered formalin and embedded in paraffin. Tissue sections (10 μm) were deparaffinized followed by antigen retrieval (10 mmol/L citrate buffer, pH 6.0, microwave 500 W; 15 minutes). The samples were stained for PKC-δ (sc-937, 1:100, Santa Cruz Biotechnology, Santa Cruz, CA) and HK-14 antibody (PRB-155P, Covance, Princeton, NJ), by immunohistochemical methods using the Vectastain ABC kit (Vector Laboratories, Burlingame, CA). All human tissue samples were obtained with approval from the Loyola University Medical Center's Institutional Review Board.
Laser Capture Microdissection
Laser capture microdissection (LCM) was performed using the PixCell II apparatus (Arcturus Biosciences, Moutain View, CA) as per manufacturer's instructions. Formaldehyde-fixed, paraffin-embedded tissue sections (10 μm) were deparaffinized and stained with H&E. Stained sections were dehydrated in 100% xylene immediately before performing LCM. For each sample, LCM was performed on 8 to 10 tissue sections and isolated approximately 300 to 500 cells per section, the sections were pooled to yield ∼3000 to 5000 cells per sample.
RNA Isolation and Quantitative Reverse Transcription-PCR
Total RNA was isolated using the PicoPure RNA Isolation Kit (Arcturus Biosciences) from LCM samples, and by Trizol (Gibco, Chagrin Falls, OH) from HaCaT, HaCaT Ras I-7 and HaCaT Ras-II-4 cells. The amount of RNA isolated was on the order of 50 to 500 ng per sample. Complementary DNA was synthesized by reverse transcription (RT) of total isolated RNA (Superscript First Strand Synthesis, Invitrogen, Carlsbad, CA). Quantitative (q)RT-PCR for PKC-δ and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was performed using a GeneAmp 5700 sequence detection system (Applied Biosystems, Foster City, CA) with Platinum SYBR Green PCR reagents (Invitrogen). The PKC-δ RNA levels were normalized to the GAPDH RNA for each sample. Relative mRNA expression was calculated using the ΔΔCt method. Some samples were normalized to actin to confirm relative PKC-δ mRNA expression. The sequences of PKC-δ and GAPDH specific primers are as follows: PKC-δ forward: 5′-AAAGGCAGCTTCGGGAAGGT-3′; PKC-δ reverse: 5′-TGGATGTGGTACATCAGGTC-3′; GAPDH forward: 5′-GCACCGTCAAGGCTGAGAAC-3′; and, GAPDH reverse: 5′-GCCTTCTCCATGGTGGTGAA-3′.
PCR-Based Gene Deletion Analysis
Total DNA was purified from LCM samples using the PicoPure DNA Isolation Kit (Arcturus Biosciences). The amount of DNA isolated was on the order of 10 to 50 ng per sample. Gene-specific primers were designed with identical annealing temperatures to amplify 100 bp regions of intron-1 in the PKC-δ gene or the GAPDH gene. qPCR was performed using a GeneAmp 5700 sequence detection system (Applied Biosystems) with Platinum SYBR Green PCR reagents (Invitrogen). The initial denaturation was performed at 95°C for 10 minutes, followed by 50 cycles each consisting of denaturation at 95°C for 25 s, annealing at 56°C for 1 minute and extension at 72°C for 1 minute, followed by a final extension at 72°C for 7 minutes. A single 100 bp amplicon was confirmed by postamplification dissociation curve analysis and by 3% agarose gel electrophoresis. DNA from each LCM sample was amplified in three to four independent qPCR reactions for quantitation. Relative PKC-δ gene levels were calculated by the ΔΔCt method. The sequences of PKC-δ and GAPDH gene specific primers are as follows: PKC-δ forward: 5′-ACGAAGAAGGTCAGCACAGTTAG-3′; PKC-δ reverse: 5′-TGTCCTTACCACTCCTCAACACT-3′; GAPDH forward: 5′-AGTGAGTGGAAGACAGAATGGAA-3′; GAPDH reverse: 5′-CCATATTGAGGGACACAAGGTTA-3′; PKCδ-intron 16 forward: 5′- TCAATCTTTAGCTGGGTATTTGC-3′; PKCδ-intron 16 reverse: 5′- TGAGTTCCTAGTTCCTGAAGCTG −3′; PKCδ-exon 14 forward: 5′- TACACATTCTCTGTGGACTGGTG-3′; and, PKCδ- exon 14 reverse: 5′- CTCGAAGAGTTCATCCTCATCAT −3′.
Cell Culture
HaCaT, HaCaT Ras I-7, and HaCaT Ras II-4 (provided by Norbert Fusenig and Mihaela Skobe) were grown in Dulbecco's Modified Eagle Medium (Invitrogen) with 10% fetal bovine serum and penicillin/streptomycin antibiotics (1:100).
PKC-δ Promoter Analysis
A BAC clone (RPCI-11–82B23, BACPAC Resources, Children's Hospital Oakland Research Institute) containing the human PKC-δ gene was digested with XhoI/EcoRV (New England Biolabs, Ipswitch, MA) to generate a 7.4-kb fragment containing the PKC-δ promoter and 3 kb of the PKC-δ gene. This was cloned into a pKS-Bluescript vector to generate pKS-PKCδ-Bac. Digestion of pKS-PKCδ-Bac with XhoI/SacII (New England Biolabs) generated a 4400-bp fragment that was subcloned into the firefly luciferase reporter pGL3-Basic vector (Promega) to generate pGL3-hPKCδ−4.4. pGL3-Basic or pGL3-hPKCδ−4.4 was transiently transfected, in triplicate, using FuGENE-6 transfection reagent (Roche) into HaCaT and HaCaT-Ras cells. The pRL-TK vector, encoding Renilla luciferase acted as an internal control for transfection efficiency and was co-transfected with the pGL3 constructs at a 1:10 ratio. After 48 hours, luciferase activity in cell lysates was measured with the Dual Luciferase assay kit (Promega, Madison, WI). Relative luciferase activity of cell extracts is represented as firefly luciferase value/Renilla luciferase value.
Results
LCM and RNA Analysis
To determine whether PKC-δ gene expression is reduced in human SCCs, we used LCM to isolate epithelial cells from three normal human epidermises and 14 human SCC samples. The selected SCCs all had reduced PKC-δ protein as determined by immunohistochemistry.2 LCM was used to exclude stromal cells, inflammatory cells and other normal cells, which can express high levels of PKC-δ, allowing us to isolate individual cancerous cells for expression studies.16 Histological analysis of the 14 human SCC samples was performed to identify the tumor regions and nontumor regions (Figure 1A). All tumor biopsies were confirmed as SCCs with a highly variable histology, ranging from invasive to well-differentiated. Parallel immunohistochemical staining was performed for PKC-δ and an epithelial marker (Keratin-14) as shown in Figure 1A. We identified regions of Keratin-14 positive SCC cells with reduced PKC-δ staining, as compared with normal epidermis (Figure 1A).
Figure 1.
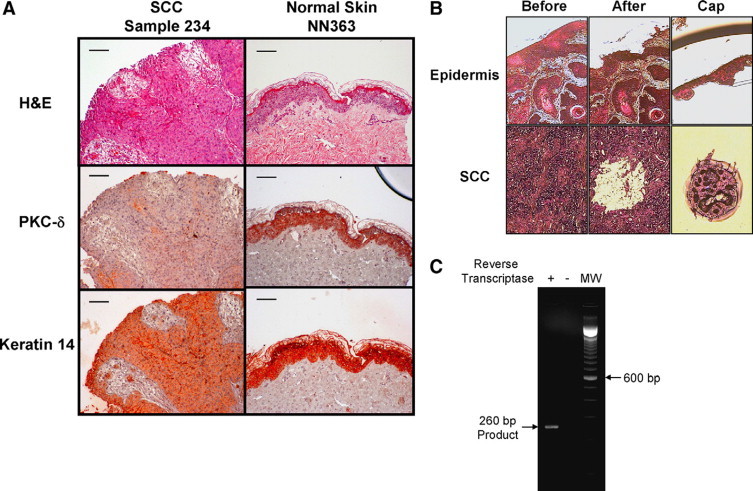
PKC-δ immunostaining in SCCs and LCM. A: Loss of PKC-δ protein in human SCCs. Hematoxylin and eosin staining of normal human skin (NN363) and human SCC (case 234) is shown on top. PKC-δ staining of normal human epidermis and human skin SCCs by immunohistochemistry is shown in the middle row. Keratin-14 staining of sections of normal human epidermis and human skin SCCs is shown at the bottom. Keratin-14 staining was used as an epithelial marker to differentiate between cells of epithelial and non-epithelial origin. Scale bars = 30 μm. B: Isolation of pure SCC and normal epidermis cells by LCM. The tissue before and after LCM are shown. The isolated region containing normal epidermal cells and SCC cells on the cap are shown. C: Detection of PKC-δ RNA. RNA was purified from normal human epidermis isolated by LCM. The integrity and specificity of the PKC-δ RNA sample was verified by performing RT-PCR in presence and absence of reverse-transcriptase as shown. The predicted 260 bp PCR fragment was obtained on performing PKC-δ RT-PCR in the presence of reverse transcriptase.
We observed that most tumors had regions intermixed with normal cells, such as inflammatory immune infiltrates and normal epidermis, which stained very strongly for PKC-δ. We performed LCM to specifically isolate SCC cells and normal epidermal cells from tumor sections and control normal skin sections, respectively (Figure 1B). For every SCC and normal epidermis sample, ∼104 cells were captured, total RNA was isolated, and PKC-δ mRNA was quantitated by qRT-PCR. To ensure the integrity of the PKC-δ mRNA was preserved and the specificity of the PCR, we performed PKC-δ RT-PCR from total RNA isolated from a normal epidermis, collected by LCM, in presence and absence of reverse transcriptase. A single, specific RT-PCR product of the expected ∼260 bp was obtained only in the presence reverse transcriptase, thus ruling out genomic DNA contamination (Figure 1C).
PKC-δ mRNA Is Lost in Human SCCs
We analyzed a total of 14 human SCC clinical samples and three normal epidermises for PKC-δ RNA levels. The PKC-δ RNA levels were normalized to GAPDH RNA levels for each tumor and normal epidermis sample as determined by qRT-PCR (Figure 2A). PKC-δ mRNA was undetectable or reduced in all of the tumors analyzed compared with normal epidermis samples (Figure 2A). On an average, PKC-δ RNA levels were reduced by 90% in human SCCs compared with normal epidermis samples. One SCC (sample 158) had PKC-δ RNA levels reduced only 20% below the lowest PKC-δ expressing normal epidermis (NN364) despite having almost undetectable PKC-δ protein by immunohistochemistry. Thus post-transcriptional mechanisms may contribute to the reduced PKC-δ protein levels in some human SCCs. To rule out the possibility that GAPDH expression might be deregulated in SCCs, we confirmed our findings by using actin normalization on six tumors and one normal human epidermis. A similar reduction in PKC-δ mRNA was observed on normalization against actin RNA levels in the LCM samples (see Supplemental Figure S1 at http://ajp.amjpathol.org). Thus, human SCCs with low PKC-δ protein have reduced PKC-δ mRNA levels, suggesting either gene deletion/loss or transcriptional silencing.
Figure 2.
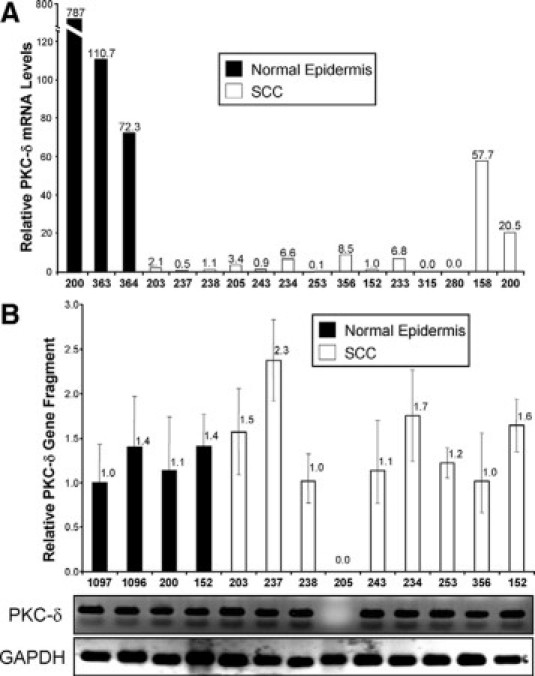
Quantification of PKC-δ mRNA and gene in human SCCs. A: PKC-δ RNA is reduced in human SCC cells compared with normal human epidermis. Keratinocytes from 14 human SCC samples and 3 normal epidermises were isolated by LCM and analyzed for PKC-δ RNA by qRT-PCR. The PKC-δ RNA levels were normalized to the GAPDH RNA for each sample. The sample numbers are shown on the x axis, and the relative PKC-δ mRNA levels above each bar. B: PCR-based gene deletion assay was performed on cells isolated by LCM from 9 human SCCs and 4 normal human epidermises. PKC-δ gene fragment was detected by specific primers for a 100 bp region inside intron 1. GAPDH gene fragment was amplified to normalize for DNA integrity and amount. The sample numbers are shown on the x axis, and the relative PKC-δ gene levels are indicated above each bar. Error bars denote SD for 3–4 independent qPCR reactions. Products from a representative qPCR reaction for PKC-δ and GAPDH were run on agarose gels and are shown below the bar graph. Note that only one SCC (205) had a deletion in the PKC-δ gene.
PKC-δ Gene Deletion Analysis
PKC-δ resides at 3p21.31 and the 3p region has been reported to be deleted in many cancers including cutaneous SCC.17 Thus loss of PKC-δ by gene deletion in human SCCs is a possible mechanism for reduced PKC-δ protein and mRNA levels. To determine the status of PKC-δ gene, we extracted genomic DNA from LCM-isolated keratinocytes from the same 14 human SCC samples and four normal epidermis samples and performed qPCR based gene deletion analysis. Only 9 of the 14 tumors were informative for the genomic DNA analysis. We found that PKC-δ gene was present in eight out of nine tumors, while the GAPDH fragment was detected and reproducibly amplified in all samples (Figure 2B). Partial gene deletion is also a possibility that could account for reduced PKCδ RNA in the PKC-δ deficient SCCs. To address this, we amplified fragments of intron 16 and exon 14 near the 3′-end of PKC-δ gene, from isolated SCC cells and confirmed that that full length PKC-δ gene was intact in the five randomly selected tumors analyzed (see Supplemental Figure S2 at http://ajp.amjpathol.org). These findings suggest that the mechanism of down-regulation of PKC-δ in SCCs is likely to be primarily at the level of gene transcription and not gene deletion.
Mechanism of Loss of PKC-δ Gene Expression
Ras activation has been shown to down-regulate PKC-δ protein and RNA in human keratinocytes2,13 and activation of Ras oncogene was found in 58% of human SCCs.18 Furthermore, EGFR activation correlates with PKC-δ protein down-regulation in human SCCs, supporting an inverse relationship between the EGFR/Ras pathway and PKC-δ.2 To explore the potential transcription mechanism of PKC-δ down-regulation in human SCCs, we used the HaCaT cell line and Ras transformed HaCaT cells (HaCaT Ras II-4 & HaCaT-Ras I-7).19,20 The tumorigenic HaCaT Ras II-4 and HaCaT Ras I-7 cells have been shown to have reduced PKC-δ protein levels and their tumorigenicity is significantly inhibited by re-expression of PKC-δ.2 We analyzed PKC-δ RNA levels in HaCaT Ras II-4 and HaCaT Ras I-7 by qRT-PCR and confirmed that PKC-δ RNA is reduced relative to HaCaT cells (Figure 3A).
Figure 3.
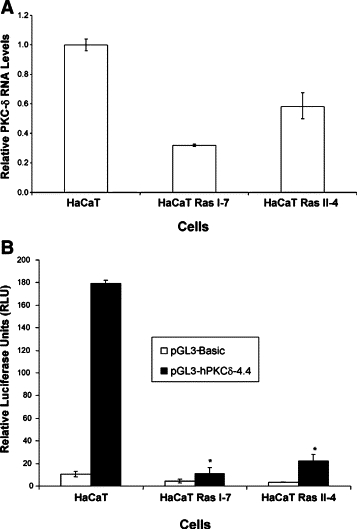
PKC-δ gene expression is reduced in Ras transformed keratinocytes. A: PKC-δ RNA is reduced in Ras transformed HaCaT cells. Total RNA was isolated from HaCaT, HaCaT Ras I-7 and HaCaT Ras II-4 cells and analyzed for PKC-δ RNA by qRT-PCR. B: PKC-δ promoter activity is down-regulated in Ras transformed HaCaT cells. pGL3-Basic or pGL3-hPKCδ−4.4 was transiently co-transfected with pRL-TK into HaCaT and HaCaT-Ras cells. After 48 hours, luciferase activity in cell lysates was measured. Shown is the mean and SD from experiments performed in triplicate. *P < 0.001.
We then performed PKC-δ promoter reporter assays in HaCaT and Ras-transformed HaCaT cells using 4.4 kb of the human PKC-δ promoter. We found that PKC-δ promoter activity was significantly (P < 0.001) reduced in HaCaT I-7 and HaCaT Ras II-4, as compared with control HaCaT cells (Figure 3B). We also found that PKC-δ reporter activity is decreased in HaCaT cells that were treated with EGF or transduced with a Ha-Ras(G12V) retrovirus (see Supplemental Figure S3 at http://ajp.amjpathol.org). These results suggest that transcriptional repression of PKC-δ might be responsible for down-regulation of PKC-δ in human SCCs as well.
Discussion
Tumor suppressor genes are lost or inactivated in tumors by a variety of mechanisms, including gene deletion, gene repression (ie, promoter methylation), and protein destabilization. Definitive understanding of the mechanism of tumor suppressor gene loss is complicated by tumor heterogeneity and the presence of other cell types within and adjacent to the tumor which may express the tumor suppressor gene at high levels. Indeed, when we analyzed one region of a SCC (case 203) that contained an immune infiltrate strongly positive for PKC-δ by immunohistochemistry, we found 30-fold higher levels of PKCδ mRNA by qRT-PCR (data not shown). To circumvent this caveat, we used LCM to isolate relatively pure SCC and epidermal cells for RNA and DNA analysis. Using this more selective approach, we found the tumor suppressor PKC-δ is lost at the mRNA level in human SCCs, and that the PKC-δ gene is rarely deleted. Furthermore, in Figure 3 we showed that the human PKC-δ promoter is negatively regulated in Ras-transformed keratinocytes. Finally, mRNA half-life studies found that Ras did not increase the turn-over of PKC-δ mRNA (data not shown). Together, these results strongly support a transcriptional repression mechanism for silencing PKC-δ expression.
The PKC-δ gene resides at 3p21.31, and the 3p region has been reported to be deleted in many cancers, including cutaneous SCC.17,21 Based on the qPCR data in Figure 2 and Supplemental Figure S2 (http://ajp.amjpathol.org), we have ruled out PKC-δ gene deletion as a major mechanism of PKC-δ loss in human SCCs. PKC-δ protein is also reduced in high grade endometrial and bladder cancers, but the mechanism of PKCδ loss in these cancers has not been reported.22–24
The PKC-δ gene has been previously shown to be up-regulated by a variety of transcription factors.25–29 The mechanism of reduced PKC-δ gene expression in human SCCs is unknown, but is likely to involve Ras/EGFR signaling. Ras oncogene or EGFR signaling are activated in a high percentage of human cancers, including cutaneous SCCs, and regulates transcription of many pro-survival and tumor suppressor genes via activation of a variety of transcription factors such as nuclear factor-κB, Ets, and activator protein-1.30,31 Computational analysis of PKC-δ promoter sequence reveals potential binding sites for proteins such as AP-1 and Ets, which can repress the target gene expression in response to Ras activation.31,32
We have previously shown that re-expression of PKC-δ induces apoptotic cell death in human SCC cells and inhibits their growth,2 therefore understanding the mechanism of PKC-δ down-regulation has strong implications for the development of therapeutic drugs targeting signaling pathway(s) capable of up-regulating the pro-apoptotic PKC-δ. Drugs blocking PKC-δ gene repression may be especially efficacious against skin cancers due to topical route of administration, thus localizing drug delivery to the tumor cells and minimizing systemic toxicities.
Acknowledgements
We thank everyone from Skin Cancer Research Program for their help with this project. Special thanks go to Emily Fitch for constructing the pGL3-hPKCδ4.4 vector, and to Dr. June K. Robinson (Northwestern University) for providing human tumor tissue. We thank Dr. Norbert Fusenig (German Cancer Research Center, Heidelberg, Germany) and Mihaela Skobe (Harvard School of Medicine, Boston, MA) for providing HaCaT cells and HaCaT-Ras clones.
Footnotes
Supported by NIH grant CA083784 (M.F.D.).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
Web Extra Material
References
- 1.Padgett JK, Hendrix JD., Jr Cutaneous malignancies and their management. Otolaryngol Clin North Am. 2001;34:523–553. doi: 10.1016/s0030-6665(05)70004-9. [DOI] [PubMed] [Google Scholar]
- 2.D'Costa AM, Robinson JK, Maududi T, Chaturvedi V, Nickoloff BJ, Denning MF. The proapoptotic tumor suppressor protein kinase C-δ is lost in human squamous cell carcinomas. Oncogene. 2006;25:378–386. doi: 10.1038/sj.onc.1209065. [DOI] [PubMed] [Google Scholar]
- 3.Reddig PJ, Dreckschimdt NE, Ahrens H, Simsiman R, Tseng CP, Zou J, Oberley TD, Verma AK. Transgenic mice overexpressing protein kinase C δ in the epidermis are resistant to skin tumor promotion by 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1999;59:5710–5718. [PubMed] [Google Scholar]
- 4.Gschwendt M. Protein kinase C delta. Eur J Biochem. 1999;259:555–564. doi: 10.1046/j.1432-1327.1999.00120.x. [DOI] [PubMed] [Google Scholar]
- 5.Ohba M, Ishino K, Kashiwagi M, Kawabe S, Chida K, Huh NH, Kuroki T. Induction of differentiation in normal human keratinocytes by adenovirus-mediated introduction of the eta and delta isoforms of protein kinase C. Mol Cell Biol. 1998;18:5199–5207. doi: 10.1128/mcb.18.9.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watanabe T, Ono Y, Taniyama Y, Hazama K, Igarashi K, Ogita K, Kikkawa U, Nishizuka Y. Cell division arrest induced by phorbol ester in CHO cells overexpressing protein kinase C-δsubspecies. Proc Natl Acad Sci USA. 1992;89:10159–10163. doi: 10.1073/pnas.89.21.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Efimova T, Eckert RL. Regulation of human involucrin promoter activity by novel protein kinase C isoforms. J Biol Chem. 2000;275:1601–1607. doi: 10.1074/jbc.275.3.1601. [DOI] [PubMed] [Google Scholar]
- 8.D'Costa AM, Denning MF. A caspase-resistant mutant of PKC-δ protects keratinocytes from UV-induced apoptosis. Cell Death Differ. 2005;12:224–232. doi: 10.1038/sj.cdd.4401558. [DOI] [PubMed] [Google Scholar]
- 9.Denning MF, Wang Y, Tibudan S, Nickoloff BJ, Qin JZ. Caspase activation and disruption of mitochondrial membrane potential during UV radiation-induced apoptosis of human keratinocyte requires activation of protein kinase C. Cell Death Diff. 2002;9:40–52. doi: 10.1038/sj.cdd.4400929. [DOI] [PubMed] [Google Scholar]
- 10.Sitailo LA, Tibudan SS, Denning MF. Bax activation and induction of apoptosis in human keratinocytes by the protein kinase C δ catalytic domain. J Invest Dermatol. 2004;123:434–443. doi: 10.1111/j.0022-202X.2004.23403.x. [DOI] [PubMed] [Google Scholar]
- 11.Sitailo LA, Tibudan SS, Denning MF. The protein kinase Cδ catalytic fragment targets Mcl-1 for degradation to trigger apoptosis. J Biol Chem. 2006;281:29703–29710. doi: 10.1074/jbc.M607351200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aziz MH, Wheeler DL, Bhamb B, Verma AK. Protein kinase C δ overexpressing transgenic mice are resistant to chemically but not to UV radiation-induced development of squamous cell carcinomas: a possible link to specific cytokines and cyclooxygenase-2. Cancer Res. 2006;66:713–722. doi: 10.1158/0008-5472.CAN-05-2684. [DOI] [PubMed] [Google Scholar]
- 13.Geiges D, Marks F, Gschwendt M. Loss of protein kinase C delta from human HaCaT keratinocytes upon ras transfection is mediated by TGF alpha. Exp Cell Res. 1995;219:299–303. doi: 10.1006/excr.1995.1231. [DOI] [PubMed] [Google Scholar]
- 14.Denning MF, Dlugosz AA, Howett MK, Yuspa SH. Expression of an oncogenic rasHa gene in murine keratinocytes induces tyrosine phosphorylation and reduced activity of protein kinase C δ. J Biol Chem. 1993;268:26079–26081. [PubMed] [Google Scholar]
- 15.Lu Z, Liu D, Hornia A, Devonish W, Pagano M, Foster DA. Activation of protein kinase C triggers its ubiquitination and degradation. Mol Cell Biol. 1998;18:839–845. doi: 10.1128/mcb.18.2.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Espina V, Wulfkuhle JD, Calvert VS, VanMeter A, Zhou W, Coukos G, Geho DH, Petricoin EF, III, Liotta LA. Laser-capture microdissection. Nat Protoc. 2006;1:586–603. doi: 10.1038/nprot.2006.85. [DOI] [PubMed] [Google Scholar]
- 17.Sikkink SK, Rehman I, Rees JL. Deletion mapping of chromosome 3p and 13q and preliminary analysis of the FHIT gene in human nonmelanoma skin cancer. J Invest Dermatol. 1997;109:801–805. doi: 10.1111/1523-1747.ep12340991. [DOI] [PubMed] [Google Scholar]
- 18.Pierceall WE, Goldberg LH, Tainsky MA, Mukhopadhyay T, Ananthaswamy HN. Ras gene mutation and amplification in human nonmelanoma skin cancers. Mol Carcinog. 1991;4:196–202. doi: 10.1002/mc.2940040306. [DOI] [PubMed] [Google Scholar]
- 19.Henseleit U, Zhang J, Wanner R, Haase I, Kolde G, Rosenbach T. Role of p53 in UVB-induced apoptosis in human HaCaT keratinocytes. J Invest Dermatol. 1997;109:722–727. doi: 10.1111/1523-1747.ep12340708. [DOI] [PubMed] [Google Scholar]
- 20.Breitkreutz D, Boukamp P, Ryle CM, Stark HJ, Roop DR, Fusenig NE. Epidermal morphogenesis and keratin expression in c-Ha-ras-transfected tumorigenic clones of the human HaCaT cell line. Cancer Res. 1991;51:4402–4409. [PubMed] [Google Scholar]
- 21.Dobler M, Schuh J, Kiesewetter F, Schell H, Liehr T, Gebhart E. Deletion monitoring in skin tumors by interphase-FISH using band-specific DNA probes. Int J Oncol. 1999;14:571–576. doi: 10.3892/ijo.14.3.571. [DOI] [PubMed] [Google Scholar]
- 22.Reno EM, Haughian JM, Dimitrova IK, Jackson TA, Shroyer KR, Bradford AP. Analysis of protein kinase C delta (PKC δ) expression in endometrial tumors. Hum Pathol. 2008;39:21–29. doi: 10.1016/j.humpath.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langzam L, Koren R, Gal R, Kugel V, Paz A, Farkas A, Sampson SR. Patterns of protein kinase C isoenzyme expression in transitional cell carcinoma of bladder. Relation to degree of malignancy. Am J Clin Pathol. 2001;116:377–385. doi: 10.1309/1VKK-HWH7-YVJN-7UF7. [DOI] [PubMed] [Google Scholar]
- 24.Varga A, Czifra G, Tallai B, Nemeth T, Kovacs I, Kovacs L, Biro T. Tumor grade-dependent alterations in the protein kinase C isoform pattern in urinary bladder carcinomas. Eur Urol. 2004;46:462–465. doi: 10.1016/j.eururo.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 25.Horovitz-Fried M, Jacob AI, Cooper DR, Sampson SR. Activation of the nuclear transcription factor SP-1 by insulin rapidly increases the expression of protein kinase C delta in skeletal muscle. Cell Signal. 2007;19:556–562. doi: 10.1016/j.cellsig.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 26.Liu J, Yang D, Minemoto Y, Leitges M, Rosner MR, Lin A. NF-κB is required for UV-induced JNK activation via induction of PKCδ. Mol Cell. 2006;21:467–480. doi: 10.1016/j.molcel.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 27.Ponassi R, Terrinoni A, Chikh A, Rufini A, Lena AM, Sayan BS, Melino G, Candi E. p63 and p73, members of the p53 gene family, transactivate PKCδ. Biochem Pharmacol. 2006;72:1417–1422. doi: 10.1016/j.bcp.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 28.Gavrielides MV, Gonzalez-Guerrico AM, Riobo NA, Kazanietz MG. Androgens regulate protein kinase Cδ transcription and modulate its apoptotic function in prostate cancer cells. Cancer Res. 2006;66:11792–11801. doi: 10.1158/0008-5472.CAN-06-1139. [DOI] [PubMed] [Google Scholar]
- 29.Suh KS, Tatunchak TT, Crutchley JM, Edwards LE, Marin KG, Yuspa SH. Genomic structure and promoter analysis of PKC-δ. Genomics. 2003;82:57–67. doi: 10.1016/s0888-7543(03)00072-7. [DOI] [PubMed] [Google Scholar]
- 30.Finco TS, Westwick JK, Norris JL, Beg AA, Der CJ, Baldwin AS., Jr Oncogenic Ha-Ras-induced signaling activates NF-κB transcriptional activity, which is required for cellular transformation. J Biol Chem. 1997;272:24113–24116. doi: 10.1074/jbc.272.39.24113. [DOI] [PubMed] [Google Scholar]
- 31.Mavrothalassitis G, Ghysdael J. Proteins of the ETS family with transcriptional repressor activity. Oncogene. 2000;19:6524–6532. doi: 10.1038/sj.onc.1204045. [DOI] [PubMed] [Google Scholar]
- 32.Alvarez-Salas LM, Benitez-Hess ML, Dipaolo JA. YY-1 and c-Jun transcription factors participate in the repression of the human involucrin promoter. Int J Oncol. 2005;26:259–266. doi: 10.3892/ijo.26.1.259. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


