Abstract
Rationale
Multiple protein kinases have been implicated in cardiovascular disease; however, little is known about the role of their counterparts — the protein phosphatases.
Objective
To test the hypothesis that Mitogen-Activated Protein Kinase Phosphatase-1 (MKP-1) is actively involved in atherogenesis.
Methods and Results
Mice with homozygous deficiency in MKP-1 (MKP-1−/−) were bred with ApoE-deficient mice (ApoE−/−) and the three MKP-1 genotypes (MKP-1+/+ /ApoE−/−; MKP-1+/− /ApoE−/− and MKP-1−/− /ApoE−/−) were maintained on a normal chow diet for 16-week. The three groups of mice exhibited similar body weight and serum lipid profiles; however, both MKP-1+/− and MKP-1−/− mice had significantly less aortic root atherosclerotic lesion formation than MKP-1+/+ mice. Less en face lesion was observed in 8-month old MKP-1−/− mice. The reduction in atherosclerosis was accompanied by decreased plasma levels of IL-1α and TNFα, and preceded by increased anti-inflammatory cytokine IL-10. In addition, MKP-1-null mice had higher levels of plasma SDF-1α, which negatively correlated with atherosclerotic lesion size. Immuno-histochemical analysis revealed that MKP-1 expression was enriched in macrophage-rich areas versus smooth muscle cell regions of the atheroma. Furthermore, macrophages isolated from MKP-1-null mice showed dramatic defects in their spreading/migration and impairment in ERK, but not JNK and p38, pathway activation. In line with this, MKP-1-null atheroma exhibited less macrophage content. Finally, transplantation of MKP-1-intact bone marrow into MKP-1-null mice fully rescued the wild type atherosclerotic phenotype.
Conclusion
These findings demonstrate that chronic deficiency of MKP-1 leads to decreased atherosclerosis via mechanisms involving impaired macrophage migration and defective ERK signalling.
Keywords: Mitogen-activated protein kinases, protein phosphatases, atherosclerosis, macrophage, cell migration
INTRODUCTION
Atherosclerosis is a chronic inflammatory disease involving complex interactions among multiple modified lipoproteins, monocyte-derived macrophages, T lymphocytes, endothelial cells (EC) and smooth muscle cells.1 It is generally believed that endothelial dysfunction is one of the key initiating steps in the pathogenesis of atherosclerosis.1 Specifically, activation of vascular endothelial cells by various stimuli, including oxidatively modified lipoproteins and inflammatory cytokines, increases the expression of adhesion molecules such as E-selectin, VCAM-1 and ICAM-1 on the EC surface, leading to increased rolling, adhesion and trans-migration of monocytes into the vascular wall.2 Infiltrated monocytes then differentiate into macrophages, which produce more inflammatory mediators and become foam cells after uptake of oxidized LDL via scavenger receptors SR-A and CD36.2
The above scenario of atherogenesis requires cell signaling mechanisms involving multiple protein kinases, including the mitogen-activated protein kinases (MAPKs), such as ERK, JNK and p38.3 Although much information has been obtained regarding the roles of various protein kinases in the pathogenesis of atherosclerosis, little is known about the role of their counterparts—protein phosphatases.4 Among the ~147 human protein phosphatases,5 none have been rigorously demonstrated to play a role in atherogenesis, although many have been implicated in cancer.6 A recent study showed that the mitogen-activated protein kinase phosphatase-1 (MKP-1) is required for oxidized LDL-induced monocyte adhesion to vascular endothelial cells.7 In line with this observation, MKP-1 has been shown to be highly expressed in the atherosclerotic lesions of mouse aorta.8 These findings suggest a potential role of MKP-1 in atherogenesis.
MKP-1 belongs to a family of dual-specificity protein phosphatases that differ in their substrate specificity, tissue distribution, inducibility by extracellular stimuli and cellular localization.9 An established function of MKP-1 is inactivating MAPKs by causing dephosphorylation of ERK, JNK and p38 at specific tyrosine and threonine residues.10 MKP-1 is an immediate early gene and its encoding protein is primarily localized to the nucleus.11 It is up-regulated by many factors, including oxidative stress,12 heat shock,12 lipopolysaccharide (LPS)13 and some peptide ligands, such as angiotensin14 and ANP15 in different non-vascular cells. We and others have recently shown that stimulation of vascular endothelial cells with thrombin16, VEGF17 or TNFα29 leads to up-regulation of MKP-1, which plays roles in the transcriptional regulation of pathologically important genes such as PDGF,VCAM-116 and E-selectin,16, 29 and in the control of endothelial cell migration and angiogenesis in vitro.17 In addition, several independent studies have demonstrated that MKP-1 is a negative regulator of acute inflammation by suppression of LPS-induced endotoxic shock in MKP-1-null mice.18–21 In view of these observations, one might expect that MKP-1 deficiency would lead to increased atherosclerosis if in fact MKP-1 is exclusively anti-inflammatory.
The principal aim of the current study was to determine whether MKP-1 is causally involved in the development of experimental atherosclerosis and, if so, to identify the potential underlying cellular mechanism(s). Our findings demonstrate that in ApoE-null mice, MKP-1 deficiency leads to a decrease in atherosclerotic lesion size, which is accompanied by a decrease in inflammatory cytokines in the circulation and by dramatic defects in macrophage functions, including decreased spreading, migration and ERK signaling.
METHODS
An expanded Methods section is available in the Online Data Supplement at http://circres.ahajournals.org.
Animal Procedures
Mice homozygous for inactivation of MKP-1 were intercrossed with the ApoE-deficient mice (Jackson Laboratory) to generate mice heterozygous at both loci. These MKP-1+/− ApoE+/− mice were back-crossed with ApoE−/− mice to produce MKP-1+/− ApoE−/− mice. Subsequently, the MKP-1+/− ApoE−/− offspring were bred to obtain mice with the following three genotypes: MKP-1+/+ ApoE−/−, MKP-1+/− ApoE−/−, and MKP-1−/− ApoE−/−.
Atherosclerotic Lesion Analysis
Because MKP-1-null mice have been reported to be resistant to high fat diet-induced obesity,22 and to exclude the confounding factors of obesity on atherosclerosis formation, we fed mice with normal chow diet and then evaluated atherosclerosis lesion size at aortic sinus and en face entire aorta. The mouse heart and aorta were perfused, dissected, and subjected to quantification of atherosclerosis as previously described.23, 24
PCR Genotyping
Primers: MKP-1 forward-1: 5′-CCAGGTACTGTGTCGGTGGT-GC-3′, MKP-1 forward-2: 5′-TGCCTGCTCTTTACTGAAGGCTC-3′, MKP-1 reverse: 5′-CCTGGCACAATCCTCCTAGAC-3′; ApoE forward-1: 5′-GCCTAGCCGAGGGAGAGCC-G-3′, ApoE forward-2: 5′-TGTGACTTGGGAGCTCTGCAGC-3′, and ApoE reverse: 5′-GCCGCCC-CGACTGCATCT-3′.
Lipid Analysis and Lipoprotein Profile Measurement
Mouse plasma was fractionated by protein liquid chromatography. Cholesterol in the column eluate was combined with Infinity cholesterol reagent (Thermo Electron, Melbourne, Australia) as previously described 25. Areas under the cholesterol elution curve were integrated and indentified as VLDL, IDL, LDL or HDL based on their co-elution with human lipoproteins. Plasma total cholesterol was measured with the same reagent following the manufacturers’ instructions.
Mouse Cytokine/Chemokine Array Assay
Mouse plasma levels of 40 cytokines/chemokines were screened and determined using the “Mouse Cytokine Array Panel A Array Kit” (R&D Systems, Minneapolis), according to the user manual.
Luminex Bead-based Multiplexing Assay
A customized “Mouse Cytokine 6-Plex” kit (LINCOplex, MILLIPORE) was used according to the user manual to quantify interleukin-1α (IL-1α), IL-1β, IL-10, IP-10, MIP-1α, and TNFα levels in mouse plasma.
ELISA Assay
Mouse plasma SDF-1α and IL-10 concentrations were determined using mouse SDF-1 and IL-10 Quantikine ELISA kits (R&D Systems, Minneapolis) according to the respective user manuals.
Immuno-histochemical Analysis
Mouse hearts were sectioned, fixed and processed for antibody staining. The following antibodies were used: anti-MKP-1 (V-15, Santa Cruz., 1:50 dilution); anti-Mac-3 (BD Biosciences, 1:500 dilution); and anti-αsmooth muscle actin (Sigma, 1:500 dilution).
Macrophage Infiltration Assay
Peritoneal macrophages from MKP-1−/−ApoE−/− mice and MKP-1+/+ApoE−/− mice were harvested with 5 mL PBS 3 days after the intra-peritoneal injection of thioglycollate. Cells that had infiltrated the peritoneal area in response to thioglycollate were counted.
Boyden Chamber Cell Migration Assay
Cell migration was performed with a modified Boyden chamber system as previously described.26 Peritoneal macrophages added to the upper chambers of the Boyden chambers were attracted overnight by 10% FBS in the lower chamber, after which the number of cells that migrated to the underside of the membrane were counted.
Cell Adhesion and Spreading Assay
Peritoneal macrophages were seeded into six-well plates. After seeding for 2 h, the number of macrophages attached to the plates was counted and compared between MKP-1−/−ApoE−/− mice and MKP-1+/+ApoE−/− mice. The cell morphology (spreading) difference between wild type and MKP-1-null macrophages was observed after 2 d in culture.
Western Blot Analysis
Peritoneal macrophages were seeded in 6-well plates for 48 h (10% FBS in DMEM), after which the cells were serum-starved (0.1% FBS in DMEM) for 4 h. The cells were then treated with 10% FBS for different time points. Cells were then lysed and standard western blotting was performed as previously described.26
Bone Marrow Transplantation
At 6 weeks of age, ApoE-null and MKP-1/ApoE-double null female mice were lethally irradiated (9 Gy) using a cesium gamma source. Four hours later, 1 × 107 bone marrow cells from the donor mice were injected into the tail veins of the recipient mice. The bone marrow-transplanted mice received normal chow diet for an additional 16 weeks. At the end of 22 weeks, the mice were euthanized and atherosclerotic lesions in the aortic sinus were determined as described above. Animal procedures were performed in a facility accredited by the Association for Assessment and Accreditation of LaboratoryAnimal Care International.
Data Analysis
Data are expressed as means ± S.E.M. Means of two groups were compared using Student’s t-test (unpaired, two-tailed) and one-way ANOVA was used for comparison of more than two groups with p<0.05 considered to be statistically significant.
RESULTS
Metabolic Characteristics of MKP-1+/+ApoE−/−, MKP-1+/−ApoE−/− and MKP-1−/−ApoE−/− Mice
Since MKP-1-null mice are known to be resistant to high fat diet-induced obesity,22 we fed mice normal chow diet to avoid potential confounding factors from differences in body weights. As shown in figure 1, mice of the three genotypes fed with normal chow diet for 16 weeks showed no difference in body weights for males or females (figure 1A and 1B). In addition, the heart weights among the three groups of mice also were not statistically different in males or females (figure 1C and 1D). Serum total cholesterol as well as HDL, LDL, VLDL and IDL levels were nearly identical among the three groups of the male or female mice (figure 1E and 1F). No difference was observed in cholesterol distributions of HDL, LDL, VLDL and IDL among the three groups of male or female mice (data not shown).
Figure 1. Effect of MKP-1 deficiency on metabolic characteristics of ApoE-null mice.
Body (A, B) and heart (C, D) weights in male and female mice fed a normal chow diet for 16-week. Serum level of total cholesterol and its different components were determined as described in “METHODS” for male (E) and female (F) mice fed a normal diet for 16-week. Numbers in columns indicate the No. of mice in that group.
Analysis of Atherosclerosis in MKP-1+/+ApoE−/−, MKP-1+/−ApoE−/− and MKP-1−/−ApoE−/− Mice
After 16 weeks of normal chow diet, aortic sinus and en face assays were performed to evaluate lesion formation in MKP-1 deficient mice. We observed limited atherosclerotic lesion formation using an en face staining approach in the aortic arch, thoracic and abdominal aorta after 16 weeks of normal diet; this was not affected by MKP-1 deficiency (online supplement figure I). However, atherosclerotic lesion size in the aortic sinus was markedly reduced in both the MKP-1+/−ApoE−/− and MKP-1−/−ApoE−/− mice as compared to MKP-1+/+ApoE−/− mice (~50% reduction), but only in the female mice (figure 2B and 2C). To test whether this gender difference was due to a low level of lesion formation in the male ApoE-null control mice, we extended the feeding time to 32 weeks for the male mice. MKP-1-intact male mice at 32 weeks (figure 2D) exhibited almost 10-fold greater lesion size than at 16 weeks (figure 2B) in their aortic sinus. In this set of experiments, compared to controls, we observed an ~50% reduction in lesion size in the MKP-1−/−ApoE−/− mice, but not in the MKP-1+/−ApoE−/− mice (figure 2D). Further analysis of the entire aorta from 8-month old male mice showed a significant reduction (~60%) of en face lesion area in MKP-1−/−ApoE−/− mice compared with MKP-1+/+ApoE−/− mice (figure 2E).
Figure 2. Effect of MKP-1 deficiency on aortic atherosclerosis formation in ApoE-null mice.
Representative Oil red O staining of aortic sinus sections from 16-week-old female mice with indicated genotypes (A). Quantification of atherosclerotic lesion areas in aortic sinus from the 16-week-old male (B) and female (C) mice on the ApoE-null background with indicated MKP-1 genotypes. D, aortic sinus lesion of 8-month-old male mice. E, en face lesion area of entire aorta from 8-month-old male mice, n=7 mice in each group.
Plasma Levels of Cytokines/Chemokines in MKP-1+/+ApoE−/−, MKP-1+/−ApoE−/− and MKP-1−/−ApoE−/− Mice
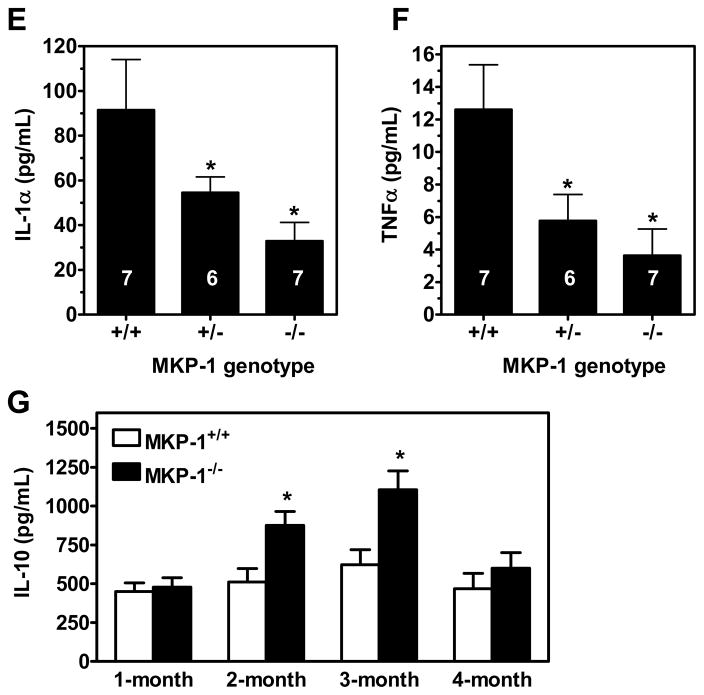
To determine whether the reduced atherosclerotic lesion is associated with less inflammation, we screened 40 cytokines/chemokines in mouse plasma using a micro-array approach. We found that 20 out of the 40 plasma cytokines/chemokines were down-regulated in MKP-1−/−ApoE−/− mice as compared to MKP-1+/+ApoE−/− (figure 3A and online supplement figure II). In MKP-1+/−ApoE−/− mice, many cytokines/chemokines were also down-regulated (35 out of the 40) (figure 3A and online supplement figure II). Notably, there was only one chemokine, SDF-1α, that was expressed at a much higher level in both MKP-1+/−ApoE−/− mice and MKP-1−/−ApoE−/− mice than in the control MKP-1+/+ApoE−/− mice (figure 3A and online supplement figure II). To confirm this observation, we used an ELISA assay to quantify the plasma levels of SDF-1. We found that the plasma SDF-1 level was nearly 3-fold higher in MKP-1+/−ApoE−/− and MKP-1−/−ApoE−/− mice than in the control mice in the female but not male groups (figure 3B and 3C). Analysis of 32 plasma samples from the 3 groups of mice at 16-week revealed that the plasma level of SDF-1 was negatively correlated with atherosclerotic lesion size (figure 3D). Luminex bead-based multiplexing assay showed that the absence of MKP-1 decreased plasma levels of IL-1α and TNFα (figure 3E and 3F), but not IP-10, MIP-1α and IL-1β (online supplement figure III). Interestingly, MKP-1 deficiency did not change the level of anti-inflammatory cytokine IL-10 at 4-month, but IL-10 levels were significantly higher at 2- & 3-month in MKP-1-null mice as compared to MKP-1-intact mice (figure 3G).
Figure 3. MKP-1 deficiency alters plasma levels of chemokines/cytokines in ApoE-null mice.
The relative levels of 40 chemokines/cytokines were tested by a micro-array approach using plasma samples pooled from 3 female mice of each genotype (A). Plasma levels of SDF-1α were determined in male (B) and female (C) mice by ELISA assay. The relationship between plasma SDF-1α level and atherosclerotic lesion area was determined by a correlation analysis (D). The plasma levels of IL-1α (E) and TNFα (F) were determined by a Luminex bead-based multiplexing assay. Numbers in columns indicate the No. of mice in that group. Plasma levels of IL-10 (G) in ApoE-null mice of indicated ages were determined by ELISA, n=6 mice in each group. *, p<0.05 to respective controls.
MKP-1 Expression in Atherosclerotic Lesions
To determine the localization of MKP-1 protein in the mouse aortic sinus, we performed an Immuno-histochemical study. We found that MKP-1 protein expression was enriched in the atheroma of 16-week old ApoE-null mice (figure 4) while virtually no staining was observed in MKP-1-null mice (online supplement figure IV), indicating the specificity of the MKP-1 antibody. Figure 4 also shows that MKP-1 protein expression was highly concentrated in the macrophage-rich (Mac-3 staining), versus smooth muscle-rich (SMC actin staining) regions, suggesting a potential link between macrophage function and MKP-1-defiency-mediated attenuation of atherosclerosis.
Figure 4. MKP-1 expression in atherosclerotic plaque and macrophages.
Immuno-histochemical staining of MKP-1 protein in aortic sinus sections of representative 16-week-old ApoE-null mice. Macrophage-rich area was indicated by Mac-3 staining and SMC actin staining revealed smooth muscle-rich areas. The green arrows point to the localization of atheroma, MKP-1 protein and macrophage-rich versus smooth muscle-rich areas, respectively.
Macrophage Function of MKP-1−/−ApoE−/− Mice versus MKP-1+/+ApoE−/− Mice
To assess possible functional defects in macrophages from MKP-1-null mice, we focused on cell adhesion and migration since we have recently shown that MKP-1 deficiency leads to decreased vascular endothelial cell migration.17 Figure 5A shows that macrophages isolated from MKP-1−/−ApoE−/− mice exhibited much less spreading capacity than macrophages from MKP-1+/+ApoE−/− mice. However, macrophage adhesion at 2 h was not different between these two genotypes (data not shown). Because cell spreading is highly related to cell migration, we further examined macrophage migration in vitro and in vivo. Figure 5C shows that peritoneal injection of thioglycollate for 3 days elicited macrophage infiltration to the peritoneum with mean cell number 3.3 × 106/mL in MKP-1+/+ApoE−/− mice, which was reduced to 1.8 × 106/mL in MKP-1−/−ApoE−/− mice. To further confirm a defect in macrophage migration in MKP-1−/−ApoE−/− mice, we isolated peritoneal macrophages and seeded them into the Boyden chamber system to test macrophage migration in vitro. Figure 5B shows that the number of MKP-1-null macrophages migrating toward serum was significantly less than the number of MKP-1-intact macrophages. In line with these observations, we further found that MKP-1 deficiency led to decreased macrophage content in the atheroma as evidenced by reduced Mac-3 staining (figure 6).
Figure 5. MKP-1 deficiency affects macrophage spreading and migration.
Macrophage spreading capacity was observed by cell morphology after 2 d in culture (A). Macrophage migration in vitro in response to 10% FBS was determined using a Boyden chamber system (B); left panel are representative pictures of Boyden chamber membranes containing migrated cells (B). The number of thioglycollate-elicited macrophages in the peritoneum was counted and compared between the indicated groups of mice (C). Numbers in columns indicate the No. of mice in that group. *, p<0.05 to respective controls.
Figure 6. Macrophage content in atheroma of MKP-1-intact versus MKP-1-null mice.
Macrophage accumulation in the aortic sinus of indicated genotype was examined by Immunohistochemical staining of Mac-3. Brown color in representative images (A) stands for Mac-3 positive macrophages. Relative macrophage numbers in the plaque areas of indicated mice (n=5 in each group) were quantified (B). *, p<0.05 vs. control.
Activation of MAPK Pathways in Macrophages of MKP-1−/−ApoE−/− Mice versus MKP-1+/+ApoE−/− Mice
We compared the time courses of MAPK pathway activation, including ERK, JNK and p38, in macrophages with or without MKP-1 in the ApoE-null background. Figure 7 shows that macrophages isolated from MKP-1−/− and ApoE−/− mice exhibited no serum stimulation of the ERK pathway, whereas ERK was transiently activated by serum as expected in macrophages from MKP-1+/+ and ApoE−/− mice. No significant difference in the kinetics of either JNK or p38 activation by serum between MKP-1+/+ and MKP-1−/− macrophages was observed (figure 7A). To assess the connection between defective ERK signaling and impaired macrophage migration, peritoneal macrophages were pretreated with U0126, a selective MEK1/2 inhibitor, and then stimulated with serum. Figure 7B shows that the ERK pathway inhibitor decreased serum-induced migration of MKP-1-intact cells, with a negligible effect on MKP-1-null cells, suggesting that defective ERK signaling may be one of the mechanisms responsible for impaired cell migration.
Figure 7. MAPKs activation in macrophage of MKP-1-intact versus MKP-1-null mice with ApoE-null background.
The time courses of ERK, JNK and p38 phosphorylation in response to serum were determined by western blotting in macrophages of female mice fed normal chow diet for 16-week. Data are expressed as fold increase over control after normalization to the respective controls. The experiment was repeated 3 times (3 mice in each genotype) with similar results (A). The effect of U0126 on migration capacity of MKP-1-intact versus MKP-1-null macrophages was determined using a Boyden chamber system (B). *, p<0.05 vs. respective controls.
Analysis of Atherosclerotic Lesions in Mice Having Undergone Bone Marrow Transplantation
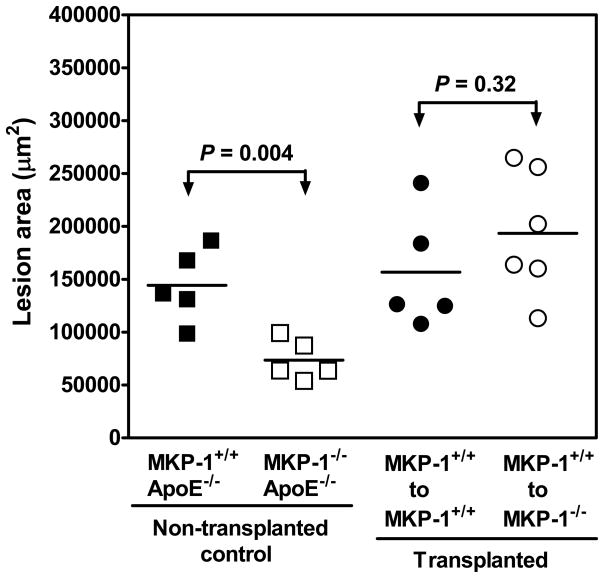
Since a recent study showed a potential role of endothelial MKP-1 in controlling adhesion molecule expression,28 bone marrow transplantation was employed to determine the contribution of MKP-1 in blood cells to the development of atherosclerosis. Figure 8 shows that in non-transplanted age-matched (22 weeks) control female mice, the lack of the MKP-1 gene led to a ~50% reduction in aortic sinus atherosclerotic lesion formation as expected. The lesser atherosclerotic lesion phenotype in the MKP-1-null mice was fully restored to the level of MKP-1-intact mice by transplanting MKP-1-intact bone marrow into the MKP-1-null mice at 6-week (maintained for additional 16 weeks, figure 8). These results suggest that macrophage MKP-1 may be more important than endothelial MKP-1 in optimal initiation and progression of atherosclerosis.
Figure 8. Contribution of MKP-1 in bone marrow-derived cells to atherosclerotic lesion formation.
Female MKP-1+/+ /ApoE−/− and MKP-1−/− /ApoE−/− mice were lethally irradiated at 6-week, and transplanted with donor bone marrow (MKP-1+/+ /ApoE−/−) as indicated. Mice were continuously fed with normal chow diet for additional 16 weeks after bone marrow transplantation, after which they were sacrificed for evaluation of atherosclerotic lesion size in the aortic sinus. Age-matched (22 weeks) female mice served as non-transplanted controls.
DISCUSSION
In the present study, we show for the first time that genetic deletion of the MKP-1 gene reduced atherosclerosis in ApoE-null mice fed with normal chow diet. We also found that MKP-1 deficiency led to decreased plasma levels of pro-inflammatory cytokines IL-1a and TNFa, preceded by increased anti-inflammatory cytokine IL-10. In addition, we unexpectedly observed that the absence of MKP-1 yielded a “normal” plasma level of SDF-1 in ApoE-null mice, and that SDF-1 levels negatively correlated with atherosclerotic lesion size. Furthermore, we demonstrated that MKP-1 deficiency prevented macrophage spreading in culture and attenuated migration in vitro and in vivo. Finally, we found a macrophage signaling defect in the ERK MAPK pathway in the absence of MKP-1.
MKP-1 is highly expressed in atheroma 8 and is required for monocyte adhesion to cultured endothelial cells activated by oxidized LDL.7 However, it has not been determined whether lesional MKP-1 is a potential therapeutic target, i.e., is increased MKP-1 part of a compensatory mechanism limiting lesion development or is it fundamentally pro-atherogenic? The results of the current study reveal that loss of MKP-1 results in a favorable outcome in an animal model of atherosclerosis. Thus, in the absence of MKP-1, mice on the ApoE-null background on a normal chow diet for 16 weeks (32 weeks for male mice) had a 50% reduction in lesion size compared with ApoE-null mice with intact MKP-1. This is in spite of similarly elevated levels of total cholesterol and comparable levels of LDL and HDL in the two groups of mice. It is also striking to see that even deletion of one copy of the MKP-1 gene led to a similar degree of reduction in atherosclerosis compared with MKP-1-null homozygous mice, at least in females, suggesting that MKP-1 may be a vital molecule for maximal progression of atherosclerosis. On the other hand, we found that MKP-1-intact ApoE-null mice fed with normal chow diet for 16 weeks exhibited limited aortic en face lesion (less than 1.0%), which was not statistically different from the MKP-1-null mice. Though this result differs from our observations in the aortic sinus and we are aware that not all sites of the aorta show the same degree of lesion development,30 it should be noted that normal chow diet for 16 weeks is of limited value for assessing a potential anti-atherogenic effect on the entire aorta because of the low number and small size of lesions in the control mice. This notion is supported by our finding that when significant aortic lesions (~7.0%) were developed at 8-month time point on normal chow diet, MKP-1 deficiency led to ~60% reduction of en face lesion area of the entire aorta. Overall, these results clearly indicate that lack of MKP-1 prevents atherosclerosis not only in aortic sinus but also in the entire aorta.
Multiple risk factors are implicated in the pathogenesis of atherosclerosis, including metabolic abnormalities, such as hyperlipidemia, obesity and diabetes.1, 2 In this study, we did not find significant difference in the lipid profile between MKP-1-null and MKP-1-intact mice in the ApoE-deficient background, suggesting that reduced atherosclerosis caused by MKP-1 deficiency is not due to a change in lipid metabolism. This is further supported by the fact that MKP-1 deletion did not change the body and heart weights in the ApoE-null mice. It should be noted, however, that mice lacking MKP-1 are resistant to high fat diet-induced obesity.22 This apparent discrepancy could be explained by multiple differences in experimental conditions, including normal chow diet vs. high fat diet, young vs. old mice, and ApoE-null vs. ApoE-intact background.
It is well established that atherosclerosis is a chronic inflammatory disease.1, 2 In line with this concept, our current study showed that loss of MKP-1 led to decreases of multiple pro-inflammatory cytokines in MKP-1-null mice, including TNFa and IL-1a, whereas the anti-inflammatory cytokine IL-10 was increased at 2- & 3-month, but not in the 4-month. These results suggest that MKP-1 deletion leads to up-regulation of anti-inflammatory cytokine during atherosclerosis initiation phase, and may explain in part decreased expression of pro-inflammatory cytokine. These data are closely associated and consistent with the reduced atherosclerosis results. However, this result does differ from other studies. For example, several studies showed that MKP-1 deletion render mice more vulnerable to LPS-induced endotoxic shock and death, which was accompanied by up-regulation of pro-inflammatory cytokines such as TNFα and IL-1β in serum.18–21 The exact mechanism(s) responsible for this apparent discrepancy remains to be determined, however, a key difference between the current study and the prior studies is the use of mice lacking ApoE. Total serum cholesterol was about 4-fold higher in these mice versus wild type mice, and this pathological level of cholesterol and its derived oxidized lipids may be considered chronic inflammatory stimuli on the mice. In addition, we measured the cytokine/chemokine level at 16-week, whereas prior studies tested within hours or less than 2 days after LPS injection.18–21 Thus, it is conceivable that whether MKP-1 deficiency leads to a pro-inflammatory or an anti-inflammatory phenotype may depend on the experimental model used, particularly acute versus chronic models. Indeed, Chi et al. have shown that LPS challenge of the MKP-1-null mice first led to an acute increase of the pro-inflammatory cytokine TNFα, but later, TNFα decreased and the anti-inflammatory cytokine IL-10 became dominant.18 We believe this interpretation may also explain the disparity between the current study and a recent study in an acute inflammation model by Zakkar et al., who showed that endothelial MKP-1 suppresses proinflammatory gene activation at sites in the aorta that are resistant to atherosclerosis.28 The finding by Zakkar et al. would suggest the possibility that aortic atherosclerosis in the MKP-1-null mice could be increased due to increased adhesion molecule expression. It should be noted that Zakkar et al. used ApoE-intact mice without assessment of atherosclerotic lesions, and they determined endothelial adhesion molecule expression within just several hours of LPS injection.28 Although we did not measure endothelial adhesion molecule expression in our animal model, our study showed that transplantation of MKP-1-intact bone marrow into MKP-1-null mice fully rescued the wild type atherosclerotic phenotype, suggesting that the macrophage MKP-1 may be more important than the endothelial MKP-1 in optimal formation of atherosclerotic lesions.
Another intriguing finding of this study is that MKP-1 deficiency appears to maintain a normal plasma level of SDF-1a in female ApoE-null mice, and more importantly, the plasma level of SDF-1a was negatively correlated with the lesion size of atherosclerosis. These results are consistent with a report showing that in healthy men, the average plasma level of SDF-1a is about 3.5 ng/ml, whereas it is less than 1.0 ng/ml in patients with unstable angina pectoris, a complication of advanced atherosclerotic lesion/plaque rupture.27 Although the exact role of SDF-1a in atherogenesis is not well understood and beyond the scope of the current study, our finding, together with the reports of others, point to a role for SDF-1a in the pathogenesis of atherosclerosis. Furthermore, plasma SDF-1a levels may represent a new prognostic factor for atherosclerosis. SDF-1a is primarily expressed in pancreas and liver, with low expression in vascular smooth muscle and endothelial cells.27 Thus, it is reasonable to see a relatively high level of circulating SDF-1a in blood in physiological condition. We propose that circulating SDF-1a may be athero-protective by keeping leukocytes inside the bloodstream, preventing them from transmigrating into the tissues/organs. In addition, SDF-1a may also have some protective effects on endothelium.
It is well known that early stage fatty lesions are predominantly composed of lipid-enriched macrophages differentiated from infiltrated monocytes.1, 2 Our results showed that MKP-1 was mainly expressed in the macrophage-rich, rather than smooth muscle-rich, areas of the aortic sinus. This observation led us to propose that macrophage functions may be altered in the MKP-1-null mice. Evidence supporting this hypothesis include: (1) macrophages isolated from MKP-1-null mice showed a defect in their spreading capacity when compared to macrophages from control mice; (2) macrophage infiltration into the peritoneum in response to thioglycollate was decreased about 50% in MKP-1-null mice compared with MKP-1-intact mice; (3) our Boyden chamber cell migration study showed that the number of MKP-1-null macrophages migrating toward serum was significantly less than the number of MKP-1-intact macrophages; (4) we found that the ERK MAPK signaling pathway was impaired in MKP-1-null macrophages; and (5) importantly, we confirmed that macrophage content was much lower than that in the controls, and that ERK pathway inhibition decreased the migration capacity of MKP-1-intact cells to the level of MKP-1-null cells. These results strongly suggest that our observed reduction in atherosclerotic lesions in MKP-1-null mice is, at least in part, due to defective spreading and migration of macrophages. Of note, a similar defective migration capacity was observed in MKP-1-null vascular EC17 and more recently reported in MKP-1-null lymphocytes.31 In addition, our finding that transplantation of MKP-1-intact bone marrow into the MKP-1-null mice fully rescued the wild type atherosclerotic phenotype provided another line of strong evidence in support of our contention. The molecular basis of defective ERK signaling in MKP-1-null mice needs to be explored in the future. It is plausible that deletion of MKP-1 in a chronic inflammation condition, such as ApoE-null background, may compensatorily up-regulate other phosphatase(s) that selectively interfere with the ERK pathway. Also, we cannot exclude the possibility of an involvement of histone H3, which recently was identified as a new substrate for MKP-1.32
In summary, we report the first evidence that in ApoE-null mice fed normal chow diet, the absence of MKP-1 leads to reduced atherosclerotic lesion formation, which is accompanied by a decreased expression of multiple cytokines, including TNFa and IL-1a. In addition, MKP-1 deficiency maintains a normal plasma level of SDF-1a, which is negatively associated with the degree of atherosclerosis. Furthermore, our results suggest that defective macrophage migration and ERK signaling underlie MKP-1-deficiency-mediated reduction in atherosclerosis. These findings highlight a new view that protein phosphatases may be as important as protein kinases in the pathogenesis of atherosclerosis.
NOVELTY AND SIGNIFICANCE
What is known?
Several protein kinases have been shown to be s involved in experimental atherogenesis, but the roles of protein phosphatases are less well known
MKP-1 has been shown as a negative regulator of MAPK signaling and acute inflammation; and
MKP-1 deletion leads to impaired endothelial cell migration.
What new information does this article contribute?
This study shows for the first time that the prototype phosphatase, MKP-1, is actively involved in atherogenesis through a macrophage based mechanism;
In a model of chronic inflammationl, MKP-1 acts as a positive regulator of MAPK signaling and inflammation;
MKP-1 deficiency impairs macrophage adhesion and migration..
Although many studies show involvement of protein kinases in the formation of atherosclerosis, less is known about protein phosphatases. Using mice deficient in both MKP-1 and ApoE, we found that: 1) genetic deletion of the prototype phosphatase MKP-1 decreases aortic atherosclerosis; 2) contrary to previous work, lack of MKP-1 in this chronic inflammation model (ApoE-null background) result in the development of an anti-inflammatory phenotype; 3) MKP-1 deficiency impaires macrophage migration and infiltration; and interestingly, 4) ERK1/2 signaling pathway is attenuated in MKP-1-null macrophages. These findings demonstrate that MKP-1 is a positive regulator of inflammation in vivo and cell signaling pathways that are causally involved in the etiology of atherosclerosis. These findings demonstrate the importance of regulating protein phosphatases in atherogenesis and defined MKP-1 as a potential novel drug target for atherosclerosis therapy.
Supplementary Material
Acknowledgments
The authors would like to thank Julie Baglione and Lana Pollock, and Zhiping Chen for their excellent technical assistance.
Sources of Funding: This study was supported by NIH grant HL29582 (P.E.D.) and the Morganthaler Postdoctoral Fellowship (J.S.). A presentation ofsome of this research was supported by a Junior Investigator Award from the North American Vascular Biology Organization to the 2008 EB meeting in San Diego
Non-standard Abbreviations and Acronyms
- ApoE
apolipoprotein E
- MKP-1
Mitogen-activated protein kinase phosphatase-1
- EC
endothelial cells
- LDL
low-density lipoprotein
- ERK
extracellular signal-regulated kinases
- MAPK
mitogen-activated protein kinases
- SDF-1
stromal cell derived factor-1
Footnotes
Disclosures: None.
References
- 1.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 3.Senokuchi T, Matsumura T, Sakai M, Matsuo T, Yano M, Kiritoshi S, Sonoda K, Kukidome D, Nishikawa T, Araki E. Extracellular signal-regulated kinase and p38 mitogen-activated protein kinase mediate macrophage proliferation induced by oxidized low-density lipoprotein. Atherosclerosis. 2004;176:233–245. doi: 10.1016/j.atherosclerosis.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 4.DiCorleto PE. Protein tyrosine phosphatases in the vessel wall: counterpoint to the tyrosine kinases. Arterioscler Thromb Vasc Biol. 2000;20:1179–1181. doi: 10.1161/01.atv.20.5.1179. [DOI] [PubMed] [Google Scholar]
- 5.Moorhead GB, Trinkle-Mulcahy L, Ulke-Lemée A. Emerging roles of nuclear protein phosphatases. Nat Rev Mol Cell Biol. 2007;8:234–244. doi: 10.1038/nrm2126. [DOI] [PubMed] [Google Scholar]
- 6.Alonso A, Sasin J, Bottini N, Friedberg I, Friedberg I, Osterman A, Godzik A, Hunter T, Dixon J, Mustelin T. Protein tyrosine phosphatases in the human genome. Cell. 2004;117:699–711. doi: 10.1016/j.cell.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 7.Reddy S, Hama S, Grijalva V, Hassan K, Mottahedeh R, Hough G, Wadleigh DJ, Navab M, Fogelman AM. Mitogen-activated protein kinase phosphatase 1 activity is necessary for oxidized phospholipids to induce monocyte chemotactic activity in human aortic endothelial cells. J Biol Chem. 2001;276:17030–17035. doi: 10.1074/jbc.M011663200. [DOI] [PubMed] [Google Scholar]
- 8.Reddy ST, Nguyen JT, Grijalva V, Hough G, Hama S, Navab M, Fogelman AM. Potential role for mitogen-activated protein kinase phosphatase-1 in the development of atherosclerotic lesions in mouse models. Arterioscler Thromb Vasc Biol. 2004;24:1676–1681. doi: 10.1161/01.ATV.0000138342.94314.64. [DOI] [PubMed] [Google Scholar]
- 9.Farooq A, Zhou MM. Structure and regulation of MAPK phosphatases. Cell Signal. 2004;16:769–779. doi: 10.1016/j.cellsig.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Dickinson RJ, Keyse SM. Diverse physiological functions for dual-specificity MAP kinase phosphatases. J Cell Sci. 2006;119:4607–4615. doi: 10.1242/jcs.03266. [DOI] [PubMed] [Google Scholar]
- 11.Sun H, Charles CH, Lau LF, Tonks NK. MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell. 1993;75:487–493. doi: 10.1016/0092-8674(93)90383-2. [DOI] [PubMed] [Google Scholar]
- 12.Keyse SM, Emslie EA. Oxidative stress and heat shock induce a human gene encoding a protein-tyrosine phosphatase. Nature. 1992;359:644–647. doi: 10.1038/359644a0. [DOI] [PubMed] [Google Scholar]
- 13.Stawowy P, Goetze S, Margeta C, Fleck E, Graf K. LPS regulate ERK1/2-dependent signaling in cardiac fibroblasts via PKC-mediated MKP-1 induction. Biochem Biophys Res Commun. 2003;303:74–80. doi: 10.1016/s0006-291x(03)00301-2. [DOI] [PubMed] [Google Scholar]
- 14.Liu PQ, Lu W, Wang TH, Pan JY. MKP-1 regulates the cardiomyocyte hypertrophic responses induced by angiotensin II. Sheng Li Xue Bao. 2000;52:365–370. [PubMed] [Google Scholar]
- 15.Sugimoto T, Haneda M, Togawa M, Isono M, Shikano T, Araki S, Nakagawa T, Kashiwagi A, Guan KL, Kikkawa R. Atrial natriuretic peptide induces the expression of MKP-1, a mitogen-activated protein kinase phosphatase, in glomerular mesangial cells. J Biol Chem. 1996;271:544–547. doi: 10.1074/jbc.271.1.544. [DOI] [PubMed] [Google Scholar]
- 16.Chandrasekharan UM, Yang L, Walters A, Howe P, DiCorleto PE. Role of CL-100, a dual specificity phosphatase, in thrombin-induced endothelial cell activation. J Biol Chem. 2004;279:46678–46685. doi: 10.1074/jbc.M406441200. [DOI] [PubMed] [Google Scholar]
- 17.Kinney CM, Chandrasekharan UM, Mavrakis L, DiCorleto PE. VEGF and thrombin induce MKP-1 through distinct signaling pathways: role for MKP-1 in endothelial cell migration. Am J Physiol Cell Physiol. 2008;294:C241–250. doi: 10.1152/ajpcell.00187.2007. [DOI] [PubMed] [Google Scholar]
- 18.Chi H, Barry SP, Roth RJ, Wu JJ, Jones EA, Bennett AM, Flavell RA. Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc Natl Acad Sci U S A. 2006;103:2274–2279. doi: 10.1073/pnas.0510965103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salojin KV, Owusu IB, Millerchip KA, Potter M, Platt KA, Oravecz T. Essential role of MAPK phosphatase-1 in the negative control of innate immune responses. J Immunol. 2006;176:1899–1907. doi: 10.4049/jimmunol.176.3.1899. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Q, Wang X, Nelin LD, Yao Y, Matta R, Manson ME, Baliga RS, Meng X, Smith CV, Bauer JA, Chang CH, Liu Y. MAP kinase phosphatase 1 controls innate immune responses and suppresses endotoxic shock. J Exp Med. 2006;203:131–140. doi: 10.1084/jem.20051794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammer M, Mages J, Dietrich H, Servatius A, Howells N, Cato AC, Lang R. Dual specificity phosphatase 1 (DUSP1) regulates a subset of LPS-induced genes and protects mice from lethal endotoxin shock. J Exp Med. 2006;203:15–20. doi: 10.1084/jem.20051753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu JJ, Roth RJ, Anderson EJ, Hong EG, Lee MK, Choi CS, Neufer PD, Shulman GI, Kim JK, Bennett AM. Mice lacking MAP kinase phosphatase-1 have enhanced MAP kinase activity and resistance to diet-induced obesity. Cell Metab. 2006;4:61–73. doi: 10.1016/j.cmet.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Baglione J, Smith JD. Quantitative assay for mouse atherosclerosis in the aortic root. Methods Mol Med. 2006;129:83–95. doi: 10.1385/1-59745-213-0:83. [DOI] [PubMed] [Google Scholar]
- 24.Chandrasekharan UM, Mavrakis L, Bonfield TL, Smith JD, DiCorleto PE. Decreased atherosclerosis in mice deficient in tumor necrosis factor-alpha receptor-II (p75) Arterioscler Thromb Vasc Biol. 2007;27:e16–17. doi: 10.1161/01.ATV.0000255551.33365.22. [DOI] [PubMed] [Google Scholar]
- 25.Garber DW, Kulkarni KR, Anantharamaiah GM. A sensitive and convenient method for lipoprotein profile analysis of individual mouse plasma samples. J Lipid Res. 2000;41:1020–1026. [PubMed] [Google Scholar]
- 26.Shen J, DiCorleto PE. ADP stimulates human endothelial cell migration via P2Y1 nucleotide receptor-mediated mitogen-activated protein kinase pathways. Circ Res. 2008;102:448–456. doi: 10.1161/CIRCRESAHA.107.165795. [DOI] [PubMed] [Google Scholar]
- 27.Damås JK, Waehre T, Yndestad A, Ueland T, Müller F, Eiken HG, Holm AM, Halvorsen B, Frøland SS, Gullestad L, Aukrust P. Stromal cell-derived factor-1a in unstable angina: potential antiinflammatory and matrix-stabilizing effects. Circulation. 2002;106:36–42. doi: 10.1161/01.cir.0000020001.09990.90. [DOI] [PubMed] [Google Scholar]
- 28.Zakkar M, Chaudhury H, Sandvik G, Enesa K, Luong le A, Cuhlmann S, Mason JC, Krams R, Clark AR, Haskard DO, Evans PC. Increased endothelial mitogen-activated protein kinase phosphatase-1 expression suppresses proinflammatory activation at sites that are resistant to atherosclerosis. Circ Res. 2008;103:726–32. doi: 10.1161/CIRCRESAHA.108.183913. [DOI] [PubMed] [Google Scholar]
- 29.Wadgaonkar R, Pierce JW, Somnay K, Damico RL, Crow MT, Collins T, Garcia JG. Regulation of c-Jun N-terminal kinase and p38 kinase pathways in endothelial cells. Am J Respir Cell Mol Biol. 2004;31:423–31. doi: 10.1165/rcmb.2003-0384OC. [DOI] [PubMed] [Google Scholar]
- 30.VanderLaan PA, Reardon CA, Getz GS. Site specificity of atherosclerosis: site-selective responses to atherosclerotic modulators. Arterioscler Thromb Vasc Biol. 2004;24:12–22. doi: 10.1161/01.ATV.0000105054.43931.f0. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Reynolds J, Chang SH, Martin-Orozco N, Chung Y, Nurieva RI, Dong C. MAP kinase phosphatase 1 is necessary for T cell activation and function. J Biol Chem. 2009;284:30815–24. doi: 10.1074/jbc.M109.052472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kinney CM, Chandrasekharan UM, Yang L, Shen J, Kinter M, McDermott MS, DiCorleto PE. Histone H3 as a novel substrate for MAP kinase phosphatase-1. Am J Physiol Cell Physiol. 2009;296:C242–9. doi: 10.1152/ajpcell.00492.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.