SUMMARY
Coordinated apical constriction (AC) in epithelial sheets drives tissue invagination [1, 2] and is required for diverse morphogenetic movements such as gastrulation [3], neurulation [4, 5] and organogenesis [6]. We showed previously that actomyosin contractility drives AC in Xenopus bottle cells [7]; however, it remained unclear whether it does so in concert with other processes. Here, we report that endocytosis-driven membrane remodeling is required for efficient AC. We found endosomes exclusively in bottle cells in the early gastrula. Disrupting endocytosis with dominant negative Dynamin or Rab5 perturbed AC, with a significant decrease in constriction rate late in the process, suggesting that endocytosis operates downstream of actomyosin contractility to remove excess membrane. Additionally, disrupting endocytosis during neurulation inhibits AC in hingepoint cells, resulting in neural tube closure defects. Thus, membrane remodeling during AC could be a general mechanism to achieve efficient invagination in embryos.
RESULTS AND DISCUSSION
Endosomes are present only in bottle cells and traffic along microtubules
In Xenopus laevis embryos, cells in the dorsal marginal zone (DMZ) undergo apical constriction (AC) to form bottle cells, initiating blastopore invagination [8, 9]. Previously, we showed that taxol, which stabilizes microtubules, does not affect bottle cell formation, whereas nocodazole, a microtubule depolymerizing agent, disrupts AC; therefore microtubules must be intact, but need not be dynamic for AC [7]. As microtubules are necessary for vesicular trafficking [10], we hypothesized that microtubules may facilitate AC by functioning as tracks for endosomes.
To determine whether endocytosis occurs in bottle cells, we employed a cell impermeable activated-biotin that was previously used to surface label Xenopus embryos [11, 12]; subsequently, internalized membrane can be visualized with fluorescent streptavidin. We observed biotin-labeled vesicles exclusively in bottle cells (Figure 1A), starting in stage 10 gastrulae (data not shown); therefore, the appearance of vesicles accompanies the onset of constriction.
Figure 1.
Biotinylated vesicles are present only in bottle cells in Xenopus early gastrula stage embryos. (A) Vegetal view (left, epifluorescence), midsagittal section (center, confocal), and higher magnification of midsagittal section (right) of Stage 10 embryos labeled with NHS-LC-sulfo biotin and anti-DM1α (tubulin). Arrow indicates bottle cells. Scale bar = 25μm. (B) Confocal midsagittal sections of embryos stained with anti-EEA1 and biotin. (C) Confocal midsagittal sections of embryos injected with Rab5 EGFP mRNA, then fixed and stained with anti-GFP and biotin. Arrow(s) in B and C indicate colocalization of biotin with early endosome markers. (D) Confocal midsagittal sections of embryos following pulse-chase labeling with biotin. Embryos were fixed at the indicated timepoints and stained with anti-DM1α and streptavidin. Arrows indicate endosomes that appear to be on or near the basolateral membrane. In all midsagittal sections, images are oriented vegetal to the lower left; scale bars for B,C,D = 50μm. See also Figure S1.
To confirm that biotinylated vesicles represented endosomes, we used endosome markers EEA1 [13] and Rab5-EGFP [14]; labeled vesicles co-localized with both markers, confirming that they are endosomes (Figures 1B and 1C). We therefore exploited the specificity and low background of biotinylation to assay endocytosis.
To determine where endosomes traffic in bottle cells, we labeled stage 9 embryos with biotin, quenched the reaction, and fixed the embryos at 30-minute intervals (“chase”). As cells underwent AC, the number of endosomes also increased, strengthening the correlation between endocytosis and AC. Endosomes trafficked away from the apical membrane and some vesicles colocalized with the basolateral membrane (Figure 1D).
Previously, we showed that intact but not dynamic microtubules were required for AC [7]. If intact microtubules function as tracks for endosomes, then nocodazole treatment should prevent endocytosis, whereas taxol treatment should not. Indeed, in nocodazole-treated embryos, endosomes remained at the apical membrane (Figure S1). In contrast, taxol-treated embryos exhibited internalized vesicles indistinguishable from controls (Figure S1). Our data confirm that intact microtubules are required for endocytosis and show that endosomes move towards the basolateral membrane in bottle cells.
Perturbing endocytosis disrupts AC
As intact microtubules are required for both AC [7] and endocytosis (Figure S1), we postulated that endocytosis could be required for AC. To test this, we perturbed the initial step of endocytosis with dominant negative (DN) Dynamin, which weakly binds GTP, preventing endosome scission [15, 16]. Targeting DN-Dynamin to the DMZ perturbed blastopore formation (Figure 2A) but did not affect cell morphology when expressed in non-DMZ cells (Figure S2A). Despite the significant effect on AC (see below) and delay in blastopore formation, DN- Dynamin-injected embryos (referred to as DN-Dynamin embryos) developed with no obvious defects (Figure S2F). This is consistent with the finding that development occurs normally even after surgical removal of bottle cells [9].
Figure 2.
Dominant Negative (DN) Dynamin and DN Rab5 disrupt bottle cell formation by affecting apical constriction. (A) Epifluorescence images of stage 10.5 embryos, vegetal views. All embryos were injected with membrane-EGFP mRNA, plus DN Dynamin, WT Dynamin, or WT + DN. Arrows point to blastopore forming in the DMZ. Scale bar = 250μm. (B) Confocal midsagittal sections of embryos, all injected with membrane-EGFP, plus DN, WT, or WT + DN. Embryos were stained with anti-GFP and streptavidin. Scale bar = 50μm. (C) Quantification of blastopore depth and bottle cell morphometrics in DN Dynamin-injected embryos. *, p<0.01; **, p<0.001; ‡, p<0.05 for GFP versus rescue, p<0.001 for DN versus rescue. Each bar represents the mean of five experiments. For all graphs, error bars represent ± SEM. (D) Confocal midsagittal sections of uninjected control embryos or embryos injected with DN Rab5 mRNA and stained with anti-DM1α tubulin and streptavidin. Scale bar = 50μm. Bottom panels (D’) show higher magnification of biotin-labeled membrane. Scale bar = 10μm. (E) Quantification of blastopore depth (n=88 control embryos; 53 DN Rab5 embryos) and bottle cell morphometrics (n=187 control cells; 113 DN Rab5 cells). *, p<0.001. Each bar represents the mean of six experiments. See also Figures S2 and S3.
To confirm that DN-Dynamin disrupts endocytosis, we counted endosomes in GFP and DN-Dynamin embryos. DN-Dynamin embryos had significantly fewer endosomes than control embryos (Figures 2B and S2B), confirming that endocytosis was inhibited.
To determine the effect of inhibiting endocytosis on AC, we measured blastopore depth and bottle cell morphometric parameters, including apical width, apicobasal length, and Apical Index (length-over-width ratio, AI) [7]. DN-Dynamin blastopores were 36% shallower than GFP blastopores (Figures 2B and 2C). Additionally, DN-Dynamin bottle cells were significantly less constricted than control embryos, whereas apicobasal length was indistinguishable between the two groups (Figure 2C). The wider apices in DN-Dynamin bottle cells accounted for the significant decrease in AI compared to control (Figure 2C). These effects were rescuable with wild-type Dynamin, illustrating specificity (Figures 2A, 2B, and 2C). Interestingly, these measurements recapitulate what we previously observed in nocodazole-treated embryos [7], suggesting an overlapping function between endocytosis and microtubules during AC. These results suggest that Dynamin-mediated endocytosis is required for efficient AC in bottle cells.
To test whether DN-Dynamin affects bottle cell AC by perturbing cell fate [16], we examined chordin, xbra, and sox17 expression as readouts of organizer, mesoderm, and endoderm fate, respectively. Expression was indistinguishable between controls and DN-Dynamin embryos (Figure S2E). Thus, DN-Dynamin does not affect AC indirectly through altering cell fate.
To independently assess the role of Dynamin during AC, we injected translation-blocking antisense morpholino oligonucleotides (MO) targeting Dnm-like and Dnm-2, the two most abundant transcripts during gastrulation [17]. Whereas single MO injections at 160ng did not affect endocytosis or AC, double injections of 80ng of each MO significantly perturbed both processes (Figures S3A, S3B, and S3C). Since only the double morphants exhibited endocytosis and AC defects, we suggest that both isoforms are involved in bottle cell AC; moreover, the effect on endocytosis and AC is specific and not due to off-target effects. However, both single and double morphants exhibited a range of developmental phenotypes, such as failure to close the blastopore, defective convergent extension, and embryonic lethality, presumably caused by MOrelated toxicity (data not shown). Because DN-Dynamin effectively inhibited endocytosis without these deleterious side effects, we chose to use DN-Dynamin in our experiments as an effective, specific reagent to block Dynamin function.
To further elaborate the role of endocytosis during AC, we inhibited Rab5, a small GTPase that is required for early endosome sorting, by injection of DN-Rab5, which binds GTP weakly [18]. Internalized biotin was nearly undetectable in DN-Rab5 embryos, confirming inhibition of endocytosis (Figures 2D and S2C). DN-Rab5 embryos had 33% shallower blastopores and less constricted bottle cells relative to uninjected embryos, with no differences in apicobasal length (Figure 2E). As with DN-Dynamin, cell fates were unaltered by DN-Rab5 (Figure S2E), consistent with the finding that DN-Rab5 does not affect Activin signaling [19]. Compared to controls, DN-Rab5 tailbud stage embryos were short and kinked (Figure S2G), indicative of defective convergent extension [20]. Interestingly, overexpression of constitutively active Rab5 had no effect on blastopore formation or bottle cell morphometrics (Figures S3D and S3E), suggesting that upregulating endocytosis at the level of Rab5 cannot increase the rate or extent of AC. Together, the DN-Dynamin and DN-Rab5 results strongly argue that endocytosis is required for AC in bottle cells.
Endocytosis is required for AC downstream of actomyosin contractility
Although endocytosis is required for efficient AC, it did not completely abolish contraction. Thus, we hypothesized that actomyosin contractility folds the apical membrane into microvilli and endocytosis is then required to remove the excess membrane.
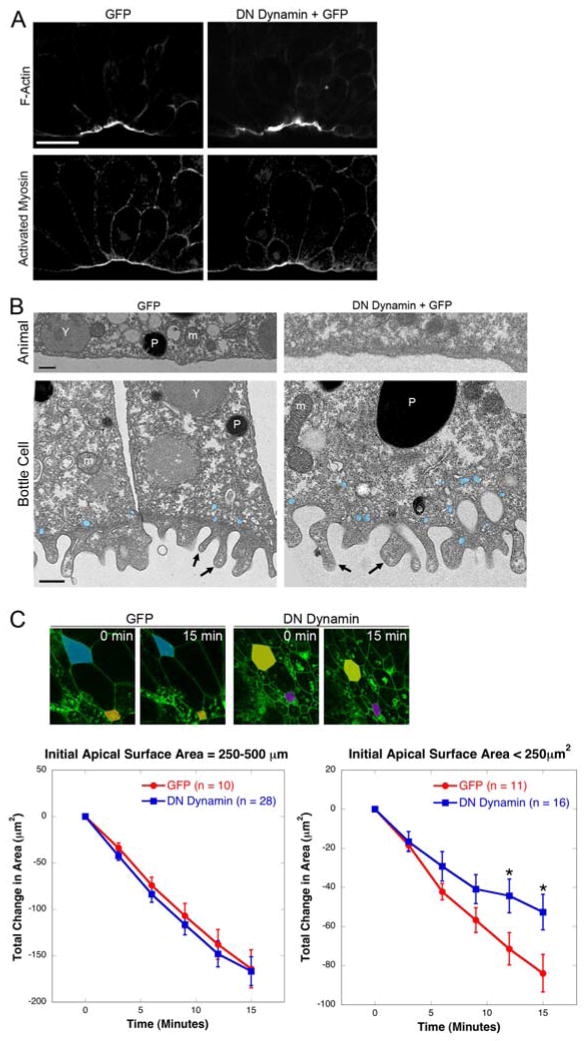
This hypothesis makes three predictions. First, actin and myosin should be apically localized in both GFP and DN-Dynamin bottle cells. Indeed, both GFP and DN-Dynamin bottle cells exhibited apical localization of F-actin and activated myosin (Figure 3A), suggesting that endocytosis acts downstream of the establishment of the apical actomyosin machinery. The correct apical localization of F-actin and myosin in Xenopus bottle cells also indicates that endocytosis is not required for some aspects of polarity, as it is in C. elegans [21] and Drosophila [22].
Figure 3.
Apical membrane remodeling is required for efficient apical constriction downstream of actomyosin contractility. (A) Apical accumulation of F-actin and Myosin do not require endocytosis. Confocal midsagittal sections of embryos injected with membrane-EGFP alone or with membrane-EGFP plus DN Dynamin, then stained with phalloidin to visualize F-actin or anti-pMLC. Scale bar = 50μm. (B) Transmission electron micrographs of GFP and DN Dynamin-injected embryos. Animal cells do not have microvilli, while bottle cells have microvilli. Arrows, microvilli; m, mitochondria; P, pigment granule; Y, yolk platelet. Vesicles are pseudocolored in blue. Scale bars = 0.5μm. (C) Rate of constriction is the same between wild-type and DN Dynamin bottle cells until cells become very constricted. Top panels are stills from two timepoints (0 minutes, 15 minutes) of time-lapse movies (Movies 1 and 2). Larger cells (>250μm2) pseudocolored in blue and mustard; smaller cells (<250μm2) in orange and purple. Line graphs indicate the total decrease in apical surface area over 15 minutes. n, number of cells measured; *, p < 0.05. See also Table S1.
The second prediction is that if endocytosis is required downstream of actomyosin contractility and membrane folding, then microvilli should be present at the apical surface of both GFP and DN-Dynamin bottle cells. Microvilli have been observed in many apically constricting cells, including bottle cells [23], where they contribute to reduction in apical surface area. Transmission electron microscopy revealed that while animal cells exhibited unfolded apical membrane, both GFP and DN-Dynamin bottle cells contained numerous microvilli (Figure 3B). Apart from the difference in apical width, there were no apparent disparities between GFP and DN-Dynamin bottle cells, suggesting that membrane folding is not significantly disrupted in DN-Dynamin bottle cells.
The final prediction is that both GFP and DN-Dynamin bottle cells should initially constrict at similar rates, but as membrane accumulates, the DN-Dynamin bottle cells should slow down. To test this prediction, we monitored the apical surface area at the blastopores of embryos injected with membrane-EGFP alone or GFP plus DN-Dynamin (Movies 1 and 2; Figure 3C). Measurement of apical surface areas at three-minute intervals over the course of 15 minutes revealed that both GFP and DN-Dynamin bottle cells with apices greater than 250 μm2 exhibited nearly identical rates of AC, but as expected, the constriction rate decreased as cells became smaller (Figure 3C and Table S1). In cells with apical surfaces smaller than 250 μm2, constriction rates were initially indistinguishable between GFP and DN-Dynamin bottle cells. However, as AC proceeded, GFP bottle cells continued to constrict at a constant rate, while DN-Dynamin bottle cells stalled, constricting significantly slower than GFP cells (Figure 3C and Table S1). Therefore, as we predicted, DN-Dynamin cells initially constrict at the same rate as their GFP counterparts, but then stall as they get smaller, presumably due to excess membrane that impedes constriction. Our results suggest that AC involves a dual mechanism: actomyosin contraction accompanied by membrane removal to drive efficient invagination.
Endocytosis occurs in the apically constricting neural tube
We propose that actomyosin contractility initiates AC, folding apical plasma membrane into microvilli (Figure 4A). As the apical surface area decreases, excess membrane must be removed by endocytosis to accommodate further shrinkage. When endocytosis is inhibited, either by microtubule depolymerization or by blocking core members of the endocytic pathway, AC initiates properly but slows due to a build-up of excess membrane.
Figure 4.
Endocytosis occurs the apically constricting dorsal lateral hingepoint cells during neurulation, and perturbing endocytosis results in apical constriction and neural tube closure defects. (A) Model of apical membrane dynamics during bottle cell apical constriction. (B) Endocytosis occurs in the neural tube. Cross-section of a stage 18 embryo, dorsal side up, stained with streptavidin. Scale bar = 50μm. (C) Injection of DN Dynamin results in a range of neural tube closure defects. Top panels show unilateral injection of membrane-EGFP in whole stage 24 embryos, dorsal views, anterior to the left. Bottom panels show higher magnification of the anterior neural tube, anterior up. Asterisks indicate injected side. (D) DN Dynamin-injected hingepoint cells appear less apically constricted than GFP-injected cells. Stage 18 embryos were stained with anti-DM1α (tubulin) and anti-GFP. Scale bar = 50μm. See also Figure S4 and Movie 3.
To determine if this mechanism is specific to bottle cells or if it is applicable elsewhere, we examined the role of endocytosis during Xenopus neural tube (NT) closure, where cells undergo AC to bend the neural plate [24]. In mice, defective AC results in NT closure defects such as exencephaly and spina bifida [25]. To confirm that endocytosis occurs in the Xenopus NT [12], we biotinylated neurula stage embryos and observed endocytic vesicles exclusively in the NT (Figure 4B). Next, we perturbed endocytosis by targeting DN-Dynamin mRNA to the anterior NT. Unilaterally-injected embryos were selected so that the uninjected side could serve as an internal control. Neural plates expressing GFP alone exhibited NT morphology and closure rates indistinguishable from the uninjected side, whereas DN-Dynamin neural folds were irregularly shaped and formed slower than the uninjected side (Movie 3). At stage 24, all GFP embryos had completed NT closure (58/58 embryos), whereas DN-Dynamin embryos exhibited a range of phenotypes, varying from wild-type (15/50 embryos), to kinked (21/50 embryos), to open anterior NT or mild exencephaly (28/50 embryos)(Figure 4C). The kinked phenotype probably results from the uninjected side crossing over the midline to compensate for less efficient folding on the DN-Dynamin-injected side. In cross-section, DN-Dynamin hingepoint cells appeared less constricted and less elongated compared to GFP-injected cells (Figure 4D) and to their uninjected counterparts (Figure S4). Together, our results show that inhibiting endocytosis with DN-Dynamin causes NT closure defects and suggests that inefficient AC in the dorsolateral hingepoint cells may be the underlying cause of these defects.
Endocytosis and morphogenesis
Endocytosis is important for many cellular process during development, such as maintenance of epithelial polarity [22], signaling during convergent extension [20, 26–28], and cellularization [29]. In particular, endocytosis at the apical membrane is crucial for Drosophila tracheal morphogenesis to modulate membrane tension [30], to clear proteins from the lumen [31], and to remodel adherens junctions during intercalation [32]. Whereas previous reports showed that endocytosis is important for organ shape, our results point to an additional mechanism where endocytosis is required during AC to affect cell shape.
In the context of AC, endocytosis may be a general mechanism to accelerate constriction during bending and invagination. Interestingly, the two C. elegans endoderm percursors that ingress during gastrulation do so via AC [33], but they do not undergo obvious shape changes or contain microvilli [34], suggesting that membrane folding and endocytosis may only be necessary in cases of AC associated with cell sheet invagination. Furthermore, efficient AC may be more important for embryos that rely on ingression en masse to drive morphogenesis, such as in the chick primitive streak [35] and in the gastrulating axolotl [36]. That inefficient AC is not more deleterious for Xenopus embryos is consistent with the view that several engines cooperate to drive gastrulation, and removal of just one may have modest outcomes [37]. Nonetheless, our results implicate endocytosis-driven membrane remodeling as a mechanism essential for efficient AC and subsequent invagination of cell sheets.
EXPERIMENTAL PROCEDURES
Embryo culture and manipulation
Embryo culture and inhibitor experiments were performed as described [7, 38]. Capped mRNA was injected dorsal-vegetally in two cells at the four-cell stage. Amounts injected were 250pg GFP (membrane-tethered EGFP) [39]; 250–500pg DN Dynamin1-K44E, 1ng DN Rab5-S34N, and 500pg Rab5-EGFP [16]. For rescue experiments, equal amounts of WT and DN-Dynamin were co-injected. For neural tube experiments, 100pg membrane-EGFP and 200pg DN-Dynamin1 mRNA were injected into one animal-dorsal cell at the 8-cell stage.
Staining
Embryos were labeled with 2.5mg/ml sulfo-NHS-LC-biotin (Pierce) [11]. Biotin was detected with 1:500 AlexaFluor 555 Streptavidin (Molecular Probes). Embryos were stained [7] using primary antibodies against mouse DM1α (1:500; Sigma); rabbit EEA1 (1:200; Abcam); rabbit phospho20 myosin light chain (1:500; Abcam); chicken GFP (1:500; Abcam). Goat AlexaFluor secondary antibodies (Molecular Probes) were used at 1:500. Standard antibody specificity controls were performed.
Imaging and morphometrics
Embryos were imaged and quantified as described [7]. Statistical significance was determined by two-tailed T-tests. For time-lapse movies, images were captured every 30 seconds with the 63x objective on a Leica DM RE confocal microscope with Leica TCS software (Leica Microsystems). Apical surface areas were measured using ImageJ (NIH).
Transmission electron microscopy
Embryos were fixed in 2.5% glutaraldehyde plus 2% formaldehyde (Electron Microscopy Sciences) in cacodylate buffer, 4°C overnight. One hour into fixation, DMZs were cut out. Samples were prepared [23], omitting the low-melt agarose step and using Epon-Araldite resin. Ultrathin sections were imaged on a FEI Tecnai 12.
Supplementary Material
Acknowledgments
We thank J. Jullien and L. Jan for reagents, R. Zalpurni for TEM assistance, M. Sohaskey, S. Haigo, and L. Christiaen for critically reading the manuscript, and Harland lab members for helpful discussions. This work was supported by NIH grants GM42341 to RMH and F32HD052374 to JYL.
Footnotes
Supplemental Data include four figures, one table, methods, and three movies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lewis W. Mechanics of Invagination. Anat Rec. 1947;97:139–156. doi: 10.1002/ar.1090970203. [DOI] [PubMed] [Google Scholar]
- 2.Odell GM, Oster G, Alberch P, Burnside B. The mechanical basis of morphogenesis. I. Epithelial folding and invagination. Dev Biol. 1981;85:446–462. doi: 10.1016/0012-1606(81)90276-1. [DOI] [PubMed] [Google Scholar]
- 3.Kimberly EL, Hardin J. Bottle cells are required for the initiation of primary invagination in the sea urchin embryo. Dev Biol. 1998;204:235–250. doi: 10.1006/dbio.1998.9075. [DOI] [PubMed] [Google Scholar]
- 4.Burnside B. Microtubules and microfilaments in newt neuralation. Dev Biol. 1971;26:416–441. doi: 10.1016/0012-1606(71)90073-x. [DOI] [PubMed] [Google Scholar]
- 5.Haigo SL, Hildebrand JD, Harland RM, Wallingford JB. Shroom induces apical constriction and is required for hingepoint formation during neural tube closure. Curr Biol. 2003;13:2125–2137. doi: 10.1016/j.cub.2003.11.054. [DOI] [PubMed] [Google Scholar]
- 6.Myat MM, Andrew DJ. Organ shape in the Drosophila salivary gland is controlled by regulated, sequential internalization of the primordia. Development. 2000;127:679–691. doi: 10.1242/dev.127.4.679. [DOI] [PubMed] [Google Scholar]
- 7.Lee JY, Harland RM. Actomyosin contractility and microtubules drive apical constriction in Xenopus bottle cells. Dev Biol. 2007;311:40–52. doi: 10.1016/j.ydbio.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holtfreter J. A Study of the Mechanics of Gastrulation, Part I. J Exp Zool. 1943;94:261–318. [Google Scholar]
- 9.Keller RE. An experimental analysis of the role of bottle cells and the deep marginal zone in gastrulation of Xenopus laevis. J Exp Zool. 1981;216:81–101. doi: 10.1002/jez.1402160109. [DOI] [PubMed] [Google Scholar]
- 10.Matteoni R, Kreis TE. Translocation and clustering of endosomes and lysosomes depends on microtubules. J Cell Biol. 1987;105:1253–1265. doi: 10.1083/jcb.105.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Minsuk SB, Keller RE. Surface mesoderm in Xenopus: a revision of the stage 10 fate map. Dev Genes Evol. 1997;207:389–401. doi: 10.1007/s004270050128. [DOI] [PubMed] [Google Scholar]
- 12.Shook DR, Majer C, Keller R. Pattern and morphogenesis of presumptive superficial mesoderm in two closely related species, Xenopus laevis and Xenopus tropicalis. Dev Biol. 2004;270:163–185. doi: 10.1016/j.ydbio.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 13.Mu FT, Callaghan JM, Steele-Mortimer O, Stenmark H, Parton RG, Campbell PL, McCluskey J, Yeo JP, Tock EP, Toh BH. EEA1, an early endosome-associated protein. EEA1 is a conserved alpha-helical peripheral membrane protein flanked by cysteine "fingers" and contains a calmodulin-binding IQ motif. J Biol Chem. 1995;270:13503–13511. doi: 10.1074/jbc.270.22.13503. [DOI] [PubMed] [Google Scholar]
- 14.Chavrier P, Parton RG, Hauri HP, Simons K, Zerial M. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990;62:317–329. doi: 10.1016/0092-8674(90)90369-p. [DOI] [PubMed] [Google Scholar]
- 15.Damke H, Baba T, Warnock DE, Schmid SL. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jullien J, Gurdon J. Morphogen gradient interpretation by a regulated trafficking step during ligand-receptor transduction. Genes & Development. 2005;19:2682–2694. doi: 10.1101/gad.341605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilchrist MJ, Zorn AM, Voigt J, Smith JC, Papalopulu N, Amaya E. Defining a large set of full-length clones from a Xenopus tropicalis EST project. Dev Biol. 2004;271:498–516. doi: 10.1016/j.ydbio.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 18.Li G, Stahl PD. Structure-function relationship of the small GTPase rab5. J Biol Chem. 1993;268:24475–24480. [PubMed] [Google Scholar]
- 19.Hagemann AI, Xu X, Nentwich O, Hyvonen M, Smith JC. Rab5-mediated endocytosis of activin is not required for gene activation or long-range signalling in Xenopus. Development. 2009;136:2803–2813. doi: 10.1242/dev.034124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ulrich F, Krieg M, Schötz EM, Link V, Castanon I, Schnabel V, Taubenberger A, Mueller D, Puech PH, Heisenberg CP. Wnt11 functions in gastrulation by controlling cell cohesion through Rab5c and E-cadherin. Dev Cell. 2005;9:555–564. doi: 10.1016/j.devcel.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Balklava Z, Pant S, Fares H, Grant BD. Genome-wide analysis identifies a general requirement for polarity proteins in endocytic traffic. Nat Cell Biol. 2007;9:1066–1073. doi: 10.1038/ncb1627. [DOI] [PubMed] [Google Scholar]
- 22.Lu H, Bilder D. Endocytic control of epithelial polarity and proliferation in Drosophila. Nat Cell Biol. 2005;7:1232–1239. doi: 10.1038/ncb1324. [DOI] [PubMed] [Google Scholar]
- 23.Kurth T, Hausen P. Bottle cell formation in relation to mesodermal patterning in the Xenopus embryo. Mech Dev. 2000;97:117–131. doi: 10.1016/s0925-4773(00)00428-7. [DOI] [PubMed] [Google Scholar]
- 24.Schoenwolf GC, Franks MV. Quantitative analyses of changes in cell shapes during bending of the avian neural plate. Dev Biol. 1984;105:257–272. doi: 10.1016/0012-1606(84)90284-7. [DOI] [PubMed] [Google Scholar]
- 25.Copp AJ. Neurulation in the cranial region--normal and abnormal. J Anat. 2005;207:623–635. doi: 10.1111/j.1469-7580.2005.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kida YS, Sato T, Miyasaka KY, Suto A, Ogura T. Daam1 regulates the endocytosis of EphB during the convergent extension of the zebrafish notochord. Proc Natl Acad Sci USA. 2007;104:6708–6713. doi: 10.1073/pnas.0608946104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogata S, Morokuma J, Hayata T, Kolle G, Niehrs C, Ueno N, Cho KW. TGF-beta signaling-mediated morphogenesis: modulation of cell adhesion via cadherin endocytosis. Genes & Development. 2007;21:1817–1831. doi: 10.1101/gad.1541807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim GH, Her JH, Han JK. Ryk cooperates with Frizzled 7 to promote Wnt11-mediated endocytosis and is essential for Xenopus laevis convergent extension movements. The Journal of Cell Biology. 2008;182:1073–1082. doi: 10.1083/jcb.200710188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pelissier A, Chauvin JP, Lecuit T. Trafficking through Rab11 endosomes is required for cellularization during Drosophila embryogenesis. Curr Biol. 2003;13:1848–1857. doi: 10.1016/j.cub.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 30.Kerman BE, Cheshire AM, Myat MM, Andrew DJ. Ribbon modulates apical membrane during tube elongation through Crumbs and Moesin. Dev Biol. 2008;320:278–288. doi: 10.1016/j.ydbio.2008.05.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsarouhas V, Senti KA, Jayaram SA, Tiklova K, Hemphala J, Adler J, Samakovlis C. Sequential pulses of apical epithelial secretion and endocytosis drive airway maturation in Drosophila. Dev Cell. 2007;13:214–225. doi: 10.1016/j.devcel.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 32.Shaye DD, Casanova J, Llimargas M. Modulation of intracellular trafficking regulates cell intercalation in the Drosophila trachea. Nat Cell Biol. 2008;10:964–970. doi: 10.1038/ncb1756. [DOI] [PubMed] [Google Scholar]
- 33.Lee JY, Goldstein B. Mechanisms of cell positioning during C. elegans gastrulation. Development. 2003;130:307–320. doi: 10.1242/dev.00211. [DOI] [PubMed] [Google Scholar]
- 34.Krieg C, Cole T, Deppe U, Schierenberg E, Schmitt D, Yoder B, con Ehrenstein G. The cellular anatomy of embryos of the nematode Caenorhabditis elegans. Analysis and reconstruction of serial section electron micrographs. Dev Biol. 1978;65:193–215. doi: 10.1016/0012-1606(78)90190-2. [DOI] [PubMed] [Google Scholar]
- 35.Voiculescu O, Bertocchini F, Wolpert L, Keller RE, Stern CD. The amniote primitive streak is defined by epithelial cell intercalation before gastrulation. Nature. 2007;449:1049–1052. doi: 10.1038/nature06211. [DOI] [PubMed] [Google Scholar]
- 36.Shook DR, Keller R. Epithelial type, ingression, blastopore architecture and the evolution of chordate mesoderm morphogenesis. J Exp Zoolog B Mol Dev Evol. 2008;310:85–110. doi: 10.1002/jez.b.21198. [DOI] [PubMed] [Google Scholar]
- 37.Keller R, Davidson LA, Shook DR. How we are shaped: the biomechanics of gastrulation. Differentiation. 2003;71:171–205. doi: 10.1046/j.1432-0436.2003.710301.x. [DOI] [PubMed] [Google Scholar]
- 38.Sive HL, Grainger RM, Harland RM. Early Development of Xenopus laevis: A Laboratory Manual. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2000. [Google Scholar]
- 39.Wallingford JB, Harland RM. Neural tube closure requires Dishevelled-dependent convergent extension of the midline. Development. 2002;129:5815–5825. doi: 10.1242/dev.00123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.