Abstract
Aims
Bone marrow-derived smooth muscle cells (BM-SMCs) have high potential as an autologous cell source of vascular progenitors but normal cell function and turnover frequency may decline with age. In this study we set out to study the effects of organismal ageing on the molecular and functional properties of BM-SMCs.
Methods and results
To address this issue, we employed a smooth muscle α-actin promoter (αSMA) driving expression of enhanced green fluorescence protein (EGFP) to isolate SMCs from bone marrow of neonatal (nBM-SMCs) or adult (aBM-SMCs) sheep and examined their proliferation potential and contractility. Compared with nBM-SMCs, aBM-SMCs exhibited lower clonogenicity and proliferation potential that could be improved significantly by addition of basic fibroblast growth factor. Vascular constructs from aBM-SMCs showed reduced ability to generate force and contract fibrin hydrogels and this function could be partially restored by addition of transforming growth factor-β1. They also exhibited lower receptor- and non-receptor-mediated vascular contractility and mechanical strength, which was comparable to that of tissue constructs prepared with vascular SMCs from neonatal umbilical veins. In agreement with the contractile properties and mechanical strength of vascular constructs, aBM-SMCs displayed significantly lower expression of αSMA, smoothelin, desmin, type I collagen, and tropoelastin transcripts compared with nBM-SMCs.
Conclusion
Understanding the effects of organismal ageing on BM-SMCs and the properties of the resulting vascular constructs may lead to innovative ways to facilitate application of these cells in the treatment of cardiovascular disease which is especially prevalent in the elderly.
Keywords: Ageing, Bone marrow, Mesenchymal stem cells, Smooth muscle, Cardiovascular tissue regeneration, Vascular reactivity, Extracellular matrix profile
1. Introduction
Adult mesenchymal stem cells (MSCs) from bone marrow provide a promising cell source for tissue regeneration. However, stem cell senescence due to donor age or culture conditions appears to limit clinical application of MSCs.1 Some studies showed that the growth potential of MSCs declines with donor age2–4 and this dependence may be species-specific, with human cells displaying lower proliferation potential than mouse cells.5 In addition to proliferation, the differentiation potential of MSCs was also studied as a function of ageing but the results were variable. Although some studies suggested that osteogenic3,6,7 and chondrogenic8 differentiation of MSCs decreased with donor age, others reported that the potential of MSCs for osteogenic or adipogenic differentiation9 as well as tendon regeneration in vivo was independent of ageing.10 Clearly, more studies are required to understand the effect of organismal ageing on the potential of MSCs for multilineage differentiation and tissue regeneration.
Previous studies clearly demonstrated the importance of ageing in the behaviour of cardiovascular cells.11 In addition to proliferation, donor ageing had a significant effect on mechanical properties of engineered vascular grafts generated from porcine12 or human smooth muscle cells.13 Constructs prepared with cells from aged donors showed impaired mechanical strength unless they were modified to express human telomerase reverse transcriptase.14–16 These studies clearly demonstrated that a clinically important challenge in vascular tissue engineering is the ability to engineer blood vessels from an easily accessible, autologous source of adult or even elderly donors.
Recently, our laboratory showed that bone marrow-derived smooth muscle cells (BM-SMCs) were isolated from neonatal ovine using fluorescence-activated cell sorting (FACS) to sort out BM-MSCs that expressed EGFP under the control of a smooth muscle α-actin (αSMA) promoter.17,18 The BM-SMCs exhibited high proliferation potential and expressed vascular smooth muscle cell (V-SMC) markers.18 Fibrin-based vascular constructs prepared with BM-SMCs displayed receptor- and non-receptor-mediated contractility and could be implanted into the jugular veins of lambs where they remained patent for 5 weeks. BM-SMC-derived tissue-engineered vessel (BM-TEV) exhibited similar structure and collagen deposition to native veins. In contrast to TEV with V-SMCs from ovine umbilical veins,19 BM-SMCs expressed significantly higher amount of elastin that was deposited in well-organized fibres. Our results suggested that bone marrow-derived progenitor cells can be an autologous source of highly proliferative and functional cells for vascular tissue regeneration.
In this study, we explored the effect of organismal ageing on BM-SMCs and the resulting vascular constructs prepared with these cells. To this end, we employed the αSMA promoter driving EGFP expression to isolate SMCs from BM-MSCs derived from adult or neonatal animals (aBM-SMCs vs. nBM-SMCs). These cells were compared in terms of proliferation potential, clonogenicity, and expression of gene transcripts related to extracellular matrix (ECM) molecules. We also measured the mechanical properties of resulting TEV, i.e. the ability to generate force and respond to vasoactive agonists. Finally, we evaluated the potential of basic fibroblast growth factor (bFGF) to increase proliferation and transforming growth factor-β1 (TGF-β1) to restore contractility of aBM-SMCs vs. nBM-SMCs.
2. Methods
2.1. Isolation of smooth muscle progenitor cells from neonatal and adult ovine bone marrow
Bone marrow samples were aspirated from three neonatal lambs (<3 days old) and three adult sheep (4–4.5 years old). Isolation of BM-SMCs from BM-MSCs was described previously.18 Proliferation and clonogenic assays were described before.17 For details, see Supplementary material online.
The Institutional Animal Care and Use Committee of the University at Buffalo, State University of New York, approved the use of animals for this project (IACUC#PGY15112N). The investigation conformed with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
2.2. Contractility and mechanical properties of tissue equivalents
Cylindrical vascular tissue equivalents containing BM-SMCs in fibrin hydrogels were cultured around a 6.0 mm mandrel of poly(di-methyl siloxane) as described previously.17–19 After 2 weeks in culture, the tissue constructs were released from the mandrel and vascular reactivity was measured in an isolated tissue bath. For details, see Supplementary material online.
2.3. Quantitative real-time PCR
Total RNA was isolated using the RNeasy kit (Qiagen, Chatsworth, CA, USA) and reverse transcribed using a cDNA synthesis kit (Qiagen) according to the manufacturer's instructions. Quantitative PCR was performed using the iCycler (Bio-Rad Laboratories). The reaction was carried out in a volume of 25 µL containing 1 µL of cDNA, 0.4 µM of each primer (Sigma Genosys, Woodlands, TX, USA), and 12.5 µL of 2× IQ TM SYBR Green Supermix (Bio-Rad Laboratories). The primer sequences for each gene are shown in Supplementary material online, Table S1. Each reaction comprised of 40 cycles each with melting at 95°C for 10 s, annealing at 55°C for 10 s, and extension at 72°C for 20 s. The fluorescence intensity was recorded at 72°C for 20 s after the extension step of each cycle. The specificity of the PCR products was verified using the melting curve generated by MyiQ software and by electrophoresis on 1% agarose gels. The PCR data analysis was performed as described before.20
2.4. Statistical analysis
All experiments were conducted with cells from three adult and three neonatal animals in triplicate or quadruplicate (n = 9–12 per group). Pair-wise statistical analysis of the data was performed using a two-tailed Student's t-test using Microsoft Excel software. The data were considered statistically different when P < 0.05.
3. Results
3.1. nBM-SMCs displayed higher proliferation and clonogenic potential compared with aBM-SMCs
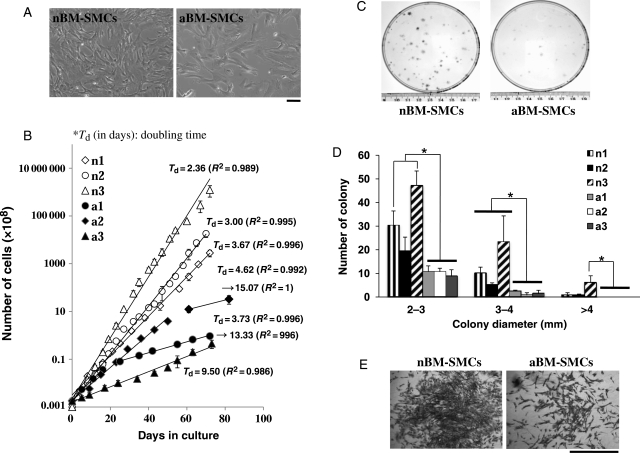
BM-SMCs were derived from BM-MSCs by FACS based on expression of EGFP (upper 50% of expressers) under the control of smooth muscle α-actin promoter (PαSMA) as we described previously.18 To study the effects of ageing, BM-SMCs were derived from three neonatal (n1, n2, and n3) and three 4- to 4.5-year-old adult lambs (a1, a2, and a3), corresponding to humans between 35 and 40 years old (the lifespan of sheep is about 9 years21). As shown in Figure 1A, cell morphology and size were significantly different between nBM-SMCs and aBM-SMCs. Neonatal cells were smaller and more elongated, whereas adult cells appeared larger with flat morphology.
Figure 1.
nBM-SMCs showed higher proliferation and clonogenic potential compared with aBM-SMCs. Cells were seeded at 1.8 × 104 cells/cm2 and grown to near confluence, trypsinized, and counted at the indicated times. (A) Bright-field photomicrographs of n1 or a3 BM-SMC (bar = 100 µm). (B) The results are plotted as cumulative cell number over time for nBM-SMCs (n1, n2, and n3) or aBM-SMCs (a1, a2, and a3). (C) Cells were plated onto 100 mm dishes at clonal density (400 cells/dish) and cultured for 10 days. Photographs of cell culture dishes after 10 days in culture display colonies that originated from single cells. (D) Colony size distribution for nBM-SMCs and aBM-SMCs. *P < 0.05. (E) Representative colonies originating from nBM-SMCs or aBM-SMCs (bar = 1 mm).
To examine the proliferation potential of BM-SMCs, cells from three neonatal and three adult animals were plated in 6-well plates at 1.8 × 104 cells/cm2. When confluence was reached (at the indicated times), cells were trypsinized, counted, and replated at the same density. Cell growth was significantly affected by the age of donor animals (Figure 1B). Although the population doubling time of nBM-SMCs varied among cells from different animals, all neonatal cells exhibited higher proliferation rate compared with cells from adult donors. Specifically, the average doubling time was 3.67, 3.00, and 2.36 days for n1, n2, and n3, respectively. Cells from one adult donor (a3) exhibited slow proliferation throughout the experiment with an average doubling time of 9.5 days. Cells from the other two adult donors (a1 and a2) divided fast in the beginning of the culture (doubling time: 3.73 and 4.36 days) but their proliferation rate decreased significantly at later passages reaching a doubling time of 13 days or longer. During 10 weeks in culture, neonatal cells were expanded at least a million-fold, whereas adult cells were expanded at most 10 000-fold.
To measure the clonogenic capacity of aBM-SMCs and nBM-SMCs, cells were seeded at clonal density (400 cells in 100 mm dish) and allowed to grow for 10 days. At that time, the cells were fixed and stained with Trypan Blue to visualize and count the number of colonies. Only colonies with diameter >2 mm were counted as those originate from highly proliferative cells, which are more likely to be stem/progenitor cells. As shown in Figure 1C, nBM-SMCs were enriched in highly clonogenic cells compared with aBM-SMCs. Specifically, the fraction of cells that formed >2 mm colonies was 10.3 ± 2.5, 6.3 ± 1.9, and 19.2 ± 5.0% for n1, n2, and n3 vs. 2.8 ± 0.7, 2.5 ± 1.1, and 3.3 ± 0.8% for a1, a2, and a3, respectively. Similar results were found for smaller (2–3 mm) and larger colonies (3–4 or >4 mm in diameter) as shown in Figure 1D. In addition, colonies originating from neonatal cells contained smaller cells at a higher density compared with their counterparts from adult cells (Figure 1E).
We also examined expression of SMC specific markers in nBM-SMCs and aBM-SMCs (see Supplementary material online, Figure S1). Immunostaining showed that both neonatal and adult cells expressed early, intermediate, and late SMC markers such as α-actin, calponin, and myosin heavy chain (MHC).
3.2. Higher force generation and vascular contractility by nBM-SMCs compared with aBM-SMCs
Next, we examined the ability of BM-SMCs to generate force by measuring the compaction of three-dimensional (3D) fibrin hydrogels. To this end, nBM-SMCs or aBM-SMCs were embedded in fibrin gels (106 cell/mL) that contained fibrinogen (2.5 mg/mL) and thrombin (2.5 U/mL). One hour after polymerization, gels were released from the plate walls and were allowed to compact the gels over time in the presence of smooth muscle differentiation factors TGF-β1 (2 ng/mL) and ascorbic acid (300 µM).19,22,23 To quantify gel compaction, gels were photographed at the indicated times and their area was measured using Image J software.
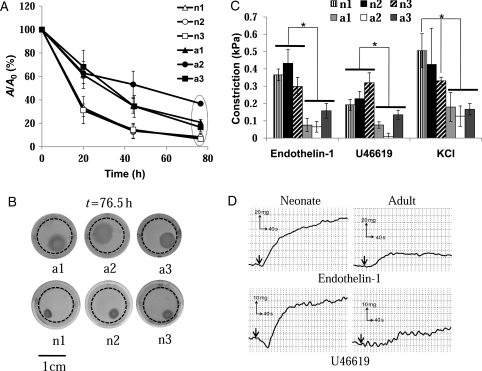
Although both neonatal and adult cells showed significant contractile properties, the rate of gel compaction was significantly higher for neonatal compared with adult cells (Figure 2A). Specifically, nBM-SMCs compacted gels to half of their original size within 15 h when compared with 30–50 h for aBM-SMCs. The ultimate extent of gel compaction was also higher in nBM-SMCs containing hydrogels (Figure 2A and B).
Figure 2.
Vascular contractility of BM-TEVs from nBM-SMCs vs. aBM-SMCs. (A) BM-SMCs were embedded in fibrin, which was allowed to polymerize in 24-well plates to form disks. One hour after polymerization, the gels were detached from the walls and allowed to compact. At the indicated times, the gels were photographed and their area was measured using Image J software. The ratio of the area of each gel (A) at the indicated times over the initial area (A0) was plotted as a function of time. (B) Representative pictures of fibrin hydrogels containing BM-SMCs at t >76.5 h. Dotted circles indicate initial hydrogel perimeter. (C) BM-SMCs were embedded in fibrin hydrogels and cultured around 6 mm mandrels for 2 weeks to form cylindrical tubes. Vascular reactivity (kPa) was measured in response to endothelin-1 (20 nM), U46619 (10−6 M), or KCl (118 mM). (D) Representative graphs of isometric contraction in response endothelin-1 and U46619. *P < 0.05.
We also evaluated the isometric tension of nBM-SMCs or aBM-SMCs fibrin-based tissue constructs in response to vasoreactive agonists. To this end, we prepared small-diameter cylindrical tissue equivalents by embedding cells in fibrin hydrogels that were allowed to polymerize around 6.5 mm-diameter cylindrical mandrels. After 2 weeks in culture in the presence of TGF-β1 (2 ng/mL) and ascorbic acid (300 µM), cells compacted the gels yielding cylindrical constructs with a wall thickness of 0.49 ± 0.06 mm for n1 (n = 8), 0.42 ± 0.02 mm for n2 (n = 4), 0.52 ± 0.03 mm for n3 (n = 9), 0.54 ± 0.05 mm for a1 (n = 7), 0.62 ± 0.13 mm for a2 (n = 4), and 0.56 ± 0.04 mm for a3 (n = 6).
The isometric tension was measured in response to receptor- and non-receptor-mediated agonists and representative plots of force vs. time are shown in Figure 2C. Tissue equivalents based on nBM-SMCs (n1: n = 4, n2: n = 3, and n3: n = 3) exhibited significantly higher isometric tension in response to endothelin-1 (20 nM), U46619 (1 µM), or KCl (118 mM) compared with those prepared with aBM-SMCs (a1: n = 3, a2: n = 3, and a3: n = 3) (Figure 2D). These data suggested that donor age may decrease the force-generating ability of BM-SMCs.
3.3. Differential SMC-specific marker expression in aBM-SMCs and nBM-SMCs
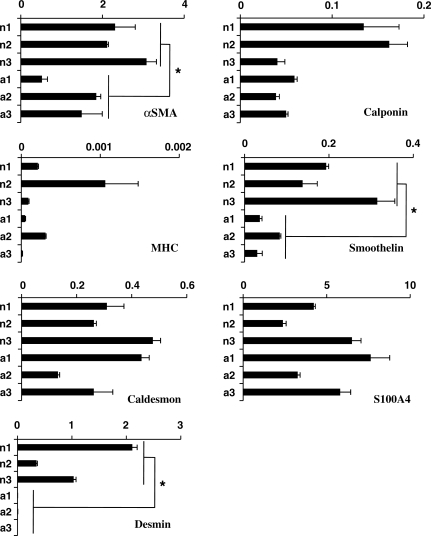
The higher contractility exhibited by nBM-SMCs prompted us to examine the expression level of several SMC markers including αSMA, calponin, MHC, smoothelin, desmin, and S100A4. To this end, cells were cultured under differentiation conditions [in the presence of TGF-β1 (2 ng/ml) and ascorbic acid (300 µM)] for 5 days and mRNA expression was quantified using qRT–PCR (Figure 3). The expression level of calponin and MHC varied among cells from different animals. Calponin was significantly higher in two neonatal samples (n1 and n2) but the third one (n3) showed comparable level to adult cells. Similarly, MHC was higher in some (n2) but not all neonatal cells. In contrast, other SMC markers that have been associated with SMC contractile phenotype such as αSMA,24,25 smoothelin,26,27 and desmin28 were expressed at 2 ± 0.8-fold (n = 6, P = 0.006), 4.3 ± 0.09-fold (n = 6, P = 0.004), and 647 ± 0.7-fold (n = 9, P = 0.002) higher level in nBM-SMCs compared with aBM-SMCs, respectively (fold increase was based on the average values of all nBM-SMCs or aBM-SMCs).
Figure 3.
nBM-SMCs expressed higher level of SMC contractility-related genes. Total RNA was isolated from nBM-SMCs or aBM-SMCs cultured to near confluence in the presence of TGF-β1 (2 ng/mL) and ascorbic acid (300 µM) and reverse transcribed to cDNA. Expression of the indicated transcripts was measured by real-time RT–PCR and normalized relative to GAPDH. Each experiment was done with triplicate samples. *P < 0.05.
3.4. TEVs from nBM-SMCs exhibited superior mechanical properties
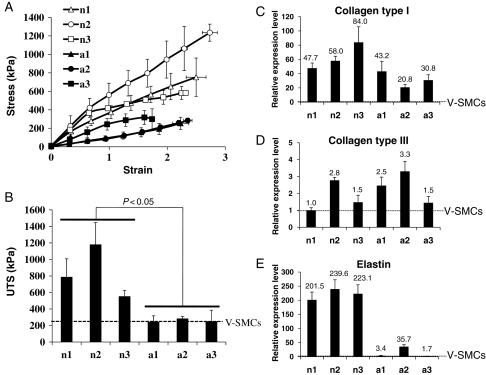
Next, we examined the mechanical properties of vascular constructs prepared from nBM-SMCs or aBM-SMCs. Stress–strain curve showed that elastic modulus of nBM-SMC-based tissue constructs was significantly higher (P < 0.05) than those based on aBM-SMCs (Figure 4A). Specifically, the elastic modulus for neonatal constructs was 327.9 ± 69.8 kPa (n1, n = 4), 487.8 ± 103.1 kPa (n2, n = 4), and 304.6 ± 6.9 kPa (n3, n = 3), whereas adult cells yielded tissues with 103.6 ± 27.7 kPa (a1, n = 4), 112.0 ± 10.3 kPa (a2, n = 3), and 201.7 ± 43.6 kPa (a3, n = 3). Neonatal constructs also demonstrated significantly higher ultimate tensile stress [787.1 ± 221.2 kPa (n1, n = 4), 1181.5 ± 265.9 kPa (n2, n = 4), and 554.0 ± 72.8 kPa (n3, n = 3)] compared with constructs grown from adult cells [244.7 ± 72.1 kPa (a1, n = 4), 284.3 ± 24.3 kPa (a2, n = 3), and 251.5 ± 134.1 kPa (a3, n = 3)] (Figure 4B).
Figure 4.
Mechanical properties of BM-TEV and ECM synthesis in BM-SMCs. (A) Stress–strain curves generated with TEV from nBM-SMCs or aBM-SMCs. (B) Ultimate tensile stress (kPa). The dotted line denotes the value of UTS for V-SMCs from umbilical veins of neonatal lambs, which served as a control. (C–E) Total RNA was isolated from nBM-SMCs or aBM-SMCs cultured to near confluence and expression of the indicated transcripts was measured by real-time RT–PCR and normalized relative to GAPDH. The relative expression level was defined as the ratio of the normalized expression level of each gene in BM-SMCs to that in V-SMCs from umbilical veins of neonatal lambs. RT–PCR experiment was repeated with RNA from three independent experiments. (C) Collagen type I, (D) collagen type III, and (E) elastin.
3.5. Differential gene expression of ECM molecules
Previous studies provided evidence that matrix remodelling and secretion of new ECM by cells affects the mechanical properties of engineered tissue constructs. These studies along with the data presented above prompted us to examine the expression level of several ECM molecules including tropoelastin, collagen type I, and collagen type III using quantitative real-time PCR. V-SMCs from the umbilical veins of neonatal sheep were used as reference.
The expression level of collagen I and III varied among cells from different animals. One of the adult cells (a1) showed similar collagen I level as neonatal cells but the others (a2 and a3) exhibited lower expression (Figure 4C). Overall, the average expression level of collagen I in nBM-SMCs was significantly higher than that in aBM-SMCs (n = 9, P = 0.009). Collagen type III expression varied between samples and there was no statistically significant difference between neonatal and adult cells (Figure 4D). Notably, all three nBM-SMCs expressed tropoelastin transcripts to a much higher level—6- to 100-fold higher—than aBM-SMCs (Figure 4E; n = 9, P < 0.000001). Interestingly, aBM-SMCs showed significantly higher tropoelastin and collagen type I levels compared with V-SMCs from neonatal animals, suggesting that BM-derived cells—even when originating from adult animals—express higher levels of these ECM molecules compared with neonatal SMCs from veins.
3.6. bFGF enhanced proliferation of aBM-SMCs
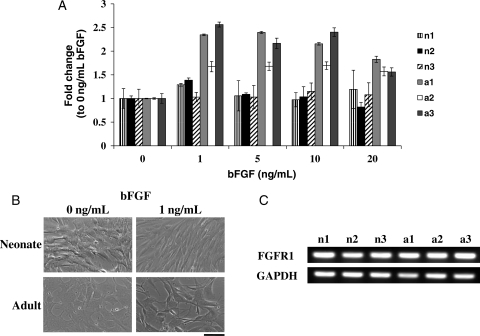
As bFGF has been shown to promote proliferation and prevent differentiation of adult and embryonic stem cells,29–31 we examined whether bFGF could also increase the growth rate of the slowly proliferating aBM-SMCs. Addition of 1 ng/mL bFGF increased the number of aBM-SMCs by 1.9- to 2.5-fold, whereas nBM-SMCs increased only by 1.2- to 1.4-fold (Figure 5A). The number of both nBM-SMCs and aBM-SMCs displayed the highest increase at 1 ng/mL bFGF and no additional effect was observed up to10 ng/mL. At higher concentration (20 ng/mL), the positive effect of bFGF on aBM-SMCs was less pronounced. Cell morphology also changed dramatically within 24 h of bFGF treatment with both neonatal and adult cells appearing smaller and more elongated (Figure 5B). Finally, RT–PCR showed that both aBM-SMCs and nBM-SMCs expressed a similar FGF receptor-1 (FGFR1) level (Figure 5C), suggesting that the higher response of aBM-SMCs to bFGF could not be due to higher number of FGFR1 on the surface of these cells.
Figure 5.
FGF augmented proliferation of aBM-SMCs to a larger extent than nBM-SMCs. (A) nBM-SMCs or aBM-SMCs were cultured with the indicated bFGF concentrations and cell number was determined 3 days later and normalized each to cell number without bFGF. (B) Phase contrast microscopy showed that bFGF induced significant morphological changes to aBM-SMCs and nBM-SMCs (bar = 100 µm). (C) aBM-SMCs and nBM-SMCs expressed similar level of FGFR1 as determined by RT–PCR. GAPDH served as a loading control.
3.7. TGF-β1 enhanced hydrogel compaction by aBM-SMCs
Previous work in our laboratory and others showed that TGF-β1 enhanced V-SMC contractility.17,22,23 Therefore, we examined whether TGF-β1 could improve the force-generating ability of aBM-SMCs. To this end, aBM-SMCs and nBM-SMCs were embedded in fibrin hydrogels that were allowed to compact in the presence or absence of TGF-β1 (2 ng/mL). Fibrinolytic inhibitor ε-aminocaproic acid was added to all samples to eliminate the effects of fibrinolysis on compaction.
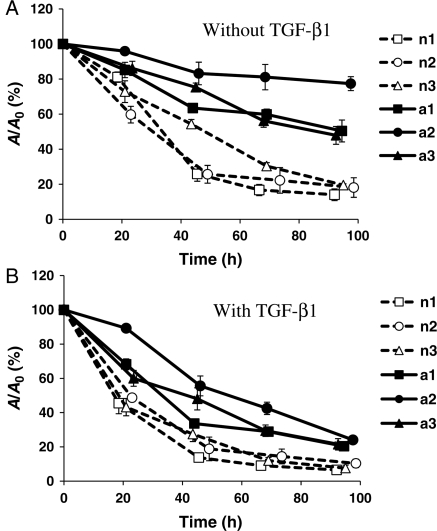
In the absence of TGF-β1, nBM-SMCs contracted fibrin hydrogels significantly, reaching 50% of their initial area within 30–50 h (t50%) and 14–20% of the initial area within 96–100 h (final compaction). In contrast, aBM-SMCs showed impaired gel compaction ability, reaching to only 50–77% of the initial area after 95–100 h (Figure 6A). Addition of TGF-β1 to nBM-SMCs reduced t50% to 20–25 h and increased the final extent of compaction to 7–10% of the initial area. Similarly, for aBM-SMCs, TGF-β1 reduced t50% to 35–40 h and improved the final extent of compaction to 21–24% of the initial hydrogel area, suggesting that TGF-β1 can restore the force generation deficit of aBM-SMCs to a similar level as that of nBM-SMCs under control conditions (no TGF-β1) (Figure 6B).
Figure 6.
TGF-β1 increased fibrin gel contractility in nBM-SMCs and aBM-SMCs. Cells were embedded and polymerized in fibrin and allowed to compact gels in the absence (A) or presence (B) of TGF-β1. At the indicated times, the gels were photographed and their area was measured using Image J software. The ratio of the area of each gel (A) at the indicated time over the initial area (A0) was plotted as a function of time.
4. Discussion
Lack of an easily accessible, autologous cell source is a major barrier for the development of tissue-engineered vascular grafts, especially for elder patients who are most likely to suffer from vascular diseases. Previously, we demonstrated a novel method to isolate pure population of SMCs from BM-MSCs using the αSMA promoter and cell sorting.18 BM-SMCs showed high proliferation potential and displayed V-SMC-like phenotype and functionality. Moreover, when BM-TEVs were implanted into the jugular veins of lambs, they showed significant matrix remodelling with expression of collagen and elastin that was organized in a fibrillar network similar to native vessels. These results suggested that nBM-SMCs are a promising cell source for development of cell therapeutics and raised the question whether aBM-SMCs would have similar potential. This is an important issue as older patients are more likely to suffer from cardiovascular disease and in need of vascular grafts. To this end, we examined the effects of organismal ageing on BM-SMCs with respect to proliferation potential and vascular function.
We found that aBM-SMCs exhibited longer doubling time and limited clonogenic potential compared with nBM-SMCs. Interestingly, bFGF increased proliferation of aBM-SMCs by up to 2.5-fold but had no significant effect on nBM-SMCs, despite a similar level of FGFR1 expression by both cells. This result is in agreement with previous studies showing that bFGF accelerated proliferation and maintained the undifferentiated state of BM stem cells29,32,33 and slow-cycling human multipotent adipose-derived stem (hMADS) cells.34 In contrast to slow-cycling hMADS cells, which expressed lower amount of bFGF than their fast proliferating counterparts,34 aBM-SMCs and nBM-SMCs expressed similar levels of bFGF (data not shown) and FGFR1 receptor mRNA, suggesting that other factors may be responsible for the observed difference. Although the mechanism of bFGF's action remains elusive, inhibition of cell cycle arrest factors and down-regulation of TGF-β235 may account—at least in part—for such effects.
Functional experiments with TEVs prepared from nBM-SMCs or aBM-SMCs revealed differences that depended on donor ageing. Specifically, TEVs from nBM-SMCs exhibited higher hydrogel compaction and vascular contractility compared with aBM-TEVs. These results prompted us to examine whether aBM-SMC function could be further improved by addition of TGF-β1, which was previously shown to promote SMC differentiation of embryonic stem cells,36,37 BM-MSCs,38–40 bone marrow multipotent stem cells41 and hair follicle stem cells.17 TGF-β1 also enhanced the mechanical strength42 and vascular reactivity of fibrin-based V-TEV.23 In agreement with these studies, TGF-β1 improved fibrin compaction by aBM-SMCs significantly to the level of nBM-SMCs under control (no TGF-β1) conditions. Collectively, our results suggest that sequential treatment of bFGF in cell culture followed by TGF-β1 during generation of 3D contractile TEV constructs may allow cell expansion and engineering TEVs with enhanced vascular function.
The higher contractility of nBM-SMCs correlated with higher expression of SMC markers such as αSMA, smoothelin, and desmin. Expression of αSMA has been correlated with contractility in several studies,24,25 whereas smoothelin expression is thought to be an indicator of contractile SMCs.27 Specifically, smoothelin expression decreased during neointima formation following endothelial injury when SMCs de-differentiated and resumed proliferation. At later times, as proliferation decreased and SMCs re-gained contractility, the level of smoothelin increased again suggesting that smoothelin expression mirrors the state of SMC differentiation.26 Finally, studies with knockout mice suggested that desmin expression is required for efficient control of vascular contractile and dilatory functions in small resistance arteries.28,43 Taken collectively, these results suggest that nBM-SMCs have higher potential to develop a contractile phenotype than aBM-SMCs.
Although the SMC phenotype is still under debate, it is generally accepted that the contractile and proliferative (or synthetic) phenotypes are two ends of a spectrum.44 SMCs can either proliferate and produce ECM components or develop a contractile phenotype that enables them to respond to mechanical and biochemical stimuli and to control blood pressure and blood flow. Our data showed that nBM-SMCs were not only more proliferative but that they also displayed higher contractility. Although these results may seem to contradict the accepted view of SMC phenotype, it must be noted that SMC contractility was examined after the cells were induced to differentiate in the presence of two soluble factors—TGF-β1 and ascorbic acid—which have been previously shown to promote the contractile SMC phenotype.17,22,23 Culture in a 3D environment—within a fibrin scaffold polymerized around a mandrel—might also have contributed to the development of contractile phenotype by constraining hydrogel compaction and inducing cellular alignment in the circumferential direction.45 Therefore, our experiments suggest the following: (i) under growth-promoting conditions, nBM-SMCs proliferate faster and have higher clonogenic potential, indicating that they are not terminally differentiated SMCs but perhaps SMC-lineage committed progenitors; (ii) under differentiation-promoting conditions, nBM-SMCs exhibited higher SMC differentiation potential than aBM-SMCs, as exemplified by development of superior contractile properties.
Our results are in agreement with recent reports demonstrating that ageing decreased not only the proliferation potential but also the myogenic46 and osteogenic6,47 differentiation capacity of BM-MSCs. In contrast to MSCs, Zhang et al.46 reported that ageing did not affect the cardiac regeneration ability of SMCs isolated from the rat aorta. The reason for this result is unclear but the several reports in the literature suggest that the cardiac regeneration potential of injected MSCs is more likely due to indirect effects such as secretion of growth factors that may induce proliferation of resident cardiac cells rather than direct tissue regeneration due to MSC infiltration.48 Such indirect effects may not be affected by cell ageing. However, when cells are used to prepare vascular grafts or other engineered tissues, proliferation and development of contractile properties are critical, necessitating development of strategies to restore age-dependent deterioration of cellular function.
The differences in mechanical properties and vascular contractility between aBM-SMCs and nBM-SMCs may be at least in part due to differences in matrix remodelling. To examine this possibility, we measured the expression level of ECM proteins such as elastin and collagen type I and III, the main ECM components affecting the mechanical properties of arteries and veins. Interestingly, both aBM-SMCs and nBM-SMCs expressed higher amounts of type I collagen than V-SMCs from neonatal animals. Similar results were observed with type III collagen with the exception of one nBM-SMC sample, which showed similar expression levels as V-SMCs. Notably, nBM-SMCs expressed over 200 times higher level of elastin transcripts compared with V-SMCs. This result supports our previous in vivo experiments,18 which showed that after implantation into jugular veins of lambs, TEVs from nBM-SMCs synthesized high amounts of elastin that was organized in a fibrillar network very similar to native vessels. In contrast, TEVs from V-SMCs exhibited very little elastin deposition even at 15-week post-implantation.19 Although inferior to nBM-SMCs, aBM-SMCs expressed significantly higher elastin levels than V-SMCs from neonatal animals, suggesting that BM-SMCs may be a superior cell source for vascular tissue engineering even when they originate from adult donors. We are currently working towards testing this hypothesis in vivo using the ovine animal model that we previously developed in our laboratory.18,19
In summary, our study demonstrated that organismal ageing affects the proliferation and clonogenic ability of BM-SMCs as well as the mechanical properties of the resulting fibrin-based vascular constructs. These results correlated with expression of key SMC-specific markers and ECM molecules suggesting a direct relationship between matrix remodelling and vascular function. Interestingly, BM-SMCs from aged animals exhibited higher ECM expression levels compared with V-SMCs from the veins of neonatal animals, suggesting that vascular constructs prepared from these cells may have the potential for implantation and in vivo remodelling. High-throughput genomics and proteomic studies may determine the signalling networks that account for the functional differences between aBM-SMCs and nBM-SMCs and provide potential intervention targets to enhance proliferation and function of vascular cells from adult donors.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This work was supported by grants from the National Institutes of Health (R01 HL086582) and the John R. Oishei Foundation to S.T.A.
Supplementary Material
References
- 1.Roobrouck VD, Ulloa-Montoya F, Verfaillie CM. Self-renewal and differentiation capacity of young and aged stem cells. Exp Cell Res. 2008;314:1937–1944. doi: 10.1016/j.yexcr.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Stenderup K, Justesen J, Clausen C, Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33:919–926. doi: 10.1016/j.bone.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, Bellantuono I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells. 2004;22:675–682. doi: 10.1634/stemcells.22-5-675. [DOI] [PubMed] [Google Scholar]
- 4.Hacia JG, Lee CC, Jimenez DF, Karaman MW, Ho VV, Siegmund KD, et al. Age-related gene expression profiles of rhesus monkey bone marrow-derived mesenchymal stem cells. J Cell Biochem. 2008;103:1198–1210. doi: 10.1002/jcb.21498. [DOI] [PubMed] [Google Scholar]
- 5.Meirelles Lda S, Nardi NB. Murine marrow-derived mesenchymal stem cell: isolation, in vitro expansion, and characterization. Br J Haematol. 2003;123:702–711. doi: 10.1046/j.1365-2141.2003.04669.x. [DOI] [PubMed] [Google Scholar]
- 6.Zhou S, Greenberger JS, Epperly MW, Goff JP, Adler C, Leboff MS, et al. Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell. 2008;7:335–343. doi: 10.1111/j.1474-9726.2008.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Ippolito G, Schiller PC, Ricordi C, Roos BA, Howard GA. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res. 1999;14:1115–1122. doi: 10.1359/jbmr.1999.14.7.1115. [DOI] [PubMed] [Google Scholar]
- 8.Zheng H, Martin JA, Duwayri Y, Falcon G, Buckwalter JA. Impact of aging on rat bone marrow-derived stem cell chondrogenesis. J Gerontol A Biol Sci Med Sci. 2007;62:136–148. doi: 10.1093/gerona/62.2.136. [DOI] [PubMed] [Google Scholar]
- 9.Roura S, Farre J, Soler-Botija C, Llach A, Hove-Madsen L, Cairo JJ, et al. Effect of aging on the pluripotential capacity of human CD105(+) mesenchymal stem cells. Eur J Heart Fail. 2006;8:555–563. doi: 10.1016/j.ejheart.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Dressler MR, Butler DL, Boivin GP. Effects of age on the repair ability of mesenchymal stem cells in rabbit tendon. J Orthop Res. 2005;23:287–293. doi: 10.1016/j.orthres.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 11.Ruiz-Torres A, Gimeno A, Melon J, Mendez L, Munoz FJ, Macia M. Age-related loss of proliferative activity of human vascular smooth muscle cells in culture. Mech Ageing Dev. 1999;110:49–55. doi: 10.1016/s0047-6374(99)00042-1. [DOI] [PubMed] [Google Scholar]
- 12.Solan A, Niklason L. Age effects on vascular smooth muscle: an engineered tissue approach. Cell Transplant. 2005;14:481–488. doi: 10.3727/000000005783982918. [DOI] [PubMed] [Google Scholar]
- 13.Gong Z, Niklason LE. Blood vessels engineered from human cells. Trends Cardiovasc Med. 2006;16:153–156. doi: 10.1016/j.tcm.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 14.McKee JA, Banik SS, Boyer MJ, Hamad NM, Lawson JH, Niklason LE, et al. Human arteries engineered in vitro. EMBO Rep. 2003;4:633–638. doi: 10.1038/sj.embor.embor847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poh M, Boyer M, Solan A, Dahl SL, Pedrotty D, Banik SS, et al. Blood vessels engineered from human cells. Lancet. 2005;365:2122–2124. doi: 10.1016/S0140-6736(05)66735-9. [DOI] [PubMed] [Google Scholar]
- 16.Klinger RY, Blum JL, Hearn B, Lebow B, Niklason LE. Relevance and safety of telomerase for human tissue engineering. Proc Natl Acad Sci USA. 2006;103:2500–2505. doi: 10.1073/pnas.0508184103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu JY, Peng HF, Andreadis ST. Contractile smooth muscle cells derived from hair-follicle stem cells. Cardiovasc Res. 2008;79:24–33. doi: 10.1093/cvr/cvn059. [DOI] [PubMed] [Google Scholar]
- 18.Liu JY, Swartz DD, Peng HF, Gugino SF, Russell JA, Andreadis ST. Functional tissue-engineered blood vessels from bone marrow progenitor cells. Cardiovasc Res. 2007;75:618–628. doi: 10.1016/j.cardiores.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 19.Swartz DD, Russell JA, Andreadis ST. Engineering of fibrin-based functional and implantable small-diameter blood vessels. Am J Physiol Heart Circ Physiol. 2005;288:H1451–H1460. doi: 10.1152/ajpheart.00479.2004. [DOI] [PubMed] [Google Scholar]
- 20.Koria P, Brazeau D, Kirkwood K, Hayden P, Klausner M, Andreadis ST. Gene expression profile of tissue engineered skin subjected to acute barrier disruption. J Invest Dermatol. 2003;121:368–382. doi: 10.1046/j.1523-1747.2003.12364.x. [DOI] [PubMed] [Google Scholar]
- 21.An YH, Freidman RJ, editors. Animal Models in Orthopaedic Research. Boca Raton, FL: CRC Press; 1999. [Google Scholar]
- 22.Grainger DJ. Transforming growth factor beta and atherosclerosis: so far, so good for the protective cytokine hypothesis. Arterioscler Thromb Vasc Biol. 2004;24:399–404. doi: 10.1161/01.ATV.0000114567.76772.33. [DOI] [PubMed] [Google Scholar]
- 23.Yao L, Swartz DD, Gugino SF, Russell JA, Andreadis ST. Fibrin-based tissue-engineered blood vessels: differential effects of biomaterial and culture parameters on mechanical strength and vascular reactivity. Tissue Eng. 2005;11:991–1003. doi: 10.1089/ten.2005.11.991. [DOI] [PubMed] [Google Scholar]
- 24.Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell. 2001;12:2730–2741. doi: 10.1091/mbc.12.9.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J, Li H, SundarRaj N, Wang JH. Alpha-smooth muscle actin expression enhances cell traction force. Cell Motil Cytoskeleton. 2007;64:248–257. doi: 10.1002/cm.20178. [DOI] [PubMed] [Google Scholar]
- 26.Bar H, Wende P, Watson L, Denger S, van Eys G, Kreuzer J, et al. Smoothelin is an indicator of reversible phenotype modulation of smooth muscle cells in balloon-injured rat carotid arteries. Basic Res Cardiol. 2002;97:9–16. doi: 10.1007/s395-002-8382-z. [DOI] [PubMed] [Google Scholar]
- 27.van der Loop FT, Gabbiani G, Kohnen G, Ramaekers FC, van Eys GJ. Differentiation of smooth muscle cells in human blood vessels as defined by smoothelin, a novel marker for the contractile phenotype. Arterioscler Thromb Vasc Biol. 1997;17:665–671. doi: 10.1161/01.atv.17.4.665. [DOI] [PubMed] [Google Scholar]
- 28.Loufrani L, Matrougui K, Li Z, Levy BI, Lacolley P, Paulin D, et al. Selective microvascular dysfunction in mice lacking the gene encoding for desmin. FASEB J. 2002;16:117–119. doi: 10.1096/fj.01-0505fje. [DOI] [PubMed] [Google Scholar]
- 29.Tsutsumi S, Shimazu A, Miyazaki K, Pan H, Koike C, Yoshida E, et al. Retention of multilineage differentiation potential of mesenchymal cells during proliferation in response to FGF. Biochem Biophys Res Commun. 2001;288:413–419. doi: 10.1006/bbrc.2001.5777. [DOI] [PubMed] [Google Scholar]
- 30.Solchaga LA, Penick K, Porter JD, Goldberg VM, Caplan AI, Welter JF. FGF-2 enhances the mitotic and chondrogenic potentials of human adult bone marrow-derived mesenchymal stem cells. J Cell Physiol. 2005;203:398–409. doi: 10.1002/jcp.20238. [DOI] [PubMed] [Google Scholar]
- 31.Vallier L, Alexander M, Pedersen RA. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J Cell Sci. 2005;118:4495–4509. doi: 10.1242/jcs.02553. [DOI] [PubMed] [Google Scholar]
- 32.Bianchi G, Banfi A, Mastrogiacomo M, Notaro R, Luzzatto L, Cancedda R, et al. Ex vivo enrichment of mesenchymal cell progenitors by fibroblast growth factor 2. Exp Cell Res. 2003;287:98–105. doi: 10.1016/s0014-4827(03)00138-1. [DOI] [PubMed] [Google Scholar]
- 33.Mastrogiacomo M, Cancedda R, Quarto R. Effect of different growth factors on the chondrogenic potential of human bone marrow stromal cells. Osteoarthritis Cartilage. 2001;9(Suppl. A):S36–S40. doi: 10.1053/joca.2001.0442. [DOI] [PubMed] [Google Scholar]
- 34.Zaragosi LE, Ailhaud G, Dani C. Autocrine fibroblast growth factor 2 signaling is critical for self-renewal of human multipotent adipose-derived stem cells. Stem Cells. 2006;24:2412–2419. doi: 10.1634/stemcells.2006-0006. [DOI] [PubMed] [Google Scholar]
- 35.Ito T, Sawada R, Fujiwara Y, Seyama Y, Tsuchiya T. FGF-2 suppresses cellular senescence of human mesenchymal stem cells by down-regulation of TGF-beta2. Biochem Biophys Res Commun. 2007;359:108–114. doi: 10.1016/j.bbrc.2007.05.067. [DOI] [PubMed] [Google Scholar]
- 36.Sinha S, Hoofnagle MH, Kingston PA, McCanna ME, Owens GK. Transforming growth factor-beta1 signaling contributes to development of smooth muscle cells from embryonic stem cells. Am J Physiol Cell Physiol. 2004;287:C1560–C1568. doi: 10.1152/ajpcell.00221.2004. [DOI] [PubMed] [Google Scholar]
- 37.Hirschi KK, Rohovsky SA, D'Amore PA. PDGF, TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J Cell Biol. 1998;141:805–814. doi: 10.1083/jcb.141.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kinner B, Zaleskas JM, Spector M. Regulation of smooth muscle actin expression and contraction in adult human mesenchymal stem cells. Exp Cell Res. 2002;278:72–83. doi: 10.1006/excr.2002.5561. [DOI] [PubMed] [Google Scholar]
- 39.Wang D, Park JS, Chu JS, Krakowski A, Luo K, Chen DJ, et al. Proteomic profiling of bone marrow mesenchymal stem cells upon transforming growth factor beta1 stimulation. J Biol Chem. 2004;279:43725–43734. doi: 10.1074/jbc.M407368200. [DOI] [PubMed] [Google Scholar]
- 40.Gong Z, Niklason LE. Small-diameter human vessel wall engineered from bone marrow-derived mesenchymal stem cells (hMSCs) FASEB J. 2008;22:1635–1648. doi: 10.1096/fj.07-087924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ross JJ, Hong Z, Willenbring B, Zeng L, Isenberg B, Lee EH, et al. Cytokine-induced differentiation of multipotent adult progenitor cells into functional smooth muscle cells. J Clin Invest. 2006;116:3139–3149. doi: 10.1172/JCI28184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Long JL, Tranquillo RT. Elastic fiber production in cardiovascular tissue-equivalents. Matrix Biol. 2003;22:339–350. doi: 10.1016/s0945-053x(03)00052-0. [DOI] [PubMed] [Google Scholar]
- 43.Loufrani L, Li Z, Levy BI, Paulin D, Henrion D. Excessive microvascular adaptation to changes in blood flow in mice lacking gene encoding for desmin. Arterioscler Thromb Vasc Biol. 2002;22:1579–1584. doi: 10.1161/01.atv.0000032652.24932.1a. [DOI] [PubMed] [Google Scholar]
- 44.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 45.Barocas VH, Girton TS, Tranquillo RT. Engineered alignment in media equivalents: magnetic prealignment and mandrel compaction. J Biomech Eng. 1998;120:660–666. doi: 10.1115/1.2834759. [DOI] [PubMed] [Google Scholar]
- 46.Zhang H, Fazel S, Tian H, Mickle DA, Weisel RD, Fujii T, et al. Increasing donor age adversely impacts beneficial effects of bone marrow but not smooth muscle myocardial cell therapy. Am J Physiol Heart Circ Physiol. 2005;289:H2089–H2096. doi: 10.1152/ajpheart.00019.2005. [DOI] [PubMed] [Google Scholar]
- 47.Zhang ZY, Teoh SH, Chong MS, Schantz JT, Fisk NM, Choolani MA, et al. Superior osteogenic capacity for bone tissue engineering of fetal compared to perinatal and adult mesenchymal stem cells. Stem Cells. 2009;27:126–137. doi: 10.1634/stemcells.2008-0456. [DOI] [PubMed] [Google Scholar]
- 48.Psaltis PJ, Zannettino AC, Worthley SG, Gronthos S. Concise review: mesenchymal stromal cells: potential for cardiovascular repair. Stem Cells. 2008;26:2201–2210. doi: 10.1634/stemcells.2008-0428. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.