Abstract
Reversible protein phosphorylation is a key regulatory mechanism of mitotic progression. Importantly, protein kinases themselves are also regulated by phosphorylation-dephosphorylation processes; hence, phosphorylation dynamics of kinases hold a wealth of information about phosphorylation networks. Here, we investigated the site-specific phosphorylation dynamics of human kinases during mitosis using synchronization of HeLa suspension cells, kinase enrichment, and high resolution mass spectrometry. In biological triplicate analyses, we identified 206 protein kinases and more than 900 protein kinase phosphorylation sites, including 61 phosphorylation sites on activation segments, and quantified their relative abundances across three specific mitotic stages. Around 25% of the kinase phosphorylation site ratios were found to be changed by at least 50% during mitotic progression. Further network analysis of jointly regulated kinase groups suggested that Cyclin-dependent kinase- and mitogen-activated kinase-centered interaction networks are coordinately down- and up-regulated in late mitosis, respectively. Importantly, our data cover most of the already known mitotic kinases and, moreover, identify attractive candidates for future studies of phosphorylation-based mitotic signaling. Thus, the results of this study provide a valuable resource for cell biologists and provide insight into the system properties of the mitotic phosphokinome.
Reversible phosphorylation is a ubiquitous posttranslational protein modification that is involved in the regulation of almost all biological processes (1–3). In human, 518 protein kinases have been identified in the genome that phosphorylate the majority of cellular proteins and increase the diversity of the proteome by severalfold (4). Addition of a phosphate group to a protein can alter its structural, catalytic, and functional properties; hence, kinases require tight regulation to avoid unspecific phosphorylation, which can be deleterious to cells (5–7). As a result, cells use a variety of mechanisms to ensure proper regulation of kinase activities (8). Importantly, most kinases are also in turn regulated through autophosphorylation and phosphorylation by other kinases, thus generating complex phosphorylation networks. In particular, phosphorylation on activation segments is a common mechanism to modulate kinase activities (9–11), but additional phosphorylation sites are also frequently required for fine tuning of kinase localizations and functions (12). Some kinases contain phosphopeptide binding domains that recognize prephosphorylated sites on other kinases, resulting in processive phosphorylation and/or targeting of kinases to distinct cellular locations (13–16). Because such priming phosphorylation events depend on the activities of the priming kinases, these motifs act as conditional docking sites and restrict the interaction with docking kinases to a particular point in time and physiological state. In addition, phosphorylation sites may act through combinatorial mechanisms or through cross-talk with other posttranslational modifications (PTMs)1 (17, 18), thus further increasing the complexity of kinase regulatory networks.
Regulation of kinases is of particular interest in mitosis as most of the mitotic events are regulated by reversible protein phosphorylation (19). During mitosis, error-free segregation of sister chromatids into the two daughter cells is essential to ensure genomic stability. Physically, this process is carried out by the mitotic spindle, a highly dynamic microtubule-based structure. After entry into mitosis, the major microtubule-organizing centers in animal cells, the centrosomes, start to increase microtubule nucleation and move to opposite poles of the cell. Throughout prometaphase, microtubules emanating from centrosomes are captured by kinetochores, protein complexes assembled on centromeric chromosomal DNA. This eventually leads to the alignment of all chromosomes in a metaphase plate. Because proper bipolar attachment of chromosomes to spindle microtubules is essential for the correct segregation of chromosomes, this critical step is monitored by a signaling pathway known as the spindle assembly checkpoint (SAC) (20). This checkpoint is silenced only after all chromosomes have attached to the spindle in a bioriented fashion, resulting in the synchronous segregation of sister chromatids during anaphase. Simultaneously, a so-called central spindle is formed between the separating chromatids, and the formation of a contractile ring initiates cytokinesis. Finally, in telophase, the chromosomes decondense and reassemble into nuclei, whereas remnants of the central spindle form the midbody, marking the site of abscission. Cyclin-dependent kinase 1 (Cdk1), an evolutionarily conserved master mitotic kinase, is activated prior to mitosis and initiates most of the mitotic events. Cdk1 works in close association with other essential mitotic kinases such as Plk1, Aurora A, and Aurora B for the regulation of mitotic progression (19, 21–24). Plk1 and Aurora kinases dynamically localize to different subcellular locations to perform multiple functions during mitosis and are phosphorylated at several conserved sites. Although little is known about the precise roles of these phosphorylation sites, emerging data indicate that they are involved in regulating localization-specific functions (25, 26). Furthermore, the kinases Bub1, BubR1, and TTK (Mps1) and kinases of the Nek family play important roles in maintaining the fidelity and robustness of mitosis (19). Recently, a genome-wide RNA-mediated interference screen identified M phase phenotypes for many kinases that have not previously been implicated in cell cycle functions, indicating that additional kinases have important mitotic functions (27).
Although protein phosphorylation plays a pivotal role in the regulation of cellular networks, many phosphorylation events remain undiscovered mainly because of technical limitations (28). The advent of mass spectrometry-based proteomics along with developments in phosphopeptide enrichment methods has enabled large scale global phosphoproteomics studies (29, 30). However, the number of phosphorylation sites identified on kinases is limited compared with other proteins because of their frequently low expression levels. To overcome this problem, small inhibitor-based kinase enrichment strategies were developed, resulting in the identification of more than 200 kinases from HeLa cell lysates (31, 32). This method was also used recently to compare the phosphokinomes during S phase and M phase of the cell cycle, resulting in the identification of several hundreds of M phase-specific kinase phosphorylation sites (31). In the present study, we address the dynamics of the phosphokinome during mitotic progression using large scale cell synchronization at three distinct mitotic stages, small inhibitor-based kinase enrichment, and stable isotope labeling by amino acids in cell culture (SILAC)-based quantitative mass spectrometry. Thus, we determined the mitotic phosphorylation dynamics of more than 900 kinase phosphorylation sites and identified distinctly regulated kinase interaction networks. Our results provide a valuable resource for the dynamics of the kinome during mitotic progression and give insight into the system properties of kinase interaction networks.
EXPERIMENTAL PROCEDURES
Antibodies
Anti-Ser(P)-676 BubR1 was raised in house (33); anti-Thr(P)-180/Tyr(P)-182 p38 (catalog number 9211), anti-p38 (catalog number 9212), anti-Thr(P)173/Tyr(p)-175 ERK1/2 (catalog number 4376), and anti-ERK1/2 (catalog number 9102) were from Cell Signaling Technology; anti-Ser(P)-10 Histone H3 (catalog number 06570) and anti-Cyclin B (catalog number 05373) were from Upstate; anti-Securin (catalog number ab3305) was from Abcam; and anti-α-Tubulin (catalog number T9026) was from Sigma-Aldrich. Fluorescein labeled anti-α-Tublin antibody was from Sigma-Aldrich (catalog number F2168).
Cell Culture, SILAC Labeling, and Synchronization
For SILAC, HeLa suspension (S) cells were grown in Dulbecco's modified Eagle's medium containing 10% dialyzed fetal calf serum (PAA Laboratories); 50 units/ml penicillin; 50 μg/ml streptomycin; and unlabeled l-arginine (R0) and l-lysine (K0) (Sigma) (SILAC light), l-[U-13C6,14N4]arginine (R6) and l-[2H4]lysine (K4) (SILAC medium), or l-[U-13C6,15N4]arginine (R10) and l-[U-13C6,15N2]lysine (K8) (SILAC heavy) (Cambridge Isotope Laboratories). Cells were grown at 37 °C in a humidified incubator with 5% CO2 atmosphere. After five cell doublings on culture dishes, cell cultures were expanded in spinner flasks (INTEGRA Biosciences). Spinner flasks were operated at 45 rpm and were kept inside the cell culture incubator. Prior to synchronization, cells were transferred to 400 ml of fresh medium with 20% FCS, and the cell density was adjusted to 0.6 × 106 cells/ml. HeLa S cells were first synchronized at the G1/S phase boundary by treatment with 4 mm thymidine (Sigma) for 16 h. They were then released for 12 h into fresh medium. After 4 h of release, 40 ng/ml nocodazole (Sigma) was added to arrest the cells in prometaphase. One population of the prometaphase-arrested cells was harvested by centrifugation, whereas the two other populations were washed once, centrifuged at 300 × g in PBS, and released in fresh medium containing 20 μm MG132 (Calbiochem) to induce metaphase arrest. The population of metaphase-arrested cells was then harvested by centrifugation, whereas the last population of cells was washed and released in fresh medium for 90 min when most of them had reached telophase. Harvested cells were snap frozen by liquid nitrogen and stored at −80 °C until cell lysis.
Immunofluorescence Staining of Cells
Before harvesting the cells at different mitotic stages, a small aliquot of around 0.5 ml of cell suspension was taken into a 1.5-ml tube and incubated with fixing solution (1% paraformaldehyde and 3% sucrose). Approximately 10% of the fixed cells were transferred to a fresh 1.5-ml tube and treated with Triton X-100 (final concentration, 0.1%) at room temperature for 15 min with constant shaking. Cells were then centrifuged at 300 × g, and the supernatant was discarded. The cell pellet was resuspended in 100 μl of PBS with 2% BSA. 4′,6-Diamidino-2-phenylindole and Fluorescein labeled anti-α-Tubulin antibody were added. After 60 min of incubation, unbound 4′,6-diamidino-2-phenylindole and antibody were removed, and cells were washed once with PBS to reduce background staining. Cells were kept at 4 °C, and the mitotic stages of fixed cells were examined by immunofluorescence microscopy.
Kinase Enrichment
Affinity chromatography with the immobilized kinase inhibitors VI16832 (31), bisindolylmaleimide X (Alexis Biochemicals), AX14596 (34), SU6668 (35), and purvalanol B (Tocris) was done on an ÄKTA Explorer system (GE Healthcare) as described except that 2 volumes of 1.5 mm and 5 mm inhibitor solutions were incubated with 1 volume of epoxy-activated Sepharose beads to generate the VI16832 and bisindolylmaleimide X affinity resins, respectively (31, 36). Cell lysate was prepared as reported previously (36), and equal protein amounts of prometaphase, metaphase, and telophase HeLa S cell extracts were combined prior to multiresin affinity chromatography. Combined total cell extracts were loaded on the five different inhibitor resins filled into three consecutive columns at a flow rate of 70 μl/min. The first and third columns contained 0.5 ml of the V16832 and purvalanol B affinity resins, whereas the second column was packed with 0.33 ml of the SU6668, AX14596, and bisindolylmaleimide X matrices on top of each other. After sample loading and column washing, resin-bound proteins were eluted and concentrated as described previously (31).
In-gel Protein Digestion
Around 700 μg of the kinase-enriched fraction was dissolved in lithium dodecyl sulfate loading buffer and separated by SDS-PAGE using NuPAGE Novex Bis-Tris gels (Invitrogen) according to the manufacturer's instructions. The gel was stained with Coomassie Blue, and protein bands were visualized. The resulting lane was cut into around 15 slices, which were then subjected to in-gel digestion essentially as described previously (37). Gel slices were cut into small cubes of 1 mm3 and washed with 50 mm ammonium bicarbonate (ABC) and 30% ACN until the cubes were fully destained. Gel cubes were dehydrated with 100% ACN and rehydrated with 50 mm ABC containing 10 mm DTT. Proteins were reduced for 1 h at 56 °C. Resulting free thiol groups were then alkylated by adding 55 mm iodoacetamide in 50 mm ABC for 30 min at 25 °C in the dark. Gel pieces were washed twice with a 50 mm ABC solution, dehydrated with 100% ACN, and dried in a vacuum concentrator. Each gel fraction was rehydrated in 50 mm ABC solution containing 15 ng/μl trypsin, and samples were incubated at 37 °C overnight. Supernatants were transferred to new tubes, and residual peptides were extracted out of the gel pieces by incubation with 30% ACN in 5% formic acid and subsequent incubation with 100% ACN. All extracts were combined, and ACN was evaporated in a vacuum concentrator. In the case of experiment 3, peptides were analyzed twice (technical replicate).
In-solution Protein Digestion
One-third of the precipitated kinase-enriched fraction was directly dissolved in 20 mm HEPES buffer (pH 7.5) containing 7 m urea and 2 m thiourea. Proteins were reduced by adding 2 mm DTT (final concentration) for 45 min at 25 °C, and thiols were carboxymethylated with 5.5 mm iodoacetamide for 30 min at room temperature. Endoproteinase Lys-C (Wako) was added in an enzyme/substrate ratio of 1:50 (5–6 μg of Lys-C), and the proteins were digested for 4 h at room temperature. The resulting peptide mixtures were diluted with water (1:4) to reduce the final urea concentration below 2 m. For double digestion, modified trypsin (sequencing grade, Roche Applied Science) was added in an enzyme/substrate ratio of 1:50 (5–6 μg of trypsin), and the digest was incubated at 30 °C overnight. Trypsin activity was quenched by adding TFA to a final concentration of 1%.
Strong Cation Exchange (SCX)
The in-solution digests were loaded onto an SCX column (polysulfoethyl, 1 × 150 mm, 5-μm particles, 200-Å pore size; PolyLC Inc.) using a Jasco 2000-series HPLC system (Jasco). Peptides were separated by a linear 30-min gradient between 100% buffer A (5 mm KH2PO4, pH 2.7, 33% ACN) and 30% buffer B (350 mm KCl, 5 mm KH2PO4, pH 2.7, 33% ACN) with a flow rate of 120 μl/min. 60-s fractions were collected using an automated fraction collector.
Phosphopeptide Enrichment and Desalting
Titanium dioxide beads were used to selectively enrich for phosphorylated peptides with glycolic acid as a modifier (29). About 3 mg of titanium dioxide beads were transferred to a GELoader tip (plugged at the constricted end by a small piece of C8 material) and washed with 40 μl of 80 μg/μl glycolic acid in a mixture of 80% ACN and 0.2% TFA. Digested peptides were dissolved in 20 μl of 80 μg/μl glycolic acid in a mixture of 80% ACN and 2% TFA and applied to microcolumns, allowing phosphopeptides to bind to the titanium dioxide phase. Microcolumns were then washed with 40 μl of 80 μg/μl glycolic acid in a mixture of 80% ACN and 0.2% TFA and 40 μl of a mixture of 80% ACN and 0.2% TFA (collected as flow-through). Finally, phosphorylated peptides were eluted slowly with 40 μl of 0.6% NH4OH and subsequently 40 μl of 60% ACN. Flow-through fractions were desalted with C18 reversed-phase material prior to LC-MS/MS analysis essentially as described (38).
Nano-LC-MS/MS Analysis
All peptide samples were separated by on-line reverse phase nano-LC and analyzed by electrospray MS/MS. Using a nanoACQUITY ultra performance liquid chromatography system (Waters), samples were injected onto a 14-cm fused silica capillary column with an inner diameter of 75 μm and a tip of 8 μm (New Objective) packed in house with 3-μm ReproSil-Pur C18-AQ (Dr. Maisch GmbH). The LC setup was connected to an LTQ-Orbitrap mass spectrometer (Thermo Fisher Scientific) equipped with a nanoelectrospray ion source (Proxeon Biosystems). Peptides were separated and eluted by a stepwise 180-min gradient of 0–100% between buffer A (0.2% formic acid in water) and buffer B (0.2% formic acid in ACN). Data-dependent acquisition was performed on the LTQ-Orbitrap using Xcalibur software in the positive ion mode. Survey full-scan MS spectra (from m/z 300 to 2000) were acquired in the FT-Orbitrap with a resolution of 60,000 at m/z 400. A maximum of five peptides was sequentially isolated for fragmentation in the linear ion trap using CID. The Orbitrap lock mass feature was applied to improve mass accuracy as described (39). To improve phosphopeptide analysis, the multistage activation option in the software was enabled, and the neutral loss species at 97.97, 48.99, or 32.66 m/z below the precursor ion were activated for 30 ms during fragmentation (pseudo-MS3) (40).
Data Processing and Analysis
Raw data files were processed using the MaxQuant software suite (version 1.0.12.5), which performs, among other tasks, peak list generation, mass recalibration, SILAC-based quantitation, estimation of false discovery rates based on Mascot search results, and assembling of peptides to protein groups (41). Generation of peak lists was performed with the following MaxQuant parameters: top 12 MS/MS peaks for 100 Da, three data points for centroid, Gaussian centroid determination, and slice peaks at local minima. During the peak list generation, MaxQuant identified potential SILAC pairs based on mass differences of specified labeled amino acids, intensity correlation over elution time, etc. Mascot (version 2.2.0, Matrix Science) was used for peptide identifications (42). The initial precursor mass tolerance was set to ±7 ppm, whereas an accuracy of ±0.5 Da was used for MS/MS fragmentation spectra. Carbamidomethylation was set as fixed modification, and methionine oxidation, protein N-terminal acetylation, and phosphorylation (STY) were considered as variable modifications. Putative SILAC pairs were searched with their respective labeled amino acids as fixed modification, whereas peaks that were not assigned to any of the SILAC pairs were searched using R6, R10, K4, and K8 as variable modifications. Enzyme specificity was set to trypsin/P, i.e. allowing cleavage N-terminal to proline in the context of (K/R)P. Up to two missed cleavages were allowed. The minimum required peptide length was set to 6 amino acids. Searches were performed against International Protein Index human (version 3.48; 71,400 protein entries) that was concatenated with reverse database sequences (142,800 protein entries in total) (43). Furthermore, MaxQuant filtered Mascot results using additional parameters such as the number of labeled amino acids (maximum of 3) in the identified peptide sequence and the measured mass accuracy as a function of intensity (41). As an additional quality measure to increase identification stringencies, we only accepted phosphorylation site identifications with Mascot scores of at least 12 or PTM scores of at least 30. Quantitation of SILAC pairs was performed with the following parameters; requantify; for protein quantitation, discard unmodified counterpart peptides except for oxidation and acetyl protein N-terminal; use razor and unique peptides; minimum ratio count, 1; minimum score, 0; and minimum peptides, 1 (41). The initial maximum false discovery rates (FDRs) were set to 0.02 and 0.05 for peptides and proteins, respectively, and further reduced by Mascot score filtering as described above. FDRs were calculated as (number of hits in the reversed database/number of hits in the forward database) × 100% (44). Whenever the set of identified peptides in one protein was equal to or completely contained in the set of identified peptides of another protein, these two proteins were joined in a single protein group. Shared peptides are most parsimoniously associated with the group with the highest number of identified peptides (“razor” peptides (45)) but remain in all groups where they occur (41). In cases where the peptides have more than one phosphorylation site, some of these phosphorylation sites are identified as multiply phosphorylated peptides, whereas others are identified on multiple singly phosphorylated peptides. Different ratios of phosphorylation sites within the same peptide can only be determined if the different singly phosphorylated peptides eluted at different time points. In general, singly phosphorylated peptides are preferred for quantitation purposes compared with multiply phosphorylated peptides because the individual contributions of multiple phosphorylation sites on the observed ratio of the phosphopeptide cannot be determined. The MS/MS spectra of phosphopeptides (based on peak lists) are shown in supplemental Fig. S11, whereas the MS/MS spectra based on profile data, all peak lists, and the Orbitrap raw data were submitted to the Tranche data repository (please see below for access information).2
Interaction Networks and Functional Clustering
Protein interaction networks were obtained from the STRING database version 8 (46) using the web interface with the following parameters: required confidence, medium; network depth, 1; and experiments and databases as prediction methods. Interaction data obtained from the STRING database were loaded to Cytoscape (47) for drawing the images. Functional annotation clustering was done using DAVID Bioinformatics Resources (48). Default parameters with medium classification stringency were used.
RESULTS AND DISCUSSION
Experimental Strategy
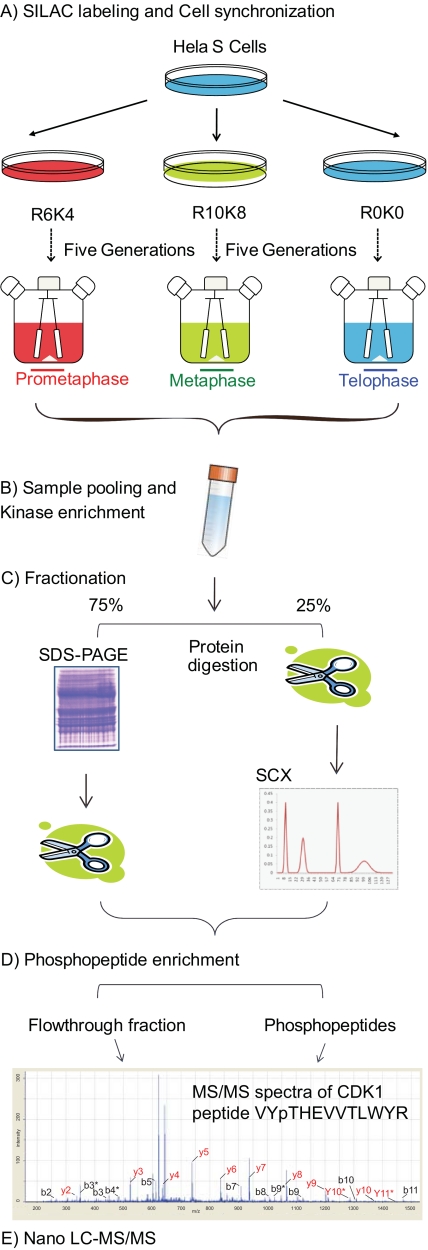
SILAC is among the preferred methods for comparative proteomics studies in mammalian cell lines and provides easy and accurate quantitation (49, 50). In the present study, we used SILAC triple labeling in combination with small inhibitor-based kinase enrichment and large scale cell synchronization methods (Fig. 1). In brief, three populations of HeLa cells were grown in large scale under identical conditions using spinner cultures in light, medium, and heavy SILAC media containing different isotopic forms of arginine and lysine. Next, cells were synchronized in prometaphase, metaphase, and telophase using the drugs nocodazole and MG132 (Fig. 1), and the three cell populations were lysed and mixed in equal proportions. From the lysate, cellular protein kinases were enriched using kinase affinity chromatography and fractionated by one-dimensional SDS-PAGE followed by tryptic digestion of the proteins. Alternatively, the enriched kinase fraction was in-solution digested by trypsin and fractionated using SCX chromatography. All fractions were enriched for phosphopeptides using TiO2 and measured by nano-LC-MS/MS. Flow-through fractions remaining after phosphopeptides enrichment were also analyzed for calculating protein ratios. To address the biological reproducibility, experiments were carried out in triplicate.
Fig. 1.
Schematic overview of experimental strategy.
Large Scale Synchronization of HeLa Cells in Different Mitotic Stages
The HeLa cell line is a popular model system for mammalian cell cycle research and often used in large scale proteomics studies (31, 51). Established protocols for HeLa synchronization at specific mitotic stages, however, are optimized for adherent HeLa cell lines, which are not compatible with the large amounts of cells needed for kinase enrichment. Therefore, we developed mitotic synchronization protocols for HeLa S cells, which can be grown on a sufficiently large scale in suspension (spinner) cultures. To assure HeLa S undergoes a normal cell cycle, growth kinetics were studied, and immunofluorescence pictures after DNA and α-Tubulin staining were taken across the cell cycle. These results demonstrate that HeLa S cells faithfully mimic the normal cell cycle and can be synchronized with traditional mitotic inhibitors (supplemental Fig. S1).
Double synchronization with thymidine in S phase followed by treatment with microtubule-destabilizing drugs such as nocodazole is the preferred method to enrich HeLa cells in mitosis. Nocodazole is a reversible inhibitor that efficiently arrests the cells in a prometaphase-like state with an active SAC. Generally, drug-induced mitotic states mimic natural mitotic stages but may not perfectly represent unperturbed mitosis. However, synchronization with thymidine and nocodazole are accepted methods in biological studies of mitosis, and our current knowledge of mitotic progression is to a large extent based on the use of these drugs for synchronization. To reduce possible side effects to a minimum, we used a single thymidine block instead of the double thymidine arrest. Also, for the nocodazole and MG132 synchronization steps, we have carefully determined the minimal concentrations and incubation times of the drugs to reduce possible side effects.
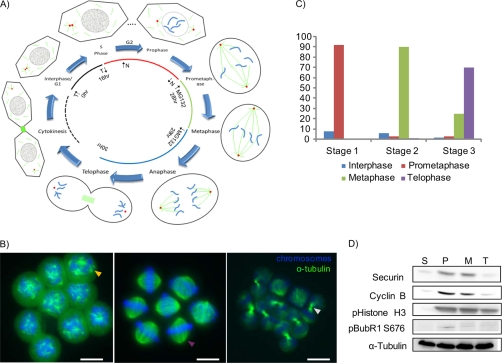
Various nocodazole concentrations were tested, and 40–50 ng/ml was found sufficient to block HeLa S cells in mitosis. For adherent nocodazole-treated HeLa cell cultures, the purity of the mitotic cell fraction can be strongly increased by shake-off of the rounded mitotic cells. Unfortunately, this shake-off method cannot be applied to cells grown in suspension, and we therefore tried other means to increase the purity of mitotic HeLa S cells. Assuming that an increase of the growth stimulus may help cells reach prometaphase faster and thus more synchronously, nocodazole was applied to media with increased concentrations of FCS. As expected, cells supplemented with higher concentrations of FCS (20%) entered into mitosis more coherently with more than 90% of cells reaching prometaphase within 12 h of thymidine release (Fig. 2B). Next, to enrich cells in metaphase, MG132, a reversible cell-permeable proteasome inhibitor, was used. This drug blocks proteasome-mediated degradation of Securin, which is required for progression to anaphase. Importantly, cells blocked by MG132 are characterized by an inactivated SAC, providing the opportunity to study SAC signaling by comparison with nocodazole-blocked cells with an active SAC. Thus, ∼90% of the MG132-blocked cells were determined to be in metaphase. Finally, to enrich for the telophase state, cells were released from MG132, and the optimal time to harvest telophase cells was determined by immunofluorescence microscopy of DNA-stained cell fractions at various time points. Synchronization in telophase turned out to be the most difficult and least reproducible task because this stage is rather short (typically 20–30 min), and there are no drugs available to block cells efficiently in telophase. HeLa cells released from MG132 enter telophase after ∼90–120 min (release time from the metaphase block and duration of anaphase). This duration is slightly longer than in unperturbed mitosis as the cells take some time to recover from the MG132 block. After optimization of the release period, 45, 60, and 70% telophase cells were counted in experiment 1, experiment 2, and experiment 3, respectively (supplemental Figs. S2 and S3).
Fig. 2.
Outline and quality control of cell synchronization procedure. A, eukaryotic cell cycle with emphasis on mitotic stages. Centrosomes are shown as red dots, chromosomes are depicted in blue, and microtubules are displayed in green. The synchronization steps used are indicated in the diagram with inward and outward directed arrows marking the addition and release of drugs. T and N refer to thymidine and nocodazole, respectively. B, representative pictures from prometaphase, metaphase, and anaphase cells. The yellow arrow highlights a microtubule cluster, the pink arrow marks a mitotic spindle, and the white arrow points at a midbody. Scale bars represent 10 μm. C, synchronization efficiencies were plotted as counted from the images. D, Western blot analyses of the samples using various mitotic markers. P stands for prometaphase samples obtained from nocodazole block (Stage 1), M represents metaphase samples from a MG132 (Stage 2) block, T marks telophase (Stage 3) samples from MG132 release, and S stands for thymidine-blocked HeLa cells in S phase. The S phase stage is not part of the proteomics experiment but was included in the Western blot analyses as a reference. pHistone, phosphorylate Histone.
We further benchmarked the samples using Western blot analysis of various mitotic markers (Fig. 2D). The concentrations of Cyclin B and Securin are known to be low in S phase, to peak in prometaphase and metaphase, and to be quickly diminished in anaphase after inactivation of the SAC. As shown in Fig. 2D, Western blot analyses of the synchronized HeLa S cells are fully consistent with the expected expression levels. Phosphorylation of Ser-10 of Histone H3 is a marker for chromosome condensation. Because anti-Ser(P)-10 staining is not diminished in the telophase sample (Fig. 2D), we conclude that cells did not enter the next G1 cycle yet. Furthermore, BubR1 Ser(P)-676 has been shown to be phosphorylated in prometaphase but not in metaphase (33), so the Western blots in Fig. 2D demonstrate a good separation between prometaphase- and metaphase-enriched cells. In summary, the Western blot results along with the immunofluorescence pictures (Fig. 2B) show that the samples faithfully represent the mitotic stages prometaphase, metaphase, and telophase.
Identification of Proteins and Phosphorylation Sites
The experiments carried out in biological triplicates resulted in 117 raw files with 555,444 MS/MS spectra, making data analysis computationally and technically challenging. Analysis of SILAC data was done in a fully automated manner using the MaxQuant software suite (version 1.0.12.5) (41) in association with the Mascot search engine (42). To ensure the fidelity of protein and phosphorylation site identifications, the decoy database strategy was used to minimize FDRs (44). Peptide sequences were assigned for one-third of the MS/MS spectra at an FDR of 0.01. We used a conservative approach for calculating the number of proteins and phosphopeptides identified in our study. All the peptide isoforms (SILAC and PTMs) matching to a single sequence were counted as one, and all the proteins that share common peptides were grouped into one protein group (41). Distinguishing protein kinases from other proteins turned out to be a complex task as multiple isoforms and splice variants are annotated for many kinases in the International Protein Index. Therefore, we strictly adhered to the kinase sequences provided by Manning et al. (KinBase database version 08.02) (4), and kinase peptides were identified by matching identified peptide sequences to kinase sequences. Later, these peptides were assembled into proteins essentially as described for the MaxQuant algorithm. The efficiency of the enrichment for kinases is demonstrated by the large number of kinase identified in this study. From the three biological replicates, 206 protein kinases could be detected of which 163 were phosphorylated (Table I). On average, each kinase was identified by 19 tryptic peptides, and at least two unphosphorylated peptides were identified for 164 kinases. For phosphopeptides with more than one Ser/Thr/Tyr residue, the MaxQuant PTM scoring algorithm was used to calculate localization probabilities of phosphorylation sites for each of these residues (31). Regarding the grouping of phosphorylation sites according to the confidence level of phosphorylation site identifications within the phosphopeptide sequences, we adapted the classification scheme of Olsen et al. (53). In total, 944 high confidence phosphorylation sites on protein kinases were detected (class I sites), whereas 277 phosphorylation sites fell into lower confidence classes (class II and III sites). Kinase phosphopeptides accounted for 68% of the total phosphopeptide ion count, further demonstrating the efficiency of the kinase enrichment strategy. The use of SCX separation after in-solution digestion as an alternative strategy to SDS-PAGE (in combination with in-gel digestion) is beneficial as only 34% of the total phosphorylation sites were identified by both methods, thus indicating that the two methods are highly complementary (supplemental Fig. S4). However, we did not detect significant differences between the pools of phosphopeptides detected by the SCX and SDS-PAGE strategies in terms of peptide length and the percentage of multiply phosphorylated peptides. To compare the kinase coverage of this work with the related prior studies of Daub et al. (31) and Dephoure et al. (51), we calculated the numbers and overlaps of the detected kinase phosphorylation sites (supplemental Fig. S5). Compared with the Daub et al. (31) data set, we detected about the same number of kinase phosphorylation sites, whereas about 40% more kinase phosphorylation sites were detected compared with the study by Dephoure et al. (51), thus justifying the use of a kinase enrichment strategy.
Table I. Summary of mitotic kinome.
A summary of the numbers of detected kinases and phosphorylation sites is given. PK, protein kinase.
| Total number of PKs | 206 |
| PK phosphorylation sites | |
| Class I | 944 |
| Class II | 169 |
| Class III | 108 |
| FDR, peptides (%) | 1.0 |
| Non-PK phosphorylation sites (class I) | 1374 |
Mitotic Dynamics of Kinase Phosphorylation Sites
SILAC triple labeling along with cell synchronization techniques allowed us to compare three specific mitotic stages. Phosphorylation site ratios were normalized to protein ratios to eliminate variations resulting from different expression levels at the analyzed stages, unequal sample mixing, and labeling efficiency of different isotopic forms of arginine and lysine (41). If no unphosphorylated peptides were detected to calculate protein ratios (7% of the detected kinase phosphorylation sites), global correction factors were used (41) (supplemental Table 1). To choose optimal thresholds for identifying regulated phosphorylation sites, we analyzed a 1:1 mixture of mitotic lysates (identically prepared but distinctly SILAC-labeled samples) in a separate experiment. Less than 5 and 1% of the detected phosphorylation site ratios differed by more than 35 and 50%, respectively (supplemental Table 2). Based on these values, we decided to accept regulation levels above 1.5 (3/2) for up-regulation and below 0.66 (2/3) for down-regulation as significant. Reproducibility of the results between the experiments was found to be high, even though the samples were biological replicates (supplemental Table 2). Less than 11 and 19% of the phosphorylation site ratios differed from each other by more than 35% for prometaphase-metaphase and metaphase-telophase transitions, respectively, between experiment 2 and experiment 3 for example. Of the three replicates, experiment 3 was found to be best in terms of synchronization efficiency and number of detected phosphorylation sites (supplemental Fig. S3), so results from experiment 3 are shown in all figures unless otherwise mentioned.
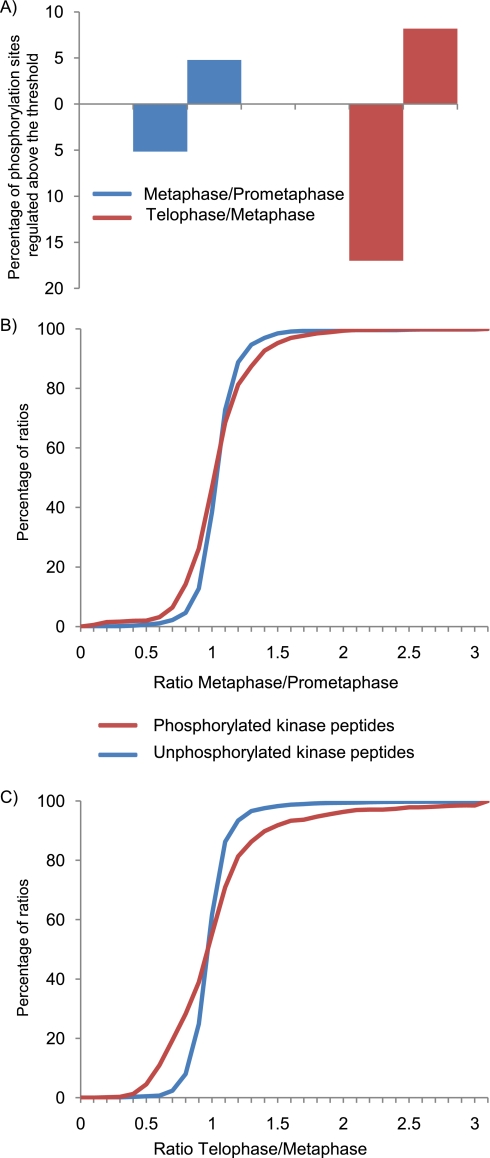
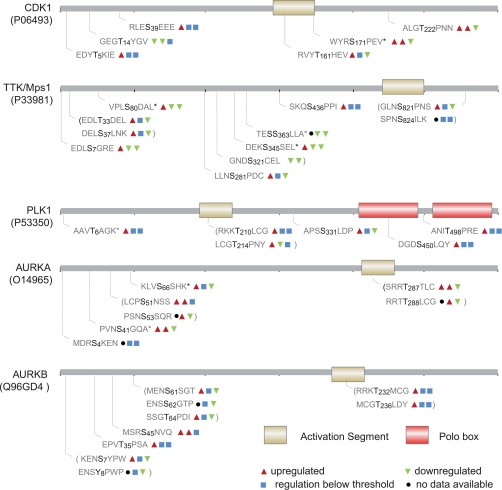
During the prometaphase to metaphase transition, phosphorylation site regulation exceeding the above defined threshold was found to be confined to few kinases that comprise important known regulators of the SAC such as TTK (Mps1) and Plk1 (Figs. 3A and 4 and supplemental Table 1). Notably, although the percentage of significantly regulated phosphorylation sites did not exceed 10%, a stronger regulation of phosphorylated peptides compared with non-phosphorylated peptides was observed (Fig. 3B), thus demonstrating a small but significant regulation of the phosphokinome between these mitotic stages. In contrast, a pronounced regulation of the phosphokinome was observed in telophase with 17% down-regulated and 8% up-regulated phosphorylation sites (Fig. 3, A and C). Importantly, the identified phosphoproteins comprise key mitotic kinases, such as Cdk1, Plk1, Aurora A (AURKA), Aurora B (AURKB), and TTK (Mps1), allowing insight into their phosphorylation-based regulation during mitosis (Fig. 4). The activating phosphorylation site of the key mitotic kinase Cdk1, Thr-161, for example, stayed constant in metaphase, whereas the inhibitory phosphorylation site Thr-14 continued to be dephosphorylated (Fig. 4). Inactivation of Cdk1, which is required for mitotic exit, is reflected by the down-regulation of the activating Cdk1 T-loop phosphorylation site Thr-161 in the telophase sample. Plk1, another crucial mitotic kinase, contains two conserved threonines, Thr-210 and Thr-214, on its activation segment (Fig. 4 and Table II) that were both phosphorylated in mitosis. Thr-210 has been previously described as the major activating phosphorylation site of Plk1 in mitotic HeLa cells (10), and in agreement with the essential role of Plk1 in cytokinesis, this site remained phosphorylated throughout mitosis. In contrast, Thr-214 was found to be dephosphorylated after silencing of the spindle checkpoint in metaphase, suggesting that this phosphorylation site may have other functions than regulating Plk1 activity. During mitosis, Aurora A is localized at spindle poles and the mitotic spindle where it regulates the functions of centrosomes and spindles and is required for proper mitotic progression (23). Aurora A activation segment phosphorylation sites (Fig. 4 and Table II) as well as four other phosphorylation sites of this kinase continued to be phosphorylated during the prometaphase-metaphase transition, indicating that complete activation of Aurora A is not achieved until metaphase. Interestingly, the Aurora A activation segment was dephosphorylated in telophase, whereas phosphorylation of the Aurora B activation segment along with other phosphorylation sites stayed constant in mitosis, which is in agreement with the reported role of Aurora B in cytokinesis.
Fig. 3.
Quantitative dynamics of phosphokinome across mitosis. A, percentage of class I phosphorylation sites that are up-regulated (upward directed bars) above a ratio of 1.5 or down-regulated below a ratio of 0.66 (downward directed bars) between metaphase and prometaphase and telophase and metaphase, respectively. Percentages of the measured ratios between metaphase and prometaphase (B) and between telophase and metaphase (C) are depicted separately for phosphorylated kinase peptides (red) and unphosphorylated kinase peptides (blue).
Fig. 4.
Phosphorylation dynamics of key mitotic kinases. The regulation of phosphorylation sites (red triangle, up-regulated; green triangle, down-regulated; blue square, regulation below threshold; black circle, no data available) is shown for the ratios between prometaphase and S phase (first position; data taken from Daub et al. (31)), between metaphase and prometaphase (second position), and between telophase and metaphase. Data were taken from experiment 3 (best synchronization) if available. Phosphorylation sites marked with a star were only detected in experiment 1 or 2. AURK, Aurora kinase.
Table II. Dynamics of protein kinase activation segment phosphorylation.
The regulation of kinase activation segment phosphorylation sites (red triangle, up-regulated; green triangle, down-regulated; blue square, regulation below threshold; black circle, no data available) is shown for the ratios between prometaphase and S phase (first position; data taken from Daub et al. (31)), between metaphase and prometaphase (second position), and between telophase and metaphase (third position). Data were taken from experiment 3 (best synchronization) if available. Phosphorylation sites marked with an asterisk were only detected in experiment 1 or 2. Non-class I sites are underlined. AURK, Aurora kinase; FAK, focal adhesion kinase; PKC, protein kinase C; PKA, cAMP-dependent protein kinase.
Maternal embryonic leucine zipper kinase (MELK) inhibits pre-mRNA splicing by binding NIPP1 in a cell cycle-regulated manner. In vitro studies have shown that phosphorylation of Thr-460, Thr-466, and Thr-478 residues is critical for MELK-NIPP1 binding (54) and pre-mRNA splicing inhibition. Importantly, our in vivo data confirm these results as the phosphorylated MELK residues were highly dephosphorylated in telophase (supplemental Table 1). Similarly, protein kinase C δ, which was recently shown to be important for meiotic spindle formation, was found to be dephosphorylated on multiple sites in metaphase (55) (supplemental Table 1), suggesting that these phosphorylation sites may be interesting in the context of potential mitotic roles of this kinase.
Network Analysis
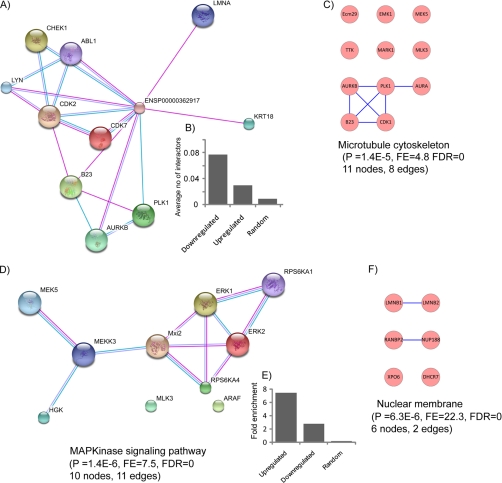
Proteins work rarely in isolation but rather function in complexes as multiprotein machines. Importantly, members of protein interaction networks are frequently regulated in a similar fashion, thus enhancing the robustness of signaling events (46). To detect regulatory networks involved in mitotic exit, we therefore asked whether phosphoproteins with similar phosphorylation dynamics in telophase were enriched for members of specific interaction networks. To this end, we extracted a group of proteins containing at least one up-regulated phosphorylation site (group U, 59 proteins) and another group with proteins containing at least one down-regulated phosphorylation event (group D, 130 proteins). Importantly, the overlap between the groups was modest (22 proteins), illustrating the specificity of the applied approach. To gain further insight into underlying mitotic phosphorylation networks, interaction network analyses were performed separately for the up-regulated and down-regulated groups, and the results were visualized (supplemental Figs. S6 and Fig. S7). Strikingly, a Cdk1 interaction network was found to be enriched in group D (Fig. 5A), which is in agreement with the deactivation of Cdk1 in late mitosis (25). To compensate for the unequal number of proteins in each group, average numbers of interactors were computed (number of interactions in a particular group of proteins/total number of proteins in that group). 2.5-fold more Cdk1 interactors were found in group D compared with group U, and 9-fold more were found compared with a randomly chosen set of proteins (Fig. 5B). Along similar lines, we observed that 54% of the phosphorylation sites matching a stringent Cdk1 motif ((S/T)PXR) were down-regulated (supplemental Table 1) compared with the 17% of sites in the total data set. This demonstrates that although the overall number of down-regulated phosphorylation sites is relatively small a large number of Cdk1 phosphorylation sites are down-regulated nevertheless. Regarding the group of proteins containing up-regulated phosphorylation sites, the MAPK signaling pathway was found to be highly enriched (7.5-fold, p value, 1.4e−6) (Fig. 5D) (see next paragraph for further discussion). Furthermore, we analyzed the functional annotations of proteins in the D and U groups. As may be expected, “protein kinase” and “cell cycle” were highly enriched terms in both the groups (supplemental Figs. S8 and S9). Apart from these, the term “microtubule cytoskeleton” was enriched 4.8-fold (p value, 1.4e−5) in group D (Fig. 5C). Interestingly, the enrichment of the term “nuclear membrane part” in group U (22.3-fold; p value, 6.3e−6) (Fig. 5F) suggests that phosphorylation may also have a role in nuclear envelope reformation (Fig. 5F). In summary, network analysis of subgroups of proteins with similar phosphorylation dynamics identifies mitotic interaction networks of proteins and is therefore useful for the detection of the pathways targeted by phosphorylation-based signaling.
Fig. 5.
Interaction networks and enriched functional annotations. A, selected part of an interaction network extracted from a group of proteins with down-regulated phosphorylation sites centered on Cdk1; inset B shows the enrichment of Cdk1 interactors in the complete interaction network of proteins with down-regulated phosphorylation sites (supplemental Fig. S6) compared with proteins with up-regulated phosphorylation sites (supplemental Fig. S7) and a random group from the UniProt data set. C highlights that the functional annotation term “microtubule skeleton” is enriched in this network. D, selected part of an interaction network extracted from a group of proteins with up-regulated phosphorylation sites in telophase, containing many MAP kinases; inset E shows the enrichment of MAPK interactors in the complete interaction network of proteins with up-regulated phosphorylation sites (supplemental Fig. S7) compared with proteins with down-regulated phosphorylation sites (supplemental Fig. S6) and a random group from the UniProt data set. The enrichment of the functional annotation “nuclear membrane” in this network is shown in F. AURK, Aurora kinase.
MAPK Pathways Are Activated during Late Mitosis
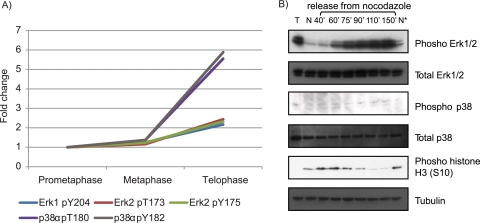
Although mitotic exit is thought to be mostly driven by dephosphorylation events, some kinases were nevertheless found to be phosphorylated in telophase. As shown above, functional annotation revealed that most of these kinases either belong to the MAPK family or represent components of MAPK pathways (Fig. 5D). MAPKs have been studied extensively because of their strong association with many important cellular processes such as cell proliferation, survival, growth, and differentiation (56). As part of key signaling pathways, MAPKs are activated in response to various mitogenic signals and stress factors. The role of MAPKs during the G1-S transition is relatively well understood, but their function in mitotic progression has been discussed controversially. Initial studies suggested that ERK1 and ERK2 localize to centrosomes and kinetochores and that their activities are required for proper spindle formation and timely metaphase-anaphase transition in mouse fibroblast-like 3T3 cells (57). These findings, however, could not be confirmed by a successive study in normal and transformed human cells (58). Another member of the MAP kinase family, p38, was observed to localize to centrosomes during HeLa cell mitosis (26, 59) and to be required for the metaphase-anaphase transition in developing retina (60). However, a subsequent study demonstrated that the timing of the metaphase-anaphase transition was not affected in the absence of p38 activity (61). Although these conflicting results may be partially explained by the use of different model systems and experimental techniques, further elucidation of the role of MAPK during mitotic progression is clearly needed. Because our study tracked the phosphorylation of kinases during mitosis through a kinome approach, a wide range of MAPKs could be analyzed simultaneously (Fig. 6A). We found that the MAP kinases ERK1/2 and p38 were phosphorylated on their activation segments and that the relative abundances of these phosphorylation sites peak during late mitosis (telophase). p38 showed an ∼5-fold up-regulation of TXY motif phosphorylation in late mitosis, whereas ERK1 and ERK2 phosphorylation increased by 2-fold. Importantly, these results were found consistently in all three biological replicates (supplemental Table 1) and were further confirmed using a phosphospecific antibody against the pTXpY (where pT is phosphothreonine and pY is phosphotyrosine) motif of p38 and ERK1/2 with additional time points in mitosis (Fig. 6B). MG132 treatment of cells is known to activate programmed cell death response, and p38 α is one of the prototypical stress-activated kinases. To address the concern that the observed pathway activation was related to drug-related side effects, we released cells directly from nocodazole and still observed phosphorylation on p38 and ERK1/2, thus ruling out MG132 treatment as the primary cause of phosphorylation. To rule out nocodazole as the cause of phosphorylation, we kept one batch of cells in nocodazole, whereas another batch of cells was released from this drug. We observed phosphorylation on p38 as well as ERK1/2 only in the cells that were released into telophase. Taken together, we speculate that the detected up-regulation of phosphorylation sites on a MAPK interaction network during late mitosis indicates a role of these kinases in late mitotic events, a view that is consistent with a recent report showing that phosphorylation of protein kinase C ε by p38 is required for cytokinesis (15).
Fig. 6.
Activation segment phosphorylation of MAP kinases in mitosis. A, dynamics of activation segment phosphorylation of different MAP kinases identified in the study. B, Western blots depicting the activation segment phosphorylation of p38 and ERK1/2 MAP kinases. T stands for thymidine-blocked HeLa cells in S phase. N represents the prometaphase sample obtained from a nocodazole block. Upon release from nocodazole, cells were harvested at six different time points. N* represents the sample for which the nocodazole block was continued for 150 min (′) after the initial nocodazole block. Phosphorylation on p38 and ERK1/2 is observed only in the cells that were released into telophase.
Changes Made during Mitotic Entry Are Restored at End of Mitosis
Whereas the current study was focused on the dynamics of the phosphokinome during different phases of mitotic progression, a previous study has quantitatively analyzed the phosphorylation of the kinome during S phase and prometaphase (31). Because one common time point (nocodazole block in prometaphase) was used in both experiments, it became possible to combine the results of the two studies to broaden the monitored time window (Fig. 4) and to analyze the correlation between the observed phosphokinome dynamics (Fig. 7).
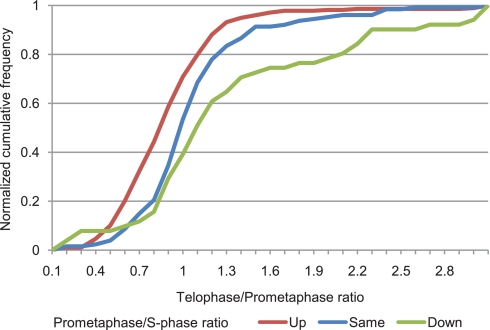
Fig. 7.
Correlation between kinase phosphorylation site regulation at mitotic entry and mitotic exit. The proportions of the class I kinase phosphorylation sites that were found up-regulated (red), not significantly changed (blue), or down-regulated (green) between S phase and prometaphase (the ratios were taken from Daub et al. (31)) in relation to their regulation in telophase are shown.
Interestingly, phosphorylation sites that were found to be up-regulated between S phase and prometaphase were significantly more down-regulated between prometaphase and telophase than phosphorylation sites that either stayed constant during the prometaphase to S phase transition or were down-regulated (Fig. 7). This correlation is reminiscent of the cycling behavior of Cdk activities that drive the “cell cycle oscillator” (25, 62). According to the current perception of mitosis, Cdk1 activity initiates mitosis, and mitotic exit requires Cdk1 inactivation, thus restoring the initial state (63). In line with this view, the observed correlation between up-regulation during mitotic entry and down-regulation at mitotic exit (and vice versa) can also be interpreted as a partial reconstitution of a “non-mitotic” phosphokinome state toward the end of mitosis.
Regulation of Non-kinases in Mitosis
Although a strong enrichment for kinases could be achieved through our experimental strategy (see above), other “contaminating” proteins were also detected. These “non-kinases” may form stable complexes with kinases, as may be the case for the detected Cyclins, or bind to the kinase affinity columns through undesired selectivities (64). Thus, a total of 1730 phosphorylation sites (Table I), including 1374 class I sites, could be identified on these proteins in addition to the above discussed kinase phosphorylation sites (supplemental Table 1). Although the detection of these other phosphorylation sites was not the purpose of this study, closer analysis revealed that key mitotic proteins were also covered, thus providing additional information on the regulation of mitotic progression dynamics. For instance, we identified more than 12 phosphorylation sites on Borealin, an important component of the chromosomal passenger complex (CPC). Of those, three of the conserved pTP sites, located close to the Borealin C terminus, were consistently down-regulated in telophase in all three replicates. During the metaphase to anaphase transition, the CPC moves from centromeres to the spindle midzone, which can be inhibited by constitutively active Cdk1 (65). Moreover, the C terminus of Borealin was already previously shown to be important for CPC targeting to centromeres (66). It is therefore conceivable that the three down-regulated phosphorylation sites at the C terminus of Borealin may play a role in targeting the CPC to centromeres, so these sites may constitute attractive targets for future mutational analyses. Another detected phosphorylation substrate, protein regulator of cytokinesis 1 (PRC1), is a key protein required for cytokinesis and was found to be phosphorylated on 12 sites during mitosis. Previously, it was reported that Plk1 phosphorylates PRC1 at the two sites Thr-578 and Thr-602, thus promoting the interaction of PRC1 with several kinesin motor proteins required for cytokinesis (67). Interestingly, both these reported sites were identified in our study. Because the Thr-602-containing peptide covers the C terminus of PRC1 and does not contain the SILAC-labeled amino acids lysine and arginine, this peptide could unfortunately not be quantified. The peptide spanning the other reported phosphorylation site, Thr-578, however, was found to be up-regulated once all chromosomes were captured by microtubules (ratio, 1.45 metaphase/prometaphase). Interestingly, also the protein level of PCR1 varied during mitosis, peaking in metaphase and decreasing again in telophase (ratios in experiments 1, 2, and 3, 1.3, 1.63, and 1.88 metaphase/prometaphase and 1.22, 0.62, and 0.67 telophase/metaphase, respectively). As previous studies had suggested that the level of PRC1 remains constant throughout mitosis (67), we speculate that the PRC1 level is maintained by equilibrium between synthesis and degradation so that MG132-mediated proteasome inhibition as used in this study would result in elevated protein levels. It is also interesting in this context that cap-independent protein translation during mitosis was shown to be important for recruiting Plk1 to the central spindle (68). Because PRC1 plays a crucial role in targeting Plk1 to the central spindle, it may be rewarding to test whether PRC1 is translated through a cap-independent mechanism during mitosis.
In addition to the identification of potentially important regulatory phosphorylation sites, the detection of “non-kinase” proteins also allowed us to further evaluate synchronization efficiencies. The protein level of Cyclin B, the regulatory subunit of Cdk1, stayed constant between prometaphase and metaphase, whereas it was found to drop significantly in telophase, a finding that is in agreement with Western blot data (supplemental Fig. S10A) and the dynamics of mitotic Cdk1 activity. In particular, Cyclin B levels were found to be reduced by more than 3-fold, thus indicating that the synchronization efficiency of telophase cells exceeded 70%. Furthermore, the levels of α-Tubulin, a commonly used loading control for mitotic samples, remained almost constant throughout mitosis (ratios in experiments 1, 2, and 3, 0.98, 0.98, and 1.07 metaphase/prometaphase and 0.92, 1.00, and 1.00 telophase/metaphase, respectively) (supplemental Fig. S10B), highlighting the accuracy of SILAC-based quantitation and downstream data analysis steps.
In summary, the large number of identified kinase phosphorylation sites enables an unbiased and quantitative view of the regulation of kinase networks and thus constitutes a valuable resource to study the system properties of the kinome during mitosis. Importantly, known key mitotic kinases such as Plk1, TTK (Mps1), Aurora A, and Aurora B as well as many kinases that have not been implied yet in mitotic functions were captured in the survey. Thus, the results of this study recapitulate many of the previously known mitotic events but also identify attractive candidates for future studies of phosphorylation-based mitotic signaling. As an example, interaction network analysis of jointly regulated kinase groups revealed that a MAP kinase-dependent pathway is up-regulated in telophase, thus suggesting a biological role in late mitosis. As an outlook, the number of identified kinase phosphorylation sites may be further increased, taking advantage of recently published advances in sample separation and peptide fractionation methods such as improved SCX protocols (69), methods orthogonal to reverse phase chromatography (70), and alternative proteases (52). Furthermore, given that the cell synchronization method developed here is not specific for kinase studies, it may be used for other large scale comparative studies addressing specific mitotic stages.
Acknowledgments
We thank Jürgen Cox and Matthias Mann for early access to the MaxQuant program and Anna Santamaria and Rainer Malik for fruitful discussions during the preparation of this manuscript. We acknowledge Albert Ries for excellent technical assistance. H. D. and R. H. thank Axel Ullrich for generous support. Furthermore, we thank Zuzana Storchova and Krishna Moorthy Sreenivasan for support during the revisions of this manuscript.
* This work was supported by the Max Planck Society as well as by Experimental Network for Functional Integration (ENFIN) Contract LSHG-CT-2005-518254 funded by the European Commission within its FP6 Program under the thematic area “Life sciences, genomics and biotechnology for health.”
 This article contains supplemental Figs. S1–S11 and Tables 1 and 2.
This article contains supplemental Figs. S1–S11 and Tables 1 and 2.
2 The following supporting data are saved at Tranche (https://proteomecommons.org/tranche/). They can be accessed using their corresponding hash: supplemental Data S1: raw files, L4TzF0GZAYPyYmI+3GL6/WJ5JEyHg4uxtGNefDjSBlW9q2l1zxEVq1ZPkOj8wK9qvE0dLcrqawRw3yVmqvGuXx/KLRYAAAAAAABYPA==; supplemental Data S2: MS/MS peak lists of the 117 raw data files, XZXixrydqHkFY0t6jDa25U0LuTQJ4E+7jnZK18O69QHLOwjoSdQitT04lGvBwOunWPB5upvnLq8Ha6kl+MK2OZ02NWIAAAAAAAACOA==; and supplemental Data S3: annotated MS/MS spectra (based on full resolution profile data) of all phosphopeptides exported from MaxQuant, r1SCPFUELrBZO0/8UnYyvIgOrIQA1LneEOdcWX4QpIp6yPA6VILahGA3WH0ctK8RFACLd1ZOeVeR9vhCHoLMr5QokDoAAAAAAAmLhA==.
1 The abbreviations used are:
- PTM
- posttranslational modification
- SAC
- spindle assembly checkpoint
- Cdk
- Cyclin-dependent kinase
- SILAC
- stable isotope labeling by amino acids in cell culture
- ERK
- extracellular signal-regulated kinase
- Bis-Tris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- ABC
- ammonium bicarbonate
- SCX
- strong cation exchange
- LTQ
- linear triple quadrupole
- FDR
- false discovery rate
- MAPK
- mitogen-activated protein kinase
- S
- suspension
- MELK
- maternal embryonic leucine zipper kinase
- MAP
- mitogen-activated protein
- CPC
- chromosomal passenger complex
- PRC1
- protein regulator of cytokinesis 1.
REFERENCES
- 1.Cohen P. (2002) The origins of protein phosphorylation. Nat. Cell Biol 4, E127–E130 [DOI] [PubMed] [Google Scholar]
- 2.Pawson T., Scott J. D. (2005) Protein phosphorylation in signaling–50 years and counting. Trends Biochem. Sci 30, 286–290 [DOI] [PubMed] [Google Scholar]
- 3.Graves J. D., Krebs E. G. (1999) Protein phosphorylation and signal transduction. Pharmacol. Ther 82, 111–121 [DOI] [PubMed] [Google Scholar]
- 4.Manning G., Whyte D. B., Martinez R., Hunter T., Sudarsanam S. (2002) The protein kinase complement of the human genome. Science 298, 1912–1934 [DOI] [PubMed] [Google Scholar]
- 5.Draviam V. M., Orrechia S., Lowe M., Pardi R., Pines J. (2001) The localization of human cyclins B1 and B2 determines CDK1 substrate specificity and neither enzyme requires MEK to disassemble the Golgi apparatus. J. Cell Biol 152, 945–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bischoff J. R., Anderson L., Zhu Y., Mossie K., Ng L., Souza B., Schryver B., Flanagan P., Clairvoyant F., Ginther C., Chan C. S., Novotny M., Slamon D. J., Plowman G. D. (1998) A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. EMBO J 17, 3052–3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mundt K. E., Golsteyn R. M., Lane H. A., Nigg E. A. (1997) On the regulation and function of human polo-like kinase 1 (PLK1): effects of overexpression on cell cycle progression. Biochem. Biophys. Res. Commun 239, 377–385 [DOI] [PubMed] [Google Scholar]
- 8.Ubersax J. A., Ferrell J. E., Jr. (2007) Mechanisms of specificity in protein phosphorylation. Nat. Rev. Mol. Cell Biol 8, 530–541 [DOI] [PubMed] [Google Scholar]
- 9.Nolen B., Taylor S., Ghosh G. (2004) Regulation of protein kinases; controlling activity through activation segment conformation. Mol. Cell 15, 661–675 [DOI] [PubMed] [Google Scholar]
- 10.Jang Y. J., Ma S., Terada Y., Erikson R. L. (2002) Phosphorylation of threonine 210 and the role of serine 137 in the regulation of mammalian polo-like kinase. J. Biol. Chem 277, 44115–44120 [DOI] [PubMed] [Google Scholar]
- 11.Krek W., Nigg E. A. (1992) Cell cycle regulation of vertebrate p34cdc2 activity: identification of Thr161 as an essential in vivo phosphorylation site. New Biol 4, 323–329 [PubMed] [Google Scholar]
- 12.Krek W., Nigg E. A. (1991) Differential phosphorylation of vertebrate p34cdc2 kinase at the G1/S and G2/M transitions of the cell cycle: identification of major phosphorylation sites. EMBO J 10, 305–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elia A. E., Cantley L. C., Yaffe M. B. (2003) Proteomic screen finds pSer/pThr-binding domain localizing Plk1 to mitotic substrates. Science 299, 1228–1231 [DOI] [PubMed] [Google Scholar]
- 14.Qi W., Tang Z., Yu H. (2006) Phosphorylation- and polo-box-dependent binding of Plk1 to Bub1 is required for the kinetochore localization of Plk1. Mol. Biol. Cell 17, 3705–3716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saurin A. T., Durgan J., Cameron A. J., Faisal A., Marber M. S., Parker P. J. (2008) The regulated assembly of a PKCepsilon complex controls the completion of cytokinesis. Nat. Cell Biol 10, 891–901 [DOI] [PubMed] [Google Scholar]
- 16.Biondi R. M., Komander D., Thomas C. C., Lizcano J. M., Deak M., Alessi D. R., van Aalten D. M. (2002) High resolution crystal structure of the human PDK1 catalytic domain defines the regulatory phosphopeptide docking site. EMBO J 21, 4219–4228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watanabe N., Arai H., Nishihara Y., Taniguchi M., Watanabe N., Hunter T., Osada H. (2004) M-phase kinases induce phospho-dependent ubiquitination of somatic Wee1 by SCFbeta-TrCP. Proc. Natl. Acad. Sci. U.S.A 101, 4419–4424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunter T. (2007) The age of crosstalk: phosphorylation, ubiquitination, and beyond. Mol. Cell 28, 730–738 [DOI] [PubMed] [Google Scholar]
- 19.Nigg E. A. (2001) Mitotic kinases as regulators of cell division and its checkpoints. Nat. Rev. Mol. Cell Biol 2, 21–32 [DOI] [PubMed] [Google Scholar]
- 20.Musacchio A., Salmon E. D. (2007) The spindle-assembly checkpoint in space and time. Nat. Rev. Mol. Cell Biol 8, 379–393 [DOI] [PubMed] [Google Scholar]
- 21.Li J. J., Li S. A. (2006) Mitotic kinases: the key to duplication, segregation, and cytokinesis errors, chromosomal instability, and oncogenesis. Pharmacol. Ther 111, 974–984 [DOI] [PubMed] [Google Scholar]
- 22.Barr F. A., Silljé H. H., Nigg E. A. (2004) Polo-like kinases and the orchestration of cell division. Nat. Rev. Mol. Cell Biol 5, 429–440 [DOI] [PubMed] [Google Scholar]
- 23.Marumoto T., Zhang D., Saya H. (2005) Aurora-A—a guardian of poles. Nat. Rev. Cancer 5, 42–50 [DOI] [PubMed] [Google Scholar]
- 24.Meraldi P., Honda R., Nigg E. A. (2004) Aurora kinases link chromosome segregation and cell division to cancer susceptibility. Curr. Opin. Genet. Dev 14, 29–36 [DOI] [PubMed] [Google Scholar]
- 25.Morgan D. (2007) The Cell Cycle: Principles of Control, 1st Ed., New Science Press Ltd., London [Google Scholar]
- 26.Tang J., Yang X., Liu X. (2008) Phosphorylation of Plk1 at Ser326 regulates its functions during mitotic progression. Oncogene 27, 6635–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kittler R., Pelletier L., Heninger A. K., Slabicki M., Theis M., Miroslaw L., Poser I., Lawo S., Grabner H., Kozak K., Wagner J., Surendranath V., Richter C., Bowen W., Jackson A. L., Habermann B., Hyman A. A., Buchholz F. (2007) Genome-scale RNAi profiling of cell division in human tissue culture cells. Nat. Cell Biol 9, 1401–1412 [DOI] [PubMed] [Google Scholar]
- 28.Johnson S. A., Hunter T. (2005) Kinomics: methods for deciphering the kinome. Nat. Methods 2, 17–25 [DOI] [PubMed] [Google Scholar]
- 29.Sugiyama N., Masuda T., Shinoda K., Nakamura A., Tomita M., Ishihama Y. (2007) Phosphopeptide enrichment by aliphatic hydroxy acid-modified metal oxide chromatography for nano-LC-MS/MS in proteomics applications. Mol. Cell. Proteomics 6, 1103–1109 [DOI] [PubMed] [Google Scholar]
- 30.Schreiber T. B., Mäusbacher N., Breitkopf S. B., Grundner-Culemann K., Daub H. (2008) Quantitative phosphoproteomics—an emerging key technology in signal-transduction research. Proteomics 8, 4416–4432 [DOI] [PubMed] [Google Scholar]
- 31.Daub H., Olsen J. V., Bairlein M., Gnad F., Oppermann F. S., Körner R., Greff Z., Kéri G., Stemmann O., Mann M. (2008) Kinase-selective enrichment enables quantitative phosphoproteomics of the kinome across the cell cycle. Mol. Cell 31, 438–448 [DOI] [PubMed] [Google Scholar]
- 32.Bantscheff M., Eberhard D., Abraham Y., Bastuck S., Boesche M., Hobson S., Mathieson T., Perrin J., Raida M., Rau C., Reader V., Sweetman G., Bauer A., Bouwmeester T., Hopf C., Kruse U., Neubauer G., Ramsden N., Rick J., Kuster B., Drewes G. (2007) Quantitative chemical proteomics reveals mechanisms of action of clinical ABL kinase inhibitors. Nat. Biotechnol 25, 1035–1044 [DOI] [PubMed] [Google Scholar]
- 33.Elowe S., Hümmer S., Uldschmid A., Li X., Nigg E. A. (2007) Tension-sensitive Plk1 phosphorylation on BubR1 regulates the stability of kinetochore microtubule interactions. Genes Dev 21, 2205–2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brehmer D., Greff Z., Godl K., Blencke S., Kurtenbach A., Weber M., Müller S., Klebl B., Cotten M., Kéri G., Wissing J., Daub H. (2005) Cellular targets of gefitinib. Cancer Res 65, 379–382 [PubMed] [Google Scholar]
- 35.Godl K., Gruss O. J., Eickhoff J., Wissing J., Blencke S., Weber M., Degen H., Brehmer D., Orfi L., Horváth Z., Kéri G., Müller S., Cotten M., Ullrich A., Daub H. (2005) Proteomic characterization of the angiogenesis inhibitor SU6668 reveals multiple impacts on cellular kinase signaling. Cancer Res 65, 6919–6926 [DOI] [PubMed] [Google Scholar]
- 36.Wissing J., Jänsch L., Nimtz M., Dieterich G., Hornberger R., Kéri G., Wehland J., Daub H. (2007) Proteomics analysis of protein kinases by target class-selective prefractionation and tandem mass spectrometry. Mol. Cell. Proteomics 6, 537–547 [DOI] [PubMed] [Google Scholar]
- 37.Shevchenko A., Wilm M., Vorm O., Mann M. (1996) Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem 68, 850–858 [DOI] [PubMed] [Google Scholar]
- 38.Rappsilber J., Ishihama Y., Mann M. (2003) Stop and go extraction tips for matrix-assisted laser desorption/ionization, nanoelectrospray, and LC/MS sample pretreatment in proteomics. Anal. Chem 75, 663–670 [DOI] [PubMed] [Google Scholar]
- 39.Olsen J. V., de Godoy L. M., Li G., Macek B., Mortensen P., Pesch R., Makarov A., Lange O., Horning S., Mann M. (2005) Parts per million mass accuracy on an Orbitrap mass spectrometer via lock mass injection into a C-trap. Mol. Cell. Proteomics 4, 2010–2021 [DOI] [PubMed] [Google Scholar]
- 40.Schroeder M. J., Shabanowitz J., Schwartz J. C., Hunt D. F., Coon J. J. (2004) A neutral loss activation method for improved phosphopeptide sequence analysis by quadrupole ion trap mass spectrometry. Anal. Chem 76, 3590–3598 [DOI] [PubMed] [Google Scholar]
- 41.Cox J., Mann M. (2008) MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol 26, 1367–1372 [DOI] [PubMed] [Google Scholar]
- 42.Perkins D. N., Pappin D. J., Creasy D. M., Cottrell J. S. (1999) Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20, 3551–3567 [DOI] [PubMed] [Google Scholar]
- 43.Elias J. E., Gygi S. P. (2007) Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat. Methods 4, 207–214 [DOI] [PubMed] [Google Scholar]
- 44.Choi H., Nesvizhskii A. I. (2008) False discovery rates and related statistical concepts in mass spectrometry-based proteomics. J. Proteome Res 7, 47–50 [DOI] [PubMed] [Google Scholar]
- 45.Nesvizhskii A. I., Aebersold R. (2005) Interpretation of shotgun proteomic data: the protein inference problem. Mol. Cell. Proteomics 4, 1419–1440 [DOI] [PubMed] [Google Scholar]
- 46.Jensen L. J., Kuhn M., Stark M., Chaffron S., Creevey C., Muller J., Doerks T., Julien P., Roth A., Simonovic M., Bork P., von Mering C. (2009) STRING 8—a global view on proteins and their functional interactions in 630 organisms. Nucleic Acids Res 37, D412–D416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shannon P., Markiel A., Ozier O., Baliga N. S., Wang J. T., Ramage D., Amin N., Schwikowski B., Ideker T. (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13, 2498–2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang da W., Sherman B. T., Lempicki R. A. (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc 4, 44–57 [DOI] [PubMed] [Google Scholar]
- 49.Mann M. (2006) Functional and quantitative proteomics using SILAC. Nat. Rev. Mol. Cell Biol 7, 952–958 [DOI] [PubMed] [Google Scholar]
- 50.Ong S. E., Blagoev B., Kratchmarova I., Kristensen D. B., Steen H., Pandey A., Mann M. (2002) Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol. Cell. Proteomics 1, 376–386 [DOI] [PubMed] [Google Scholar]
- 51.Dephoure N., Zhou C., Villén J., Beausoleil S. A., Bakalarski C. E., Elledge S. J., Gygi S. P. (2008) A quantitative atlas of mitotic phosphorylation. Proc. Natl. Acad. Sci. U.S.A 105, 10762–10767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang B., Malik R., Nigg E. A., Körner R. (2008) Evaluation of the low-specificity protease elastase for large-scale phosphoproteome analysis. Anal. Chem 80, 9526–9533 [DOI] [PubMed] [Google Scholar]
- 53.Olsen J. V., Blagoev B., Gnad F., Macek B., Kumar C., Mortensen P., Mann M. (2006) Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell 127, 635–648 [DOI] [PubMed] [Google Scholar]
- 54.Vulsteke V., Beullens M., Boudrez A., Keppens S., Van Eynde A., Rider M. H., Stalmans W., Bollen M. (2004) Inhibition of spliceosome assembly by the cell cycle-regulated protein kinase MELK and involvement of splicing factor NIPP1. J. Biol. Chem 279, 8642–8647 [DOI] [PubMed] [Google Scholar]
- 55.Ma W., Koch J. A., Viveiros M. M. (2008) Protein kinase C delta (PKCdelta) interacts with microtubule organizing center (MTOC)-associated proteins and participates in meiotic spindle organization. Dev. Biol 320, 414–425 [DOI] [PubMed] [Google Scholar]
- 56.Sturgill T. W. (2008) MAP kinase: it's been longer than fifteen minutes. Biochem. Biophys. Res. Commun 371, 1–4 [DOI] [PubMed] [Google Scholar]
- 57.Willard F. S., Crouch M. F. (2001) MEK, ERK, and p90RSK are present on mitotic tubulin in Swiss 3T3 cells: a role for the MAP kinase pathway in regulating mitotic exit. Cell. Signal 13, 653–664 [DOI] [PubMed] [Google Scholar]
- 58.Shinohara M., Mikhailov A. V., Aguirre-Ghiso J. A., Rieder C. L. (2006) Extracellular signal-regulated kinase 1/2 activity is not required in mammalian cells during late G2 for timely entry into or exit from mitosis. Mol. Biol. Cell 17, 5227–5240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cha H., Wang X., Li H., Fornace A. J., Jr. (2007) A functional role for p38 MAPK in modulating mitotic transit in the absence of stress. J. Biol. Chem 282, 22984–22992 [DOI] [PubMed] [Google Scholar]
- 60.Campos C. B., Bédard P. A., Linden R. (2002) Activation of p38 mitogen-activated protein kinase during normal mitosis in the developing retina. Neuroscience 112, 583–591 [DOI] [PubMed] [Google Scholar]
- 61.Mikhailov A., Shinohara M., Rieder C. L. (2004) Topoisomerase II and histone deacetylase inhibitors delay the G2/M transition by triggering the p38 MAPK checkpoint pathway. J. Cell Biol 166, 517–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kauffman S., Wille J. J. (1975) The mitotic oscillator in Physarum polycephalum. J. Theor. Biol 55, 47–93 [DOI] [PubMed] [Google Scholar]
- 63.Murray A. W., Solomon M. J., Kirschner M. W. (1989) The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature 339, 280–286 [DOI] [PubMed] [Google Scholar]
- 64.Oppermann F. S., Gnad F., Olsen J. V., Hornberger R., Greff Z., Kéri G., Mann M., Daub H. (2009) Large-scale proteomics analysis of the human kinome. Mol. Cell. Proteomics 8, 1751–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Murata-Hori M., Tatsuka M., Wang Y. L. (2002) Probing the dynamics and functions of aurora B kinase in living cells during mitosis and cytokinesis. Mol. Biol. Cell 13, 1099–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gassmann R., Carvalho A., Henzing A. J., Ruchaud S., Hudson D. F., Honda R., Nigg E. A., Gerloff D. L., Earnshaw W. C. (2004) Borealin: a novel chromosomal passenger required for stability of the bipolar mitotic spindle. J. Cell Biol 166, 179–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Neef R., Gruneberg U., Kopajtich R., Li X., Nigg E. A., Sillje H., Barr F. A. (2007) Choice of Plk1 docking partners during mitosis and cytokinesis is controlled by the activation state of Cdk1. Nat. Cell Biol 9, 436–444 [DOI] [PubMed] [Google Scholar]
- 68.Wilker E. W., van Vugt M. A., Artim S. A., Huang P. H., Petersen C. P., Reinhardt H. C., Feng Y., Sharp P. A., Sonenberg N., White F. M., Yaffe M. B. (2007) 14–3-3sigma controls mitotic translation to facilitate cytokinesis. Nature 446, 329–332 [DOI] [PubMed] [Google Scholar]
- 69.Villén J., Gygi S. P. (2008) The SCX/IMAC enrichment approach for global phosphorylation analysis by mass spectrometry. Nat. Protoc 3, 1630–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McNulty D. E., Annan R. S. (2008) Hydrophilic interaction chromatography reduces the complexity of the phosphoproteome and improves global phosphopeptide isolation and detection. Mol. Cell. Proteomics 7, 971–980 [DOI] [PubMed] [Google Scholar]