Abstract
(+)-Methamphetamine (MA) is an illicit psychostimulant that can be synthesized from the nonprescription nasal decongestant, (+)-pseudoephedrine (PE). While MA is widely abused, PE appears to have little or no abuse liability in currently available formulations. However, PE produces centrally-mediated dopaminergic effects that are linked to the reinforcing effects of MA and other illicit psychostimulants and has been reported to function as a positive reinforcer in non-human primates. There has yet to be an assessment of the relative reinforcing effects of MA and PE. Therefore, the current study compared the reinforcing potency and strength of MA and PE, alone and combined, in four rhesus monkeys that were allowed to self-administer MA (0.003-0.3 mg/kg/inj), PE (0.1-3.0 mg/kg/inj), or combinations of the two under a progressive-ratio schedule of reinforcement. (+)-Methamphetamine functioned as a positive reinforcer in a dose-dependent manner. (+)-Pseudoephedrine also functioned as a positive reinforcer, but was less potent than MA. There were no differences in maximum injections between MA, PE, or any of the combinations of the two. Dose-addition analysis and the interaction index indicated that combinations of PE and MA were either additive or sub-additive in their reinforcing effects. These results suggest that, while MA is a more potent reinforcer than PE, the two drugs are comparable in terms of reinforcing strength. However, MA and PE do not appear to interact in a manner that enhances their relative reinforcing effects.
Keywords: (+)-methamphetamine, (+)-pseudoephedrine, rhesus monkey, self-administration, progressive-ratio
Introduction
(+)-Methamphetamine (MA) is one of the most widely abused psychostimulants in the world (World Drug Report, 2007). However, unlike many drugs of abuse that must be cultivated from crops and exported for distribution (e.g., marijuana, cocaine, heroin), MA can be produced domestically using chemical precursors that are available over-the-counter. One of these precursors is the nonprescription nasal decongestant, (+)-pseudoephedrine (PE; Logan et al., 2001). The widespread use of PE in the production of MA has, in numerous countries, led to regulations on its purchase that include limitations on the amount that can be bought and the tracking of sales to individual purchasers (Logan, 2001).
Despite the widespread availability of PE and its structural similarity to MA, it does not appear to have significant abuse liability in currently available formulations. However, results from in vitro and in vivo testing suggest that PE may have abuse potential under certain conditions. In terms of neurochemistry, it has been widely reported that the reinforcing effects of psychostimulants are mediated by their actions on brain dopamine systems, particularly within the mesocorticolimbic circuit (Gold et al., 1989; Koob et al., 1994), and like the illicit psychostimulants, PE acts on these systems. For example, although much less potent than cocaine, PE binds to the dopamine transporter and blocks dopamine uptake in HEK-293 cells transfected with the human dopamine transporter (Wee et al., 2004). Furthermore, in rats, PE increases Fos-like activity in the nucleus accumbens, a brain area that is critical for the reinforcing effects of psychostimulants (Kumarnsit et al., 1999). Moreover, cross-tolerance for Fos-like expression in the nucleus accumbens occurs reciprocally between PE and amphetamine (Ruskee et al., 2008), suggesting that these compounds induce their effects in this area through similar mechanisms. Lastly, like the illicit psychostimulants, Fos-like expression in the nucleus accumbens induced by PE appears to be dopaminergically mediated because it can be blocked by pretreatment with the dopaminergic antagonist SCH23390 (Kumarnsit et al., 1999).
Similar to PE’s in vitro potency, the behavioral potency of PE is relatively low. For example, PE produces amphetamine-like discriminative stimulus effects in rats (Tongjaroenbuangam et al., 1998) and non-human primates (Anderson et al., 2001), but only when administered at relatively high doses. Furthermore, PE is self-administered by non-human primates under fixed-ratio schedules of reinforcement (Anderson et al., 2001; Wee et al., 2004), indicating that it can function as a positive reinforcer, but its potency is at least ten times lower than cocaine (Wee et al., 2004). Taken together, these results suggest that PE has a similar behavioral and pharmacological profile to the illicit psychostimulants, but is generally less potent.
There has yet to be a direct comparison between PE and MA on any measure of reinforcement. Given the use of PE as a precursor for MA synthesis, it is of interest to know the relative reinforcing effects of PE and MA. Furthermore, given the widespread use of PE as an over-the-counter medication, it is also of interest to know whether and how it affects the reinforcing effects of MA when the two are concurrently taken. To examine these issues, rhesus monkeys were allowed to self-administer MA and PE, both alone and in combination, under a progressive-ratio (PR) schedule of reinforcement. A PR schedule was used because it allows for the measurement of both the potency and the strength (maximum reinforcing effect) of a reinforcer by including both a dose-response function and an extinction criterion (breakpoint), the latter of which is thought to index the reinforcing strength of a drug (Richardson and Roberts, 1996; Rowlett et al., 1996). It was hypothesized that, similar to a previous report comparing PE to cocaine (Wee et al., 2004), PE would function as a reinforcer in monkeys but would be less potent than MA. Furthermore, if PE and MA produced their reinforcing effects through a common mechanism of action, it was hypothesized that the two would be additive in terms of reinforcing strength.
Method
All animal-use procedures were approved by the University of Mississippi Medical Center’s Animal Care and Use Committee and were in accordance with the National Research Council’s Guide for Care and Use of Laboratory Animals (1996).
Subjects and Apparatus
The subjects were four male rhesus monkeys (Macaca mulatta) weighing between 8.2 and 11.2 kg at the beginning of the study. All the monkeys had histories of drug self-administration under conditions similar to those used here. Most recently, monkeys CK47and M1388 had histories of cocaine and pentobarbital self-administration (Woolverton and Wang, 2009). Monkey M341 had a history of self-administration of MDMA and its isomers (Wang and Woolverton, 2007) and monkey R463 had as a history of self-administering cocaine and antihistamine mixtures (Wang and Woolverton, 2009). There was no drug-free period between experiments. All monkeys were provided with sufficient food to maintain stable body weight (Teklad 25% Monkey Diet, Herlan/Teklad, Madison, WI) and had unlimited access to water. Fresh fruit and a vitamin supplement were provided daily and three times a week, respectively.
The monkeys were individually housed in the experimental cubicles (1.0 m3, Plaslabs, Lansing, MI). Each monkey was fitted with a stainless-steel harness attached by a tether to the rear wall of the cubicle. The front door of the cubicle was made of transparent plastic and the remaining walls were opaque plastic. Two response levers (PRL-001, BRS/LVE, Beltsville, MD) were mounted on the inside of the door. Four jeweled stimulus lights, two red and two white, were mounted above each lever. Drug injections were delivered by a peristaltic infusion pump (Cole-Parmer Co., Chicago, IL). A Macintosh computer with custom interface and software controlled all events in an experimental session.
Procedure
Each monkey was implanted with a single-lumen silastic catheter (0.26 cm o.d. × 0.076 cm i.d.; Cole-Parmer Co., Chicago, IL) into a jugular (internal or external) or femoral vein. Brachial veins were implanted with a silicone catheter (0.17 cm o.d. × 0.07 cm i.d.; Access Technologies, Skokie, IL). For catheter implantation, the monkey was injected with a combination of atropine sulfate (0.04 mg/kg, i.m.) and ketamine hydrochloride (10 mg/kg, i.m.) followed 20-30 min later by inhaled isoflurane. The proximal end of the catheter was inserted into the vein and terminated in or near the vena cava. The distal end was threaded subcutaneously to exit the back of the monkey, threaded through the spring arm, out the rear of the cubicle and connected to the peristaltic pump. In the event of catheter failure, surgery was repeated using another vein after the veterinarian confirmed the health of the monkey.
Experimental sessions began at 11:00 hours each day and were conducted seven days per week. Thirty minutes before each session started, each monkey’s catheter was filled with the solution available for that day’s session without infusing the solution into the monkey. At the start of a session, the white lights were illuminated above both levers and pressing the right lever resulted in the delivery of a drug injection for 10 seconds. During the injection, the white lights were extinguished and the red lights were illuminated. Pressing the left lever was counted but had no other programmed consequence. After the session, catheters were filled with 0.9% saline containing heparin (40 units/ml).
Lever pressing for baseline and test sessions was maintained under a PR schedule of reinforcement as previously described (Wilcox et al., 2000). Briefly, the PR schedule consisted of five components, each made up of four trials, for a possible total of 20 trials. The response requirement was fixed for the four trials within a component and doubled with each progression to a new component. For most monkeys, the response requirement started at 100 responses per injection (i.e., the response requirement for the first component was 100 responses per injection). For monkey R463, responding was not well maintained under these conditions and the response requirement started at 50. A subject had 30 minutes to complete each trial (limited hold 30 min: LH 30′). A trial ended with a 10-sec drug injection or the expiration of the LH. There was a 30 minute-timeout (TO 30′) after each trial. If the response requirement was not completed for two consecutive trials (i.e., the LH expired), or the animal self-administered all 20 injections, the session ended.
In baseline sessions, cocaine or saline was available for injection. The baseline dose of cocaine or saline was initially available under a double-alternation schedule. That is, two consecutive cocaine sessions were followed by two consecutive saline sessions. When responding was stable (running mean for each type of baseline session within ± 2 injections, and four or fewer injections/session in saline sessions) for at least two consecutive double-alternation sequences (i.e., eight sessions), test sessions were inserted into the daily sequence between two saline and two cocaine sessions. Additionally, to prevent monkeys from learning this session sequence, a randomly determined saline or cocaine baseline session was inserted after every other test session. Thus, the daily sequence of sessions was C, S, T, S, C, T, R, C, S, T, S, C, T, R, where “C”, “S”, “R” and “T”, respectively, represent a cocaine, a saline, a randomly determined cocaine/saline and a test session. The baseline dose of cocaine for all monkeys was the lowest that maintained maximum injections, 0.2 mg/kg/inj. During test sessions, one of various doses of MA (0.003-0.3 mg/kg/inj or PE (0.1-3.0 mg/kg/inj) was available for self-administration under conditions identical to baseline sessions. All doses were tested at least twice in each monkey, once with a saline session the day before and once with a cocaine session the day before. When the two test sessions of a dose showed high variability (the number of injections exceeded ± 3 injections of the mean), the dose was tested twice as before, once after saline session and once after cocaine baseline session. If the redetermined effects were less variable and comparable to one of the original test sessions, then those three sessions of four were used for data analysis. If the redetermined effects were variable like the initially determined effects, all four sessions were used to calculate the mean. MA was tested first in M1388, M341 and CK47, while (+)-PE was tested first in R463. Doses of each drug were tested that ranged between one or two with no effect and one or two with the maximum observed effect After testing drugs alone, mixtures of MA and (+)-PE were tested using a “fixed-dose” method (Tallarida, 2000). That is, monkeys were tested with combinations of various doses of MA with fixed (+)-PE doses of 0.1, 0.3 and 1.0 mg/kg/injection. In all cases, doses of drugs alone and mixtures were available in an irregular order across monkeys.
Data analysis
The mean number of injections per session was calculated individually from the two test sessions at a dose. A single drug or combination was considered to be a positive reinforcer in a monkey if the mean value for these two sessions exceeded the mean value for saline test sessions and the ranges did not overlap. For each monkey, the mean dose-effect data for MA alone, PE alone, and for MA mixed with 0.1 and 0.3 mg/kg PE were fitted by non-linear regression (Graphpad Prism 4.0), and ED50 values were calculated for each function. An ED50 for MA mixed with 1.0 mg/kg PE could not be determined because this mixture resulted in a horizontal dose-effect function (see Results).
Interactions between MA and PE were examined using dose-addition analysis and the interaction index (Tallarida, 2000). The dose addition analysis was used to predict the ED50 value of MA mixed with 0.1 and 0.3 mg/kg PE if MA and PE were additive in their reinforcing effects (termed ED50Add). The ED50Add for the mixtures were then compared to the experimentally-determined ED50s of the mixtures (ED50Exp) to generate an interaction index as follows:
where an interaction index value of 1 indicates additivity and values above and below 1 indicate sub-additivity and super-additivity, respectively. Interaction indices for MA mixed with 0.1 and 0.3 mg/kg PE were individually determined in each monkey. The interaction indices for each mixture were then averaged across monkeys and 95% confidence intervals were determined for the mean interaction index of each mixture. Confidence intervals that included the value of 1 indicated that the mixture was additive. Intervals that spanned a range above and below 1 indicated that the mixtures were sub-additive and super-additive, respectively.
To assess the relative reinforcing strength of MA and PE, alone and combined, mean maximum injections per session for each drug condition (i.e., the highest mean breakpoint for each monkey in each drug condition: MA alone, PE alone, or MA mixed with 0.1, 0.3, or 1.0 mg/kg/inj of PE) were averaged for all monkeys and compared using a one-way repeated-measures analysis of variance (ANOVA) test with the single factor of drug condition. It should be noted that, between monkeys, the maximum injections per session for a particular drug condition could vary by dose for MA or PE in the single drug tests and the dose of MA in the mixture tests. Significance was set at p ≤ 0.05.
Results
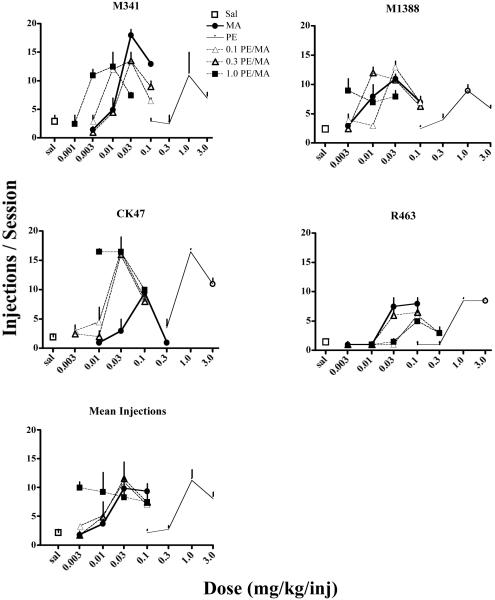
Methamphetamine and PE functioned as positive reinforcers in a dose-dependent manner in all monkeys, and in each case MA was a more potent reinforcer than PE (see Figure 1). Adding PE to MA shifted the dose-response function for MA to the left in two monkeys (M341 and CK47) and to the right in one (R463), while the fourth monkey (M1388) showed no systematic effects of PE additions on MA self-administration. When the data were averaged for all monkeys (Figure 1, bottom graph), MA functioned as a positive reinforcer in a dose-dependent manner when mixed with 0.1 and 0.3 mg/kg PE. However, adding 1.0 mg/kg PE to MA resulted in a dose-response function that was shifted upward and did not vary by MA dose, an effect that can be explained by the fact that PE was maximally reinforcing at this dose when taken alone.
Fig. 1.
Dose-response functions for self-administration of methamphetamine (MA; bold solid line, solid circles), (+)-pseudoephedrine (PA; thin solid line, open circles) and MA mixed with various concentrations of PE (hatched lines) under a progressive-ratio schedule of reinforcement. The top four graphs represent the data for individual monkeys, identified by numbers on the graphs. Each point is the mean value for two test sessions at each dose or combination. The bottom graph represents the data averaged across all four monkeys. Vertical lines are the range (SEM).
The 95% confidence interval of the mean interaction index of MA mixed with PE 0.1 mg/kg spanned a range that included the value of 1 (see Table 1), indicating that MA and PE were additive in their reinforcing effects when the dose of PE was fixed at 0.1 mg/kg. However, the 95% confidence interval for MA mixed with PE 0.3 mg/kg spanned a range above the value of 1, indicating that MA and PE were sub-additive when the PE dose was fixed at 0.3 mg/kg.
Table 1.
ED50 values for combinations of (+)-methamphetamine and fixed doses of 0.1 and 0.3 mg/kg of (+)-pseudoephedrine (MA-PE 0.1 and MA-PE 0.3, respectively) for individual monkeys as predicted by additivity (ED50Add) and as determined experimentally (ED50Exp). The three right columns are the individual interaction indices for each monkey and the mean and 95% confidence intervals (C.I.) for the interaction indices for the two drug combinations
| 95% C.I. |
||||||
|---|---|---|---|---|---|---|
| ED50Add | ED50Exp | Interaction Index |
Lower | Upper | ||
| MA-PE 0.1 | M341 | 0.009 | 0.008 | 0.89 | ||
| R463 | 0.020 | 0.054 | 2.70 | |||
| CK47 | 0.024 | 0.012 | 0.50 | |||
| M1388 | 0.006 | 0.013 | 2.17 | |||
| Mean | 1.57 | −0.09 | 3.22 | |||
|
| ||||||
| MA-PE 0.3 | M341 | 0.006 | 0.011 | 1.83 | ||
| R463 | 0.010 | 0.024 | 2.40 | |||
| CK47 | 0.006 | 0.017 | 2.83 | |||
| M1388 | 0.001 | 0.004 | 4.00 | |||
| Mean | 2.77 | 1.30 | 4.23 * | |||
Range of the 95% confidence interval does not include 1.
Mean maximum injections/session of MA and PE, alone and combined, are presented for each subject in Figure 2. There were no significant differences in maximum injections between MA, PE, or any of the mixtures (F [4,19] = 0.188; p = 0.94), indicating that MA and PE were equivalent in reinforcing strength and that adding PE to MA did not change the reinforcing strength of MA.
Fig. 2.
Maximum number of injections per session for methamphetamine (MA), (+)-pseudoephedrine (PE), or MA mixed with 0.1, 0.3, or 1.0 mg/kg PE, respectively, regardless of MA dose(s). Each bar is the mean value for four monkeys and vertical lines are the SEM.
Discussion
As previously reported using a variant of the present procedure (Wang and Woolverton, 2007), MA functioned as a positive reinforcer in a dose-dependent manner. (+)-Pseudoephedrine also functioned as a positive reinforcer, but was less potent than MA. The fact that PE was a less potent reinforcer than MA is consistent with previous reports comparing PE to other psychostimulants on behavioral and pharmacological processes related to drug reinforcement. In self-administration tests with rhesus monkeys, PE has been reported to be a less potent reinforcer than cocaine (Wee et al., 2004). Furthermore, in drug discrimination tests, PE substituted for amphetamine in rats (Tongjaroenbuangam et al., 1998) and monkeys (Anderson et al., 2001), but only did so at relatively high doses, indicating that it was less potent than amphetamine at producing amphetamine-like discriminative stimulus effects. These potency differences in behavior are consistent with PE’s low potency at binding to the dopamine transporter and inhibiting dopamine reuptake relative to amphetamine and cocaine (Ruskee et al., 2008; Wee et al., 2004). Currently, there are no reports of direct potency comparisons between PE and MA on dopaminergic neurochemistry. However, given that MA’s potency as an inhibitor of dopamine reuptake falls in between amphetamine and cocaine, and that its dopamine-releasing potency is comparable to amphetamine (Rothman et al., 2001), it is likely that PE’s low reinforcing potency relative to MA is related to its low potency as a modulator of dopaminergic neurochemistry.
The fact that the maximum number of injections per session were comparable for PE and MA indicates that, under the current conditions, the two drugs were comparable in terms of reinforcing strength. This somewhat surprising finding raises the possibility that, in humans, PE taken at sufficiently high doses can have reinforcing effects comparable to the maximum reinforcing effects produced by MA. Accepting this, it would be predicted that PE might have significant abuse liability. However, reports of PE abuse are rare. While this discrepancy could be accounted for by species differences (i.e., human vs. non-human primates), it could also be due to differences in routes of administration. In the current study, monkeys took PE intravenously while humans typically take PE orally as an over-the-counter medication. It is well-known that intravenous drug administration results in faster peak plasma concentration and delivery to the central nervous system than oral administration. Furthermore, direct relationships between the reinforcing potency and strength of drugs and their rates of infusion and delivery into the central nervous system are widely reported (Gorelick, 1998; Gossop et al., 1992; Panlilio et al., 1998; Woolverton & Wang, 2004). Therefore, given the relatively low reinforcing potency of PE, it is possible that its reinforcing effects are only apparent using a rapid form of drug delivery. Interestingly, while the reinforcing effects of intravenous PE have yet to be tested in humans, there has been one case report of a man presenting with acute symptoms consistent with psychostimulant-induced psychosis after self-administering PE intravenously (Sullivan, 1996). Notably, this individual injected a single pill’s dosage of PE (Sudafed, 60 mg, dissolved in water) which, if taken orally, should not have caused psychotic symptoms. This further suggests that the psychoactive effects of PE are route-dependent. If so, it would be of interest to test the reinforcing effects of intravenous PE in humans. It would also be interesting to see how the subjective ratings of intravenous PE compare to illicit psychostimulants like MA or cocaine.
The current results, when taken together with previous studies comparing PE to other psychostimulants (Anderson et al., 2001; Ruskee et al., 2008; Tongjaroenbuangam et al., 1998; Wee et al., 2004), indicate that PE and MA may function as positive reinforcers through a common mechanism of action. The relative positioning of the dose-response functions for PE and MA, taken together with their similarity of shape (see Figure 1, bottom graph), are consistent with drugs that have common mechanisms of action but different relative potencies (Tallarida, 1979). However, when the PE dose was fixed at 0.3 mg/kg, the drugs interacted in a manner that was sub-additive. That is, the reinforcing effect of the drug combination was less than what was predicted by dose-additivity. It has been previously reported that reinforcing drugs with similar mechanisms of action are additive in their reinforcing effects (Woolverton et al., 2008). If MA and PE both produce their reinforcing effect through similar actions at the dopamine transporter, it would be predicted that they would be additive in their reinforcing effects at all dose combinations. Why they were not at the fixed dose of PE 0.3 mg/kg is not certain. It seems reasonable to suppose, however, that at low doses the dopaminergic actions of PE are limited and/or that penetration of the BBB is limited. At the very least, the fact that MA and PE did not interact in a super-additive fashion indicates that PE use does not enhance the reinforcing effects of MA.
In summary, the current results indicate that intravenous MA and PE are both reinforcing in monkeys and differ in terms of reinforcing potency but not strength. While PE appears to have little or no abuse liability in its current over-the-counter formulation, the fact that, in monkeys, its reinforcing strength was comparable to MA suggests that PE may have abuse potential if taken intravenously. However, there is no indication that PE and MA interact in a way that enhances their relative reinforcing effects. As such, the co-abuse of these drugs does not seem likely.
Acknowledgments
This research was supported by National Institute on Drug Abuse grant R01 DA-01947 to W.L.W. We gratefully acknowledge Lee Hutson for his technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson KG, Winger G, Woods J, Woolverton WL. Reinforcing and discriminative-stimulus effects of ephedrine isomers in rhesus monkeys. Drug Alc Dep. 2001;65:45–53. doi: 10.1016/s0376-8716(01)00143-0. [DOI] [PubMed] [Google Scholar]
- Gold LH, Geyer MA, Koob GF. Neurochemical mechanisms involved in behavioral effects of amphetamines and related designer drugs. NIDA Res Monogr. 1989;94:101–26. [PubMed] [Google Scholar]
- Gorelick DA. The rate hypothesis and agonist substitution approaches to drug use. Adv Pharmacol. 1998;42:995–7. doi: 10.1016/s1054-3589(08)60914-x. [DOI] [PubMed] [Google Scholar]
- Gossop M, Griffiths P, Powis B, Strang J. Severity of dependence and route of administration of heroin, cocaine and amphetamines. Br J Addict. 1992;87:1527–36. doi: 10.1111/j.1360-0443.1992.tb02660.x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Caine B, Markou A, Pulvirenti L, Weiss F. Role for mesocortical dopamine system in the motivating effects of cocaine. NIDA Res Monagr. 1994;145:1–18. [PubMed] [Google Scholar]
- Kumarnsit E, Harnyuttanakorn P, Meksuriyen D, Govitrapong P, Baldwin BA, Kotchabhakdi N, Casalotti SO. Pseudoephedrine, a sympathomimetic agent, induces Fos-like immunoreactivity in rat nucleus accumbens and striatum. Neuropharmacol. 1999;38:1381–7. doi: 10.1016/s0028-3908(99)00054-4. [DOI] [PubMed] [Google Scholar]
- Logan BK. Amphetamines: An update on forensic issues. J Anal Toxicol. 2001;25:400–4. doi: 10.1093/jat/25.5.400. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Goldberg SR, Gilman JP, Jufer R, Cone EJ, Schindler CW. Effects of delivery rate and non-contingent infusion of cocaine on cocaine self-administration in rhesus monkeys. Psychopharm. 1998;137:253–8. doi: 10.1007/s002130050618. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DCS. Progressive ratio schedules of drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll I, Partilla JS. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Massey BW, Kleven MS, Woolverton WL. Parametric analysis of cocaine self-administration under a progressive-ratio schedule in rhesus monkeys. Psychopharm. 1996;125:361–70. doi: 10.1007/BF02246019. [DOI] [PubMed] [Google Scholar]
- Ruskee N, Tongjaroenbuangam W, Casalotti SO, Govitrapong P. Amphetamine and pseudoephedrine cross-tolerance measured by c-Fos protein expression in brains of chronically treated rats. BMC Neurosci. 2008;9:99. doi: 10.1186/1471-2202-9-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan G. Acute psychosis following intravenous abuse of pseudoephedrine: a case report. J Psychopharm. 1996;10:324–5. doi: 10.1177/026988119601000413. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ. The Dose-Response Relation in Pharmacology. Springer-Verlag; 1979. [Google Scholar]
- Tallarida RJ. Drug synergism and dose-effect data analysis. Chapman & Hall/CRC; 2000. [Google Scholar]
- Tongjaroenbuangam W, Meksuriyen D, Govitrapong P, Kotchabhakdi N, Baldwin BA. Drug discrimination analysis of pseudoephedrine in rats. Pharmacol Biochem Behav. 1998;59:505–10. doi: 10.1016/s0091-3057(97)00459-0. [DOI] [PubMed] [Google Scholar]
- Wang Z, Woolverton WL. Estimating the relative reinforcing strength of (±)-3,4-methylenedioxymethamphetamine (MDMA) and its isomers in rhesus monkeys: comparison to (+)-methamphetamine. Psychopharm. 2007;189:483–8. doi: 10.1007/s00213-006-0599-5. [DOI] [PubMed] [Google Scholar]
- Wang Z, Woolverton WL. Super-additive interaction of the reinforcing effects of cocaine and H1-antihistamines in rhesus monkeys. Pharmacol Biochem Behav. 2009;91:590–5. doi: 10.1016/j.pbb.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Ordway GA, Woolverton WL. Reinforcing effect of pseudoephedrine isomers and the mechanism of action. Eur J Pharmacol. 2004;493:117–25. doi: 10.1016/j.ejphar.2004.04.030. [DOI] [PubMed] [Google Scholar]
- Wilcox KM, Rowlett JK, Paul IA, Ordway GA, Woolverton WL. On the relationship between the dopamine transporter and the reinforcing effects of local anesthetics in rhesus monkeys: practical and theoretical concerns. Psychopharm. 2000;153:139–47. doi: 10.1007/s002130000457. [DOI] [PubMed] [Google Scholar]
- World Drug Report. United Nations, Office on Drugs and Crime; 2007. [Google Scholar]
- Woolverton WL, Wang Z. Relationship between injection duration, transporter occupancy and reinforcing strength of cocaine. Br J Pharmacol. 2004;486:251–7. doi: 10.1016/j.ejphar.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Wang Z. Self-administration of cocaine-pentobarbital mixtures by rhesus monkeys. Drug Alc Dep. 2009;100:272–6. doi: 10.1016/j.drugalcdep.2008.10.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolverton WL, Wang Z, Vasterling T, Carroll FI, Tallarida R. Self administration of drug mixtures by monkeys: combining drugs with comparable mechanisms of action. Psychopharm. 2008;196:575–82. doi: 10.1007/s00213-007-0991-9. [DOI] [PMC free article] [PubMed] [Google Scholar]