Abstract
The present study resolves some of the discrepancies in the literature by correlating the effects of tobacco smoking on hormone release with venous plasma nicotine levels. Cortisol, prolactin, and β-endorphin concentrations were measured. Habitual male tobacco users smoked denicotinized (very low nicotine) and average nicotine cigarettes in the A.M. after overnight tobacco abstinence. Several venous blood samples were withdrawn before and during the smoking sessions for subsequent analyses. The increases in plasma nicotine correlated well with plasma cortisol and prolactin levels (correlation coefficients r = 0.66 and 0.53, respectively, p< 0.05). This study quantifies the well known increase in plasma cortisol and prolactin after nicotine postsmoking for about 1 hr with peak plasma levels up to 35 ng/ml. Contrary to most abused drugs which release dopamine and decrease prolactin, nicotine concentration correlated with increased prolactin release. Increases in maximal plasma β-endorphin levels following tobacco smoking were barely statistically significant with insufficient data to obtain a correlation coefficient.
Keywords: cigarette smoking, nicotine, cortisol, prolactin, β-endorphin
1. Background
Over the years, a great deal of research has been published on nicotine/tobacco smoking releasing various hormones in animals or humans. However, not all effects are the same between species. For example, rats trained to self-administer nicotine have increased levels of adrenocorticotropin (ACTH) and corticosterone on day 1 but not by day 3. After 20 days of self-administered nicotine, the rats’ hormonal response is augmented 2–3 fold to mild foot shock stress but not to moderate shock, lipopolysaccharide or immobilization stress (Chen et al., 2008). Childs and de Wit (2008) compared cortisol, progesterone, allopregnanolone, and catecholamine responses to public speaking stress and a control nonstressful task in male nonsmokers and smokers. Two hr after smoking a cigarette at 8:00 a.m., compared to nonsmokers the smokers had blunted cortisol responses to the speaking stress as well as greater and prolonged agitation. Stress induced progesterone was similar but lower in the smokers. The allopregnanolone levels were also lower in the smokers. Stress did not alter the levels of the latter neurosteroid. Plasma norepinephrine levels increased in both groups with speaking induced stress. Heart rate and blood pressure increased in both groups subjected to stress. Diastolic blood pressure was less in the stressed smokers. The authors concluded that smoking dampens the hormonal response to stress and prolongs subjective distressed mood. The results of this study in humans appear to be opposite to the findings in rats that nicotine self-administration cross-sensitizes to mild stress.
In rats, nicotine inhibits the release of prolactin (Muraki et al., 1979; Andersson, 1985), whereas it increases plasma levels of prolactin in humans. Nicotine also produces increased levels of ACTH, vasopressin, β-endorphin, growth hormone, and cortisol in human subjects (Wilkins, et al., 1982; Seyler, et al., 1986; Fuxe, et al., 1989; Pomerleau and Rosecrans, 1989; Kirschbaum et al., 1994). Increases in ACTH, corticosterone, β-endorphin, and growth hormone after nicotine have also been observed in rats (Conte-Devolx et al., 1982).
With animal research, the dose of nicotine administered is known. When studying human tobacco users the dose of nicotine absorbed varies markedly even when the nicotine content of the tobacco product is reported. In order to obtain a greater variation in nicotine dose, investigators have studied tobacco with different nicotine content. Imprecise terms such as denicotinized, low, average, or high nicotine tobacco are used for dose-effect studies with differing results. For example, Wilkins et al. (1982) compared levels of cortisol, growth hormone, and prolactin after smoking either low (0.2 mg) or high nicotine (2.0 mg) cigarettes. All three hormone levels were found to be significantly greater after high nicotine cigarettes. Meliska and Gilbert (1991) studied the effects of nicotine on cortisol and β-endorphin in both male and female smokers. Smoking caused increases in cortisol and β-endorphin. However, β-endorphin was elevated after two, but no further increase was observed after four or five tobacco cigarettes. Gilbert et al. (1992) found increases in cortisol and β-endorphin after smoking, but only when using 2.4 mg cigarettes. Subjects’ own brand of cigarettes (1 mg nicotine) did not significantly increase cortisol or β-endorphin levels, even though plasma nicotine levels were significantly increased when compared to nicotine free cigarette smoking. The reports by Mendelson et al. (2005, 2008) are among the best to date on tobacco smoking, hypothalamic, pituitary adrenal axis and cortisol increases, and the significance of these changes.
Prolactin release after tobacco smoking has also been established in research using high, but not low nicotine cigarettes. Seyler et al. (1986) found hormone release after 2.87 mg (high) nicotine cigarettes were smoked, but not after 0.48 mg (medium) nicotine cigarettes. Wilkins et al. (1982) did not find a prolactin response with low (0.2 mg) nicotine cigarettes, but did with high (2.0 mg) nicotine cigarettes. In a more recent study (Mendelson et al., 2003), it was again shown that high nicotine cigarettes (2.3 mg) elicited a prolactin response, but low nicotine cigarettes (0.1 mg) failed to do so. Circulating levels of prolactin have also been observed to be higher in tobacco smokers than in nonsmoking controls, even without smoking before the measurement was taken. Tobacco smokers also have differing plasma concentrations of hormones depending on the number of cigarettes they smoke per day. In a study done by del Arbol et al. (2000), β-endorphin, cortisol, and ACTH plasma concentrations were compared between subjects who consumed different amounts of cigarettes per day vs nonsmoking controls. Blood was drawn in the morning in all subjects after an overnight fast and smoking abstinence. Cortisol levels were significantly higher compared to controls only in subjects who smoked over 20 cigarettes per day. β-endorphin levels were only significantly higher than controls in subjects smoking fewer than 10 cigarettes per day, suggesting that increases in β-endorphin are sustained longer after smoking in lighter smokers. No significant differences in ACTH concentrations were found between groups. ACTH was found by Pomerleau et al. (1983) to have no correlation with plasma nicotine after tobacco smoking. They did, however, find a significant correlation between β-endorphin-β-lipoprotein and plasma nicotine after smoking.
Although nicotine is the major ingredient in tobacco smoke that releases many hormones, precise plasma nicotine concentration-effect relationships bear further scrutiny. The present study quantifies the relationship between plasma nicotine levels and circulating levels of plasma cortisol, prolactin and β-endorphin in overnight abstinent chronic smokers who participated in a brain imaging study.
2. Methods
This study was part of a larger ongoing project involving tobacco smoking effects on human dopamine D2 and mu-opioid brain receptors using positron emission tomography (PET). The entire project was approved by the University of Michigan Institutional Review Board for human studies. The PET project required a counterbalanced use of radioisotopes on two separate days. Hence, the endocrine data reported herein were replicated, on the first day in the PET scanner, and then again on day 2. The day 1 and then day 2 endocrine results were obtained with the hope of measuring scanner stress objectively. A total of 24 healthy male subjects, between 20 and 36 yrs of age (mean 25.8 ± S.E. 0.93) who smoked 15–40 cigarettes per day were recruited for this portion of the project. When a smaller number of subjects from this group were used for specific hormone analysis, their total number is listed. All subjects were instructed to cease tobacco use overnight, approximately 12 hrs before study. To assure compliance, exhaled CO levels were < 10 parts per million prior to the experiment. During the experiment, the subjects were supine and restrained in a PET scanner from about 8:00 a.m. to 12:30 p.m. A counterbalanced day 1 and within a week or so day 2 design of PET scans with [11C]carfentanil and [11C]raclopride in trace nonpharmacological doses was used. The subjects smoked as rapidly as possible two pairs of cigarettes for 5 min each while in the PET scanner. All subjects received both radiotracers, in randomized and counterbalanced order. Subjects first smoked denicotinized (0.08 mg nicotine) cigarettes, then smoked average nicotine (1.01 mg nicotine) cigarettes. Through a series of fortunate circumstances, the two different research cigarettes were obtained through the courtesy of Dr. Frank P. Gullota (now retired) and Ms. Cynthia S. Hayes of the Philip Morris Research Center, Richmond, VA. Unfortunately, these cigarettes are no longer available because Philip Morris stopped producing them. The nicotine containing cigarette was prepared with unextracted tobacco (nicotine 1.01 mg/cigarette and tar 9.5 mg/cigarette). The denicotinized cigarette was made with almost 100% extracted tobacco (nicotine 0.08 mg/cigarette and tar 9.1 mg/cigarette). Hence, the word denicotinized refers to very low nicotine containing cigarettes. Both cigarettes contained identical filter tips and were made from the same blend of tobacco with no flavors added. Thus, their tar content was almost identical (9.5 vs 9.1 mg) and only the mg of nicotine per cigarette was markedly different (1.01 vs 0.08 mg). Heart rate was monitored during both scans. Blood samples (5 ml) were collected repeatedly in vacutainer tubes before and after the onset of smoking both cigarettes. Blood for nicotine and hormone samples was always drawn at the same time points – at 0, 15, 30, 43, 49, 59, 65, 75, and 95 min into each scan. Cigarettes were smoked at 45 and 55 min into each scan. The samples were placed on ice and centrifuged. The plasma was removed and frozen at −70°C for nicotine and hormone analyses. Nicotine assay was done by gas chromatography/nitrogen phosphorus detection (GC-NPD) in the Medtox Laboratory, St. Paul, MN. Cortisol and prolactin analyses were performed at the University of Michigan Clinical Ligand Assay Service Satellite Laboratory (CLASS Lab, Ann Arbor, MI). The cortisol assay was a competitive immunoassay performed on Siemen’s ADVIA Centaur automated analyzer using chemiluminescent technology. This method had a minimum detectable concentration of 0.2 μg/dl and the assay measured up to 75 μg/dl. The assay was standardized analytically and confirmed by gas chromatography-mass spectroscopy. Prolactin was determined as a two-site chemiluminometric (sandwich) immunoassay run on Siemen’s ADVIA Centaur automated analyzer. The minimum detectable concentration was 0.3 ng/ml and the assay measured up to 200 ng/ml. The test results were determined from a calibration curve derived from the standard, WHO 3rd IRP 84/500. β-endorphin was assayed using radioimmunoassay kits (Phoenix Pharmaceuticals, Inc., Burlingame, CA) with a minimum sensitivity of 1 pg/ml.
2.1 Statistical Analysis and Calculations
The hormone data from two separate experimental days were collected in a parametric format. The SPSS 17.0 System for Windows was used for statistical analysis. Descriptive procedures were used to obtain mean ± SE A two-way analysis of variance (ANOVA) with repeated measures in a mixed procedure was conducted to compare the differences between the two experimental days. The post hoc comparison and the Sidak multiple comparison adjustment were options used to indicate the significant time points of endocrine change. Linear regression was conducted to establish the relationships between nicotine cortisol and prolactin levels. The latter were in a continuous variable format without predicting the outcome from the nicotine variable. Therefore, a simple correlation coefficient is sufficient for quantifying the relationship of nicotine and cortisol and prolactin. All data were normally distributed and a probability level of less than 5% (p <0.05) was considered significant.
3. Results
3.1- Plasma Nicotine Levels
The baseline and peak mean ± SE of venous plasma nicotine levels for the two separate conditions (see Methods: counterbalanced design) smoking denicotinized or average nicotine tobacco cigarettes are given in Table 1. Inasmuch as there were no significant nicotine differences between the two PET imaging results, the overall data were combined. It is obvious that smoking denicotinized cigarettes produced a minor delta venous plasma nicotine increase of only 0.88 ng/ml, whereas smoking average nicotine cigarettes produced a 12.9 ng/ml increase. As described in the Methods Section, the denicotinized cigarettes are actually very low nicotine cigarettes. All of the after smoking data shown in the table below were statistically significant (p<0.001) including the denicotinized vs average nicotine cigarettes.
Table 1.
Effects of tobacco smoking on venous plasma nicotine levels after overnight abstinence
| Condition | Plasma Nicotine After Smoking Denicotinized Cigarettes (ng/ml) | Plasma Nicotine After Smoking Average Cigarettes (ng/ml) | ||
|---|---|---|---|---|
| Borderline Baseline | After Peak | Before Baseline | After Peak | |
| [11C] Carfentanil | 2.58 ± 0.30 | 3.58 ± 0.41 | 2.52 ± 0.25 | 15.9 ± 1.72 |
| [11C]Raclopride | 2.78 ± 0.34 | 3.57 ± 0.41 | 2.59 ± 0.25 | 15.0 ± 1.63 |
| Combined | 2.69 ± 0.23 | 3.57 ± 0.29 | 2.56 ± 0.18 | 15.5 ± 1.19 |
Note that the baseline levels of venous plasma nicotine are very low, indicating that the subject volunteer smokers were indeed tobacco abstinent overnight. The fact that the plasma nicotine boost after smoking the average nicotine cigarette is > 10 ng/ml is important (see Discussion).
3.2. Plasma Cortisol Levels
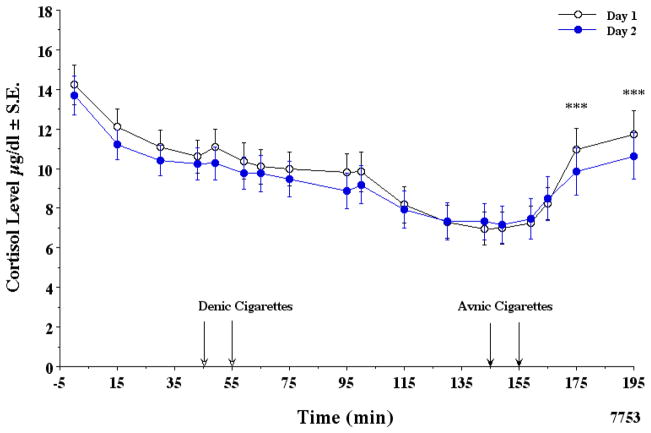
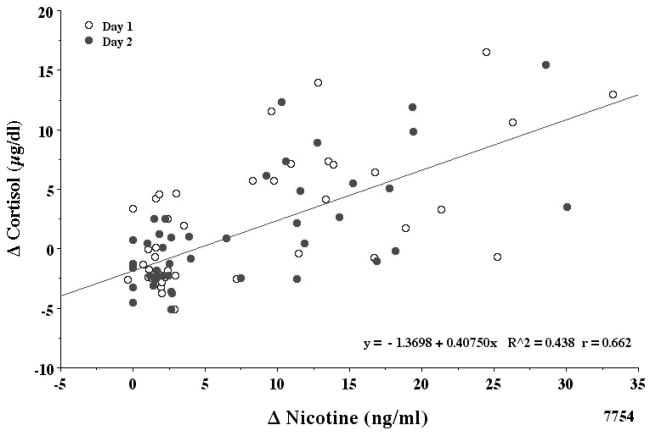
The normal levels of plasma cortisol related to its diurnal decrease in the a.m. were absent in a large number of the subjects, indicating possible PET scanner related stress. However, the mean ± SE of all the data indicated that the early a.m. decrease in plasma cortisol was relatively unaffected by denicotinized cigarette smoking. None of the differences seen in Fig. 1 were statistically significant. In contrast, smoking average nicotine cigarettes clearly increased plasma cortisol levels (Fig. 1). The two way ANOVA with repeated measures with a mixed model analysis indicated that all of the day 1 and day 2 cortisol values of 23 subjects were not different. In order to adjust for all possible time point comparisons, the Sidak adjustment method was used. There was no interaction effect (p = 0.797). The trajectory of cortisol by min was similar between the two days. However, the min effect was highly significant (F1,17= 17.23, p <0.000). Based on this mixed model analysis, there is no interaction effect. The two lines of the two days are statistically similar. Therefore, a reduced mixed model analysis was done without the day * min interaction. Again, the day effect at any min was not significant (F1,1=0.660, p = 0.424). The before/after denicotine smoking data from −5 to 130 min indicated a significant drop in cortisol on both days (p= <0.000). In contrast, a pair wise comparison of before/after average nicotine smoking from 130 to 195 min indicated an increase in cortisol (p < 0.000). The increases were observed at 175 min and 195 min. The increase in cortisol levels was significantly correlated with the increase in plasma nicotine levels (r = 0.66) as shown in Fig. 2 correlation coefficients (day 1 r= 0.64, day 2 r= 0.68). Therefore, the data were combined as illustrated. A 10 ng/ml boost in venous plasma nicotine gave about a 2.5 μg/dl increase in plasma cortisol. The maximal change (mean ± SE) in plasma cortisol for smoking denicotinized and average nicotine cigarettes was −1.18 ± 0.38 and +4.68 ± 0.81 μg/dl, respectively.
Figure 1.
Effects of smoking tobacco cigarettes on plasma cortisol.
In this and subsequent figures, the A.M. data from day 1 (open circles) and day 2 (closed circles) sessions (usually within one week or so) in overnight abstinent tobacco smokers are illustrated from about 8:00 a.m. Time −5 min is when the first venous blood samples were drawn and 195 min when the last samples were drawn. A total of 16–23 subjects’ data were included. Note that day 1 cortisol levels are statistically significant and consistently slightly higher than on day 2 from −5 to 115 min in the PET scanner. In this and in Fig. 3 significant time differences are noted by **p < .01, ***p < .001.
Figure 2.
Correlation of plasma nicotine and cortisol levels.
Note that day 1 and day 2 data are very similar. Plasma nicotine and cortisol levels after denic and avnic cigarette smoking were combined. A total of 16–23 subjects with complete data were studied. The delta increase in nicotine and cortisol levels were both calculated by using the peak value after smoking minus the value just before smoking. The correlation coefficient r = 0.66. Time 0 in this and Fig. 4 is immediately after smoking.
3.3. Plasma Prolactin Levels
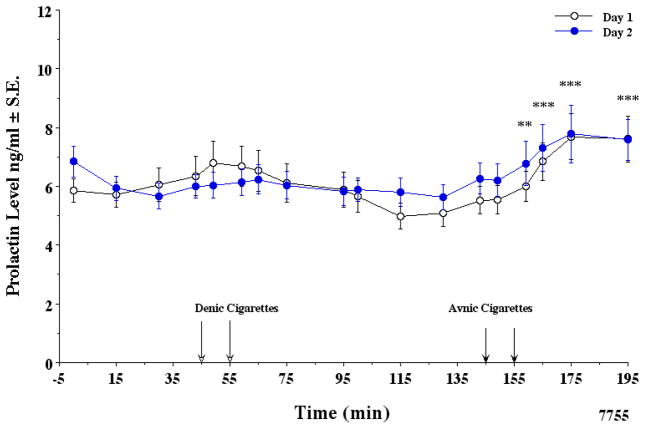
As shown in Fig. 3, smoking average nicotine cigarettes increased prolactin to an average of 7.74 ng/ml within 30 min. Prolactin levels did not increase after smoking denicotinized cigarettes. The two way ANOVA with repeated measures in a mixed procedure indicated that days 1 and 2 prolactin values of 17 subjects were not different. There was no interaction effect (p=0.287). The trajectory of prolactin by min was similar between the two days. Therefore, a reduced mixed model analysis was done. The day effect at any min was not significant (F1,1=0.475, p=0.498). The min effect was significant (F1,17=5.770, p < 0.000). The before/after denicotinized smoking data from −5 to 130 min was not significant. The before/after average nicotine smoking increase in prolactin from 130 to 195 min was highly significant (p < 0.005). The increase in combined prolactin levels correlated with the increase in plasma nicotine levels (r = 0.53) shown in Fig. 4 (correlation coefficients, day 1 r= 0.53, day 2 r= 0.62). A 10 ng/ml boost in venous plasma nicotine gave about a 1 ng/ml increase in plasma prolactin. The maximal change (mean ± SE) for plasma prolactin after smoking denicotinized and average nicotine cigarettes was 0.20 ± 0.14 and 2.20 ± 0.38 ng/ml, respectively.
Figure 3.
Effects of tobacco cigarette smoking on plasma prolactin.
A total of 16 subjects were included with complete data. Note that there is a trend for a slight increase after smoking denic and a greater increase after avnic cigarettes on the two different days.
Figure 4.
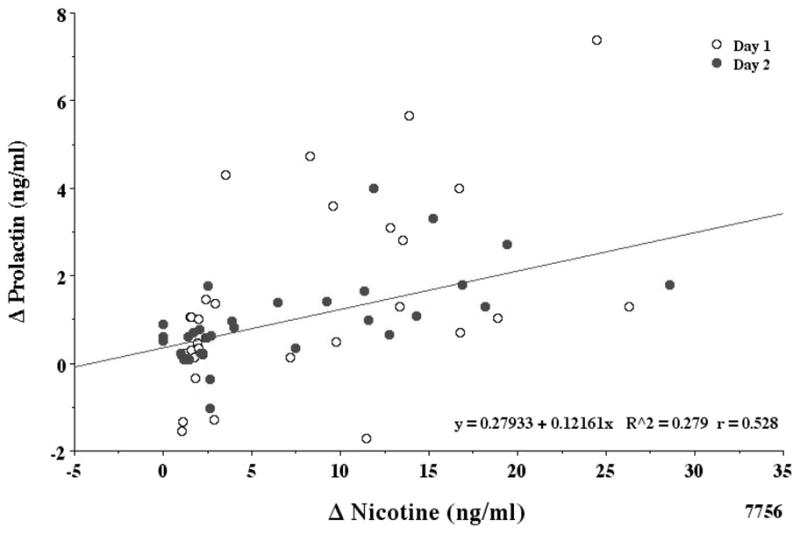
Correlation of plasma nicotine and prolactin levels.
Plasma nicotine and prolactin levels after denic and avnic cigarette smoking are correlated (r = 0.53) but less than with plasma cortisol.
3.4. Plasma β Endorphin
Before smoking average nicotine cigarettes, mean ± SE peak plasma β-endorphin levels were 20.45 ± 1.93, and after smoking 26.97 ± 2.18 pg/ml, which was surprisingly significant (p< 0.05). There was a great deal of individual variability after smoking so the data were not as consistent as with cortisol and prolactin. Only maximal changes are reported. Due to the minor and variable effects, the effects of denicotinized cigarettes were not determined.
4. Discussion
Plasma cortisol levels increased after smoking average nicotine compared to denicotinized cigarettes. These data are consistent with previous reports that cigarette smoking induces an increase in plasma cortisol levels (Cryer et al., 1976; Gossain et al., 1986; Mendelson et al., 2008; Mendelson et al., 2005; Pickworth and Fant, 1998; Seyler et al., 1984; Spohr et al., 1979; Wilkins et al., 1982; Winternitz and Quillen, 1977).
The absolute increase in plasma cortisol on day 1 was 4.74; on day 2 it was 3.31 μg/dl (both days p<.001). These absolute changes are relatively small in comparison to the increases in corticosterone in rats given nicotine. The biological significance of this minimal increase deserves further study. The extent of nicotine dependence, tolerance, diurnal cycle, and behaviorally equivalent doses of nicotine, etc. need to be controlled.
The increase in cortisol levels was less closely correlated with the increase in plasma nicotine levels (r = 0.66) than expected, but clearly significant. Perhaps the additional stress effect of the PET scanner on the first compared to the second day was a factor. A review of the literature on the cortisol response to stressors indicates that most physical and psychological challenges produce increased cortisol concentrations (Kirschbaum and Hellhammer, 1994). Tasks that included social-evaluative threat or uncontrollable conditions consistently elevate cortisol levels (Dickerson and Kemeny, 2004). Furthermore, marked increases in hypothalamic-pituitary activity have been observed in animals and humans exposed to novel environments (Davis et al., 1999; Hennessy et al., 2000). The novel radiologic environment associated with magnetic resonance imaging (MRI) and PET scanners is well known to induce claustrophobia, fear, and anxiety (Melendez and McCrank, 1993; Goyen and Klever, 1997; McIsaac et al., 1998; Westerman et al., 2004; Eshed et al., 2007; Thorpe et al., 2008). Tessner et al. (2006) also demonstrated that the MRI scanning environment can induce cortisol elevations. Individuals undergoing MRI for the first time exhibited increased post-scan salivary cortisol levels compared to subjects familiar with the MRI environment and procedure. Our observation that plasma cortisol release on day 1 was slightly greater than that on day 2 is consistent with their findings. However, it is surprising that the cortisol changes observed are so similar in this small group of male tobacco smokers exposed to the PET facilities on day 1 vs day 2.
Pickworth et al. (1996) reported that during tobacco abstinence smokers with a 0 mg control path had no significant change in ACHT, cortisol, or prolactin. However, Frederick et al. (1998) found that cortisol levels dropped significantly two weeks post-quit and returned to a level below baseline one month later. The initial drop in cortisol was strongly related to post-quit distress. Subsequently, Ussher et al. (2006) and al’Absi and coworkers (2004) showed decreases in cortisol after smoking abstinence. In the latter study, these were associated with a higher rate of relapse during the first week. The importance of cortisol for the reinforcing effects of cocaine has also been demonstrated in rodents (Goeders, 2002).
Most drugs of abuse that release brain dopamine inhibit prolactin release. These include cocaine (Mendelson et al., 2003), amphetamine (Lurie and O’Quinn, 1991; Overtoom et al., 2003) and methylphenidate (Overtoom et al., 2003; Shaywitz et al., 1990; Weizman et al., 1987). In contrast, in our study average nicotine cigarette smoking produced significant and sustained elevations in venous prolactin. The contribution of high prolactin levels to the maintenance of smoking behavior is still debated. Seyler et al. showed that malaise or nausea induced by smoking could increase prolactin release (Seyler et al., 1986). Although it is possible that discomfort associated with high nicotine cigarette smoking may produce increased prolactin levels, the subjects in our study never reported feeling sick or uncomfortable. Furthermore, some animal studies showed that acute administration of nicotine by intravenous, intracerebroventricular, and intraperitoneal routes each consistently resulted in dose-dependent increases in prolactin (Hulihan-Giblin et al., 1990; Sharp and Beyer, 1986). Average nicotine cigarette smoking may stimulate release of prolactin by increasing endogenous opioids (Pomerleau et al., 1983; Seyler et al., 1986), which in turn may inhibit dopamine release (Shah et al., 1988). Chronic use of nicotine may dysregulate the hypothalamic-pituitary-adrenocortical (HPA) axis. This dysregulation may have an important relationship to addiction and relapse (Lovallo, 2006), as well as adverse health consequences (Rohleder and Kirschbaum, 2006). Cortisol levels are generally higher throughout the day in smokers on both working and weekend days, and cortisol responses to waking are also greater in smokers (Steptoe and Ussher, 2006; Badrick et al., 2007). As mentioned above, cortisol levels usually decrease in smokers upon quitting smoking. It is possible that a greater decline in cortisol after quitting may be used to predict relapse (Ussher et al., 2006). Badrick et al. (2007) reported that eventually ex-smokers’ and never-smokers’ cortisol levels do not differ significantly, indicating that nicotine’s effect on the endocrine system is not permanent. Yu et al. (2008) have demonstrated that nicotine self-administration in rats differentially regulates hypothalamic corticotrophin-releasing factor and arginine vasopressin mRNAs. This is a key mechanism for chronic nicotine to cross-sensitize the hypothalamic-pituitary-adrenal (HPA) response to stress. This rat study suggests that tobacco smoking may augment HPA responsiveness to specific human stressors as a longer term consequence to its continued use.
The relationship between endogenous opioids and nicotine has been studied intensively. In mice exposed to nicotine, endogenous opioid levels are increased in the nucleus accumbens and striatum (Davenport et al., 1990; Dhatt et al., 1995; Houdi et al., 1991; Pierzchala et al., 1987; Pomerleau and Pomerleau, 1984). Several clinical studies have demonstrated that levels of β-endorphin were elevated in response to differences in nicotine exposure (Backon, 1989; Gilbert et al., 1992; Meliska and Gilbert, 1991; Seyler et al., 1986). Interestingly, plasma levels of β-endorphin in light but not heavy smokers were reported to be significantly higher than in non-smokers (del Arbol et al., 2000). However, Osaki et al. (2003) showed that acute exposure of rats to cocaine, amphetamine, and alcohol increases β-endorphin in the nucleus accumbens, but acute exposure to nicotine does not. Moreover, in the mediobasohypothalamus of rats, mRNA for pro-opiomelanocortin decreases following long-term nicotine administration, a decrease that persists for 21 days after withdrawal (Rasmussen, 1998). In another study, nicotine exposure caused no change in β-endorphin plasma levels (Wewers et al., 1994). In the present study, after smoking average nicotine cigarettes, plasma β-endorphin changes were highly variable and barely significant. In view of previously published findings cited above, this was an unexpected but not to be ignored lack of dramatic effect. A limitation to the present study is that the volunteers were not interested in quitting smoking so we were not able to examine the effects of smoking on hormone levels.
4.1 Conclusions
This study confirms an increase in plasma cortisol, prolactin, and nicotine following tobacco smoking. A good plasma nicotine-hormone (cortisol, prolactin) correlation was obtained. Contrary to most studies, the effects of smoking average nicotine cigarettes on increased plasma β-endorphin levels were very variable and barely statistically significant. Additional work is needed to further clarify the precise concentration effect relationship between plasma nicotine and β-endorphin release.
Acknowledgments
The authors would like to thank Lingling Zhang, M.A., Statistical Consultant, University of Michigan Center for Statistical Consultation and Research, for her consultation on data analysis. Funding for this study was supplied in part by NIH grant 5RO1 DA-18974-03 and the University of Michigan Education and Research Development Fund 276157.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- al’Absi M, Hatsukami D, Davis GL, Wittmers LE. Prospective examination of effects of smoking abstinence on cortisol and withdrawal symptoms as predictors of early smoking relapse. Drug Alcohol Depen. 2004;73:267–78. doi: 10.1016/j.drugalcdep.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Andersson K. Mecamylamine pretreatment counteracts cigarette smoke induced changes in hypothalamic catecholamine neuron systems and in anterior pituitary function. Acta Physiologica Scandinavica. 1985;125(3):445–52. doi: 10.1111/j.1748-1716.1985.tb07741.x. [DOI] [PubMed] [Google Scholar]
- Backon J. Negative correlation of cigarette smoking and dysmenorrhea: reduced prostaglandin synthesis due to beta-endorphin, nicotine, or acrolein antagonism. Med Hypotheses. 1989;28:213–4. doi: 10.1016/0306-9877(89)90054-6. [DOI] [PubMed] [Google Scholar]
- Badrick E, Kirschbaum C, Kumari M. The relationship between smoking status and cortisol secretion. J Clin Endocrinol Metab. 2007;92(3):819–24. doi: 10.1210/jc.2006-2155. [DOI] [PubMed] [Google Scholar]
- Chen H, Fu Y, Sharp BM. Chronic nicotine self-administration augments hypothalamic-pituitary-adrenal responses to mild acute stress. Neuropschopharmacol. 2008;33:721–730. doi: 10.1038/sj.npp.1301466. [DOI] [PubMed] [Google Scholar]
- Childs E, de Wit H. Hormonal, cardiovascular, and subjective responses to acute stress in smokers. Psychopharmacol. 2009;203:1–12. doi: 10.1007/s00213-008-1359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte-Devolx B, Oliver C, Giraud P, Castanas E, Boudouresque F, Gillioz P, et al. Adrenocorticotropin, and corticosterone secretion in brattleboro rats. Endocrinol. 1982;110(6):2097–2100. doi: 10.1210/endo-110-6-2097. [DOI] [PubMed] [Google Scholar]
- Cryer PE, Haymond MW, Santiago JV, Shah SD. Norepinephrine and epinephrine release and adrenergic mediation of smoking-associated hemodynamic and metabolic events. New Engl J Med. 1976;295:573–7. doi: 10.1056/NEJM197609092951101. [DOI] [PubMed] [Google Scholar]
- Davenport KE, Houdi AA, Van Loon GR. Nicotine protects against mu-opioid receptor antagonism by beta-funaltrexamine: evidence for nicotine-induced release of endogenous opioids in brain. Neurosci Lett. 1990;113:40–6. doi: 10.1016/0304-3940(90)90491-q. [DOI] [PubMed] [Google Scholar]
- Davis EP, Donzella B, Krueger WK, Gunnar MR. The start of a new school year: individual differences in salivary cortisol response in relation to child temperament. Develop Psychobiol. 1999;35:188–96. doi: 10.1002/(sici)1098-2302(199911)35:3<188::aid-dev3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- del Arbol JL, Munoz JR, Ojeda L, Cascales AL, Irles JR, Miranda MT, Ruiz Requena ME, Aguirre JC. Plasma concentrations of beta-endorphin in smokers who consume different numbers of cigarettes per day. Pharmacol Biochem Behav. 2000;67:25–8. doi: 10.1016/s0091-3057(00)00291-4. [DOI] [PubMed] [Google Scholar]
- Dhatt RK, Gudehithlu KP, Wemlinger TA, Tejwani GA, Neff NH, Hadjiconstantinou M. Preproenkephalin mRNA and methionine-enkephalin content are increased in mouse striatum after treatment with nicotine. J Neurochem. 1995;64:1878–83. doi: 10.1046/j.1471-4159.1995.64041878.x. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–91. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Eshed I, Althoff EI, Hamm B, Hermann KG. Claustrophobia and premature termination of magnetic resonance imaging examinations. J Magn Reson Imaging. 2007;26:401–404. doi: 10.1002/jmri.21012. [DOI] [PubMed] [Google Scholar]
- Frederick SL, Reus VI, Ginsberg D, Hall SM, Munoz RF, Ellman G. Cortisol and response to dexamethasone as predictors of withdrawal distress and abstinence success in smokers. Biol Psychiat. 1998;43:525–530. doi: 10.1016/S0006-3223(97)00423-X. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Andersson K, Eneroth P, Harfstrand A, Agnati LF. Neuroendocrine actions of nicotine and of exposure to cigarette smoke: Medical implications. Psychoneuroendocrinol. 1989;14(1–2):19–41. doi: 10.1016/0306-4530(89)90054-1. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, Meliska CJ, Williams CL, Jensen RA. Psychopharmacol. Vol. 106. Berl: 1992. Subjective correlates of cigarette-smoking-induced elevations of peripheral beta-endorphin and cortisol; pp. 275–81. [DOI] [PubMed] [Google Scholar]
- Goeders NE. The HPA axis and cocaine reinforcement. Psychoneuroendocrinol. 2002;27:13–33. doi: 10.1016/s0306-4530(01)00034-8. [DOI] [PubMed] [Google Scholar]
- Gossain VV, Sherma NK, Srivastava L, Michelakis AM, Rovner DR. Hormonal effects of smoking--II: Effects on plasma cortisol, growth hormone, and prolactin. Am J Med Sci. 1986;291:325–7. doi: 10.1097/00000441-198605000-00007. [DOI] [PubMed] [Google Scholar]
- Goyen M, Klewe J. The anxious patient during magnetic resonance tomography (MRI) examination. Health care economic aspects of patient education. Z Arztl Fortbild Qualitatssich. 1997;91(4):319–22. [PubMed] [Google Scholar]
- Hennessy MB, Maken DS, Graves FC. Consequences of the presence of the mother or unfamiliar adult female on cortisol, ACTH, testosterone and behavioral responses of periadolescent guinea pigs during exposure to novelty. Psychoneuroendocrinol. 2000;25:619–32. doi: 10.1016/s0306-4530(00)00014-7. [DOI] [PubMed] [Google Scholar]
- Houdi AA, Pierzchala K, Marson L, Palkovits M, Van Loon GR. Nicotine-induced alteration in Tyr-Gly-Gly and Met-enkephalin in discrete brain nuclei reflects altered enkephalin neuron activity. Peptides. 1991;12:161–66. doi: 10.1016/0196-9781(91)90183-p. [DOI] [PubMed] [Google Scholar]
- Hulihan-Giblin BA, Lumpkin MD, Kellar KJ. Acute effects of nicotine on prolactin release in the rat: agonist and antagonist effects of a single injection of nicotine. J Pharmacol Exper Therap. 1990;252:15–20. [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: recent developments and applications. Psychoneuroendocrinol. 1994;19:313–33. doi: 10.1016/0306-4530(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Scherer G, Strasburger CJ. Pituitary and adrenal hormone responses to pharmacological, physical, and psychological stimulation in habitual smokers and nonsmokers. Clin Invest. 1994;72:804–10. doi: 10.1007/BF00180552. [DOI] [PubMed] [Google Scholar]
- Lovallo WR. Cortisol secretion patterns in addiction and addiction risk. Int J Psychophysiol. 2006;59(3):195–202. doi: 10.1016/j.ijpsycho.2005.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurie S, O’Quinn A. Neuroendocrine responses to methylphenidate and d-amphetamine: applications to attention-deficit disorder. J Neuropsychiat Clin Neurosci. 1991;3:41–50. doi: 10.1176/jnp.3.1.41. [DOI] [PubMed] [Google Scholar]
- McIsaac HK, Thordarson DS, Shafran R, Rachman S, Pool G. Claustrophobia and the magnetic resonance imaging procedure. J Behav Med. 1998;21:255–68. doi: 10.1023/a:1018717016680. [DOI] [PubMed] [Google Scholar]
- Melendez JC, McCrank E. Anxiety-related reactions associated with magnetic resonance imaging examinations. JAMA. 1993;270:745–82. doi: 10.1001/jama.1993.03510060091039. [DOI] [PubMed] [Google Scholar]
- Meliska CJ, Gilbert DG. Hormonal and subjective effects of smoking the first five cigarettes of the day: a comparison in males and females. Pharmacol Biochem Behav. 1991;40:229–35. doi: 10.1016/0091-3057(91)90544-c. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Goletiani N, Sholar MB, Siegel AJ, Mello NK. Effects of smoking successive low- and high-nicotine cigarettes on hypothalamic-pituitary-adrenal axis hormones and mood in men. Neuropsychopharmacol. 2008;33:749–60. doi: 10.1038/sj.npp.1301455. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Sholar MB, Goletiani N, Siegel AJ, Mello NK. Effects of low- and high-nicotine cigarette smoking on mood states and the HPA axis in men. Neuropsychopharmacol. 2005;30:1751–63. doi: 10.1038/sj.npp.1300753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson JH, Sholar MB, Mutschler NH, Jaszyna-Gasior M, Goletiani NV, Siegel AJ, Mello NK. Effects of intravenous cocaine and cigarette smoking on luteinizing hormone, testosterone, and prolactin in men. J Pharmacol Exper Therap. 2003;307:339–48. doi: 10.1124/jpet.103.052928. [DOI] [PubMed] [Google Scholar]
- Muraki T, Tokunaga Y, Nakadate T, Kato R. Inhibition by cholinergic agonists of the prolactin release induced by morphine. Naunyn-Schmiedeberg’s Arch Pharmacol. 1979;308(3):249–54. doi: 10.1007/BF00501389. [DOI] [PubMed] [Google Scholar]
- Osaki MY, Castellan-Baldan L, Calvo F, Carvalho AD, Felippotti TT, de Oliveira R, Ubiali WA. Neuroanatomical and neuropharmacological study of opioid pathways in the mesencephalic tectum: effect of mu(1)- and kappa-opioid receptor blockade on escape behavior induced by electrical stimulation of the inferior colliculus. Brain Res. 2003;992:179–92. doi: 10.1016/j.brainres.2003.08.040. [DOI] [PubMed] [Google Scholar]
- Overtoom CC, Verbaten MN, Kemner C, Kenemans JL, van Engeland H, Buitelaar JK, et al. Effects of methylphenidate, desipramine, and L-dopa on attention and inhibition in children with Attention Deficit Hyperactivity Disorder. Behav Brain Res. 2003;145:7–15. doi: 10.1016/s0166-4328(03)00097-4. [DOI] [PubMed] [Google Scholar]
- Pickworth WB, Baumann MH, Fant RV, Rothman RB, Henningfield JE. Endocrine responses during acute nicotine withdrawal. Pharmacol Biochem Behav. 1996;55:433–437. doi: 10.1016/s0091-3057(96)00114-1. [DOI] [PubMed] [Google Scholar]
- Pickworth WB, Fant RV. Endocrine effects of nicotine administration, tobacco and other drug withdrawal in humans. Psychoneuroendocrinol. 1998;23:131–41. doi: 10.1016/s0306-4530(97)00075-9. [DOI] [PubMed] [Google Scholar]
- Pierzchala K, Houdi AA, Van Loon GR. Nicotine-induced alterations in brain regional concentrations of native and cryptic Met- and Leu-enkephalin. Peptides. 1987;8:1035–43. doi: 10.1016/0196-9781(87)90133-1. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Fertig JB, Seyler LE, Jaffe J. Psychopharmacol. Vol. 81. Berl: 1983. Neuroendocrine reactivity to nicotine in smokers; pp. 61–7. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Pomerleau CS. Neuroregulators and the reinforcement of smoking: towards a biobehavioral explanation. Neurosci Biobehav Rev. 1984;85:3–13. doi: 10.1016/0149-7634(84)90007-1. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Rosecrans J. Neuroregulatory effects of nicotine. Psychoneuroendocrinol. 1989;14:407–23. doi: 10.1016/0306-4530(89)90040-1. [DOI] [PubMed] [Google Scholar]
- Rasmussen DD. Effects of chronic nicotine treatment and withdrawal on hypothalamic proopiomelanocortin gene expression and neuroendocrine regulation. Psychoneuroendocrinol. 1998;23:245–59. doi: 10.1016/s0306-4530(98)00003-1. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Kirschbaum C. The hypothalamic-pituitary-adrenal (HPA) axis in habitual smokers. Int J Psychophysiol. 2006;59(3):236–43. doi: 10.1016/j.ijpsycho.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Seyler LE, Jr, Fertig J, Pomerleau O, Hunt D, Parker K. The effects of smoking on ACTH and cortisol secretion. Life Sci. 1984;34:57–65. doi: 10.1016/0024-3205(84)90330-8. [DOI] [PubMed] [Google Scholar]
- Seyler LE, Jr, Pomerleau OF, Fertig JB, Hunt D, Parker K. Pituitary hormone response to cigarette smoking. Pharmacol Biochem Behav. 1986;24:159–62. doi: 10.1016/0091-3057(86)90062-6. [DOI] [PubMed] [Google Scholar]
- Shah GV, Shyr SW, Grosvenor CE, Crowley WR. Hyperprolactinemia after neonatal prolactin (PRL) deficiency in rats: evidence for altered anterior pituitary regulation of PRL secretion. Endocrinol. 1988;122:1883–89. doi: 10.1210/endo-122-5-1883. [DOI] [PubMed] [Google Scholar]
- Sharp BM, Beyer HS. Rapid desensitization of the acute stimulatory effects of nicotine on rat plasma adrenocorticotropin and prolactin. J Pharmacol Exper Therap. 1986;238:486–91. [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Sebrechts MM, Anderson GM, Cohen DJ, Jatlow P, Young JG. Growth hormone and prolactin response to methylphenidate in children with attention deficit disorder. Life Sci. 1990;46:625–33. doi: 10.1016/0024-3205(90)90131-a. [DOI] [PubMed] [Google Scholar]
- Spohr U, Hofmann K, Steck W, Harenberg J, Walter E, Hengen N, et al. Evaluation of smoking-induced effects on sympathetic, hemodynamic and metabolic variables with respect to plasma nicotine and COHb levels. Atherosclerosis. 1979;33:271–83. doi: 10.1016/0021-9150(79)90179-5. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Ussher M. Smoking, cortisol and nicotine. Int J Psychophysiol. 2006;59(3):228–35. doi: 10.1016/j.ijpsycho.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Tessner KD, Walker EF, Hochman K, Hamann S. Cortisol responses of healthy volunteers undergoing magnetic resonance imaging. Human Brain Map. 2006;27:889–95. doi: 10.1002/hbm.20229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe S, Salkovskis PM, Dittner A. Claustrophobia in MRI: the role of cognitions. Magn Reson Imaging. 2008;26:1081–8. doi: 10.1016/j.mri.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Ussher M, West R, Evan P, Steptoe A, McEwen A, Clow A, et al. Reduction in cortisol after smoking cessation among users of nicotine patches. Psychosomatic Med. 2006;68(2):299–306. doi: 10.1097/01.psy.0000204926.27215.a1. [DOI] [PubMed] [Google Scholar]
- Weizman R, Dick J, Gil-Ad I, Weitz R, Tyano S, Laron Z. Effects of acute and chronic methylphenidate administration on beta-endorphin, growth hormone, prolactin and cortisol in children with attention deficit disorder and hyperactivity. Life Sci. 1987;40:2247–52. doi: 10.1016/0024-3205(87)90060-9. [DOI] [PubMed] [Google Scholar]
- Westerman K, Aubrey B, Gauthier D, Aung M, Beanlands RS, Ruddy TD, Davies RA, De Kemp RA, Soodend K. Positron emission tomography: a study of PET test-related anxiety. Can J Cardiovasc Nurs. 2004;14:42–8. [PubMed] [Google Scholar]
- Wewers ME, Tejwani GA, Anderson J. Psychopharmacol. Vol. 116. Berl: 1994. Plasma nicotine, plasma beta-endorphin and mood states during periods of chronic smoking, abstinence and nicotine replacement; pp. 98–102. [DOI] [PubMed] [Google Scholar]
- Wilkins JN, Carlson HE, Van Vunakis H, Hill MA, Gritz E, Jarvik ME. Psychopharmacol. Vol. 78. Berl: 1982. Nicotine from cigarette smoking increases circulating levels of cortisol, growth hormone, and prolactin in male chronic smokers; pp. 305–8. [DOI] [PubMed] [Google Scholar]
- Winternitz W, Quillen D. Acute hormonal response to cigarette smoking. J Clin Pharmacol. 1977;17:389–97. doi: 10.1002/j.1552-4604.1977.tb04621.x. [DOI] [PubMed] [Google Scholar]
- Yu G, Chen H, Zhao W, Matta SG, Sharp BM. Nicotine self-administration differentially regulates hypothalamic corticotropin-releasing factor and arginine vasopressin mRNAs and facilitates stress-induced neuronal activation. J Neurosci. 2008;28:2773–2778. doi: 10.1523/JNEUROSCI.3837-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]