Abstract
Objective
An intact ability to mount preparatory emotional, cognitive and bodily responses to anticipated environmental change is necessary for adaptive responding. Although abnormal insula activity during aversive anticipation has been observed in Major Depressive Disorder (MDD) individuals, the extent to which shifts in homeostatic state during anticipation affect insular activity in MDD subjects has not been reported. The aim of this study was to use functional Magnetic Resonance Imaging (fMRI) to examine how shifts in homeostatic state affect anticipatory insular activity in MDD.
Methods
Cued hot and warm stimuli were delivered while subjects either passively viewed a fixation cross or performed an attentional task during fMRI. The task was designed so that anticipatory brain activation related to the following three types of shifts could be measured: (1) anticipatory shifts in stimulus intensity, (2) anticipatory shifts in cognitive demand, (3) dual anticipatory shifts (i.e., shifts in both stimulus intensity and cognitive demand). Brain activation related to each of these three contrasts was compared between 15 (12F) unmedicated subjects with current MDD and 17 (10F) age- and education-comparable healthy control (HC) subjects.
Results
MDD versus HC subjects showed lower right anterior insula activity related to anticipatory shifts in stimulus intensity, and altered brain activation during anticipatory shifts in cognitive demand and dual anticipatory shifts.
Conclusions
These results indicate that MDD individuals show altered brain responses to shifts in homeostatic state during anticipation, and may suggest that MDD is associated with an impaired ability to effectively prepare for changes in the environment.
Keywords: Homeostatsis, emotion, interoception, fmri, emotional allodynia, heat, pain
Introduction
Everyday events that we need to prepare for are constantly changing. An intact ability to mount preparatory emotional, cognitive and bodily responses to anticipated environmental change is necessary for adaptive responding. Recent evidence indicates that individuals with Major Depressive Disorder (MDD) demonstrate a reduced range of emotional and physiological responding (1–3), which suggests that this disorder may be associated with an impaired ability to mount preparatory responses to anticipated changes in the environment.
Recent evidence suggests that the right anterior insular region (RAI) plays an important role in emotional anticipation by acting as an integrator of current physiological, cognitive and emotional experiences, and as a neural substrate of subjective emotional awareness (4–6). Although abnormal activation of RAI in MDD is likely the result of a heightened awareness of negative emotional events (7–10), it may also be due to an inability to adjust emotionally and physiologically to changing environmental cues, or to an incorrect interpretation of these cues by attributing a negative emotional valence to neutral and non-threatening conditions (11). Specifically, MDD individuals show decreased activation of this region during errors committed during an inhibition task and during affective set shifting in reversal-learning tasks (12–14), suggesting that negative biasing of neutral and non-threatening conditions creates a restricted physiological response range in MDD. Taken together, this evidence suggests that MDD is associated with altered physiological responding and brain activity within the insula specifically, during anticipatory processing. Unknown however is the extent to which shifts in homeostatic state during anticipation affect insular activity in MDD.
The aim of this study was to build on existing evidence that MDD is associated with altered activity in neural circuitry involved in monitoring and altering the physiological condition of the body (8), by determining the degree to which MDD affects insula activity related to shifts in homeostatic state during anticipation. We hypothesized that MDD relative to healthy control subjects would show altered RAI activity during shifts in homeostatic state during anticipation.
Materials and Methods
Subjects
Fifteen unmedicated (no pharmacological treatments > 30 days) subjects with current MDD (12F, mean age ± SD: 24.5±5.5) were recruited via flyers and internet bulletin boards. Each individual fulfilled diagnostic criteria for current MDD according to a structured clinical interview for DSM IV (SCID-P) (15), which was administered by a board certified psychiatrist (SCM). Participants completed the Beck Depression Inventory (BDI-2) (16) to establish the severity of current depressive symptoms (Table 1). Ten out of fifteen subjects were naïve to psychotropic medication. As well as meeting criteria for current MDD, seven of the MDD individuals also met criteria for lifetime (but not current) comorbid depressive and/or anxiety disorders. Specifically, three MDD subjects (one male) met criteria for past but not current dysthymia, two female MDD subjects met criteria for past but not current PTSD, one female MDD subject met criteria for past but not current generalized anxiety disorder and panic disorder and one female MDD subject met criteria for past but not current dysthymia and panic disorder. Seventeen medically healthy comparison (CON) subjects (10F, mean age 24.3±5.0) with no current or past history of psychiatric disorders according to a structured clinical interview for DSM IV (SCID-P) and no first-degree relatives with current or past psychiatric disorders were matched to the MDD subjects for age (t(30)=0.1, p=0.92) and level of education (t(30)=0.4, p=0.65). The groups did not differ significantly on gender distributions (χ2=1.7, p=0.20) (Table 1). All subjects also completed the Spielberger State Anxiety Inventory (17) in order to assess current anxiety symptoms. Subjects were excluded from both groups if they: 1) met DSM-IV criteria for lifetime alcohol or substance dependence; 2) fulfilled DSM-IV criteria for alcohol or substance abuse within 30 days of study participation; 3) were experiencing suicidal ideation; 4) had a lifetime history of bipolar or psychotic disorder; 5) had clinically significant comorbid medical conditions, such as cardiovascular and/or neurological abnormality; 6) had a history of current or past chronic pain condition. Written informed consent was obtained from each individual following a detailed description of the study, which was approved by the University of California San Diego Institutional Review Board. All procedures complied with national legislation and the Code of Ethical Principles for Medical Research Involving Human Subjects of the World Medical Association (Declaration of Helsinki). These data were collected from January 2005 to September 2006.
Table 1.
Demographics and Psychological Variables
| CON | MDD | Stats | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t/χ2 | p | |
| Demographic Variables | ||||||
| Gender | 10F | 7M | 12F | 3M | 1.7 | 0.20 |
| Age (yrs) | 24.3 | 5.0 | 24.5 | 5.5 | 0.1 | 0.92 |
| Education (yrs) | 16.0 | 1.6 | 16.2 | 1.7 | 0.4 | 0.65 |
| Comorbid Diagnosis (lifetime not current) | ||||||
| Post-Traumatic Stress Disorder | N=2 | |||||
| Panic Disorder | N=2 | |||||
| Generalized Anxiety Disorder | N=1 | |||||
| Disthymic Disorder | N=4 | |||||
| Psychological Variables | ||||||
| Beck Depression Inventory 2 | 1.0 | 1.4 | 27.8 | 7.6 | 14.2 | <0.01 |
| Spielberger’s Trait Anxiety$ | 31.9 | 9.1 | 57.8 | 6.9 | 7.2 | <0.01 |
| Spielberger’s State Anxiety | 28.6 | 6.2 | 47.7 | 9.1 | 7.0 | <0.01 |
CON – healthy controls; MDD – Major Depressive Disorder; $ – available in 10/15 MDD and 10/17 CON participants
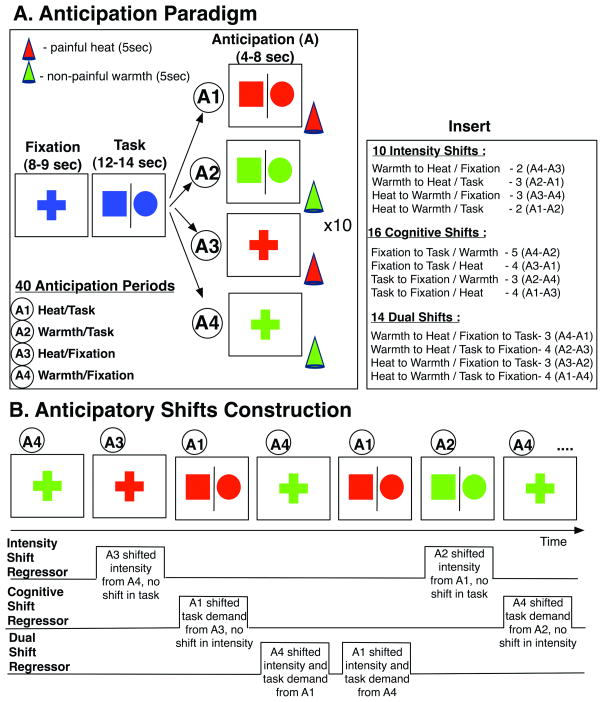
Paradigm Design (see Figure 1): We used two different types of temperature stimuli (i.e., moderately painful heat and non-painful warmth) and two different cognitive contexts (i.e., fixation and continuous performance task, referred to as “task” from now on) and analyzed the response to stimulus anticipation across subjects. This resulted in four different anticipation conditions: 1) painful heat − task; 2) painful heat + task; 3) non-painful warmth − task; and 4) non-painful warmth + task (Figure 1a). Each anticipation condition was presented 10 times, resulting in total of 40 anticipation periods. The analyses of the main effects of stimulus anticipation and administration, i.e., the between-group differences in brain activation during stimulus anticipation and administration with and without task, were recently published (8). The nature of this paradigm, however, allowed us to also examine between-group differences in brain activation during shifts in anticipatory conditions, i.e., when the current anticipatory condition was different in temperature intensity and/or cognitive demand from the prior anticipatory condition (Figure 1b). In order to increase statistical power we collected data in two more healthy male participants and used an approach similar to (18) to analyze this dataset. We tested the hypothesis that MDD is associated with an altered RAI response to shifts in homeostatic state induced by shifts in temperature intensity. To thoroughly test this hypothesis, three different planned contrasts were examined between the groups (Figure 1b): 1) shift in stimulus intensity during anticipation, whereby the conditions differed in stimulus intensity but not in cognitive demand (i.e., a shift from painful heat to non-painful warmth and vice versa, with the task condition not changing; e.g., shifting from painful heat + task to non-painful warmth + task); 2) shift in cognitive demand during anticipation, whereby the conditions differed in cognitive demand but not in stimulus intensity (i.e., a shift from “fixation” to “task” and vice versa, with no shift in stimulus intensity; e.g., shifting from painful heat + task to painful heat − task); and 3) shift in both stimulus intensity and cognitive demand during anticipation, whereby the conditions differed in both stimulus intensity and cognitive demand (i.e., a shift in both the temperature and the task; e.g., shifting from painful heat + task to non-painful warmth − task). All 40 anticipation conditions were categorized into stimulus intensity (n=10), cognitive (n=16), and dual shifts (n=14). To increase statistical power, painful to non-painful and non-painful to painful shifts were combined and we did not examine direction-specific stimulus intensity anticipatory shifts. Further examination showed that the split between different conditions was well-balanced and neither the direction of the shift in intensity (i.e., heat to warmth or warmth to heat) nor the direction of the shift it task (i.e., task to fixation or fixation to task) biased the stimulus intensity, cognitive or dual anticipatory shift conditions (χ2’s<0.4, p’s>0.50) (Figure 1 – insert). Due to a proposed lateralized effect in the right anterior insula (RAI)(19), the direction of the anticipatory shift in stimulus intensity was investigated for this region. The cognitive task required pressing the LEFT button (using index finger on the right hand) when a circle appeared and pressing the RIGHT (using middle finger on the right hand) button when a square appeared on the screen. Percent correct and reaction time data were recorded continuously during scanning sessions and compared between the groups. Due to equipment failure, data from one female MDD and one female CON subjects were not recorded. Performance on this task was not significantly different between the two groups in terms of percent correct (p’s > 0.05, t(28)<2.00) and reaction time (p’s > 0.20, t(28)<1.2). Visual stimuli were presented at a rate of 0.5Hz. The two stimulation intensities (i.e., moderately painful heat and non-painful warmth) were individualized to each participant prior to scanning in order to establish similar perceptual intensities between the groups. The temperature stimuli were administered in a pseudo-random and counterbalanced order using a 9cm2 thermode (Medoc TSA-II, Ramat-Yishai, Israel), which was securely fastened to each subject’s left volar forearm. Each temperature was presented 20 times. The following average temperatures (mean ± SD) that resulted in similar ratings of intensity were used: 1) MDD: painful − 46.4 ± 0.6°C, non-painful − 38.9 ± 0.2°C; 2) CON: painful − 46.9±0.7°C, non-painful − 39.0±0.2°C. Painful temperatures were slightly lower in the MDD compared to the CON group (p=0.03, t(30)=2.2), whereas the non-painful temperatures were not significantly different (p=0.77, t(30)=0.29). Subjects were cued to an upcoming painful stimulus whenever the color of the shape changed to RED, and to an upcoming non-painful warm stimulus whenever the color of the shape changed to GREEN. This design allowed us to contrast between groups brain activation during anticipatory periods in which: 1) the stimulus intensity changed compared to the prior anticipatory period (but the cognitive demand did not), 2) the cognitive demand changed compared to the prior anticipatory period (but the stimulus intensity did not), and 3) both the stimulus intensity and the cognitive demand changed compared to the prior anticipatory period.
Figure 1. Experimental Paradigm.
(A) All subjects completed the anticipation paradigm in the scanner. In order to manipulate cognitive demand, subjects were asked to engage in the continuous performance task (“Task”) [circle – LEFT, square – RIGHT button, 1 trial/2 secs]. The stimuli change color (red – anticipate pain, green –anticipate warmth, 4–8 seconds) for the anticipation condition. Stimulus consisted of a hot-painful or warm-non-painful stimulus for 5 seconds and followed anticipation. Four different anticipatory trial types for a total of 40 anticipatory periods (10 times/trial type) were delivered: A1: painful heat + task, A2: non-painful warmth + task, A3: painful heat + fixation, A4: non-painful warmth + fixation. (B) In order to construct anticipatory shift regressors all 40 original anticipation periods were divided into three different types of anticipatory shifts relative to the preceding anticipatory period: 1) Intensity Shift Anticipation shift in intensity of the stimulus only (from painful heat to non-painful warmth and vice versa) and no shift in cognitive demand (task condition stays the same), 2) Cognitive Shift Anticipation – shift in cognitive demand only (from “fixation” to “task” condition and vice versa) and no shift in stimulus intensity (temperature stays the same), and 3) Dual Shift Anticipation – shift in stimulus intensity and cognitive demand (temperature stimulus and task demand both change). Insert indicates the distribution of shifting trials relative to the original anticipatory trials (see text for details)
fMRI Protocol
Four fMRI runs (for a total of 952 brain volumes) sensitive to blood oxygenation level-dependent (BOLD) contrast (21) were collected for each subject using 3.0 Tesla GE scanner (T2* weighted echo planar imaging, TR=2000 ms, TE=32ms, flip angle= 90, FOV=23cm, 64×64 matrix, 30 2.6-mm 1.4-mm gap axial slices, 238 scans) while they performed the paradigm described above. After each functional run subjects provided an average intensity and unpleasantness rating for warm and hot temperatures (20). FMRI acquisitions were time-locked to the onset of the task. During the same experimental session, a high-resolution T1-weighted image (FSPGR, TR=8ms, TE=3ms, TI=450 ms, flip angle=12, FOV=25cm, 172 sagital slices, 1×0.97×0.97 mm voxels) was obtained for anatomical reference.
Statistical Analysis
All imaging data were analyzed with the Analysis of Functional NeuroImages (AFNI) software package (22). Preprocessed time series data for each individual were analyzed using a multiple regression model. To examine brain areas involved in mounting preparatory responses during anticipatory shifts, we divided all anticipation periods into three separate conditions, represented in the regression model by three different regressors (described above): 1) Stimulus intensity anticipatory shift regressor, 2) Cognitive anticipatory shift regressor, and 3) Dual anticipatory shift regressor (Figure 1). Regressors representing stimulation periods were added to the model as regressors of no interest to reduce the variance in brain activation attributable to the main effect of anticipation. These results are not discussed in the present manuscript as they were examined in detail in a prior publication (8). Eight additional regressors were included in the model as nuisance regressors: two cue regressors (to signal an upcoming temperature stimulus), one outlier regressor to account for physiological and scanner noise, each individual’s white matter regressor to account for activation that is not spatially specific, three movement regressors to account for residual motion (in the roll, pitch, and yaw directions), and regressors for baseline and linear trends to account for signal drifts. A Gaussian filter with a full width-half maximum of 4mm was applied to the voxel-wise percent signal change data to account for individual variation in the anatomical landmarks (8). Data from each subject were normalized to Talairach coordinates (23).
We examined activation differences between the groups using two-sample t-tests for the following three planned contrasts: 1) Stimulus intensity anticipatory shifts, 2) Cognitive anticipatory shifts, and 3) Dual anticipatory shifts (Figure 1). A threshold adjustment method based on Monte-Carlo simulations was used to guard against identifying false positive areas of activation (24). Based on the whole brain analysis, an a priori voxel-wise probability of p< 0.05 in a cluster of 704 μL resulted in an a posteriori cluster-wise probability of p <0.05. The average percent signal in the brain regions that survived this threshold/cluster method was extracted from each individual subject’s data using the group functional mask. All post-hoc statistical analyses were performed with StatistiXL (Nedlands, Australia).
Results
Behavioral Ratings between fMRI runs
All thirty-two subjects rated the average intensity and unpleasantness of the painful and non-painful stimuli following each functional run. On average, all subjects rated high temperatures as painful, and low temperatures as non-painful. The MDD and CON groups did not differ in their intensity ratings of either painful heat or non-painful warmth, and in their unpleasantness ratings of painful heat (p’s > 0.20, t(30) <1.29). MDD relative to CON subjects rated non-painful warm stimuli as slightly more unpleasant, i.e., the average unpleasantness rating for non-painful warm stimuli was significantly higher in the MDD group than in the CON group (p=0.03, t(30)=2.35). As expected, all subjects experienced painful heat as significantly more unpleasant than non-painful warmth (p’s < 0.01). Importantly, when subjective ratings were examined across functional runs, no significant effects of group (F’s < 0.07; p’s > 0.80) or significant group by temperature interactions (F’s <1.5; p’s>0.20) were observed suggesting that groups did not differ in habituation/sensitization rates to applied temperatures, which could have potentially influenced between-group differences in shift-related brain activation.
fMRI Results
Stimulus Intensity Anticipatory Shift
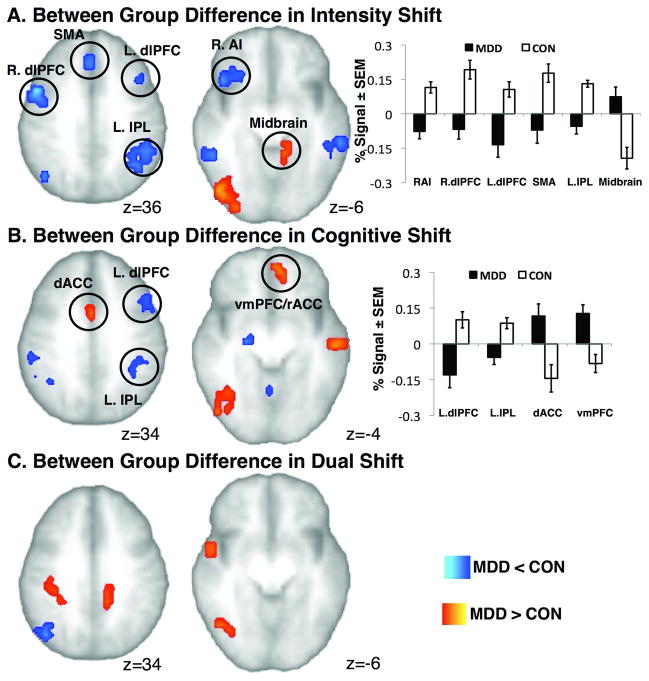
Table 2 and Figure 2a show significant group differences in BOLD signal during stimulus intensity anticipatory shifts. Whole brain analyses revealed that MDD compared to CON subjects showed decreased activation in several brain regions, including clusters within bilateral dorsolateral (dlPFC) and dorsomedial (dmPFC) prefrontal cortices, right anterior insula region (RAI), bilateral inferior parietal lobules (IPL), bilateral mid insula, as well as clusters in supplementary motor area (SMA), bilateral temporal lobes and cerebellar regions. In contrast, MDD compared to CON subjects showed increased activation within temporal, occipital and midbrain areas.
Table 2.
Group Differences in Brain Activation
| Brain Region | Talairach Coordinates | Volume μL | T-val | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Emotional Shift Anticipation | |||||
| CON > MDD | |||||
| R. DLPFC (BA 45/44) | 51 | 11 | 14 | 1152 | 4.5 |
| R. DLPFC (BA 9) | 49 | 4 | 35 | 2176 | 4.1 |
| L. DLPFC (BA9) | −39 | 18 | 31 | 1280 | 4 |
| R. DMPFC (BA 8) | 23 | 20 | 49 | 2176 | 4.8 |
| L. DMPFC (BA 8) | −23 | 9 | 53 | 2816 | 5.9 |
| SMA (BA 6) | 3 | 19 | 50 | 1728 | 4.6 |
| SMA (BA 6) | 4 | 31 | 41 | 1152 | 3.7 |
| R. IPL | 41 | −63 | 31 | 1280 | 4.4 |
| 55 | −21 | 28 | 896 | 4.1 | |
| L. IPL | −43 | −51 | 33 | 6080 | 5.5 |
| R. AI | 37 | 24 | −7 | 1536 | 5 |
| L. Mid Insula | −34 | 3 | 25 | 1024 | 3.7 |
| R. Mid Insula | 37 | 4 | 21 | 960 | 4 |
| R. MTG | 53 | −42 | −7 | 704 | 3.3 |
| L. MTG | −54 | −37 | −4 | 1600 | 4.1 |
| Cerebellum | 12 | −70 | −34 | 3136 | 5 |
| −31 | −62 | −37 | 1728 | 4.7 | |
| −37 | −78 | 20 | 768 | 3.9 | |
| MDD > CON | |||||
| R. MOG | 39 | −75 | −6 | 2240 | 4.3 |
| L. Lingual Gyrus | −3 | −83 | 4 | 1216 | 3.4 |
| L. MTG | −5 | −35 | −56 | 832 | 3.5 |
| Midbrain (PAG) | −12 | −39 | −8 | 704 | 4.3 |
DLPFC – dorsolateral prefrontal; DMPFC – dorsomedial prefrontal; SMA –supplementary motor area; IPL – inferior parietal lobule; RAI – right anterior insula; MTG – medial temporal; MOG – middle occipital; PAG – periaqueductal grey
Figure 2.
Significant Group Differences in BOLD activation during A) Intensity Shift Anticipation; B) Cognitive Shift Anticipation and C) Dual Shift Anticipation. Bar graphs show % BOLD changes for the Intensity and Cognitive Shifts for MDD and CON groups. C.f. Table 1, 2, and 3 for details. Images are shown in neurological orientation.
Cognitive Anticipatory Shift
Table 3 and Figure 2b show significant group differences in BOLD signal during cognitive anticipatory shifts. Visual comparison of the whole brain analyses revealed that fewer brain regions showed differential between-group BOLD activation during cognitive anticipatory shifts than during stimulus intensity anticipatory shifts. MDD compared to CON subjects showed decreased activation within left dlPFC, bilateral IPL, SMA, right mid insula, basal ganglia and cerebellum. Interestingly, during cognitive anticipatory shifts (and not during stimulus intensity anticipatory shifts) MDD compared to CON subjects showed increased BOLD activation within ventromedial prefrontal cortex (vmPFC)/rostral ACC, dorsal anterior (dACC) and posterior cingulate cortex, as well as one cluster in the temporal and occipital lobes.
Table 3.
Group Differences in Brain Activation
| Talairach Coordinates | Volume μL | T-val | |||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Cognitive Shift Anticipation | |||||
| MDD < CON | |||||
| L. DLPFC (BA 9) | −45 | 14 | 31 | 1472 | 3.8 |
| L. DLPFC (BA 46) | −36 | 21 | 12 | 704 | 4.7 |
| SMA | 4 | 11 | 45 | 832 | 3.9 |
| R. IPL | 42 | −51 | 42 | 1728 | 4 |
| L. IPL | −40 | −39 | 35 | 1728 | 4 |
| R. Mid IC | 45 | 5 | 11 | 704 | 4.6 |
| R. Lentiform nucleus | 20 | −12 | −3 | 768 | 5.1 |
| Cerebellum | 0 | −54 | −19 | 4096 | 5.7 |
| −13 | −56 | −36 | 896 | 3 | |
| 28 | −71 | −32 | 832 | 3.2 | |
| MDD > CON | |||||
| MPFC/rACC | −8 | 46 | −7 | 960 | 4.1 |
| dACC | 3 | 6 | 33 | 704 | 3.5 |
| PCC | −5 | −67 | 12 | 896 | 3.4 |
| L. STG | −57 | −13 | −1 | 1216 | 4.6 |
| R. MOG | 41 | −64 | −6 | 1472 | 3.5 |
IPL – inferior parietal lobule,
Dual Anticipatory Shift
Table 4 and Figure 2c show significant group differences in BOLD signal during anticipation of dual anticipatory shifts. Relatively few brain regions showed differential between-group activation during dual anticipatory shifts. MDD compared to CON subjects showed decreased BOLD signal within right mid insula and medial temporal gyrus, and increased BOLD signal within PCC, right IPL, occipital and temporal regions.
Table 4.
Group Differences in Brain Activation (cont’d)
| Brain Region | Talairach Coordinates | VolumeμL | T-val | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Dual Shift Anticipation | |||||
| CON > MDD | |||||
| R. Mid IC | 35 | 2 | 22 | 896 | 4 |
| R. MTG/Angular | 44 | −66 | 29 | 1728 | 3.6 |
| MDD > CON | |||||
| PCC | −11 | −35 | 35 | 1664 | 5.1 |
| R. IPL (BA 40) | 31 | −36 | 40 | 3392 | 4.8 |
| R. STG (BA 22) | 55 | 0 | −1 | 1344 | 4.9 |
| 54 | −21 | 4 | 832 | 3.6 | |
| L. Precuneus | −1 | −54 | 49 | 704 | 3.9 |
| −2 | −71 | 50 | 896 | 3.7 | |
| R. MOG | 42 | −62 | −5 | 1088 | 3.9 |
MTG – medial temporal; STG – superior temporal; MOG – middle occipital
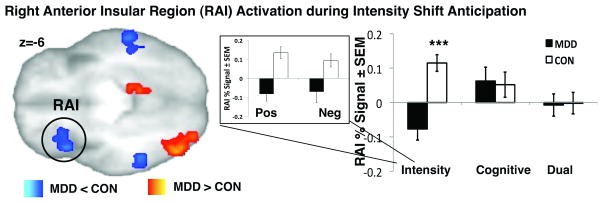
Valence-Dependent Stimulus Intensity Shift
To increase statistical power in the main analysis, which examined anticipation of stimulus intensity anticipatory shifts, trials when the temperature changed from non-painful warmth to painful heat were combined with the trials when the temperature changed from painful heat to non-painful warmth. To further examine how valence affected group differences within RAI activation, we compared percent signal during shifts from painful to non-painful temperatures (or “positive” shifts) to percent signal during shifts from non-painful to painful temperatures (or “negative” shifts), see Figure 3. We found similar relationships within RAI during positive to negative and negative to positive shifts in both groups, i.e., CON subjects showed increased RAI activation, while MDD subjects showed decreased RAI activation during anticipation of both types of temperature change.
Figure 3. Right AI activation during Stimulus Intensity Shift Anticipation.
A) Significant group differences in RAI activation. Bar graphs show % BOLD changes for the Intensity, Cognitive and Dual Shifts for MDD and CON groups. Exploratory examination of RAI activation during positive (i.e., shift from painful heat to non-painfully warm temperature stimulus) and negative (i.e., shift from non-painful warmth to painfully hot temperature stimulus) separately showed no valence-specific activation within this region.
Discussion
This report provides initial evidence of altered brain responses in unmedicated MDD subjects during three specific types of anticipatory shifts. Anticipation-related brain activation to shifts in stimulus intensity, shifts in cognitive demand and shifts in both stimulus intensity and cognitive demand (i.e., dual anticipatory shifts) were compared between unmedicated MDD and healthy subjects. Consistent with our a priori hypothesis, we observed that MDD compared to CON showed: (1) decreased activity in RAI, as well as bilateral dorsomedial prefrontal, dorsolateral prefrontal and parietal cortices and increased activity within midbrain and occipital and temporal regions during anticipatory shifts in stimulus intensity, (2) decreased activation within left dorsolateral prefrontal and bilateral parietal cortices and increased activation within ventromedial prefrontal cortex/rostral cingulate, as well as dorsal and posterior cingulate, occipital and temporal regions during anticipatory shifts in cognitive demand, and (3) decreased activation within right mid insula and medial temporal gyrus, and increased BOLD signal within posterior cingulate, right parietal, and occipital and temporal regions during dual anticipatory shifts. Although each of these three shifts required a unique amount of affective and cognitive processing, all three perturbed homeostasis because each anticipatory condition differed from the prior anticipatory condition, thereby necessitating adaptation to a new state. Therefore, our findings, which are directly in line with two recent fMRI studies in unmedicated MDD subjects who performed affective set shifting tasks (13, 14), may suggest that MDD individuals are unable to effectively prepare for anticipated environmental change.
Temperature is a physiologically relevant and highly affective stimulus (4). Therefore, we infer that the anticipatory shifts in stimulus intensity in our paradigm required emotional, cognitive and motivational adjustments. Consistent with the cognitive model of depression (25), MDD individuals demonstrate a pre-existing negative bias, focus more on negative stimuli (26, 27), and are less easily distracted from negative emotion processing (28–31). Related evidence suggests that emotional and physiological reactivity to external stimuli is blunted in MDD (1–3). Taken together these data suggest that the range of emotional/physiological response may be attenuated in MDD due to pre-existing negative bias (2). Therefore, the altered brain responses that the MDD subjects displayed in the current study during anticipatory shifts in stimulus intensity, which also corresponded to changes in the affective value of the anticipatory stimulus, could have resulted from a limited range of emotional/physiological responding (1). Alternatively, these findings may have resulted from an inability to accurately integrate external cues with signals from the body (i.e., impaired interoception) (5), from decreased motivational drive to incur change (32), from dysfunctional cognitive control (29, 33), and/or from emotional allodynia (8).
During anticipatory shifts in cognitive demand, MDD compared to CON subjects showed decreased activation of dorsolateral prefrontal and parietal regions and increased activation within rostral and dorsal ACC. Unlike anticipatory shifts in stimulus intensity, no differential activation within RAI was observed during anticipatory shifts in cognitive demand. It is well known that emotional experiences, including pain, can be modulated by attentional load (34). However, shifts in attentional load, which occurred in the current paradigm during anticipatory shifts in cognitive demand, may have been perceived as more stressful by MDD individuals, resulting in decreased dlPFC and increased ACC activity in this group (35, 36). The lack of differential RAI activation in the dual anticipatory shifts in our study may further indicate that changes in attentional load can modulate the affective aspects of the aversive anticipatory experience (37).
Several influential models support the notion that emotions arise from interoception of physiological changes in the organism (4, 5, 38–43), and that feelings from the body and emotions interact. Specifically, autonomic responses substantially contribute to awareness (44, 45). Abnormal baseline physiological arousal (1, 2) and decreased heart rate variability (HRV) (46) in MDD may increase focus on internal condition and thus allow for less adaptation during anticipation of environmental change. This abnormal physiological arousal even during safety conditions would prevent MDD subjects from attributing accurate emotional/physiological value to safe or neutral stimuli, thereby creating a negative bias when looking at neutral images (47) or perceiving non-painful warmth (20). Such biasing of the external environment can be a major contributing factor to the emotional context insensitivity model in MDD proposed by Rottenberg et al. (2005) (48) and decreased physiological reactivity (2, 3). Consistent with this interpretation, we propose that a core deficit in MDD may be an impaired ability to recognize and modulate one’s interoceptive state.
We acknowledge that our findings are based on a sample of modest size. Although we observed large statistical differences between the MDD and CON groups, further studies confirming our results are required. Specifically, in the current design we were unable to examine in detail how valence-specific shifting contributes to the observed between-group differences in brain activation and behavior during anticipatory shifts. Although our findings are similar to Simmons et al. (2009) (18) who also combined across valences, this is an important topic for future research. In addition, future studies should examine how different subpopulations of MDD individuals (i.e., older vs. younger age, many vs. few comorbidities and prior episodes, earlier vs. later age of MDD onset) respond to various types of environmental shifts. Finally, thorough physiological monitoring in future studies would increase understanding regarding how decreased physiological and emotional responding in MDD (1) contributes to blunted brain responding to homeostatic shifts in this disorder.
In summary, we found that unmedicated MDD individuals showed decreased right anterior insula activation related to anticipatory shifts in stimulus intensity but not anticipatory shifts in cognitive demand. These findings taken in conjunction with prior literature may suggest that MDD is associated with an impaired ability to adjust homeostatic state and prepare for anticipated environmental change.
Acknowledgments
This study was supported by the National Institute of Mental Health (MH080003; MH077205; IAS), by the National Association for Research in Schizophrenia and Depression (NARSAD) (IAS, ANS and SCM), and by the UCSD Center of Excellence for Stress and Mental Health (ANS);
Abbreviations
- MDD
major depressive disorder
- fMRI
functional magnetic resonance imaging
- RAI
right anterior insula
- ACC
anterior cingulate cortex
- dlPFC
dorsolateral prefrontal cortex
- dmPFC
dorsomedial prefrontal cortex
References
- 1.Bylsma LM, Morris BH, Rottenberg J. A meta-analysis of emotional reactivity in major depressive disorder. Clin Psychol Rev. 2008;28:676–91. doi: 10.1016/j.cpr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Salomon K, Clift A, Karlsdottir M, Rottenberg J. Major depressive disorder is associated with attenuated cardiovascular reactivity and impaired recovery among those free of cardiovascular disease. Health Psychol. 2009;28:157–65. doi: 10.1037/a0013001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carroll D, Phillips AC, Hunt K, Der G. Symptoms of depression and cardiovascular reactions to acute psychological stress: evidence from a population study. Biol Psychol. 2007;75:68–74. doi: 10.1016/j.biopsycho.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. NatRevNeurosci. 2002;3:655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 5.Craig AD. Interoception and emotion: a neuroanatomical perspective. In: Lewis M, Haviland-Jones JM, Barrett LF, editors. Handbook of Emotions. 3. New York: Guilford Publications; 2008. pp. 272–88. [Google Scholar]
- 6.Craig AD. How do you feel [mdash] now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 7.Giesecke T, Gracely RH, Williams DA, Geisser ME, Petzke FW, Clauw DJ. The relationship between depression, clinical pain, and experimental pain in a chronic pain cohort. Arthritis Rheum. 2005;52:1577–84. doi: 10.1002/art.21008. [DOI] [PubMed] [Google Scholar]
- 8.Strigo IA, Simmons AN, Matthews SC, Craig AD, Paulus MP. Association of major depressive disorder with altered functional brain response during anticipation and processing of heat pain. Arch Gen Psychiatry. 2008;65:1275–84. doi: 10.1001/archpsyc.65.11.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herwig U, Bruhl AB, Kaffenberger T, Baumgartner T, Boeker H, Jancke L. Neural correlates of ‘pessimistic’ attitude in depression. Psychol Med. 2009:1–12. doi: 10.1017/S0033291709991073. [DOI] [PubMed] [Google Scholar]
- 10.Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27:8877–84. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strigo IA, Simmons AN, Matthews SC, Craig AD, Paulus MP. Increased affective bias reveald using experimental graded heat stimuli in young depressed adults: evidence of “emotional allodynia”. Psychosom Med. 2008 doi: 10.1097/PSY.0b013e3181656a48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthews S, Simmons A, Strigo I, Gianaros P, Yang T, Paulus M. Inhibition-related activity in subgenual cingulate is associated with symptom severity in major depression. Psychiatry Res. 2009;172:1–6. doi: 10.1016/j.pscychresns.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Remijnse PL, Nielen MM, van Balkom AJ, Hendriks GJ, Hoogendijk WJ, Uylings HB, Veltman DJ. Differential frontal-striatal and paralimbic activity during reversal learning in major depressive disorder and obsessive-compulsive disorder. Psychol Med. 2009:1–16. doi: 10.1017/S0033291708005072. [DOI] [PubMed] [Google Scholar]
- 14.Taylor Tavares JV, Clark L, Furey ML, Williams GB, Sahakian BJ, Drevets WC. Neural basis of abnormal response to negative feedback in unmedicated mood disorders. Neuroimage. 2008;42:1118–26. doi: 10.1016/j.neuroimage.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders - Clinician Version (SCID-1) Washington, D.C: American Psychiatric Press Inc; 1997. [Google Scholar]
- 16.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–97. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 17.Spielberger CD. Manual for the State-Trait Anxiety Inventory (Form Y) 1983. [Google Scholar]
- 18.Simmons A, Strigo IA, Matthews SC, Paulus MP, Stein MB. Initial Evidence of a Failure to Activate Right Anterior Insula During Affective Set Shifting in Posttraumatic Stress Disorder. Psychosom Med. 2009 doi: 10.1097/PSY.0b013e3181a56ed8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Craig AD. Forebrain emotional asymmetry: a neuroanatomical basis? Trends Cogn Sci. 2005;9:566–71. doi: 10.1016/j.tics.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Strigo IA, Simmons AN, Matthews SC, Craig AD, Paulus MP. Increased affective bias revealed using experimental graded heat stimuli in young depressed adults: evidence of “emotional allodynia”. Psychosom Med. 2008;70:338–44. doi: 10.1097/PSY.0b013e3181656a48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA. 1990;87:9868–72. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 23.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- 24.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn ResonMed. 1995;33:636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 25.Beck AT. Depression: clinical, experimental and theorietical aspects. New York: Hoeber Medical Division, Harper & Row; 1967. [Google Scholar]
- 26.Mogg K, Bradley BP, Williams R. Attentional bias in anxiety and depression: the role of awareness. Br J Clin Psychol. 1995;34 ( Pt 1):17–36. doi: 10.1111/j.2044-8260.1995.tb01434.x. [DOI] [PubMed] [Google Scholar]
- 27.Scher CD, Ingram RE, Segal ZV. Cognitive reactivity and vulnerability: empirical evaluation of construct activation and cognitive diatheses in unipolar depression. Clin Psychol Rev. 2005;25:487–510. doi: 10.1016/j.cpr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Ellenbogen MA, Schwartzman AE, Stewart J, Walker CD. Stress and selective attention: the interplay of mood, cortisol levels, and emotional information processing. Psychophysiology. 2002;39:723–32. doi: 10.1111/1469-8986.3960723. [DOI] [PubMed] [Google Scholar]
- 29.Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can’t shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- 30.Lyubomirsky S, Caldwell ND, Nolen-Hoeksema S. Effects of ruminative and distracting responses to depressed mood on retrieval of autobiographical memories. J Pers Soc Psychol. 1998;75:166–77. doi: 10.1037//0022-3514.75.1.166. [DOI] [PubMed] [Google Scholar]
- 31.Wenzlaff RM, Bates DE. Unmasking a cognitive vulnerability to depression: how lapses in mental control reveal depressive thinking. J Pers Soc Psychol. 1998;75:1559–71. doi: 10.1037//0022-3514.75.6.1559. [DOI] [PubMed] [Google Scholar]
- 32.Kumar P, Waiter G, Ahearn T, Milders M, Reid I, Steele JD. Abnormal temporal difference reward-learning signals in major depression. Brain. 2008;131:2084–93. doi: 10.1093/brain/awn136. [DOI] [PubMed] [Google Scholar]
- 33.Whitmer AJ, Banich MT. Inhibition versus switching deficits in different forms of rumination. Psychol Sci. 2007;18:546–53. doi: 10.1111/j.1467-9280.2007.01936.x. [DOI] [PubMed] [Google Scholar]
- 34.Valet M, Sprenger T, Boecker H, Willoch F, Rummeny E, Conrad B, Erhard P, Tolle TR. Distraction modulates connectivity of the cingulo-frontal cortex and the midbrain during pain--an fMRI analysis. Pain. 2004;109:399–408. doi: 10.1016/j.pain.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 35.Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003;126:1079–91. doi: 10.1093/brain/awg102. [DOI] [PubMed] [Google Scholar]
- 36.Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–71. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- 37.Erk S, Abler B, Walter H. Cognitive modulation of emotion anticipation. Eur J Neurosci. 2006;24:1227–36. doi: 10.1111/j.1460-9568.2006.04976.x. [DOI] [PubMed] [Google Scholar]
- 38.James W. The principles of psychology. 2007. p. 1890. [Google Scholar]
- 39.Wiens S. Interoception in emotional experience. Curr Opin Neurol. 2005;18:442–7. doi: 10.1097/01.wco.0000168079.92106.99. [DOI] [PubMed] [Google Scholar]
- 40.Philippot P, Chapelle G, Blairy S. Respiratory feedback in the generation of emotion. Cognition & Emotion. 2002;16:605–27. [Google Scholar]
- 41.Barrett LF, Quigley KS, Bliss-Moreau E, Aronson KR. Interoceptive sensitivity and self-reports of emotional experience. J Pers Soc Psychol. 2004;87:684–97. doi: 10.1037/0022-3514.87.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Damasio AR. Descarte’s Error. New York, NY: Avon Books, Inc; 1994. [Google Scholar]
- 43.Thayer JF, Lane RD. Claude Bernard and the heart-brain connection: Further elaboration of a model of neurovisceral integration. Neuroscience & Biobehavioral Reviews. 2009;33:81–8. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 44.Klein TA, Endrass T, Kathmann N, Neumann J, von Cramon DY, Ullsperger M. Neural correlates of error awareness. Neuroimage. 2007;34:1774–81. doi: 10.1016/j.neuroimage.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 45.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. NatNeurosci. 2004;7:189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 46.Thayer JF, Brosschot JF. Psychosomatics and psychopathology: looking up and down from the brain. Psychoneuroendocrinology. 2005;30:1050–8. doi: 10.1016/j.psyneuen.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 47.Leppanen JM, Milders M, Bell JS, Terriere E, Hietanen JK. Depression biases the recognition of emotionally neutral faces. Psychiatry Res. 2004;128:123–33. doi: 10.1016/j.psychres.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 48.Rottenberg J, Gross JJ, Gotlib IH. Emotion context insensitivity in major depressive disorder. J Abnorm Psychol. 2005;114:627–39. doi: 10.1037/0021-843X.114.4.627. [DOI] [PubMed] [Google Scholar]