Summary
Dendritic cell (DC) apoptosis has been observed in patients with cancer and sepsis, and defects in DC apoptosis have been implicated in development of autoimmune diseases. The mechanisms of how DC apoptosis affects immune responses however, are unclear. In this study we showed that immature viable DC have the ability to uptake apoptotic DC as well as necrotic DC without it being recognized as an inflammatory event by immature viable DC. However, the specific uptake of apoptotic DC converted immature viable DC into tolerogenic DC, which were resistant to LPS induced maturation. In contrast, these tolerogenic DC secreted increased levels of TGF-β1, which induced differentiation of naïve T cells into Foxp3+ regulatory T cells (Treg). Furthermore, induction of Treg differentiation only occurred upon uptake of apoptotic DC and not upon uptake of apoptotic splenocytes by viable DC, indicating that it is specifically the uptake of apoptotic DC that gives viable immature DC the potential to induce Foxp3+ Treg. Taken together, these findings identify uptake of apoptotic DC by viable immature DC as an immunologically tolerogenic event.
Keywords: Dendritic cells, Autoimmunity, Tolerance, Apoptosis
Introduction
Dendritic cells (DC) are professional antigen-presenting cells, which are well positioned in peripheral tissues to capture foreign antigens. DC are phagocytic and can ingest apoptotic cells, and hence are affected by the death of other cells in close proximity [1–3]. Clearance of apoptotic cells results in their removal from tissues, and provides protection from release of pro-inflammatory contents. Necrotic cells impact the immune response by acting as “danger signals” whereas apoptotic cells are cleared without an immunological response [3, 4]. Studies have identified necrotic cells acting as adjuvants whereas apoptotic cells have been reported as immunogenic [5–7] or immunosuppressive [8, 9].
DC apoptosis in itself is an important event for maintenance of tolerance. Defects in DC apoptosis have been linked to development of autoimmunity with systemic autoimmune diseases modeled in transgenic mice harboring defects in DC apoptosis [10] but not in mice with apoptosis defects in T and B cells [11–13]. However, it is unclear how defects in DC apoptosis can trigger autoimmune responses. Furthermore, spontaneous DC apoptosis has been reported in sepsis as well as breast cancer patients with its significance being unclear [14–16]. Most patient deaths associated with sepsis occur at later time points and are associated with prolonged immunosuppression [17]. In this later stage there is marked apoptosis of DC, with no effects on macrophage and neutrophil apoptosis. In addition, immunostimulants such as CpG DNA inhibit DC apoptosis [18], while the deficiency of pro-apoptotic Bim protein in DC results in autoimmunity [19].
Immature DC have the ability to acquire protein complexes or soluble antigen using many different pathways such as macropinocytosis, endocytosis and even through ingestion of entire cells. Despite the importance of DC apoptosis in the immune response, studies have not investigated the effects of DC death on viable DC.
In this study we show that viable immature DC have the ability to uptake apoptotic DC. The uptake of apoptotic DC or necrotic DC is recognized as an immunologically null event. However, it is the uptake of apoptotic DC that suppresses subsequent maturation of viable DC in response to LPS and results in upregulation of TGF-β2 and preferential secretion of TGF-β1, which mediates induction of naïve T cells into Foxp3+ Treg. In contrast, the uptake of apoptotic splenocytes by viable immature DC does not result in TGF-β1 secretion, nor does it result in induction of Foxp3+ Treg. Therefore, it is likely the uptake of apoptotic DC by viable DC that provides a potential to induce Foxp3+ Treg.
Results
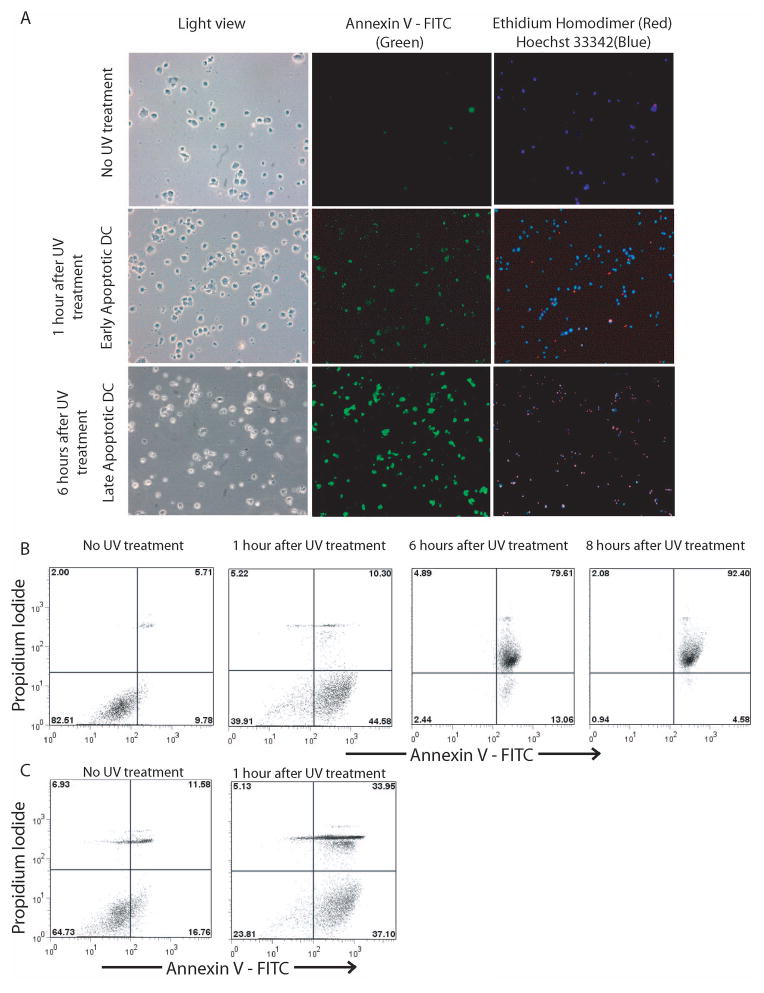
Induction of DC apoptosis by UV radiation
Bone-marrow-derived DC were treated with UV light and apoptosis induction was assessed at 1 hour and 6 hours after UV treatment. Prior to UV treatment, cells were mostly positive for Hoechst 3342 (a cell permeant DNA binding stain, blue) with very few cells being positive for annexin V (a phosphatidylserine binding protein, green), indicative of live DC. One hour after UV treatment, majority of cells were positive for both annexin V as well as Hoechst 3342, with very few cells positive for Ethidium homodimer (EH) (a nuclei probe, impermeant to live or apoptotic cells, red) (Fig. 1A). In these cells, there was translocation of phosphatidylserine on the membrane as indicated by positive annexin V staining, but the membrane integrity was still maintained, since they were mostly negative for EH stain; hence they can be classified as apoptotic cells. In contrast, 6 hours after UV treatment, there was a pronounced increase in EH positive cells, indicating that the membrane integrity was compromised. However, these cells were also positive for annexin V (Fig. 1A). Therefore, these cells can be classified as late apoptotic cells. In order to further confirm apoptosis in a quantitative manner, 1 or 6 hours after UV treatment, DC were stained with annexin V and propidium iodide (PI), and apoptosis was assessed via FACS analysis. Prior to UV treatment, approximately 10% of DC were annexin V+PI−, whereas, 1 hour after UV treatment approximately 45% of DC were annexin V+PI−, indicative of apoptotic cells and confirming our above findings (Fig. 1B). At 6 hours post UV treatment, approximately 80% of cells were annexin V+PI+, indicating that these cells were in late apoptosis (Fig. 1B). This proportion of annexin V+PI+ cells further increased to close to 92.4%, 8 hours after UV treatment (Fig. 1B). Therefore, we chose to use cells immediately after UV treatment as apoptotic DC for further experiments. Similarly, apoptosis was induced in splenocytes via UV radiation and 1 hour after UV treatment, approximately 40% of splenocytes were annexin V+PI−, indicative of apoptotic splenocytes (Fig. 1C).
Figure 1.
UV radiation induces apoptosis in DC and splenocytes. (A) Detection of apoptotic DC, 1 hr, and 6 hr after UV exposure, by staining with annexin V-FITC, EH and Hoechst 33342. (B) FACS analysis for assessment of late and apoptotic DC by staining with annexin V-FITC and PI. (C) Splenocytes were UV-irradiated and FACS analysis was conducted for assessment of apoptotic splenocytes by staining with annexin V-FITC and PI. Data are representative of 4–5 independent experiments.
Uptake of apoptotic DC by live DC
In order to assess uptake of apoptotic DC by viable DC, apoptotic DC were labelled with CFSE and incubated with immature viable DC. Eight hours later, FACS analysis was performed to assess uptake of CFSE-labelled apoptotic DC by live DC (PI−CD11c+) (Fig. 2A). Results indicate that approximately 50% of viable DC had taken up apoptotic DC (Fig. 2). In order to confirm that there were no contaminating CFSE+ PI− apoptotic DC, a parallel experiment was performed where apoptotic DC were labelled with CFSE, cultured for 8 hours, and subsequently stained with PI; approximately 98% of the DC were PI+ (data not shown), indicating that gating for PI− cells, would gate out any CFSE+ apoptotic DC. Furthermore, in order to distinguish binding of apoptotic DC to live DC from uptake of apoptotic DC by live DC, the co-culture experiments were carried out in the presence of cytochalasin D, a known inhibitor of phagocytosis (Fig. 2). In the presence of cytochalasin D, only 12% of the cells were CFSE+, which is probably indicative of apoptotic DC that bound to live DC. Collectively, the results indicate that immature viable DC have the ability to phagocytose apoptotic DC.
Figure 2.
Viable DC uptake apoptotic DC in vitro and this uptake is inhibited by cytochalasin D. CFSE-labelled apoptotic DC were incubated with viable immature DC with or without cytochalasin D at a ratio of 10:1 and 8 hours later, FACS analyses were conducted to assess uptake of CFSE+ apoptotic DC by viable DC. Viable DC were gated based on PI-exclusion (see gate in top panel) and the proportion of CFSE+ cells was assessed among PI− viable DC (middle panel). DC phenotype is confirmed by staining with CD11c and MHC class II (bottom panel). Data are representative of 3 independent experiments.
Viable DC do not undergo maturation upon uptake of apoptotic or necrotic DC
In order to assess the effects of apoptotic or necrotic DC on viable DC, viable immature DC were incubated with mature apoptotic, immature apoptotic and necrotic DC respectively. In order to generate mature apoptotic DC, bone-marrow-derived DC were treated with LPS for 24 hours to induce maturation followed by exposure to UV radiation.
Viable immature DC were characterized as CD11c+ DC with low levels of CD86, CD80 and MHC II expression. LPS treatment of viable immature DC resulted in upregulation of CD86, CD80 and MHC II (Fig. 3A). Furthermore viable immature DC do not produce any IL-12; however, in response to LPS, approximately 30% of DC were IL-12+, as expected (Fig. 3B). However, treatment with immature or mature apoptotic DC did not result in upregulation of CD86, CD80 or MHC II; nor was there any induction of IL-12 production. Similar results were also observed upon treatment of immature viable DC with necrotic DC. Taken together, these findings indicate that immature/mature apoptotic or necrotic DC do not induce maturation of viable immature DC.
Figure 3.
Immature/mature apoptotic DC or necrotic DC do not induce maturation of viable DC. Viable immature DC were incubated with immature apoptotic DC, mature apoptotic DC, LPS or necrotic DC and 24 hours later FACS analysis was performed to assess expression of CD86, CD80, MHC II on PI− CD11c+ viable DC (A) along with the proportion of IL-12+ cells among CD11c+ DC (B). Data are representative of 3 independent experiments, with n=3–4 for every experiment.
Viable DC upon uptake of apoptotic DC but not necrotic DC are resistant to LPS induced maturation
We next assessed the effects of uptake of necrotic/apoptotic DC by viable immature DC on subsequent treatment with LPS (Fig. 4A). In absence of inflammatory stimuli, viable immature DC express very low levels of CD86, with approximately only 20% cells being CD86+. This proportion increases to 50–60% upon treatment with LPS with a concomitant increase in the intensity of CD86 expression (Fig. 4B). Similar results are also observed upon incubation of viable immature DC with necrotic DC followed by treatment with LPS. However, upon incubation of viable immature DC with apoptotic DC followed by LPS treatment, only 20–25% of viable immature DC become CD86+, which is in fact similar to the levels seen in viable immature DC without any LPS treatment (Fig. 4B, C). Furthermore, incubation of viable immature DC with apoptotic splenocytes also resulted in suppression of LPS induced subsequent DC maturation. However, the extent of immunosuppression induced by apoptotic splenocytes was not as potent as apoptotic DC (Fig. 4B, C). These results indicate that uptake of apoptotic DC by viable immature DC prevents subsequent upregulation of CD86 in response to LPS.
Figure 4.
Viable DC fail to upregulate CD86 expression and IL-12 production in response to LPS upon uptake of apoptotic DC. Viable immature DC were cultured as follows: without LPS (DC only), with LPS (DC+LPS), incubated with apoptotic DC and then subsequently cultured with LPS (DC+ApoDC+LPS), incubated with necrotic DC and subsequently cultured with LPS (DC+NecDC+LPS), incubated with apoptotic splenocytes and subsequently cultured with LPS (DC+ApoSplen+LPS). (A) Representative dot plots depicting gating strategy for viable CD11c+ DC and exclude apoptotic/necrotic DC. (B) Comparison of proportion of CD86+CD11c+ DC 24 hours after culture. (C) Representative histograms of CD86 expression on viable CD11c+ DC in response to indicated treatments. (D) Representative histograms of IL-12 production by CD11c+ DC in response to indicated treatments. (E) Comparison of proportion of IL-12+ DC 24 hours after culture. Data show mean ± SD, and are representative of 4–5 independent experiments, with n=3–4 in every experiment.*p<0.05, DC+ApoDC+LPS vs. all other groups except DC only. #p<0.05, DC+ApoSplen+LPS vs. all other groups.
In absence of inflammatory stimuli, viable immature DC do not produce any IL-12. However, in response to LPS, approximately 22% of cells become IL-12+ (Fig. 4D and E). Similarly, viable immature DC incubated with necrotic DC followed by treatment with LPS show similar proportion of IL-12+ DC. In contrast, viable DC incubated with apoptotic splenocytes followed by LPS treatment, showed a slight reduction in IL-12 production, as only 8–11% of the cells became IL-12+. However, viable immature DC incubated with apoptotic DC followed by treatment with LPS failed to induce IL-12, as only 1–2% of DC become IL-12+ (Fig. 4D and E). The uptake of apoptotic DC by viable immature DC is critically important for suppression of CD86 upregulation and IL-12 induction in response to LPS for no suppression is observed in response to LPS if apoptotic DC and viable DC are separated in culture via transwell (data not shown).
In addition to IL-12, DC maturation is also characterized by upregulation of other inflammatory cytokines. In order to assess the effects of apoptotic or necrotic DC uptake by viable immature DC on induction of inflammatory cytokines in response to LPS, we looked at the mRNA expression levels of inflammatory cytokines, including IL-1β (Fig. 5A), IL-6 (Fig. 5B), TNF-α (Fig. 5C), IL-12p35 (Fig. 5D) and IL-12p40 (Fig. 5E). These inflammatory cytokines are expressed at very low levels in viable immature DC at basal levels. However, in response to LPS, there is massive and rapid induction of these cytokines at mRNA levels (Fig. 5A–E). However, incubation of viable immature DC with apoptotic DC but not necrotic DC suppressed induction of the aforementioned inflammatory cytokines in response to LPS. These findings collectively indicate that the specific uptake of apoptotic DC converts viable immature DC into tolerogenic DC.
Figure 5.
Viable DC become tolerogenic DC upon uptake of apoptotic DC. Viable DC were cultured as follows: without LPS (DC only), with LPS (DC+LPS), incubated with apoptotic DC and then subsequently with LPS (DC+ApoDC+LPS), or incubated with necrotic DC and subsequently cultured with LPS (DC+NecDC+LPS). Real-time RT-PCR analysis to look at expression levels of IL-1β (A), IL-6 (B), TNF-α (C), IL-12p35 (D), and IL-12p40 (E). Results show relative expression of different cytokines normalized to expression levels in viable immature DC without any treatment (DC only). (F) Naïve CD4+CD25− T cells isolated from OT-II mice were cultured with viable DC (DC- No OVA), viable DC pulsed with OVA (DC - OVA), viable DC incubated with apoptotic DC and then pulsed with OVA (DC+ApoDC – OVA) or viable DC incubated with necrotic DC and then pulsed with OVA (DC + NecDC –OVA). 3 days later, proliferation was assessed via BrdU incorporation assay. Data show mean ± SD, obtained from 4–5 independent experiments, with n=2–3 in every experiment.*p<0.05, DC+ApoDC+LPS vs all other groups except DC only for D–E, DC+Apo-OVA vs. all other groups except DC-No OVA for F. #p<0.05, DC+ApoDC+LPS vs. all other groups for A–C.
Next, we looked at the ability of viable DC to prime ovalbumin (OVA)-specific T cell proliferation upon apoptotic DC uptake (Fig. 5F). Viable immature DC were incubated with apoptotic or necrotic DC and then pulsed with OVA in the presence of LPS. Next, these were cultured with naïve T cells to assess their ability to induce OVA-specific T cell proliferation. Extensive T cell proliferation was observed upon culture of naïve T cells with viable immature DC that were pulsed with OVA in the presence of LPS or with viable immature DC that were first incubated with necrotic DC and then pulsed with OVA. However, OVA-pulsed viable DC that had taken up apopotic DC failed to induce OVA-specific T cell proliferation (Fig. 5F). These results indicate that upon uptake of apoptotic DC but not necrotic DC, viable DC are refractory to LPS-induced maturation.
Viable DC upon uptake of apoptotic DC induce differentiation of naïve T cells into Treg
Since, viable DC acquired a tolerogenic phenotype upon apoptotic DC uptake; we next went on to assess the ability of viable DC to induce Treg differentiation upon apoptotic DC uptake. Culture of naïve CD4+CD25− OT-II T cells with OVA-pulsed viable DC resulted in approximately 4–5% of naïve T cells differentiating into Foxp3+ Treg, which increased to approximately 23–24% upon culture with OVA-pulsed viable DC that had taken up apoptotic DC. In contrast, culture of naïve CD4+CD25− T cells with OVA-pulsed viable DC that had taken up necrotic DC only resulted in approximately 5–6% Foxp3+ Treg (Fig. 6A and B). The increase in the proportion of Foxp3+ Treg was not paralleled by an increase in the absolute T cell count, indicating that it was likely the induced expression of Foxp3 and not expansion, which mediated the observed increase in the proportion of Foxp3+ Treg among T cells cultured with OVA-pulsed viable DC that had taken up apoptotic DC (data not shown). In order to test, if the induction of Foxp3+ Treg was induced specifically upon uptake of apoptotic DC by viable immature DC and not by uptake of other types of apoptotic cells, we looked at the effects of apoptotic splenocyte uptake on the ability of viable DC to induce Foxp3+ Treg. Results indicate that the uptake of apoptotic splenocytes did not enhance the ability of viable DC to induce Treg, as only 7–8% of naïve T cells differentiated into Foxp3+ Treg, which was similar to the control group. Furthermore, we also assessed the ability of in vitro generated Foxp3+ Treg to suppress T cell proliferation. Our findings identify that the CD4+CD25+ T cell subset only from the co-culture of naïve T cells and OVA-pulsed viable DC that had taken up apoptotic DC, was in fact enriched for suppressor T cells, since they were able to inhibit T cell proliferation in a dose-dependent manner (Fig. 6C). Overall, these results indicate that it was specifically the uptake of apoptotic DC which was primarily responsible for induction of Foxp3+ Treg by viable DC.
Figure 6.
Viable DC take up apoptotic DC and induce differentiation of naïve T cells into Foxp3+ Treg in vitro. Naïve OT-II CD4+CD25− T cells were cultured with the following: live DC pulsed with OVA (Live DC), necrotic DC (NecDC), live DC incubated with necrotic DC and then pulsed with OVA (Live + NecDC), apoptotic splenocytes (ApoSplen), live DC incubated with apoptotic splenocytes and then pulsed with OVA (Live + ApoSplen), apoptotic DC (ApoDC), or live DC incubated with apoptotic DC and then pulsed with OVA (Live + Apo DC). Five days later, T cells were analyzed for Foxp3 expression. (A) Representative dot plots of CD4+Foxp3+ Treg in OT-II T cells cultured with DC under various conditions. (B) Histogram comparing percentages of Treg. Percentages are normalized to total CD4+ T cells in the culture. (C) 5 days after culture, CD4+CD25hi T cells were isolated from the co-culture and were added to a co-culture of naïve OT-II CD4+ T cells and OVA-pulsed DC at different ratios. 4 days later, cell proliferation was assessed by BrdU incorporation assay and data are presented as % suppression of T cell proliferation compared to that of OT-II CD4+ T cells cultured in the presence of OVA-pulsed DC without addition of any CD4+CD25hi T cells. (D–E) Naïve wild-type CD4+CD25− T cells were cultured for 5 days with plate-bound anti-CD3 Ab and soluble anti-CD28 Ab, under a transwell containing treatments as described above without pulsing live DC with OVA. 5 days later, FACS analysis was performed to assess percentages of CD4+ Foxp3+ Treg. (D) Representative histogram of Foxp3 on CD4+ T cells cultured under a transwell containing Live DC or Live+ApoDC. (E) Comparison of % Treg induced as a proportion of CD4+ T cells. (F) Naïve CD4+CD25− T cells were cultured under Th17 inducing conditions in presence of indicated treatments. Representative histogram of the proportion of IL-17+ cells after 4 days of culture. Data show mean ± SD and are representative of 4 independent experiments, with n=3 for each experiment.*p<0.05 for Live+ApoDC vs. all the other groups.
Next, we wanted to assess whether the ability to induce Foxp3+ Treg by viable DC upon apoptotic DC uptake, dependent on interaction with naïve T cells or soluble factors. This was tested by separating T cells from DC using a transwell plate followed by an assessment of Foxp3+ Treg induction. Naïve CD4+CD25− T cells from wild-type mice were placed in the lower chamber, stimulated with plate-bound anti-CD3 as well as soluble CD28 antibody, and with viable DC and apoptotic DC in the upper chamber, Foxp3 induction was observed in approximately 20% of T cells, indicating that soluble factors secreted by viable DC that have taken up apoptotic DC may be involved in Foxp3 induction (Fig. 6D and E). In contrast, addition of only viable DC, necrotic DC, viable DC and necrotic DC, or apoptotic DC alone or viable DC and apoptotic splenocytes, even with a very high ratio of apoptotic splenocytes to the upper well of the transwell only resulted in approximately 5–6% of naïve CD4+CD25− T cells differentiating into Foxp3+ Treg. Overall, these findings indicate that only upon uptake of apoptotic DC, viable DC acquire the ability to induce Foxp3+ Treg, which is mediated by soluble factors released by viable DC upon apoptotic DC uptake.
Additionally, since tolerance is a balance between effector and suppressor T cells, we looked at the effect of apoptotic/necrotic DC on the ability of viable DC to induce Th17. Our findings demonstrated that it is only upon apoptotic DC uptake, that viable DC had a diminished ability to induce Th17 (Fig. 6F).
Viable DC secrete TGF-β1 upon apoptotic DC uptake
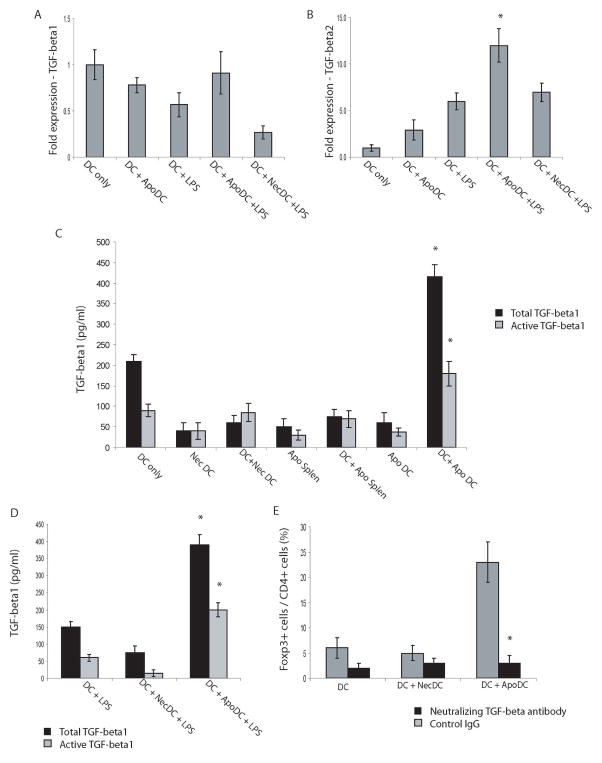
Since TGF-β is a known inducer of Foxp3, we looked at the induction of TGF-β1 and TGF-β2 at the mRNA level in viable DC that had taken up apoptotic DC in the presence/absence of LPS. Our findings indicate that at basal levels without any stimulation there is some expression of TGF-β1 in DC which is suppressed in response to LPS. This suppression is also observed in viable DC incubated with necrotic DC followed by LPS exposure (Fig. 7A). However, no suppression of TGF-β1 expression is observed in viable DC incubated with apoptotic DC prior to LPS exposure. At the same time, no induction of TGF-β1 is observed in this group. In contrast to TGF-β1, TGF-β2 levels were upregulated approximately 12–13 fold in viable DC incubated with apoptotic DC followed by LPS exposure compared to viable immature DC without any treatment (Fig. 7B).
Figure 7.
In response to LPS, viable DC that have taken up apoptotic DC, induce secretion of TGF-β1 and upregulate TGF-β2 gene expression, which mediates generation of Foxp3+ Treg. (A–B) Real-time RT-PCR analysis was conducted to detect expression levels of TGF-β1 (A) and TGF-β2 (B) from cultured DC with the following treatments: no LPS (DC only), incubation with apoptotic DC with no LPS (DC+ApoDC), LPS (DC+LPS), incubation with apoptotic DC followed by culturing in the presence of LPS (DC+ApoDC+LPS) or incubation with necrotic DC followed by culturing in the presence of LPS (DC +NecDC +LPS). (C) Concentration of total TGF-β1 and active TGF-β1 released into medium by viable DC (DC only), necrotic DC (NecDC), viable DC incubated with necrotic DC (DC + NecDC), apoptotic splenocytes (ApoSplen), viable DC incubated with apoptotic splenocytes (DC + ApoSplen), apoptotic DC (ApoDC), or viable DC incubated with apoptotic DC (DC + ApoDC). (D) Concentration of total TGF-β1 and active TGF-β1 released into the media by: viable DC cultured in the presence of LPS (DC + LPS), viable DC incubated with necrotic DC and then cultured in the presence of LPS (DC + NecDC + LPS) or viable DC incubated with apoptotic DC and then cultured in the presence of LPS (DC + ApoDC + LPS). (E) Naïve CD4+CD25− T cells, isolated from spleens of OT-II mice, were cultured with the following: live DC pulsed with OVA (DC), live DC incubated with necrotic DC and then pulsed with OVA (DC + NecDC), or live DC incubated with apoptotic DC and then pulsed with OVA (DC + ApoDC). Furthermore, TGF-β neutralizing antibody or a control antibody was added to the culture. Five days later, T cells were analyzed for Foxp3 expression. Data show mean ± SD, obtained and pooled from 4 independent experiments, n=2–3 for each experiment. *p<0.05, DC+ApoDC+LPS vs. all other groups for B, DC+ApoDC vs. all other groups for total TGF-β1 or active TGF-β1 levels for C, DC+ApoDC+LPS vs. all other groups for total TGF-β1 or active TGF-β1 levels for D, DC + ApoDC with control antibody versus DC + ApoDC with neutralizing TGF-β antibody for E.
Since, cytokines are also regulated at translational level; we also looked at the protein levels of total as well as active TGF-β1 by ELISA. Results show that upon uptake of apoptotic DC, there was a significant increase in secretion of total as well active TGF-β1 by viable DC (Fig. 7C and D). However, this was not observed upon uptake of necrotic DC or apoptotic splenocytes by viable DC. In addition, viable immature DC upon incubation with apoptotic DC followed by LPS exposure, also secreted significantly higher levels of both total as well as active TGF-β1 compared to viable immature DC treated with LPS or viable immature DC incubated with necrotic DC and then treated with LPS. Collectively, these findings clearly show that only upon uptake of apoptotic DC, viable DC secrete increased levels of TGF-β1, which is regulated at the protein level. In order to confirm that it was specifically the release of TGF-β upon uptake of apoptotic DC by live DC, which was mediating induction of Foxp3+ Treg, we repeated Treg differentiation experiments in the presence of TGF-β neutralizing antibody (Fig. 7E). Our results indicate that addition of TGF-β neutralizing antibody to the culture was able to reduce the proportion of Foxp3+ Treg from 23% to 3%, indicating that it is in fact the release of TGF-β upon uptake of apoptotic DC by live DC, which mediates differentiation of naïve T cells into Foxp3+ Treg.
The release of TGF-β1 by live DC upon apoptotic DC uptake was regulated at the translational level, since no upregulation of TGF-β1 mRNA was observed. In order to investigate the underlying mechanism, we looked at the role of the mammalian target of rapamycin (mTOR). mTOR, a serine/threonine protein kinase, is a regulator of translation and its major substrates include p70S60K serine/threonine kinase and 4E Binding protein (4EBP-1). Live DC were co-cultured with apoptotic DC in presence of rapamycin, a known inhibitor of mTOR pathway. Next, we looked at the levels of total and active TGF-β1 released in the media (Fig. 8A). Our findings indicate that pre-treatment with rapamycin resulted in significant reduction of both total as well as active TGF-β1 released in the media, indicating a role of mTOR in the observed TGF-β1 release upon uptake of apoptotic DC by viable DC. Furthermore, TGF-β1 secretion in response to LPS stimulation of viable DC that had taken apoptotic DC, was also suppressed in presence of rapamycin (Fig. 8B).
Figure 8.
mTOR pathway is involved in induction of TGF-β1secretion upon uptake of apoptotic DC by viable DC. (A) Total and active TGF-β1 levels released in the media upon uptake of apoptotic DC by live DC in the absence/presence of rapamycin. (B) Total and active TGF-β1 levels released in the media in response to LPS stimulation upon uptake of apoptotic DC by live DC in the presence/absence of rapamycin. Data show mean ± SD, representative of 3 independent experiments. *p<0.05, rapamycin treated groups vs. untreated groups.
Discussion
Taken together, our results show that the impact of dying DC on the immune system is dependent on the manner in which DC die. If DC undergo apoptosis and viable DC take them up, then viable DC transform into tolerogenic DC. These tolerogenic DC are resistant to stimuli-induced maturation, secrete TGF-β1, which is dependent on mTOR pathway and induce generation of Foxp3+ Tregs. Surprisingly, our findings show that necrotic DC, irrespective of their maturation status, are not immuno-stimulatory, which may be due to the paucity of presence of certain immunosuppressive factors in primary DC, which renders them non-immunogenic even after the cellular contents are released into the extracellular milieu. However, such factors still need to be identified.
Studies have shown that DC can take up antigen from dying cells and cross-present the antigenic material onto both MHC I as well as MHC II [20, 21]. However, these studies relied on the use of mature DC to phagocytose apoptotic cells. We can speculate that perhaps in a physiological setting, if the causative agent of DC apoptosis is an infection, then it is usually the semi-mature or mature viable DC in close proximity that take up apoptotic DC. Thereby, these viable DC can cross-present the antigen and then prime a T cell response rather than induction of tolerance, as seen in our study.
Previous studies have indicated that phosphatidylserine, an anionic aminophospholipid, which is exposed to cell surface as cells undergo apoptosis, plays an important role in the recognition and clearance of apoptotic cells by macrophages. Studies have also shown that this interaction with phosphatidylserine results in suppression of macrophage activation and induction of TGF-β1 gene expression [8, 22] [23]. It is tempting to argue that conversion of viable immature DC to tolerogenic DC with a potential to induce Treg via secretion of TGF-β1, is largely phosphatidylserine dependent. However, in our study, when viable immature DC were exposed to apoptotic splenocytes, no increase in TGF-β1 secretion was observed and previous studies have also indicated that exposure of murine DC to apoptotic cells or phosphatidylserine does not induce TGF-β1 secretion [24–26]. Therefore, it is likely that the ability to secrete TGF-β1 and to induce Foxp3+ Treg, may be dependent on the uptake of apoptotic DC by viable DC, which has not been described previously, and could be independent of phosphatidylserine. It is feasible that as DC undergo apoptosis, there is exposure of phosphatidylserine, which may play a passive role in suppression of DC by suppressing the ability of DC to undergo maturation without any induction of Foxp3+ Treg. We propose that uptake of apoptotic DC in particular, triggers signalling through a previously unidentified receptor in viable DC that induces TGF-β1 secretion.
Our findings identify that the release of TGF-β1 upon uptake of apoptotic DC by viable DC is regulated at translational level via mTOR pathway. Mammalian target of rapamycin (mTOR), a serine/threonine protein kinase is a regulator of translation and its major substrates include p70S60K serine/threonine kinase and 4E Binding protein (4EBP-1). mTOR phosphorylates 4EBP-1 which results in release of protein translation initiation factor eIF4E. eIF4E plays a role in enhancing rates of translation of capped mRNA which also includes TGF-β1. mTOR is likely regulated upstream by PI3/Akt pathway and Rho A has previously been shown to induce PI3 pathway to prevent myoblast death[27]. Therefore it is likely that RhoA induces PI3K which phosphorylates mTOR resulting in release of eIF4E, which further results in increased translation of TGF-β1 mRNA. Some studies have indicated that another mechanism whereby DC can acquire tolerogenic potential is through induction of indoleamine-2,3-deoxygenase (IDO) [28, 29]. Our results show no upregulation of IDO upon uptake of apoptotic DC by viable DC, indicating that induction of IDO is likely not the underlying mechanism for tolerance induction (data not shown).
The hallmarks of sepsis include impaired immune function along with immunosuppression [30]. Concominantly, there is is substantial depletion of DC along with increased levels of circulating Treg [31, 32] [33]. However, the mechanism of how DC apoptosis can contribute to immunosuppression in sepsis is unclear. Our findings suggest that perhaps enhanced DC apoptosis in sepsis may result in their uptake by viable DC, resulting in immunosuppression and Treg induction/expansion. We need to be cautious in interpreting our findings because our data indicates that several fold higher amounts of apoptotic DC are required than live DC for tolerance induction. However, in certain pathologies such as sepsis, there could be local environments within lymphoid organs where apoptotic DC could potentially outnumber live DC.
Systemic autoimmune diseases can be modeled in transgenic mice harboring defects in DC apoptosis [10] but not in mice with apoptosis defects in T cells and B cells [11–13]. Our study shows that in addition to the dogma of DC apoptosis as a mechanism to eliminate activated DC to prevent hyper-activation of the immune response, DC apoptosis also plays an active role in induction and maintenance of tolerance through induction of Treg, whereby defects in DC apoptosis may trigger autoimmunity.
High levels of spontaneous DC apoptosis have also been observed in breast cancer patients, with its significance being unclear [15, 16]. Our study indicates that DC apoptosis in cancer patients may play a role in suppressing immune responses against the tumor by inducing immunosuppression and tolerance. Therefore, prevention of DC apoptosis may enhance the therapeutic effects of chemotherapy in tumor eradication [15, 16]..
Our findings may also represent a therapeutic approach in prevention of unwanted immune responses in autoimmune diseases and transplantation along with inhibition of DC apoptosis to assist in tumor eradication.
Materials and Methods
Mice, antibodies and other reagents
C57BL/6 mice were purchased from Charles River Laboratories (St. Constant, QC) and maintained as per guidelines of SickKids animal facilities. All the animal studies were reviewed and approved by the SickKids Institutional Committee for humane use of laboratory animals. OT-II mice were purchases from Jackson Laboratories (Bar Harbor, ME). The following antibodies were purchased from eBioscience (San Diego, CA): CD11c PE, CD86 PE, CD80 PE, MHC II PE, IL10 Alexa647, IL12 APC, IL 17 PE, Foxp3 PE along with neutralizing IL 4 and IFN γ antibody and the following from BD Biosciences (Mississauga, ON): CD11c-FITC, CD4-FITC, CD3-PE. Anti-TGF-β neutralizing antibody (MAB1835) was obtained from R&D Systems (Minneapolis, MN). Isotype control IgGs were obtained from eBioscience and/or Serotec (Raleigh, NC). CFSE was obtained from Molecular Probes (Burlington, ON); BrdU, Ovalbumin, Cytochalasin D, Rapamycin and PI were obtained from Sigma-Aldrich (Oakville, ON). GM-CSF was obtained from R&D Systems (Minneapolis, MN). IL-6 and TGF-β were obtained from Peprotech (Rocky Hill, NJ).
Generation of bone marrow derived dendritic cells
Bone marrow cells were isolated from tibia and femurs of adult mice and cultured in the presence of GM-CSF for 7 days as described previously [34].
CFSE labelling of DC
DC were harvested and stained with 1 μM CFSE as described previously [35].
Isolation of naïve CD4+CD25− T cells
Naïve CD4+CD25− CD62L+ T cells were isolated from spleens of mice using CD4+ CD62L+ naïve T-cell isolation kit in conjunction with MACS columns from Miltenyi Biotec Inc. (Auburn, CA), following manufacturer’s instructions.
Induction of dendritic cell apoptosis and necrosis
Dendritic cells were cultured on a 6-well dish and irradiated for 2 minutes with a UV transilluminator, with a peak intensity of 9000 mW/cm2 at the filter surface and a peak emission of 313 nm. Induction of apoptosis was confirmed using apoptosis, necrosis and healthy cell quantification kit (Biotium, Hayward, CA), following manufacturer’s instructions. Necrosis was induced by pelleting cells followed by 3 cycles of freeze and thaw. Similar protocol was used for induction of splenocyte apoptosis, which were isolated from spleens of C57BL/6 mice as described previously [34].
Live DC and apoptotic DC/splenocytes or necrotic DC co-culture experiments
Bone marrow derived immature live DC (100,000 cells/well) were co-cultured with apoptotic/necrotic DC or apoptotic splenocytes (1,000,000 cells/well). In some experiments, Cytochalasin D (0.8 μg/ml) was added to inhibit phagocytosis. In order to inhibit mTOR signalling pathway, rapamycin (100nm) was added to the co-culture of apoptotic DC with viable DC. 24 hours later, cells were exposed to 1μg/ml LPS and FACS analysis was performed.
In vitro generation of Treg
Live DC (100,000 /well) were incubated with apoptotic / necrotic DC or apoptotic splenocytes (1,000,000 cells/well) at a ratio of 1:10 and then pulsed with OVA, followed by co-culture with naïve CD4+ T cells (250,000/well) from OT-II mice. Five days later, CD4+ T cells were analyzed for foxp3 expression via FACS. In some experiments, neutralizing TGF-β antibody was added (50μg/ml). In transwell experiments, DC were added to the top chamber and naïve CD4+ T cells from C57BL/6 mice were placed in the lower chamber and stimulated with plate bound CD3 and soluble CD28 antibodies.
In vitro suppression assay
OVA-pulsed (0.5 mg/ml) DC were used as stimulators and naïve OT-II CD4+ T cells were used as responders. The stimulators (2.5 × 105 cells/well) and responder cells (2.5 × 104 cells/well) were cultured in 96 well round bottom plates at a ratio of 10:1 and suppressors (CD25+) isolated from co-culture of OT-II naïve T cells and OVA-pulsed viable DC that had taken up apoptotic DC were added. Proliferation was assessed at day 4 of co-culture using BrdU cell proliferation assay following manufacturer’s instructions (Roche, QC).
In vitro generation of Th17 cells
Naïve CD4+CD25− T cells were cultured for 4 days in presence of LPS treated live DC, LPS treated live DC incubated with necrotic DC or LPS treated live DC incubated with apoptotic DC, and were activated with plate bound anti-CD3 and soluble anti-CD28 antibodies in presence of 5 ng/ml IL-6, 2.5 ng/ml TGF-β, 10 μg/ml anti-IL-4 and 10 μg/ml anti-IFN-γ.
Cytokine assays
We quantified levels of total/active TGF-β1 in culture supernatants by ELISA using commercial kit following manufacturer’s instructions (TGF-β1 kit, R&D Systems, Minneapolis, MN). However, for measurements of TGF-β, cells were cultured in X-VIVO 20 serum-free medium (Cambrex).
Real-time PCR
TaqMan real-time RT-PCR was carried out as described previously using primer sequences listed in table 1 [36].
Table 1.
List of primers used for real-time RT-PCR analysis in this study
| NAME | SEQUENCE | REFERENCE |
|---|---|---|
| TGF-beta1 | forward: 5′-CAACAATTCCTGGCGTTACCTTGG-3′ | Am J Physiol Renal Physiol 295: F118–F127, 2008 |
| reverse: 5′-GAAAGCCCTGTATTCCGTCTCCTT-3′ | ||
| TGF-beta2 | forward: 5′-CTTAACATCTCCCACCCAGC-3′ | J. Immunol., 179: 6325–6335, 2007 |
| reverse: 5′-TCACCACTGGCATATGTAGA-3′ | ||
| IL-6 | forward: 5′-CCCAACAGACCTGTCTATACC-3′ | This study |
| reverse: 5′-CTGCAAGTGCATCATCGTTGTTC-3′ | ||
| IL-1beta | forward: 5′-GACAGTGATGAGAATGACCTG-3′ | This study |
| reverse: 5′-CCACAGCCACAATGAGTGATA-3′ | ||
| IL-12p35 | forward: 5′-CACCCTTGCCCTCCTAAACC-3′ | JEM, vol201, No. 9, 1435–1446, 2005 |
| reverse: 5′-CACCTGGCAGGTCCAGAGA-3′ | ||
| IL-12p40 | forward: 5′-ACAGCACCAGCTTCTTCATCAG-3′ | JEM, vol201, No. 9, 1435–1446, 2005 |
| reverse: 5′-TCTTCAAAGGCTTCATCTGCAA-3′ | ||
| TNF-alpha | forward: 5′-CATCTTCTCAAAATTCGAGTGACAA-3′ | JEM, vol204, No. 6, 1487–1501 |
| reverse: 5′-TGGGAGTAGACAAGGTACAACCC-3′ |
Statistical analysis
Statistical analyses were performed using Student t-test to compare two groups and ANOVA to compare multiple groups (SPSS 16.0). Significance was set at P<0.05.
Acknowledgments
This work was supported in part by Operating Grants from the Canadian Institutes of Health Research, the Canadian Cystic Fibrosis Foundation, and the Foundation Fighting Blindness-Canada to J.H. J.H. was a CCFF Scholar and recipient of the CCFF Zellers Senior Scientist Award, and holds a Premier’s Research Excellence Award of Ontario, Canada. R.K. is a recipient of CCFF doctoral award. We thank Dr. Michel C. Nussenzweig (Rockefeller University) for reading the manuscript.
Abbreviations
- EH
ethidium homodimer
- PI
Propidium iodide
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 2.Huang FP, Platt N, Wykes M, Major JR, Powell TJ, Jenkins CD, MacPherson GG. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J Exp Med. 2000;191:435–444. doi: 10.1084/jem.191.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peng Y, Martin DA, Kenkel J, Zhang K, Ogden CA, Elkon KB. Innate and adaptive immune response to apoptotic cells. J Autoimmun. 2007;29:303–309. doi: 10.1016/j.jaut.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winau F, Weber S, Sad S, de Diego J, Hoops SL, Breiden B, Sandhoff K, Brinkmann V, Kaufmann SH, Schaible UE. Apoptotic vesicles crossprime CD8 T cells and protect against tuberculosis. Immunity. 2006;24:105–117. doi: 10.1016/j.immuni.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Feng H, Zeng Y, Graner MW, Katsanis E. Stressed apoptotic tumor cells stimulate dendritic cells and induce specific cytotoxic T cells. Blood. 2002;100:4108–4115. doi: 10.1182/blood-2002-05-1389. [DOI] [PubMed] [Google Scholar]
- 7.Johansson U, Walther-Jallow L, Smed-Sorensen A, Spetz AL. Triggering of dendritic cell responses after exposure to activated, but not resting, apoptotic PBMCs. J Immunol. 2007;179:1711–1720. doi: 10.4049/jimmunol.179.3.1711. [DOI] [PubMed] [Google Scholar]
- 8.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen W, Frank ME, Jin W, Wahl SM. TGF-beta released by apoptotic T cells contributes to an immunosuppressive milieu. Immunity. 2001;14:715–725. doi: 10.1016/s1074-7613(01)00147-9. [DOI] [PubMed] [Google Scholar]
- 10.Chen M, Wang YH, Wang Y, Huang L, Sandoval H, Liu YJ, Wang J. Dendritic cell apoptosis in the maintenance of immune tolerance. Science. 2006;311:1160–1164. doi: 10.1126/science.1122545. [DOI] [PubMed] [Google Scholar]
- 11.Doerfler P, Forbush KA, Perlmutter RM. Caspase enzyme activity is not essential for apoptosis during thymocyte development. J Immunol. 2000;164:4071–4079. doi: 10.4049/jimmunol.164.8.4071. [DOI] [PubMed] [Google Scholar]
- 12.Walsh CM, Wen BG, Chinnaiyan AM, O’Rourke K, Dixit VM, Hedrick SM. A role for FADD in T cell activation and development. Immunity. 1998;8:439–449. doi: 10.1016/s1074-7613(00)80549-x. [DOI] [PubMed] [Google Scholar]
- 13.Newton K, Harris AW, Bath ML, Smith KG, Strasser A. A dominant interfering mutant of FADD/MORT1 enhances deletion of autoreactive thymocytes and inhibits proliferation of mature T lymphocytes. Embo J. 1998;17:706–718. doi: 10.1093/emboj/17.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito M, Minamiya Y, Kawai H, Saito S, Saito H, Nakagawa T, Imai K, Hirokawa M, Ogawa J. Tumor-derived TGFbeta-1 induces dendritic cell apoptosis in the sentinel lymph node. J Immunol. 2006;176:5637–5643. doi: 10.4049/jimmunol.176.9.5637. [DOI] [PubMed] [Google Scholar]
- 15.Pinzon-Charry A, Maxwell T, McGuckin MA, Schmidt C, Furnival C, Lopez JA. Spontaneous apoptosis of blood dendritic cells in patients with breast cancer. Breast Cancer Res. 2006;8:R5. doi: 10.1186/bcr1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinzon-Charry A, Ho CS, Maxwell T, McGuckin MA, Schmidt C, Furnival C, Pyke CM, Lopez JA. Numerical and functional defects of blood dendritic cells in early- and late-stage breast cancer. Br J Cancer. 2007;97:1251–1259. doi: 10.1038/sj.bjc.6604018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeni F, Freeman B, Natanson C. Anti-inflammatory therapies to treat sepsis and septic shock: a reassessment. Crit Care Med. 1997;25:1095–1100. doi: 10.1097/00003246-199707000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Park Y, Lee SW, Sung YC. Cutting Edge: CpG DNA inhibits dendritic cell apoptosis by up-regulating cellular inhibitor of apoptosis proteins through the phosphatidylinositide-3′-OH kinase pathway. J Immunol. 2002;168:5–8. doi: 10.4049/jimmunol.168.1.5. [DOI] [PubMed] [Google Scholar]
- 19.Chen M, Huang L, Wang J. Deficiency of Bim in dendritic cells contributes to overactivation of lymphocytes and autoimmunity. Blood. 2007;109:4360–4367. doi: 10.1182/blood-2006-11-056424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inaba K, Turley S, Yamaide F, Iyoda T, Mahnke K, Inaba M, Pack M, Subklewe M, Sauter B, Sheff D, Albert M, Bhardwaj N, Mellman I, Steinman RM. Efficient presentation of phagocytosed cellular fragments on the major histocompatibility complex class II products of dendritic cells. J Exp Med. 1998;188:2163–2173. doi: 10.1084/jem.188.11.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 22.De SR, Ajmone-Cat MA, Nicolini A, Minghetti L. Expression of phosphatidylserine receptor and down-regulation of pro-inflammatory molecule production by its natural ligand in rat microglial cultures. J Neuropathol Exp Neurol. 2002;61:237–244. doi: 10.1093/jnen/61.3.237. [DOI] [PubMed] [Google Scholar]
- 23.Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen X, Doffek K, Sugg SL, Shilyansky J. Phosphatidylserine regulates the maturation of human dendritic cells. J Immunol. 2004;173:2985–2994. doi: 10.4049/jimmunol.173.5.2985. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi M, Kobayashi Y. Cytokine production in association with phagocytosis of apoptotic cells by immature dendritic cells. Cell Immunol. 2003;226:105–115. doi: 10.1016/j.cellimm.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Morelli AE, Larregina AT, Shufesky WJ, Zahorchak AF, Logar AJ, Papworth GD, Wang Z, Watkins SC, Falo LD, Jr, Thomson AW. Internalization of circulating apoptotic cells by splenic marginal zone dendritic cells: dependence on complement receptors and effect on cytokine production. Blood. 2003;101:611–620. doi: 10.1182/blood-2002-06-1769. [DOI] [PubMed] [Google Scholar]
- 27.Reuveny M, Heller H, Bengal E. RhoA controls myoblast survival by inducing the phosphatidylinositol 3-kinase-Akt signaling pathway. FEBS Lett. 2004;569:129–134. doi: 10.1016/j.febslet.2004.05.035. [DOI] [PubMed] [Google Scholar]
- 28.Williams CA, Harry RA, McLeod JD. Apoptotic cells induce dendritic cell-mediated suppression via interferon-gamma-induced IDO. Immunology. 2008;124:89–101. doi: 10.1111/j.1365-2567.2007.02743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orabona C, Pallotta MT, Volpi C, Fallarino F, Vacca C, Bianchi R, Belladonna ML, Fioretti MC, Grohmann U, Puccetti P. SOCS3 drives proteasomal degradation of indoleamine 2,3-dioxygenase (IDO) and antagonizes IDO-dependent tolerogenesis. Proc Natl Acad Sci U S A. 2008;105:20828–20833. doi: 10.1073/pnas.0810278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 31.Hotchkiss RS, Tinsley KW, Swanson PE, Grayson MH, Osborne DF, Wagner TH, Cobb JP, Coopersmith C, Karl IE. Depletion of dendritic cells, but not macrophages, in patients with sepsis. J Immunol. 2002;168:2493–2500. doi: 10.4049/jimmunol.168.5.2493. [DOI] [PubMed] [Google Scholar]
- 32.Tinsley KW, Grayson MH, Swanson PE, Drewry AM, Chang KC, Karl IE, Hotchkiss RS. Sepsis induces apoptosis and profound depletion of splenic interdigitating and follicular dendritic cells. J Immunol. 2003;171:909–914. doi: 10.4049/jimmunol.171.2.909. [DOI] [PubMed] [Google Scholar]
- 33.Monneret G, Debard AL, Venet F, Bohe J, Hequet O, Bienvenu J, Lepape A. Marked elevation of human circulating CD4+CD25+ regulatory T cells in sepsis-induced immunoparalysis. Crit Care Med. 2003;31 :2068–2071. doi: 10.1097/01.CCM.0000069345.78884.0F. [DOI] [PubMed] [Google Scholar]
- 34.Kushwah R, Cao H, Hu J. Characterization of pulmonary T cell response to helper-dependent adenoviral vectors following intranasal delivery. J Immunol. 2008;180:4098–4108. doi: 10.4049/jimmunol.180.6.4098. [DOI] [PubMed] [Google Scholar]
- 35.Angelov GS, Tomkowiak M, Marcais A, Leverrier Y, Marvel J. Flt3 ligand-generated murine plasmacytoid and conventional dendritic cells differ in their capacity to prime naive CD8 T cells and to generate memory cells in vivo. J Immunol. 2005;175:189–195. doi: 10.4049/jimmunol.175.1.189. [DOI] [PubMed] [Google Scholar]
- 36.Wu J, Duan R, Cao H, Field D, Newnham CM, Koehler DR, Zamel N, Pritchard MA, Hertzog P, Post M, Tanswell AK, Hu J. Regulation of epithelium-specific Ets-like factors ESE-1 and ESE-3 in airway epithelial cells: potential roles in airway inflammation. Cell Res. 2008;18:649–663. doi: 10.1038/cr.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]